ABSTRACT
Hemophagocytic lymphohistiocytosis (HLH) is a severe or even fatal inflammatory status caused by a hereditary or acquired immunoregulatory abnormality. Lymphoma-associated hemophagocytic lymphohistiocytosis (LAHS) is a kind of secondary HLH (sHLH). It suffers the worst outcome among sHLH. However, there is no standard treatment strategy. The argument mainly focuses on whether an HLH-directed or malignancy-directed approach should initially be adopted. Etoposide is one of the key drugs in HLH treatment and also shows activity in lymphomas. We sought to identify the importance of containing etoposide in the initial treatment of LAHS. 66 patients diagnosed with LAHS in our center during the three years were divided into two groups according to whether the initial treatment involved etoposide or lymphoma-directed chemotherapy without etoposide. The remission rate of the initial etoposide group (52 patients) is significantly better than that of the no initial etoposide group (14 patients) (73.1% vs. 42.9%, p = .033). The two-month survival rate (79.8% vs. 46.8%, p = .035) and overall survival (median survival time 25.8 w vs. 7.8 w, p = .048) of the initial etoposide contained group is significantly better. Multivariate cox analysis revealed that for patients without EBV infection (37 cases), initial treatment with etoposide could significantly improve prognosis (p = .010, Exp(B) = 0.183), but for patients with positive EBV, it shows a tendency. Containing etoposide is beneficial in the initial treatment of LAHS, whether in the HLH-directed or lymphoma-directed strategy. It provides higher response rate, lower mortality rate, and better survival, especially for EBV negative patients.
KEYWORDS: Lymphoma, hemophagocytic lymphohistiocytosis, etoposide, treatment, Epstein-Barr virus
Background
Hemophagocytic lymphohistiocytosis (HLH) is a severe or even fatal inflammatory status caused by a hereditary or acquired immunoregulatory abnormality.1–3 It is divided into two categories: primary and secondary. Secondary HLH (sHLH) is often associated with and caused by infections, malignant tumors, and autoimmune diseases. Malignancy HLH (M-HLH) is a kind of sHLH, and lymphoma associated hemophagocytic lymphohistiocytosis (LAHS) is the most common kind of M-HLH. The prevalence is approximately 20% among patients with LBCIVL (large B cell intravascular lymphoma) and T cell lymphoma.4 M-HLH suffers the worst prognosis in secondary HLH,5 which has a high early mortality, and the median survival is considered to be less than two months.6 That is because the treatment of LAHS is often faced with many difficulties, such as HLH controlling problem, the high recurrence rate, and the difficulty in relieving lymphoma. However, there is still no standard strategy for LAHS’s treatment up until now. There are no universal conclusions on whether an HLH directed, malignancy-directed or combined approach should initially be adopted.7 In the study of adult M-HLH by Gevorg Tamamyan in 2016, they noted a very aggressive and rapid progression of HLH with M-HLH, with a majority of patients dying from HLH within 2–4 weeks in spite of continued treatment of the underlying malignancy.6 In the consensus review on malignancy-HLH in adults in 2017, it is suggested that once HLH-triggered organ damage occurred, the application of lympholytic agents must be considered, including etoposide, corticosteroids, polyvalent immunoglobulins, which targets the cytokine storm and T-cell proliferation, and DEP regimen as salvage therapy. Neoplastic specific treatment should be put after the organ function is reestablished.7 According to the HLH Steering Committee of the Histiocyte Society, a corticosteroid should be used to treat inflammation followed by CHOP based treatment (with or without rituximab).8 However, it is noted only as an expert opinion, prospective, randomized or controlled clinical trials is still needed to support. Etoposide is one of the key drugs in HLH treatment,9 and its importance and necessity, especially in initial treatment of some types of HLH, are getting a lot of attention. In previous reports, the initial treatment with etoposide is believed to improve the prognosis of EBV-HLH patients, especially in adult patients.10,11 In the Alison S. and Nancy B.’s experience of HLH treatment, they suggested that the etoposide containing chemotherapy regimen in LAHS should be strongly considered.12 Also, in 2018, Camille Bigenwald et al. reanalyzed 71 cases of LAHS and found out that etoposide contained is an independent factor in improving prognosis.13 Besides the controlling HLH cytokine storm, etoposide also has a certain anti-tumor effect, especially in lymphoma.14 By comparing the etoposide-containing regimen and the lymphoma specialized chemotherapy without etoposide sought to identify the importance of including etoposide in the initial treatment of LAHS. Can etoposide control HLH at an early stage and also play a certain anti-lymphoma effect so that it may address the treatment of HLH and lymphoma at the same time? This may lay a foundation for the future better treatment strategy of LAHS.
Methods
Patients
534 patients diagnosed with HLH (according to HLH-2004 diagnostic criteria9) in our center during these three years were screened. Among the 534 patients, 80 were diagnosed with a hematological malignancy, and of these, 75 were determined to be lymphoma. The pathological criteria of the diagnosis of the lymphoma were according to the World Health Organization classification of lymphoid neoplasms. All pathological biopsies were reviewed double blinded by two pathologists. Six patients were excluded because of missing data. Three patients with HLH occurring during chemotherapeutic treatment referred to as Ch-HLH were excluded.7,15 Finally, 66 patients were included in the analysis. The medical records were reviewed by investigators, and they were responsible for selecting cases and collecting data.
According to the differences in the initial treatment regimen, the patients were divided into two groups according to whether the initial treatment contained etoposide or not. There were 52 patients with initial etoposide and 14 without one. The treatment regimens that contained initial etoposide include HLH-94/2004 regimen (20 cases), DEP (doxorubicin-etoposide-methylprednisolone) (17 cases), L-DEP (PEG-aspargase and DEP regimen) (8 cases), E-CHOP (etoposide, cyclophosphamide, hydroxydaunorubicin, vincristine, prednisone) (5 cases), and RE-CHOP (rituximab and E-CHOP regimen) (2 cases); for those that had lymphoma-directed chemotherapy but without etoposide, the regimens include CHOP/COP (5 cases), R-CHOP/COP (5 cases), L-GDP (2 cases), and P-Gemox (PEG-aspargase, gemcitabine, oxaliplatin) (2 cases). The dose of etoposide is 75–100 mg/m2 for HLH-94/2004 regimen, 100 mg/m2 for DEP/L-DEP regimen once a week, and 60–100 mg/m2 d1–d3 for E-CHOP regimen.
The positive EBV is defined as the EBV-DNA copy number of peripheral blood ≥ 500 copies/ml (pass the EBV international standard, namely: NIBSC number 09/260 for detection16) or EBV-encoded small RNA (EBER)+ in tissues.
Efficacy evaluation criteria and observed indicators
Age, gender, pathological type of lymphoma, IPI (International prognostic index) scores, initial clinical symptoms as well as laboratory findings, initial treatment regimen, response rate, transplantation, and outcome of each patient.
As this study is mainly focusing on the HLH part of LAHS, the assessment of the efficacy of LAHS followed the criteria proposed by Marsh et al. and revised by Yini Wang et al.17,18 A complete response (CR) was defined as the normalization of all quantifiable symptoms and laboratory markers of HLH, including levels of soluble CD25, ferritin, and triglyceride; hemoglobin levels; neutrophil and platelet counts; and alanine aminotransferase (ALT) levels. A partial response (PR) was defined as improvement in two or more of the following quantifiable symptoms and laboratory markers by two weeks: 1.5-fold decrease in soluble CD25 response; ferritin and triglyceride decreases of at least 25%; an increase of at least 100% to >0.5*109/L in patients with an initial neutrophil count of <0.5*109/L; an increase of at least 100% to >2.0*109/L in patients with an initial neutrophil count of 0.5 to 2.0*109/L; and a decrease of at least 50% in patients with initial ALT levels >400 U/L. Additionally, the subject’s body temperature must have reverted to normal ranges for either CR or PR to be diagnosed. Failure to achieve PR was defined as no response. All patients who have relapsed or failed to reached remission were treated with HLH salvage treatment.
Besides the clinical manifestation mentioned above and laboratory findings for efficacy evaluation, considering the main side effects of etoposide and lymphoma-directed chemotherapy, the level of myelosuppression was graded with the World Health Organization (WHO) Toxicity Grading Scale for determining the severity of adverse events.191919
Survival and statistical analysis
Follow-up started at the date of LAHS diagnosis and ended at the date of death or last examination. Overall survival (OS) was calculated from the diagnosis of HLH to death of any cause. When the latter date was not reached, the date was censored at the time of the last follow-up evaluation. Cases that underwent allogeneic hematopoietic stem cell transplantation were censored on the date of transplantation.
SPSS 22.0 (IBM, New York/USA) statistical software was adopted, and data that did not fit a normal distribution are presented as median and range. The T-test was used for data that fit a normal distribution and homogeneity of variance, and the Wilcoxon rank sum test was used for others. Survival functions were estimated by the Kaplan–Meier method. Confidence intervals were calculated using log-transformation for survival analysis. The log-rank test was used to compare survival between different groups. The Cox proportional hazards model was used in the multivariate analyses of prognostic factors. The selection of variables included in the proportional hazards regression analysis was according to the results of univariate and the clinical experiences. The factors included the age of onset, the gender of the patient, with/without EBV infection, the type of lymphomas (B or T/NK), the IPI scores, and whether the initial treatment contained etoposide. P < .05 was considered to denote a significant difference.
Results
Patients characteristics
In the 66 patients, 39 were male, and 27 were female. The male to female ratio was 1.4:1. The median age of the patients was 48 y (15–76 y). Only two patients were under 16 yold, and both of them were 15 yold. As for the type of lymphoma, 32 patients were B cell lymphomas (29 B-cell non-Hodgkin lymphoma, 3 Hodgkin lymphoma) and the other 34 were T/NK cell lymphomas (18 NK/T cell lymphoma, 16 other T-cell non-Hodgkin lymphoma). 29 (43.9%) patients were complicated with EBV infection. The patients’ characteristics of the whole population were listed in Table 1.
Table 1.
Initial clinical characteristics of the patients of two groups
| Clinical features | With initial etoposide | Without initial etoposide | p valve | Total |
|---|---|---|---|---|
| (n = 52) | (n = 14) | (n = 66) | ||
| Age, years | ||||
| Median | 48 | 44.5 | 0.881 | 48 |
| Range | [15, 76] | [29, 65] | [15, 76] | |
| Gender | 0.544 | |||
| Male (n) | 32 | 7 | 39 | |
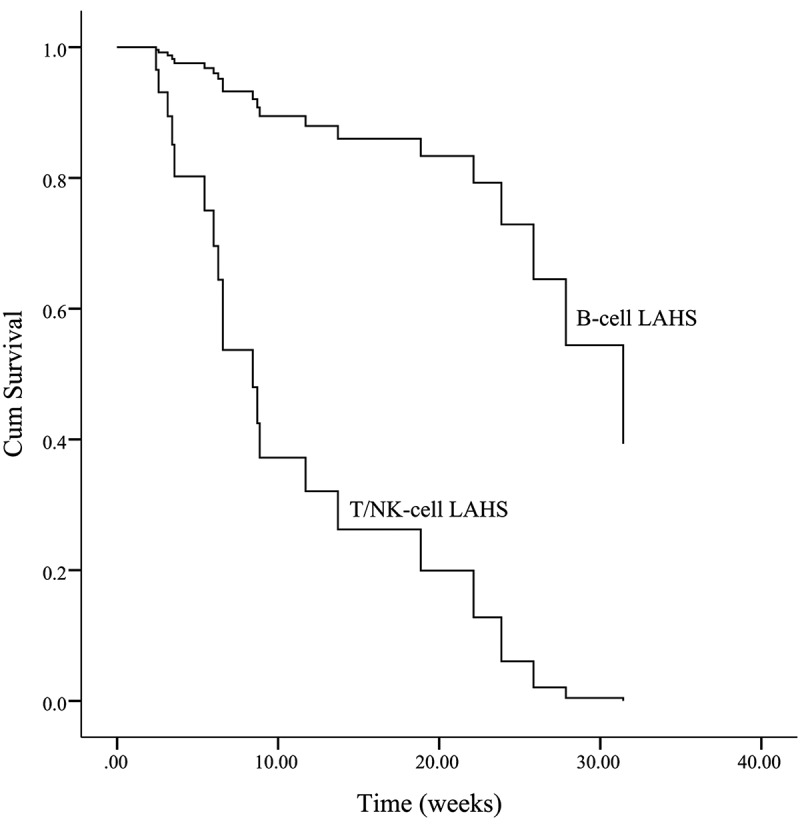
| Female (n) | 20 | 7 | 27 | |
| Lymphoma classification | 0.635 | |||
| B cell | 26 | 6 | 32 | |
| T/NK cell | 26 | 8 | 34 | |
| Fever (T > 38.5°C) | 52 (100%) | 14 (100%) | 66 (100%) | |
| Splenomegaly (n) | 38 (73.1%) | 11 (78.6%) | 1.00 | 49 (74.2%) |
| Haemophagocytosis (n) | 45 (86.5%) | 11 (78.6%) | 0.431 | 56 (84.8%) |
| WBC (*109/L) | 2.25 [0.06, 17.00] | 3.25 [0.4, 8.75] | 0.165 | 2.55 [0.06, 17.00] |
| HGB (g/L) | 90.05 ± 21.84 | 81.71 ± 15.11 | 0.184 | 88.29 ± 20.781 |
| PLT (*109/L) | 47.5 [3, 344] | 61.5 [9, 392] | 0.252 | 50.5 [3, 392] |
| ALT (U/L) | 52.5 [7.0, 798.0] | 41.5 [13, 235] | 0.753 | 51.5 [7.0, 798.0] |
| AST (U/L) | 56.4 [3.3, 520.2] | 43.9 [14.3, 990.0] | 0.869 | 55.2 [3.3, 990.0] |
| LDH | 556 [137, 4581] | 990 [76, 1986] | 0.569 | 537 [76, 4581] |
| Total bilirubin (µmol/L) | 24.77 [6.29, 348.15] | 20.70 [6.50, 213.58] | 0.718 | 22.9 [6.29, 348.15] |
| ALB | 28.5 [19.2, 44.0] | 28.7 [20.1, 34.8] | 0.389 | 28.2 ± 5.27 |
| Cr (umol/L) | 59.7 [35.5, 125] | 64.4 [37.7, 272.3] | 0.725 | 60 [35.5, 272.3] |
| TG (mmol/L) | 2.45 ± 1.22 | 2.95 ± 1.49 | 0.215 | 2.30 [0.69, 5.68] |
| Fbg (g/L) | 1.76 [0.16, 8.47] | 3.7 [0.73, 8.68] | 0.017 | 1.96 [0.16, 8.68] |
| Ferritin (ng/mL) | 2307.5 [204.3, 75000] | 2574 [557.3, 45487] | 0.734 | 2447 [204.3, 75000] |
| sCD25 (pg/mL) | 36605 [661, 44000] | 34749 [3827, 44000] | 0.903 | 36517 [661, 44000] |
| IPI scores | 3.00 ± 0.913 | 3.29 ± 0.994 | 0.315 | 3.06 ± 0.931 |
| EBV-positive | 23 (44.2%) | 6 (42.8%) | 0.927 | 29 (43.9%) |
| Corticosteroid contained | 51 (98.1%) | 13 (92.9%) | 0.382 | 64 (97.0%) |
| Outcome | ||||
| Death | 28 (53.8%) | 12 (85.7%) | 0.030 | 40 (60.6%) |
| Survival | 25.8 w [95%CI (13.2, 38.5)] | 7.6 w [95% (0, 19.8)] | 0.048 | 23.8 w [95%CI (15.5, 32.2)] |
The valve was expressed as median [range] or mean±standard deviation.
Treatment and outcome
The baseline level and the level of patients under corticosteroids between two group show no differences (p > .05) (Table 1). The response rates of the 66 patients were 66.7% (44/66), with a CR rate of 24.2% (16/66) and a PR rate of 42.4% (28/66). A total of 38 cases with initial etoposide achieved remission, and the remission rate was 73.1% (38/52), with the CR rate 28.8% (15/52) and PR 44.2% (23/52). Six cases used chemotherapy without etoposide achieved remission, and the remission rate was 42.9% (6/14), with a CR rate of 7.1% (1/14) and a PR rate of 35.7% (5/14). A significant difference was noted between the two groups (p = .033). The level of myelosuppression shows no differences between two groups of patients (p = .122) (29 out of 52 cases in the initial etoposide group and 9 out of 14 in the no-initial etoposide group). In the 44 patients that achieved CR/PR, there were 13 patients suffering from HLH relapse (10 out of 38 cases in the initial etoposide group and 3 out of 6 in the no-initial etoposide group, p = .339). Seven patients finally went through allo-HSCT.
A total of 40 (60.6%) deaths occurred. Almost half of the deaths occurred in the first twomonths (42.5%, 17/40), and most of them occurred in the first six months after diagnosis (77.5%, 31/40). There were 28 (53.8%) deaths in patients with initial etoposide and 12 (85.7%) deaths in the without-initial-etoposide group. A significant difference in mortality was noted between the two groups (p = .030). For the cause of death, in the initial-etoposide group, 24 patients died of HLH progression (85.7%, 24/28) and 4 died of complications. In the without-initial-etoposide group, 11 patients died of HLH progression (91.7%, 11/12), and one patient died during the conditioning before allo-HSCT.
Survival
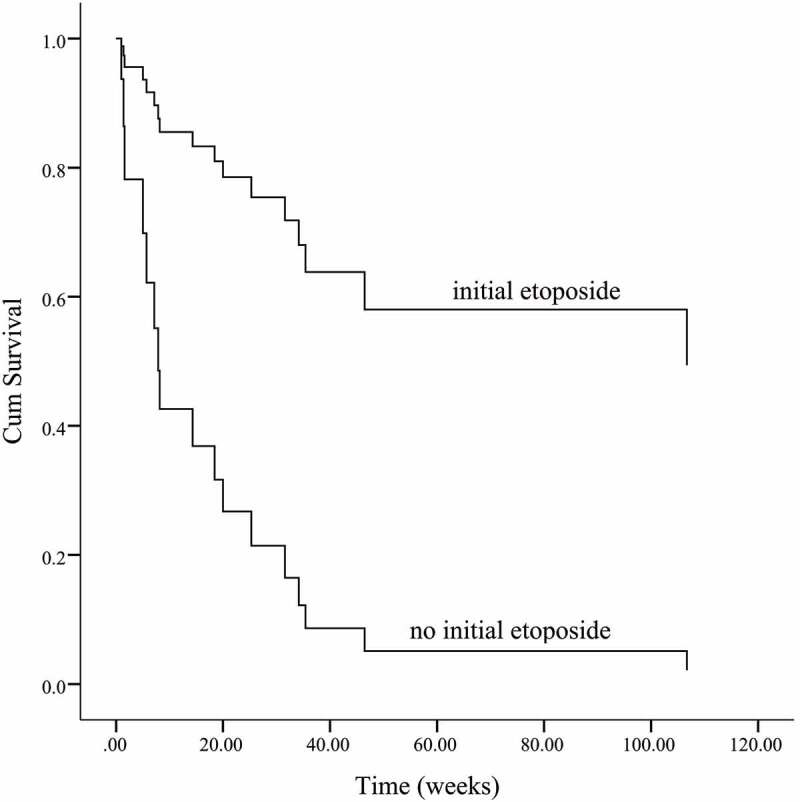
The median survival of all patients is 23.8 w [95%CI (15.5, 32.2)]. For the with-initial-etoposide group, the median survival time is 25.8 w [95%CI (13.2, 38.5)], and for the without-initial-etoposide group, it is 7.8 w [95% (0, 19.8)]. The overall survival of the with-initial-etoposide group is significantly better than that of the other one (p = .048) (Figure 1). Considering that almost half of the patients died within 8 weeks (2 months), the overall survival at 2 months was estimated as 79.8% vs. 46.8%, with a significant difference (p = .035). The treatment efficacy evaluation suggested that the overall survival was significantly prolonged in patients who reached CR/PR compared with patients who did not reach response (p < .01) (Figure 2). Also, patients with positive EBV suffered a worse prognosis (p = .035) (Figure 3)(Figure 4).
Figure 1.
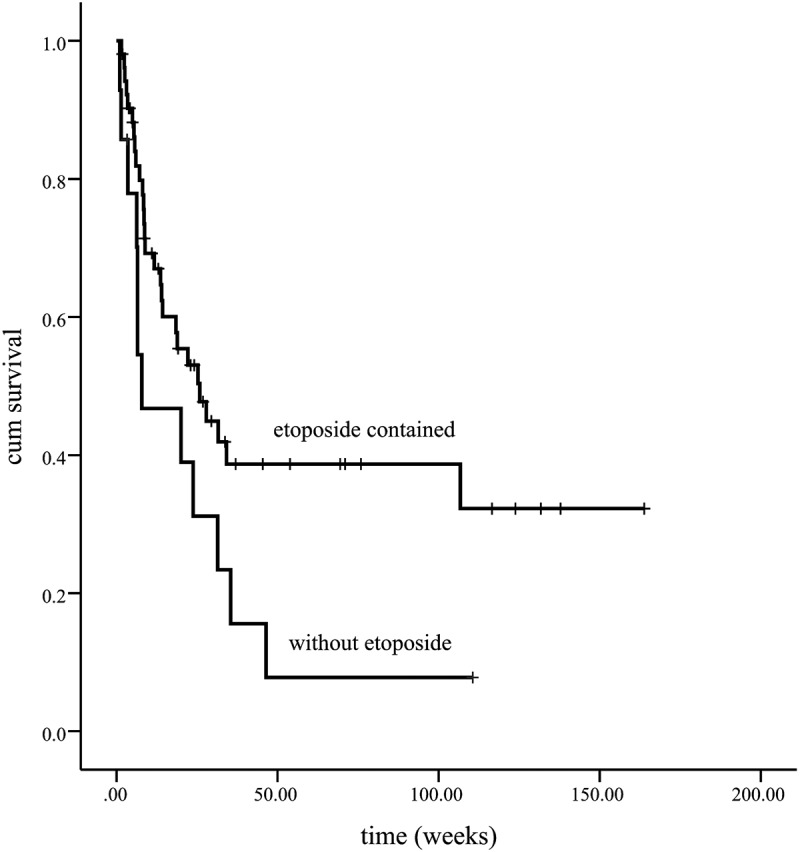
Etoposide contained in initial treatment significantly improves the prognosis of LAHS compared to the only lymphoma directed therapy one (p = .048)
Figure 2.
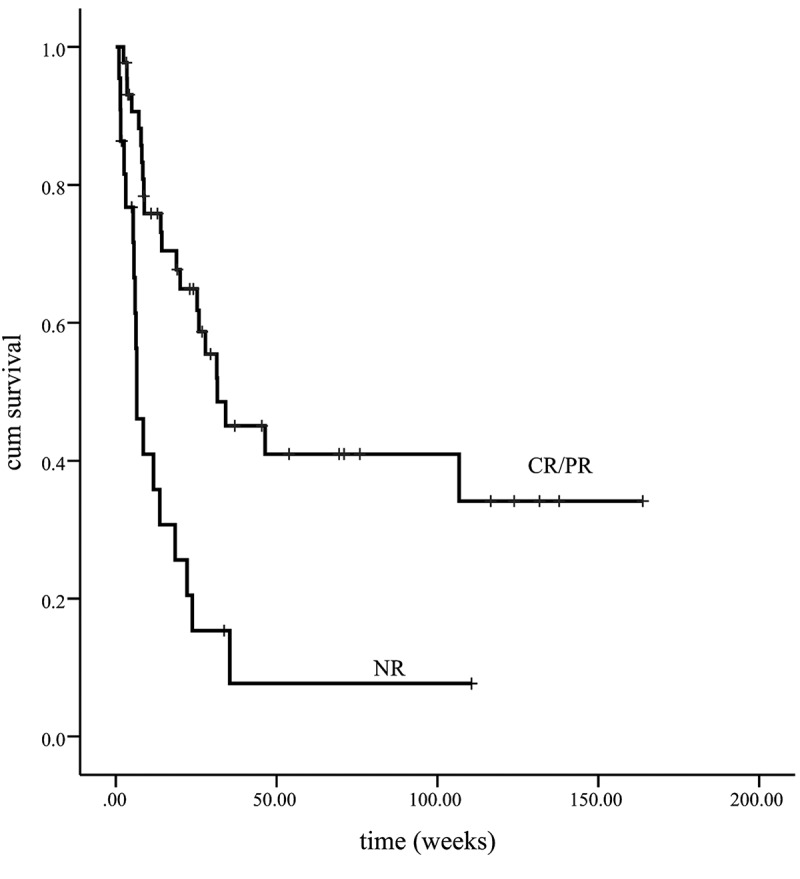
The treatment efficacy evaluation suggested that the overall survival was significantly prolonged in patients who reached CR/PR compared with patients who did not reached response (p < .01)
Figure 3.
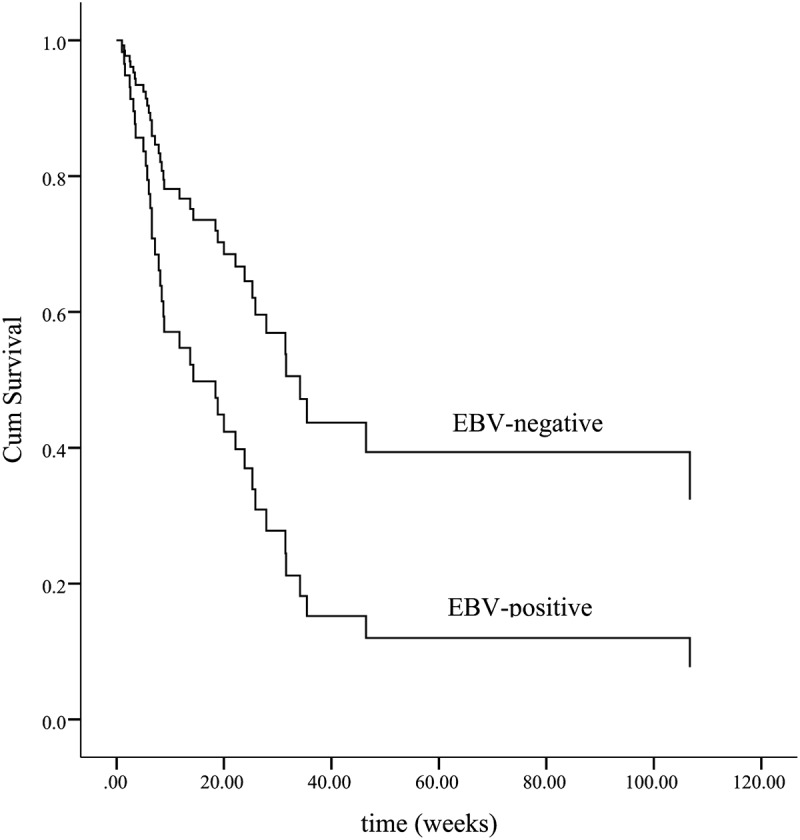
Adjusted survival curves of patients with or without EBV infection. Patients with positive EBV suffered a worse prognosis (p = .035)
Figure 4.
For EBV-positive patients, COX analysis: age of onset, the gender of the patient, the type of lymphomas (B or T/NK), the IPI scores of lymphomas, and whether the initial treatment contained etoposide. The results showed the type of lymphoma (P = .011, ExpB = 0.113) and the IPI scores (p = .004, ExpB = 2.419) were associated with prognosis
Figure 4.
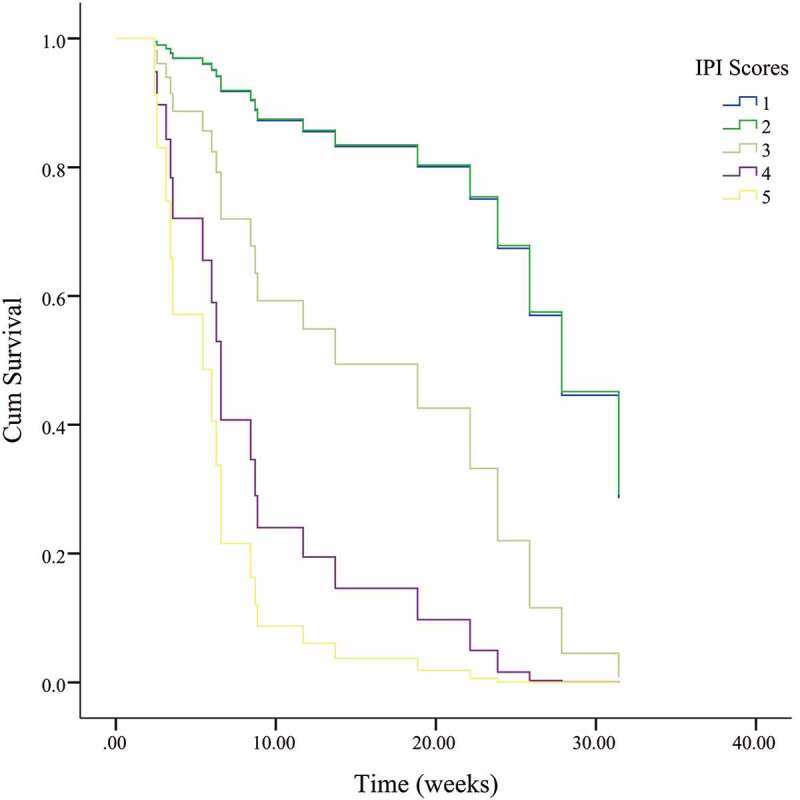
Continued
Multivariate cox analysis revealed that for patients with EBV infection (29 cases), the type of lymphoma (B or T/NK cell) (p = .011) and the IPI score (p = .004) was associated with prognosis (Figure 4), while whether etoposide was included in the initial treatment had no significant effect on prognosis (P = .474). However, in patients without EBV infection (37 cases), multivariate cox analysis showed that initial treatment with etoposide was significantly related to prognosis (p = .010, Exp(B) = 0.183), while the type of lymphoma (p = .259) and IPI score (p = .419) did not show significant effects (Figure 5) (Table 2).
Figure 5.
For EBV-negative patients, COX analysis: age of onset, the gender of the patient, the type of lymphomas (B or T/NK), the IPI scores of lymphomas, and whether the initial treatment contained etoposide. The results showed that whether initial treatment contained etoposide (p = .010, ExpB = 0.183) was associated with prognosis
Table 2.
Univariable and multivariable analyses of prognosis with different factors
| Variables | Univariate Analysis | Multivariate Analysis |
||
|---|---|---|---|---|
| Exp(B) | 95% CI | p valve | ||
| Age | 0.981 | 1.008 | 0.979–1.037 | 0.614 |
| Gender (male vs. female) | 0.810 | 0.729 | 0.370–1.438 | 0.362 |
| EBV infection (with vs. without) | 0.002 | 2.272 | 1.060–4.867 | 0.035 |
| Lymphoma classification (T/NK vs. B) | 0.010 | 2.898 | 1.167–7.197 | 0.022 |
| IPI score | 0.107 | 1.459 | 1.032–2.165 | 0.033 |
| Treatment (initial etoposide vs. without) | 0.048 | 0.682 | 0.324–1.433 | 0.312 |
Discussion
Malignancy HLH is one of the most common secondary HLHs, especially in adult patients. M-HLH may occur in up to 1% of the patients with hematologic malignancies, and the lymphoma associated HLH (LAHS) is the most common type in M-HLH.20 M-HLH is considered to suffer the worst outcome among the secondary HLH.5,21 The mortality rate of M-HLH is >80%5 and the median survival time is only 2 months.6,7,21 However, there is currently no standard treatment strategy for LAHS. The priority of HLH-directed treatment and lymphoma-directed treatment is still controversial,6,7,15 even though in some clinical observation, many patients died of HLH within 2–4 weeks in spite of continued treatment of the underlying malignancy.7 Etoposide is one of the core drugs in the HLH-94/2004 standard protocol, and its importance in the treatment and prognosis of HLH has been confirmed repeatedly.10,11,22 However, its significance in LAHS’s treatment has not yet been discussed. In the “How I treat” article in adult HLH, Alison S. and Nancy B. reported one case of a LAHS patient who was effectively treated with etoposide combined with CHOP regimen.12 In the 2017 adult M-HLH expert consensus review, it is recommended to prioritize the treatment for cytokine storms and T cell proliferation, such as etoposide, for patients who have experienced severe organ damages.7 In this study, it was evident that the early response rate, short-term survival, and overall survival of the treatment group containing etoposide were superior to those of lymphoma directed chemotherapy without etoposide.
The mechanism of etoposide in HLH is that it reduces the production of excessive proinflammatory factors by selectively ablating over-activated T cells and inhibiting mononuclear-macrophage activation.23 This effect is different from immunosuppressive effects of corticosteroids and the immunomodulatory effects of IVIG or cyclosporin, which makes it indispensable in some kind of HLH treatment strategy. Camille B et al. have an opinion that the long-term LAHS prognosis may be similar to the simple lymphoma not associated with HLH, once the initial and crucial early period is overcome.13 In our study, it was observed that nearly half of the deaths occurred within 2 months after diagnosis. In addition to the early response rate, the improvement of the 2-month survival rate was also significant in the etoposide-contained group, and we believe that the improvement in short-term survival is also the reason the etoposide group contributes to a better long-term survival. The underlining malignancy plays a crucial role in the development of HLH. Etoposide is a chemotherapy medication used for the treatment of a number of malignancies.24 Although etoposide is not a commonly used first-line regimen in lymphoma, it has been reported that it can improve EFS and PFS in some patients with T-cell lymphoma under 60 y of age,14,25 also used in some B-cell lymphoma,26 indicating that the usage of etoposide in LAHS not only affects the controlling HLH but also may affect the underlining lymphoma.
For the intervention time of etoposide, considering that the prognosis of LAHS patients who achieved early remission was significantly better, it is more appropriate to include etoposide in the initial treatment. However, the sever cytopenia caused by HLH may bring some concern for the usage of etoposide. One of the main reasons why less than 50% of LAHS patients received HLH directed treatment is the concern of myelosuppression deterioration after cytotoxic therapy. However, in fact, etoposide’s efficiency occurs within 24–48 h, and in HLH, etoposide actually prevented the development of cytopenia and marrow hypocellularity in HLH instead.9,22,27 This study evaluated the level of myelosuppression and there was no significant difference between the two groups, but the dose of etoposide in this study was indeed adjusted according to adult intolerance and taking into account the cytopenia. It seems that as long as they are administered with appropriate doses, concerns about myelosuppression are not enough for holding etoposide back.
In the multivariate analysis, it was found that in patients with EBV infection, whether etoposide was included in the initial treatment had little effect on the prognosis, but in patients who did not suffer EBV infection, the initial treatment that included etoposide could clearly improve the prognosis. In previous reports, whether it is T/NK-cell or B-cell lymphoma, the presence of EBV infection is often a poor prognostic factor.28,29 Also, as we all know, the type of lymphoma in LAHS is closely related to the prognosis. In the study of Sano H et al., the rate of early death was higher in patients with T/NK-cell LAHS than in those with B-cell LAHS (62.5 vs. 10.5%).30 However, in this study, for patients without positive EBV, the type of lymphoma was not significantly correlated with prognosis. This result indicates that the poor prognosis of T/NK cell LAHS may be attributed to the fact that EB virus infection is more common in T/NK cell lymphoma. EBV infection itself is an independent prognostic factor. A possible conjecture is that when lymphoma is secondary to HLH and combined with positive EBV, EBV influences the prognosis from both aspects of lymphoma and HLH. In this EBV+ LAHS, the inclusion of etoposide in the initial treatment is not sufficient to improve the outcome. However, for EBV− LAHS, initial treatment with etoposide is clearly helpful for prognosis.
Although this study mainly focusses on the value of etoposide in the initial treatment of LAHS, attention should be paid to the relapsed and refractory LAHS31 because this group of patients often suffers a very poor outcome. In addition to the widely accepted DEP regimen (29 M-HLH cases of 63),17 many new drugs have emerged in recent years for the treatment of HLH, such as the JAK1/2 inhibitor and Ruxolitinib. In a multicenter study using Ruxolitinib combined with DEP chemotherapy to treat Relapse/Refractory HLH, 2/3 of LAHS achieved remission.32 However, ruxolitinib is a drug whose main mechanism of effects in HLH is to control the inflammatory factor storm,33 and its role in LAHS still needs to be further confirmed. The PD-1 inhibitor, Nivolumab, has been effective in NK/T lymphoma and reported effective in relapsing/refractory EBV-associated HLH.34 Considering the therapeutic effect of anti-PD-1 antibody on lymphoma, its therapeutic effect in LAHS, especially EBV+ LAHS, deserves further study. Of course, adverse effects that immune checkpoint inhibitors may induce HLH must be noted. This study has some limitations. Most of all, it is a retrospective study, and the results still need to be verified by prospective studies. Also, the number of cases is rather small, especially the number of cases in the control group without initial etoposide, which may cause statistical bias.
Conclusion
As one of the most common secondary HLHs, LAHS suffers the worst outcome and most of patients died within a short term after diagnosis. However, no clear and unified initial treatment plan is currently available, and the debate about the priority of HLH-directed or lymphoma-directed is still going on. This study found that containing etoposide in the initial treatment plan significantly improved the early response rate, two-month survival, and overall survival, especially prominent in EBV negative patients. Also, the level of bone marrow suppression shows no significant differences in whether etoposide is contained. So, containing etoposide is suggested in the initial treatment of LAHS, whether using the HLH-directed or lymphoma-directed strategy, especially for EBV negative patients.
Acknowledgments
We thank the patients and their families for participating in our study.
Funding Statement
This work was supported by the National Natural Science Foundation of China (no. 81871633); the Beijing Natural Science Foundation (no. 7181003); and the Beijing Municipal Administration of Hospitals’ Ascent Plan (Beno. DFL20180101). The funding body did not contribute to the design of the study and collection, analysis, and interpretation of data, or to writing of the manuscript.
Abbreviations
Allo-HSCT: allogeneic hematopoietic stem cell transplantation; WBC: white blood cells; HGB hemoglobin; PLT: platelets; LDH: lactate dehydrogenase; ALB: albumin; Cr: creatinine; TG: triglycerides; ALT: alanine aminotransferase; CR: complete response; DEP regimen: doxorubicin hydrochloride liposome, etoposide, and methylprednisolone; EBER: EBV encoded small RNA; EBV: Epstein–Barr virus; HLH: hemophagocytic lymphohistiocytosis; L-DEP regimen: PEG-aspargase plus DEP regimen; NK: natural killer; OS: overall survival; PR: partial response; VP-16: etoposide.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Ethics Committee at Beijing Friendship Hospital.
Data availability statements
The datasets generated during and/or analyzed during the current study are available from the corresponding author (wangzhao@ccmu.edu.cn) upon reasonable request.
Authors’ contributions
Z.W. contributed to the design of the study. Y.N.W., J.S.W., and L.W. helped with the study design and data analyses. Y.S. conducted the data analysis and wrote the manuscript. All authors approved the final manuscript.
References
- 1.Henter JI, Samuelsson-Horne A, Arico M, Egeler RM, Elinder G, Filipovich AH, Samuelsson-Horne A, Arico M, Egeler RM, Elinder G, et al. 2002. Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood. 100(7):2367–2373. doi: 10.1182/blood-2002-01-0172. [DOI] [PubMed] [Google Scholar]
- 2.Arico M, Janka G, Fischer A, Henter JI, Blanche S, Elinder G, Martinetti, M, and Rusca, M, 1996. Hemophagocytic lymphohistiocytosis. Report of 122 children from the International Registry. FHL Study Group of the Histiocyte Society. Leukemia. 10:197–203. [PubMed] [Google Scholar]
- 3.Jordan MB, Allen CE, Weitzman S, Filipovich AH, McClain KL.. 2011. How I treat hemophagocytic lymphohistiocytosis. Blood. 118(15):4041–4052. doi: 10.1182/blood-2011-03-278127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yildiz H, Van Den Neste E, Defour JP, Danse E, Yombi JC. Adult haemophagocytic lymphohistiocytosis: a Review. QJM 2020. [DOI] [PubMed]
- 5.Otrock ZK, Eby CS. 2015. Clinical characteristics, prognostic factors, and outcomes of adult patients with hemophagocytic lymphohistiocytosis. Am J Hematol. 90(3):220–224. doi: 10.1002/ajh.23911. [DOI] [PubMed] [Google Scholar]
- 6.Tamamyan GN, Kantarjian HM, Ning J, Jain P, Sasaki K, McClain KL, Allen CE, Pierce SA, Cortes JE, Ravandi F, et al. 2016. Malignancy-associated hemophagocytic lymphohistiocytosis in adults: relation to hemophagocytosis, characteristics, and outcomes. Cancer. 122(18):2857–2866. doi: 10.1002/cncr.30084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daver N, McClain K, Allen CE, Parikh SA, Otrock Z, Rojas-Hernandez C, Blechacz B, Wang S, Minkov M, Jordan MB, et al. 2017. A consensus review on malignancy-associated hemophagocytic lymphohistiocytosis in adults. Cancer. 123(17):3229–3240. doi: 10.1002/cncr.30826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.La Rosee P, Horne A, Hines M, von Bahr Greenwood T, Machowicz R, Berliner N, Birndt S, Gil-Herrera J, Girschikofsky M, Jordan MB, et al. 2019. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 133(23):2465–2477. doi: 10.1182/blood.2018894618. [DOI] [PubMed] [Google Scholar]
- 9.Henter JI, Horne A, Arico M, Egeler RM, Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski J, et al. 2007. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 48(2):124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 10.Song Y, Wang Y, Wang Z. 2019. Requirement for etoposide in the initial treatment of Epstein-Barr virus-associated haemophagocytic lymphohistiocytosis. Br J Haematol. 186(5):717–723. doi: 10.1111/bjh.15988. [DOI] [PubMed] [Google Scholar]
- 11.Imashuku S, Kuriyama K, Teramura T, Ishii E, Kinugawa N, Kato M, Sako M, Hibi S. 2001. Requirement for etoposide in the treatment of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 19(10):2665–2673. doi: 10.1200/JCO.2001.19.10.2665. [DOI] [PubMed] [Google Scholar]
- 12.Schram AM, Berliner N. 2015. How I treat hemophagocytic lymphohistiocytosis in the adult patient. Blood. 125(19):2908–2914. doi: 10.1182/blood-2015-01-551622. [DOI] [PubMed] [Google Scholar]
- 13.Bigenwald C, Fardet L, Coppo P, Meignin V, Lazure T, Fabiani B, Kohn M, Oksenhendler E, Boutboul D, Uzzan M, et al. 2018. A comprehensive analysis of Lymphoma-associated haemophagocytic syndrome in a large French multicentre cohort detects some clues to improve prognosis. Br J Haematol. 183(1):68–75. doi: 10.1111/bjh.15506. [DOI] [PubMed] [Google Scholar]
- 14.Schmitz N, Trumper L, Ziepert M, Nickelsen M, Ho AD, Metzner B, Peter N, Loeffler M, Rosenwald A, Pfreundschuh M, et al. 2010. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 116(18):3418–3425. doi: 10.1182/blood-2010-02-270785. [DOI] [PubMed] [Google Scholar]
- 15.Lehmberg K, Sprekels B, Nichols KE, Woessmann W, Muller I, Suttorp M, Bernig T, Beutel K, Bode SFN, Kentouche K, et al. 2015. Malignancy-associated haemophagocytic lymphohistiocytosis in children and adolescents. Br J Haematol. 170(4):539–549. doi: 10.1111/bjh.13462. [DOI] [PubMed] [Google Scholar]
- 16.Collaborative study to evaluate the proposed 1st WHO International.
- 17.Wang Y, Huang W, Hu L, Cen X, Li L, Wang J, Shen, JL, Wei, N, and Wang, Z, 2015. Multicenter study of combination DEP regimen as a salvage therapy for adult refractory hemophagocytic lymphohistiocytosis. Blood. 126:2186–2192. doi: 10.1182/blood-2015-05-644914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marsh RA, Allen CE, McClain KL, Weinstein JL, Kanter J, Skiles J, Lee ND, Khan SP, Lawrence J, Mo JQ, et al. 2013. Salvage therapy of refractory hemophagocytic lymphohistiocytosis with alemtuzumab. Pediatr Blood Cancer. 60(1):101–109. doi: 10.1002/pbc.24188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organisation . The WHO toxicity grading scale for determining the severity of adverse events. 2003. [Accessed 2015 Mar 22]. Available from URL: http://wwwicsscorg/Documents/Resources/AEManual2003AppendicesFebruary_06_2003%20finalpdf.
- 20.Machaczka M, Vaktnas J, Klimkowska M, Hagglund H. 2011. Malignancy-associated hemophagocytic lymphohistiocytosis in adults: a retrospective population-based analysis from a single center. Leuk Lymphoma. 52(4):613–619. doi: 10.3109/10428194.2010.551153. [DOI] [PubMed] [Google Scholar]
- 21.Parikh SA, Kapoor P, Letendre L, Kumar S, Wolanskyj AP. 2014. Prognostic factors and outcomes of adults with hemophagocytic lymphohistiocytosis. Mayo Clinic Proceedings. 89(4):484–492. doi: 10.1016/j.mayocp.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Arca M, Fardet L, Galicier L, Riviere S, Marzac C, Aumont C, Lambotte, O, and Coppo, P, 2015. Prognostic factors of early death in a cohort of 162 adult haemophagocytic syndrome: impact of triggering disease and early treatment with etoposide. Br J Haematol. 168(1):63–68. doi: 10.1111/bjh.13102. [DOI] [PubMed] [Google Scholar]
- 23.Johnson TS, Terrell CE, Millen SH, Katz JD, Hildeman DA, Jordan MB. 2014. Etoposide selectively ablates activated T cells to control the immunoregulatory disorder hemophagocytic lymphohistiocytosis. The Journal of Immunology. 192(1):84–91. doi: 10.4049/jimmunol.1302282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pommier Y, Leo E, Zhang H, Marchand C. 2010. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol. 17(5):421–433. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellin F, Landstrom J, Jerkeman M, Relander T. 2014. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: a study from the Swedish lymphoma registry. Blood. 124(10):1570–1577. doi: 10.1182/blood-2014-04-573089. [DOI] [PubMed] [Google Scholar]
- 26.Dodero A, Guidetti A, Tucci A, Barretta F, Novo M, Devizzi L, Re A, Passi A, Pellegrinelli A, Pruneri G, et al. 2019. Dose-adjusted EPOCH plus rituximab improves the clinical outcome of young patients affected by double expressor diffuse large B-cell lymphoma. Leukemia. 33(4):1047–1051. doi: 10.1038/s41375-018-0320-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizrahi M, Ben-Chetrit E. 2009. Relapsing macrophage activating syndrome in a 15-year-old girl with Still’s disease: a case report. J Med Case Rep. 3(1):138. doi: 10.1186/1752-1947-3-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fei Q, Tian XK, Wu J, Zhu HM, Wang Y, Peng FY, Zhang, WJ, Yin, L, and He, X. 2018. Prognostic significance of Epstein-Barr virus DNA in NK/T-cell lymphoma: a meta-analysis. Onco Targets Ther. 11:997–1004. doi: 10.2147/OTT.S153942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castillo JJ, Beltran BE, Miranda RN, Young KH, Chavez JC, Sotomayor EM 2016. EBV-positive diffuse large B-cell lymphoma of the elderly: 2016 update on diagnosis, risk-stratification, and management Am J Hematol 91(5)529–537 doi: 10.1002/ajh.24370 [DOI] [PubMed] [Google Scholar]
- 30.Sano H, Kobayashi R, Tanaka J, Hashino S, Ota S, Torimoto Y, Kakinoki Y, Yamamoto S, Kurosawa M, Hatakeyama N, et al. 2014. Risk factor analysis of non-Hodgkin lymphoma-associated haemophagocytic syndromes: a multicentre study. Br J Haematol. 165(6):786–792. doi: 10.1111/bjh.12823. [DOI] [PubMed] [Google Scholar]
- 31.Yildiz H, Bailly S, Van Den Neste E, Yombi JC. 2021. Clinical management of relapsed/refractory hemophagocytic lymphohistiocytosis in adult patients: a review of current strategies and emerging therapies. Ther Clin Risk Manag. 17:293–304. doi: 10.2147/TCRM.S195538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, Zhang R, Wu X, Li F, Yang H, Liu L, Guo H, Zhang X, Mai H, Li H, et al. 2021. Ruxolitinib-combined doxorubicin-etoposide-methylprednisolone regimen as a salvage therapy for refractory/relapsed haemophagocytic lymphohistiocytosis: a single-arm, multicentre, phase 2 trial. Br J Haematol. 193(4):761–768. doi: 10.1111/bjh.17331. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Wang Y, Wu L, Wang X, Jin Z, Gao Z, and Wang, Z. 2019. Ruxolitinib for refractory/relapsed hemophagocytic lymphohistiocytosis. Haematologica 105(5): e210–e212. doi: 10.3324/haematol.2019.222471. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu P, Pan X, Chen C, Niu T, Shuai X, Wang J, Chen X, Liu J, Guo Y, Xie L, et al. 2020. Nivolumab treatment of relapsed/refractory Epstein-Barr virus–associated hemophagocytic lymphohistiocytosis in adults. Blood. 135(11):826–833. doi: 10.1182/blood.2019003886. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author (wangzhao@ccmu.edu.cn) upon reasonable request.


