Abstract
Background:
Traumatic brainstem injury has yet to be incorporated into widely used imaging classification systems for traumatic brain injury (TBI), and questions remain regarding prognostic implications for this TBI subgroup. To address this, retrospective data on patients from the multicenter prospective Transforming Research and Clinical Knowledge in TBI study were studied.
Methods:
Patients with brainstem and cerebrum injury (BSI+) were matched by age, sex, and admission Glasgow Coma Scale (GCS) score to patients with cerebrum injuries only. All patients had an interpretable head computed tomography (CT) scan from the first 48 hours after injury and a 6-month Glasgow Outcome Scale Extended (GOSE) score. CT scans were reviewed for brainstem lesions and, when present, characterized by location, size, and type (traumatic axonal injury, contusion, or Duret hemorrhage). Clinical, demographic, and outcome data were then compared between the two groups.
Results:
Mann–Whitney U-tests showed no significant difference in 6-month GOSE scores in patients with BSI+ (mean 2.7) compared with patients with similar but only cerebrum injuries (mean 3.9), although there is a trend (p = 0.10). However, subclassification by brainstem lesion type, traumatic axonal injury (mean 4.0) versus Duret hemorrhage or contusion (mean 1.4), did identify a proportion of BSI+ with significantly less favorable outcome (p = 0.002). The incorporation of brainstem lesion type (traumatic axonal injury vs. contusion/Duret), along with GCS into a multivariate logistic regression model of favorable outcome (GOSE score 4–8) did show a significant contribution to the prognostication of this brainstem injury subgroup (odds ratio 0.08, 95% confidence interval 0.00–0.67, p = 0.01).
Conclusions:
These findings suggest two groups of patients with brainstem injuries may exist with divergent recovery potential after TBI. These data support the notion that newer CT imaging classification systems may augment traditional clinical measures, such as GCS in identifying those patients with TBI and brainstem injuries that stand a higher chance of favorable outcome.
Keywords: Traumatic brain injury, Brainstem injury, Computed tomography, Outcomes, Traumatic axonal injury
Introduction
In the United States, traumatic brain injury (TBI) accounts for a substantial portion of medical cost, disability and death. In 2014, 2.53 million Americans visited emergency departments for TBI; 288,000 were admitted to hospitals and 56,800 died as a result of their TBI [1]. In 2010, direct and indirect costs related to TBI in the United States were estimated to be $76.5 billion, 90% of which stemmed from hospitalization and death related to TBI [2, 3]. Nonfatal severe TBI can lead to extended depressed levels of consciousness or coma and/or amnesia after the injury. Forty-three percent of individuals have a related disability 1 year after injury [4]. Compared with all other injuries, TBI admissions are longer and more costly on average [5]. TBI is frequently associated with ongoing deficits in cognitive function, motor function, sensation, and emotional/psychiatric parameters, such as depression, aggression, personality change, and impulse control [6]. Despite the costs of brain injury, prognostication of severe TBI based on early imaging and biomarkers has been universally challenging [7-9].
In combination with the neurological examination, nonenhanced computed tomography (CT) remains a vital tool for triage of TBI. CT can be obtained rapidly without screening and is highly sensitive for injuries requiring neurosurgical intervention and/or intensive care [1]. CT has adequate sensitivity to detect acute life-threatening intracranial hemorrhage, contusion, mass effect, and fracture. Sufficient information can be obtained about injury type, location, and severity to guide medical and surgical care in the acute post-TBI treatment phase [4]. For these reasons, nonenhanced head CT is a class I recommendation for patients with moderate to severe TBI [2]. The Marshall, Rotterdam, Stockholm, and Helsinki CT scores were developed to use acute head CT images to predict mortality at 6 months [3].
The Marshall Classification system appeared in 1991 and has been used widely to inform TBI outcome studies. It categorizes TBI based on focal versus diffuse lesions, basal cistern compression, midline shift, and volume of mass lesions [5]. However, the Marshall classification was not originally designed as a prognostic tool, and more recent analysis has called its use into question [4]. Newer classifications, including the Stockholm and Helsinki CT scores have emerged, incorporating more imaging elements. The Stockholm CT score includes midline shift as a continuous variable and an additional score for traumatic subarachnoid hemorrhage and is the only system to include diffuse axonal injury visible on CT [4]. The Helsinki score expands on the Marshall score with the addition of components that focus on the type of intracranial injury present [6].
In Thelin et al.’s [3] recent evaluation of the latter scoring systems, they found both added significant independent predictive value with the Stockholm CT classification system showing the highest predictive value of all classifications with a pseudo-R2 of 0.35, similar to results in the original development cohort [6]. Further research by Yuh et al. [8] demonstrated quantitative analysis of acute head CT in TBI can augment previously described CT classification systems’ ability to predict 6-month Glasgow Outcome Scale Extended (GOSE) scores. Although these classification systems have become increasingly valuable in their contribution to predicting outcome in TBI, they remain imperfect and with enough predictive variation to hamper their utility in real-world clinical decision-making. Notably, none include criteria related to traumatic brainstem injury (BSI).
The relative lack of available literature using traumatic BSI on CT as a predictor of outcome may be related to its lack of sensitivity in detecting these lesions when compared with magnetic resonance imaging (MRI) [9, 10]. Multiple studies indicate added precision in prognostication when brainstem lesions are included in models, even when controlling for other clincal, demographic, and imaging factors [11, 12]. A 2017 meta-analysis showed traumatic BSI on MRI was associated with significantly increased risk of all-cause mortality (RR 1.78) and unfavorable GOSE score at 6 months or greater (RR 2.49) [13].
However, insights into a more optimistic prognosis for some patients with traumatic BSI are emerging through study of traumatic axonal injury (TAI). The development and utilization of Gradient echo and susceptibility weighted sequences on MRI have increased the detection rate of microhemorrhage associated with TAI to more than 70% [14-16]. Increased detection has allowed for improved understanding of TAI as a prognostic biomarker in patients presenting with severe TBI [9, 14, 16, 17]. Initially believed to portend a devastating neurologic prognosis, literature has emerged describing patients with brainstem TAI who experienced better than expected outcomes [14, 18, 19].
Despite ubiquitous use of CT imaging in the acute critical care enviornment for TBI, ongoing efforts to refine prognostic tools based on head CT and increasing evidence that traumatic BSI on MRI is associated with more variable outcomes than previously assumed, minimal data are available that relate acute traumatic BSI on CT to long-term functional outcome. The objective of this study was to compare the long-term outcome of patients with BSI indentifed on CT with that of patients with similar TBI injury but without BSI in an effort to identify subsets of patients with divergent probabilities of functional recovery.
Methods
Study population
All retrospective data on patients were identified in conjunction with the National Institute of Neurological Disorders and Stroke (NINDS)-funded multicenter initiative Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI). TRACK-TBI is a large-scale longitudinal observational multicenter research collaboration committed to assessing outcomes related to the entire TBI spectrum [20]. Patients were identified from the larger pateint pool of the TRACK-TBI cohort (for additional information and full details of the primary study inclusion and exclusion criteria, please see the study Web site at http://tracktbi.ucsf.edu/). Imaging for enrolled patients was analyzed in a standardized fashion using consensus-based common data elements (CDEs) for TBI pathoanatomic lesions outlined by the global Working Group on Demographics and Clinical Assessment and refined by the National Institutes of Health and NINDS-lead multidisciplinary team in 2010 [21]. All head CT scans were interpreted by a board-certified neuroradiologist (EY, PM) using these methods.
In total, the CT scans of 2697 patientswith TBI were reviewed (Fig. 1). Of those, 145 were excluded for selection of analysis for the current study focused on adult patients with TBI because they were pediatric cases, 114 were excluded because they did not have an acute head CT, and another 477 were omitted because they had incomplete outcome data. This left 1961 adult patients with TBI across the severity spectrum with pathoanatomic lesion-confirmed CT scan data available for this current study. Of the 1961 patients, 29 were identified by the board-certified neuroradiologists to have brainstem lesions (BSI+). From the remaining 1932 patients with TBI, we matched each patient with BSI+ by age, sex, and admission Glasgow Coma Scale (GCS) score to an adult patient with TBI without brainstem lesions (BSI−). Nineteen of the patients with BSI+ were matched exactly on all three factors, nine nonexact matches were matched within 5 years of age, and two within 1 point on the admission GCS score, staying within the same severity range (moderate or severe). Matching on admission GCS score was done to ensure comparable levels of brain injury severity in consideration of long-term outcome as the fairest possible way to investigate any unique contribution of brainstem lesions. In total, 58 adult patients with TBI, 29 with BSI+ and 29 with BSI−, were used for the analysis in this study.
Fig. 1.
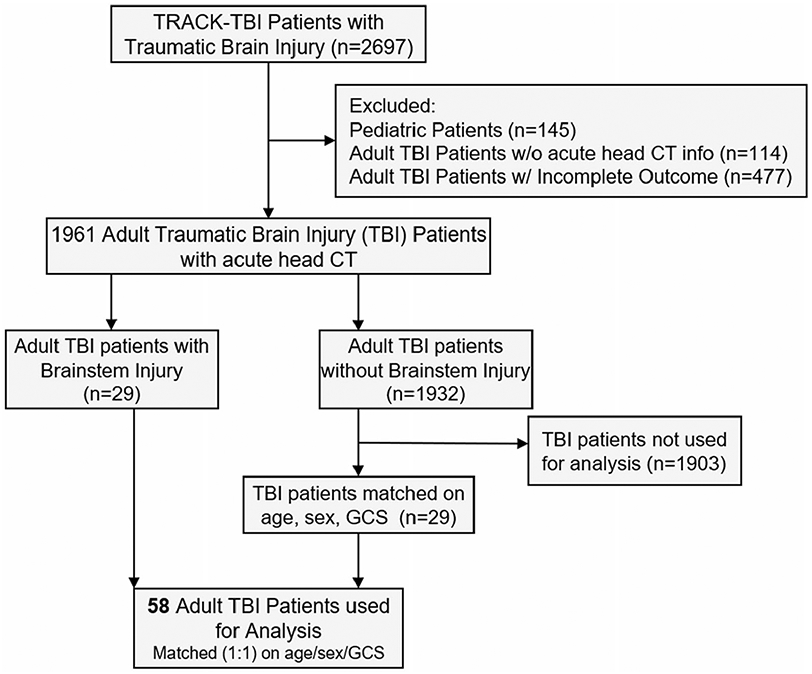
Consort diagram for patient selection. All adult patients with traumatic brain injury (TBI) identified by a team of board-certified neuroradiologists as having a brainstem lesion were utilized from this cohort and matched by age, sex, and admission Glasgow Coma Scale (GCS) score to adult patients with TBI but without injuries to the brainstem. TRACK-TBI Transforming Research and Clinical Knowledge in Traumatic Brain Injury
Outcomes
The primary outcome of interest was observed GOSE score at 6 months. The GOSE is a global outcome scale designed to assess basic components of functional independence. It is an 8-point scale with scores in ascending numeric value ranging from 1 (death) to 8 (upper good recovery, no problems). It is assessed by a semistructured interview and can be administered via phone or mail. It is has established interrater reliability and is the most widely used outcome measure in neurotrauma literature [22]. Favorable GOSE scores were defined as 4, upper severe disability (independent at home for up to 8 h/day), to 8 (see above), and unfavorable scores were 1 (see above) to 3 (lower severe disability).
CT imaging
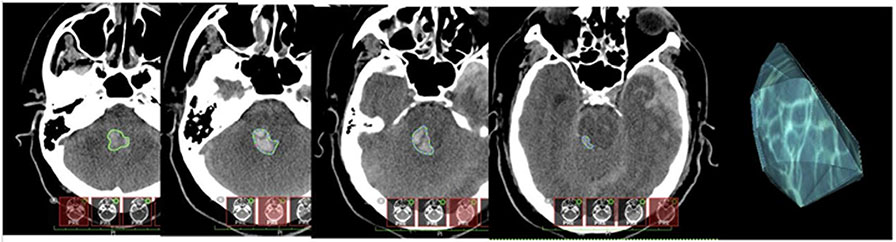
The earliest head CT of interpretable quality from within 48 h of recorded injury was selected for detailed qualitative and quantitative analysis. Because the interpretation by the board-certified neuroradiologists only indicated “presence” or “absence” of brainstem lesions, additional work was done to further characterize these lesions. All pathoanatomic TBI lesions were demarcated, measured, and recorded based on the National Institutes of Health-defined and TRACK-TBI-validated neuroimaging CDEs for TBI [21, 23]. Regions of interest (ROIs) were demarcated and measured using OsiriX MD DICOM Viewer (v. 11.0.2; Pixmeo SARL, Bernex, Switzerland). ROIs were outlined by hand, and relevant measures were computed using OsiriX MD software by a fifth-year neurological surgery resident (Fig. 2). Intrarater reliability was tested by comparing primary CDE measures (n = 23 core and n = 23 supplementary) of five CT scans from two evaluations separated by 8 weeks, and 96.3% agreement was observed.
Fig. 2.
Brainstem lesion segmentation. Left to right: hand-traced borders on ascending axial head computed tomography (CT) slices through the brainstem area in Osirix MD software. Demarcated cuts are merged to create a 3-dimensional region of interest (ROI) used for further quantitative analysis
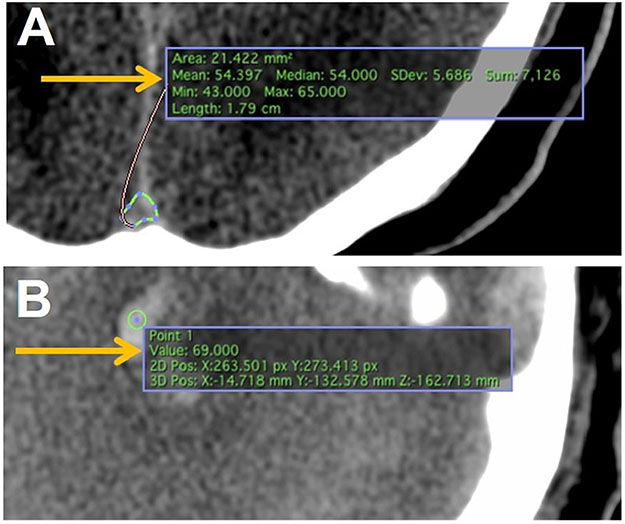
To account for variation in CT slicing and penetration across institutions, a threshold for size and density as a function of Hounsfield units was based on internal measurements. Pixel cross-sectional area was measured for each CT used for analysis. The average Hounsfield unit measurement for the cross section of the superior sagittal sinus just superior to its convergence with the torcula was also calculated. A suspected lesion was only recorded and used for analysis if it (1) appeared qualitatively to represent hemorrhage over other hyperdense elements, such as bone in the skull base, artifact, intracranial monitoring devices, etc.; (2) had at least three contiguous pixels with Hounsfield unit measurements equal to or greater than the measured average Hounsfield units of the superior sagittal sinus just rostral to the torcula; and (3) had a measured volume greater than or equal to 0.025 ml (cm3) (Fig. 3). Brainstem lesions that met inclusion criteria were classified as TAI, contusion, or Duret based on size and associated injury patterns (Fig. 4). To delineate whether a lesion was in the anterior, posterior, or both regions of the brainstem, lesions were classified based on their relationship to a line drawn perpendicular to the midpoint measured from the center of any given brainstem slice on axial CT where the axial plane was parallel to the tuberculum sellae-occipital protuberance line (Fig. 5).
Fig. 3.
Hounsfield unit normalization across computed tomography (CT) images. a Tracing feature in Osirix MD allows the user to trace the superior sagittal sinus just before entry into the torcula. “Mean” (arrow) indicates the mean Hounsfield unit value for the tracing, which was used as the baseline threshold for suspected lesions. b The point-measure feature allows the user to test individual pixels within an image. “Value” (arrow) indicates the Hounsfield unit value for that pixel. Three contiguous pixels above threshold qualified as lesions for measurement. Volume larger than 0.025 cm3 qualified as lesions for inclusion in statistical analysis
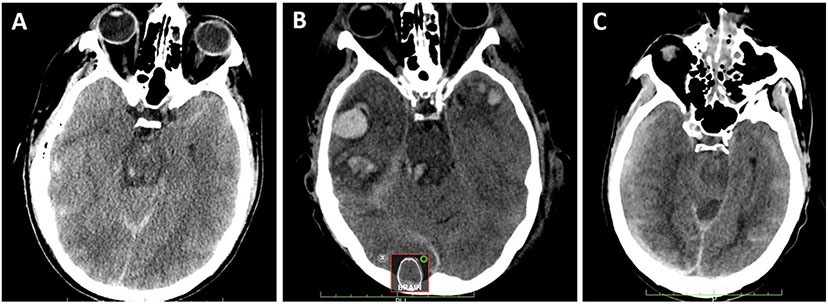
Fig. 4.
Computed tomography (CT) lesion exemplars. a Small, isolated hemorrhagic lesions without associated mass effect or edema most consistent with traumatic axonal injury (TAI). b Larger petechial hemorrhagic lesion with associated edema consistent with brainstem contusion. c Larger, diffuse hemorrhage associated with trans-tentorial mass effect and dusky intervening nonhemorrhagic parenchyma consistent with Duret hemorrhage
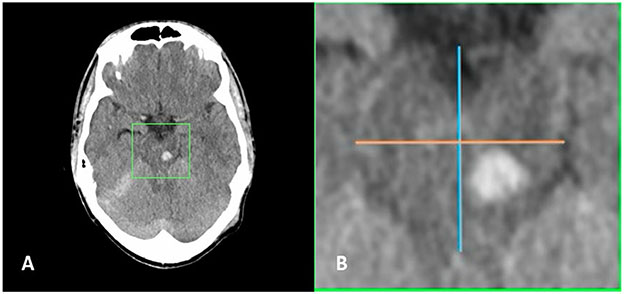
Fig. 5.
Deliniation of anterior versus posterior brainstem injuries. a Axial head computed tomography (CT) slice demonstrating left midbrain traumatic brainstem injury. b Magnified view of area within green box demonstrated in panel a showing midline anterior–posterior line (blue) with perpendicular bisecting line (orange) situating the injury in the posterior half of the brainstem
Statistical analysis
The distribution of patients with BSI+ and BSI− within subgroups of variables were analyzed using Fisher’s exact and Mann–Whitney U-tests, as appropriate. The difference between long-term GOSE scores among groups in variables of interest were analyzed using Mann–Whitney and Spearman correlation, as appropriate. Similarly, bivariate differences in long-term GOSE scores among different subtypes of BSI+ were analyzed using Mann–Whitney and Spearman correlation, as appropriate. Significance tests in all bivariate analysis were conducted without assumptions about underlying distributions (i.e., nonparametric analysis).
BSI+ lesions were analyzed by volume, location and lesion subtype (TAI, contusion, or Duret). Separation of lesions into location by specific brainstem structures was not possible because of bony artifact and low tissue resolution. To improve statistical confidence, location delimitation was limited to anterior and posterior. This also allowed for some analysis of posterior brainstem lesions, as dorsal pons and midbrain lesions have predicted worse outcomes in related literature [9, 14, 24]. Lesions were classified as anterior if the majority of the measured volume was situated anterior to a lateral line drawn perpendicular to the anterior–posterior midpoint of involved brainstem structures on relevant axial CT cuts. Patients with BSI+ were then divided into groups based on those with only one or more posterior, those with only one or more anterior, and those with at least one brainstem lesion in both the anterior and posterior halves of the brainstem.
The association between lesion volume and outcome was analyzed by grouping BSI+ lesions above and below a cutoff point of 1 ml (1 cm3), whereas lesion type was consider as either TAI or Duret/contusion. For lesion volume, this cutoff appeared to best segment lesions resulting from microscopic versus macroscopic mechanisms (i.e., isolated TAI versus arterial injury, venous stasis hemorrhage, etc.). Patients with BSI+ with one or more lesion of at least 1 ml in size were compared with those with all lesions less than 1 ml with regard to observed 6-month GOSE scores using the same statistical tests as location or type for analysis. Given the small sample size, multivariate regression analysis was conducted with exact logistic regression and was limited to no more than three parameters. A sensitivity analyses was performed by extending the match ratios to 3:1 and 5:1. Matches from the BSI− cohort were found for all 29 patients with BSI+, although this required relaxing of the matching rules for sex, age (within 10 years), and GCS score (within 1 point while still matching on mild/moderate/severe). The resulting model parameter estimates in these analyses did not markedly differ from those from the 1:1 matched analysis, suggesting that the selection algorithm used for the matched BSI− cohort was not biasing the results. All statistical analysis was carried out using SPSS version 26 and SAS version 9.4 software.
Results
Twenty-nine patients with BSI+ and 29 patients with BSI− were evaluated. The mean age for all patients was 49.1 years (standard deviation 19.5). Of 58 total patients, 52 patients evaluated were men (90%) and 6 were women (10%; Table 1). There was no significant difference in education, insurance, admission GCS score, hypoxia, hypotension, neuromonitoring, non-central-nervous-system injury severity score, hematocrit, hemoglobin, or the percent of each group who underwent withdrawal of life-sustaining measures within 5 days of injury (p range = 0.26–1.00 across all measures).
Table 1.
TRACK-TBI patient characteristics by BSI status
| n (%) or mean (SD) | BSI status |
|||
|---|---|---|---|---|
| BSI− | BSI+ | p value | ||
| Participants, n | 58 | 29 | 29 | |
| Age (year) | ||||
| Mean (SD) | 49.1 (19.5) | 49.0 (19.5) | 49.2 (19.8) | 0.96 |
| 18–34, n (%) | 16 (28) | 8 (28) | 8 (28) | 1.00 |
| 35–64, n (%) | 29 (50) | 15 (53) | 14 (48) | |
| 65+, n (%) | 13 (22) | 6 (21) | 7 (24) | |
| Sex [n (%)] | ||||
| Male | 52 (90) | 26 (90) | 26 (90) | 1.00 |
| Female | 6 (10) | 3 (10) | 3 (10) | |
| Education | ||||
| Mean (SD) | 12.6 (2.4) | 12.6 (2.4) | 12.6 (2.6) | 0.83 |
| Less than high school, n (%) | 15 (26) | 8 (28) | 7 (24) | 1.00 |
| High school only, n (%) | 14 (24) | 7 (24) | 7 (24) | |
| Some college, n (%) | 17 (29) | 9 (31) | 8 (28) | |
| Unknown, n (%) | 12 (21) | 5 (17) | 7 (24) | |
| Insurance, n (%) | ||||
| Private insurance/Medicare | 33 (57) | 15 (53) | 18 (62) | 0.60 |
| Medicaid/other | 3 (5) | 1 (3) | 2 (7) | |
| Seif-pay | 10 (17) | 6 (21) | 4 (14) | |
| Unknown | 12 (21) | 7 (24) | 5 (17) | |
| Admission GCS | ||||
| Mean (SD) | 5.4 (3.9) | 5.4 (3.9) | 5.4 (3.9) | 1.00 |
| Severe (3–8), n (%) | 46 (79) | 23 (79) | 23 (80) | 1.00 |
| Moderate (9–12), n (%) | 2 (3) | 1 (3) | 1 (3) | |
| Mild (13–15), n (%) | 8 (14) | 4 (14) | 4 (14) | |
| Unknown, n (%) | 2 (3) | 1 (3) | 1 (3) | |
| Hypoxia, n (%) | ||||
| No | 50 (86) | 25 (86) | 25 (86) | 1.00 |
| Yes | 8 (14) | 4 (14) | 4 (14) | |
| Hypotension, n (%) | ||||
| No | 48 (83) | 25 (86) | 23 (80) | 0.73 |
| Yes | 10 (17) | 4 (14) | 6 (20) | |
| Required neuromonitoring, n (%) | ||||
| No | 19 (33) | 12 (41) | 7 (24) | 0.26 |
| Yes | 39 (67) | 17 (59) | 22 (76) | |
| ISS nonhead | ||||
| Mean (SD) | 7.0 (6.3) | 6.0 (6.2) | 7.9 (6.3) | 0.21 |
| 0–3, n(%) | 25 (43) | 15 (52) | 10 (34) | 0.49 |
| 4–8, n (%) | 10 (17) | 4 (14) | 6 (21) | |
| 9+, n (%) | 20 (34) | 9 (31) | 11 (38) | |
| Unknown, n (%) | 3 (5) | 1 (3) | 2 (7) | |
| Hematocrit, n (%) | ||||
| <21 | 50 (86) | 24 (83) | 26 (90) | 0.71 |
| 21+ | 8 (14) | 5 (17) | 3 (10) | |
| Hemoglobin, n (%) | ||||
| <7 | 51 (88) | 22 (76) | 25 (86) | 0.50 |
| 7+ | 7 (12) | 7 (24) | 4 (14) | |
| Withdrawal of life-sustaining measureswithin 5 days, n (%) | ||||
| No | 51 (88) | 26 (90) | 25 (86) | 1.00 |
| Yes | 7 (12) | 3 (10) | 4 (14) | |
Comparison of the distribution of patients with and without BSI on initial CT imaging within demographic groups of interest. Statistical comparison by Fisher’s exact and Mann–Whitney U-tests as appropriate
BSI, brainstem injury, BSI−, cerebrum injuries only, BSI+, brainstem and cerebrum injury, CT, computed tomography, GCS, Glasgow Coma Scale, ISS, injury severity score, SD, standard deviation, TBI, traumatic brain injury, TRACK-TBI, Transforming Research and Clinical Knowledge in Traumatic Brain Injury
Outcome, as evidenced by 6-month GOSE score, did not identify a significant difference between BSI+ and BSI− groups, although there was a trend toward the BSI+ group having less favorable outcome (BSI+ mean GOSE score 2.7, BSI− mean GOSE score 3.9, p = 0.10). This was also the case when removing the patients with withdrawal of life-sustaining treatment (WOLST) from each group and rerunning the analysis to ensure that the patients with WOLST were not driving the results (BSI+ mean GOSE score 3.0, BSI− mean GOSE score 4.2, p = 0.09). To examine this further, we next completed subgroup analysis of the BSI+ group by lesion size, lesion location, and lesion type (Table 2). Assessment of patients with BSI+ by lesion volume above or below 1 cm3 revealed significant differences in 6-month outcome, with 41% of patients with lesions less than 1 cm3 achieving GOSE scores of 4–8, whereas 8% of patients with brainstem lesions greater than 1 cm3 made it into this range (p = 0.004). Supplemental analysis by lesion size removing patients with BSI+ and with WOLST remained significant (p = 0.03). Assessment of patient outcome by brainstem lesion location in anterior or posterior or both halves of the brainstem did not identify any difference in outcome among those subgroups (p = 0.99). Supplemental analysis by lesion location removing patients with BSI+ and with WOLST remained nonsignificant (p = 0.78). In contrast, differentiation by lesion type found that 47% of patients with BSI+ with TAI of the brainstem had favorable outcome compared with 0–13% for those with brainstem contusions or Duret hemorrhage (p = 0.002). Supplemental analysis by lesion type removing patients with BSI+ and with WOLST remained significant (p = 0.02). All 15 of the participants with TAI/shear had a lesion size less than 1 cm3.
Table 2.
Bivariate analysis of mean 6-month GOSE score by brainstem injury subtypes: volume, location, and type
| All BSI+ had an observed 6-mo GOSE score |
n (%) or mean (SD) | 6-mo GOSE score |
||
|---|---|---|---|---|
| Mean | 4–8 (%) | p value | ||
| Patients who were brainstem positive | 29 | 2.7 | 28 | |
| Brainstem lesion volume | ||||
| Mean (SD) | 1.55 (2.22) | <0.001a | ||
| < 1 cm3 | 17 (59%) | 3.6 | 41 | 0.004a |
| ≥ 1 cm3 | 12 (41%) | 1.4 | 8 | |
| Lesion location | ||||
| (A) Anterior only | 8 (28%) | 3.1 | 38 | 0.99 |
| (B) Posterior only | 18 (62%) | 2.6 | 28 | |
| (C) Both | 3 (10%) | 2.3 | 0 | |
| Lesion type | ||||
| (1) TAI/shear | 15 (52%) | 4.0 | 47 | 0.002a |
| (2) Contusion | 6 (21%) | 1.3 | 0 | |
| (3) Duret | 8 (28%) | 1.4 | 13 | |
Comparison of mean 6-mo GOSE scores of patients with brainstem injury by brainstem injury characteristics. Statistical significance computed using Mann–Whitney and Spearman correlation as appropriate. The proportion of patients with GOSE scores of 4–8 is also listed
BSI+, brainstem and cerebrum injury, GOSE, Glasgow Outcome Scale Extended, SD, standard deviation, TAI, traumatic axonal injury
Denotes p value reaching significance
Exact logistic regression analysis was then used to evaluate the predictive ability of brainstem injury characteristics in an exploratory fashion compared to other clinical and demographic parameters previously reported to be predictive (Table 3). First, univariate exact logistic regression was employed to test the predictive ability of admission GCS score, age, sex, BSI volume, and BSI type. Admission GCS score by itself was not significantly predictive of favorable outcome (odds ratio [OR] 1.07, confidence interval [CI] 0.93–1.23, p = 0.37). Age was subdivided into three groups, 18–34 years, 35–64 years, and 65+ years, and was found to only be significant for the oldest age group (OR 0.06, CI 0.00–0.55, p = 0.01), indicating older age by itself was predictive of less favorable outcome. Consideration of sex was not found to be significant (OR 0.30, CI 0.00–2.97, p = 0.51), whereas subgroups of BSI volume and BSI type were found to significantly predict less favorable outcome, specifically, BSI volume greater than 1 cm3 (OR 0.10, CI 0.00–0.87, p = 0.02) and BSI lesion type of contusion/Duret hemorrhage (OR 0.09, CI 0.00–0.72, p = 0.02). It should be noted the near-perfect overlap in the patients with larger lesions and those identified with contusion or Duret hemorrhage, so only BSI type was used for further exporatory analysis.
Table 3.
Predicting favorable outcome at 6 months after TBI in patients with brainstem lesions using exact logistic regression
| Comparison | OR | 95% CI | p value | |
|---|---|---|---|---|
| Univariate | ||||
| Admission GCSa | 1.07 | 0.93, 1.23 | 0.37 | |
| Age (year) | 0.01b | |||
| (35–64) vs. (18–34) | 0.38 | 0.08, 1.52 | 0.20 | |
| 65+ vs. (18–34) | 0.06 | 0.00, 0.55 | 0.01b | |
| Sex | Female vs. male | 0.30 | 0.00, 2.97 | 0.51 |
| BSI volume | 0.04b | |||
| <1 cm3 vs. no BSI | 0.76 | 0.19, 2.94 | 0.88 | |
| >1 cm3 vs. no BSI | 0.10 | 0.00, 0.87 | 0.02b | |
| Type | 0.02b | |||
| TAI vs. no BSI | 0.94 | 0.22, 3.89 | 1.00 | |
| Contusion/Duret vs. no BSI | 0.09 | 0.00, 0.72 | 0.02b | |
| Multivariate | Admission GCS, BSI type | OR | 95% Confidence interval | p value |
| Admission GCSa | 1.09 | 0.94, 1.29 | 0.28 | |
| Type | 0.01b | |||
| TAI vs. No BSI | 1.03 | 0.24, 4.52 | 1.00 | |
| Contusion/Duret vs. no BSI | 0.08 | 0.00, 0.67 | 0.01b | |
| Multivariate | Admission GCS, age, BSI type | OR | 95% CI | p value |
| Admission GCSa | 1.23 | 1.01, 1.58 | 0.04 | |
| Age (yr) | 0.02b | |||
| (35–64) vs. (18–34) | 0.31 | 0.05, 1.52 | 0.18 | |
| 65+ vs. (18–34) | 0.04 | 0.00, 0.68 | 0.02b | |
| Type | 0.09 | |||
| TAI vs. no BSI | 0.68 | 0.11, 3.55 | 0.86 | |
| Contusion/Duret vs. no BSI | 0.12 | 0.00, 1.08 | 0.06 |
Univariate and multivariate analysis of variables of interest ability to predict GOSE scores of 4–8 at 6 months. All analyses carried out using exact logistic regression BSI, brainstem injury, GCS, Glasgow Coma Scale, GOSE, Glasgow Outcome Scale Extended, OR, odds ratio, TAI, traumatic axonal injury, TBI, traumatic brain injury
Estimate of OR corresponds to the change in odds due to a 1-pt increase in GCS; one subject is missing their GCS score and is thus excluded from all multivariate models
Denotes p value reaching statistical significance
Given these univariate findings, we last explored whether multivariate modeling of the significant univariate elements would strengthen the overall prediction of favorable outcome and whether there would be a unique contribution of brainstem leisons. Given the groups sizes, only two models were employed: one considering GCS and brainstem lesion type, the second adding in age given it was also significant in univariate analysis. GCS was “forced” into both models given its clinical relevance in the acute critical care environment for medical decision-making. Multivariate modeling of admission GCS score and brainstem lesion type identified a significant and unique contribution of brainstem lesion type, again contusion/Duret hemorrhage to less favorable outcome (OR 0.08, CI 0.00–0.67, p = 0.01). Multivariate modeling of admission GCS score, age subdivided into three groups, and BSI type revealed significant associations to favorable outcome in admission GCS score (OR 1.23, CI 1.01–1.58, p = 0.04) and the oldest age group of 65+ years (OR 0.04, CI 0.00–0.68, p = 0.02), whereas BSI type of contusion/Duret hemorrhage (OR 0.12, CI 0.00–1.08, p = 0.06) remained very close to significance, suggesting still an important contribution to the model. We interpret the slight lessoning of predictive strength of BSI type when age is considered to reflect the predilication for older patients with BSI to also more likely be specifically those with contusion/Duret hemorrhage injuries (five of the seven pateitns with BSI who were 65+ years had contusion/Duret hemorrhage).
Discussion
This study provides evidence that patients with brainstem lesions evident on acute head CT have the potential for favorable outcome and do not differ significantly when compared directly with patient with TBI with similar injury severity who do not have brainstem lesions. In fact, 28% of BSI+ met criteria for 6-month favorable outcome on GOSE. A lower 6-month GOSE profile was observed in our BSI+ cohort with lesions above a volume threshold of 1 ml. BSI volume greater than 1 ml was associated with a mean 6-month GOSE score of 1.4, implying most patients in that group did not survive. Conversely, patients with hemorrhage volume less than 1 ml had a significantly better mean outcome score of 3.6 (p = 0.004), and a reasonable chance of recovering functional independence, with 41% recovering to a 6-month GOSE score of 4 or higher. In parallel with lesions of larger volume, the mean 6-month GOSE scores for patients with Duret or contusion-type hemorrhage was 1.4 and 1.3. Patients with brainstem TAI/shear type injury had a significantly higher mean outcome score of 4.0 (p = 0.002), and nearly half of these patients reached GOSE scores of 4 or higher at 6 months (47%).
Admission GCS scores have been validated as an independent predictor of long-term outcomes in two well-designed prediction models, one developed by the Corticosteroid Randomization After Significant Head injury trial investigators and the other from the International Mission for Prognosis and Analysis of Clinical Trials in TBI group, both of which have been externally validated by numerous groups [25-28]. Although we saw no difference in admission GCS score because we intentionally matched on it as a variable of interest, we did observe that admission GCS score was a significant predictor of outcome in multivariate analysis (p = 0.04) even when the presence of age and BSI of any kind was controlled for. However, admission GCS score is often confounded by sedation for intubation, metabolic nervous system suppression secondary to extracranial injury, and patient intoxication, limiting its independent predictive utility [26].
When patients with BSI+ were isolated for logistic regression analysis, both lesion volume less than 1 ml and TAI lesion types were significantly predictive of favorable outcome at 6 months (p = 0.004 and p = 0.002, respectively). In contrast, Duret hemorrhage has been reported to be associated with severely elevated intracranial pressure leading to transtentorial herniation and brainstem distortion [29]. Duret hemorrhage and brainstem contusion may be the result of more complex intracranial phenomena than isolated rotational acceleration injury, leading to compound and more severe BSI. This is consistent with the poor long-term outcomes observed in the subset of patients with BSI+ with lesions larger than 1 ml and/or contusion or Duret hemorrhage injury patterns.
The majority of brainstem lesions observed to be less than 1 ml in volume were consistent with shear injury from predominantly rotational acceleration, the mechanism underlying TAI [30-32]. Advances in neurocritical care, rehabilitation medicine and MRI have shed a new light on the possibility of recovery even in high-grade diffuse axonal injury (4 or more instances of TAI) involving brainstem structures [9, 14, 16-19]. TAI lesions tend to be smaller in size on imaging, and they may be less devastating than Duret hemorrhage and hemorrhagic brainstem contusion because they are less likely to injure bilateral structures in the dorsal, rostral brainstem, the seat of the arousal network [33, 34]. In this study and others, patients with brainstem TAI have a reasonable chance for functional recovery, and for this reason, traumatic BSI should not be interpreted as a categorical entity from a mechanistic, pathologic, or prognostic standpoint.
MRI remains superior in its ability to detect traumatic BSI [9, 10]. Multiple studies indicate added precision in prognostication when brainstem lesions are included in models, even when controlling for other clinical, demographic and imaging factors [8, 12, 13, 17, 23]. Although MRI acquisition in the early stages of post-TBI care is often not feasible, a study designed to compare early CT with early MRI in patients with traumatic BSI would allow more insight into the best characterization of BSI subtype and other TBI lesions outside the brainstem that may be contributing to observed long-term outcomes.
This study’s findings should be balanced by its limitations, which include a small sample size, restricting statistical analysis and confidence, lack of further patient follow-up, inability to control for post acute care and other patient comorbidities not collected by the TRACK-TBI study, some missing clinical and demographic data, incomplete more advanced neuroimaging (such as MRI collected on these patients), and other unmeasured parameters that may inform outcome. Furthermore, the data were analyzed in a retrospective and unblinded fashion, and detailed information about medical decision-making is not known. As such, we acknowledge that the findings of this study may be heavily influenced by confirmation bias. Further prospective study of BSI lesions on early head CT in their relation to additional imaging, clinical parameters, and long-term outcome is needed before they can be incorporated into widely used prognostic models.
Conclusions
The current study provides early evidence that a subset of patients with TBI with BSI can achieve favorable outcomes. Specifically, there appear to be two distinct groups of patients with acute traumatic BSI identifiable with early head CT: one group with smaller lesions consistent with TAI with a reasonable chance of recovery and another group with larger lesions consistent with Duret hemorrhage or contusion with less likelihood of regaining functional independence. This division may be helpful in designing larger studies to provide more robust evidence for the inclusion of BSI in prognostic models. However, until those studies are available, the data presented in this study should be interpreted with caution given the limitations.
Acknowledgments
We would like to thank the patients and their families for their participation and support of the TRACK-TBI study. Funding for the original TRACK-TBI study was awarded to GTM by the National Institutes of Health.
Source of support
Funding was provided by National Institute of Neurological Disorders and Stroke (Grant No. U01NS086090) and U.S. Department of Defense (Grant No. W81XWH-14-2-0176).
Appendix: TRACK-TBI Investigators
Opeolu Adeoye, MD, University of Cincinnati; Neeraj Badjatia, MD, University of Maryland; Kim Boase, University of Washington; Yelena Bodien, PhD, Massachusetts General Hospital; M. Ross Bullock, MD PhD, University of Miami; Randall Chesnut, MD, University of Washington; John D. Corrigan, PhD, ABPP, Ohio State University; Karen Crawford, University of Southern California; Ramon Diaz-Arrastia, MD PhD, University of Pennsylvania; Sureyya Dikmen, PhD, University of Washington; Ann-Christine Duhaime, MD, MassGeneral Hospital for Children; Richard Ellenbogen, MD, University of Washington; V Ramana Feeser, MD, Virginia Commonwealth University; Adam R. Ferguson, PhD, University of California, San Francisco; Brandon Foreman, MD, University of Cincinnati; Raquel Gardner, University of California, San Francisco; Etienne Gaudette, PhD, University of Southern California; Dana Goldman, PhD, University of Southern California; Luis Gonzalez, TIRR Memorial Hermann; Shankar Gopinath, MD, Baylor College of Medicine; Rao Gullapalli, PhD, University of Maryland; J Claude Hemphill, MD, University of California, San Francisco; Gillian Hotz, PhD, University of Miami; Sonia Jain, PhD, University of California, San Diego; C. Dirk Keene, MD PhD, University of Washington; Frederick K. Korley, MD, PhD, University of Michigan; Joel Kramer, PsyD, University of California, San Francisco; Natalie Kreitzer, MD, University of Cincinnati; Harvey Levin, MD, Baylor College of Medicine; Chris Lindsell, PhD, Vanderbilt University; Joan Machamer, MA, University of Washington; Christopher Madden, MD, UT Southwestern; Alastair Martin, PhD, University of California, San Francisco; Thomas McAllister, MD, Indiana University; Michael McCrea, PhD, Medical College of Wisconsin; Randall Merchant, PhD, Virginia Commonwealth University; Lindsay Nelson, PhD, Medical College of Wisconsin; Laura B. Ngwenya, MD, PhD, University of Cincinnati; Florence Noel, PhD, Baylor College of Medicine; Amber Nolan, MD PhD, University of California, San Francisco; Eva Palacios, PhD, University of California, San Francisco; Daniel Perl, MD, Uniformed Services University; Ava Puccio, PhD, University of Pittsburgh; Miri Rabinowitz, PhD, University of Pittsburgh; Claudia Robertson, MD, Baylor College of Medicine; Jonathan Rosand, MD, MSc, Massachusetts General Hospital; Angelle Sander, PhD, Baylor College of Medicine; Gabriella Satris, University of California, San Francisco; David Schnyer, PhD, UT Austin; Seth Seabury, PhD, University of Southern California; Murray Stein, MD MPH, University of California, San Diego; Sabrina Taylor, PhD, University of California, San Francisco; Arthur Toga, PhD, University of Southern California; Alex Valadka, MD, Virginia Commonwealth University; Mary Vassar, RN MS, University of California, San Francisco; Paul Vespa, MD, University of California, Los Angeles; Kevin Wang, PhD, University of Florida; John K. Yue, MD, University of California, San Francisco; Ross Zafonte, Harvard Medical School.
Footnotes
Conflicts of interest
Dr. Williams reports Grants from Neurosurgery Research and Education Foundation Fellowship, Grants from Adler Giersch Law Firm Endowed Fund for Traumatic Brain Injury Research, outside the submitted work. Mr. Nieblas-Bedolla has nothing to disclose. Dr. Feroze has nothing to disclose. Dr. Young has nothing to disclose. Dr. Temkin reports Grants from US Federal Government, during the conduct of the study. Dr. Giacino reports other funding from University of California at San Francisco, during the conduct of the study. Dr. Okonkwo has nothing to disclose. Dr. Manley reports Grants from United States Department of Defense, and a contract from United States Department of Defense/MTEC, Grants from NIH-NINDS, other from United States Department of Energy, other from One Mind, and other from NeuroTruama Sciences LLC, during the conduct of the study; other from National Football League Scientific Advisory Board, outside the submitted work. Mr. Barber has nothing to disclose. Dr. Durfy has nothing to disclose. Ms. Markowitz reports Grants and contracts from US Department of Defense/MTEC, and salary support from United States Department of Energy and One Mind during the conduct of the study. Dr. Yuh reports Grant support through NIH, DoD during the conduct of the study. Dr. Mukherjee reports Grants from NIH, DoD, outside the submitted work; In addition, Dr. Mukherjee has a patent US PTO Serial No. 15/782,005 pending to University of California Regents, and a patent PCT/US No. 20/42811 pending to University of California Regents. Dr. Mac Donald reports Grants from National Institute of Neurological Disorders, Grants from Department of Defense, outside the submitted work.
Ethical approval/informed concent
We further confirm adherence to ethical guidelines and investigated whether we needed institutional review board approval, but as this was a retrospective analysis of deidentified patient data, it was deemed “nonhuman subjects” and was not required to have formal institutional review board approval.
References
- 1.Schweitzer AD, Niogi SN, Whitlow CT, Tsiouris AJ. Traumatic brain injury: imaging patterns and complications. Radiographics. 2019;39:1571–95. [DOI] [PubMed] [Google Scholar]
- 2.Wintermark M, Sanelli PC, Anzai Y, Tsiouris AJ, Whitlow CT. Imaging evidence and recommendations for traumatic brain injury: advanced neuro- and neurovascular imaging techniques. AJNR Am J Neuroradiol. 2015;36:E1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thelin EP, Nelson DW, Vehvilainen J, et al. Evaluation of novel computerized tomography scoring systems in human traumatic brain injury: an observational, multicenter study. PLoS Med. 2017;14:e1002368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown AW, Pretz CR, Bell KR, et al. Predictive utility of an adapted Marshall head CT classification scheme after traumatic brain injury. Brain Inj. 2019;33:610–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubin R. Traumatic brain injury hospital stays are longer, more costly. JAMA. 2020;323:1998. [DOI] [PubMed] [Google Scholar]
- 6.Raj R, Siironen J, Skrifvars MB, Hernesniemi J, Kivisaari R. Predicting outcome in traumatic brain injury: development of a novel computerized tomography classification system (Helsinki computerized tomography score). Neurosurgery. 2014;75:632–46; discussion 46–7. [DOI] [PubMed] [Google Scholar]
- 7.Nelson DW, Nyström H, MacCallum RM, et al. Extended analysis of early computed tomography scans of traumatic brain injured patients and relations to outcome. J Neurotrauma. 2010;27:51–64. [DOI] [PubMed] [Google Scholar]
- 8.Yuh EL, Cooper SR, Ferguson AR, Manley GT. Quantitative CT improves outcome prediction in acute traumatic brain injury. J Neurotrauma. 2012;29:735–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenblum WI. Immediate, irreversible, posttraumatic coma: a review indicating that bilateral brainstem injury rather than widespread hemispheric damage is essential for its production. J Neuropathol Exp Neurol. 2015;74:198–202. [DOI] [PubMed] [Google Scholar]
- 10.Chew BG, Spearman CM, Quigley MR, Wilberger JE. The prognostic significance of traumatic brainstem injury detected on T2-weighted MRI. J Neurosurg. 2012;117:722–8. [DOI] [PubMed] [Google Scholar]
- 11.Yuh EL, Mukherjee P, Lingsma HF, et al. Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Ann Neurol. 2013;73:224–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cicuendez M, Castaño-León A, Ramos A, Hilario A, Gñmez PA, Lagares A. The added prognostic value of magnetic resonance imaging in traumatic brain injury: the importance of traumatic axonal injury when performing ordinal logistic regression. J Neuroradiol. 2019;46:299–306. [DOI] [PubMed] [Google Scholar]
- 13.Haghbayan H, Boutin A, Laflamme M, et al. The prognostic value of MRI in moderate and severe traumatic brain injury: a systematic review and meta-analysis. Crit Care Med. 2017;45:e1280–8. [DOI] [PubMed] [Google Scholar]
- 14.Izzy S, Mazwi NL, Martinez S, et al. Revisiting grade 3 diffuse axonal injury: not all brainstem microbleeds are prognostically equal. Neurocrit Care. 2017;27:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gentry LR, Godersky JC, Thompson B, Dunn VD. Prospective comparative study of intermediate-field MR and CT in the evaluation of closed head trauma. AJR Am J Roentgenol. 1988;150:673–82. [DOI] [PubMed] [Google Scholar]
- 16.Skandsen T, Kvistad KA, Solheim O, Strand IH, Folvik M, Vik A. Prevalence and impact of diffuse axonal injury in patients with moderate and severe head injury: a cohort study of early magnetic resonance imaging findings and 1-year outcome. J Neurosurg. 2010;113:556–63. [DOI] [PubMed] [Google Scholar]
- 17.Skandsen T, Kvistad KA, Solheim O, Lydersen S, Strand IH, Vik A. Prognostic value of magnetic resonance imaging in moderate and severe head injury: a prospective study of early MRI findings and one-year outcome. J Neurotrauma. 2011;28:691–9. [DOI] [PubMed] [Google Scholar]
- 18.Mannion RJ, Cross J, Bradley P et al. Mechanism-based MRI classification of traumatic brainstem injury and its relationship to outcome. J Neurotrauma. 2007;24:128–35. [DOI] [PubMed] [Google Scholar]
- 19.Edlow BL, Giacino JT, Hirschberg RE, Gerrard J, Wu O, Hochberg LR. Unexpected recovery of function after severe traumatic brain injury: the limits of early neuroimaging-based outcome prediction. Neurocrit Care. 2013;19:364–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brain and Spinal Injury Center. Transforming research and clinical knowledge in TBI. Brain and Spinal Injury Center, UCSF. 2014. Accessed July 13, 2020. https://tracktbi.ucsf.edu/transforming-research-and-clinical-knowledge-tbi. [Google Scholar]
- 21.Maas AI, Harrison-Felix CL, Menon D, et al. Common data elements for traumatic brain injury: recommendations from the interagency working group on demographics and clinical assessment. Arch Phys Med Rehabil. 2010;91:1641–9. [DOI] [PubMed] [Google Scholar]
- 22.Shukla D, Devi BI, Agrawal A. Outcome measures for traumatic brain injury. Clin Neurol Neurosurg. 2011;113:435–41. [DOI] [PubMed] [Google Scholar]
- 23.Yue JK, Vassar MJ, Lingsma HF, et al. Transforming research and clinical knowledge in traumatic brain injury pilot: multicenter implementation of the common data elements for traumatic brain injury. J Neurotrauma. 2013;30:1831–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parvizi J, Damasio AR. Neuroanatomical correlates of brainstem coma. Brain. 2003;126:1524–36. [DOI] [PubMed] [Google Scholar]
- 25.Roozenbeek B, Lingsma HF, Lecky FE, et al. Prediction of outcome after moderate and severe traumatic brain injury: external validation of the International Mission on Prognosis and Analysis of Clinical Trials (IMPACT) and Corticoid Randomisation After Significant Head injury (CRASH) prognostic models. Crit Care Med. 2012;40:1609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lingsma H, Andriessen TM, Haitsema I, et al. Prognosis in moderate and severe traumatic brain injury: external validation of the IMPACT models and the role of extracranial injuries. J Trauma Acute Care Surg. 2013;74:639–46. [DOI] [PubMed] [Google Scholar]
- 27.Gómez PA, de la Cruz J, Lora D, et al. Validation of a prognostic score for early mortality in severe head injury cases. J Neurosurg. 2014;121:1314–22. [DOI] [PubMed] [Google Scholar]
- 28.Murray GD, Butcher I, McHugh GS, et al. Multivariable prognostic analysis in traumatic brain injury: results from the IMPACT study. J Jeurotrauma. 2007;24:329–37. [DOI] [PubMed] [Google Scholar]
- 29.Duret RL. A rare and little known hemorrhagic syndrome. Brux Med. 1955;35:797–800. [PubMed] [Google Scholar]
- 30.Meany DF, Cullen DK. Biomechanical basis of traumatic brain injury. Youmans and Winn’s Neurological Surgery, 7th edition. 2016. 2755–64.e1. [Google Scholar]
- 31.Gennarelli TA, Thibault LE, Adams JH, Graham DI, Thompson CJ, Marcincin RP. Diffuse axonal injury and traumatic coma in the primate. Ann Neurol. 1982;12:564–74. [DOI] [PubMed] [Google Scholar]
- 32.Adams JH, Doyle D, Ford I, Gennarelli TA, Graham DI, McLellan DR. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology. 1989;15:49–59. [DOI] [PubMed] [Google Scholar]
- 33.Edlow BL, Takahashi E, Wu O, et al. Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. J Neuropathol Exp Neurol. 2012;71:531–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edlow BL, Haynes RL, Takahashi E, et al. Disconnection of the ascending arousal system in traumatic coma. J Neuropathol Exp Neurol. 2013;72:505–23. [DOI] [PMC free article] [PubMed] [Google Scholar]