Abstract
Lower back pain is a leading cause of disability and is one of the reasons for the substantial socioeconomic burden. The etiology of intervertebral disc (IVD) degeneration is complicated, and its mechanism is still not completely understood. Factors such as aging, systemic inflammation, biochemical mediators, toxic environmental factors, physical injuries, and genetic factors are involved in the progression of its pathophysiology. Currently, no therapy for restoring degenerated IVD is available except pain management, reduced physical activities, and surgical intervention. Therefore, it is imperative to establish regenerative medicine-based approaches to heal and repair the injured disc, repopulate the cell types to retain water content, synthesize extracellular matrix, and strengthen the disc to restore normal spine flexion. Cellular therapy has gained attention for IVD management as an alternative therapeutic option. In this review, we present an overview of the anatomical and molecular structure and the surrounding pathophysiology of the IVD. Modern therapeutic approaches, including proteins and growth factors, cellular and gene therapy, and cell fate regulators are reviewed. Similarly, small molecules that modulate the fate of stem cells for their differentiation into chondrocytes and notochordal cell types are highlighted.
Keywords: Stem cell, Intervertebral disc, Degeneration, Inflammation, Cell therapy, Gene modification
Core Tip: In this review, we presented a precise overview of the anatomical and molecular structure and surrounding pathophysiology of the intervertebral disc (IVD). Modern therapeutic approaches including proteins and growth factors, cellular and gene therapy, and cell fate regulators are highlighted. In addition, different types of stem cells used for the implantation in IVD are reviewed. Furthermore, small molecules that modulate the fate of stem cells for their differentiation into chondrocytes and notochordal cell types are presented. In conclusion, this review highlights regenerative medicine-based approaches for the regeneration of intervertebral disc degeneration.
INTRODUCTION
Intervertebral disc (IVD) degeneration is a progressive, inflammation-driven cascade that leads to structural and mechanical failure, strongly associated with lower back pain (LBP), representing a global health burden. The worst aspect(s) of degenerative disc disease (DDD) is/are pain, discomfort, emotional distress, and functional disability, affecting the quality of life and causing socioeconomic burden[1]. Altered cellular microenvironment within the disc, reduced cell viability due to structural failure, and functional inadequacy are the leading causes of the adverse condition in LBP[2,3]. IVD degeneration (IVDD) treatments can only mitigate painful symptoms and improve flexibility and body movements[4].
Around 84% of the population experience an event of LBP sooner or later in their life span; 50% of them are younger age group (18 to 44 years), otherwise adulthood (45 to 64-years), and generate almost 80% of health care expenditure[5]. Even though the correct etiology of LBP remains obscure[6], IVDD results due to the loss of nucleus pulposus (NP) and/or annulus fibrosus (AF), which leads to the reduction in water content, diminished glycosaminoglycans (GAGs), and extracellular matrix (ECM), and collagen II deterioration in the NP region[7]. This remodeling results in reduced IVD height, osteophyte development, facet joint arthritis, and bending of vertebral bodies, which are reflected through magnetic resonance imaging (MRI)[8]. Spine fusion is the only available option, but it greatly reduces the flexion of the body. With the disease advancement, pharmaceutical or otherwise postoperative intervention is needed to reduce symptomatic pain and reserve the flexion of the spine[9]. Despite the innovations in IVD surgery, patients with the progressive disorder cannot receive the benefits of surgical intervention because of the associated morbidities.
Perinatal stem cells and their derivatives can offer an improved therapeutic approach for the treatment of disc degenerated diseases. Mesenchymal stem cells (MSCs) are being utilized to rectify the pathogenesis of DDD[10]. This review presents an overview of IVD biology and how cellular signaling plays a role in IVD homeostasis. We also review the opportunities and challenges for the utilization of cell-based therapy for IVD regeneration.
CELLULAR SIGNAL IN IVD
The development of IVD in embryogenesis relies on the coordinated network of molecular signals arising in the notochord and neural tube plate[11]. Following signaling pathways are involved in the IVD.
Sonic hedgehog
Sonic hedgehog (Shh) signaling plays a vital role in tissue morphogenesis, regulation, presenting information about embryonic patterning, and degree of cell fate differentiation and proliferation[12,13]. Somite stalks evolve in response to Shh and Wnt (wingless-related integration site) dependent regulatory pathways, while a sclerotome tissue generates only under the activating impact of the Shh pathway[14]. A specific attribute of the Shh intracellular signaling cascade works through synergistic interaction with Noggin-cascade, a direct antagonist of the bone morphogenetic proteins (BMPs) pathway in the induction of sclerotome growth[14,15]. Noggin molecules are primitively expressed by the notochord cells blocking BMP signaling from developing vertebral bodies till the formation of the AF[16,17].
Paired box genes
Paired box (Pax) genes encode transcription regulators for proliferation, differentiation, apoptosis, and migration of pluripotent cells during embryogenesis. Expression of Pax genes plays an essential role in subsequent cell differentiation of distinct populations of IVD[18-20]. It is proved that Pax1 and Pax9 genes are entirely involved in the IVD formation. When these genes are obliterated, IVD and vertebral bodies do not develop, forming an irregular cartilaginous core[21]. Pax1 gene expression in all sclerotome tissues is intervened by the activity of Shh and Noggin regulatory pathways in the notochord cells[22,23]. After IVD development, expression of the Pax1 gene arises exclusively in the tissue of IVD primordium (precursor of the AF) enclosing the notochord. Hence, the Pax1 gene impacts the notochord advancement by activating cell expansion which turns into the NP.
SRY-box genes
The SRY-box (Sox) family is involved in developing the vertebral column[24,25]. Sox5, Sox6, and Sox9 genes are of significant importance for IVD development and growth. Sox5 and Sox6 are present in the cells of the notochord and the sclerotome[26]. In the mice deprived of Sox5 and Sox6 genes, the development of the notochordal membrane was weakened. This is associated with the evidence that these genes are key players in genesis IVD and intercellular proteins, including collagen II and aggrecan[26,27]. Lack of notochordal membrane prompts apoptosis of the notochordal cells (NCs) and disrupts the development of IVD segments. In the cells with knockout Sox9, notochord development starts, which is degraded due to the deprivation of the notochordal membrane matrix and inhibits the formation of sclerotome[28].
Transforming growth factor-β genes
Transforming growth factor-β (TGF-β) signaling pathways are effectively involved in advancing IVD and vertebral bodies. TGF-β intercellular signaling cascade stimulates cellular migration, proliferation, differentiation, and IVD matrix synthesis[29]. TGF-β3 is actively synthesized in the perichordal membrane during the condensation stage of embryogenesis and promotes the development of the AF and vertebral bodies. Blockage of the TGF-β2 receptors inhibits the synthesis of type II collagen leading to defective NP, the exterior part of the AF, and inadequate IVD mineralization. TGF-β2 receptors participate in the differentiation of IVD tissue and vertebral bodies, producing spine[30].
IVDD
DDD is a complex, multifactorial process, the etiology of which is not well known. Thus, there are no particular criteria to differentiate the IVDD from the physiological retardation of development, maturation, or adaptive tissue remodeling[31]. IVDD has perhaps been best defined as an “aberrant cell-mediated response to progressive structural failure”[32]. Heredities, ecological causes, mechanical factors, aging, systemic and toxic mediators are identified as risk factors[33]. This mechanism begins with alterations to the cellular IVD microenvironment leading to structural and functional failure[34]. Interestingly, evidence showed that the early disappearance of NC density in NP is crucial for IVD stability and induces impairment in the ECM anabolic/catabolic proportion, resulting in the change of the IVD mechanical properties[25,35]. IVDD is related to expanded ECM disruption[36], abnormal matrix formation[37], cellular apoptosis[38], inflammation[39], and regulation of sensory nerve and blood vessel in-growth into a normal avascular and neural tissue[40].
The onset of the IVDD is believed to be mainly in the NP[41]. The decline of the key essential proteoglycan, aggrecan[42], reduces additional ECM production in the NP, and causes decreased hydration[43], a deficit of IVD height, and general failure to resist compressive burden[44]. Compression pressures are hence dispensed through the NP to the adjacent AF, which leads to altered biomechanical function of AF and structural failure with radial and circumferential tears in the AF[45]. These fissures and tears facilitate the in-growth of nociceptive nerves and blood vessels, resulting in the secretion of inflammatory pain-related mediators, thus leads to radial disc bulges or herniation of the NP into the contiguous spine, causing LBP[34].
Although the IVDs degenerate with aging and can be asymptomatic, a pathological process of IVDD is followed by pain. It has been revealed that a large number of people with no pain show degenerative disc changes that further complicate the differentiation of typical age-related degeneration from pathological conditions[46]. An increase in catabolic action of matrix-degrading proteases, pro-inflammatory cytokines, and contemporary immune cell infiltration is proposed to define disc degeneration factors[39]. Furthermore, lower disc pH, reduced nutrition, and calcified cartilaginous endplate (CEP) create an unfavorable environment for restoring the disc[47]. Presently, there are symptomatic cures for advanced phases of DDD but no effective disease-modifying therapies[48].
Inflammation in degenerated IVD
Degenerated IVD cells produce higher concentrations of pro-inflammatory mediators, which suggest their role in the pathogenesis of IVD. A variety of cytokines, chemokines, and enzymes have been associated with IVDD, including interleukins (IL), interferons, tumor necrosis factor-alpha (TNF-α), matrix metalloproteinases (MMPs), prostaglandin E2 (PGE2), nitric oxide (NO), and aggrecanase. Among these, TNF-α and cytokines of the IL-1 family have been most widely investigated. Both TNF-α and IL-1β are produced by IVD cells, and they acquire strong association in the pathogenesis of IVDD[49,50]. Degenerated and herniated discs exhibit upregulated expression of both pro-inflammatory chemokines, TNF-α and IL-1β[51]. Both have been found to activate ECM degrading enzymes and reduce ECM constituent synthesis in vitro[49,52]. Recent studies showed that both TNF-α and IL-1β molecules induce increased MMP expression, particularly MMP-1, -2, -3, -7, -8, and -13. These MMPs are well recognized for their proteolytic activity towards collagen and proteoglycans (PGs)[53]. Also, IL-1β, as a pro-inflammatory cytokine, upregulates the vascular endothelial growth factor (VEGF), brain-derived neurotrophic factor, and nerve growth factor expressions to stimulate the neovascularization and neoinnervation of IVD that eventually lead to inflammation and discogenic pain[24]. Another study concluded that IL-1β is a master regulator in the disc cells that influence other cytokines and chemokines[54]. IL-1β and TNF-α in NP cells contribute to the secretion of chemoattractant molecules such as C-C motif ligand 5/regulated 5 (CCL5/CCR5), regulated upon activation, normal T cell expressed and presumably secreted (CCL5/RANTES) or chemokine C-X-C motif ligand 6 (CXCL6)[55], and are involved in the migration of MSCs.
Another pro-inflammatory cytokine that has been involved in the pathogenesis of IVDD is IL-6, which is also secreted by NP cells[56]. Indeed, degenerated IVD tissue samples contain a significantly higher expression of IL-6[57]. Notably, numerous genetic variations in cytokine genes have been correlated with IVD degeneration. Traditionally, inflammation has mainly been considered as a primary reaction to infection at the site of tissue injury; however, it is not sure if it is a cause or outcome of IVD degeneration and herniation[58]. During degeneration, increased aggrecan and collagen breakdown occur within the disc tissue with significant changes in IVD cell phenotype and increased levels of inflammatory cytokines[47]. With an advanced degeneration phase, clefts and tears are developed in the AF and NP, which leak into the external environment. This allows immune cell activation and the invading blood vessels to pervade the IVD through the clefts and tears of the AF[59].
THERAPEUTICS FOR DEGENERATIVE INTERVERTEBRAL DISCS
Modern treatments for IVDD remain a subject of debate. Despite the known consequences of the IVD pathological cascade, the treatment options for IVDD are limited. The traditional conservative therapy for chronic LBP involves a wide range of treatment modalities, including bed rest, physiotherapy, analgesic and anti-inflammatory medications, acupuncture, and chiropractic[60]. Approximately, 75%-90% of chronic LBP patients obtain satisfactory results with conservative treatment[34,61]. The pain symptoms can be overcome by administering anti-inflammatory mediators, for example, opioids, steroids, non-steroidal anti-inflammatory drugs, and muscle relaxants[39]. These anti-inflammatory drugs have effective short-term alleviation for back pain, but they cannot reverse the progression of IVDD[62]. If conservative management does not have the desired effect, the constant pain sensation progresses because of the nerve compression[63].
Interventional procedures for IVDD include spinal surgical interventions, such as discectomy, spine fusion, and total disc replacement to manage the degenerated disc. The main surgical treatment alternatives for IVDD are spinal fusion and the replacement of the whole disc. Spinal fusion surgery, fusing two vertebrae, provides stability to the spine, which can be attained by a range of surgical interventions, such as posterolateral fusion, anterior and posterior lumbar interbody fusion. The minimally invasive methods to the lumbar spine for interbody fusion include lateral lumbar interbody fusion[64]. Spinal fusion is considered as a gold standard treatment option for LBP[65]. The results of three randomized controlled trials, which compared spinal fusion with conservative treatment, showed substantial clinical improvement in only a limited number of patients[45].
Moreover, spinal fusion could accelerate the degenerative process in adjacent vertebrae[66], and it mitigates painful symptoms, irrespective of repairing disc structure and mechanics; therefore, its efficacy remains controversial. Disc arthroplasty has the advantage of removing the degenerated IVD and restoring it with a prosthesis that can permit flexibility between the discs[67]. Moreover, disc arthroplasty does not restore the mechanical movement of the native joint[61]. The additional motion-preserving surgical procedure includes posterior dynamic stabilization. These surgical procedures contain the installation of pedicle screws over a motion segment associated with a flexible graft. These devices intend to limit motion over the interspace to control discogenic pain[68]. The disadvantages of the surgical therapies can be extreme invasiveness, the increased possibility of recurrences, and failure of mechanical properties with contiguous segment degeneration. In most cases, some surgical intrusions and conservative treatments have low efficiency with lack of sustainable long-term effects. Instead of targeting the pathophysiology of the degenerative progression, they target the clinical symptoms[69].
Recent surgical treatment options for symptomatic degenerated IVD are still far from optimal outcomes. Hence, there is a substantial necessity for new therapies that focus on relieving painful symptoms and reestablishing IVD structure and mechanical loading capacity by explicitly addressing the underlying biological causes of DDD.
NOVEL THERAPEUTIC APPROACHES
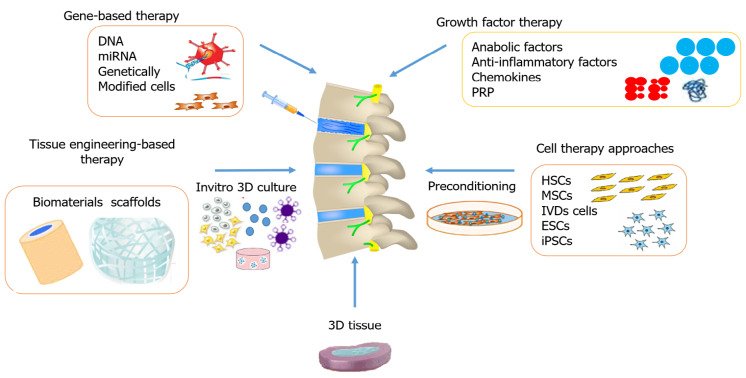
The advancements in research and development have encouraged scientists to search for innovative pharmacological therapies in the regeneration of the IVD that mitigate painful symptoms by restoring and maintaining mechanical function. Depending on the stage of degeneration, different biological treatment options are used that alter the cascaded events at the molecular level. Figure 1 summarizes various therapeutic options for disc degeneration diseases. The three major groups of biological approaches for disc regeneration are divided as follows: (1) In the early stage of IVDD (grade II-III), growth factor injections may be effective; (2) In the intermediate stage of degeneration (grade IV), gene therapy or cell therapy may be required; and (3) In the advanced stage of IVDD (grade V), tissue engineering approaches are needed[70].
Figure 1.
Different approaches used for restoring a degenerated disc. MSCs: Mesenchymal stem cells; ESCs: Embryonic stem cells; iPSCs: Induced pluripotent stem cells; IVD: Intervertebral disc; HSCs: Hematopoietic stem cells; PRP: Platelet-rich plasma.
Growth factor therapy
The therapeutic use of growth factors enhances the matrix synthesis and delay degeneration by reducing inflammation[71,72]. Growth factors are the peptides or polypeptides that target specific receptors present on the surface of the cell, thereby influencing cell proliferation, differentiation and increasing their ability to synthesize the ECM[73,74]. Specific growth factors that include BMPs and TGF-β family members are used to stimulate osteogenic and chondrogenic differentiation[75,76].
The first successful exogenous administration of TGF-β1 in animal models showed the enhanced synthesis of PGs in the NP. Several in vitro and in vivo analyses on BMP-2 and -7, TGF-β, epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), growth and differentiate factor 5 (GDF-5), and insulin-like growth factor 1 (IGF-1) revealed that they stimulate the synthesis of ECM[77-82].
In chronic conditions of IVDD, cocktails of growth factors may be needed because the growth factors have a short half-life and are unstable that limits their use as direct injection into the IVD. The administration of multiple injections of growth factors could enhance gradual release at target site or gene-based delivery system to obtain the desired effect. Currently, the primary focus is on platelet-rich plasma (PRP) that is used as a possible therapeutic option to promote IVD regeneration[83-86]. Some limitations like the absence of standardization of the dosage, the process of preparation, and identification of mode of action need to be settled[87].
Gene-based therapy
In the last few decades, gene-based therapy has achieved wide research applications to focus on the regeneration of the IVD structures. The introduction of genes encoding the chondrocyte-specific proteins is directly transferred into the effectual host tissues[88]. The gene-dose impact needs to be characterized for a safe and effective treatment. In contrast, certain findings have revealed inadequate outcomes of direct gene approach into the host cells[89]. Nonetheless, there are limited investigations that support the efficacy of this approach[90-93]. Recently, lentiviruses are believed to be competent vectors for gene transfer because they can deliver a substantial quantity of genetic material into the host cell's genome. The most frequently studied factors are TGF-β3, Sox-9, GDF-5, BMP family including 2, 7, and 12, connective tissue growth factor (CTGF), Wnt, IL-1, tissue inhibitor of metalloproteinases (TIMP-1), and LIM mineralization protein 1 (LMP-1), that are reported to enhance the synthesis of collagen type II and aggrecan in NP cells[94-106]. Genes involved in the development of IVD are summarized in Table 1.
Table 1.
Modifying genes essential for the development of intervertebral disc
|
Ref.
|
Protein (Gene)
|
Key findings
|
| Choi et al[14], 2012 | Sonic Hedgehog (SHH) | Sclerotome tissue formation, annulus fibrosus formation, chondrogenesis of sclerotome cells |
| Wijgerde et al[15], 2005 | Noggin (NOG) | Antagonist of the BMP pathway, promotes Shh intracellular signaling cascade and Pax1 gene activation |
| Murtaugh et al[16], 1999 | Bone Morphogenetic Protein (BMP) family | In the presence of Shh, promotes chondrocyte differentiation of somite-derived IVD progenitors |
| Peters et al[21], 1999 | Paired Box 1 (PAX1) | Chondrogenic commitment of sclerotome cells |
| Sugimoto et al[27], 2013 | SRY-Box 9 (SOX) | Regulates IVD tissue growth and development |
| Sohn et al[30], 2010 | Transforming growth factor-β (TGF-β) | Development of vertebral bodies |
| Pearson et al[31], 2005 | Homeodomain Protein (HOX) | Somite Patterning |
IVD: Intervertebral disc.
Cell therapy approaches
Regardless of the development of various treatment alternatives, the conservative and surgical therapeutic approaches are not exceptionally valuable for treating degenerated disc disease. These are usually incapable of delivering any solution to reestablish the structural and mechanical function of degenerated IVD. This situation has prompted the advancement of a regenerative medicine-based approach that substitutes the apoptotic and necrotic cells and limits cell death in IVD by targeting different cellular and molecular events[107]. Out of several approved cellular and molecular approaches, the utilization of stem cell therapies has shown superior outcomes, and stem cell transplantation is being used to restore the degenerated IVDs[70]. Stem cells are undifferentiated cells that can differentiate into particular cell types and are broadly utilized as a cell therapy approach. Stem cells exist in a quiescent condition, and they self-renew in the propagation process. Stem cells are being researched in vitro and in vivo according to the need for the desired effect. Stem cell research has reformed the eventual fate of regenerative medicine because of its capability to recover impaired and damaged organs from treating various debilitating syndromes. The sources of stem cells and their properties are summarized in Table 2. Investigations are being made to comprehend the mechanism of regeneration at the molecular level to address the possible solutions for degenerative diseases and understand the basic pathogenesis and progression of different disorders.
Table 2.
Variation in properties of different sources of stem cell types
|
Properties
|
MSCs
|
ESCs
|
iPSCs
|
| Sources | Perinatal and adult tissues | Embryo at blastocyst stage | Genetically reprogrammed specialized cells |
| Plasticity | Multipotent | Pluripotent | Pluripotent |
| Teratoma formation | No | Yes | Yes |
| Growth | Limited | High | High |
| Ethical concerns | No | Yes | No |
| Immune rejection | No | Yes | No |
| Cell transplantation | Autologous and allogenic | Allogenic | Autologous |
| Clinical trials in human patients | Ongoing | Limited | In vitro/in vivo only |
| Use in genetic disorder | Deficient (Carry mutated gene) | Superior | Deficient (Carry mutated gene) |
| Ease of isolation | Yes | No | No |
MSCs: Mesenchymal stem cells; ESCs: Embryonic stem cells; iPSCs: Induced pluripotent stem cells.
STEM CELLS FOR IVD REGENERATION
Stem cells from different sources are involved in the regeneration of disc diseases. A comparison of MSCs and other cell types is presented in Table 3. Different cellular approaches used for the regeneration of IVDs are highlighted in Table 4.
Table 3.
Human umbilical cord-derived mesenchymal stem cells compared with other stem cells sources
|
Properties
|
Perinatal
|
Adult
|
Embryonic
|
| Ability to differentiate into various cell type | √ | √ | √ |
| Plastic adherence | √ | √ | |
| High in vitro proliferation ability | √ | √ | |
| Low risk of tumorigenicity | √ | √ | |
| Ethical issues | √ | ||
| Lower risk of viral contamination | √ | √ | |
| Capacity for autologous transplantation | √ | √ | |
| Established/proven treatment in human patients | √ | √ | |
| Ease of collection | √ | √ | |
| Less need for stringent antigen typing | √ | √ |
Table 4.
Summary of studies on cellular therapeutic approaches for regenerative potential of the degenerated disc
|
Type of stem cells
|
Gene
|
Preconditioning outcomes
|
Ref.
|
| In vitro human cultured NP cells and MSCs | TGF-β1 | TGF-β1 stimulates collagen-1 expression in cultured NP cells and in MSCs, increased collagen-1 and sox-9 expression. Co-cultured MSCs with NP cells showed high expression of collagen-1, aggrecan and sox-9 expression via TGF-β-dependent effect | [126] |
| Chick periosteum-derived MSCs Rabbit bone marrow-derived MSCs Rat MSCs | TGF-β1 | Stimulate chondrogenesis and inhibits osteogenesis. Facilitates in vitro chondrogenic differentiation of rabbit BM-MSCs. Increased MAPK activity and upregulation of mRNA expression of sox-9, aggrecan, and collagen type II | [190,122,123] |
| Human adipose-derived MSCs and bone marrow-derived MSCs | TGF-β3, GDF-5, or GDF-6 | In the presence of GDF-6, AD-MSCs leads to differentiation into an NP-like phenotype and results in a richer proteoglycan matrix with low rigidity | [158] |
| Human bone marrow-derived MSCs | TGF-β1, and GDF-5 | Hypoxic TGF-β1 and GDF-5 both increased aggrecan and collagen II mRNA levels and GAGs accumulation | [159] |
| In vitro human bone marrow-derived MSCs | TGF-β3, dexamethasone, and ascorbate | Preconditioned BM-MSCs expressed higher level of chondrocytes differentiation markers than culture-expanded human IVD cells and articular chondrocytes | [193] |
| In vivo murine IVD cells | TGF-β3, GDF-5, FGF, or IGF-1 | After four weeks of GDF-5 treatment, showed significantly increase in IVD height | [72] |
| Human adipose-derived MSCs | TGF-β1 and GDF-5 | Both distinctly efficient in promoting an NP cell phenotype | [160] |
| Human cultured NP cells | TGF-β1, and IL-1β | TGF-β1 improved NP cell proliferation, downregulation of mRNA expression of ADAMTS-4 and -5, upregulation expression of TIMP-3. IL-1β inhibited NP cells proliferation, increase of ADAMTS-4 and -5 | [161] |
| Canine cultured NP cells | TGF-β, and IL-10 | Suppressed IL1-β and TNF-α expression inhibiting inflammatory reaction | [200] |
| In vitro human cultured NP cells. E19 rat cultured AF cell | TGF-β1, and IGF-1 | Stimulation of human NP cells in a dose and time-dependent manner. TGF-β1 pushed AF cells to fibrocartilaginous phenotype. IGF-1 showed an upregulation of ECM | [79,162] |
| Murine ESCs | TGF-β, IGF, ascorbic acid, and cis-retinoic acid | All promotes differentiation toward chondrogenic lineage | [175] |
| Human bone marrow-derived stromal cells | TGF-β1, rhGDF-5, or bovine NPCs | Stimulates cytokeratin-19 and aggrecan/type II collagen ratio distinguish chondrogenic from IVD cell phenotype | [163] |
| Human bone marrow-derived MSCs | TGF-β3, and dexamethasone | Notochordal cell conditioned medium expressed higher level of NP-like phenotype markers and GAGs deposition than chondrogenic medium or TGF-β groups | [194] |
| Human cultured NP cells | TGF-β3, and dexamethasone | Enhanced NP proliferation, cell metabolism and reduce catabolism | [195] |
| Rabbit cultured NP cells | TGF-β1, and BMP-2 | Robust restoration of ECM. Increased mRNA expression of aggrecan, type I and type II collagen | [133] |
| In vitro porcine cultured AF cells | BMP-2, and TGF-β1 | Decrease in MMP-1 and increase in aggrecan synthesis | [73] |
| Mouse MSCs | BMP-2, 7, 13 | Proliferate and differentiate into osteoblastic and chondrogenic lineages and no adverse effects on proliferation on undifferentiated MSCs | [164] |
| Human bone marrow-derived MSCs | BMP-7 | Promotes both chondrogenic and osteogenic differentiation of MSCs | [165] |
| In vitro rat cultured AF cells | BMP-2 | Increased mRNA expression of aggrecan and type II collagen. Also, up-regulates BMP-7 and TGFβ-3 mRNA expression | [166] |
| Mouse embryonic-derived MSCs | BMP-4, Insulin, triiodothyronine, or TGF-β3 | All BMP-4, Insulin, and triiodothyronine suppressed adipogenesis and develop osteogenic phenotype. TGFβ-3 promotes chondrogenesis | [128] |
| In vitro human bone marrow-derived MSC cocultured with human cultured NP cells | BMP2, BMP4, BMP6, and BMP7 | BMP4 showed a high potential for IVDs regeneration. Although, BMP2 and BMP7 showed no potent inducer for degenerated human NP cell’s regeneration | [167] |
| Human bone marrow-derived MSCs | BMP-13 | Inhibited osteogenic differentiation of human BM-MSCs and increased proteoglycan synthesis | [168] |
| Human adult MSCs | BMP-3, and TGFβ-1 | Enhanced cell proliferation, GAGs content and differentiation into NP-like phenotype. Upregulated smad-3 signaling pathway | [126] |
| Human adipose tissue-derived MSCs | BMP-2, BMP-6, BMP-7, and TGF-β2 | Both TGFβ-2 and BMP-7 induces chondrogenic potential | [76] |
| Human cultured NP and AF IVD cells | rhBMP-2, rhBMP-12, and adenoviralBMP-12 | Both rhBMP-2 and rhBMP-12 increased NP collagen and proteoglycan but least effects on AF. Though, adenoviral BMP-12 increased ECM protein formation in equally NP and AF | [99] |
| Human and bovine cultured NP cells | BMP-7/OP-1 with BMP-2 | Enhanced GAGs production and NP cells proliferation | [77] |
| Human cultured NP cells | rhBMP-7 | Inhibited apoptotic effects, decreased caspase-3 activity and maintained ECM production | [169] |
| Bovine cultured NP cells | BMP-7, and IGF-1 | Both BMP-7 and IGF-1 induces Smad signaling pathways and suppresses noggin expression via PI3-kinase/Akt pathways | [170] |
| Human cultured NP and AF IVD cells | BMP-2 | Improved newly synthesized proteoglycan and increased mRNA expression of aggrecan, type I and type II collagen | [171] |
| In vitro cultured NP cells | IGF-1 | Increase of matrix synthesis in well-nourished regions | [180] |
| In vitro canine cultured IVD cells | IGF-1, FGF, EGF, or TGF-β3 | TGF-β3 and EGF both produced higher proliferative responses than FGF. Also, IGF-1 showed a slightly significant responses in NP but no contribution in AF and transition zone | [74] |
| Horse cultured articular cartilage cells. Bovine cultured NP cells | IGF-1 | Maintained differentiated chondrocyte morphology and enhanced synthesis of ECM molecules. Increased proteoglycan synthesis | [178,191] |
| Bovine cultured AF and NP cells | IGF-1, bFGF, and PDGF | Strengthened cell proliferation | [81] |
| Human cultured AF cells | IGF-1, and PDGF | Significant reduced in apoptotic cell level | [182] |
| Chondroitinase ABC injection rabbit model | OP-1 | Increase in disk height and matrix synthesis | [172] |
| Rabbit cultured NP and AF IVD cells | OP-1 | Restored collagens and upregulated proteoglycan synthesis | [173] |
| Human cultured NP and AF cells | OP-1 | Improved in the proteoglycan contents, total DNA, and collagen | [174] |
| Human cultured NP cells | OP-1 | Partially repaired GAGs content, depends on a very high doses | [175] |
| Gene therapy, in vitro human IVD cells. Gene therapy, in vivo rabbit IVD | TIMP-1 | Increased proteoglycan synthesis. Less MRI and histologic evidence of degeneration | [102,103] |
| In vitro cultured AF cells and chondrocytes | LMP-1 | Increased proteoglycan synthesis, upregulation of mRNA expression of aggrecan, collagen types I and II, BMP-2 and -7 | [105] |
| Human synovium derived stem cells | FGF-2, and FGF-10 | FGF-2 stimulates chondrogenic gene expression, GAGs deposition and promotes both chondrogenic and osteogenic lineages | [176] |
| Ovine bone marrow-derived MSCs | FGF-2, and FGF-18 | Promotes both chondrogenic and osteogenic lineages of MSCs | [177] |
| In vitro cultured human NP cells | FGF2 | Increased proliferative potential, redifferentiation gene expression and GAGs deposition | [178] |
| Bone marrow-derived MSCs | bFGF, TGFβ-1 and TCH gel | Greater survival and repair effect on the degenerated IVDs | [179] |
| In vitro rat cultured NP cells | rGDF-5 | Dose-dependency high expression of aggrecan and collagen type II genes was induced by rGDF-5 disc cells from GDF-5-deficient mouse | [82] |
| In vitro bovine cultured. NP and AF cells, in vivo rabbit IVD model | rhGDF-5 | Increased DNA and proteoglycan level in vitro. In vivo, rhGDF-5 injection improved IVD height, MRI and histological grade score | [183] |
| In vivo mice and rabbit model | GDF-5 | Structural and functional maintenance of IVD | [184] |
| Canine BM peri-adipocyte cells (BM-PACs) | GDF-5, TGFβ-1, BMP-2, and IGF-1 | GDF-5 promoted GAGs production and collagen type II without increasing collagen-10 mRNA expression | [199] |
| Adult bone marrow-derived MSCs | EGF | In the presence of EGF, promotes osteogenic differentiation and enhance paracrine secretion of BM-MSCs both in vitro and in vivo | [80] |
| In vivo rat bone marrow-derived MSCs | rhGCSF | Increase of end plates cell proliferation but no contribution in IVD regeneration or maintenance | [185] |
| Human synovium-derived MSCs | IL-1β, and TNF-α | Enhanced synovial MSCs proliferation and chondrogenic ability | [205,206] |
| Human bone marrow-derived MSCs. In vitro cultured porcine AF cells | IL-1β, and TNF-α | Both IL-1β and TNF-α suppressed chondrogenesis in a dose-selective manner. Increased expression of MMP-1 | [73,207] |
| Gene therapy, in vitro cultured NP cells | IL-1 and IL-1Ra | IL-1Ra decreased extracellular matrix degradation | [101] |
| Mouse bone marrow-derived MSCs | SOX-9 | Stimulate chondrogenesis | [95] |
| Gene therapy, in vivo in rabbit IVD | SOX-9 | Chondrocyte phenotype of IVD, restored architecture of NP | [96] |
| Gene therapy, in vitro bovine AF cells | Sox-9, and BMP | Increased proteoglycan and/or collagen type II synthesis | [97] |
| Gene therapy, in vitro human NP cells | WNT-3A, WNT-5A, and WNT-11 | Increased expression of redifferentiation NP genes and GAGs accumulation | [100] |
| Human bone marrow-derived MSCs | WNT-3A and FGF2 | Synergistically both promoted MSC proliferation, chondrogenesis and cartilage formation | [186] |
| VEGFR-1 and VEGFR-2 lacZ/+ NP cells | VEGF | Raise NP survival | [208] |
| Rhesus monkey cultured NP cells | CTGF | Stimulation of collagen type II and proteoglycan synthesis | [187] |
| Human cultured NP cells | PRP | Enhanced NP proliferation and differentiation into chondrogenic lineage | [134] |
| Porcine cultured NP and AF cells; Porcine IVDD organ | PRP | Stimulation of IVDD cells proliferation. Increased mRNA expression levels of chondrogenesis and matrix formation | [83,84] |
| Bovine cultured AF cells | PRP | Upregulation of cell numbers and matrix synthesis | [85] |
| In vitro porcine cultured AF cells | PRP and other cytokines | Decreased enzymes expression causing degradation and increased matrix proteins synthesis | [86] |
IVD: Intervertebral disc; BMP: Bone morphogenetic protein; EGF: Epidermal growth factor; FGF: Fibroblast growth factor; IGF-1: Insulin-like growth factor-1; OP-1: Osteogenic protein-1; PDGF: Platelet-derived growth factor; TGF-β1: Transforming growth factor-β1; ADAMTS: A disintegrin and metalloproteinase with thrombospondin motifs; TIMP: Tissue inhibitor of metalloproteinases; TNF-α: Tumor necrosis factor-α; MMP: Matrix metalloproteinase; IL-1β: Interleukin-1 beta; IL-1Ra: IL-1 receptor antagonist; SOX-9: SRY-box transcription factor-9; rhGDF-5: Recombinant human growth and differentiation factor-5; LMP-1: LIM mineralization protein-1; WNTs: Wingless-related integration site; VEGFR: Vascular endothelial growth factor receptor; LacZ: β-galactosidase; CTGF: Connective tissue growth factor; GCSF: Granulocyte colony-stimulating factor; PRP: Platelet-rich plasma; AF: Annulus fibrosus; GAGs: Glycosaminoglycans; NP: Nucleus pulposus; ECM: Extracellular matrix; IVDD: Intervertebral disc degeneration; MSCs: Mesenchymal stem cells; BM: Bone marrow; AD: Adipose tissue; ESCs: Embryonic stem cells; NPCs: Nucleus pulposus cells; MRI: Magnetic resonance imaging; DNA: Deoxyribonucleic acid; mRNA: Messenger ribonucleic acid; TCH: Temperature-responsive chitosan hydrogel; MAPK: Mitogen-activated protein kinase; PI3: Phosphatidylinositol 3; Akt: Protein kinase B.
Hematopoietic stem cells
Hematopoietic stem cells (HSCs) possess the capability to differentiate into blood cells. HSCs express CD34 molecules, while non-hematopoietic stem cells, including MSCs, do not show CD34 expression. These cells were injected into the rat IVDD model to investigate which population of cells might acquire disc-identical cells for treating IVDD. It is reported that HSCs can survive in the NP of host IVDs up to 42 d, while non-HSCs were detected up to 21 d only[108]. However, this was nullified by further confirmation that HSCs cannot cure DDD. Although HSCs can only induce blood cells and cannot differentiate into chondrocyte-like cells and repair disintegrated NP, this has begun a novel era of scientific investigation for tissue regeneration. It is demonstrated that HSC transplantation of autologous pelvic bone marrow (BM) cells for the degenerated disc in clinical trials yielded no efficient recovery[109].
MSCs
The therapeutic use of MSCs is based on their two basic characteristics, i.e., they can be used to treat different diseases and can be isolated from the autologous source. MSCs are considered as a treatment choice for several diseases like DDD, stroke, myocardial ischemia, diabetes, and neurodegenerative diseases[110-113]. MSCs can be readily isolated due to their adherent property. MSCs possess the excellent capability to differentiate into three mature lineages, namely bone, adipose, and cartilage, as well as into endothelial, myogenic[114-116], epithelial[117], and neural cell types[118] under specific conditions when guided by appropriate growth factors or pharmacological inducers. They possess the remarkable proliferative capability in cell culture with excellent stability in their phenotype and differentiation potential[119].
Furthermore, they can be smoothly transformed with the ability to home at the transplantation site. MSCs are immunologically inactive, which makes them ideal candidates for transplantation[120]. MSCs have great capability to differentiate into chondrocyte-like cells that phenotypically resemble NP cells in chondrogenic induction conditions[121-123]. MSCs promote the regeneration of endogenous tissue by secreting cell survival factors[124].
Tissue-specific stem cells
CEP, AF, and NP-derived stem cells are isolated from the adult IVD, namely cartilage endplate stem cells, AF stem cells, and nucleus pulposus stem cells (NPSCs), respectively. These cells are effective candidates for IVD recovery. Trials with disc stem cells revealed remarkable advantages in homing and retention in the IVD niche, differentiation capability, and functional competency. However, limitations in harvesting, separation, and proliferation of disc stem cells and low potency hinder researchers from using them for therapy[125]. Studies to overcome IVD injury using disc derived stem cells showed their ability to replace affected tissue by producing disc-specific collagen type II and proteoglycan, and restoring disc hydration to physiological state[126,127].
Embryonic stem cells
Embryonic stem cells (ESCs) originate from the inner cell mass of blastula and possess an excellent tendency to differentiate into different cell types. They proved themselves as stable and relatively better source for disc regeneration involving in vitro production of NCs. These NCs are the first to form NP during the embryogenesis of the disc. Researchers have successfully differentiated ESCs into chondrocyte-like cells[128]. However, ESCs display tumorigenic properties, can cause teratoma formation, and also pose ethical concerns because of their embryonic origin, which limit their application for IVDD therapy[69].
Induced pluripotent stem cells
Induced pluripotent stem cells (iPSCs) are derived from genetically reprogrammed somatic cells to an embryonic-like state. The introduction of pluripotency genes and factors in adult terminally differentiated cells is a major discovery of this era. In 2006, mouse iPSCs were first reported by Shinya Yamanaka together with his co-investigators who revealed that fibroblasts might be reprogrammed to an ESC-like cells by four pluripotent gene-induced expressions i.e. Sox2, octamer-binding transcription factor 3/4 (Oct3/4), Kruppel-like factor 4 (Klf4) and Myelocytomatosis (c-myc). These iPSCs were identical to the mouse ESCs because they express pluripotent markers and can differentiate into any cell lineage[119,129]. In subsequent years, they performed several experiments using human fibroblasts and successfully reprogrammed them to iPSCs by applying the same factors. A different team of researchers attained a similar achievement with minor alterations of Lin-28 and Nanog rather than c-myc and Klf4[130]. iPSCs possess a great tendency to differentiate into each of the three germ layer cells containing NCs[131,132]. Despite their ability to induce chondrogenesis, iPSCs might be susceptible to tumorigenesis because of their extreme pluripotent nature.
Tissue engineering-based therapy
MSCs face challenges like survival following transplantation, inadequate paracrine secretion, and limitations in cell homing. These hindrances in the effectiveness of MSCs can be overcome by improving their potential of migration, homing, propagation, and differentiation into the preferred cell type. Thus, selecting an appropriate scaffold for stem cells can better serve for the re-development of the lost tissue. Injectable bio-materials or micro and nanoscale scaffolds are preferable for biocompatibility, cell infiltration, and remodeling of the transplanted cells. Upon preconditioning, the fully biocompatible material can also target cell attachment, proliferation, normal morphology, and elevated expression of desired factors. Thus, the strategy has the advantage of inducing differentiation in vitro and transplanting cells in vivo[133,134].
CURRENT ISSUES RELATED TO TREATING DEGENERATIVE INTERVERTEBRAL DISC
IVD is the largest avascular structure in the human body that has limited efficiency for regeneration. Due to a vascular nature of IVD, tendency to develop strategy for their treatment and regeneration is low[135]. Rehabilitation, surgical interventions, post-trial treatment, and standardized procedures for the subjects should be deemed mandatory. In the case of the local treatment, a small incision should be made[136]. Therefore, surgeries for injecting therapeutic cells should be minimally invasive. In addition, safety concerns such as high intensity of neuropathic pain and secondary infections and genuine diagnosis of complications are significant. One of the critical aspects of designing clinical trials with lower back injuries is the level of injury-induced cases[137]. In selecting subjects with an exclusively specific level of damage, the distance of the injured spinal segment, route of administration, and phenomenal interaction of cell or drug action should be considered[138]. Therefore, long term patient follow-up with standardized measurement scales, such as the American Spinal Injury Association Scale for neurological levels, Normal Rating Scale (pain and spinal cord independence level), Modified Ashworth Scale (for spasticity), and International Association of Neurorestoratology Spinal Cord Injury Functional Rating Scale (for the report of functionality) are essential[139]. Current IVDD animal models are of limited significance as most are different from human disc degeneration[140]. Factual information can be obtained from animal models; however, the limitations are that the studies were generally applied on young rodents with the recently damaged disc in which normal tissue repair mechanisms are still active to heal the degeneration. It is also difficult to quantify the amount of pain. Therefore, researchers use alternate methods to examine disc regeneration or repair success by performing biochemical, molecular, and histological assessments.
Few ethical concerns should be considered while performing pre-clinical studies to translate into clinical trials. Using scientific validity, fair subject selection, favorable distribution of risks-benefits ratio, and informed consent is necessary to make clinical research ethical, which is considered challenging in disc diseases[141]. Typical successful measurements comprise proportions of morphology (e.g., IVDs height, AF delamination, and IVD degeneration grade through MRI and histology), cellularity, ECM quality and quantity, cytokine levels, and biomechanics (e.g. pressure/volume testing, compressive strength, and range of motion)[142]. Further, leakage of the delivering cells or drugs is a concern because small escape is possible while injecting. Cell therapy may upregulate the production of some growth factors, which may not be suitable for disc repair, as the cells intrinsically express a high level of growth factors, for example, TGF-β1 and bFGF, that can mediate blood vessel formation, trigger inflammatory mechanism and regulate abnormal disc cell differentiation. Therefore, extensive studies related to the toxicity of biochemical factors in the intervertebral disc are necessary before they are applied in clinical trials. Furthermore, safety with any type of gene therapy is a major consideration. These limitations make direct application of biological approaches difficult to treat disc injuries from animals to humans[143,144].
ENHANCING THE IVD REGENERATION POTENTIAL BY HUMAN PERINATAL MSCs
The implantation of MSCs is considered a promising therapeutic approach for IVD regeneration. MSCs are primarily found in adipose tissue, dental pulp, BM, and peripheral blood. Recent advances with MSCs have shown that they can be isolated from a variety of postnatal organs such as skin, bone, cartilage, periodontium, pancreatic islets, skeletal muscle, periosteum, and synovial membrane/fluid as well as from perinatal tissues like umbilical cord tissue, umbilical cord blood (UCB), AF, and placenta[107,145,146]. The human perinatal umbilical cord is an optimistic source of MSCs. Like BM stem cells, human umbilical cord-derived MSCs (hUC-MSCs) are the noncontroversial source. The cells have rapid self-renewal properties and possess various advantages, making them promising therapeutic candidates[147]. Some of the advantages are as follows: (1) They are accessible in massive amounts, considering plenty of umbilical cord (UC) with around 135 million births globally every year; (2) They can be effectively collected and manipulated without any adverse effect on the infant or mother; (3) There are no predetermined ethical issues that need to be managed in contrast with ESCs; (4) They show more significant proliferative potential compared to BM-MSCs[148]; (5) They possess minimal immunogenicity[149]; (6) There is minimal possibility of viral contamination[150]; (7) They possess a relatively large harvest size as compared to MSCs from BM[151]; and (8) They need less stringent antigenic typing, and there may be less rejection[152].
Studies have shown that MSC isolation and characterization from Wharton’s jelly (WJ) tissue can be easily performed[153,154]. In addition, several current clinical trials explain the utilization of UC matrix-derived MSCs. It is early to relate in vivo research of tissue regeneration utilizing MSCs derived from UCB compared to other sources to understand better the capability of hUC-MSCs to regenerate degenerative discs. Clinical trials showed that hUC-MSC transplantation could be a promising substitute for the treatment of prolonged discogenic LBP[155] due to better survival in the avascular niche of the IVD[156] with differently manipulating transplanting cells[157].
DIFFERENTIATION of MSCs TOWARDS CHONDROGENESIS
Stem cells have been treated with small molecules to improve their renewing capability. Numerous proteins and small molecules have been examined in this perspective such as TGF-β[158-163], BMPs[164-171], osteogenic protein (OP)[172-175], bFGF[176-179], IGF[180-182], GDF-5[183,184], granulocyte colony-stimulating factor (GCSF)[185], Wnt[186], CTGF[187], decalpenic acid, β-glycerophosphate, isobutyl methylxanthine, purmorphamine, ascorbic acid, and heparin-binding growth-associated molecule (HB-GAM)[188,189]. TGF-β has been found to lead periosteum-derived stem cells towards chondrogenic lineage and inhibit osteogenic differentiation in extreme density culture[190]. High concentrations of IGF-1 can impose the expression of chondrogenic proteins in BM-derived MSCs[191]. Ascorbic acid, non-organic phosphates, and dexamethasone increase the differentiation potential of BM-derived stem cells towards osteoblasts in CEPs[192-195]. Similarly, pleiotrophin (PTN) has also been reported to differentiate stem cells derived from human BM into chondrocytes[196]. Dexamethasone, insulin, and soluble factors have also been shown to stimulate chondrogenic differentiation of MSCs in vitro[197].
Chemical treatment to improve cell survival
Cell survival at the transplantation site is the most critical challenge. Numerous cells die soon after implantation at the site of injury[156]. Direct stimulation of stem cells into specific lineage by using growth factors and small molecules to increase their survival in host tissue is the most practical approach. Investigations showed that the expression of particular cell survival factors could enhance cell feasibility and survival in diseased tissue[198,199]. TGF-β is a growth factor associated with several cellular processes including cell proliferation and differentiation[200]. The rabbit model of IVDD induced through nucleus aspiration and infused with a combination of TGF-β1, fibrin glue, and rabbit MSCs, produced improved results[201]. Similarly, in vitro trans-differentiation phenomenon of MSCs into different cell types showed that transplanted cells could combine with native cells to give better performance in the damaged tissue[202].
Chemical treatment to improve stem cell homing
For enhanced regeneration, proficient cell homing is essential because the curative impact primarily depends on the effective cell engraftment following transplantation. Various investigators have utilized chemokine/cytokines receptors associated with MSC homing to enhance cell attachment at degenerated tissues[203], including CCR1, 2, 4, 7, 9, and in addition, CXC chemokine receptor-5, -6[204]. CCL5/RANTES has been identified as a chemoattractant secreted by degenerative IVD in organ culture[55]. Moreover, the possibility of different cytokines associated with the pathogenesis of IVD degeneration, specifically TNF-α and IL-1β, play an important role in controlling MSC recruitment to the IVD[101,205-207]. In vitro and in vivo research studies showed that molecular pre-requisite of MSCs with growth factors like TNF-α and stromal-derived-factor-1 (SDF-1) represent primary signaling cues to elevate VEGF production[208]. MSC conditioned medium improved neuronal survival in several neurological disorders such as neurodegenerative diseases, stroke, and spinal injuries[209]. Moreover, the conditioned medium acquired from articular cartilage stimulated the chondrogenic potential of MSCs and ECM development. The paracrine influence of prominin-1 or CD133+ endothelial progenitor cells from cord blood releases biologically active molecules in the conditioned medium along with microvesicles, which stimulate cell growth and homing. CD133+ cell derivatives with microvesicles possess messenger RNAs for various pro-angiopoietins and anti-apoptotic factors, containing bFGF, receptor tyrosine kinase (c-kit) ligand, IGF-1, VEGF, and IL-8, contributing to withstand harsh microenvironment of the disc[210].
CONCLUSION
In conclusion, this review highlights regenerative medicine-based approaches for the regeneration of IVDD. Numerous potential therapeutic options were identified for the development of cellular therapies. The harsh microenvironment of the degenerative disc poses challenge to the survival of implanted cells. Therefore, possible strategies are needed to enhance the ability of the transplanted cells by preconditioning, chemical modification, genetic manipulation, and augmentation of growth and survival factors to help cells withstand the harsh disc microenvironment. The ultimate goal is to ensure that the transplanted cells survive, integrate and differentiate into desired cell types to regenerate and restore the normal physiological function of the IVD.
Footnotes
Conflict-of-interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: June 4, 2021
First decision: June 23, 2021
Article in press: November 15, 2021
Specialty type: Cell and tissue engineering
Country/Territory of origin: Pakistan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu L S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
Contributor Information
Sobia Ekram, Dr. Panjwani Center for Molecular Medicine and Drug Research, International Center for Chemical and Biological Sciences, University of Karachi, Karachi 75270, Sindh, Pakistan.
Shumaila Khalid, Dr. Panjwani Center for Molecular Medicine and Drug Research, International Center for Chemical and Biological Sciences, University of Karachi, Karachi 75270, Sindh, Pakistan.
Asmat Salim, Dr. Panjwani Center for Molecular Medicine and Drug Research, International Center for Chemical and Biological Sciences, University of Karachi, Karachi 75270, Sindh, Pakistan.
Irfan Khan, Dr. Panjwani Center for Molecular Medicine and Drug Research, International Center for Chemical and Biological Sciences, University of Karachi, Karachi 75270, Sindh, Pakistan. khan@iccs.edu.
References
- 1.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fèvre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gosselin R, Grainger R, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Ma J, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O'Donnell M, O'Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA 3rd, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leòn FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi Y, Park MH, Lee K. Tissue Engineering Strategies for Intervertebral Disc Treatment Using Functional Polymers. Polymers (Basel) 2019;11 doi: 10.3390/polym11050872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith LJ, Nerurkar NL, Choi KS, Harfe BD, Elliott DM. Degeneration and regeneration of the intervertebral disc: lessons from development. Dis Model Mech. 2011;4:31–41. doi: 10.1242/dmm.006403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581–585. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 5.Löwhagen GB, Tunbäck P, Andersson K, Bergström T, Johannisson G. First episodes of genital herpes in a Swedish STD population: a study of epidemiology and transmission by the use of herpes simplex virus (HSV) typing and specific serology. Sex Transm Infect. 2000;76:179–182. doi: 10.1136/sti.76.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DePalma MJ, Ketchum JM, Saullo T. What is the source of chronic low back pain and does age play a role? Pain Med. 2011;12:224–233. doi: 10.1111/j.1526-4637.2010.01045.x. [DOI] [PubMed] [Google Scholar]
- 7.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10:44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford) 2009;48:5–10. doi: 10.1093/rheumatology/ken396. [DOI] [PubMed] [Google Scholar]
- 9.Hodge Jr SD. A Medical-Legal Guide to Spinal Surgery. J Heal Bio Law. 2021;17:169–208. [Google Scholar]
- 10.Dowdell J, Erwin M, Choma T, Vaccaro A, Iatridis J, Cho SK. Intervertebral disk degeneration and repair. Neurosurgery. 2017;80:S46–S54. doi: 10.1093/neuros/nyw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Placzek M. The role of the notochord and floor plate in inductive interactions. Curr Opin Genet Dev. 1995;5:499–506. doi: 10.1016/0959-437x(95)90055-l. [DOI] [PubMed] [Google Scholar]
- 12.Ehlen HW, Buelens LA, Vortkamp A. Hedgehog signaling in skeletal development. Birth Defects Res C Embryo Today. 2006;78:267–279. doi: 10.1002/bdrc.20076. [DOI] [PubMed] [Google Scholar]
- 13.McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol. 2003;53:1–114. doi: 10.1016/s0070-2153(03)53002-2. [DOI] [PubMed] [Google Scholar]
- 14.Choi KS, Lee C, Harfe BD. Sonic hedgehog in the notochord is sufficient for patterning of the intervertebral discs. Mech Dev. 2012;129:255–262. doi: 10.1016/j.mod.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wijgerde M, Karp S, McMahon J, McMahon AP. Noggin antagonism of BMP4 signaling controls development of the axial skeleton in the mouse. Dev Biol. 2005;286:149–157. doi: 10.1016/j.ydbio.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 16.Murtaugh LC, Chyung JH, Lassar AB. Sonic hedgehog promotes somitic chondrogenesis by altering the cellular response to BMP signaling. Genes Dev. 1999;13:225–237. doi: 10.1101/gad.13.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ireland D. Molecular mechanisms involved in intervertebral disc degeneration and potential new treatment strategies. Biosc Hori. 2009;2:83–89. [Google Scholar]
- 18.Frost V, Grocott T, Eccles MR, Chantry A. Self-regulated Pax gene expression and modulation by the TGFbeta superfamily. Crit Rev Biochem Mol Biol. 2008;43:371–391. doi: 10.1080/10409230802486208. [DOI] [PubMed] [Google Scholar]
- 19.Wallin J, Wilting J, Koseki H, Fritsch R, Christ B, Balling R. The role of Pax-1 in axial skeleton development. Development. 1994;120:1109–1121. doi: 10.1242/dev.120.5.1109. [DOI] [PubMed] [Google Scholar]
- 20.Smith CA, Tuan RS. Human PAX gene expression and development of the vertebral column. Clin Orthop Relat Res. 1994:241–250. [PubMed] [Google Scholar]
- 21.Peters H, Wilm B, Sakai N, Imai K, Maas R, Balling R. Pax1 and Pax9 synergistically regulate vertebral column development. Development. 1999;126:5399–5408. doi: 10.1242/dev.126.23.5399. [DOI] [PubMed] [Google Scholar]
- 22.Fan CM, Tessier-Lavigne M. Patterning of mammalian somites by surface ectoderm and notochord: evidence for sclerotome induction by a hedgehog homolog. Cell. 1994;79:1175–1186. doi: 10.1016/0092-8674(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 23.Furumoto TA, Miura N, Akasaka T, Mizutani-Koseki Y, Sudo H, Fukuda K, Maekawa M, Yuasa S, Fu Y, Moriya H, Taniguchi M, Imai K, Dahl E, Balling R, Pavlova M, Gossler A, Koseki H. Notochord-dependent expression of MFH1 and PAX1 cooperates to maintain the proliferation of sclerotome cells during the vertebral column development. Dev Biol. 1999;210:15–29. doi: 10.1006/dbio.1999.9261. [DOI] [PubMed] [Google Scholar]
- 24.Schepers GE, Teasdale RD, Koopman P. Twenty pairs of sox: extent, homology, and nomenclature of the mouse and human sox transcription factor gene families. Dev Cell. 2002;3:167–170. doi: 10.1016/s1534-5807(02)00223-x. [DOI] [PubMed] [Google Scholar]
- 25.Daniel M. Wegner: Award for Distinguished Scientific Contributions. Am Psychol. 2011;66:669–671. doi: 10.1037/a0024639. [DOI] [PubMed] [Google Scholar]
- 26.Smits P, Dy P, Mitra S, Lefebvre V. Sox5 and Sox6 are needed to develop and maintain source, columnar, and hypertrophic chondrocytes in the cartilage growth plate. J Cell Biol. 2004;164:747–758. doi: 10.1083/jcb.200312045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugimoto Y, Takimoto A, Akiyama H, Kist R, Scherer G, Nakamura T, Hiraki Y, Shukunami C. Scx+/Sox9+ progenitors contribute to the establishment of the junction between cartilage and tendon/Ligament. Development. 2013;140:2280–2288. doi: 10.1242/dev.096354. [DOI] [PubMed] [Google Scholar]
- 28.Barrionuevo F, Taketo MM, Scherer G, Kispert A. Sox9 is required for notochord maintenance in mice. Dev Biol. 2006;295:128–140. doi: 10.1016/j.ydbio.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 29.Dünker N, Krieglstein K. Targeted mutations of transforming growth factor-beta genes reveal important roles in mouse development and adult homeostasis. Eur J Biochem. 2000;267:6982–6988. doi: 10.1046/j.1432-1327.2000.01825.x. [DOI] [PubMed] [Google Scholar]
- 30.Sohn P, Cox M, Chen D, Serra R. Molecular profiling of the developing mouse axial skeleton: a role for Tgfbr2 in the development of the intervertebral disc. BMC Dev Biol. 2010;10:29. doi: 10.1186/1471-213X-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pearson JC, Lemons D, McGinnis W. Modulating Hox gene functions during animal body patterning. Nat Rev Genet. 2005;6:893–904. doi: 10.1038/nrg1726. [DOI] [PubMed] [Google Scholar]
- 32.Lyu FJ, Cui H, Pan H, Mc Cheung K, Cao X, Iatridis JC, Zheng Z. Painful intervertebral disc degeneration and inflammation: from laboratory evidence to clinical interventions. Bone Res. 2021;9:7. doi: 10.1038/s41413-020-00125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hadjipavlou AG, Tzermiadianos MN, Bogduk N, Zindrick MR. The pathophysiology of disc degeneration: a critical review. J Bone Joint Surg Br. 2008;90:1261–1270. doi: 10.1302/0301-620X.90B10.20910. [DOI] [PubMed] [Google Scholar]
- 34.Maidhof R, Alipui DO, Rafiuddin A, Levine M, Grande DA, Chahine NO. Emerging trends in biological therapy for intervertebral disc degeneration. Discov Med. 2012;14:401–411. [PubMed] [Google Scholar]
- 35.Erwin WM. The Notochord, Notochordal cell and CTGF/CCN-2: ongoing activity from development through maturation. J Cell Commun Signal. 2008;2:59–65. doi: 10.1007/s12079-008-0031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dou Y, Sun X, Ma X, Zhao X, Yang Q. Intervertebral Disk Degeneration: The Microenvironment and Tissue Engineering Strategies. Front Bioeng Biotechnol. 2021;9:592118. doi: 10.3389/fbioe.2021.592118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng Y, Lv FJ. Fibrosis in intervertebral disc degeneration: Knowledge and gaps. Austin J Orthopade Rheumatol . 2014;1:3. [Google Scholar]
- 38.Zhao CQ, Wang LM, Jiang LS, Dai LY. The cell biology of intervertebral disc aging and degeneration. Ageing Res Rev. 2007;6:247–261. doi: 10.1016/j.arr.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Ekram S, Khalid S, Bashir I, Salim A, Khan I. Human umbilical cord-derived mesenchymal stem cells and their chondroprogenitor derivatives reduced pain and inflammation signaling and promote regeneration in a rat intervertebral disc degeneration model. Mol Cell Biochem. 2021;476:3191–3205. doi: 10.1007/s11010-021-04155-9. [DOI] [PubMed] [Google Scholar]
- 40.Freemont AJ, Peacock TE, Goupille P, Hoyland JA, O'Brien J, Jayson MI. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350:178–181. doi: 10.1016/s0140-6736(97)02135-1. [DOI] [PubMed] [Google Scholar]
- 41.Sobajima S, Kim JS, Gilbertson LG, Kang JD. Gene therapy for degenerative disc disease. Gene Ther. 2004;11:390–401. doi: 10.1038/sj.gt.3302200. [DOI] [PubMed] [Google Scholar]
- 42.Boxberger JI, Orlansky AS, Sen S, Elliott DM. Reduced nucleus pulposus glycosaminoglycan content alters intervertebral disc dynamic viscoelastic mechanics. J Biomech. 2009;42:1941–1946. doi: 10.1016/j.jbiomech.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tavana S, Masouros SD, Baxan N, Freedman BA, Hansen UN, Newell N. The Effect of Degeneration on Internal Strains and the Mechanism of Failure in Human Intervertebral Discs Analyzed Using Digital Volume Correlation (DVC) and Ultra-High Field MRI. Front Bioeng Biotechnol. 2020;8:610907. doi: 10.3389/fbioe.2020.610907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou M, Lim S, O'Connell GD. A Robust Multiscale and Multiphasic Structure-Based Modeling Framework for the Intervertebral Disc. Front Bioeng Biotechnol. 2021;9:685799. doi: 10.3389/fbioe.2021.685799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baptista JD, Fontes RB, Liberti EA. Aging and degeneration of the intervertebral disc: review of basic science. Coluna/Columna. 2015;14:144–148. [Google Scholar]
- 46.Brinjikji W, Luetmer PH, Comstock B, Bresnahan BW, Chen LE, Deyo RA, Halabi S, Turner JA, Avins AL, James K, Wald JT, Kallmes DF, Jarvik JG. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR Am J Neuroradiol. 2015;36:811–816. doi: 10.3174/ajnr.A4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sampara P, Banala RR, Vemuri SK, Av GR, Gpv S. Understanding the molecular biology of intervertebral disc degeneration and potential gene therapy strategies for regeneration: a review. Gene Ther. 2018;25:67–82. doi: 10.1038/s41434-018-0004-0. [DOI] [PubMed] [Google Scholar]
- 48.Risbud MV, Schaer TP, Shapiro IM. Toward an understanding of the role of notochordal cells in the adult intervertebral disc: from discord to accord. Dev Dyn. 2010;239:2141–2148. doi: 10.1002/dvdy.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthr Res The . 2005;7:1–4. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoyland JA, Le Maitre C, Freemont AJ. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatology (Oxford) 2008;47:809–814. doi: 10.1093/rheumatology/ken056. [DOI] [PubMed] [Google Scholar]
- 51.Sakai D, Grad S. Advancing the cellular and molecular therapy for intervertebral disc disease. Adv Drug Deliv Rev. 2015;84:159–171. doi: 10.1016/j.addr.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Che M, Xin J, Zheng Z, Li J, Zhang S. The role of IL-1β and TNF-α in intervertebral disc degeneration. Biomed Pharmacother. 2020;131:110660. doi: 10.1016/j.biopha.2020.110660. [DOI] [PubMed] [Google Scholar]
- 53.Wang WJ, Yu XH, Wang C, Yang W, He WS, Zhang SJ, Yan YG, Zhang J. MMPs and ADAMTSs in intervertebral disc degeneration. Clin Chim Acta. 2015;448:238–246. doi: 10.1016/j.cca.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 54.Phillips KL, Cullen K, Chiverton N, Michael AL, Cole AA, Breakwell LM, Haddock G, Bunning RA, Cross AK, Le Maitre CL. Potential roles of cytokines and chemokines in human intervertebral disc degeneration: interleukin-1 is a master regulator of catabolic processes. Osteoarthritis Cartilage. 2015;23:1165–1177. doi: 10.1016/j.joca.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 55.Grad S, Bow C, Karppinen J, Luk KD, Cheung KM, Alini M, Samartzis D. Systemic blood plasma CCL5 and CXCL6: Potential biomarkers for human lumbar disc degeneration. Eur Cell Mater. 2016;31:1–10. doi: 10.22203/ecm.v031a01. [DOI] [PubMed] [Google Scholar]
- 56.Li H, Zou X, Baatrup A, Lind M, Bünger C. Cytokine profiles in conditioned media from cultured human intervertebral disc tissue. Implications of their effect on bone marrow stem cell metabolism. Acta Orthop. 2005;76:115–121. doi: 10.1080/00016470510030436. [DOI] [PubMed] [Google Scholar]
- 57.Jungen MJ, Ter Meulen BC, van Osch T, Weinstein HC, Ostelo RWJG. Inflammatory biomarkers in patients with sciatica: a systematic review. BMC Musculoskelet Disord. 2019;20:156. doi: 10.1186/s12891-019-2541-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Molinos M, Almeida CR, Caldeira J, Cunha C, Gonçalves RM, Barbosa MA. Inflammation in intervertebral disc degeneration and regeneration. J Royal Soc Inter . 2015;12:20141191. doi: 10.1098/rsif.2015.0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shamji MF, Setton LA, Jarvis W, So S, Chen J, Jing L, Bullock R, Isaacs RE, Brown C, Richardson WJ. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum. 2010;62:1974–1982. doi: 10.1002/art.27444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bydon M, De la Garza-Ramos R, Macki M, Baker A, Gokaslan AK, Bydon A. Lumbar fusion vs nonoperative management for treatment of discogenic low back pain: a systematic review and meta-analysis of randomized controlled trials. J Spinal Disord Tech. 2014;27:297–304. doi: 10.1097/BSD.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 61.Hughes SP, Freemont AJ, Hukins DW, McGregor AH, Roberts S. The pathogenesis of degeneration of the intervertebral disc and emerging therapies in the management of back pain. J Bone Joint Surg Br. 2012;94:1298–1304. doi: 10.1302/0301-620X.94B10.28986. [DOI] [PubMed] [Google Scholar]
- 62.Kadow T, Sowa G, Vo N, Kang JD. Molecular basis of intervertebral disc degeneration and herniations: what are the important translational questions? Clin Orthop Relat Res. 2015;473:1903–1912. doi: 10.1007/s11999-014-3774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Navani A, Ambach MA, Wei JJ, Gupta D. Biologic Therapies for Intervertebral Degenerative Disc Disease: A Review of Novel Applications. J Stem Cells Res. 2017;4:1023. [Google Scholar]
- 64.Frost BA, Camarero-Espinosa S, Foster EJ. Materials for the Spine: Anatomy, Problems, and Solutions. Materials (Basel) 2019;12 doi: 10.3390/ma12020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blumenkrantz G, Lindsey CT, Dunn TC, Jin H, Ries MD, Link TM, Steinbach LS, Majumdar S. A pilot, two-year longitudinal study of the interrelationship between trabecular bone and articular cartilage in the osteoarthritic knee. Osteoarthritis Cartilage. 2004;12:997–1005. doi: 10.1016/j.joca.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 66.Rutges JP, Duit RA, Kummer JA, Oner FC, van Rijen MH, Verbout AJ, Castelein RM, Dhert WJ, Creemers LB. Hypertrophic differentiation and calcification during intervertebral disc degeneration. Osteoarthritis Cartilage. 2010;18:1487–1495. doi: 10.1016/j.joca.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 67.Hollenbeck JFM, Fattor JA, Patel V, Burger E, Rullkoetter PJ, Cain CMJ. Validation of Pre-operative Templating for Total Disc Replacement Surgery. Int J Spine Surg. 2019;13:84–91. doi: 10.14444/6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ebrahimkhani M, Arjmand N, Shirazi-Adl A. Biomechanical effects of lumbar fusion surgery on adjacent segments using musculoskeletal models of the intact, degenerated and fused spine. Sci Rep. 2021;11:17892. doi: 10.1038/s41598-021-97288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vadalà G, Russo F, Ambrosio L, Loppini M, Denaro V. Stem cells sources for intervertebral disc regeneration. World J Stem Cells. 2016;8:185–201. doi: 10.4252/wjsc.v8.i5.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fernandez-Moure J, Moore CA, Kim K, Karim A, Smith K, Barbosa Z, Van Eps J, Rameshwar P, Weiner B. Novel therapeutic strategies for degenerative disc disease: Review of cell biology and intervertebral disc cell therapy. SAGE Open Med. 2018;6:2050312118761674. doi: 10.1177/2050312118761674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Knezevic NN, Mandalia S, Raasch J, Knezevic I, Candido KD. Treatment of chronic low back pain - new approaches on the horizon. J Pain Res. 2017;10:1111–1123. doi: 10.2147/JPR.S132769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.An HS, Masuda K, Inoue N. Intervertebral disc degeneration: biological and biomechanical factors. J Orthop Sci. 2006;11:541–552. doi: 10.1007/s00776-006-1055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cho H, Lee S, Park SH, Huang J, Hasty KA, Kim SJ. Synergistic effect of combined growth factors in porcine intervertebral disc degeneration. Connect Tissue Res. 2013;54:181–186. doi: 10.3109/03008207.2013.775258. [DOI] [PubMed] [Google Scholar]
- 74.Haddadi K. Degenerative Disc Disease: A Review of Cell Technologies and Stem Cell Therapy. Iranian J Neur. 2016;1:6–10. [Google Scholar]
- 75.Ju DG, Kanim LE, Bae HW. Intervertebral Disc Repair: Current Concepts. Global Spine J. 2020;10:130S–136S. doi: 10.1177/2192568219872460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim HJ, Im GI. Combination of transforming growth factor-beta2 and bone morphogenetic protein 7 enhances chondrogenesis from adipose tissue-derived mesenchymal stem cells. Tissue Eng Part A. 2009;15:1543–1551. doi: 10.1089/ten.tea.2008.0368. [DOI] [PubMed] [Google Scholar]
- 77.Leung VYL, Zhou L, Tam WK, Sun Y, Lv F, Zhou G, Cheung KMC. Bone morphogenetic protein-2 and -7 mediate the anabolic function of nucleus pulposus cells with discrete mechanisms. Connect Tissue Res. 2017;58:573–585. doi: 10.1080/03008207.2017.1282951. [DOI] [PubMed] [Google Scholar]
- 78.May RD, Frauchiger DA, Albers CE, Tekari A, Benneker LM, Klenke FM, Hofstetter W, Gantenbein B. Application of Cytokines of the Bone Morphogenetic Protein (BMP) Family in Spinal Fusion - Effects on the Bone, Intervertebral Disc and Mesenchymal Stromal Cells. Curr Stem Cell Res Ther. 2019;14:618–643. doi: 10.2174/1574888X14666190628103528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hayes AJ, Ralphs JR. The response of foetal annulus fibrosus cells to growth factors: modulation of matrix synthesis by TGF-β1 and IGF-1. Histochem Cell Biol. 2011;136:163–175. doi: 10.1007/s00418-011-0835-x. [DOI] [PubMed] [Google Scholar]
- 80.Tamama K, Kawasaki H, Wells A. Epidermal growth factor (EGF) treatment on multipotential stromal cells (MSCs). Possible enhancement of therapeutic potential of MSC. J Biomed Biotechnol. 2010;2010:795385. doi: 10.1155/2010/795385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pratsinis H, Kletsas D. PDGF, bFGF and IGF-I stimulate the proliferation of intervertebral disc cells in vitro via the activation of the ERK and Akt signaling pathways. Eur Spine J. 2007;16:1858–1866. doi: 10.1007/s00586-007-0408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li X, Leo BM, Beck G, Balian G, Anderson GD. Collagen and proteoglycan abnormalities in the GDF-5-deficient mice and molecular changes when treating disk cells with recombinant growth factor. Spine (Phila Pa 1976) 2004;29:2229–2234. doi: 10.1097/01.brs.0000142427.82605.fb. [DOI] [PubMed] [Google Scholar]
- 83.Akeda K, An HS, Pichika R, Attawia M, Thonar EJ, Lenz ME, Uchida A, Masuda K. Platelet-rich plasma (PRP) stimulates the extracellular matrix metabolism of porcine nucleus pulposus and anulus fibrosus cells cultured in alginate beads. Spine (Phila Pa 1976) 2006;31:959–966. doi: 10.1097/01.brs.0000214942.78119.24. [DOI] [PubMed] [Google Scholar]
- 84.Chen WH, Liu HY, Lo WC, Wu SC, Chi CH, Chang HY, Hsiao SH, Wu CH, Chiu WT, Chen BJ, Deng WP. Intervertebral disc regeneration in an ex vivo culture system using mesenchymal stem cells and platelet-rich plasma. Biomaterials. 2009;30:5523–5533. doi: 10.1016/j.biomaterials.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 85.Pirvu TN, Schroeder JE, Peroglio M, Verrier S, Kaplan L, Richards RG, Alini M, Grad S. Platelet-rich plasma induces annulus fibrosus cell proliferation and matrix production. Eur Spine J. 2014;23:745–753. doi: 10.1007/s00586-014-3198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cho H, Holt DC 3rd, Smith R, Kim SJ, Gardocki RJ, Hasty KA. The Effects of Platelet-Rich Plasma on Halting the Progression in Porcine Intervertebral Disc Degeneration. Artif Organs. 2016;40:190–195. doi: 10.1111/aor.12530. [DOI] [PubMed] [Google Scholar]
- 87.Monfett M, Harrison J, Boachie-Adjei K, Lutz G. Intradiscal platelet-rich plasma (PRP) injections for discogenic low back pain: an update. Int Orthop. 2016;40:1321–1328. doi: 10.1007/s00264-016-3178-3. [DOI] [PubMed] [Google Scholar]
- 88.Lee HM, Park MS, Kim H, Kim HS, Kang ES, Kim NH, Moon SH. Responsiveness of Human Intervertebral Disc Cells in Matrix Synthesis to Adenovirus-Mediated Gene Transfer (IGF-1, TGF-β1 Encoding Genes) J Korean Soc Spine Sur. 2001;8:195–201. [Google Scholar]
- 89.Mobasheri A, Richardson SM. Cell and Gene Therapy for Spine Regeneration: Mammalian Protein Production Platforms for Overproduction of Therapeutic Proteins and Growth Factors. Neurosurg Clin N Am. 2020;31:131–139. doi: 10.1016/j.nec.2019.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Takeoka Y, Yurube T, Nishida K. Gene Therapy Approach for Intervertebral Disc Degeneration: An Update. Neurospine. 2020;17:3–14. doi: 10.14245/ns.2040042.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Han IB. Moving Forward: Gene Therapy for Intervertebral Disc Degeneration. Neurospine. 2020;17:17–18. doi: 10.14245/ns.2040108.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moriguchi Y, Alimi M, Khair T, Manolarakis G, Berlin C, Bonassar LJ, Härtl R. Biological Treatment Approaches for Degenerative Disk Disease: A Literature Review of In Vivo Animal and Clinical Data. Global Spine J. 2016;6:497–518. doi: 10.1055/s-0036-1571955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nishida K, Maeno K, Kenichiro K, Yurube T, Kurosaka M. Potential Gene Therapy for Intervertebral Disc Degeneration. Gene Therapy Applications. 2011:129. [Google Scholar]
- 94.Liu Y, Yu T, Ma XX, Xiang HF, Hu YG, Chen BH. Lentivirus-mediated TGF-β3, CTGF and TIMP1 gene transduction as a gene therapy for intervertebral disc degeneration in an in vivo rabbit model. Exp Ther Med. 2016;11:1399–1404. doi: 10.3892/etm.2016.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tsuchiya H, Kitoh H, Sugiura F, Ishiguro N. Chondrogenesis enhanced by overexpression of sox9 gene in mouse bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2003;301:338–343. doi: 10.1016/s0006-291x(02)03026-7. [DOI] [PubMed] [Google Scholar]
- 96.Zhao Z, Li S, Huang H, Fang J, Wei H, Xi Y. In vivo delivery of MMP3-shRNA and Sox9 Lentivirus cocktail enhances matrix synthesis to prevent lumbar disc degeneration. Adv Clin Exp Med. 2020;29:639–647. doi: 10.17219/acem/121509. [DOI] [PubMed] [Google Scholar]
- 97.Zhang Y, Markova D, Im HJ, Hu W, Thonar EJ, He TC, An HS, Phillips FM, Anderson DG. Primary bovine intervertebral disc cells transduced with adenovirus overexpressing 12 BMPs and Sox9 maintain appropriate phenotype. Am J Phys Med Rehabil. 2009;88:455–463. doi: 10.1097/PHM.0b013e3181a5f0aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang H, Kroeber M, Hanke M, Ries R, Schmid C, Poller W, Richter W. Release of active and depot GDF-5 after adenovirus-mediated overexpression stimulates rabbit and human intervertebral disc cells. J Mol Med (Berl) 2004;82:126–134. doi: 10.1007/s00109-003-0507-y. [DOI] [PubMed] [Google Scholar]
- 99.Gilbertson L, Ahn SH, Teng PN, Studer RK, Niyibizi C, Kang JD. The effects of recombinant human bone morphogenetic protein-2, recombinant human bone morphogenetic protein-12, and adenoviral bone morphogenetic protein-12 on matrix synthesis in human annulus fibrosis and nucleus pulposus cells. Spine J. 2008;8:449–456. doi: 10.1016/j.spinee.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 100.Pizzute T, He F, Zhang XB, Pei M. Impact of Wnt signals on human intervertebral disc cell regeneration. J Orthop Res. 2018;36:3196–3207. doi: 10.1002/jor.24115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Le Maitre CL, Hoyland JA, Freemont AJ. Interleukin-1 receptor antagonist delivered directly and by gene therapy inhibits matrix degradation in the intact degenerate human intervertebral disc: an in situ zymographic and gene therapy study. Arthritis Res Ther. 2007;9:R83. doi: 10.1186/ar2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wallach CJ. Gene transfer of the catabolic inhibitor TIMP-1 increases measured proteoglycans in human intervertebral disc cells. Spine . 2003;28:2331–2337. doi: 10.1097/01.BRS.0000085303.67942.94. [DOI] [PubMed] [Google Scholar]
- 103.Leckie SK, Bechara BP, Hartman RA, Sowa GA, Woods BI, Coelho JP, Witt WT, Dong QD, Bowman BW, Bell KM, Vo NV, Wang B, Kang JD. Injection of AAV2-BMP2 and AAV2-TIMP1 into the nucleus pulposus slows the course of intervertebral disc degeneration in an in vivo rabbit model. Spine J. 2012;12:7–20. doi: 10.1016/j.spinee.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu Y, Zhou W, Chen FF, Xiao F, Zhu HY, Zhou Y, Guo GC. Overexpression of LMP-1 Decreases Apoptosis in Human Nucleus Pulposus Cells via Suppressing the NF-κB Signaling Pathway. Oxid Med Cell Longev. 2020;2020:8189706. doi: 10.1155/2020/8189706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kuh SU, Zhu Y, Li J, Tsai KJ, Fei Q, Hutton WC, Yoon ST. The AdLMP-1 transfection in two different cells; AF cells, chondrocytes as potential cell therapy candidates for disc degeneration. Acta Neurochir (Wien) 2008;150:803–810. doi: 10.1007/s00701-008-1617-7. [DOI] [PubMed] [Google Scholar]
- 106.Chen S, Luo M, Kou H, Shang G, Ji Y, Liu H. A Review of Gene Therapy Delivery Systems for Intervertebral Disc Degeneration. Curr Pharm Biotechnol. 2020;21:194–205. doi: 10.2174/1389201020666191024171618. [DOI] [PubMed] [Google Scholar]
- 107.Vasiliadis ES, Pneumaticos SG, Evangelopoulos DS, Papavassiliou AG. Biologic treatment of mild and moderate intervertebral disc degeneration. Mol Med. 2014;20:400–409. doi: 10.2119/molmed.2014.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yuan M, Yeung CW, Li YY, Diao H, Cheung KMC, Chan D, Cheah K, Chan PB. Effects of nucleus pulposus cell-derived acellular matrix on the differentiation of mesenchymal stem cells. Biomaterials. 2013;34:3948–3961. doi: 10.1016/j.biomaterials.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 109.Haufe SM, Mork AR. Intradiscal injection of hematopoietic stem cells in an attempt to rejuvenate the intervertebral discs. Stem Cells Dev. 2006;15:136–137. doi: 10.1089/scd.2006.15.136. [DOI] [PubMed] [Google Scholar]
- 110.Drazin D, Rosner J, Avalos P, Acosta F. Stem cell therapy for degenerative disc disease. Adv Orthop. 2012;2012:961052. doi: 10.1155/2012/961052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hanabusa K, Nagaya N, Iwase T, Itoh T, Murakami S, Shimizu Y, Taki W, Miyatake K, Kangawa K. Adrenomedullin enhances therapeutic potency of mesenchymal stem cells after experimental stroke in rats. Stroke. 2005;36:853–858. doi: 10.1161/01.STR.0000157661.69482.76. [DOI] [PubMed] [Google Scholar]
- 112.Lalu MM, Mazzarello S, Zlepnig J, Dong YYR, Montroy J, McIntyre L, Devereaux PJ, Stewart DJ, David Mazer C, Barron CC, McIsaac DI, Fergusson DA. Safety and Efficacy of Adult Stem Cell Therapy for Acute Myocardial Infarction and Ischemic Heart Failure (SafeCell Heart): A Systematic Review and Meta-Analysis. Stem Cells Transl Med. 2018;7:857–866. doi: 10.1002/sctm.18-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Päth G, Perakakis N, Mantzoros CS, Seufert J. Stem cells in the treatment of diabetes mellitus - Focus on mesenchymal stem cells. Metabolism. 2019;90:1–15. doi: 10.1016/j.metabol.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 114.Lee BC, Kang KS. Functional enhancement strategies for immunomodulation of mesenchymal stem cells and their therapeutic application. Stem Cell Res Ther. 2020;11:397. doi: 10.1186/s13287-020-01920-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, Branco RV, Oliveira EM, He R, Geng YJ, Willerson JT, Perin EC. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 116.de la Garza-Rodea AS, van der Velde-van Dijke I, Boersma H, Gonçalves MA, van Bekkum DW, de Vries AA, Knaän-Shanzer S. Myogenic properties of human mesenchymal stem cells derived from three different sources. Cell Transplant. 2012;21:153–173. doi: 10.3727/096368911X580554. [DOI] [PubMed] [Google Scholar]
- 117.Spees JL, Olson SD, Ylostalo J, Lynch PJ, Smith J, Perry A, Peister A, Wang MY, Prockop DJ. Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc Natl Acad Sci U S A. 2003;100:2397–2402. doi: 10.1073/pnas.0437997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pacary E, Legros H, Valable S, Duchatelle P, Lecocq M, Petit E, Nicole O, Bernaudin M. Synergistic effects of CoCl2 and ROCK inhibition on mesenchymal stem cell differentiation into neuron-like cells. J Cell Sci. 2006;119:2667–2678. doi: 10.1242/jcs.03004. [DOI] [PubMed] [Google Scholar]
- 119.García-Bernal D, García-Arranz M, Yáñez RM, Hervás-Salcedo R, Cortés A, Fernández-García M, Hernando-Rodríguez M, Quintana-Bustamante Ó, Bueren JA, García-Olmo D, Moraleda JM, Segovia JC, Zapata AG. The Current Status of Mesenchymal Stromal Cells: Controversies, Unresolved Issues and Some Promising Solutions to Improve Their Therapeutic Efficacy. Front Cell Dev Biol. 2021;9:650664. doi: 10.3389/fcell.2021.650664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Najar M, Krayem M, Meuleman N, Bron D, Lagneaux L. Mesenchymal Stromal Cells and Toll-Like Receptor Priming: A Critical Review. Immune Netw. 2017;17:89–102. doi: 10.4110/in.2017.17.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Long D, Liang S, Liu H, Wu X, Li Z, Wang H, Huang S, Zeng J. Mesenchymal stem cell in the intervertebral disc. China: Mesenchymal Stem Cells-Isolation, Characterization and Applications. Phuc Van Pham. 2017 [Google Scholar]
- 122.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 123.Strassburg S, Richardson SM, Freemont AJ, Hoyland JA. Co-culture induces mesenchymal stem cell differentiation and modulation of the degenerate human nucleus pulposus cell phenotype. Regen Med. 2010;5:701–711. doi: 10.2217/rme.10.59. [DOI] [PubMed] [Google Scholar]
- 124.Cofano F, Boido M, Monticelli M, Zenga F, Ducati A, Vercelli A, Garbossa D. Mesenchymal Stem Cells for Spinal Cord Injury: Current Options, Limitations, and Future of Cell Therapy. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20112698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Du Y, Wang Z, Wu Y, Liu C, Zhang L. Intervertebral Disc Stem/Progenitor Cells: A Promising "Seed" for Intervertebral Disc Regeneration. Stem Cells Int. 2021;2021:2130727. doi: 10.1155/2021/2130727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lehmann TP, Jakub G, Harasymczuk J, Jagodziński PP. Transforming growth factor β mediates communication of co-cultured human nucleus pulposus cells and mesenchymal stem cells. J Orthop Res. 2018;36:3023–3032. doi: 10.1002/jor.24106. [DOI] [PubMed] [Google Scholar]
- 127.De Luca P, Castagnetta M, de Girolamo L, Coco S, Malacarne M, Ragni E, Viganò M, Lugano G, Brayda-Bruno M, Coviello D, Colombini A. Intervertebral disc and endplate cell characterisation highlights annulus fibrosus cells as the most promising for tissue-specific disc degeneration therapy. Eur Cell Mater. 2020;39:156–170. doi: 10.22203/eCM.v039a10. [DOI] [PubMed] [Google Scholar]
- 128.Kawaguchi J, Mee PJ, Smith AG. Osteogenic and chondrogenic differentiation of embryonic stem cells in response to specific growth factors. Bone. 2005;36:758–769. doi: 10.1016/j.bone.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 129.Shi Y, Desponts C, Do JT, Hahm HS, Schöler HR, Ding S. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell. 2008;3:568–574. doi: 10.1016/j.stem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 130.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 131.Sheyn D, Ben-David S, Tawackoli W, Zhou Z, Salehi K, Bez M, De Mel S, Chan V, Roth J, Avalos P, Giaconi JC, Yameen H, Hazanov L, Seliktar D, Li D, Gazit D, Gazit Z. Human iPSCs can be differentiated into notochordal cells that reduce intervertebral disc degeneration in a porcine model. Theranostics. 2019;9:7506–7524. doi: 10.7150/thno.34898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xia K, Zhu J, Hua J, Gong Z, Yu C, Zhou X, Wang J, Huang X, Yu W, Li L, Gao J, Chen Q, Li F, Liang C. Intradiscal Injection of Induced Pluripotent Stem Cell-Derived Nucleus Pulposus-Like Cell-Seeded Polymeric Microspheres Promotes Rat Disc Regeneration. Stem Cells Int. 2019;2019:6806540. doi: 10.1155/2019/6806540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Buckley CT, Hoyland JA, Fujii K, Pandit A, Iatridis JC, Grad S. Critical aspects and challenges for intervertebral disc repair and regeneration-Harnessing advances in tissue engineering. JOR Spine. 2018;1:e1029. doi: 10.1002/jsp2.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen WH, Lo WC, Lee JJ, Su CH, Lin CT, Liu HY, Lin TW, Lin WC, Huang TY, Deng WP. Tissue-engineered intervertebral disc and chondrogenesis using human nucleus pulposus regulated through TGF-beta1 in platelet-rich plasma. J Cell Physiol. 2006;209:744–754. doi: 10.1002/jcp.20765. [DOI] [PubMed] [Google Scholar]
- 135.Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, Fehlings MG. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci. 2006;26:3377–3389. doi: 10.1523/JNEUROSCI.4184-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Illes J, Reimer JC, Kwon BK. Stem cell clinical trials for spinal cord injury: readiness, reluctance, redefinition. Stem Cell Rev Rep. 2011;7:997–1005. doi: 10.1007/s12015-011-9259-1. [DOI] [PubMed] [Google Scholar]
- 137.Guest J, Harrop JS, Aarabi B, Grossman RG, Fawcett JW, Fehlings MG, Tator CH. Optimization of the decision-making process for the selection of therapeutics to undergo clinical testing for spinal cord injury in the North American Clinical Trials Network. J Neurosurg Spine. 2012;17:94–101. doi: 10.3171/2012.5.AOSPINE1289. [DOI] [PubMed] [Google Scholar]
- 138.Tuszynski MH, Steeves JD, Fawcett JW, Lammertse D, Kalichman M, Rask C, Curt A, Ditunno JF, Fehlings MG, Guest JD, Ellaway PH, Kleitman N, Bartlett PF, Blight AR, Dietz V, Dobkin BH, Grossman R, Privat A International Campaign for Cures of Spinal Cord Injury Paralysis. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP Panel: clinical trial inclusion/exclusion criteria and ethics. Spinal Cord. 2007;45:222–231. doi: 10.1038/sj.sc.3102009. [DOI] [PubMed] [Google Scholar]
- 139.Saberi H, Derakhshanrad N, Ghajarzadeh M. Overview of ethical issues for conducting neuroprotective clinical trials in patients with spinal cord injury. J Neurorestorat. 2015;3:97–100. [Google Scholar]
- 140.Daly C, Ghosh P, Jenkin G, Oehme D, Goldschlager T. A Review of Animal Models of Intervertebral Disc Degeneration: Pathophysiology, Regeneration, and Translation to the Clinic. Biomed Res Int. 2016;2016:5952165. doi: 10.1155/2016/5952165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Willett NJ, Boninger ML, Miller LJ, Alvarez L, Aoyama T, Bedoni M, Brix KA, Chisari C, Christ G, Dearth CL, Dyson-Hudson TA, Evans CH, Goldman SM, Gregory K, Gualerzi A, Hart J, Ito A, Kuroki H, Loghmani MT, Mack DL, Malanga GA, Noble-Haeusslein L, Pasquina P, Roche JA, Rose L, Stoddart MJ, Tajino J, Terzic C, Topp KS, Wagner WR, Warden SJ, Wolf SL, Xie H, Rando TA, Ambrosio F. Taking the Next Steps in Regenerative Rehabilitation: Establishment of a New Interdisciplinary Field. Arch Phys Med Rehabil. 2020;101:917–923. doi: 10.1016/j.apmr.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Masuda K, Lotz JC. New challenges for intervertebral disc treatment using regenerative medicine. Tissue Eng Part B Rev. 2010;16:147–158. doi: 10.1089/ten.teb.2009.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fiani B, Covarrubias C, Jarrah R. Genetic Predictors of Early-Onset Spinal Intervertebral Disc Degeneration: Part One of Two. Cureus. 2021;13:e15182. doi: 10.7759/cureus.15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kennon JC, Awad ME, Chutkan N, DeVine J, Fulzele S. Current insights on use of growth factors as therapy for Intervertebral Disc Degeneration. Biomol Concepts. 2018;9:43–52. doi: 10.1515/bmc-2018-0003. [DOI] [PubMed] [Google Scholar]
- 145.Beeravolu N, Khan I, McKee C, Dinda S, Thibodeau B, Wilson G, Perez-Cruet M, Bahado-Singh R, Chaudhry GR. Isolation and comparative analysis of potential stem/progenitor cells from different regions of human umbilical cord. Stem Cell Res. 2016;16:696–711. doi: 10.1016/j.scr.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 146.Chen JY, Mou XZ, Du XC, Xiang C. Comparative analysis of biological characteristics of adult mesenchymal stem cells with different tissue origins. Asian Pac J Trop Med. 2015;8:739–746. doi: 10.1016/j.apjtm.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 147.Ding DC, Chang YH, Shyu WC, Lin SZ. Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant. 2015;24:339–347. doi: 10.3727/096368915X686841. [DOI] [PubMed] [Google Scholar]
- 148.Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384–1392. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 149.Rocha V, Wagner JE Jr, Sobocinski KA, Klein JP, Zhang MJ, Horowitz MM, Gluckman E. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. Eurocord and International Bone Marrow Transplant Registry Working Committee on Alternative Donor and Stem Cell Sources. N Engl J Med. 2000;342:1846–1854. doi: 10.1056/NEJM200006223422501. [DOI] [PubMed] [Google Scholar]
- 150.Dilxat D, Li H, Liu L. Unique Features and Clinical Application Prospect of Human Umbilical Cord Mesenchymal Stem Cells. Int J Orthop. 2016;3:627–631. [Google Scholar]
- 151.Stefańska K, Ożegowska K, Hutchings G, Popis M, Moncrieff L, Dompe C, Janowicz K, Pieńkowski W, Gutaj P, Shibli JA, Prado WM, Piotrowska-Kempisty H, Mozdziak P, Bruska M, Zabel M, Kempisty B, Nowicki M. Human Wharton's Jelly-Cellular Specificity, Stemness Potency, Animal Models, and Current Application in Human Clinical Trials. J Clin Med. 2020;9 doi: 10.3390/jcm9041102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.McGuirk JP, Smith JR, Divine CL, Zuniga M, Weiss ML. Wharton's Jelly-Derived Mesenchymal Stromal Cells as a Promising Cellular Therapeutic Strategy for the Management of Graft-versus-Host Disease. Pharmaceuticals (Basel) 2015;8:196–220. doi: 10.3390/ph8020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Beeravolu N, McKee C, Alamri A, Mikhael S, Brown C, Perez-Cruet M, Chaudhry GR. Isolation and Characterization of Mesenchymal Stromal Cells from Human Umbilical Cord and Fetal Placenta. J Vis Exp. 2017 doi: 10.3791/55224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Secunda R, Vennila R, Mohanashankar AM, Rajasundari M, Jeswanth S, Surendran R. Isolation, expansion and characterisation of mesenchymal stem cells from human bone marrow, adipose tissue, umbilical cord blood and matrix: a comparative study. Cytotechnology. 2015;67:793–807. doi: 10.1007/s10616-014-9718-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pang X, Yang H, Peng B. Human umbilical cord mesenchymal stem cell transplantation for the treatment of chronic discogenic low back pain. Pain Physician. 2014;17:E525–E530. [PubMed] [Google Scholar]
- 156.Sakai D, Andersson GB. Stem cell therapy for intervertebral disc regeneration: obstacles and solutions. Nat Rev Rheumatol. 2015;11:243–256. doi: 10.1038/nrrheum.2015.13. [DOI] [PubMed] [Google Scholar]
- 157.Hu C, Li L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J Cell Mol Med. 2018;22:1428–1442. doi: 10.1111/jcmm.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Clarke LE, McConnell JC, Sherratt MJ, Derby B, Richardson SM, Hoyland JA. Growth differentiation factor 6 and transforming growth factor-beta differentially mediate mesenchymal stem cell differentiation, composition, and micromechanical properties of nucleus pulposus constructs. Arthritis Res Ther. 2014;16:R67. doi: 10.1186/ar4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stoyanov JV, Gantenbein-Ritter B, Bertolo A, Aebli N, Baur M, Alini M, Grad S. Role of hypoxia and growth and differentiation factor-5 on differentiation of human mesenchymal stem cells towards intervertebral nucleus pulposus-like cells. Eur Cell Mater. 2011;21:533–547. doi: 10.22203/ecm.v021a40. [DOI] [PubMed] [Google Scholar]
- 160.Colombier P, Clouet J, Boyer C, Ruel M, Bonin G, Lesoeur J, Moreau A, Fellah BH, Weiss P, Lescaudron L, Camus A, Guicheux J. TGF-β1 and GDF5 Act Synergistically to Drive the Differentiation of Human Adipose Stromal Cells toward Nucleus Pulposus-like Cells. Stem Cells. 2016;34:653–667. doi: 10.1002/stem.2249. [DOI] [PubMed] [Google Scholar]
- 161.Wang SL, Yu YL, Tang CL, Lv FZ. Effects of TGF-β1 and IL-1β on expression of ADAMTS enzymes and TIMP-3 in human intervertebral disc degeneration. Exp Ther Med. 2013;6:1522–1526. doi: 10.3892/etm.2013.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang R, Ruan D, Zhang C. Effects of TGF-beta1 and IGF-1 on proliferation of human nucleus pulposus cells in medium with different serum concentrations. J Orthop Surg Res. 2006;1:9. doi: 10.1186/1749-799X-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gantenbein-Ritter B, Benneker LM, Alini M, Grad S. Differential response of human bone marrow stromal cells to either TGF-β(1) or rhGDF-5. Eur Spine J. 2011;20:962–971. doi: 10.1007/s00586-010-1619-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dorman LJ, Tucci M, Benghuzzi H. In vitro effects of bmp-2, bmp-7, and bmp-13 on proliferation and differentation of mouse mesenchymal stem cells. Biomed Sci Instrum. 2012;48:81–87. [PubMed] [Google Scholar]
- 165.Shen B, Wei A, Whittaker S, Williams LA, Tao H, Ma DD, Diwan AD. The role of BMP-7 in chondrogenic and osteogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in vitro. J Cell Biochem. 2010;109:406–416. doi: 10.1002/jcb.22412. [DOI] [PubMed] [Google Scholar]
- 166.Li J, Yoon ST, Hutton WC. Effect of bone morphogenetic protein-2 (BMP-2) on matrix production, other BMPs, and BMP receptors in rat intervertebral disc cells. J Spinal Disord Tech. 2004;17:423–428. doi: 10.1097/01.bsd.0000112084.85112.5d. [DOI] [PubMed] [Google Scholar]
- 167.Krouwels A, Iljas JD, Kragten AHM, Dhert WJA, Öner FC, Tryfonidou MA, Creemers LB. Bone Morphogenetic Proteins for Nucleus Pulposus Regeneration. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21082720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shen B, Bhargav D, Wei A, Williams LA, Tao H, Ma DD, Diwan AD. BMP-13 emerges as a potential inhibitor of bone formation. Int J Biol Sci. 2009;5:192–200. doi: 10.7150/ijbs.5.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gong C, Pan W, Hu W, Chen L. Bone morphogenetic protein-7 retards cell subculture-induced senescence of human nucleus pulposus cells through activating the PI3K/Akt pathway. Biosci Rep. 2019;39 doi: 10.1042/BSR20182312. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 170.Kim JS, Ellman MB, An HS, van Wijnen AJ, Borgia JA, Im HJ. Insulin-like growth factor 1 synergizes with bone morphogenetic protein 7-mediated anabolism in bovine intervertebral disc cells. Arthritis Rheum. 2010;62:3706–3715. doi: 10.1002/art.27733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang SZ, Rui YF, Tan Q, Wang C. Enhancing intervertebral disc repair and regeneration through biology: platelet-rich plasma as an alternative strategy. Arthritis Res Ther. 2013;15:220. doi: 10.1186/ar4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bae WC, Masuda K. Emerging technologies for molecular therapy for intervertebral disk degeneration. Orthop Clin North Am. 2011;42:585–601, ix. doi: 10.1016/j.ocl.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fang H, Li X, Shen H, Sun B, Teng H, Li P. Osteogenic protein-1 attenuates apoptosis and enhances matrix synthesis of nucleus pulposus cells under high-magnitude compression though inhibiting the p38 MAPK pathway. Biosci Rep. 2018 doi: 10.1042/BSR20180018. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 174.Masuda K, Takegami K, An H, Kumano F, Chiba K, Andersson GB, Schmid T, Thonar E. Recombinant osteogenic protein-1 upregulates extracellular matrix metabolism by rabbit annulus fibrosus and nucleus pulposus cells cultured in alginate beads. J Orthop Res. 2003;21:922–930. doi: 10.1016/S0736-0266(03)00037-8. [DOI] [PubMed] [Google Scholar]
- 175.van Dijk BGM, Potier E, van Dijk M, Creemers LB, Ito K. Osteogenic protein 1 does not stimulate a regenerative effect in cultured human degenerated nucleus pulposus tissue. J Tissue Eng Regen Med. 2017;11:2127–2135. doi: 10.1002/term.2111. [DOI] [PubMed] [Google Scholar]
- 176.Pizzute T, Li J, Zhang Y, Davis ME, Pei M. Fibroblast Growth Factor Ligand Dependent Proliferation and Chondrogenic Differentiation of Synovium-Derived Stem Cells and Concomitant Adaptation of Wnt/Mitogen-Activated Protein Kinase Signals. Tissue Eng Part A. 2016;22:1036–1046. doi: 10.1089/ten.tea.2016.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shu C, Smith SM, Little CB, Melrose J. Use of FGF-2 and FGF-18 to direct bone marrow stromal stem cells to chondrogenic and osteogenic lineages. Future Sci OA. 2016;2:FSO142. doi: 10.4155/fsoa-2016-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pizzute TT, Emery S, Zhang Y, Waris S, Pei M. Preconditioning strategies unequally impact regeneration of nucleus pulposus cells from human herniated discs: a proof of principle study. Nucleus Pulposus and Mesenchymal Stem Cell Preconditioning: A Focus on Redifferentiation, Wnt Signaling, and Immunity 2017; 1001: 171. [Google Scholar]
- 179.Jiang C, Li DP, Zhang ZJ, Shu HM, Hu L, Li ZN, Huang YH. [Effect of Basic Fibroblast Growth Factor and Transforming Growth Factor-Β1 Combined with Bone Marrow Mesenchymal Stem Cells on the Repair of Degenerated Intervertebral Discs in Rat Models] Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2015;37:456–465. doi: 10.3881/j.issn.1000-503X.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 180.Travascio F, Elmasry S, Asfour S. Modeling the role of IGF-1 on extracellular matrix biosynthesis and cellularity in intervertebral disc. J Biomech. 2014;47:2269–2276. doi: 10.1016/j.jbiomech.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 181.Li B, Zheng XF, Ni BB, Yang YH, Jiang SD, Lu H, Jiang LS. Reduced expression of insulin-like growth factor 1 receptor leads to accelerated intervertebral disc degeneration in mice. Int J Immunopathol Pharmacol. 2013;26:337–347. doi: 10.1177/039463201302600207. [DOI] [PubMed] [Google Scholar]
- 182.Xu G, Zhang C, Zhu K, Ye Y, Bao Z. [Effects of lentivirus-mediated insulin-like growth factor 1 and platelet derived growth factor genes on nucleus pulposus tissue of human degenerated intervertebral disc] Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2020;34:907–914. doi: 10.7507/1002-1892.201910101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chujo T, An HS, Akeda K, Miyamoto K, Muehleman C, Attawia M, Andersson G, Masuda K. Effects of growth differentiation factor-5 on the intervertebral disc--in vitro bovine study and in vivo rabbit disc degeneration model study. Spine (Phila Pa 1976) 2006;31:2909–2917. doi: 10.1097/01.brs.0000248428.22823.86. [DOI] [PubMed] [Google Scholar]
- 184.Feng C, Liu H, Yang Y, Huang B, Zhou Y. Growth and differentiation factor-5 contributes to the structural and functional maintenance of the intervertebral disc. Cell Physiol Biochem. 2015;35:1–16. doi: 10.1159/000369670. [DOI] [PubMed] [Google Scholar]
- 185.Tzaan WC, Chen HC. Investigating the possibility of intervertebral disc regeneration induced by granulocyte colony stimulating factor-stimulated stem cells in rats. Adv Orthop. 2011;2011:602089. doi: 10.4061/2011/602089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Narcisi R, Cleary MA, Brama PA, Hoogduijn MJ, Tüysüz N, ten Berge D, van Osch GJ. Long-term expansion, enhanced chondrogenic potential, and suppression of endochondral ossification of adult human MSCs via WNT signaling modulation. Stem Cell Reports. 2015;4:459–472. doi: 10.1016/j.stemcr.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Liu Y, Kong J, Chen BH, Hu YG. Combined expression of CTGF and tissue inhibitor of metalloprotease-1 promotes synthesis of proteoglycan and collagen type II in rhesus monkey lumbar intervertebral disc cells in vitro. Chin Med J (Engl) 2010;123:2082–2087. [PubMed] [Google Scholar]
- 188.Sheikh H, Zakharian K, De La Torre RP, Facek C, Vasquez A, Chaudhry GR, Svinarich D, Perez-Cruet MJ. In vivo intervertebral disc regeneration using stem cell-derived chondroprogenitors. J Neurosurg Spine. 2009;10:265–272. doi: 10.3171/2008.12.SPINE0835. [DOI] [PubMed] [Google Scholar]
- 189.Dreyfus J, Brunet-de Carvalho N, Duprez D, Raulais D, Vigny M. HB-GAM/pleiotrophin but not RIHB/midkine enhances chondrogenesis in micromass culture. Exp Cell Res. 1998;241:171–180. doi: 10.1006/excr.1998.4040. [DOI] [PubMed] [Google Scholar]
- 190.Iwasaki M, Nakata K, Nakahara H, Nakase T, Kimura T, Kimata K, Caplan AI, Ono K. Transforming growth factor-beta 1 stimulates chondrogenesis and inhibits osteogenesis in high density culture of periosteum-derived cells. Endocrinology. 1993;132:1603–1608. doi: 10.1210/endo.132.4.8462458. [DOI] [PubMed] [Google Scholar]
- 191.Fortier LA, Nixon AJ, Lust G. Phenotypic expression of equine articular chondrocytes grown in three-dimensional cultures supplemented with supraphysiologic concentrations of insulin-like growth factor-I. J Am Vet Med Assoc. 2002;63:301–305. doi: 10.2460/ajvr.2002.63.301. [DOI] [PubMed] [Google Scholar]
- 192.Fujisawa K, Hara K, Takami T, Okada S, Matsumoto T, Yamamoto N, Sakaida I. Evaluation of the effects of ascorbic acid on metabolism of human mesenchymal stem cells. Stem Cell Res Ther. 2018;9:93. doi: 10.1186/s13287-018-0825-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Steck E, Bertram H, Abel R, Chen B, Winter A, Richter W. Induction of intervertebral disc-like cells from adult mesenchymal stem cells. Stem Cells. 2005;23:403–411. doi: 10.1634/stemcells.2004-0107. [DOI] [PubMed] [Google Scholar]
- 194.Korecki CL, Taboas JM, Tuan RS, Iatridis JC. Notochordal cell conditioned medium stimulates mesenchymal stem cell differentiation toward a young nucleus pulposus phenotype. Stem Cell Res Ther. 2010;1:18. doi: 10.1186/scrt18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Abbott RD, Purmessur D, Monsey RD, Iatridis JC. Regenerative potential of TGFβ3 + Dex and notochordal cell conditioned media on degenerated human intervertebral disc cells. J Orthop Res. 2012;30:482–488. doi: 10.1002/jor.21534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bouderlique T, Henault E, Lebouvier A, Frescaline G, Bierling P, Rouard H, Courty J, Albanese P, Chevallier N. Pleiotrophin commits human bone marrow mesenchymal stromal cells towards hypertrophy during chondrogenesis. PLoS One. 2014;9:e88287. doi: 10.1371/journal.pone.0088287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Jakobsen RB, Østrup E, Zhang X, Mikkelsen TS, Brinchmann JE. Analysis of the effects of five factors relevant to in vitro chondrogenesis of human mesenchymal stem cells using factorial design and high throughput mRNA-profiling. PLoS One. 2014;9:e96615. doi: 10.1371/journal.pone.0096615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wei A, Shen B, Williams L, Diwan A. Mesenchymal stem cells: potential application in intervertebral disc regeneration. Transl Pediatr. 2014;3:71–90. doi: 10.3978/j.issn.2224-4336.2014.03.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Endo K, Fujita N, Nakagawa T, Nishimura R. Comparison of the effect of growth factors on chondrogenesis of canine mesenchymal stem cells. J Vet Med Sci. 2019;81:1211–1218. doi: 10.1292/jvms.18-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li W, Liu T, Wu L, Chen C, Jia Z, Bai X, Ruan D. Blocking the function of inflammatory cytokines and mediators by using IL-10 and TGF-β: a potential biological immunotherapy for intervertebral disc degeneration in a beagle model. Int J Mol Sci. 2014;15:17270–17283. doi: 10.3390/ijms151017270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yang H, Wu J, Liu J, Ebraheim M, Castillo S, Liu X, Tang T, Ebraheim NA. Transplanted mesenchymal stem cells with pure fibrinous gelatin-transforming growth factor-beta1 decrease rabbit intervertebral disc degeneration. Spine J. 2010;10:802–810. doi: 10.1016/j.spinee.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 202.Vadalà G, Ambrosio L, Russo F, Papalia R, Denaro V. Interaction between Mesenchymal Stem Cells and Intervertebral Disc Microenvironment: From Cell Therapy to Tissue Engineering. Stem Cells Int. 2019;2019:2376172. doi: 10.1155/2019/2376172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Schäfer R, Spohn G, Baer PC. Mesenchymal Stem/Stromal Cells in Regenerative Medicine: Can Preconditioning Strategies Improve Therapeutic Efficacy? Transfus Med Hemother. 2016;43:256–267. doi: 10.1159/000447458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Baek SJ, Kang SK, Ra JC. In vitro migration capacity of human adipose tissue-derived mesenchymal stem cells reflects their expression of receptors for chemokines and growth factors. Exp Mol Med. 2011;43:596–603. doi: 10.3858/emm.2011.43.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Matsumura E, Tsuji K, Komori K, Koga H, Sekiya I, Muneta T. Pretreatment with IL-1β enhances proliferation and chondrogenic potential of synovium-derived mesenchymal stem cells. Cytotherapy. 2017;19:181–193. doi: 10.1016/j.jcyt.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 206.Shioda M, Muneta T, Tsuji K, Mizuno M, Komori K, Koga H, Sekiya I. TNFα promotes proliferation of human synovial MSCs while maintaining chondrogenic potential. PLoS One. 2017;12:e0177771. doi: 10.1371/journal.pone.0177771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wehling N, Palmer GD, Pilapil C, Liu F, Wells JW, Müller PE, Evans CH, Porter RM. Interleukin-1beta and tumor necrosis factor alpha inhibit chondrogenesis by human mesenchymal stem cells through NF-kappaB-dependent pathways. Arthritis Rheum. 2009;60:801–812. doi: 10.1002/art.24352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Fujita N, Imai J, Suzuki T, Yamada M, Ninomiya K, Miyamoto K, Iwasaki R, Morioka H, Matsumoto M, Chiba K, Watanabe S, Suda T, Toyama Y, Miyamoto T. Vascular endothelial growth factor-A is a survival factor for nucleus pulposus cells in the intervertebral disc. Biochem Biophys Res Commun. 2008;372:367–372. doi: 10.1016/j.bbrc.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 209.Perez-Cruet M, Beeravolu N, McKee C, Brougham J, Khan I, Bakshi S, Chaudhry GR. Potential of Human Nucleus Pulposus-Like Cells Derived From Umbilical Cord to Treat Degenerative Disc Disease. Neurosurgery. 2019;84:272–283. doi: 10.1093/neuros/nyy012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ratajczak J, Kucia M, Mierzejewska K, Marlicz W, Pietrzkowski Z, Wojakowski W, Greco NJ, Tendera M, Ratajczak MZ. Paracrine proangiopoietic effects of human umbilical cord blood-derived purified CD133+ cells--implications for stem cell therapies in regenerative medicine. Stem Cells Dev. 2013;22:422–430. doi: 10.1089/scd.2012.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]