Abstract
Most of the currently marketed drugs consist of heterocyclic scaffolds containing nitrogen and or oxygen as heteroatoms in their structures. Several research groups have synthesized diversely substituted 1,2,4-oxadiazoles as anti-infective agents having anti-bacterial, anti-viral, anti-leishmanial, etc. activities. For the first time, the present review article will provide the coverage of synthetic account of 1,2,4-oxadiazoles as anti-infective agents along with their potential for SAR, activity potential, promising target for mode of action. The efforts have been made to provide the chemical intuitions to the reader to design new chemical entity with potential of anti-infective activity. This review will mark the impact as the valuable, comprehensive and pioneered work along with the library of synthetic strategies for the organic and medicinal chemists for further refinement of 1,2,4-oxadiazole as anti-infective agents.
Graphical abstract
Keywords: 1,2,4-Oxadiazoles; Anti-infective; Anti-bacterial; Anti-malarial; Synthesis
Introduction
Most of the parts of the world have been affected from deadly infectious disease including tuberculosis (Mycobacterium tuberculosis, Mtb) [1, 2], malaria (Plasmodium falciparum) [3], Chagas disease or American trypanosomiasis (Trypanosoma cruzi) [4], nosocomial infections (Staphylococcus aureus or methicillin-resistant S. aureus, MRSA) [5] and also the recent severe acute respiratory syndrome coronavirus-2 (SARS CoV-2, caused by Coronavirus) [6–8]. The scarcity of curative action of antibiotics and anti-infective agents and complex genomic structure of microorganism has led to resistance among them leading to ineffectiveness of the currently used treatment regimen [9]. The resistance to antibiotics has become a threat to the world in the absence of effective anti-infective therapeutics with the demand of new hybrid drugs acting against resistant microorganism [10]. These all facts have necessitated the urge of new chemical entities to act against these microorganisms.
4-Oxadiazoles as important heterocyclic scaffolds
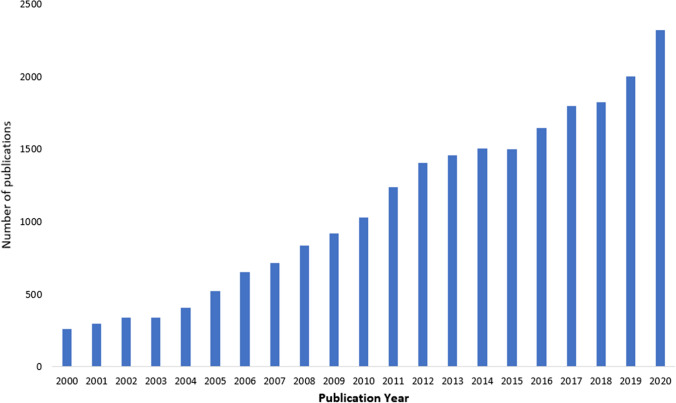
The synthesis of nitrogen- and oxygen-containing scaffolds has gained momentum [11–15] due to their versatility in the arsenal of drug discovery [16–18]. Oxadiazoles, being five-membered heterocyclic scaffold with an oxygen and two nitrogen atoms constitute four regioisomeric structures such as 1,2,3-oxadiazole, 1,2,5-oxadiazole, 1,2,4-oxadiazole and 1,3,4-oxadiazole. Oxadiazoles possess hydrogen bond acceptor properties [19] owing to electronegativities of nitrogen and oxygen, wherein nitrogen has been stronger hydrogen bond acceptor than oxygen [20]. Additionally, oxadiazoles have been recognized as the bioisosteres of (hydroxamic)esters, carboxamides and carbamates leading to metabolic stability to parent scaffolds [21]. Among these isomeric oxadiazoles, 1,2,4-oxadiazoles have found many applications in pharmaceutical industry for drug discovery, scintillating materials and dyestuff industry [22]. Oxadiazoles are also synthesized from green chemistry approach which microwave-assisted synthesis which is low cost and also they are not producing any toxic material during the preparation of oxadiazoles [23]. Following the search through Scopus using the keywords “1,2,4-oxadiazoles,” research on 1,2,4-oxadiazoles has been expanding exponentially year by year (Fig. 1) [24].
Fig. 1.
Exponential growth of on-going research on 1,2,4-oxadiazoles retrieved by Scopus on Sept 27, 2021 using the keywords “1,2,4-oxadiazoles.” [24]
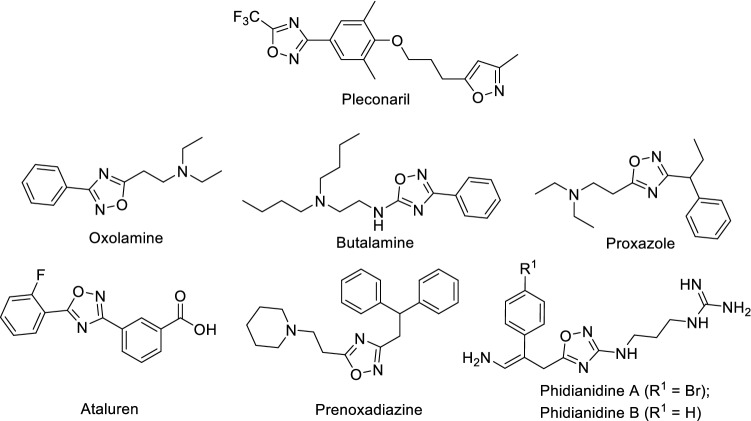
Nowadays, there are a few commercially available drugs containing 1,2,4-oxadiazole nucleus [25] such as pleconaril (anti-infective) [26], oxolamine (anti-inflammatory) [27], prenoxdiazine (cough suppressant), butalamine (vasodilator) [28], ataluren (use in Duchenne muscular dystrophy) [29] and proxazole (used for anti-tussive and anti-plasmodic) [30] (Fig. 2). Additionally, a few marine natural products such as phidianidine A and B possess 1,2,4-oxadiazole as key pharmacophoric features [31]. Apart from this, 1,2,4-oxadiazoles have been recognized as the important scaffold of biological importance [32] along with anti-cancer [33–37], anti-inflammatory [38–40], analgesic [41], anti-Alzheimer [42], monoamine oxidase-B (MAO-B) inhibitor [43] and anti-oxidant activities [44].
Fig. 2.
Chemical structures of commercial drugs and natural products based on a 1,2,4-oxadiazole scaffold
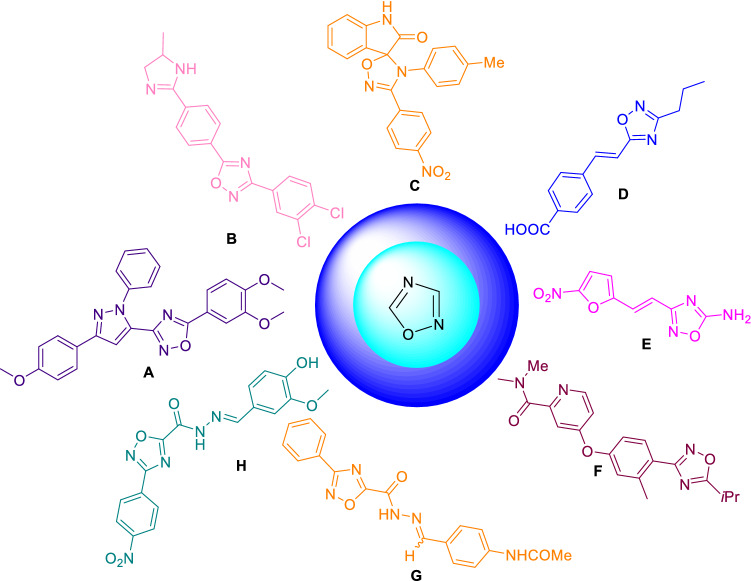
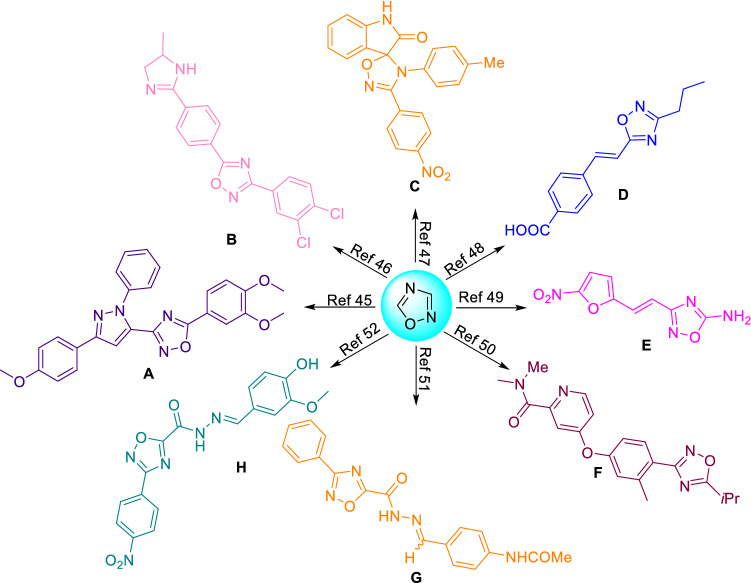
The 1,2,4-oxadiazoles (A–H) have been recognized as the scaffold of biological importance for anti-parasitic activity (Fig. 3) [45–52]. These 1,2,4-oxadiazoles have been reported as anti-fungal [45], anti-microbial [53], anti-inflammatory, anti-trypanosomal [51, 54], anti-malarial [52], anti-mycobacterial [48], and anti-viral [55]. In 2018, Lima and co-workers have reported the importance of 1,2,4-oxadiazoles as anti-parasitic agents along with other isomeric 1,3,4-oxadiazoles [56]. Recently, de Souza et al. have reported the synthetic applications of nitriles for the synthesis of 1,2,4-oxadiazoles along with 1,2,5-oxadiazoles, 1,3-oxazoles and 2-oxazolines reported during 2014–2021 [57]. To the best of our knowledge, there is no specific synthetic account on synthetic strategies for 1,2,4-oxadiazoles as anti-infective agents till date. In the present work, we made the efforts to summarize the reported synthetic strategies of 1,2,4-oxadiazoles as anti-parasitic agents with the emphasis on identified hits, and their key SARs, etc.
Fig. 3.
Scaffolds of the 1,2,4-oxadiazoles with different anti-infective activities [45–52]
Synthetic account of 1,2,4-oxadizoles with anti-infective activity
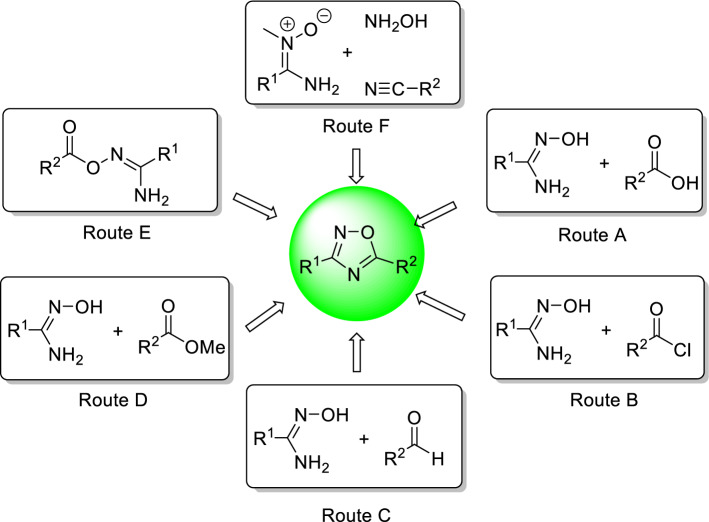
The oxadiazole scaffolds having anti-infective activity were prepared from different synthetic strategies involving the use of amidoxime with carbonyl compounds such as carboxylic acids (Route A, Fig. 4) [45, 48], aldehydes (Route B) [53], 2-chloro-2-oxoacetate (Route C) [51, 55, 58], and ester (Route D) [46]. Along with these, other routes such as intramolecular cyclization of ester clubbed with amidoxime (Route E) have been reported by Raval et al. [59] with high atom economy. Another excellent approach involving use of aminonitrone, hydroxylamine and isocyanide at rt to yield 1,2,4-oxadiazole (Route F) has been also attempted for the synthesis of anti-infective 1,2,4-oxadiazoles [60]. The present review article has been organized with the focus on the synthetic strategies using all these routes A–F.
Fig. 4.
Synthetic strategies for accessing anti-infective 1,2,4-oxadiazole scaffold
Reported synthesis of 1,2,4-oxadizoles as anti-infective agents
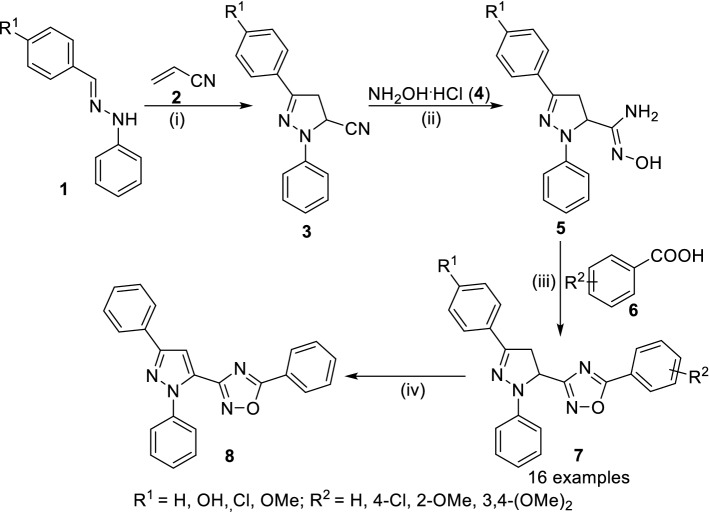
Bhadraiah et al. [45] reported 1,2,4-oxadiazoles as anti-infective agents having anti-bacterial, anti-fungal, anti-inflammatory activity. Chloramine-T mediated synthesis of N-phenyl pyrazolines was achieved by cyclocondensation of N-phenyl hydrazine (1) with acrylonitrile (2) in ethanol at reflux for 3 h. Next, 4,5-dihydro-1,3-diphenyl-1H-pyrazole-5-carbonitrile (3) was treated with hydroxyl amine hydrochloride (4) in aqueous ethanol to afford the synthesis of amidoxime (5) at reflux for 5–6 h. Next, 5 was treated with carboxylic acids (6) using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC.HCl) in dichloromethane at 0–30 °C for 6 h followed by stirring at 110 °C to synthesize 1,2,4-oxadiazoles (7, Scheme 1). Further, 7 was reacted with bromine to undergo oxidation to form aromatic pyrazoline derivative of 1,2,4-oxadiazole (8). Compounds 7 with substitutions (R1 = 4-OMe-C6H4; R2 = 3,4-(OMe)2-C6H3) were found with the best anti-microbial activity (MIC of 10 µg/mL) and 7 having R1/R2 as 4-chlorophenyl as potent anti-inflammatory activity.
Scheme 1.
Synthesis of 1,2,4-oxadiazoles (7/8) having anti-bacterial and anti-fungal activity. Conditions: (i) Chloramine-T, EtOH, reflux, 3 h; (ii) Na2CO3, aq. EtOH, reflux, 5–6 h; (iii) EDC.HCl, DCM, 0–30 °C, 1–2 h, then rt for 6 h followed by 110 °C, 6 h; (iv) Br2, DCM, reflux, 1 h
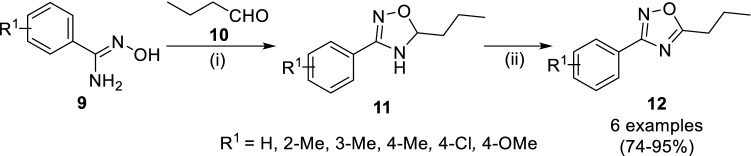
Srivastava et al. [53] reported the synthesis of 3-aryl-5-propyl-1,2,4-oxadiazole (12) by manganese dioxide mediated oxidation of 3-aryl-5-propyl-4,5-dihydro-1,2,4,-oxadiazole (11), obtained from cyclocondensation of arylamidoxamines (9) with n-butanal (10, Scheme 2). A small quantity of acidic ion-exchange resin (amberlite IRP-64) as catalyst has yielded 11 in 3 days instead of 5–8 days. Secondly, addition of sodium hypochlorite in a mixture of tetrahydrofuran and water yielded 12 (R1 = H) from 11 (R1 = H) in 89% yields. Upon the evaluation of compounds 11 against microorganisms, compounds 11 with 3-methyl and 4-methoxy substitutions were found more effective against S. aureus, M. smegmatis and C. albicans. Further, they have performed the evaluation of anti-inflammatory activity of compounds 12, however, although no compounds were found potent than aspirin during in vivo assay.
Scheme 2.
Synthetic strategy for 3-aryl-5-propyl-1,2,4,oxadiazoles (12) reported by Srivastava and co-workers. Conditions: (i) EtOH, H2O, rt, 5–8 days; (ii) MnO2, (3.8 equiv), DCM, rt, 2 h
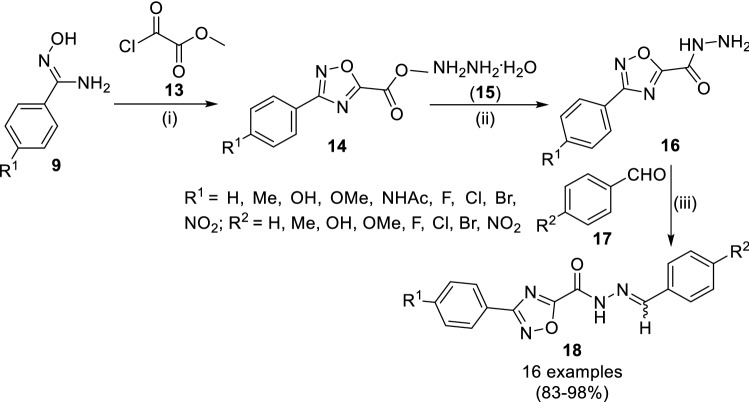
Filho et al. [51] reported the synthesis of 3-(4-susbstituted-aryl)-1,2,4-oxadiazolyl-N-acylhydrozone (18) as anti-trypanosomal agents (Scheme 3). The cyclocondensation of aryl amidoximes (9) and methyl 2-chloro-2-oxoacetate (13) under reflux in tetrahydrofuran yielded 1,2,4-oxadiazole (14) which was further treated with hydrazine hydrate (15) to get the E and Z mixture of N-acylhydrazides (16) at 0 °C for 60 min. Finally, its acid-catalyzed addition–elimination reaction with 4-substituted benzaldehydes (17) at reflux in ethanol yielded (18) in 83–98% yields. Further, they speculated the formation of E isomer as major and thermodynamically stable product considering their conformational and configurational energies rather than Z isomer. Further, they studied the probable mode of action of the synthesized compounds against Trypanosoma cruzi cysteine protease cruzain using molecular docking (PDB ID: 1U9Q) followed by evaluation of cytotoxicity and anti-trypanosomal activity. Compounds (18) with R1 = H and R2 = NHCOMe were found with the satisfactory IC50 of 3.6 µM. The evaluation of in vivo toxicity in mice revealed this compound as non-toxic to mice at the concentration of 100 mg/kg.
Scheme 3.
Synthesis of 3-(4-susbstituted-aryl)-1,2,4-oxadiazolyl-N-acylhydrozone (18) acting against T. cruzi. Conditions: (i) THF, reflux, 4.5 h; (ii) EtOH, 0 °C, 60 min; (iii) H2SO4, EtOH, rt,10 min
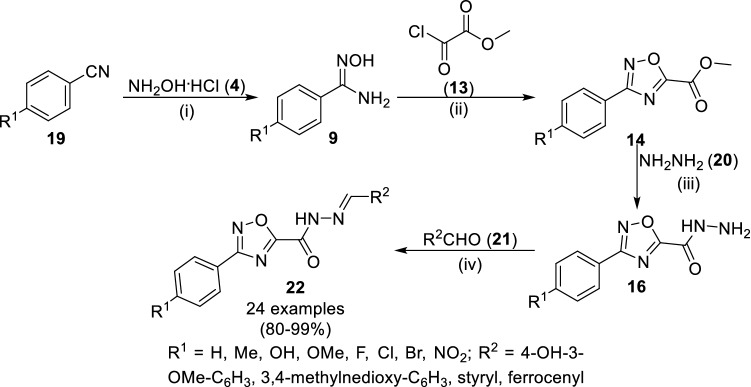
Filho et al. reported the trypanosomicidal [54] and anti-malarial [52] activity of 1,2,4-oxadiazole conjugated with N-acylhydrazone (19 and 22, Scheme 4). In 2012, they reported the treatment of 4-substituted aryl amidoximes (9) with methyl 2-chloro-2-oxoacetate (13) at reflux for 4–4.5 h to synthesize 1,2,4-oxadiazoles (14) in good yields. This was followed by their sequential reactions with hydrazine (20) and aldehydes (21) to yield 3,5-disubstitute 1,2,4-oxadiazoles (22) in 80–99% yields. Compounds 22 with R2 as methylene 3,4-methylenedioxybenzene substitutions having R1 as chloro and fluoro substitutions were found with potent IC50 of 9.5 ± 2.8 and 3.5 ± 1.8 µM, respectively, against T. cruzi [54]. The similar synthetic methodology was adopted in 2016 by them for the synthesis of 1,2,4-oxadiazoles (22) as anti-malarial compounds by using styryl and ferrocene carboxaldehydes (21) [52]. Herein, the H2SO4 catalysts did not afford the product (22) with ferrocene carboxaldehyde, so the alternative Lewis acid catalysts, cerium chloride (CeCl3.7H2O) was tried to yield thermodynamically stable E-isomers (22). These anti-malarial compounds, synthesized in the laboratory of design and synthesis applied to medicinal chemistry-SintMed,® inhibited the formation of β-hematin, which has been recognized as the mechanism of quinoline-based anti-malarial β-hematin inhibitors. Despite of diminished parasitemia, the most active compound 22 (R1 = NO2; R2 = 4–OH–3–OMe–C6H3, with 69% inhibition of chloroquine-resistant P. falciparum W2 strain) did not show any improvement in survival of Plasmodium berghei-infected mice during the in vivo studies as compared to chloroquine, marketed anti-malarial drug.
Scheme 4.
Synthetic strategy of 3,5-disubstituted oxadiazoles (16 and 22) as trypanosomicidal and anti-malarial agents. Conditions: (i) Na2CO3, H2O/MeOH, 4 h, reflux; (ii) dry THF, reflux, 4–4.5 h; (iii) EtOH, 0 °C, 2 h; (iv) H2SO4 or CeCl3.7H2O (10 mol%), EtOH, 40 °C,10–30 min
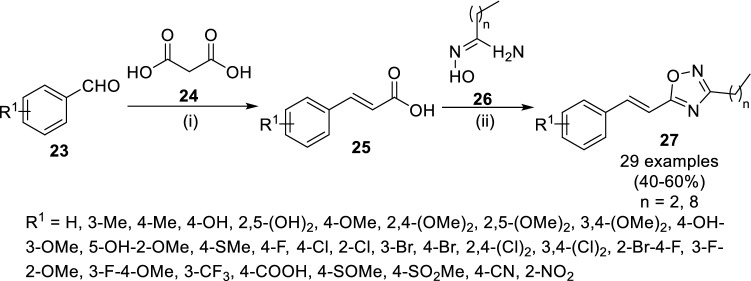
Several nitrogen- and oxygen-containing scaffolds have been found of much interest due to their potential as anti-mycobacterial agents [15, 17, 18, 61–63]. In this regard, Upare and coworkers reported the anti-tubercular activity of (E)-3-alkyl-5-styryl 1,2,4-oxadiazoles [48]. The treatment of benzaldehyde (23) with malonic acid (24) in the presence of catalytic amount of piperidine at 110 °C yielded cinnamic acid (25), which was reacted with amidoxime (26, Scheme 5) using carbonyl diimidazoles (CDI) in toluene to obtain 3,5-disubstituted 1,2,4-oxadiazole derivatives (27). Following the SAR studies of 1,2,4-oxadiazoles with different functional groups on the phenyl ring of cinnamic acid, electron-withdrawing groups and halogens were found with good anti-tubercular activity in comparison with electron-donating and bulky substituents. Whereas the chain length and bulkiness at 3rd position of 1,2,4-oxadiazoles increased anti-mycobacterial potential. The compound 27 (R1 = COOH; n = 2) was reported with promising anti-TB activity against M. tuberculosis H37Ra having higher anti-TB activity with IC50 of 0.63 and MIC of 8.45 µg/mL after 12 days of incubation. Further, the molecular modeling studies against InhA revealed their binding mode in the active site of enoyl-ACP reductase InhA.
Scheme 5.
Synthesis strategy of (E)-3 alkyl-5-styryl-1,2,4-oxadiazoles (27) as anti-tubercular agents adopted by Upare et al. Conditions: (i) piperidine, pyridine, 110 °C,10–12 h; (ii) CDI, PhMe, rt, 3–4 h followed by 100–110 °C, 5–6 h
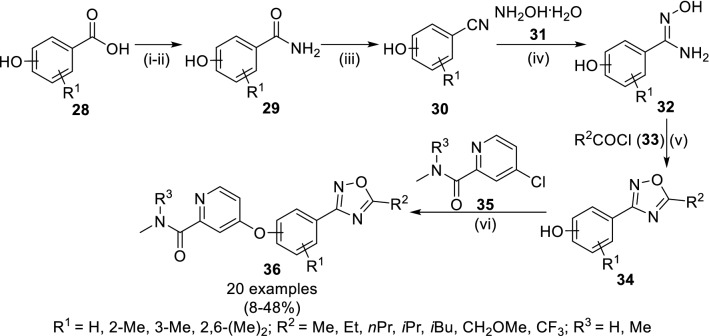
Jung and coworkers have designed the 3-aryl-1,2,4-oxadiazoles (36) [55] having activity potential against human rhinovirus from their previously identified 2,6-disubstituted benzo[d]thiophene derivative [50]. The conversion of hydroxy benzoic acid 28 into acid chloride and benzamide intermediate (29) was followed cyanation of 29 using trifluoracetic anhydride (TFAA) in pyridine to yield hydroxy benzonitriles (30, Scheme 6). The synthetic target picolinamide clubbed 3-phenyl-1,2,4-oxadiazoles (33) was obtained by treatment of 30 with hydroxyl amine hydrate (31) to get amidoxime (32) followed by cyclization using acyl chloride (33) and O-arylation using 4-chloro-N-methylpicolinamide (35). The biological investigation of compounds 36 against rat and human liver revealed compound 36 (R1 = 3-Me, R2 = iPr, R3 = Me) with highest stability under hepatic microsomal conditions (with 60 and 41% of compound being unmetabolized, respectively). Further, it showed the significant efficacy against human rhinovirus (hRV-A71, hRV-B14 and hRV-A21) with better inhibitory concentration (EC50 of 3.7, 66.0 and 22.0 nM, respectively). However, in vivo pharmacokinetic analysis of this compound in rats resulted in low systemic clearance with moderate oral bioavailability.
Scheme 6.
Strategy for the multi-step synthesis of phenyl oxadiazole compound (36) acting against human rhinovirus. Conditions: (i) SOCl2, DMF, 70 °C; (ii) NH3, H2O, THF, 0–25 °C, 5 h; (iii) TFAA, pyridine, 16 h; (iv) EtOH, 90 °C,16 h; (v) TFAA, pyridine, 0–120 °C, 16 h; (vi) neat, 150 °C, 90 h
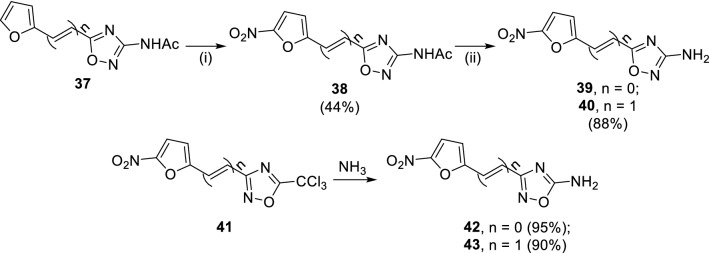
In 1969, Breuer reported that 5-nitrofuryl substituted 3-amino-1,2,4-oxadiazoles and 5-amino-1,2,4-oxadiazoles with antimicrobial activity [49]. The furyl oxadiazoles (37) upon nitration with nitric acid and acetic anhydride provided 5-nitrofuryl derivatives (38, Scheme 7), which could be cleaved by warm ethanolic HCl to afford the target compounds (39/40), wherein compound 41 was found with broad anti-microbial spectrum. The reaction of trichloromethyl substituted 1,2,4-oxadiazoles (41) with NH3 yielded 3-substituted 5-amino 1,2,4-oxadiazoles (42/43). Upon its biological evaluation, the compound 43 was found to be the most potent antimicrobial compound having minimum inhibitory concentration (MIC) against various microorganism Staphylococcus aureus (0.15 µg/mL), Salmonella schottmulleri (0.05 µg/ml), Pseudomonas aeruginosa (7.8 µg/mL), Proteus vulgaris (9.4 µg/mL), Escherichia coli (0.05 µg/mL), Candida albicans (12.5 µg/mL), Trichophyton mentagrophytes (6.3 µg/mL) and Fusarium bulbilgenum (12.5 µg/mL). Against Mycobacterium tuberculosis, compound (43) was found to be most effective with MIC of 6.3 µg/mL among all other 1,2,4-oxadiazoles.
Scheme 7.
Synthesis of nitrofuryl substituted 3-amino-1,2,4-oxadiazoles (39/40) and 5-amino-1,2,4-oxadiazoles (42/43) having potent anti-microbial activity. Conditions: (i) HNO3, Ac2O, 15 °C, 30 min; (ii) 10% HCl (ethanolic), 3 h
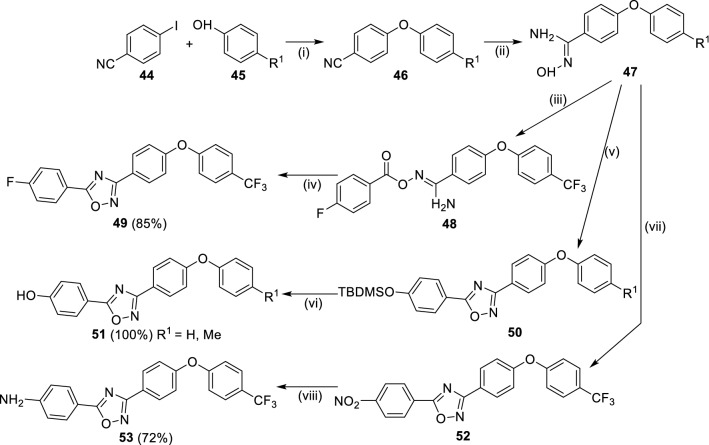
Mobashery et al. reported the use of 1,2,4-oxadiazole (Scheme 8) class of non-β-lactam antibiotics having activity towards Gram-positive bacteria along with good bioavailability [64]. Through in silico screening of 1.2 million compounds from ZINC database against penicillin-binding protein 2a (PBP2a), compound 49 has shown good MIC against S. aureus and E. faecium with better reproducibility. From the synthesized 370 structural derivatives of 49, compounds 52 and 53 were found most effective against the panel of clinical microorganism. Further, they demonstrated bactericidal activity against vancomycin and linezolid resistant MRSA along with other strains of bacterial. The synthesis of compound 49 was achieved by the reaction of 4-iodobenzonitrile (44) with 4-substituted phenol (45) in the presence of Cul and dicaesium carbonate at 90 °C followed by amidoxime formation using di-isopropyl ethyl amine (DIPEA) in THF and cyclocondensation to yield 1,2,4-oxadiazole using tetrabutylammonium fluoride (TBAF) in tetrahydrofuran (THF). To synthesize, intermediate 47 was reacted with 4-tert-butyl dimethyl silyl benzoyl chloride at 120 °C in toluene to yield 50 followed by desilylation using TBAF to yield 51. In a similar fashion, 53 was obtained by treatment of 52 with 4-nitrobenzoyl chloride in toluene under reflux followed by catalytic reduction of nitro functional group to its amino counterpart (53). Further, they have studied these compounds through in vivo mouse model and revealed 100% oral bioavailability.
Scheme 8.
Synthesis of 1,2,4-oxadiazoles (49/51/53) as non-β-lactam inhibitors. Conditions: (i) Cul, Cs2CO3, N,N-dimethyl, glycine HCl, 1,4-dioxane, 90 °C; (ii) NH2OH, EtOH, reflux, 3 h; (iii) DIPEA, DCM, 0 °C—> rt; (iv) TBAF, THF, rt, 24 h; (v) 4-TBDMS-BzCl, PhMe, 120 °C, overnight; (vi) TBAF, (1 equiv), THF, < 1 min; (vii) 4-NO2-BzCl, PhMe, reflux, 5.5 h; (viii) Fe, H2O, HCl, EtOH, 95 °C, 2 h
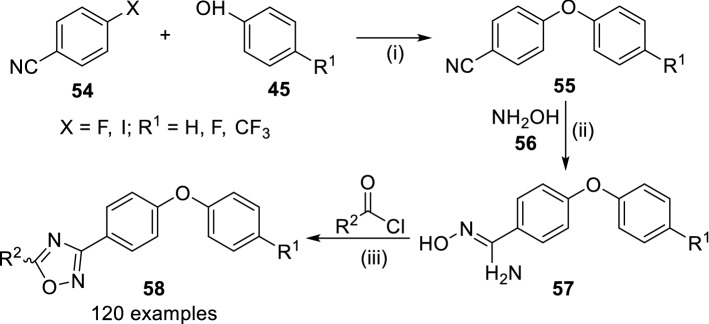
In continuation of their efforts for exploration of SAR of 1,2,4-oxadiazoles [65], they reported the use of about 120 synthetic oxadiazole derivatives (52) as antibiotics acting against several microorganisms and established their structure–activity relationships (Scheme 9) [66]. The reaction of 4-fluro or 4-iodobenzonitrile (54) with 4-substituted phenol derivatives (45) via nucleophilic aromatic substitution or Ullmann coupling resulted in the formation of 4,4’-diphenyl ether (55), which upon the treatment with hydroxylamine (56) in ethanol at reflux followed by cyclization with acyl chloride (obtained by reacting substituted benzoic acids or heteroaromatic carboxylic acids with oxalyl chloride or thionyl chloride) in pyridine/toluene/1,4-dioxane to obtain 1,2,4-oxadiazoles (58). The para position substitution (R1) improved metabolic stability and diminished the rate of excretion. The synthesized oxadiazoles have been found active against gram-positive bacteria including drug sensitive and methicillin/vancomycin/linezolid-resistant S. aureus. Replacement of pyrazole with indole broadened the spectrum against gram-positive organisms. The highest activity (MIC of 4 µg/mL) was observed for antibiotic 58 having indol-5-yl (R2) and 4-trifluoromethyl substitutions against S. aureus ATCC. The same compound was also found effective against in vivo mouse peritonitis model of infection with better pharmacokinetic and bioavailability profile. Other 4-trifluoromethyl (R1) substituted antibiotics with 4-N-isopropyl amino-pyrazol-5-yl (R2, MIC of 0.5 µg/mL) and 4-ethynyl-pyrazol-5-yl (R2, MIC of 0.25 µg/mL) showed good anti-bacterial activity with the highest toxicity towards mammalian cells. However, these potent antibiotics were found ineffective against other microbial strains.
Scheme 9.
Structure activity relationship of 1,2,4-oxadiazole antibiotics 58. Conditions: (i) K2CO3, DMF, 60–100 °C or Cul, Cs2CO3, N,N-dimethylglycine.HCl 1,4-dioxane, 90 °C; (ii) NH2OH, EtOH and (iii) pyridine/toluene/1,4-dioxane
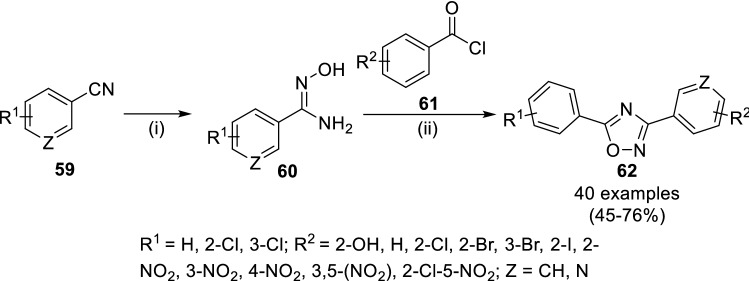
Aguiar and colleagues evaluated the antimicrobial properties of 3,5-diphenyl or 3-pyridino-5-phenyl 1,2,4-oxadiazoles (62) [58]. These derivatives were synthesized (Scheme 10) by cyclization of amidoxime derivatives (60), synthesized from hydroxylamine hydrochloride and anhydrous sodium carbonate with acyl chloride (61, obtained from corresponding substituted carboxylic acid and thionyl chloride). Upon the biological evaluation of all the synthesized compounds against E. coli, P. aeruginosa, S. aureus, P. mirabilis, E. faecalis, the compounds 62a (R1 = 4-Cl, R2 = 2-NO2, Z = C), 62b (R1 = 4-Cl, R2 = 4-NO2, Z = C) and 62c (R1 = 4-Cl, R2 = 2-Cl-5-NO2, Z = C) have been found with the best anti-bacterial activity. The compounds with nitro as electron-donating group have shown potent anti-bacterial activity. Further, they anticipated the mode of action of these active hits through inhibition of promising enzyme of citric acid cycle through free radical formation involving reduction of nitro to amino counterparts via enzymatic bio-reduction approach.
Scheme 10.
Synthesis of 1,2,4-oxadiazole derivatives (62) having anti-microbial property. Conditions: (i) EtOH, H2O, reflux, 5 h and (ii) PhMe, reflux, 5 h
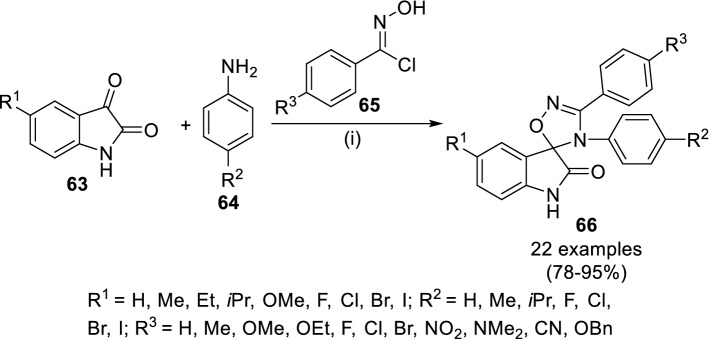
Du et al. [47] synthesized 3′,4′-diaryl-4′H-spiro[indoline-3,5′-[1′,2′,4’]oxadiazol]-2-ones (66) having antibacterial activity (Scheme 11) by the reaction of isatin (63) and aniline (64) in ethanol at rt followed by 1,3-dipolar cycloaddition of the intermediates with N-hydroxybenzimidoyl chloride (65) in the presence of catalytic amount of DMAP (10 mol%) at rt for 3 h. Compound 66 (R1 = H; R2 = Me; R3 = NO2) was found to be most effective against S. epidermidis, wherein the anti-bacterial activity could be attributed to the electron-withdrawing group on phenyl ring. However, none of the compounds were found more effective than the anti-bacterial drug, levofloxacin against Staphylococcus epidermidis, Escherichia coli, Staphylococcus aureus and Klebsiella pneumoniae.
Scheme 11.
Synthesis of 3′,4′-diaryl-4′H-spiro[indoline-3,5′-[1′,2′,4′]oxadiazol]-2-ones (66) via DMAP-catalyzed domino reaction. Conditions: (i) EtOH, rt, 3 h, DMAP (10 mol%), rt, 3 h
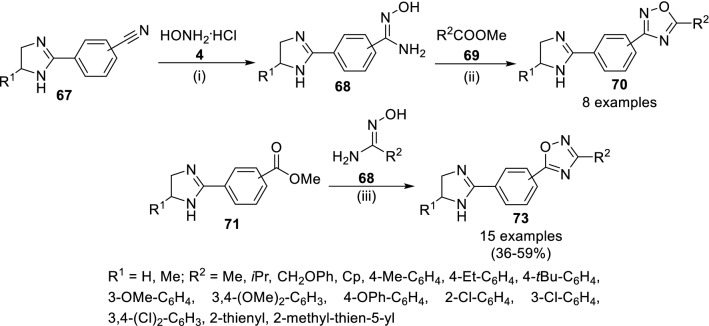
The synthetic strategy of 1,2,4-oxadiazole hybrids as anti-bacterial agents acting against both gram-positive and gram-negative bacteria has been presented in Scheme 12 reported by Baykov et al. [46] The substituted 2-imidazoline (67) on reaction with hydroxylamine hydrochloride (4) in alkaline ethanolic medium at 80 °C formed N-aryl amidoxime (68), which was reacted with methyl carboxylate (69) in DMSO added with powdered caustic soda to yield 70. In the reverse way, treatment of N-aryl methyl ester (71) and N-alkyl amidoxime (68) yielded 1,2,4-oxadiazoles. Compound 70 being p-phenylene derivative with R1 = Me, R2 = 3,4-(Cl)2-C6H3 resulted in most potent compound having MIC in 8–16 µg/mL where bacteriostatic property has been also observed with p-phenylene derivative 73 in 8–32 µg/mL (R1 = H, R2 = 3-Cl-C6H4 and R1 = Me, R2 = 4-Me-C6H4).
Scheme 12.
Synthesis of 1,2,4-oxadiazoles (73) from N-aryl amidoxime (72) and alkyl ester (71). Conditions: (i) NaHCO3, EtOH, 80 °C, 6 h, (ii) NaOH, DMSO, rt; (iii) NaOH, DMSO, rt, 4 h
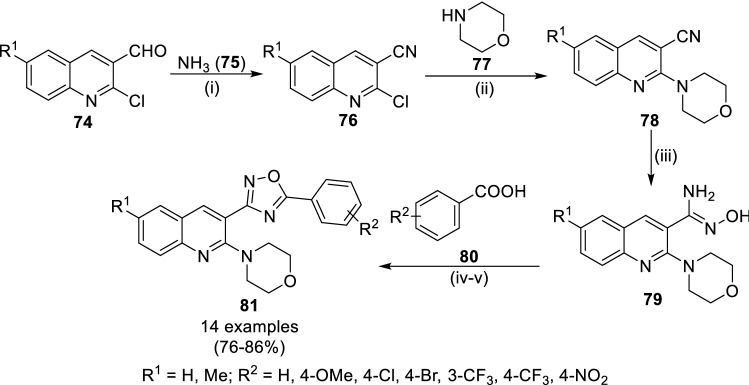
In 2017, Raval and co-workers have synthesized novel 2-morpholinoquinoline nucleus appended 1,2,4-oxadiazoles having antimicrobial and antifungal activity (Scheme 13) [59]. The treatment of 2-chloroquinoline-3-carbaldehydes (74) in THF (30%) with aqueous ammonia (75) and sublimed iodine at rt for 30 min yielded 2-chloro-6-substituted quinoline-3-carbonitrile (76). The next reaction of 76 with morpholine (77) in presence of K2CO3 in DMF followed by reaction with hydroxylamine hydrochloride in alkaline mixture of ethanol:water (7:3) to synthesize amidoximes (79). Finally, the condensation of aromatic acids (80) in dichloromethane using coupling reagent ethyl-(N,N′-dimethylamino)propylcarbodiimide hydrochloride (EDC.HCl) under nitrogen atmosphere to obtain the target compounds, 1,2,4-oxadiazoles (81). All the target compounds were evaluated for assessment of drug likeness using Lipinski’s rule of five indicating their druggability. The evaluation of anti-microbial potential of final compounds revealed compounds 81a-d (R1 = Me and R2 = 4-NO2, 4-Cl, 4-CF3, 3-CF3, respectively) as most potent compounds against gram-positive and gram-negative microbial and compounds fungal strains having better potency than ampicillin and griseofulvin. The presence of electron-withdrawing groups such nitro and trifluoromethyl on 5-phenyl ring have increased the anti-infective potential of the 1,2,4-oxadiazoles.
Scheme 13.
Synthesis of 2-morpholinoquinoline integrated 1,2,4-oxadiazole scaffolds (81). Conditions: (i) I2, THF, rt, 30 min; (ii) DMF, K2CO3, reflux, 2 h; (iii) NH2OH.HCl, Na2CO3, EtOH, reflux, 5 h and (iv) EDC.HCl, DCM, rt, 30 min then reflux, 2 h; (v) NaOAc, EtOH, reflux, 3 h
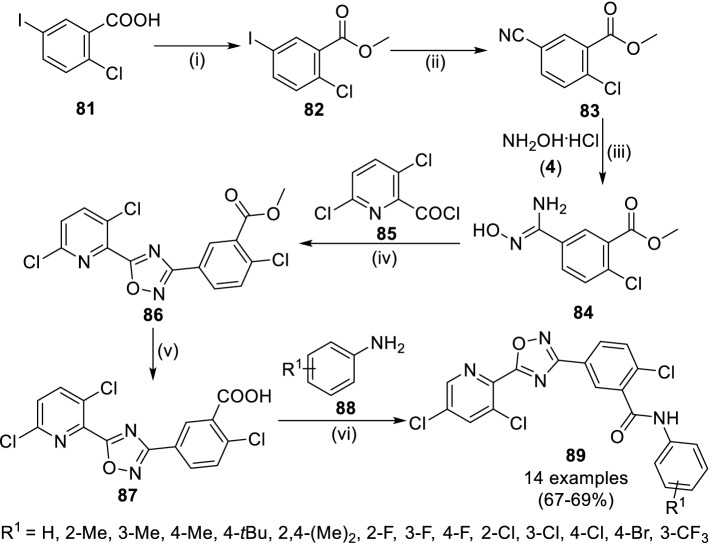
Recently, Tan et al. reported the synthesis of benzamide coupled pyridine anchored 1,2,4-oxadiazoles (89) as anti-fungal agents (Scheme 14) [67]. The esterification of 2-chloro-5-iodobenzoic acid (81) was achieved in methanol at reflux followed by cyanation using copper cyanide in the presence of L-proline and treatment with hydroxyl amine hydrochloride (4) yielded benzamidoxime (84). Compound 84 was further reacted with 3,6-dichloropicolinoyl chloride (85) to 2-chloro-5-(5-(3,6-dichloropyridin-2-yl)-1,2,4-oxadiazol-3-yl)benzoate (86) via cyclization followed by alkali catalyzed hydrolysis to yield carboxylic acid (87). The amidation reaction of 86 with substituted aniline (88) at 0 °C for 7 h yielded (89). All the synthetic targets were evaluated through in vitro mycelia growth inhibition method against fungal strains including Alternaria solani, FusaHum graminearum, Cercospora arachidicola, Phytophthora capsica, Sclerotinia sclerotiorum, Botrytis cinereal, Thanatephorus cucumeris and Fusarium oxysporum and these results were compared with fluxapyroxad, a broad-spectrum fungicide. Compounds 87 (R1 = 2-F) showed the good inhibitory activity (90.5%) against Botrytis cinereal, respectively.
Scheme 14.
Synthetic scheme for 3,5-disusbtituted 1,2,4-oxadiazoles (89) as anti-fungal agents. Conditions: (i) MeOH, reflux, 8 h; (ii) CuCN, L-proline, DMF, 11 h; (iii) EtOH, rt; (iv) PhMe; (v) NaOH, THF, 2 h; (vi) 0 °C, 8 h
Kukushkin and coworkers reported 5-amino-1,2,4-oxadiazoles (93) with higher anti-bacterial activity against multidrug-resistant strains such as Staphylococcus aureus and Klebsiella pneumonia (Scheme 15) [60]. These were prepared from different synthetic strategies involving the use of aminonitrone (90), hydroxylamine (91) and isocyanide (92) at rt in 29–86% yields. However, none of the compounds were found superior in activity as compared to ciprofloxacin, the currently marketed anti-bacterial drug.
Scheme 15.
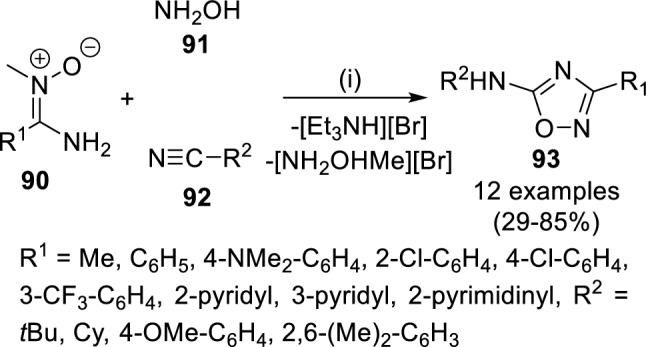
Synthesis of 1,2,4-oxadiazoles (93) active as anti-bacterial agents. Conditions: (i) Br2, Et3N, CHCl3, MeOH, rt, 10 min
Summary and future directions
In a nutshell, the synthesis of 1,2,4-oxadiazoles has been recognized as the key pharmacophore for broad-spectrum anti-infective or anti-microbial activities including anti-fungal, anti-bacterial, anti-trypanosomal, anti-malarial, anti-tubercular and anti-viral activities. In general, these scaffolds can be synthesized from amidoxime (obtained from reaction of nitrile and hydroxylamine) with carbonyl derivatives such as acyl chloride, ester, carboxylic acid or isatin. Mechanistically, the reaction of amidoxime with aldehyde yields the 1,2,4-oxadiazoline, which can be oxidized to 1,2,4-oxadiazole with the stoichiometric assistance of oxidizing agents. The objective behind the present work has been set to create the spotlight around 1,2,4-oxadiazole considering their synthetic strategies and usefulness as anti-parasitic activity for the benefit of medicinal chemists to design new scaffolds with potential activity of interest.
Abbreviations
- CDI
Carbonyl diimidazole
- DIPEA
Di-isopropyl ethyl amine
- DMAP
Dimethylaminopyridine
- DMF
Dimethylformamide
- DMSO
Dimethylsulfoxide
- EDC.HCl
(3-Dimethylaminopropyl)carbodiimide hydrochloride
- EtOH
Ethanol
- SARS CoV-2
Severe acute respiratory syndrome coronavirus-2
- TBAF
Tetra-n-Butyl ammonium fluoride
- TBDMS
tert-Butyl dimethylsilyl
- TFAA
Trifluoroacetic anhydride
- THF
Tetrahydrofuran
Authors Contribution
SJC Literature review, Writing-Original draft and revised manuscript preparation, Reviewing and Editing; TMP Writing-Original draft preparation; BPD Writing-Original draft preparation; TMD Conceptualization, Supervision, Writing- Original draft and revised manuscript preparation, Reviewing and Editing.
Declarations
Conflict of interest
The authors declare no competing financial interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jadhavar PS, Vaja MD, Dhameliya TM, Chakraborti AK. Oxazolidinones as anti-tubercular agents: Discovery, development and future perspectives. Curr Med Chem. 2015;22:4379–4397. doi: 10.2174/0929867323666151106125759. [DOI] [PubMed] [Google Scholar]
- 2.Global Tuberculosis Report 2021. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2021. Accessed 27 Oct 2021
- 3.Malaria. https://www.who.int/news-room/fact-sheets/detail/malaria. Accessed 15 Oct 2021
- 4.Barrett MP, Burchmore RJS, Stich A, et al. The trypanosomiases. Lancet. 2003;362:1469–1480. doi: 10.1016/S0140-6736(03)14694-6. [DOI] [PubMed] [Google Scholar]
- 5.Ryu S, Song PI, Seo CH, et al. Colonization and infection of the skin by S. aureus: immune system evasion and the response to cationic antimicrobial peptides. Int J Mol Sci. 2014;15:8753–8772. doi: 10.3390/ijms15058753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashour HM, Elkhatib WF, Rahman MM, Elshabrawy HA. Insights into the recent 2019 novel coronavirus (SARS-CoV-2) in light of past human coronavirus outbreaks. Pathogens. 2020;9:e186. doi: 10.3390/pathogens9030186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gajjar ND, Dhameliya TM, Shah GB. In search of RdRp and Mpro inhibitors against SARS CoV-2: molecular docking, molecular dynamic simulations and ADMET analysis. J Mol Struct. 2021;1239:130488. doi: 10.1016/j.molstruc.2021.130488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagar PR, Gajjar ND, Dhameliya TM. In search of SARS CoV-2 replication inhibitors: virtual screening, molecular dynamics simulations and ADMET analysis. J Mol Struct. 2021;1246:131190. doi: 10.1016/j.molstruc.2021.131190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikaido H. Multidrug resistance in bacteria. Annu Rev Biochem. 2009;78:119–146. doi: 10.1146/annurev.biochem.78.082907.145923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verma T, Aggarwal A, Singh S, et al. Current challenges and advancements towards discovery and resistance of antibiotics. J Mol Struct. 2022;1248:131380. doi: 10.1016/j.molstruc.2021.131380. [DOI] [Google Scholar]
- 11.Dhameliya TM, Chourasiya SS, Mishra E, et al. Rationalization of benzazole-2-carboxylate versus benzazine-3-one/benzazine-2,3-dione selectivity switch during cyclocondensation of 2-aminothiophenols/phenols/anilines with 1,2-biselectrophiles in aqueous medium. J Org Chem. 2017;82:10077–10091. doi: 10.1021/acs.joc.7b01548. [DOI] [PubMed] [Google Scholar]
- 12.Bhakhar KA, Sureja DK, Dhameliya TM. Synthetic account of indoles in search of potential anti-mycobacterial agents: a review and future insights. J Mol Struct. 2022;1248:131522. doi: 10.1016/j.molstruc.2021.131522. [DOI] [Google Scholar]
- 13.Dhameliya TM, Donga HA, Vaghela PV, et al. A decennary update on applications of metal nanoparticles (MNPs) in the synthesis of nitrogen- and oxygen-containing heterocyclic scaffolds. RSC Adv. 2020;10:32740–32820. doi: 10.1039/D0RA02272A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Othman AA, Kihel M, Amara S. 1,3,4-Oxadiazole, 1,3,4-thiadiazole and 1,2,4-triazole derivatives as potential antibacterial agents. Arab J Chem. 2019;12:1660–1675. doi: 10.1016/j.arabjc.2014.09.003. [DOI] [Google Scholar]
- 15.Dhameliya TM, Bhakhar KA, Gajjar ND, et al. Recent advancements and developments in search of anti-tuberculosis agents: a quinquennial update and future directions. J Mol Struct. 2022;1248:131473. doi: 10.1016/j.molstruc.2021.131473. [DOI] [Google Scholar]
- 16.Kerru N, Gummidi L, Maddila S, et al. A review on recent advances in nitrogen-containing molecules and their biological applications. Molecules. 2020;25:1909. doi: 10.3390/molecules25081909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jadhavar PS, Patel KI, Dhameliya TM, et al. Benzimidazoquinazolines as new potent anti-TB chemotypes: design, synthesis, and biological evaluation. Bioorg Chem. 2020;99:103774. doi: 10.1016/j.bioorg.2020.103774. [DOI] [PubMed] [Google Scholar]
- 18.Dhameliya TM, Patel KI, Tiwari R, et al. Design, synthesis, and biological evaluation of benzo[d]imidazole-2-carboxamides as new anti-TB agents. Bioorg Chem. 2021;107:104538. doi: 10.1016/j.bioorg.2020.104538. [DOI] [PubMed] [Google Scholar]
- 19.Boström J, Hogner A, Llinàs A, et al. Oxadiazoles in medicinal chemistry. J Med Chem. 2012;55:1817–1830. doi: 10.1021/jm2013248. [DOI] [PubMed] [Google Scholar]
- 20.Nobeli I, Price SL, Lommerse JPM, Taylor R. Hydrogen bonding properties of oxygen and nitrogen acceptors in aromatic heterocycles. J Comput Chem. 1997;18:2060–2074. doi: 10.1002/(SICI)1096-987X(199712)18:16<2060::AID-JCC10>3.0.CO;2-S. [DOI] [Google Scholar]
- 21.Patani GA, LaVoie EJ. Bioisosterism: a rational approach in drug design. Chem Rev. 1996;96:3147–3176. doi: 10.1021/cr950066q. [DOI] [PubMed] [Google Scholar]
- 22.Salahuddin MA, Yar MS, et al. Updates on synthesis and biological activities of 1,3,4-oxadiazole: a review. Synth Commun. 2017;47:1805–1847. doi: 10.1080/00397911.2017.1360911. [DOI] [Google Scholar]
- 23.Banik BK, Sahoo BM, Kumar BVVR, et al. Green synthetic approach: an efficient eco-friendly tool for synthesis of biologically active oxadiazole derivatives. Molecules. 2021;26:1163. doi: 10.3390/molecules26041163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scopus. https://www.scopus.com/. Accessed 27 Sep 2021
- 25.Biernacki K, Daśko M, Ciupak O, et al. Novel 1,2,4-oxadiazole derivatives in drug discovery. Pharmaceuticals. 2020;13:111. doi: 10.3390/ph13060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pevear DC, Tull TM, Seipel ME, Groarke JM. Activity of pleconaril against enteroviruses. Antimicrob Agents Chemother. 1999;43:2109–2115. doi: 10.1128/AAC.43.9.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tadikonda R, Nakka M, Gajula MB, et al. Efficient and convenient protocol for the synthesis of 3,5-disubstituted 1,2,4-oxadiazoles using HClO4-SiO2 as a heterogeneous recyclable catalyst. Synth Commun. 2014;44:1978–1986. doi: 10.1080/00397911.2014.883633. [DOI] [Google Scholar]
- 28.Coupar IM, Hedges A, Metcalfe HL, Turner P. Effect of aminophylline, butalamine and imolamine on human isolated smooth muscle. J Pharm Pharmacol. 1969;21:474–475. doi: 10.1111/j.2042-7158.1969.tb08294.x. [DOI] [PubMed] [Google Scholar]
- 29.Ryan NJ. Ataluren: First global approval. Drugs. 2014;74:1709–1714. doi: 10.1007/s40265-014-0287-4. [DOI] [PubMed] [Google Scholar]
- 30.Gaetani E, Laureri C, Vitto M. GC-ITD detection and quantitative analysis of Proxazole in cows’ plasma and milk. J Pharm Biomed Anal. 1995;13:335–337. doi: 10.1016/0731-7085(95)01283-Q. [DOI] [PubMed] [Google Scholar]
- 31.Carbone M, Li Y, Irace C, et al. Structure and cytotoxicity of phidianidines A and B: first finding of 1,2,4-oxadiazole system in a marine natural product. Org Lett. 2011;13:2516–2519. doi: 10.1021/ol200234r. [DOI] [PubMed] [Google Scholar]
- 32.Bora OR, Dar B, Pradhan V, Farooqui M. [1,2,4]-Oxadiazoles: synthesis and biological applications. Mini Rev Med Chem. 2014;14:355–369. doi: 10.2174/1389557514666140329200745. [DOI] [PubMed] [Google Scholar]
- 33.Shamsi F, Hasan P, Queen A, et al. Synthesis and SAR studies of novel 1,2,4-oxadiazole-sulfonamide based compounds as potential anticancer agents for colorectal cancer therapy. Bioorg Chem. 2020;98:103754. doi: 10.1016/j.bioorg.2020.103754. [DOI] [PubMed] [Google Scholar]
- 34.Loboda KB, Valjavec K, Štampar M, et al. Design and synthesis of 3,5-substituted 1,2,4-oxadiazoles as catalytic inhibitors of human DNA topoisomerase IIα. Bioorg Chem. 2020;99:103828. doi: 10.1016/j.bioorg.2020.103828. [DOI] [PubMed] [Google Scholar]
- 35.Abdelhameid MK, Mohammed MR. Design, synthesis, and cytotoxicity screening of 5-aryl-3-(2-(pyrrolyl) thiophenyl)-1,2,4-oxadiazoles as potential antitumor molecules on breast cancer MCF-7 cells. Bioorg Chem. 2019;86:609–623. doi: 10.1016/j.bioorg.2019.01.067. [DOI] [PubMed] [Google Scholar]
- 36.Caneschi W, Enes KB, Carvalho de Mendonça C, et al. Synthesis and anticancer evaluation of new lipophilic 1,2,4 and 1,3,4-oxadiazoles. Eur J Med Chem. 2019;165:18–30. doi: 10.1016/j.ejmech.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Moniot S, Forgione M, Lucidi A, et al. Development of 1,2,4-oxadiazoles as potent and selective inhibitors of the human deacetylase sirtuin 2: structure-activity relationship, X-ray crystal structure, and anticancer activity. J Med Chem. 2017;60:2344–2360. doi: 10.1021/acs.jmedchem.6b01609. [DOI] [PubMed] [Google Scholar]
- 38.Mohamed MFA, Marzouk AA, Nafady A, et al. Design, synthesis and molecular modeling of novel aryl carboximidamides and 3-aryl-1,2,4-oxadiazoles derived from indomethacin as potent anti-inflammatory iNOS/PGE2 inhibitors. Bioorg Chem. 2020;105:104439. doi: 10.1016/j.bioorg.2020.104439. [DOI] [PubMed] [Google Scholar]
- 39.Youssif BGM, Mohamed MFA, Al-Sanea MM, et al. Novel aryl carboximidamide and 3-aryl-1,2,4-oxadiazole analogues of naproxen as dual selective COX-2/15-LOX inhibitors: design, synthesis and docking studies. Bioorg Chem. 2019;85:577–584. doi: 10.1016/j.bioorg.2019.02.043. [DOI] [PubMed] [Google Scholar]
- 40.Yatam S, Gundla R, Jadav SS, et al. Focused library design and synthesis of 2-mercapto benzothiazole linked 1,2,4-oxadiazoles as COX-2/5-LOX inhibitors. J Mol Struct. 2018;1159:193–204. doi: 10.1016/j.molstruc.2018.01.060. [DOI] [Google Scholar]
- 41.Chawla G. 1,2,4-Oxadiazole as a privileged scaffold for anti-inflammatory and analgesic activities: a review. Mini Rev Med Chem. 2018;18:1536–1547. doi: 10.2174/1389557518666180524112050. [DOI] [PubMed] [Google Scholar]
- 42.Wang M, Liu T, Chen S, et al. Design and synthesis of 3-(4-pyridyl)-5-(4-sulfamido-phenyl)-1,2,4-oxadiazole derivatives as novel GSK-3β inhibitors and evaluation of their potential as multifunctional anti-Alzheimer agents. Eur J Med Chem. 2021;209:112874. doi: 10.1016/j.ejmech.2020.112874. [DOI] [PubMed] [Google Scholar]
- 43.Shetnev A, Osipyan A, Baykov S, et al. Novel monoamine oxidase inhibitors based on the privileged 2-imidazoline molecular framework. Bioorg Med Chem Lett. 2019;29:40–46. doi: 10.1016/j.bmcl.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 44.Brahmi J, Ghannay S, Bakari S, et al. Unprecedented stereoselective synthesis of 3-methylisoxazolidine-5-aryl-1,2,4-oxadiazoles via 1,3-dipolar cycloaddition and study of their in vitro antioxidant activity. Synth Commun. 2016;46:2037–2044. doi: 10.1080/00397911.2016.1244692. [DOI] [Google Scholar]
- 45.Ningaiah S, Bhadraiah UK, Keshavamurthy S, Javarasetty C. Novel pyrazoline amidoxime and their 1,2,4-oxadiazole analogues: synthesis and pharmacological screening. Bioorg Med Chem Lett. 2013;23:4532–4539. doi: 10.1016/j.bmcl.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 46.Shetnev A, Baykov S, Kalinin S, et al. 1,2,4-oxadiazole/2-imidazoline hybrids: multi-target-directed compounds for the treatment of infectious diseases and cancer. Int J Mol Sci. 2019;20:1699. doi: 10.3390/ijms20071699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi G, He X, Shang Y, et al. Synthesis of 3′,4′-Diaryl-4′H-spiro[indoline-3,5′-[1′,2′,4′]oxadiazol]-2-ones via DMAP-catalyzed domino reactions and their antibacterial activity. Chin J Chem. 2016;34:901–909. doi: 10.1002/cjoc.201600285. [DOI] [Google Scholar]
- 48.Atmaram Upare A, Gadekar PK, Sivaramakrishnan H, et al. Design, synthesis and biological evaluation of (E)-5-styryl-1,2,4-oxadiazoles as anti-tubercular agents. Bioorg Chem. 2019;86:507–512. doi: 10.1016/j.bioorg.2019.01.054. [DOI] [PubMed] [Google Scholar]
- 49.Breuer H. Nitroheterocycles. I. Nitrofuryl-substituted 3-amino-1,2,4-oxadiazoles and 5-Amino-1,2,4-oxadiazoles. J Med Chem. 1969;12:708–709. doi: 10.1021/jm00304a042. [DOI] [PubMed] [Google Scholar]
- 50.Kim J, Jung YK, Kim C, et al. A novel series of highly potent small molecule inhibitors of rhinovirus replication. J Med Chem. 2017;60:5472–5492. doi: 10.1021/acs.jmedchem.7b00175. [DOI] [PubMed] [Google Scholar]
- 51.dos Santos Filho JM, Leite ACL, de Oliveira BG, et al. Design, synthesis and cruzain docking of 3-(4-substituted-aryl)-1,2,4-oxadiazole-N-acylhydrazones as anti-Trypanosoma cruzi agents. Bioorg Med Chem. 2009;17:6682–6691. doi: 10.1016/j.bmc.2009.07.068. [DOI] [PubMed] [Google Scholar]
- 52.dos Santos Filho JM, de Queiroz e Silva DMA, Macedo TS, et al. Conjugation of N-acylhydrazone and 1,2,4-oxadiazole leads to the identification of active antimalarial agents. Bioorg Med Chem. 2016;24:5693–5701. doi: 10.1016/j.bmc.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 53.Srivastava RM, De Almeida LA, Viana OS, et al. Antiinflammatory property of 3-aryl-5-(n-propyl)-1,2,4-oxadiazoles and antimicrobial property of 3-aryl-5-(n-propyl)-4,5-dihydro-1,2,4-oxadiazoles: Their syntheses and spectroscopic studies. Bioorg Med Chem. 2003;11:1821–1827. doi: 10.1016/S0968-0896(03)00035-X. [DOI] [PubMed] [Google Scholar]
- 54.Dos Santos Filho JM, Moreira DRM, De Simone CA, et al. Optimization of anti-Trypanosoma cruzi oxadiazoles leads to identification of compounds with efficacy in infected mice. Bioorg Med Chem. 2012;20:6423–6433. doi: 10.1016/j.bmc.2012.08.047. [DOI] [PubMed] [Google Scholar]
- 55.Kim J, Shin JS, Ahn S, et al. 3-Aryl-1,2,4-oxadiazole derivatives active against human rhinovirus. ACS Med Chem Lett. 2018;9:667–672. doi: 10.1021/acsmedchemlett.8b00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pitasse-santos P, Sueth-santiago V, Lima MEF. 1,2,4- and 1,3,4-Oxadiazoles as Scaffolds in the development of antiparasitic agents. J Braz Chem Soc. 2018;29:435–456. [Google Scholar]
- 57.Facchinetti V, Gomes CRB, de Souza MVN. Application of nitriles on the synthesis of 1,3-oxazoles, 2-oxazolines, and oxadiazoles: An update from 2014 to 2021. Tetrahedron. 2021;102:132544. doi: 10.1016/j.tet.2021.132544. [DOI] [Google Scholar]
- 58.Cunha FS, Nogueira JMR, De Aguiar AP. Synthesis and antibacterial evaluation of 3,5-diaryl-1,2,4-oxadiazole derivatives. J Braz Chem Soc. 2018;29:2405–2416. [Google Scholar]
- 59.Karad SC, Purohit VB, Thummar RP, et al. Synthesis and biological screening of novel 2-morpholinoquinoline nucleus clubbed with 1,2,4-oxadiazole motifs. Eur J Med Chem. 2017;126:894–909. doi: 10.1016/j.ejmech.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 60.Il’In MV, Sysoeva AA, Bolotin DS, et al. Aminonitrones as highly reactive bifunctional synthons. An expedient one-pot route to 5-amino-1,2,4-triazoles and 5-amino-1,2,4-oxadiazoles-potential antimicrobials targeting multi-drug resistant bacteria. New J Chem. 2019;43:17358–17366. doi: 10.1039/C9NJ04529E. [DOI] [Google Scholar]
- 61.Jadhavar PS, Dhameliya TM, Vaja MD, et al. Synthesis, biological evaluation and structure-activity relationship of 2-styrylquinazolones as anti-tubercular agents. Bioorg Med Chem Lett. 2016;26:2663–2669. doi: 10.1016/j.bmcl.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 62.Pancholia S, Dhameliya TM, Shah P, et al. Benzo[d]thiazol-2-yl(piperazin-1-yl)methanones as new anti-mycobacterial chemotypes: Design, synthesis, biological evaluation and 3D-QSAR studies. Eur J Med Chem. 2016;116:187–199. doi: 10.1016/j.ejmech.2016.03.060. [DOI] [PubMed] [Google Scholar]
- 63.Dhameliya TM, Tiwari R, Banerjee A, et al. Benzo[d]thiazole-2-carbanilides as new anti-TB chemotypes: design, synthesis, biological evaluation, and structure-activity relationship. Eur J Med Chem. 2018;155:364–380. doi: 10.1016/j.ejmech.2018.05.049. [DOI] [PubMed] [Google Scholar]
- 64.O’Daniel PI, Peng Z, Pi H, et al. Discovery of a new class of non-β-lactam inhibitors of penicillin-binding proteins with gram-positive antibacterial activity. J Am Chem Soc. 2014;136:3664–3672. doi: 10.1021/ja500053x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ding D, Boudreau MA, Leemans E, et al. Exploration of the structure-activity relationship of 1,2,4-oxadiazole antibiotics. Bioorg Med Chem Lett. 2015;25:4854–4857. doi: 10.1016/j.bmcl.2015.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spink E, Ding D, Peng Z, et al. Structure–activity relationship for the oxadiazole class of antibiotics. J Med Chem. 2015;58:1380–1389. doi: 10.1021/jm501661f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang S, Tian XY, Ma TY, et al. Synthesis and biological activity of benzamides substituted with pyridine-linked 1,2,4-oxadiazole. Molecules. 2020;25:3500. doi: 10.3390/molecules25153500. [DOI] [PMC free article] [PubMed] [Google Scholar]