Abstract
Lasia spinosa (L.) is used ethnobotanically for the treatment of various diseases, including rheumatoid arthritis, inflammation of the lungs, bleeding cough, hemorrhoids, intestinal diseases, stomach pain, and uterine cancer. This review is aimed at summarizing phytochemistry and pharmacological data with their molecular mechanisms of action. A search was performed in databases such as PubMed, Science Direct, and Google Scholar using the keywords: “Lasia spinosa,” then combined with “ethnopharmacological use,” “phytochemistry,” and “pharmacological activity.” This updated review included studies with in vitro, ex vivo, and in vivo experiments with compounds of known concentration and highlighted pharmacological mechanisms. The research results showed that L. spinosa contains many important nutritional and phytochemical components such as alkanes, aldehydes, alkaloids, carotenoids, flavonoids, fatty acids, ketones, lignans, phenolics, terpenoids, steroids, and volatile oil with excellent bioactivity. The importance of this review lies in the fact that scientific pharmacological evidence supports the fact that the plant has antioxidant, anti-inflammatory, antimicrobial, cytotoxic, antidiarrheal, antihelminthic, antidiabetic, antihyperlipidemic, and antinociceptive effects, while protecting the gastrointestinal system and reproductive. Regarding future toxicological and safety data, more research is needed, including studies on human subjects. In light of these data, L. spinosa can be considered a medicinal plant with effective bioactives for the adjuvant treatment of various diseases in humans.
1. Introduction
Traditional, herbal, and Ayurvedic medicine comprise an important and prestigious form of treatment for various diseases and conditions in different locations all over the world from the beginning of human civilization on Earth [1]. Several plants and their corresponding preparations have been used for various therapeutic purposes for a long time ago. The history of traditional, herbal, and Ayurvedic medicine is the eldest to establish a treatment pattern [1].
Lasia spinosa (L.) Thwaites, often known as Chengmora/Sibru in Assames, Kata-kachu in Bengali, Janum-Saru in Manipuri, Kohila/Mahakohila/Engilikohila in Sri Lanka, Zawangzang in Mizoram, and Laksmana in Sanskrit [2–4], belongs to the Araceae family [5]. It is found in Asia-Bangladesh, China, the Indian subcontinent, Myanmar, Thailand, Indo-China, Indonesia, and Papua New Guinea [6].
Briefly, L. spinosa is an aquatic or terrestrial plant, short-stemmed spiny heirs with underground rhizome that usually occurs in wet forests, open marshes, wetlands, or in permanently standing water [7]. L. spinosa is a large marsh plant with the stem stout 1 m high and the leaves broadly arrow-shaped in outlines, 20-30 cm long deeply divided into 4-6 pairs of narrow side lobes. The petiole is 30-40 cm long, veins beneath the petiole and peduncle prickly [8]. Morpho-anatomical feature of L. spinosa has been recently reported by Lakshmi et al. [9]. The plant is harvested from the wild for its edible leaves and various medicinal uses. Sometimes, it is also cultivated as a vegetable crop along ponds margins [10]. Recently, it has been reported that Fusarium fujikuroi caused leaf spot on L. spinosa in China [11].
With regards to their biological effects, the tender leaves and rhizomes of this plant, used as a vegetable and in indigenous medicine, have been recommended for a variety of conditions [12–15].
Given the multiple potentialities of this plant, this review provides up-to-date data on L. spinosa chemical composition and biological effects based on the scientific reports found in the databases.
2. Review Methodology
In this study, the literature on botanical classification of L. spinosa, ethnomedicinal applications, secondary metabolites, biological properties were compiled, reviewed, and summarized. For the compilation of all written papers on this species, scientific search engines such as PubMed, ScienceDirect, SpringerLink, Web of Science, Scopus, Wiley Online, Scifnder, and Google Scholar have been used. In this study, the literature on botanical classification of Lasia spinosa, ethnomedicinal applications, secondary metabolites, and biological properties were compiled, reviewed, and summarized. For searching, the next MeSH terms were used: “Phytotherapy”, “Plants”, “Medicinal”, “Plant Extracts/administration & dosage”, “Plant Extracts/isolation & purification”, “Plant/chemistry”, “Structure-Activity Relationship”, “Disease Models”, “Animal”, and “Plant Extracts/toxicity”. Using the Chemsketch version 12.01 program, chemical structures were drawn. The scientific names of the plants have been verified according to the PlantList [16, 17].
Inclusion criteria: works published in English on Lasia spinosa that highlighted the following data: chemical compounds isolated from each genus, preclinical pharmacological research highlighting molecular mechanisms, and in vitro/in vivo pharmacological studies that contained the concentration and dose at which the chemical compounds studied were pharmacologically active and toxicological data. The most important results obtained were summarized in the tables.
Exclusion criteria: abstracts, letters to the editor, papers in languages other than English, studies that did not have dose-effect correlations, and studies that did not have proven molecular mechanisms which underline the pharmacology.
3. Ethnopharmacology
L. spinosa is a medicinally important plant, traditionally used by different ethnic communities all over the world. There are various reports on L. spinosa medicinal and economical properties. Often used for treating colic, tuberculosis of lymph nodes, swollen lymph nodes, rheumatism/rheumatoid arthritis, injuries, snake bites, and insect bites, this plant is also recommended as effective for the treatment of sore throat, constipation, to purify the blood, on lung inflammation, bleeding cough, and uterine cancer [14, 18–20]. Rhizomes (roots) are most often used as a remedy for hemorrhoids in Sri Lanka and Malays and to confer protection for some of the above conditions, because of their high fibre content and antioxidant compounds [15].
Besides, leaves and stalks have demonstrated profound antihelminthic, anticestode, and antinematode efficacy [12, 15, 18, 19, 21, 22]. The root decoction is also useful in gastrointestinal diseases and stomachache [4], while also stimulating liver function [22]. Paste from tender leaves is externally used in burns [4].
4. Phytochemical Profile
L. spinosa whole plant contains several essential phytochemicals, including alkaloids, flavonoids, tannin, saponins, steroids, terpenoids, and varying amounts of micronutrients, like zinc (Zn), magnesium (Mg), calcium (Ca), iron (Fe), copper (Cu), manganese (Mn), and molybdenum (Mo) (Table 1).
Table 1.
Amounts of micronutrients of Lasia spinosa in ppm (parts per million) [2].
| Elements | Amounts (ppm) |
|---|---|
| Zn | 7.442 ± 0.01 |
| Mg | 6.228 ± 0.11 |
| Fe | 17.06 ± 0.87 |
| Cu | 0.316 ± 0.02 |
| Mn | 1.334 ± 0.08 |
| Mo | 1.180 ± 0.06 |
Nutritional analysis of L. spinosa showed that it contains proteins (17.6 kcal/100 g), fats (1.16 kcal/100 g), and carbohydrates (35.7 kcal/100 g), with a nutritive value of 224 kcal/100 g [2, 23]. In another study, the protein, fats, and carbohydrate content on a dry weight basis were 17.9, 3.8, and 45.5 g/100 g edible portion for protein, fats, and carbohydrate, respectively, for L. spinosa leaf, with a nutritive value of 288.5 kcal/100 g [24]. L. spinosa roots/rhizome contains dietary fibre, Ca, and provitamin A carotenoids [18, 25]. L. spinosa leaf contains 15.4 g of fibre, 250 mg of Ca, 19.2 mg of Fe, and 455 mg of vitamin C for 100 g edible portion on a dry weight basis [24, 26].
In fresh weight, other studies reported content of proteins, fats, and carbohydrates of 3.68 ± 0.28, 0.44 ± 0.03, and 4.78 ± 0.38 g/100 g, respectively, the mineral content of 158.08 ± 3.98, 321.73 ± 7.00, 73.17 ± 2.37, 53.86 ± 3.86, and 0.92 ± 0.08 mg/100 g for Ca, K, P, Mg, and Fe, and 2.99 ± 0.11 and 0.28 ± 0.01 mg/100 g of vitamins C and E, respectively [27].
When specifically addressing the different extracts prepared from L. spinosa, hexane extracts leaves and root contains the alkaloid berberine [28], lignan (e.g., lyoniresinol, meridional, secoisolariciresinol; 5,5′-dimethoxysecoiso-lariciresinol; 2-(4-hydroxy-3,5-dimethoxybenzyl)-3-(4-hydroxy-3-methoxybenzyl)-1,2-butanediol; (7′S,8S,8R)-4,4′-dihydroxy-3,3′5,5′-tetra methoxy-7′,9-eproxylignan-9′-ol-7-one; 5,5′-dimethoxy-lariciresinol; 5′-methyoxlariciresinol, dihydrodehydrodiconifery alcohol; syringaresinol) [27–29], aldehyde (e.g., p-hydroxy benzaldehyde) [30], phenolic (e.g., procyanidin A1) [31] and other compounds (e.g., 4-hydroxybenzoic acid, 2-(4′- methoxyphenyl)-ethanol, 4-methoxyphenyl alcohol, 1-tetracosane) [30], from stem carotenoids (e.g., α-carotene, β-carotene, β-carotene-5,6, 5′, 6′-diepoxide; 5, 6, 5′, 6′-diepoxy-5, 8, 5′,8′-tetrahydro-β, cis-neoxanthin, and unidentified carotenoids I, II, III, and IV) are isolated [24, 32, 33].
The aerial parts of L. spinosa contain terpenoids (e.g., limonene, aqualene, caryophyllene), volatile oil (e.g., methyl octadec-6,9-dien-12-ynoate, α-glyceryl-linolenate α-pinene, α-selinene, camphene, δ-3-carene, camphor) [21, 34], phenolic compounds (e.g., 4-hydroxybenzoic acid, morin, cinnamic acid, syringic acid, gentisic aid) [21, 28, 34], fatty acids (e.g., methyl ester of oleic acid, palmitic acid, stearic acid, epoxyoleic acid) [34], steroids (e.g., spinasterone, β-sitosterol, γ-sitosterol, stigmasterol, campesterol, crinosterol) [21, 34], alkane (e.g., hexatriacontane and heptacosane) [35].
The whole plant contains phenolics (e.g., gentisic acid, isovanilic acid, syringic acid, chlorogenic acid, p-hydroxy benzoic acid, (+)-catechin) [28], flavonoids including flavonoid glycosides and flavonoid aglycones (e.g., vitexin, vitexin-2”-O-β-D-glucopyranoside; isorhamnetin 3-O-rutinoside, morin, apigenin, 3′-methyl-quercetin-3-O-α-L-rhamnopyranosyl-(1/6) β-D-glucopyranoside; triglochinin) [28, 32, 35], and ketone (e.g., hexahydrofarnesyl acetone) (21).
The chemical structures of such compounds are shown in Table 2 and Figure 1.
Table 2.
Phytochemical profile of Lasia spinosa.
| Plant parts | Phytochemical class | Compounds | Ref. |
|---|---|---|---|
| Leave | Alkaloids | Berberine | [25] |
|
| |||
| Leaf and root/rhizome | Aldehyde | p-Hydroxy benzaldehyde | [26] |
| Other compounds | 4-Hydroxybenzoic acid, 2-(4′-methoxyphenyl)-ethanol, 4-methoxyphenethyl alcohol, 1-tetracosane | ||
|
| |||
| Stem | Carotenoid | α-Carotene, β-carotene, β-carotene-5,6, 5′, 6′-diepoxide; 5, 6, 5′, 6′-diepoxy-5, 8, 5′,8′-tetrahydro-β, cis-neoxanthin | [24, 31] Priyadarshani and Jansz, [25] |
|
| |||
| Aerial parts | Terpinoid | Limonene, β-elemene, squalene, caryophyllene | [21]; Rahman et al., [23] |
| Volatile oil | Methyl octadec-6,9-dien-12-ynoate, α-glyceryl-linolenate α-pinene, α-selinene, camphene, δ-3-carene, camphor | [21]; Rahman et al., [23] | |
| Phenolics | 4-Hydroxybenzoic acid, morin, cinnamic acid, syringic acid, gentisic acid | Rahman et al., [23]; [21, 28] | |
| Fatty acids | Methyl ester of oleic acid, palmitic acid, stearic acid, epoxyoleic acid | Rahman et al., [23] | |
| Steroid | Spinasterone, β-sitosterol, γ-sitosterol, stigmasterol, campesterol, crinosterol, | Rahman et al., [23]; [21] | |
| Alkane | Hexatriacontane, heptacosane | Rahman et al., [23] | |
|
| |||
| Root/rhizome | Lignan | Lyoniresinol, meridinol, secoisolariciresinol; 5,5′-dimethoxysecoiso-lariciresinol; 2-(4-hydroxy-3,5-dimethoxybenzyl)-3-(4-hydroxy-3-methoxybenzyl)-1,2-butanediol; (7′S,8S,8R)-4,4′-dihydroxy-3,3′5,5′-tetramethoxy-7′,9-eproxylignan-9′-ol-7-one; 5,5′-dimethoxy-lariciresinol; 5′-methyoxlariciresinol, dihydrodehydrodiconifery alcohol; syringaresinol | Alam et al., [36]; [28, 29, 32] |
| Phenolic | Procyanidin A1 | [30] | |
|
| |||
| Whole plant | Phenolic | Gentisic acid, isovanilic acid, syringic acid, chlorogenic acid, p-hydroxy benzoic acid, (+)-catechin | [28] |
| Flavonoids (glycosides and aglycones) | Vitexin, vitexin 2”-O-β-D-glucopyranoside; isorhamnetin 3-O-rutinoside, morin, apigenin, 3′-methyl quercetin-3-O-α-L-rhamnopyranosyl-(1/6) β-D-glucopyranoside; triglochinin | [27, 28, 31] | |
| Ketone | Hexahydrofarnesyl acetone | [21] | |
Figure 1.
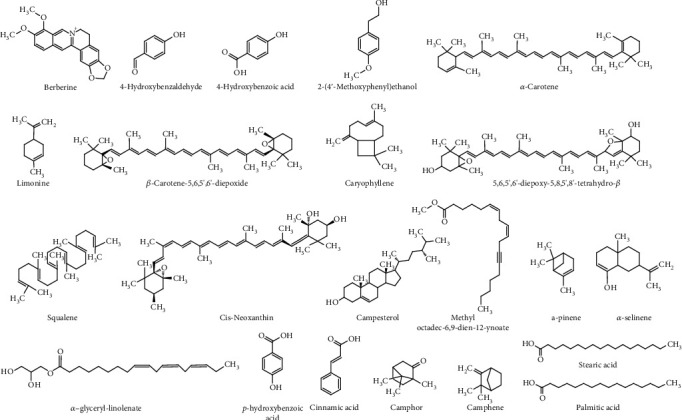
Chemical structures of the most important phytochemicals found in different parts of Lasia spinosa.
5. Pharmacological Properties: Mechanisms and Targeted Molecular Pathways
5.1. Antioxidant
Oxidative stress is the basis of premature ageing of the body, the basis of disease, and is triggered by free radicals [29] more precisely occurs as a result of the imbalance between the amount of reactive oxygen produced in the body and its ability to eliminate it [30, 31]. Oxidative stress can be alleviated by approaching a balanced lifestyle that includes a healthy diet and sports [32]. Physical exercise reduces cellular oxidation by deep oxygenating tissues, eliminating stress, and relaxing the body [33]. On the other hand, the diet has a very important role, and the best treatment against oxidative stress is antioxidants [34]. They are found in many herbs and can kill free radicals [35, 37]. Medicinal plants usually contain a high level of antioxidants that can counteract the oxidative stress process linked to a disease [38, 39].
The free radical scavenging activity of L. spinosa leaves extracts on 1,1-diphenyl-2-picrylhydrazyl (DPPH) had been assessed and showed significant antioxidant activities [40]. The ethyl acetate fraction showed the highest free radical scavenging activity (IC50 = 16.42 μg/mL) when compared to the positive control-butylated hydroxytoluene (BHT). At the same time, the aqueous fraction also exhibited moderate antioxidant potential (IC50 = 73.20 μg/mL) [40]. In DPPH and ABTS assay, ethanol extract (leaves) showed antioxidant activity (SC50 = 17.25 μg/mL and 16.47 μg/mL, respectively). Antioxidant activity is due to the presence of high levels of polyphenolic compounds [38]. In a study performed with different extracts of L. spinosa aerial parts, the highest free radical scavenging activity (DPPH) was stated to the methanol extract (IC50 = 0.48 ± 0.04 μg/mL), whereas in the metal chelating activity of ferrous ions (Fe2+) assay, the highest activity was observed for hexane extract (IC50 = 0.55 ± 0.08 μg/mL) [23]. In another study, the antiradical activity (1/EC50) of L. spinosa leaf determined by the DPPH method was 0.1 [24]. The same study reported a total phenolic content of 6.4 mg gallic equivalents/g and total flavonoid content of 4.4 retinol equivalent in L. spinosa leaf [24]. In other studies, L. spinosa showed a total phenolic content of 2.1 mg gallic equivalents/g and low antioxidant activity, through ferric reducing antioxidant power (FRAP) and oxygen radical absorbance capacity (ORAC) assays, in comparison to indigenous vegetables from Southern Thailand, such as young cashew leaves (Anacardium occidentale L.) and Mon-pu (Glochidion zeylanicum (Gaertn.) A.Juss.) [27].
5.2. Anti-Inflammatory
Inflammation is part of the complex biological response of body tissues to harmful stimuli such as pathogens, damaged cells, or irritants [41] and a protective response involving immune system cells, molecular mediators, among others [42–44].
In lipopolysaccharide-induced RAW 264.7 macrophages, the anti-inflammatory activity of L. spinosa leaf extract was addressed [45], is stated that it can activate the nuclear factor- (NF-) κappa B, and nuclear factor erythroid 2-related factor 2/heme-oxygenase-1 (Nrf2/HO-1) pathways and to suppress mitogen-activated protein kinase (MAPK) and phosphoinositide-3-kinase/protein kinase B (PI3K/Akt) pathways. Furthermore, L. spinosa leaf extract suppresses the upregulation enzyme iNOS (NOS2), COX2, and proinflammatory cytokines (TNF-α, IL-1β, and IL-6) and increases cytokines (IL-10) which produced anti-inflammatory effect [40].
In another study, the anti-inflammatory activity of L. spinosa hydroalcoholic extract in xylene-induced ear oedema model mice was assessed, being stated a significant inhibitory effect on oedema formation 17.1% at 250 mg/kg and 27.9% at 500 mg/kg. An inhibitory potential was also stated in a carrageenan-induced paw oedema model rat, and it was highest at 3 h, with 26.72% inhibition at 250 mg/kg and 38.70% at 500 mg/kg, when compared to the standard drugs (diclofenac sodium (10 mg/kg): 29.52%, and phenylbutazone (100 mg/kg): 40.47%) (Figure 2) [46].
Figure 2.
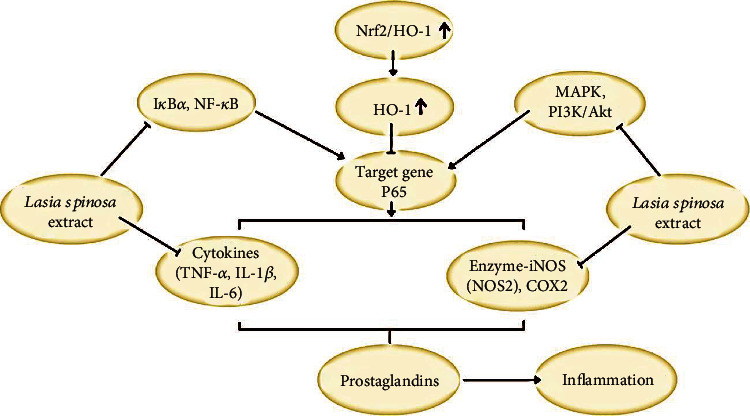
Diagram with molecular mechanisms of anti-inflammatory effect of Lasia spinosa.
5.3. Antimicrobial
An antimicrobial agent is that able to kill or stop microorganisms' growth. For that, antibacterial and antifungals are used to fight bacterial and fungal infections, respectively [47–49]. Specifically addressing antibacterial, their prolonged use is closely related to a marked decrease in the number of enteric bacteria, thus, having a major negative impact on health and wellbeing [50, 51]. In this sense, the consumption of probiotics and a prebiotics-rich diet may help to replace the destroyed gut microbiota [52]. Stool transplants may also be proposed for patients with difficulty in recovering from prolonged antibiotic treatment, as for recurrent Clostridium difficile infections [53, 54].
The organic extracts (hexane, chloroform, ethyl acetate; 300 μg/disc) and essential oil of L. spinosa aerial parts showed potent antibacterial activity against Escherichia coli, methicillin-resistant Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Enterococcus faecalis in comparison of standard antibiotics (tetracycline 30 μg/disc, streptomycin 30 μg/disc, and erythromycin 15 μg/disc), except for methanol extract [23]. In another study, L. spinosa leaves methanol extract showed moderate antimicrobial properties against Bacillus subtilis, E. coli, Bacillus cereus, S. aureus, Candida albicans, Aspergillus niger, and Vibrio para hemolyticus at 400 μg/disc by disc diffusion assay [36]. In other studies, the methanol extract of L. spinosa edible parts did not show antimicrobial activity against C. albicans [46].
5.4. Cytotoxic
Cytotoxicity destroys cancer cells or prevents them from multiplying [55–57]. This cytotoxicity is done in different ways: some bioactive compounds can affect the genetic material of cells, and others act by blocking the access of malignant cells to the nutrients needed for division and multiplication [58, 59].
The cytotoxic potential of L. spinosa extracts has also been assessed. Brine shrimp lethality bioassay technique was applied to determine the cytotoxic potential of crude extracts. The aqueous extract from L. spinosa leaves showed moderate cytotoxicity (LC50 = 98.66 μg/mL) in brine shrimp lethality bioassay [40], while the methanol extract from the whole plant led to significant cytotoxic effects (IC50 = 13.49 μg/mL) on brine shrimp [36].
5.5. Antidiarrheal
In the castor oil-induced diarrheal mice model, both standard antimotility drug loperamide and hydroalcoholic extract from L. spinosa root significantly reduced the number of stools in a dose-dependent manner compared to the negative control group. The mean number of stools found was 11.6 for 250 mg/kg and 8.2 for 500 mg/kg b.w., whereas for the standard drug (5 mg/kg) was of 5.6.L. spinosa root extract at 250 mg/kg b.w, causing a little increase in the latent period, but at 500 mg/kg b.w. led to a significant increase [46]. L. spinosa root extract exhibited a potent antidiarrheal activity, supporting their traditional use for diarrhea.
5.6. Antihelminthic
Helminthic infections continue to be the major people's health hazard, especially in those living in tropical developing countries [60]. L. spinosa leaves methanol extract significantly exhibited paralysis and triggered worms' death, especially at high doses (100 mg/mL) against Pheritimaposthuma [61], in a hymenolepisdiminuta–rat animal model [12] and infected mice with Trichinellaspiralis (800 mg/kg; p.o.) [19].
5.7. Antidiabetic
Diabetes mellitus is a chronic metabolic disease with numerous complications, like retinopathy, neuropathy, and peripheral vascular insufficiency. Several synthetic agents are available for diabetes treatment, but several side effects have been reported [62]. Plant-based medicinal products have been used since ancient times to manage diabetes in traditional medicine in many countries all over the world. L. spinosa stem hydroalcoholic extract have revealed antidiabetic activity at 200 and 400 mg/kg (p.o.) in dexamethasone (10 mg/kg s.c.)-induced diabetic albino rats by preventing serum glucose levels rise triggered by dexamethasone [63] and significantly reducing the triglycerides levels [64]. This extract also ameliorated hyperglycemia, and it likely has greater therapeutic potential as they may also exert beneficial effects on the clinical course of noninsulin-dependent diabetes mellitus (NIDDM), hypertension, and coronary artery disease [63]. A study developed by Shafie et al. [65] showed inhibitory effects of different parts (leaves, stems, and roots) of L. spinosa extracts (aqueous hot/cold, ethanol) against pancreatic lipase, α-amylase, and α-glucosidase.
5.8. Antihyperlipidemic
L. spinosa leaves have also the potential to prevent hyperlipidemia-induced pancreatitis in rats at concentrations of 400 and 800 mg/kg (p.o.) while exerting cardioprotective effects by significantly increasing serum high-density lipoprotein-cholesterol (HDL-c) at 100 mg/kg, p.o., and Triton-X 100 at 480 mg/kg, i.p. in an induced hyperlipidemic animal model [3].
5.9. Antinociceptive
Antinociception is the action or process of blocking the detection of a painful or injurious stimulus by sensory neurons, and antinociceptives are agents that block painful stimulus [66, 67]. The acetic acid-induced writhing test is used for detecting both central and peripheral analgesia, whereas the hot plate is most sensitive to centrally acting analgesics [68]. In acetic acid-induced writhing and hot plate-induced pain in mice the hydroalcoholic extract of L. spinosa roots revealed antinociceptive activity in mice, being stated 37% and 50% writhing inhibition at 250 mg/kg and 500 mg/kg b.w., respectively, while increased pain threshold [46]. On the other hand, the methanol extract from L. spinosa leaves at 400 mg/kg led to a significant decrease in the number of writhes and elongated the reaction time in the acetic acid writhing method and radiant heat tail flicking method, respectively [69].
5.10. Gastroprotective
A study revealed that L. spinosa leaves ethanol extract has gastroprotective effects. In albino rats with indomethacin (5 mg/kg, p.o.) and cold restrain stress-induced ulcers, 3 doses (100, 200, and 400 mg/kg, p.o.) of L. spinosa extract were tested, with gastroprotective effects being mainly conferred by the extractability to create a defensive layer in stomach, through scavenging free radicals and inhibiting lipid peroxidation [70]. In gastric secretion studies, L. spinosa significantly evidenced a tendency to decrease gastric juice, free acidity, and total acidity [70]. Thus, after isolation of the individual compounds present in the extract, those responsible for the observed effect can be used both to treat ulcers and to reduce their severity.
5.11. Effect on Reproductive Activity
Testosterone plays an important role in Sertoli and Leydig cell proliferation and hyperplasia that can increase the testis size [71]. Testosterone is also involved in spermatogenesis and the growth and development of testis and male accessory reproductive glands [64]. The hydroalcoholic extract of L. spinosa rhizomes was revealed to be able to increase the serum testosterone levels and sperm count in male rats at 40 g/kg b.w. These data were confirmed afterward by an increase in testicular weight and sperm count, with the increase in the absolute weight of testis being attributed to the elevation of androgen biosynthesis leading to an increase in serum testosterone levels [64].
The most important pharmacological properties are summarized in Table 3 and Figure 3.
Table 3.
Pharmacological activities of Lasia spinosa.
| Activity | Sources | Test system | Dose tested | Positive value | Results | Ref. |
|---|---|---|---|---|---|---|
| Antioxidant | Ethyl acetate extract (leaves) | DPPH | IC50 = 73.20 μg/mL | BHT IC50 = 23.19 μg/mL |
Moderate potential | [40] |
| Ethanol extract (leaves) | DPPH ABTS |
DPPH, IC50 = 17, 25 μg/mL; ABTS SC50 = 16.47 μg/mL |
Vitamin C Dose = 5.38 μg/mL Trolox Dose = 3.17 μg/mL |
↑ antioxidant activity due to the presence of high levels of polyphenolic compounds | [45] | |
| Hexane, chloroform, ethyl acetate, and methanol extracts (aerial parts) | DPPH Chelating activity of ferrous ions (Fe2+) |
IC50 = 0.48 ± 0.04 μg/mL (methanolic) IC50 = 0.55 ± 0.08 μg/mL (hexane) |
Not studied | ↑ antioxidant activity | [23] | |
|
| ||||||
| Anti-inflammatory | Ethanol extract (leaves) | Lipopolysaccharide-induced RAW 264.7 macrophages in vitro | Dose = 50, 100, 200, 400 μg/mL | L-NAME Dose = 100 μM |
↑ NF-κB, ↑ Nrf2/HO-1 ↓MAPK, ↓ PI3K/Akt |
[45] |
| Hydroalcoholic extract (roots) | Carrageenan-induced paw edema model rats and xylene-induced ear edema mice in vivo | Dose = 250, 500 mg/kg bw, i.p. n = 6 |
Nalbuphine Dose = 10 mg/kg |
↓oedema formation | [46] | |
|
| ||||||
| Antimicrobial | Methanol extract (leaves) | Disc diffusion assay in vitro | Dose = 400 μg/disc | Kanamycin Dose = 30 μg/disc |
Moderate antimicrobial activity against Bacillus subtilis, Escherichia coli, Bacillus cereus, Staphylococcus aureus, Candida albicans, Aspergillus niger, and Vibrio parahemolyticus | [36] |
| Hexane, chloroform, ethyl acetate, and methanol extracts (aerial parts) and essential oil | Disc diffusion assay in vitro | Dose = 300 μg/disc | Tetracycline Dose = 30 μg/disc Streptomycin Dose = 30 μg/disc Erythromycine Dose = 15 μg/disc |
Potent antibacterial activity against Escherichia coli, methicillin-resistant Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Enterococcus faecalis, with the exception of methanolic extract | [23] | |
|
| ||||||
| Cytotoxicity | Hydromethanolic extract (leaves) | Brine shrimp lethality bioassay in vitro | LC50 = 98.663 μg/mL | Vincristine sulphate LC50 = 0.544 μg/ml |
Moderate cytotoxic effect | [40] |
| Methanol extract (whole plant) | Triton-X 100 (480 mg/kg, i.p.) induced hyperlipidemic rat model in vivo | IC50 = 13.49 μg/mL | Vincristine sulphate LC50=20 μg/ml |
↑cytotoxicity | [36] | |
| Dose = 200, 400, 800 mg/kg, p.o. n = 6 |
Triton-X 100 Dose = 480 mg/kg |
↓triglycerides, ↓LDL-C ↓VLDL-C |
||||
|
| ||||||
| Antidiarrheal | Hydroalcoholic extract (roots) | Castor oil-induced diarrhea mice model in vivo | 250 and 500 mg/kg b.w., i.p. n = 6 |
Loperamide Dose = 5 mg/kg |
↓number of stools ↑latent period of diarrhea |
[46] |
|
| ||||||
| Anthelmintic | Methanol extract (leaves) | Pheritima posthuman in vivo | Dose = 25, 50, 100 mg/mL, n = 6 | Albendazole Dose = 10 mg/ml |
↑paralysis, ↑worm death, especially at 100 mg/ml | [61] |
| Extract (leaves) | Hymenolepis diminuta rat model in vivo | Dose = 200, 400, 800, 1600 mg/kg, p.o., n = 6 |
Praziquantel Dose = 5 − 10 mg/kg |
↓eggs per gram of feces ↓worm recovery rates |
[12] | |
|
| ||||||
| Antidiabetic | Hydroalcoholic extract (stem) | Dexamethasone 10 mg/kg s.c. induced diabetes rats in vivo | Dose = 200, 400 mg/kg, p.o. n = 6 |
Dexamethasone dose = 10 mg/kg | ↑antidiabetic activity | [63] |
|
| ||||||
| Antihyperlipidemic | Methanol extract (leaves) | Cholestero,l 100 mg/kg p.o, induced hyperlipidemic rat model in vivo | Dose = 200, 400, 800 mg/kg, p.o. n = 6 |
Cholesterol Dose = 100 mg/kg |
↓cholesterol | [3] |
| Triton-X 100, 480 mg/kg, i.p. induced hyperlipidemic rat model in vivo | Dose = 200, 400, 800 mg/kg, p.o. n = 6 |
Triton-X 100 Dose = 480 mg/kg |
↓triglycerides ↓LDL-C ↓VLDL-C |
|||
|
| ||||||
| Antinociceptive | Hydroalcoholic extract (roots) | Acetic acid-induced writhing and hot plate-induced pain in mice in vivo | Dose = 250, 500 mg/kg b.w., i.p. n = 6 |
Diclofenac sodium Dose = 10 mg/kg |
50% writhing inhibition ↑pain threshold |
[46] |
| Methanol extract (leaves) | Acetic acid writhing method and radiant heat tail flicking method in vivo | Dose = 200, 400 mg/kg, p.o. n = 5 |
Diclofenac sodium Dose = 50 mg/kg |
↓number of writhes ↑reaction time at dose 400 mg/kg | [69] | |
|
| ||||||
| Gastroprotective | Ethanol extract (leaves) | Indomethacin, 5 mg/kg b.w., p.o. Cold restraint stress-induced ulcers in rats in vivo |
Dose = 100, 200, 400 mg/kg, p.o. n = 5 |
Indomethacin dose = 5 mg/kg | Development of a defensive layer; ↑free radical scavenging activity; ↓LPO | [70] |
|
| ||||||
| Reproductive activity | Hydroalcoholic extract (rhizomes) | Male rats in vivo | Dose = 5, 10, 20, 40 g/kg b.w., p.o. n = 5 |
Distilled water | ↑serum testosterone | [64] |
Abbreviations: ABTS: 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid; BHT: butylatedhydroxytoluene; DPPH: 1,1-diphenyl-2-pecrylhydrazyl; HO-1: heme-oxygenase-1; L-NAME: N-Nitro-L-arginine methyl ester; LDL-C: low-density lipoprotein cholesterol; MAPK: mitogen-activated protein kinase; NF-κB: kappa B; Nrf2: nuclear factor erythroid 2-related factor 2; PI3K/Akt: phosphoinositide-3-kinase/protein kinase B; VLDL-C: very-low-density lipoprotein cholesterol.
Figure 3.
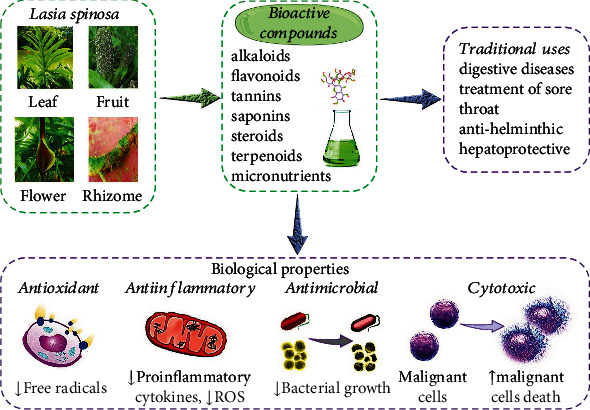
Summarized scheme with traditional uses and the most representative biological properties of L. spinosa.
6. Toxicological Data and Clinical Gaps
Oral administration in an acute analysis of 5, 10, 20, and 40 gm/kg of an extract, there were no mortality or physiological changes demonstrated. In the subchronic assay for 28 gm/kg, administration of 5 or 20 gm/kg of extract for 28 gm/kg, no animal deaths were announced that day. No differences in haematological parameters were noticed in either case [52].
Therapeutic limitation of natural bioactive compounds from Lasia spinosa results from the relatively reduced bioavailability of bioactive compounds. In addition, numerous interactions with other prescription drugs may occur. Interactions between medicinal plants interfere with the metabolism or elimination of the drug/chemotherapy from the body. Drug metabolism/elimination is mediated by enzymes that metabolize drugs in the cytochrome P450 (CYP) family and drug transport proteins. These interactions can change the concentration of drugs in the body [72].
Interactions between plants and drugs may occur due to inhibition or activation by plant phytochemicals of CYP enzymes or drug transport proteins that metabolize the drug [73]. Some therapeutic pharmacological agents must be activated by CYP to be effective. Once CYPs are inhibited, such drugs that need to be activated will be ineffective. There may be interactions between plants and drugs that lead to increased elimination of drugs due to CYP activation, which could lead to subtherapeutic exposure to drugs and could lead to therapy failure [74]. Some plant-drug interactions due to CYP inhibition may lead to the accumulation of cytotoxic drugs due to delayed clearance and may increase drug toxicity due to high doses of drugs. Cancer patients are already taking several medications at the same time due to other conditions associated with cancer and comorbidities, which present a risk of drug interactions [75]. The use of herbs/herbal products may further increase the risk of these potentially harmful interactions that interfere with the impact of the drug.
7. Overall Conclusions and Future Perspectives
Natural plant sources have contributed to many drug developments. In this study, we comply with the traditional uses, pharmacological properties, and chemical constituents of L. spinosa, information that can be useful for further research. Many phytochemicals present in L. spinosa may be responsible for its biological effects in various test systems, but more studies are needed to identify and characterize the active compounds responsible for the pharmacological activities of this hopeful medicinal plant. Future directions must be oriented to toxicological studies which are scarce, and there are necessary new reports to ensure the safety of this plant. Besides, clinical studies are required to confirm the preclinical biological effects in humans.
Acknowledgments
This work was supported by Comisión Nacional de Investigación Científica y Tecnológica (CONICYT) PIA/APOYO CCTE AFB170007. N.C.-M. acknowledges the Portuguese Foundation for Science and Technology under the Horizon 2020 Program (PTDC/PSI-GER/28076/2017).
Contributor Information
Natália Cruz-Martins, Email: ncmartins@med.up.pt.
Javad Sharifi-Rad, Email: javad.sharifirad@gmail.com.
Daniela Calina, Email: calinadaniela@gmail.com.
William C. Cho, Email: chocs@ha.org.hk.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- 1.Yuan H., Ma Q., Ye L., Piao G. The traditional medicine and modern medicine from natural products. Molecules . 2016;21(5):p. 559. doi: 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahma J., Chakravarty S., Rethy P. Qualitative estimation of the presence of bioactive and nutritional compound in Lasia spinosa: an important vegetable plant used by the Bodos of Kokrajhar District. International Journal of ChemTech Research . 2014;6:1405–1412. [Google Scholar]
- 3.Mahmood S. B., Atif M., Ali S. R., Ahmed M. I., Rahman S. A. Evaluation of antihyperlipidemic activity of methanolic leaves extract of Lasia spinosa and its role in prevention of hyperlipidemia induced pancreatitis in rats. International Journal of Pharmaceutical Sciences and Research . 2015;6(4):p. 1502. [Google Scholar]
- 4.Majumdar K., Datta B. A study on ethnomedicinal usage of plants among the folklore herbalists and Tripuri medical practitioners: part-II. Natural Product Radiance . 2007;6:66–73. [Google Scholar]
- 5.Gupta A. K., Spinosa L. 18th Meeting of the conference of the Parties . Geneva, Switzerland: 2019. IUCN Red List of Threatened Species, [Google Scholar]
- 6. Useful Tropical Plants . 2019. http://tropical.theferns.info/
- 7.Keating R. C. Acoraceae and Araceae. In: Gregory M., Cutler D. F., editors. Anatomy of the Monocotyledons . Oxford, UK: Oxford University Press; 2002. pp. 1–327. [Google Scholar]
- 8.Hiong Y. Y. School of Food Science and Nutrition . University Malaysia Sabah; 2009. Anti-oxidant activity and total phenolic contents in Cucurbita moschtata, Lasia spinosa and Limnocharis flava, 2005-2009. [Google Scholar]
- 9.Lakshmi M. A., Priya G. V., Rao B. G. Morpho-anatomical feature and phytochemical assessments of Lasia spinosa (L)Thwaites. Indian Journal of Pharmaceutical Sciences . 2020;82(5):891–901. doi: 10.36468/pharmaceutical-sciences.718. [DOI] [Google Scholar]
- 10. Flora of China . 1999. http://www.efloras.org/
- 11.Shen Y. N., Xiao D., Hu X. X., et al. First report of leaf spot on Lasia spinosa Caused by Fusarium fujikuroi in China. Plant Disease . 2020;104(9):2525–2525. doi: 10.1094/PDIS-01-20-0013-PDN. [DOI] [Google Scholar]
- 12.Temjenmongla Y. A. K. Anticestodal efficacy of Lasia spinosa. Extract against experimental Hymenolepis diminuta. Infections in rats. Pharmaceutical Biology . 2006;44(7):499–502. [Google Scholar]
- 13.Reddy K. N., Pattanaik C., Reddy C. S., Raju V. S. Traditional knowledge on wild food plants in Andhra Pradesh. Indian Journal of Traditional Knowledge . 2007;6(1):223–229. [Google Scholar]
- 14.Das H. B., Majumdar K., Datta B. K., Ray D. Ethnobotanical uses of some plants by Tripuri and Reang tribes of Tripura. Natural Product Radiance . 2009;8(2):172–180. [Google Scholar]
- 15.Shefana A., Ekanayake S. Some nutritional aspects of Lasia spinosa (kohila) Vidyodaya Journal of Science . 2010;14(1):59–64. [Google Scholar]
- 16. The Plant List (TPL) 2021. http://www.theplantlist.org/
- 17.Heinrich M., Appendino G., Efferth T., et al. Best practice in research - Overcoming common challenges in phytopharmacological research. Journal of Ethnopharmacology . 2020;246 doi: 10.1016/j.jep.2019.112230. [DOI] [PubMed] [Google Scholar]
- 18.Gautam A. K. The wealth of India: an encyclopedia of India's raw material resources. Pakistan Journal of Biological Sciences . 2001;15(5):449–507. [Google Scholar]
- 19.Yadav A. K., Temjenmongla Efficacy of Lasia spinosa leaf extract in treating mice infected with Trichinella spiralis. Parasitology Research . 2012;110(1):493–498. doi: 10.1007/s00436-011-2551-9. [DOI] [PubMed] [Google Scholar]
- 20.Temjenmongla T., Yadav A. K. Anticestodal efficacy of folklore medicinal plants of Naga tribes in north-east India. African Journal of Traditional, Complementary, and Alternative Medicines . 2005;2(2):129–133. doi: 10.4314/ajtcam.v2i2.31111. [DOI] [Google Scholar]
- 21.Deka L., Majumdar R., Dutta A. M. Some ayurvedic important plants from district Kamrup (Assam) Ancient Science of Life . 1983;3(2):108–115. [PMC free article] [PubMed] [Google Scholar]
- 22.IAMM, Institute of Aryuveda and Alternative Medicine (IAAM) 2013. http://www.instituteofayurveda.org/
- 23.Rahman A., Siddiqui S. A., Oke-Altuntas F., OKAY S., GÜL F., Demirtas I. Phenolic profile, essential oil composition and bioactivity of Lasia spinosa (L.) Thwaites. Brazilian Archives of Biology and Technology . 2019;62 doi: 10.1590/1678-4324-2019170757. [DOI] [Google Scholar]
- 24.Maisuthisakul P., Pasuk S., Ritthiruangdej P. Relationship between antioxidant properties and chemical composition of some Thai plants. Journal of Food Composition and Analysis . 2008;21(3):229–240. doi: 10.1016/j.jfca.2007.11.005. [DOI] [Google Scholar]
- 25.Priyadarshani A. M. B., Jansz E. R. The effect of maturity on carotenoids of Lasia spinosa stem and the effects of cooking on in-vitro bioaccessibility of carotenoids. Journal of the National Science Foundation of Sri Lanka . 2006;34(3):131–136. [Google Scholar]
- 26.Islam M. S., Rashid M. M., Ahmed A. A., Reza A. A., Rahman M. A., Choudhury T. R. The food ingredients of different extracts of Lasia spinosa (L.) Thwaites can turn it into a potential medicinal food. NFS Journal . 2021;25:56–69. doi: 10.1016/j.nfs.2021.11.002. [DOI] [Google Scholar]
- 27.Kongkachuichai R., Charoensiri R., Yakoh K., Kringkasemsee A., Insung P. Nutrients value and antioxidant content of indigenous vegetables from southern Thailand. Food Chemistry . 2015;173:838–846. doi: 10.1016/j.foodchem.2014.10.123. [DOI] [PubMed] [Google Scholar]
- 28.Kumar M., Mondal P., Borah S., Mahato K. Physico-chemical evaluation, preliminary phytochemical investigation, fluorescence and TLC analysis of leaves of the plant Lasia spinosa (Lour) Thwaites. International Journal of Pharmacy and Pharmaceutical Sciences . 2013;5(2):306–310. [Google Scholar]
- 29.Salehi B., Sharifi-Rad J., Cappellini F., et al. The therapeutic potential of anthocyanins: current approaches based on their molecular mechanism of action. Frontiers in Pharmacology . 2020;11:p. 20. doi: 10.3389/fphar.2020.01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mititelu R. R., Pădureanu R., Băcănoiu M., et al. Inflammatory and oxidative stress markers-mirror tools in rheumatoid arthritis. Biomedicine . 2020;8(5):p. 125. doi: 10.3390/biomedicines8050125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salehi B., Sharifi-Rad, Capanoglu, et al. Cucurbita plants: from farm to industry. Applied Sciences-Basel . 2019;9(16):p. 3387. doi: 10.3390/app9163387. [DOI] [Google Scholar]
- 32.Sharifi-Rad M., Anil Kumar N. V., Zucca P., et al. Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Frontiers in Physiology . 2020;11:p. 21. doi: 10.3389/fphys.2020.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharifi-Rad J., Rodrigues C. F., Sharopov F., et al. Diet, lifestyle and cardiovascular diseases: linking pathophysiology to cardioprotective effects of natural bioactive compounds. International Journal of Environmental Research and Public Health . 2020;17(7):p. 2326. doi: 10.3390/ijerph17072326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Islam M. T., Quispe C., Martorell M., et al. Dietary supplements, vitamins and minerals as potential interventions against viruses: perspectives for COVID-19. International Journal for Vitamin and Nutrition Research . 2021:1–18. doi: 10.1024/0300-9831/a000694. [DOI] [PubMed] [Google Scholar]
- 35.Salehi B., Rescigno A., Dettori T., et al. Avocado-soybean unsaponifiables: a panoply of potentialities to be exploited. Biomolecules . 2020;10(1):p. 130. doi: 10.3390/biom10010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alam F., Haque M., Sohrab H., Monsur M. A., Hasan C. M., Ahmed N. Antimicrobial and cytotoxic activity from Lasia spinosa and isolated lignan. Latin American Journal of Pharmacy . 2011;30(3):550–553. [Google Scholar]
- 37.Salehi B., Prakash Mishra A., Nigam M., et al. Ficus plants: state of the art from a phytochemical, pharmacological, and toxicological perspective. Phytotherapy Research . 2021;35(3):1187–1217. doi: 10.1002/ptr.6884. [DOI] [PubMed] [Google Scholar]
- 38.Salehi B., Quispe C., Chamkhi I., et al. Pharmacological properties of chalcones: a review of preclinical including molecular mechanisms and clinical evidence. Frontiers in Pharmacology . 2021;11:592654–592654. doi: 10.3389/fphar.2020.592654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Docea A. O., Calina D., Buga A. M., et al. The effect of silver nanoparticles on antioxidant/pro-oxidant balance in a murine model. International Journal of Molecular Sciences . 2020;21(4):p. 1233. doi: 10.3390/ijms21041233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goshwami D., Rahman M. M., Muhit M. A., Islam M. S., Anasri M. Antioxidant property, cytotoxicity and antimicrobial activity of Lasia spinosa leaves. Nepal Journal of Science and Technology . 2013;13(2):215–218. doi: 10.3126/njst.v13i2.7739. [DOI] [Google Scholar]
- 41.Scheau C., Caruntu C., Badarau I. A., et al. Cannabinoids and inflammations of the gut-lung-skin barrier. Journal of Personalized Medicine . 2021;11(6):p. 494. doi: 10.3390/jpm11060494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Padureanu R., Albu C. V., Mititelu R. R., et al. Oxidative stress and inflammation interdependence in multiple sclerosis. Journal of Clinical Medicine . 2019;8(11):p. 1815. doi: 10.3390/jcm8111815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Georgiadis G., Zisis I. E., Docea A. O., et al. Current concepts on the reno-protective effects of phosphodiesterase 5 inhibitors in acute kidney injury: systematic search and review. Journal of Clinical Medicine . 2020;9(5):p. 1284. doi: 10.3390/jcm9051284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calina D., Buga A. M., Mitroi M., et al. The treatment of cognitive, behavioural and motor impairments from brain injury and neurodegenerative diseases through cannabinoid system modulation-evidence from in vivo studies. Journal of Clinical Medicine . 2020;9(8):p. 2395. doi: 10.3390/jcm9082395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen T. Q. C., Duy Binh T., Pham T. L. A., et al. Anti-inflammatory effects of Lasia spinosa leaf extract in lipopolysaccharide-induced RAW 264.7 macrophages. International Journal of Molecular Sciences . 2020;21(10):p. 3439. doi: 10.3390/ijms21103439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deb D., Dev S., Das A. K., et al. Antinociceptive, anti-inflammatory and anti-diarrheal activities of the hydroalcoholic extract of Lasia spinosa Linn. (Araceae) roots. The American Journal of Pharmacy . 2010;29(8):1269–1276. [Google Scholar]
- 47.Taheri Y., Joković N., Vitorović J., Grundmann O., Maroyi A., Calina D. The burden of the serious and difficult-to-treat infections and a new antibiotic available: cefiderocol. Frontiers in Pharmacology . 2021;11:p. 1922. doi: 10.3389/fphar.2020.578823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zlatian O., Balasoiu A. T., Balasoiu M., et al. Antimicrobial resistance in bacterial pathogens among hospitalised patients with severe invasive infections. Experimental and Therapeutic Medicine . 2018;16(6):4499–4510. doi: 10.3892/etm.2018.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharifi-Rad J., Dey A., Koirala N., et al. Cinnamomum species: bridging phytochemistry knowledge, pharmacological properties and toxicological safety for health benefits. Frontiers in Pharmacology . 2021;12:600139–600139. doi: 10.3389/fphar.2021.600139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghenea A. E., Cioboată R., Drocaş A. I., et al. Prevalence and antimicrobial resistance of Klebsiella strains isolated from a county hospital in Romania. Antibiotics . 2021;10(7):p. 868. doi: 10.3390/antibiotics10070868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharifi-Rad J., Quispe C., Rahavian A., et al. Bioactive compounds as potential agents for sexually transmitted diseases management: a review to explore molecular mechanisms of action. Frontiers in Pharmacology . 2021;12(1886) doi: 10.3389/fphar.2021.674682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharifi-Rad J., Rodrigues C. F., Stojanović-Radić Z., et al. Probiotics: versatile bioactive components in promoting human health. Medicina-Lithuania . 2020;56(9):p. 433. doi: 10.3390/medicina56090433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brandt L. J. American journal of gastroenterology lecture: intestinal microbiota and the role of fecal microbiota transplant (FMT) in treatment of C. difficile infection. The American Journal of Gastroenterology . 2013;108(2):177–185. doi: 10.1038/ajg.2012.450. [DOI] [PubMed] [Google Scholar]
- 54.Kellermayer R. Prospects and challenges for intestinal microbiome therapy in pediatric gastrointestinal disorders. World Journal of Gastrointestinal Pathophysiology . 2013;4(4):91–93. doi: 10.4291/wjgp.v4.i4.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Docea A. O., Mitruţ P., Grigore D., Pirici D., Călina D. C., Gofiţă E. Immunohistochemical expression of TGF beta (TGF-beta), TGF beta receptor 1 (TGFBR1), and Ki67 in intestinal variant of gastric adenocarcinomas. Romanian Journal of Morphology and Embryology . 2012;53(3):683–692. [PubMed] [Google Scholar]
- 56.Zlatian O. M., Comănescu M. V., Roşu A. F., et al. Histochemical and immunohistochemical evidence of tumor heterogeneity in colorectal cancer. Romanian Journal of Morphology and Embryology . 2015;56(1):175–181. [PubMed] [Google Scholar]
- 57.Sharifi-Rad J., Quispe C., Butnariu M., et al. Chitosan nanoparticles as a promising tool in nanomedicine with particular emphasis on oncological treatment. Cancer Cell International . 2021;21(1):318–318. doi: 10.1186/s12935-021-02025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharifi-Rad J., Bahukhandi A., Dhyani P., et al. Therapeutic potential of neoechinulins and their derivatives: an overview of the molecular mechanisms behind pharmacological activities. Frontiers in Nutrition . 2021;8, article 664197 doi: 10.3389/fnut.2021.664197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharifi-Rad J., Quispe C., Patra J. K., et al. Paclitaxel: application in modern oncology and nanomedicine-based cancer therapy. Oxidative Medicine and Cellular Longevity . 2021;2021 doi: 10.1155/2021/3687700.3687700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tandon V., Yadav A. K., Roy B., Das B. Phytochemicals as cure of worm infections in traditional medicine systems. Emerging trends in zoology . New Delhi: Narendra Publishing House; 2011. [Google Scholar]
- 61.Goshwami D. U., Rahman M. M., Muhit M. A., Islam S. In-vitro evaluation of anthelmintic activity of Lasia spinosa leaves. International Journal of Current Pharmaceutical Research . 2013;5(1):34–35. [Google Scholar]
- 62.Pandey A., Tripathi P., Pandey R., Srivatava R., Goswami S. Alternative therapies useful in the management of diabetes: a systematic review. Journal of Pharmacy & Bioallied Sciences . 2011;3(4):504–512. doi: 10.4103/0975-7406.90103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Das S., Baruah M., Shill D. Evaluation of antidiabetic activity from the stem of Lasia spinosa in dexamethasone induced diabetic albino rats. Journal of Pharmaceutical, Chemical and Biological Sciences . 2014;1(1):12–17. [Google Scholar]
- 64.Kaewamatawong T., Suthikrai W., Bintvihok A., Banlunara W. Acute to subchronic toxicity and reproductive effects of aqueous ethanolic extract of rhizomes of Lasia spinosa Thw. in male rats. Thai. Journal of Veterinary Medicine . 2013;43(1):p. 74. [Google Scholar]
- 65.Shafie N. H., Idris S. L., Hamdan N. N., et al. Nutritional composition, antioxidative and inhibitory effects against pancreatic lipase, α-amylase and α -glucosidase of Lasia spinosa. Journal of Engineering and Applied Science . 2018;13(11):8898–8905. [Google Scholar]
- 66.Salehi B., Sestito S., Rapposelli S., et al. Epibatidine: a promising natural alkaloid in health. Biomolecules . 2019;9(1):p. 6. doi: 10.3390/biom9010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharifi-Rad J., Quispe C., Imran M., et al. Genistein: an integrative overview of its mode of action, pharmacological properties, and health benefits. Oxidative Medicine and Cellular Longevity . 2021;2021 doi: 10.1155/2021/3268136.3268136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shanmugasundaram P., Venkataraman S. Anti-nociceptive activity of Hygrophila auriculata (Schum) Heine. African Journal of Traditional, Complementary, and Alternative Medicines . 2006;2(1):62–69. [Google Scholar]
- 69.Goshwami D., Rahman M. M., Muhit M. A., Islam M. S. Antinociceptive activity of leaves of Lasia spinosa. Archives of Applied Science Research . 2012;4(6):2431–2434. [Google Scholar]
- 70.Atif M., Azharuddin M., Mahmood S. B. Gastroprotective potential of Lasia spinosa in albino rats. International Journal of Pharmacy and Pharmaceutical Sciences . 2015;7(3):254–257. [Google Scholar]
- 71.Calina D., Docea A., Golokhvast K., Sifakis S., Tsatsakis A., Makrigiannakis A. Management of endocrinopathies in pregnancy: a review of current evidence. International Journal of Environmental Research and Public Health . 2019;16(5):p. 781. doi: 10.3390/ijerph16050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Orellana-Paucar A., Vintimilla-Rojas D. Interactions of clinical relevance associated with concurrent administration of prescription drug and food or medicinal plants: a systematic review protocol. Systematic Reviews . 2020;9(1):p. 1. doi: 10.1186/s13643-019-1259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsoukalas D., Fragkiadaki P., Docea A., et al. Association of nutraceutical supplements with longer telomere length. International Journal of Molecular Medicine . 2019;44(1):218–226. doi: 10.3892/ijmm.2019.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsoukalas D., Zlatian O., Mitroi M., et al. A novel nutraceutical formulation can improve motor activity and decrease the stress level in a murine model of middle-age animals. Journal of Clinical Medicine . 2021;10(4):p. 624. doi: 10.3390/jcm10040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wanwimolruk S., Prachayasittikul V. Cytochrome P450 enzyme mediated herbal drug interactions (part 1) EXCLI Journal . 2014;13:347–391. [PMC free article] [PubMed] [Google Scholar]


