Abstract
Recent evidence has shown that gut microbiota dysbiosis is associated with development of gestational diabetes mellitus (GDM). However, the gut microbiota composition of non-obese women with GDM, which accounts for a relatively large percentage of Asian GDM, is unknown. We investigated the characteristics of gut microbiota of Japanese pregnant women with GDM. Fecal samples from Japanese pregnant women with GDM (n=20) and normal glucose tolerance (NGT, n=16) were collected at the time of GDM diagnosis (T1), at 35–37 weeks of gestation (T2), and at 4 weeks postpartum (T3). Gut microbiota composition was characterized from fecal DNA by sequencing of 16S rRNA genes. Serum samples were collected late in the third trimester, and the circulating levels of adiponectin and IL-6 were measured by ELISA. At the genus level, Peptostreptococcaceae Romboutsia was enriched in GDM women at T1 (p=0.008) and T2 (p=0.047). The women with lower serum adiponectin tended to have more Romboutsia. The Shannon index was significantly lower in the GDM women at T3 than in the NGT women (p=0.008), and that of the GDM women decreased significantly from T2 to T3 (p=0.02). No significant difference in bacterial community structure was found in a beta diversity analysis. The non-obese GDM women (body mass index <25.0 kg/m2) showed a lower abundance of Coriobacteriaceae Collinsella at T1 (p=0.03) and higher abundance of Akkermansia at T2 (p=0.04) than the normal control. The non-obese GDM women had the distinctive gut microbiota profiles. Analysis of gut microbiota is potentially useful for risk assessment of GDM in non-obese pregnant women.
Keywords: gut microbiota, gestational diabetes mellitus, non-obesity, Collinsella, Akkermansia
INTRODUCTION
Recent evidence has shown that gut microbiota dysbiosis is associated with a wide variety of metabolic diseases, including type 2 diabetes mellitus (DM) [1]. Some studies have also reported the importance of the gut microbiota composition in the development of gestational diabetes mellitus (GDM), a transient form of glucose intolerance in pregnant women [2, 3]. GDM is characterized by an imbalance between insulin secretion and insulin resistance during pregnancy. Obese women already have elevated levels of insulin resistance before conception, and they develop relative insulin deficiency due to the physiologically increased in insulin resistance caused by pregnancy. On the other hand, there are also GDM women incapable of producing enough insulin to meet even the physiological insulin demand during pregnancy, possibly because of impairment of their ability to secrete insulin [4]. According to a previous report, there is a high proportion of non-obese women with poor insulin secretion ability among Japanese GDM women [5]. Most of the previous reports on the gut microbiota of GDM women have mainly included the overweight or obese women [6,7,8,9,10]. Therefore, the significance of gut microbiota in the development of GDM in the non-obese women with impaired insulin secretion has been poorly investigated.
In the current study, we aimed to reveal the characteristics of gut microbiota of Japanese pregnant women with GDM, especially focusing on the non-obese cases.
MATERIALS AND METHODS
Study population and sample collection
We conducted a prospective observational study on Japanese pregnant women who received prenatal care and delivered at Kyorin University Hospital, a tertiary perinatal center in Tokyo, Japan, from April 2018 to November 2019. We enrolled pregnant women who underwent 75 g oral glucose tolerance tests (OGTTs) before 27 weeks of gestation for GDM diagnosis. The patients were diagnosed with GDM based on International Association of the Diabetes and Pregnancy Study Groups criteria [11], whereas those who were diagnosed as negative were categorized as having normal glucose tolerance (NGT). Women who met the following criteria were excluded from the study: multiple pregnancy, use of antibiotics during pregnancy, and any pathological intestinal conditions which required medication, such as inflammatory bowel diseases. Participants with a pre-pregnancy body mass index (BMI) ≥25.0 kg/m2 were categorized as OW/OB (overweight/obese); the rest were categorized as non-OW/OB. This study was approved by the institutional review board of Kyorin University School of Medicine (No. 712), and all included women provided a written informed consent.
Stool samples were collected from all participants a total of three times, at the time of GDM diagnosis (T1), at 35–37 weeks of gestation (T2), and at 4 weeks postpartum period (T3). The stool samples were self-collected by the participants at home using a specimen collection kit (TechnoSuruga Laboratory, Shizuoka, Japan) and brought to the hospital within 2 days.
Blood samples were collected at the time of admission for delivery and centrifuged at 1,500 × g for 10 min at room temperature. The serum was collected and stored at −80°C.
Fecal DNA extraction and 16S rRNA amplicon target sequencing
DNA extraction from stool samples was carried out using NucleoSpin® DNA Stool kit (MACHEREY-NAGEL GmbH & Co., UK MACHEREY-NAGEL, Fisher Scientific UK, Loughborough, UK) based on the manufacturer’s instructions. The DNA concentration and quality of purified DNA were analyzed with a Qubit fluorometer (Life Technologies) and TapeStation (Agilent). A 16S library was constructed according to the “16S Metagenomic Sequencing Library Preparation” protocol recommended by Illumina. Polymerase chain reaction (PCR) was performed with a TaKaRa Cycler Dice Touch (TaKaRa) and 2 × KAPA HiFi HotStart ReadyMix (Kapa Biosystems, Wilmington, MA, USA) under the following conditions: initial denaturation at 95°C for 3 min, followed by 25 cycles of 95°C for 30 sec, 55°C for 30 sec, and 72°C for 30 sec, and finally an extension step at 72°C for 5 min. DNA concentration and size distribution of ready libraries were analyzed with a Qubit fluorometer and TapeStation. PCR products were purified using AMPure XP magnetic beads (Beckman Coulter Inc., Carlsbad, CA, USA) diluted into an equimolar concentration and pooled according to their unique barcode sequences, which enabled multiplexing. Next, Illumina dual-index barcodes were added to the pooled PCR products with a Nextera XT Index Kit (Illumina, San Diego, CA, USA). Indexed PCR products were purified and pooled in equimolar amounts prior to paired-end sequencing with MiSeq Reagent Kit v3 (600 – cycles; Illumina) following the manufacturer’s directions.
Sequence data analysis
For microbial sequence analysis, the assembled reads were processed using the QIIME2 platform (v. 2019.10). Sequence reads were imported into QIIME2 with quality assessment, filtering, barcode trimming, and chimera detection performed using the DADA2 pipeline. A taxonomic classification was assigned to amplicon sequence variants using the SILVA release 132 with taxonomic classification at >99% confidence. Sequencing data for phyla, family, and genus amplicon sequence variants were calculated by dividing the number of reads for each taxon by the number of reads in the sample. All amplicon sequence variants with an average of >0.5% relative abundance were included for data analysis. Alpha-diversity indices calculations including the Shannon index, Faith’s phylogenic diversity (PD), and observed operational taxonomic units (OTUs), were calculated and visualized with QIIME scripts. The cross-sectional difference in beta diversity between the groups was assessed by permutational analysis of variance (PERMANOVA) of un-weighted and weighted UniFrac distances and illustrated by PCoA models.
ELISA
The concentrations of adiponectin and IL-6 in the serum samples were assayed by ELISA kits (R & D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. The sensitivities of the assays for adiponectin and IL-6 were 0.989 ng/mL and 0.7 pg/mL, respectively. Based on the previous reports on the cytokines in GDM women [12,13,14,15,16], the levels of adiponectin and IL-6 were categorized as follows: high adiponectin, ≥5.0 ng/mL; low adiponectin, <5.0 ng/mL; high IL-6, >5.0 pg/mL; and low IL-6, ≤5.0 pg/mL.
Statistical analysis
Statistical analysis was performed in R version 3.6.3 (The R Foundation, Vienna, Austria; http://www.r-project.org). All results are presented as medians (ranges). Maternal characteristics were compared between groups using χ2 (or Fisher’s exact) or Mann–Whitney U tests. Differences in the gut microbiota analysis were tested with the Wilcoxon signed-rank test. Bonferroni’s correction was applied for multiple comparisons, and a p-value <0.05 was considered as statistically significant. For beta diversity, the Benjamini-Hochberg procedure was applied in QIIME2.
RESULTS
Clinical characteristics
We ultimately enrolled 20 GDM and 16 NGT pregnant women. The demographic data of the participants are shown in Table 1. There were only two OW/OB cases in the NGT group. Non-OW/OB women accounted for 40% of the GDM group (8/20). All GDM patients maintained good glycemic control with HbA1c <6.0% at 36 weeks of gestations. There was no difference in maternal age or proportion of primipara between the GDM and NGT groups overall. The T3 postpartum period was shorter than in the NGT group, but the difference in mean numbers of days was only two days (NGT, 33 days; GDM, 35 days).
Table 1. Clinical characteristics of the participants.
| NGT | GDM | p-value† | |||||
|---|---|---|---|---|---|---|---|
| All | Non-OW/OB | All | Non-OW/OB | OW/OB | All | Non-OW/OB | |
| N | 16 | 14 | 20 | 8 | 12 | ||
| Age (years) | 40 (24–43) | 40 (24–43) | 38 (29–45) | 39 (29–45) | 38 (29–45) | 0.77 | 0.94 |
| Primipara | 11 (69%) | 9 (64%) | 7 (35%) | 4 (50%) | 3 (25%) | 0.09 | 0.66 |
| BMI (kg/m2) | 21.3 (16.2–37.2) | 20.7 (16.2–24.4) | 24.3 (17.8–34.4) | 20.0 (27.8–23.5) | 28.4 (25.0–34.4) | 0.06 | 0.87 |
| OGTT (weeks) | 14 (12–18) | 14 (12–18) | 15 (12–26) | 16 (14–26) | 14 (12–26) | 0.15 | 0.03 |
| T1 (weeks) | 16 (13–24) | 17.5 (13–24) | 17.5 (15–22) | 17.5 (15–20) | 18 (15–22) | 0.96 | 0.35 |
| T2 (weeks) | 35 (35–37) | 35.5 (35–37) | 35 (35–37) | 35.5 (35–37) | 35 (35–37) | 0.99 | 0.99 |
| T3 (days) | 33 (27–42) | 33 (27–42) | 35 (29–45) | 35 (29–43) | 38 (32–45) | 0.02 | 0.06 |
| Delivery weeks | 39 (36–41) | 39 (36–41) | 38 (33–41) | 38.5 (37–40) | 38 (33–41) | 0.44 | 0.24 |
| Cesarean section | 7 (44%) | 5 (36%) | 8 (40%) | 1 (13%) | 7 (58%) | 0.99 | 0.35 |
| Weight gain (kg) | 9.1 (1.3–18.2) | 9.3 (5.9–18.2) | 7 (–4.2–13.9) | 8.5 (5.3–13.9) | 5.2 (–4.2–13.8) | 0.11 | 0.34 |
Data are presented as medians (range) or numbers (%).
BMI, pre-pregnancy body mass index; OGTT, 75 g oral glucose tolerance test; T1, test at the time of diagnosis; T2, test at 35–37 weeks of gestation; T3, test in the postpartum period. † NGT vs GDM.
There was no major difference in the maternal backgrounds of the non-OW/OB patients between the GDM and NGT groups. The timing of the OGTTs was earlier in the NGT group, with the mean difference being only two weeks (non-OW/OB NGT, 14 weeks; non-OW/OB GDM, 16 weeks), and there was no difference in the number of gestational weeks for T1.
Differential abundance analysis
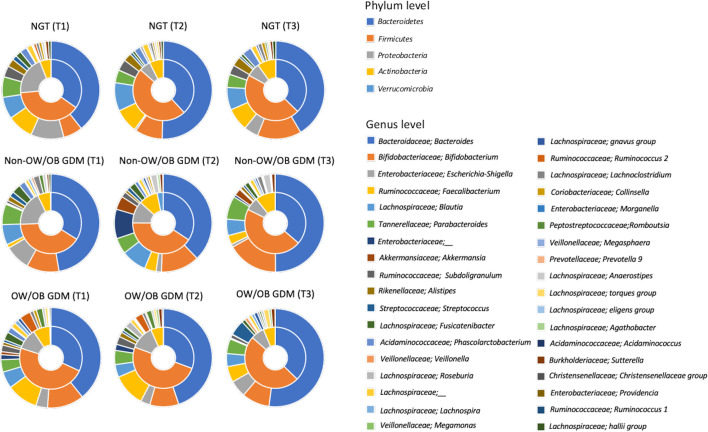
The gut microbiota compositions of three groups (NGT, non-OW/OB GDM, and OW/OB GDM) are shown in Fig. 1. The gut microbiota composition of each group showed longitudinal changes from the pregnancy to postpartum periods. Differences in gut microbiota composition were observed in the non-OW/OB GDM group compared with the NGT group and OW/OB GDM group, especially in late pregnancy (T2).
Fig. 1.
Relative abundance of taxa in fecal microbiota.
Pie charts show the phylum (pie) and genus (outer ring) level distribution. In this analysis, only phyla and genera with relative abundances of more than 0.5% were included. The data of NGT is from all samples.
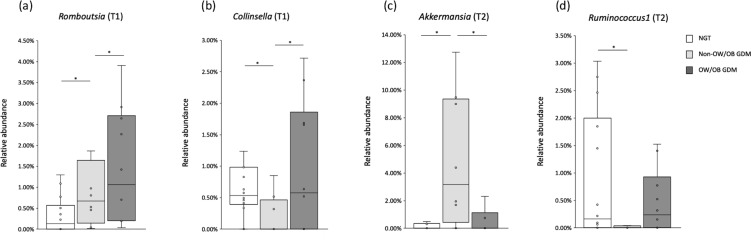
Initially, we compared the relative abundances of OTUs for all subjects between the NGT and GDM groups (Table 2). No significantly different OTUs were detected at the phylum or family level. At the genus level, the relative abundance of Romboutsia was significantly higher in the GDM group during pregnancy (T1, p=0.008; T2, p=0.047) (Fig. 2). Prevotella 9 was more abundant in the NGT group in T3 (p=0.03); however, only 4 of 30 samples had relative abundances >0.5. Furthermore, the genus was not detected in 23 samples.
Table 2. Relative abundance of differentially enriched genera between the NGT and GDM women (all subjects).
| All | NGT (n=16) | GDM (n=20) | ||||
|---|---|---|---|---|---|---|
| Taxon | Median (%) | Min–Max (%) | Median (%) | Min–Max (%) | p-value | |
| T1 | Peptostreptococcaceae; Romboutsia | 0.04 | 0.00–1.29 | 0.76 | 0.01–4.28 | 0.01 |
| Lachnospiraceae; Lachnospira | 0.15 | 0.00–1.78 | 0.45 | 0.00–5.94 | 0.11 | |
| Lachnospiraceae; Blautia | 3.72 | 1.36–16.41 | 2.94 | 0.06–9.49 | 0.12 | |
| Prevotellaceae; Prevotella 9 | 0.00 | 0.00–20.94 | 0.00 | 0.00–0.02 | 0.14 | |
| Lachnospiraceae; Lachnoclostridium | 0.49 | 0.13–1.91 | 0.93 | 0.08–2.57 | 0.14 | |
| T2 | Peptostreptococcacea; Romboutsia | 0.09 | 0.00–2.03 | 0.32 | 0.00–12.47 | 0.04 |
| Enterobacteriaceae;_ | 0.02 | 0.00–7.35 | 2.93 | 0.00–17.12 | 0.05 | |
| Veillonellaceae; Megamonas | 0.00 | 0.00–1.70 | 0.00 | 0.00–21.33 | 0.11 | |
| Prevotellaceae ; Prevotella 9 | 0.00 | 0.00–16.00 | 0.00 | 0.00–0.01 | 0.11 | |
| Lachnospiraceae; Agathobacter | 0.00 | 0.00–4.16 | 0.52 | 0.00–3.86 | 0.11 | |
| Lachnospiraceae;_ | 1.04 | 0.00–2.33 | 0.74 | 0.28–2.14 | 0.13 | |
| Ruminococcaceae; Ruminococcus 2 | 0.00 | 0.00–4.29 | 0.62 | 0.00–4.89 | 0.13 | |
| T3 | Prevotellacea; Prevotella 9 | 0.00 | 0.00–18.27 | 0.00 | 0.00–0.01 | 0.03 |
| Ruminococcaceae; Ruminococcus 1 | 0.18 | 0.00–5.57 | 0.01 | 0.00–1.52 | 0.06 | |
| Veillonellaceae; Megasphaera | 0.00 | 0.00–7.23 | 0.00 | 0.00–1.92 | 0.09 | |
| Lachnospiraceae; Blautia | 0.00 | 0.00–10.43 | 2.69 | 0.63–10.33 | 0.10 | |
| Ruminococcaceae; Subdoligranulum | 1.93 | 0.00–8.03 | 0.52 | 0.00–4.34 | 0.13 | |
| Enterobacteriaceae;_ | 0.00 | 0.00–31.21 | 0.42 | 0.00–21.25 | 0.14 | |
Taxa with a p-value <0.15 are shown.
Fig. 2.
Representative genera identified as differentially abundant in GDM women.
Box plots show the relative abundances of (a) genus Romboutsia at T1, (b) genus Collinsella at T1, (c) genus Akkermansia at T2, and (d) genus Ruminococcus1 at T2. They also show the 25th and 75th percentiles with a line at the median. Blank boxes represent NGT women. Light gray boxes represent non-OW/OB GDM women. Dark gray boxes represent OW/OB GDM women. *p<0.05.
We also compared OTUs between the NGT and GDM women in the non-OW/OB subgroups. At the phylum level, the relative abundance of Verrucomicrobia was significantly higher in the GDM women (p=0.04). At the family level, Coriobacteriaceae (Test1, p=0.03) and Akkermansiaceae (Test2, p=0.04) were significantly more abundant in the GDM women. At genus level (Table 3, Fig. 2), the GDM women showed a significantly lower abundance of Collinsella in T1 (p=0.03). The relative abundance of Ruminococcus1 in the GDM women was significantly lower in T2 (p=0.04) and T3 (p=0.02). Akkermansia was more abundant in the GDM women in T2 (p=0.04).
Table 3. Relative abundances of differentially enriched genera between the NGT and GDM women (non-OW/OB).
| Non-OW/OB | NGT (n=16) | GDM (n=20) | ||||
|---|---|---|---|---|---|---|
| Taxon | Median (%) | Min–Max (%) | Median (%) | Min–Max (%) | p-value | |
| T1 | Coriobacteriaceae; Collinsella | 0.50 | 0.00–2.05 | 0.00 | 0.00–0.085 | 0.03 |
| Peptostreptococcaceae; Romboutsia | 0.13 | 0.00–1.29 | 0.67 | 0.01–4.28 | 0.06 | |
| T2 | Ruminococcaceae; Ruminococcus 1 | 0.16 | 0.00–3.03 | 0.00 | 0.00–0.38 | 0.04 |
| Akkermansiaceae; Akkermansia | 0.00 | 0.00–4.52 | 3.18 | 0.00–12.73 | 0.04 | |
| Tannerellaceae; Parabacteroides | 2.10 | 0.00–4.52 | 3.75 | 1.61–7.41 | 0.05 | |
| Veillonellaceae; Megamonas | 0.00 | 0.00–1.70 | 0.00 | 0.00–3.52 | 0.06 | |
| Lachnospiraceae; Anaerostipes | 0.34 | 0.01–2.74 | 1.47 | 0.05–3.87 | 0.08 | |
| Enterobacteriaceae;_ | 0.02 | 0.00–7.35 | 6.89 | 0.00–17.21 | 0.12 | |
| T3 | Ruminococcaceae; Ruminococcus 1 | 0.26 | 0.00–5.57 | 0.00 | 0.00–0.35 | 0.02 |
| Prevotellacea; Prevotella 9 | 0.00 | 0.00–18.27 | 0.00 | 0.00–0.001 | 0.13 | |
| Ruminococcaceae; Faecalibacterium | 4.86 | 0.00–17.59 | 1.62 | 0.01–10.32 | 0.13 | |
Taxa with p-value <0.15 were shown.
Association between maternal cytokines and gut microbiota
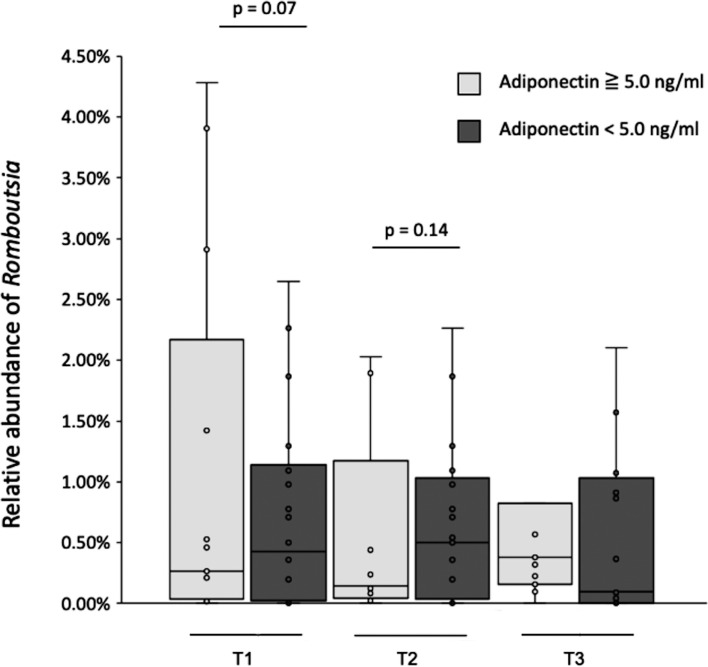
The participants with low adiponectin (<5.0 ng/mL) showed higher abundances of Romboutsia during pregnancy, although the differences did not reach statistical significance (T1, p=0.07; T2, p=0.15; Supplementary Table 1, Fig. 3). There was no association between serum adiponectin and the abundances of Collinsella, Ruminococcus1, or Akkermansia. The participants with low IL-6 (≤5.0 ng/mL) showed a higher abundance of Romboutsia in T3 (p=0.03; Supplementary Table 4).
Fig. 3.
Relation between serum adiponectin and Romboutsia.
Box plots show the relative abundances of genus Romboutsia of the groups. They show the 25th and 75th percentiles with a line at the median. Light gray boxes represent the subjects with serum adiponectin ≥5.0 ng/mL. Gray boxes represent the subjects with serum adiponectin <5.0 ng/mL.
Alpha-diversity
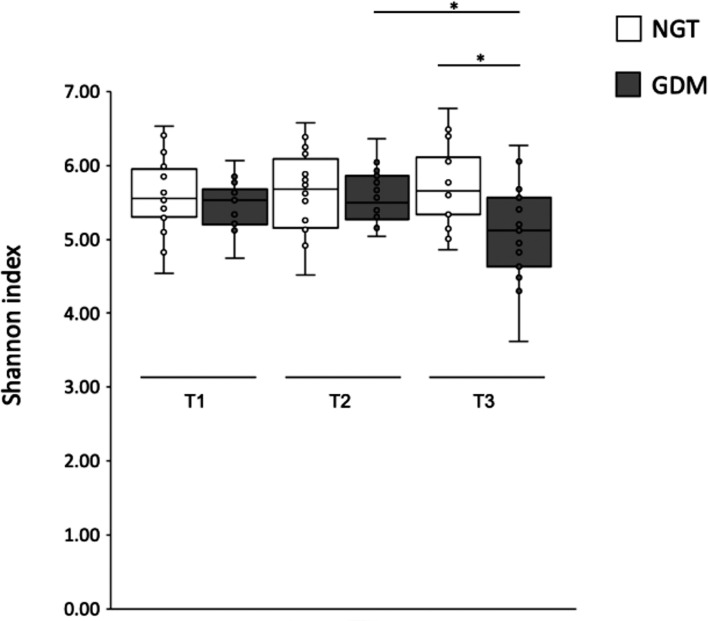
In the GDM group, the Shannon index was significantly lower in T3, and there were no significant differences at other time points (p=0.008; Fig. 4, Supplementary Table 3). While the Shannon indices of the NGT group did not change from T1 to T3, those of the GDM group were decreased in the postpartum period compared with the decreased from pregnancy to postpartum periods (T1 vs. T3, p=0.06; T2 vs. T3, p=0.02). Among the non-OW/OB patients, the Shannon index of those in the GDM group was also significantly lower in T3 (p=0.03). Faith’s PD and observed OTUs showed the similar trends, but without statistical significance.
Fig. 4.
Shannon index of the gut microbiota.
Box plots show the values of Shannon indices calculated based on the sequencing data (T1–T3). They show the 25th and 75th percentiles with a line at the median. Blank boxes represent NGT women. Gray boxes represent GDM women. *p<0.05.
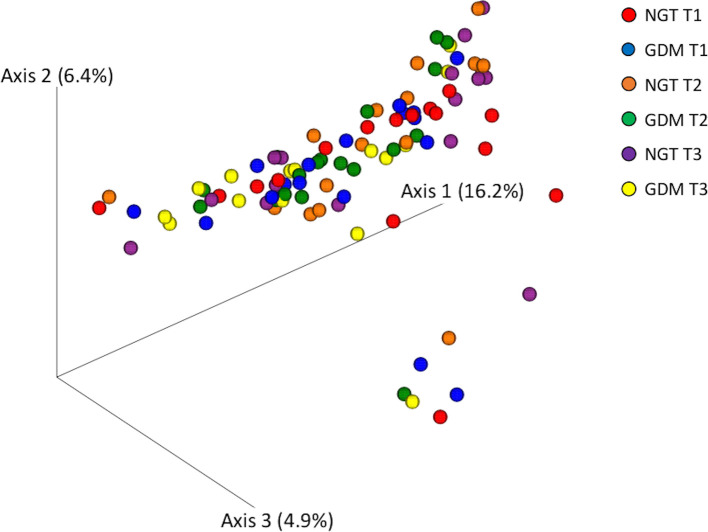
Beta-diversity
In the analysis of the bacterial community structure based on the unweighted (Fig. 5, Supplementary Table 4) and weighted UniFrac distances (Supplementary Fig. 1, Supplementary Table 4), no significant differences were detected among the groups.
Fig. 5.
PCoA models of the bacterial community composition.
The cross-sectional difference in beta diversity between the groups was assessed by permutational analysis of variance (PERMANOVA) of the un-weighted UniFrac distance and illustrated by PCoA models. No significant difference was detected among the groups.
DISCUSSION
The present study revealed that Japanese GDM women showed the different gut microbiota profiles compared with NGT women.
The relative abundances of OTUs were compared to identify the general characteristics for GDM in Japanese women. At the genus level, Peptostreptococcaceae Romboutsia was enriched in the GDM women during the pregnancy periods (T1, T2). The genus Romboutsia was created to classify the newly isolated species Romboutsia ilealis as well as Romboutsia lituseburensis, which was previously named Clostridium lituseburense [17]. Romboutsia is a member of the order Clostridiales along with the Lachnospiraceae and Ruminococcaceae families. Although they did not reach statistical significance, differences in abundance were also found for some genera of the Lachnospiraceae and Ruminococcaceae families (Table 2). Romboutsia has not been previously reported to be a genus associated with diabetic disease. There are some studies that have reported the increased abundances of Lachnospiraceae and Ruminococcaceae in subjects with an insulin resistant status, such as diabetic disease and obesity [7,8,9,10, 18,19,20]. Our results showed that the women with lower serum adiponectin levels tended to have more Romboutsia and that Romboutsia was more abundant in OW/OB GDM women than non-OW/OB women (Fig. 2). These results suggest the potential association of Romboutsia with an insulin resistant status. Another characteristic result of Romboutsia in the present study was its predominance in the GDM women only in the pregnancy periods, which suggests the possible role of Romboutsia in pregnancy-related insulin resistance. Based on our study, we believed that Peptostreptococcaceae Romboutsia comprises novel candidates for the pathobionts of GDM, in addition to other Clostridiales.
The enrichment of Prevotella in GDM women has been also reported during both the pregnancy and postpartum periods [9, 10]. In contrast, our results showed a higher abundance of Prevotella9 in postpartum NGT women. However, the detection rate of the genus was extremely low and, therefore, the clinical significance of the results is unknown.
Our study further revealed that the gut microbiota of the non-obese GDM women showed different characteristics from those of the obese GDM women when they were compared with the NGT women.
The relative abundance of Akkermansia was significantly higher in the non-OW/OB GDM subgroup in late pregnancy (T2). Contrary to our results, a reduction of Akkermansia has been previously reported in the gut microbiota of subjects with T2DM and obesity has been previously reported [1, 2, 6, 19, 21], and the supplementation with Akkermansia rather contributed to the improvement of insulin resistance [22, 23]. The richness of Akkermansia in the gut is highly influenced by the dietary interventions. According to the previous reports, a calory restriction diet [24] and the supplementation with dietary fibers [25] or polyphenols [26, 27] increased the abundance of Akkermansia. At our institution, all GDM patients were educated about their individualized diabetic diets by dieticians according to the national guidelines for obstetric practice in Japan. A calory-controlled diet comprised of vegetable-rich Japanese food may have contributed to the enrichment of Akkermansia in the non-OW/OB GDM women in the present study. The OW/OB GDM women with low insulin sensitivity were probably more resistant to the dietary therapy, as the calorie restriction of the diet during pregnancy was relatively mild.
A significantly reduced abundance of Coriobacteriaceae Collinsella was found in the non-OW/OB GDM women in the second trimester (T1). Collinsella is one of the representative genera enriched in GDM and has been reported to be correlated with insulin secretion [6, 7, 18, 28]. As previously reported [4], most of the Japanese non-OW/OB GDM women in the current study probably had the type of GDM with impaired insulin secretion, which reflected reduced Collinsella. Insulin secretion is usually enhanced in obese women in response to increased insulin resistance. It is speculated that GDM women in previous studies showed an enriched abundance of Collinsella because the studies included mostly obese cases [6, 7, 10].
In the present study, the results for the gut microbiota of the non-obese GDM women showed distinctive characteristics from those previously reported for GDM gut microbiota. Non-obese women develop GDM with a different pathophysiology from that of obese women, which possibly reflects the difference in the gut microbiota profiles.
No significant difference in alpha diversity was found between the GDM and NGT women during pregnancy. The alpha diversity of the NGT women did not significantly change from the pregnancy to postpartum periods. Koren et al. reported that the gut microbiota richness was reduced as pregnancy progressed and that GDM women showed less alpha diversity than NGT women [29]. One of the possible reasons for these contradictions is that most of the pregnant women in this study gained a small amount of weight during pregnancy. Approximately 60% of the subjects (NGT women, 10/16; GDM women, 13/20) were categorized as having “poor weight gain” according to the IOM criteria [30]. Weight gain can be one of the factors associated with a reduction of alpha diversity during pregnancy. Therefore, poor weight gain may have abrogated the physiological change in gut microbiota richness. Furthermore, the GDM women received a dietary intervention, which also possibly eliminated the difference between GDM and NGT during pregnancy. It is speculated that most of the GDM women no longer adhered to the diet therapy after delivery and that the alpha diversity was reduced in the postpartum period. There was no difference in alpha diversity between the OW/OB and non-OW/OB GDM women, and it is suggested that some factors other than insulin resistance are responsible for the gut microbiota richness.
We acknowledge that the current study had the several limitations. One of the limitations was the lack of the detailed data about the dietary intervention. The dietary education was individualized, and the extent of the adherence to the recommended diet was not precisely known. However, the glycemic control of the GDM patients was basically good, and the weight gain during pregnancy was not excessive throughout the whole study. Another limitation of the present study was the relatively small number of the subjects included in the present study compared with the previous reports. The lack of statistical significance in the correlation between the serum adiponectin levels and the abundance of Romboutsia was presumably due to the limited number of the subjects. A study with a larger population size would provide more precise evidence about the gut microbiota of Japanese pregnant women. However, the proportion of non-obese women in the GDM group was consistent with a previous report [5], and we believe that the patients in the present study sufficiently represented Japanese pregnant women. In addition, the influence of the drugs other than antibiotics cannot be ruled out, since we could not completely control the medication taken by subjects in this study, especially OTC drugs and the commercially available supplements. The gut microbiota could be also altered by the use of other drug, such as proton pump inhibitors [31].
In conclusion, some genera associated with insulin resistance, including Romboutsia, were differentially identified in the guts of Japanese GDM women. Non-obese GDM women showed a distinctive gut microbiota profile compared with the previously reported microbiota of obese GDM women, including the abundance of Collinsella and Akkermansia. Our study has provided, for the first time, the important data for gut microbiota in Japanese GDM women. Furthermore, the present study has revealed a novel insight about the distinctive gut microbiota of non-obese GDM women, which possibly reflected the difference in pathophysiology. Asians are relatively lean and known to have high insulin sensitivity with low insulin secretion ability [32]. We should be aware of the differences according to ethnicity and race, which can affect how patients are classified into patient types, when investigating the gut microbiota of GDM women.
CONFLICT OF INTEREST
G. Harata, K. Miyazawa, and F. He are employees of Takanashi Milk Products Co., Ltd.; K. Tanaka, S. Tanigaki and Y. Kobayashi declare no conflicts of interest.
Supplementary Material
REFERENCES
- 1.Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, Shulzhenko N. 2020. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 51: 102590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ponzo V, Fedele D, Goitre I, Leone F, Lezo A, Monzeglio C, Finocchiaro C, Ghigo E, Bo S. 2019. Diet-gut microbiota interactions and gestational diabetes mellitus (GDM). Nutrients 11: 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasain Z, Mokhtar NM, Kamaruddin NA, Mohamed Ismail NA, Razalli NH, Gnanou JV, Raja Ali RA. 2020. Gut microbiota and gestational diabetes mellitus: a review of host-gut microbiota interactions and their therapeutic potential. Front Cell Infect Microbiol 10: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kusunoki Y, Katsuno T, Nakae R, Watanabe K, Ochi F, Tokuda M, Akagami T, Miuchi M, Miyagawa J, Namba M. 2015. Insulin resistance and β-cell function influence postprandial blood glucose levels in Japanese patients with gestational diabetes mellitus. Gynecol Endocrinol 31: 929–933. [DOI] [PubMed] [Google Scholar]
- 5.Furukawa S, Kobayashi Y. 2019. Leaner women with impaired insulin secretion accounts for about 40% of gestational diabetes mellitus in Japan. J Pregnancy 2019: 7578403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crusell MKW, Hansen TH, Nielsen T, Allin KH, Rühlemann MC, Damm P, Vestergaard H, Rørbye C, Jørgensen NR, Christiansen OB, Heinsen FA, Franke A, Hansen T, Lauenborg J, Pedersen O. 2018. Gestational diabetes is associated with change in the gut microbiota composition in third trimester of pregnancy and postpartum. Microbiome 6: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrocino I, Ponzo V, Gambino R, Zarovska A, Leone F, Monzeglio C, Goitre I, Rosato R, Romano A, Grassi G, Broglio F, Cassader M, Cocolin L, Bo S. 2018. Changes in the gut microbiota composition during pregnancy in patients with gestational diabetes mellitus (GDM). Sci Rep 8: 12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mokkala K, Houttu N, Vahlberg T, Munukka E, Rönnemaa T, Laitinen K. 2017. Gut microbiota aberrations precede diagnosis of gestational diabetes mellitus. Acta Diabetol 54: 1147–1149. [DOI] [PubMed] [Google Scholar]
- 9.Fugmann M, Breier M, Rottenkolber M, Banning F, Ferrari U, Sacco V, Grallert H, Parhofer KG, Seissler J, Clavel T, Lechner A. 2015. The stool microbiota of insulin resistant women with recent gestational diabetes, a high risk group for type 2 diabetes. Sci Rep 5: 13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortez RV, Taddei CR, Sparvoli LG, Ângelo AGS, Padilha M, Mattar R, Daher S. 2019. Microbiome and its relation to gestational diabetes. Endocrine 64: 254–264. [DOI] [PubMed] [Google Scholar]
- 11.Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva A, Hod M, Kitzmiler JL, Lowe LP, McIntyre HD, Oats JJ, Omori Y, Schmidt MI, International Association of Diabetes and Pregnancy Study Groups Consensus Panel.2010. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 33: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohammadi T, Paknahad Z. 2017. Adiponectin concentration in gestational diabetic women: a case-control study. Clin Nutr Res 6: 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Souvannavong-Vilivong X, Sitticharoon C, Klinjampa R, Keadkraichaiwat I, Sripong C, Chatree S, Sririwichitchai R, Lertbunnaphong T. 2019. Placental expressions and serum levels of adiponectin, visfatin, and omentin in GDM. Acta Diabetol 56: 1121–1131. [DOI] [PubMed] [Google Scholar]
- 14.Bossick AS, Peters RM, Burmeister C, Kakumanu N, Shill JE, Cassidy-Bushrow AE. 2016. Antenatal inflammation and gestational diabetes mellitus risk among pregnant African-American women. J Reprod Immunol 115: 1–5. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Chi H, Xiao H, Tian X, Wang Y, Yun X, Xu Y. 2017. Interleukin 6 (IL-6) and Tumor Necrosis Factor α (TNF-α) Single Nucleotide Polymorphisms (SNPs), inflammation and metabolism in gestational diabetes mellitus in Inner Mongolia. Med Sci Monit 23: 4149–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Šimják P, Cinkajzlová A, Anderlová K, Kloučková J, Kratochvílová H, Lacinová Z, Kaválková P, Krejčí H, Mráz M, Pařízek A, Kršek M, Haluzík M. 2018. Changes in plasma concentrations and mRNA expression of hepatokines fetuin A, fetuin B and FGF21 in physiological pregnancy and gestational diabetes mellitus. Physiol Res 67Suppl 3: S531–S542. [DOI] [PubMed] [Google Scholar]
- 17.Gerritsen J, Fuentes S, Grievink W, van Niftrik L, Tindall BJ, Timmerman HM, Rijkers GT, Smidt H. 2014. Characterization of Romboutsia ilealis gen. nov., sp. nov., isolated from the gastro-intestinal tract of a rat, and proposal for the reclassification of five closely related members of the genus Clostridium into the genera Romboutsia gen. nov., Intestinibacter gen. nov., Terrisporobacter gen. nov. and Asaccharospora gen. nov. Int J Syst Evol Microbiol 64: 1600–1616. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Dekker Nitert M, SPRING Trial Group2016. Connections between the gut microbiome and metabolic hormones in early pregnancy in overweight and obese women. Diabetes 65: 2214–2223. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Shen D, Fang Z, Jie Z, Qiu X, Zhang C, Chen Y, Ji L. 2013. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS One 8: e71108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salamon D, Sroka-Oleksiak A, Kapusta P, Szopa M, Mrozińska S, Ludwig-Słomczyńska AH, Wołkow PP, Bulanda M, Klupa T, Małecki MT, Gosiewski T. 2018. Characteristics of gut microbiota in adult patients with type 1 and type 2 diabetes based on next‑generation sequencing of the 16S rRNA gene fragment. Pol Arch Intern Med 128: 336–343. [DOI] [PubMed] [Google Scholar]
- 21.Santacruz A, Collado MC, García-Valdés L, Segura MT, Martín-Lagos JA, Anjos T, Martí-Romero M, Lopez RM, Florido J, Campoy C, Sanz Y. 2010. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr 104: 83–92. [DOI] [PubMed] [Google Scholar]
- 22.Zhao S, Liu W, Wang J, Shi J, Sun Y, Wang W, Ning G, Liu R, Hong J. 2017. Akkermansia muciniphila improves metabolic profiles by reducing inflammation in chow diet-fed mice. J Mol Endocrinol 58: 1–14. [DOI] [PubMed] [Google Scholar]
- 23.Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, Falony G, Raes J, Maiter D, Delzenne NM, de Barsy M, Loumaye A, Hermans MP, Thissen JP, de Vos WM, Cani PD. 2019. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med 25: 1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L, Dumas ME, Rizkalla SW, Doré J, Cani PD, Clément K, MICRO-Obes Consortium.2016. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 65: 426–436. [DOI] [PubMed] [Google Scholar]
- 25.Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Muir JG, Gibson PR. 2016. Consistent prebiotic effect on gut microbiota with altered FODMAP intake in patients with Crohn’s disease: a randomised, controlled cross-over trial of well-defined diets. Clin Transl Gastroenterol 7: e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anhê FF, Varin TV, Le Barz M, Desjardins Y, Levy E, Roy D, Marette A. 2015. Gut microbiota dysbiosis in obesity-linked metabolic diseases and prebiotic potential of polyphenol-rich extracts. Curr Obes Rep 4: 389–400. [DOI] [PubMed] [Google Scholar]
- 27.Walker JM, Eckardt P, Aleman JO, da Rosa JC, Liang Y, Iizumi T, Etheve S, Blaser MJ, Breslow JL, Holt PR. 2018. The effects of trans-resveratrol on insulin resistance, inflammation, and microbiota in men with the metabolic syndrome: a pilot randomized, placebo-controlled clinical trial. J Clin Transl Res 4: 122–135. [PMC free article] [PubMed] [Google Scholar]
- 28.Gomez-Arango LF, Barrett HL, Wilkinson SA, Callaway LK, McIntyre HD, Morrison M, Dekker Nitert M. 2018. Low dietary fiber intake increases Collinsella abundance in the gut microbiota of overweight and obese pregnant women. Gut Microbes 9: 189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Bäckhed HK, Gonzalez A, Werner JJ, Angenent LT, Knight R, Bäckhed F, Isolauri E, Salminen S, Ley RE. 2012. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 150: 470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasmussen KM, Yaktine AL. 2009. Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight gain during pregnancy: reexamining the guidelines. National Academies Press,Washington DC. [PubMed] [Google Scholar]
- 31.Imhann F, Bonder MJ, Vich Vila A, Fu J, Mujagic Z, Vork L, Tigchelaar EF, Jankipersadsing SA, Cenit MC, Harmsen HJ, Dijkstra G, Franke L, Xavier RJ, Jonkers D, Wijmenga C, Weersma RK, Zhernakova A. 2016. Proton pump inhibitors affect the gut microbiome. Gut 65: 740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. 2013. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care 36: 1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.