Abstract
Liver damage in severe acute respiratory coronavirus 2 infection occurs in patients with or without preexisting liver disorders, posing a significant complication and mortality risk. During coronavirus disease 2019 (COVID-19), abnormal liver function is typically observed. However, liver injury may occur because of the treatment as well. Ischemia, cytokine storm, and hypoxia were identified as the three major factors contributing to liver damage during COVID-19. Indeed, raised liver enzymes during hospitalizations may be attributed to medications used, as well as sepsis and shock. As a result, the proportion of hospitalized patients afflicted with COVID-19 and pathological liver biomarkers varies from 14% to 53%. Aminotransferases and bilirubin are found most often elevated. Usually, increased gamma-glutamyltransferase, alkaline phosphatase, and decreased serum albumin levels are demonstrated. Additionally, although there is no specific treatment for COVID-19, many of the drugs used to treat the infection are hepatotoxic. In this mini-review, we focus on how liver dysfunction can be one of the features associated with the COVID-19 cytokine storm. Furthermore, data show that liver injury can be an independent predictor of severe COVID-19, the need for hospitalization, and death.
Keywords: Liver dysfunction, Liver damage, Cytokine storm, Prognostic factor, COVID-19, Severe COVID-19, SARS-CoV-2, Aspartate aminotransferase, Alanine aminotrans-ferase, Bilirubin, Interleukin-6
Core Tip: Looking at the liver tests in patients with severe coronavirus disease 2019 (COVID-19), C-reactive protein (CRP) showed a strong correlation with the aspartate aminotransferase (AST) levels. This was observed in both intensive care units (ICU) and non-ICU patients. However, CRP levels were higher in non-ICU patients with liver damage, whereas alanine aminotransferase (ALT) was higher in ICU COVID-19 patients. Thus, like interleukin-6 (IL-6), ferritin, and CRP correlated directly with AST and ALT levels in non-ICU patients, there is a direct correlation of IL-6 and acute phase proteins with AST in severe COVID-19 cases. These observations confirm the critical impact of systemic inflammation and specifically elevated IL-6 during severe acute respiratory coronavirus 2 cytokine storm on liver injury.
INTRODUCTION
The newly emerged severe acute respiratory coronavirus 2 (SARS-CoV-2) and the disease that causes coronaviral disease 2019 (COVID-19) are still unclear regarding all virulence factors, immunological effects and deteriorations of human organs during infection[1]. However, it is assumed that the interaction between the SARS-CoV-2 virus and the individual's immune system substantially influences the disease's onset and progression and the pathological effects on many organs. Both humoral and cell-mediated immune mechanisms participate in the immune response to a viral infection[2].
However, in some patients, these antiviral immunological mechanisms escape the regulatory control and eventually contribute to the multiorgan failure caused by the virus, including liver failure. Furthermore, an overreaction of the host immune system triggers a systemic inflammatory state that causes significant tissue and organs damage due to high cytokine release. The latter phenomenon is known as cytokine storm, leading to extreme tissue damage[2]. Therefore, the mortality rate and the COVID-19 complications in the elderly and patients with preexisting medical comorbidities, such as diabetes, asthma and cardiovascular disease, are even higher. Furthermore, the risk of severe COVID-19 might be increased by the underlying liver disease. In addition, it can cause direct or indirect damage to the liver by creating a multisystem inflammation[3].
Liver damage in SARS-CoV-2 infection occurring during disease progression in patients with or without preexisting liver diseases is a substantial challenge for clinical practice. Abnormal liver function is expected during COVID-19 infection because of SARS-CoV-2 direct and indirect impact on the liver. Additionally, certain hepatotoxic medications, especially for COVID-19 treatment, are connected with drug-induced liver damage. However, liver injury is defined as any liver damage occurring during disease and treatment. Therefore, hospitalized patients infected with COVID-19 with abnormal liver biomarkers range from 14% to 53%; this is most often observed for aminotransferase and bilirubin[1]. In addition, increased levels of gamma-glutamyl transferase (GGT), alkaline phosphatase, and decreasing serum albumin levels are also observed[4].
As significant liver biomarkers changes are observed in patients with severe COVID-19, more frequent in adults in the intensive care unit, studies documented that elevation of liver enzymes is associated with severity of COVID-19. Additionally, male sex and CRP were demonstrated as independent risk factors of COVID-19 complicated by liver injury[5].
This mini-review discusses how liver dysfunction can be one of the manifestations of the COVID-19-associated cytokine storm. Furthermore, liver damage might be an independent prognostic factor for severe COVID-19 and hospitalization and death.
LIVER DYSFUNCTION AS A MANIFESTATION OF THE CYTOKINE STORM
Cytokine storm syndrome occurring in some of the COVID-19 infected patients involved many organs, such as lungs, kidneys, heart, and liver[2]. COVID-19 may also lead to multiorgan failure and severe consequences owing to systemic inflammatory conditions caused by a cytokine cascade with pulmonary, cardiac, and hepatic involvement, as described above[6].
Three main factors are associated with liver damage during COVID-19: ischemia, cytokine storm, and hypoxia. Other influential contributors are the direct cytopathic effect of the virus on cholangiocytes (via ACE2 receptors), preexisting liver disease (i.e., steatosis, hepatitis, cholangitis, thrombosis, Kupfer cell proliferation, liver impairment), severe inflammatory responses/sepsis[6].
Direct or indirect effects of SARS-CoV-2 on other organs are described beyond the respiratory system. In addition, it was shown that additional receptors might facilitate the virus to enter and infect the human cells via spike protein, including the liver. This suggests that there might be additional receptor pathways for infection with COVID-19 that can be targeted with specific treatment.
SARS-CoV-2 caused dysfunction and inducing a systemic inflammatory response leading to severe liver injury by binding to ACE2 receptors on cholangiocytes. In detail, spike protein binds the asialoglycoprotein receptor located on human hepatocytes. It was recently published that in vitro, SARS-CoV-2 spike protein can bind the asialoglycoprotein receptor 1 Located on primary human hepatocytes and hepatocyte-like cells[7]. In line with this, the serum GGT as a diagnostic marker for cholangiocyte injury has been found at elevated levels in up to 72% of severe COVID-19 patients[8].
Hypoxic liver injury (HLI) is not rare in patients with severe COVID-19 and has a high mortality. Its leading causes are lung and cardiac failure and may be associated with the immune-mediated inflammatory response. Patients with HLI have high mortality as a result of the deterioration of multiple organ failures. Levels of total bilirubin (TBIL), C-reactive protein (CRP), procalcitonin, and interleukin-6 (IL-6) show a statistically significant elevation in HLI cases compared with that in non-HLI cases. Besides, the median survival time of patients with HLI is significantly shorter than that of those not developing HLI[9].
Massive cytokine release causes a cytokine storm (also known as cytokine release syndrome) and is characterized by elevated CRP, IL-6, lactate dehydrogenase (LDH), and ferritin concentrations[10]. Furthermore, the subsequent organ dysfunction (i.e., acute respiratory distress syndrome, progressive liver damage, and liver failure). As a result, systemic pro-inflammatory cytokine release appears to be a driver of disease progression in COVID-19[11-13].
Notably, COVID-19 patients had hepatic lymphocyte infiltration, centrilobular sinusoidal dilation, and patchy necrosis following the SARS-CoV-2 directly binding to ACE2-expressing cholangiocytes. However, the cause of the liver damage is unknown and may be due to systemic inflammation, SARS-CoV-2 infection, or drug administration[14].
Effenberger et al[10] discovered a clear link between systemic inflammation (as measured by IL-6, CRP, and ferritin) and liver damage. IL-6 development can be attributed to immune cells, fibroblasts, endothelial cells, and hepatocytes, orchestrating an acute phase response in the liver. Though IL-6 signaling impacts hepatic regeneration, clinical trials (for example, testing the effect of IL-6 administration in cancer patients) have shown that this pathway is essential in hepatic injury and hepatotoxicity[10]. The authors also found a strong association between acute-phase proteins and IL-6 in the serum of COVID-19 patients with elevated aspartate aminotransferase (AST), which is consistent with the importance of systemic inflammation and, in particular, IL-6 on liver injury.
The main sources of IL-6, which is the chief stimulator of the production of most acute phase proteins, are macrophages and monocytes at inflammatory sites. It has been shown that macrophages and monocytes produce high amounts of IL-6 in response to SARS-CoV-2 proteins[15].
COVID-19 patients with gastrointestinal complaints (nausea, vomiting, diarrhea, etc.) had higher AST and alanine aminotransferase (ALT) levels. Furthermore, there was a significant increase in enzymes among COVID-19 patients, primarily in the intensive care unit (ICU) facilities[16]. A relationship between liver enzyme elevation and disease activity has been also demonstrated[17].
Furthermore, the incidence of elevated AST levels was found to be greater than that of ALT levels and significantly higher in patients with severe COVID-19 (45.5%) relative to non-severe cases (15.0%). Thus, Lei et al[18] established a link between liver injury and inpatient mortality in COVID-19 patients. They also found a correlation between AST abnormality and mortality risk compared to other liver injury measures during hospitalization[18].
Liver biopsies revealed moderate microvesicular steatosis with slight lobular and portal inflammation, indicating either direct viral or drug-induced liver damage[19]. It is proposed that a direct virus-mediated cytopathic effect exists. The latter can result after triggered immunological reactions and inflammatory cytokines, leading to liver injury[20,21]. Monocyte and macrophage dysfunction contribute to the progression of liver damage. Activation of liver-resident macrophages (Kupffer cells) and damage-associated molecular patterns result in recruitment of effector cells to the injured liver. Early monocyte infiltration is a major factor in the progression of local tissue destruction. Furthermore, the local inflammation results in the secretion of more and more pro-inflammatory cytokines that drive systemic inflammatory response syndrome[22].
Additionally, predominated parenchymal liver damage according to the elevated AST (23.2%) and ALT (21.2%), rather than bile duct injury, as shown by GGT (9.7%) and ALP (4.0%) levels in COVID-19 patients[16]. Patients with mild COVID-19 also have liver damage which resolves without any specific treatment. Most of the patients with liver failure during hospitalization, associated with severe COVID-19, are due to several drugs’ hepatotoxicity.
Different drugs can impair liver function. However, the hepatotoxicity of medications varies on race, sex, and age of the patients[23]. Thus, the knowledge on the potential contributors to liver failure is significant. In addition, some medications can induce asymptomatic elevations of liver enzymes, acute hepatitis.
Many of the patients required treatment with antibiotics, anti-inflammatory, and antiviral agents. Antibiotics, anti-inflammatory, and antiviral medications used to treat COVID-19 patients are among the medicines that can induce liver harm[24,25]. Some of them cause asymptomatic elevation of the liver enzymes, while others lead to acute hepatitis. In some cases (e.g., acetaminophen), these effects are dose-dependent. In contrast, in other medications, liver damage occurs independently of the drug dosage[24].
Hydroxychloroquine alone or in combination with azithromycin, lopinavir / ritonavir, remdesivir, darunavir, umifenovir, interferon beta, baricitinib, imatinib exert hepatotoxicity. Their immediate availability has led to off-label use for COVID-19 treatment in many countries[26].
There is currently no specific antiviral medication for SARS-CoV-2. Still, many COVID-19 patients are given antivirals approved for different uses (i.e., remdesivir, lopinavir, or ritonavir, and other medications[27], all of which have been linked to hepatotoxicity and liver impairment[26].
Incorrect liver metabolization may also result in COVID 19-induced liver impairment which increases the risk of poisoning. However, a combination of patient records and thorough laboratory tests is carried out to diagnose drug-induced liver impairment to exclude other hepatic diseases and identify the relationship between hepatic injuries and probable causative medications.
More COVID 19 individuals suffer from fever, and hepatotoxicity can be triggered by antipyretics and analgesics (i.e., paracetamol). This is associated with liver injuries, resulting in a potentially deadly combination, generally in the most severe phases of COVID-19. Furthermore, some antiviral drugs - remdesivir, lopinavir, ritonavir, IL-6 inhibitors (i.e., tocilizumab), antibiotics - azithromycin, may cause idiosyncratic drug-induced liver failure[26].
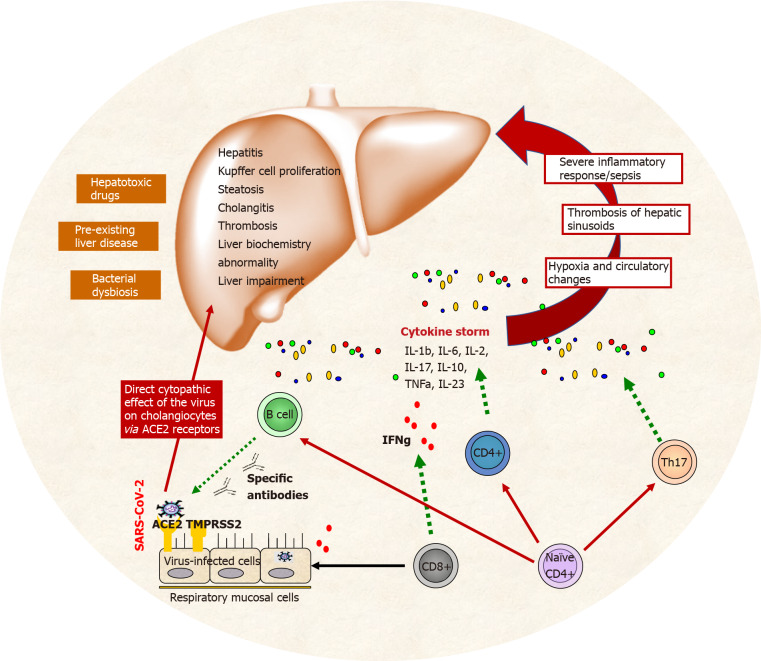
Mechanisms involved in liver injury during COVID-19 infection and cytokine storm are presented on Figure 1.
Figure 1.
Liver dysfunction defined by the negative effects of cytokine storm (severe inflammation, thrombosis, hypoxia, etc.) during coronavirus disease 2019 infection. Other contributing factors for liver injury are also presented – preexisting liver condition, direct cytopathic action of severe acute respiratory coronavirus 2 and treatment with hepatotoxic drugs.
LIVER FAILURE AS A PROGNOSTIC FACTOR IN SEVERE COVID-19 PATIENTS
Different risk factors can be associated with severe liver injury. Specifically, preexisting liver diseases - obesity with non-alcoholic fatty liver disease (NAFLD), alcoholic liver disease, cirrhosis - all of them correlate with Child-Pugh class and model for end-stage liver disease score. Moreover, autoimmune liver diseases, chronic hepatitis B infections could be reactivated and contribute to high levels of AST/ALT[28,29].
Patients with cirrhosis have a high risk of mortality from respiratory failure following severe SARS-CoV-2 infection. This risk might occur through multiple converging pathways, including contributions from cirrhosis-associated immune dysfunction, acute hepatic decompensation, and systemic inflammatory response. Cirrhosis-associated immune dysfunction could also lead to defective immune responses following future SARS-CoV-2 vaccination[20]. Patient with abnormal liver tests had a higher mortality rate (28.9% vs 9.0%, P < 0.001) and higher chance to develop systemic inflammatory response[30,31].
Interestingly, abnormal liver tests and liver injury can be associated with the progression of severe pneumonia[12]. The abnormalities can be hepatocellular, cholesteric, or mixed. Some clinical research studies show that patients with abnormal liver test results, especially in hepatocyte or mixed type ALT/AST and ALP/GGT at admission or during hospitalization, had significantly higher odds of progressing to severe COVID-19[28].
As we mentioned above, the pattern of liver injury is predominantly hepatocellular rather than cholestatic, although elevations in TBIL and ALT may be more common than reported in earlier studies. Since the ACE2 receptor is predominantly expressed in cholangiocytes than in hepatocytes, it is suggested that the most prevalent mechanism of liver impairment is not due to a direct cytopathic effect of the SARS-CoV-2 virus[32].
Raised liver enzymes during hospitalizations could be partly due to drugs used for treatment and might be due to sepsis and shock[28]. Looking at the liver tests, CRP showed a strong correlation with the AST levels, especially in hospitalized patients. Additionally, for both ICU and non-ICU patients, where this association was demonstrated at admission. However, CRP levels were higher in non-ICU patients with liver damage, whereas ALT was higher in ICU COVID-19 patients[33]. IL-6, ferritin, and CRP correlated directly with AST and ALT levels in non-ICU patients.
Further analysis revealed a direct correlation of IL-6 and acute phase proteins with AST. In severe COVID-19 cases. To sum up, these observations confirm the critical impact of systemic inflammation and specifically IL-6 on liver injury. Furthermore, these observations led to the establishment of abnormal AST and direct bilirubin (DBil) at hospital admission as independent risk factors for increased COVID-19 mortality[33].
We can emphasize that the pathological examination of liver tissues from deceased patients with COVID-19 confirmed that liver involvement of COVID-19 was characterized by microvesicular steatosis, focal necrosis with lymphocytes infiltration, and micro thrombosis in the portal area[34]. Furthermore, pathological levels DBil were often found during the hospitalization of deceased COVID-19 patients. Both baseline and higher AST and DBil levels were independently associated with in-hospital death in patients with COVID-19. While liver anomalies are typical in COVID-19, these findings indicate that the liver is unlikely to be the primary organ driving COVID-19 mortality.
Since the number of people who develop severe and fatal COVID-19 is increased in elderly patients and those with liver failure and NAFLD, it is typically advised that older COVID-9 patients on hepatotoxic medication be closely followed up. Moreover, NAFLD can make the liver more sensitive to the most recommended and widespread antipyretic medication treatment for symptomatic diseases, such as acetaminophen[35,36]. However, while the association of the COVID-19 with the liver steatosis disease is still unknown, a recent histological study of a COVID-19 patient's liver revealed microvesicular liver steatosis[19,37].
CONCLUSION
We can conclude that the pathological mechanisms of liver damage during COVID-19 confirmed that liver involvement was often observed with an increased risk for complications and death. Furthermore, the incidence of abnormal liver enzymes, significantly elevated AST and ALT levels were observed in patients with severe COVID-19 than non-severe cases. Additionally, a link between liver injury and inpatient mortality in COVID-19 patients was established. Moreover, recent studies confirmed that if liver dysfunction, preexisting or acquired during COVID-19 treatment, is a prognostic factor for severe COVID-19, development of complications and death.
Footnotes
Conflict-of-interest statement: None disclosed.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: April 22, 2021
First decision: June 15, 2021
Article in press: November 18, 2021
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: Bulgaria
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ratajewski M S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
Contributor Information
Gergana Taneva, Department of Gastroenterology, Sveta Sofia Hospital, Sofia 1618, Bulgaria.
Dimitar Dimitrov, Department of Gastroenterology, Sveta Sofia Hospital, Sofia 1618, Bulgaria.
Tsvetelina Velikova, Department of Clinical Immunology, University Hospital Lozenetz, Sofia 1407, Bulgaria; Medical Faculty, Sofia University St. Kliment Ohridski, Sofia 1407, Bulgaria. tsvelikova@medfac.mu-sofia.bg.
References
- 1.Sun J, Aghemo A, Forner A, Valenti L. COVID-19 and liver disease. Liver Int. 2020;40:1278–1281. doi: 10.1111/liv.14470. [DOI] [PubMed] [Google Scholar]
- 2.Velikova TV, Kotsev SV, Georgiev DS, Batselova HM. Immunological aspects of COVID-19: What do we know? World J Biol Chem. 2020;11:14–29. doi: 10.4331/wjbc.v11.i2.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boeckmans J, Rodrigues RM, Demuyser T, Piérard D, Vanhaecke T, Rogiers V. COVID-19 and drug-induced liver injury: a problem of plenty or a petty point? Arch Toxicol. 2020;94:1367–1369. doi: 10.1007/s00204-020-02734-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yadav DK, Singh A, Zhang Q, Bai X, Zhang W, Yadav RK, Zhiwei L, Adhikari VP, Liang T. Involvement of liver in COVID-19: systematic review and meta-analysis. Gut. 2021;70:807–809. doi: 10.1136/gutjnl-2020-322072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen JX, Zhuang ZH, Zhang QX, Huang JF, Chen GP, Fang YY, Cheng AG. Risk Factors and Prognosis in Patients with COVID-19 and Liver Injury: A Retrospective Analysis. J Multidiscip Healthc. 2021;14:629–637. doi: 10.2147/JMDH.S293378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vitiello A, La Porta R, D'Aiuto V, Ferrara F. The risks of liver injury in COVID-19 patients and pharmacological management to reduce or prevent the damage induced. Egypt Liver J . 2021;11:11. doi: 10.1186/s43066-021-00082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins DP, Steer CJ. Binding of the SARS-CoV-2 Spike Protein to the Asialoglycoprotein Receptor on Human Primary Hepatocytes and Immortalized Hepatocyte-Like Cells by Confocal Analysis. Hepat Med. 2021;13:37–44. doi: 10.2147/HMER.S301979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lagana SM, Kudose S, Iuga AC, Lee MJ, Fazlollahi L, Remotti HE, Del Portillo A, De Michele S, de Gonzalez AK, Saqi A, Khairallah P, Chong AM, Park H, Uhlemann AC, Lefkowitch JH, Verna EC. Hepatic pathology in patients dying of COVID-19: a series of 40 cases including clinical, histologic, and virologic data. Mod Pathol. 2020;33:2147–2155. doi: 10.1038/s41379-020-00649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang H, Li H, Chen S, Zhou X, Dai X, Wu J, Zhang J, Shao L, Yan R, Wang M, Wang J, Tu Y, Ge M. Prevalence and Characteristics of Hypoxic Hepatitis in COVID-19 Patients in the Intensive Care Unit: A First Retrospective Study. Front Med (Lausanne) 2020;7:607206. doi: 10.3389/fmed.2020.607206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Effenberger M, Grander C, Grabherr F, Griesmacher A, Ploner T, Hartig F, Bellmann-Weiler R, Joannidis M, Zoller H, Weiss G, Adolph TE, Tilg H. Systemic inflammation as fuel for acute liver injury in COVID-19. Dig Liver Dis. 2021;53:158–165. doi: 10.1016/j.dld.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 13.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, Damoraki G, Gkavogianni T, Adami ME, Katsaounou P, Ntaganou M, Kyriakopoulou M, Dimopoulos G, Koutsodimitropoulos I, Velissaris D, Koufargyris P, Karageorgos A, Katrini K, Lekakis V, Lupse M, Kotsaki A, Renieris G, Theodoulou D, Panou V, Koukaki E, Koulouris N, Gogos C, Koutsoukou A. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe. 2020;27:992–1000.e3. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karwaciak I, Sałkowska A, Karaś K, Dastych J, Ratajewski M. Nucleocapsid and Spike Proteins of the Coronavirus SARS-CoV-2 Induce IL6 in Monocytes and Macrophages-Potential Implications for Cytokine Storm Syndrome. Vaccines (Basel) 2021;9 doi: 10.3390/vaccines9010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wijarnpreecha K, Ungprasert P, Panjawatanan P, Harnois DM, Zaver HB, Ahmed A, Kim D. COVID-19 and liver injury: a meta-analysis. Eur J Gastroenterol Hepatol. 2021;33:990–995. doi: 10.1097/MEG.0000000000001817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, Li J, Zhao D, Xu D, Gong Q, Liao J, Yang H, Hou W, Zhang Y. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, Zhang XJ, Cai J, Lin L, Ouyang S, Wang X, Yang C, Cheng X, Liu W, Li H, Xie J, Wu B, Luo H, Xiao F, Chen J, Tao L, Cheng G, She ZG, Zhou J, Wang H, Lin J, Luo P, Fu S, Ye P, Xiao B, Mao W, Liu L, Yan Y, Chen G, Huang X, Zhang BH, Yuan Y. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology. 2020;72:389–398. doi: 10.1002/hep.31301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu J, Han B, Wang J. COVID-19: Gastrointestinal Manifestations and Potential Fecal-Oral Transmission. Gastroenterology. 2020;158:1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Triantafyllou E, Woollard KJ, McPhail MJW, Antoniades CG, Possamai LA. The Role of Monocytes and Macrophages in Acute and Acute-on-Chronic Liver Failure. Front Immunol. 2018;9:2948. doi: 10.3389/fimmu.2018.02948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Björnsson ES. Hepatotoxicity by Drugs: The Most Common Implicated Agents. Int J Mol Sci. 2016;17:224. doi: 10.3390/ijms17020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cascella M, Rajnik M, Cuom , A , Dulebohn SC, Di Napoli R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In: StatPearls. Treasure Island: StatPearls Publishing, 2020. [PubMed] [Google Scholar]
- 25.Cao R, Hu Y, Wang Y, Gurley EC, Studer EJ, Wang X, Hylemon PB, Pandak WM, Sanyal AJ, Zhang L, Zhou H. Prevention of HIV protease inhibitor-induced dysregulation of hepatic lipid metabolism by raltegravir via endoplasmic reticulum stress signaling pathways. J Pharmacol Exp Ther. 2010;334:530–539. doi: 10.1124/jpet.110.168484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olry A, Meunier L, Délire B, Larrey D, Horsmans Y, Le Louët H. Drug-Induced Liver Injury and COVID-19 Infection: The Rules Remain the Same. Drug Saf. 2020;43:615–617. doi: 10.1007/s40264-020-00954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zha BS, Wan X, Zhang X, Zha W, Zhou J, Wabitsch M, Wang G, Lyall V, Hylemon PB, Zhou H. HIV protease inhibitors disrupt lipid metabolism by activating endoplasmic reticulum stress and inhibiting autophagy activity in adipocytes. PLoS One. 2013;8:e59514. doi: 10.1371/journal.pone.0059514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu ZH, Yang DL. A meta-analysis of the impact of COVID-19 on liver dysfunction. Eur J Med Res. 2020;25:54. doi: 10.1186/s40001-020-00454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marjot T, Webb GJ, Barritt AS 4th, Moon AM, Stamataki Z, Wong VW, Barnes E. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021;18:348–364. doi: 10.1038/s41575-021-00426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen LY, Chu HK, Bai T, Tu SJ, Wei Y, Li ZL, Hu LL, Zhu R, Zhang L, Han CQ, Xiao L, He Q, Song J, Liu WH, Zhu QJ, Chen H, Yang L, Hou XH. Liver damage at admission is an independent prognostic factor for COVID-19. J Dig Dis. 2020;21:512–518. doi: 10.1111/1751-2980.12925. [DOI] [PubMed] [Google Scholar]
- 32.Hundt MA, Deng Y, Ciarleglio MM, Nathanson MH, Lim JK. Abnormal Liver Tests in COVID-19: A Retrospective Observational Cohort Study of 1,827 Patients in a Major U.S. Hospital Network. Hepatology. 2020;72:1169–1176. doi: 10.1002/hep.31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding ZY, Li GX, Chen L, Shu C, Song J, Wang W, Wang YW, Chen Q, Jin GN, Liu TT, Liang JN, Zhu P, Zhu W, Li Y, Zhang BH, Feng H, Zhang WG, Yin ZY, Yu WK, Yang Y, Zhang HQ, Tang ZP, Wang H, Hu JB, Liu JH, Yin P, Chen XP, Zhang B Tongji Multidisciplinary Team for Treating COVID-19 (TTTC) Association of liver abnormalities with in-hospital mortality in patients with COVID-19. J Hepatol. 2021;74:1295–1302. doi: 10.1016/j.jhep.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Health Commission of China. Guidance for COVID-19: Prevention, Control, Diagnosis and Management. Version 7.0. 2020. [accessed 2021 Nov 5]. Available from: https://www.yoifos.com/sites/default/files/covid_19_guideline_chn.pdf .
- 35.Massart J, Begriche K, Moreau C, Fromenty B. Role of nonalcoholic fatty liver disease as risk factor for drug-induced hepatotoxicity. J Clin Transl Res. 2017;3:212–232. doi: 10.18053/jctres.03.2017S1.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michaut A, Moreau C, Robin MA, Fromenty B. Acetaminophen-induced liver injury in obesity and nonalcoholic fatty liver disease. Liver Int. 2014;34:e171–e179. doi: 10.1111/liv.12514. [DOI] [PubMed] [Google Scholar]
- 37.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]