ABSTRACT
Standard chemotherapy regimens for gastric adenocarcinoma (GAC) have limited efficacy and considerable toxicity profiles. Nab-paclitaxel has shown promising antitumor benefits in previous GAC preclinical studies. Dovitinib inhibits members of the receptor tyrosine kinase family including FGFR, VEGFR and PDGFR, and has exhibited antitumor effects in many solid tumors including GAC. Based on the antimitotic, antistromal and EPR effects of nab-paclitaxel, we investigated augmentation of nab-paclitaxel response by dovitinib in multiple GAC preclinical models. In MKN-45 subcutaneous xenografts, inhibition in tumor growth by nab-paclitaxel and dovitinib was 75% and 76%, respectively. Dovitinib plus nab-paclitaxel had an additive effect on tumor growth inhibition and resulted in tumor regression (85% of its original value). Dovitinib monotherapy resulted in minimal improvement in animal survival (25 days) compared to control (23 days), while nab-paclitaxel monotherapy or dovitinib plus nab-paclitaxel combination therapy led to a clinically significant lifespan extension of 83% (42 days) and 187% (66 days), respectively. IHC analysis of subcutaneous tumors exhibited reduced tumor cell proliferation and tumor vasculature by dovitinib. In vitro studies demonstrated that dovitinib and nab-paclitaxel individually reduced tumor cell proliferation, with an additive effect from combination therapy. Immunoblot analyses of MKN-45 and KATO-III cells revealed that dovitinib decreased phospho-FGFR, phospho-AKT, phospho-ERK, phospho-p70S6K, phospho-4EBP1, Bcl-2 and increased cleaved PARP-1, cleaved-caspase-3, p27, Bax, Bim, with an additive effect from combination therapy. These results demonstrate that the FGFR/VEGFR/PDGFR inhibitor, dovitinib, has the potential to augment the antitumor effects of nab-paclitaxel, with implications for use in the advancement of clinical GAC therapy.
KEYWORDS: Combination therapy, dovitinib, nab-paclitaxel, gastric cancer
Introduction
Gastric adenocarcinoma (GAC) has remained one of the leading cancers globally, placed at third for mortality and fifth for incidence.1 Many patients are diagnosed with an advanced stage of GAC, with the most common method of metastasis being peritoneal dissemination.2–4 The prognosis of GAC patients is typically poor despite treatment with available standard therapies. In most areas of the world, the estimated 5-year survival rate of gastric cancer is around 20%, except for Japan and South Korea where rigorous screening programs led to the early-stage diagnosis and the resulting 5-year survival rate ranges from 65% to 71.5%.5 Dependent upon patients’ age and treatment modalities, advanced GAC has a median survival of ~ 4–13 months.6–8 A recently approved standard first-line therapy for GAC includes the FLOT regimen, consisting of docetaxel, oxaliplatin, fluorouracil and leucovorin.9 The second-line treatment of GAC includes irinotecan or taxane-based therapies.10–13 The likelihood of toxicity with these therapies remains high, especially with an increased number of agents added to the treatment regimen. In GAC, there is high chemoresistance associated with available therapies.14 Thus, more efficacious therapies with less toxicity are needed to improve the mortality and morbidity associated with GAC.
Nanoparticle albumin-bound paclitaxel (nab-paclitaxel, NPT), a solvent-free formulation of paclitaxel, is a microtubule inhibitor with anti-stromal properties. Nab-paclitaxel has a favorable toxicity profile and improved drug delivery compared to solvent-based paclitaxel.15 It was first approved in the US to treat metastatic breast cancer.16 Nab-paclitaxel has since been approved to treat advanced non-small cell lung cancer and pancreatic cancer.17,18 Previous studies in our laboratory have demonstrated stronger antitumor effects of nab-paclitaxel in gastric cancer compared to standard cytotoxic therapies.19
Recently, comprehensive molecular characterization of GAC led to the advancement of targeted therapies that have improved the prognosis for patients with advanced GAC. Trastuzumab, a human epidermal growth factor receptor 2 (HER2) antibody, and ramucirumab, a vascular endothelial growth factor receptor 2 (VEGFR2) antibody, are approved targeted therapies for GAC.20,21 Recently, rilotumumab, a monoclonal antibody against mesenchymal-epithelial transition (MET) protein, demonstrated improved median survival for patients with high MET expression.22 The success of these therapies exemplifies the therapeutic benefit of targeted treatment for GAC, along with the increased efficacy associated with combination therapy.
Like HER2 and VEGFR2, other growth factor signaling such as fibroblast growth factor receptor (FGFR) and platelet-derived growth factor receptor (PDGFR) also play a critical role in GAC tumorigenesis. Aberrant FGFR signaling has been proven to mediate oncogenesis, cell proliferation, differentiation, mitogenesis, angiogenesis, invasion, and drug resistance.23 FGFR2 gene amplification has been implicated specifically in gastric cancer development.24 The VEGF pathway is well-established as a critical regulator of the growth and metastatic dissemination of several solid tumors.25 VEGFR1 expression in gastric cancer is associated with poor survival due to its ability to promote hematogenous metastasis.26 VEGFR2 signaling plays a major role in tumor angiogenesis including GAC.21 The overexpression of PDGF-β and PDGFR-β has been shown to promote tumor growth and angiogenesis and its association with poor prognosis in GAC, implying the importance of this pathway as a therapeutic target.27–29 Further, the VEGF, FGF and PDGF signaling pathways seem to be highly connected in terms of inducing several similar oncogenic pathways including PI3K/AKT, MAPK, PLC-γ and Src leading to increased tumor angiogenesis, suggesting the implication of FGF and PDGF signaling pathways in anti-VEGF therapy resistance.30,31 These findings suggest the significance of focusing on simultaneous inhibition of FGFR/PDGFR/VEGFR pathways in order to attain successful antitumor effects in advanced GAC.
Dovitinib is an antiangiogenic agent that inhibits multiple RTKs, including FGFRs, PDGFRs and VEGFRs with IC50 8–40 nM. It also inhibits FLT3, c-Kit, CSF-1 R/c-Fms, EGFR, c-MET, IGFR1 and HER2 (Supplementary Table 1).32,33 Dovitinib has shown significant antitumor potential in a number of cancer types in preclinical models.34,35 This drug has been evaluated in some phase II clinical trials for its antitumor effects in gastric cancer (Clinicaltrials.gov). Several clinical trials have evaluated the effectiveness of dovitinib in other types of cancer, including advanced melanoma, renal cell carcinoma, and urothelial carcinoma.36–38 This drug is safe based on its evaluated pharmacokinetic and pharmacodynamic profile.39
Based on the antimitotic, antistromal and enhanced permeability and retention (EPR) effect of nab-paclitaxel, and the potential inhibitory effect of dovitinib on several GAC oncogenic pathways, this study aims to demonstrate the antitumor effects of dovitinib and its ability to augment nab-paclitaxel in preclinical GAC models.
Materials and methods
Reagents
Nab-paclitaxel was purchased from the Goshen Center for Cancer Care Pharmacy (Goshen, IN) and was dissolved in 0.9% saline. Dovitinib was obtained from LC Laboratories (Woburn, MA). The cell proliferation agent, WST-1, was acquired from Roche Diagnostic Corporation (Indianapolis, IN).
Cell culture
Human GAC cell lines KATO-III and SNU-1 were obtained from American Type Culture Collection (ATCC, Rockville, MD). Human GAC MKN-45 cell line was purchased from Creative Bioarray (Shirley, Ny). The characteristics of these GAC cell lines are presented in Supplementary Table 2. These cells were cultured in RPMI 1640 medium (Sigma Chemical Co. St. Louis, MO) with 10% or 20% fetal bovine serum (FBS) at 37° Celsius in an incubator with 5% CO2. Human gastric fibroblasts were purchased from ScienCell Research Laboratories (Carlsbad, CA) and cultured in a fibroblast medium.
Cell viability assay
Cell viability assays were conducted in 96-well plates as previously described.40 Briefly, 4000 cells were plated in each well of a 96-well plate in a regular growth medium. After 24 hours, the medium was replaced with low serum (2% FBS) medium without phenol red. The cells were treated with different concentrations of nab-paclitaxel, dovitinib, or nab-paclitaxel plus dovitinib. After 72 hours incubation, 10 μl WST-1 reagent was added to each well and incubated for additional 2 hours. The absorbance was measured at a wavelength of 450 nm using Microplate Reader, and cell viability was calculated.
Western blot analysis
Subconfluent monolayers of KATO-III and MKN-45 cells were treated with nab-paclitaxel and dovitinib, incubated for 16 hours, and whole cell lysates were prepared. Tumor lysates were prepared as previously described.41 Briefly, tumor tissues from subcutaneous xenografts were snap-frozen and stored at −80°C. These tissues were suspended in lysis buffer containing protease and phosphatase inhibitors and homogenized in a Bullet Blender Homogenizer (Next Generation, Averill Park, NY), and extracts were sonicated. Protein concentration was determined using a Bradford assay. Proteins were separated on 10% polyacrylamide gels via electrophoresis and transferred to a PVDF membrane (Bio-Rad, Hercules, CA) for immobilization. These membranes were then incubated at 4° Celsius overnight with specific antibodies: c-Met, phospho-c-Met, FGFR, phospho-FGFR, AKT, phospho-AKT, ERK, phospho-ERK, cleaved PARP-1, cleaved caspase-3, p27, Bcl-2, Bax, Bim, P70S6K, phospho-P70S6K, 4EBP1, phospho-4EBP1, and GAPDH (Cell Signaling Technology, Beverly, MA). This was followed by a 1-2-hour incubation with the corresponding HRP-conjugated secondary antibodies (Cell Signaling). Protein bands were visualized using an enhanced chemiluminescence reagent in an Image360 system and densitometry analysis was conducted. The intensity of the protein bands was quantified using Image J software. The mean densitometric values of independent protein bands were normalized to mean densitometric values of their respective total protein or GAPDH and plotted to present the relative protein expression levels. The standard curves were not employed for the quantitation of different antigens.
In vivo studies
Animal experiments were conducted according to the Institutional Animal Care and Use Committee (IACUC) guidelines of the Indiana University School of Medicine. Mice were housed in a specific pathogen-free facility and supplied an ad libitum diet including food and water. Female 4-6-week-old NOD/SCID mice were purchased from Charles River Laboratories (Wilmington, MA).
In the cell-derived subcutaneous xenograft (CDX) model, MKN-45 (7.5 x 106) cells were injected subcutaneously into the right flank region of the mice. Ten days after tumor cell injection, mice were randomly assigned to four groups (n = 5 mice/group) and injected intraperitoneally with PBS (control), nab-paclitaxel (10 mg/kg, twice weekly), dovitinib (30 mg/kg, three times weekly), or a combination of nab-paclitaxel and dovitinib for 2 weeks. The tumor size was measured twice weekly and tumor volume was determined using the following formula: Volume = ½ (Length x Width2). Two weeks later, mice were euthanized, and the tumors were removed, weighed, and prepared for immunohistochemical analysis.
Immunohistochemical analysis
Subcutaneous tumors were fixed in 4% paraformaldehyde, dehydrated with sequential ethanol (25% to 100%), embedded in paraffin, and sectioned. Tumor sections (5 μm) were deparaffinized and rehydrated, and heat-mediated antigen retrieval was performed in citrate buffer. Tumor sections were then blocked in CAS buffer for 20 minutes. Tumor cell proliferation was determined with an overnight incubation at 4° Celsius with anti-Ki67 antibody (Abcam, Cambridge, MA), followed by incubation for 40 minutes at room temperature with Cy3 secondary antibody. Fluorescence mounting solution was used to mount slides that were then viewed under a fluorescence microscope. Tumor cell proliferation was determined by counting Ki67-positive cells from 5 high-power fields (HPF). Microvessel density was determined by staining tumor sections with endomucin antibody (Millipore; MAB2624) overnight at 4° Celsius followed by incubation with Cy3 secondary antibody at room temperature for 40 minutes. Using a fluorescence mounting solution and fluorescence microscope, microvessel density was calculated by counting endomucin positive vessels in five HPF. Fluorescence microscopy was performed with the Olympus microscope IX81 and images were obtained with a Hamamatsu Orca digital camera (Hamamatsu Corporation, Bridgewater, NJ) with a DSU spinning confocal unit using cellSens Dimension software (Olympus, Center Valley, PA).
Animal survival analysis
A peritoneal dissemination xenograft model, using 4-6-week-old female NOD/SCID mice, was used for the animal survival studies. Human GAC MKN-45 cells (10 x 106) were injected into the peritoneal cavity of mice. Ten days later, mice were randomly placed in four groups (n = 6 mice/group). These groups included treatment with PBS (control), nab-paclitaxel, dovitinib, and nab-paclitaxel plus dovitinib over a 2-week period, as described previously in the subcutaneous xenograft experiment. Moribund mice were euthanized following predefined criteria, which include tumors exceeding 2 cm in any direction, rapid weight loss of 15–20%, lack of strength, lethargy or inability to remain upright. Survival was assessed from treatment initiation day until death.
Statistical analysis
A two-tailed Student’s t-test (GraphPad Prism 6.0 Software, San Diego, CA) was used to determine statistical significance for the comparison of individual groups. For in vivo tumor growth studies, the Student’s t-test was used for individual group comparisons, and the one-way ANOVA was used for multiple group comparisons. For the survival studies, nonparametric testing was utilized for log-rank group comparisons (GraphPad Prism 6.0). In vitro cell proliferation data was reported as a mean with standard deviation. Values that had a p-value of <0.05 were considered statistically significant.
Results
Synergy of dovitinib and nab-paclitaxel leads to tumor regression
In order to determine a novel therapeutic approach for GAC, we first evaluated the effect of dovitinib and nab-paclitaxel therapy on tumor growth in MKN-45 cell-derived subcutaneous xenografts. Tumor growth was most impacted by combination therapy of nab-paclitaxel and dovitinib compared to nab-paclitaxel and dovitinib monotherapies (Figure 1a). Net tumor size reduction, calculated by subtracting tumor volume on therapy initiation day from that on the final day, for nab-paclitaxel was 75% and for dovitinib was 76%, compared to control (PBS treated). When used in combination, nab-paclitaxel and dovitinib caused the tumor to regress, and the total size reduced to 85% of its original value (Figure 1b). Tumor weight data in Figure 1c displays similar results, with the largest reduction in tumor weight after combined treatment of dovitinib and nab-paclitaxel at 84%. Tumor weight was reduced by 73% with dovitinib monotherapy and 56% with nab-paclitaxel monotherapy. Mouse body weight did not change significantly in any of the treatment groups (Figure 1d). This experiment suggests that dovitinib and nab-paclitaxel have significant tumor growth inhibitory effects in GAC subcutaneous xenografts, and their combination was more effective.
Figure 1.
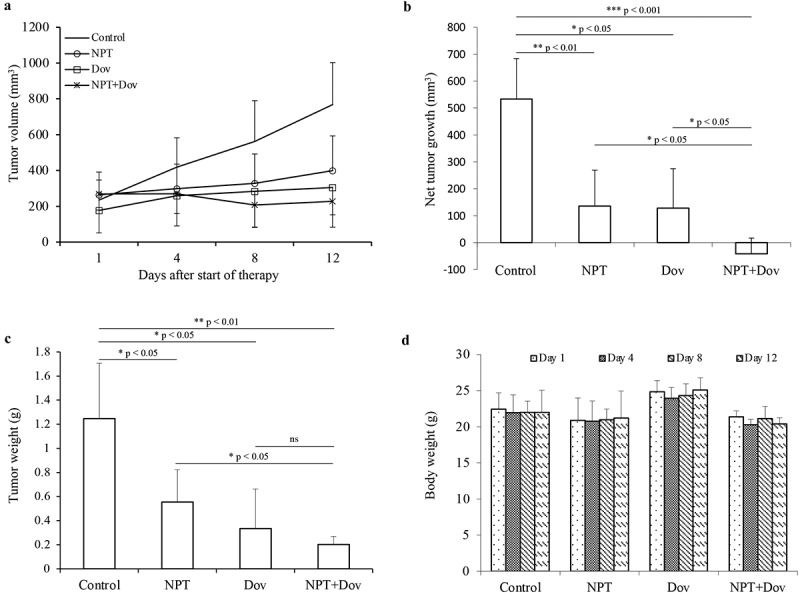
Dovitinib and nab-paclitaxel synergistically inhibit GAC cell-derived xenograft tumor growth: Subcutaneous xenograft tumor growth model utilizing MKN-45 cells (a-d). (a) Ten days after tumor cell injection, mice were randomized (N = 5) and treated with PBS (control), dovitinib, nab-paclitaxel, or nab-paclitaxel plus dovitinib over a 2-week period. Tumor size was measured twice weekly on days 1, 4, 8 and 12, and tumor volume was calculated. (b) Net tumor growth over the 2-week treatment period was calculated by using mean tumor volume on the last day minus tumor volume on the first day. * p < .05; ** p < .01; *** p < .001 by t-test. (c) At the end of the 2-week therapy period, mice were sacrificed, tumors were excised and weighed. * p < .05; ** p < .01 by t-test. (d) Mice were weighed twice weekly during the 2-week therapy period. Mouse body weight is presented as a bar chart and the individual bars represent mean values on days 1, 4, 8 and 12 in different treatment groups. This data is expressed as mean values ± standard deviation for each treatment group. Statistical analysis was conducted by Student’s t-test for the individual group comparison and one-way ANOVA for multiple group comparisons
Synergy of dovitinib and nab-paclitaxel significantly improves animal survival
Peritoneal metastasis is a hallmark of advanced GAC that is associated with a poor prognosis. Therefore, we next sought to identify the effect of dovitinib and nab-paclitaxel therapy in enhancing animal survival in MKN-45 cell-derived peritoneal dissemination xenografts. The median survival for the mice treated with dovitinib alone was 25 days, not statistically different from the median survival of the control mice (PBS treated) at 23 days. When the control mice died, tumors were present in all parts of the stomach, and metastases were found in the liver, peritoneum, lung and gallbladder. The mice treated with nab-paclitaxel alone had a median survival of 42 days and a lifespan extension of 83% compared to control with a p-value of 0.0014. The mice treated with both nab-paclitaxel and dovitinib had a median survival of 66 days and a 187% extension in lifespan with a p-value of 0.0006 (Figure 2). These findings indicate that single-agent dovitinib has no significant effect on increasing animal survival, but it was able to improve nab-paclitaxel response in the GAC metastatic peritoneal dissemination model.
Figure 2.
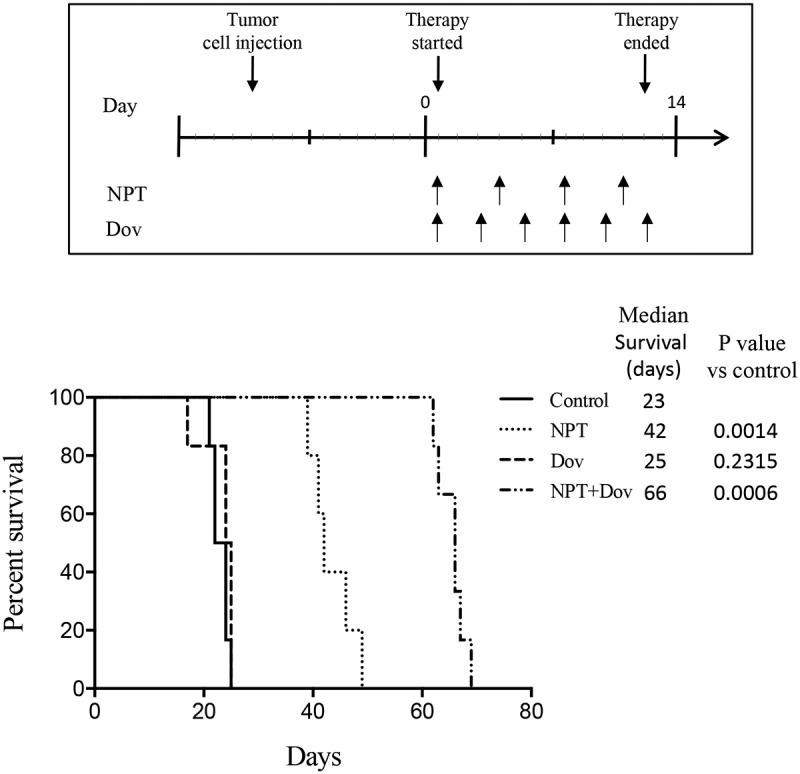
Dovitinib and nab-paclitaxel combination therapy significantly improves animal survival through a synergistic approach: Intraperitoneal dissemination model utilizing MKN-45 cells. Ten days after tumor cell injection, mice were randomized (N = 6) and treated with PBS (control), dovitinib, nab-paclitaxel, and nab-paclitaxel plus dovitinib over a 2-week period. A Kaplan-Meier survival curve is shown representing mice survival from the start of treatment until death. Log-rank testing was used to determine statistical differences between groups in terms of survival time
Dovitinib reduces tumor cell proliferation, vasculature and alters the expression of marker proteins
To investigate the biological impact of dovitinib and nab-paclitaxel therapy in GAC tissues, we performed IHC analysis of MKN-45 xenograft tumor sections. We observed that dovitinib combined with nab-paclitaxel had the greatest impact on tumor cell proliferation reduction (by 75%) after Ki67 staining compared to controls. Reduction in tumor cell proliferation by single-agent therapy with nab-paclitaxel and dovitinib was 58% and 39%, respectively (Figure 3a).
Figure 3.
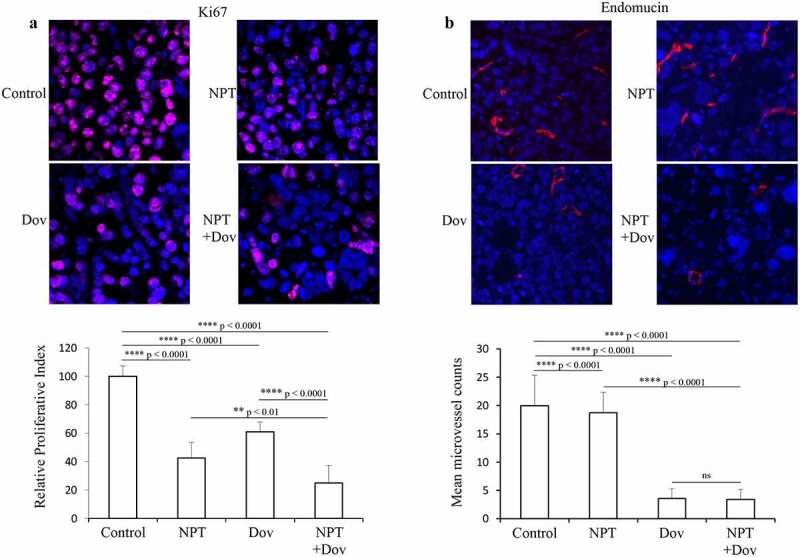
Dovitinib and nab-paclitaxel reduce tumor cell proliferation and tumor vasculature in GAC cell-derived xenograft: Tumor sections derived from MKN-45 xenografts after 2-week therapy with control, dovitinib, nab-paclitaxel, and nab-paclitaxel plus dovitinib were analyzed by IHC. (a) Tumor cell proliferation was determined after incubating tumor sections with anti-Ki67 antibody followed by counting the Ki67-positive cells from 5 high-power fields (HPF). Cell nuclei stained with Ki67 (red) and DAPI (blue) are illustrated at 20X magnification. ** p < .01; **** p < .0001 by t-test. (b) Microvessel density was determined by incubating with endomucin antibody followed by calculating endomucin positive vessels in 5 HPF. Endomucin positive microvessel (red) and cell nuclei (DAPI, blue) are illustrated at 20X magnification. **** p < .0001 by t-test. The results are displayed as mean values ± standard deviation for each treatment group
In MKN-45 subcutaneous tumors, dovitinib exhibited significant alleviation in tumor vasculature and reduction in microvessel density as monotherapy and in combination with nab-paclitaxel was 82% and 83%, respectively. Nab-paclitaxel monotherapy had no significant impact on tumor vasculature, and it reduced microvessel density by only 6.3% compared to control (Figure 3b).
Further investigation of dovitinib’s intratumoral mechanism of action by Immunoblot analysis of MKN-45 subcutaneous tumors demonstrated that dovitinib treatment led to a dramatic reduction in phospho-c-Met and phospho-FGFR protein levels and the PI3K/MAPK pathway proteins phospho-AKT, phospho-ERK, phospho-P70S6K and phospho-4EBP1. Dovitinib also led to an increase in apoptosis-marker protein cleaved caspase-3 (Figure 4).
Figure 4.
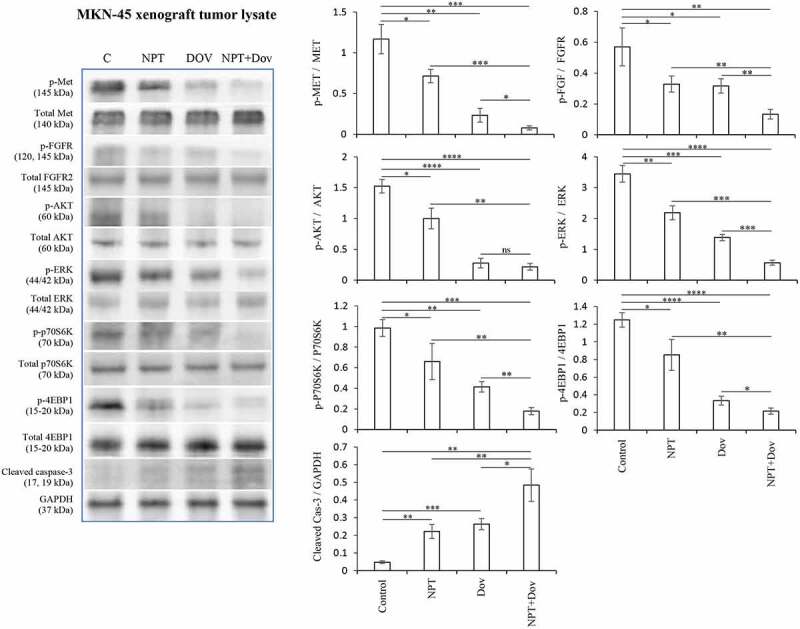
Dovitinib alters marker protein expression in MKN-45 xenografts: Tumor lysates were prepared from MKN-45 subcutaneous xenograft tumors. The images represent Western blot data of at least three independent experiments with identical outcomes. The intensity of protein bands was quantitated by densitometry and represented in the bar graph after normalizing values with corresponding total protein or GAPDH. * p < .05; ** p < .01; *** p < .001; **** p < .0001 by t-test
Dovitinib diminishes gastric cancer cell viability in vitro
The potential clinical relevance of dovitinib and nab-paclitaxel combination therapy was further confirmed by analyzing their effect on cell viability of multiple mutationally different GAC epithelial cells and gastric fibroblasts. We observed that dovitinib treatment resulted in growth inhibitory effects and its combination with nab-paclitaxel exhibited additive response in GAC associated cells (Figure 5). In the MKN-45 cell line at 1 μM and 10 μM concentrations, reduction in cell viability was 13% and 63% (dovitinib), 59% and 53% (nab-paclitaxel), and 72% and 81% (nab-paclitaxel plus dovitinib), respectively (Figure 5a). In the KATO-III cell line at 1 μM and 10 μM concentrations, decrease in cell viability was 75% and 85% (dovitinib), 65% and 69% (nab-paclitaxel), and 84% and 88% (nab-paclitaxel plus dovitinib), respectively (Figure 5b). In the SNU-1 cell line at 1 μM and 10 μM concentrations, reduction in cell viability was 45% and 65% (dovitinib), 53% and 61% (nab-paclitaxel), and 64% and 74% (nab-paclitaxel plus dovitinib), respectively (Figure 5c). In gastric fibroblasts, a marker of GAC stromal cells, dovitinib lessened cell viability by 33% at 1 μM and 74% at 10 μM; nab-paclitaxel lessened cell viability by 25% at 10 nM and 71% at 100 nM. Cell viability reduction for nab-paclitaxel plus dovitinib was the greatest compared to all of the other treatments with 52% and 81%, respectively (Figure 5d). These in vitro results indicate a more generalized higher inhibitory effect of dovitinib and nab-paclitaxel combination on GAC cell viability irrespective of their mutational and molecular characterization.
Figure 5.
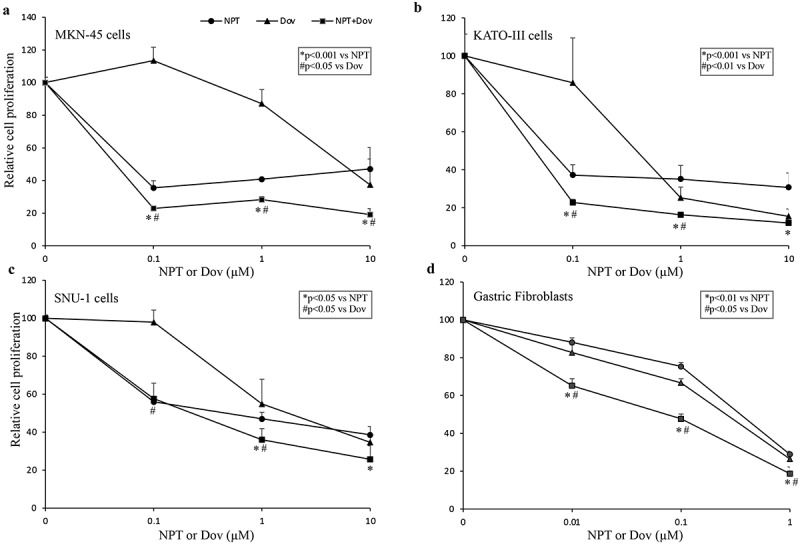
Dovitinib and nab-paclitaxel diminish GAC cell viability in vitro: GAC epithelial cells (MKN-45, SNU-1, KATO-III) and gastric fibroblasts were plated on 96-well plates and treated with varying concentrations of dovitinib and nab-paclitaxel (a-d). After 72 hours, WST-1 reagent was added to each well and incubated for an additional 2 hours. Cell viability was calculated from absorbance measurements at a wavelength of 450 nm. This data is expressed as mean values ± standard deviation of triplicate determinations for each treatment group
Dovitinib alters the expression of marker oncogenic protein
To further delineate the molecular mechanisms underlying the efficacy of dovitinib, either alone or in combination with nab-paclitaxel, we performed Immunoblot analysis of mutationally different human GAC MKN-45 and KATO-III cells. Consistent with the inhibition in cell viability observed in vitro, dovitinib decreased the expression of pro-oncogenic proteins in the RTK family, including phospho-Met, phospho-FGFR, phospho-AKT, phospho-ERK, phospho-p70S6K and phospho-4EBP1. Dovitinib demonstrated an increase in expression of proapoptotic proteins including cleaved PARP-1, cleaved caspase-3, Bax and Bim, and a reduction in expression of the antiapoptotic protein Bcl-2. Dovitinib also induced the expression of cyclin-dependent kinase inhibitor p27 (Figure 6, Supplementary Figure S1 and S2).
Figure 6.
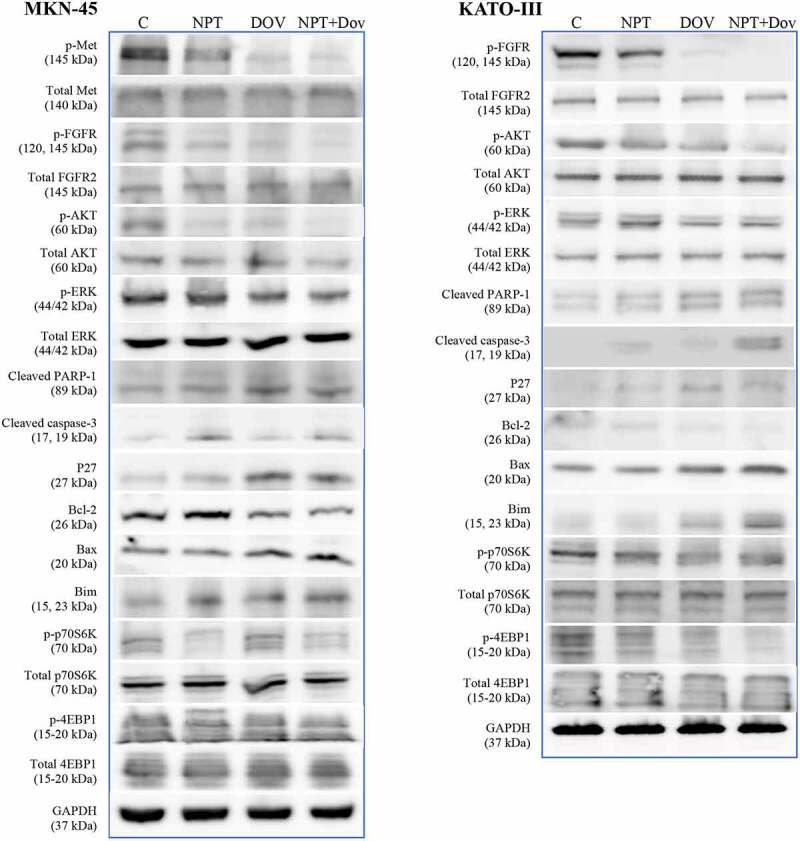
Dovitinib and nab-paclitaxel alter the expression of key oncogenic proteins in GAC cells: Subconfluent cultures of MKN-45 and KATO-III cells were treated with dovitinib and nab-paclitaxel and incubated for 16 hours. Whole cell lysates were prepared then protein expressions were determined via Western blot analysis. The images represent Western blot data of at least three independent experiments with identical outcomes
Discussion
Treating GAC has been challenging due to the poor clinical efficacy, chemoresistance and toxicities associated with current standard therapies. Due to the rise of molecular characterization of GAC, there are newer avenues consisting of targeted therapies to improve clinical outcomes of GAC patients. There is limited efficacy of single-target therapies in this disease due to the multifactorial nature of GAC and the development of drug resistance is commonplace. GAC is characterized by the aberrant signaling of multiple growth factors, including FGF/FGFR, VEGF/VEGFR, PDGF/PDGFR and HGF/HGFR. The significant role that these growth factors and their receptors play in GAC progression demonstrates the need for multi-targeted therapies to effectively treat GAC, in addition to chemotherapeutic agents.
Due to the critical role of peritoneal metastasis associated with a poor prognosis in advanced GAC, we tested several GAC cell lines to establish a peritoneal dissemination animal survival model. Among these cell lines, MKN-45 cells were most suitable based on their aggressive metastatic progression pattern in the peritoneal dissemination survival model and subcutaneous tumor growth pattern. Furthermore, MKN-45 cells are clinically relevant in GAC studies as these cells have c-MET amplification, p15 & p16 mutations, and FGFR/VEGFR /PDGFR/EGFR expression.42–45
In this study, treatment with dovitinib showed that simultaneous targeted inhibition of FGFR, PDGFR and VEGFR pathways can improve outcomes in GAC, specifically when used in combination with the nanoparticle-based chemotherapeutic agent, nab-paclitaxel. When dovitinib was added to nab-paclitaxel in the GAC peritoneal dissemination mouse xenograft model, there was a significant improvement in mice survival. Dovitinib monotherapy was unsuccessful at prolonging animal survival, indicating that when used alone, the targeted effects against multiple growth factors signaling may be less effective in this setting. The presence of metastasis to the liver, peritoneum, lung and gallbladder, in the peritoneal dissemination model, might explain the limited benefit of dovitinib monotherapy in this setting. Previous studies have demonstrated difficulties in targeting cancer metastasis due to the fact that the signaling pathways driving metastasis can vary between primary and secondary tumors.46 Consistent with our findings, prior preclinical and clinical studies of different targeted therapies have exhibited limited benefit when used as monotherapy.47–49
Dovitinib exhibited strong antitumor effects in subcutaneous GAC models by resulting in tumor regression when added to nab-paclitaxel therapy. Dovitinib reduced tumor size equal to that of nab-paclitaxel monotherapy, indicating that its targeted effects may be more effective in the tumor microenvironment associated with subcutaneous xenografts. Dovitinib augments nab-paclitaxel in reducing tumor cell proliferation and tumor vasculature, supporting its beneficial role as an antiangiogenic and antiproliferative agent through targeting growth factor-induced angiogenesis. This information supports the addition of targeted therapy to a systemic chemotherapeutic agent in treating advanced GAC to provide the best outcome in terms of both tumor size and survival. Although MKN-45 cells do not harbor driver mutation/amplification for the main targets of dovitinib, this cell line has been shown to express several oncogenic growth factors and their receptors including FGFR, PDGFR, VEGFR, EGFR and c-Met that are targets of this drug.42–45 Analysis of MKN-45-cell derived tumor tissues demonstrated that dovitinib not only inhibits the expression of phospho-FGFR but it also inhibits phospho-c-Met expression. Although, no detectable expression of VEGFR or PDGFR was observed in the tumor tissues but importantly, dovitinib inhibited PI3K/AKT and MAPK signaling that are the most common downstream signaling of FGFR/VEGFR/FGFR/c-Met. Further, in addition to the direct impact of dovitinib on tumor epithelial cells, the antiangiogenic stromal effect might be playing an important role in the overall antitumor efficacy of this drug.
Based on the in vitro cell viability analysis results, dovitinib had a differential antiproliferative effect in mutationally different human GAC cell lines MKN-45, KATO-III and SNU-1, and gastric fibroblasts, indicating its diversified impact on gastric cancer epithelial and stromal cells. Dovitinib had the greatest antiproliferative effects in the FGFR2 overexpressing KATO-III cell line compared with FGFR2 low-expressing MKN-45 and SNU-1 cell lines. Immunoblot analysis results support that dovitinib inhibits FGFR pathways, including PI3K/AKT and MAPK/ERK. These findings suggest the potential of dovitinib therapy in GAC patient population with FGFR2 amplification. The expression of VEGFR and PDGFR was below the detection limit in MKN-45 and KATO-III cells. Therefore, the impact of dovitinib on these targets couldn’t be determined. However, the downstream effectors of VEGFR and PDGFR pathways such as PI3K/AKT and MAPK were significantly alleviated by dovitinib treatment.
Nab-paclitaxel has proven to have stronger antitumor effects on GAC than contemporary, cytotoxic agents including docetaxel, oxaliplatin and epirubicin.9,19 The benefits of nab-paclitaxel compared to other agents include enhanced transport, permeability and retention resulting in increased tissue distribution and tumor penetration, which can be attributed to its anti-mitotic and anti-stromal properties.50–53 Using drugs that inhibit PI3K has been shown to make tumors more susceptible to paclitaxel.54–56 Due to its solvent-free, nanoparticle formulation property, nab-paclitaxel results in higher tumor retention and lower toxicity compared to standard paclitaxel.51 Nab-paclitaxel has been shown to increase tumoral delivery of targeted drugs when used in combination.53,57 Both nab-paclitaxel and dovitinib have the ability to enhance other therapeutic agents when treating GAC, supporting the synergistic outcome evidenced by this study. How dovitinib enhances nab-paclitaxel response has still not been solidified, however, some of the possible explanations include the inhibition of angiogenesis, decrease in stromal growth, and enhancement of tumoral delivery of the drugs.52,58
The development of advanced GAC depends on several factors, including cell proliferation, migration, angiogenesis, invasion, and metastasis. A combination therapeutic regimen should have the ability to target most, if not all, of these factors with limited toxicity, in order to provide the best outcome for patients with advanced GAC. This study presents the multi-kinase inhibitor, dovitinib, as an efficacious targeted therapy for GAC, with the ability to augment the effects of new-generation chemotherapy, nab-paclitaxel, in preclinical models, providing another gateway for future clinical treatment of advanced GAC.
Supplementary Material
Funding Statement
This research project was supported by Indiana University School of Medicine funds to R.E. Schwarz and N. Awasthi; School of Medicine, Indiana University
Disclosure statement
The authors do not declare any conflicts of interest.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F.. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Okugawa Y, Toiyama Y, Hur K, Toden S, Saigusa S, Tanaka K, Inoue Y, Mohri Y, Kusunoki M, Boland CR, et al. Metastasis-associated long non-coding RNA drives gastric cancer development and promotes peritoneal metastasis. Carcinogenesis. 2014;35:2731–2739. 10.1093/carcin/bgu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takashima A, Shitara K, Fujitani K, Koeda K, Hara H, Nakayama N, Hironaka S, Nishikawa K, Kimura Y, Amagai K, et al. Peritoneal metastasis as a predictive factor for nab-paclitaxel in patients with pretreated advanced gastric cancer: an exploratory analysis of the phase III ABSOLUTE trial. Gastric Cancer. 2019;22:155–163. 10.1007/s10120-018-0838-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lazar DC, Avram MF, Romosan I, Cornianu M, Taban S, Goldis A. Prognostic significance of tumor immune microenvironment and immunotherapy: novel insights and future perspectives in gastric cancer. World J Gastroenterol. 2018;24:3583–3616. 10.3748/wjg.v24.i32.3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Etemadi A, Safiri S, Sepanlou SG, Ikuta K, Bisignano C, Shakeri R, Amani M, Fitzmaurice C, Nixon M, Abbasi N, et al. The global, regional, and national burden of stomach cancer in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol Hepatol. 2020;5:42–54. 10.1016/s2468-1253(19)30328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang YR, Han DS, Kong SH, Lee HJ, Kim SH, Kim WH, Yang HK. The value of palliative gastrectomy in gastric cancer with distant metastasis. Ann Surg Oncol. 2012;19:1231–1239. 10.1245/s10434-011-2056-x. [DOI] [PubMed] [Google Scholar]
- 7.Koizumi W, Kim YH, Fujii M, Kim HK, Imamura H, Lee KH, Hara T, Chung HC, Satoh T, Cho JY, et al. Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START). J Cancer Res Clin Oncol. 2014;140:319–328. 10.1007/s00432-013-1563-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, Nishina T, Amagai K, Chin K, Niwa Y, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol. 2015;26:141–148. 10.1093/annonc/mdu472. [DOI] [PubMed] [Google Scholar]
- 9.Al-Batran SE, Hofheinz RD, Pauligk C, Kopp HG, Haag GM, Luley KB, Meiler J, Homann N, Lorenzen S, Schmalenberg H, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016;17:1697–1708. 10.1016/S1470-2045(16)30531-9. [DOI] [PubMed] [Google Scholar]
- 10.Kanat O, Evrensel T, Manavoglu O, Demiray M, Kurt E, Gonullu G, Kiyici M, Arslan M. Single-agent irinotecan as second-line treatment for advanced gastric cancer. Tumori. 2003;89:405–407. https://www.ncbi.nlm.nih.gov/pubmed/14606644. [DOI] [PubMed] [Google Scholar]
- 11.Chun JH, Kim HK, Lee JS, Choi JY, Lee HG, Yoon SM, Choi IJ, Ryu KW, Kim YW, Bae JM. Weekly irinotecan in patients with metastatic gastric cancer failing cisplatin-based chemotherapy. Jpn J Clin Oncol. 2004;34:8–13. 10.1093/jjco/hyh006. [DOI] [PubMed] [Google Scholar]
- 12.Kim ST, Kang WK, Kang JH, Park KW, Lee J, Lee SH, Park JO, Kim K, Kim WS, Jung CW, et al. Salvage chemotherapy with irinotecan, 5-fluorouracil and leucovorin for taxane- and cisplatin-refractory, metastatic gastric cancer. Br J Cancer. 2005;92:1850–1854. 10.1038/sj.bjc.6602575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kunisaki C, Imada T, Yamada R, Hatori S, Ono H, Otsuka Y, Matsuda G, Nomura M, Akiyama H, Kubo A, et al. 2005. Phase II study of docetaxel plus cisplatin as a second-line combined therapy in patients with advanced gastric carcinoma. Anticancer Res. 25:2973–2977. https://www.ncbi.nlm.nih.gov/pubmed/16080554 [PubMed] [Google Scholar]
- 14.Marin JJG, Perez-Silva L, Macias RIR, Asensio M, Peleteiro-Vigil A, Sanchez-Martin A, Cives-Losada C, Sanchon-Sanchez P, Sanchez De Blas B, Herraez E, et al. Molecular bases of mechanisms accounting for drug resistance in gastric adenocarcinoma. Cancers (Basel). 2020;12. 10.3390/cancers12082116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kundranda MN, Niu J. Albumin-bound paclitaxel in solid tumors: clinical development and future directions. Drug Des Devel Ther. 2015;9:3767–3777. 10.2147/DDDT.S88023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moreno-Aspitia A, Perez EA, Kanat O, Evrensel T, Manavoglu O, Demiray M, Kurt E, Gonullu G, Kiyici M, Arslan M. Nanoparticle albumin-bound paclitaxel (ABI-007): a newer taxane alternative in breast cancer. Future Oncol. 2005;1:755–762. 10.2217/14796694.1.6.755. [DOI] [PubMed] [Google Scholar]
- 17.Socinski MA, Bondarenko I, Karaseva NA, Makhson AM, Vynnychenko I, Okamoto I, Hon JK, Hirsh V, Bhar P, Zhang H, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012;30:2055–2062. 10.1200/JCO.2011.39.5848. [DOI] [PubMed] [Google Scholar]
- 18.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang C, Awasthi N, Schwarz MA, Hinz S, Schwarz RE, . Superior antitumor activity of nanoparticle albumin-bound paclitaxel in experimental gastric cancer. PLoS One. 2013;8:e58037. 10.1371/journal.pone.0058037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry DR, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–39. 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 22.Iveson T, Donehower RC, Davidenko I, Tjulandin S, Deptala A, Harrison M, Nirni S, Lakshmaiah K, Thomas A, Jiang Y, et al. Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: an open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study. Lancet Oncol. 2014;15:1007–1018. 10.1016/S1470-2045(14)70023-3. [DOI] [PubMed] [Google Scholar]
- 23.Yashiro M, Matsuoka T. Fibroblast growth factor receptor signaling as therapeutic targets in gastric cancer. World J Gastroenterol. 2016;22:2415–2423. 10.3748/wjg.v22.i8.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunii K, Davis L, Gorenstein J, Hatch H, Yashiro M, Di Bacco A, Elbi C, Lutterbach B. FGFR2-amplified gastric cancer cell lines require FGFR2 and Erbb3 signaling for growth and survival. Cancer Res. 2008;68:2340–2348. 10.1158/0008-5472.CAN-07-5229. [DOI] [PubMed] [Google Scholar]
- 25.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. 10.1200/jco.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 26.Mimori K, Fukagawa T, Kosaka Y, Kita Y, Ishikawa K, Etoh T, Iinuma H, Sasako M, Mori M. Hematogenous metastasis in gastric cancer requires isolated tumor cells and expression of vascular endothelial growth factor receptor-1. Clin Cancer Res. 2008;14:2609–2616. 10.1158/1078-0432.CCR-07-4354. [DOI] [PubMed] [Google Scholar]
- 27.Kodama M, Kitadai Y, Sumida T, Ohnishi M, Ohara E, Tanaka M, Shinagawa K, Tanaka S, Yasui W, Chayama K. Expression of platelet-derived growth factor (PDGF)-B and PDGF-receptor β is associated with lymphatic metastasis in human gastric carcinoma. Cancer Sci. 2010;101:1984–1989. 10.1111/j.1349-7006.2010.01639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higuchi A, Oshima T, Yoshihara K, Sakamaki K, Aoyama T, Suganuma N, Yamamoto N, Sato T, Cho H, Shiozawa M, et al. Clinical significance of platelet-derived growth factor receptor-β gene expression in stage II/III gastric cancer with S-1 adjuvant chemotherapy. Oncol Lett. 2017;13:905–911. 10.3892/ol.2016.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim R, Emi M, Arihiro K, Tanabe K, Uchida Y, Toge T. Chemosensitization by STI571 targeting the platelet-derived growth factor/platelet-derived growth factor receptor-signaling pathway in the tumor progression and angiogenesis of gastric carcinoma. Cancer. 2005;103:1800–1809. 10.1002/cncr.20973. [DOI] [PubMed] [Google Scholar]
- 30.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trudel S, Li ZH, Wei E, Wiesmann M, Chang H, Chen C, Reece D, Heise C, Ak S. CHIR-258, a novel, multitargeted tyrosine kinase inhibitor for the potential treatment of t(4;14) multiple myeloma. Blood. 2005;105:2941–2948. 10.1182/blood-2004-10-3913. [DOI] [PubMed] [Google Scholar]
- 33.Lee SH, Lopes de Menezes D, Vora J, Harris A, Ye H, Nordahl L, Garrett E, Samara E, Sl A, Ab G, et al. In vivo target modulation and biological activity of CHIR-258, a multitargeted growth factor receptor kinase inhibitor, in colon cancer models. Clin Cancer Res. 2005;11:3633–3641. 10.1158/1078-0432.CCR-04-2129. [DOI] [PubMed] [Google Scholar]
- 34.Gaur S, Chen L, Ann V, Lin WC, Wang Y, Chang VH, Hsu NY, Shia HS, Yen Y. Dovitinib synergizes with oxaliplatin in suppressing cell proliferation and inducing apoptosis in colorectal cancer cells regardless of RAS-RAF mutation status. Mol Cancer. 2014;13:21. 10.1186/1476-4598-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huynh H, Chow PK, Tai WM, Choo SP, Chung AY, Ong HS, Soo KC, Ong R, Linnartz R, Shi MM. Dovitinib demonstrates antitumor and antimetastatic activities in xenograft models of hepatocellular carcinoma. J Hepatol. 2012;56:595–601. 10.1016/j.jhep.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 36.Kim KB, Chesney J, Robinson D, Gardner H, Shi MM, Kirkwood JM. Phase I/II and pharmacodynamic study of dovitinib (TKI258), an inhibitor of fibroblast growth factor receptors and VEGF receptors, in patients with advanced melanoma. Clin Cancer Res. 2011;17:7451–7461. 10.1158/1078-0432.CCR-11-1747. [DOI] [PubMed] [Google Scholar]
- 37.Angevin E, Lopez-Martin JA, Lin CC, Gschwend JE, Harzstark A, Castellano D, Soria JC, Sen P, Chang J, Shi M, et al. Phase I study of dovitinib (TKI258), an oral FGFR, VEGFR, and PDGFR inhibitor, in advanced or metastatic renal cell carcinoma. Clin Cancer Res. 2013;19:1257–1268. 10.1158/1078-0432.CCR-12-2885. [DOI] [PubMed] [Google Scholar]
- 38.Milowsky MI, Dittrich C, Duran I, Jagdev S, Millard FE, Sweeney CJ, Bajorin D, Cerbone L, Quinn DI, Stadler WM, et al. Phase 2 trial of dovitinib in patients with progressive FGFR3-mutated or FGFR3 wild-type advanced urothelial carcinoma. Eur J Cancer. 2014;50:3145–3152. 10.1016/j.ejca.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 39.Sarker D, Molife R, Evans TR, Hardie M, Marriott C, Butzberger-Zimmerli P, Morrison R, Fox JA, Heise C, Louie S, et al. A phase I pharmacokinetic and pharmacodynamic study of TKI258, an oral, multitargeted receptor tyrosine kinase inhibitor in patients with advanced solid tumors. Clin Cancer Res. 2008;14:2075–2081. 10.1158/1078-0432.CCR-07-1466. [DOI] [PubMed] [Google Scholar]
- 40.Awasthi N, Schwarz MA, Verma V, Cappiello C, Schwarz RE. Endothelial monocyte activating polypeptide II interferes with VEGF-induced proangiogenic signaling. Lab Invest. 2009;89:38–46. 10.1038/labinvest.2008.106. [DOI] [PubMed] [Google Scholar]
- 41.Awasthi N, Zhang C, Ruan W, Schwarz MA, Schwarz RE. Evaluation of poly-mechanistic antiangiogenic combinations to enhance cytotoxic therapy response in pancreatic cancer. PLoS One. 2012;7:e38477. 10.1371/journal.pone.0038477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt K, Moser C, Hellerbrand C, Zieker D, Wagner C, Redekopf J, Schlitt HJ, Geissler EK, Lang SA. Targeting fibroblast growth factor receptor (FGFR) with BGJ398 in a gastric cancer model. Anticancer Res. 2015;35:6655–6665. [PubMed] [Google Scholar]
- 43.Kataoka Y, Mukohara T, Tomioka H, Funakoshi Y, Kiyota N, Fujiwara Y, Yashiro M, Hirakawa K, Hirai M, Minami H. Foretinib (GSK1363089), a multi-kinase inhibitor of MET and VEGFRs, inhibits growth of gastric cancer cell lines by blocking inter-receptor tyrosine kinase networks. Invest New Drugs. 2012;30:1352–1360. 10.1007/s10637-011-9699-0. [DOI] [PubMed] [Google Scholar]
- 44.Du S, Miao J, Zhu Z, Xu E, Shi L, Ai S, Wang F, Kang X, Chen H, Lu X, et al. NADPH oxidase 4 regulates anoikis resistance of gastric cancer cells through the generation of reactive oxygen species and the induction of EGFR. Cell Death Dis. 2018;9:948. 10.1038/s41419-018-0953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lian L, Li XL, Xu MD, Li XM, Wu MY, Zhang Y, Tao M, Li W, Shen XM, Zhou C, et al. VEGFR2 promotes tumorigenesis and metastasis in a pro-angiogenic-independent way in gastric cancer. BMC Cancer. 2019;19:183. 10.1186/s12885-019-5322-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson RL, Balasas T, Callaghan J, Coombes RC, Evans J, Hall JA, Kinrade S, Jones D, Jones PS, Jones R, et al. A framework for the development of effective anti-metastatic agents. Nat Rev Clin Oncol. 2019;16:185–204. 10.1038/s41571-018-0134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Awasthi N, Schwarz MA, Schwarz RE. Antitumour activity of sunitinib in combination with gemcitabine in experimental pancreatic cancer. HPB (Oxford). 2011;13:597–604. 10.1111/j.1477-2574.2011.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Awasthi N, Zhang C, Hinz S, Schwarz MA, Schwarz RE. Enhancing sorafenib-mediated sensitization to gemcitabine in experimental pancreatic cancer through EMAP II. J Exp Clin Cancer Res. 2013;32:12. 10.1186/1756-9966-32-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ellis LM, Hicklin DJ. Resistance to targeted therapies: refining anticancer therapy in the era of molecular oncology. Clin Cancer Res. 2009;15:7471–7478. 10.1158/1078-0432.Ccr-09-1070. [DOI] [PubMed] [Google Scholar]
- 50.Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, Tao C, De T, Beals B, Dykes D, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res. 2006;12:1317–1324. 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]
- 51.Gardner ER, Dahut WL, Scripture CD, Jones J, Aragon-Ching JB, Desai N, Hawkins MJ, Sparreboom A, Figg WD. Randomized crossover pharmacokinetic study of solvent-based paclitaxel and nab-paclitaxel. Clin Cancer Res. 2008;14:4200–4205. 10.1158/1078-0432.CCR-07-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Awasthi N, Zhang C, Schwarz AM, Hinz S, Schwarz MA, Schwarz RE. Enhancement of nab-paclitaxel antitumor activity through addition of multitargeting antiangiogenic agents in experimental pancreatic cancer. Mol Cancer Ther. 2014;13:1032–1043. 10.1158/1535-7163.MCT-13-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Awasthi N, Zhang C, Schwarz AM, Hinz S, Wang C, Williams NS, Schwarz MA, Schwarz RE. Comparative benefits of Nab-paclitaxel over gemcitabine or polysorbate-based docetaxel in experimental pancreatic cancer. Carcinogenesis. 2013;34:2361–2369. 10.1093/carcin/bgt227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen D, Lin X, Zhang C, Liu Z, Chen Z, Li Z, Wang J, Li B, Hu Y, Dong B, et al. Dual PI3K/mTOR inhibitor BEZ235 as a promising therapeutic strategy against paclitaxel-resistant gastric cancer via targeting PI3K/Akt/mTOR pathway. Cell Death Dis. 2018;9:123. 10.1038/s41419-017-0132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu L, Hofmann J, Lu Y, Mills GB, Jaffe RB. Inhibition of phosphatidylinositol 3ʹ-kinase increases efficacy of paclitaxel in in vitro and in vivo ovarian cancer models. Cancer Res. 2002;62:1087–1092. [PubMed] [Google Scholar]
- 56.Zhang C, Awasthi N, Schwarz MA, Schwarz RE. The dual PI3K/mTOR inhibitor NVP-BEZ235 enhances nab-paclitaxel antitumor response in experimental gastric cancer. Int J Oncol. 2013;43:1627–1635. 10.3892/ijo.2013.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim H, Samuel S, Lopez-Casas P, Grizzle W, Hidalgo M, Kovar J, Oelschlager D, Zinn K, Warram J, Buchsbaum D. SPARC-Independent delivery of nab-paclitaxel without depleting tumor stroma in patient-derived pancreatic cancer xenografts. Mol Cancer Ther. 2016;15:680–688. 10.1158/1535-7163.MCT-15-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Awasthi N, Schwarz MA, Zhang C, Schwarz RE. Augmentation of nab-paclitaxel chemotherapy response by mechanistically diverse antiangiogenic agents in preclinical gastric cancer models. Mol Cancer Ther. 2018;17:2353–2364. 10.1158/1535-7163.MCT-18-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


