Abstract
The prevalence of food allergy has increased in recent years, especially among the pediatric population. Differences in the gut microbiota composition between children with FA and healthy children have brought this topic into the spotlight as a possible explanation for the increase in FA. The gut microbiota characteristics are acquired through environmental interactions starting early in life, such as type of delivery during birth and breastfeeding. The microbiota features may be shaped by a plethora of immunomodulatory mechanisms, including a predominant role of Tregs and the transcription factor FOXP3. Additionally, a pivotal role has been given to vitamin A and butyrate, the main anti-inflammatory metabolite. These observations have led to the study and development of therapies oriented to modifying the microbiota and metabolite profiles, such as the use of pre- and probiotics and the determination of their capacity to induce tolerance to allergens that are relevant to FA. To date, evidence supporting these approaches in humans is scarce and inconclusive. Larger cohorts and dose-titration studies are mandatory to evaluate whether the observed changes in gut microbiota composition reflect medical recovery and increased tolerance in pediatric patients with FA. In this article, we discuss the establishment of the microbiota, the immunological mechanisms that regulate the microbiota of children with food allergies, and the evidence in research focused on its regulation as a means to achieve tolerance to food allergens.
1. Introduction
Food allergy (FA) or food allergies are pathologies triggered by exposure to food allergens [1]. The prevalence of immunoglobulin (Ig) E-mediated FA in children has increased in recent years, with figures ranging from 1 to 2.53% in the USA [2] and Canada [3] to 5.5% in Chile [4]. Higher proportions are observed in self-report studies, reaching up to 25% in some regions [5]. The most common allergens include peanuts, walnuts, eggs, milk, fish, and soy, varying between countries and age groups [3, 6].
Risk factors for the development of FA include vitamin D deficiency, delayed exposure to food allergens, reduced exposure to microorganisms (as suggested by the hygiene hypothesis), and changes in the microbiota [7]. The gut microbiota corresponds to the group of microorganisms that colonize the intestine [8]. The loss of the gut microbiota homeostasis due to changes in their relative abundance and diversity is known as dysbiosis [9]. This condition has been observed in children with FA, whose gut microbiota profiles differ from those without FA [10]. Food allergies developed by mechanisms not mediated by IgE, present in approximately one-third of the population, also present differences in the intestinal microbiota, and a greater relative abundance of Bacteroides and Alistipes has been observed, in addition to presenting changes associated with probiotic supplementation. Therefore, the alteration of the microbiota would not be associated only with IgE [11].
Currently, the only available treatment for FA consists of the strict exclusion of the allergen from the diet. Nevertheless, this approach may impact the nutritional state of the patient depending on the type and number of allergens involved and the age of the patient at the time of the diagnosis [12]. Therefore, it is necessary to explore novel therapies to induce food-specific immune tolerance and decrease FA symptoms in these patients.
2. Differences between the Adult and Infant Immune Systems
The immune system of infants is thought to be under active development and training, making it inherently susceptible to react to microbial agents and generate atopic reactions [13]. Throughout neonatal life, the immune system of the infant relies on the immunity of the mother transferred through the placenta, the exposure during childbirth (the birth canal), and breastfeeding [14]. Although hereditary factors also influence the type of immune response that an infant may develop [15], several studies have suggested that nonhereditary factors are the most relevant for shaping the immune system and developing immunity during the first year of life [16]. For example, a recent study on twins characterized 204 immunity-related parameters and showed that 77% of them were heavily influenced by nonhereditary factors [17]. Additionally, it has been reported that immune cells from infants possess high intraindividual heterogeneity, opposed to what has been described for adults [18]. This observation highlights the relevance of environmental exposure during neonatal life [19].
Most of the components of the immune system of newborns are formed but immature in their function [20]. Regarding cell types, neutrophils appear increased in the fetus but fall to levels that will prevail in adulthood a few days after birth, cytotoxic T lymphocytes present low activity compared to adults, and monocytes and macrophages also appear immature [21]. Valiathan et al. measure the concentration of immune cells in different age groups and observed a predominance of lymphocytes, platelets, and B cells that decrease significantly with age, neutrophils and CD8+ T cells increase in adulthood, and natural killer (NK) cells, which are part of the innate immune response, increase mainly in adolescence [22].
Compared to adults, mononuclear cells from children display a reduced capacity to secrete IL-12p70, which plays a pivotal role in the polarization of Th1 cells [23]. Consequently, children show a predominantly Th2 immune response [24].
Functional B and T cells in the gastrointestinal tract are newly expressed at 12 weeks of the newborn, not with the maturity present in adults. However, the transforming growth factor-β (TGF-β) favors the expression of Tregs [25].
Breast milk plays a fundamental role in the maturation of the immune system since it supplies components that may be absent or immature, such as IgA that is absent, therefore the only source is breast milk, and others such as IgG that has been identified that by being associated with food allergens improves their tolerance, reinforcing that the introduction of allergens together with breast milk would be a protective factor against FA [26].
Due to environmental stimulation, adults (previously exposed to tobacco smoke, altered nutritional status, diabetes, dyslipidemia, or insulin resistance) may display a predominantly proinflammatory immune profile [27].
The differences between the adult and the infant immune system will determine the development of the immune response. Of particular relevance is maternal immunity during gestation and environmental exposure during the neonatal and infant life [28].
3. Development of the Microbiota in Infants
Early gut microbiota marks the health of the individual in later life, where it has been associated with pathological conditions triggered later during old age [29] (Figure 1). However, little is known on the specific time point at which disease-generating dysbiosis is generated, the exact composition of a “healthy” microbiota, and whether its alteration is due to pathological conditions, such as food allergies [30], irritable bowel syndrome [31], celiac disease [32], or physiological status, including pregnancy, dietary changes, or age (Figure 1) [33, 34].
Figure 1.
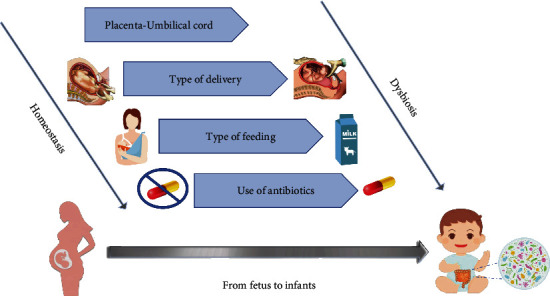
Factors that determine the development of the microbiota in infants. The development of the microbiota begins during pregnancy and continues after birth with exposure to environmental factors. To the left of the figure are factors associated with homeostasis. To the right of the figure are factors related to dysbiosis.
3.1. Factors Associated with the Prenatal and Neonatal Periods
The microbiota colonization begins during gestation, a period in which maternal nutrition and health status influence the type of microorganisms present in the placenta and umbilical cord [35–37]. These factors contribute to the generation of a microbiota profile even before childbirth [38]. Although it was previously thought that the gestational period hosted a sterile environment [39], it is currently known that the establishment of the gut microbiota in infants initiates during pregnancy by the presence of maternal microorganisms that translocate through the vagina, maternal gut, placental tissue, and meconium, thus discarding the concept of a sterile placenta [40, 41].
A report studied the composition of the microbiota of the placenta, amniotic fluid, meconium, newborn stool samples, and maternal stool samples from patients that underwent optional C-sections, but there was no similarity between the microbial populations of amniotic fluid and placenta, where there were nondiverse populations of Proteobacteria (Enterobacter and Escherichia/Shigella), also present in colostrum and meconium but less abundantly [42]. Contrary to this report, another study reported no microbiota in the placenta or amniotic fluid in C-sections [41]. However, 100% of the patients received prophylactic antibiotic treatment that may have affected the results of this study [41, 43]. It remains a matter of discussion whether the presence of placental or amniotic microorganisms results from the development of the fetus or is only detected following microbial translocation from the mother to the fetus [42].
The mode of delivery is known to impact the development of the gut microbiota [44]. A sevenfold higher abundance in Bifidobacterium, Proteobacteria, and the genus Enterobacter-Escherichia-Klebsiella, Clostridium, and Enterococcus has been reported in newborns delivered by vaginal birth compared to C-section, the latter being deprived of exposure to the birth canal, thus presenting a greater abundance of Bacteroidetes and a reduced fraction of Streptococcus [45]. A second study from Korea compared the microbiota of stool samples from infants born of vaginal birth and C-section at 3, 7, and 14 days postpartum. Reduced bacterial diversity was observed with time. Microbiota composition of C-section infants varied from day 14, suggesting that this mode of delivery is associated with the delayed establishment of the gut microbiota, associated with obesity and asthma later in life [46]. Newborns via vaginal birth displayed a higher abundance of Bifidobacterium, Bacteroides, Lactobacillus, and Lachnospiraceae at day 7 compared to C-section, in which Enterococcaceae and Enterobacteriaceae were more abundant [46].
3.2. Factors Associated with the Postnatal Period
Breastfeeding also influences microbiota development because breast milk contains bioactive compounds, such as oligosaccharides that nourish intestinal bacteria, molding the gut microbiota [47]. A recent study of Chilean newborns compared the intestinal microbiota of infants fed with breast milk to that of infants fed with formula at months 1 and 3 and showed that the microbiota of infants fed with breast milk displayed a higher Bacteroidetes abundance [48]. In contrast, those fed with formula showed a higher proportion of Firmicutes. At the genus level, the Enterococcus, Streptococcus, Enterobacter, Lactococcus, and Propionibacterium communities were enriched in the breast milk group at the first month. These differences in bacterial diversity were no longer present in the third month [48, 49]. Another study evaluated the fecal microbiota composition at 40 days, 3 months, and 6 months postpartum; it reported that Bifidobacterium and Enterobacteriaceae were the most abundant in breastfed infants at all time points and were more abundant in this group than formula-fed infants; to differ the formula group, Streptococcus and Enterococcus were the most abundant [50]. In the same study, when evaluating the introduction of complementary feeding, the abundance of Bacteroides increased in the formula group, which did not occur in breastfed infants, which was associated with increased diversity of the microbiota in the formula group [50].
4. Mechanisms of Microbiota Dysbiosis Associated with Food Allergy
The association between microbiota and FA was first reported in germ-free mice displaying elevated IgE levels in the intestinal mucosa without additional alterations of the other immunoglobulins [51]. Based on this observation, it is believed that the microbiota may contribute to maintaining the homeostasis of IgE and the control of allergic responses triggered by these immunoglobulins [52]. Further, the population of microorganisms in the intestine of mice with FA would favor a Th2-type response, shifting the balance from Th1 to Th2 immune profile [53]. It was shown that Citrobacter sp., abundant in FA models, induces the expression of IL-33, promoting a Th2-type immune response [54].
Short-chain fatty acids (SCFA) are fundamental to the microbiota, as they provide metabolites that serve as nourishment to bacterial communities. Of great importance are butyrate, propionate, and acetate that result from the fermentation of dietary carbohydrates by intestinal bacteria [55, 56]. In infants younger than 6 months, dietary carbohydrates correspond to breast milk oligosaccharides, which are not physiologically digested by the infant but serve as sustenance for the microbiota, mainly the genus Bifidobacterium and Bacteroides [57].
The most prevalent SCFA in 3-month-old infants is acetate, where it can be found between 70 and 90% of the total SCFA, followed by propionate and butyrate, with an increase of up to 4 times with the start of feeding, complementary at 6 months [58, 59]. Although it is not the metabolite present in the greatest quantity, butyrate has been more studied and associated with the production of regulating microorganisms of the microbiota and with a lower probability of developing asthma and food allergies in infants [60, 61].
Nilsen et al. observed correlations between bacterial species and relative amount of SCFA, for example, the presence of the E. rectocele and F. prausnitzii network with a higher relative abundance of butyrate measured at 12 months of the infant. These networks have been identified as prominent producers of butyrate in adults. The authors suggest that eating habits between 6 and 12 months are crucial for establishing the adult microbiota [58]. Butyrate fulfills regulatory functions in the immune system as an inhibitor of histone deacetylase, reducing the release of proinflammatory cytosines through G protein-coupled receptors, among other pathways. In addition, this molecule also contributes to regulating the function of T cells. Therefore, factors such as incorporated foods and lifestyles during the early stages could affect immune mechanisms in later stages [60].
4.1. Effect of the Microbiota on the Innate Immune System
Although the epithelial barrier prevents the contact of allergens with the immune system, in some cases, the antigens can cross this barrier and cause sensitivity to food allergens [62]. During the innate immune response, at the skin level, exposure to the allergen in a defective epithelial barrier causes keratinocytes to synthesize alarmins such as IL-33, thymic stromal lymphopoietin (TSLP), and IL-25, cytokines with the function of sending an “alarm signal” and activating type 2 innate lymphoid cells (ILC-2) [63–65]. ILC-2 will stimulate the production of Th2 cytokines, especially IL-5, IL-14, IL-4, and IL-9, which are characteristic of food allergies [66, 67]. A study carried out in mouse models for FA showed that mice deficient in the IL-33 receptor (IL-33R) do not develop FA because they cannot generate ILC-2 differentiation, and IL-4 is capable of suppressing Treg differentiation through increased mast cell activation [68]. Therefore, differentiation to ILC2 contributes to the development of FA by promoting the bias of the immune response towards a Th2 and proinflammatory phenotype.
The intestinal microbiota is capable of inducing the innate immune response [69, 70]. A recent study evaluating the effect of SCFA on the innate lymphoid cell (ILC) response in mice found that SCFA administration suppressed the IL-33-induced ILC2 response in WT mice and Ffar2-/- mice [71].
4.2. Butyrate as the Main Metabolite in Microbiota Regulation
Butyrate has been identified as the metabolite with the most significant effect on immunity due to the capacity of this molecule to promote anti-inflammatory pathways by inducing CD4+ T cell differentiation into the Treg cells (with a fundamental role in allergen tolerance) mediated by the inhibition of histone deacetylases (HDAC) through the GPR109A receptor expressed on the surface of these cells, which among all metabolites is activated only by butyrate and also by niacin (Figure 2) [66]. This receptor acts on CD103+ cells capable of promoting the proliferation of Treg cells [68, 69].
Figure 2.
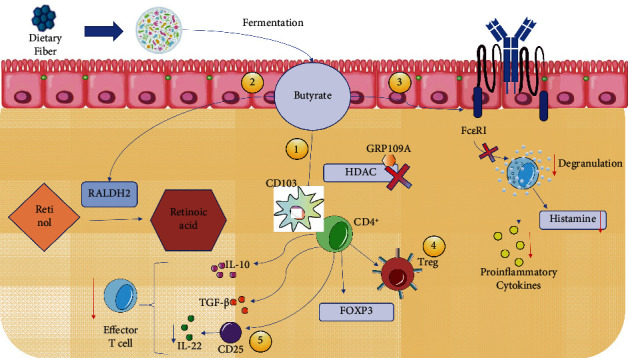
Intestinal microbiota immunological mechanism against FA. 1: inhibits HDAC through the GRP109A receptor, acting on CD103 and promoting Treg differentiation; 2: induces RALPH activity, also acting on CD103; 3: inhibits the FcεRI receptor, decreasing degranulation and thereby the expression of proinflammatory cytokines; 4: Treg expresses FOXP3 and synthesizes inhibitory cytokines such as IL-10 and TGF-β; 5: CD25, expressed by Treg FOXP3, is capable of decreasing effector T cell proliferation. All these mechanisms contribute to microbiota homeostasis and the gaining of tolerance.
In addition, butyrate can induce the activity of retina dehydrogenase (RALDH2), an enzyme responsible for the conversion of vitamin A into retinoic acid (RA) and that is expressed by CD103+ DCs. It has been suggested that RALDH2 would promote Treg cell differentiation, which favors oral tolerance (Figure 2) [70].
It has been observed that during intestinal pathologies, the presence of a proinflammatory cytokine in conjunction with retinoic acid leads to the loss of tolerance, such as IL-15; there is a lower abundance of butyrate-producing bacteria and therefore a lower concentration of the total levels of the metabolite in the epithelium, promoting microbiota dysbiosis and decreasing Treg differentiation [67, 71]. Finally, butyrate could inhibit the high-affinity IgE receptor- (FcεRI-) triggered degranulation of mast cells, which leads to a decrease in the release of inflammatory mediators and histamine, reducing the development of allergic reactions [72].
4.3. Effect of Treg Cells on Microbiota
Treg cells have an anti-inflammatory effect that contributes to immune tolerance, counteracting the function of follicular T helper (THF) cells, which are needed for IgE synthesis. Therefore, Treg cells can downmodulate IgE synthesis and reduce allergic reactions [73].
In addition, it has been observed in mouse models that the synthesis of colonic Treg cells increases in mice colonized with benign microbiota compared to germ-free mice, which is why they would be highly related to the gut microbiota [74]. Following this observation, other researchers identified a lower frequency of Treg cells in mice treated with vancomycin, an antibiotic targeting Gram-positive bacteria, compared to mice that were administered polymyxin, associated with Gram-negative bacteria, suggesting a predominant role of Gram (+) in the accumulation of Treg, such as Clostridium [75].
A factor that has been identified as an important inducer of allergen tolerance is forkhead box P3 (FOXP3), a transcription factor expressed by FOXP3+Treg cells together with CD25 (IL-2 high-affinity receptor), and according to research, a greater inflammatory response associated with allergies has been observed in knockout FOXP3 mice [76, 77]. Furthermore, significant inflammatory responses have already been observed in FOXP3 KO mice [78], while children with IgE-mediated FA have also shown a lower expression of FOXP3 as compared to healthy controls [79].
Tregs can also modulate the immune response by means of the production of IL-10 and TGF-β, which are cytokines that in general suppress immune responses [76]. In addition, a mechanism associated with IL-2 has been identified, through the supplementation of IL-2 to mice with peanut allergy, where the supplemented mice presented increased function and number of Treg cells, therefore prevention of food allergy for a period of 7 months [80].
4.4. Modulation of the Mastocyte Function by the Microbiota
Th2 lymphocytes activate mast cells after exposure to an allergen, which in turn are responsible for releasing histamine and other mediators of inflammation, such as cytokine tumor necrosis factor alpha (TNF-α), and for generating the characteristic symptoms of IgE-mediated FA [81].
The intestinal microbiota also appears to regulate mast cell expression and functionality, as observed in a study with mice free of minor germs and low mast cell functionality; however, the mechanism is not yet clear [82]. Another proposed mechanism is that acetate produced by Bifidobacterium spp induces mast cell apoptosis in mouse mast cells, reducing allergic symptoms [83].
Finally, evaluating therapeutic options, an investigation administered Bifidobacterium longum KACC91563 to Balb/c mice with induction of FA and observed an increase in mast cell apoptosis with consequent reduction of allergy symptoms, providing additional evidence for the role of these cells in the regulation of FA [84].
5. Future Treatments for FA with a Focus on Microbiota
Currently, available evidence addressing the impact of the microbiota on FA regulation encouraged researchers to formulate interventions with compounds that can correct the dysbiosis state, favoring the improvement of symptoms and resulting in tolerance to allergens [85].
Probiotics and prebiotics with different fiber types are some of the current approaches to improve microbiota composition and reduce dysbiosis (Table 1).
Table 1.
Evidence regarding microbiota interventions in children with FA.
| Type of FA | Age | n | Prebiotic/probiotic | Form of administration | Doses | Main result | Reference |
|---|---|---|---|---|---|---|---|
| CMA | 10-25 months | 12 | Lactobacillus rhamnosus GG | Extensively hydrolyzed commercial milk formula supplement | N/A | Enriched fecal microbiota with LGG formula consumption | [110] |
| CMA | 4-6 years | 330 | Lactobacillus rhamnosus GG | Extensively hydrolyzed commercial formula | LGG 2.5 × 107 to 5 × 108 CFU/g | Less functional dyspepsia, functional constipation, and functional abdominal pain in children with probiotics | [111] |
| CMA | 1-12 months | 220 | Lactobacillus rhamnosus GG | Extensively hydrolyzed commercial formula | N/A | Less allergy-associated symptoms and faster tolerance acquisition at 12, 24, and 36 months | [112] |
| CMA | 1-12 months | 39 | Lactobacillus rhamnosus GG | Extensively hydrolyzed commercial formula | 4.5 × 107‐8.5 × 107 CFU by gram of powder | Higher production of butyrate and related to higher production of butyrate in tolerant patients | [113] |
| Peanut allergy | 1-10 years | 62 children | Coadministration of Lactobacillus rhamnosus CGMCC 1.3724 (NCC4007) and oral immunotherapy with peanuts | Lyophilized powder | 2 × 1010 CFU once a day together with peanut OIT for 18 months | Reduced peanut sensitization in combination with immunotherapy | [106] |
| CMA | <6 months | 119 | Lactobacillus casei CRL431 and Bifidobacterium lactis Bb-12 | Extensively hydrolyzed commercial formula | N/A | No differences were observed regarding severity of atopic dermatitis | [114] |
| CMA | <1 year | 260 | Lactobacillus rhamnosus GG | Extensively hydrolyzed commercial formula | N/A | Earlier acquisition of tolerance in supplemented children | [103] |
| CMA | 3-12 months | 60 | Bifidobacterium lactis BB-12 and Streptococcus thermophilus TH-4 | Extensively hydrolyzed commercial formula | Bifidobacterium lactis BB-12 (1 × 109 CFU) and Streptococcus thermophilus TH-4 (1 × 108 CFU) | Reduced clinical symptoms with supplementation | [115] |
| CMA | 0-12 months | 26 | Lactobacillus rhamnosus GG | Extensively hydrolyzed commercial formula | 2.50 × 107 to 5 × 108 CFU/g | Higher decrease of calprotectin and reduction of fecal hematochezia in supplemented participants | [116] |
| CMA | <6 months | 111 | Lactobacillus casei CRL431 (Lactobacillus paracasei subspecies paracasei) and Bifidobacterium lactis Bb-12 | Extensively hydrolyzed formula | 1 × 107 colony-forming units/g formula for each of the probiotic bacteria used | No difference was observed in the age of acquisition of allergen tolerance | [104] |
The focus on the microbiota has been present for several years, starting with interventions carried out using yogurt to reduce gastrointestinal symptoms, which, however, had low therapeutic action [86, 87]. Nonetheless, latter experiences were performed with the first uses of probiotic strains, while today, other microorganisms that can regulate the intestinal environment have been evaluated [88].
Research performed on animals has successfully used various probiotics and prebiotics at different times during childhood, yielding promising results in modifying the microbiota towards a healthy profile and decreasing IgE levels, proinflammatory cytokines, and anaphylaxis symptoms [89–92]. However, in humans, the type and dose of probiotic or prebiotic and the proper time for their prescription have yet to be defined.
5.1. Prebiotics
Prebiotics are defined as a substrate for host microorganisms to which health benefits are attributed [93]. In animal models, a study performed in fiber-supplemented mice showed a reduction in anaphylaxis symptoms and greater tolerance to allergens with the intervention [70].
In humans, prebiotics used to modulate the microbiota are short-chain galactooligosaccharides (GOS) and short-chain fructooligosaccharides (FOS), and their use as supplements in milk formula has been evaluated for food allergy prevention in susceptible infants [94] and in pregnant women to prevent infant allergy [95].
Milk formula supplementation with prebiotics has been evaluated from birth in children with high risk of atopy, where the appearance of symptoms such as atopic dermatitis and infectious episodes were evaluated in a prospective cohort, where infants were supplemented fewer infectious episodes and cumulative incidence of atopic dermatitis [96].
In addition, in infants with a low risk of atopy in follow-up up to 12 months, supplementation with GOS/FOS resulted in a lower incidence of atopic dermatitis compared to infants without prebiotics and a tendency for the clinical presentation to be less severe [97].
Using other prebiotics, children with atopic dermatitis were supplemented using Kestosa, a FOS capable of stimulating the activity of Bifidobacteria, and observed a decrease in clinical symptoms [98], and a clinical trial used a mixture of polydextrose (a prebiotic present in breast milk) and GOS in infants at high risk of atopy; however, no differences were observed in the development of atopic dermatitis between the groups at 2 years of age [99]. Although reviews have been published on the effect of prebiotics on the development of immunological diseases in infants, so far, no interventions have been carried out where the use of prebiotics is considered a scientifically proven treatment for food allergy [94, 100].
5.2. Probiotics
Probiotics are defined as living microorganisms that, in suitable doses, provide benefits for their host [101]. Unlike prebiotics, the use of probiotics in research has been more extensive, and the most commonly studied strain is Lactobacillus GG (LGG), a component of dairy formulas for the pediatric population. Although research has evaluated changes in the gut microbiota profile in infants after probiotic use, not all have followed up to assess changes in clinical symptoms [102].
A prospective clinical trial in 329 children with cow's milk allergy (CMA) evaluated the acquisition of tolerance in children with ingestion of a formula extensively hydrolyzed with LGG probiotics, and 80% of the children treated with this formula had acquired tolerance at 12 months, with significant differences at 12 months as compared to the other types of formula without probiotics [103].
However, opposite results have been observed for some studies, in which no acquisition of early immune tolerance has been observed [104]. A systematic review that sought to evaluate the efficacy of probiotic supplementation in CMA in children under 5 years of age observed a higher proportion of children who reached tolerance after 36 months with a RR 1.47, with no significant difference at 6 and 12 months [105].
In addition, the effect on tolerance of oral immunotherapy (ITO) in coadministration with probiotics has been studied in children with peanut allergy, where a sustained lack of response was observed in 82% of children operated after 2-5 weeks; however, this study does not evaluate whether the effect is maintained when probiotics are withdrawn [106]. For that purpose, a protocol for a randomized controlled trial in phase 2 was recently published to intervene with OTI and probiotics in children with peanut allergy and to be able to evaluate the contribution that probiotics have in this therapy [107].
Other studies have shown similar results in modifying intestinal microbiota profiles; however, they differ in the probiotics used and the dosage, and some are performed in a small number of patients (Table 1). Due to these limitations, it is not yet possible to make clinical recommendations regarding the use and effectiveness of probiotics as a treatment for food allergy.
6. Concluding Remarks
Human research on the use of prebiotics or probiotics to modify the intestinal microbiota and therefore induce FA tolerance is limited for probiotics or absent in the case of prebiotics and with a low number of participants. The latter does not allow making clinically applicable recommendations. Although there is a trend leaning towards research on LGG 74-77, other populations should be identified that may significantly impact the microbiota and have a more clinically evident benefit.
The lack of research on exclusively breastfed children under 6 months is striking, while the main recommendation is of this being a protective factor and an immune mediator [108], in addition to providing FOS/GOS as a part of its nutritional composition [109]. Although the studies carried out have not evidenced adverse effects or tolerance to supplementation, more robust evidence is needed to support the use of prebiotics and probiotics as a general recommendation.
7. Conclusion
The impact of the intestinal microbiota on the development of FA is well known. However, the mechanisms involved are not yet clear. The modification of intestinal microbiota has shown promise in animal models regarding both immunological and clinical parameters. However, human research is still scarce and should be carried out with a more significant number of participants. Probiotics and prebiotics are proposed as innovative, safe, and economical therapy once the most effective agents and their appropriate doses are precisely identified.
Acknowledgments
This study was supported by grants from the “Fondo Nacional de Ciencia y Tecnología de Chile” (FONDECYT) (# 1190830 and # 11706964), Millennium Institute on Immunology and Immunotherapy, ANID - Millennium Science Initiative Program ICN09_016 (former P09/016-F), and Regional Government of Antofagasta through the Innovation Fund for Competitiveness FIC-R 2017 (BIP Code: 30488811-0).
Abbreviations
- CFU:
Colony-forming unit
- CMA:
Cow's milk allergy
- FA:
Food allergy
- FcεRI:
High-affinity IgE receptor
- FOS:
Short-chain fructooligosaccharides
- FOXP3:
Forkhead box P3
- GOS:
Galactooligosaccharides
- HDAC:
Histone deacetylases
- IG:
Immunoglobulin
- IL-33R:
IL-33 receptor
- ILC:
Innate lymphoid cells
- ILC-2:
Type 2 innate lymphoid cells
- ITO:
Oral immunotherapy
- LGG:
Lactobacillus GG
- NK:
Natural killer
- RA:
Retinoic acid
- RALDH2:
Retina dehydrogenase
- SCFA:
Short-chain fatty acids
- TGF-β:
Transforming growth factor-β
- THF:
Follicular T helper cells
- TNF-α:
Tumor necrosis factor alpha
- Tregs:
Regulatory T cells
- TSLP:
Thymic stromal lymphopoietin.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Boyce J. A., Assa'ad A., Burks A. W., et al. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored Expert Panel report. The Journal of Allergy and Clinical Immunology . 2010;126(6):1105–1118. doi: 10.1016/j.jaci.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilaver L. A., Kanaley M. K., Fierstein J. L., Gupta R. S. Prevalence and correlates of food allergy among Medicaid-enrolled United States children. Academic Pediatrics . 2021;21(1):84–92. doi: 10.1016/j.acap.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Singer A. G., Kosowan L., Soller L., et al. Prevalence of physician-reported food allergy in Canadian children. The Journal of Allergy and Clinical Immunology. In Practice . 2021;9(1):193–199. doi: 10.1016/j.jaip.2020.07.039. [DOI] [PubMed] [Google Scholar]
- 4.Hoyos-Bachiloglu R., Ivanovic-Zuvic D., Álvarez J., et al. Prevalence of parent-reported immediate hypersensitivity food allergy in Chilean school-aged children. Allergol Immunopathol (Madr) . 2014;42(6):527–532. doi: 10.1016/j.aller.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Lyons S. A., Clausen M., Knulst A. C., et al. Prevalence of food sensitization and food allergy in children across Europe. The Journal of Allergy and Clinical Immunology. In Practice . 2020;8(8):2736–2746.e9. doi: 10.1016/j.jaip.2020.04.020. [DOI] [PubMed] [Google Scholar]
- 6.Sicherer S. H., Sampson H. A. Food allergy: a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. The Journal of Allergy and Clinical Immunology . 2018;141(1):41–58. doi: 10.1016/j.jaci.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Du Toit G., Tsakok T., Lack S., Lack G. Prevention of food allergy. The Journal of Allergy and Clinical Immunology . 2016;137(4):998–1010. doi: 10.1016/j.jaci.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Riccio P., Rossano R. The human gut microbiota is neither an organ nor a commensal. FEBS Letters . 2020;594(20):3262–3271. doi: 10.1002/1873-3468.13946. [DOI] [PubMed] [Google Scholar]
- 9.Brüssow H. Problems with the concept of gut microbiota dysbiosis. Microbial Biotechnology . 2020;13(2):423–434. doi: 10.1111/1751-7915.13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nance C. L., Deniskin R., Diaz V. C., Paul M., Anvari S., Anagnostou A. The role of the microbiome in food allergy: a review. Children . 2020;7(6):p. 50. doi: 10.3390/children7060050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berni Canani R., de Filippis F., Nocerino R., et al. Gut microbiota composition and butyrate production in children affected by non-IgE-mediated cow's milk allergy. Scientific Reports . 2018;8(1, article 12500) doi: 10.1038/s41598-018-30428-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer R. Nutritional disorders resulting from food allergy in children. Pediatric Allergy and Immunology . 2018;29(7):689–704. doi: 10.1111/pai.12960. [DOI] [PubMed] [Google Scholar]
- 13.Yu H. R., Huang L. H., Li S. C. Roles of microRNA in the immature immune system of neonates. Cancer Letters . 2018;433:99–106. doi: 10.1016/j.canlet.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 14.de Moraes-Pinto M. I., Suano-Souza F., Aranda C. S. Immune system: development and acquisition of immunological competence. Jornal de Pediatria . 2021;97:S59–S66. doi: 10.1016/j.jped.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kivistö J. E., Clarke A., Dery A., et al. Genetic and environmental susceptibility to food allergy in a registry of twins. The Journal of Allergy and Clinical Immunology. In Practice . 2019;7(8):2916–2918. doi: 10.1016/j.jaip.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 16.Gallay C., Meylan P., Mermoud S., et al. Genetic predisposition and environmental factors associated with the development of atopic dermatitis in infancy: a prospective birth cohort study. European Journal of Pediatrics . 2020;179(9):1367–1377. doi: 10.1007/s00431-020-03616-5. [DOI] [PubMed] [Google Scholar]
- 17.Brodin P., Jojic V., Gao T., et al. Variation in the human immune system is largely driven by non-heritable influences. Cell . 2015;160(1-2):37–47. doi: 10.1016/j.cell.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carr E. J., Dooley J., Garcia-Perez J. E., et al. The cellular composition of the human immune system is shaped by age and cohabitation. Nature Immunology . 2016;17(4):461–468. doi: 10.1038/ni.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olin A., Henckel E., Chen Y., et al. Stereotypic immune system development in newborn children. Cell . 2018;174(5):1277–1292.e14. doi: 10.1016/j.cell.2018.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gleeson M., Cripps A. W. Development of mucosal immunity in the first year of life and relationship to sudden infant death syndrome. FEMS Immunology and Medical Microbiology . 2004;42(1):21–33. doi: 10.1016/j.femsim.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Dawod B., Marshall J. S., Azad M. B. Breastfeeding and the developmental origins of mucosal immunity: how human milk shapes the innate and adaptive mucosal immune systems. Current Opinion in Gastroenterology . 2021;37(6):547–556. doi: 10.1097/MOG.0000000000000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valiathan R., Ashman M., Asthana D. Effects of ageing on the immune system: infants to elderly. Scandinavian Journal of Immunology . 2016;83(4):255–266. doi: 10.1111/sji.12413. [DOI] [PubMed] [Google Scholar]
- 23.Upham J. W., Lee P. T., Holt B. J., et al. Development of interleukin-12-producing capacity throughout childhood. Infection and Immunity . 2002;70(12):6583–6588. doi: 10.1128/IAI.70.12.6583-6588.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X., Zhivaki D., Lo-Man R. Unique aspects of the perinatal immune system. Nature Reviews Immunology . 2017;17(8):495–507. doi: 10.1038/nri.2017.54. [DOI] [PubMed] [Google Scholar]
- 25.Mold J. E., Venkatasubrahmanyam S., Burt T. D., et al. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science . 2010;330(6011):1695–1699. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohsaki A., Venturelli N., Buccigrosso T. M., et al. Maternal IgG immune complexes induce food allergen-specific tolerance in offspring. The Journal of Experimental Medicine . 2018;215(1):91–113. doi: 10.1084/jem.20171163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrucci L., Corsi A., Lauretani F., et al. The origins of age-related proinflammatory state. Blood . 2005;105(6):2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H. J., Dong X. L., Zhang Y. F., Fang Y. F., Zhang H. Y. Effect of maternal immune level at different pregnancy stages on cow’s milk protein allergy in infants. Zhongguo Dang Dai Er Ke Za Zhi . 2020;22(11):1221–1225. doi: 10.7499/j.issn.1008-8830.2006070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yatsunenko T., Rey F. E., Manary M. J., et al. Human gut microbiome viewed across age and geography. Nature . 2012;486(7402):222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Łoś-Rycharska E., Gołębiewski M., Sikora M., et al. A combined analysis of gut and skin microbiota in infants with food allergy and atopic dermatitis: a pilot study. Nutrients . 2021;13(5):p. 1682. doi: 10.3390/nu13051682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shukla R., Ghoshal U., Dhole T. N., Ghoshal U. C. Fecal microbiota in patients with irritable bowel syndrome compared with healthy controls using real-time polymerase chain reaction: an evidence of dysbiosis. Digestive Diseases and Sciences . 2015;60(10):2953–2962. doi: 10.1007/s10620-015-3607-y. [DOI] [PubMed] [Google Scholar]
- 32.Quagliariello A., Aloisio I., Bozzi Cionci N., et al. Effect of Bifidobacterium breve on the intestinal microbiota of coeliac children on a gluten free diet: a pilot study. Nutrients . 2016;8(10):p. 660. doi: 10.3390/nu8100660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milani C., Duranti S., Bottacini F., et al. The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiology and Molecular Biology Reviews . 2017;81(4) doi: 10.1128/MMBR.00036-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Penders J., Thijs C., Vink C., et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics . 2006;118(2):511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 35.Antony K. M., Ma J., Mitchell K. B., Racusin D. A., Versalovic J., Aagaard K. The preterm placental microbiome varies in association with excess maternal gestational weight gain. American Journal of Obstetrics and Gynecology . 2015;212(5):653.e1–653.e16. doi: 10.1016/j.ajog.2014.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng J., Xiao X.-H., Zhang Q., et al. Correlation of placental microbiota with fetal macrosomia and clinical characteristics in mothers and newborns. Oncotarget . 2017;8(47):82314–82325. doi: 10.18632/oncotarget.19319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nowland T. L., Kirkwood R. N., Plush K. J., Barton M. D., Torok V. A. Exposure to maternal feces in lactation influences piglet enteric microbiota, growth, and survival preweaning. Journal of Animal Science . 2021;99(7):1–10. doi: 10.1093/jas/skab170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Selma-Royo M., Tarrazó M., García-Mantrana I., Gómez-Gallego C., Salminen S., Collado M. C. Shaping microbiota during the first 1000 days of life. Advances in Experimental Medicine and Biology . 2019;1125:3–24. doi: 10.1007/5584_2018_312. [DOI] [PubMed] [Google Scholar]
- 39.Stark P. L., Lee A. The microbial ecology of the large bowel of breastfed and formula-fed infants during the first year of life. Journal of Medical Microbiology . 1982;15(2):189–203. doi: 10.1099/00222615-15-2-189. [DOI] [PubMed] [Google Scholar]
- 40.Nyangahu D. D., Jaspan H. B. Influence of maternal microbiota during pregnancy on infant immunity. Clinical and Experimental Immunology . 2019;198(1):47–56. doi: 10.1111/cei.13331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Theis K. R., Romero R., Winters A. D., et al. Does the human placenta delivered at term have a microbiota? Results of cultivation, quantitative real-time PCR, 16S rRNA gene sequencing, and metagenomics. American Journal of Obstetrics and Gynecology . 2019;220(3):267.e1–267.e39. doi: 10.1016/j.ajog.2018.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collado M. C., Rautava S., Aakko J., Isolauri E., Salminen S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Scientific Reports . 2016;6(1):1–13. doi: 10.1038/srep23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyoshi J., Bobe A. M., Miyoshi S., et al. Peripartum antibiotics promote gut dysbiosis, loss of immune tolerance, and inflammatory bowel disease in genetically prone offspring. Cell Reports . 2017;20(2):491–504. doi: 10.1016/j.celrep.2017.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Podlesny D., Fricke W. F. Strain inheritance and neonatal gut microbiota development: a meta-analysis. International Journal of Medical Microbiology . 2021;311(3, article 151483) doi: 10.1016/j.ijmm.2021.151483. [DOI] [PubMed] [Google Scholar]
- 45.Murata C., Gutiérrez-Castrellón P., Pérez-Villatoro F., et al. Delivery mode-associated gut microbiota in the first 3 months of life in a country with high obesity rates: a descriptive study. Medicine (Baltimore) . 2020;99(40, article e22442) doi: 10.1097/MD.0000000000022442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim G., Bae J., Kim M. J., et al. Delayed establishment of gut microbiota in infants delivered by cesarean section. Frontiers in Microbiology . 2020;11 doi: 10.3389/fmicb.2020.02099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lyons K. E., Ryan C. A., Dempsey E. M., Ross R. P., Stanton C. Breast milk, a source of beneficial microbes and associated benefits for infant health. Nutrients . 2020;12(4):p. 1039. doi: 10.3390/nu12041039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ossa J. C., Yáñez D., Valenzuela R., Gallardo P., Lucero Y., Farfán M. J. Intestinal inflammation in Chilean infants fed with bovine formula vs. breast milk and its association with their gut microbiota. Frontiers in Cellular and Infection Microbiology . 2018;8:p. 190. doi: 10.3389/fcimb.2018.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oba P. M., Holscher H. D., Mathai R. A., Kim J., Swanson K. S. Diet influences the oral microbiota of infants during the first six months of life. Nutrients . 2020;12(11):3400–3417. doi: 10.3390/nu12113400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma J., Li Z., Zhang W., et al. Comparison of gut microbiota in exclusively breast-fed and formula-fed babies: a study of 91 term infants. Scientific Reports . 2020;10(1):1–11. doi: 10.1038/s41598-020-72635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herbst T., Sichelstiel A., Schär C., et al. Dysregulation of allergic airway inflammation in the absence of microbial colonization. American Journal of Respiratory and Critical Care Medicine . 2012;184(2):198–205. doi: 10.1164/rccm.201010-1574OC. [DOI] [PubMed] [Google Scholar]
- 52.Kreft L., Hoffmann C., Ohnmacht C. Therapeutic potential of the intestinal microbiota for immunomodulation of food allergies. Frontiers in Immunology . 2020;11:p. 1853. doi: 10.3389/fimmu.2020.01853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mauras A., Wopereis H., Yeop I., et al. Gut microbiota from infant with cow’s milk allergy promotes clinical and immune features of atopy in a murine model. Allergy . 2019;74(9):1790–1793. doi: 10.1111/all.13787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsui S., Kataoka H., Tanaka J.-I., et al. Dysregulation of intestinal microbiota elicited by food allergy induces IgA-mediated oral dysbiosis. Infection and Immunity . 2020;88(1) doi: 10.1128/IAI.00741-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoo J. Y., Groer M., Dutra S., Sarkar A., McSkimming D. Gut microbiota and immune system interactions. Microorganisms . 2020;8(10):p. 1587. doi: 10.3390/microorganisms8101587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bach Knudsen K., Lærke H. N., Hedemann M. S., et al. Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients . 2018;10(10):p. 1499. doi: 10.3390/nu10101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Underwood M. A., German J. B., Lebrilla C. B., Mills D. A. Bifidobacterium longum subspecies infantis: champion colonizer of the infant gut. Pediatric Research . 2015;77(1-2):229–235. doi: 10.1038/pr.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nilsen M., Madelen Saunders C., Leena Angell I., et al. Butyrate levels in the transition from an infant- to an adult-like gut microbiota correlate with bacterial networks associated with Eubacterium rectale and Ruminococcus gnavus. Genes (Basel) . 2020;11(11):p. 1245. doi: 10.3390/genes11111245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Differding M. K., Benjamin-Neelon S. E., Hoyo C., Østbye T., Mueller N. T. Timing of complementary feeding is associated with gut microbiota diversity and composition and short chain fatty acid concentrations over the first year of life. BMC Microbiology . 2020;20(1):p. 56. doi: 10.1186/s12866-020-01723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu L.-B., Zhang Y. C., Huang H. H., Lin J. Prospects for clinical applications of butyrate-producing bacteria. World Journal of Clinical Pediatrics . 2021;10(5):84–92. doi: 10.5409/wjcp.v10.i5.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roduit C., Frei R., Ferstl R., et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy . 2019;74(4):799–809. doi: 10.1111/all.13660. [DOI] [PubMed] [Google Scholar]
- 62.Brough H. A., Nadeau K. C., Sindher S. B., et al. Epicutaneous sensitization in the development of food allergy: what is the evidence and how can this be prevented? Allergy . 2020;75(9):2185–2205. doi: 10.1111/all.14304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schmiechen Z., Weissler K. A., Frischmeyer-Guerrerio P. A. Recent developments in understanding the mechanisms of food allergy. Current Opinion in Pediatrics . 2019;31(6):807–814. doi: 10.1097/MOP.0000000000000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iweala O. I., Nagler C. R. The microbiome and food allergy. Beneficial Microbes . 2019;37(1):377–403. doi: 10.1146/annurev-immunol-042718-041621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cayrol C., Girard J. P. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Current Opinion in Immunology . 2014;31:31–37. doi: 10.1016/j.coi.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 66.Furusawa Y., Obata Y., Fukuda S., et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature . 2013;504(7480):446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 67.Caminero A., Meisel M., Jabri B., Verdu E. F. Mechanisms by which gut microorganisms influence food sensitivities. Nature Reviews. Gastroenterology & Hepatology . 2018;16(1):7–18. doi: 10.1038/s41575-018-0064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Singh N., Gurav A., Sivaprakasam S., et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity . 2014;40(1):128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arpaia N., Campbell C., Fan X., et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature Cell Biology . 2013;504(7480):451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tan J., McKenzie C., Vuillermin P. J., et al. Dietary fiber and bacterial SCFA enhance oral tolerance and protect against food allergy through diverse cellular pathways. Cell Reports . 2016;15(12):2809–2824. doi: 10.1016/j.celrep.2016.05.047. [DOI] [PubMed] [Google Scholar]
- 71.Yap Y. A., Mariño E. An insight into the intestinal web of mucosal immunity, microbiota, and diet in inflammation. Frontiers in Immunology . 2018;9:p. 2617. doi: 10.3389/fimmu.2018.02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luu M., Monning H., Visekruna A. Exploring the molecular mechanisms underlying the protective effects of microbial SCFAs on intestinal tolerance and food allergy. Frontiers in Immunology . 2020;11:p. 1225. doi: 10.3389/fimmu.2020.01225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yao Y., Chen C. L., Yu D., Liu Z. Roles of follicular helper and regulatory T cells in allergic diseases and allergen immunotherapy. Allergy . 2021;76(2):456–470. doi: 10.1111/all.14639. [DOI] [PubMed] [Google Scholar]
- 74.Geuking M. B., Cahenzli J., Lawson M. A. E., et al. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity . 2011;34(5):794–806. doi: 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 75.Atarashi K., Tanoue T., Shima T., et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science . 2011;331(6015):337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Satitsuksanoa P., Jansen K., Głobińska A., van de Veen W., Akdis M. Regulatory immune mechanisms in tolerance to food allergy. Frontiers in Immunology . 2018;9:p. 2939. doi: 10.3389/fimmu.2018.02939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin W., Truong N., Grossman W. J., et al. Allergic dysregulation and hyperimmunoglobulinemia E in Foxp3 mutant mice. The Journal of Allergy and Clinical Immunology . 2005;116(5):1106–1115. doi: 10.1016/j.jaci.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 78.Mondoulet L., Dioszeghy V., Busato F., et al. Gata3 hypermethylation and Foxp3 hypomethylation are associated with sustained protection and bystander effect following epicutaneous immunotherapy in peanut-sensitized mice. Allergy . 2019;74(1):152–164. doi: 10.1111/all.13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krogulska A., Polakowska E., Wąsowska-Królikowska K., Małachowska B., Młynarski W., Borowiec M. Decreased FOXP3 mRNA expression in children with atopic asthma and IgE- mediated food allergy. Annals of Allergy, Asthma & Immunology . 2015;115(5):415–421. doi: 10.1016/j.anai.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 80.Bonnet B., Vigneron J., Levacher B., et al. Low-dose IL-2 induces regulatory T cell–mediated control of experimental food allergy. Journal of Immunology . 2016;197(1):188–198. doi: 10.4049/jimmunol.1501271. [DOI] [PubMed] [Google Scholar]
- 81.Kraneveld A. D., Sagar S., Garssen J., Folkerts G. The two faces of mast cells in food allergy and allergic asthma: the possible concept of Yin Yang. Biochimica et Biophysica Acta, Reviews on Cancer . 2012;1822(1):93–99. doi: 10.1016/j.bbadis.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 82.Schwarzer M., Hermanova P., Srutkova D., et al. Germ-free mice exhibit mast cells with impaired functionality and gut homing and do not develop food allergy. Frontiers in Immunology . 2019;10:p. 205. doi: 10.3389/fimmu.2019.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fukuda S., Toh H., Hase K., et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature Cell Biology . 2011;469(7331):543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 84.Kim J. H., Jeun E. J., Hong C. P., et al. Extracellular vesicle-derived protein from Bifidobacterium longum alleviates food allergy through mast cell suppression. The Journal of Allergy and Clinical Immunology . 2016;137(2):507–516.e8. doi: 10.1016/j.jaci.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 85.Tan-Lim C. S. C., Esteban-Ipac N. A. R. Probiotics as treatment for food allergies among pediatric patients: a meta- analysis. World Allergy Organization Journal . 2018;11(1):p. 25. doi: 10.1186/s40413-018-0204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li X.-R., Liu C.-J., Tang X.-D., et al. Gut microbiota alterations from three-strain yogurt formulation treatments in slow-transit constipation. Canadian Journal of Infectious Diseases and Medical Microbiology . 2020;2020:9. doi: 10.1155/2020/4583973.4583973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Noorbakhsh H., Yavarmanesh M., Mortazavi S. A., Adibi P., Moazzami A. A. Metabolomics analysis revealed metabolic changes in patients with diarrhea-predominant irritable bowel syndrome and metabolic responses to a synbiotic yogurt intervention. European Journal of Nutrition . 2018;58(8):3109–3119. doi: 10.1007/s00394-018-1855-2. [DOI] [PubMed] [Google Scholar]
- 88.Nagler C. R. Drugging the microbiome. The Journal of Experimental Medicine . 2020;217(4) doi: 10.1084/jem.20191642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fu L., Song J., Wang C., Fu S., Wang Y. Bifidobacterium infantis potentially alleviates shrimp tropomyosin-induced allergy by tolerogenic dendritic cell-dependent induction of regulatory t cells and alterations in gut microbiota. Frontiers in Immunology . 2017;8:p. 1536. doi: 10.3389/fimmu.2017.01536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cheng R. Y., Yao J. R., Wan Q., et al. Oral administration of Bifidobacterium bifidum TMC3115 to neonatal mice may alleviate IgE-mediated allergic risk in adulthood. Beneficial Microbes . 2018;9(5):815–828. doi: 10.3920/BM2018.0005. [DOI] [PubMed] [Google Scholar]
- 91.Bouchaud G., Castan L., Chesné J., et al. Maternal exposure to GOS/inulin mixture prevents food allergies and promotes tolerance in offspring in mice. Allergy . 2016;71(1):68–76. doi: 10.1111/all.12777. [DOI] [PubMed] [Google Scholar]
- 92.Vonk M. M., Engen P. A., Naqib A., et al. Altered microbial community structure and metabolism in cow’s milk allergic mice treated with oral immunotherapy and fructo-oligosaccharides. Beneficial Microbes . 2020;11(1):19–32. doi: 10.3920/BM2019.0024. [DOI] [PubMed] [Google Scholar]
- 93.Gibson G. R., Hutkins R., Sanders M. E., et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nature Reviews. Gastroenterology & Hepatology . 2017;14(8):491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 94.Osborn D. A., Sinn J. K. H. Prebiotics in infants for prevention of allergy. Cochrane Database of Systematic Reviews . 2013;2013(3) doi: 10.1002/14651858.CD006474.pub3. [DOI] [PubMed] [Google Scholar]
- 95.Pretorius R. A., Bodinier M., Prescott S. L., Palmer D. J. Maternal fiber dietary intakes during pregnancy and infant allergic disease. Nutrients . 2019;11(8):p. 1767. doi: 10.3390/nu11081767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arslanoglu S., Moro G. E., Schmitt J., Tandoi L., Rizzardi S., Boehm G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. The Journal of Nutrition . 2008;138(6):1091–1095. doi: 10.1093/jn/138.6.1091. [DOI] [PubMed] [Google Scholar]
- 97.Grüber C., Stuijvenberg M., Mosca F., et al. Reduced occurrence of early atopic dermatitis because of immunoactive prebiotics among low-atopy-risk infants. The Journal of Allergy and Clinical Immunology . 2010;126(4):791–797. doi: 10.1016/j.jaci.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 98.Shibata R., Kimura M., Takahashi H., et al. Clinical effects of kestose, a prebiotic oligosaccharide, on the treatment of atopic dermatitis in infants. Clinical and Experimental Allergy . 2009;39(9):1397–1403. doi: 10.1111/j.1365-2222.2009.03295.x. [DOI] [PubMed] [Google Scholar]
- 99.Ranucci G., Buccigrossi V., Borgia E., et al. Galacto-oligosaccharide/polidextrose enriched formula protects against respiratory infections in infants at high risk of atopy: a randomized clinical trial. Nutrients . 2018;10(3):p. 286. doi: 10.3390/nu10030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brosseau C., Selle A., Palmer D. J., Prescott S. L., Barbarot S., Bodinier M. Prebiotics: mechanisms and preventive effects in allergy. Nutrients . 2019;11(8):p. 1841. doi: 10.3390/nu11081841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hill C., Guarner F., Reid G., et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews. Gastroenterology & Hepatology . 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 102.Mennini M., Reddel S., del Chierico F., et al. Gut microbiota profile in children with IgE-mediated cow’s milk allergy and cow’s milk sensitization and probiotic intestinal persistence evaluation. International Journal of Molecular Sciences . 2021;22(4):p. 1649. doi: 10.3390/ijms22041649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Berni Canani R., Nocerino R., Terrin G., et al. Formula selection for management of children with cow's milk allergy influences the rate of acquisition of tolerance: a prospective multicenter study. The Journal of Pediatrics . 2013;163(3):771–777.e1. doi: 10.1016/j.jpeds.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 104.Hol J., van Leer E. H. G., Elink Schuurman B. E. E., et al. The acquisition of tolerance toward cow's milk through probiotic supplementation: a randomized, controlled trial. The Journal of Allergy and Clinical Immunology . 2008;121(6):1448–1454. doi: 10.1016/j.jaci.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 105.Qamer S., Deshmukh M., Patole S. Probiotics for cow’s milk protein allergy: a systematic review of randomized controlled trials. European Journal of Pediatrics . 2019;178(8):1139–1149. doi: 10.1007/s00431-019-03397-6. [DOI] [PubMed] [Google Scholar]
- 106.Tang M. L. K., Ponsonby A.-L., Orsini F., et al. Administration of a probiotic with peanut oral immunotherapy: a randomized trial. The Journal of Allergy and Clinical Immunology . 2015;135(3):737–744.e8. doi: 10.1016/j.jaci.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 107.Loke P., Chebar Lozinsky A., Orsini F., et al. Study protocol of a phase 2, dual-centre, randomised, controlled trial evaluating the effectiveness of probiotic and egg oral immunotherapy at inducing desensitisation or sustained unresponsiveness (remission) in participants with egg allergy compared with placebo (Probiotic Egg Allergen Oral Immunotherapy for Treatment of Egg Allergy: PEAT study) BMJ Open . 2021;11(7, article e044331) doi: 10.1136/bmjopen-2020-044331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Matsumoto N., Yorifuji T., Nakamura K., Ikeda M., Tsukahara H., Doi H. Breastfeeding and risk of food allergy: a nationwide birth cohort in Japan. Allergology International . 2020;69(1):91–97. doi: 10.1016/j.alit.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 109.Han S. M., Binia A., Godfrey K. M., el-Heis S., Cutfield W. S. Do human milk oligosaccharides protect against infant atopic disorders and food allergy? Nutrients . 2020;12(10):3212–3212. doi: 10.3390/nu12103212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guadamuro L., Diaz M., Jiménez S., et al. Fecal changes following introduction of milk in infants with outgrowing non-IgE cow’s milk protein allergy are influenced by previous consumption of the probiotic LGG. Frontiers in Immunology . 2019;10:p. 1819. doi: 10.3389/fimmu.2019.01819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nocerino R., di Costanzo M., Bedogni G., et al. Dietary treatment with extensively hydrolyzed casein formula containing the probiotic Lactobacillus rhamnosus GG prevents the occurrence of functional gastrointestinal disorders in children with cow's milk allergy. The Journal of Pediatrics . 2019;213:137–142.e2. doi: 10.1016/j.jpeds.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 112.Berni Canani R., di Costanzo M., Bedogni G., et al. Extensively hydrolyzed casein formula containing Lactobacillus rhamnosus GG reduces the occurrence of other allergic manifestations in children with cow's milk allergy: 3-year randomized controlled trial. The Journal of Allergy and Clinical Immunology . 2017;139(6):1906–1913.e4. doi: 10.1016/j.jaci.2016.10.050. [DOI] [PubMed] [Google Scholar]
- 113.Berni Canani R., Sangwan N., Stefka A. T., et al. Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. The ISME Journal . 2016;10(3):742–750. doi: 10.1038/ismej.2015.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dupont C., Hol J., Nieuwenhuis E. E. S., the Cow's Milk Allergy Modified by Elimination and Lactobacilli study group An extensively hydrolysed casein-based formula for infants with cows’ milk protein allergy: tolerance/hypo-allergenicity and growth catch-up. The British Journal of Nutrition . 2015;113(7):1102–1112. doi: 10.1017/S000711451500015X. [DOI] [PubMed] [Google Scholar]
- 115.Ivakhnenko E. S., Nian'kovskiĭ S. L. Effect of probiotics on the dynamics of gastrointestinal symptoms of food allergy to cow’s milk protein in infants. Georgian Medical News . 2013;219:46–52. [PubMed] [Google Scholar]
- 116.Baldassarre M. E., Laforgia N., Fanelli M., Laneve A., Grosso R., Lifschitz C. Lactobacillus GG improves recovery in infants with blood in the stools and presumptive allergic colitis compared with extensively hydrolyzed formula alone. The Journal of Pediatrics . 2010;156(3):397–401. doi: 10.1016/j.jpeds.2009.09.012. [DOI] [PubMed] [Google Scholar]


