ABSTRACT
The increasing prevalence of metabolic diseases has become a severe public health problem. Gut microbiota play important roles in maintaining human health by modulating the host’s metabolism. Recent evidences demonstrate that Akkermansia muciniphila is effective in improving metabolic disorders and is thus considered as a promising “next-generation beneficial microbe”. In addition to the live A. muciniphila, similar or even stronger beneficial effects have been observed in pasteurized A. muciniphila and its components, including the outer membrane protein Amuc_1100, A. muciniphila-derived extracellular vesicles (AmEVs), and secreted protein P9. Hence, this paper presents a systemic review of recent progress in the effects and mechanisms of A. muciniphila and its components in the treatment of metabolic diseases, including obesity, type 2 diabetes mellitus, cardiovascular disease, and nonalcoholic fatty liver disease, as well as perspectives on its future study.
KEYWORDS: Akkermansia muciniphila, metabolic diseases, pasteurized A. muciniphila, Amuc_1100, AmEVs, P9
1. Introduction
The prevalence of metabolic diseases such as obesity, type 2 diabetes mellitus (T2DM), cardiovascular diseases (CVD), and nonalcoholic fatty liver disease (NAFLD) has becoming a severe public health problem:1, 2 Gut microbiota can regulate host metabolism by influencing immune maturation and homeostasis, protecting against pathogen overgrowth, regulating intestinal endocrine functions and neurologic signaling, modulating energy metabolism, and producing functional metabolites.3,4 The compositional and metabolic changes of intestinal microbiota (dysbiosis) is closely involved in the occurrence of metabolic diseases.5,6 Thus, considerable attention has been paid to gut microbiota-targeted therapies on metabolic diseases with diverse approaches, including probiotics, prebiotics, fecal microbiota transplantation, and antibiotics.7–12
Akkermansia muciniphila, a commensal bacterium, is an oval-shaped, non-motile bacterium with no endospore formation, and a microaerophilic microbe that was first isolated from human feces in 2004.13–15 It colonizes in the intestinal tract early in life, and accounts for approximately 1–3% of the total intestinal microbiota in healthy adults.16 A. muciniphila resides in the intestinal mucus layer, utilizing the mucin as the sole source of carbon, nitrogen, and energy.13 In recent years, A. muciniphila has attracted much attention for its comprehensive roles in maintaining host wellbeing,17 which is regarded as a promising “next-generation beneficial microbe” for metabolic disease prevention or therapy owing to its various properties, including producing short-chain fatty acids (SCFAs),13,15,18 improving intestinal integrity,19,20 and reducing endotoxemia through inhibiting the translocation of lipopolysaccharide (LPS) from the intestine to circulation.19–21 However, the exact mechanisms underlying the benefits of A. muciniphila on metabolic diseases are complicated because similar effects have been observed on either live A. muciniphila or pasteurized A. muciniphila, their outer membrane proteins or secreted proteins, as well as extracellular vesicles.19–42 In these papers, we systemically reviewed the updated progress of A. muciniphila with respect to its role in metabolic diseases, and discussion on its future research direction as well.
2. Role of A. muciniphila in metabolic diseases
2.1. A. muciniphila and obesity
In recent years, with the changes in people’s dietary habits and the increased availability of high calorie diet, overweightness and obesity have become one of the most serious health problems in the world. Manipulation of gut microbiota is a promising strategy for obesity prevention or therapy.43–46 The relative abundance of A. muciniphila is significantly reduced in high-fat diet (HFD)-fed obese mice or rats compared with their lean littermates, and negatively correlated with body fat mass and glucose intolerance (Table 1).19,47–52 Nevertheless, inconsistent results have also been reported. For example, the copy number of A. muciniphila had an increased trend in high-fat and high-sucrose diet (HFHS)-fed mice without reaching a statistical significance when analyzed by qPCR.53 In addition, by 16s rRNA sequencing, Arias et al. found significantly increased relative abundance of Akkermansia genus in HFD-fed female C3HeB/FeJ mice.54 Interestingly, the dietary impacts on Akkermansia levels were found to be genetically dependent in mice: Akkermansia was decreased in 129S1/SvImJ mice, but was increased in A/J, NOD/LtJ, C57BL/6 J, and NZO/HILtJ mice after short-term HFHS intake.55 Thus, the divergent genetically susceptible to intestinal microenvironment might account for the different response of A. muciniphila abundance to diet in the host. In clinical studies, a decreased abundance of A. muciniphila occurred in adults and children with obesity of both sexes.56–68 Furthermore, studies indicated that the reduced A. muciniphila in patients with obesity was independent of other metabolic diseases, including diabetes and NAFLD.65,66,68,69 In contrast, an increase in A. muciniphila abundance was observed in children with obesity compared to children with normal-weight.70 Despite some inconsistent findings, the majority of studies in both animal and clinical studies support the negative correlation between A. muciniphila and obesity.
Table 1.
The differential changes of A. muciniphila in metabolic diseases
| Ref. | Disease condition | Sample type | Sample detection | Study group | Beneficial changes achieved |
|---|---|---|---|---|---|
| Mice | |||||
| Everard et al (2013)19 | Obesity | Cecal sample | qPCR | 1. ob/ob mice (n = 5) 2. High fat-fed obese mice (n = 10)3. Lean littermates (n = 5) |
The abundance of A. muciniphila was 3,300-fold lower in leptin-deficient obese mice than in their lean littermates. It was also observed that a 100-fold decrease of this bacterium in high fat-fed mice. |
| Everard et al (2014)147 | HFD induced obesity | Cecal sample | Metagenomic sequencing | 1. Control diet (n = 9)2. HFD (n = 7) | HFD treatment profoundly affected the abundance of Verrucomicrobia. Meanwhile, Akkermansia was not detectable under HFD treatment but was detected in control diet-fed mice. |
| Schneeberger et al (2015)148 | Diet-induced obesity | Cecal sample, at 3, 6, 12 and 16 weeks | qPCR | 1. Chow diet (n = 6 mice/diet/time point) 2. High-fat diet (n = 6 mice/diet/time point) | The abundance of A. muciniphila progressively decline with prolonged dietary treatment in chow diet fed mice, and that this effect is exacerbated upon HFD. |
| Hussain et al (2016)47 | HFD induced obesity | Feces | qPCR | 1. Normal chow diet (NCD) (n = 7)2. High fat diet (HFD) (n = 7) | The relative abundance of A. muciniphila was lower in HFD group than that in NCD group. |
| Li et al (2016)20 | Atherosclerosis | Feces, at a selected time point | qPCR | 1. Normal chow diet (NCD) (n = 8–10)2. Daily oral gavage with live A. muciniphila (WD+AKK) (n = 8–10)3. Heat-killed A. muciniphila (WD+hk-AKK) (n = 8–10) | The fecal abundance of A. muciniphila was significantly reduced by western diet. |
| Mehrpouya-Bahrami et al (2017)149 | Diet-induced obesity | Feces | qPCR | 1. Low fat diet (LFD) (n = 10)2. High fat diet (HFD) (n = 10) | The abundance of A. muciniphila was lower in HFD fed mice than that in LFD fed mice. |
| Lee et al (2018)48 | Diet-induced obesity | Cecal sample | 16S rRNA sequencing, qPCR | 1. Normal diet (n = 5)2. HFD (n = 5) | Compared with normal diet group mice, the relative abundance of A. muciniphila was lower in HFD group. |
| Natividad et al (2018)49 | HFD induced metabolic dysfunctions | Feces | 16S rRNA sequencing | 1. Control diet (CD) (n = 5–6)2. High fat diet enriched with milk-fat (n = 5–6) | Compared to CD, HFD-fed mice had lower abundance of bacteria belonging to the Akkermansia muciniphila species. |
| Villamil et al (2018)150 | HFD | Feces, T1 = at weaning, T2 = after eight weeks of dietary intervention | 16S rRNA sequencing | 1. Control diet (n = 12)2. HFD (n = 12) | The HFD diet decreased the level of A. muciniphila. |
| Hänninen et al (2018)28 | T1DM | Feces or cecal and colon content | 16S rRNA sequencing | 1. NOD/MrkTac (n = 6) 2. NOD/Jax (n = 6) | NOD/MrkTac mice develop diabetes less often and later than NOD/Jax mice because their microbiota is more diverse and more favorably balanced. The level of A. muciniphila was lower in NOD/Jax mice than that in NOD/MrkTac. Akkermansia may defer diabetes development in NOD/MrkTac mice. |
| Arias et al (2019)54 | HFD induced obesity | Cecal sample | 16S rRNA sequencing | 1. Normal chow diet (NCD) (n = 20)2. HFD (n = 20) | The relative abundance of A. muciniphila was higher in HFD group than that in NCD group. |
| Anhê et al (2019)53 | High fat/high sucrose (HFHS) induced obesity | Feces | qPCR | 1. Chow-fed (Chow, n = 9) 2. HFHS (n = 11) |
When compared with the chow-fed group, the copy number of A. muciniphila had an increased trend in HFHS group without reaching a statistical significance. |
| Wang et al (2019)50 | HFD induced obesity | Feces | 16S rRNA sequencing, qPCR | 1 Normal control (NC) (n = 6)2 HFD (n = 6) | The abundance of A. muciniphila was significantly lower in the HFD group than in NC group. |
| Yang et al (2019)51 | HFD-induced cognitive deficits | Feces | 16S rRNA sequencing, qPCR | 1. Chow diet (n = 8)2. HFD (n = 8) | The relative abundance of A. muciniphila was lower in HFD group than that in chow diet group. |
| Fujisaka et al (2020)151 | HFD induced obesity | Feces | qPCR | 1. Normal chow diet (n = 4)2. HFD (n = 4) | The level of A. muciniphila decreased after a week of administration of an HFD. |
| Régnier et al (2020)152 | Diet-induced obesity and diabetes | Feces | 16S rRNA sequencing, qPCR | 1. Control diet (n = 9–10)2. High-fat and high-sucrose diet (n = 9–10) | The 16S rRNA sequencing result showed the relative abundance of Akkermansia had an increased trend in high-fat and high-sucrose group without reaching a statistical significance. However, qPCR analysis showed the copy number of A. muciniphila was comparable between two groups. |
| Wu et al (2020)153 | HFD induced obesity | Cecal sample | 16S rRNA sequencing | 1. Low fat diet (LFD) (n = 12)2. HFD (n = 12) | The abundance of A. muciniphila was lower in HFD fed mice than that in LFD fed mice. |
| Rats | |||||
| Fåk et al (2015)154 | HFD induced obesity | Cecal sample | qPCR | 1. Low fat (n = 7) 2. High fat (n = 7) |
The abundance of A. muciniphila was increased with high-fat feeding. |
| Wang et al (2015)52 | HFD induced obesity | Feces | qPCR | 1. Normal (n = 8)2. HFD (n = 8) | The level of A. muciniphila in the HFD-fed rats was lower than that in the normal group rats. |
| Human | |||||
| Santacruz et al (2010)61 | Overweight pregnant women | Feces, at 24 weeks of pregnancy | qPCR | 1. Normal-weight (n = 34)2. Overweight (n = 16) | A. muciniphila numbers were lower in women with excessive weight gain than in women with normal weight gain during pregnancy. |
| Karlsson et al (2012)62 | Overweight and obese children | Feces, at a selected time point | qPCR | 1. Normal weight (n = 20)2. Overweight (n = 20)3. Obesity (n = 20) | The abundance of A. muciniphila was less in overweight and obese children than that in normal weight children. |
| Zhang et al (2013)77 | T2DM | Feces | 16S rRNA sequencing | 1. Normal glucose tolerance (n = 44) 2. Pre-diabetes (n = 64) 3. Type 2 diabetes (n = 13) |
The abundance of A. muciniphila was reduced in the subjects with pre-diabetes and type 2 diabetes compared to subjects with normal glucose tolerance. |
| Teixeira et al (2013)59 | Obese females | Feces, at a selected time point | qPCR | 1. Lean females (n = 17)2. Obese females (n = 15) | The level of A. muciniphlia was higher in lean females than that in obese females. |
| Chatelier et al (2013)155 | Obesity | Feces | Metagenomic sequencing | 1. Low gene count (LGC) (n = 68)2. High gene count (HGC) (n = 224) | Obese LGC individuals gained on average significantly more weight than HGC individuals during the past 9 years. The level of A. muciniphila decreased in LGC group compared to HGC group. |
| Escobar et al (2014)56 | Obesity | Feces | 16S rRNA sequencing | 30 volunteers from Colombians | The level of Akkermansia was decreased with the increasing BMI in the Colombian dataset. |
| Remely et al (2015)156 | Obesity | Feces | qPCR | Obese individuals (n = 33) | The level of A. muciniphila in obese individuals was higher than that before intervention, after 16-week weight loss diet. |
| Brahe et al (2015)157 | Postmenopausal women with obesity | Feces | Metagenomic sequencing | 53 women with obesity | The species A. muciniphila was negatively associated with markers for insulin resistance or dyslipidaemia. |
| Yassour et al (2016)58 | Obesity and T2DM | Feces, at up to two time points each (12–44 months apart) | Metagenomic sequencing | 20 monozygotic Korean twins | The abundance of A. muciniphila was negatively correlated with BMI, fasting blood sugar, and insulin levels. |
| Dao et al (2016)78 | Overweight and Obesity | Feces, at baseline, 6 weeks after calorie restriction and 12 weeks after stable body weight | qPCR | 1. Overweight (n = 11)2. Obesity (n = 38) | The abundance of A. muciniphila at baseline was negatively correlated with fasting blood glucose, waist-to-hip ratio, and subcutaneous fat cell diameter. |
| Wang et al (2017)158 | T2DM | Feces | 16S rRNA sequencing | 1. Patients with short durations of diabetes (n = 18)2. Patients with medium durations of diabetes (n = 35)3. Patients with long durations of diabetes (n = 21) | There was a significantly higher abundance of A. muciniphila in patients with short and medium durations than those with long duration of diabetes. |
| Liu et al (2017)63 | Obesity | Feces | Metagenomic sequencing | 1. Lean controls (n = 105) 2. Obese individuals (n = 95) |
A. muciniphila were highly enriched in lean controls. |
| Li et al (2017)159 | Hypertension | Feces | Metagenomic sequencing | 1. Healthy controls (n = 41)2. pre-hypertension (pHTNs) (n = 56)3. Hypertension (HTN) (n = 99) | The abundance of A. muciniphila was higher in the healthy controls than in the patients with pHTNs and HTN. |
| Borgo et al (2017)64 | Obese children | Feces | 16S rRNA amplification followed by denaturing gradient gel electrophoresis (DGGE) analysis and sequencing, qPCR | 1. Obese children (n = 28) 2. Normal-weight children (n = 33) |
The abundance of A. muciniphila in obese children was significantly lower than that in normal weight children. |
| Cuesta-Zuluaga et al (2018)69 | Obese and cardiometabolically abnormal subjects |
Feces | 16S rRNA sequencing | 1. Normal weight subjects (n = 138)2. Overweight subjects (n = 171)3. Obese subjects (n = 132)(According to the BMI categories, each group was divided into cardiometabolically healthy and cardiometabolically abnormal) | A. muciniphila has higher prevalence in cardiometabolically healthy and normal weight participants when compared with obese and cardiometabolically abnormal subjects. |
| Chelakkot et al (2018)41 | T2DM | Feces | Metagenomic sequencing | 1. T2DM patients (n = 12)2. Healthy controls (n = 8) | There are more AmEVs in the fecal samples of healthy controls compared with those of patients with T2DM. |
| Thingholm et al (2019)65 | Obese individuals with and without T2DM | Feces | 16S rRNA sequencing, Metagenomic sequencing | 1. Lean non-diabetic (n = 95) 2. Obese non-diabetic (n = 55) 3. Obese individuals with T2DM (n = 51) |
The abundance of A. muciniphila decreased in obese individuals. |
| Singh et al (2019)160 | Aging | Feces | 16S rRNA sequencing | 1. Healthy aging (HA) (n = 33)2. Non-healthy aging (NHA) (n = 32) | The abundance of Akkermansia was lower in the HA group than that in the NHA group. |
| Salah et al (2019)66 | Obesity and T2DM | Feces | 16S rRNA sequencing | 1. Controls without obesity or T2DM (n = 5)2. Obese adults without T2DM (n = 25)3. T2DM without obesity (n = 5)4. Adults with both obese and T2DM (n = 25) | The abundance of A. muciniphila was decreased in obese adults when compared with control individuals. |
| Mitsou et al (2019)67 | Obesity | Feces | qPCR | 1. Normal weight subjects (n = 30)2. Overweight/obese subjects (n = 80) | Overweight/obese subjects were more prone in low bimodal levels of A. muciniphila compared to normal-weight individuals. Low bimodal levels of A. muciniphila were positively associated with fasting blood glucose. |
| Medina-Vera et al (2019)161 | T2DM | Feces | 16S rRNA sequencing | 1. T2DM patients (n = 151)2. Healthy controls (n = 50) | The level of A. muciniphila decreased in T2DM, compared to healthy subjects. |
| Dao et al (2019)60 | Obesity | Feces, at 1, 3, and 12 months | Metagenomic sequencing, qPCR | 65 women with severe obesity | The relative abundance of A. muciniphila was significantly lower in severe obesity than in moderate obesity. |
| Nistal et al (2019)68 | Patients with nonalcoholic fatty liver disease (NAFLD) associated with obesity | Feces | 16S rRNA sequencing | 1. Healthy adults (n = 20)2. Obese patients with NAFLD (n = 36)3. Obese patients without NAFLD (n = 17) | When compared to healthy controls, the relative abundance of A. muciniphila was reduced in obese patients, both with or without NAFLD. |
| Marvasti et al (2020)57 | Obesity | Feces | qPCR | 1. Normal weight subjects (n = 50)2. Obese subjects (n = 50) | The abundance of A. muciniphila significantly decreased in obese group, compared to the normal weight group. |
| Liang et al (2020)70 | Obese children | Feces | 16S rRNA sequencing | 1. Obesity (n = 42)2. Normal weight (n = 57) | The relative abundance of A. muciniphila in children with obesity was higher than that in children with normal-weight. |
| Journey et al (2020)162 | Obesity | Feces, at the beginning and end of the 2015–2016 academic year | qPCR | 42 college freshmen living in dormitories at Arizona State University (24 female and 15 male adolescents) | The abundance of A. muciniphila was negatively associated with both the increasement of percent waist circumference and percent body mass index. |
| Hsu et al (2020)163 | Chronic kidney disease (CKD) (children and adolescents) | Feces | 16S rRNA sequencing | 1.G1 (n = 79)2.G2 (n = 27)3.G3 (n = 7)4.G4 (n = 2)(Participants were categorized according to eGFR (mL/min/1.73 m2): G1 ≥ 90, G2 60–89, G3 30–59, or G4 15–29.) | CKD children with an abnormal ambulatory BP monitoring profile had decreased abundance of A. muciniphila. |
Given this negative correlation, the role of A. muciniphila in obesity has been widely investigated in both mice and human subjects. Everard et al. reported that administration of A. muciniphila reversed a series of disorders in HFD-fed mice, including reducing body weight, relieving insulin resistance, and fasting hyperglycemia, as well as increasing the mRNA expression of genes involved in the regulation of adipocyte differentiation and lipid oxidation.19 From that on, many research teams further found live or even pasteurized A. muciniphila as well as its components, including the outer membrane protein Amuc_1100, A. muciniphila-derived extracellular vesicles (AmEVs), and secreted protein P9, were effective in improving diet-induced obesity.19,21,22,24,26,27,29,32–34,36,39,42 These findings were summarized in Table 2. The benefits of A. muciniphila in obesity intervention has also been investigated in one clinical trial. Depommier et al. compared the safety and efficacy of live and pasteurized A. muciniphila in adults with overweight or obesity. Their results showed that daily oral supplementation of 1010 CFU of live or pasteurized A. muciniphila for three months was safe and well tolerated. However, improved insulin sensitivity, reduced insulinemia, and plasma total cholesterol was only present in patients given pasteurized A. muciniphila supplementation.37 This prospective study showed the feasibility to administer A. muciniphila to obese humans, however, further study is needed to demonstrate the relationship between supplement of A. muciniphila and improvement of metabolic parameters on a larger scale of subjects.
Table 2.
The efficacy of A. muciniphila supplementation on metabolic diseases
| Ref. | Bacterial status | Diet | Disease model | Dosage and period | study group | Treatment outcome | |
|---|---|---|---|---|---|---|---|
| Animals | |||||||
| Everard et al (2013)19 | Live A. muciniphila, heat-killed A. muciniphila | CT or HFD | HFD-fed obese mice | 1. CT and HFD group mice orally administrated with 0.2 ml sterile anaerobic PBS containing a similar end concentration of glycerol (2.5% vol/vol) for 4 weeks.2. CT + AKK and HFD + AKK group mice orally administrated with 2 × 108 CFU/0.2 ml A. muciniphila suspended in sterile anaerobic PBS.3. HFD + K-AKK group mice orally administrated with 2 × 108 CFU/0.2 ml heat-killed A. muciniphila for 4 weeks. | 1. Control diet (CT) (n = 10)2. HFD (n = 10)3. CT + AKK (n = 10)4. HFD + AKK (n = 10)5. HFD + K-AKK (n = 10) | A. muciniphila treatment reversed HFD including fat-mass gain, metabolic endotoxemia, adipose tissue inflammation, and insulin resistance. It also increased the intestinal levels of endocannabinoids and gut peptide secretion. And all these effects required viable A. muciniphila because treatment with heat-killed cells did not improve the metabolic profile or the mucus layer thickness. | |
| Shin et al (2014)22 | Live A. muciniphila | NCD or HFD | HFD induced obese mice | 1. HFD group treated with PBS for the 6 weeks.2. HFD + AKK group treated with 4.0 × 108 CFU A. muciniphila for 6 weeks. | 1. HFD (n = 6)2. HFD + AKK (n = 6) | Oral administration of A. muciniphila to HFD-fed mice significantly enhanced glucose tolerance and attenuated adipose tissue inflammation by inducing Foxp3 regulatory T cells in the visceral adipose tissue. | |
| Org et al (2015)71 | HFD/high-sucrose diet (HSD) induced obesity | High-fat, high-sucrose (HF/HS) diet | (HF/HS) diet induced obese mice | 1. HF/HS-AKK group was treated five times per week with A. muciniphila by oral gavage at a dose of 1.44 × 109 CFU/0.2 mL for five weeks. 2. HF/HS group was treated with an oral gavage of an equivalent volume of heat-killed A. muciniphila for five weeks. |
1. HF/HS-AKK (n = 5)2. HF/HS (n = 5) | Oral administration of A. muciniphila significantly reduced body weight and total body fat as well as improved metabolic parameters in HF/HS fed mice. | |
| Plovier et al (2017)21 | Live A. muciniphila, pasteurized A. muciniphila, and Amuc_1100 | ND or HFD | HFD-induced obese mice | 1. Control groups (ND and HFD) were treated with an oral gavage of an equivalent volume of sterile PBS containing 2.5% glycerol for 5 weeks. 2. HFD + AKK M group mice orally administered 2 × 108 CFU/150 μl A. muciniphila grown on the mucus-based medium for 5 weeks.3. HFD + AKK S group mice orally administrated 2 × 108 CFU/150 μl A. muciniphila grown on the synthetic medium for 5 weeks.4. HFD + AKK P group mice orally administrated 2 × 108 CFU/150 μl pasteurized A. muciniphila for 5 weeks.5. Amuc_1100 group mice orally administrated 3 μg of the protein Amuc_1100 for 5 weeks. |
1. ND (n = 10) 2. HFD (n = 9) 3. HFD + AKK M (mucus) (n = 9) 4. HFD + AKK S (synthetic) (n = 8) 5. HFD + AKK P (pasteurized) (n = 9) 6. Amuc_1100 (n = 9) |
A. muciniphila retains its beneficial effects when grown on the synthetic medium. Pasteurization of A. muciniphila enhanced its capacity to reduce fat mass development, insulin resistance and dyslipidemia in mice. Amuc_1100 can interact with Toll-like receptor 2 and improve the gut barrier. | |
| Li et al (2016)20 | Live A. muciniphila, heat-killed A. muciniphila | NCD or WD | Western diet-induced atherosclerosis in Apoe−/− mice on a C57BL background | 1. WD + PBS group treated PBS for 8 weeks.2. WD + AKK group treated 5 × 109 CFU live A. muciniphila for 8 weeks. 3. WD + hk-AKK group treated 5 × 109 heat-killed A. muciniphila for 8 weeks. |
1. NCD (n = 8–10)2. WD (n = 8–10)3. WD + AKK (n = 8–10)4. WD + hk-AKK (n = 8–10)5. WD + PBS (n = 8–10) | Oral gavage with A. muciniphila protected against WD-induced atherosclerotic lesion formation in Apoe−/− mice. It also ameliorated both aortic and systemic inflammation, decreased intestinal permeability and reduced the penetration of gut-derived lipopolysaccharide into circulation in WD-fed Apoe−/− mice. | |
| Shen et al (2016)23 | Live A. muciniphila | CD | CREBH-null mice | 1. WT + Veh and KO + Veh mice treated with PBS that included 25% (vol/vol) glycerol for 2 weeks. 2. WT + AKK and KO + AKK mice treated with AKK for 2 weeks. 3. KO + inactive-AKK mice treated with heat-inactivated AKK for 2 weeks. | 1. WT + AKK (n = 6–12)2. WT + Veh (n = 6–12)3. KO (CREBH-null mice) + AKK (n = 6–12)4. KO + Veh (n = 6–12)5. KO + inactive-AKK (n = 6–12) | A. muciniphila administration protected mice from an acute fat load–induced hyperlipidemia compared with vehicle-treated mice. It also significantly ameliorated chronic hypertriglyceridemia, improved insulin sensitivity, and prevented overproduction of postprandial chylomicrons in CREBH-null mice. Treatment with A. muciniphila further improved hepatic endoplasmic reticulum stress and metabolic inflammation in CREBH-null mice. | |
| Zhao et al (2017)24 | Live A. muciniphila | NCD | NCD induced obese mice | 1. NCD + PBS group orally administrated sterile PBS (NCD + PBS) for five weeks. 2. NCD + AKK group orally administrated 2.0 × 108 CFU/200 µl A. muciniphila every day for five weeks. | 1. NCD + PBS (n = 10)2. NCD + AKK (n = 10) | A. muciniphila supplementation significantly alleviated body weight gain, reduced fat mass and improved glucose tolerance and insulin sensitivity when compared with the vehicle group. It also reduced gene expression related to fatty acid synthesis and transport in liver and muscle as well as alleviated the endoplasmic reticulum stress in liver and muscle. A. muciniphila decreased plasma levels of lipopolysaccharide (LPS)-binding protein (LBP) and leptin, inactivated LPS/LBP downstream signaling in liver and muscle as well as increased anti-inflammatory factors. | |
| Gao et al (2017)25 | Live A. muciniphila | NCD or HFD | HFD induced metabolic syndrome in mice | 1. The NCD + AKK and HFD + AKK groups mice were treated daily with oral doses of 1 × 109 CFU/300 μl A. muciniphila for 14 weeks.2. NCD- and HFD-fed control mice received a gavage with the corresponding sterile culture medium for A. muciniphila for 14 weeks. | 1. NCD (n = 6–8)2. HFD (n = 6–8)3. NCD + AKK (n = 6–8)4. HFD + AKK (n = 6–8) | A. muciniphila administration altered body composition and energy efficiency, promoted the browning of white fat tissue, and improved the lipid and glucose metabolism disorder in the HFD-fed mice. | |
| Sheng et al (2018)26 | Live A. muciniphila | WD | WD induced obese mice | 1. WD-fed WT mice + PBS group orally administrated PBS for 1 month.2. WD-fed WT mice + AKK group orally administrated A. muciniphila 109 CFU/mouse per day for 1 month. | 1. WD-fed WT mice + PBS (n = 3–4)2. WD-fed WT mice + AKK (n = 3–4) | Supplementation of A. muciniphila reduces body weight and regulates lipid metabolism. | |
| Lee et al (2018)27 | AmEVs | HFD | Six-week-old mice were fed a HFD for 39 weeks to induce metabolic disorders, including obesity and T2DM. | 1. HFD + PBS mice were orally administrated PBS for 5 weeks.2. HFD + AmEVs were orally administrated 20 µg AmEVs daily for 5 weeks. | 1. HFD + PBS (n = 4) 2. HFD + AmEVs (n = 3) | AmEVs significantly decreased the body weight gain, when compared with HFD-fed group. The total cholesterol level in the AmEVs-fed group was lower than that in the HFD-fed group. The epididymal fat pad weight in the AmEVs-fed group was lower than that in the NC group, albeit not significantly so. | |
| Bodogai et al (2018)40 | Live A. muciniphila | / | Young (10 to 12 weeks) and aged (18 to 24 months) female C57BL/6 mice (n = 400) | 1. Young and aged group mice orally administrated vehicle every second day for 20 days.2. Aged mice + AKK group orally administrated 1 × 108 A. muciniphila every second day for 20 days. | 1. Young (n = 7)2. Aged (n = 7)3. Aged mice+AKK (n = 7) | Supplementation with A. muciniphila could restore normal insulin response in aged mice and macaques. | |
| Chelakkot et al (2018)41 | AmEVs | NCD or HFD | HFD induced a diabetic phenotype | 1. NCD and HFD groups treated with PBS for 2 weeks.2. NCD+AmEVs and HFD+AmEVs groups EVs were orally administered for 2 weeks. | 1. NCD (n = 5–7)2. NCD + AmEVs (n = 5–7)3. HFD (n = 5–7)4. HFD + AmEVs (n = 5–7) | AmEVs improved body weight and glucose tolerance in diabetic mice. It also reduced HFD-induced barrier permeability. | |
| Hänninen et al (2018)28 | Live A. muciniphila | / | Non-obese diabetic (NOD) mice | 1. Vehicle control group treated with PBS three times a week for 7 weeks.2. A. muciniphila group treated with 2 × 108 CFU of A. muciniphila three times a week for 7 weeks. | 1. Vehicle control (n = 25)2. A. muciniphila (n = 24) | Oral gavage of female NOD/Jax mice with A. muciniphila delayed diabetes significantly as compared with gavage with vehicle only. | |
| Shin et al (2019)39 | Live A. muciniphila | HFD | HFD induced obese mice | 1. HFD group orally administrated with 25% glycerol in sterile PBS for 4 weeks.2. HFD + AKK mucin (+) group orally administrated with A. muciniphila (1.0 × 108 CFU/day) grown on mucus-based for 4 weeks.3. HFD + AKK mucin (-) group orally administrated with A. muciniphila (1.0 × 108 CFU/day) grown on mucus-depleted medium for 4 weeks. | 1. HFD group (n = 4–5) 2. HFD + AKK mucin (+) group (n = 4–5) 3. HFD + AKK mucin (-) group (n = 4–5) |
Administration of A. muciniphila grown under mucin-depleted conditions to high-fat diet-induced diabetic mice reduced obesity and improved intestinal barrier integrity more efficiently than administration of A. muciniphila grown under mucin-containing conditions. | |
| Ashrafian et al (2019)29 | Live A. muciniphila, AmEVs | ND or HFD | HFD-induced obese mice | 1. ND or HFD-fed mice treated with 200 μl PBS for 5 weeks.2. HFD + Live A. muciniphila and ND + Live A. muciniphila groups treated with 109 CFU/200 μl live A. muciniphila.3. HFD + AmEVs and ND + AmEVs groups treated with 10 μg protein/200 μl AmEVs. | 1. HFD + PBS (HPBS) (n = 5)2. HFD + Live A. muciniphila (n = 5)3. HFD + AmEVs (n = 5)4. ND + PBS (NPBS) (n = 5)5. ND + Live A. muciniphila (n = 5)6. ND + AmEVs (n = 5) | A. muciniphila and AmEVs reduced food intake and body weight gain. They can also alleviate adipose Inflammation, ameliorate HFD-induced intestinal barrier dysfunction, regulate inflammation and energy homeostasis in the colon of obese mice, and regulate gene expression involved in FA oxidation and energy metabolism of adipose tissues. | |
| Everard et al (2019)30 | Live A. muciniphila | ND or HFD | Napepld∆IEC mice fed with ND or HFD | 1. WT ND, WT HFD and Napepld∆IEC HFD group mice treated daily with 150 μl PBS.2. WT ND AKK and Napepld∆IEC HFD AKK groups mice treated daily with an oral gavage of either 2.0 × 108 CFU of Akkermansia muciniphila. | 1. WT ND (n = 8–10)2. WT ND AKK (n = 8–10)3. WT HFD (n = 8–10)4. Napepld∆IEC HFD (n = 8–10)5. Napepld ∆IEC HFD AKK (n = 8–10) | Napepld∆IEC mice are hyperphagic upon first HFD exposure, and develop exacerbated obesity and steatosis. A. muciniphila administration partly counteracts the gene deletion effects. | |
| Van der Lugt et al (2019)31 | Live A. muciniphila | An ad libitum purified diet | Aging Ercc1 −/Δ7 mice | 1. The control group simultaneously received oral gavages PBS. 2. Ercc1−/Δ7 + AKK group mice were supplemented with Akkermansia muciniphila by oral gavage at a dose of 2 × 108 CFU/200 μL, three times a week, for a total of 10 weeks. Oral gavages were given in the morning. | 1. Control group (n = 18)2. Ercc1−/Δ7 + AKK (n = 18) | Supplementation with A. muciniphila prevented the age-related decline in thickness of the colonic mucus layer and attenuated inflammation and immune-related processes at old age. | |
| Wu et al (2020)72 | An Akkermansia muciniphila subtype (A. muciniphilasub) |
NCD or HFD | HFD-induced obesity and diabetes | 1. The mice in the HFD + PBS and NCD + PBS groups were administered the PBS vehicle.2. The mice in the HFD + AKKsub and NCD + AKKsub groups were orally administered AKKsub daily at a dose of 109 CFU/200 μl. | 1. HFD + PBS (n = 10)2. HFD + AKKsub (n = 10)3. NCD + PBS (n = 10)4. NCD + AKKsub (n = 10) | A. muciniphilasub reduced body weight and food consumption, improved blood glucose control, and prevented memory decay but not depression induced by high fat diet. A. muciniphilasub can also decrease systemic inflammation and improve tryptophan metabolism in mice fed HFD, produce high concentrations of acetic acid, propionic acid and isovaleric acid, and restore gut microbiota altered by HFD. | |
| Depommier et al (2020)32 | Pasteurized A. muciniphila | HFD | HFD-induced obesity | 1. ND and HFD groups treated with 180 µl of vehicle solution (PBS containing 2.5% glycerol) for 5 weeks.2. HFD pasteurized A. muciniphila group mice were treated daily with an oral gavage of either 2 × 108 CFU/180 µl of pasteurized A. muciniphila. | 1. ND (n = 7)2. HFD (n = 7)3. HFD pasteurized A. muciniphila (n = 7) | Daily oral administration of pasteurized A. muciniphila alleviated diet-induced obesity and decreased food energy efficiency. This effect was associated with an increase in energy expenditure and spontaneous physical activity. | |
| Huo et al (2020)33 | Live A. muciniphila | ND or HFD | HFD induced obesity | 1. The mice in the HFD group treated with 0.9% saline solution for 9 weeks.2. The mice in the HFD + AKK group treated with 109 CFU/kg per day for 9 weeks. | 1. ND (n = 6)2. HFD (n = 6)3. HFD + AKK (n = 6) | A. muciniphila decreased body weight, relative fat weight, and serum LPS. It can also increase lipid catabolism in epididymal adipose tissues. | |
| Deng et al (2020)42 | A. muciniphila I (Amuc_GP01), A. muciniphila II (Amuc_GP25) | NCD or HFD | HFD induced obese mice | 1. HFD + PBS and NCD + PBS groups mice orally gavaged with 200 µL sterile PBS daily for 16 weeks.2. The remaining groups orally gavaged with A muciniphila (5 × 109 CFU/mL) in 200 µL sterile PBS for 16 weeks. | 1. HFD + A. muciniphila I (n = 10)2. HFD + A. muciniphila II (n = 10)3. HFD + PBS group (n = 10)4. NCD + A. muciniphila I (n = 10)5. NCD + A. muciniphila II (n = 10)6. NCD + PBS group (n = 10) | A. muciniphila I and II exert different impacts on blood glucose and lipid metabolism. Both A. muciniphila I and II could alleviate brown adipose tissue inflammation and whitening induced by HFD, which were regulated much better under A. muciniphila I intervention; A. muciniphila I could alleviate endotoxemia in HFD mice while II could not. | |
| Kim et al (2020)34 | Live A. muciniphila | NCD or HFD (45% fat diet) | HFD induced obesity in mice | 1. The NC + PBS and HFD + PBS groups mice are daily treated with 108 to 109 CFU/ml A. muciniphila by oral gavage for 10 weeks.2. Mice in the ND + PBS and HFD + PBS groups were fed with the same volume of PBS by oral gavage for 10 weeks. | 1. NC + PBS (n = 4)2. NC + AKK (n = 4)3. HFD + PBS (n = 4)4. HFD + AKK (n = 4) | A. muciniphila treatment prevented fatty liver disease in obese mice. | |
| Katiraei et al (2020)35 | Live A. muciniphila | Atherogenic Western‐type diet containing 1% cholesterol and 0.05% cholate | Hyperlipidemic APOE*3‐Leiden (E3L). CETP mice | 1. The AKK group mice are daily treated with 2 × 108 CFU A. muciniphila by oral gavage for 4 weeks.2. The Control mice orally gavaged daily for 4 weeks with anaerobic PBS. | 1. Control (n = 8)2. AKK (n = 8) | A. muciniphila administration decreased body weight as well as plasma TC and TG levels. | |
| Yoon et al (2021)36 | P9 | HFD | HFD induced obese mice | 1. HFD group mice treated with 200 μl anaerobic PBS.2. HFD + Am group mice treated with 4.0 × 108 CFU/200 μl A. muciniphila.3. HFD + P9 group mice treated with 100 μg per mouse for 8 weeks. | 1. HFD (n = 7–10)2. HFD + A. muciniphila (Am) (n = 7–10)3. HFD + P9 (n = 7) | A. muciniphila increases thermogenesis and glucagon-like peptide-1 (GLP-1) secretion in HFD-induced C57BL/6 J mice by induction of uncoupling protein 1 in brown adipose tissue and systemic GLP-1 secretion. Purified P9 alone is sufficient to induce GLP-1 secretion and brown adipose tissue thermogenesis. | |
| Human | |||||||
| Depommier et al (2019)37 | Live A. muciniphila, pasteurized A. muciniphila | / | Overweight/obese insulin resistant | 1. Placebo group received placebo per day for 3 months. 2. Alive group received 1010 CFU alive A. muciniphila per day for 3 months.3. Pasteurized group received 1010 pasteurized A. muciniphila per day for 3 months. | 1. Placebo (n = 11)2. Pasteurized (n = 12)3. Alive (n = 9) | The supplemention of A. muciniphila was safe and well-tolerated, it can reduce the levels of relevant blood markers of liver dysfunction and inflammation while the overall gut microbiome structure was unaffected. | |
| Perraudeau et al (2021)38 | Live A. muciniphila | / | T2DM | 1. Placebo group received colloidal silicon dioxide for 12 weeks.2. WBF-010 group received WBF-010 (which contains inulin, Clostridium beijerinckii, Clostridium butyricum and Bifidobacterium infantis).3. WBF-011 group received WBF-011 (which contains inulin, Akkermansia muciniphila, Clostridium beijerinckii, Clostridium butyricum, Bifidobacterium infantis and Anaerobutyricum hallii). | 1. Placebo (n = 26)2. WBF-010 (n = 27)3. WBF-011 (n = 23) | Compared with placebo, a statistically significant decrease in total glucose AUC0-180 min was observed in WBF-011 group. Incremental glucose AUC0-180 min was also lower in WBF-011 group. The validated measure of long-term glucose control, A1c, was reduced by 0.6 in WBF-011 group when compared with placebo. | |
2.2. A. muciniphila and T2DM
T2DM is a common metabolic disease, which is genetic susceptible and obesity-oriented.73–75 In addition to well-recognized genetic and environmental risk factors, gut dysbiosis has emerged as a new risk factor for T2DM development,2,76 in which the decreased abundance of A. muciniphila was frequently observed in either diabetic mice,19 or patients with pre-diabetes or T2DM.77 Our previous study demonstrated that administration of A. muciniphila reduced the fasting blood glucose level in western diet-fed mice, suggesting that A. muciniphila contributes to T2DM recovery.26 Moreover, a series of studies have shown that A. muciniphila supplementation may regulate host lipoprotein metabolism, improve insulin sensitivity, and alleviate hepatic metabolic inflammation in mice.23,29,42 A recent clinical trial of a new probiotic formulation WBF-011, which contains A. muciniphila and another four bacterial strains as well as inulin, found that WBF-011 improved postprandial blood glucose in T2DM patients.38 This is the first randomized controlled trial to show the effect of A. muciniphila on improving T2DM in human subjects. In addition, the baseline abundance of A. muciniphila also affected the metabolic outcomes of calorie restriction: individuals with higher baseline A. muciniphila showed better responses toward calorie restriction than those who had low baseline A. muciniphila.78
Interestingly, the alteration of A. muciniphila was also found to be involved in the anti-T2DM effect of metformin, a widely used first-line medicine for T2DM.79 Shin et al. reported that metformin significantly increased the abundance of A. muciniphila in HFD-fed mice, while oral supplementation of A. muciniphila to HFD-fed mice without metformin also improved glucose tolerance and reduced inflammation in adipose tissue.22 Cuesta-Zuluaga et al. found higher abundance of A. muciniphila in T2DM patients with metformin therapy than healthy subjects (Table 3).80 These results suggest that the elevated A. muciniphila contributes to the anti-T2DM effect of metformin, providing new understanding on the role of A. muciniphila. Overall, existing evidence highlights the significance of A. muciniphila in T2DM development, as well as its involvement in the anti-T2DM activity of clinical medicines.
Table 3.
The interventions aiming at improving metabolic disorders in humans accompanied by the increase of A. muciniphila.
| Ref. | Disease condition | Intervention | Intervention period | Diet | Study group | Sample type | Sample detection | Beneficial changes achieved |
|---|---|---|---|---|---|---|---|---|
| Obesity | ||||||||
| Kim et al (2014)164 |
Obesity | Ephedra sinica (Ma Huang) | 8 weeks | Subjects maintain usual daily diet, limiting caloric intake to 20–25 kcal/kg, according to subject’s weight. | Subjects should be obese (BMI ≥ 25 kg/m2) and female between the ages of 40 and 65 (n = 7). | Feces | 16S rRNA sequencing | Body weights, body mass index, and body fat percentage of subjects were reduced after intake Ephedra sinica. Negative correlation of Akkermansia with waist circumference, body weight, and BMI indicates an association of Akkermansia genus with weight loss. |
| Dao et al (2016)78 | Overweight or obesity | Calorie restriction 112(CR) | 12 weeks | CR diet enriched with fibers and protein. | 1. AKK LO (n = 24)2. AKK HI (n = 25) | Feces | qPCR | The Akk HI group remained metabolically healthier throughout the CR intervention when compared with AKK LO. While there was a decrease in A. muciniphila abundance in the Akk HI group after CR and the total intervention period, it remained consistently and significantly higher than the Akk LO group. |
| Palleja et al (2016)165 | Obesity | Roux-en-Y gastric bypass (RYGB) | / | / | 13 morbidly obese patients who underwent RYGB | Feces, before RYGB (n = 13) and 3 months (n = 12) and 12 months (n = 8) after RYGB | Metagenomic sequencing | In parallel with the weight loss and metabolic improvements, the abundance of A. muciniphila increased within the first 3 months after RYGB and remained high 1 year later. |
| Payahoo et al (2019)166 | Obesity | Oleoylethanolamide (OEA) supplementation | 8 weeks | 1. The placebo group who received two capsules containing 125 mg of starch daily similar to the intervention group.2. The OEA group who received 125 mg of OEA daily before lunch and dinner meals. | 1. Placebo group (n = 30)2. OEA group (n = 30) | Feces | qPCR | After eight weeks of OEA supplementation, the abundance of A. muciniphila bacterium increased significantly for OEA group compared to placebo group. |
| Pedret et al (2019)167 | Abdominal obesity | 1. Placebo group received 300 mg of maltodextrin.2. Ba8145 group received 100 mg of the live strain, 1010 CFU/capsule containing maltodextrin 200 mg.3. H-k Ba8145 group received 100 mg of heat-killed CECT 8145 strain at a concentration of 1010 CFU before the heat treatment/capsule containing maltodextrin 200 mg. | 3 months | Dietary recommendations were provided according to guidelines of the 2013 Adult Treatment Panel (ATP III). Diet and physical activity were similar among groups, but fiber intake was greater in the h-k Ba8145 group versus the placebo group. | 1. Placebo group (n = 40)2. Ba8145 group (n = 42)3. H-k Ba8145 group (n = 44) | Feces | Metagenomic sequencing | Ba8145 decreased the body mass index compared with baseline and placebo group. The decrease in visceral fat area after Ba8145 treatments reached significance only after h-k Ba8145. Ba8145 treatments also increased the incidence of Akkermansia. Consistent with this fact, the maximum increase in Akkermansia spp. was observed after the Ba8145 live-form administration, when the maximum decrease in BMI occurred. Also, Akkermansia content was 1.8% lower in participants over 90 kg after Ba8145 treatments. |
| Zhang et al (2019)168 | Normal body weight | Resistant starch (RS) | 4 weeks | HAM-RS2 (Ingredion Inc., Bridgewater, NJ, USA) at 255.4 kcal/day (2.8 kcal/g, 91.2 g, containing 40 g of RS) | 1. Resistant starch (n = 19 2. Control starch (n = 19) | Feces | 16S rRNA sequencing | RS was found to reduce abdominal adiposity in normal-weight subjects. Intra-abdominal visceral and abdominal subcutaneous fat were significantly reduced by taking RS at 40 g/d in the 4-week study. Moreover, an increasing trend of abundance of Akkermania was observed after RS intake compared to that at baseline. |
| Dong et al (2020)169 | Obesity | A dietary intervention trial of overweight and obese subjects who were randomized to a calorie-restricted high protein diet (HPD) (30% calorie intake) or calorie-restricted normal protein diet (NPD) (15%) for 8 weeks. | 8 weeks | An HPD (30% protein, 40% carbohydrate, 30% fat by calorie intake) or an NPD (15% protein, 55% carbohydrate, 30% fat) | 1. HPD (n = 31)2. NPD (n = 29) | Feces, at baseline, week 1, week 2, week 4, week 6, and week 8 | 16S rRNA sequencing | At the end of 8 weeks, the HPD lost more weight on average than the NPD group, though the results were not statistically significant. The three genera with the highest relative abundance were Akkermansia, Bifidobacterium, and Prevotella_9. Akkermansia spp. and Bifidobacterium spp. were elevated at 8-weeks as compared to baseline. |
| T2DM | ||||||||
| Cortez et al (2018)170 | T2DM | Duodenal-jejunal bypass surgery with minimal gastric resection | 12 months | The diet was formulated using Total Energy Expenditure data according to the Cunningham Equation. | 1. Duodenal-jejunal bypass surgery with minimal gastric resection (DJBm) (n = 11)2. Standard care group (n = 11) | Feces, before the operation and after 6 and 12 months (DJBm group), at baseline and after 6 and 12 months (Standard care group) | 16S rRNA sequencing | The abundance of Akkermansia was increased in duodenal-jejunal bypass surgery with minimal gastric resection group compared with that in the standard care group. |
| Wu et al (2017)79 | T2DM | Metformin | 4 months | A calorie-restricted diet | 1. Placebo group (n = 18)2. Metformin group (n = 22) | Feces | Metagenomic sequencing | Metformin treatment promotes the growth of A. muciniphila in vitro. The abundance of A. muciniphila was increased in individuals who received metformin for 4 months. |
| Cuesta-Zuluaga et al (2017)80 | T2DM | Metformin | / | Dietary intake was evaluated through 24-h dietary recalls. | 1. T2DM using metformin (T2D-met+) (n = 14)2. T2DM not using metformin (T2D-met−) (n = 14) | Feces | 16S rRNA sequencing | Compared with participants without diabetes, participants with diabetes taking metformin had higher relative abundance of A. muciniphila. |
| Wang et al (2018)158 | T2DM | Liraglutide | 42 days | Subjects were on the diet recommended by their primary care physician. | 1. Metformin (n = 18)2. Liraglutide (n = 19) | Feces | 16S rRNA sequencing | At baseline, the genus Akkermansia showed a significant increase in liraglutide relative to metformin subjects. At Day 42, when controlling for placebo treatments in the statistical model, a significant increase in Akkermansia were observed in liraglutide relative to metformin subjects. |
| Guevara-Cruz et al (2019)171 | Metabolic syndrome (MetS) |
A lifestyle intervention with functional foods and energy reduction (−500 kcal) for 75 days | 75 days | A low‐saturated‐fat diet, reduced‐energy intake, with functional foods | 1. Class III obesity (OCIII)+MetS+functional foods (FF) (n = 18)2. Class III obesity (OCIII)+MetS+placebo (P) (n = 17) | Feces | 16S rRNA sequencing | The level of A. muciniphila was increased after intervened with FF. |
| Shin et al (2020)172 | T2DM | Scutellaria baicalensis (SB) combined with metformin | 20 weeks | / | 1. SB (n = 6)2. Placebo (n = 6) | Feces | 16s rRNA sequencing | SB with metformin treatment may improve the glucose tolerance and inflammation and influence the gut microbiota community in T2DM, the level of Akkermansia remarkable increased after SB treatment. |
2.3. A. muciniphila and CVD
CVD remains the leading cause of death worldwide, especially in western countries.81,82 The relationship between gut microbiota dysbiosis and CVD has been well determined.83,84 Dietary phosphatidylcholine or L-carnitine can be metabolized into trimethylamine (TMA) by the gut microbiota,85–87 and then transported to the liver, where TMA is converted into trimethylamine N-oxide (TMAO) by hepatic flavin monooxygenase 3 (FMO3).88–90 TMAO has been shown to be a potent trigger and biomarker for CVD.91,92 Recently, Plovier et al. reported that supplementation with A. muciniphila significantly increased the excretion of TMAO and TMA in urine, resulting in decreased plasma TMAO and TMA levels.21 In addition, they found that HFD induced two-fold higher FMO3 expression compared with that in control-diet fed mice, whereas treatment with pasteurized A. muciniphila could offset this change, suggesting pasteurized A. muciniphila intervention may also reduce TMAO production.21 Li et al. discovered that oral administration of live A. muciniphila reduced exacerbation of atherosclerotic lesion formation, as well as aortic and systemic inflammation induced by a western diet, and improved intestinal integrity in antherosclerotic Apoe−/− mice.20 These evidences indicate that A. muciniphila, live or pasteurized, has a protective effect against CVD development.
In addition to the co-metabolized TMA/TMAO pathway by host and gut microbiota, short-chain fatty acids (SCFAs), which can be generated by A. muciniphila, are also essential metabolites for bridging the crosstalk between A. muciniphila and host.93,94 The beneficial effects of SCFAs on host metabolism have been extensively investigated and reviewed in CVD.95,96 In summary, A. muciniphila may play a protective role in CVD development directly or through producing metabolites, and via crosstalk with host and commensal bacteria as well.
2.4. A. muciniphila and NAFLD
NAFLD is a chronic liver disease and hepatic manifestation of metabolic syndrome. The homeostasis of commensal bacteria and bacteria-derived molecules have been increasingly recognized as a key determinant of NAFLD.6 The association of A. muciniphila with NAFLD development was recently investigated in obese mice with NAFLD, in which a decreased abundance of A. muciniphila was observed.97 The administration of anti-obesity drug, such as liraglutide, decreased the levels of total cholesterol and triacylglycerol in the liver while increasing the abundance of A. muciniphila.98 A. muciniphila supplementation also decreased the levels of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST), and alleviated liver histopathological damage in a mouse model .99 Kim et al. recently reported that oral administration of A. muciniphila prevented fatty liver disease by regulating the expression of genes that regulate fat synthesis and inflammation in the liver.34 Moreover, different genotypes of A. muciniphila, isolated from human stool samples, played different roles in HFD-induced hyperlipidemia and liver steatosis. Specifically, A. muciniphila I (Amuc_GP01, strain GP01 of A. muciniphila I) was more effective for alleviating hyperlipidemia, liver steatosis, and glucose tolerance than A. muciniphila II (Amuc_GP25, strain GP25 of A. muciniphila II) in dietary obese mice. Both two genotypes could improve the intestinal barrier, but the effect of A. muciniphila II on improving endotoxemia was not apparent, possibly because they have different characteristics of genes and functions, leading to the identification of specific target pathways and disparate roles.42 Overall, these results indicate that A. muciniphila may alleviate NAFLD by regulating lipid metabolism and reducing inflammation.
3. Mechanisms of A. muciniphila action on metabolic diseases
3.1. Production of SCFAs and cross-feeding with butyrate-producing bacteria
SCFAs, mainly acetate, propionate, and butyrate, are the principal products of carbohydrate and protein fermentation by gut microbiota.100 There are a large number of investigations on the diverse roles of SCFAs in host metabolism.101,102 A. muciniphila is also a potent generator of acetate, propionate, and oligosaccharides by fermenting mucin,13,18 resulting in the activation of fatty acid receptors FFAR2/GPR41 and FFAR3/GPR43.103 Interestingly, GPR41 is involved in the microbiota-associated adiposity process, as regularly raised Gpr41−/− mice are leaner than wild-type mice, whereas this difference is not observed under germ-free (GF) conditions.104 Activation of GPR41 and GPR43 induces intestinal L cells to produce peptide YY (PYY), glucagon-like peptide-1 (GLP-1), and glucagon-like peptide-2 (GLP-2).105–107 PYY acts on the gastrointestinal tract by modulating a series of physiological actions. It is a satiety signal released following meals, and decreasing food intake.108 GLP-1, one of the principal incretin hormones, promotes glucose-dependent insulinotropic activity, inhibits appetite and food intake, delays gastric emptying, and restores the impaired “incretin effect” in T2DM patients.108–110 Acetate could also promote anti-lipolytic activity through GPR43 in white adipose tissue (WAT).111 GPR43 stimulation by acetate in the WAT, rather than muscle or liver, also improves glucose and lipid metabolism.112 Propionate can be converted into glucose by intestinal gluconeogenesis (IGN), resulting in satiety and reduced hepatic glucose production.113 In addition, Lukovac et al. found that many transcription factors regulating lipid metabolism and proliferation, such as Hnf4α and p53 family members (Tp53 and Tp73), were affected by both A. muciniphila and propionate.114
The biological function of A. muciniphila is also associated with cross-feeding activity with other butyrate-producing bacteria such as Faecalibacterium prausnitzii and Anaerostipes caccae, resulting in the increased production of butyrate.115,116 Moreover, acetate can also stimulate the growth of butyrate-producing bacteria within the same mucosal niche.117 Butyrate is not only a preferred energy source for colon cells,118 it also has various beneficial functions for the host, especially in metabolic diseases,119–121 and is a more potent agonist for GPR41 than acetate or propionate.103
Overall, considering the facts that pasteurization of A. muciniphila enhanced its capacity to improve body weight, reduce fat mass development and dyslipidemia,21 and administration of Amuc_1100, AmEVs, and secreted P9 protein replicated part of the biological functions of live bacteria (Table 2), the beneficial effects of A. muciniphila might depend in part on its capacity of SCFAs production, as well as the cross-feeding relationship with other butyrate-producing bacteria in the gut.
3.2. Maintaining the integrity of gut barrier
A number of studies have revealed the correlation between obesity-related metabolic diseases and increased gut permeability, which induces metabolic endotoxemia and inflammation.122–125 A. muciniphila can considerably improve gut barrier integrity in obese mice by restoring the thickness of the intestinal mucus layer,19 and oral supplementation of A. muciniphila can increase the number of goblet cells, normalize the mucus thickness of the inner layer, and increase the expression of tight-junction proteins in the gut of both HFD-induced obese mice and mice with alcoholic fatty liver.19,41,126 Moreover, Li et al. discovered that A. muciniphila reduced intestinal permeability by increasing the expression of occludin and ZO-1 in Apoe−/− mice.20 Similarly, Zhao et al. reported that administration of A. muciniphila could reduce chronic low-grade inflammation by decreasing the permeability of the gut and lipopolysaccharide (LPS)-binding protein (LBP) downstream signaling in the liver and muscle.24 Reunanen et al. found that A. muciniphila could adhere to the intestinal epithelium and enhance enterocyte monolayer integrity in vitro, suggesting the ability of A. muciniphila to repair the damaged gut barrier.14 Furthermore, a large number of studies have shown that bacteria-derived SCFAs maintain the integrity of the intestinal tract and prevent the translocation of LPS across the intestinal wall to alleviate the systemic inflammatory response.63,127,128 Not only live A. muciniphila, but also pasteurized A. muciniphila has been found to enhance the gut barrier function, leading to attenuation of metabolic endotoxemia.21 In addition, Amuc_1100 elevated the development of transepithelial electrical resistance in Caco2-cells and increased the expression of tight junction genes in HFD-fed mice to improve intestinal barrier function.21,129 Moreover, administration of AmEVs also protected mice from HFD-induced leaky gut (Table 2).29,41
In addition, gut barrier function is disrupted in inflammatory bowel disease (IBD). As a mucin-degrader, A. muciniphila decreased significantly in dextran sulfate sodium (DSS)-induced colitis in mice and in IBD patients,130,131 while administration of A. muciniphila or AmEVs have been reported to protect the progression of DSS-induced colitis.132–135 On the contrary, the abundance of A. muciniphila was found to be increased in the spontaneous colitis in the Il10−/− mice model of IBD, while supplementation of A. muciniphila further promoted colitis in this model.136 However, it should be noticed that these findings in immune compromised or genetic editing mouse models cannot be translated into the human situation directly. Although the role of A. muciniphila in colitis is somewhat contradictory based on these reports, most studies supported the potential benefits of A. muciniphila or its derived metabolites in respect to their functions of reducing metabolic endotoxemia and systemic inflammation of host, or improving the integrity of gut barrier. Further studies are also needed to determine the exact role of A. muciniphila, live or pasteurized, or its metabolites, in colitis.
4. Active components of A. muciniphila on metabolic diseases
4.1. Pasteurized A. muciniphila in metabolic disease
Although the beneficial effects of A. muciniphila on metabolic diseases have been extensively investigated,19,20,78 their clinical application is still challenging, owing to its microaerophilic requirements and the loss of activity after heat-killing.19 Meanwhile, its growth media probably contain animal-derived compounds, which may have viruses, allergens or bacterial contaminants, thus limiting the usage for clinical study. Plovier et al. showed that A. muciniphila retained its efficacy in improving metabolic disorders when grown on a synthetic medium, a replacement for animal derived mucins.21 Ottman et al. identified 79 putative outer membrane and membrane-associated extracellular proteins and 23 of those had different abundance between cells of A. muciniphila grown on mucin-containing media and those grown on the non-mucus glucose-containing media.137 Moreover, Shin et al. found that A. muciniphila grown under mucin-containing media upregulated genes encoding mucin-degrading enzymes. In contrast, A. muciniphila grown under mucin-depleted conditions upregulated the genes involved in glycolysis, energy metabolic pathways, and 79 genes encoding extracellular protein candidates including Amuc_1100, which, in turn, reduced obesity and improved intestinal barrier more efficiently than administration of A. muciniphila grown under mucin-containing conditions.39 These findings by different teams suggest mucin in the medium might affect the expression of outer membrane protein and subsequently influence the function of A. muciniphila. Interestingly, the recent study discovered that pasteurized A. muciniphila was more potent than live A. muciniphila for reducing body weight and improving glucose tolerance in HFD-induced obese mice.21 This is of great significance for clinical applications, and therefore, increased attention has been paid to the effect of pasteurized A. muciniphila on metabolic diseases in recent years. Zhang et al. found that oral administration of live or pasteurized A. muciniphila significantly increased the levels of plasma high-density lipoprotein (HDL) and decrease hepatic glycogen, as well as reduced inflammatory markers of LPS and TNF-α to alleviate systemic inflammation. However, oral administration of live or pasteurized A. muciniphila did not improve glucose levels in diabetic rats.138 Depommier et al. reported that daily oral supplementation of pasteurized A. muciniphila in a small number of subjects improved insulin sensitivity and decreased insulinemia and plasma total cholesterol compared to the placebo group, and the effects of pasteurized A. muciniphila were better than those of live A. muciniphila.37 Although this study only included a small number of participants, the results highlight the potential of pasteurized A. muciniphila in clinical applications. It is hypothesized that the effects of pasteurized A. muciniphila are attributed to increased energy excretion in feces, reduced carbohydrate absorption, and enhanced intestinal epithelial turnover, but without impacts on intestinal lipid absorption or chylomicron synthesis.32 In conclusion, these studies demonstrate that pasteurized A. muciniphila is superior to live ones for improving metabolic disorders in mice, rats, and humans; however, the underlying mechanism warrants further investigation.
4.2. A. muciniphila outer membrane protein enhances the gut barrier
In addition to the A. muciniphila itself, the identification of active components of A. muciniphila for the treatment of metabolic diseases is also valued recently. Cell derived fragments of A. muciniphila have been shown to activate Toll-like receptor 2 (TLR2), in which a highly abundant outer membrane pili-like protein of A. muciniphila, named Amuc_1100, with specific activating capacity for TLR2 has been identified by proteomics. TLRs regulate bacterial recognition, intestinal homeostasis, and shape host metabolism.129 Ottman et al. found that Amuc_1100 activated TLR 2 and TLR4 and significantly increased transepithelial electrical resistance in vitro.129 In line with the enhanced effects of pasteurized A. muciniphila, Amuc_1100 was found to be active after pasteurization.21 It has also been found that the expression of Cnr1, which codes cannabinoid receptor 1 (CB1) in the jejunum, was lower in Amuc_1100 treated mice.21 The downregulation of CB1 was associated with improved gut integrity and lipid accumulation induced by LPS in both liver and adipose tissue.139 Therefore, Amuc_1100 might contribute in part to the beneficial effect of live or pasteurized A. muciniphila on gut barrier function.
In addition to Amuc_1100, several other proteins of A. muciniphila have also been identified including Amuc_1434, Amuc_1686, Amuc_0771, and Amuc_1666. Meng et al. reported that Amuc_1434, a member of the aspartic protease family,140 degraded mucin2 protein secreted by LS174T and suppresses LS174T cell viability,141 suggesting its potential involvement in controlling colon cancer. Nevertheless, the roles of some β-galactosidases with mucin degradation capacity, such as Amuc_1686, Amuc_0771, and Amuc_1666, in regulating metabolic disorders remain unclear so far.142,143
4.3. A. muciniphila-derived extracellular vesicles (AmEVs) improve metabolic disorders
Emerging evidence shows that bacteria-derived extracellular vesicles, especially AmEVs, play important roles in mediating host-bacteria interactions.41,144,145 Chelakkot et al. analyzed the fecal extracellular vesicles of healthy people and individuals with obesity, and discovered that the feces of healthy individuals contained higher levels of AmEVs than individuals with obesity. They also revealed that oral gavage of AmEVs decreased HFD-induced body weight gain and fat mass, and improved metabolic functions and gut integrity.41 Ashrafian et al. also found that AmEVs ameliorated intestinal barrier impairment in obese mice.29 Moreover, AmEVs regulated inflammation and energy homeostasis in the colon of obese mice. Compared with A. muciniphila, oral gavage of AmEVs (10 μg/mouse) alleviated more body and fat weight gain as well as blood glucose and cholesterol levels in HFD-induced obese mice. In addition, AmEVs administration significantly reduced the expression of TLR-4 and induced lower TLR-2 expression in the colon tissue of obese mice.29 It is, however, improtant to note that AmEVs administration reducd daily food intake in this study. Additionally, the dose of AmEVs derived from how many A. muciniphila is unclear, and the relevant of the oral dose of AmEVs to physiological levels of AmEVs secreted by A. muciniphila in the gut is also unknown. Thus, the rationale for the dose used for AmEVs administration need to be further explored. Moreover, whether AmEVs contain Amuc_1100 or other effectors need further investigation. It has been reported that oral administration of AmEVs alleviated DSS-induced inflammatory bowel disease, characterized by reduced infiltration of inflammatory cells through the colon wall.132 Overall, these results suggest that AmEVs may protect the host by decreasing intestinal permeability and reducing inflammation in the gut. Therefore, the beneficial effects of the live bacteria may be, at least partly, due to AmEVs.
4.4. A. muciniphila-secreted protein ameliorates metabolic disease
Since administration of either the cell-free supernatantlive or live A. muciniphila, but not bacterial pellet, increased systemic GLP-1 secretion, Yoon et al. identified an 84 kDa protein in the culture supernatant, named P9, which accounts for the induction of GLP-1 secretion in HFD-fed mice and L cells.36 Administration of P9 to HFD-fed mice prevented obesity and improved glucose tolerance by regulating GLP-1 secretion and inducing brown adipose tissue thermogenesis. In terms of mechanism, ICAM-2 can bind to P9 and modulate P9-induced secretion of GLP-1. In addition, P9 strongly induced IL-6 expression and IL-6 dose-dependently increased GLP-1 secretion, wheares IL-6 deficiency downregulated the expression of ICAM-2 and blocked the response toward P9-induced GLP-1 secretion in mice, demostrating that P9 may improve metabolic diseases through an IL-6-GLP-1 signaling axis.36 Since pasteurized A. muciniphila and Amuc_1100 also have beneficial effects on regulating blood glucose,21,32 P9 is not the only way for this bacterium to regulate glucose homeostasis. Cani and Knauf commented on this research and raised several important questions, such as how P9 acts on L cells to stimulate GLP-1 secretion and whether P9 is specific to A. muciniphila, etc.146 Further, in addtion to stimulating GLP-1 secretion, whether P9-mediated induction of IL-6 promotes inflammtaion in the gut and how it affects gut barrier function need to be illustrated.
5. Conclusions and perspectives
The current focus on improving health using gut microbiota-targeted strategies is overwhelming in the context of accumulating experimental and clinical evidence. A. muciniphila has emerged as a uniquely promising “next-generation beneficial microbe”, especially for metabolic disease management. A large number of studies have confirmed the alteration of A. muciniphila in both animal models and human patients with metabolic diseases (summarized in Table 1), its therapeutic benefits (summarized in Table 2), as well as the efficiency of interventions to boost its abundance (summarized in Table 3). However, most current animal studies with A. muciniphila supplementation were performed with A. muciniphila grown under mucin-containing conditions. The animal-derived mucin may introduce contaminants and cause compromised beneficial effect of A. muciniphila on alleviating metabolic diseases. Therefore, mucin-depleted media should be explored and given more attention for both animal and human investigations. Notably, most of the mechanistic studies on the effects of A. muciniphila were performed in animal models. Given the differences between animal models and humans in genetic and environmental elements, it is critical to investigate the real effects and mechanisms of A. muciniphila in clinical study. Recently, two randomized controlled trials confirmed that administration of A. muciniphila or A. muciniphila containing formulation WBF-011 to human subjects with obesity or T2DM were safe and well tolerated in a 12-week period with significant improvement in several metabolic paramaters.37,38 This paves the way for more clinical applications of A. muciniphila in the near future. The mechanisms underlying the effects of A. muciniphila on metabolic diseases have been extensively investigated and are summarized in Figure 1.
Figure 1.
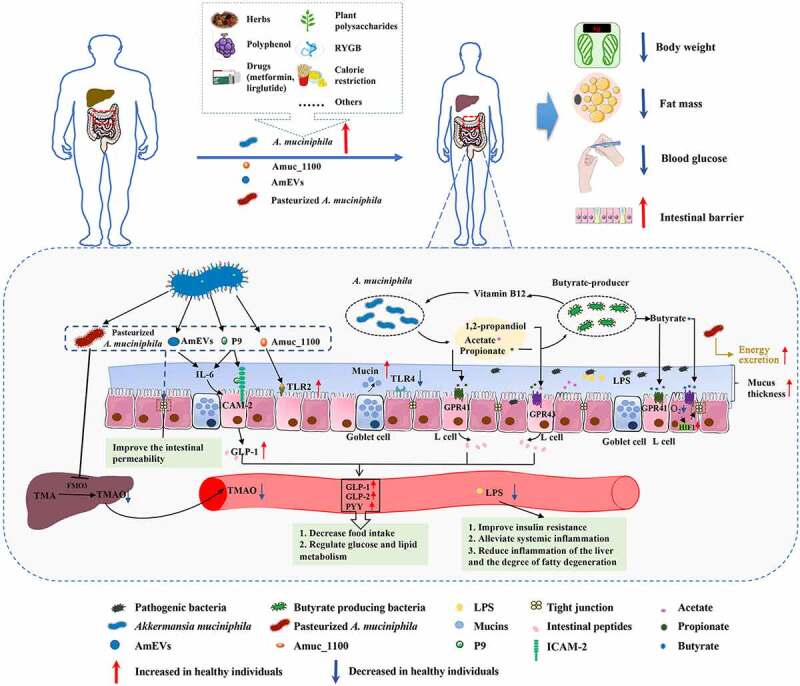
Effects of Akkermansia muciniphila and its derived parts on ameliorating metabolic disorders. The level of A. muciniphila decreased in several metabolic diseases, including obesity, type 2 diabetes mellitus (T2DM), cardiovascular diseases (CVD), and nonalcoholic fatty liver disease (NAFLD). Many interventions based on the diet and surgery have been reported for improving the human health in context of metabolic disorder, which accompanied by the increase of A. muciniphila. A. muciniphila and its different parts, including live or pasteurized A. muciniphila, Amuc_1100, P9, as well as AmEVs, have shown to reduce body weight and fat mass gain, and regulate glucose homeostasis and intestinal barrier. Mechanistically, A. muciniphila and its different parts have shown to improve the intestinal barrier through up-regulating the expression of tight-junction proteins and reducing the leakage of LPS, thus reducing inflammation. In addition, live A. muciniphila produces acetate, propionate, and 1,2-propandiol through the fermentation of mucin. It has a nutritional interaction with butyrate-producing bacteria to stimulate the production of butyrate. These SCFAs can active GPR41 and GPR43 to affect glucose and lipid metabolism. The activation of GPR41 and GPR43 induces the intestinal L cells producing peptide YY (PYY), glucagon-like peptide-1 (GLP-1), and glucagon-like peptide-2 (GLP-2) to decrease food intake. Butyrate promotes the epithelial barrier function by increasing the expression of hypoxia-inducible factor-1α (HIF-1α). Moreover, A. muciniphila, live or pasteurized, can normalize the mucus thickness and increase the number of goblet cells. Pasteurized A. muciniphila specifically decreases the expression of hepatic flavin monooxygenase 3 (FMO3), increases the excretion of TMAO and TMA in urine, and decreases the level of plasma TMAO. It may also increase fecal energy excretion to reduce obesity. Amuc_1100 can act on TLR2 to regulate intestinal homeostasis. Furthermore, the newly identified secreted protein P9 can bind to ICAM-2 to trigger the secretion of GLP-1 by L cells. Both P9 and AmEVs can simulate IL-6, leading to further secretion of GLP-1. The above mechanism was summarized based on existing articles; however, there may be other mechanisms
Given the similar, or even superior efficacy of pasteurized A. muciniphila and its outer membrane proteins such as Amuc_1100 or extracellular vesicles and secreted proteins, the exact mechanisms of A. muciniphila activity in the real world of complicated commensal systems is only beginning to be discovered. In this sense, we envisage several critical aspects for future studies on A. muciniphila. First, a complete understanding is required with regards to the common or differential mechanisms between live A. muciniphila and its derived products, including pasteurized A. muciniphila, or the active components such as proteins, vesicles, or metabolites released from the bacteria. Since it is unclear the equivalence of the doses used for AmEVs, P9, and other effectors with the physiological levels of A. muciniphila found in the gut, the physiological relevance of exact mechanisms of A. muciniphila activity need further exploration. Second, more efforts should be focused on elucidating the complex crosstalk between A. muciniphila and commensal bacteria, which may help to explain the discrepant results that have been observed in preclinical and clinical studies. Third, a deeper exploration of the relationship between the specificity in various conditions and the strains of A. muciniphila, rather than at the species level. Finally, scientists should always hold a reasonable dose of expectation and skepticism in terms of the overwhelming “good effects” of any potential beneficial microbe, including A. muciniphila, if the scientific basis has not been well established. Altogether, given the accumulating evidence of A. muciniphila on ameliorating metabolic disorders in both animals and humans, A. muciniphila is widely supposed to be one of the most promising microbes with multiple benefits for host metabolism. Although the mechanisms underlying the effects of A. muciniphila are largely unclear, identification and isolation of specific effectors and biomolecules that derived from A. muciniphila will pave the way for understanding the mechanisms of their action, which is essential and full of challenges for translation of the positive findings in animals to clinic application.
Funding Statement
This work has received grant/research support from the National Natural Science Foundation of China (No. 81873059), Natural Science Foundation of Shanghai (No. 20ZR1453900), Project of Traditional Chinese Medicine of Shanghai Municipal Health Commission (No. 2020JP016), Shanghai Pujiang Program (No. 20PJ1413100), and Clinical Research Plan of SHDC (No. SHDC2020CR2049B).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author Contributions
Juan Yan retrieved all the references and drafted the manuscript, and Lili Sheng and Houkai Li designed and revised the manuscript.
References
- 1.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ.. Prevalence of the metabolic syndrome in the United States, 2003-2012. Jama. 2015;313(19):1973–30. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- 2.Adeva-Andany MM, Rañal-Muíño E, Vila-Altesor M, Fernández-Fernández C, Funcasta-Calderón R, Castro-Quintela E.. Dietary habits contribute to define the risk of type 2 diabetes in humans. Clin Nutr ESPEN. 2019:34:8-17. doi: 10.1016/j.clnesp.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19(1):55–71. doi: 10.1038/s41579-020-0433-9. [DOI] [PubMed] [Google Scholar]
- 4.Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375:2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 5.Saad MJ, Santos A, Prada PO. Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology (Bethesda, Md). 2016;31:283–293. doi: 10.1152/physiol.00041.2015. [DOI] [PubMed] [Google Scholar]
- 6.Aron-Wisnewsky J, Vigliotti C, Witjes J, Le P, Holleboom AG, Verheij J, Nieuwdorp M, Clément K. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol. 2020;17(5):279–297. doi: 10.1038/s41575-020-0269-9. [DOI] [PubMed] [Google Scholar]
- 7.Angelakis E, Merhej V, Raoult D. Related actions of probiotics and antibiotics on gut microbiota and weight modification. Lancet Infect Dis. 2013;13:889–899. doi: 10.1016/s1473-3099(13)70179-8. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez J, Hiel S, Neyrinck AM, Le Roy T, Pötgens SA, Leyrolle Q, Pachikian BD, Gianfrancesco MA, Cani PD, Paquot N, et al. Discovery of the gut microbial signature driving the efficacy of prebiotic intervention in obese patients. Gut. 2020;69(11):1975–1987. doi: 10.1136/gutjnl-2019-319726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicolucci AC, Hume MP, Martínez I, Mayengbam S, Walter J, Reimer RA. Prebiotics reduce body fat and alter intestinal microbiota in children who are overweight or with obesity. Gastroenterology. 2017;153:711–722. doi: 10.1053/j.gastro.2017.05.055. [DOI] [PubMed] [Google Scholar]
- 10.Hong Y, Li B, Zheng N, Wu G, Ma J, Tao X, Chen L, Zhong J, Sheng L, Li H, et al. Integrated metagenomic and metabolomic analyses of the effect of astragalus polysaccharides on alleviating high-fat diet-induced metabolic disorders. Front Pharmacol. 2020;11:833. doi: 10.3389/fphar.2020.00833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu TR, Lin CS, Chang CJ, Lin TL, Martel J, Ko YF, Ojcius DM, Lu -C-C, Young JD, Lai H-C, et al. Gut commensal parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from hirsutella sinensis. Gut. 2019;68:248–262. doi: 10.1136/gutjnl-2017-315458. [DOI] [PubMed] [Google Scholar]
- 12.Smits LP, Bouter KE, de Vos WM, Borody TJ, Nieuwdorp M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology. 2013;145(5):946–953. doi: 10.1053/j.gastro.2013.08.058. [DOI] [PubMed] [Google Scholar]
- 13.Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. 2004;54(5):1469–1476. doi: 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- 14.Reunanen J, Kainulainen V, Huuskonen L, Ottman N, Belzer C, Huhtinen H, de Vos WM, Satokari R. Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of the epithelial cell layer. Appl Environ Microbiol. 2015;81(11):3655–3662. doi: 10.1128/AEM.04050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouwerkerk JP, Van Der Ark KCH, Davids M, Claassens NJ, Finestra TR, de Vos WM, Belzer C. Adaptation of Akkermansia muciniphila to the oxic-anoxic interface of the mucus layer. Appl Environ Microbiol. 2016;82(23):6983–6993. doi: 10.1128/AEM.01641-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Derrien M, Collado MC, Ben-Amor K, Salminen S, de Vos WM. The mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl Environ Microbiol. 2008;74(5):1646–1648. doi: 10.1128/aem.01226-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhai Q, Feng S, Arjan N, Chen W. A next generation probiotic. Crit Rev Food Sci Nutr. 2019;59:3227–3236. doi: 10.1080/10408398.2018.1517725. [DOI] [PubMed] [Google Scholar]
- 18.Ottman N, Davids M, Suarez-Diez M, Boeren S, Schaap PJ, Martins Dos Santos VAP,Smidt H, Belzer C, de Vos WM. Genome-scale model and omics analysis of metabolic capacities of reveal a preferential mucin-degrading lifestyle. Appl Environ Microbiol. 2017;83(18):e01014-17. doi: 10.1128/AEM.01014-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110(22):9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Lin S, Vanhoutte PM, Woo CW, Xu A. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe-/- mice. Circulation. 2016;133:2434–2446. doi: 10.1161/CIRCULATIONAHA.115.019645. [DOI] [PubMed] [Google Scholar]
- 21.Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, Chilloux J, Ottman N, Duparc T, Lichtenstein L, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23(1):107–113. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- 22.Shin N-R, Lee J-C, Lee H-Y, Kim M-S, Whon TW, Lee M-S, Bae J-W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63(5):727–735. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- 23.Shen J, Tong X, Sud N, Khound R, Song Y, Maldonado-Gomez MX, Walter J, Su Q. Low-Density lipoprotein receptor signaling mediates the triglyceride-lowering action of Akkermansia muciniphila in genetic-induced hyperlipidemia. Arterioscler Thromb Vasc Biol. 2016;36:1448–1456. doi: 10.1161/ATVBAHA.116.307597. [DOI] [PubMed] [Google Scholar]
- 24.Zhao S, Liu W, Wang J, Shi J, Sun Y, Wang W, Ning G, Liu R, Hong J. Akkermansia muciniphila improves metabolic profiles by reducing inflammation in chow diet-fed mice. J Mol Endocrinol. 2017;58(1):1-14. doi: 10.1530/JME-16-0054 [DOI] [PubMed] [Google Scholar]
- 25.Gao X, Xie Q, Kong P, Liu L, Sun S, Xiong B, Huang B, Yan L, Sheng J, Xiang H. Polyphenol- and caffeine-rich postfermented Pu-erh tea improves diet-induced metabolic syndrome by remodeling intestinal homeostasis in mice. Infect Immun. 2018;86(1):e00601-17. doi: 10.1128/IAI.00601-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheng L, Jena PK, Liu H-X, Hu Y, Nagar N, Bronner DN, Settles ML, Bäumler AJ, Wan Y-JY. Obesity treatment by epigallocatechin-3-gallate-regulated bile acid signaling and its enriched Akkermansia muciniphila. FASEB J. 2018;32(12):fj201800370R. doi: 10.1096/fj.201800370R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee H, Lee Y, Kim J, An J, Lee S, Kong H, Song Y, Lee C-K, Kim K. Modulation of the gut microbiota by metformin improves metabolic profiles in aged obese mice. Gut Microbes. 2018;9(2):155–165. doi: 10.1080/19490976.2017.1405209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hänninen A, Toivonen R, Pöysti S, Belzer C, Plovier H, Ouwerkerk JP, Emani R, Cani PD, De Vos WM. Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoimmunity in NOD mice. Gut. 2018;67(8):1445–1453. doi: 10.1136/gutjnl-2017-314508. [DOI] [PubMed] [Google Scholar]
- 29.Ashrafian F, Shahriary A, Behrouzi A, Moradi HR, Keshavarz Azizi Raftar S, Lari A, Hadifar S, Yaghoubfar R, Ahmadi Badi S, Khatami S, et al. Derived extracellular vesicles as a mucosal delivery vector for amelioration of obesity in mice. Front Microbiol. 2019;10:2155. doi: 10.3389/fmicb.2019.02155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Everard A, Plovier H, Rastelli M, Van Hul M, De Wouters D’oplinter A, Geurts L, Druart C, Robine S, Delzenne NM, Muccioli GG, et al. Intestinal epithelial N-acylphosphatidylethanolamine phospholipase D links dietary fat to metabolic adaptations in obesity and steatosis. Nat Commun. 2019;10(1):457. doi: 10.1038/s41467-018-08051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Lugt B, van Beek AA, Aalvink S, Meijer B, Sovran B, Vermeij WP, Brandt RMC, de Vos WM, Savelkoul HFJ, Steegenga WT, et al. Akkermansia muciniphila ameliorates the age-related decline in colonic mucus thickness and attenuates immune activation in accelerated aging Ercc1−/Δ7 mice. Immun Ageing. 2019;16(1):6. doi: 10.1186/s12979-019-0145-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Depommier C, Van Hul M, Everard A, Delzenne NM, De Vos WM, Cani PD. Pasteurized Akkermansia muciniphila increases whole-body energy expenditure and fecal energy excretion in diet-induced obese mice. Gut Microbes. 2020;11(5):1231–1245. doi: 10.1080/19490976.2020.1737307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huo Y, Lu X, Wang X, Wang X, Chen L, Guo H, Zhang M, Li Y. Bifidobacterium animalis subsp. lactis A6 alleviates obesity associated with promoting mitochondrial biogenesis and function of adipose tissue in mice. Molecules. 2020;25(7):1490. doi: 10.3390/molecules25071490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim S, Lee Y, Kim Y, Seo Y, Lee H, Ha J, Lee J, Choi Y, Oh H, Yoon Y, et al. Akkermansia muciniphila prevents fatty liver disease, decreases serum triglycerides, and maintains gut homeostasis. Appl Environ Microbiol. 2020;86(7):e03004-19. doi: 10.1128/AEM.03004-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katiraei S, De Vries MR, Costain AH, Thiem K, Hoving LR, van Diepen JA, Smits HH, Bouter KE, Rensen PCN, Quax PHA, et al. Akkermansia muciniphila exerts lipid-lowering and immunomodulatory effects without affecting neointima formation in hyperlipidemic APOE*3-Leiden.CETP mice. Mol Nutr Food Res. 2020;64(15):e1900732. doi: 10.1002/mnfr.201900732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoon HS, Cho CH, Yun MS, Jang SJ, You HJ, Kim J-H, Han D, Cha KH, Moon SH, Lee K, et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat Microbiol. 2021;6(5):563–573. doi: 10.1038/s41564-021-00880-5. [DOI] [PubMed] [Google Scholar]
- 37.Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, Falony G, Raes J, Maiter D, Delzenne NM, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. 2019;25:1096–1103. doi: 10.1038/s41591-019-0495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perraudeau F, McMurdie P, Bullard J, Cheng A, Cutcliffe C, Deo A, Eid J, Gines J, Iyer M, Justice N, et al. Improvements to postprandial glucose control in subjects with type 2 diabetes: a multicenter, double blind, randomized placebo-controlled trial of a novel probiotic formulation. BMJ Open Diabetes Res Care. 2020;8(1):e001319. doi: 10.1136/bmjdrc-2020-001319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shin J, Noh J-R, Chang D-H, Kim Y-H, Kim MH, Lee ES, Cho S, Ku BJ, Rhee M-S, Kim B-C, et al. Elucidation of akkermansia muciniphila probiotic traits driven by mucin depletion. Front Microbiol. 2019;10:1137. doi: 10.3389/fmicb.2019.01137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bodogai M, O’Connell J, Kim K, Kim Y, Moritoh K, Chen C, Gusev F, Vaughan K, Shulzhenko N, Mattison JA, et al. Commensal bacteria contribute to insulin resistance in aging by activating innate B1a cells. Sci Transl Med. 2018;10(467):eaat4271. doi: 10.1126/scitranslmed.aat4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chelakkot C, Choi Y, Kim D-K, Park HT, Ghim J, Kwon Y, Jeon J, Kim M-S, Jee Y-K, Gho YS, et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med. 2018;50(2):e450. doi: 10.1038/emm.2017.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deng L, Ou Z, Huang D, Li C, Lu Z, Liu W, Wu F, Nong C, Gao J, Peng Y, et al. Diverse effects of different Akkermansia muciniphila genotypes on brown adipose tissue inflammation and whitening in a high-fat-diet murine model. Microb Pathog. 2020;147:104353. doi: 10.1016/j.micpath.2020.104353. [DOI] [PubMed] [Google Scholar]
- 43.Okeke F, Roland BC, Mullin GE. The role of the gut microbiome in the pathogenesis and treatment of obesity. Glob Adv Health Med. 2014;3:44–57. doi: 10.7453/gahmj.2014.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aguirre M, Venema K. The art of targeting gut microbiota for tackling human obesity. Genes Nutr. 2015;10:472. doi: 10.1007/s12263-015-0472-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delzenne NM, Neyrinck AM, Bäckhed F, Cani PD. Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat Rev Endocrinol. 2011;7:639–646. doi: 10.1038/nrendo.2011.126. [DOI] [PubMed] [Google Scholar]
- 46.Cani PD. Targeting gut microbiota with a complex mix of dietary fibers improves metabolic diseases. Kidney Int. 2019;95:14–16. doi: 10.1016/j.kint.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 47.Hussain A, Yadav MK, Bose S, Wang J-H, Lim D, Song Y-K, Ko S-G, Kim H. Daesiho-Tang is an effective herbal formulation in attenuation of obesity in mice through alteration of gene expression and modulation of intestinal microbiota. PLoS One. 2016;11(11):e0165483. doi: 10.1371/journal.pone.0165483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee J, Jang J-Y, Kwon M-S, Lim SK, Kim N, Lee J, Park HK, Yun M, Shin M-Y, Jo HE, et al. Mixture of two lactobacillus plantarum strains modulates the gut microbiota structure and regulatory T cell response in diet-induced obese mice. Mol Nutr Food Res. 2018;62(24):e1800329. doi: 10.1002/mnfr.201800329. [DOI] [PubMed] [Google Scholar]
- 49.Natividad JM, Lamas B, Pham HP, Michel M-L, Rainteau D, Bridonneau C, Da Costa G, van Hylckama Vlieg J, Sovran B, Chamignon C, et al. Bilophila wadsworthia aggravates high fat diet induced metabolic dysfunctions in mice. Nat Commun. 2018;9(1):2802. doi: 10.1038/s41467-018-05249-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang L, Wu Y, Zhuang L, Chen X, Min H, Song S, Liang Q, Li A-D, Gao Q. Puerarin prevents high-fat diet-induced obesity by enriching Akkermansia muciniphila in the gut microbiota of mice. PLoS One. 2019;14(6):e0218490. doi: 10.1371/journal.pone.0218490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang Y, Zhong Z, Wang B, Xia X, Yao W, Huang L, Wang Y, Ding W. Early-life high-fat diet-induced obesity programs hippocampal development and cognitive functions via regulation of gut commensal Akkermansia muciniphila. Neuropsychopharmacology. 2019;44(12):2054–2064. doi: 10.1038/s41386-019-0437-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J-H, Bose S, Kim H-G, Han K-S, Kim H. Fermented rhizoma atractylodis macrocephalae alleviates high fat diet-induced obesity in association with regulation of intestinal permeability and microbiota in rats. Sci Rep. 2015;5(1):8391. doi: 10.1038/srep08391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anhê FF, Nachbar RT, Varin TV, Trottier J, Dudonné S, Le Barz M, Feutry P, Pilon G, Barbier O, Desjardins Y, et al. Treatment with camu camu (Myrciaria dubia) prevents obesity by altering the gut microbiota and increasing energy expenditure in diet-induced obese mice. Gut. 2019;68(3):453–464. doi: 10.1136/gutjnl-2017-315565. [DOI] [PubMed] [Google Scholar]
- 54.Arias L, Goig GA, Cardona P, Torres-Puente M, Díaz J, Rosales Y, Garcia E, Tapia G, Comas I, Vilaplana C, et al. Influence of gut microbiota on progression to tuberculosis generated by high fat diet-induced obesity in C3HeB/FeJ mice. Front Immunol. 2019;10:2464. doi: 10.3389/fimmu.2019.02464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carmody RN, Gerber GK, Luevano JM, Gatti DM, Somes L, Svenson KL, Turnbaugh P. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe. 2015;17(1):72–84. doi: 10.1016/j.chom.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Escobar JS, Klotz B, Valdes BE, Agudelo GM. The gut microbiota of Colombians differs from that of Americans, Europeans and Asians. BMC Microbiol. 2014;14:311. doi: 10.1186/s12866-014-0311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ettehad Marvasti F, Moshiri A, Taghavi MS, Riazi S, Taati M, Sadati SF, Ghaheri A, Masoomi M, Vaziri F, Fateh A, et al. The first report of differences in gut microbiota composition between obese and normal weight Iranian subjects. Iran Biomed J. 2020;24(3):148–154. doi: 10.29252/ibj.24.3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yassour M, Lim MY, Yun HS, Tickle TL, Sung J, Song Y-M, Lee K, Franzosa EA, Morgan XC, Gevers D, et al. Sub-clinical detection of gut microbial biomarkers of obesity and type 2 diabetes. Genome Med. 2016;8(1):17. doi: 10.1186/s13073-016-0271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teixeira FS, Grześkowiak T, Salminen LM, Laitinen S, Bressan J, Gouveia Peluzio M. Faecal levels of bifidobacterium and clostridium coccoides but not plasma lipopolysaccharide are inversely related to insulin and HOMA index in women. Clin Nutr. 2013;32:1017–1022. doi: 10.1016/j.clnu.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 60.Dao MC, Belda E, Prifti E, Everard A, Kayser BD, Bouillot J-L, Chevallier J-M, Pons N, Le Chatelier E, Ehrlich SD, et al. Akkermansia muciniphila abundance is lower in severe obesity, but its increased level after bariatric surgery is not associated with metabolic health improvement. Am J Physiol Endocrinol Metab. 2019;317(3):E446–E59. doi: 10.1152/ajpendo.00140.2019. [DOI] [PubMed] [Google Scholar]
- 61.Santacruz A, Collado MC, García-Valdés L, Segura MT, Martín-Lagos JA, Anjos T, Martí-Romero M, Lopez RM, Florido J, Campoy C, et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr. 2010;104(1):83–92. doi: 10.1017/S0007114510000176. [DOI] [PubMed] [Google Scholar]
- 62.Karlsson CLJ, Onnerfält J, Xu J, Molin G, Ahrné S, Thorngren-Jerneck K. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity (Silver Spring). 2012;20(11):2257–2261. doi: 10.1038/oby.2012.110. [DOI] [PubMed] [Google Scholar]
- 63.Liu R, Hong J, Xu X, Feng Q, Zhang D, Gu Y, Shi J, Zhao S, Liu W, Wang X, et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med. 2017;23(7):859–868. doi: 10.1038/nm.4358. [DOI] [PubMed] [Google Scholar]
- 64.Borgo F, Verduci E, Riva A, Lassandro C, Riva E, Morace G, Borghi E. Relative abundance in bacterial and fungal gut microbes in obese children: a case control study. Child Obes. 2017;13:78–84. doi: 10.1089/chi.2015.0194. [DOI] [PubMed] [Google Scholar]
- 65.Thingholm LB, Rühlemann MC, Koch M, Fuqua B, Laucke G, Boehm R, et al. Obese individuals with and without type 2 diabetes show different gut microbial functional capacity and composition. Cell Host Microbe. 2019;26(2):252-264.e10. doi: 10.1016/j.chom.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salah M, Azab M, Ramadan A, Hanora A. New insights on obesity and diabetes from gut microbiome alterations in egyptian adults. OMICS. 2019;23:477–485. doi: 10.1089/omi.2019.0063. [DOI] [PubMed] [Google Scholar]
- 67.Mitsou EK, Detopoulou M, Kakali A, Fragopoulou E, Nomikos T, Antonopoulou S, Panagiotakos DB, Kyriacou A. Mining possible associations of faecal A. muciniphila colonisation patterns with host adiposity and cardiometabolic markers in an adult population. Benef Microbes. 2019;10(7):741–749. doi: 10.3920/BM2019.0033. [DOI] [PubMed] [Google Scholar]
- 68.Nistal E, Sáenz de Miera LE, Ballesteros PM, Sánchez-Campos S, García-Mediavilla MV, Álvarez-Cuenllas B, Linares P, Olcoz JL, Arias Loste MT, García Lobo JM, et al. An altered fecal microbiota profile in patients with non-alcoholic fatty liver disease (NAFLD) associated with obesity. Rev Esp Enferm Dig. 2019;111:275–282. doi: 10.17235/reed.2019.6068/2018. [DOI] [PubMed] [Google Scholar]
- 69.de la Cuesta-Zuluaga J, Corrales-Agudelo V, Carmona JA, Abad JM, Escobar JS. Body size phenotypes comprehensively assess cardiometabolic risk and refine the association between obesity and gut microbiota. Int J Obes (Lond). 2018;42:424–432. doi: 10.1038/ijo.2017.281. [DOI] [PubMed] [Google Scholar]
- 70.Liang C, Guo M, Liu T, Zhou X, Gong P, Lyu L, Niu H, Wu Y, Chen S, Han X, et al. Profiles of gut microbiota in children with obesity from Harbin, China and screening of strains with anti-obesity ability in vitro and in vivo. J Appl Microbiol. 2020;129(3):728–737. doi: 10.1111/jam.14639. [DOI] [PubMed] [Google Scholar]
- 71.Org E, Parks BW, Joo JWJ, Emert B, Schwartzman W, Kang EY, Mehrabian M, Pan C, Knight R, Gunsalus R, et al. Genetic and environmental control of host-gut microbiota interactions. Genome Res. 2015;25(10):1558–1569. doi: 10.1101/gr.194118.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu F, Guo X, Zhang M, Ou Z, Wu D, Deng L, Lu Z, Zhang J, Deng G, Chen S, et al. An Akkermansia muciniphila subtype alleviates high-fat diet-induced metabolic disorders and inhibits the neurodegenerative process in mice. Anaerobe. 2020;61:102138. doi: 10.1016/j.anaerobe.2019.102138. [DOI] [PubMed] [Google Scholar]
- 73.Prasad RB, Groop L. Genetics of type 2 diabetes-pitfalls and possibilities. Genes. 2015;6:87–123. doi: 10.3390/genes6010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tuomi T, Nagorny CLF, Singh P, Bennet H, Yu Q, Alenkvist I, Isomaa B, Östman B, Söderström J, Pesonen A-K, et al. Increased melatonin signaling is a risk factor for type 2 diabetes. Cell Metab. 2016;23(6):1067–1077. doi: 10.1016/j.cmet.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 75.Huang T, Qi Q, Zheng Y, Ley SH, Manson JE, Hu FB, Qi L. Genetic predisposition to central obesity and risk of type 2 diabetes: two independent cohort studies. Diabetes Care. 2015;38(7):1306–1311. doi: 10.2337/dc14-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barrès R, Zierath JR. The role of diet and exercise in the transgenerational epigenetic landscape of T2DM. Nat Rev Endocrinol. 2016;12:441–451. doi: 10.1038/nrendo.2016.87. [DOI] [PubMed] [Google Scholar]
- 77.Zhang X, Shen D, Fang Z, Jie Z, Qiu X, Zhang C, Chen Y, Ji L. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS One. 2013;8(8):e71108. doi: 10.1371/journal.pone.0071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65(3):426–436. doi: 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- 79.Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Mannerås-Holm L, Ståhlman M, Olsson LM, Serino M, Planas-Fèlix M, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23(7):850–858. doi: 10.1038/nm.4345. [DOI] [PubMed] [Google Scholar]
- 80.de la Cuesta-Zuluaga J, Mueller NT, Corrales-Agudelo V, Velásquez-Mejía EP, Carmona JA, Abad JM, Escobar JS. Metformin is associated with higher relative abundance of mucin-degrading Akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut. Diabetes Care. 2017;40:54–62. doi: 10.2337/dc16-1324. [DOI] [PubMed] [Google Scholar]
- 81.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després J-P, Fullerton HJ, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 82.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982-3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peng J, Xiao X, Hu M, Zhang X. Interaction between gut microbiome and cardiovascular disease. Life Sci. 2018;214:153–157. doi: 10.1016/j.lfs.2018.10.063. [DOI] [PubMed] [Google Scholar]
- 84.Tang WH, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res. 2017;120:1183–1196. doi: 10.1161/circresaha.117.309715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Al-Waiz M, Mikov M, Mitchell SC, Smith RL. The exogenous origin of trimethylamine in the mouse. Metabolism. 1992;41:135–136. doi: 10.1016/0026-0495(92)90140-6. [DOI] [PubMed] [Google Scholar]
- 86.Rebouche CJ, Seim H. Carnitine metabolism and its regulation in microorganisms and mammals. Annu Rev Nutr. 1998;18:39–61. doi: 10.1146/annurev.nutr.18.1.39. [DOI] [PubMed] [Google Scholar]
- 87.Zeisel SH, Wishnok JS, Blusztajn JK. Formation of methylamines from ingested choline and lecithin. J Pharmacol Exp Ther. 1983;225:320–324. [PubMed] [Google Scholar]
- 88.Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17:49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhu W, Buffa JA, Wang Z, Warrier M, Schugar R, Shih DM, Gupta N, Gregory JC, Org E, Fu X, et al. Flavin monooxygenase 3, the host hepatic enzyme in the metaorganismal trimethylamine N-oxide-generating pathway, modulates platelet responsiveness and thrombosis risk. J Thromb Haemost. 2018;16(9):1857–1872. doi: 10.1111/jth.14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Treacy EP, Akerman BR, Chow LM, Youil R, Bibeau C, Lin J, Bruce AG, Knight M, Danks DM, Cashman JR, et al. Mutations of the flavin-containing monooxygenase gene (FMO3) cause trimethylaminuria, a defect in detoxication. Hum Mol Genet. 1998;7:839–845. doi: 10.1093/hmg/7.5.839. [DOI] [PubMed] [Google Scholar]
- 91.Ettinger G, MacDonald K, Reid G, Burton JP. The influence of the human microbiome and probiotics on cardiovascular health. Gut Microbes. 2014;5:719–728. doi: 10.4161/19490976.2014.983775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung Y-M, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tang WHW, Bäckhed F, Landmesser U, Hazen SL. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:2089–2105. doi: 10.1016/j.jacc.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ahmadmehrabi S, Tang WHW. Gut microbiome and its role in cardiovascular diseases. Curr Opin Cardiol. 2017;32:761–766. doi: 10.1097/HCO.0000000000000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ma J, Li H. The role of gut microbiota in atherosclerosis and hypertension. Front Pharmacol. 2018;9:1082. doi: 10.3389/fphar.2018.01082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chambers ES, Preston T, Frost G, Morrison DJ. Role of gut microbiota-generated short-chain fatty acids in metabolic and cardiovascular health. Curr Nutr Rep. 2018;7:198–206. doi: 10.1007/s13668-018-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moreira GV, Azevedo FF, Ribeiro LM, Santos A, Guadagnini D, Gama P, Liberti EA, Saad M, Carvalho C. Liraglutide modulates gut microbiota and reduces NAFLD in obese mice. J Nutr Biochem. 2018;62:143–154. doi: 10.1016/j.jnutbio.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 98.Wang H, Wang L, Li Y, Luo S, Ye J, Lu Z, Li X, Lu H. The HIF-2α/PPARα pathway is essential for liraglutide-alleviated, lipid-induced hepatic steatosis. Biomed Pharmacother = Biomedecine Pharmacotherapie. 2021;140:111778. doi: 10.1016/j.biopha.2021.111778. [DOI] [PubMed] [Google Scholar]
- 99.Wu W, Lv L, Shi D, Ye J, Fang D, Guo F, Li Y, He X, Li L. Protective effect of against immune-mediated liver injury in a mouse model. Front Microbiol. 2017;8:1804. doi: 10.3389/fmicb.2017.01804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Macfarlane GT, Macfarlane S. Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int. 2012;95:50–60. doi: 10.5740/jaoacint.sge_macfarlane. [DOI] [PubMed] [Google Scholar]
- 101.Kimura I, Ichimura A, Ohue-Kitano R, Igarashi M. Free fatty acid receptors in health and disease. Physiol Rev. 2020;100:171–210. doi: 10.1152/physrev.00041.2018. [DOI] [PubMed] [Google Scholar]
- 102.Yu C, Liu S, Chen L, Shen J, Niu Y, Wang T, Zhang W, Fu L. Effect of exercise and butyrate supplementation on microbiota composition and lipid metabolism. J Endocrinol. 2019;243(2):125–135. doi: 10.1530/joe-19-0122. [DOI] [PubMed] [Google Scholar]
- 103.Le Poul E, Loison C, Struyf S, Springael J-Y, Lannoy V, Decobecq M-E, Brezillon S, Dupriez V, Vassart G, Van Damme J, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278(28):25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 104.Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105(43):16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Christiansen CB, Gabe MBN, Svendsen B, Dragsted LO, Rosenkilde MM, Holst JJ. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am J Physiol Gastrointest Liver Physiol. 2018;315(1):G53–G65. doi: 10.1152/ajpgi.00346.2017. [DOI] [PubMed] [Google Scholar]
- 106.Psichas A, Sleeth ML, Murphy KG, Brooks L, Bewick GA, Hanyaloglu AC, Ghatei MA, Bloom SR, Frost G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes (Lond). 2015;39(3):424–429. doi: 10.1038/ijo.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou D, Chen Y-W, Zhao Z-H, Yang R-X, Xin F-Z, Liu X-L, Pan Q, Zhou H, Fan J-G. Sodium butyrate reduces high-fat diet-induced non-alcoholic steatohepatitis through upregulation of hepatic GLP-1R expression. Exp Mol Med. 2018;50(12):1-12. doi: 10.1038/s12276-018-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Steinert RE, Feinle-Bisset C, Asarian L, Horowitz M, Beglinger C, Ghrelin GN. CCK, GLP-1, and PYY(3-36): secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol Rev. 2017;97:411–463. doi: 10.1152/physrev.00031.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8(12):728–742. doi: 10.1038/nrendo.2012.140. [DOI] [PubMed] [Google Scholar]
- 110.Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L, Anastasovska J, Ghourab S, Hankir M, Zhang S, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5(1):3611. doi: 10.1038/ncomms4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Robertson MD, Bickerton AS, Dennis AL, Vidal H, Frayn KN. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am J Clin Nutr. 2005;82:559–567. doi: 10.1093/ajcn.82.3.559. [DOI] [PubMed] [Google Scholar]
- 112.Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani T, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4(1):1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156(1–2):84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 114.Lukovac S, Belzer C, Pellis L, Keijser BJ, de Vos WM, Montijn RC, Roeselers G. Differential modulation by Akkermansia muciniphila and faecalibacterium prausnitzii of host peripheral lipid metabolism and histone acetylation in mouse gut organoids. mBio. 2014;5(4):e01438-14. doi: 10.1128/mBio.01438-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Belzer C, Chia LW, Aalvink S, Chamlagain B, Piironen V, Knol J, de Vos WM. Microbial metabolic networks at the mucus layer lead to diet-independent butyrate and vitamin B production by intestinal symbionts. mBio. 2017;8(5):e00770-17. doi: 10.1128/mBio.00770-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chia LW, Hornung BVH, Aalvink S, Schaap PJ, de Vos WM, Knol J, Belzer C. Deciphering the trophic interaction between Akkermansia muciniphila and the butyrogenic gut commensal anaerostipes caccae using a metatranscriptomic approach. Antonie Van Leeuwenhoek. 2018;111(6):859–873. doi: 10.1007/s10482-018-1040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Belzer C, de Vos WM. Microbes inside–from diversity to function: the case of Akkermansia. ISME J. 2012;6:1449–1458. doi: 10.1038/ismej.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Donohoe DR, Garge N, Zhang X, Sun W, O’Connell TM, Bunger MK, Bultman S. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13(5):517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Roshanravan N, Mahdavi R, Alizadeh E, Jafarabadi MA, Hedayati M, Ghavami A, Alipour S, Alamdari N, Barati M, Ostadrahimi A, et al. Effect of butyrate and inulin supplementation on glycemic status, lipid profile and glucagon-like peptide 1 level in patients with type 2 diabetes: a randomized double-blind, placebo-controlled trial. Horm Metab Res. 2017;49(11):886–891. doi: 10.1055/s-0043-119089. [DOI] [PubMed] [Google Scholar]
- 120.Moran BM, Flatt PR, McKillop AM. G protein-coupled receptors: signalling and regulation by lipid agonists for improved glucose homoeostasis. Acta Diabetol. 2016;53(2):177–188. doi: 10.1007/s00592-015-0826-9. [DOI] [PubMed] [Google Scholar]
- 121.Bach Knudsen KE, Lærke HN, Hedemann MS, Nielsen TS, Ingerslev AK, Gundelund Nielsen DS, Theil PK, Purup S, Hald S, Schioldan AG, et al. Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients. 2018;10(10):1499. doi: 10.3390/nu10101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 123.Frazier TH, DiBaise JK, McClain CJ. Gut microbiota, intestinal permeability, obesity-induced inflammation, and liver injury. JPEN J Parenter Enteral Nutr. 2011;35(5_suppl):14S–20S. doi: 10.1177/0148607111413772. [DOI] [PubMed] [Google Scholar]
- 124.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58(8):1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bastard J-P, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17(1):4-12. [PubMed] [Google Scholar]
- 126.Grander C, Adolph TE, Wieser V, Lowe P, Wrzosek L, Gyongyosi B, Ward DV, Grabherr F, Gerner RR, Pfister A, et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut. 2018;67(5):891–901. doi: 10.1136/gutjnl-2016-313432. [DOI] [PubMed] [Google Scholar]
- 127.Gonzalez A, Krieg R, Massey HD, Carl D, Ghosh S, Gehr TWB, Ghosh SS. Sodium butyrate ameliorates insulin resistance and renal failure in CKD rats by modulating intestinal permeability and mucin expression. Nephrol Dial Transplant. 2019;34(5):783–794. doi: 10.1093/ndt/gfy238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cheng D, Xu J-H, Li J-Y, Wang S-Y, Wu T-F, Chen Q-K, Yu T. Butyrate ameliorated-NLRC3 protects the intestinal barrier in a GPR43-dependent manner. Exp Cell Res. 2018;368(1):101–110. doi: 10.1016/j.yexcr.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 129.Ottman N, Reunanen J, Meijerink M, Pietilä TE, Kainulainen V, Klievink J, Huuskonen L, Aalvink S, Skurnik M, Boeren S, et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS One. 2017;12(3):e0173004. doi: 10.1371/journal.pone.0173004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Png CW, Lindén SK, Gilshenan KS, Zoetendal EG, McSweeney CS, Sly LI, McGuckin MA, Florin THJ. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol. 2010;105(11):2420–2428. doi: 10.1038/ajg.2010.281. [DOI] [PubMed] [Google Scholar]
- 131.Vigsnæs LK, Brynskov J, Steenholdt C, Wilcks A, Licht TR. Gram-negative bacteria account for main differences between faecal microbiota from patients with ulcerative colitis and healthy controls. Benef Microbes. 2012;3:287–297. doi: 10.3920/BM2012.0018. [DOI] [PubMed] [Google Scholar]
- 132.Kang C-S, Ban M, Choi E-J, Moon H-G, Jeon J-S, Kim D-K, Park S-K, Jeon SG, Roh T-Y, Myung S-J, et al. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS One. 2013;8:e76520. doi: 10.1371/journal.pone.0076520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bian X, Wu W, Yang L, Lv L, Wang Q, Li Y, Ye J, Fang D, Wu J, Jiang X. Administration of Akkermansia muciniphila Ameliorates dextran sulfate sodium-induced ulcerative colitis in mice. Front Microbiol. 2019;10:2259. doi: 10.3389/fmicb.2019.02259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhai R, Xue X, Zhang L, Yang X, Zhao L, Zhang C. Strain-specific anti-inflammatory properties of two strains on chronic colitis in mice. Front Cell Infect Microbiol. 2019;9:239. doi: 10.3389/fcimb.2019.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang L, Tang L, Feng Y, Zhao S, Han M, Zhang C, Yuan G, Zhu J, Cao S, Wu Q, et al. A purified membrane protein from akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8 + T cells in mice. Gut. 2020;69:1988–1997. doi: 10.1136/gutjnl-2019-320105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Seregin SS, Golovchenko N, Schaf B, Chen J, Pudlo NA, Mitchell J, Baxter NT, Zhao L, Schloss PD, Martens EC et al. NLRP6 protects Il10 Mice from colitis by limiting colonization of Akkermansia muciniphila. Cell Rep. 2017;19:733–745. doi: 10.1016/j.celrep.2017.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ottman N, Huuskonen L, Reunanen J, Boeren S, Klievink J, Smidt H, Belzer C, de Vos WM. Characterization of outer membrane proteome of Akkermansia muciniphila reveals sets of novel proteins exposed to the human intestine. Front Microbiol. 2016;7:1157. doi: 10.3389/fmicb.2016.01157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang L, Qin Q, Liu M, Zhang X, He F, Wang G. Akkermansia muciniphila can reduce the damage of gluco/lipotoxicity, oxidative stress and inflammation, and normalize intestine microbiota in streptozotocin-induced diabetic rats. Pathog Dis. 2018;76(4). doi: 10.1093/femspd/fty028. [DOI] [PubMed] [Google Scholar]
- 139.Muccioli GG, Naslain D, Bäckhed F, Reigstad CS, Lambert DM, Delzenne NM, Cani PD. The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol. 2010;6(1):392. doi: 10.1038/msb.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Meng X, Wang W, Lan T, Yang W, Yu D, Fang X, Wu H. A purified aspartic protease from plays an important role in degrading Muc2. Int J Mol Sci. 2019;21(1):72. doi: 10.3390/ijms21010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Meng X, Zhang J, Wu H, Yu D, Fang X. Aspartic protease Amuc_1434* inhibits human colorectal cancer LS174T cell viability via TRAIL-mediated apoptosis pathway. Int J Mol Sci. 2020:21(9):3385. doi: 10.3390/ijms21093385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kosciow K, Deppenmeier U. Characterization of a phospholipid-regulated β-galactosidase from Akkermansia muciniphila involved in mucin degradation. MicrobiologyOpen. 2019;8(8):e00796. doi: 10.1002/mbo3.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kosciow K, Deppenmeier U. Characterization of three novel β-galactosidases from Akkermansia muciniphila involved in mucin degradation. Int J Biol Macromol. 2020;149:331–340. doi: 10.1016/j.ijbiomac.2020.01.246. [DOI] [PubMed] [Google Scholar]
- 144.Kuipers ME, Hokke CH, Smits HH, Nolte-’t Hoen ENM. Pathogen-derived extracellular vesicle-associated molecules that affect the host immune system: an overview. Front Microbiol. 2018;9:2182. doi: 10.3389/fmicb.2018.02182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Macia L, Nanan R, Hosseini-Beheshti E, Grau GE. Host- and microbiota-derived extracellular vesicles, immune function, and disease development. Int J Mol Sci. 2019:21(1):107. doi: 10.3390/ijms21010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cani PD, Knauf C. A newly identified protein from Akkermansia muciniphila stimulates GLP-1 secretion. Cell Metab. 2021;33(6):1073–1075. doi: 10.1016/j.cmet.2021.05.004. [DOI] [PubMed] [Google Scholar]
- 147.Everard A, Lazarevic V, Gaïa N, Johansson M, Ståhlman M, Backhed F, Delzenne NM, Schrenzel J, François P, Cani PD, et al. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J. 2014;8(10):2116–2130. doi: 10.1038/ismej.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schneeberger M, Everard A, Gómez-Valadés AG, Matamoros S, Ramírez S, Delzenne NM, Gomis R, Claret M, Cani PD. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep. 2015;5(1):16643. doi: 10.1038/srep16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mehrpouya-Bahrami P, Chitrala KN, Ganewatta MS, Tang C, Murphy EA, Enos RT, Velazquez KT, McCellan J, Nagarkatti M, Nagarkatti P, et al. Blockade of CB1 cannabinoid receptor alters gut microbiota and attenuates inflammation and diet-induced obesity. Sci Rep. 2017;7(1):15645. doi: 10.1038/s41598-017-15154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Villamil SI, Huerlimann R, Morianos C, Sarnyai Z, Maes GE. Adverse effect of early-life high-fat/high-carbohydrate (“Western”) diet on bacterial community in the distal bowel of mice. Nutr Res (New York, NY). 2018;50:25–36. doi: 10.1016/j.nutres.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 151.Fujisaka S, Usui I, Nawaz A, Igarashi Y, Okabe K, Furusawa Y, Watanabe S, Yamamoto S, Sasahara M, Watanabe Y, et al. Bofutsushosan improves gut barrier function with a bloom of Akkermansia muciniphila and improves glucose metabolism in mice with diet-induced obesity. Sci Rep. 2020;10(1):5544. doi: 10.1038/s41598-020-62506-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Régnier M, Rastelli M, Morissette A, Suriano F, Le Roy T, Pilon G, Delzenne NM, Marette A, Van Hul M, Cani PD. Rhubarb supplementation prevents diet-induced obesity and diabetes in association with increased in mice. Nutrients. 2020;12(10):2932. doi: 10.3390/nu12102932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wu T, Gao Y, Hao J, Geng J, Zhang J, Yin J, Liu R, Sui W, Gong L, Zhang M, et al. Capsanthin extract prevents obesity, reduces serum TMAO levels and modulates the gut microbiota composition in high-fat-diet induced obese C57BL/6J mice. Food Res Int. 2020;128:108774. doi: 10.1016/j.foodres.2019.108774. [DOI] [PubMed] [Google Scholar]
- 154.Fåk F, Jakobsdottir G, Kulcinskaja E, Marungruang N, Matziouridou C, Nilsson U, Stålbrand H, Nyman M. The physico-chemical properties of dietary fibre determine metabolic responses, short-chain fatty acid profiles and gut microbiota composition in rats fed low- and high-fat diets. PLoS One. 2015;10(5):e0127252. doi: 10.1371/journal.pone.0127252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto J-M, Kennedy S, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 156.Remely M, Tesar I, Hippe B, Gnauer S, Rust P, Haslberger AG. Gut microbiota composition correlates with changes in body fat content due to weight loss. Benef Microbes. 2015;6(4):431–439. doi: 10.3920/BM2014.0104. [DOI] [PubMed] [Google Scholar]
- 157.Brahe LK, Le Chatelier E, Prifti E, Pons N, Kennedy S, Hansen T, Pedersen O, Astrup A, Ehrlich SD, Larsen LH, et al. Specific gut microbiota features and metabolic markers in postmenopausal women with obesity. Nutr Diabetes. 2015;5(6):e159. doi: 10.1038/nutd.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang Z, Saha S, Van Horn S, Thomas E, Traini C, Sathe G, Rajpal DK, Brown JR. Gut microbiome differences between metformin- and liraglutide-treated T2DM subjects. Endocrinol Diabetes Metab. 2018;1(1):e00009. doi: 10.1002/edm2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, Wu S, Liu W, Cui Q, Geng B, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5(1):14. doi: 10.1186/s40168-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Singh H, Torralba MG, Moncera KJ, DiLello L, Petrini J, Nelson KE, Pieper R. Gastro-intestinal and oral microbiome signatures associated with healthy aging. Geroscience. 2019;41(6):907–921. doi: 10.1007/s11357-019-00098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Medina-Vera I, Sanchez-Tapia M, Noriega-López L, Granados-Portillo O, Guevara-Cruz M, Flores-López A, Avila-Nava A, Fernández ML, Tovar AR, Torres N, et al. A dietary intervention with functional foods reduces metabolic endotoxaemia and attenuates biochemical abnormalities by modifying faecal microbiota in people with type 2 diabetes. Diabetes Metab. 2019;45:122–131. doi: 10.1016/j.diabet.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 162.Journey EK, Ortega-Santos CP, Bruening M, Whisner CM. Changes in weight status and the intestinal microbiota among college freshman, aged 18 years. J Adolesc Health. 2020;66(2):166–171. doi: 10.1016/j.jadohealth.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hsu C-N, Chang-Chien G-P, Lin S, Hou C-Y, Lu P-C, Tain Y-L. Association of trimethylamine, trimethylamine N-oxide, and dimethylamine with cardiovascular risk in children with chronic kidney disease. J Clin Med. 2020:9(2):336. doi: 10.3390/jcm9020336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kim B-S, Song M-Y, Kim H. The anti-obesity effect of ephedra sinica through modulation of gut microbiota in obese Korean women. J Ethnopharmacol. 2014;152(3):532–539. doi: 10.1016/j.jep.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 165.Palleja A, Kashani A, Allin KH, Nielsen T, Zhang C, Li Y, Brach T, Liang S, Feng Q, Jørgensen NB, et al. Roux-en-Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. Genome Med. 2016;8(1):67. doi: 10.1186/s13073-016-0312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Payahoo L, Khajebishak Y, Alivand MR, Soleimanzade H, Alipour S, Barzegari A, Ostadrahimi A. Investigation the effect of oleoylethanolamide supplementation on the abundance of Akkermansia muciniphila bacterium and the dietary intakes in people with obesity: a randomized clinical trial. Appetite. 2019;141:104301. doi: 10.1016/j.appet.2019.05.032. [DOI] [PubMed] [Google Scholar]
- 167.Pedret A, Valls RM, Calderón-Pérez L, Llauradó E, Companys J, Pla-Pagà L, Moragas A, Martín-Luján F, Ortega Y, Giralt M, et al. Effects of daily consumption of the probiotic bifidobacterium animalis subsp. lactis CECT 8145 on anthropometric adiposity biomarkers in abdominally obese subjects: a randomized controlled trial. Int J Obes (Lond). 2019;43(9):1863–1868. doi: 10.1038/s41366-018-0220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang L, Ouyang Y, Li H, Shen L, Ni Y, Fang Q, Wu G, Qian L, Xiao Y, Zhang J, et al. Metabolic phenotypes and the gut microbiota in response to dietary resistant starch type 2 in normal-weight subjects: a randomized crossover trial. Sci Rep. 2019;9(1):4736. doi: 10.1038/s41598-018-38216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dong TS, Luu K, Lagishetty V, Sedighian F, Woo S-L, Dreskin BW, Katzka W, Chang C, Zhou Y, Arias-Jayo N, et al. A high protein calorie restriction diet alters the gut microbiome in obesity. Nutrients. 2020;12(10):3221. doi: 10.3390/nu12103221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cortez RV, Petry T, Caravatto P, Pessôa R, Sanabani SS, Martinez MB, Sarian T, Salles JE, Cohen R, Taddei CR, et al. Shifts in intestinal microbiota after duodenal exclusion favor glycemic control and weight loss: a randomized controlled trial. Surg Obes Relat Dis. 2018;14(11):1748–1754. doi: 10.1016/j.soard.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 171.Guevara-Cruz M, Flores-López AG, Aguilar-López M, Sánchez-Tapia M, Medina-Vera I, Díaz D, Tovar AR, Torres N. Improvement of lipoprotein profile and metabolic endotoxemia by a lifestyle intervention that modifies the gut microbiota in subjects with metabolic syndrome. J Am Heart Assoc. 2019;8:e012401. doi: 10.1161/JAHA.119.012401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shin NR, Gu N, Choi HS, Kim H. Combined effects of Scutellaria baicalensis with metformin on glucose tolerance of patients with type 2 diabetes via gut microbiota modulation. Am J Physiol Endocrinol Metab. 2020;318(1):E52–E61. doi: 10.1152/ajpendo.00221.2019. [DOI] [PubMed] [Google Scholar]


