Abstract
Background: Recent research has shown that a high percentage of treatment-seeking transgender adults who were assigned female at birth (AFAB) reported scores above the clinical cutoff for autistic traits. It is unclear whether those scores reflect a stable trait or may be inflated by the high levels of anxiety typically associated with transgender people attending clinical services.
Aims: This longitudinal study aims to explore the impact of Cross-sex Hormone Treatment (CHT) on levels autistic traits, independent of changes in anxiety.
Method: Transgender adults who were assessed at a national transgender health service in the UK, who had not previously received CHT and who had completed the AQ-Short as a measure of autistic traits pre- and one-year post-CHT were included in the study (n = 118). Anxiety was assessed at the same time points using the Hospital Anxiety and Depression Scale.
Results: AQ-Short scores remained very stable over time (ICC = 0.7; CIs 0.591-0.779) but anxiety showed little consistency (ICC = 0.386; CIs 0.219 to 0.531). Repeated measures ANOVA found a main effect of assigned sex with AFAB having higher AQ-Short scores. There was no change in AQ-Short scores and no significant interaction between assigned sex and change in AQ-Short scores.
Conclusion: This study confirmed that treatment seeking transgender AFAB people have higher levels of autistic traits at follow-up compared to AMAB transgender people and that these traits are stable following one year of CHT regardless of assigned sex. This may have clinical implications regarding the support that transgender people may require following medical transition.
Keywords: anxiety, autistic traits, autism spectrum conditions, cross-sex hormone treatment, gender dysphoria, transgender
Introduction
A number of studies suggest a link between the presence of Autistic Spectrum Conditions (ASC) in children and young people and higher rates of gender dysphoria: a discrepancy between sex assigned at birth and gender identity (Glidden et al., 2016). To-date six studies have investigated the prevalence of autistic traits in transgender adults (Heylens et al., 2018; Jones et al., 2012; Kristensen & Broome, 2015; Nobili et al., 2018b; Pasterski et al., 2014; Vermaat, van der Miesen, de Vries, Steensma, Popma, Cohen-Kettenis, Kreukels; 2018), with all six using versions of the Autism Spectrum Quotient (AQ) (Baron-Cohen et al., 2001a). Overall, findings suggest that there is a significantly higher percentage of transgender Assigned Female At Birth (AFAB) scoring above the cutoff for clinically significant autistic traits compared to both transgender Assigned Male At Birth (AMAB) and cisgender people.
Autistic Spectrum Conditions (ASC) such as Asperger syndrome and autism are neurodevelopmental conditions characterized by social and communication difficulties as well as restricted interests, highly repetitive behavioral patterns, and sensory hyper-sensitivity (APA, 2013). It has been argued that elevated autistic traits in transgender adults might reflect high levels of anxiety and increased social difficulties associated with gender dysphoria and experiences associated with being transgender such as bullying and transphobia, rather than ASC (Turban & van Schalkwyk, 2018).
However, clinically the perception that autism and gender dysphoria are co-occurring conditions has strong support although the mechanisms for the association are poorly understood (Strang et al., 2018). The finding that transgender AFAB people are more likely to have elevated levels of autistic traits compared to cisgender women (45.4% vs. 30%) (Nobili et al., 2018a) could relate to prenatal sex differences in the brain more typical of the male brain. The Extreme Male Brain (EMB) theory argues that males are at higher risk of autism as a result of in-utero exposure to androgens which accentuates systemizing behavior and diminish the drive to empathize (Auyeung et al., 2009; Baron-Cohen, 2002, 2010; Baron-Cohen et al., 2015; Greenberg et al., 2018). Additionally, the EMB theory posits that on average, allistic (i.e., non-autistic) females and males differ in their profiles on the psychological domains of empathizing and systemizing with females having a stronger drive to empathize than systemize than males, and vice versa (Baron-Cohen, 2010). Past research showed that people with ASC score higher than both allistic females and males in the domains of intuitive physics (Baron-Cohen et al., 2001b), systemizing (Baron-Cohen et al., 2003), and attention to detail (Jolliffe & Baron-Cohen, 1997), thus they are thought to be on the extreme end of the male brain spectrum (Baron-Cohen, 2010; Greenberg et al., 2018). High levels of prenatal androgens have also been suggested to be responsible for a masculinized gender identity (Hines, 2006; Knickmeyer et al., 2006).
ASC traits tend to be stable over time (Robinson et al., 2011) and much of this stability is attributable to genetic factors (Taylor et al., 2017). If self-reported ASC traits in transgender populations reflect stable and heritable traits, then we should expect to find strong consistency over time regardless of Cross-sex Hormone Treatment (CHT). In contrast, levels of anxiety are more likely to be responsive to external factors and in transgender populations anxiety appears to be lower in those on CHT (Bouman et al., 2016, 2017; Millet et al., 2017).
Aims
This study aims to investigate the stability of autistic traits in transgender people following CHT, when controlling for the impact of change in anxiety levels and age. It also aims to explore the impact of assigned sex at birth and change in anxiety on any change in levels of autistic traits.
The present study explored the following research questions; do levels of autistic traits remain stable from pretreatment to one-year post-CHT, when controlling for change in anxiety and age? If autistic traits change from baseline to follow-up, what is the impact of sex assigned at birth and change in anxiety on changes in levels of autistic traits? As this is an exploratory study and the first research longitudinally investigating the impact of CHT upon levels of autistic traits, no hypotheses were formulated.
Methods
Participants
Participants were consecutive attenders at a national adult transgender healthcare service in the UK between November 2014 and June 2018. Inclusion criteria were initiation of CHT at the clinic and completion of the AQ-Short prior to treatment (T0 - baseline) and at one-year post CHT (T1 - follow-up). The study focusses on those who have been on treatment for over 12 months as this allows for enough time for hormone treatment to produce physical, bodily changes but before surgical procedures have taken place, which could bias the results. Unfortunately, due to the long waiting list for gender affirming surgical treatment in the United Kingdom (UK), it is unlikely that people will have undergone these interventions before this time.
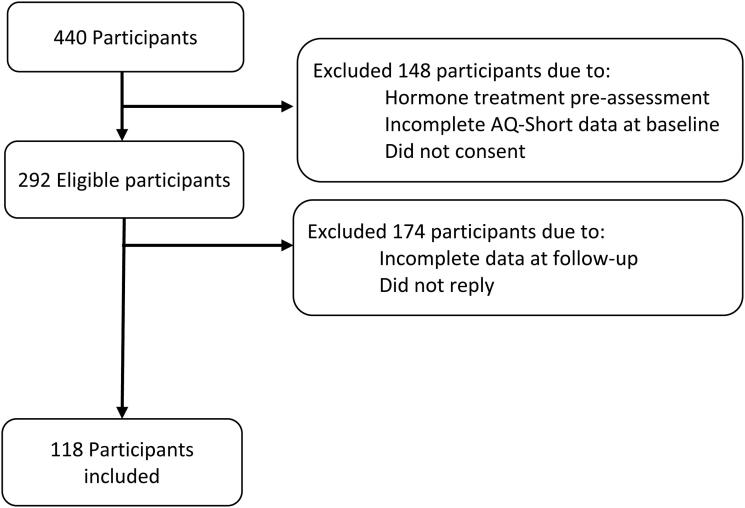
Exclusion criteria were having undergone any type of gender-affirming treatment prior to the first assessment at the clinic (in order to create a more homogeneous sample and to better control for participants’ treatment status), failure to give consent to participation and incomplete completion of AQ at T0. Participant with a diagnosis of ASC were not excluded from the study. The participant flow is shown in Figure 1.
Figure 1.
Flow chart of exclusion of participants.
Setting
The center is a national transgender health service that offers gender affirming medical treatment to people living in England and Wales. The service offers assessment for treatment suitability, psychological support, endocrinological treatment and referrals for gender affirming surgeries.
Design and procedure
A longitudinal design was adopted for the present study with data collection pretreatment and one-year post-CHT. Questionnaire packs were sent out by a research assistant one-month prior to the first assessment together with the information sheet and consent form. The pack consisted of socio-demographic information, as well as the AQ-Short to measure autistic traits and the Hospital Anxiety and Depression Scale (HADS-A) to measure anxiety levels. However, information regarding a diagnosis of ASC was not available. Participants who returned completed questionnaires and the signed consent form were included in the study. One-year post-CHT, patients were provided with a second questionnaire pack either face-to-face or sent by post. A research assistant checked the medical notes to determine when patients reached one-year post-CHT in order to identify those who were eligible to participate.
Sex assigned at birth was used as the gender descriptor to allow comparison with previous research (Nobili et al., 2018b) and to increase the study’s power to explore gender differences in ASC traits due to the use of a binary variable rather than a categorical one. Participants were therefore grouped into those assigned female at birth (AFAB) and those assigned male at birth (AMAB).
Ethical approval for the research was received from the NHS Ethics Committee (14/EM/0092) and by the Nottinghamshire Healthcare NHS Foundation Trust Research and Development Department in agreement with the Health Research Authority guidance (HRA, 2013).
Main outcome measures
Autism Spectrum Quotient Short Version – AQ-Short (Hoekstra et al., 2008)
The AQ-Short is a self-reported 28-items 4-point Likert-scale ranging from 1 ‘definitely agree’ to 4 ‘definitely disagree’. It is used to assess autistic traits in adult individuals with average intelligence and to indicate where a person falls in the autistic spectrum ranging from not-autistic to autistic (Woodbury-Smith et al., 2005). The measure assesses difficulties and core traits related to autism, such as attention switching, social and communication skills and imagination (Hoekstra et al., 2008). The AQ-Short is an abridged validated version of the well-established AQ-50 (Baron-Cohen et al., 2001a). The AQ has been used in a large population of half a million people (Ruzich et al., 2015), confirming sex differences on average (males scoring higher than females) and those working in STEM (Science, Technology, Engineering, and Maths) scoring higher on average than those not working in STEM. Total scores range from 28 to 112 and at clinical cutoff of ≥70, the sensitivity and specificity of the AQ-Short are high (.94 and .91, respectively). Cronbach’s alphas for this measure range between .77 and .86 (Hoekstra et al., 2008).
Anxiety subscale of the Hospital Anxiety and Depression Scale, (HADS-A) (Zigmond & Snaith, 1983)
The HADS-A is a 7-item subscale of the Hospital Anxiety and Depression scale. Respondents rate their emotions over the previous week on 4-point Likert-scales. Total scores range between 0 and 21. The HADS is a reliable measure in assessing symptoms’ caseness and severity of anxiety disorders in both clinical and general populations (Bjelland et al., 2002) as well as in clinical transgender populations (Bouman et al., 2016, 2017; Witcomb et al., 2018). The measure’s specificity is 0.78, sensitivity is 0.9 and reliability is excellent with Cronbach’s alphas ranging from 0.68 to 0.93 (Bjelland et al., 2002).
Data analysis
Data analyses were performed using the Statistical Software Package SPSS 24 (IBM Corporation, 2016). As data regarding only a few variables was normally distributed, and appropriate univariate analyses, such as t-tests and Mann Whitney U tests were carried outdepending on the variables distribution. McNemar test was adopted to assess if the percentage of clinically significant autistic traits differed from pre- to post-CHT, overall as well as for both gender groups. Additionally, repeated measures ANOVA was used to explore change in AQ-Short scores over time and interactions between assigned sex at birth and change in autistic trait scores over time, with anxiety change and age included as covariates. The significance level used for the statistical analyses was p < 0.05.
Results
A total of 440 patients were assessed during the studied period, of whom 292 were eligible to participate. One hundred-and eighteen patients returned a fully completed AQ-Short questionnaire, giving a response rate of 40.4%. Responders did not differ from non-responders in terms of either demographic characteristics or baseline AQ-Short scores, but they were less anxious at baseline than non-responders (median 9 vs 8, p = 0.001; z = 3.225).
Of the 118 participants who completed the questionnaires at follow-up, there were the equal numbers of AFAB and AMAB participants (n = 59, 50%). AFAB transgender participants were significantly younger (Mage=22.53 ± 8.04) than AMAB transgender participants (Mage=33.37 ± 14.9), (t = 4.921, df = 89.158, p < 0.001) (see Table 1).
Table 1.
Descriptive statistics for overall sample as well as divided by sex assigned at birth.
| Birth assigned females [N (%)] or [M±SD] (N = 59) | Birth assigned males [N (%)] or [M±SD] (N = 59) | Total sample [N (%)] or [M±SD] (N = 118) | |
|---|---|---|---|
| Mean age (SD)* | 22.53 ± 8.04 | 33.37 ± 14.90 | 27.95 ± 13.11 |
| Employment status | |||
| Unemployed | 8 (13.6) | 4 (6.8) | 12 (10.2) |
| Employed | 11 (18.6) | 21 (35.6) | 32 (27.1) |
| Student | 25 (42.4) | 14 (23.7) | 39 (33.1) |
| Volunteer Work | 0 (0) | 1 (1.7) | 1 (0.8) |
| Disabled | 1 (1.7) | 1 (1.7) | 2 (1.7) |
| Retired | 0 (0) | 2 (3.4) | 2 (1.7) |
| No answer | 14 (23.7) | 16 (27.1) | 30 (25.4) |
| Civil status* | |||
| Single | 84 (71.2) | 37 (62.7) | 47 (79.7) |
| Married / | 10 (8.5) | 9 (12.8) | 1 (1.7) |
| Civil partnership | 3 (2.5) | 0 (0) | 3 (5.1) |
| Divorced | 1 (0.8) | 8 (13.6) | 1 (1.7) |
| Widowed | 1 (0.8) | 1 (1.7) | 0 (0) |
| In a relationship | 1 (0.8) | 0 (0) | 1 (1.7) |
| Other | 1 (0.8) | 0 (0) | 1 (1.7) |
| No answer | 9 (7.6) | 4 (6.8) | 5 (6.6) |
p < 0.05.
Confidence interval = 95%.
Change in autistic traits and in anxiety symptoms post-CHT
Over a third of participants (34.7%) scored above the cutoff (>70) indicating clinically significant autistic traits at baseline. Post-CHT, the proportion of participants who scored above the AQ cutoff remained stable (32.2%) and there was no significant change in AQ-Short scores (see Tables 2 and 3).
Table 2.
ASC clinical caseness at baseline and follow-up for total sample and by sex assigned at birth.
| Clinical [n (%)] | Non-clinical [n (%)] | |
|---|---|---|
| Total sample (n = 118) | ||
| T0 | 41 (34.7) | 77 (65.3) |
| T1 | 38 (32.2) | 80 (67.8) |
| Assigned Females at Birth (AFAB) (n = 59) | ||
| T0 | 22 (37.3) | 37 (62.7) |
| T1 | 25 (42.4) | 34 (57.6) |
| Assigned Males at Birth (AMAB) (n = 59) | ||
| T0 | 19 (32.2) | 40 (67.8) |
| T1 | 13 (22.0) | 46 (78.0) |
Confidence interval = 95%.
Table 3.
Paired samples t-test examining change for total sample in total AQ-short total and anxiety scores from T0 to T1, for the overall sample as well as split by assigned gender at birth.
| T0 (M ± SD) | T1 (M ± SD) | t (df) | Effect size (d) | p | |
|---|---|---|---|---|---|
| AQ-Short - Total (n = 118) | 64.78 ± 12.26 | 65.55 ± 12.51 | −.876 (117) | 0.062 | .388 |
| Anxiety (n = 116) | 8.00 ± 4.23 | 8.05 ± 3.76 | −.125 (115) | 0.012 | .9 |
| Assigned Females at Birth (AFAB) | |||||
| AQ-Short - Total (n = 59) | 66.54 ± 11.81 | 68.41 ± 12.60 | −1.448 (58) | 0.153 | .153 |
| Anxiety (n = 57) | 8.39 ± 4.33 | 8.49 ± 3.79 | −.167 (56) | 0.023 | .868 |
| Assigned Males at Birth (AMAB) | |||||
| AQ-Short - Total (n = 59) | 63.02 ± 12.55 | 62.69 ± 11.85 | .264 (58) | 0.027 | .793 |
| Anxiety (n = 59) | 7.63 ± 4.14 | 7.63 ± 3.71 | −.000 (58) | 0.069 | 1 |
p < 0.05.
Repeating the analyses for each gender group also found no significant changes in the proportion of participants scoring above the cutoff for AQ caseness and no significant change in AQ-Short scores. However, for AFAB transgender participants, the percentage of people with clinically significant autistic traits rose from 37.3% to 42.4% and for AMAB transgender participants it fell from 32.2% to 22%. Thus, at follow-up AFAB participants were significantly more likely to score above the cutoff for clinically significant autistic traits compared to AMAB participants (χ2=5.589; p = 0.018). They also had significantly higher AQ-Short scores at follow-up (t = 2.537; p = 0.013).
There was a strong agreement between AQ-Short scores at time 0 and at time 1 (ICC = 0.696, CIs 0.591 to 0.779, p < 0.001) for the group as a whole. Anxiety ratings had only poor agreement over time (ICC = 0.386, CIs 0.219 to 0.531, p < 0.001): a significantly lower agreement compared to AQ-Short scores as indicated by the lack of overlap in the CIs.
A HADS-A change score was calculated with higher scores indicating increased levels of anxiety over time. The median HADS-A change scores was 0 with a range from –12 to 10. There were no significant changes in anxiety scores over time either for the whole group or when looking at AMAB and AFAB transgender participants separately (Table 3).
Change in AQ-Short scores controlling for age and change in anxiety
A two-way repeated measures ANOVA was conducted to assess change in AQ-Short scores after CHT, whilst controlling for age and change in anxiety, and to explore possible interactions between gender group and change in AQ-Short over time. Total AQ-Short scores pre- and post-treatment were the dependent variables. Sex assigned at birth was an independent factor with age and anxiety change scores entered as co-variants. The within-subjects factor was non-significant confirming that AQ-Short scores remained stable over time.
There was a main effect of assigned sex at birth suggesting that AFAB transgender people have higher scores than AMAB transgender people independently of any other factor, [F(1,112) = 3.912, p = 0.05, partial η2=0.034]. The gap in AQ-Short scores between genders appeared to widen after CHT but the interaction between change in AQ-Short scores and gender group was non-significant. Age was also unrelated to AQ-Short scores. There was no main effect for change in anxiety, but there was a strong significant interaction with greater increase in anxiety associated with greater increase in AQ-Short scores over time [F(1,112)=23.338, P < 0.001. η2=0.172].
Discussion
In the group as a whole, autistic traits, as measured by the AQ-Short, remained stable following gender affirming hormone treatment: neither the AQ-Short scores nor the proportion of AQ-Short cases changed significantly over time. The stability of AQ-Short scores, confirmed by repeated measure ANOVA, is in conflict with previous research suggesting the presence of autistic traits and social difficulties in transgender populations reflecting feelings of anxiety possibly due to having undergone negative past experiences (Turban, 2018; Turban & van Schalkwyk, 2018). As found in previous research (Jones et al., 2012; Nobili et al., 2018b; Vermaat et al., 2018) transgender participants who had been assigned female at birth had higher AQ-Short scores overall.
Over a third of treatment seeking transgender individuals scored above the cut off for clinically significant autistic traits. The key finding from this study is that AQ-Short scores appear to reflect stable traits rather than a state response to the social and psychological challenges of their transgender status. The study also found that although the difference in the proportion of ASC cases by sex assigned at birth was non-significant pre-CHT, post-CHT a greater proportion of participants who were AFAB scored above the cutoff for ASC caseness compared to AMAB participants. This could suggest that testosterone treatment enhances the expression of male brain characteristics. Previous research suggested that testosterone has an impact upon social connections as fluctuations in hormonal levels can divert people’s interests away from social interactions (Pennebaker et al., 2004). Thus, there is need for more research into the psychological effects of cross-sex hormone treatment.
The study also found that, contrary to previous research (Colizzi et al., 2014), anxiety levels did not reduce following CHT. In fact there was considerable variation in the profile of anxiety form pre- to post-CHT, as evidenced by the low ICC score in comparison to the stability in AQ-Short scores (0.39 vs 0.7) and the lack of overlap in CIs. However, changes in anxiety were associated with corresponding changes in levels of autistic traits suggesting that any increases in levels of anxiety post-CHT may elevate the expression of autistic traits and thus increase social impairment. The association between the trajectory of anxiety and autistic traits is to be expected sincethe presence of a correlation between AQ and HADS was reported by past research on both adults (Kanai et al., 2011) and youth (Uljarević et al., 2018) populations, showing thatthere is a significant overlap in symptomatology between anxiety and ASC (Cath et al., 2008).
This study represents an important advance in the research on autism in transgender populations, as it is the first study that longitudinally explores the effect of cross-sex hormone treatment on autistic traits. However, there are also limitations to the study such as low response rate at follow-up. Participants may have also downplayed their autistic traits before their first assessment at the clinic for fear of not being treated. Consequently, participants may have attenuated their mental health symptoms concerned about not being accepted for cross-sex hormone treatment. This could indicate that the changes between T0 and T1 may be more significant than recorded. Additionally, poor completion of the questionnaire and an under-representation of more anxious participants in the sample may have limited the study’s capacity to demonstrate a reduction in anxiety symptoms post-treatment.
Nonetheless, participants’ AQ-Short scores were representative of the wider clinical population. The AQ-Short is a self-report tool to measure autistic traits and is not an autism diagnostic instrument, but it has a number of strengths as a valid and reliable measure that is widely used in clinical research.
The findings of this study can support the extreme male brain theory of autism (Baron-Cohen, 2002) as it suggests the presence of gender differences in the expression of autistic traits. Additionally, this theory suggests that individuals who were AMAB report higher percentages of ASC (Baron-Cohen, 2002) whilst the results of the present study indicate that AFAB transgender individuals (or possible trans men) report enhanced rates of clinically significant autistic traits. Therefore, these findings seem to suggest that transgender assigned females at birth may have a similar autistic profile to cisgender men rather than cisgender women.
Conclusion
Autistic traits remained stable following cross-sex hormone treatment for both AMAB and AFAB transgender individuals. However, the apparent widening gap in the proportion of AFAB participants and AMAB participants scoring above the cut off for clinically significant autistic traits suggests that the pattern of response to hormone treatment may differ but this needs further investigation in a larger sample. It may simply reflect the fact that living as the experienced gender allows stronger expression of male traits.
These results highlight the need to properly support transgender people (especially AFAB transgender people) when transitioning, as autistic traits in this population may be at a clinically significant level for at least a third of people, and seems to be independent of hormone treatment. Support can be specifically tailored and delivered with online interventions as they might be particularly suited for this population (Perry et al., 2018).
Thus, a better understanding of the psychological impact of hormone treatment is needed in order to properly support transgender people through transition and to enable them to make informed decisions about gender-affirming medical treatment.
References
- APA . (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Association. [DOI] [PubMed] [Google Scholar]
- Auyeung, B., Baron-Cohen, S., Ashwin, E., Knickmeyer, R., Taylor, K., & Hackett, G. (2009). Fetal testosterone and autistic traits. British Journal of Psychology (Psychology), 100(Pt 1), 1–22. 10.1348/000712608X311731 [DOI] [PubMed] [Google Scholar]
- Baron-Cohen, S. (2002). The extreme male brain theory of autism. Trends in Cognitive Sciences, 6(6), 248–254. 10.1016/S1364-6613(02)01904-6 [DOI] [PubMed] [Google Scholar]
- Baron-Cohen, S. (2010). Empathizing, systemizing, and the extreme male brain theory of autism. In Progress in brain research (Vol. 186, pp. 167–175). Elsevier. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen, S., Auyeung, B., Nørgaard-Pedersen, B., Hougaard, D. M., Abdallah, M. W., Melgaard, L., Cohen, A. S., Chakrabarti, B., Ruta, L., & Lombardo, M. V. (2015). Elevated fetal steroidogenic activity in autism. Molecular Psychiatry, 20(3), 369–376. 10.1038/mp.2014.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen, S., Richler, J., Bisarya, D., Gurunathan, N., & Wheelwright, S. (2003). The Systemising Quotient (SQ): An investigation of adults with Asperger syndrome or high functioning autism and normal sex differences. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 358(1430), 361–374. 10.1098/rstb.2002.1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen, S., Wheelwright, S., Skinner, R., Martin, J., & Clubley, E. (2001a). The autism-spectrum quotient (AQ): Evidence from asperger syndrome/high-functioning autism, malesand females, scientists and mathematicians. Journal of Autism and Developmental Disorders, 31(1), 5–17. 10.1023/B:JADD.0000022607.19833.00 [DOI] [PubMed] [Google Scholar]
- Baron-Cohen, S., Wheelwright, S., Scahill, V., Lawson, J., & Spong, A. (2001b). Are intuitive physics and intuitive psychology independent? Journal of Developmental and Learning Disorders, 5, 47–78. [Google Scholar]
- Bjelland, I., Dahl, A. A., Haug, T. T., & Neckelmann, D. (2002). The validity of the Hospital Anxiety and Depression Scale: an updated literature review. Journal of Psychosomatic Research, 52(2), 69–77. 10.1016/S0022-3999(01)00296-3 [DOI] [PubMed] [Google Scholar]
- Bouman, W. P., Claes, L., Brewin, N., Crawford, J. R., Millet, N., Fernandez-Aranda, F., & Arcelus, J. (2017). Transgender and anxiety: A comparative study between transgender people and the general population. International Journal of Transgenderism, 18(1), 16–26. 10.1080/15532739.2016.1258352 [DOI] [Google Scholar]
- Bouman, W. P., Claes, L., Marshall, E., Pinner, G. T., Longworth, J., Maddox, V., Witcomb, G., Jimenez-Murcia, S., Fernandez-Aranda, F., & Arcelus, J. (2016). Socio-demographic variables, clinical features and the role of pre-assessment cross-sex hormones in older trans people. The Journal of Sexual Medicine, 13(4), 711–719. 10.1016/j.jsxm.2016.01.009 [DOI] [PubMed] [Google Scholar]
- Cath, D. C., Ran, N., Smit, J. H., Van Balkom, A. J., & Comijs, H. C. (2008). Symptom overlap between autism spectrum disorder, generalized social anxiety disorder and obsessive-compulsive disorder in adults: a preliminary case-controlled study. Psychopathology, 41(2), 101–110. 10.1159/000111555 [DOI] [PubMed] [Google Scholar]
- Colizzi, M., Costa, R., & Todarello, O. (2014). Transsexual patients' psychiatric comorbidity and positive effect of cross-sex hormonal treatment on mental health: results from a longitudinal study. Psychoneuroendocrinology, 39, 65–73. 10.1016/j.psyneuen.2013.09.029 [DOI] [PubMed] [Google Scholar]
- Glidden, D., Bouman, W. P., Jones, B. A., & Arcelus, J. (2016). Gender dysphoria and autism spectrum disorder: A systematic review of the literature. Sexual Medicine Reviews, 4(1), 3–14. 10.1016/j.sxmr.2015.10.003 [DOI] [PubMed] [Google Scholar]
- Greenberg, D. M., Warrier, V., Allison, C., & Baron-Cohen, S. (2018). Testing the empathizing–systemizing theory of sex differences and the extreme male brain theory of autism in half a million people. Proceedings of the National Academy of Sciences, 115(48), 12152–12157. 10.1073/pnas.1811032115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heylens, G., Aspeslagh, L., Dierickx, J., Baetens, K., Van Hoorde, B., De Cuypere, G., & Elaut, E. (2018). The co-occurrence of gender dysphoria and autism spectrum disorder in adults: An analysis of cross-sectional and clinical chart data. Journal of Autism and Developmental Disorders, 48(6), 2217–2223. 10.1007/s10803-018-3480-6 [DOI] [PubMed] [Google Scholar]
- Hines, M. (2006). Prenatal testosterone and gender-related behaviour. European Journal of Endocrinology, 155(suppl_1), S115–S121. 10.1530/eje.1.02236 [DOI] [PubMed] [Google Scholar]
- Hoekstra, R. A., Bartels, M., Cath, D. C., & Boomsma, D. I. (2008). Factor structure, reliability and criterion validity of the Autism-Spectrum Quotient (AQ): a study in Dutch population and patient groups. Journal of Autism and Developmental Disorders, 38(8), 1555–1566. 10.1007/s10803-008-0538-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corporation . (2016). IBM SPSS Statistics for Windows. Version 24.0. IBM Corporation. [Google Scholar]
- Jolliffe, T., & Baron-Cohen, S. (1997). Are people with autism and Asperger syndrome faster than normal on the Embedded Figures Test?. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 38(5), 527–534. 10.1111/j.1469-7610.1997.tb01539.x [DOI] [PubMed] [Google Scholar]
- Jones, R., Wheelwright, S., Farrell, K., Martin, E., Green, R., Di Ceglie, D., & Baron-Cohen, S. (2012). Brief report: Female-to-male transsexual people and autistic traits. Journal of Autism and Developmental Disorders, 42(2), 301–306. 10.1007/s10803-011-1227-8 [DOI] [PubMed] [Google Scholar]
- Kanai, C., Iwanami, A., Hashimoto, R., Ota, H., Tani, M., Yamada, T., & Kato, N. (2011). Clinical characterization of adults with Asperger’s syndrome assessed by self-report questionnaires based on depression, anxiety, and personality. Research in Autism Spectrum Disorders, 5(4), 1451–1458. 10.1016/j.rasd.2011.02.005 [DOI] [Google Scholar]
- Knickmeyer, R., Baron-Cohen, S., Fane, B., Wheelwright, S., Mathews, G., Conway, G., Brook, C., & Hines, M. (2006). Androgens and autistic traits: a study of individuals with congenital adrenal hyperplasia. Hormones and Behavior, 50(1), 148–153. 10.1016/j.yhbeh.2006.02.006 [DOI] [PubMed] [Google Scholar]
- Kristensen, Z. E., & Broome, M. R. (2015). Autistic traits in an internet sample of gender variant UK adults. International Journal of Transgenderism, 16(4), 234–245. 10.1080/15532739.2015.1094436 [DOI] [Google Scholar]
- Millet, N., Longworth, J., & Arcelus, J. (2017). Prevalence of anxiety symptoms and disorders in the transgender population: A systematic review of the literature. International Journal of Transgenderism, 18(1), 27–38. 10.1080/15532739.2016.1258353 [DOI] [Google Scholar]
- Nobili, A., Glazebrook, C., & Arcelus, J. (2018a). Quality of life of treatment-seeking transgender adults: A systematic review and meta-analysis. Reviews in Endocrine & Metabolic Disorders, 19(3), 199–220. 10.1007/s11154-018-9459-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobili, A., Glazebrook, C., Bouman, W. P., Glidden, D., Baron-Cohen, S., Allison, C., Smith, P., & Arcelus, J. (2018b). Autistic traits in treatment-seeking transgender adults. Journal of Autism and Developmental Disorders, 48(12), 3984–3994. 10.1007/s10803-018-3557-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterski, V., Gilligan, L., & Curtis, R. (2014). Traits of autism spectrum disorders in adults with gender dysphoria. Archives of Sexual Behavior, 43(2), 387–393. 10.1007/s10508-013-0154-5 [DOI] [PubMed] [Google Scholar]
- Pennebaker, J. W., Groom, C. J., Loew, D., & Dabbs, J. M. (2004). Testosterone as a social inhibitor: two case studies of the effect of testosterone treatment on language. Journal of Abnormal Psychology, 113(1), 172–175. 10.1037/0021-843X.113.1.172 [DOI] [PubMed] [Google Scholar]
- Perry, Y., Strauss, P., & Lin, A. (2018). Online interventions for the mental health needs of trans and gender diverse young people. The Lancet. Psychiatry, 5(2), e6. 10.1016/S2215-0366(18)30017-8 [DOI] [PubMed] [Google Scholar]
- Robinson, E. B., Munir, K., Munafò, M. R., Hughes, M., McCormick, M. C., & Koenen, K. C. (2011). Stability of autistic traits in the general population: further evidence for a continuum of impairment. Journal of the American Academy of Child & Adolescent Psychiatry, 50(4), 376–384. 10.1016/j.jaac.2011.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzich, E., Allison, C., Chakrabarti, B., Smith, P., Musto, H., Ring, H., & Baron-Cohen, S. (2015). Sex and STEM occupation predict Autism Spectrum Quotient (AQ) scores in half a million people. PLoS One, 10(10), e0141229. 10.1371/journal.pone.0141229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang, J. F., Janssen, A., Tishelman, A., Leibowitz, S., Kenworthy, L., McGuire, J., Edwards-Leeper, L., Mazefsky, C., Rofey, D., Bascom, J., Caplan, R., Gomez-Lobo, V., Berg, D., Zaks, Z., Wallace, G., Wimms, H., Pine-Twaddell, E., Shumer, D., Register-Brown, K., Sadikova, E., & Anthony, L. (2018). Revisiting the link: Evidence of the rates of autism in studies of gender diverse individuals. Journal of the American Academy of Child & Adolescent Psychiatry, 57(11), 885–887. 10.1016/j.jaac.2018.04.023 [DOI] [PubMed] [Google Scholar]
- Taylor, M. J., Gillberg, C., Lichtenstein, P., & Lundström, S. (2017). Etiological influences on the stability of autistic traits from childhood to early adulthood: evidence from a twin study. Molecular Autism, 8(1), 5. 10.1186/s13229-017-0120-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turban, J. L. (2018). Potentially reversible social deficits among transgender youth. Journal of Autism and Developmental Disorders, 48(12), 4007–4009. 10.1007/s10803-018-3603-0 [DOI] [PubMed] [Google Scholar]
- Turban, J. L., & van Schalkwyk, G. I. (2018). Gender dysphoria” and autism spectrum disorder: Is the link real? Journal of the American Academy of Child and Adolescent Psychiatry, 57(1), 8–9. 10.1016/j.jaac.2017.08.017 [DOI] [PubMed] [Google Scholar]
- Uljarević, M., Richdale, A. L., McConachie, H., Hedley, D., Cai, R. Y., Merrick, H., Parr, J., & Le Couteur, A. (2018). The hospital anxiety and depression scale: Factor structure and psychometric properties in older adolescents and young adults with autism spectrum disorder. Autism Research, 11(2), 258–269. 10.1002/aur.1872 [DOI] [PubMed] [Google Scholar]
- Vermaat, L. E., van der Miesen, A. I., de Vries, A. L., Steensma, T. D., Popma, A., Cohen-Kettenis, P. T., & Kreukels, B. P. (2018). Self-reported autism spectrum disorder symptoms among adults referred to a gender identity clinic. LGBT Health, 5(4), 226–233. 10.1089/lgbt.2017.0178 [DOI] [PubMed] [Google Scholar]
- Witcomb, G. L., Bouman, W. P., Claes, L., Brewin, N., Crawford, J., & Arcelus, J. (2018). Levels of depression in transgender people and its predictors: Results of a large matched control study with transgender people accessing clinical services. Journal of Affective Disorders, 235, 308–315. 10.1016/j.jad.2018.02.051 [DOI] [PubMed] [Google Scholar]
- Woodbury-Smith, M. R., Robinson, J., Wheelwright, S., & Baron-Cohen, S. (2005). Screening adults for Asperger syndrome using the AQ: A preliminary study of its diagnostic validity in clinical practice. Journal of Autism and Developmental Disorders, 35(3), 331–335. 10.1007/s10803-005-3300-7 [DOI] [PubMed] [Google Scholar]
- Zigmond, A. S., & Snaith, R. P. (1983). The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica, 67(6), 361–370. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]