Abstract
Background and Objectives
To report final, 36-month safety and clinical outcomes from the PD-1101 trial of NBIb-1817 (VY-AADC01) in participants with moderately advanced Parkinson disease (PD) and motor fluctuations.
Methods
PD-1101 was a phase 1b, open-label, dose escalation trial of VY-AADC01, an experimental AAV2 gene therapy encoding the human aromatic l-amino acid decarboxylase (AADC) enzyme. VY-AADC01 was delivered via bilateral, intraoperative MRI-guided putaminal infusions to 3 cohorts (n = 5 participants per cohort): cohort 1, ≤7.5 × 1011 vector genomes (vg); cohort 2, ≤1.5 × 1012 vg; cohort 3, ≤4.7 × 1012 vg.
Results
No serious adverse events (SAEs) attributed to VY-AADC01 were reported. All 4 non-vector–related SAEs (atrial fibrillation and pulmonary embolism in 1 participant and 2 events of small bowel obstruction in another participant) resolved. Requirements for PD medications were reduced by 21%–30% in the 2 highest dose cohorts at 36 months. Standard measures of motor function (PD diary, Unified Parkinson's Disease Rating Scale III “off”-medication and “on”-medication scores), global impressions of improvement (Clinical Global Impression of Improvement, Patient Global Impression of Improvement), and quality of life (39-item Parkinson's Disease Questionnaire) were stable or improved compared with baseline at 12, 24, and 36 months following VY-AADC01 administration across cohorts.
Discussions
VY-AADC01 and the surgical administration procedure were well-tolerated and resulted in stable or improved motor function and quality of life across cohorts, as well as reduced PD medication requirements in cohorts 2 and 3 over 3 years.
Trial Registration Information
Classification of Evidence
This study provides Class IV evidence that, in patients with moderately advanced PD and motor fluctuations, putaminal infusion of VY-AADC01 is well tolerated and may improve motor function.
Parkinson disease (PD), a progressive neurodegenerative disorder, results in significant motor and nonmotor impairments.1 Levodopa, converted to dopamine by l-amino acid decarboxylase (AADC), is the most effective treatment for PD motor symptoms.1,2 In PD, dopaminergic nigrostriatal neurons that express AADC degenerate, resulting in progressive loss of AADC in nigrostriatal terminals in the putamen and reduced synthesis of dopamine from levodopa.3,4 As PD progresses, escalating levodopa doses and adjunct medications are required to manage increasingly disabling symptoms, including motor fluctuations and dyskinesias.2,5
Gene therapy may provide durable, possibly lifelong, clinical benefits in PD following a single administration.6 Previous studies of gene therapies targeting the basal ganglia in PD have shown promise in preclinical and early clinical studies, but efficacy results have been mixed.6-12 NBIb-1817 (VY-AADC01) is an experimental adeno-associated virus serotype 2 (AAV2) gene therapy encoding the human AADC (hAADC) enzyme designed to increase dopamine production by delivering the AADC gene directly to the putamen,2 where transduced cells are expected to express AADC, allowing for localized dopamine production from the prodrug levodopa. Resulting dopamine levels are expected to depend on levodopa dosing.13 Another gene therapy approach using a lentiviral vector encoding the 3 enzymes required for endogenous dopamine synthesis (tyrosine hydroxylase, cyclohydrolase 1, and AADC) is also under development.14
PD-1101 was a phase 1b, open-label, dose-escalation trial of VY-AADC01 delivered bilaterally to the putamen in participants with moderately advanced PD and motor fluctuations.2 We report final 36-month data from PD-1101; interim safety and efficacy results have been published.2
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
A detailed description of the methods used in PD-1101 has been published.2 The trial protocol and participant informed consent forms were approved by the institutional review boards of the University of California, San Francisco [UCSF], the University of Pittsburgh, and the US Food and Drug Administration (FDA). The protocol was also reviewed and approved by the Recombinant DNA Advisory Committee of the NIH; all participants provided written informed consent prior to trial participation. A data safety monitoring board reviewed safety data throughout the trial. The clinicaltrials.gov identifier is NCT01973543.
Trial Design and Participants
PD-1101 was a phase 1b, open-label, dose escalation trial intended to provide Class IV evidence that, in patients with moderately advanced PD and motor fluctuations, putaminal infusion of VY-AADC01 is well tolerated and may improve motor function. Participants were enrolled at 2 clinical sites (UCSF and University of Pittsburgh Medical Center [UPMC]) and received surgery between May 7, 2014, and December 9, 2016. Eligible participants were 40–70 years of age with moderately advanced PD and a history of responsiveness to dopaminergic therapy, disabling motor fluctuations despite optimal medical therapy, disease duration ≥5 years, modified Hoehn & Yahr (mH&Y) stage ≥2.5 “off”-medication, and a stable PD medication regimen for ≥4 weeks before screening. Full inclusion and exclusion criteria are detailed on ClinicalTrials.gov. By cohort, for cohort 1, 5/5 participants were treated and followed at UCSF; for cohort 2, 4 participants were treated and followed at UCSF and 1 at UPMC; and for cohort 3, 2 participants were treated and followed at UCSF and 3 at UPMC.
VY-AADC01 Preparation, Dosing, and Administration
VY-AADC01, a recombinant AAV2 vector carrying complementary DNA encoding the human AADC enzyme under control of a cytomegalovirus promoter, was prepared as previously described2 in accordance with FDA good manufacturing practice. VY-AADC01 was delivered at escalating maximum total doses across 3 trial cohorts: cohort 1 received VY-AADC01 at a concentration of 8.3 × 1011 vector genomes (vg)/mL and up to 450 µL per putamen for a maximum total dose of up to 7.5 × 1011 vg; cohort 2 received the same concentration as cohort 1 and up to 900 µL per putamen for a total dose of up to 1.5 × 1012 vg; in cohort 3, the concentration was increased to 2.6 × 1012 vg/mL and the volume (up to 900 µL per putamen) was kept identical to cohort 2 for a maximum total dose of up to 4.7 × 1012 vg. Full details of the surgical administration of VY-AADC01 have been published.2,12 In brief, participants were placed under general anesthesia and positioned supine within the bore of an MRI scanner. The ClearPoint system (ClearPoint Neuro, Inc.), which consists of an MRI-compatible skull-mounted aiming device (SmartFrame) and MRI-integrated software, was used to plan and guide cannula placement. VY-AADC01 was administered via 2–3 trajectories to the putamen using SmartFlow stepped tip cannulae (ClearPoint Neuro, Inc.) and convection-enhanced delivery (CED), with a primary goal of achieving maximal possible coverage of the postcommissural putamen, as it is the motor region of the putamen and shows greater loss of dopaminergic terminals than the anterior putamen in PD.12,15 Cannula trajectories were chosen to center the cannula within the putamen, with the tip at least 3 mm from any putaminal borders, and to avoid direct contact with perivascular spaces. Prior to administration, VY-AADC01 was admixed with the contrast agent gadoteridol to allow for real-time MRI monitoring of infusate distribution and assessment of putamen coverage. Gadoteridol distribution has been shown to be a reliable predictor of vector distribution following CED of AAV2-AADC in primates.16 The infusion technique was refined over the course of the trial to maximize putamen coverage and mitigate “off-target” distribution.12 Participants were monitored postoperatively in the hospital for at least 1 night.
Putamen Coverage Analysis
VY-AADC01 volumetric coverage of the putamen was determined using iPlan Flow software (Brainlab AG) as previously described.2 Gadoteridol-enhanced magnetic resonance (MR) images obtained at the end of infusion were compared with preinfusion MR images to determine the percentage of total putamen volume to which drug was administered. The overlap between pre- and postinfusion MR images was used to calculate percent coverage of each putamen, with total putamen coverage expressed as a single value representing the average across right and left putamen.
18F-DOPA PET to Assess AADC Activity
The distribution and change in AADC activity at 6 months postadministration was a secondary outcome. (18)F-fluoro-l-dihydroxyphenylalanine (18F-DOPA) PET scans were acquired at baseline prior to administration of VY-AADC01 and 5–6 months postadministration as previously described.2 On each occasion, participants were given oral carbidopa (2.5 mg/kg; 200 mg maximum dose) 60–90 minutes prior to 18F-DOPA administration, and acquisition frames were captured 65–75 minutes later for subsequent analysis. The striatum (putamen) to occipital cortex standardized uptake ratio (SOR-1) was calculated for the right and left putamen, with the 2 values averaged to provide a single value. The 5- to 6-month postadministration value was expressed as percent change from baseline.
Safety and Clinical Outcomes
The primary outcome measure was the safety and tolerability of AADC gene transfer (Class IV evidence) as assessed by frequency of adverse events (AEs) and serious AEs (SAEs) and their relationship to VY-AADC01 or the trial procedure. Secondary outcome measures included changes from baseline in PD medications measured as daily levodopa equivalent dose (LED)17,18 and motor function as assessed by PD diary19 good “on” time (defined as the sum of “on” time with no dyskinesia and “on” time with nontroublesome dyskinesia) and “off” time, Unified Dyskinesia Rating Scale (UDysRS) total scores, Unified Parkinson's Disease Rating Scale Part III (UPDRS III) “off”-medication and “on”-medication scores, and mH&Y scale “off”-medication scores. Changes to PD medications were made at the discretion of the investigator. Patient Global Impression of Improvement (PGI-I) and Clinical Global Impression of Improvement (CGI-I) were assessed throughout follow-up. Changes in quality of life were assessed using the 39-item Parkinson's Disease Questionnaire (PDQ-39). The “off” medication state was defined as ≥12 hours after stopping PD medications; the “on” medication state was defined as 30–120 minutes after taking the first daily dose of PD medications when participants and investigators both agreed that benefits of PD medications had occurred. If the participant was not fully “on” as judged by the participant and investigator, an additional ½ to 1 carbidopa/levodopa 25/100 mg tablet was administered.
Statistical Analysis
A sample size of up to 20 participants was chosen to provide preliminary evidence of safety and potential efficacy; this estimate was not based on statistical power calculations. Primary and secondary outcome measures were analyzed using descriptive methods. Postsurgical mean absolute changes from baseline or percent changes from baseline were summarized by cohort. Safety analyses were based on the safety population, which included all participants who initiated any trial procedure, starting from the beginning of preinfusion anesthesia. The intent-to-treat (ITT) population included all enrolled participants who had efficacy assessment data at baseline and at ≥1 postsurgical visit. The ITT population was used for analyses of vector coverage of the putamen, AADC expression, and clinical efficacy. PD diary data were normalized to a 16-hour waking day. Vector coverage of the putamen and changes from baseline in AADC expression and clinical outcomes are reported as mean ± SEM. One participant in cohort 1 underwent bilateral globus pallidus interna (GPi) deep brain stimulation (DBS) placement 34 months after VY-AADC01. The indication for DBS was gradual worsening of PD motor symptoms including rest tremor, dyskinesias, and wearing off. All these features preceded participation in this trial and were considered consistent with typical PD progression. In this participant, 36-month assessments were performed with DBS stimulation “on.” A sensitivity analysis of 36-month outcomes in cohort 1 excluding this participant's data was conducted.
Data Availability
NBIb-1817 (VY-AADC01) is an investigational product that has not yet been approved for any indication; we do not plan to share the study data or related documents at this time.
Results
Baseline Characteristics
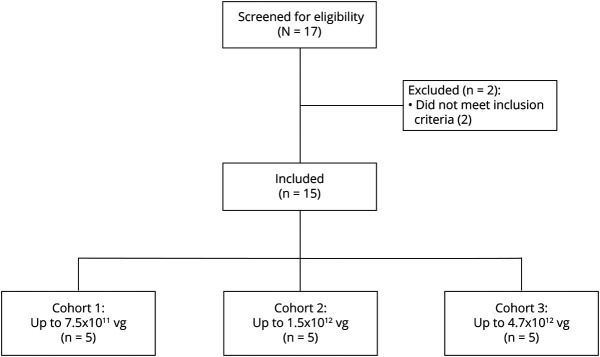
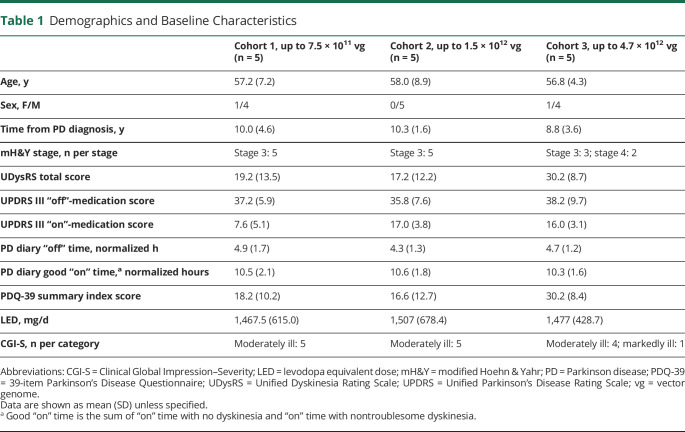
The ITT and safety populations included 15 participants (5 participants in each cohort; Figure 1). Demographic and baseline characteristics were generally similar across cohorts and consistent with moderately advanced PD (Table 1). At baseline, the UPDRS III “on”-medication score was lower in cohort 1; in cohort 3, the UDysRS total score and PDQ-39 summary index score were higher than the other cohorts. Cohort 3 included 2 participants with mH&Y stage 4 “off” medication and 3 participants with mH&Y stage 3 “off” medication, whereas all participants in cohorts 1 and 2 were stage 3.
Figure 1. Participant Flow Diagram for the PD-1101 Trial.
PD-1101 was a phase 1b, open-label, dose escalation trial of VY-AADC01 in participants with moderately advanced Parkinson disease. Of the 2 participants excluded from enrollment, 1 participant had an elevated anti-AAV2 antibody titer and the other participant had a modified Hoehn & Yahr score below the inclusion criteria. Participants were screened for eligibility within 60 days of VY-AADC01 administration and followed for 36 months after administration. All participants were included in analyses.
Table 1.
Demographics and Baseline Characteristics
Safety Outcomes
VY-AADC01 and the surgical procedure were well-tolerated over the 3-year trial. A total of 4 SAEs were reported in 2 participants, all of which resolved and were assessed by the investigator as not related to VY-AADC01. As previously reported,2 1 participant experienced a pulmonary embolism and subsequent atrial fibrillation due to deep vein thrombosis (DVT). The investigator assessed the DVT as unrelated to VY-AADC01. The DVT was considered likely attributable to immobilization during surgery. Intraoperative sequential leg compression for DVT prophylaxis was used during subsequent surgical procedures, and no other AEs of thromboembolic events occurred during the trial. Another participant experienced 2 SAEs of small bowel obstruction, which occurred 6 days apart, 29 months after VY-AADC01 administration. A summary of SAEs and treatment-emergent AEs (TEAEs) occurring in >2 participants is reported in Table 2. Most events were either grade 1 or grade 2 (97%) in severity and considered unrelated to VY-AADC01 (91%). The most common TEAE was headache in the immediate postoperative period, which affected 11 participants (73%) overall. Dyskinesia was reported in 4 participants (27%) and was considered probably related to treatment in all 4 participants. Each participant was carefully monitored for the development of worsening dyskinesias (including runaway dyskinesias) but in all cases, dyskinesias were transient and resolved with dose reduction of PD medications or the addition of amantadine. No participants discontinued evaluations following administration of VY-AADC01 due to AEs.
Table 2.
Number of Participants With Any Serious Adverse Event and Treatment-Emergent Adverse Events That Occurred in >2 Participants by MedDRA Preferred Term
VY-AADC01 Coverage of the Putamen and AADC Activity Changes
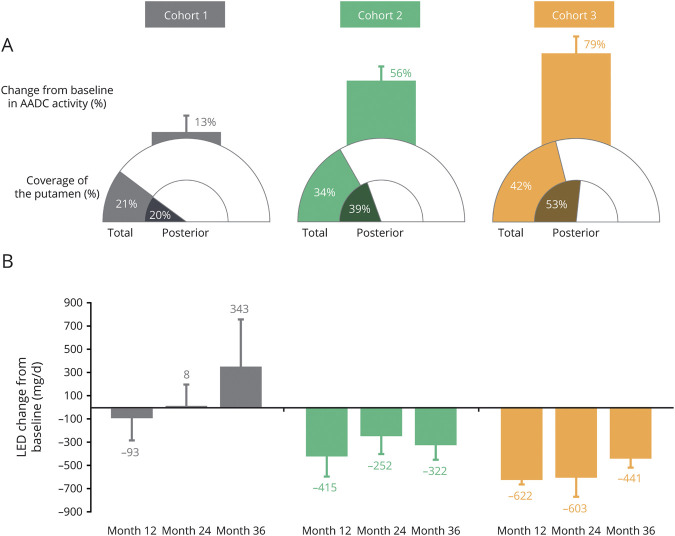
As previously reported,2 VY-AADC01 coverage of the total putamen was 21% ± 1%, 34% ± 2%, and 42% ± 3% in cohorts 1, 2, and 3, respectively. Coverage of the preferentially-targeted postcommissural putamen was 20% ± 6%, 39% ± 5%, and 53% ± 5%, respectively (Figure 2A). Changes in AADC enzyme activity as measured by 18F-DOPA PET 5–6 months after VY-AADC01 administration followed a dose-dependent pattern, with increases of 13% ± 7%, 56% ± 13%, and 79% ± 15% in cohorts 1, 2, and 3, respectively (Figure 2A). Increases from baseline in AADC enzyme activity as measured by 18F-DOPA PET and total putamen coverage were positively correlated.2
Figure 2. AADC Enzyme Activity, VY-AADC01 Putaminal Coverage, and LED Change From Baseline by Cohort in PD-1101.
(A) Coverage and l-amino acid decarboxylase (AADC) enzyme activity data shown are mean percent coverage (total putamen coverage and posterior putamen coverage) and percent AADC increase from baseline. Error bars represent SEM. Change from baseline in AADC enzyme activity was measured using 18F-DOPA PET 5–6 months after VY-AADC01 administration. The change in AADC enzyme activity as measured by 18F-DOPA PET correlated with total putamen coverage. (B) Levodopa equivalent dose (LED) changes from baseline at months 12, 24, and 36 are shown as mean change in mg/day ± SEM. Daily PD medication requirements were decreased from baseline in cohorts 2 and 3 throughout the trial. A sensitivity analysis that excluded the participant in cohort 1 who underwent deep brain stimulation resulted in a mean ± SEM change from baseline of 531 ± 465 mg/day at month 36.
Changes in PD Medication Requirements
Daily PD medication requirements as measured by LED were decreased from baseline in cohorts 2 and 3 throughout the trial (Figure 2B). At end of trial (36 months after VY-AADC01 administration), mean changes from baseline in LED of −322 ± 124 mg/day (21% decrease) and −441 ± 73 mg/day (30% decrease) were documented in cohorts 2 and 3, respectively. In cohort 1, LED remained stable at 12 and 24 months, but then increased by 343 ± 406 mg/day (23% increase) above baseline at 36 months; excluding data from the participant who underwent DBS resulted in an increase of 531 ± 465 mg/day from baseline.
Changes in Motor Function, Global Impressions, and Quality of Life
All other clinical outcomes were stable or improved compared with baseline in the “on”-medication and “off”-medication states across all 3 cohorts throughout this 36-month trial. Excluding data from the participant who underwent DBS had a minimal effect on the reported results.
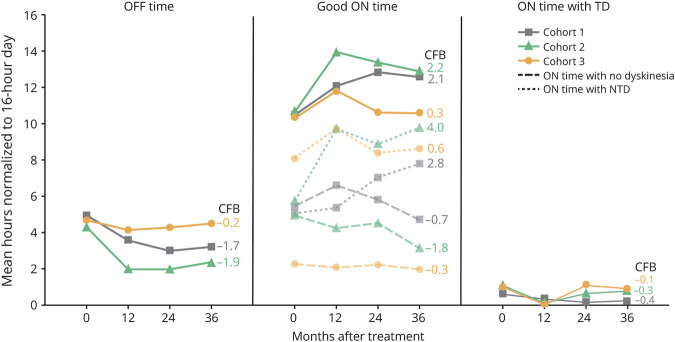
PD diary data are shown in Figure 3. Improvements in PD diary good “on” time were reported in cohorts 1 and 2 at all annual follow-up assessments, with increases from baseline at the final 36-month follow-up of 2.1 ± 0.6 and 2.2 ± 1.7 hours, respectively. In cohort 3 there was an improvement in good “on” time at 12 months (1.5 ± 0.5 hours) that returned to baseline at both 24 months (0.3 ± 1.2 hours) and 36 months (0.3 ± 0.7 hours). PD diary “on” time with troublesome dyskinesia and UDysRS total score remained relatively stable and comparable to baseline over the 36-month trial in each of the 3 cohorts. At 36 months, changes from baseline in PD diary “on” time with troublesome dyskinesia and UDysRS total score in cohorts 1, 2, and 3, respectively, were −0.4 ± 0.3 hours and −2.4 ± 3.7 points, −0.3 ± 1.3 hours and 4.4 ± 2.5 points, and −0.1 ± 0.6 hours and 4.0 ± 5.0 points. Improvements in PD diary “off” time were similar to those observed for good “on” time throughout the trial. Specifically, improvements in “off” time were reported in cohorts 1 and 2 at 12 months (−1.4 ± 0.9 hours and −2.3 ± 0.6 hours, respectively) that were maintained through the final 36-month follow-up (−1.7 ± 0.8 hours and −1.9 ± 0.5 hours, respectively). An improvement in “off” time was reported in cohort 3 at 12 months (−0.5 ± 0.8 hours) that was maintained at 24 months (−0.4 ± 0.7 hours) but returned to baseline at 36 months (−0.2 ± 0.9 hours).
Figure 3. PD Diary “Off” Time and “On” Times at Baseline and Months 12, 24, and 36.
Data shown are mean hours normalized to a 16-hour waking day. Change from baseline (CFB) at 36 months is reported to the right of each panel. PD diary “off” time and good “on” time (sum of “on” time with no dyskinesia and “on” time with nontroublesome dyskinesia [NTD]) were stable or improved from baseline throughout the trial. In a sensitivity analysis that excluded the participant in cohort 1 who underwent deep brain stimulation, “off” time and good “on” time (mean ± SEM) at month 36 were 3.4 ± 1.5 hours and 12.3 ± 1.6 hours, respectively. TD = troublesome dyskinesia.
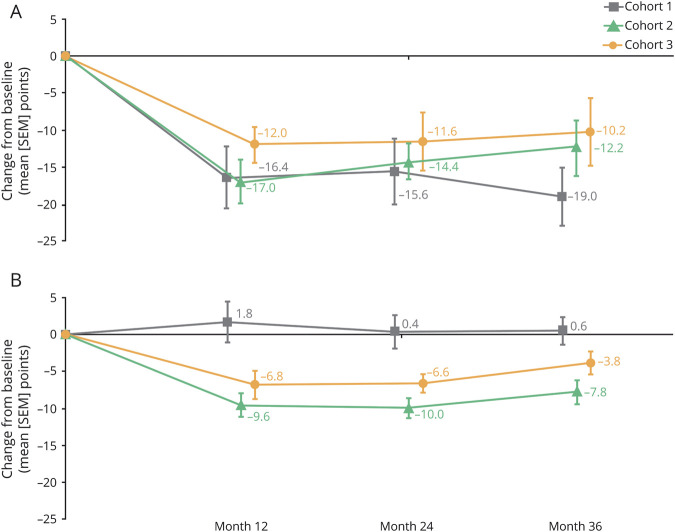
UPDRS III “off”-medication scores improved from baseline in all cohorts at all annual follow-up assessments (Figure 4A). Improvements in cohorts 1, 2, and 3 at 12 months were maintained through 36 months, with changes from baseline of −19.0 ± 3.9 points, −12.2 ± 3.3 points, and −10.2 ± 4.6 points, respectively, at the final 36-month follow-up. Improvements in UPDRS III “on”-medication scores were observed in cohorts 2 and 3 at 12 months, which persisted at the final 36-month follow-up (−7.8 ± 1.6 points and −3.8 ± 1.5 points, respectively; Figure 4B). The UPDRS III “on”-medication score in cohort 1 remained low and comparable to baseline at all annual follow-up assessments.
Figure 4. Changes From Baseline in UPDRS III Scores.
(A) “Off” medication. (B) “On” medication. Data shown are mean change in points ± SEM. Unified Parkinson's Disease Rating Scale (UPDRS) III “off”-medication scores improved in all cohorts throughout the trial, and “on”-medication scores improved in cohorts 2 and 3 throughout the trial. A sensitivity analysis that excluded the participant in cohort 1 who underwent deep brain stimulation resulted in a mean ± SEM change from baseline of −17.3 ± 4.5 points (“off” medication) and 0.8 ± 2.4 points (“on” medication) at month 36.
Improvement in mH&Y stage (“off” medication) at the final 36-month follow-up assessment was observed in 14/15 participants, and 1 participant in cohort 3 had no change. In cohort 1, of 5 participants with mH&Y stage 3 at baseline, 4 participants improved to stage 2 and 1 participant improved to stage 2.5 at 36 months. All 5 participants in cohort 2 improved from mH&Y stage 3 at baseline to stage 2 at 36 months. In cohort 3, of 3 participants with mH&Y stage 3 and 2 participants with stage 4 at baseline, 3 participants improved to stage 2 and 1 participant improved to stage 2.5.
CGI-I scale scores indicated improvement from baseline in all 15 participants. At 36 months, clinicians rated 1 participant as “very much improved,” 11 participants as “much improved,” and 3 participants as “minimally improved.” PGI-I scale score results were similar and indicated improvement from baseline in 12 of 15 participants at 36 months, with 1 participant “very much improved,” 10 participants “much improved,” and 1 participant “minimally improved.” Three of 15 participants reported being “minimally worse.”
In cohort 1, quality of life as assessed by the PDQ-39 summary index score remained stable and comparable to baseline at all annual follow-up assessments (Figure 5). PDQ-39 summary index scores were improved in cohort 2 throughout the trial, and in cohort 3, were improved at 12 and 24 months but were returning toward baseline at 36 months.
Figure 5. Changes From Baseline in PDQ-39 Summary Index Score at Months 12, 24, and 36.
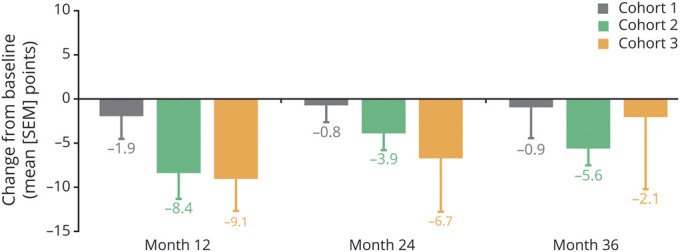
Data shown are mean change in points ± SEM. Quality of life (39-item Parkinson's Disease Questionnaire [PDQ-39]) scores were improved throughout the trial in cohort 2, and at months 12 and 24 in cohort 3. A sensitivity analysis that excluded the participant in cohort 1 who underwent deep brain stimulation resulted in a mean ± SEM change from baseline of −0.6 ± 4.6 points at month 36.
Classification of Evidence
This trial provides Class IV evidence that VY-AADC01 and the associated surgical delivery procedure were well-tolerated in a population 40–70 years of age with moderately advanced PD, and that in the 2 higher-dose cohorts (at total doses of up to 1.5 × 1012 vg and 4.7 × 1012 vg), stable or improved clinical outcomes were observed along with reduced PD medication requirements for at least 3 years.
Discussion
The final 3-year results from the PD-1101 trial demonstrate that VY-AADC01 and the surgical delivery procedure were well-tolerated with no vector-related SAEs reported. The safety and tolerability of VY-AADC01 was observed with infusion volumes that were several-fold higher than those in previous AADC gene therapy trials in PD.5,20 The most common TEAEs were mostly transient, including dyskinesias, which resolved with PD medication dose reductions or addition of amantadine.
All cohorts showed stability or improvement in motor function, global impressions of change, and quality of life throughout the 3 years. In cohorts 2 and 3, the stability or improvement in outcomes was observed with reduced LED, which may indicate an increased responsiveness to therapeutic levodopa after treatment with VY-AADC01 as previously reported from the levodopa infusion substudy of this trial.21 Indications of improved motor function following VY-AADC01 were observed across multiple endpoints. Specifically, PD diary good “on” and “off” times improved throughout the trial in cohorts 1 and 2, whereas cohort 3 improved at month 12 before returning to baseline at month 36. UPDRS III “off”-medication and “on”-medication scores also improved from baseline in all cohorts at all annual follow-up assessments, except for the “on”-medication score in cohort 1, which remained low and comparable to baseline throughout the trial (note that the UPDRS III mean “on”-medication score in cohort 1 was 7.6 ± 2.3 at baseline). Similarly, improvement in mH&Y stage (“off” medication) at the final 3-year follow-up assessment was observed in 14/15 participants, and 1 participant in cohort 3 remained stable. Importantly, PD diary “on” time with troublesome dyskinesia and UDysRS total score remained relatively stable and comparable to baseline in each of the 3 cohorts, indicating a lack of increased dyskinesia over the 3-year trial. The clinician- and participant-rated global impressions of change (CGI-I and PGI-I) scores indicated improvement in 100% and 80% of participants, respectively, and quality of life as assessed by the PDQ-39 summary index score indicated improvement in cohort 2 and stability in cohort 1 throughout the trial, with a transient benefit in cohort 3 that returned toward baseline at the final assessment. Collectively, these results suggest that VY-AADC01 may provide clinical and functional benefits in moderately advanced PD. Potential clinical and functional benefits should be considered in the context of the study design, which was phase 1b, open label, and intended to provide Class IV evidence that putaminal infusion of VY-AADC01 is well tolerated in patients with moderately advanced PD and motor fluctuations and may improve motor function and did not include a control group. A sham surgery–controlled trial will be needed to demonstrate robust efficacy of VY-AADC01.
The durability of motor function stability or improvement throughout the 3-year trial, with concurrent LED reduction in cohorts 2 and 3, suggests that a single infusion of VY-AADC01 may have been associated with a persistent increase in putaminal AADC enzyme activity.22 Consistent with PD-1101, persistent increases in AADC enzyme activity have been described in a previous phase 1 clinical trial of AAV2-hAADC that reported a dose-dependent increase in AADC activity in the putamen that was maintained through 4 years of follow-up,22 and in a preclinical nonhuman primate (NHP) study, which demonstrated that treatment with AAV2-hAADC restored enzyme activity to normal levels and enhanced behavioral responses to levodopa through 8 years of follow-up.23 The primary cellular source of the persistently increased AADC activity in the NHP putamen was found to be medium spiny neurons,23 which do not degenerate in PD.13 AADC expression in these cells may provide an alternate path for dopamine production to supplement the loss from the nigrostriatal neurons that degenerate in PD.
The levels of putamen coverage achieved in cohorts 2 and 3 of PD-1101 (34% and 42%, respectively) exceeded putamen coverage in cohort 1 (21%). A similar pattern was observed for AADC activity as measured by 18F-DOPA PET (56% and 79% increases in cohorts 2 and 3, respectively, vs an increase of 13% in cohort 1). Unlike PD-1101, prior AAV2-hAADC clinical trials likely did not achieve putamen coverage above ∼10%.12 In the prior phase 1 clinical trial of AAV2-hAADC, despite modest early clinical benefit and persistent enhancement of AADC activity, durable clinical efficacy (>12 months) was not observed.5,22 The potential benefit of achieving sufficient coverage and AADC activity enhancement in PD-1101 was evident across multiple clinical assessments and outcomes indicating an improvement from baseline that was generally greater in cohorts 2 and 3 compared with cohort 1, including LED reduction, UPDRS III “on”-medication score, and PDQ-39 summary index. However, we did not observe a clear dose response between cohorts 2 and 3. The lack of an evident dose response between the 2 higher-dose cohorts may be attributable to the small number of participants, variability of baseline characteristics (i.e., cohort 3 had higher baseline mH&Y, UDysRS, and PDQ-39 scale scores, indicating greater overall PD severity than cohort 2), or the possibility of a “ceiling effect,” whereby exceeding the dose required for response does not provide a greater magnitude of benefit.
Prior to PD-1101, the abovementioned open-label, phase 1 clinical trial investigated the effects of AAV2-hAADC gene therapy administered to the putamen in PD and followed participants for 4 years.5,22 That trial confirmed the safety and tolerability of the AAV2-hAADC vector delivered to the putamen using CED, and reported that treatment persistently increased AADC enzyme activity throughout the follow-up period.22 However, no reductions in LED were observed, and benefits to motor function (total UPDRS “on”-medication and “off”-medication scores) were present for the first 12 months before deteriorating thereafter.22 Another trial of 6 months duration that included participants with PD in Japan investigated the effects of AAV2-hAADC administered to the putamen and reported a favorable safety/tolerability profile, increase of AADC enzyme activity, modest improvements in motor function, and no reduction in LED.20 Although these trials used a different surgical approach than PD-1101, the low infusion volumes (100 µL per putamen), total vector doses (up to 3 × 1011 vg), and target coverage (<10%) likely played a role in the limited efficacy reported.12 Neither trial was equipped to monitor infusate distribution during the infusions to assess target coverage and off-target spread. In contrast, PD-1101 employed innovative techniques, specifically, real-time MRI-monitored infusions, stepped tip cannula design, and progressive cannula advancement during infusion, to achieve greater infusion volumes and target tissue coverage than previous AAV2-hAADC trials.12 Other gene therapy trials in PD assessed the effects of neurotrophic factors or other enzymes (for a comprehensive review, see Richardson et al.12). Although methodologic differences between these prior trials and PD-1101 preclude direct comparison, few of the prior trials reported long-term (>12 months) clinical benefit with reduced LED.
DBS is a standard therapy used to improve motor symptoms in advanced PD when these are no longer adequately managed with medication.24 Long-term follow-up of participants with PD who received GPi or subthalamic nucleus DBS reported stable improvements in the primary outcome of UPDRS III scores in the “on” stimulation/“off” medication state with associated reductions in LED over 36 months.24 These results appear similar to PD-1101 in that benefits to motor function were observed concurrent with reduced LED through 3 years of follow-up.
PD-1101 did not include a control group and its primary objective was to assess the safety and tolerability of AADC gene therapy. Although there are limited data on the natural progression of motor impairment in moderately advanced PD to help contextualize the observed effects of VY-AADC01 on motor function in the current trial, long-term extension studies of therapies designed for use in moderately advanced PD can provide insight into the expected progression in such participants under medical management. For example, a long-term extension study of levodopa-carbidopa intestinal gel reported initial improvements over the first year of treatment in PD diary “off” time and good “on” time (∼4 hours each) that were maintained over ∼3 years of follow-up.25 However, UPDRS (total and part III on medication) and PDQ-39 scores worsened even as LED remained constant or increased throughout follow-up. Similarly, long-term studies (CLEOPATRA-PD and PREFER) of rotigotine, a dopamine receptor agonist administered via a transdermal delivery system, reported initial improvement in UPDRS II and III scores during a 5- to 7-week dose titration period in participants with advanced PD that then steadily declined over the subsequent 3-year maintenance period and LED increased ∼40%–100%.26 These findings, combined with worsening mH&Y stage scores, suggest continuing disease progression over the course of both studies despite continued medical therapy. In PD-1101, stability or improvements in clinician- and participant-reported outcomes were observed, along with reduced LED in the 2 higher-dose cohorts, over a similar 3-year period. Although these PD-1101 results suggest potential long-term clinical benefit following a single administration of VY-AADC01, a sham surgery controlled trial will be needed to provide a robust assessment of treatment efficacy given the placebo effects observed in previous drug and surgical trials in PD.7,27
The PD-1101 trial supports the long-term safety and potential durability of VY-AADC01 clinical effects following a single administration. No vector-related SAEs were reported and clinical outcomes of motor function and quality of life were stable or improved throughout the 3-year trial duration in a PD population that would otherwise be expected to decline over this time period. PD-1101, along with a companion IV administered levodopa response substudy21 and a second, ongoing open-label phase 1b trial (PD-1102, NCT03065192),28 suggest that AAV2-hAADC gene therapy may confer meaningful benefits to patients with PD.
Acknowledgment
The authors thank the people with Parkinson disease and their families who participated in this trial, as well as the trial support teams, and Kyle B. Herbert and Patricia Porter for study support and coordination.
Glossary
- AADC
L-amino acid decarboxylase
- AAV2
adeno-associated virus serotype 2
- AE
adverse event
- CED
convection-enhanced delivery
- CGI-I
Clinical Global Impression–Improvement
- DBS
deep brain stimulation
- DVT
deep vein thrombosis
- 18F-DOPA
(18)F-fluoro-l-dihydroxyphenylalanine
- FDA
Food and Drug Administration
- GPi
globus pallidus interna
- hAADC
human amino acid decarboxylase
- ITT
intent-to-treat
- LED
levodopa equivalent dose
- mH&Y
modified Hoehn & Yahr
- MR
magnetic resonance
- NHP
nonhuman primate
- PD
Parkinson disease
- PDQ-39
39-item Parkinson's Disease Questionnaire
- PGI-I
Patient Global Impression of Improvement
- SAE
serious adverse event
- SEM
standard error of the mean
- TEAE
treatment-emergent adverse event
- UCSF
University of California, San Francisco
- UDysRS
Unified Dyskinesia Rating Scale
- UPDRS
Unified Parkinson's Disease Rating Scale
- UPMC
University of Pittsburgh Medical Center
- vg
vector genome
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Study Funding
Financial support for this trial and trial materials were provided by the Michael J. Fox Foundation, Voyager Therapeutics, Inc., and Neurocrine Biosciences, Inc.
Disclosure
C.W. Christine has received institutional research support from Voyager Therapeutics, Neurocrine Biosciences, the Michael J. Fox Foundation, and Brain Neurotherapy Bio. R.M. Richardson has received institutional research support from Voyager Therapeutics and consulting fees or honoraria from Voyager Therapeutics and Neurocrine Biosciences. A.D. Van Laar has received institutional research support and travel support from Voyager Therapeutics. She is an employee of Brain Neurotherapy Bio and has received consulting fees and travel support from Brain Neurotherapy Bio. M.E. Thompson has received institutional research support from Voyager Therapeutics, Brain Neurotherapy Bio, and UniQure, and travel support from Voyager Therapeutics. Drs Khwaja, Li, and Meier are full-time employees of Voyager Therapeutics and hold stock/stock options in Voyager Therapeutics. E.M. Fine is a former employee of Voyager Therapeutics. O.S. Khwaja is a board member of Korro Bio and Pureos Bioventures and has received consulting fees from Vico Therapeutics for serving as a scientific advisory board member. G.S. Liang and E.W. Roberts are full-time employees of Neurocrine Biosciences and hold stock/stock options in Neurocrine Biosciences. M.L. Pfau and J.R. Rodman are employees of ApotheCom and provided editorial and writing support for this publication, which was funded by Neurocrine Biosciences. K.S. Bankiewicz has received institutional research support from the National Institute of Neurologic Disorders and Stroke and the Michael J. Fox Foundation and receives consulting fees from AskBio. P.S. Larson has received institutional research support from the Michael J. Fox Foundation, Voyager Therapeutics, Neurocrine Biosciences, and Brain Neurotherapy Bio and consulting fees or honoraria from Sio Gene Therapies, Aspen Neuroscience, and Cellular Dynamics. Go to Neurology.org/N for full disclosures.
References
- 1.Fahn S, Oakes D, Shoulson I, et al. Levodopa and the progression of Parkinson's disease. N Engl J Med. 2004;351(24):2498-2508. [DOI] [PubMed] [Google Scholar]
- 2.Christine CW, Bankiewicz KS, Van Laar AD, et al. Magnetic resonance imaging-guided phase 1 trial of putaminal AADC gene therapy for Parkinson's disease. Ann Neurol. 2019;85(5):704-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kordower JH, Olanow CW, Dodiya HB, et al. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain. 2013;136(Pt 8):2419-2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciesielska A, Samaranch L, San Sebastian W, et al. Depletion of AADC activity in caudate nucleus and putamen of Parkinson's disease patients; implications for ongoing AAV2-AADC gene therapy trial. PLoS One. 2017;12(2):e0169965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christine CW, Starr PA, Larson PS, et al. Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology. 2009;73(20):1662-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sudhakar V, Richardson RM. Gene therapy for neurodegenerative diseases. Neurotherapeutics. 2019;16(1):166-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olanow CW, Bartus RT, Baumann TL, et al. Gene delivery of neurturin to putamen and substantia nigra in Parkinson disease: a double-blind, randomized, controlled trial. Ann Neurol. 2015;78(2):248-257. [DOI] [PubMed] [Google Scholar]
- 8.LeWitt PA, Rezai AR, Leehey MA, et al. AAV2-GAD gene therapy for advanced Parkinson's disease: a double-blind, sham-surgery controlled, randomised trial. Lancet Neurol. 2011;10(4):309-319. [DOI] [PubMed] [Google Scholar]
- 9.Heiss JD, Lungu C, Hammoud DA, et al. Trial of magnetic resonance-guided putaminal gene therapy for advanced Parkinson's disease. Mov Disord. 2019;34(7):1073-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palfi S, Gurruchaga JM, Ralph GS, et al. Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson's disease: a dose escalation, open-label, phase 1/2 trial. Lancet. 2014;383(9923):1138-1146. [DOI] [PubMed] [Google Scholar]
- 11.Palfi S, Gurruchaga JM, Lepetit H, et al. Long-term follow-up of a phase I/II study of ProSavin, a lentiviral vector gene therapy for Parkinson's disease. Hum Gene Ther Clin Dev. 2018;29(3):148-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richardson RM, Bankiewicz KS, Christine CW, et al. Data-driven evolution of neurosurgical gene therapy delivery in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2020;91(11):1210-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bankiewicz KS, Eberling JL, Kohutnicka M, et al. Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp Neurol. 2000;164(1):2-14. [DOI] [PubMed] [Google Scholar]
- 14.Stewart HJ, Ralph GS, Fong-Wong L, et al. Optimizing transgene configuration and protein fusions to maximize dopamine production for the gene therapy of Parkinson's disease. Hum Gene Ther Clin Dev. 2016;27(3):100-110. [DOI] [PubMed] [Google Scholar]
- 15.Oh M, Kim JS, Kim JY, et al. Subregional patterns of preferential striatal dopamine transporter loss differ in Parkinson disease, progressive supranuclear palsy, and multiple-system atrophy. J Nucl Med. 2012;53(3):399-406. [DOI] [PubMed] [Google Scholar]
- 16.Su X, Kells AP, Salegio EA, et al. Real-time MR imaging with Gadoteridol predicts distribution of transgenes after convection-enhanced delivery of AAV2 vectors. Mol Ther. 2010;18(8):1490-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25(15):2649-2653. [DOI] [PubMed] [Google Scholar]
- 18.Espay AJ, Pagan FL, Walter BL, et al. Optimizing extended-release carbidopa/levodopa in Parkinson disease: consensus on conversion from standard therapy. Neurol Clin Pract. 2017;7(1):86-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauser RA, Friedlander J, Zesiewicz TA, et al. A home diary to assess functional status in patients with Parkinson's disease with motor fluctuations and dyskinesia. Clin Neuropharmacol. 2000;23(2):75-81. [DOI] [PubMed] [Google Scholar]
- 20.Muramatsu S, Fujimoto K, Kato S, et al. A phase I study of aromatic L-amino acid decarboxylase gene therapy for Parkinson's disease. Mol Ther. 2010;18(9):1731-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nutt JG, Curtze C, Hiller A, et al. Aromatic L-amino acid decarboxylase gene therapy enhances levodopa response in Parkinson's disease. Mov Disord. 2020;35(5):851-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mittermeyer G, Christine CW, Rosenbluth KH, et al. Long-term evaluation of a phase 1 study of AADC gene therapy for Parkinson's disease. Hum Gene Ther. 2012;23(4):377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hadaczek P, Eberling JL, Pivirotto P, Bringas J, Forsayeth J, Bankiewicz KS. Eight years of clinical improvement in MPTP-lesioned primates after gene therapy with AAV2-hAADC. Mol Ther. 2010;18(8):1458-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weaver FM, Follett KA, Stern M, et al. Randomized trial of deep brain stimulation for Parkinson disease: thirty-six-month outcomes. Neurology. 2012;79(1):55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez HH, Boyd JT, Fung VSC, et al. Long-term safety and efficacy of levodopa-carbidopa intestinal gel in advanced Parkinson's disease. Mov Disord. 2018;33(6):928-936. [DOI] [PubMed] [Google Scholar]
- 26.LeWitt PA, Boroojerdi B, Surmann E, Poewe W. Rotigotine transdermal system for long-term treatment of patients with advanced Parkinson's disease: results of two open-label extension studies, CLEOPATRA-PD and PREFER. J Neural Transm. 2013;120(7):1069-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marks WJ Jr., Bartus RT, Siffert J, et al. Gene delivery of AAV2-neurturin for Parkinson's disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2010;9(12):1164-1172. [DOI] [PubMed] [Google Scholar]
- 28.Factor S, Van Laar A, Richardson R, et al. AADC gene therapy administered via a posterior approach: 18-month results from the PD-1102 trial in advanced Parkinson's disease. Mov Disord. 2020;35(suppl 1); Abstract 889. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
NBIb-1817 (VY-AADC01) is an investigational product that has not yet been approved for any indication; we do not plan to share the study data or related documents at this time.