Abstract
Coronavirus disease 2019 (COVID-19) disease affects multiple organs, including anomalies in liver function. In this review we summarize the knowledge about liver injury found during severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection with special attention paid to possible mechanisms of liver damage and abnormalities in liver function tests allowing for the evaluation of the severity of liver disease. Abnormalities in liver function observed in COVID-19 disease are associated with the age and sex of patients, severity of liver injury, presence of comorbidity and pre-treatment. The method of antiviral treatment can also impact on liver function, which manifests as increasing values in liver function tests. Therefore, analysis of variations in liver function tests is necessary in evaluating the progression of liver injury to severe disease.
Keywords: COVID-19, Pathogenesis of liver injury, Angiotensin-converting enzyme 2 receptor, Liver function tests, Severe COVID-19, Treatment effect
Core Tip: The frequency of abnormalities in liver function tests (LFTs) in coronavirus disease 2019 (COVID-19) infected patients increases with age and is observed in males more than females. A pre-existing history of liver disease and comorbidity increases LFT abnormality and the likelihood of severe liver damage in COVID-19 infection. Antiviral treatment and treatment of comorbid diseases intensifies the hepatotoxic effect on the liver, which often manifests itself in higher levels in LFTs.
INTRODUCTION
Pulmonary disease is the primary clinical manifestation in patients with coronavirus disease 2019 (COVID-19) disease. There is increasing evidence of the involvement of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in multiple organs including the heart, kidneys, central nervous system and liver. In this paper we summarize data concerning liver injury in COVID-19 patients with special attention paid to the possible mechanisms of liver damage and laboratory tests to monitor liver injury during SARS-CoV-2 infection.
GENERAL CHARACTERISTICS
COVID-19 is an acute respiratory infectious disease caused by SARS-CoV-2[1,2]. The SARS-CoV-2 belongs to the Coronaviridae family of enveloped, single-stranded RNA viruses[3]. There is evidence that SARS-CoV-2 shares nearly 80% of its genomic sequence with SARS-CoV and about 50% with Middle East respiratory syndrome coronavirus[2,4]. COVID-19 is a viral infectious disease affecting all age groups (from infants to the elderly) resulting in a wide range of clinical manifestations[5-7]. The incubation period of COVID-19 tends to vary from 1 d to 14 d[8].
Multiple organ involvement
Furthermore, COVID-19 infection can present itself with differing degrees of severity, varying from asymptomatic and mild disease to viral pneumonia, in addition to various other extra-pulmonary manifestations, including for example heart, kidney, central nervous system or liver affection, with a risk of fatality[5-7]. Thus, the virus is capable of affecting any organ in the body, and in critically ill patients multiple organs are often affected. Mild cases of COVID-19 infection exhibit symptoms such as fever, dry cough, fatigue, vomiting, diarrhea, muscle weakness, and chest pain[5,7,8]. Patients may also suffer from headaches, as well as loss of smell and taste. While, in severe cases, respiratory distress and/or hypoxemia occur one week after the onset of the disease leading to deterioration into acute respiratory distress syndrome (ARDS), metabolic acidosis, septic shock, and in some cases, even death[5,7,8]. SARS-CoV-2 presents primarily as a lower tract respiratory infection transmitted via air droplets, but evidence of the multisystemic nature of COVID-19 is still significantly increasing[5,7,8]. The complications of COVID-19 are associated with several risk factors, namely, advancing age (> 65 years old), cardiovascular disease, hypertension, chronic respiratory disease, diabetes, and obesity[5,8]. The most common reported complication is ARDS, but other severe or even fatal complications are pneumonia, sepsis, metabolic acidosis, heart failure, and acute kidney injury[5,9-11].
Main pulmonary manifestations
Pulmonary affection is the most common serious COVID-19 manifestation[7]. There is evidence that the severity of pulmonary affection caused by SARS-CoV-2 ranges from lack of symptoms or mild pneumonia in 81% of cases, to severe cases associated with hypoxia - in 14% of cases; critical disease associated with shock, respiratory failure and multiple-organ failure - in 5% of cases; or death - in 2.3% of cases[7,12]. SARS-CoV-2 infection induces alveolar damage and interstitial inflammation. During the course of inflammation, the dendritic cells and alveolar macrophages phagocytose epithelial cells infected by SARS-CoV-2, whilst at the same time, the immune mechanisms with T cell responses are activated[7,13].
So, in patients with COVID-19 infection levels of proinflammatory cytokines and chemokines e.g., interleukin 6 (IL-6), IL-1β, tumor necrosis factor, interferon γ, granulocyte stimulating factor are increased[7,8,14]. There is a suggestion that cytokine storms play a crucial role in the immunopathology of the COVID-19 infection.
Cardiac manifestations
Cardiac injury is a common characteristic of patients with COVID-19 infection. Furthermore, despite the fact that cardiovascular diseases might significantly worsen the clinical outcome of COVID-19 patients, SARS-CoV-2 infection might also induce new cardiac complications[5,15]. Additionally, this cardiac damage might even occur without of any signs or symptoms of pneumonia and with an absence of other complications[5-7]. The major effects of SARS-CoV-2 infection on cardiomyocytes, include for example, acute myocardial injury, heart failure, impaired renal function, arrhythmias, cardiac arrest, myocarditis, sepsis, and septic shock[5,8,16]. The most frequently presented cardiac complication associated with COVID-19 infection is an acute myocardial injury with an estimated prevalence of 8%-12%[5,6,17]. Additionally, the most prevalent complications, with an estimated incidence of 16.7%, are brady- or tachyarrhythmias, also blood pressure abnormalities and dysfunction of the left ventricular[5,6,18]. Importantly, cardiac complications may occur long after viral clearance and recovery, because the inflammation can persist and evolve silently[6,7]. Confirmation of this thesis is exemplified by pulmonary fibrosis, avascular necrosis or dyslipidemia which have evolved over the long term in many survivors of SARS infection, which is closely related to COVID-19 caused by SARS-CoV-2[6]. There is evidence that about one-half of fatal cases show acute cardiac injury and heart failure[6]. These conditions are more probable in elderly patients, while in younger patients myocarditis is the more likely cause.
Although pulmonary disease is the primary clinical manifestation in patients with COVID-19, with cardiac and kidney injury also being common, as we mentioned above there is increasing evidence of its involvement in multiple organs. In this paper we summarize data concerning liver injury in COVID-19 patients.
POSSIBLE PATHOMECHANISMS OF LIVER INJURY
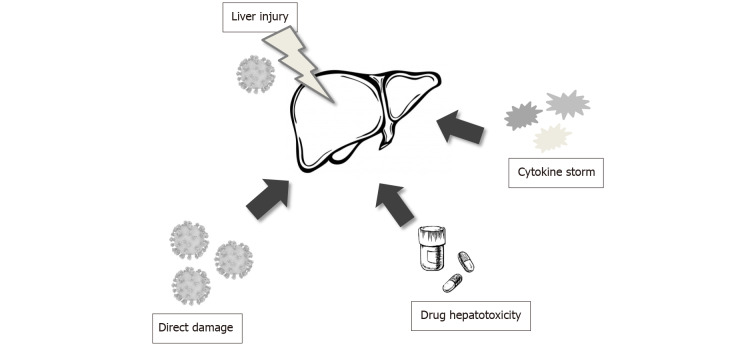
The alteration of hepatocyte damage biomarkers, such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, and bilirubin is a common laboratory finding in patients with COVID-19 infection. However, the pathomechanism of liver injury during infection is convoluted and not yet fully understood[8,19]. Is not clear if the liver damage is caused by the direct viral effect or if it perhaps reflects a more severe inflammatory response with hepatic injury[20,21]. The possible major pathomechanisms of liver damage are presented in Figure 1. It has been reported that the angiotensin-converting enzyme 2 (ACE2) was identified as the SARS-CoV binding site[19,20,22]. This data facilitated confirmation that SARS-CoV-2 may also directly enter the host cells through binding of its S protein to ACE2 on the surface of the host cell, although with a 10-20-fold higher affinity[2]. The ACE2 receptor expression is higher in many organs, such as lungs, heart, kidney, and it is widely expressed across a variety of cell types[8,22]. Hepatocytes and bile duct epithelial cells also express the ACE2 receptor[7,8,19]. Nevertheless, no significant altered histopathological features have been detected in such cells from COVID-19 patients[8,23]. Only single studies have claimed that the derangement of liver function is usually mild and there is not enough evidence that late-onset symptoms are related to increasing liver damage in patients with COVID-19 infection[2,19]. Additionally, recent data has suggested that SARS-CoV-2 may directly bind to ACE2 expressed in cholangiocytes, because there is evidence that ACE2 expression is displayed in 2.6% of hepatocytes and 59.7% of cholangiocytes[2,19]. Moreover, the alteration of cholangiocyte injury biomarkers, such as alkaline phosphatase (ALP) and γ-glutamyl transferase (GGT) has been observed in some cases, and consistent with biliary epithelial cell damage, and about 10% of patients with COVID-19 infection have an elevated total level of bilirubin[2,24]. There is evidence that specific expression of ACE2 in bile duct epithelial cells was about 20 times higher than in hepatocyte. Furthermore, the bile duct epithelial cells play a substantial role in immune response and liver regeneration. So, this data suggests that liver damage in COVID-19 infection results from bile duct cell injury rather than a direct viral effect in liver cells[19].
Figure 1.
Possible pathomechanism of liver injury in patients with severe acute respiratory syndrome coronavirus 2 infection.
On the other hand, the liver is a vital organ for the metabolism of drugs. It is well known that patients suffering from certain viral infections caused for example, by the human immunodeficiency virus or hepatitis C virus are more prone to develop drug-induced liver injury, particularly when it is associated with highly active anti-retroviral therapy[25-27]. Therefore, nowadays it is postulated that the same mechanism of liver injury could be present in COVID-19 as a result of the SARS-CoV-2 virus. Thus, hepatotoxicity during the course of the COVID-19 infection, may be initiated by the different types of antiviral drugs, antibiotics and steroids which are currently used to treat COVID-19 patients[25,28]. However, there is a lack of evidence for liver damage in chronic COVID-19 patients being completely drug-induced. A potential example of the relationship between the use of certain drugs and resulting liver damage is found in the study of Fan et al[29]. They reported that a high percentage of patients with abnormal values in liver function tests (LFTs) were treated with lopinavir and ritonavir during hospitalization. Similar results appeared in the study of Cai et al[30]. Moreover, they reported an almost four-fold increase in liver injury after lopinavir/ritonavir were used in the treatment of severe COVID-19 infection. This finding is consistent with some liver biopsy findings[31]. Certain studies have reported mild lobular and portal activity and moderate microvascular steatosis in patients who died from COVID-19[23]. Further evidence also showed minimal lymphocytic infiltration and mild sinusoidal dilatation in COVID-19 patients[24]. However, these alterations are nonspecific and may be caused by drug-induced liver injury, not excluding the possibility of hypoxemia or having come directly from the SARS-CoV-2 virus[19,23]. Considering these facts, it is very important that these patients be treated with drugs that can inhibit inflammatory response while at the same time protecting hepatic functions.
Another possible reason for liver damage in patients with COVID-19 infection may be dysregulation of the innate immune response[2,19,22]. There is evidence that inflammatory cytokine storms were found in chronically ill patients. The increased values of inflammatory indices, such as C-reactive protein (CRP), IL-6, neutrophils and lymphocytes can be observed in patients with COVID-19 infection, which suggests a relationship between liver damage and inflammatory response induced by severe COVID-19 infection.
ABNORMALITIES IN LABORATORY TESTS
There are many studies showing abnormal laboratory test results in patients with severe COVID-19 disease[32-35]. The first cases of COVID-19 patients from China with liver abnormality were documented by Chen et al[32]. Elevations in ALT, AST and lactate dehydrogenase (LDH) were present in 43 out of 99 patients, while most of these cases showed some mild abnormality, whilst one patient exhibited a large increase in test results (ALT of 7590 U/L and AST of 1445 U/L). Most of the participants were male, half of them with chronic diseases. LFTs not only showed abnormalities such as aminotransferases, but also noted were decreased haemoglobin, platelets, an increase of creatine kinase, LDH, ferritin, CRP and a decrease/increase in leucocytes[32].
Cai et al[30] conducted laboratory tests on a population of 417 patients with COVID-19 in Shenzhen hospital, China. Three hundred and eighteen patients were confirmed with abnormal liver test results, whilst another 90 had liver injury during hospitalization. The patients were qualified to the appropriate types. Abnormalities such as: hepatocellular type [elevated ALT and/or AST more than 3 × the upper limit unit of normal (ULN)], cholangiocyte type (raised ALP or GGT 2 × ULN ) or mixed type (elevated ALT and/or AST more than 3 × the upper limit ULN and raised ALP or GGT 2 × ULN). The highest increase (3 × ULN) in liver enzymes such as ALT (23.4 % of patients), AST (14.8%), total bilirubin (TBIL) (11.5%) and GGT (24.4%) was noticed during the second week of hospitalization. Out of 318 cases, the mixed type dominated and there was a noted increase in all the above tests, except for ALP. In relation to the population of 90 patients, an increase was seen in ALT and GGT, while AST and TBIL were hardly visible. Mixed type patients or those with abnormal test results are at a greater risk of advanced to severe disease. Patients treated with lopinavir/ritonavir had much higher levels of TBIL and GGT, with an associated four-fold increase in the risk of liver damage[30].
A Study carried out on 292 patients in Italy led researchers to different conclusions than Cai et al[30]. In their opinion, LFTs are not associated with the patient’s condition deteriorating to a severe form of pneumonia. Elevations in AST (18.5%), ALT (26.7%), GGT (36.2%), TBIL (10.6%) and ALP (9.2%) were inconsiderable[36]. Only ALP was not ruled out as a predictive factor, however, it may be associated with bad patient condition, systemic inflammatory response or SARS-CoV-2 tropism for the liver and ACE2 converting enzyme expression in cholangiocytes and hepatocytes. Although 250 patients were treated with lopinavir/ritonavir and 56 patients died, 82 deteriorated and 56 were admitted to intensive care, this was not in any way related to LFTs. Researchers recommended drawing conclusions carefully in the context of a complex multi-organ disease[36].
Wang et al[37] conducted an experiment on 156 people diagnosed with the SARS-CoV-2 virus from 2 chosen centers in China, in which they tested the correlation between the prognosis of patients and liver enzyme abnormalities, or lack of such abnormalities. Sixty-four of them had elevated AST and ALT which correlated with disease severity, higher alveolar-arterial oxygen partial pressure difference, growth of GGT, lower albumin and CD4+ T cells and B lymphocytes. The histological trial revealed severe liver apoptosis. Cytopathy in hepatocytes showed ultrastructural features such as endoplasmic reticulum dilatation, mitochondrial swelling and an impaired cell membrane. The above evidence shows that the virus has an influence on the increase in the value of liver enzymes. The most important observation was an association between a very high level of alveolar-arterial oxygen tension difference (A-aDO2) and elevated transaminases. According to this study, SARS-CoV-2 virus infection is a direct factor in liver disease[37].
Conclusions from a study carried out on 5771 adult patients from 10 hospitals in Wuhan indicated a need for monitoring hepatic parameters during hospitalization[38]. On admission to the hospital, chronically ill patients had AST levels significantly higher than ALT. Abnormalities in LFTs have been additionally associated with males, treatment, chronic liver disease, lymphocyte, neutrophil and platelet count. Abnormalities in LFTs, such as AST, ALT, TBIL, GGT, were related to mortality, however AST had the highest correlation. A significantly higher level of AST compared to ALT was also confirmed in the study of Guan et al[39] and Chu et al[40].
The medical records of 838 patients hospitalized in China indicated an increased level of AST and GGT[40]. Anomalies in LFTs (AST, GGT) were associated with organ injuries, hypoxia, inflammation and the use of antiviral drugs. The level of AST, ALT, GGT and total bilirubin displayed no significant difference between patients who were treated or not treated with umifenovir. By way of contrast, patients who underwent lopinavir/ritonavir treatment had higher levels of AST and GGT. Among the total number of COVID-19 patients, 48.8% showed normal liver function and 51.2% liver injury. Fan et al[29] observed abnormal liver function defined as increased LFTs in 57.8% of SARS-CoV-2 patients treated with lopinavir/ritonavir. Moreover, research in Italy suggested that remdesivir may be significant in the origin of hepatocellular injury[41]. Four out of five patients who switched from lopinavir/ritonavir to remdesivir had a reduced level of bilirubin, and significantly increased levels of AST and ALT.
In a study of 2115 people conducted in China, a more notable level of liver injury was uncovered in the group treated with lopinavir/ritonavir than in the untreated group[42]. Patients with COVID-19 and with pre-existing liver injury had more severe disease and a higher prevalence of mortality. However, the observed changes did not mimic the so-called ‘cytokine storm’ because the absolute lymphocyte count was lower and ESR was higher in the liver injury group than that of the non-liver injury group.
Hundt et al[43] observed abnormal liver tests at admission (AST 66.9%, ALT 41.6%, ALP 13.5%, and TBIL 4.3%) and peak of hospitalization (AST 83.4%, ALT 61.6%, ALP 22.7%, and TBIL 16.1%). Moreover, the type of treatment used (hydroxychloroquine, lopinavir/ritonavir, remdesivir, tocilizumab) was associated with abnormal liver transaminase elevations during hospitalization. The results of liver tests were associated with intensive care unit (ICU) admission, mechanical ventilation and death, as well as age, sex and comorbidities. Patients with severe COVID-19 showed an increase in the total of bilirubin and regardless of severity, a significant rise in transaminases and decrease in albumin was observed[43]. Studies conducted on the Indian population also confirmed the link between laboratory test abnormalities and the severity of the disease[44]. Kumar et al[44] included 91 patients in their study, excluding those with pre-existing liver disease (hepatitis B and C, alcoholics, those on known hepatotoxic treatment). The analysis of patients divided into groups (I. asymptomatic, II. mild, III. moderate, IV. severe) showed that the level of transaminases was highest in group IV, ALP was highest in group III but for total bilirubin growth there was no difference between the groups. This study showed that AST and ALP are better tests for indicating the severity of liver damage in COVID-19 than ALT and TBIL.
LFT abnormality was confirmed in 17.6% of Chinese patients with the COVID-19 infection (a population of 159 patients)[45]. The authors concluded that frequency of LFT abnormality was greater in patients with chronic disease than those with mild/moderate illness, especially in older patients. In the another study (148 cases) abnormal liver function was noted in 37.2% of patients on admission and nearly half of those were over 50 years old, half of the 37.2% being men[44]. The patients with abnormal liver function had higher inflammatory indexes (CRP and procalcitonin). On admission, patients who received lopinavir/ritonavir treatment displayed a higher frequency of abnormal LFTs than those with normal liver function. The effect of antiviral treatment on liver function was observed in the study of Zampino et al[41]. Treatment of COVID-19 patients with remdesivir can cause hepatocellular injury with aminotransferase elevation, in contrast to the trend of bilirubin elevation with lopinavir/ritonavir treatment.
Abnormally raised liver enzymes were seen in about half of patients with COVID-19 disease[46]. AST and/or ALT > 3 × ULN, and/or ALP and/or GGT > 2 × ULN was seen in 53.5% of patients with hepatocellular injury. In addition, an association between LFTs and markers of inflammation (CRP and ferritin) was observed. Total protein and albumin, were significantly reduced in patients with abnormal liver enzymes and in patients with liver injury, in contrast to the total bilirubin level, which was significantly increased in these patients. Hepatocellular and cholestatic liver injury was more frequent in patients below the age of 50, whereas in patients over 50 years old, more common was the mixed type of liver injury.
Among a French cohort of 281 patients, 102 of them had increased liver enzymes (36.3%)[47]. The most common was an increase in GGT, followed by AST and ALT. Cases with elevated LFTs and CRP value were associated with higher rates of admission to ICU and mortality. Age, sex, diabetes and hypertension were not associated with disease severity. High levels of ALT or AST are associated with disease severity. The authors suggested that liver abnormalities are due to sepsis and tissue hypoxemia, which is documented by apoptotic injuries visualized in the histological examination (vesicular steatosis and watery degeneration). In summary, liver test abnormalities are associated with a poorer prognosis in patients with the coronavirus disease 2019[47].
A study conducted in Istanbul confirmed that liver test abnormalities, especially the AST/ALT ratio, was a good marker of mortality risk and the need for ICU admission[48]. A poorer prognosis rate was associated with higher levels of AST and ALT in the mixed pattern group followed by the hepatocellular injury group and the cholestatic injury group. Mortality in patients with abnormal AST and ALT was higher than that of patients with normal results. The patients with increased AST and ALT showed elevated levels of CRP, procalcitonin, ferritin, D-dimer, lactate and TBIL, which ultimately extended the hospitalization period[48]. The percentage of people in the ICU with elevated aminotransferases was higher than those with normal test results. Patients with ratio AST/ALT > 1 had a higher level of CRP, fibrinogen, LDH, APTT, d-dimer and lower levels of lymphocyte, albumin and GGT. This study showed that low albumin may be marker of severity in SARS-CoV-2 during the hospital admission. Abnormalities in LFTs are more common in men compared to women.
Comorbidities in people with liver diseases are a huge problem, which may have an impact on the severity of COVID-19. A prime example is obesity, in which a person is more prone to develop non-alcoholic fatty liver diseases (NAFLD)[49]. In adipose tissue, there may be a greater expression of ACE2, which increases the risk of severe COVID-19. Chronic liver disease also affects the severity of the disease. This may be related to low levels of blood platelets and lymphocytes[50]. A higher index of cytokines has also been reported, which may influence the progression of NAFLD[51]. In the course of liver cirrhosis, attention should be paid to the activation of cytokines, which leads to hepatocyte necrosis. A study population, from 9 hospitals in Lombardy showed higher mortality (17 out of 50 respondents died)[52]. There was a decrease in albumin in patients and a significant increase in bilirubin, creatinine and prothrombin. Zou et al[53] detected elevated LFTs in 105 Wuhan patients with chronic HBV infection and coexisting SARS-CoV-2 (ALT 20.95%, AST 27.62%, TBIL and GGT 6.67%). These values changed during hospitalization, where 28.57% of the subjects developed acute or chronic liver failure[53]. Research carried out on 9 pregnant women showed lymphopenia (< 10 × 109 cells per L) in 5 of them, elevated CRP (> 10 mg/L) in 6 and 3 had raised AST and ALT[54]. One patient demonstrated a very high level of AST (1263 U/L) and ALT (2093 U/L).
Liver injury in severe COVID-19
The liver test abnormalities mentioned above are more frequently found in severe COVID-19 infection than in mild courses of the same infection. A few studies have demonstrated a relationship between liver test abnormalities, disease severity and mortality of patients with COVID-19[30,55]. A higher rate of LFT abnormalities was observed in severe COVID-19 infection. The higher liver test markers such as ALT, AST, GGT and total bilirubin were reported more in severe patients than in non-severe ones[56,57]. A large cohort study totalling 1099 patients, reported a much higher level of ALT and AST in severe patients (28% and 39%, respectively) than in non-severe patients (20% and 18%, respectively)[39]. So-called weighted mean difference for AST, ALT, total bilirubin and for albumin were associated with a significant increase in the severity of COVID-19 infection[58]. Among the 3381 patients included in the retrospective cohort study, 67.2% of them who were positive for SARS-CoV-2 had higher initial and peak of ALT than those who were negative[59]. Additionally, severe acute liver injury was significantly associated with elevated inflammatory markers including ferritin and IL-6. Besides ferritin and IL-6, other tests such as WBC count, lymphocyte count and platelet count were strong discriminators for severe disease[60].
There is a discrepancy between the frequency of liver test abnormalities and the liver injury in COVID-19 patients. For example, elevated liver damage markers were present in 76.3% of hospitalised patients but only 21.5% of them had liver injury[30]. This variance can be explained by pre-existing liver diseases, which contributed to the severity of liver injury during COVID-19 infection[61,62]. Finally, patients with severe liver injury are more likely to have a poorer prognosis[21]. On the other hand, pre-existing liver disease can increase the risk of COVID-19 infection[63].
CONCLUSION
Not all COVID-19 patients have liver injury and abnormalities in LFTs. However, after measuring the wide variations in these tests, the clinicians can come to some conclusions about the severity of the liver disease and improve the prognosis for patients with liver damage.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: February 25, 2021
First decision: May 3, 2021
Article in press: November 24, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Poland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lin W S-Editor: Liu M L-Editor: A P-Editor: Liu M
Contributor Information
Dagmara Przekop, Diagnostics-Experimental Center of Sexually Transmissible Diseases, Bialystok 15-879, Poland.
Ewa Gruszewska, Department of Biochemical Diagnostics, Medical University of Bialystok, Bialystok 15-269, Poland.
Lech Chrostek, Department of Biochemical Diagnostics, Medical University of Bialystok, Bialystok 15-269, Poland. chrostek@umb.edu.pl.
References
- 1.Bogoch II, Watts A, Thomas-Bachli A, Huber C, Kraemer MUG, Khan K. Potential for global spread of a novel coronavirus from China. J Travel Med. 2020;27 doi: 10.1093/jtm/taaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tian D, Ye Q. Hepatic complications of COVID-19 and its treatment. J Med Virol. 2020;92:1818–1824. doi: 10.1002/jmv.26036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baj J, Karakuła-Juchnowicz H, Teresiński G, Buszewicz G, Ciesielka M, Sitarz E, Forma A, Karakuła K, Flieger W, Portincasa P, Maciejewski R. COVID-19: Specific and Non-Specific Clinical Manifestations and Symptoms: The Current State of Knowledge. J Clin Med. 2020;9 doi: 10.3390/jcm9061753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain U. Effect of COVID-19 on the Organs. Cureus. 2020;12:e9540. doi: 10.7759/cureus.9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gavriatopoulou M, Korompoki E, Fotiou D, Ntanasis-Stathopoulos I, Psaltopoulou T, Kastritis E, Terpos E, Dimopoulos MA. Organ-specific manifestations of COVID-19 infection. Clin Exp Med. 2020;20:493–506. doi: 10.1007/s10238-020-00648-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciaccio M, Agnello L. Biochemical biomarkers alterations in Coronavirus Disease 2019 (COVID-19) Diagnosis (Berl) 2020;7:365–372. doi: 10.1515/dx-2020-0057. [DOI] [PubMed] [Google Scholar]
- 9.Chellasamy G, Arumugasamy SK, Govindaraju S, Yun K. Analytical insights of COVID-19 pandemic. Trends Analyt Chem. 2020;133:116072. doi: 10.1016/j.trac.2020.116072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu L, Wang B, Yuan T, Chen X, Ao Y, Fitzpatrick T, Li P, Zhou Y, Lin YF, Duan Q, Luo G, Fan S, Lu Y, Feng A, Zhan Y, Liang B, Cai W, Zhang L, Du X, Li L, Shu Y, Zou H. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis. J Infect. 2020;80:656–665. doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 13.Channappanavar R, Zhao J, Perlman S. T cell-mediated immune response to respiratory coronaviruses. Immunol Res. 2014;59:118–128. doi: 10.1007/s12026-014-8534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang SH. What We Know So Far (As of March 26, 2020) About COVID-19-An MRT Point of View. J Med Imaging Radiat Sci. 2020;51:200–203. doi: 10.1016/j.jmir.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr. 2020;14:247–250. doi: 10.1016/j.dsx.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63:390–391. doi: 10.1016/j.pcad.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang Y, Chen T, Mui D, Ferrari V, Jagasia D, Scherrer-Crosbie M, Chen Y, Han Y. Cardiovascular manifestations and treatment considerations in COVID-19. Heart. 2020;106:1132–1141. doi: 10.1136/heartjnl-2020-317056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020;5:831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 19.Ali N, Hossain K. Liver injury in severe COVID-19 infection: current insights and challenges. Expert Rev Gastroenterol Hepatol. 2020;14:879–884. doi: 10.1080/17474124.2020.1794812. [DOI] [PubMed] [Google Scholar]
- 20.Garrido I, Liberal R, Macedo G. Review article: COVID-19 and liver disease-what we know on 1st May 2020. Aliment Pharmacol Ther. 2020;52:267–275. doi: 10.1111/apt.15813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng G, Zheng KI, Yan QQ, Rios RS, Targher G, Byrne CD, Poucke SV, Liu WY, Zheng MH. COVID-19 and Liver Dysfunction: Current Insights and Emergent Therapeutic Strategies. J Clin Transl Hepatol. 2020;8:18–24. doi: 10.14218/JCTH.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alqahtani SA, Schattenberg JM. Liver injury in COVID-19: The current evidence. United European Gastroenterol J. 2020;8:509–519. doi: 10.1177/2050640620924157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40:2095–2103. doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 25.Boeckmans J, Rodrigues RM, Demuyser T, Piérard D, Vanhaecke T, Rogiers V. COVID-19 and drug-induced liver injury: a problem of plenty or a petty point? Arch Toxicol. 2020;94:1367–1369. doi: 10.1007/s00204-020-02734-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naidoo K, Hassan-Moosa R, Mlotshwa P, Yende-Zuma N, Govender D, Padayatchi N, Abdool-Karim SSS. High Rates of Drug-induced Liver Injury in People Living With HIV Coinfected With Tuberculosis (TB) Irrespective of Antiretroviral Therapy Timing During Antituberculosis Treatment: Results From the Starting Antiretroviral Therapy at Three Points in TB Trial. Clin Infect Dis. 2020;70:2675–2682. doi: 10.1093/cid/ciz732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonacini M. Liver injury during highly active antiretroviral therapy: the effect of hepatitis C coinfection. Clin Infect Dis. 2004;38 Suppl 2:S104–S108. doi: 10.1086/381453. [DOI] [PubMed] [Google Scholar]
- 28.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luglio M, Tannuri U, de Carvalho WB, Bastos KLM, Rodriguez IS, Johnston C, Delgado AF. COVID-19 and Liver Damage: Narrative Review and Proposed Clinical Protocol for Critically ill Pediatric Patients. Clinics (Sao Paulo) 2020;75:e2250. doi: 10.6061/clinics/2020/e2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang W, Cao Q, Qin L, Wang X, Cheng Z, Pan A, Dai J, Sun Q, Zhao F, Qu J, Yan F. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19):A multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80:388–393. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, Akdis CA, Gao YD. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 35.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vespa E, Pugliese N, Piovani D, Capogreco A, Danese S, Aghemo A Humanitas Covid-19 Task Force. Liver tests abnormalities in COVID-19: trick or treat? J Hepatol. 2020;73:1275–1276. doi: 10.1016/j.jhep.2020.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807–816. doi: 10.1016/j.jhep.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, Zhang XJ, Cai J, Lin L, Ouyang S, Wang X, Yang C, Cheng X, Liu W, Li H, Xie J, Wu B, Luo H, Xiao F, Chen J, Tao L, Cheng G, She ZG, Zhou J, Wang H, Lin J, Luo P, Fu S, Ye P, Xiao B, Mao W, Liu L, Yan Y, Chen G, Huang X, Zhang BH, Yuan Y. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology. 2020;72:389–398. doi: 10.1002/hep.31301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chu H, Bai T, Chen L, Hu L, Xiao L, Yao L, Zhu R, Niu X, Li Z, Zhang L, Han C, Song S, He Q, Zhao Y, Zhu Q, Chen H, Schnabl B, Yang L, Hou X. Multicenter Analysis of Liver Injury Patterns and Mortality in COVID-19. Front Med (Lausanne) 2020;7:584342. doi: 10.3389/fmed.2020.584342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zampino R, Mele F, Florio LL, Bertolino L, Andini R, Galdo M, De Rosa R, Corcione A, Durante-Mangoni E. Liver injury in remdesivir-treated COVID-19 patients. Hepatol Int. 2020;14:881–883. doi: 10.1007/s12072-020-10077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yadav DK, Singh A, Zhang Q, Bai X, Zhang W, Yadav RK, Zhiwei L, Adhikari VP, Liang T. Involvement of liver in COVID-19: systematic review and meta-analysis. Gut. 2021;70:807–809. doi: 10.1136/gutjnl-2020-322072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hundt MA, Deng Y, Ciarleglio MM, Nathanson MH, Lim JK. Abnormal Liver Tests in COVID-19: A Retrospective Observational Cohort Study of 1,827 Patients in a Major U.S. Hospital Network. Hepatology. 2020;72:1169–1176. doi: 10.1002/hep.31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar A, Kumar P, Dungdung A, Kumar Gupta A, Anurag A, Kumar A. Pattern of liver function and clinical profile in COVID-19: A cross-sectional study of 91 patients. Diabetes Metab Syndr. 2020;14:1951–1954. doi: 10.1016/j.dsx.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li S, Li J, Zhang Z, Tan L, Shao T, Li M, Li X, Holmes JA, Lin W, Han M. COVID-19 induced liver function abnormality associates with age. Aging (Albany NY) 2020;12:13895–13904. doi: 10.18632/aging.103720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saini RK, Saini N, Ram S, Soni SL, Suri V, Malhotra P, Kaur J, Verma I, Sharma S, Zohmangaihi D. COVID-19 associated variations in liver function parameters: a retrospective study. Postgrad Med J. :2020 epub ahead of print. doi: 10.1136/postgradmedj-2020-138930. [DOI] [PubMed] [Google Scholar]
- 47.Chaibi S, Boussier J, Hajj WE, Abitbol Y, Taieb S, Horaist C, Jouannaud V, Wang P, Piquet J, Maurer C, Lahmek P, Nahon S. Liver function test abnormalities are associated with a poorer prognosis in Covid-19 patients: Results of a French cohort. Clin Res Hepatol Gastroenterol. 2021;45:101556. doi: 10.1016/j.clinre.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Medetalibeyoglu A, Catma Y, Senkal N, Ormeci A, Cavus B, Kose M, Bayramlar OF, Yildiz G, Akyuz F, Kaymakoglu S, Tukek T. The effect of liver test abnormalities on the prognosis of COVID-19. Ann Hepatol. 2020;19:614–621. doi: 10.1016/j.aohep.2020.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng KI, Gao F, Wang XB, Sun QF, Pan KH, Wang TY, Ma HL, Chen YP, Liu WY, George J, Zheng MH. Letter to the Editor: Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108:154244. doi: 10.1016/j.metabol.2020.154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qi X, Liu Y, Wang J, Fallowfield JA, Li X, Shi J, Pan H, Zou S, Zhang H, Chen Z, Li F, Luo Y, Mei M, Liu H, Wang Z, Li J, Yang H, Xiang H, Liu T, Zheng MH, Liu C, Huang Y, Xu D, Kang N, He Q, Gu Y, Zhang G, Shao C, Liu D, Zhang L, Kawada N, Jiang Z, Wang F, Xiong B, Takehara T, Rockey DC COVID-Cirrhosis-CHESS Group. Clinical course and risk factors for mortality of COVID-19 patients with pre-existing cirrhosis: a multicentre cohort study. Gut. 2021;70:433–436. doi: 10.1136/gutjnl-2020-321666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prins GH, Olinga P. Potential implications of COVID-19 in non-alcoholic fatty liver disease. Liver Int. 2020;40:2568. doi: 10.1111/liv.14484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iavarone M, D'Ambrosio R, Soria A, Triolo M, Pugliese N, Del Poggio P, Perricone G, Massironi S, Spinetti A, Buscarini E, Viganò M, Carriero C, Fagiuoli S, Aghemo A, Belli LS, Lucà M, Pedaci M, Rimondi A, Rumi MG, Invernizzi P, Bonfanti P, Lampertico P. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73:1063–1071. doi: 10.1016/j.jhep.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zou X, Fang M, Li S, Wu L, Gao B, Gao H, Ran X, Bian Y, Li R, ShanshanYu , Ling J, Li D, Tian D, Huang J. Characteristics of Liver Function in Patients With SARS-CoV-2 and Chronic HBV Coinfection. Clin Gastroenterol Hepatol. 2021;19:597–603. doi: 10.1016/j.cgh.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, Li J, Zhao D, Xu D, Gong Q, Liao J, Yang H, Hou W, Zhang Y. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bloom PP, Meyerowitz EA, Reinus Z, Daidone M, Gustafson J, Kim AY, Schaefer E, Chung RT. Liver Biochemistries in Hospitalized Patients With COVID-19. Hepatology. 2021;73:890–900. doi: 10.1002/hep.31326. [DOI] [PubMed] [Google Scholar]
- 56.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW the Northwell COVID-19 Research Consortium, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parohan M, Yaghoubi S, Seraji A. Liver injury is associated with severe coronavirus disease 2019 (COVID-19) infection: A systematic review and meta-analysis of retrospective studies. Hepatol Res. 2020;50:924–935. doi: 10.1111/hepr.13510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, Fox AN, Zucker J, Verna EC. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large U.S. Cohort. Hepatology. 2020;72:807–817. doi: 10.1002/hep.31404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 61.Singh S, Khan A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology. 2020;159:768–771.e3. doi: 10.1053/j.gastro.2020.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moon AM, Webb GJ, Aloman C, Armstrong MJ, Cargill T, Dhanasekaran R, Genescà J, Gill US, James TW, Jones PD, Marshall A, Mells G, Perumalswami PV, Qi X, Su F, Ufere NN, Barnes E, Barritt AS, Marjot T. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: Preliminary results from an international registry. J Hepatol. 2020;73:705–708. doi: 10.1016/j.jhep.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liang W, Guan W, Chen R, Wang W, Li J, Xu K, Li C, Ai Q, Lu W, Liang H, Li S, He J. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]