Abstract
Dok-1 (p62Dok) is a multiple-site docking protein that acts downstream of receptor and nonreceptor tyrosine kinases. Although it has been proposed to contribute to the control of cell growth and migration through association with the Ras GTPase-activating protein and the adapter protein Nck, the role of Dok-1 remains largely unknown. The functions of Dok-1 have now been investigated by the generation of two different COOH-terminal truncation mutants of this protein: one (DokPH+PTB) containing the pleckstrin homology and phosphotyrosine-binding domains, and the other (DokPH) composed only of the pleckstrin homology domain. Both of these mutant proteins were shown to act in a dominant negative manner. Overexpression of each of the mutants in highly metastatic B16F10 mouse melanoma cells thus both inhibited the tyrosine phosphorylation of endogenous Dok-1 induced by cell adhesion as well as reduced the association of the endogenous protein with cellular membranes and the cytoskeleton. Overexpression of DokPH+PTB in these cells also markedly reduced both the rates of cell spreading, migration, and growth as well as the extent of Ras activation. The effects of DokPH on these processes were less pronounced than were those of DokPH+PTB, indicating the importance of the phosphotyrosine-binding domain. These results suggest that at least in B16F10 cells, Dok-1 positively regulates not only cell spreading and migration but also cell growth and Ras activity.
The abilities to metastasize and to invade tissue are important characteristics of transformed cells, often complicating cancer therapy and becoming critical determinants of patient prognosis. Although metastasis and tissue invasion are complex, an enhanced motility of cancer cells is thought to contribute to both processes. Cell motility is itself controlled by a complex mechanism; however, reorganization of the actin cytoskeleton and adhesion to extracellular matrix (ECM) proteins have been shown to play crucial roles (20, 26, 35).
The p62Dok (Dok-1) protein was first identified as a common substrate for activated protein tyrosine kinases (PTKs) such as v-Abl, v-Src, v-Fps, and v-Fms (7). Subsequent studies revealed that Dok-1 is constitutively phosphorylated on tyrosine residues in chronic myelogenous leukemia cells expressing the oncoprotein p210bcr-abl (2, 44). The extent of tyrosine phosphorylation of Dok-1 in cells also correlates with the transforming activity of activated PTKs (1, 5, 25, 27). These observations suggest a causal role for Dok-1 in the acquired features of transformed cells, such as the aberrant growth and multiple abnormalities in cytoskeletal function (13). However, the physiological significance of tyrosine phosphorylation of Dok-1 has remained unclear.
The NH2-terminal region of Dok-1 contains a pleckstrin homology (PH) domain and a putative phosphotyrosine-binding (PTB) domain, which are thought to mediate the association of this protein with the cell membrane and with phosphotyrosine-containing NPXpY motifs, respectively (2, 45). Dok-1 also contains several consensus sequences for tyrosine phosphorylation by cytosolic and receptor PTKs (2, 45). These tyrosine residues, if phosphorylated, may constitute docking sites for various Src homology 2 (SH2) domain-containing signaling molecules such as the Ras GTPase-activating protein (RasGAP), the adapter protein Nck, the cytosolic PTK Csk, and the product of the X-linked lymphoproliferative syndrome gene, SH2D1A (2, 31, 39, 41, 42, 45). The structural organization of Dok-1 thus resembles that of multiple-site docking proteins, such as insulin receptor substrate (IRS) proteins (43), that act downstream of PTKs. Proteins related to Dok-1, including Dok-2 (6) (also known as Dok-R or FRIP [16, 29]) and Dok-L (also known as Dok-3 [4, 22]), have also recently been identified.
Dok family proteins have been implicated in negative regulation of cell growth in hematopoietic cell lines. Thus, overexpression of these proteins inhibited activation of extracellular signal-regulated kinases (ERKs), induction of c-Myc, or cell proliferation triggered either by coaggregation of the B-cell antigen receptor and the FcγRIIB receptor for immunoglobulin G, by cytokine stimulation, or by v-Abl (29, 38, 40). Furthermore, cross-linking of the B-cell receptor in primary B cells derived from Dok-1-deficient mice fails to result in FcγRIIB-dependent inhibition of cell proliferation and ERK activation (46). Dok family proteins have also been shown either to inhibit ERK activation induced by epidermal growth factor in COS 1 cells (17) or to block c-Src-induced transformation in NIH 3T3 cells (37). However, less is known about the biological function of this family of proteins in nonhematopoietic cells.
We have previously shown that cell adhesion to ECM proteins induces marked tyrosine phosphorylation of Dok-1 as well as the binding of this protein to RasGAP and Nck (31). Furthermore, overexpression of wild-type Dok-1 increased the rate of migration of Chinese hamster ovary (CHO) cells, suggesting that Dok-1 regulates cytoskeletal reorganization triggered by integrins (31). To gain further insight into the biological role of Dok-1, we have now generated two different COOH-terminal truncation mutants of this protein that act in a dominant negative manner. We introduced these mutant proteins into highly metastatic B16F10 melanoma cells (8, 14) by stable transfection; characterization of the resulting established cell lines not only provided support for a role of Dok-1 in cell migration but also revealed a function in cell growth.
MATERIALS AND METHODS
Expression vectors.
The wild-type human Dok-1 cDNA (2) was kindly provided by B. Stillman (Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.). PCR was performed with this cDNA as a template to prepare vectors encoding full-length Dok-1 (DokWT; amino acids 1 to 481), a Dok-1 mutant lacking the PH domain (DokΔPH: amino acids 120 to 481), a Dok-1 mutant composed only of the PH domain (DokPH; amino acids 1 to 122), and a Dok-1 mutant consisting only of the PH domain and the PTB domain (DokPH+PTB; amino acids 1 to 237). The amplification products were digested with EcoRI and SalI and then inserted in frame into the EcoRI and SalI sites of a pCl-neo vector (Invitrogen) that had been engineered to add the coding sequence for the Myc epitope to the 5′ end of the inserted cDNA. The pSRα vector encoding hemagglutinin epitope (HA) tagged mutant IRS 1, which consists of the PH and PTB domains and lacks COOH-terminal tyrosine phosphorylation sites (IRS-1PH+PTB; amino acids 2 to 400), was also generated with a human IRS-1 cDNA as a template. The pRc/CMV vector encoding HA-tagged mouse Dok-1 was described previously (31).
Cells, antibodies, and transfection.
B16F10 cells (∼4 × 105 cells per 60-mm-diameter dish) were transfected with 5 μg of pCl-neo containing Dok-1 cDNA with the use of a Lipofectamine transfection kit (Gibco-BRL). The resulting G418-resistant colonies were isolated 14 days after transfection, and the stable transfectants were identified by immunoblot analysis with a monoclonal antibody (MAb) to the Myc epitope tag as described below. The established cell lines were maintained in modified Eagle's medium (MEM) supplemented with 10% fetal bovine serum (FBS; Gibco-BRL). Horseradish peroxidase (HRP)-conjugated monoclonal antibody (MAb) PY20 to phosphotyrosine, mouse MAbs to Dok-1 (A-3), RasGAP (B4F8), and RhoA, and rabbit polyclonal antibody to focal adhesion kinase (FAK) (C-20) were obtained from Santa Cruz Biotechnology; mouse MAbs to H-Ras and to Shc were from Transduction Laboratories; rabbit polyclonal antibody to FAK (06-543) was from Upstate Biotechnology; and rabbit polyclonal antibodies that react specifically with tyrosine-phosphorylated (activated) ERK or with total ERK protein were from New England Biolabs. MAb 9E10 to the Myc tag and MAb 12CA5 to the HA tag were purified from the culture supernatants of mouse hybridoma cells. Rabbit polyclonal antibodies generated in response to the COOH-terminal portion of Dok-1 were described previously (31).
Immunoprecipitation and immunoblot analysis.
Cells in one 100-mm-diameter dish were lysed on ice in 1 ml of ice-cold lysis buffer (20 mM Tris-HCl [pH 7.6], 150 mM NaCl, 1 mM EDTA, 1% [vol/vol] Nonidet P-40) containing 5 mM NaF, 1 mM phenylmethylsulfonyl fluoride (PMSF), aprotinin (10 μg/ml), and 1 mM sodium vanadate. The cell lysates were centrifuged at 10,000 × g for 15 min at 4°C, and the resulting supernatants were incubated for 3 h at 4°C with antibody-coupled protein G-Sepharose beads (20 μl of beads; Amersham Pharmacia Biotech). The beads were then washed three times with lysis buffer and suspended in Laemmli sample buffer, and the eluted proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Immunoblot analysis was performed with an enhanced chemiluminescence detection system (Amersham Pharmacia Biotech).
Subcellular fractionation.
The following procedures were all performed at 4°C. Cells from four 100-mm-diameter dishes were scraped into 2 ml of an ice-cold solution containing 20 mM HEPES-NaOH (pH 7.6), 5 mM sodium pyrophosphate, 5 mM EGTA, 250 mM sucrose, 5 mM NaF, 1 mM PMSF, aprotinin (10 μg/ml), and 1 mM sodium vanadate. The cells were homogenized with a Dounce homogenizer, and the homogenate was centrifuged at 900 × g for 10 min. The postnuclear supernatant was then centrifuged at 100,000 × g for 60 min. The resulting supernatant was saved as the cytosolic fraction, and the pellet was suspended in 1 ml of membrane solubilization buffer (20 mM Tris-HCl [pH 7.5], 1% [vol/vol] Triton X-100, 100 mM NaCl, 1 mM MgCl2, 1 mM CaCl2) containing 5 mM NaF, 1 mM PMSF, aprotinin (10 μg/ml), and 1 mM sodium vanadate. The resulting extract was centrifuged at 10,000 × g for 10 min, yielding a pellet referred to as the cytoskeletal fraction and a supernatant that was centrifuged again at 100,000 × g for 60 min. The final supernatant and pellet were saved as the membrane fraction and membrane-skeletal fraction, respectively.
Cell spreading assay.
Cells were detached from culture dishes by treatment with 0.025% trypsin, collected by centrifugation, washed once with serum-free MEM, and transferred at a density of 105 cells/ml to 60-mm-diameter culture dishes coated with fibronectin (10 μg/ml; Sigma). After incubation in serum-free MEM for 30 to 60 min at 37°C in a humidified incubator containing 5% CO2, the cells were examined with a light microscope equipped with phase-contrast optics (model IX 70; Olympus), and random fields were photographed.
Cell migration assay.
Cell migration was assessed with a Boyden chamber assay. In brief, 8-μm-pore-size polyvinylpyrrolidine-free polycarbonate filters (Neuroprobe) coated with fibronectin (10 μg/ml), vitronectin (10 μg/ml; Sigma), or type 4 collagen (25 μg/ml; Falcon) were placed over the lower wells of a Boyden multiwell chemotactic chamber that had been filled with serum-free MEM. Cells (1.5 × 105 in 0.2 ml of serum-free MEM) were added to each of the upper wells. The chamber was placed in a humidified incubator containing 5% CO2 and incubated for 3 h at 37°C. Cells that had migrated were fixed in methanol, washed with phosphate-buffered saline, and exposed to Giemsa stain (Nakarai Tesque) for 15 s. The number of migrated cells was counted in at least six fields under a microscope fitted with a grid eyepiece at a total magnification of ×200.
Cell growth assay.
Cells were seeded in six-well culture plates at a density of 105 per well and cultured in MEM containing 10 or 0.5% FBS. The culture medium was changed every 2 days, and the number of cells was counted every 24 h.
GTPase activity assay.
The Ras-binding domain (amino acids 1 to 149) of human c-Raf-1 and the Rho-binding domain (amino acids 7 to 89) of mouse Rhotekin were expressed as glutathione S-transferase (GST) fusion proteins in bacteria and bound to glutathione-Sepharose beads (20 μg of protein per 15 μl of packed beads) (Amersham Pharmacia Biotech). For Rho activity assays, cells in one 100-mm-diameter dish were lysed in 600 μl of a solution containing 25 mM HEPES-NaOH (pH 7.4), 100 mM NaCl, 0.5% Nonidet P-40, 10 mM MgCl2, 10 mM β-glycerophosphate, 10% (vol/vol) glycerol, 1 mM PMSF, and aprotinin (10 μg/ml). For assay of Ras activity, cells were lysed in the same volume of a solution containing 25 mM HEPES-NaOH (pH 7.5), 150 mM NaCl, 1% Nonidet P-40, 0.25% sodium deoxycholate, 10% glycerol, 25 mM NaF, 10 mM MgCl2, 1 mM EDTA, 1 mM PMSF, aprotinin (10 μg/ml), and 1 mM sodium vanadate. Cell lysates were incubated at 4°C with the GST fusion protein-coupled beads for 30 min (Ras assay) or 45 min (Rho assay). Proteins that bound to the beads were resolved on a 12.5% polyacrylamide gel and subjected to immunoblot analysis with antibodies specific for the corresponding GTPase. The total abundance of each GTPase was determined by immunoblot analysis of cell lysates. Activated Ras was quantified by scanning densitometry with the NIH Image program.
Assay of ERK activation.
Cells (60-mm-diameter dishes) were deprived of serum for 24 h and then incubated for 5 min with hepatocyte growth factor (40 ng/ml; Calbiochem). Cells were then lysed in 400 μl of a solution containing 50 mM HEPES-NaOH (pH 7.8), 150 mM NaCl, 1.5 mM MgCl2, 1 mM EDTA, 0.1% Triton X-100, 20 mM β-glycerophosphate, 100 mM NaF, 10 mM sodium pyrophosphate, 1 mM PMSF, aprotinin (10 μg/ml), and 1 mM sodium vanadate. Cell lysates were subjected to immunoblot analysis with antibodies specific for activated ERK or for total ERK protein.
RESULTS
Expression of DokWT and mutant Dok-1 proteins in B16F10 melanoma cells.
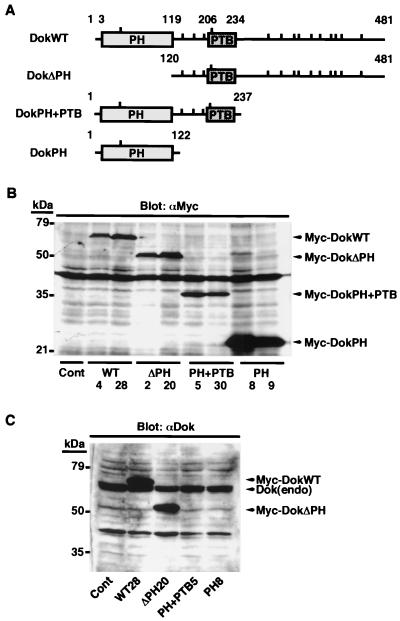
We generated three different Dok-1 mutants: one (DokΔPH) that lacks the PH domain, one (DokPH+PTB) composed of the PH and PTB domains, and one (DokPH) consisting only of the PH domain (Fig. 1A). The latter two mutants lack COOH-terminal tyrosine residues that, if phosphorylated, might form docking sites for SH2 domain-containing signaling molecules (2, 45). DokWT and mutant Dok-1 cDNAs, cloned into an expression vector downstream of a sequence encoding the Myc epitope tag, were introduced individually into B16F10 murine melanoma cells by transfection. We obtained several independent transfectants that stably expressed exogenous Dok-1 as revealed by immunoblot analysis with MAb 9E10 to the Myc tag (Fig. 1B). The established cell lines (WT28, ΔPH20, PH+PTB5, and PH8) that expressed the highest amount of each recombinant Dok-1 protein were studied most extensively, although the other cell clones showed similar respective phenotypes (data not shown). The abundance of exogenous Dok-1 was about twice that of the endogenous protein in WT28, ΔPH20, and PH+PTB5 cells and five times that of endogenous Dok-1 in PH8 cells, as estimated by immunoblot analysis with polyclonal antibodies to the COOH-terminal portion of Dok 1 (Fig. 1C). The amounts of endogenous Dok-1 were similar among these various cell lines (Fig. 1C).
FIG. 1.
Generation and expression of Dok-1 mutants. (A) Schematic representation of recombinant Dok-1 proteins. The numbers correspond to amino acid positions of human Dok-1, and the locations of tyrosine residues are indicated by vertical bars. (B) Cell lysates were prepared from control B16F10 cells transfected with the empty vector (Cont) as well as from two independent clones of B16F10 cells expressing each of the recombinant Dok-1 proteins. Lysates (20 μg of protein) were subjected to immunoblot analysis with MAb 9E10 to the Myc tag (αMyc). (C) Cell lysates (20 μg of protein) prepared from the indicated cell lines were subjected to immunoblot analysis with polyclonal antibodies specific for the COOH-terminal region of Dok-1 (αDok). The positions of recombinant Dok-1 proteins, endogenous Dok-1 [Dok(endo)], and molecular size standards are indicated. The ∼40-kDa immunoreactive material in panels B and C comprises nonspecific cross-reactive polypeptides of unknown origin. Data in all figures are representative of three independent experiments.
Dominant negative action of Dok-1 mutants lacking COOH-terminal tyrosine phosphorylation sites.
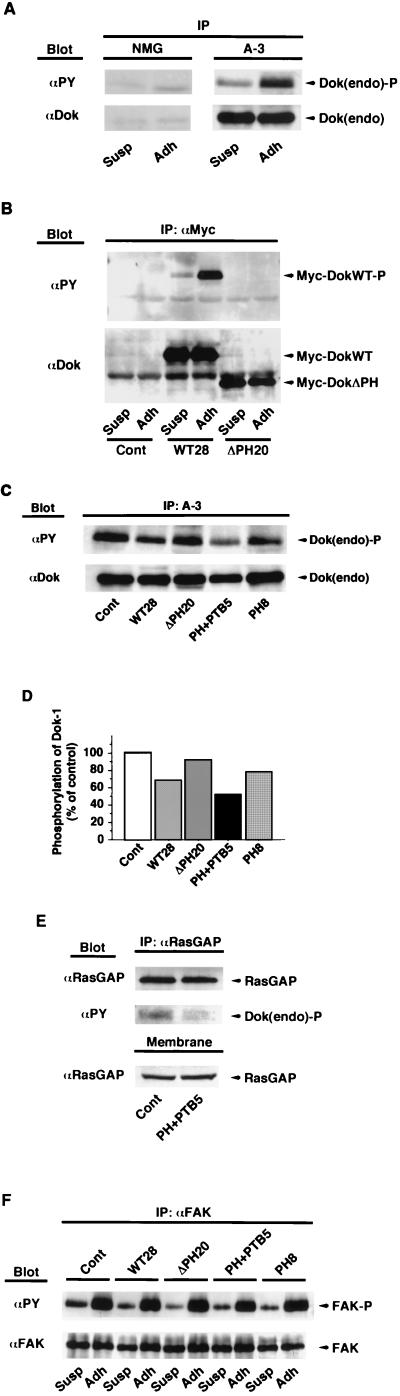
Immunoprecipitation with MAb A-3, which is specific for the NH2-terminal portion of mouse Dok-1, and subsequent immunoblot analysis with MAb PY20 to phosphotyrosine revealed that replating of suspended B16F10 cells onto fibronectin induced marked tyrosine phosphorylation of endogenous Dok-1 (Fig. 2A). Recombinant DokWT also underwent adhesion-dependent tyrosine phosphorylation, whereas DokΔPH did not (Fig. 2B). These results are consistent with our previous observation that tyrosine phosphorylation of Dok-1 in CHO cells depends on both cell-substratum adhesion and the presence of the PH domain (31).
FIG. 2.
Adhesion-induced tyrosine phosphorylation of endogenous Dok-1 and FAK in established B16F10 cell lines expressing recombinant Dok-1 proteins. (A) B16F10 cells were detached from culture dishes and either maintained in suspension (Susp) or replated on fibronectin-coated dishes (Adh). After incubation for 30 min at 37°C, cells were lysed and subjected to immunoprecipitation (IP) with MAb A-3 to Dok-1 or with normal mouse immunoglobulin G (NMG). The immunoprecipitates were then subjected to immunoblot analysis with HRP-conjugated MAb PY20 to phosphotyrosine (αPY). Duplicate immunoprecipitates were probed with polyclonal antibodies to Dok-1 (αDok) to verify the presence of equal amounts of Dok-1 in each sample. (B) The indicated cell lines (Cont [control], WT28, and ΔPH20) were treated as in panel A, and the resulting cell lysates were subjected to immunoprecipitation with MAb 9E10 to the Myc tag (αMyc). The phosphotyrosine content of each recombinant Dok-1 protein was assessed as in panel A. (C) The extent of adhesion-induced tyrosine phosphorylation of endogenous Dok-1 in the indicated cell lines was determined as in panel A. (D) The phosphotyrosine content of endogenous Dok-1 in the experiment shown in panel C was quantified by scanning densitometry with the NIH Image program, normalized for the amount of Dok-1 protein in each sample, and was expressed as a percentage of the value for control cells transfected with the empty vector. (E) The indicated cell lines were treated as in panel A and the resulting detargent-solubilized membrane fraction was subjected to immunoprecipitation with MAb B4F8 to RasGAP (αRasGAP). The immunoprecipitates were then subjected to immunoblot analysis with HRP-conjugated MAb PY20 to detect tyrosine-phosphorylated Dok-1 bound to RasGAP. Duplicate immunoprecipitates were probed with the MAb to RasGAP to verify the presence of equal amounts of RasGAP in each sample. Aliquots of each membrane fraction (Membrane) were also directly probed with the MAb to RasGAP. (F) The indicated cell lines were treated as in panel A and subjected to immunoprecipitation with polyclonal antibody C-20 to FAK (αFAK). The immunoprecipitates were then subjected to immunoblot analysis with HRP-conjugated MAb PY20 to phosphotyrosine. Duplicate immunoprecipitates were probed with polyclonal antibody 06-543 to FAK (αFAK) to verify the presence of equal amounts of FAK in each sample. The positions of endogenous Dok-1, exogenous Dok-1, RasGAP, FAK, and the phosphorylated forms of these various proteins [Dok(endo)-P, Myc-DokWT-P, and FAK-P] are indicated.
Because MAb A-3 does not react with human Dok-1, with the use of this antibody we were able to assess the phosphotyrosine content of endogenous Dok-1 separate from that of the exogenous Dok-1 proteins in the established cell lines. Overexpression of DokPH+PTB and, to a lesser degree, that of DokPH reduced the extent of adhesion-induced tyrosine phosphorylation of endogenous Dok-1 (Fig. 2C and D). In addition, overexpression of DokPH+PTB reduced the association of endogenous Dok-1 with RasGAP in the membrane fraction with no marked effect on the amount of RasGAP in this fraction (Fig. 2E). Overexpression of DokWT also exhibited an inhibitory effect on tyrosine phosphorylation of the endogenous protein, presumably by altering its subcellular localization, whereas overexpression of DokΔPH had no such effect (Fig. 2C and D; see Fig. 4). These effects of exogenous Dok-1 proteins appeared specific, given that the overexpression of these proteins did not substantially affect tyrosine phosphorylation of FAK (Fig. 2F) or of p130Cas (data not shown). When transiently expressed, DokPH+PTB inhibited tyrosine phosphorylation of coexpressed wild-type mouse Dok-1 in a manner dependent on its expression level (Fig. 3A), consistent with the inhibitory effect of this mutant on tyrosine phosphorylation of endogenous Dok-1. In contrast, overexpression of IRS-1PH+PTB failed to inhibit tyrosine phosphorylation of coexpressed DokWT (Fig. 3B), suggesting that the effects observed are unique to the DokPH+PTB mutant. This observation is also in agreement with the previous report showing that the Dok-1 PTB domain and the IRS-1 PTB domain each recognize distinct phosphotyrosine-containing sequences (37).
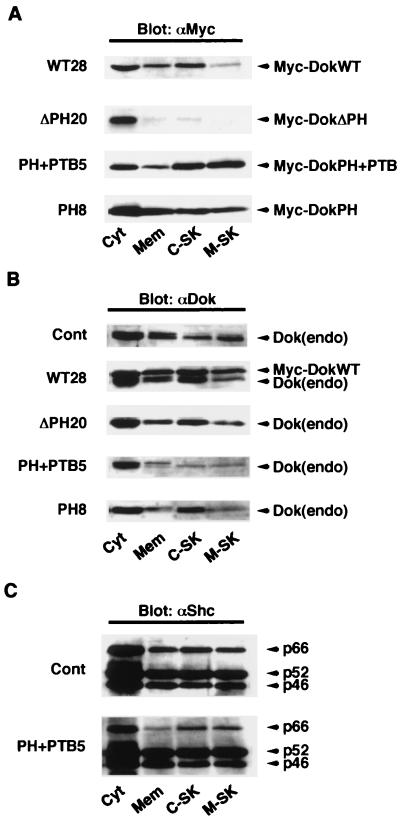
FIG. 4.
Effects of overexpression of Dok-1 mutants on the subcellular localization of endogenous Dok-1. (A) The indicated cell lines were fractionated into cytosolic (Cyt), membrane (Mem), cytoskeletal (C-SK), and membrane-skeletal (M-SK) components, and each fraction was then subjected to immunoblot analysis with MAb 9E10 to the Myc tag (αMyc). (B and C) Subcellular fractions from each cell line were subjected to immunoblot analysis either with polyclonal antibodies to Dok-1 (αDok) (B) or with a MAb to Shc (αShc) (C) to examine the subcellular localization of endogenous Dok-1 and Shc, respectively. The positions of exogenous Dok-1 proteins, endogenous Dok-1, and three isoforms of Shc (p66, p52, and p46) are indicated. Cont, control.
FIG. 3.
Effects of transient overexpression of DokPH+PTB and IRS-1PH+PTB on tyrosine phosphorylation of Dok-1. (A) B16F10 cells were transiently cotransfected with 0.5 μg of pRc/CMV encoding HA-tagged wild-type mouse Dok-1 (HA-DokWT) and the indicated amount of pCl-neo encoding Myc-tagged DokPH+PTB. Forty-eight hours after transfection, cell lysates were prepared and subjected to immunoprecipitation (IP) with MAb 12CA5 to the HA tag (αHA). The immunoprecipitates were then subjected to immunoblot analysis with HRP-conjugated MAb PY20 to phosphotyrosine (αPY). Duplicate immunoprecipitates were probed with polyclonal antibodies to Dok-1 (αDok) to verify the presence of equal amounts of Dok-1 in each sample. Total cell lysates (Lysate) were also probed with a MAb to the Myc tag (αMyc) to determine the amount of the mutant Dok-1 protein expressed. (B) Cells were transiently cotransfected with 1 μg of pCl-neo encoding Myc-tagged wild-type human Dok-1 (Myc-DokWT) and the indicated amount of pSRα encoding HA-tagged IRS-1PH+PTB. Cell lysates prepared as in panel A were subjected to immunoprecipitation with a MAb to the Myc tag and subsequent immunoblot analysis either with MAb PY20 or with polyclonal antibodies to Dok-1. Total cell lysates were also probed with a MAb to the HA tag to determine the amount of the mutant IRS-1 protein expressed.
We next examined the effect of overexpression of each recombinant Dok-1 protein on the subcellular localization of endogenous Dok-1. DokWT was detected in all subcellular fractions analyzed, exhibiting a distribution pattern similar to that of endogenous Dok-1 (Fig. 4A and B). Unlike the wild-type protein and consistent with our previous data (31), DokΔPH was localized almost exclusively to the cytosolic fraction (Fig. 4A). In contrast, DokPH+PTB was preferentially localized to the cytoskeletal and membrane-skeletal fractions. DokPH showed a subcellular distribution similar to that of DokPH+PTB, although it was more preferentially associated with the membrane fraction than was DokPH+PTB (Fig. 4A). Overexpression of DokPH+PTB or DokPH substantially reduced the amount of endogenous Dok-1 in all subcellular fractions but the cytosolic fraction (Fig. 4B), although the effect of DokPH was less marked than that of DokPH+PTB in the cytoskeletal fraction. Overexpression of DokWT reduced the amount of the endogenous protein in the membrane and membrane-skeletal fractions, whereas DokΔPH had no marked effect on the subcellular distribution of endogenous Dok-1 (Fig. 4B). None of the recombinant Dok-1 proteins substantially affected the subcellular localization of Shc, another PTB domain-containing docking protein (Fig. 4C and data not shown). Both tyrosine phosphorylation and proper subcellular localization of Dok-1 are required for its function (18, 31, 47). These results, together with those shown in Fig. 2 and 3, therefore suggest that DokPH+PTB and DokPH act in a dominant negative manner and that DokΔPH is functionally impaired.
Effects of Dok-1 mutants on cell spreading on fibronectin.
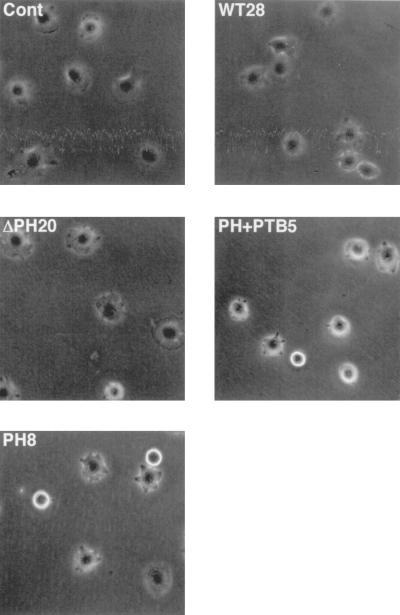
We have previously proposed that Dok-1 promotes cell migration by facilitating reorganization of the actin cytoskeleton (31). To further test this hypothesis, we detached B16F10 cells expressing the various recombinant Dok-1 proteins from their culture dishes and then monitored with phase-contrast microscopy their spreading on fibronectin, a phenomenon associated with cytoskeletal reorganization. After 30 min on fibronectin, 91% ± 0.3% (mean ± standard error [SE], n = 3) of the control cells transfected with the empty vector had become phase-dark and exhibited well-defined membranous ruffles at the cell periphery, characteristics of the early stage of cell spreading (Fig. 5). The rate of spreading and the morphology of cells expressing DokWT or DokΔPH were similar to those of the control cells. In contrast, 45% ± 2% (mean ± SE, n = 3) of the cells expressing DokPH+PTB as well as 30% ± 2% (mean ± SE, n = 3) of those expressing DokPH exhibited a phase-bright, rounded morphology with no sign of ruffle formation 30 min after plating (Fig. 5). By 60 min, the numbers of spread cells were similar among all cell lines (data not shown). Thus, inhibition of Dok-1 function appeared to delay spreading of B16F10 cells on fibronectin.
FIG. 5.
Effects of overexpression of Dok-1 mutants on cell spreading on fibronectin. The indicated cell lines were detached from their culture dishes and replated in serum-free MEM on dishes coated with fibronectin. The cells were then allowed to adhere and spread at 37°C for 30 min, after which they were photographed in random fields with the use of phase-contrast optics. Cont, control. Original magnification, ×200.
Effects of Dok-1 mutants on cell migration on ECM proteins.
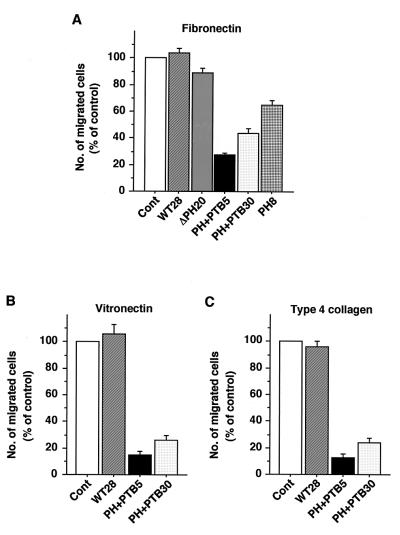
The rate of migration of B16F10 cells expressing the various recombinant Dok-1 proteins was analyzed quantitatively with the use of a Boyden chamber assay. The number of cells that migrated through a membrane coated with either fibronectin, vitronectin, or type 4 collagen was markedly reduced for two independent cell lines expressing DokPH+PTB compared with that for control cells (Fig. 6). The migration rate of cells expressing DokPH on each of these ECM proteins was also substantially reduced (Fig. 6A and data not shown), although to a lesser extent than was that of cells expressing DokPH+PTB. Thus, inhibition of Dok-1 function reduced the rate of migration of B16F10 cells that was triggered by engagement of integrins by the ECM. In contrast, the migration rates of cells expressing either DokWT or DokΔPH were similar to those of the control cells (Fig. 6 and data not shown).
FIG. 6.
Effects of overexpression of Dok-1 mutants on cell migration on ECM proteins. The indicated cell lines were seeded onto porous membranes that had been both coated with fibronectin (A), vitronectin (B), or type 4 collagen (C) and placed in Boyden multiwell chambers. After incubation at 37°C for 3 h, cells that had migrated were stained with Giemsa solution. The number of migrated cells was counted and expressed as a percentage of the value for control cells transfected with the empty vector (Cont). Data are means ± SE of triplicate determinations from three independent experiments.
Effects of Dok-1 mutants on cell growth.
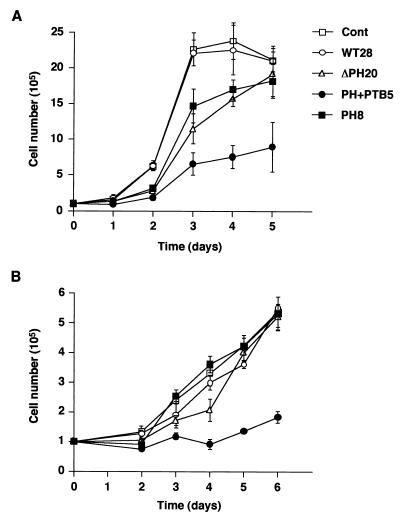
Dok family proteins have been implicated as negative regulators of cell growth in various hematopoietic cell lines (29, 38, 40, 46). To examine the role of Dok-1 in the growth of B16F10 cells, we monitored the growth rates of the various cell lines expressing recombinant Dok-1 proteins. Under normal culture conditions (10% FBS), the growth rate of cells expressing DokPH+PTB was about one-third of that of control cells (Fig. 7A). The cells expressing DokPH or DokΔPH also exhibited reduced growth rates, although the final cell number after incubation of these cells for 5 days was similar to that for control cells. In contrast, the growth rate of cells expressing DokWT was similar to that of the control cells. The cells expressing DokPH+PTB also grew more slowly than did control cells in the presence of a low concentration (0.5%) of FBS (Fig. 7B). These results indicate that inhibition of Dok-1 function, but not overexpression of this protein, reduced the growth rate of B16F10 cells.
FIG. 7.
Effects of overexpression of Dok-1 mutants on cell growth. The rate of cell growth was monitored for the indicated cell lines in the presence of 10% (A) or 0.5% (B) FBS. Data are means ± SE of triplicate determinations from three independent experiments. Cont, control.
Effects of Dok-1 mutants on the activity of Ras and ERK.
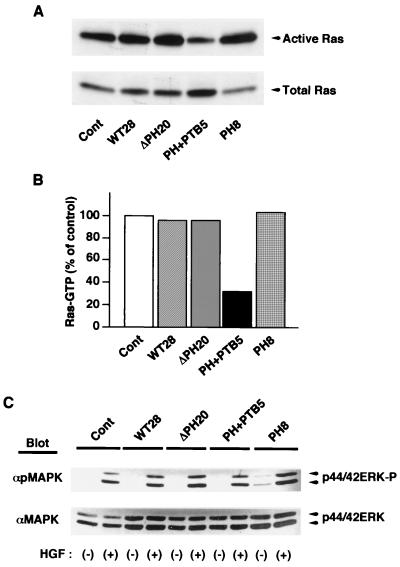
Dok family proteins are thought to regulate in either a positive or a negative manner the activity of Ras (18, 47), which is itself an important regulator of cell motility and cell growth (11, 19, 28, 30, 33). The activation state of Ras has been shown to correlate with invasiveness, proliferation, and anchorage-independent growth of melanoma cells (12, 36). To investigate the possible role of Ras in the effects of the dominant negative mutants of Dok-1, we therefore examined Ras activity in the various B16F10 cell lines with the use of a binding assay in which the GTP-bound (activated) form of Ras was precipitated from cell lysates with a GST fusion protein containing the Ras-binding domain of c-Raf-1. Adherent control cells contained a substantial amount of activated Ras, which was not changed significantly in response to adhesion to the ECM or to growth factor stimulation (Fig. 8A and data not shown). The amount of activated Ras in cells expressing DokPH+PTB was markedly reduced compared with that in control cells (Fig. 8A and B), indicating that inhibition of Dok-1 function decreases Ras activity in B16F10 cells. In contrast, no marked difference in the amount of activated Ras was apparent between control cells and cells expressing DokWT, DokΔPH, or DokPH.
FIG. 8.
Effects of overexpression of Dok-1 mutants on the activity of Ras and ERK. (A) The active (GTP-bound) form of Ras was precipitated from lysates of the indicated cell lines with a GST fusion protein containing the Ras-binding domain of c-Raf-1. The precipitates were then subjected to immunoblot analysis with a MAb to H-Ras (top). Whole-cell lysates were also subjected directly to immunoblot analysis with the same MAb to determine the total amount of Ras (bottom). Cont, control. (B) The amount of activated Ras in panel A was quantified by scanning densitometry with the NIH Image program, normalized for the amount of total Ras protein, and expressed as a percentage of the value for control cells transfected with the empty vector. (C) The indicated cell lines were incubated for 5 min in the absence (−) or presence (+) of hepatocyte growth factor (HGF; 40 ng/ml). Total cell lysates prepared were subjected to immunoblot analysis with antibodies specific for tyrosine-phosphorylated ERK (αpMAPK) or for total ERK protein (αMAPK).
We also examined the effects of overexpression of each recombinant Dok-1 protein on growth factor-induced ERK activation. In control B16F10 cells, hepatocyte growth factor (Fig. 8C) and lysophosphatidic acid (LPA) (data not shown) each induced marked activation of ERKs. However, we did not detect a substantial difference in the extent of ERK activation among the established cell lines exposed to these growth factors (Fig. 8C and data not shown). These results suggest that formation of a complex between Dok-1 and RasGAP does not play a major role in growth factor-induced ERK activation in B16F10 cells, yet it may positively regulate steady-state Ras activity.
Effects of Dok-1 mutants on the activity of Rho.
Members of the Rho family of small GTPases also regulate cell spreading and migration on the ECM through their effects on rearrangement of the actin-based cytoskeleton (3, 30, 33, 34). We therefore finally examined whether inhibition of Dok-1 function affected activation of Rho in response to cell adhesion (Fig. 9A) or to LPA (Fig. 9B). Each stimulus activated Rho to similar extents in control B16F10 cells and cells expressing DokPH+PTB.
FIG. 9.
Effects of inhibition of Dok-1 function on the activity of Rho. (A) B16F10 cells transfected with the empty vector (Cont, [control]) or expressing DokPH+PTB (PH+PTB5) were detached from their culture dishes and then either maintained in suspension (Susp) or replated on fibronectin-coated dishes (Adh). After incubation of cells for 30 min in MEM supplemented with 1% FBS, the active form of Rho was precipitated from cell lysates with a GST fusion protein containing the Rho-binding domain of Rhotekin. The resulting precipitates were then subjected to immunoblot analysis with a MAb to RhoA (top). Whole-cell lysates were also directly subjected to immunoblot analysis with the same MAb to determine the total amount Rho (bottom). (B) The same two cell lines were deprived of serum for 12 h and then incubated for 1 min in the absence (−) or presence (+) of 4 μM LPA (Sigma). The active form of Rho was then precipitated and analyzed as in panel A.
DISCUSSION
We have generated two different COOH-terminal truncation mutants of Dok-1 (DokPH+PTB and DokPH) to explore the biological role of this protein. Overexpression of these mutants competitively inhibited tyrosine phosphorylation of endogenous Dok-1 as well as altered its subcellular localization, indicating that the mutant proteins act in a dominant negative manner. The expression of these mutants in highly metastatic B16F10 melanoma cells has now revealed that Dok-1 positively regulates not only cell spreading and migration but also cell growth. These dominant negative mutants of Dok-1 did not significantly affect various cellular responses, including tyrosine phosphorylation of FAK and p130Cas as well as activation of ERKs and Rho in response either to cell adhesion or to growth factor stimulation. These results argue that the mutant Dok-1 proteins might not have nonspecific effects on cytoskeletal function or growth factor signaling. However, we cannot rule out the possibility that these mutants also interfere with other PH domain- or PTB domain-containing signaling molecules, thus affecting cellular function.
We have shown that inhibition of Dok-1 function by the dominant negative mutants markedly reduced the rate of spreading of B16F10 cells on fibronectin. Overexpression of the mutants also markedly reduced the rate of migration of these cells on various ECM proteins. These observations, together with our previous data showing that Dok-1 promotes the migration of CHO cells (31), establish a positive regulatory role for this protein in cell migration. In the present study, however, overexpression of wild-type Dok-1 did not further enhance cell migration. This apparent discrepancy with our previous results (31) is most likely due to the difference in the cell line studied; unlike CHO cells, B16F10 cells are highly motile, so that their basal migration rate might already be maximal.
Another important finding of our present study is that the dominant negative mutants of Dok-1 reduced the growth rate of B16F10 cells, suggesting that Dok-1 may positively regulate the growth of certain types of cancer cells. On the other hand, overexpression of wild-type Dok-1 did not affect the growth rate of this cell line. These observations appeared somewhat unexpected since previous data obtained with hematopoietic cell lines have suggested that Dok family proteins inhibit cell growth (29, 38, 40, 46). A likely explanation for this apparent discrepancy is that the relative contribution of Dok-1 to cell growth may differ between hematopoietic cells and nonhematopoietic cells.
Whereas the DokPH+PTB and DokPH mutants each reduced the proportion of endogenous Dok-1 localized to the membrane, membrane-skeletal, and cytoskeletal fractions, the effect of DokPH on localization of the endogenous protein to the cytoskeletal fraction was less marked than that of DokPH+PTB. This difference suggests that DokPH+PTB competes with endogenous Dok-1 for upstream regulators in the cytoskeleton more effectively than does DokPH. The active forms of members of the Src family of PTKs, such as Lyn and c-Src, that have been suggested to phosphorylate Dok-1 (23, 31, 46) preferentially localize to the cytoskeleton (9, 48). Together, these results might explain why the dominant negative effect on tyrosine phosphorylation of endogenous Dok-1 is substantially greater for DokPH+PTB than for DokPH. The effects of DokPH+PTB on cytoskeletal function and cell growth were also more pronounced than were those of DokPH. The extents of these latter effects thus correlated with the extents to which the mutants inhibited the tyrosine phosphorylation of endogenous Dok-1.
With regard to the mechanism by which Dok-1 regulates cell migration and growth, we showed that overexpression of DokPH+PTB reduced the activity of Ras. The DokPH+PTB mutant affected neither the subcellular localization nor the tyrosine phosphorylation of Shc, a key docking protein responsible for Ras activation (Fig. 4C and data not shown), indicating that this mutant indeed inactivates Ras through inhibition of Dok 1 function. Based on the observation that overexpression of the wild-type protein inactivates Ras, Dok-1 has been proposed to negatively regulate Ras activity by recruiting RasGAP to cell membrane (37, 47). According to this model, overexpression of DokPH+PTB would be expected to prevent RasGAP from localizing to cell membrane. However, we found no marked effect of this mutant on the amount of RasGAP in the membrane fraction, yet it did reduce the association of RasGAP with endogenous Dok-1 in this fraction. Thus, our results may be inconsistent with the proposed model (37, 47); in contrast, they appear to be in agreement with the previous observation suggesting that tyrosine-phosphorylated Dok-1 might up-regulate Ras signaling pathway by inhibiting RasGAP activity (18). Although the functional significance of complex formation between Dok-1 and RasGAP in the activation of Ras remains unclear, our results indicate that at least in B16F10 cells, Dok-1 may normally down-regulate rather than enhance RasGAP activity in a tyrosine phosphorylation-dependent manner. However, overexpression of wild-type Dok-1 alone did not increase the accumulation of Ras-GTP, raising the possibility that Ras activation requires not only Dok-1-mediated down-regulation of RasGAP activity but also the presence of active guanine nucleotide exchange factors such as SOS. It is also possible that such overexpression may allow RasGAP to cluster nearby Ras, thereby overriding Dok-1-mediated down-regulation of this enzyme. If this latter possibility holds true, the net effect of exogenous wild-type Dok-1 on Ras activity would vary with its expression level. Activation of the Ras signaling pathway has been implicated in cytoskeletal reorganization and cell motility as well as in cell growth triggered by growth factor receptors or integrins (11, 19, 28, 30, 33). Thus, Dok-1 may positively regulate these cellular responses in B16F10 cells through inhibition of RasGAP activity and the subsequent activation of Ras. However, it is also likely that Ras-independent mechanisms contribute to these effects, given that DokPH, which did not reduce Ras activity, was still able to impair cell spreading and migration as well as cell growth.
The small GTPase Rho also regulates cell spreading and migration on the ECM (3, 30, 33, 34). Rho has been suggested to act downstream of RasGAP to regulate cytoskeletal reorganization (21). Furthermore, the association of Dok-1 with RasGAP might affect the activity of p190RhoGAP by modulating the interaction between p190RhoGAP and RasGAP (10, 15). It is therefore also possible that Rho participates in the promotion of cell migration by Dok-1. However, this conclusion appears inconsistent with our observation that inhibition of Dok-1 function by DokPH+PTB did not affect the activation of Rho either by cell adhesion or by LPA. The SH2 domain-containing adapter protein Nck, which also regulates actin organization (24), is a possible mediator of the effect of Dok-1 on cell migration (31, 41). However, with the use of a coimmunoprecipitaion assay, we did not detect formation of a complex between Dok-1 and Nck in B16F10 cells (T. Hosooka and T. Noguchi, unpublished data).
In conclusion, we have shown that Dok-1 positively regulates the spreading, migration, and growth of B16F10 cells and that these effects of Dok-1 may be mediated, at least in part, through activation of Ras. Our data thus provide a potential new target (Dok-1) for therapeutic intervention in the treatment of highly metastatic cancers such as malignant melanoma.
ACKNOWLEDGMENTS
This study was supported by a grant-in-aid for cancer research and a grant-in-aid for scientific research from the Ministry of Education, Science, Sports, and Culture of Japan and by a grant-in-aid from the Research for the Future Program of the Japan Society for the Promotion of Science.
We thank B. Stillman for providing the human Dok-1 cDNA; we thank W. Ogawa and M. Matsumoto for providing the mutant IRS-1 construct.
REFERENCES
- 1.Bouton A H, Kanner S B, Vines R R, Wang H C, Gibbs J B, Parsons J T. Transformation by pp60src or stimulation of cells with epidermal growth factor induces the stable association of tyrosine-phosphorylated cellular proteins with GTPase-activating protein. Mol Cell Biol. 1991;11:945–953. doi: 10.1128/mcb.11.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carpino N, Wisniewski D, Strife A, Marshak D, Kobayashi R, Stillman B, Clarkson B. p62(dok): a constitutively tyrosine-phosphorylated, GAP-associated protein in chronic myelogenous leukemia progenitor cells. Cell. 1997;88:197–204. doi: 10.1016/s0092-8674(00)81840-1. [DOI] [PubMed] [Google Scholar]
- 3.Clark E A, King W G, Brugge J S, Symons M, Hynes R O. Integrin-mediated signals regulated by members of the Rho family of GTPases. J Cell Biol. 1998;142:573–586. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cong F, Yuan B, Goff S P. Characterization of a novel member of the DOK family that binds and modulates Abl signaling. Mol Cell Biol. 1999;19:8314–8325. doi: 10.1128/mcb.19.12.8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeClue J E, Vass W C, Johnson M R, Stacey D W, Lowy D R. Functional role of GTPase-activating protein in cell transformation by pp60v-src. Mol Cell Biol. 1993;13:6799–6809. doi: 10.1128/mcb.13.11.6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Cristofano A, Carpino N, Dunant N, Friedland G, Kobayashi R, Strife A, Wisniewski D, Clarkson B, Pandolfi P P, Resh M D. Molecular cloning and characterization of p56dok-2 defines a new family of RasGAP-binding proteins. J Biol Chem. 1998;273:4827–4830. doi: 10.1074/jbc.273.9.4827. [DOI] [PubMed] [Google Scholar]
- 7.Ellis C, Moran M, McCormick F, Pawson T. Phosphorylation of GAP and GAP-associated proteins by transforming and mitogenic tyrosine kinases. Nature. 1990;343:377–381. doi: 10.1038/343377a0. [DOI] [PubMed] [Google Scholar]
- 8.Fidler I. Selection of successive tumour lines for metastasis. Nat New Biol. 1973;242:148–149. doi: 10.1038/newbio242148a0. [DOI] [PubMed] [Google Scholar]
- 9.Fincham V J, Unlu M, Brunton V G, Pitts J D, Wyke J A, Frame M C. Translocation of Src kinase to the cell periphery is mediated by the actin cytoskeleton under the control of the Rho family of small G proteins. J Cell Biol. 1996;135:1551–1564. doi: 10.1083/jcb.135.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fincham V J, Chudleigh A, Frame M C. Regulation of p190 Rho-GAP by v-Src is linked to cytoskeletal disruption during transformation. J Cell Sci. 1999;112:947–956. doi: 10.1242/jcs.112.6.947. [DOI] [PubMed] [Google Scholar]
- 11.Fox P L, Sa G, Dobrowolski S F, Stacey D W. The regulation of endothelial cell motility by p21 ras. Oncogene. 1994;9:3519–3526. [PubMed] [Google Scholar]
- 12.Fujita M, Norris D A, Yagi H, Walsh P, Morelli J G, Weston W L, Terada N, Bennion S D, Robinson W, Lemon M, Maxwell I H, Yohn J J. Overexpression of mutant ras in human melanoma increases invasiveness, proliferation and anchorage-independent growth in vitro and induces tumour formation and cachexia in vivo. Melanoma Res. 1999;9:279–291. doi: 10.1097/00008390-199906000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Hanahan D, Weinberg R A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 14.Hill R P, Chambers A F, Ling V, Harris J F. Dynamic heterogeneity: rapid generation of metastatic variants in mouse B16 melanoma cells. Science. 1984;224:998–1001. doi: 10.1126/science.6719130. [DOI] [PubMed] [Google Scholar]
- 15.Hu K Q, Settleman J. Tandem SH2 binding sites mediate the RasGAP-RhoGAP interaction: a conformational mechanism for SH3 domain regulation. EMBO J. 1997;16:473–483. doi: 10.1093/emboj/16.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones N, Dumont D J. The Tek/Tie2 receptor signals through a novel Dok-related docking protein, Dok-R. Oncogene. 1998;17:1097–1108. doi: 10.1038/sj.onc.1202115. [DOI] [PubMed] [Google Scholar]
- 17.Jones N, Dumont D J. Recruitment of Dok-R to the EGF receptor through its PTB domain is required for attenuation of Erk MAP kinase activation. Curr Biol. 1999;9:1057–1060. doi: 10.1016/s0960-9822(99)80458-8. [DOI] [PubMed] [Google Scholar]
- 18.Kashige N, Carpino N, Kobayashi R. Tyrosine phosphorylation of p62dok by p210bcr-abl inhibits RasGAP activity. Proc Natl Acad Sci USA. 2000;97:2093–2098. doi: 10.1073/pnas.040547997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klemke R L, Cai S, Giannini A L, Gallagher P J, de Lanerolle P, Cheresh D A. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lauffenburger D A, Horwitz A F. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 21.Leblanc V, Tocque B, Delumeau I. Ras-GAP controls Rho-mediated cytoskeletal reorganization through its SH3 domain. Mol Cell Biol. 1998;18:5567–5578. doi: 10.1128/mcb.18.9.5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemay S, Davidson D, Latour S, Veillette A. Dok-3, a novel adapter molecule involved in the negative regulation of immunoreceptor signaling. Mol Cell Biol. 2000;20:2743–2754. doi: 10.1128/mcb.20.8.2743-2754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lock P, Casagranda F, Dunn A R. Independent SH2-binding sites mediate interaction of Dok-related protein with RasGTPase-activating protein and Nck. J Biol Chem. 1999;274:22775–22784. doi: 10.1074/jbc.274.32.22775. [DOI] [PubMed] [Google Scholar]
- 24.Lu W, Katz S, Gupta R, Mayer B J. Activation of Pak by membrane localization mediated by an SH3 domain from the adaptor protein Nck. Curr Biol. 1997;7:85–94. doi: 10.1016/s0960-9822(06)00052-2. [DOI] [PubMed] [Google Scholar]
- 25.Lugo T G, Pendergast A M, Muller A J, Witte O N. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science. 1990;247:1079–1082. doi: 10.1126/science.2408149. [DOI] [PubMed] [Google Scholar]
- 26.Mitchison T J, Cramer L P. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- 27.Moran M F, Polakis P, McCormick F, Pawson T, Ellis C. Protein-tyrosine kinases regulate the phosphorylation, protein interactions, subcellular distribution, and activity of p21ras GTPase-activating protein. Mol Cell Biol. 1991;11:1804–1812. doi: 10.1128/mcb.11.4.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mulcahy L S, Smith M R, Stacey D W. Requirement for ras proto-oncogene function during serum-stimulated growth of NIH 3T3 cells. Nature. 1985;313:241–243. doi: 10.1038/313241a0. [DOI] [PubMed] [Google Scholar]
- 29.Nelms K, Snow A L, Hu-Li J, Paul W E. FRIP, a hematopoietic cell-specific rasGAP-interacting protein phosphorylated in response to cytokine stimulation. Immunity. 1998;9:13–24. doi: 10.1016/s1074-7613(00)80584-1. [DOI] [PubMed] [Google Scholar]
- 30.Nobes C D, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noguchi T, Matozaki T, Inagaki K, Tsuda M, Fukunaga K, Kitamura Y, Kitamura T, Shii K, Yamanashi Y, Kasuga M. Tyrosine phosphorylation of p62(Dok) induced by cell adhesion and insulin: possible role in cell migration. EMBO J. 1999;18:1748–1760. doi: 10.1093/emboj/18.7.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rafnar T, Peebles R S, Brummet M E, Catipovic B, Imani F, MacGlashan D W, Marsh D G. Stimulation of the high-affinity IgE receptor results in the tyrosine phosphorylation of a 60 kD protein which is associated with the protein-tyrosine kinase, Csk. Mol Immunol. 1998;35:249–257. doi: 10.1016/s0161-5890(98)00028-5. [DOI] [PubMed] [Google Scholar]
- 33.Ridley A J, Comoglio P M, Hall A. Regulation of scatter factor/hepatocyte growth factor responses by Ras, Rac, and Rho in MDCK cells. Mol Cell Biol. 1995;15:1110–1122. doi: 10.1128/mcb.15.2.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ridley A J, Allen W E, Peppelenbosch M, Jones G E. Rho family proteins and cell migration. Biochem Soc Symp. 1999;65:111–123. [PubMed] [Google Scholar]
- 35.Schwarzbauer J E. Cell migration: may the force be with you. Curr Biol. 1997;7:292–294. doi: 10.1016/s0960-9822(06)00140-0. [DOI] [PubMed] [Google Scholar]
- 36.Shellman Y G, Chapman J T, Fujita M, Norris D A, Maxwell I H. Expression of activated N-ras in a primary melanoma cell line counteracts growth inhibition by transforming growth factor-beta. J Investig Dermatol. 2000;114:1200–1204. doi: 10.1046/j.1523-1747.2000.00988.x. [DOI] [PubMed] [Google Scholar]
- 37.Songyang Z, Yamanashi Y, Liu D, Baltimore D. Domain-dependent function of the rasGAP-binding protein p62Dok in cell signaling. J Biol Chem. 2001;276:2459–2465. doi: 10.1074/jbc.M005504200. [DOI] [PubMed] [Google Scholar]
- 38.Suzu S, Tanaka-Douzono M, Nomaguchi K, Yamada M, Hayasawa H, Kimura F, Motoyoshi K. p56(dok-2) as a cytokine-inducible inhibitor of cell proliferation and signal transduction. EMBO J. 2000;19:5114–5122. doi: 10.1093/emboj/19.19.5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sylla B S, Murphy K, Cahir-McFarland E, Lane W S, Mosialos G, Kieff E. The X-1inked lymphoproliferative syndrome gene product SH2D1A associates with p62dok (Dok1) and activates NF-κB. Proc Natl Acad Sci USA. 2000;97:7470–7475. doi: 10.1073/pnas.130193097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamir I, Stolpa J C, Helgason C D, Nakamura K, Bruhns P, Daeron M, Cambier J C. The RasGAP-binding protein p62dok is a mediator of inhibitory FcγRIIB signals in B cells. Immunity. 2000;12:347–358. doi: 10.1016/s1074-7613(00)80187-9. [DOI] [PubMed] [Google Scholar]
- 41.Tang J, Feng G-S, Li W. Induced direct binding of the adapter protein Nck to the GTPase-activating protein-associated protein p62 by epidermal growth factor. Oncogene. 1997;15:1823–1832. doi: 10.1038/sj.onc.1201351. [DOI] [PubMed] [Google Scholar]
- 42.Vuica M, Desiderio S, Schneck J P. Differential effects of B cell receptor and B cell receptor-FcγRIIB1 engagement on docking of Csk to GTPase-activating protein (GAP)-associated p62. J Exp Med. 1997;186:259–267. doi: 10.1084/jem.186.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White M F. The IRS-signalling system: a network of docking proteins that mediate insulin action. Mol Cell Biochem. 1998;182:3–11. [PubMed] [Google Scholar]
- 44.Wisniewski D, Strife A, Wojciechowicz D, Lambek C, Clarkson B. A 62-kilodalton tyrosine phosphoprotein constitutively present in primary chronic phase chronic myelogenous leukemia enriched lineage negative blast populations. Leukemia. 1994;8:688–693. [PubMed] [Google Scholar]
- 45.Yamanashi Y, Baltimore D. Identification of the Abl- and rasGAP-associated 62 kDa protein as a docking protein, Dok. Cell. 1997;88:205–211. doi: 10.1016/s0092-8674(00)81841-3. [DOI] [PubMed] [Google Scholar]
- 46.Yamanashi Y, Tamura T, Kanamori T, Yamane H, Nariuchi H, Yamamoto T, Baltimore D. Role of the rasGAP-associated docking protein p62(dok) in negative regulation of B cell receptor-mediated signaling. Genes Dev. 2000;14:11–16. [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshida K, Yamashita Y, Miyazato A, Ohya K, Kitanaka A, Ikeda U, Shimada K, Yamanaka T, Ozawa K, Mano H. Mediation by the protein-tyrosine kinase Tec of signaling between the B cell antigen receptor and Dok-1. J Biol Chem. 2000;275:24945–24952. doi: 10.1074/jbc.M909012199. [DOI] [PubMed] [Google Scholar]
- 48.Zaffran Y, Escallier J C, Ruta S, Capo C, Mege J L. Zymosan-triggered association of tyrosine phosphoproteins and lyn kinase with cytoskeleton in human monocytes. J Immunol. 1995;154:3488–3497. [PubMed] [Google Scholar]