Abstract
Activated Ras, but not Raf, causes transformation of RIE-1 rat intestinal epithelial cells, demonstrating the importance of Raf-independent effector signaling in mediating Ras transformation. To further assess the contribution of Raf-dependent and Raf-independent function in oncogenic Ras transformation, we evaluated the mechanism by which oncogenic Ras blocks suspension-induced apoptosis, or anoikis, of RIE-1 cells. We determined that oncogenic versions of H-, K-, and N-Ras, as well as the Ras-related proteins TC21 and R-Ras, protected RIE-1 cells from anoikis. Surprisingly, our analyses of Ras effector domain mutants or constitutively activated effectors indicated that activation of Raf-1, phosphatidylinositol 3-kinase (PI3K), or RalGDS alone is not sufficient to promote Ras inhibition of anoikis. Treatment of Ras-transformed cells with the U0126 MEK inhibitor caused partial reversion to an anoikis-sensitive state, indicating that extracellular signal-regulated kinase activation contributes to inhibition of anoikis. Unexpectedly, oncogenic Ras failed to activate Akt, and treatment of Ras-transformed RIE-1 cells with the LY294002 PI3K inhibitor did not affect anoikis resistance or growth in soft agar. Thus, while important for Ras transformation of fibroblasts, PI3K may not be involved in Ras transformation of RIE-1 cells. Finally, inhibition of epidermal growth factor receptor kinase activity did not overcome Ras inhibition of anoikis, indicating that this autocrine loop essential for transformation is not involved in anoikis protection. We conclude that a PI3K- and RalGEF-independent Ras effector(s) likely cooperates with Raf to confer anoikis resistance upon RIE-1 cells, thus underscoring the complex nature by which Ras transforms cells.
Anoikis means “homelessness” in Greek (18). It is a term used to describe the observation that normal epithelial cells are dependent upon an appropriate extracellular basement membrane, or home, to be viable. When epithelial cells lose contact with their basement membrane, they undergo anoikis, also known as suspension-induced apoptosis (17). This allows the body to rid itself of cells that are no longer needed and, presumably, protects tissues from inappropriate colonization by nonadherent cells. In adult organisms, suspension-induced apoptosis is commonly observed during regeneration of skin or colonic epithelia or during involution of the mammary gland (6, 23, 40).
Gaining resistance to anoikis may be a general prerequisite for the development and progression of cancers of epithelial origin, or carcinomas. Acquiring independence from adhesion is a hallmark of the transformed cell, and most cell lines derived from human tumors are capable of growing in the absence of adhesion (49). This characteristic of transformation likely imparts a significant, and clearly abnormal, survival advantage to cells. Cells in primary tumors, for example, often lack contact with an organized basement membrane and thus must adapt to growth in matrix-poor or disorganized extracellular environments (39). Traversing the blood and lymph systems during metastasis also requires that cells survive in the absence of appropriate matrix contacts.
In vitro, a variety of immortalized but phenotypically normal cell lines can be made adhesion independent by expression of the dominant positive oncoprotein Ras. Aberrant activation of Ras is common in human cancers, both by direct mutation and by indirect stimulation via deregulated cell surface receptor signaling (1, 4, 10). Thus, understanding how Ras signal transduction imparts adhesion independence in vitro may reveal crucial targets for pharmacologic intervention and cancer treatment in vivo.
Understanding the mechanisms by which Ras promotes adhesion independence is complicated by the fact that Ras signal transduction is much more complex than originally envisioned (51). First, there are currently over 18 known proteins that bind Ras in its GTP-bound or activated state and thus have the potential to serve as downstream effectors of Ras (7, 33). These proteins include lipid kinases, protein kinases, GTPase-activating proteins, guanine nucleotide exchange factors (GEFs), and proteins with no known enzymatic function. For many of these proteins, it is unknown what role they play in Ras transformation. Second, oncogenic Ras can exert different biological effects depending on the genetic context in which it is expressed. For example, while primary mouse fibroblasts undergo senescence in response to activated Ras expression, the additional loss of p53 or Rb-1 tumor suppressor function allows Ras to cause growth transformation (22, 50). Third, the mechanisms of Ras transformation may vary as a function of cellular context. For example, the signaling pathways by which Ras causes transformation of NIH 3T3 mouse fibroblasts and RIE-1 rat intestinal epithelial cells are strikingly different (20, 31, 35). While aberrant activation of the Ras effector Raf alone is sufficient to transform fibroblasts, Raf activation alone is insufficient to transform RIE-1 cells. Furthermore, Ras transformation of RIE-1 cells is critically dependent on a Raf-independent transforming growth factor α (TGF-α)–epidermal growth factor receptor (EGFR) autocrine signaling mechanism not required for fibroblast transformation.
Despite the complexity of Ras signal transduction, there are three well-characterized Ras effectors that play established roles in Ras transformation of rodent fibroblasts. The best characterized are the Raf serine/threonine protein kinases c-Raf-1, A-Raf, and B-Raf (7). These kinases activate MEK1/2 and in turn activate the extracellular signal-regulated kinase (ERK) mitogen-activated protein kinases (MAPKs). The Raf-MEK-ERK pathway has been shown to be necessary and sufficient to promote Ras transformation of rodent fibroblasts. The second best characterized effectors of Ras are the class I phosphatidylinositol 3-kinases (PI3Ks) p110α, p110β, p110γ, and p110δ (42–44). A major function for these lipid kinases is the phosphorylation of phosphatidylinositol (4,5)-bisphosphate (PIP2) to produce phosphatidylinositol (3,4,5)-triphosphate (PIP3). Accumulation of PIP3 in Ras-transformed cells can facilitate activation of the Akt/protein kinase B (PKB) protein kinases. Akt, in turn, promotes cell survival by directly regulating the machinery of apoptosis as well as by causing changes in gene expression (12, 30, 45). PIP3 levels are elevated in Ras-transformed rodent fibroblasts, and dominant negative PI3K can block Ras transformation of NIH 3T3 cells (43). The third best characterized effectors are GEFs for the Ral small GTPases (RalGDS, Rgl, and Rlf/Rgl2) (15, 57). These proteins stimulate formation of the active, GTP-bound forms of RalA and RalB, and dominant negative Ral can block Ras transformation (54). Aside from these three main classes of effectors, the roles of other proteins in Ras transformation are less well characterized.
Given that ras is most commonly mutated in carcinomas (4, 10), we sought to expand our understanding of the role of Ras in epithelial cell transformation. Previous studies had documented that oncogenic Ras blocks anoikis of MDCK canine kidney epithelial cells (17, 29). Furthermore, it was determined that the Ras effector PI3K and its downstream target Akt were both necessary and sufficient for Ras-mediated anoikis protection of these cells. To assess whether these results are applicable to other Ras-transformed epithelial cell lines and whether other Ras effectors contribute to anoikis resistance, we evaluated the mechanism of anoikis resistance in Ras-transformed RIE-1 epithelial cells. Surprisingly, we found that PI3K-Akt signaling was neither necessary nor sufficient for Ras-mediated anoikis resistance, or growth transformation, of RIE-1 cells. Instead, we determined that anoikis resistance of Ras-transformed RIE-1 cells is complex and caused by the combined actions of Raf and an unknown PI3K- and RalGEF-independent effector(s). Cumulatively, these data indicate that the Ras oncoprotein has multiple signaling properties that promote anoikis resistance and transformation of epithelial cells.
MATERIALS AND METHODS
Molecular constructs.
cDNA sequences encoding constitutively activated Ras proteins [H-Ras(12V), H-Ras(61L), K-Ras(12V), and N-Ras(13D)]; activated Ras-related proteins [TC21(23V) and R-Ras(38V)]; hemagglutinin (HA) epitope-tagged and activated Rho family GTPases [Rac1(61L) and RhoA(63L)]; and activated Ras effectors Raf-1 (Raf-22W and Raf-CAAX), PI3K (p110-CAAX), and RalGDS (RalGDS-CAAX) and HA-tagged Rlf (HA-Rlf-CAAX) were cloned into the BamHI or EcoRI sites of the pBabe-puro and pZIP-NeoSV(x)1 retroviral expression vectors. The amino acid substitutions shown for the GTPases render them constitutively GTP bound and thus activated. Activation of Raf-1 was achieved by NH2-terminal truncation (designated Raf-22W) or membrane targeting (Raf-CAAX) (52) and the PI3K, RalGDS, and Rlf were activated by addition of the COOH-terminal plasma membrane targeting sequences from H- or K-Ras4B and designated p110-CAAX (43), RalGDS-CAAX (41), and HA-Rlf-CAAX (58), respectively. These Ras sequences signal posttranslational modifications that cause constitutive membrane localization. Membrane localization, in turn, promotes activation of the catalytic functions of Raf-1 and p110. The pDCR eukaryotic expression vectors encoding effector domain mutants of H-Ras(12V) (provided by M. White), which are impaired in specific effector interactions, have been described previously (28, 43, 56). In brief, the E37G mutant retains binding of RalGEFs but is reduced in its ability to bind Raf or PI3K. Similarly, the Y40C mutant retains PI3K binding but is reduced in Raf and RalGEF binding, while the T35S mutant retains Raf binding but is reduced in PI3K and RalGEF binding.
Inhibitors.
Chemical inhibitors used in this study are specific to MEK1/2 (U0126) (provided by J. Trzaskos, Dupont) (13), PI3K (LY294002) (A.G. Scientific) (55), caspase-1-like proteases (Z-VAD-FMK) (Calbiochem) (14), and the EGFR kinase (PD153035) (Tocris Cookson) (19). All inhibitors were dissolved in dimethyl sulfoxide for use, and their effects were measured relative to dimethyl sulfoxide (vehicle)-treated controls.
Cell culture, retroviral infection, and transfection.
RIE-1 cells were obtained from Robert J. Coffey (Vanderbilt University, Nashville, Tenn.) (3). RIE-1 or Bosc23 and ROSE 199 cells (provided by R. Schäfer) were grown in Dulbecco's modified Eagle's medium supplemented with 5 or 10% fetal calf serum, respectively. Mass populations of stably infected [pBabe-puro and pZIP-NeoSV(x)1] or transfected (pDCR) cell populations were selected by the supplementation of the growth medium with 400 μg of G418/ml [for pDCR and pZIP-NeoSV(x)1 expression constructs] or 2 μg of puromycin/ml (for pBabe-puro expression constructs).
Production of infectious, replication-incompetent retrovirus was achieved by transfection of pZIP-NeoSV(x)1 and pBabe-puro expression constructs into the Bosc23 ecotropic packaging cell line (37). RIE-1 cells seeded 24 h in advance at a density of 105 cells per 60-mm dish were infected by exposure to 1.5 ml of retroviral supernatant, together with 1.5 ml of growth medium, and Polybrene was added to a final concentration of 4 μg/ml. After 5 h, fresh growth medium was added, and drug selection was initiated 24 h later. Cells expressing pDCR constructs were obtained by transfection with Effectene per the manufacturer's instructions (Qiagen).
SDS-polyacrylamide gel electrophoresis and Western blot analyses.
Cell lysates were generated by lysis directly into sodium dodecyl sulfate (SDS) sample buffer (for Akt analysis) or buffer containing 20 mM Tris (pH 7.4), 0.5% NP-40, and 250 mM NaCl (for Akt analysis and other analyses). The latter lysates were clarified by centrifugation at 12,000 × g for 10 min at 4°C prior to use. Proteins were separated by SDS-polyacrylamide gel electrophoresis in 14, 7.5, or 10% gels; transferred to Immobilon P (Millipore) membranes; and incubated with primary antibodies per the manufacturer's instructions. Antibodies used for immunoblotting are specific to ERK (sc93R; Santa Cruz Biotechnology), Akt, phospho-Akt, phospho-ERK (New England Biolabs, Inc./Cell Signaling Technology), Ras (LA045; Viromed Biosafety), and the HA epitope tag (BabCO). Secondary antibodies were either horseradish peroxidase or alkaline phosphatase conjugated for detection by enhanced chemiluminescence (Amersham Pharmacia Biotech) or phosphorimaging (Molecular Dynamics), respectively.
Apoptosis assays.
RIE-1 cells were plated 36 h in advance of suspension at a density of 4 × 106 cells per T185 flask (Nalge Nunc). To suspend cells, cells were treated with 0.25% trypsin dissolved in phosphate-buffered saline containing 2.5 mM EDTA for 8 min at 37°C. Cells were washed with either growth medium or 0.5 mg of soybean trypsin inhibitor (Sigma)/ml depending on whether cells were to be suspended in growth medium or in Dulbecco's modified Eagle's medium supplemented with 0.5 mg of bovine serum albumin/ml, respectively.
Cells were plated in poly(2-hydroxyethyl methacrylate) (poly-HEME)-coated dishes, prepared as described previously, in the presence of growth medium unless otherwise noted (17). Treatment with the LY294002 inhibitor involved suspending cells in the absence of serum for 30 min prior to the addition of 10 μM LY294002. After an additional 30 min of incubation, cells were stimulated with 5% fetal bovine serum when indicated. The MEK and EGFR kinase inhibitors were used at 30 and 2 μM, respectively, and added to cells after a 30-min incubation in growth medium in suspension.
We used three types of assays to measure anoikis: DNA laddering, [3-(4,5- dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H- tetrazolium) (MTS) tetrazolium-based viability assays, and DNA fragmentation enzyme-linked immunosorbent assay (ELISA). For viability assays, 106 cells were suspended in poly-HEME-coated petri dishes for 15 h prior to replating a fraction of the suspended samples in 96-well dishes. Viability was measured by the conversion of MTS tetrazolium to formazan per the manufacturer's instructions (CellTiter AQueous kit; Promega). For DNA fragmentation ELISA, cells were suspended as described above and subsequently lysed in 1.5 ml of a lysis buffer containing 10 mM Tris (pH 8.0), 10 mM EDTA, and 0.5% Tx-100. Twenty microliters of this lysate was analyzed as recommended by the manufacturer (DNA Death ELISA 10x; Boehringer Mannheim). For DNA laddering, 3 × 106 cells were processed by extraction and processing of low-molecular-weight DNA essentially as described previously (29). In brief, cells were lysed in the same buffer used for DNA fragmentation ELISAs. Subsequently, the soluble fraction was subjected to phenol-chloroform extraction and RNase A treatment prior to electrophoresis of the samples in a 1.5% agarose gel.
Soft agar colony formation.
To assess growth of RIE-1 cells in soft agar, cells were seeded at 103 to 104 cells per 60-mm dish in growth medium containing 0.3% agar over a base layer of 0.6% agar (9). LY294002 was added at a final concentration of 10 μM when indicated.
RESULTS
Oncogenic Ras promotes anoikis resistance of RIE-1 cells.
Previous studies showed that oncogenic Ras conferred anoikis resistance upon MDCK canine kidney epithelial cells (17, 29). It was determined that Ras activation of PI3K, and not Raf, was necessary and sufficient to block anoikis. We showed previously that oncogenic Ras caused anchorage-independent growth of RIE-1 rat intestinal epithelial cells by activation of Raf-dependent and Raf-independent effectors (35). Furthermore, we determined that the anchorage-independent growth of Ras-transformed RIE-1 cells was also dependent on the activation of TGF-α via Raf-dependent and Raf-independent pathways (20). These observations suggested that Ras may block anoikis, a critical requirement for anchorage-independent growth, by a more complex mechanism in RIE-1 cells. Therefore, we initiated studies to determine if oncogenic Ras rendered RIE-1 cells insensitive to anoikis, and if so, what effectors were important in mediating this activity.
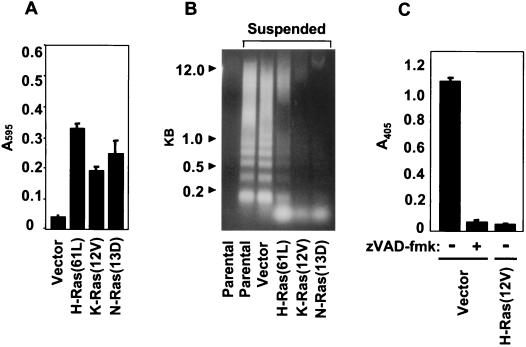
We first determined if untransformed RIE-1 cells responded to loss of matrix attachment by undergoing anoikis. We utilized three assays to evaluate this. The first indirectly measures anoikis by quantitating decreases in cellular viability. The second is specific to apoptosis and visualizes internucleosomal DNA degradation as a “ladder” of low-molecular-weight DNA in agarose gels. The third quantitates DNA fragmentation that results from apoptosis by ELISA. As shown in Fig. 1, RIE-1 cells suffered a dramatic loss of cell viability when held in suspension for 12 h (Fig. 1A). Loss of viability was a consequence of detachment-induced apoptosis (Fig. 1B). DNA fragmentation in response to suspension was blocked by treatment with a general caspase inhibitor, Z-VAD-FMK, indicating that RIE-1 cells underwent apoptosis in a caspase-dependent manner (Fig. 1C).
FIG. 1.
Ras blocks anoikis of RIE-1 cells. (A and B) RIE-1 cells stably expressing activated H-, K-, or N-Ras proteins were analyzed for viability by the MTS assay after being held in suspension for 15 h (A) or 12 h prior to assessing apoptosis by DNA laddering (B). (C) Cells stably infected with vector were incubated with vehicle or a broad-spectrum caspase inhibitor for 15 h in suspension prior to assessing apoptosis by DNA fragmentation ELISA. Data shown are representative of at least two independent experiments. Bars represent the standard deviations resulting from triplicate samples (A) or duplicate reading of the same sample (C).
To determine whether oncogenic Ras is capable of inhibiting anoikis of RIE-1 cells, expression plasmids encoding constitutively active versions of the three Ras proteins, H-Ras(12V), N-Ras(13D), and K-Ras4B(12V), were introduced by retroviral infection followed by drug selection to establish mass populations of RIE-1 cells stably expressing activated Ras. The resulting cell populations were suspended and assayed for apoptosis and viability. All oncogenic versions of Ras blocked anoikis of RIE-1 cells (Fig. 1A and B). Thus, while differences in the function of Ras proteins have been described previously (51), no differences were seen for inhibition of anoikis.
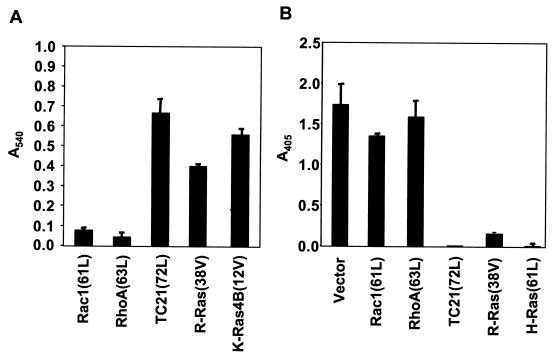
We also evaluated the ability of other Ras superfamily GTPases to protect RIE-1 cells from anoikis. Activated forms of the Ras family proteins R-Ras and TC21/R-Ras2 and the Rho family proteins RhoA and Rac1 have been shown previously to promote the anchorage-independent growth of NIH 3T3 cells (11, 27). We found that RIE-1 cells stably expressing activated R-Ras and TC21, but not RhoA or Rac1, were also resistant to anoikis (Fig. 2). Since we showed previously that R-Ras and TC21 are not activators of Raf (21, 26), these results suggest that Ras activation of Raf is not required for inhibition of anoikis. Support for this conclusion was also provided by our observation that ERK activation was seen in suspended RIE-1 cells expressing activated Ras, but not R-Ras or TC21 (data not shown).
FIG. 2.
Unlike Ras and Ras-related proteins, activated Rho family members fail to protect RIE-1 cells from anoikis. (A) RIE-1 cells stably expressing Rac1(61L), RhoA(63L), TC21(72L), R-Ras(38V), or K-Ras4B(12V) were evaluated for viability in response to a 15-h suspension by the MTS viability assay. (B) The cells described for panel A and H-Ras(61L)-expressing cells were also evaluated for apoptosis by DNA fragmentation ELISA. Data shown are representative of two independent experiments. Bars represent the standard deviations resulting from triplicate samples (A) or duplicate reading of the same sample (B).
Ras activation of the Raf, PI3K, or RalGEF effector pathways is not sufficient to promote anoikis resistance.
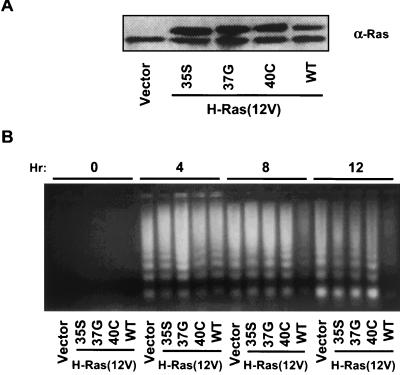
Oncogenic Ras signals through multiple effectors, including PI3K, Raf, and RalGEFs. We sought to determine if activation of individual Ras effector signaling pathways was sufficient to block anoikis of Ras-transformed RIE-1 cells. To do this, we first evaluated the ability of effector domain mutants of activated H-Ras(12V), which are differentially impaired in effector utilization, to block anoikis (28, 43, 56). The H-Ras(12V/35S) mutant retains the ability to activate Raf but not PI3K or RalGDS. The H-Ras(12V/37G) mutant no longer activates Raf or PI3K but can activate RalGDS and related proteins. The H-Ras(12V/40C) mutant retains the ability to activate PI3K but no longer activates Raf or RalGDS. Surprisingly, mass populations of RIE-1 cells stably expressing the three Ras effector domain mutants were as sensitive to anoikis as were the empty vector-infected control RIE-1 cells (Fig. 3B). This result suggests that Ras activation of Raf, PI3K, or RalGDS alone is not sufficient to block anoikis.
FIG. 3.
Effector domain mutants of H-Ras(12V) fail to block anoikis. (A) RIE-1 cells stably infected with H-Ras(12V), H-Ras(12V/S35), H-Ras(12V/G37), or H-Ras(12V/C40) were assayed for Ras expression levels by Western blot analysis with pan-Ras antibody. The upper band represents the HA epitope-tagged exogenous H-Ras(12V) proteins, and the lower band represents endogenous Ras proteins. (B) The cell populations described for panel A were held in suspension for the indicated time (hours) prior to assessing anoikis by DNA laddering. WT, wild type.
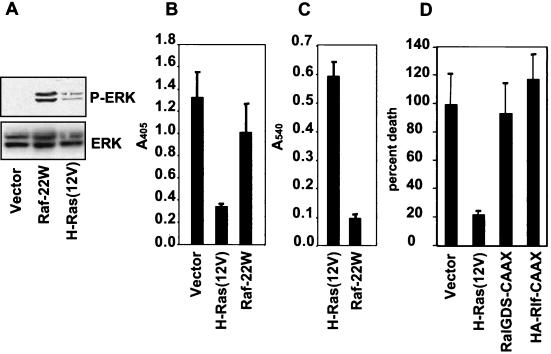
To further evaluate whether activation of different effectors of Ras is sufficient to block anoikis, we also established RIE-1 cells stably expressing constitutively activated versions of Raf, PI3K, or two RalGEF family members, RalGDS and Rlf. Similar to what had been observed previously with MDCK cells (29), expression of the amino-terminally truncated and constitutively activated mutant of the Raf-1 kinase (Raf-22W) did not protect RIE-1 cells from anoikis. While RIE-1 cells expressing Raf-22W had elevated levels of activated ERK in suspension (Fig. 4A), these cells were not anoikis resistant as determined by both a viability assay and an apoptosis-specific DNA fragmentation ELISA (Fig. 4B and C). These results are also consistent with our previous observation that Raf-22W-expressing RIE-1 cells failed to form colonies in soft agar (35).
FIG. 4.
Activated Raf-1 or RalGEFs alone are not sufficient to block anoikis. (A) RIE-1 cells expressing activated Raf-1 (Raf-22W) or H-Ras(12V) were assayed for ERK activation by immunoblotting cell lysates with phospho-ERK antibodies. Cell lysates were made from cells suspended for 6 h. Blots were stripped and reprobed with anti-ERK antibodies. (B and C) The Raf-expressing RIE-1 cells were evaluated for anoikis resistance after suspension for 8 or 15 h by performing DNA fragmentation ELISA (B) or an MTS assay (C), respectively. (D) RIE-1 cells stably infected with membrane-targeted and constitutively activated RalGDS protein, RalGDS-CAAX, or Rlf protein, HA-Rlf-CAAX, were maintained in suspension for 12 h and subsequently analyzed for anoikis by DNA fragmentation ELISA. Data shown are representative of two independent experiments. Bars represent the standard deviations resulting from triplicate samples (B) or duplicate reading of the same sample (C and D). The extent of cell death in the vector line is arbitrarily set to 100%.
Similarly, we found that RIE-1 cells stably infected with an expression vector encoding a plasma membrane-targeted and constitutively activated form of RalGDS (RalGDS-CAAX) were also sensitive to anoikis (Fig. 4D). To extend our evaluation to an additional RalGEF family member, namely, Rlf, we also expressed HA-tagged, membrane-targeted Rlf (HA-Rlf-CAAX). HA-Rlf-CAAX expression was confirmed in suspended cells by anti-HA immunoblotting (data not shown). Activated Rlf, like RalGDS, failed to block anoikis of RIE-1 cells (Fig. 4D). When this finding is taken together with the failure of the H-Ras(12V/37G) effector domain mutant to block anoikis, we conclude that Ras activation of RalGEFs alone is not sufficient to allow cells to survive in suspension.
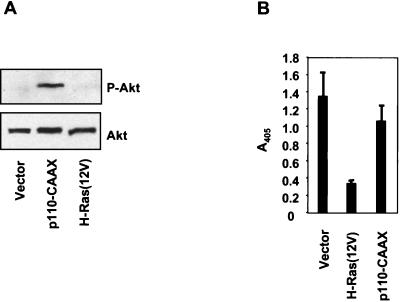
When assessed in MDCK cells, expression of activated PI3K (p110-CAAX) or the PI3K target Akt/PKB conferred protection against anoikis (29). To determine if PI3K activation alone was sufficient to overcome anoikis of RIE-1 cells, we established a mass population stably expressing a plasma membrane-targeted form of the catalytic subunit of PI3K (designated p110-CAAX). Western blot analyses with phospho-specific antibodies that recognize activated Akt verified that these cells possessed elevated PI3K activity (Fig. 5A). However, as we had seen with H-Ras(12V/40C), expression of activated PI3K was not sufficient to prevent suspension-induced apoptosis (Fig. 5B). These results contrast with those made with MDCK cells, where Akt activation alone was sufficient to block anoikis (29).
FIG. 5.
PI3K activation of Akt alone is not sufficient to protect RIE-1 cells from anoikis. (A) RIE-1 cells stably infected with the plasma membrane-targeted and constitutively activated p110α subunit of PI3K were assayed for Akt activation by Western blot analysis with phospho-specific Akt antibodies after being held in suspension for 6 h in the absence of serum. Blots were stripped and reprobed with anti-Akt antibodies to determine total Akt levels. (B) Cells expressing p110-CAAX were assessed for anoikis resistance by DNA fragmentation ELISA after 12 h of suspension. Data shown are representative of two independent experiments. Bars represent the standard deviations resulting from duplicate reading of the same sample.
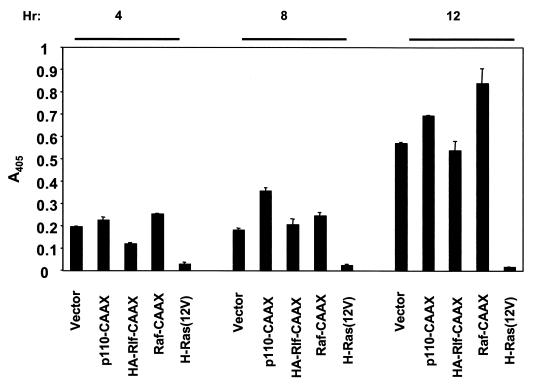
Given the central role for PI3K-Akt signaling in Ras-transformed MDCK cells, we sought to determine if our results with PI3K were not restricted to a single cell line. We thus chose to perform anoikis studies with another epithelial model of transformation, namely, the ROSE 199 rat ovarian surface epithelial cell line. Mass populations of the cells expressing either H-Ras(12V), activated PI3K (p110-CAAX), activated Rlf (HA-Rlf-CAAX) or activated Raf (Raf-CAAX) were established. While expression of H-Ras(12V) blocked anoikis of ROSE cells, activated PI3K or activated versions of the other two main Ras effectors, Raf and the RalGEF Rlf, failed to block anoikis of ROSE cells (Fig. 6). These results suggest that, unlike what has been observed with MDCK cells, PI3K signaling is insufficient to block anoikis of at least two other epithelial cell lines, namely, RIE-1 and ROSE 199 cells.
FIG. 6.
Activated PI3K, Raf-1, or Rlf alone is not sufficient to block anoikis of ROSE 199 cells. ROSE 199 cells stably infected with pBabe-puro retrovirus vectors encoding oncogenic H-Ras or the plasma membrane-targeted and constitutively activated versions of the p110α subunit of PI3K, Raf-1, or HA-tagged Rlf were assayed for anoikis resistance after suspension for 4, 8, and 12 h by performing DNA fragmentation ELISA. Data shown are representative of two independent experiments. Bars represent standard deviations resulting from duplicate reading of the same sample.
The Raf-MEK-ERK signaling pathway partially contributes to anoikis resistance of RIE-1 cells.
Although expression of Raf-22W was previously shown to be insufficient to support growth of RIE-1 cells in soft agar (35), inhibition of ERK activation in Ras-transformed cells blocked growth in soft agar (36). Therefore, we sought to test if Raf activation was necessary for Ras-mediated anoikis resistance.
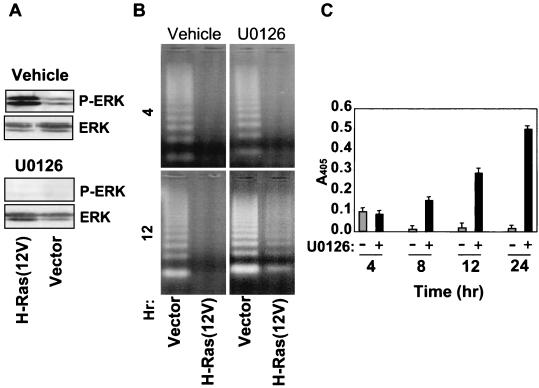
H-Ras(12V)-transformed RIE-1 cells showed elevated levels of activated ERK when grown in suspension. Treatment with 30 μM U0126 MEK inhibitor reduced the level of ERK activation in control and H-Ras(12V)-transformed cells to below basal activity (Fig. 7A). Interestingly, inhibition of ERK activation partially sensitized Ras-transformed RIE-1 cells to anoikis. The response to inhibition of MEK was a gradual and partial induction of apoptosis in suspended Ras-transformed cells, which was substantially delayed relative to the apoptosis observed for the control empty vector-infected cells (Fig. 7B). Using DNA fragmentation ELISA, a more sensitive assay than DNA laddering, the effects of U0126 were observed as early as 8 h postsuspension and continued to increase through the latest time point tested, namely, 24 h (Fig. 7C).
FIG. 7.
ERK activation partially contributes to anoikis resistance of Ras-transformed RIE-1 cells. (A) RIE-1 cells expressing activated Ras were suspended for 6 h in the presence of vehicle or 30 μM U0126 and assayed for ERK activation by immunoblotting with phospho-specific ERK antibodies. Blots were stripped and reprobed with anti-ERK antibodies to determine total ERK levels. (B and C) The effects of pharmacologic inhibition of ERK activation on anoikis were assessed by treating suspended cells with either U0126 (dark bars) or vehicle (light bars) for the indicated time (hours) and performing DNA laddering (B) and DNA fragmentation ELISA (C) analyses. For panel C, only H-Ras(12V)-expressing cells were assayed. Data shown are representative of at least two independent experiments. Bars represent the standard deviations resulting from duplicate reading of the same sample.
One concern with these analyses is that the U0126 inhibitor was overly effective at blocking MEK function and inhibited basal levels of ERK phosphorylation that may be generally required for cell viability. However, treatment of adherent Ras-transformed RIE-1 cells with the same concentration of U0126 did not cause any induction in apoptosis relative to vehicle-treated controls (data not shown). Thus, while ERK activation alone clearly is not sufficient to confer anoikis resistance, the Raf-ERK signaling pathway partially contributes to Ras-mediated inhibition of anoikis. This is in contrast to the complete independence from Raf-MEK-ERK signaling described previously for the resistance of MDCK cells to anoikis (29).
Ras activation of the PI3K-Akt pathway is not involved in transformation of RIE-1 cells.
As described above, we found that activation of the PI3K-Akt pathway alone was not sufficient to block anoikis. Surprisingly, we found that Ras-transformed RIE-1 cells did not possess up-regulated Akt activity compared to empty vector-infected cells. The vector-infected and H-Ras-transformed RIE-1 cells showed low levels of activated Akt when suspended in serum-free growth medium, and the level of activated Akt increased comparably when these cells were suspended in serum-containing growth medium (Fig. 8A). Interestingly, while we found that the Akt activation seen for vector-infected cells in the presence of serum was inhibited by treatment with 10 μM LY29004, Akt activation remained elevated in Ras-transformed cells. Nevertheless, Ras-transformed RIE-1 cells were equally resistant to anoikis when suspended either in the presence or in the absence of serum (Fig. 8B). Thus, these data suggest that Ras fails to activate Akt above vector controls and that activation of Akt is not the means by which Ras-transformed RIE-1 cells become resistant to anoikis.
FIG. 8.
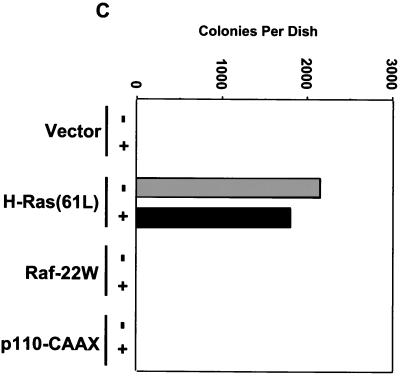
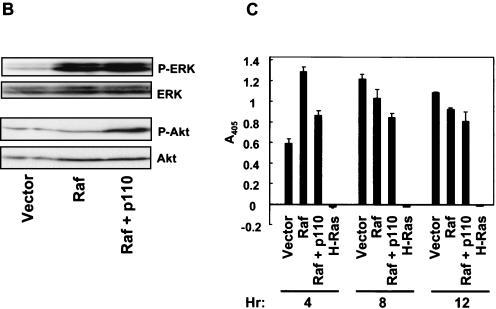
Akt activation is not caused by Ras and is not involved in protection against anoikis. (A) H-Ras(12V)-expressing RIE-1 cells were held in suspension for 6 h in either the presence or the absence of serum and treated with either 10 μM LY294002 or vehicle. Cells were analyzed for activation of Akt by Western blot analysis with phospho-specific Akt antibodies. Blots were stripped and reprobed with anti-Akt antibodies to assess total Akt protein levels. (B) DNA laddering was evaluated in cells suspended for 4 and 12 h in the presence or absence of serum and treated with LY294002 or vehicle. (C) RIE-1 cells stably infected with the empty pZIP-NeoSV(x)1 expression vector or vector encoding H-Ras(61L), Raf-22W, or p110-CAAX were seeded in soft agar and assessed for colony formation after 10 days. The dark bar represents cells treated with 10 μM LY294002, and the light bar represents cells treated with vehicle.
Since we determined that PI3K activity does not appear to play a role in anoikis protection of Ras-transformed cells, we wanted to ascertain if PI3K signaling was also dispensable for the growth of Ras-transformed RIE-1 cells in soft agar. Ras-transformed RIE-1 cells were suspended in soft agar in the presence of 10 μM LY294002. After 10 days in suspension, the number of colonies formed in the presence or absence of the inhibitor was determined. As is apparent in Fig. 8C, LY294002 treatment failed to have a significant effect on the number or size of the colonies formed. As expected, neither RIE-1 cells stably expressing activated Raf nor such cells expressing PI3K were capable of forming any colonies under these conditions. These data emphasize that additional signaling properties of Ras, independent of PI3K and Raf, are clearly required for transformation of RIE-1 cells. Furthermore, these data suggest that PI3K, which may play a central role for Ras transformation of some cell types, may be dispensable for transformation of RIE-1 cells.
Combinatorial inhibition or coexpression of the Ras effectors PI3K and Raf fails to affect anoikis resistance of RIE-1 cells.
Previous work analyzing transforming properties of Ras has shown that activated Raf and PI3K can cooperate and cause synergistic transformation of fibroblast cells (43). Given the precedent for a role for PI3K activation in anoikis protection in MDCK cells, and the partial role of ERK activation in protecting Ras-transformed RIE-1 cells from anoikis, we sought to determine if PI3K and Raf might cooperate to facilitate Ras-mediated anoikis resistance of RIE-1 cells.
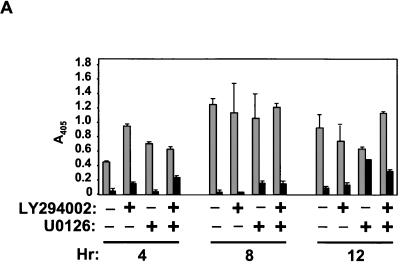
We utilized two approaches to determine if Ras inhibition of anoikis required the coordinate activation of Raf and PI3K. First, we determined if the inhibition of both PI3K and Raf signaling would cause a loss of anoikis resistance in Ras-transformed RIE-1 cells. For these analyses, we treated control and H-Ras(12V)-transformed RIE-1 cells in suspension with 30 μM U0126, 10 μM LY294002, or both inhibitors or with vehicle for up to 12 h. Anoikis was measured by DNA fragmentation ELISA. The limited reversal of anoikis protection seen with U0126 treatment alone was not enhanced by concurrent treatment with LY294002 (Fig. 9A). Thus, PI3K activation, either alone or together with ERK activation, is not required for Ras inhibition of anoikis.
FIG. 9.
Coordinate activation of Raf and PI3K is not necessary or sufficient to block anoikis. (A) RIE-1 cells stably infected with empty vector pBabe-puro (light bars) or encoding H-Ras(12V) (dark bars) were treated with either vehicle or 10 μM LY294002 and/or 30 μM U0126 for the indicated time in suspension (hours) prior to evaluating anoikis by DNA fragmentation ELISA. (B) RIE-1 cells stably infected with pBabe-p110-CAAX and pZIP-raf-22W were established by selection in growth medium supplemented with puromycin and G418. To assay for the activation state of ERK and Akt, Western blot analyses were performed with cells suspended for 6 h in the absence of serum. Cells stably infected with the pZIP-NeoSV(x)1 and pBabe-puro empty vectors were established as controls for these analyses. (C) The resulting cell populations were evaluated for anoikis by DNA fragmentation ELISA after suspension for the indicated time (hours). Data shown are representative of at least two independent experiments. Bars represent the standard deviations resulting from duplicate reading of the same sample.
For our second approach, we established mass populations of RIE-1 cells stably expressing activated Raf-22W, p110-CAAX, or both and compared their anoikis resistance to that of Ras-transformed cells. Coexpression of constitutively active PI3K and Raf was achieved by coinfection with retrovirus expression vectors encoding Raf-22W (pZIP-raf-22W; G418 resistant) and p110-CAAX (pBabe-p110-CAAX; puromycin resistant) and isolation of doubly infected cells by selection in growth medium supplemented with G418 and puromycin. Western blot analyses with phospho-specific antibodies verified that the doubly infected cells possessed enhanced Akt and ERK activity (Fig. 9B). However, despite coactivation of ERK and Akt, these cells were found to be anoikis sensitive (Fig. 9C). Interestingly, RIE-1 cells coexpressing either RalGDS-CAAX or H-Ras(12V/G37) with Raf-22W were also found to be as sensitive to anoikis as the vector controls (data not shown). These data suggest that the effector signaling pathway(s) that cooperates with Raf to cause Ras-mediated anoikis resistance of RIE-1 cells is both PI3K and RalGEF independent.
The EGFR autocrine signaling pathway critical to Ras transformation of RIE-1 cells is dispensable for anoikis resistance.
We determined previously that oncogenic Ras transformation of RIE-1 cells involves upregulation of the expression of TGF-α and related peptide growth factors that cause the subsequent activation of the EGFR (20, 35). This autocrine signaling loop is mediated by Raf-independent effector signaling and is required for Ras transformation of RIE-1 cells. Thus, we sought to determine if activation of this loop might contribute to anoikis resistance of Ras-transformed RIE-1 cells.
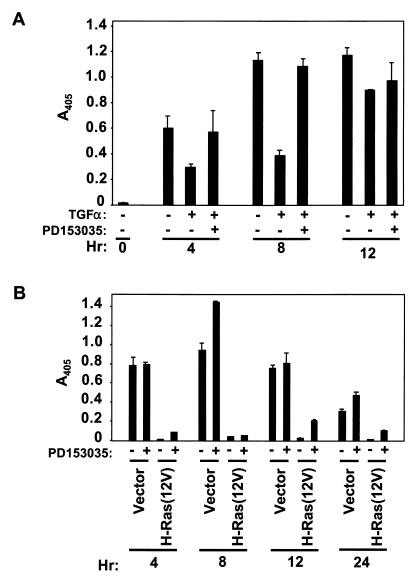
We first determined if TGF-α stimulation of the EGFR would provide any protection against anoikis. We treated suspended parental RIE-1 cells with TGF-α in the presence or absence of 2 μM PD153035, an EGFR kinase inhibitor, and assayed for apoptosis. TGF-α treatment did cause a partial inhibition of anoikis, and this activity was reversed by treatment with the inhibitor (Fig. 10A). These results suggest that activation of an EGFR autocrine loop alone has the potential to contribute to Ras inhibition of anoikis.
FIG. 10.
The TGF-α–EGFR autocrine loop is dispensable for anoikis resistance mediated by Ras. (A) Parental RIE-1 cells were treated with 10 ng of TGF-α/ml, 2 μM PD153035 EGFR kinase inhibitor, and/or vehicle for the indicated time (hours) in suspension prior to evaluating the level of anoikis by DNA fragmentation ELISA. (B) Cells expressing H-Ras(12V) or infected with the empty pBabe-puro vector were assayed for anoikis by DNA fragmentation ELISA in response to treatment with 2 μM PD153035 or vehicle.
Next, we determined if the EGFR autocrine loop was necessary for Ras inhibition of anoikis. For these analyses, Ras-transformed RIE-1 cells were treated with the PD153035 EGFR inhibitor as a function of time in suspension. The PD153035 inhibitor was effective in blocking EGFR activity as measured by its ability to block the anoikis-inhibitory signal generated by TGF-α treatment (Fig. 10A). However, DNA fragmentation ELISA revealed no significant induction of apoptosis of Ras-transformed cells, compared with vector-transfected control RIE-1 cells, over the course of 24 h (Fig. 10B). Thus, activation of an EGFR autocrine loop does not contribute significantly to the anoikis-resistant phenotype of Ras-transformed RIE-1 cells.
DISCUSSION
Oncogenic Ras utilizes multiple downstream effectors to cause tumorigenic and malignant transformation of cells (7). Hence, it is likely that distinct effectors will be involved in mediating distinct aspects of neoplastic transformation. In this study, we evaluated the role of the three best-characterized effectors of Ras transformation, namely, Raf-1, PI3K, and RalGEFs, in mediating inhibition of matrix deprivation-induced apoptosis, or anoikis (18), of Ras-transformed RIE-1 cells. First, we showed that parental RIE-1 cells underwent rapid caspase-dependent anoikis, readily detectable within 4 h of being placed in suspension, and that this response was overcome by stable expression of oncogenic Ras. Second, we found that constitutive activation of Raf-1 alone, or together with PI3K or RalGDS activation, is not sufficient to inhibit anoikis of RIE-1 cells. Inhibiting MEK activation with U0126, however, did partially restore anoikis sensitivity in Ras-transformed cells, indicating that while the Raf-MEK-ERK pathway is not sufficient, it does serve a necessary, albeit minor, role in anoikis resistance. Third, we found that treatment with the LY294002 PI3K inhibitor did not restore sensitivity to anoikis, nor did it inhibit morphological or growth transformation, indicating that PI3K may not be an important effector for Ras transformation of RIE-1 cells. Finally, we found that the EGFR autocrine growth loop, important for morphological and growth transformation of RIE-1 cells, is not a significant contributor to Ras inhibition of anoikis. Taken together, our observations contrast with previous studies with MDCK canine kidney epithelial cells, where the PI3K-Akt pathway was found to be necessary and sufficient to block anoikis (29). Our results emphasize the importance of cell context, even with respect to two epithelial cell lines, in influencing the mechanisms by which Ras promotes transformation.
Similar to observations with other epithelial cells, we found that RIE-1 cells undergo anoikis and that activated Ras blocks this apoptotic response. We found that the different Ras proteins all show equivalent abilities to render RIE-1 cells resistant to anoikis. Thus, while it has been reported previously that H-Ras and K-Ras differentially activate Raf and PI3K (59), these differences do not influence their abilities to block anoikis in RIE-1 cells. Additionally, we found that activated R-Ras and TC21/R-Ras2 also block anoikis but that activated RhoA and Rac1 do not, providing some initial clues as to the mechanism by which Ras might block anoikis. R-Ras has been shown elsewhere to activate PI3K, but not Raf and RalGEFs (34, 54). Similarly, we have found that TC21 can activate PI3K, but not Raf or RalGDS (reference 21 and unpublished observations), although other investigators have reported that TC21 can activate Raf. Thus, the ability of TC21 and R-Ras to rescue RIE-1 cells from anoikis suggests that a Raf- and RalGDS-independent effector pathway(s) plays a critical role in blocking anoikis. Since Rho GTPases are activators of NF-κB (38, 53), it appears unlikely that this survival signal is sufficient to mediate Ras inhibition of anoikis. The failure of activated RhoA and Rac1 to block anoikis is also consistent with the inability of these small GTPases to promote the anchorage-independent growth of RIE-1 cells (data not shown).
To evaluate the specific role of Raf, PI3K, and RalGEFs in mediating Ras inhibition of anoikis, we utilized both effector domain mutants of Ras that are impaired in specific effector function and constitutively activated versions of these effectors. Consistent with our observation that R-Ras and TC21 can protect RIE-1 cells against anoikis, we found that activation of Raf (Raf-22W and the 12V/35S effector domain mutant) or PI3K (p110α-CAAX and the 12V/40C effector domain mutant) alone provided no protection against anoikis. Similarly, expression of the 12V/37G effector domain mutant, an activator of RalGEFs, or constitutively activated RalGDS or Rlf also failed to block anoikis. Thus, we conclude that the activation of the Raf, PI3K, or RalGEF effector pathway alone is not sufficient to block anoikis. We also found that activated Raf, together with either activated PI3K or RalGDS, is still not sufficient to block anoikis.
While other studies have also found that Raf activation alone is not sufficient to block anoikis (29), our observations with PI3K contrast with other studies that show a primary or sole role for PI3K signaling in protecting Ras-transformed epithelial cells from anoikis. Cumulatively, these data are more likely to reflect cell-specific differences in the mechanisms of Ras transformation than differences in experimental approach. For example, our analyses of RIE-1 cells are essentially the same as those used previously to implicate the key role for PI3K in MDCK cells (29). We each expressed the H-Ras(12V/C40) effector domain mutant that retains its ability to bind PI3K or the plasma membrane-targeted p110α catalytic subunit of PI3K in the respective cell lines and assayed for anoikis resistance. While both constructs caused morphological transformation of MDCK cells, we found that neither caused morphological transformation of RIE-1 cells, suggesting that the two epithelial cell lines respond fundamentally differently to activation of the Ras effector PI3K. Finally, our use of the inhibitor LY294002 failed to sensitize Ras-transformed RIE-1 cells to anoikis. This is in contrast to results obtained elsewhere with MDCK and IEC-18 cells (29, 46), which were both made sensitive to anoikis by treatment with the LY294002 compound after transformation by Ras. We conclude that PI3K activation is neither sufficient nor necessary for protection of RIE-1 cells from anoikis.
Interestingly, while Downward and colleagues showed that oncogenic Ras activates Akt in MDCK cells (29), we found no such activation in RIE-1 cells, suggesting that Ras may not activate PI3K robustly in this cell line. Furthermore, we also saw no activation of Akt in suspended Ras-transformed ROSE 199 cells versus control empty-vector-transfected cells (data not shown), despite the fact that expression of oncogenic Ras confers anoikis resistance upon these cells as well. Interestingly, some preliminary work with human carcinoma cell lines also suggests that Ras may not utilize PI3K-Akt signaling to confer anoikis resistance upon cancer cells. DLD-1 cells, a human colon cell line with an endogenously mutated K-ras allele, did not possess elevated levels of activated Akt relative to DkS8 cells (which are derived from DLD-1 cells but have lost the oncogenic ras allele by homologous recombination) (data not shown). In fact, both DLD-1 and DkS8 cells have barely detectable levels of Akt phosphorylation. DkS8 cells have nonetheless regained some sensitivity to anoikis relative to DLD-1 cells, suggesting that Ras promotes PI3K-independent survival signaling in this human cell line. Correspondingly, MDA 231 cells, a breast carcinoma cell line that also contains an endogenously activated K-ras allele, show barely detectable levels of Akt phosphorylation in suspension and yet are anoikis resistant (L. B. Eckert and C. J. Der, unpublished data). Furthermore, when treated with LY294002, these cells are not sensitized to anoikis. Although it remains to be determined what signaling pathways contribute to anoikis resistance of MDA 231 cells, these data clearly indicate that targeting PI3K-Akt signaling in carcinomas that contain Ras mutations may not cause inhibition of oncogenic Ras function. Other researchers have also noted that Ras activation of PI3K and Akt may be cell type specific and/or be sensitive to the level and timing of expression of Ras and its effectors (16). Thus, in contrast to the important contribution of PI3K to Ras transformation of NIH 3T3 cells (43), PI3K may not be a critical effector for Ras transformation of RIE-1 or ROSE cells. Furthermore, our analyses of several human colon and breast carcinoma cell lines indicate that Ras signaling to PI3K-Akt does not play a role in anoikis resistance of at least some cancers that suffer Ras mutations in vivo.
Like the Ras effector PI3K, we observed a different role for Raf in mediating anoikis resistance of Ras-transformed RIE-1 cells compared to other epithelial cells. Unlike what has been reported elsewhere for MDCK and IEC-18 rat intestinal epithelial cells (29, 46), inhibiting MEK in Ras-transformed RIE-1 cells caused a partial reversion to an anoikis-sensitive state. Expression of activated Raf, however, was not sufficient to cause anoikis resistance of RIE-1 cells, even when coexpressed with activated PI3K or RalGEFs. Thus, Raf is likely to cooperate with an unknown PI3K- and RalGEF-independent effector(s) to block anoikis. Interestingly, cooperation between two or more Ras effectors also appears to be required for Ras-mediated anoikis resistance of IEC-18 cells (47), although PI3K, and not Raf, appears to play a pivotal role in anoikis resistance of these cells. Cooperation between Ras effectors has also been observed for mediating other facets of transformation, for example, focus formation (43, 56). Thus, perhaps it is not surprising that cooperativity exists between Ras effectors in mediating anoikis resistance.
We found previously that a TGF-α–EGFR autocrine loop is critical for complete transformation of RIE-1 cells, including growth in soft agar (20). Consistent with this observation, we found that activation of the EGFR with TGF-α is sufficient to cause partial resistance to anoikis of suspended parental RIE-1 cells. Surprisingly, however, inhibition of EGFR signaling has very little effect on anoikis resistance of Ras-transformed RIE-1 cells, at least over the span of 24 h. Perhaps this signifies that EGFR signaling plays a redundant role with a Ras effector(s) in inhibiting anoikis of Ras-transformed RIE-1 cells.
We have not yet identified what Ras effector(s) cooperates with Raf to induce anoikis resistance of RIE-1 cells. In addition to Raf, PI3K, and RalGEFs, there is an expanding roster of other proteins that interact with Ras in its activated, GTP-bound state (51). These include AF-6, Nore-1, Rin1, PKCζ, MEKK1, RASSF1, and the Ras GTPase-activated proteins (p120 and NF1). Although some of these proteins are unlikely to play a role in inhibition of anoikis, for example, MEKK1, whose activation is associated with induction of apoptosis (8), none have thus far been tested for their ability to cooperate with Raf in our study or other anoikis studies. However, since Rin1 association is retained in the 37G effector domain mutant (24) and AF-6 binding is retained in the 37G and 40C mutants (28), it is unlikely that engagement of Rin1 and AF-6 is sufficient to block anoikis. Perhaps the key effector for Ras inhibition of anoikis in RIE-1 cells remains to be identified.
How might Raf activity promote anoikis resistance of RIE-1 cells? Activation of the MAPK signaling pathway can inhibit apoptosis in response to growth factor withdrawal, death receptor activation, and, as very recently described for fibroblasts and MDCK cells, detachment from extracellular matrix (25, 32). Given the gradual and delayed sensitization of Ras-transformed RIE-1 cells to anoikis in response to MEK inhibition, one possible mechanism of action of this inhibitor is to cause changes in gene expression that regulate apoptosis. Along these lines, inhibition of MEK in mammalian cells can reduce expression of several antiapoptotic proteins of the Bcl-2 family as well as reduce expression of the antiapoptotic gene par-4 (2, 5). Alternatively, MEK signaling may have immediate effects on the apoptosis machinery, for example, by modulating the activation state of Bad (48). Whether the above-mentioned consequences of MEK activation play a role in anoikis resistance of Ras-transformed RIE-1 cells remains to be determined.
In summary, we have determined that PI3K is not a key effector for Ras inhibition of anoikis or transformation in RIE-1 cells. Furthermore, oncogenic Ras did not cause upregulation of the PI3K-Akt pathway in RIE-1 cells. Our results emphasize that Ras promotes transformation in multiple ways in a cell-context-dependent fashion. We are currently assessing the importance of the Raf-ERK, PI3K-Akt, and other Ras signaling pathways in protecting human tumor cells from anoikis. We suspect that our studies will find a similar complexity, with different Ras effector pathways playing important roles in some, but not all, tumor cells. Thus, ultimately inhibiting oncogenic Ras function in various human tumors may require effectively targeting a variety of Ras effector signaling pathways.
ACKNOWLEDGMENTS
We thank Julian Downward for providing the p110α-CAAX cDNA construct, Johannes Bos for providing the pMT2-HA-Rlf-CAAX construct, James Trzaskos (Dupont) for providing U0126, Heena Mehta and Staeci Morita for technical support, and Misha Rand for preparation of figures.
Our research was supported by grants from the National Institutes of Health (NIH) to C.J.D. (CA42978, CA55008, and CA63071). A.M. was additionally supported by an NIH training grant fellowship and an NIH National Research Service Award (CA84633-02).
REFERENCES
- 1.Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- 2.Barradas M, Monjas A, Diaz-Meco M T, Serrano M, Moscat J. The downregulation of the pro-apoptotic protein Par-4 is critical for Ras-induced survival and tumor progression. EMBO J. 1999;18:6362–6369. doi: 10.1093/emboj/18.22.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blay J, Brown K D. Characterization of an epithelioid cell line derived from the rat small intestine. Cell Biol Int Rep. 1984;8:551–560. doi: 10.1016/0309-1651(84)90054-7. [DOI] [PubMed] [Google Scholar]
- 4.Bos J L. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 5.Boucher M J, Morisset J, Vachon P H, Reed J C, Laine J, Rivard N. MEK/ERK signaling pathway regulates the expression of bcl-2, bcl-X(L), and mcl-1 and promotes survival of human pancreatic cancer cells. J Cell Biochem. 2000;79:355–369. [PubMed] [Google Scholar]
- 6.Boudreau N, Sympson C J, Werb Z, Bissell M J. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell S L, Khosravi-Far R, Rossman K L, Clark G J, Der C J. Increasing complexity of Ras signaling. Oncogene. 1998;17:1395–1413. doi: 10.1038/sj.onc.1202174. [DOI] [PubMed] [Google Scholar]
- 8.Cardone M H, Salvesen G S, Widmann C, Johnson G, Frisch S M. The regulation of anoikis: MEKK-1 activation requires cleavage by caspases. Cell. 1997;90:315–323. doi: 10.1016/s0092-8674(00)80339-6. [DOI] [PubMed] [Google Scholar]
- 9.Clark G J, Cox A D, Graham S M, Der C J. Biological assays for Ras transformation. Methods Enzymol. 1995;255:395–412. doi: 10.1016/s0076-6879(95)55042-9. [DOI] [PubMed] [Google Scholar]
- 10.Clark G J, Der C J. Oncogenic activation of Ras proteins. In: Dickey B F, Birnbaumer L, editors. GTPases in biology I. Berlin, Germany: Springer Verlag; 1993. pp. 259–288. [Google Scholar]
- 11.Cox A D, Brtva T R, Lowe D G, Der C J. R-Ras induces malignant, but not morphologic, transformation of NIH3T3 cells. Oncogene. 1994;9:3281–3288. [PubMed] [Google Scholar]
- 12.Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- 13.Favata M F, Horiuchi K Y, Manos E J, Daulerio A J, Stradley D A, Feeser W S, Van Dyk D E, Pitts W J, Earl R A, Hobbs F, Copeland R A, Magolda R L, Scherle P A, Trzaskos J M. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 14.Fearnhead H O, Dinsdale D, Cohen G M. An interleukin-1 beta-converting enzyme-like protease is a common mediator of apoptosis in thymocytes. FEBS Lett. 1995;375:283–288. doi: 10.1016/0014-5793(95)01228-7. [DOI] [PubMed] [Google Scholar]
- 15.Feig L A, Urano T, Cantor S. Evidence for a Ras/Ral signaling cascade. Trends Biochem Sci. 1996;21:438–441. doi: 10.1016/s0968-0004(96)10058-x. [DOI] [PubMed] [Google Scholar]
- 16.Franke T F, Yang S I, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 17.Frisch S M, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frisch S M, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701–706. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- 19.Fry D W, Kraker A J, McMichael A, Ambroso L A, Nelson J M, Leopold W R, Connors R W, Bridges A J. A specific inhibitor of the epidermal growth factor receptor tyrosine kinase. Science. 1994;265:1093–1095. doi: 10.1126/science.8066447. [DOI] [PubMed] [Google Scholar]
- 20.Gangarosa L M, Sizemore N, Graves-Deal R, Oldham S M, Der C J, Coffey R J. A Raf-independent epidermal growth factor receptor autocrine loop is necessary for Ras transformation of rat intestinal epithelial cells. J Biol Chem. 1997;272:18926–18931. doi: 10.1074/jbc.272.30.18926. [DOI] [PubMed] [Google Scholar]
- 21.Graham S M, Vojtek A B, Huff S Y, Cox A D, Clark G J, Cooper J A, Der C J. TC21 causes transformation by Raf-independent signaling pathways. Mol Cell Biol. 1996;16:6132–6140. doi: 10.1128/mcb.16.11.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hahn W C, Counter C M, Lundberg A S, Beijersbergen R L, Brooks M W, Weinberg R A. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 23.Hall P A, Coates P J, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107:3569–3577. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 24.Han L, Wong D, Dhaka A, Afar D, White M, Xie W, Herschman H, Witte O, Colicelli J. Protein binding and signaling properties of RIN1 suggest a unique effector function. Proc Natl Acad Sci USA. 1997;94:4954–4959. doi: 10.1073/pnas.94.10.4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmstrom T H, Chow S C, Elo I, Coffey E T, Orrenius S, Sistonen L, Eriksson J E. Suppression of Fas/APO-1-mediated apoptosis by mitogen-activated kinase signaling. J Immunol. 1998;160:2626–2636. [PubMed] [Google Scholar]
- 26.Huff S Y, Quilliam L A, Cox A D, Der C J. R-Ras is regulated by activators and effectors distinct from those that control Ras function. Oncogene. 1997;14:133–143. doi: 10.1038/sj.onc.1200815. [DOI] [PubMed] [Google Scholar]
- 27.Khosravi-Far R, Solski P A, Clark G J, Kinch M S, Der C J. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol Cell Biol. 1995;15:6443–6453. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khosravi-Far R, White M A, Westwick J K, Solski P A, Chrzanowska-Wodnicka M, Van Aelst L, Wigler M H, Der C J. Oncogenic Ras activation of Raf/mitogen-activated protein kinase-independent pathways is sufficient to cause tumorigenic transformation. Mol Cell Biol. 1996;16:3923–3933. doi: 10.1128/mcb.16.7.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khwaja A, Rodriguez-Viciana P, Wennström S, Warne P H, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kops G J, de Ruiter N D, de Vries-Smits A M, Powell D R, Bos J L, Burgering B M. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 31.Leevers S J, Paterson H F, Marshall C J. Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature. 1994;369:411–414. doi: 10.1038/369411a0. [DOI] [PubMed] [Google Scholar]
- 32.Le Gall M, Chambard J C, Breittmayer J P, Grall D, Pouyssegur J, Obberghen-Schilling E. The p42/p44 MAP kinase pathway prevents apoptosis induced by anchorage and serum removal. Mol Biol Cell. 2000;11:1103–1112. doi: 10.1091/mbc.11.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marshall C J. Ras effectors. Curr Opin Cell Biol. 1996;8:197–204. doi: 10.1016/s0955-0674(96)80066-4. [DOI] [PubMed] [Google Scholar]
- 34.Marte B M, Rodriguez-Viciana P, Wennström S, Warne P H, Downward J. R-Ras can activate the phosphoinositide 3-kinase but not the MAP kinase arm of the Ras effector pathways. Curr Biol. 1996;7:63–70. doi: 10.1016/s0960-9822(06)00028-5. [DOI] [PubMed] [Google Scholar]
- 35.Oldham S M, Clark G J, Gangarosa L M, Coffey R J, Jr, Der C J. Activation of the Raf-1/MAP kinase cascade is not sufficient for Ras transformation of RIE-1 epithelial cells. Proc Natl Acad Sci USA. 1996;93:6924–6928. doi: 10.1073/pnas.93.14.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oldham S M, Cox A D, Reynolds E R, Sizemore N S, Coffey R J, Jr, Der C J. Ras, but not Src, transformation of RIE-1 epithelial cells is dependent on activation of the mitogen-activated protein kinase cascade. Oncogene. 1998;16:2565–2573. doi: 10.1038/sj.onc.1201784. [DOI] [PubMed] [Google Scholar]
- 37.Pear W S, Nolan G P, Scott M L, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perona R, Montaner S, Saniger L, Sánchez-Pérez I, Bravo R, Lacal J C. Activation of the nuclear factor-KB by Rho, CDC42, and Rac-1 proteins. Genes Dev. 1997;11:463–475. doi: 10.1101/gad.11.4.463. [DOI] [PubMed] [Google Scholar]
- 39.Petersen O W, Ronnov-Jessen L, Weaver V M, Bissell M J. Differentiation and cancer in the mammary gland: shedding light on an old dichotomy. Adv Cancer Res. 1998;75:135–161. doi: 10.1016/s0065-230x(08)60741-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polakowska R R, Piacentini M, Bartlett R, Goldsmith L A, Haake A R. Apoptosis in human skin development: morphogenesis, periderm, and stem cells. Dev Dyn. 1994;199:176–188. doi: 10.1002/aja.1001990303. [DOI] [PubMed] [Google Scholar]
- 41.Ramocki M B, White M A, Konieczny S F, Taparowsky E J. A role for RalGDS and a novel Ras effector in the Ras-mediated inhibition of skeletal myogenesis. J Biol Chem. 1998;273:17696–17701. doi: 10.1074/jbc.273.28.17696. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez-Viciana P, Warne P H, Dhand R, Vanhaesebroeck B, Gout I, Fry M J, Waterfield M D, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez-Viciana P, Warne P H, Khwaja A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez-Viciana P, Warne P H, Vanhaesebroeck B, Waterfield M D, Downward J. Activation of phosphoinositide 3-kinase by interaction with Ras and by point mutation. EMBO J. 1996;15:2442–2451. [PMC free article] [PubMed] [Google Scholar]
- 45.Romashkova J A, Makarov S S. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 46.Rosen K, Rak J, Jin J, Kerbel R S, Newman M J, Filmus J. Downregulation of the pro-apoptotic protein Bak is required for the ras-induced transformation of intestinal epithelial cells. Curr Biol. 1998;8:1331–1334. doi: 10.1016/s0960-9822(07)00564-7. [DOI] [PubMed] [Google Scholar]
- 47.Rosen K, Rak J, Leung T, Dean N M, Kerbel R S, Filmus J. Activated Ras prevents downregulation of Bcl-X(L) triggered by detachment from the extracellular matrix. A mechanism of Ras-induced resistance to anoikis in intestinal epithelial cells. J Cell Biol. 2000;149:447–456. doi: 10.1083/jcb.149.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scheid M P, Schubert K M, Duronio V. Regulation of bad phosphorylation and association with Bcl-x(L) by the MAPK/Erk kinase. J Biol Chem. 1999;274:31108–31113. doi: 10.1074/jbc.274.43.31108. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz M A. Integrins, oncogenes, and anchorage independence. J Cell Biol. 1997;139:575–578. doi: 10.1083/jcb.139.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Serrano M, Lin A W, McCurrach M E, Beach D, Lowe S W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 51.Shields J M, Pruitt K, McFall A, Shaub A, Der C J. Understanding Ras: “it ain't over 'til it's over.”. Trends Cell Biol. 2000;10:147–153. doi: 10.1016/s0962-8924(00)01740-2. [DOI] [PubMed] [Google Scholar]
- 52.Stanton V P, Jr, Nichols D W, Laudano A P, Cooper G M. Definition of the human raf amino-terminal regulatory region by deletion mutagenesis. Mol Cell Biol. 1989;9:639–647. doi: 10.1128/mcb.9.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sulciner D J, Irani K, Yu Z-X, Ferrans V J, Goldschmidt-Clermont P, Finkel T. rac1 regulates a cytokine-stimulated, redox-dependent pathway necessary for NF-κB activation. Mol Cell Biol. 1996;16:7115–7121. doi: 10.1128/mcb.16.12.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Urano T, Emkey R, Feig L A. Ral-GTPases mediate a distinct downstream signaling pathway from Ras that facilitates cellular transformation. EMBO J. 1996;15:810–816. [PMC free article] [PubMed] [Google Scholar]
- 55.Vlahos C J, Matter W F, Hui K Y, Brown R F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 56.White M A, Nicolette C, Minden A, Polverino A, Van Aelst L, Karin M, Wigler M H. Multiple Ras functions can contribute to mammalian cell transformation. Cell. 1995;80:533–541. doi: 10.1016/0092-8674(95)90507-3. [DOI] [PubMed] [Google Scholar]
- 57.Wolthuis R M, Bos J L. Ras caught in another affair: the exchange factors for Ral. Curr Opin Genet Dev. 1999;9:112–117. doi: 10.1016/s0959-437x(99)80016-1. [DOI] [PubMed] [Google Scholar]
- 58.Wolthuis R M, de Ruiter N D, Cool R H, Bos J L. Stimulation of gene induction and cell growth by the Ras effector Rlf. EMBO J. 1997;16:6748–6761. doi: 10.1093/emboj/16.22.6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan J, Roy S, Apolloni A, Lane A, Hancock J F. Ras isoforms vary in their ability to activate Raf-1 and phosphoinositide 3-kinase. J Biol Chem. 1998;273:24052–24056. doi: 10.1074/jbc.273.37.24052. [DOI] [PubMed] [Google Scholar]