Abstract
Background
The evaluation of the natural history prevalence of adverse local tissue reactions (ALTRs) using MRI has focused only on metal-on-metal (MoM) bearing surfaces without comparison to nonMoM bearing surfaces.
Questions/purposes
To determine (1) the longitudinal changes and differences in blood metal ion levels in patients with hip resurfacing arthroplasty (HRA), ceramic-on-ceramic (CoC) THA, and metal-on-polyethylene (MoP) THA compared with those undergoing ceramic-on-polyethylene (CoP) THA; (2) how the longitudinal change of synovial reaction classification in patients with HRA, CoC THA, and MoP THA compares with those undergoing CoP THA, and whether there is an association between the presence of an ALTR or metallosis on MRI with corresponding patient-reported outcomes, or the presence of capsular dehiscence; and (3) differences in blood metal ion levels between patients undergoing HRA with an ALTR or metallosis on MRI and those with HRA without these conditions.
Methods
Between March 2014 and February 2019, 22,723 patients underwent primary HRA and THA at one center. Patients received an HRA based on their desired athletic level after surgery and the presence of normal acetabular and proximal femoral bone morphology without osteopenia or osteoporosis. Two percent (342 of 22,723) of patients were contacted to participate, and 71% (243 of 342 hips in 206 patients) were enrolled for analysis at baseline. The patients underwent arthroplasty for degenerative joint disease, and 25 patients withdrew over the course of the study. We included patients who were more than 1 year postarthroplasty. All participants had an MRI examination and blood serum ion testing and completed a Hip Disability and Osteoarthritis Outcome Score survey annually for four years (baseline, year 1, year 2, year 3). Morphologic and susceptibility-reduced MR images were evaluated by a single radiologist not involved in the care of patients for the presence and classification of synovitis (Gwet AC1: 0.65 to 0.97), synovial thickness, and volume (coefficient of repeatability: 1.8 cm3). Linear mixed-effects models were used to compare the mean synovial thickness, synovial volume, and Hip Disability and Osteoarthritis Outcome Score subscales between bearing surfaces at each timepoint and within each bearing surface over time. Marginal Cox proportional hazards models were used to compare the time to and the risk of developing ALTR only, metallosis only, and ALTR or metallosis between bearing surfaces. All models were adjusted for age, sex, BMI, and length of implantation based on known confounders for hip arthroplasty. Adjustment for multiple comparisons was performed using the Dunnett-Hsu method.
Results
Patients with unilateral HRA had higher cobalt and chromium serum ion levels (baseline: 1.8 ± 0.8 ppb, year 1: 2.0 ± 1.5 ppb, year 2: 2.1 ± 1.2 ppb, year 3: 1.6 ± 0.7 ppb) than those with unilateral CoP bearings (baseline: 0.0 ± 0.1 ppb, year 1: 0.1 ± 0.3 ppb, year 2: 0.0 ± 0.2 ppb, year 3: 0.0 ± 0.0 ppb) at all timepoints (p < 0.001 for each time point). More patients who received an HRA developed ALTR or metallosis on MRI than did patients with CoP bearings (hazard ratio 4.8 [95% confidence interval 1.2 to 18.4]; p = 0.02). There was no association between the longitudinal change of synovial reaction to ALTR or metallosis on MRI with patient-reported outcomes. In addition, there was no association between the presence of dehiscence at baseline and the subsequent development of ALTR or metallosis, as seen on MRI. There were elevated cobalt (4.7 ± 3.5 ppb) and chromium (4.7 ± 2.6 ppb) serum levels in patients with unilateral HRA who had an ALTR or metallosis present on MRI at year 1 compared with patients without an ALTR or metallosis on MRI (cobalt: 1.8 ± 1.0 ppb, mean difference 4.7 ppb [95% CI 3.3 to 6.0]; p < 0.001; chromium: 2.3 ± 0.5 ppb, mean difference 3.6 ppb [95% CI 2.2 to 5.0]; p < 0.001) as well as for chromium at year 3 (3.9 ± 2.4 ppb versus 2.2 ± 1.1 ppb, mean difference 1.3 ppb [95% CI 0.3 to 2.4]; p 0.01).
Conclusion
We found a higher proportion of ALTR or metallosis on MRI in patients with HRA compared with patients with CoP, even when patient self-assessed symptomatology of those with an ALTR or metallosis on MRI was not different than the absence of these features. MRI detected ALTRs in high-function patients, emphasizing that an annual clinical assessment dependent on survey or blood ion testing alone may not detect soft tissue complications. The results of this study are in line with prior consensus recommendations of using MRI as part of a routine follow-up protocol for this patient population.
Level of Evidence
Level III, therapeutic study.
Introduction
Interest in the development of adverse local tissue reactions (ALTRs) near THA implants has been revived recently because of recalled metal-on-metal (MoM) implants, hip resurfacing arthroplasty (HRA), and dual-modular metal-on-polyethylene (MoP) implants [6, 15, 26]. Generally, ALTRs are associated with elevated metal ion levels [4, 26], but metal ion testing alone has shown poor sensitivity in predicting failure of MoM bearing surfaces. Indeed, studies have found no association between elevated metal ion levels and the prevalence of ALTRs [3, 32]. Similarly, others have found no correlation between metal ion levels and the magnitude of local tissue damage [14] commonly seen in patients diagnosed with ALTR [36]. Of clinical concern is the presence of ALTRs in asymptomatic individuals [12, 16, 20, 26, 32]. These studies suggest that although ALTRs occur in patients with metal bearing surfaces, using metal ion levels alone may be an insufficient clinical method to detect or monitor the presence of an ALTR, especially in patients for whom an ALTR is clinically silent [3, 32]. Early detection of ALTRs is important because patients undergoing complex revision surgery tend to have longer operative times, greater operative costs [2], greater rehabilitation needs [48], and worse clinical outcomes [13]. It would be beneficial to evaluate the natural history of ALTR development in asymptomatic people with MoM implants using methods that are more effective than metal ion testing because negative metal ion test results have been found in asymptomatic individuals even at 5 to 7 years of follow-up [20].
THA implants have been evaluated longitudinally using MRI to determine the natural history of ALTRs, but previous studies have limited their evaluation to the MoM bearing surfaces of THAs [5, 11, 17], HRAs [46], or both [24, 27, 42]. These studies did not directly compare these surfaces with MoP, ceramic-on-ceramic (CoC), or ceramic-on-polyethylene (CoP) constructs. A longitudinal evaluation of nonMoM bearing surfaces is important because ALTRs occur in these more commonly used constructs [7, 10, 44, 49], either with dissimilar [9, 29, 45] or similar head-neck alloys [10, 45]. Enrollment in these previous studies may have been relatively limited, with enrollment of fewer than 40 total hip devices [17, 24], based on the availability of previously acquired data [46] or enrollment that was focused on symptomatic hips [5]. In addition, few studies have used a validated patient-reported outcome measure to report longitudinal changes of symptomatology of enrolled patients [5, 27, 46].
The purpose of this prospective, longitudinal study was to determine: (1) the longitudinal changes and differences in blood metal ion levels in patients with HRA, CoC THA, and MoP THA compared with those undergoing CoP THA; (2) how the longitudinal change of synovial reaction classification in patients with HRA, CoC THA, and MoP THA compares with those undergoing CoP THA, and whether there is an association between the presence of an ALTR or metallosis on MRI with corresponding patient-reported outcomes, or the presence of capsular dehiscence; and (3) differences in blood metal ion levels between patients undergoing HRA with an ALTR or metallosis on MRI and those with HRA without these conditions.
Patients and Methods
Study Design
We used a cohort study to evaluate the development of ALTR or metallosis on clinical morphologic MRI at four study visits, each with 1-year intervals.
Setting
Between March 2014 and February 2019, patients were recruited at one center (Hospital for Special Surgery) for a prospective study. In this cohort, we acquired morphologic MRI, blood serum ion levels, and the Hip Disability and Osteoarthritis Outcome Score (HOOS) survey [38] at four time points (baseline, year 1, year 2, and year 3), each with 1-year intervals.
Participants
In this cohort study, we approached patients from 25 different participating surgeons. A total of 2% (342) of the available 22,723 patients during the enrollment period were approached for enrollment (Fig. 1). Of these, 71% (243 of 342 hips) were enrolled. Patients chose not to participate due to: claustrophobia (14% [14 of 99]), having a revision and not primary THA (8% [8 of 99]), concern about safety (2% [2 of 99]), being too ill (2% [2 of 99]), or not being interested (74% [73 of 99]). We included patients who underwent primary THA and excluded those undergoing THA with less than 1 year of implantation (Table 1). Patients with unilateral and bilateral HRA (23 unilateral and 21 bilateral) or THA (104 unilateral and 95 bilateral) were enrolled. Patients were indicated to have HRA if their desired athletic level after surgery included impact activities such as running or jumping or if their desired activities would put a traditional hip replacement at risk for dislocation. Patients indicated for HRA were required to have normal acetabular and proximal femoral bone morphology without osteopenia or osteoporosis present.
Fig. 1.
This STROBE flow diagram demonstrates patient recruitment during the study period.
Table 1.
Demographics by implant bearing surface
| MoM | HRA | Modular MoP | MoP | CoP | CoC | CoM | ||
| Baseline | Number | 4 | 44 | 7 | 31 | 138 | 18 | 1 |
| Age in years | 68.8 ± 6.7 | 57.8 ± 7.3 | 65.0 ± 11.2 | 69.4 ± 8.0 | 64.8 ± 9.1 | 63.8 ± 12.6 | 60.0 ± 0.0 | |
| Males/females | 3/1 | 36/8 | 5/2 | 7/24 | 61/77 | 11/7 | 1/0 | |
| BMI, kg/m2 | 24.3 ± 4.7 | 25.6 ± 3.7 | 26.6 ± 5.1 | 26.2 ± 5.3 | 27.1 ± 5.4 | 25.7 ± 3.4 | 34.1 ± 0.0 | |
| LOI in years | 4.9 (4.6, 6.1) | 3.1 (1.6, 5.4) | 3.8 (1.1, 4.4) | 4.0 (2.2, 7.7) | 3.0 (1.3, 5.2) | 8.7 (8.1, 11.0) | 1.1 (1.1, 1.1) | |
| Year 1 | Number | 4 | 43 | 5 | 29 | 67 | 14 | 0 |
| Age in years | 68.8 ± 6.7 | 57.6 ± 7.2 | 64.0 ± 9.6 | 69.9 ± 7.7 | 66.2 ± 9.0 | 62.4 ± 13.3 | 0.0 ± 0.0 | |
| Males/females | 3/1 | 35/8 | 4/1 | 6/23 | 31/36 | 9/5 | 0/0 | |
| BMI, kg/m2 | 24.3 ± 4.7 | 25.6 ± 3.7 | 27.2 ± 4.4 | 26.1 ± 5.4 | 26.1 ± 5.1 | 26.2 ± 3.6 | 0.0 ± 0.0 | |
| LOI in years | 4.9 (4.6, 6.1) | 3.0 (1.6, 5.7) | 2.6 (1.1, 4.4) | 4.3 (2.7, 7.7) | 3.2 (1.5, 6.1) | 8.7 (8.2, 10.4) | 0.0 (0.0, 0.0) | |
| Year 2 | Number | 4 | 38 | 4 | 24 | 48 | 11 | 0 |
| Age in years | 68.8 ± 6.7 | 57.6 ± 7.6 | 62.8 ± 10.6 | 70.4 ± 7.9 | 65.9 ± 9.1 | 64.2 ± 13.5 | 0.0 ± 0.0 | |
| Males/females | 3/1 | 31/7 | 3/1 | 5/19 | 25/23 | 6/5 | 0/0 | |
| BMI, kg/m2 | 24.3 ± 4.7 | 25.0 ± 3.1 | 27.7 ± 5.0 | 25.9 ± 4.5 | 26.3 ± 5.3 | 25.9 ± 3.7 | 0.0 ± 0.0 | |
| LOI in years | 4.9 (4.6, 6.1) | 3.0 (1.6, 5.7) | 2.7 (1.1, 4.7) | 3.7 (2.7, 7.3) | 3.0 (1.3, 4.8) | 8.7 (8.2, 11.0) | 0.0 (0.0, 0.0) | |
| Year 3 | Number | 3 | 33 | 5 | 20 | 41 | 9 | 0 |
| Age in years | 68.3 ± 8.1 | 58.2 ± 7.0 | 64.0 ± 9.6 | 68.7 ± 7.2 | 65.0 ± 9.3 | 64.3 ± 15.0 | 0.0 ± 0.0 | |
| Males/females | 2/1 | 26/7 | 4/1 | 4/16 | 21/20 | 4/5 | 0/0 | |
| BMI, kg/m2 | 25.6 ± 4.8 | 24.7 ± 3.1 | 27.2 ± 4.4 | 25.7 ± 4.8 | 26.3 ± 5.3 | 25.5 ± 3.5 | 0.0 ± 0.0 | |
| LOI in years | 4.7 (4.4, 7.2) | 3.1 (2.0, 6.3) | 2.6 (1.1, 4.4) | 3.4 (2.4, 7.0) | 2.9 (1.3, 4.6) | 8.7 (8.2, 9.3) | 0.0 (0.0, 0.0) |
Data are presented as mean ± SD; length of implantation (LOI) is shown as the median (Q1, Q3); MoM = metal-on-metal; HRA = hip resurfacing area; MoP = metal-on-polyethylene; CoP = ceramic-on-polyethylene; CoC = ceramic-on-ceramic; CoM = ceramic-on-metal.
A radiograph of each hip at the time of the first MRI examination was evaluated by independent board-certified, musculoskeletal fellowship–trained radiologist (AJB) for bone resorption (osteolysis or a fibrous membrane) without loosening, cortical thickening, heterotopic ossification or eccentric position of the femoral head, or a combination thereof.
At the baseline visit, 202 hips (83% [202 of 243]) had radiographs that did not show abnormalities, while 21 hips (9% [21 of 243]) had heterotopic ossification, nine hips (4% [9 of 243]) had bone resorption without loosening, seven hips (3% [7 of 243]) had cortical thickening attributable to a possible stress reaction or remodeling without a fracture line, two hips (1% [2 of 243]) had an eccentric position of the femoral head consistent with linear wear, one hip (1% [1 of 243]) had bone resorption and heterotopic ossification, and one hip (1% [1 of 243]) had cortical thickening and heterotopic ossification.
Ten participants withdrew from the study after the baseline visit; one patient received a cardiac pacemaker and the remaining were not interested in returning for an examination. Eleven patients withdrew from the study after year 1; two patients had revision surgery and the remaining were not interested in further participation. A 65-year-old woman with an MoP construct underwent revision because of extensive osteolysis from progressive polyethylene wear at 22 years postoperatively, and a 62-year-old woman with HRA underwent revision for progressive femoral osteolysis under the resurfacing femoral head at 9 years postoperatively (Fig. 2). Finally, four patients withdrew from the study after year 2; one had revision surgery and the remaining were not interested in further participation. A 71-year-old man with an MoM THA implant underwent revision at 8 years postoperatively because of progressively elevated blood serum cobalt levels (7.3 ppb at baseline, 16.4 ppb at year 1, and 29.8 ppb at year 2), which the treating surgeon attributed to corrosion at the implant’s head-neck trunnion connection. The patient’s corresponding chromium levels at each timepoint were 5.1 ppb, 6.0 ppb, and 7.1 ppb, respectively.
Fig. 2.
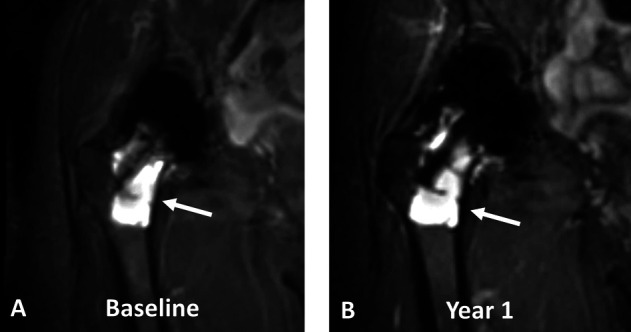
A-B Coronal multiacquisition variable resonance image combination short tau inversion recovery images of a 62-year-old woman with HRA (7.5 years after implantation at baseline) display progressive osteolysis around the femoral stem (arrows) from (A) baseline to (B) year 1. In addition, the synovium had a 33% volumetric increase between the timepoints. The patient underwent revision surgery after imaging at year 1.
Primary Outcome Variable
The primary outcome in the study was the classification of synovial reaction based on the morphologic MRIs at each study visit. Synovial reactions were classified as normal: low capsular signal intensity and thin capsular tissue [41]; ALTR: thickened, hyperintense capsule, with poor demarcation from the surrounding muscle signal and muscle architecture, indicating necrosis [37]; metallosis: low signal intensity deposits in the capsular lining; infection: lamellated synovial lining with pericapsular edema [40]; polymeric: foci of particulate, intermediate signal intensity debris [41]; and mildly abnormal: more fluid than “normal” but without the characteristics of the above-noted classifications. A primary, and secondary as needed, synovial classification was assigned to each hip.
Secondary Outcome Variables
The secondary outcomes in this study were synovial volume measurements from the acquired MRIs, blood metal ions levels, and HOOS scores at each study visit.
Data Sources/Measurement
The annual MRI examinations (interval time [mean ± SD]: 12.4 ± 2.8 months) were performed using clinical 1.5-T scanners (GE Healthcare) with an eight-channel phased-array cardiac coil (GE Healthcare). Three-plane two-dimensional fast-spin echo images and coronal slab-selective multiacquisition variable resonance image combination (MAVRIC-SL) and MAVRIC-SL short tau inversion recovery (STIR) images [21] (Supplementary Table 1; http://links.lww.com/CORR/A603) were evaluated by a single radiologist (HGP) with more than 20 years of experience of imaging near arthroplasty implants and who was not involved in the care of the patients. The images were evaluated for the maximal synovial thickness in the coronal plane at the inferomedial aspect of the head-neck junction (continuous measure), synovial volume (continuous measure), presence of synovial decompression (yes or no), and location of synovial decompression (into the iliopsoas bursa or greater trochanteric bursa), when present. The location of synovial thickness is in line with a concomitant institutional study correlating the synovial lining on MRI of patients undergoing THA who then underwent revision surgery with corresponding histologic evaluation (NCT02255331).
On the same day after the MRI examination, blood was drawn from all patients to evaluate serum cobalt and chromium levels, regardless of implant design (Arup Laboratories). All testing was performed using standard institutional methods.
All patients completed a HOOS survey [38] to assess hip pain, symptoms, activities of daily living, sport and recreation function, and hip-related quality of life on the same date that the MRI and blood work were performed. Patients with bilateral hips completed a separate HOOS survey for each hip.
Bias
The repeatability of the synovial classification was assessed based on 187 hip arthroplasties [22] included in a previous study conducted by the same radiologists participating in this study; they found an intrarater agreement of moderate to almost perfect (Gwet AC1 range 0.59 to 0.99) and an interrater agreement of substantial to almost perfect (Gwet AC1 range 0.65 to 0.97). The repeatability of the synovial volume measurements from 25 THAs in a previous study by the same radiologists found an intraclass correlation coefficient of 0.99 and a coefficient of repeatability of 1.8 cm3 [19].
We attempted to implement the methods published by MacDonald et al. [31], such as the use of a plastic needle rather than a metal needle and use of plastic tubes rather than glass tubes because of the concern that leaching metal content may affect the serum ion levels; however, serum data were obtained as part of standard clinical care at our institution, for which a metal needle and glass tube is used for many of the patients who underwent HRA.
Study Size
The sample size was estimated using the Lachin and Foulkes log-rank method [28]. MoM, HRA, MoP, CoP, CoC, and modular design bearing surfaces were anticipated. Patients were enrolled during an accrual period of 6 years with a follow-up period of 3 years. We assumed that the development of ALTR for different bearing surfaces would be between 10% and 40% over the study period, and that the difference in the ALTR development between a low-risk and a high-risk bearing surface group would be 30%. Taking CoP as the low-risk reference group, we anticipated five comparisons, each with a significance level of 1% after adjustment for multiple comparisons using the Bonferroni method. With these assumptions, we estimated that at least 36 patients per bearing surface would be needed to achieve 80% power at a 1% significance level to detect a difference of 30%, with a conservative 15% loss to follow-up in each group.
Ethical Approval
Ethical approval for this study was obtained from the Hospital for Special Surgery, New York, NY, USA (study number 2015-442-CR5). Written informed consent was obtained from all participants.
Statistical Analysis
Continuous outcomes are presented as means with SDs or medians with first and third quartiles. We used linear mixed-effects models with random subject intercepts and a first-order autoregressive covariance structure to compare the mean synovial volume and HOOS subscale scores for patients with unilateral and bilateral arthroplasty between bearing surfaces at each timepoint and within each bearing surface over time as well as to compare the mean metal ions for patients with unilateral arthroplasty and HOOS subscale scores between the status of developing an ALTR or metallosis at each time point and within each bearing surface over time. Adjustment for multiple comparisons was performed using the Dunnett-Hsu method. We used a Kaplan-Meier estimator to demonstrate the probability of developing ALTR only, metallosis only, and ALTR or metallosis, as seen on MRI, over the four timepoints. Marginal Cox proportional hazards models were used to compare the time to and the rate of developing ALTR only, metallosis only, and ALTR or metallosis between bearing surfaces. HRs were estimated using CoP as the reference group, and a log-rank test was used when the HR could not be estimated (that is, when there were no events in the CoP group). Secondary analyses to evaluate the association between dehiscence and the development of ALTR or metallosis included a log-rank test stratified by dehiscence status at baseline and a marginal Cox proportional hazards model with dehiscence as a time-varying covariate.
All models were adjusted for age, sex, BMI, and length of implantation based on known confounders for hip arthroplasty. All analyses used available data of patients who were enrolled and up to 3 years of follow-up data. Because there were small numbers of MoM, modular MoP, and ceramic-on-metal surfaces, these bearing surfaces were excluded from the analysis. Statistical significance was set at p < 0.05. Analyses were performed using SAS version 9.4 (SAS Institute).
Results
Differences in Blood Metal Ion Levels Over Time
The evaluation of the unilateral implantations revealed elevated cobalt (Table 2) and chromium levels (Table 3) in patients with HRA (baseline: 1.8 ± 0.8 ppb; year 1: 2.0 ± 1.5 ppb; year 2: 2.1 ± 1.2 ppb; year 3: 1.6 ± 0.7 ppb) compared with patients with CoP (baseline: 0.0 ± 0.1 ppb; year 1: 0.1 ± 0.3 ppb; year 2: 0.0 ± 0.2 ppb; year 3: 0.0 ± 0.0 ppb) at all timepoints (mean difference baseline: 1.6 ppb [95% CI 1.0 to 2.1]; p < 0.001; year 1: 1.8 ppb [95% CI 1.2 to 2.5]; p < 0.001; year 2: 2.0 ppb [95% CI 1.3 to 2.7]; p < 0.001; year 3: 1.6 ppb [95% CI 0.9 to 2.3]; p < 0.001). There were also elevated mean cobalt levels in patients with MoP (0.8 ± 1.9 ppb) compared with patients with CoP (0.0 ± 0.1 ppb) at baseline (mean difference 0.9 ppb [95% CI 0.3 to 1.5]; p = 0.002), year 2 (1.0 ppb [95% CI 0.3 to 1.7]; p = 0.003), and year 3 (0.9 ppb [95% CI 0.1 to 1.7]; p = 0.02).
Table 2.
Changes and differences in the serum cobalt levels over time by primary bearing surface for patients with unilateral arthroplasty
| Bearing surface | HRA vs COP at timepoint | |||||||||
| Time point | HRA | MoP | CoP | CoC | Difference in means (95% CI) | p value | ||||
| Number | Cobalt level | Number | Cobalt level | Number | Cobalt level | Number | Cobalt level | |||
| Baseline | 22 | 1.8 ± 0.8 | 16 | 0.8 ± 1.9 | 66 | 0.0 ± 0.1 | 11 | 0.1 ± 0.4 | 1.6 (1.0 to 2.1) | < 0.001 |
| Year 1 | 22 | 2.0 ± 1.5 | 15 | 0.4 ± 1.0 | 28 | 0.1 ± 0.3 | 8 | 0.5 ± 1.0 | 1.8 (1.2 to 2.5) | < 0.001 |
| Year 2 | 20 | 2.1 ± 1.2 | 14 | 0.9 ± 1.4 | 25 | 0.0 ± 0.2 | 7 | 0.0 ± 0.0 | 2.0 (1.3 to 2.7) | < 0.001 |
| Year 3 | 16 | 1.6 ± 0.7 | 11 | 0.8 ± 1.4 | 22 | 0.0 ± 0.0 | 6 | 0.2 ± 0.5 | 1.6 (0.9 to 2.3) | < 0.001 |
| MoP (> 1 year) versus CoP at timepoint | CoC vs CoP at timepoint | HRA follow-up vs baseline | MoP follow-up vs baseline | CoP follow-up vs baseline | CoC follow-up vs baseline | |||||||
| Time point | Difference in means (95% CI) | p value | Difference in means (95% CI) | p value | Difference in means (95% CI) | p value | Difference in means (95% CI) | p value | Difference in means (95% CI) | p value | Difference in means (95% CI) | p value |
| Baseline | 0.9 (0.3 to 1.5) | 0.002 | 0 (-0.9 to 0.9) | > 0.99 | ||||||||
| Year 1 | 0.5 (-0.2 to 1.2) | 0.24 | 0.4 (-0.6 to 1.4) | 0.68 | 0.2 (-0.2 to 0.7) | 0.50 | -0.4 (-1 to 0.1) | 0.20 | 0 (-0.4 to 0.4) | > 0.99 | 0.4 (-0.4 to 1.1) | 0.51 |
| Year 2 | 1 (0.3 to 1.7) | 0.003 | -0.2 (-1.2 to 0.8) | > 0.99 | 0.4 (-0.1 to 1) | 0.16 | 0.1 (-0.5 to 0.8) | > 0.99 | 0 (-0.4 to 0.4) | > 0.99 | -0.2 (-1.1 to 0.7) | 0.91 |
| Year 3 | 0.9 (0.1 to 1.7) | 0.02 | 0.1 (-1 to 1.1) | > 0.99 | 0.0 (-0.6 to 0.6) | > 0.99 | 0.0 (-0.7 to 0.7) | > 0.99 | 0.0 (-0.5 to 0.4) | > 0.99 | 0.0 (-0.9 to 1) | > 0.99 |
Data presented as mean ± SD or mean difference (95% CI); all measurements are in the units of ppb.
Table 3.
Changes and differences in serum chromium levels over time by primary bearing surface for patients with unilateral arthroplasty
| Bearing surface | HRA vs CoP at timepoint | |||||||||
| Timepoint | HRA | MoP | CoP | CoC | Difference in means (95% CI) | p value | ||||
| Number | Chromium level | Number | Chromium level | Number | Chromium level | Number | Chromium level | |||
| Baseline | 22 | 2.1 ± 1.0 | 16 | 0.4 ± 0.8 | 66 | 0.3 ± 0.6 | 11 | 0.2 ± 0.8 | 1.8 (1.3-2.3) | < 0.001 |
| Year 1 | 22 | 2.5 ± 1.0 | 15 | 0.1 ± 0.3 | 28 | 0.3 ± 0.6 | 8 | 0.4 ± 1.1 | 2.2 (1.6-2.8) | < 0.001 |
| Year 2 | 20 | 2.4 ± 1.1 | 14 | 0.2 ± 0.5 | 25 | 0.2 ± 0.5 | 7 | 0.0 ± 0.0 | 2.3 (1.7-2.9) | < 0.001 |
| Year 3 | 16 | 2.4 ± 1.3 | 11 | 0.2 ± 0.5 | 22 | 0.1 ± 0.4 | 6 | 0.0 ± 0.0 | 2.4 (1.7-3.1) | < 0.001 |
| MoP (> 1 year) vs CoP at timepoint | CoC vs CoP at timepoint | HRA follow-up vs baseline | MoP follow-up vs baseline | CoP follow-up vs baseline | CoC follow-up vs baseline | |||||||
| Timepoint | Difference in means (95% CI) | p value | Difference in means (95% CI) | p value | Difference in means (95% CI) | p value | Difference in means (95% CI) | p value | Difference in means (95% CI) | p value | Difference in means (95% CI) | p value |
| Baseline | 0.0 (-0.6 to 0.6) | > 0.99 | 0.1 (-0.7 to 0.9) | > 0.99 | ||||||||
| Year 1 | -0.3 (-0.9 to 0.3) | 0.56 | 0.2 (-0.7 to 1.1) | 0.90 | 0.4 (-0.1 to 0.8) | 0.16 | -0.3 (-0.9 to 0.2) | 0.36 | 0.0 (-0.4 to 0.3) | > 0.99 | 0.1 (-0.6 to 0.9) | > 0.99 |
| Year 2 | 0.0 (-0.7 to 0.6) | > 0.99 | -0.2 (-1.1 to 0.7) | > 0.99 | 0.4 (-0.2 to 0.9) | 0.24 | -0.1 (-0.8 to 0.5) | 0.93 | -0.1 (-0.5 to 0.3) | 0.93 | -0.3 (-1.2 to 0.5) | 0.68 |
| Year 3 | 0.2 (-0.5 to 0.9) | 0.85 | 0.1 (-0.9 to 1) | > 0.99 | 0.3 (-0.4 to 0.9) | 0.60 | -0.1 (-0.8 to 0.6) | > 0.99 | -0.3 (-0.8 to 0.2) | 0.28 | -0.3 (-1.3 to 0.6) | 0.75 |
Data presented as the mean ± SD or mean difference (95% CI); all measurements are in the units of ppb.
Longitudinal Change of Synovial Reaction Classification, and Association of ALTR or Metallosis on MRI and Capsular Dehiscence and Patient-Reported Outcomes
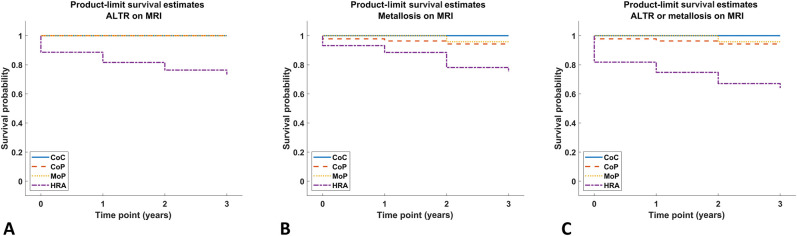
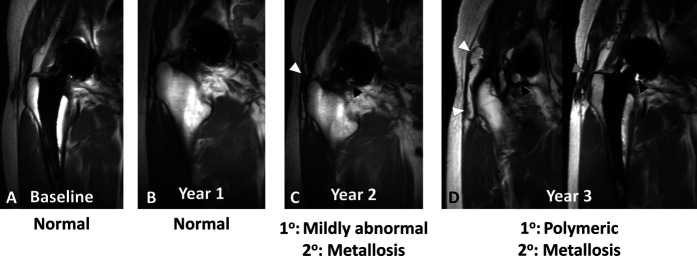
The mean ± SD time to change in the synovial classification from a nonALTR classification (normal, mildly abnormal, infection, or polymeric) to ALTR on MRI was 2.5 ± 0.2 years for HRA (log-rank p < 0.001). HRA survivorship free from ALTR on MRI was 76% (95% CI 63% to 89%) at 2 years (Fig. 3A). The risk of a metallosis synovial classification in patients with HRA was not different than the risk for patients with CoP (Table 4). HRA survivorship free from metallosis on MRI was 75% (95% CI 62% to 89%) at 2 years (Fig. 3B). More patients who received an HRA developed ALTR or metallosis on MRI than patients who had CoP bearings (hazard ratio 4.8 [95% CI 1.2 to 18.4]; p = 0.02) (Table 4), although more than half (66% [29 of 44]) of patients with HRA did not have synovial classifications of ALTR or metallosis. HRA survivorship free from ALTR or metallosis on MRI was 67% (95% CI 53% to 81%) at 2 years (Fig. 3C). Longitudinal changes in the synovial classification were found in patients with HRA (Fig. 4), patients with MoP (Fig. 5), and patients with CoP (Fig. 6)
Fig. 3.
A-B This Kaplan-Meier estimator demonstrates the probability of developing (A) ALTR, (B) metallosis, and (C) ALTR or metallosis, as seen on MRI, over the four timepoints.
Table 4.
Hazard ratios of the presence of ALTR or metallosis on MRI
| ALTR (only) on MRI | Metallosis (only) on MRI | ALTR or metallosis on MRI | |||||||||||||||||
| Bearing | No | Yes | Hazard ratio | 95% CI | p value | No | Yes | Hazard ratio | 95% CI | p value | No | Yes | Hazard ratio | 95% CI | p value | ||||
| CoC | 18 | 0 | 18 | 0 | 18 | 0 | |||||||||||||
| CoP | 138 | 0 | Reference | 138 | 5 | Reference | 133 | 5 | Reference | ||||||||||
| HRA | 33 | 11 | 34 | 10 | 2.9 | 0.7-12.6 | 0.16 | 29 | 15 | 4.8 | 1.2-18.4 | 0.02 | |||||||
| MoP | 31 | 0 | 31 | 1 | 0.6 | 0.1-5.8 | 0.68 | 30 | 1 | 0.6 | 0.1-6.3 | 0.70 | |||||||
Fig. 4.
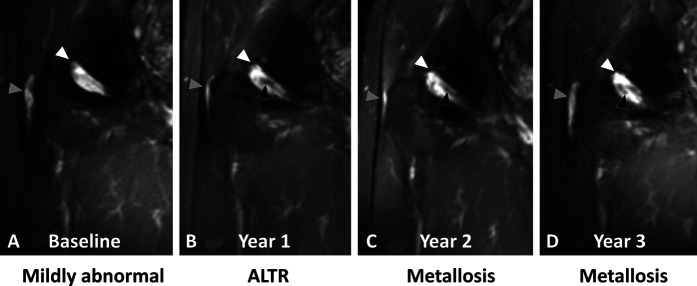
A-D Coronal multiacquisition variable resonance image combination short tau inversion recovery images of a 64-year-old man with HRA (5.1 years after implantation at baseline) display longitudinal progression in synovial classification from (A) mildly abnormal at baseline by evidence of mild nonspecific synovitis (white arrowhead) decompressing into the trochanteric bursa (gray arrowhead) to (B) ALTR at year 1 to metallosis (C) at year 2 and (D) at year 3, displayed as persistent synovitis (white arrowheads) decompressing into the trochanteric bursa (gray arrowheads) with interval development of isointense-to-hypointense synovial debris (black arrowheads), consistent with metal deposition.
Fig. 5.
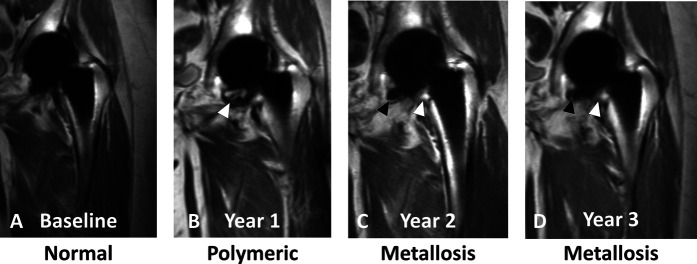
A-D Coronal multiacquisition variable resonance image combination proton density images of a 71-year-old woman with an MoP THA (4 years after implantation at baseline) display longitudinal progression in synovial classification from normal (A) at baseline with no synovial or bursal expansion to (B) mild synovial expansion with a small amount of synovial debris (white arrowhead), consistent with polymeric wear-related synovitis at year 1 to metallosis (C) at year 2 and (D) at year 3 as evident by persistent, mild synovial expansion (white arrowheads) with interval development of low-signal-intensity deposits (black arrowheads), consistent with metal wear debris.
Fig. 6.
A-D Coronal multiacquisition variable resonance image combination proton density images of a 65-year-old woman with a CoP THA implant (2.2 years implantation at baseline) display longitudinal progression in synovial classification from normal (A) at baseline and (B) year 1 with no synovial or bursal expansion to a primary classification of (C) mildly abnormal at year 2 with mild synovial expansion (black arrowhead), decompression of synovitis into the trochanteric bursa, and hypointense staining (white arrowhead) suggestive of metal deposition. (D) The primary classification at year 3 was polymeric and the secondary classification was metallosis, with evidence of progressive synovial expansion (black arrowheads) and bursal fluid distension (white arrowheads) with bulky hypointense metallic debris (gray arrowhead).
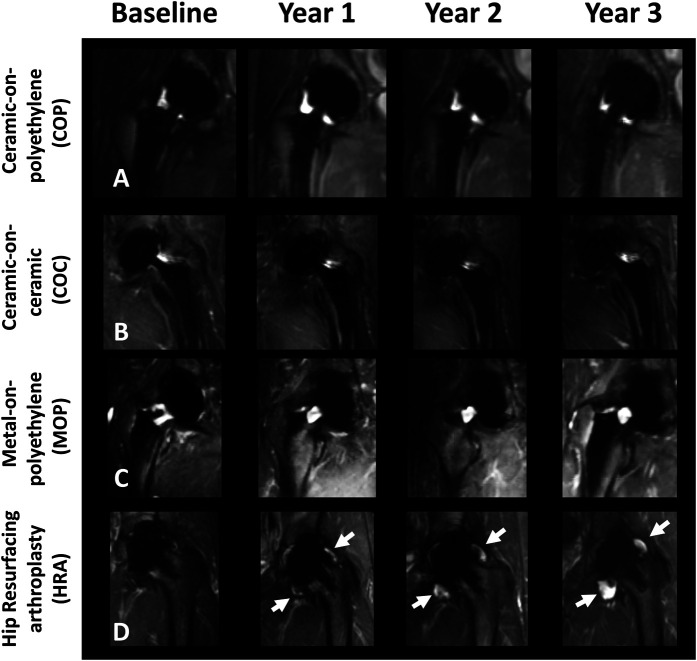
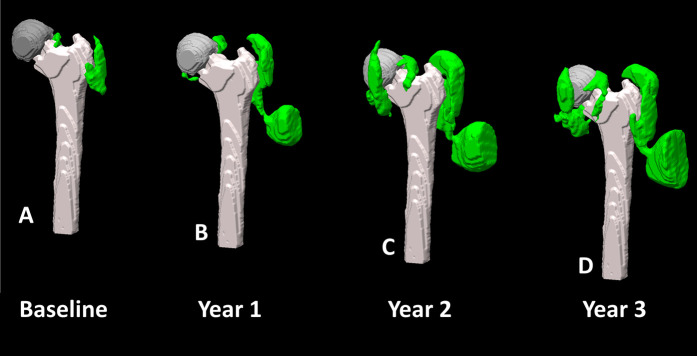
There was no difference in the magnitude of mean synovial volume of patients with HRA and CoP bearing surfaces at any time point with the numbers available (Table 5), but the variability seen in morphologic images (Fig. 7) may be fully discerned in a three-dimensional rendering (Fig. 8). Similarly, the mean synovial volume of patients with MoP was not different than patients with CoP at all timepoints. The synovial volume of patients with HRA was greater only at year 2 (baseline: 4.8 ± 7.5 cm3; year 2: 10.5 ± 40.0 cm3; mean difference 6.1 cm3 [95% CI 0.08 to 12.1]; p = 0.046). The synovial volume of patients with CoP and CoC increased at year 1 compared with baseline; at later timepoints, there were no differences with the numbers available. Additionally, there were no differences in longitudinal changes in synovial volume between patients with dehiscence and those without (Table 6).
Table 5.
Changes and differences in synovial volume over time by primary bearing surface
| Bearing surface | HRA vs CoP at timepoint | |||||||||
| Timepoint | HRA | MoP | CoP | CoC | Difference in means (95% CI) | p value | ||||
| Count | Synovial volume | Count | Synovial volume | Count | Synovial volume | Count | Synovial volume | |||
| Baseline | 44 | 4.8 ± 7.6 | 31 | 3.6± 4.9 | 129 | 4.8 ± 8.5 | 18 | 5.4 ± 10.2 | 0.3 (-7.7 to 8.2) | > 0.99 |
| Year 1 | 43 | 6.4 ± 15.5 | 29 | 2.5 ± 3.3 | 64 | 4.7 ± 5.3 | 14 | 12.2 ± 27.1 | 1.9 (-6.5 to 10.2) | 0.93 |
| Year 2 | 38 | 10.5 ± 40.0 | 24 | 1.8 ± 2.6 | 46 | 3.8 ± 4.8 | 11 | 4.0 ± 4.4 | 7.0 (-1.9 to 15.9) | 0.17 |
| Year 3 | 33 | 10.4 ± 44.5 | 20 | 1.3 ± 2.0 | 41 | 4.1 ± 6.7 | 8 | 2.6 ± 3.6 | 5.9 (-3.6 to 15.4) | 0.35 |
| MoP (> 1 year) vs CoP at timepoint | CoC vs CoP at timepoint | HRA follow-up vs baseline | MoP follow-up vs baseline | CoP follow-up vs baseline | CoC Follow-up vs baseline |
|||||||
| Timepoint | Difference in means (95% CI) | p value | Difference in means (95% CI) | p value | Difference in means (95% CI) | p value | Difference in means (95% CI) | p value | Difference in means (95% CI) | p value | Difference in means (95% CI) | p value |
| Baseline | -2.1 (-10.7 to 6.4) | 0.91 | -0.8 (-12.8 to 11.1) | > 0.99 | ||||||||
| Year 1 | -3.3 (-12.3 to 5.6) | 0.74 | 6.4 (-6.2 to 19.1) | 0.53 | 1.9 (-2.6 to 6.3) | 0.60 | -1.0 (-6.4 to 4.4) | 0.94 | 0.2 (-3.2 to 3.6) | > 0.99 | 7.5 (-0.7 to 15.7) | 0.08 |
| Year 2 | -3.2 (-12.8 to 6.3) | 0.80 | -3.4 (-16.8 to 10.0) | 0.90 | 6.1 (0.1-12.1) | 0.05 | -1.8 (-9.1 to 5.6) | 0.88 | -0.6 (-5.5 to 4.2) | > 0.99 | -3.2 (-14.2 to 7.8) | 0.81 |
| Year 3 | -3.6 (-13.9 to 6.7) | 0.78 | -4.8 (-19.2 to 9.7) | 0.81 | 5.3 (-1.9 to 12.5) | 0.19 | -1.8 (-10.6 to 6.9) | 0.92 | -0.4 (-6.1 to 5.4) | > 0.99 | -4.3 (-17.5 to 8.9) | 0.76 |
Data presented as the mean ± SD or mean difference (95% CI).
Fig. 7.
A-D These multiacquisition variable resonance image combination short tau inversion recovery images at baseline and at years 1, 2, and 3 are shown of (A) a patient with a CoP implant (length of implantation at baseline = 2.0 years), (B) a patient with a CoC implant (length of implantation at baseline = 8.8 years), (C) a patient with an MoP implant (length of implantation at baseline = 1.1 years), and (D) a patient with an HRA implant (length of implantation at baseline = 6.6 years) at baseline and at the four longitudinal timepoints, each with a 1-year interval. There was adverse synovial expansion (white arrows) over time for the HRA implant and no progressive synovial expansion for the CoP and MoP implants. The low-signal intensity debris in the patient who underwent HRA indicates a metallosis synovial reaction.
Fig. 8.
A-D This figure shows an example of gradual synovial expansion in a 77-year-old man with an HRA implant (length of implantation at baseline 9.6 years) at timepoints evaluated in the study. The synovial volume (green) progressed from (A) 42 cm3 at baseline to (B) 99 cm3 at year 1 to (C) 246 cm3 at year 2 to (D) 256 cm3 at year 3 based on manual segmentation of the synovial reactions, bony anatomy, and implant hardware, as rendered in Meshlab [8].
Table 6.
Longitudinal changes in synovial volume based on the presence of synovial dehiscence
| Timepoint comparison | Presence of dehiscence | Difference in synovial volume | Standard error | 95% confidence limit | p value |
| Year 1 – baseline | Yes | -0.5 | 1.8 | -5.8 to 4.8 | > 0.99 |
| No | 1.7 | 1.2 | -1.8 to 5.3 | > 0.99 | |
| Year 2 – year 1 | Yes | 2.8 | 1.9 | -2.7 to 8.2 | > 0.99 |
| No | -1.8 | 1.3 | -5.7 to 2.0 | > 0.99 | |
| Year 3 – year 2 | Yes | 1.3 | 2.3 | -5.3 to 8.0 | > 0.99 |
| No | -0.5 | 1.5 | -4.7 to 3.7 | > 0.99 |
Data presented in units of cm3.
Periprosthetic fluid collection posterior to the hip has been described as potential ALTR, and we assessed the longitudinal effect of posterior capsule dehiscence as a function of ALTR. The presence or absence of dehiscence was not associated with a synovial classification of ALTR or metallosis for patients with HRA (HR 2.5 [95% CI 0.8 to 8.0]; p = 0.13) or for patients with CoP (HR 1.2 [95% CI 0.2 to 8.4]; p = 0.84). There was no association between the presence of dehiscence at baseline and the subsequent development of ALTR or metallosis as seen on MRI at all timepoints (Table 7).
Table 7.
Probability of developing ALTR or metallosis as seen on MRI if dehiscence is present at baseline
| Bearing surface | Presence of dehiscence at baseline | Total | MRI synovial classification of ALTR or metallosis | Percentage | Log-rank p value |
| CoC | No | 17 | 0 | 0% | N/A |
| Yes | 1 | 0 | 0% | ||
| CoP | No | 87 | 3 | 3% | 0.98 |
| Yes | 51 | 2 | 4% | ||
| HRA | No | 33 | 9 | 27% | 0.10 |
| Yes | 11 | 6 | 55% | ||
| MoP | No | 22 | 1 | 5% | 0.56 |
| Yes | 9 | 0 | 0% | ||
| Overall | No | 159 | 13 | 8% | 0.47 |
| Yes | 72 | 8 | 11% |
Finally, we found no differences of any HOOS subscale at any timepoint between patients with or without an ALTR or metallosis on MRI for any bearing surface (Supplementary Table 2; http://links.lww.com/CORR/A604).
Patients with HRA: Blood Metal Ion Levels Between Patients With and Without an ALTR or Metallosis
There were elevated cobalt (4.7 ± 3.5 ppb) and chromium (4.7 ± 2.6 ppb) serum levels in patients with unilateral HRA who had an ALTR or metallosis present on MRI at year 1 (Table 8) compared with patients without an ALTR or metallosis on MRI (cobalt: 1.8 ± 1.0 ppb, mean difference 4.7 ppb [95% CI 3.3 to 6.0]; p < 0.001; chromium: 2.3 ± 0.5 ppb, mean difference 3.6 ppb [95% CI: 2.2 to 5.0]; p < 0.001) as well as for chromium at year 3 (3.9 ± 2.4 ppb versus 2.2 ± 1.1 ppb, mean difference 1.3 ppb [95% CI 0.3 to 2.4]; p = 0.01).
Table 8.
Differences in serum ion levels for patients with unilateral HRA
| Timepoint | Variable | ALTR or metallosis present on MRI? | Difference in means (95% CI) | p value | |
| No | Yes | ||||
| Baseline | Count | 18 | 4 | ||
| Cobalt | 1.7 ± 0.7 | 2.2 ± 1.0 | 0.4 (-0.5 to 1.3) | 0.36 | |
| Chromium | 2.0 ± 1.0 | 2.5 ± 1.1 | 0.5 (-0.3 to 1.4) | 0.22 | |
| Year 1 | Count | 20 | 2 | ||
| Cobalt | 1.8 ± 1.0 | 4.7 ± 3.5 | 4.7 (3.3 to 6.0) | < 0.001 | |
| Chromium | 2.3 ± 0.5 | 4.7 ± 2.6 | 3.6 (2.2 to 5.0) | < 0.001 | |
| Year 2 | Count | 17 | 3 | ||
| Cobalt | 2.2 ± 1.3 | 2.0 ± 0.3 | -0.3 (-1.2 to 0.5) | 0.42 | |
| Chromium | 2.4 ± 1.2 | 2.6 ± 0.6 | 0.0 (-0.8 to 0.9) | 0.94 | |
| Year 3 | Count | 14 | 2 | ||
| Cobalt | 1.5 ± 0.6 | 2.4 ± 1.3 | 0.1 (-0.9 to 1.2) | 0.80 | |
| Chromium | 2.2 ± 1.1 | 3.9 ± 2.4 | 1.3 (0.3 to 2.4) | 0.01 | |
Data presented as mean ± SD or mean difference (95% CI); all measurements are in the units of ppb.
Discussion
We evaluated the natural history of ALTR or metallosis on MRI in patients with hip arthroplasty. Compared with previous longitudinal studies that used MRI to evaluate hip arthroplasty [5, 11, 17, 24, 27, 42, 46], we evaluated MoM bearing surfaces, focusing on patients with HRA implants, in conjunction with patients having more traditional CoP, MoP, and CoC bearing surfaces. In addition, we also used the HOOS survey to uniformly assess symptomatology. The results indicate a greater prevalence of ALTR or metallosis synovial reactions on MRI in MoM articulations than in other bearing surfaces, although patients reported overall similar joint function at each timepoint. Furthermore, we found an inconsistent association between elevated blood serum ion levels and the presence of an ALTR or metallosis on MRI, which emphasizes the inability of serum ion testing alone to assess local synovial reactions.
Limitations
This study has several limitations. First, although the statistical analysis was adjusted for age, sex, BMI, and length of implantation, we did not evaluate individual implant design factors such as manufacturer or head size, nor did we incorporate specific implant anteversion or inclination angles into our analysis. However, we reviewed radiographs of all enrolled participants, and we did not find evidence of loosening or gross malalignment on any radiograph. Furthermore, we attempted to minimize the number of post hoc comparisons performed by designating patients with HRA, MoP, or CoC bearing surfaces as test groups and patients with a CoP bearing surface as the control group for many of the evaluations performed, and used the appropriate Dunnett-Hsu method to account for these multiple comparisons.
Second, as described above, our institutional method of assessing blood serum ions does not use syringes or needles free of metallic components, and this may have contaminated the resulting ion levels [31]. In addition, glass tubes were used for blood collection, which may have introduced a small amount of metal into the acquired sample [31]. We believe these effects on the results of our blood testing were minimal because the cobalt (Table 2) and chromium (Table 3) levels for MoP, CoP, and CoC designs displayed small values of metal ion concentration.
Third, participants were assigned a synovial classification based on a subjective interpretation of morphologic MR images. The repeatability of this synovial classification was assessed, and we found moderate-to-almost perfect intrarater agreement and substantial-to-almost perfect interrater agreement. However, imaging was performed at a single institution that specializes in all aspects of orthopaedic care. Our institution also uses advanced MRI acquisition protocols daily, including high-resolution, high-bandwidth fast-spin echo techniques and the MAVRIC-SL multispectral imaging technique. Even with this limitation, our methods are applicable to other MRI hardware vendors and institutions that sufficiently modify their acquisition protocol to minimize metallic susceptibility artifacts, as has been done in studies using MRI to evaluate THAs [5, 11, 18].
Fourth, the time difference between the initial arthroplasty implantation and the baseline timepoint of this study varied among all patients and could affect the outcome measures in this study, including the prevalence of ALTR or metallosis on MRI, cobalt and chromium ion levels, and HOOS; however, our statistical modeling accounted for age, sex, BMI, and length of implantation because these are known confounders for hip arthroplasty. Future studies may benefit from the use of propensity matching of subjects to account for these various patient-specific factors.
Fifth, patients with bilateral and unilateral constructs were included in the evaluation. It is possible that two hips within a single individual may not be biologically independent, but the imaging analysis of the host-mediated synovial reaction we performed was hip-specific. We also note that although all prior longitudinal MRI evaluations of THAs have focused primarily on MoM THA or HRA devices, only a single study evaluated patients with unilateral arthroplasty [27], and the remaining studies included unilateral and bilateral cases [5, 11, 17, 42]. One study did not specify the inclusion criteria, and the presented data did not clearly indicate enrollment of just unilateral cases [24]. We also note that the studies which included bilateral hips and also evaluated blood metal ion levels only reported the results of the blood testing for the unilateral hips [17], similar to what we have presented in our study, or they reported separate results for unilateral and bilateral cases [42]. Incorporation of bilateral hips into the analysis is important as many individuals who undergo primary THA will also have a contralateral THA: 22% at 10 years and 29% at 20 years [43]. It may also be anticipated that the number of contralateral THA procedures will increase with the concomitant increase in THA prevalence since 1980 [23].
Sixth, this study is not yet completed and is an interim report of our findings to date. Due to patient concerns regarding health and safety issues related to the current COVID-19 pandemic and not being able to travel, many patients have indicated their willingness to terminate their study participation. As such, we felt that an interim analysis was necessary to report the findings to date. We look forward to the completion of the study even as we address and adapt to the challenges of this research study in light of the ongoing COVID-19 pandemic.
Finally, the study had limited enrollment of MoM and modular MoP THA implants. We do not believe that their exclusion is detrimental to the study because these different bearing surfaces and implant designs have been examined extensively using MRI clinically [1, 6, 35] and provided the opportunity in the current study to focus on the prevalence of ALTR and metallosis in CoC, MoP, and CoP bearing surfaces, as seen on MRI.
Differences in Blood Metal Ion Levels Over Time
We found that patients who underwent HRA had greater cobalt and chromium ion levels than patients with CoP implants at all timepoints, and that patients with MoP had greater cobalt levels than patients with CoP at three of the four evaluated timepoints. Even as the range of ion level differences between patients with HRA and those with CoP (cobalt difference range 1.6 to 2.0 ppb; chromium difference range 1.8 to 2.4 ppb) was greater than the difference in ion levels between patients with MoP and those with CoPs (cobalt difference range 0.5 to 1.0 ppb; chromium difference range -0.3 to 0.2 ppb), the ion levels and changes in these levels for each bearing surface were less than the 7 ppb threshold proposed by the Medicines and Healthcare products Regulatory Agency for the treatment guidance of MoM hip arthroplasties [34]. We also noted that, on average, patients with unilateral HRA and an ALTR present on MRI had cobalt and chromium values less than 7 ppb (Table 8), which may indicate that a global assessment of an ALTR via serum metal ion levels may not accurately depict the severity or indicate the extent of an ALTR. The magnitude of and changes in the metal ion levels for patients with HRA in the current study are comparable with those of some prior reports [27, 42, 46] that evaluated patients who typically do not undergo revision surgery, but they were less than in patients with confirmed pseudotumors [17] or “asymptomatic” patients [24].
Longitudinal Change of Synovial Reaction Classification, and Association of ALTR or Metallosis on MRI and Capsular Dehiscence and Patient-Reported Outcomes
We found that although an ALTR or metallosis might be displayed on MRI in patients with an HRA, CoP, or MoP bearing surface, only patients with HRA had an increased HR for development of metallosis alone or in conjunction with ALTR (Table 5). Approximately 34% (15 of 44) of the HRAs displayed an ALTR or metallosis during the study period. A previous study of 37 patients with HRA found only one hip in which the disease changed from normal to moderate during an 8-month interval (median) [46], representing a change in classification of 3% of evaluated hips, while another study of 154 patients with HRA found that 10 hips progressed from a classification of normal during a 19-month interval (median) [42], representing a change in classification of 7% of evaluated hips. One study of 103 patients with MoM THAs found that four hips changed from normal to an advanced grade classification [11] between baseline and first follow-up examination while a similar change in classification was found for two hips between the baseline and second follow-up examination, representing 6% of evaluated hips. The MRI examinations were not performed at a uniform time interval. A second study of 155 patients with MoM THAs found progression from a normal to an advanced grade in 16 hips during a 14.6-month interval (mean) [5], representing a change in classification of 10% of evaluated hips. A third study of 37 patients with MoM hip arthroplasties found progression of Type II [18] pseudotumors [24]. However, all individuals had a pseudotumor at the initial timepoint, as defined by the study’s inclusion criteria, similar to another longitudinal evaluation of patients with MoM THAs [17]. The higher proportion of patients who developed an ALTR or metallosis on MRI in our current study may be attributable to the MRI evaluation method, which was used to classify synovial reactions rather than to assess the presence and severity or type of ALTR or pseudotumor. The former was selected because the latter lacks a direct correlation to biologically or clinically relevant findings [16, 18, 32, 47]. Sixty-six percent (29 of 44) of the high-functioning patients with HRAs in our study did not have any evidence of an ALTR or metallosis on MRI.
We also found that patients with HRA are at an overall higher risk of ALTR or metallosis, as seen on MRI, but the presence of dehiscence and fluid alone is not associated with ALTR or metallosis on MRI. A previous study recommended that individuals with trochanteric fluid and effusions should be assigned to a “predisease” state and a closer follow-up evaluation should be considered [5], but we did not asses the risk of this feature in relation to ALTRs. The presence of dehiscence in patients who underwent HRA may be attributed to the extent of capsular cutting necessary to obtain sufficient exposure to place an HRA implant or to incomplete suturing of the joint capsule. The current results indicate that capsular dehiscence is a normal postoperative finding and the presence of fluid without conspicuous synovial debris posterior to the arthroplasty does not indicate or predict an ALTR.
With respect to synovial volumes, the mean volumes for patients with nonMoM bearing surfaces were generally less than 12 cm3 and fluctuated over time. The measured synovial volumes are smaller than those reported in prior studies of individuals with MoM bearing surfaces [36, 37] or modular designs [35]. These studies reported mean synovial volumes of 95 cm3 and 11 cm3 for patients with an aseptic lymphocyte-dominant vasculitis-associated lesion present and those without, respectively [36]; they reported mean synovial volumes of 64 cm3 and 49 cm3 [37] for patients who underwent revision because of unexplained pain for other reasons, respectively; and they reported mean synovial volume of 91 cm3 for patients with modular MoP surfaces [35]. Another study approximated synovial reactions as a cube to measure volume [5] and found median volumes of 5 cm3 and 13 cm3 for patients with trochanteric fluid and those with effusion, respectively, which may be comparable with the current cohort. Other studies only measured the greatest cross-sectional area [17] or maximal diameter [46] or did not perform the measurement at all [11, 42]. Furthermore, we found little effect of the presence or absence of synovial dehiscence on the corresponding longitudinal changes in synovial volume, indicating that most hips will have persistent fluid, similar to what has been described [33, 39].
Finally, although we found no differences of any HOOS subscale at any timepoint between patients with or without an ALTR or metallosis on MRI for any bearing surface, the mean differences ranged between -14 and 27 points across all subcategories. This difference is larger than the minimal detectable change in the HOOS survey with 80% certainty (8 points) [30] and greater than the distribution-based minimum clinically important change in the HOOS survey (7 points) [30]. A prior study used the Oxford Hip Score and found improvement between timepoints for patients with a pseudotumor [46], and another found increased disability in individuals with a higher pseudotumor grade [5], but no change was present when individuals were separated into progressors versus nonprogressors. Another study used the Harris hip score and a VAS for pain [27], but the authors found no differences between progressors and nonprogressors. Comparing the results from our study to prior studies is difficult because the current study was not designed to longitudinally compare HOOS scores between bearing surfaces but rather to focus on the development of an ALTR or metallosis on MRI. However, similar to these previous studies, our results indicate that a patient who has an ALTR or progression of an ALTR may be highly functional with minimal pain, as reported mean values were greater than or equal to 70 points on any subscale.
Patients with HRA: Blood Metal Ion Levels Between Patients With and Without an ALTR or Metallosis
Metal ion levels were not helpful in differentiating between patients with MRI evidence of ALTR or metallosis and those who did not have those conditions. Differences were detected for the levels of cobalt and chromium at year 1 and for chromium at year 3, which represents only three of the eight ion tests across all timepoints (Table 8). The mean differences were less than 5 ppb at any timepoint. These results indicate a poor association between elevated metal ion levels and the presence of an ALTR or metallosis on MRI. The results also emphasize the inability of a test to assess whole-body metal ion levels with a direct, noninvasive assessment of the reaction of local synovial tissues. Our findings mirror those of a prior study that found development of a pseudotumor without a concomitant change in serum metal ion levels or hip pain [46]. Further, although elevated levels were found in patients with Anderson Class C1 or higher, changes of only ± 5% were reported between timepoints [46]. A direct comparison of our results with those of other studies is challenging because of the use of a synovial classification rather than a grading or staging method used by other studies, as described above. Reito et al. [42] found that longitudinal changes in blood metal ion levels were not different between patients with a progressive change in their pseudotumor on MRI and patients with no change, with median changes generally less than 1 ppb. Briant-Evans et al. [5] found that high cobalt levels, above 7 ppb, were associated with disease progression. Kwon et al. [24] found pseudotumors in all hips and did not find an increase in the median serum cobalt (4.1 to 4.5 ppb) or chromium (4.5 to 4.7 ppb) ion levels over time, and Hasegawa et al. [17] found elevated cobalt and chromium levels, both > 10 ppb, at each timepoint. However, longitudinal changes were not detected, and the authors of that study had already confirmed that the patients had pseudotumors to meet the study’s inclusion criteria. Finally, Laaksonen et al. [27] found that chromium levels greater than 5 ppb in patients with MoM HRAs were associated with progression of an ALTR.
Conclusion
We found an increase in the synovial volume and development of ALTR or metallosis in patients who underwent HRA compared with patients who had CoP bearings, even as most patients with HRA did not have an ALTR or metallosis present. The self-assessed symptomatology of patients with HRA was not different than patients with CoP and MoP. These findings indicate that MRI detected ALTRs in high-functioning individuals without complaints of pain; thus, an annual clinical assessment that depends on symptoms alone may not detect soft tissue complications in these patients. The inconsistent findings regarding the serum ion level with respect to the presence of an ALTR further supports MRI as a noninvasive imaging modality capable of assessing periprosthetic soft tissue complications and should be considered as part of the routine patient follow-up protocol to allow early detection and monitoring of ALTRs, in line with a prior consensus statement [25]. The current findings suggest that the presence of capsule dehiscence with fluid around the hip alone should be considered a normal postoperative finding because this will generally decrease over time and does not indicate an ALTR. Future longitudinal evaluations of nonMoM bearing surfaces may aid in identifying ALTRs.
Supplementary Material
Acknowledgments
We thank Parina Shah MS, MHA; Mauro Miranda MFA; Danyal Nawabi MD; Jacky Cheung BA; Kelly Zochowski BS; and Julia Sternberg BA for their assistance in participant recruitment and input during this study. We also thank the staff of the Hospital for Special Surgery’s Department of Radiology and Imaging – MRI for their assistance in scheduling and acquiring the images.
Footnotes
Research reported in this publication was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number R01AR064840.
One of the authors (MFK) certifies receipt of personal payments or benefits, during the study period, in an amount of USD 100,001 to USD 1,000,000 from Johnson and Johnson.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Ethical approval for this study was obtained from the Hospital for Special Surgery, New York, NY, USA (study number 2015-442-CR5).
This work was performed at the Hospital for Special Surgery, New York, NY, USA.
Contributor Information
Madeleine A. Gao, Email: madeleinegao@gmail.com.
John P. Neri, Email: nerij@hss.edu.
Yu-fen Chiu, Email: chiuy@hss.edu.
Bin Q. Lin, Email: linb@hss.edu.
Alissa J. Burge, Email: burgea@hss.edu.
Edwin Su, Email: sue@hss.edu.
Douglas E. Padgett, Email: padgettd@hss.edu.
Hollis G. Potter, Email: potterh@hss.edu.
References
- 1.Barlow BT, Ortiz PA, Fields KG, Burge AJ, Potter HG, Westrich GH. Magnetic resonance imaging predicts adverse local tissue reaction histologic severity in modular neck total hip arthroplasty. J Arthroplasty. 2016;31:2325-2331. [DOI] [PubMed] [Google Scholar]
- 2.Barrack RL, Sawhney J, Hsu J, Cofield RH. Cost analysis of revision total hip arthroplasty. A 5-year followup study. Clin Orthop Relat Res. 1999;369:175-178. [DOI] [PubMed] [Google Scholar]
- 3.Bayley N, Khan H, Grosso P, et al. What are the predictors and prevalence of pseudotumor and elevated metal ions after large-diameter metal-on-metal THA? Clin Orthop Relat Res. 2015;473:477-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosker BH, Ettema HB, Boomsma MF, Kollen BJ, Maas M, Verheyen CCPM. High incidence of pseudotumour formation after large-diameter metal-on-metal total hip replacement: a prospective cohort study. J Bone Joint Surg Br. 2012;94:755-761. [DOI] [PubMed] [Google Scholar]
- 5.Briant-Evans TW, Lyle N, Barbur S, et al. A longitudinal study of MARS MRI scanning of soft-tissue lesions around metal-on-metal total hip arthroplasties and disease progression. Bone Joint J. 2015;97-B:1328-1337. [DOI] [PubMed] [Google Scholar]
- 6.Burge AJ, Gold SL, Lurie B, et al. MR imaging of adverse local tissue reactions around rejuvenate modular dual-taper stems. Radiology. 2015;277:142-150. [DOI] [PubMed] [Google Scholar]
- 7.Carli A, Reuven A, Zukor DJ, Antoniou J. Adverse soft-tissue reactions around non-metal-on-metal total hip arthroplasty - a systematic review of the literature. Bull NYU Hosp Jt Dis. 2011;(69 suppl 1):S47-51. [PubMed] [Google Scholar]
- 8.Cignoni P, Callieri M, Corsini M, Dellepiane M, Ganovelli F, Ranzuglia G. MeshLab: an Open-Source Mesh Processing Tool. In: Scarano V, Chiara R De, Erra U, eds. Eurographics Italian Chapter Conference. The Eurographics Association; 2008. [Google Scholar]
- 9.Collier JP, Surprenant VA, Jensen RE, Mayor MB. Corrosion at the interface of cobalt-alloy heads on titanium-alloy stems. Clin Orthop Relat Res. 1991;271:305-312. [PubMed] [Google Scholar]
- 10.Cooper HJ, Della Valle CJ, Berger RA, et al. Corrosion at the head-neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J Bone Joint Surg Am. 2012;94:1655-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebreo D, Bell PJ, Arshad H, Donell ST, Toms A, Nolan JF. Serial magnetic resonance imaging of metalon-metal total hip replacements: follow-up of a cohort of 28 MM Ultima TPS THRs. Bone Joint J. 2013;95 B:1035-1039. [DOI] [PubMed] [Google Scholar]
- 12.Fehring TK, Odum S, Sproul R, Weathersbee J. High frequency of adverse local tissue reactions in asymptomatic patients with metal-on-metal THA. Clin Orthop Relat Res. 2014;472:517-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grammatopolous G, Pandit H, Kwon YM, et al. Hip resurfacings revised for inflammatory pseudotumour have a poor outcome. J Bone Joint Surg Br. 2009;91:1019-1024. [DOI] [PubMed] [Google Scholar]
- 14.Griffin WL, Fehring TK, Kudrna JC, et al. Are metal ion levels a useful trigger for surgical intervention? J Arthroplasty. 2012;27:32-36. [DOI] [PubMed] [Google Scholar]
- 15.Hart AJ, Sabah S, Henckel J, et al. The painful metal-on-metal hip resurfacing. J Bone Joint Surg Br. 2009;91:738-744. [DOI] [PubMed] [Google Scholar]
- 16.Hart AJ, Satchithananda K, Liddle AD, et al. Pseudotumors in association with well-functioning metal-on-metal hip prostheses: a case-control study using three-dimensional computed tomography and magnetic resonance imaging. J Bone Joint Surg Am. 2012;94:317-325. [DOI] [PubMed] [Google Scholar]
- 17.Hasegawa M, Miyamoto N, Miyazaki S, Wakabayashi H, Sudo A. Longitudinal magnetic resonance imaging of pseudotumors following metal-on-metal total hip arthroplasty. J Arthroplasty. 2014;29:2236-2238. [DOI] [PubMed] [Google Scholar]
- 18.Hauptfleisch J, Pandit H, Grammatopoulos G, Gill HS, Murray DW, Ostlere S. A MRI classification of periprosthetic soft tissue masses (pseudotumours) associated with metal-on-metal resurfacing hip arthroplasty. Skeletal Radiol. 2012;41:149-155. [DOI] [PubMed] [Google Scholar]
- 19.Hayter CLL, Gold SLL, Koff MFF, et al. MRI Findings in painful metal-on-metal hip arthroplasty. Am J Roentgenol. 2012;199:884-893. [DOI] [PubMed] [Google Scholar]
- 20.Hjorth MH, Stilling M, Soballe K, et al. No association between pseudotumors, high serum metal-ion levels and metal hypersensitivity in large-head metal-on-metal total hip arthroplasty at 5–7-year follow-up. Skeletal Radiol. 2016;45:115-125. [DOI] [PubMed] [Google Scholar]
- 21.Koch KM, Brau AC, Chen W, et al. Imaging near metal with a MAVRIC-SEMAC hybrid. Magn Reson Med. 2011;65:71-82. [DOI] [PubMed] [Google Scholar]
- 22.Koff MF, Esposito C, Shah P, et al. MRI of THA correlates with implant wear and tissue reactions: a cross-sectional study. Clin Orthop Relat Res. 2019;477:159-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kremers HM, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2014;97:1386-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon YM, Liow MHL, Dimitriou D, Tsai TY, Freiberg AA, Rubash HE. What is the natural history of “asymptomatic” pseudotumours in metal-on-metal hip arthroplasty? Minimum 4-year metal artifact reduction sequence magnetic resonance imaging longitudinal study. J Arthroplasty. 2016;31:121-126. [DOI] [PubMed] [Google Scholar]
- 25.Kwon YM, Lombardi AV, Jacobs JJ, Fehring TK, Lewis CG, Cabanela ME. Risk stratification algorithm for management of patients with metal-on-metal hip arthroplasty: consensus statement of the American Association of Hip and Knee Surgeons, the American Academy of Orthopaedic Surgeons, and the Hip Society. J Bone Joint Surg Am. 2014;96:e4. [DOI] [PubMed] [Google Scholar]
- 26.Kwon YM, Ostlere SJ, McLardy-Smith P, Athanasou NA, Gill HS, Murray DW. “Asymptomatic” pseudotumors after metal-on-metal hip resurfacing arthroplasty: prevalence and metal ion study. J Arthroplasty. 2011;26:511-518. [DOI] [PubMed] [Google Scholar]
- 27.Laaksonen I, Galea VP, Connelly JW, et al. Progression of adverse local tissue reaction in ASR metal-on-metal hip arthroplasty: a longitudinal MARS-MRI study at mid- to long-term. Hip Int. 2021;31:369-377. [DOI] [PubMed] [Google Scholar]
- 28.Lachin JM, Foulkes MA. Evaluation of sample size and power for analyses of survival with allowance for nonuniform patient entry, losses to follow-up, noncompliance, and stratification. Biometrics. 1986;42:507-519. [PubMed] [Google Scholar]
- 29.Lieberman JR, Rimnac CM, Garvin KL, Klein RW, Salvati EA. An analysis of the head-neck taper interface in retrieved hip prostheses. Clin Orthop Relat Res. 1994;300:162-167. [PubMed] [Google Scholar]
- 30.Lyman S, Lee YY, McLawhorn AS, Islam W, MacLean CH. What are the minimal and substantial improvements in the HOOS and KOOS and JR versions after total joint replacement? Clin Orthop Relat Res. 2018;476:2432-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacDonald SJ, Brodner W, Jacobs JJ. A consensus paper on metal ions in metal-on-metal hip arthroplasties. J Arthroplasty. 2004;19:12-16. [DOI] [PubMed] [Google Scholar]
- 32.Matthies AK, Skinner JA, Osmani H, Henckel J, Hart AJ. Pseudotumors are common in well-positioned low-wearing metal-on-metal hips. Clin Orthop Relat Res. 2012;470:1895-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLawhorn AS, Potter HG, Cross MB, et al. Posterior soft tissue repair after primary tha is durable at mid-term followup: prospective MRI study. Clin Orthop Relat Res. 2015;473:3183-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medicines and Healthcare Products Regulatory Agency. All metal-on-metal (MoM) hip replacements: updated advice for follow-up of patients Available at: https://www.gov.uk/drug-device-alerts/all-metal-on-metal-mom-hip-replacements-updated-advice-for-follow-up-of-patients. Accessed June 4, 2021.
- 35.Nawabi DH, Do HT, Ruel A, et al. Comprehensive analysis of a recalled modular total hip system and recommendations for management. J Bone Joint Surg Am. 2016;98:40-47. [DOI] [PubMed] [Google Scholar]
- 36.Nawabi DH, Gold S, Lyman S, Fields K, Padgett DE, Potter HG. MRI predicts ALVAL and tissue damage in MOM hip arthroplasty. Clin Orthop Relat Res. 2014;472:471-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nawabi DH, Nassif NA, Do HT, et al. What causes unexplained pain in patients with metal-on metal hip devices? A retrieval, histologic, and imaging analysis. Clin Orthop Relat Res. 2014;472:543-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nilsdotter AK, Lohmander LS, Klässbo M, Roos EM. Hip disability and osteoarthritis outcome score (HOOS) - validity and responsiveness in total hip replacement. BMC Musculoskelet Disord. 2003;4:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pellicci PM, Potter HG, Foo LF, Boettner F. MRI shows biologic restoration of posterior soft tissue repairs after THA. Clin Orthop Relat Res. 2009;467:940-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plodkowski AJ, Hayter CL, Miller TT, Nguyen JT, Potter HG. Lamellated hyperintense synovitis: potential MR imaging sign of an infected knee arthroplasty. Radiology. 2013;266:256-260. [DOI] [PubMed] [Google Scholar]
- 41.Potter HG, Nestor BJ, Sofka CM, Ho ST, Peters LE, Salvati EA. Magnetic resonance imaging after total hip arthroplasty: evaluation of periprosthetic soft tissue. J Bone Joint Surg Am. 2004;86:1947-1954. [DOI] [PubMed] [Google Scholar]
- 42.Reito A, Elo P, Puolakka T, Pajamäki J, Nieminen J, Eskelinen A. Repeated magnetic resonance imaging in 154 hips with large-diameter metal-on-metal hip replacement. Acta Orthop. 2014;85:570-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanders TL, Kremers HM, Schleck CD, Larson DR, Berry DJ. Subsequent total joint arthroplasty after primary total knee or hip arthroplasty: a 40-year population-based study. J Bone Joint Surg Am. 2017;99:396-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shulman RM, Zywiel MG, Gandhi R, Davey JR, Salonen DC. Trunnionosis: the latest culprit in adverse reactions to metal debris following hip arthroplasty. Skeletal Radiol. 2015;44:433-440. [DOI] [PubMed] [Google Scholar]
- 45.Urban RM, Jacobs JJ, Gilbert JL, Galante JO. Migration of corrosion products from modular hip prostheses. Particle microanalysis and histopathological findings. J Bone Joint Surg Am. 1994;76:1345-1359. [DOI] [PubMed] [Google Scholar]
- 46.van der Weegen W, Brakel K, Horn RJ, et al. Asymptomatic pseudotumours after metal-on-metal hip resurfacing show little change within one year. Bone Joint J. 2013;95:1626-1631. [DOI] [PubMed] [Google Scholar]
- 47.van der weegen W, Brakel K, Horn RJ, et al. Comparison of different pseudotumor grading systems in a single cohort of metal-on-metal hip arthroplasty patients. Skeletal Radiol. 2014;43:149-155. [DOI] [PubMed] [Google Scholar]
- 48.Vincent KR, Vincent HK, Lee LW, Weng J, Alfano AP. Outcomes after inpatient rehabilitation of primary and revision total hip arthroplasty. Arch Phys Med Rehabil. 2006;87:1026-1032. [DOI] [PubMed] [Google Scholar]
- 49.Walsh AJ, Nikolaou VS, Antoniou J. Inflammatory pseudotumor complicating metal-on-highly cross-linked polyethylene total hip arthroplasty. J Arthroplasty. 2012;27:324 e5-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.