Abstract
Background
Usually, the two-stage Masquelet induced-membrane technique for extremity reconstruction begins with a polymethylmethacrylate (PMMA) cement spacer–driven membrane, followed by an autologous cancellous bone graft implanted into the membrane cavity to promote healing of large bone defects. In exceptional cases, spacers made of polypropylene disposable syringes were successfully used instead of the usual PMMA spacers because of a PMMA cement shortage caused by a lack of resources. However, this approach lacks clinical evidence and requires experimental validation before being recommended as an alternative to the conventional technique.
Questions/purposes
To (1) develop and (2) validate a critical-sized femoral defect model in rats for two stages of the Masquelet technique and to (3) compare the biological and bone healing properties of polypropylene-induced membranes and PMMA-induced membranes in this model.
Methods
Fifty male Sprague Dawley rats aged 8 weeks old received a 6-mm femur defect, which was stabilized with an external fixator that was converted into an internal device. In the development phase, the defect was filled with PMMA in 16 rats to determine the most favorable timing for bone grafting. Two rats were excluded since they died of anesthetic complications. The other 14 were successively euthanized after 2 weeks (n = 3), 4 weeks (n = 4), 6 weeks (n = 4), and 8 weeks (n = 3) for induced membrane analyses. In the validation phase, 12 rats underwent both stages of the procedure using a PMMA spacer and were randomly assigned to two groups, whether the induced membrane was preserved or removed before grafting. To address our final objective, we implanted either polypropylene or PMMA spacers into the defect (Masquelet technique Stage 1; n = 11 rats per group) for the period established by the development phase. In each group, 6 of 11 rats were euthanized to compare the biological properties of polypropylene-induced membranes and PMMA-induced membranes using histological qualitative analysis, semiquantitative assessment of the bone morphogenic protein-2 content by immunostaining, and qualitative assessment of the mesenchymal stromal cell (MSC; CD31-, CD45-, CD90+, and CD73+ phenotypes) content by flow cytometry. Quantitative measurements from serum bone turnover markers were also performed. The five remaining rats of each group were used for Masquelet technique Stage 2, in which rat bone allografts were implanted in the induced membrane cavity after the polypropylene or PMMA spacers were removed. These rats recovered for 10 weeks before being euthanized for microCT quantitative measurements and bone histology qualitative assessment to evaluate and compare the extent of bone regeneration between groups.
Results
Induced membrane analyses together with serum bone turnover measurements indicated that a 4-week interval time between stages was the most favorable. Removal of the induced membrane before grafting led to almost constant early implant failures with poor bone formation. Four-week-old rats with polypropylene-triggered induced membranes displayed similar histologic organization as rats with PMMA-driven induced membranes, without any difference in the cell density of the extracellular matrix (4933 ± 916 cells per mm2 for polypropylene versus 4923 ± 1284 cells per mm2 for PMMA; p = 0.98). Induced membrane-derived MSCs were found in both groups with no difference (4 of 5 with polypropylene versus 3 of 3 with PMMA; p > 0.99). Induced membrane bone morphogenic protein-2 immunolabeling and serum bone turnover marker levels were comparable between the polypropylene and PMMA groups. MicroCT analysis found that bone regeneration in the polypropylene group seemed comparable with that in the PMMA group (29 ± 26 mm3 for polypropylene versus 24 ± 18 mm3 for PMMA; p > 0.99). Finally, qualitative histological assessment revealed a satisfactory endochondral ossification maturation in both groups.
Conclusion
Using a critical-sized femoral defect model in rats, we demonstrated that polypropylene spacers could induce membrane encapsulation with histologic characteristics and bone regenerative capacities that seem like those of PMMA spacers.
Clinical Relevance
In a same bone site, polymers with close physical properties seem to lead to similar foreign body reactions and induce encapsulating membranes with comparable bone healing properties. Polypropylene spacers made from disposable syringes could be a valuable alternative to PMMA. These results support the possibility of a cementless Masquelet technique in cases of PMMA shortage caused by a lack of resources.
Introduction
The induced-membrane technique described by Masquelet [17] in 2000 is a widely accepted method for reconstructing large segmental bone defects [9, 17, 19, 24, 25]. It is performed as a two-stage procedure involving the placement of a cement spacer in the first stage and a large bone graft in the second stage. The spacer performs a mechanical action in which it prevents invasion of fibrous tissue in the recipient site and a biological action via the induction of a surrounding membrane resulting from a foreign-body reaction [17, 19]. Within the first weeks of membrane formation, several angiogenic and osteogenic mediators are secreted, such as vascular endothelial growth factor or bone morphogenetic protein 2 (BMP-2) [16, 29]. These mediators, combined with the presence of osteoblastic and osteoclastic precursors in the membrane, promote the osteointegration of autologous cancellous bone grafts [10, 29]. Thus, the induced membrane acts as a biological chamber to revascularize the bone graft and prevent resorption [9].
Conventionally, the cement spacer used during the Masquelet technique is made of polymethylmethacrylate (PMMA), a material with a long history of use in implant fixation in orthopaedics and trauma surgery [17]. However, PMMA has several shortcomings, such as detrimental heating to the bone ends because of an exothermic reaction during polymerization, the potential toxicity of its adjuvant, and the need for surgical removal, which can be difficult and may cause trauma to the membrane [6, 16, 32]. Previous research has explored alternative spacer materials with various porosity and mechanical properties, including silicone, calcium sulfate, or titanium [6, 8, 16, 34]. These studies’ findings have important implications for improving the osteogenic properties of induced membrane. Two important findings have been demonstrated: The membrane contains a biological component that allows it to influence bone reconstruction rather than act as a simple barrier, and different membrane characteristics including histological architecture, growth factors, and progenitors’ contents can be created by changing the location, porosity, and surface typology of the spacer [8, 14, 15, 34]. Rat models of femoral defects are frequently used to study induced membrane characteristics [6, 8, 10, 11, 16, 28, 32-34]. However, only a few studies using these models completed the second stage, which is particularly challenging in rats and is mostly performed using large-animal models [1, 10, 13, 15, 34-36]. In addition, experimental conditions were highly variable between these studies, particularly regarding the bone graft timing (that is, the induced membrane maturation time), which is often empirically determined.
To date, the PMMA spacer remains the gold standard for foreign body encapsulation membrane induction in the reconstruction of critical bone defects [19]. As a result, we observed in our military and humanitarian practices that the absence of PMMA can preclude the use of Masquelet technique in developing countries [27]. Alternative materials to PMMA should be considered to make this technique possible when there are limited resources, provided these materials are low cost and readily available. Mozumder et al. [26] found that a polypropylene spacer made from disposable syringes could meet these requirements; however, to our knowledge, that work—presented at a meeting—has not been published yet as a peer-reviewed manuscript. They reported the first clinical use of this material for long-bone reconstruction with the Masquelet technique in Bangladesh and observed results comparable with those obtained with a PMMA spacer [26]. We subsequently achieved successful metacarpal bone reconstructions using polypropylene spacers from disposable syringes in a forward surgical unit where PMMA was not available [27]. However, this approach lacks clinical evidence and requires experimental validation before being recommended as an alternative to the conventional technique in low-resource settings. Although polypropylene has been successfully tested as a potential barrier for the guided bone regeneration technique in dental implantology research [5], to our knowledge, it has not been evaluated experimentally as an alternative for PMMA spacers in the Masquelet technique.
Therefore, the objectives of this study were to (1) develop and (2) validate a critical-sized femoral defect model in rats for both stages of the Masquelet technique and to (3) compare the biological and bone healing properties of polypropylene-induced membranes and PMMA-induced membranes in this model.
Materials and Methods
Overview of the Experimental Design
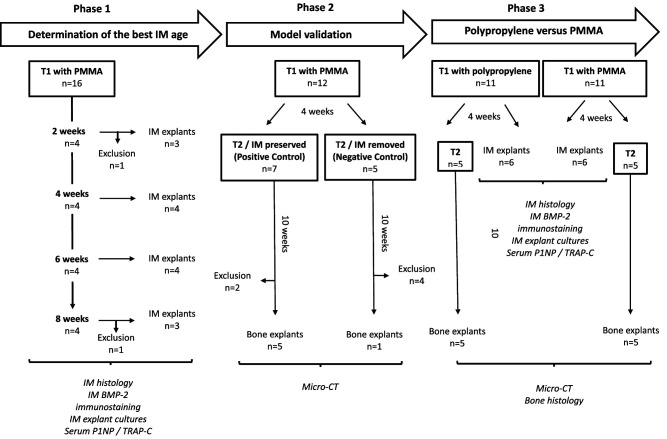
The experimental design included three phases (Fig. 1).
Fig. 1.
This flow diagram shows the experimental design. Masquelet technique Stage 1 was performed in all rats. Stage 2 was performed in the model validation phase and in half of the rats that were used to compare the properties of PMMA and polypropylene spacers. The most favorable induced membrane (IM) maturation time (4 weeks) was defined according to data collected in phase 1.
Phase 1: Determination of the Most Favorable Induced Membrane Maturation Duration
Our primary study goal was to define the best timing for bone grafting by analyzing induced membranes at different time intervals after spacer implantation. Thus, 16 rats underwent the first induced-membrane technique procedure using a PMMA spacer only. Two were excluded because of perioperative mortality related to the anesthesia. The remaining animals were successively euthanized at 2 weeks (n = 3), 4 weeks (n = 4), 6 weeks (n = 4), and 8 weeks (n = 3) after Masquelet technique Stage 1. Membranes and blood samples were collected and processed for histology, immunohistochemistry, explant cell culture, and bone turnover marker measurements (Fig. 1).
Phase 2: Masquelet Technique Model Validation
Our second study goal was to verify that the induced membrane was the key to bone healing enhancement. To this end, 12 rats underwent the entire Masquelet procedure using PMMA spacers. The time interval between stages was determined in Phase 1 of our study. Before bone grafting, rats were randomly assigned to one of two groups: the positive control group, in which the induced membrane was preserved and filled with a bone allograft, and the negative control group, in which the induced membrane was removed before bone grafting. These animals were euthanized 10 weeks after Masquelet technique Stage 2 to assess bone formation by microCT (Fig. 1).
Phase 3: Assessment of the Osteogenic Properties and Bone Healing Potential of Polypropylene Induced Membrane Versus PMMA Induced Membrane
Our third and main objective was to compare the biological and bone healing properties of membranes induced by polypropylene or PMMA. To this end, 22 rats underwent the first Masquelet technique stage and were randomly assigned to one of two groups, the polypropylene group or the PMMA group, according to the nature of the spacer filling the defect. After the time interval determined during Phase 1 of the study (development phase), these animals were either euthanized for membrane explantation or underwent the second Masquelet technique stage. Animals that underwent both surgeries were euthanized 10 weeks after Stage 2 for bone formation analysis via microCT and histology (Fig. 1).
Animals
Housing and Husbandry
Interventions were performed in an accredited animal facility. Animals were housed individually in cages in a temperature- and light-controlled environment, with food and water given ad libitum.
Sample size
Fifty male Sprague Dawley rats were used for all experiments. They were 8 weeks old (with an average weight of 200 g) when they underwent the first surgical procedure. Females were excluded to preserve group homogeneity and preclude bone turnover variations by estrogen [23]. Rats were allocated to experimental groups by randomization. To provide biological material for induced membrane properties analyses, 28 of 50 animals were euthanized after Stage 1 of the Masquelet technique. Other rats (22 of 50) underwent Stage 2 of the Masquelet technique to study bone repair. For bone graft harvesting, donor animals were littermates of the experimental animals and were 10 to 12 weeks old when euthanized. In the aftermath of bone grafting, 6 of 22 rats were excluded and euthanized before bone repair assessment due to bone fixation failure (5 of 6) or anesthesia-related mortality (1 of 6) (Fig. 1). Thus, animals were euthanized between 12 and 22 weeks old with an overdose of sodium pentobarbital (150 mg/kg) administered intraperitoneally.
Experimental procedures
Stage 1 of the Masquelet Technique
Surgical procedures were performed under general anesthesia induced by the intraperitoneal administration of a ketamine-medetomidine mixture (60 mg/kg and 0.42 mg/kg, respectively). The animals were placed in the lateral position on a heating pad after their right hind limb was prepared for aseptic surgery.
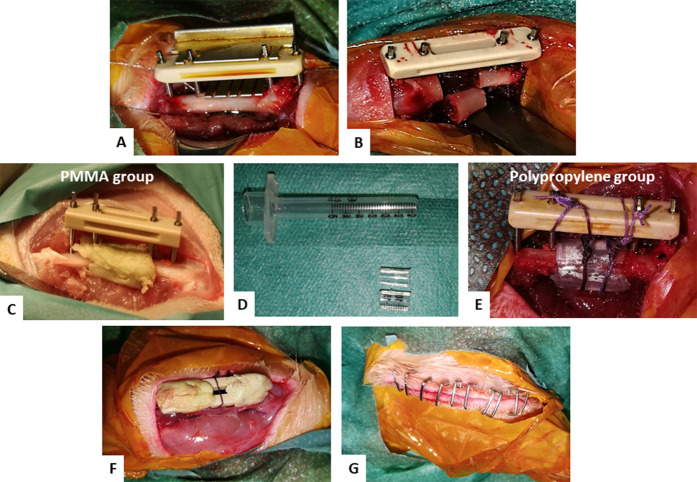
A longitudinal incision was made in the skin and fascia over the right femur. The biceps femoris and vastus lateralis muscles were bluntly separated to expose the femur. A mini external fixator (RatExFix; RISystem) was applied to the lateral aspect of the femoral shaft and secured with two 0.85-mm pins proximally and distally. Next, a critical-sized bone defect was created using a dedicated 5.0-mm-wide saw guide and a Gigli wire (Fig. 2A-B). Considering the width of the saw cut, the actual bone defect length was 6.0 mm. After the osteotomized bone was discarded, the surgical field was copiously irrigated with saline (Fig. 2B). Previous studies reported that resections smaller than 6.0 mm should not be considered for tissue engineering techniques designed to achieved bone union because of the spontaneous healing capacities observed in rats [1, 7, 34, 39].
Fig. 2.
A-G These intraoperative photographs show the first Masquelet technique stage procedure. (A) A dedicated guide saw was adapted for the external fixator frame. (B) A 6-mm femur defect was created. (C) A PMMA spacer wrapping the bone extremities was implanted. (D) A 1-mL syringe body was used to create two hemipolypropylene spacers. (E) The two hemispacers wrapped the bone extremities and were assembled using two Nice knots secured on the frame. (F) The muscles were approximated over the spacer and the pin tips were capped with cement to avoid soft tissue irritation. (G) The skin was closed without tension over the fixator.
The bone defect was filled with a PMMA or polypropylene spacer. PMMA (Palacos R + G, Heraeus) spacers were handmade during surgery, as in clinical practice; they were macroscopically smooth, cylinder-shaped, and wrapped around the bone extremities, extending 2 or 3 mm over each of the bone ends (Fig. 2C). Saline irrigation protected the surrounding tissues from heat during polymerization [19]. Polypropylene spacers were made using a 1-mL syringe barrel (Omnifix-F Solo, B. Braun), split into two parts and stabilized above the bone defect with two sliding locking Nice knots using a Vicryl 5/0 resorbable suture (Ethicon) (Fig. 2D-F) [4]. The wound was irrigated with saline before the muscles and fascia were reapproximated around the pins using Vicryl 4/0 resorbable sutures (Ethicon). Finally, the skin was closed over the external fixator using staples (Visistat Skin Stapler 35R, Teleflex Medical Inc.). The fixator was thus used as an internal fixation device to avoid secondary complications related to progressive growth of the animals during the induced membrane technique procedure (Fig. 2F-G).
Stage 2 of the Masquelet Technique
The second stage of the surgery was performed after an interval determined in Phase 1. Rats were anesthetized with isoflurane (1.5% to 2% isoflurane in 1L to 1.5 L of O2/min). An anteromedial quadriceps-splitting approach was preferred for accessing and carefully incising the membrane covering the spacer, without obstruction by the fixator. The PMMA spacers were removed by fragmentation using forceps to avoid induced membrane injuries. Conversely, polypropylene spacers were simply removed in an easy and atraumatic way, with no threat to the membrane. The fibrin clot found inside the polypropylene spacer was removed. Microtipped forceps were used to gently scrape the bone ends and open the medullary cavity. The defect was then irrigated with sterile saline and filled with a morselized corticocancellous allograft. The graft was collected from the distal femur of a littermate animal that was euthanized the same day. Wound closure was performed as in the first step of the surgery.
Postoperative Care
Animals were given a subcutaneous injection of a cephalosporin antibiotic (10 mg/kg enrofloxacin) and an opioid painkiller (0.05 mg/kg buprenorphine) during each procedure and twice per day for 3 consecutive days. Unprotected weightbearing was allowed immediately postoperatively. The animals were weighed daily. In addition, the staff of the animal facility evaluated the rats for behavior, pain, normal movements, and wound appearance each day. Radiologic follow-up evaluations were performed every 2 weeks to monitor spacer position, any implant failures, or bone regeneration (Supplemental Fig. 1; http://links.lww.com/CORR/A615).
Induced Membrane Sample Analyses
Histology
We completed a qualitative assessment of the induced membrane histological architecture and cell density. Membrane fragments were fixed for 24 hours in 4% paraformaldehyde, dehydrated in successively increasing alcohol concentrations, and embedded in paraffin. Five-micrometer-thick sections were cut from the tissue block using a Leica Microtome (Leica Microsystems GmbH) and then mounted onto silanized slides for histologic examination. Sections were stained with hematoxylin-eosin-saffron and examined using a light microscope (Leica DMRB Microscope, Leica Microsystems GmbH). Histolaboratory image analysis software (Microvision Instruments) was used to assess cell density, expressed by the number of cell nuclei per mm2. One induced membrane sample was analyzed per animal. For each sample, the cell density was determined by the mean of three densities calculated in three different areas of the membrane. All measurements were made by the same operator (LM), who was unaware of the specimen’s status.
BMP-2 Immunostaining
A semiquantitative assessment of BMP-2 was performed using immunochemistry. For the membrane fragments used in this analysis, the paraffin was first removed through sequential washes (twice in xylene for 5 minutes each, twice in 100% ethanol, twice in 95% ethanol, and then water). Immunohistochemistry was initiated by slide permeabilization using 0.5% Triton X100 (v/v) buffered with phosphate-buffered saline, followed by slide saturation using Emerald Antibody Diluent (Sigma 936B-08). Slides were overlaid with a ready-to-use rabbit polyclonal antibody specific to BMP-2 (Bioworld 90141, 1:200), a potent osteogenic growth factor. After peroxidase hydrogen blocking, slides were overlaid with a ready-to-use Anti-Rabbit IgG Horseradish Peroxidase reagent, then treated with 3,3’-Diaminobenzidine (Vector, ImmPREss MP-7451) and counterstained with hematoxylin. A brown stain denoted the presence of BMP-2. The level of BMP-2 was rated semiquantitatively as either none, low, mid, or high by the same operator (LM), who was unaware of the specimen’s status (Fig. 3).
Fig. 3.
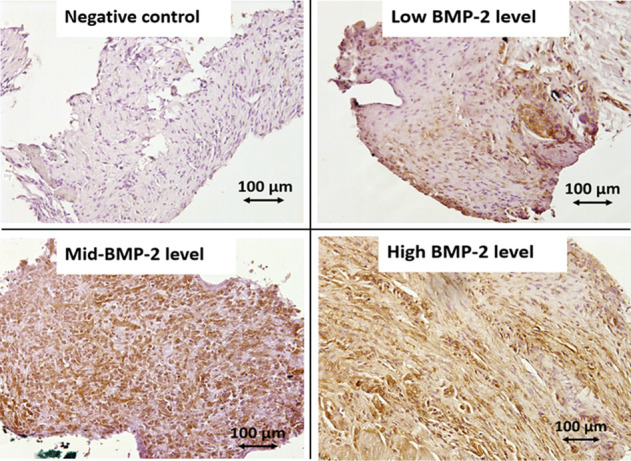
These images show the semiquantitative assessment of induced membrane BMP-2 content using immunohistochemistry (magnification x 20). A brown stain denotes the presence of BMP-2.
Explant Cultures
The presence of mesenchymal stromal cells (MSCs), the precursor cells for mature osteoblasts that are responsible for bone formation, within the induced membrane was qualitatively assessed by flow cytometry. Firstly, MSCs were isolated from the induced membrane using the explant culture method due to their plastic-adherence properties (Supplemental Fig. 2; http://links.lww.com/CORR/A616). Briefly, induced membrane fragments were transferred to culture wells. Primary explant cultures were grown in Dulbecco Modified Eagle Medium (Life Technologies, 21885-108) containing 10% (v/v) fetal bovine serum (Life Technologies, 10500-064) and 100 UI/mL of penicillin and streptomycin. MSCs were incubated at 37°C with 5% CO2 and 95% humidity for 3 weeks. At 80% to 100% confluence, cells were trypsinized, and the MSC identity was verified by flow cytometric immunophenotyping using the following antibodies: antiCD31- clone TLD-3A12, antiCD90+ clone HIS51, antiCD73+ clone 5F/B9 (BD Pharmingen), and antiCD45- clone OX-1 (Biolegend). Data were acquired and analyzed with an LSR II flow cytometer and FlowJo software, respectively (BD Biosciences).
Bone Turnover Marker Measurements
We assessed bone turnover markers with blood serum samples. Bone formation was evaluated by the procollagen-1 N terminal telopeptide (P1NP), synthesized by osteoblasts. Bone resorption was evaluated with tartrate-resistant alkaline phosphatase C (TRAP-C), released by osteoclasts during bone matrix remodeling [3]. These measurements were made using the ELISA method. For the P1NP assessments, we used the Rat/Mouse PINP EIATM assay kit (ref AC-33F1, IDS Inc.). The Rat TRAPTM (TRAcP-5b) ELISA kit (ref SB-TR102, IDS Inc.) was used for TRAP-C. For each sample, measurements were performed in duplicate and then averaged. The P1NP/TRAP-C ratio was calculated to express bone turnover after creation of the bone defects (Fig. 1).
Evaluation of Bone Regeneration
MicroCT
We used microCT to quantify bone regeneration and bone mineral density 10 weeks after Stage 2 of the Masquelet technique was performed. After the euthanasia solution was administered, the operated-on limb was collected along with the surrounding soft tissues and fixed in 10% phosphate-buffered formalin for 2 weeks. The area between the inner pins was scanned using microCT (Skyscan 1174, Bruker Micro-CT), with a voltage source of 52 keV, current of 745 mA, and isotropic resolution of 14.4 μm. Calibration was arranged before each series of images was acquired. All scans were three-dimensionally reconstructed and analyzed with the same parameter setup (NRecon v.1.6 and CTAn v.1.11 software, SkyScan) to separate mineralized elements from the background, using the software histogram tool to determine gray-level threshold values. Reconstructed images of the bone defects were analyzed by the same operator (MD), who was unaware of the specimen status and performed the analysis using the constructor’s volume tool. Because of the use of a dedicated external fixator with a guide saw, the defective region, 6 mm in length, was precisely located (considering the distance between the two adjacent pins) and was identified as the region of interest. The following data were collected within the region of interest: total defect volume (in mm3), bone volume (in mm3), bone volume fraction (in %), and bone mineral density (in g/cm3). For bone mineral density calculation, microCT calibration was performed by reference phantoms (Brucker MicroCT) containing 0.25 and 0.75 g/cm3 of calcium and hydroxyapatite evenly mixed in epoxy resin rods which were of similar diameter to the scanned bones to minimize beam hardening errors.
Histology
Qualitative assessment of bone formation was carried out by histology. After the microCT analysis, bone specimens were dehydrated in methanol and embedded in PMMA resin without decalcification. Undecalcified 5-µm-thick longitudinal bone sections were prepared using a microtome equipped with a tungsten carbide blade (Leica 2055 microtome, Leica Microsystems GmbH). Sections were stained with Masson trichrome and examined using a light microscope (Leica DMRB microscope, Leica Microsystems GmbH). Endochondral ossification maturation was rated qualitatively as either absent, limited, or satisfactory by the same operator (MD), who was unaware of the specimen’s status.
Ethical Approval
All procedures were performed with the approval of our institutional animal care and use committee at the French Military Biomedical Research Institute, Brétigny-sur-Orge, France (protocol 65 DEF_IGSSA-SP).
Statistical Analysis
Data were analyzed by the Shapiro-Wilk test to assess normal distribution. Normally distributed data are expressed as the mean ± SD. Samples in the development phase were compared using a one-way analysis of variance with a Tukey post hoc test or a nonparametric Kruskal-Wallis test, as required. The PMMA and polypropylene groups were compared using a t-test or the Mann-Whitney nonparametric test. We compared qualitative variables using a Fisher exact test. The significance for all tests was p < 0.05. Statistical analyses were performed using GraphPad Prism 5 statistical software (GraphPad Software Inc).
Results
Determination of the Most Favorable Induced Membrane Maturation Duration
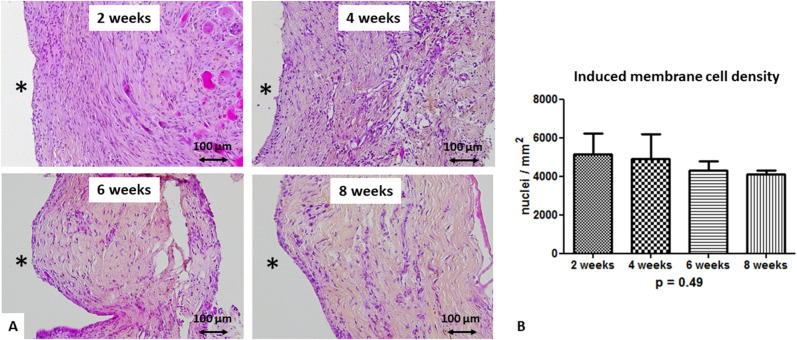
To develop a model dedicated to study the two stages of the Masquelet technique, we first sought to determine the best induced membrane age to perform bone grafting within the time period that we evaluated. The microscopic evaluation performed at 2, 4, 6, and 8 weeks after PMMA implantation consistently showed membranes that were organized in two layers: a cell-rich inner layer and a thicker outer layer consisting of loose, disorganized tissue with vessels in contact with the muscle. Regardless of the membrane’s age, the inner layer included a thin, highly cellularized layer directly in contact with PMMA. At 2 and 4 weeks, membranes were well structured, showing a clear distinction between the dense inner layer, which included fibroblast-like cells and macrophages, and the outer layer, which was mostly made of fibroblasts (Fig. 4A). Four-week-old membranes appeared to be slightly more vascularized. At 6 and 8 weeks, the membranes appeared to be more disorganized connective tissue with many fibrous tissues, including numerous collagen fibers and a dense extracellular matrix. However, the induced membrane cell density was not different (5144 ± 1093 cells per mm2 at 2 weeks, 4923 ± 1284 cells per mm2 at 4 weeks, 4289 ± 493 cells per mm2 at 6 weeks, and 4099 ± 213 cells per mm2 at 8 weeks; p = 0.49) across all induced membrane maturation times (Fig. 4B).
Fig. 4.
A-B These representative histologic sections after hematoxylin-eosin-saffron staining (magnification x 20) show (A) changes over the course of membrane maturation after PMMA spacer implantation. The black asterisk indicates the spacer location before its removal. (B) This image shows cell density (the mean number of nuclei per mm2 ± SD from three different areas for each considered membrane) at 2 weeks (n = 3), 4 weeks (n = 4), 6 weeks (n = 4), and 8 weeks (n = 3).
We assessed the osteoinductive properties of induced membrane with BMP-2 immunostaining and MSC detection using the explant culture method. The immunohistochemical qualitative analysis revealed that BMP-2 staining in induced membranes appeared to be maximal at 4 weeks, appeared to decline at 6 weeks, and was absent at 8 weeks. However, there was no difference between groups at any timepoint (Table 1). The flow cytometric analysis showed that the ex vivo explanted cells from both 2-week-old and 4-week-old membranes displayed the MSC phenotypic profile CD31-, CD45-, CD90+, and CD73+ (Supplemental Fig. 2; http://links.lww.com/CORR/A616). Conversely, cell explants of 6-week-old and 8-week-old membranes did not generate any MSCs, indicating that the induced membrane’s osteoprogenitor content was altered over time. The serum P1NP concentration peaked at 2 weeks (88 ± 27 ng/mL) and then quickly declined afterward. In contrast, the TRAP-C concentration remained almost constant up to the eighth week. Thus, the P1NP/TRAP-C ratio was higher at 2 weeks, suggesting intense bone remodeling activity after creation of the bone defect (Fig. 5).
Table 1.
Semiquantitative assessment of the induced membrane BMP-2 content by immunohistochemistry during model development
| IM BMP-2 level | 2 weeks (n = 3) | 4 weeks (n = 4) | 6 weeks (n = 4) | 8 weeks (n = 3) | p value |
| None | 1 | 2 | 3 | 0.08 | |
| Low | 2 | 2 | 2 | 0.46 | |
| Mid | 1 | > 0.99 | |||
| High | 1 | > 0.99 |
The statistical analysis indicates no difference between groups; IM = induced membrane.
Fig. 5.
A-C These graphs show the evolution of serum bone turnover markers after spacer implantation at 2 weeks (n = 3), 4 weeks (n = 4), 6 weeks (n = 4), and 8 weeks (n = 3). ELISA kits were used to measure bone formation and bone resorption activities, as well as bone remodeling turnover. We measured (A) P1NP and (B) TRAP-C serum concentrations and (C) the P1NP/TRAP-C ratio.
Altogether, these data indicated that the delay between spacer implantation and bone grafting should not exceed 4 weeks. We therefore defined a 4-week induction time as the most favorable period to perform the second stage for two reasons. Although bone turnover marker serum levels were most favorable at 2 weeks, such a short period is ethically questionable and causes a higher risk of further septic complications. Additionally, 4-week-old induced membranes displayed a high expression of BMP-2.
Model Validation
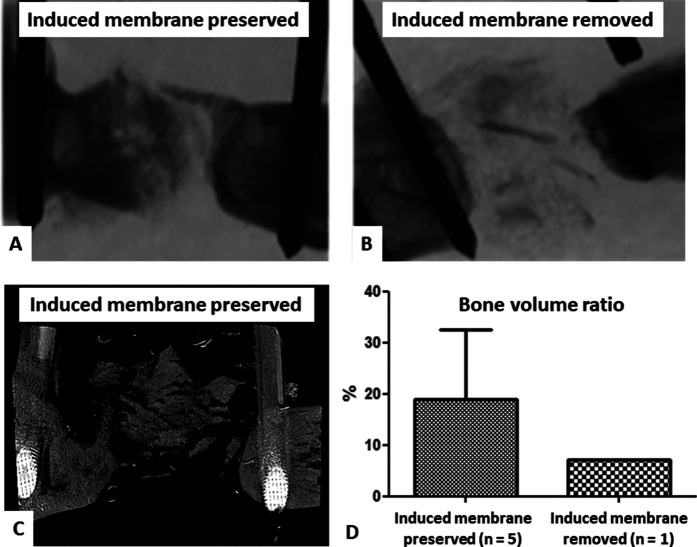
The results of this phase overall confirmed the role of the induced membrane in bone healing enhancement. MicroCT analyses were originally scheduled in negative and positive groups 10 weeks after Stage 2. However, all animals in the negative control group (in which induced membranes were removed) experienced nonunion, and 4 of 5 rats presented with early implant failure and were euthanized before the end of the experiment. Thus, no statistical analyses could be performed because of the presence of a single rat in the negative control group. In the positive control group (in which induced membranes were preserved), 2 of 7 animals were euthanized soon after bone grafting, also because of implant failure. However, the 10-week microCT analysis showed a mean bone volume ratio in the positive control group (preserved induced membranes) of 19% ± 14% (n = 5) and only a bone volume ratio of 7% (n = 1) in the negative control group (removed induced membranes) (Fig. 6).
Fig. 6.
A-D These images represent our assessment of bone regeneration during model validation. MicroCT views show (A) satisfying bone formation 10 weeks after Stage 2 in a positive control animal and (B) failure of bone graft integration with implant rupture in a negative control animal. (C) A three-dimensional reconstruction of a positive control animal with partial bone repair is shown. (D) The bone volume ratios in the positive and negative control groups are shown.
Comparing Biological and Bone Healing Properties of Polypropylene-induced and PMMA-induced Membranes
We found few, if any, substantive differences between polypropylene-induced membranes and PMMA-induced membranes in terms of histological characteristics and bone regenerative properties.
Membrane Induction
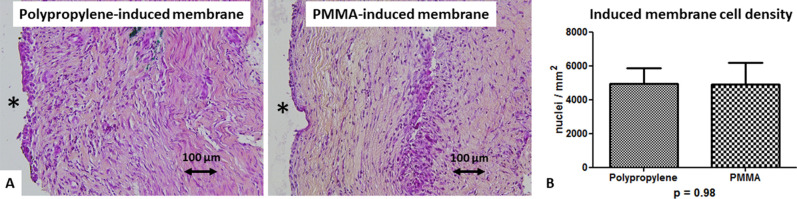
After a 4-week induction time, induced membrane biological properties were similar in the two groups. On microscopic examination, all polypropylene-induced membranes displayed the same two layers observed in the PMMA-induced membranes: a cell-rich inner layer in close contact with the polypropylene and a thick outer layer mostly composed of fibroblasts and collagen (Fig. 7). Induced membrane cell density was also comparable in both groups (4933 ± 916 cells per mm2 for polypropylene versus 4923 ± 1284 cells per mm2 for PMMA; p = 0.98). BMP-2 was detected in all induced membrane samples from both groups. There was no difference between the two groups with the numbers available (Table 2). Because of a lack of available biological material (related to technical difficulties for membrane collection), flow cytometry was possible in five animals in the polypropylene group and in only three animals in the PMMA group. Induced membrane-derived MSCs with CD31-, CD45-, CD90+, and CD73+ phenotypes were found in both groups, with no difference (4 of 5 with polypropylene versus 3 of 3 with PMMA; p > 0.99). The serum P1NP/TRAP-C ratio was not different between the groups with the numbers available at the time of membrane collection (3.2 ± 1.3 for polypropylene versus 3.4 ± 1.8 for PMMA; p = 0.85).
Fig. 7.
A-B Histologic sections stained with hematoxylin-eosin-saffron (magnification x 20) revealed that (A) PMMA-induced membranes (n = 6) and polypropylene-induced membranes (n = 6) had similar architectural organization, including a cell-rich inner layer and an outer layer mostly composed of fibroblasts and collagen. The black asterisk indicates the spacer location before its removal. (B) This graph shows the cell density (mean number of nuclei per mm2 ± SD from three different areas for each considered membrane).
Table 2.
Semiquantitative assessment of the induced membrane BMP-2 content between groups
| IM BMP-2 level | Polypropylene (n = 6) | PMMA (n = 6) | p value |
| None | > 0.99 | ||
| Low | 1 | 4 | 0.24 |
| Mid | 2 | 1 | > 0.99 |
| High | 3 | 1 | 0.54 |
IM = induced membrane; PMMA = polymethylmethacrylate.
Bone Repair
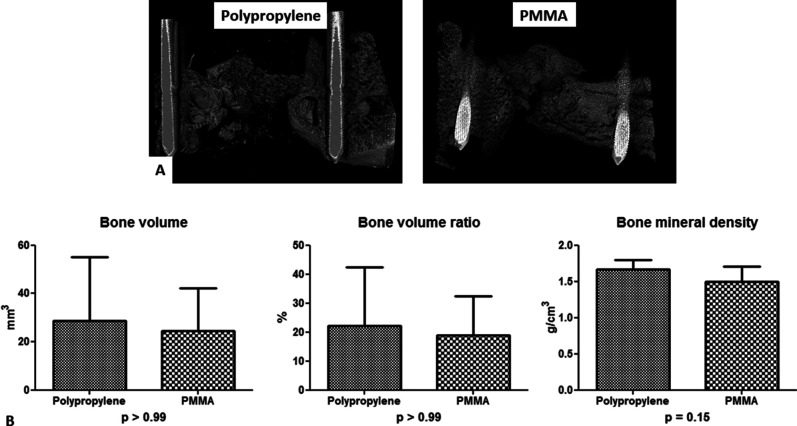
Quantitative and qualitative assessment of bone repair were similar in the two groups. The 10-week microCT analysis showed no differences with the numbers available between polypropylene and PMMA in terms of bone formation. Trabecular bone filled the entire defect in most animals, with various callus geometry. There was a persistent bone gap in one animal in each group. A quantitative analysis of the callus volume within the osteotomy region demonstrated no difference with the numbers available in bone volume (29 ± 26 mm3 for polypropylene versus 24 ± 18 mm3 for PMMA; p > 0.99), bone volume ratio (22.1% ± 20.3% for polypropylene versus 18.9% ± 13.6% for PMMA; p > 0.99), and bone mineral density (1.6 ± 0.1 g/cm3 for polypropylene versus 1.5 g/cm3 ± 0.2 g/cm3 for PMMA; p = 0.15) between the groups (Fig. 8). In each group, the qualitative histologic analysis demonstrated that the defects were filled by abundant bony callus formation and a small amount of fibrous tissue, mainly in the periphery of the calluses. Endochondral ossification maturation was rated satisfactory in all animals of the two groups (Fig. 9).
Fig. 8.
A-B Bone formation was assessed using microCT. (A) These images represent demonstrative three-dimensional reconstructions of the region of interest in both groups. (B) The quantitative and comparative analysis showed no difference between the polypropylene (n = 5) and PMMA group (n = 5) in terms of bone volume, bone volume ratio, and bone mineral density.
Fig. 9.
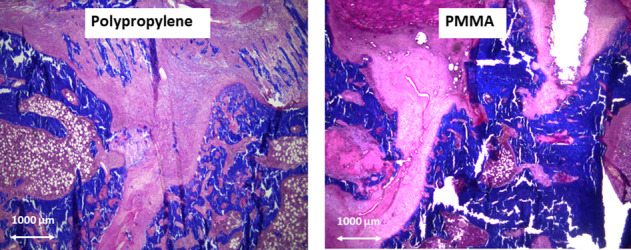
Bone formation was assessed qualitatively on histologic sections stained with Masson trichrome (magnification x 2.5). Endochondral ossification maturation (demonstrated by pink staining) was rated satisfactory in the two groups.
Discussion
The availability of PMMA is a limiting factor for performing the Masquelet technique in settings where there are minimal resources [20]. To overcome this obstacle, polypropylene disposable syringes were successfully used as an alternative to conventional PMMA spacers [26, 27]. Because this practice lacks clinical evidence, we developed an experimental critical-sized femoral defect model in rats that reproduced both stages of the Masquelet technique with the purpose to confirm that polypropylene syringes can be used as membrane inductors for bone reconstruction [27]. To confirm our clinical observation that polypropylene disposable syringes can be used as membrane inductors for bone reconstruction, we developed an experimental model in rats that reproduced both stages of the Masquelet technique [25]. Within the limits of this animal model, we found it would be possible to replace PMMA spacers with polypropylene syringes without altering the properties of membrane formation or efficiency of bone repair. This finding is important because the availability of PMMA is a limiting factor for performing the Masquelet technique in settings where there are minimal resources [19, 24, 25].
Limitations
In theory, the observations reported in this rat model cannot be extrapolated directly to humans and would require advanced preclinical assessments using large animal models [15, 35, 36]. Polypropylene syringe spacers were already used successfully in limited clinical practice [26, 27]; we believe that our results support that an animal model can be useful to continue to assess this innovative approach for clinical use. Another limitation is the limited power of our statistical analysis related to the small sample size, especially during the two first phases in which some rats were excluded because of anesthetic complications or early implant failures. In addition, the exclusive use of young male rats may be questionable since such a cohort may be not representative of the general rat population. Nevertheless, considering the small sample size in each subgroup, we gave priority to their homogeneity and comparability.
Model Development
Our rationale for choosing this model was that various authors have demonstrated the relevance of rat femoral defect models in Masquelet technique experiments [1, 6, 8, 10, 13, 16, 28, 32-34]. The model presented here exhibits two specificities: the use of an external fixator as an internal device to address some surgical issues and the prior determination of the best timing for the second Masquelet technique stage. Although the internal use of a rat external fixator is unusual, it has some advantages. In rats, bone fixation is often achieved using mini femoral plates or locked nails, which are well tolerated but have inherent disadvantages. Osteotomies are not very precise or reproducible because a guide saw is not used, and most importantly, removing the PMMA spacer is difficult, with frequent membrane alterations that compromise the completion of Stage 2 [1, 13, 32]. Instead, Toth et al. [34] used an external fixator to reproduce the full Masquelet technique in rats. They found that spacer removal was facilitated by the distance between the femur and fixator, but they experienced an implant loosening incidence of 26% after Stage 2, when the spacer was no longer present to provide mechanical support. In a preliminary study, we also experienced external fixation issues because of the rats’ sustained growth throughout life, which created friction between the skin and the device and caused subsequent infectious complications. To overcome this problem, we decided to use an external fixator as an internal device, taking care to protect the skin from the outer tip of the pins by capping the latter with PMMA (Fig. 2F). In this manner, the fixator could be placed as close as possible to the bone, providing high mechanical stability and thus limiting the occurrence of implant failure. There was no complication related to this uncommon use of external fixation. The need for a different surgical approach to the spacer during the second Masquelet technique stage was the only inconvenience.
Determination of the Most Favorable Induced Membrane Maturation Duration
The timing of the second stage of the Masquelet technique is not consistent between experimental studies and usually ranges from 4 to 8 weeks [8, 10, 16, 34]. In the present study in rats, histologic examination and flow cytometry data indicated that the interval between the two stages should not exceed 4 weeks. Beyond this period, the increased proportion of fibrous tissues, rapid decrease in BMP-2 levels, and absence of MSCs within the induced membrane suggested alteration of its osteogenic properties. Although the P1NP/TRAP-C ratio was higher at 2 weeks (showing predominant osteoblast activity), we did not choose this interval for bone grafting for various reasons. The first reason was ethical, considering that animal recovery was still incomplete at 15 days after Stage 1. We therefore chose a 4-week interval to obtain full soft tissue healing to limit the risk of infection after Stage 2. In addition, the expression of induced membrane–BMP-2 was qualitatively maximal at 4 weeks. Such an evolution in the induced membrane growth factors’ content has been demonstrated by previous studies but seems to vary according to the animal model. For instance, after studying a similar critical-sized defect model in the femurs of rats, Heinrich et al. [11] reported that osteogenic and neovascular activity in induced membranes was maximal between 2 and 4 weeks and subsided after 6 weeks. Conversely, when using a model of radial bone defects in rabbits, Wang et al. [38] found the highest concentration of BMP-2 and vascular endothelial growth factor in 6-week-old induced membranes.
Model Validation
Our validated model demonstrated the positive impact of 4-week-old induced membranes on bone repair because the mean volume of reconstructed bone was qualitatively greater in the positive control group than in the negative control group. In fact, most animals in the negative control group presented with early implant failures, attesting to the absence of bone graft integration.
Comparing Biological and Bone Healing Properties of Polypropylene-induced and PMMA-induced Membranes
We demonstrated that polypropylene and PMMA spacers led to the induction of membrane encapsulation with similar histologic characteristics and bone regenerative properties. These results support our clinical findings that disposable polypropylene syringes could be an alternative to PMMA spacers and support the possibility of a cementless Masquelet technique in cases of PMMA shortage [27].
First, we found that the polypropylene-induced membranes and PMMA-induced membranes displayed similar histologic organization, which was consistent with previous descriptions of the bone site in rats and animals of other species [6, 9, 10, 13, 15, 16, 28, 32-36]. Like Gouron et al. [10], we found that the inner layer was divided into two parts: a thin, highly cellular layer in contact with the spacer and an outer layer with a fiber network parallel to the polypropylene or PMMA. We did not identify any microscopic difference between polypropylene-induced membranes and PMMA-induced membranes in terms of membrane histology or cell density. Similarly, Gaio et al. [8] found no difference in induced membrane structure between PMMA and titanium spacers implanted in rats with femur defects. Ma et al. [16] made similar observations about PMMA and calcium sulfate spacers. Conversely, another study [22] found that intraosseous polyvinyl alcohol sponge spacers did not form a distinct membrane.
Along with these histologic aspects, BMP-2 and MSCs were present in both types of 4-week-old membranes, and serum bone turnover marker concentrations were equivalent between the groups, suggesting that the osteoinductive capacities of polypropylene-induced membranes are like those of PMMA-induced membranes. Various experimental studies demonstrated that foreign body reactions induced by polypropylene and other synthetic materials, such as polyester or polytetrafluoroethylene, are comparable in abdominal or pelvic sites [12]. It was also found that the most intense chronic inflammation was observed with heavyweight and small-pore mesh polypropylene, like the one used for syringe manufacturing [12]. In addition, impermeable polypropylene barriers can promote the bone repair process and can be used in guided bone regeneration [5]. Similarly, Toth et al. [34] found equivalent BMP-2 and other growth factor levels between PMMA- and titanium-induced membranes. The presence of BMP-2 and MSCs was also reported in membranes induced by silicone spacers in bone sites [32]. Overall, in a bone defect, it seems that spacers made of materials offering a rigid barrier with a smooth surface produce membranes with comparable characteristics in terms of histology, growth factors, and stem cell contents. Our results support that two rigid polymers with close physical properties (PMMA for surgical use and polypropylene syringes) lead to similar foreign body reactions and induce membrane encapsulation.
Induced membranes with a similar histologic composition and osteogenic properties resulted in similar bone repair and bone volume formation between the two groups. Our results were comparable to those obtained by Toth et al. [34] in a study with PMMA and titanium spacers, though most animals had partial bone regeneration, regardless of their group. This result could be because the follow-up time was insufficient or because an inappropriate graft material was used. The endpoint assessment of 10 weeks, also chosen by Toth et al. [34], may have been insufficient to allow complete bone healing in such a model. In contrast, Gouron et al. [10] reported complete union in all rats at an assessment interval of 18 weeks. They also used autologous bone grafts collected from two caudal vertebrae. Because we found that harvesting the correct amount of cancellous autograft from rats was difficult, we chose to use an allograft, like Toth et al. [34]. For the same reason, Bosemark et al. [1] focused on a bone substitute only. However, in clinical practice, it is well known that inside the membrane, a cancellous allograft or bone substitute alone is insufficient to obtain bone union [19].
Lastly, we did not evaluate the osteogenic and bone healing properties of the fibrin clot found in hollow polypropylene spacers. Indeed, we always removed this clot, which developed in the polypropylene half tubes at Masquelet technique Stage 2 because the structural properties of blood clots can significantly influence the bone healing process [37]. Thus, incorporating the fibrin clot into the bone graft could be another advantage of this technique because it could act as platelet-rich plasma, which has been described as an efficient enhancer of bone healing [2, 30].
Conclusion
We developed a novel critical-sized femoral defect model in rats by reproducing the two stages of the Masquelet technique used for bone reconstruction in humans. With this model, we demonstrated that polypropylene spacers made from disposable syringes can induce membranes with histologic characteristics and bone regenerative properties like PMMA, and they may be used instead of PMMA in low-resource settings. Thus, in a same bone site, it seems that polymers with close physical properties lead to similar foreign body reactions and membrane encapsulation.
These results support our clinical observations that polypropylene syringes seem to be a valuable alternative to PMMA spacers in developing countries with limited resources. Therefore, we think that syringes spacers could be used widely in clinical practice to allow Masquelet technique application when PMMA is not available. Polypropylene spacers, aside from being readily available, offer other advantages: (1) they allow bone ends to be wrapped easily, which is crucial to avoid nonunion between the graft and extremities; (2) dissimilar to PMMA polymerization, they do not heat the bone or the soft tissues around it, which is useful in the upper limb, where nerves and vessels are often close to the bone defect area; and (3) their removal is easy and atraumatic for the membrane [20, 27]. Polypropylene syringes could also be an alternative to PMMA tiles, which were recently used by Masquelet et al. [18] to treat recalcitrant aseptic nonunion without bone defects. Conversely, these spacers have some drawbacks compared with conventional PMMA spacers. They do not provide intrinsic mechanical stability, which allows for sequential fixation strategies, and they cannot be directly loaded with antibiotics to improve and control bone infections [21, 28]. However, outside low-resource environments, hollow polypropylene spacers could be loaded with biodegradable polymer carriers, such as hydrogels or solid sponges, permitting the delivery of antibiotics or growth factors [31]. Further studies using the same rat model or large-animal models would be beneficial to determine whether the fibrin clot found in these hollow polypropylene spacers can help enhance bone healing in the same way as platelet-rich plasma can [2, 30].
Supplementary Material
Acknowledgments
We thank Eugénie Jouve BS, Vincent Larose BS, and Florent Montespan MSc for their valuable technical support, and we thank Laurent Begot PhD for his help in bone mineral density calculation.
Footnotes
The institution of one or more of the authors (LM, JCM AdR, NdlE, DL, JMC, MD) has received funding from the Délégation Générale de l’Armement (grant numbers PDH-SAN-1-0217 and 0226) of the French Ministry of Defence.
Each author certifies that there are no funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article related to the author or any immediate family members.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Ethical approval for this study was obtained from the French Military Biomedical Research Institute, Brétigny-sur-Orge, France (protocol 65 DEF_IGSSA-SP).
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
This work was performed at the French Military Biomedical Research Institute, Brétigny-sur-Orge, France.
Contributor Information
James Charles Murison, Email: jc.murison@gmail.com.
Arnaud de Rousiers, Email: arnaud0254@gmail.com.
Nicolas de l’Escalopier, Email: ndelescalopier@gmail.com.
Didier Lutomski, Email: lutomski@univ-paris13.fr.
Jean-Marc Collombet, Email: collombet.irba@orange.fr.
Marjorie Durand, Email: marjorie-durand@hotmail.fr.
References
- 1.Bosemark P, Perdikouri C, Pelkonen M, Isaksson H, Tägil M. The Masquelet induced membrane technique with BMP and a synthetic scaffold can heal a rat femoral critical size defect. J Orthop Res . 2015;33:488-495. [DOI] [PubMed] [Google Scholar]
- 2.Bujoli B, Scimeca JC, Verron E. Fibrin as a multipurpose physiological platform for bone tissue engineering and targeted delivery of bioactive compounds. Pharmaceutics. 2019;11:556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Civitelli R, Armamento-Villareal R, Napoli N. Bone turnover markers: understanding their value in clinical trials and clinical practice. Osteoporos Int . 2009;20:843-851. [DOI] [PubMed] [Google Scholar]
- 4.Collin P, Laubster E, Denard PJ, Akué FA, Lädermann A. The Nice knot as an improvement on current knot options: a mechanical analysis. Orthop Traumatol Surg Res . 2016;102:293-296. [DOI] [PubMed] [Google Scholar]
- 5.De Lucca L, da Costa Marques M, Weinfeld I. Guided bone regeneration with polypropylene barrier in rabbit’s calvaria: a preliminary experimental study. Heliyon . 2018;4:e00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Monès E, Schlaubitz S, Oliveira H, et al. Comparative study of membranes induced by PMMA or silicone in rats, and influence of external radiotherapy. Acta Biomater. 2015;19:119-127. [DOI] [PubMed] [Google Scholar]
- 7.Drosse I, Volkmer E, Seitz S, et al. Validation of a femoral critical size defect model for orthotopic evaluation of bone healing: a biomechanical, veterinary and trauma surgical procedure. Tissue Eng Part C Methods. 2008;14:79-88. [DOI] [PubMed] [Google Scholar]
- 8.Gaio N, Martino A, Toth Z, Watson JT, Nicolaou D, McBride-Gagyi S. Masquelet technique: the effect of altering implant material and topography on membrane matrix composition, mechanical and barrier properties in a rat defect model. J Biomech . 2018;72:53-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giannoudis PV, Faour O, Goff T, Kanakaris N, Dimitriou R. Masquelet technique for the treatment of bone defects: tips-tricks and future directions. Injury. 2011;42:591-598. [DOI] [PubMed] [Google Scholar]
- 10.Gouron R, Petit L, Boudot C, et al. Osteoclasts and their precursors are present in the induced-membrane during bone reconstruction using the Masquelet technique. J Tissue Eng Regen Med. 2017;11:382-389. [DOI] [PubMed] [Google Scholar]
- 11.Heinrich D, Seebach C, Nau C, et al. Establishment and characterization of the Masquelet induced membrane technique in a rat femur critical-sized defect model. J Tissue Eng Regen Med. 2013;10:E382-E396. [DOI] [PubMed] [Google Scholar]
- 12.Kelly M, Macdougall K, Olasibi O, McGuire N. In vivo response to polypropylene following implantation in animal models: a review of biocompatibility. Int Urogynecol J. 2017;28:171-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein C, Monet M, Barbier V, et al. The Masquelet technique: current concepts, animal models, and perspectives. J Tissue Eng Regen Med. 2020;14:1349-1359. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Hu G, Shang P, et al. Histological characteristics of induced membranes in subcutaneous, intramuscular sites and bone defect. Orthop Traumatol Surg Res . 2013;99:958-964. [DOI] [PubMed] [Google Scholar]
- 15.Luangphakdy V, Pluhar E, Piuzzi NS, et al. The effect of surgical technique and spacer texture on bone regeneration: a caprine study using the Masquelet technique. Clin Orthop Relat Res. 2017;475:2575-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma YF, Jiang N, Zhang X, et al. Calcium sulfate induced versus PMMA-induced membrane in a critical-sized femoral defect in a rat model. Sci Rep. 2018;12:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masquelet AC. Induced membrane technique: pearls and pitfalls. J Orthop Trauma. 2017;31:S36-S38. [DOI] [PubMed] [Google Scholar]
- 18.Masquelet AC, Fitoussi F, Bégué T, Muller GP. Reconstruction of the long bones by the induced membrane and spongy autograft [in French]. Ann Chir Plast Esthet. 2000;45:346-353. [PubMed] [Google Scholar]
- 19.Masquelet AC, Gaillard J, Boutroux P, Beauthier-Landauer V, Cambon-Binder A. The wrapping induced membrane technique for treating recalcitrant nonunions [in French]. Ann Chir Plast Esthet. 2020;65:320-325. [DOI] [PubMed] [Google Scholar]
- 20.Mathieu L, Masquelet AC. Use of the induced membrane technique for long bone reconstruction in low-resource settings. Med Sante Trop. 2019;29:127-132. [DOI] [PubMed] [Google Scholar]
- 21.Mathieu L, Tossou-Odjo L, de l’Escalopier N, et al. Induced membrane technique with sequential internal fixation: use of a reinforced spacer for reconstruction of infected bone defects. Int Orthop. 2020;44:1647-1653. [DOI] [PubMed] [Google Scholar]
- 22.McBride-Gagyi S, Toth Z, Kim D, et al. Altering spacer material affects bone regeneration in the Masquelet technique in a rat femoral defect. J Orthop Res. Published online February 9, 2018. DOI: 10.1002/jor.23866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Metha M, Duda GN, Perka C, Strube P. Influence of gender and fixation stability on bone defect healing middle-aged rats: a pilot study. Clin Orthop Relat Res . 2011;469:3102-3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mi M, Papakostidis C, Wu X, Giannoudis PV. Mixed results with the Masquelet technique: a fact or a myth? Injury. 2020;51:132-135. [DOI] [PubMed] [Google Scholar]
- 25.Morelli I, Drago L, George DA, Gallazzi E, Scarponi S, Romanò CL. Masquelet technique: myth or reality? A systematic review and meta-analysis. Injury. 2016;47:S68-S76. [DOI] [PubMed] [Google Scholar]
- 26.Mozumder M, Chowdhury A, Islam M. Induced membrane technique (Masquelet technique) for the treatment of bone defect using piece of disposable syringe as a spacer in developing countries. 37th SICOT Orthopaedic World Congress, 2016, abstract no: 43308. Available at: http://www.sicot.org/sites/default/files/images/Rome/Abstract-Book-Free-Papers.pdf. Accessed July 1, 2020.
- 27.Murison JC, Pfister G, Amar S, Rigal S, Mathieu L. Metacarpal bone reconstruction by a cementless induced membrane technique. Hand Surg Rehab. 2019;38:83-86. [DOI] [PubMed] [Google Scholar]
- 28.Nau C, Seebach C, Trumm A, et al. Alteration of Masquelet’s induced membrane characteristics by different kinds of antibiotic enriched bone cement in a critical size defect model in the rat’s femur. Injury. 2016;47:325-334. [DOI] [PubMed] [Google Scholar]
- 29.Pelissier P, Masquelet AC, Bareille R, Mathoulin-Pelissier S, Amedee J. Induced membranes secrete growth factors including vascular and osteoconductive factors and could stimulate bone regeneration. J Orthop Res. 2004;22:73-79. [DOI] [PubMed] [Google Scholar]
- 30.Plachokova AS, van den Dolder J, van den Beucken JJJP, Jansen JA. Bone regenerative properties of rat, goat and human platelet-rich plasma. Int J Oral Maxillofac Surg 2009;38:861-869. [DOI] [PubMed] [Google Scholar]
- 31.Rothe R, Hauser S, Neuber C, et al. Adjuvant drug-assisted bone healing: advances and challenges in drug delivery approaches. Pharmaceutics . 2000;12:428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sagardoy T, Ehret C, Bareille R, Benoit J, Amedee J, de Monès E. Influence of external beam radiotherapy on the properties of polymethyl methacrylate- versus silicone-induced membranes in a bilateral segmental bone defect in rats. Tissue Eng Part A. 2018;24:703-710. [DOI] [PubMed] [Google Scholar]
- 33.Tang Q, Tong M, Zheng G, Shen L, Shang P, Liu H. Masquelet’s induced membrane promotes the osteogenic differentiation of bone marrow mesenchymal stem cells by activating the Smad ans MAPK pathways. Am J Transl Res. 2018;10:1211-1219. [PMC free article] [PubMed] [Google Scholar]
- 34.Toth Z, Roi M, Evans E, Watson JT, Nicolaou D, McBride-Gagyi S. Masquelet technique: effects of spacer material and micro-tomography on factor expression and bone regeneration. Ann Biomed Eng. 2019;47:174-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viateau V, Guillemin G, Bousson V, et al. Long-bone critical-sized defects treated with tissue-engineered grafts: a study on sheep. J Orthop Res. 2007;25:741-749. [DOI] [PubMed] [Google Scholar]
- 36.Viateau V, Guillemin G, Calando Y, et al. Induction of a barrier membrane to facilitate reconstruction of massive segmental diaphyseal bone defect: an ovine model. Vet Surg. 2006;35:445-452. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Friis T, Glatt V, Crawford R, Xiao Y. Structural properties of fracture haematoma: current status and future clinical implications. J Tissue Eng Regen Med. 2017;11:2864-2875. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Wei F, Luo F, Huang K, Xie Z. Induction of granulation tissue for the secretion of growth factors and the promotion of bone defect repair. J Orthop Surg Res. 2015;10:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Z, Yand D, Ma X, Zhao H, Nie C, Si Z. Successful repair of a critical-sized bone defect in the rat femur with a newly developed external fixator. Tohoku J Exp Med. 2009;219:115-120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.