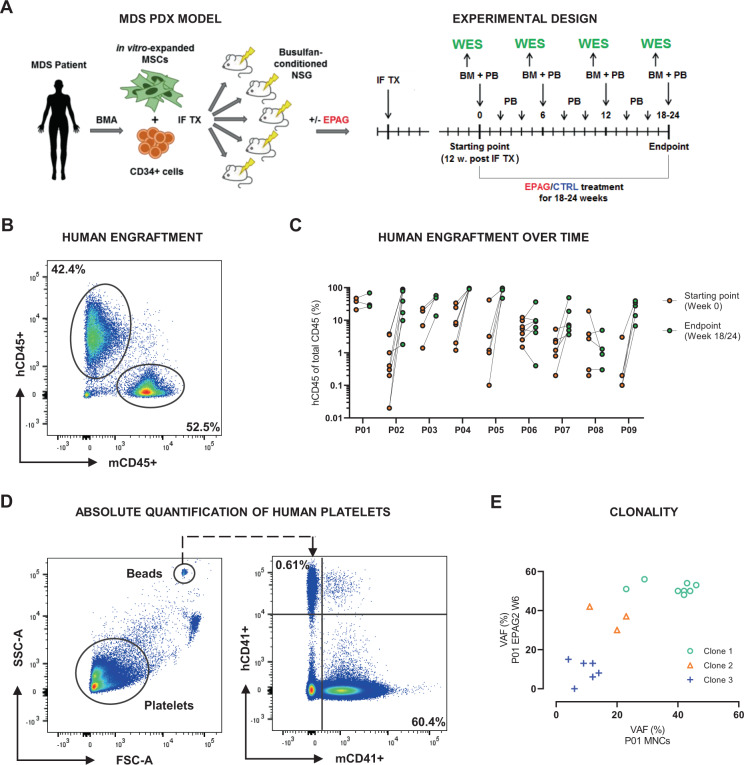
Fig. 1. Experimental setup and readouts for preclinical assessment of EPAG using a MDS patient-derived xenograft model.
A Schematic experimental setup. CD34+ cells and mesenchymal stromal cells (MSCs) derived from bone marrow aspirations (BMAs) of MDS patients were injected into Busulfan-conditioned NSG mice by bilateral intrafemoral transplantation (IF TX). Long-term engraftment was determined 12 weeks post transplant. Mice with positive human engraftment subsequently received oral treatment of eltrombopag (EPAG) or vehicle control (CTRL) for 18–24 weeks until endpoint. Starting with treatment, peripheral blood (PB) and BM were sampled every 2 and 6 weeks, respectively. B Representative flow cytometry plot showing percentage of human CD45+ (hCD45+) and mouse CD45+ (mCD45+) cells in the BM of patient-derived xenografts (PDXs). Human engraftment was defined as percentage of hCD45+ of total CD45+ cells. C Comparison of engraftment rates between patients’ PDX at starting (orange) and endpoint (green). On the x-axis, patient IDs are shown. D Representative flow cytometry plots showing gating scheme for platelets (PLTs) and beads (left), and percentage of hCD41+ and mCD41+ PLTs in PB of PDX (right). The absolute number of human PLTs per microliter PB was calculated in relation to the number of beads recorded. E Exemplary clustering of variant allele frequencies (VAFs) of mutations and copy number variations into different subclones using the bioinformatic tool SciClone. FSC-A, forward scatter area; SSC-A, side scatter area.