Abstract
Systemic Sclerosis (SSc) is an autoimmune disease marked by dysregulation of the immune system, tissue fibrosis and dysfunction of the vasculature. Vascular damage, remodeling and inadequate endothelial repair are hallmarks of the disease. Since early stages of SSc, damage and apoptosis of endothelial cells (ECs) can lead to perivascular inflammation, oxidative stress and tissue hypoxia, resulting in multiple clinical manifestations. Raynaud's phenomenon, edematous puffy hands, digital ulcers, pulmonary artery hypertension, erectile dysfunction, scleroderma renal crisis and heart involvement severely affect quality of life and survival. Understanding pathogenic aspects and biomarkers that reflect endothelial damage in SSc is essential to guide therapeutic interventions. Treatment approaches described for SSc-associated vasculopathy include pharmacological options to improve blood flow and tissue perfusion and, more recently, cellular therapy to enhance endothelial repair, promote angiogenesis and heal injuries. This mini-review examines the current knowledge on cellular and molecular aspects of SSc vasculopathy, as well as established and developing therapeutic approaches for improving the vascular compartment.
Keywords: systemic sclerosis, vasculopathy, cellular therapy, endothelial cells, vasodilator agent
Introduction
Systemic sclerosis (SSc) is an autoimmune disease marked by diffuse vasculopathy, immunological dysregulation and fibrosis of the skin and internal organs. Vascular manifestations derive mostly from impaired blood flow and tissue ischemia, and are a challenge for the management of SSc patients (1–3). In this mini-review, we examine the current and developing therapeutic interventions with pharmacological agents and cellular therapy for SSc-associated vasculopathy.
Pathophysiology of the Vascular Endothelium in Systemic Sclerosis
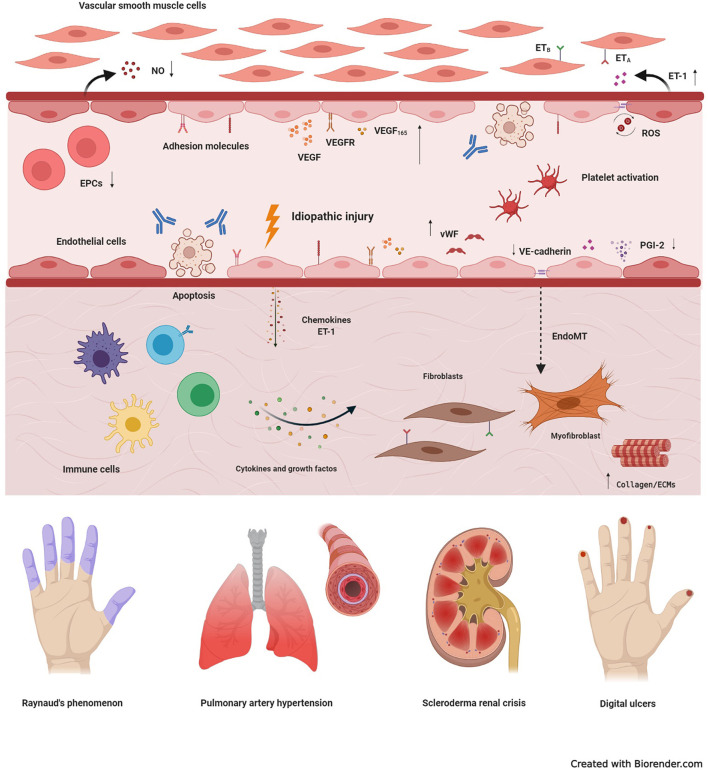
The endothelium is a metabolically active tissue that ensures regulation of vascular tone, coagulation and fibrinolysis, smooth muscle proliferation, cell adhesion and inflammation (4). Vascular injury is an early event in SSc, with damage and activation of endothelial cells (ECs) (5, 6) (Figure 1). Injured ECs in SSc produce increased levels of endothelin-1 (ET-1) and von Willebrand factor (vWF), and low levels of nitric oxide (NO) and endothelial nitric oxide synthase (5). The resulting imbalance between vasodilation and vasoconstriction modifies the vascular tone, contributing to tissue hypoxia. ET-1 also induces differentiation of fibroblasts into a myofibroblastic phenotype, promoting intimal hyperplasia, luminal narrowing, and vessel obliteration (7, 8). Myofibroblasts may also be originated through the endothelial-to-mesenchymal transition (EndoMT) (9), when ECs downregulate expression of markers such as CD31 and VE-cadherin, and assume a myofibroblast phenotype, characterized by fusiform morphology and expression of α-SMA (10). The abnormal vascular tonus and the increased expression of vWF stimulate platelet aggregation and hypercoagulation, leading to further vascular damage (11, 12). Reactive oxygen species contribute to further enhance the damage, participating in the initiation and progression of SSc (2, 5).
Figure 1.
Basic mechanisms of systemic sclerosis-related vasculopathy. Vascular injury is considered an initial event in the development of systemic sclerosis (SSc), and may be triggered by multiple factors, including autoantibodies, infectious agents, reactive oxygen species (ROS), or idiopathic stimuli. In the early stages of disease, vascular damage leads to activation of endothelial cells (ECs), with expression of adhesion molecules, production of chemokines, von Willebrand factor (vWF) and vasoconstrictor agents, such as endothelin-1 (ET-1). Molecules produced by the injured endothelium recruit immune cells, that generate a perivascular infiltrate. Prolonged inflammation leads to tissue fibrosis, with excessive activation of resident fibroblasts that transdifferentiate into myofibroblasts, the main cell type involved in excessive collagen production and other extracellular matrix components (ECMs). Myofibroblasts are also originated through the endothelial-to-mesenchymal transition (EndoMT). Dysfunction of endothelial progenitor cells (EPCs), antibody-induced ECs apoptosis, persistent platelet activation, decreased production of vasodilatory nitric oxide (NO) and prostaglandin I-2 (PGI-2), synthetized by ECs, also participate in the pathogenesis of SSc-vasculopathy. In addition, compensatory mechanisms of vasculogenesis and angiogenesis, including vascular endothelial growth factor (VEGF) and its receptor (VEGFR), are dysregulated and ineffective. High expression of VEGF165, an anti-angiogenic isoform, contributes to this scenario. Reactive oxygen species, further contribute to intensify damage and activation of the endothelium and, thus, increase tissue injury. Clinical manifestations of SSc-related vasculopathy include Raynaud's phenomenon, pulmonary arterial hypertension, scleroderma renal crisis, telangiectasias, digital ulcers and digital pitting scars, which severely affect quality of life and may compromise survival. ETA, type A endothelin receptor; ETB, type B endothelin receptor.
Cell adhesion molecules play an important role in promoting endothelial integrity, besides regulating leukocyte migration, vascular permeability and angiogenesis (13). Increased expression of adhesion molecules and their soluble levels, detected in early stages of SSc, correlate with disease severity and visceral involvement (14–18). Indeed, increased levels of E-selectin, vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM-1) lead to activation of ECs, dysregulation of angiogenesis and, as consequence, chronic and progressive vascular damage (19).
Impaired Compensatory Angiogenesis and Vasculogenesis
In SSc, damage and apoptosis of ECs result in loss of capillaries that are not repaired by compensatory mechanisms of vasculogenesis and angiogenesis (20, 21). Vascular endothelial growth factor (VEGF) regulates blood vessel growth, with key role in the process of angiogenesis (22). Serum levels of VEGF and its receptor (VEGFR) are increased in SSc (16, 23–26). Exposure to high levels of VEGF causes an exaggerated angiogenic stimulus, with proliferation of ECs, resulting in chaotic architecture of vessels, as observed by capillaroscopy (19). An anti-angiogenic isoform, VEGF165, has been described in SSc patients (27), and platelet releases containing VEGF165 impair angiogenesis in vitro (28). In addition, function and frequencies of endothelial progenitor cells (EPCs) are compromised in SSc, playing a defective role in vasculogenesis (29). Table 1 describes additional biomarkers associated with vascular damage in SSc.
Table 1.
Biomarkers associated with endothelial activation or vascular damage in SSc and clinical correlates.
| Biomarkers | Class/function | Clinical associations | References |
|---|---|---|---|
| Adhesion molecules (ICAM-1, VCAM-1, selectins) |
Cell–cell interactions | Capillaroscopic abnormalities Disease severity Pulmonary fibrosis |
(14, 15, 18, 30–35) |
| Angiopoietin system (ANG-Tie) | Angiogenesis | Disease activity Digital ulcers Esophageal dysmotility Microangiopathy Proliferative vasculopathy |
(36–41) |
| Anti-centromere (ACA) | Autoantibodies | Microangiopathy Pulmonary arterial hypertension |
(41–43) |
| Anti-AT1R and -ETAR | Autoantibodies | Digital ischemic Pulmonary arterial hypertension (PAH) |
(44, 45) |
| Anti-endothelial cell (AECA) | Autoantibodies | Pulmonary fibrosis | (46) |
| Anti-RNA polymerase III | Autoantibodies | Gastric Antral Vascular Ectasia (GAVE) Scleroderma renal crisis Diffuse skin thickening Cardiopulmonary involvement Rapid disease progression |
(34, 47–55) |
| Anti - topoisomerase I (anti-SCl70) | Autoantibodies | Digital ulcers Heart involvement Interstitial lung disease |
(56) |
| Endoglin (CD105) | Type I membrane glycoprotein. | Digital ulcers | (57) |
| Endothelin-1 | Vasoconstrictor molecule | Interstitial lung disease Right ventricle dysfunction |
(58–62) |
| Endostatin | Angiogenesis | Digital vascular damage Skin and pulmonary fibrosis |
(63, 64) |
| Thrombomodulin | Coagulation | Pulmonary hypertension | (65) |
| Thrombospondin-1 (TSP-1) | Antiangiogenic glycoprotein | Brachio-cervical inflammatory myopathy | (66) |
| Vascular endothelial cell growth (VEGF) | Angiogenesis | Diffuse skin subset Interstitial lung involvement Nailfold capillary loss Pulmonary Artery Hypertension (PAH) |
(25, 67–72) |
ETAR, endothelin receptor type A; AT1R, Ang receptor type-1.
Clinical Manifestations of SSc-Associated Vasculopathy
Raynaud's phenomenon is one of the first manifestations of the disease (8, 73). Progressive structural damage of the vessels, followed by proliferative endarteritis and consequent tissue ischemia, leads to systemic involvement, characterizing SSc as a microvascular disease. Telangiectasias and digital ulcers are frequent vascular manifestations of SSc, and associate with poor prognosis (74, 75). Scleroderma renal crisis, a severe clinical condition characterized by poor renal cortical perfusion and rapidly progressive renal failure, was a leading cause of death until the 1970s, when use of angiotensin-converting enzyme inhibitors significantly improved patient management and outcomes (76–80). Primary and secondary cardiac involvements are described as frequent and probably underestimated in SSc (81–83), and from 5 to 15% of SSc patients develop pulmonary hypertension (79, 81). Less explored, but still frequent vascular manifestations of SSc are erectile dysfunction, vascular malformations of the gastro-intestinal mucosa (gastric antral vascular ectasia - GAVE) and, to some extent, myopathy (66, 84–86). Routine assessments for vascular involvement include clinical inspections, evaluation of organ function and, when required, right-heart catheterism. Such manifestations should be actively investigated and treated early, before advanced organ damage.
Pharmacological Approaches
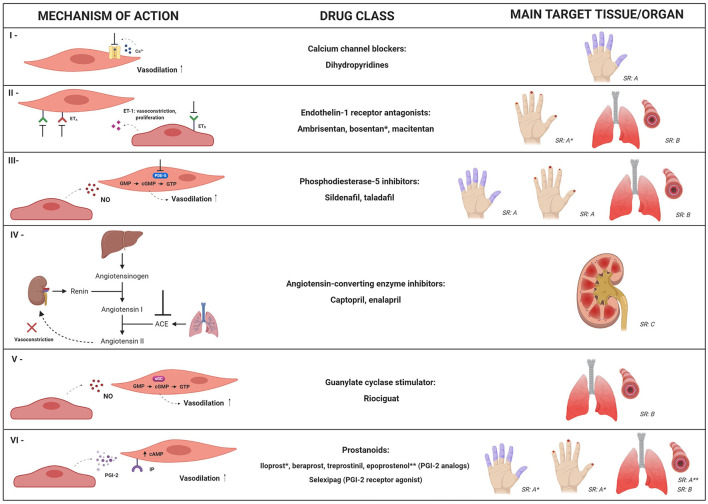
Therapeutic strategies for vasculopathy in SSc aim to improve symptoms of Raynaud's phenomenon (RP), heal and prevent development of digital ulcers (DU), and decrease the ischemic damage to internal organs. Multiple pharmacological options, with different mechanistic approaches, are available and recommended in the management of SSc patients (Figure 2) (87). New strategies, including cell therapy, have been developed to further improve this aspect of the disease.
Figure 2.
Mechanistic targets of the main pharmacological approaches for systemic sclerosis-related vasculopathy. Multiple drug options, with different mechanistic approaches, can be used for management of vascular manifestations in SSc patients. This figure summarizes mechanisms and clinical indications for: calcium channel blockers (I); endothelin-1 receptor antagonists (II); phosphodiesterase-5 (PDE-5) inhibitors (III); angiotensin-converting enzyme (ACE) inhibitors (IV); guanylate cyclase stimulator – riociguat (V) and prostanoids (VI). SR: strength of recommendation; SR: A, SR: B or SR: C according to the European League against Rheumatism, in 2017 (87). Dihydropyridines, PDE-5 inhibitors and iloprost are recommended for SSc-associated Raynaud's phenomenon (SR: A); bosentan is recommended to reduce the number of new digital ulcers (SR: A); PDE-5 inhibitors and iloprost are recommended for active digital ulcers (SR: A); PDE-5 inhibitors, endothelin-1 receptor antagonists (bosentan, ambrisentan, and macitentan) and riociguat are recommended for treatment of SSc-related pulmonary artery hypertension (SR: B); epoprostenol (SR: A) and other prostacyclin analogs (SR: B) are recommended for severe SSc-related pulmonary artery hypertension; ACE inhibitors are recommended as treatment for scleroderma renal crisis (SR: C). * and ** indicate clinical conditions in which specific drugs from the class are recommended. NO, nitric oxide; ET-1, endothelin-1; ETA, type A endothelin receptor; ETB, type B endothelin receptor; cGMP, cyclic guanosine-5-monophosphate; sGC, soluble guanylate cyclase; cAMP, cyclic adenosine monophosphate; GTP, guanosine-5′-triphosphate; IP, prostacyclin receptor; PGI-2, prostaglandin I-2.
Calcium Channel Blockers
Calcium channel blockers reduce intracellular calcium concentrations, inducing relaxation of smooth muscle and vasodilation (88). Dihydropyridines are broadly recommended to attenuate severity and frequency of uncomplicated RP in SSc (87, 89). Short and long-term use of calcium channel blockers decreased plasma markers of oxidative stress (90), and in vitro, nicardipine protected ECs against oxidative injury (91). Calcium channel blockers also decreased serum concentrations of N-terminal pro-brain natriuretic peptide (NT-proBNP) in patients with SSc-associated PAH, indicating a possible antispastic and vasodilatory effect on the pulmonary circulation, not corroborated, however, by hemodynamic changes (92). In patients with <5 years of SSc, nifedipine and nicardipine improved myocardial perfusion and left ventricle function, respectively, supporting the hypothesis of myocardial Raynaud's phenomenon in SSc (93).
Endothelin-1 Receptors Antagonists
Endothelin-1 receptor antagonists target ET-1, a crucial mediator in SSc vasculopathy. Ambrisentan is a selective type A endothelin receptor antagonist, while bosentan and macitentan are dual antagonists, targeting both type A and B receptors (88). In two randomized clinical trials, bosentan prevented the development of new DU, but did not heal active DU (94, 95). Ambrisentan reduced the number of active and new DU in SSc patients, also decreasing pain and disability (96, 97).
Bosentan and ambrisentan improved hemodynamic parameters in patients with SSc-PAH (98, 99). Bosentan also decreased serum concentrations of endothelial activation markers ICAM-1, VCAM-1, P-selectin and PECAM-1 (100). In vitro experiments with preincubation of microvascular endothelial cells (MVECs) from SSc patients with bosentan or macitentan decreased the expression of mesenchymal markers, identifying a possible pharmacological interference pathway to prevent EndoMT (101).
Phosphodiesterase-5A Inhibitors
Phosphodiesterase-5A (PDE-5A) hydrolyzes the cyclic guanosine-5-monophosphate (cGMP), associated to the nitric oxide (NO) vasodilator pathway. PDE-5A inhibitors reduce the metabolism of cGMP, intensifying the vasodilatory effects of NO (102). In SSc patients, PDE-5A inhibitors decreased frequency and duration of RP attacks, improved DU healing (103) and reduced disability and discomfort associated with RP (104). For SSc-PAH, sildenafil reduced pulmonary artery pressure, with beneficial effects on cardiopulmonary status (105). Combined therapy of tadalafil plus ambrisentan resulted in better responses for SSc-PAH than monotherapy with either agent (106). However, sildenafil did not affect the number of circulating EPCs or VEGF serum levels in SSc patients with vasculopathy (107–109). PDE-5 inhibitors have been also investigated as treatment for erectile dysfunction and, although SSc patients have poor response to on-demand administration, daily fixed doses may be effective (110).
Prostanoids
Prostacyclin, also known as prostaglandin I-2 (PGI-2), is synthetized by vascular ECs, promoting vasodilation and decreasing platelet aggregation, inflammation and vascular smooth muscle proliferation (111). Prostacyclin analogs (iloprost, beraprost, treprostinil, and epoprostenol) and the prostacyclin receptor agonist (selexipag) are available pharmaceutical agents that enhance the prostacyclin pathway and thus promote vasodilation (88).
Iloprost was effective for treatment of RP, DU and PAH in SSc patients (112–117), also decreasing serum levels of ICAM-1, VCAM-1 and E-selectin, reflecting reduced activation of ECs (115). Iloprost and bosentan combinatory therapy increased the number of nailfold capillaries (118). Beraprost did not prevent development of DU (119) and had little effect on hemodynamic parameters in SSc-PAH (120). Conversely, epoprostenol improved clinical status and hemodynamic parameters (121, 122), and increased serum levels of adiponectin (123), suggesting effects on vascular function (124) and on adipose tissue metabolic pathways (123). Treprostinil improved cutaneous blood flow (125, 126) and healing of DU (127), but recent studies failed to show changes in vascular, angiogenic and inflammatory biomarkers (128).
Prostacyclin agonists have short half-life, high frequency of administration and multiple side effects, and products with more convenient posology have been investigated. Selexipag is an oral selective prostacyclin receptor agonist that promotes vasodilation by increasing cyclic adenosine monophosphate concentrations (129) and has been effective for PAH (130). For the peripheral circulation, however, efficacy of this drug is still debated. While in a randomized, placebo-controlled study, selexipag failed to reduce the frequency of RP attacks (131), an open observational study showed considerable improvement of RP, also suggesting that selexipag may be effective for DU healing and resolution of DU related-pain (132).
Angiotensin-Converting Enzyme Inhibitors
Angiotensin-converting enzyme (ACE) inhibitors block the conversion of angiotensin I into the vasoconstrictor agent angiotensin II, promoting rapid control of the blood pressure (133, 134). Over the past decades, ACE inhibitors had a significant impact on outcomes of SSc patients with scleroderma renal crises (SRC), decreasing the need for dialysis and increasing survival (78, 135). Prophylactic use, however, did not reduce the incidence and was associated with poor prognosis and risk of death after onset of SRC (136, 137).
Riociguat
Riociguat is a soluble guanylate cyclase (sGC) stimulator that leads to strong vasodilator effects on the pulmonary arteries (138–144). Clinical trials in PAH patients, including SSc, showed improvements in pulmonary vascular resistance (145). An initial study failed to demonstrate significant reduction of active or painful DU, or changes in plasma levels of VEGF, E-selectin, VCAM-1 and ICAM-1, but long-term observations detected complete healing of the DU (146), and improvement of discomfort and disability associated with RP (147). Larger studies should determine the impact of riociguat on the peripheral vasculature (148, 149).
Cyclophosphamide
Cyclophosphamide (CYC), an immunosuppressive drug mostly used for SSc-related interstitial lung disease (150), also affects the vascular compartment, both in experimental and clinical scenarios (5). Cyclophosphamide improved nailfold capillaroscopic patterns (151), increased the number of circulating EPCs and reduced serum levels of VEGF, E-selectin and thrombomodulin, markers of endothelial injury and activation (152, 153), indicating that CYC may affect pathogenic processes associated with lung damage and fibrosis, such as re-endothelialization and re-epithelialization of the alveolar-capillary barrier (154).
Dermal MVECs exposed to the serum of CYC-treated SSc patients had better proliferation and less apoptosis than those exposed to serum of untreated SSc patients. Additionally, serum levels of antiangiogenic factors pentraxin 3 (PTX3), matrix metalloproteinase (MMP)-12, endostatin and angiostatin were significantly reduced after CYC treatment in SSc patients, suggesting a therapeutic effect on peripheral microvasculopathy (155).
Fluoxetine
Fluoxetine is a selective serotonin reuptake inhibitor that has been recommended as treatment for SSc RP attacks (87). Serotonin participates in Raynaud's phenomenon pathogenesis as a stimulator (156–158), but fluoxetine has paradoxical vasodilation effects, mediated by 5HT7 and 5HT2B receptors (159), that affect the NO and calcium pathways (160–162). Fluoxetine reduced the severity of RP attacks in SSc patients, with no impact on soluble P-selectin or wWF levels, however (163).
Less Traditional Therapeutic Interventions
Statins have been studied in immune-mediated diseases, including SSc, due to their immunomodulatory effects (164–166). Rosuvastatin improved endothelial function in SSc patients, assessed by skin microcirculation and brachial artery flow (167). Atorvastatin improved the visual analog scale for RP and DU, and was associated with reduced plasma levels of endothelial activation markers ICAM-1, E-selectin and ET-1, oxidative stress and vWF activity (159, 168). Atorvastatin led to transient increase in numbers of circulating EPCs (159), but failed to induce maturation of EPCs into ECs in vitro, indicating a limited therapeutic potential on vascular repair (169). Topical nitrate application is also effective in the treatment of RP in SSc patients. Nitrates are degraded into NO, increase cGMP concentration in the vascular smooth muscle and lead to vasodilation (170). Nitroglycerine tapes improved the peripheral circulation in SSc patients (171). Likely, MQX-503, a novel compound of nitroglicerine, was well-tolerated, improving the cutaneous blood flow in SSc patients (172). Topical application of glyceryl trinitrate increased DU perfusion, indicating supplementation of the NO pathway by nitrates as a promising strategy (173).
More recently, pirfenidone, an antifibrotic drug considered for treatment of interstitial lung disease (174), has shown vasodilatory effects. In animal models, pirfenidone induced pulmonary artery relaxation, restored renal blood flow and stimulated the NO pathway involving voltage-gated KV7 channels (175, 176). Clinical studies should further evaluate potential effects of the drug on the vascular compartment.
Local therapies are also described for SSc-associated vasculopathy. Botulinum toxin (Btx) inhibits acetylcholine release from presynaptic nerve terminals, reducing vascular smooth muscle contraction, and improving local circulation (177). A randomized controlled trial was inconclusive, since administration of Btx unexpectedly worsened blood flow in hands of SSc patients with RP, but patient perceptions of hand function and discomfort improved (178). Series of cases and one systematic review show healing of DU and reduction of pain in most patients after digital Btx applications (179, 180). Laser and intense pulsed light therapies have been investigated for digital ulcers and telagiectasies, with reports of safety and improvements of patient perception and blood flow (181, 182). In SSc patients with severe ischemic complications, especially vascular obstruction of the hands, peripheral or digital sympathectomy, microsurgical revascularization and digital artery reconstruction may be indicated. Besides limitations, these approaches are able to increase blood perfusion, decrease or eliminate pain, and may be recommended for selected cases (183).
Cellular Therapies for SSc-Associated Vasculopathy
In the last two decades, different cellular therapy approaches have been investigated for SSc patients (184). Local applications of fat graft/adipose-derived stem cells (ADSCs) or bone marrow hematopoietic stem cells show the strongest potential for regeneration of damaged tissue and vascular remodeling.
Fat Grafting and Stromal Vascular Fraction/Adipose-Derived Stem Cells-Based Therapy
Adipose-derived stem cells can be isolated from the stromal vascular fraction (SVF), located in the white adipose tissue (184), and show robust angiogenic activity (185–195). Patients with SSc treated with local administration of autologous fat grafts showed improvement of RP symptoms (188, 195), and complete healing of DU (189, 193). Treatment also led to significant increase of capillary density in fingers affected by DU (193) and enabled better pain control (189). Furthermore, autologous fat grafts increased mouth opening and vascularization in perioral areas of SSc patients (191).
Local injections of autologous SVF also improved RP, vascular flow, hand pain and finger edema in SSc patients (190, 192). Combination of autologous SVF and platelet-rich plasma, which is reported to enhance ADSC proliferation (194), also increased capillary density and decreased vascular ectasia in SSc patients, suggesting induction of neoangiogenesis (196). When locally implanted, ADSCs secrete VEGF and fibroblast growth factor, which may support local angiogenesis (197). These cells promote proliferation and inhibit apoptosis of ECs (198). Nevertheless, ADSCs isolated from SSc patients exhibit abnormal proliferation, metabolism, differentiation potential, and have a pro-fibrotic phenotype (194, 199–201), suggesting that despite beneficial effects, autologous ADSCs may not achieve full potential in tissue repair (185). More efforts are needed to investigate how they interfere with disease pathogenesis, and if there is potential for systemic therapy (185).
Hematopoietic Stem Cell Transplantation
Over the past 25 years, hundreds of patients with severe and progressive SSc have undergone autologous stem cell transplantation (AHSCT) (202), with better outcomes regarding survival, disease control and quality of life, when compared to conventional treatment (203–206). Indications for AHSCT include mainly fibrosis-related manifestations of SSc, such as skin thickening and interstitial lung disease (202–206). Patients with severe vascular manifestations, especially those with pulmonary hypertension or scleroderma renal crisis are usually excluded (143–147) and extensive cardiac assessment is recommended to avoid inclusion of patients with asymptomatic cardiac involvement (207). The procedure resets the immune system and promotes better control of autoreactivity, inflammation and fibrosis processes (208, 209).
To date, little is known about the impact of AHSCT on SSc-associated vasculopathy. Stem cell transplantation did not change dermal vessel density evaluated by immunostaining for endothelial markers CD31, VE-cadherin and vWF (210). On the other hand, AHSCT partially restored the microvascular structure assessed by nailfold video capillaroscopy (211), increased capillary counts, normalized cutaneous expression of VE-cadherin and decreased the expression of Interferon α mRNA in the skin, which is known as a potent inhibitor of angiogenesis (212, 213). Serum levels of VEGF decreased after AHSCT (214), which can be interpreted as a good result, since disrupted VEGF upregulation is associated with abnormal vessel morphology in SSc (24). Mechanisms to possibly explain the positive influence of AHSCT on the vascular compartment of SSc patients include removal of cells associated with inhibitory effects on endothelial repair, mobilization of endothelial progenitor cells from the bone marrow (212), and other still unidentified mechanisms of angiogenesis (211).
Other Cell Types Used for SSc-Vasculopathy: Bone Marrow Mesenchymal Stromal Cells
Mesenchymal stromal cells (MSC) are potential tools to treat vascular dysfunction, due to their immunosuppressive, anti-fibrotic and proangiogenic properties (215–217). Although MSCs from SSc patients display reduced capacity to differentiate into ECs in vitro (218), intramuscular injections of autologous MSCs reduced necrotic areas in one SSc patient with critical limb ischemia (219). After treatment, angiographies showed important revascularization, and histological analyses showed strong expression of angiogenic factors possibly effective through paracrine mechanisms. A SSc patient with multiple active skin ulcers was treated with intravenous infusion of allogeneic MSCs, with improvement of pain and blood flow in hands and fingers (220). In five SSc patients treated with intravenous allogeneic MSC infusions, there was healing of skin ulcers, and two of these patients also healed lesions of acral necrosis (221). An ongoing double-blind randomized placebo-controlled trial aims to evaluate safety and potential efficacy of intramuscular injections of allogeneic MSC as treatment for DU. In addition to clinical evaluations, such as DU healing and hand function, this study plans also analyze biomarkers in peripheral blood and skin biopsies (222).
Conclusions and Future Directions
Treatment of SSc-related vasculopathy remains difficult, despite the multiple available therapeutic options and targeted pathways. So far, patients seem to present advanced vascular involvement since early periods of disease, with vessel disruption and ischemic lesions. The narrow therapeutic window, associated with multiple pathophysiological presentations, makes development of new strategies a challenge. There are no reliable biomarkers of vascular severity or extension, so identification of patients with disabling or life-threatening vascular involvement is often too late. Best therapeutic effects include healing of ulcers and improvement of blood flow in pulmonary, renal and peripheral vascular beds. Cell therapy has an important potential, and may be expanded and refined in the future to achieve more substantial goals. Besides subsiding inflammation, future strategies should aim to fully repair and reverse established vascular damage.
Author Contributions
DZ-S and MS-G conceived the study. DZ-S, MS-G, and MK-V searched the literature and wrote the draft. DZ-S created the images. MO critically revised the final version of the manuscript and provided funding. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, finance code 001 and processes 88887.598001/2021-00 and 88887.597494/2021-00), by the São Paulo Research Foundation (FAPESP) (n°2013/08135-2 and 2017/09420-3), and the Fundação de Apoio ao Ensino, Pesquisa e Assistência (FAEPA).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Schniering J, Maurer B, Distler O. Vascular mechanisms of systemic sclerosis. In: Matucci-Cerinic M, Denton CP. editors. Atlas of Ulcers in Systemic Sclerosis. (2019). p. 27–37. [Google Scholar]
- 2.Denton CP, Khanna D. Systemic sclerosis. Lancet. (2017) 390:1685–99. 10.1016/S0140-6736(17)30933-9 [DOI] [PubMed] [Google Scholar]
- 3.Lambova SN, Müller-Ladner U. Nailfold capillaroscopy in systemic sclerosis – state of the art: the evolving knowledge about capillaroscopic abnormalities in systemic sclerosis. J Scleroderma Relat Disord. (2019) 4:200–11. 10.1177/2397198319833486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khaddaj Mallat R, Mathew John C, Kendrick DJ, Braun AP. The vascular endothelium: a regulator of arterial tone and interface for the immune system. Crit Rev Clin Lab Sci. (2017) 54:458–70. 10.1080/10408363.2017.1394267 [DOI] [PubMed] [Google Scholar]
- 5.Asano Y, Sato S. Vasculopathy in scleroderma. Semin Immunopathol. (2015) 37:489–500. 10.1007/s00281-015-0505-5 [DOI] [PubMed] [Google Scholar]
- 6.Matucci-Cerinic M, Kahaleh B, Wigley FM. Review: evidence that systemic sclerosis is a vascular disease. Arthritis Rheum. (2013) 65:1953–62. 10.1002/art.37988 [DOI] [PubMed] [Google Scholar]
- 7.Fonseca C, Abraham D, Renzoni EA. Endothelin in pulmonary fibrosis. Am J Respir Cell Mol Biol. (2011) 44:1–10. 10.1165/rcmb.2009-0388TR [DOI] [PubMed] [Google Scholar]
- 8.Abraham D, Distler O. How does endothelial cell injury start? The role of endothelin in systemic sclerosis. Arthritis Res Ther. (2007) 9:1–8. 10.1186/ar2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piera-Velazquez S, Mendoza F, Jimenez S. Endothelial to mesenchymal transition (EndoMT) in the pathogenesis of human fibrotic diseases. J Clin Med. (2016) 5:45. 10.3390/jcm5040045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manetti M, Guiducci S, Matucci-Cerinic M. The origin of the myofibroblast in fibroproliferative vasculopathy: does the endothelial cell steer the pathophysiology of systemic sclerosis? Arthritis Rheum. (2011) 63:2164–7. 10.1002/art.30316 [DOI] [PubMed] [Google Scholar]
- 11.Ntelis K, Solomou EE, Sakkas L, Liossis SN, Daoussis D. The role of platelets in autoimmunity, vasculopathy, and fibrosis: implications for systemic sclerosis. Semin Arthritis Rheum. (2017) 47:409–17. 10.1016/j.semarthrit.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 12.Ibrahim SE, Morad CS, Farouk N, Louis A. Platelet indices as markers of inflammation in systemic sclerosis patients: relation to vascular endothelial growth factor and flow mediated dilatation. Egypt Rheumatol. (2018) 40:239–42. 10.1016/j.ejr.2017.12.001 [DOI] [Google Scholar]
- 13.Reglero-Real N, Colom B, Bodkin JV, Nourshargh S. Endothelial cell junctional adhesion molecules: role and regulation of expression in inflammation. Arterioscler Thromb Vasc Biol. (2016) 36:2048–57. 10.1161/ATVBAHA.116.307610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valim V, Assis LSS, Simões MFJ, Trevisani VFM, Pucinelli MLC, Andrade LEC. Correlation between serum E-selectin levels and panoramic nailfold capillaroscopy in systemic sclerosis. Braz J Med Biol Res. (2004) 37:1423–7. 10.1590/S0100-879X2004000900018 [DOI] [PubMed] [Google Scholar]
- 15.Ateş A, Kinikli G, Turgay M, Duman M. Serum-soluble selectin levels in patients with rheumatoid arthritis and systemic sclerosis. Scand J Immunol. (2004) 59:315–20. 10.1111/j.0300-9475.2004.01389.x [DOI] [PubMed] [Google Scholar]
- 16.Kuryliszyn-Moskal A, Klimiuk PA, Sierakowski S. Soluble adhesion molecules (sVCAM-1, sE-selectin), vascular endothelial growth factor (VEGF) and endothelin-1 in patients with systemic sclerosis: relationship to organ systemic involvement. Clin Rheumatol. (2005) 24:111–6. 10.1007/s10067-004-0987-3 [DOI] [PubMed] [Google Scholar]
- 17.Hou Y, Rabquer BJ, Gerber ML, Del Galdo F, Jimenez SA, Haines GK, et al. Junctional adhesion molecule-A is abnormally expressed in diffuse cutaneous systemic sclerosis skin and mediates myeloid cell adhesion. Ann Rheum Dis. (2010) 69:249–254. 10.1136/ard.2008.102624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alzawawy A, Suliman I, Hamimi A, Elsawy N, Albordiny. Serum soluble vascular cell adhesion molecule-1 (sVCAM-1) in scleroderma patients and its relation to pulmonary involvement and disease activity. Egyp Rheumatol. (2011) 33:21–6. 10.1016/j.ejr.2010.06.001 [DOI] [Google Scholar]
- 19.Bruni C, Frech T, Manetti M, Rossi FW, Furst DE, De Paulis A, et al. Vascular leaking, a pivotal and early pathogenetic event in systemic sclerosis: should the door be closed? Front Immunol. (2018) 9:2045. 10.3389/fimmu.2018.02045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Distler JHW, Gay S, Distler O. Angiogenesis and vasculogenesis in systemic sclerosis. Rheumatology. (2006) 45:26–7. 10.1093/rheumatology/kel295 [DOI] [PubMed] [Google Scholar]
- 21.Rabquer BJ, Koch AE. Angiogenesis and vasculopathy in systemic sclerosis: evolving concepts. Curr Rheumatol Rep. (2012) 14:56–63. 10.1007/s11926-011-0219-1 [DOI] [PubMed] [Google Scholar]
- 22.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. (2003) 9:669–76. 10.1038/nm0603-669 [DOI] [PubMed] [Google Scholar]
- 23.Choi J-J, Min D-J, Cho M-L, Min S-Y, Kim S-J, Lee S-S, et al. Elevated vascular endothelial growth factor in systemic sclerosis. J Rheumatol. (2003) 30:1529–33. [PubMed] [Google Scholar]
- 24.Distler O, Distler HW, Scheid A, Acker T, Hirth A, Rethage J, et al. Uncontrolled expression of vascular endothelial growth factor and its receptors leads to insufficient skin angiogenesis in patients with systemic sclerosis. (2004) 95:109–16. 10.1161/01.RES.0000134644.89917.96 [DOI] [PubMed] [Google Scholar]
- 25.Shenavandeh S, Tarakemeh T, Sarvestani EK, Nazarinia MA. Serum vascular endothelial growth factor (VEGF), soluble VEGF receptor-1 (sVEGFR-1) and sVEGFR-2 in systemic sclerosis patients: relation to clinical manifestations and capillaroscopy findings. Egypt Rheumatol. (2017) 39:19–24. 10.1016/j.ejr.2016.03.004 [DOI] [Google Scholar]
- 26.Chora I, Romano E, Manetti M, Mazzotta C, Costa R, Machado V, et al. Evidence for a derangement of the microvascular system in patients with a very early diagnosis of systemic sclerosis. J Rheumatol. (2017) 44:1190–7. 10.3899/jrheum.160791 [DOI] [PubMed] [Google Scholar]
- 27.Manetti M, Guiducci S, Ibba-Manneschi L, Matucci-Cerinic M. Impaired Angiogenesis in Systemic Sclerosis: The Emerging Role of the Antiangiogenic VEGF165b Splice Variant. Trends Cardiovasc Med. (2011) 21:204–10. 10.1016/j.tcm.2012.05.011 [DOI] [PubMed] [Google Scholar]
- 28.Hirigoyen D, Burgos PI, Mezzano V, Duran J, Barrientos M, Saez CG, et al. Inhibition of angiogenesis by platelets in systemic sclerosis patients. Arthritis Res Ther. (2015) 17:332. 10.1186/s13075-015-0848-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Del Papa N, Pignataro F. The role of endothelial progenitors in the repair of vascular damage in systemic sclerosis. Front Immunol. (2018) 9:1383. 10.3389/fimmu.2018.01383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cossu M, Andracco R, Santaniello A, Marchini M, Severino A, Caronni M, et al. Serum levels of vascular dysfunction markers reflect disease severity and stage in systemic sclerosis patients. Rheumatology. (2016) 55:1112–6. 10.1093/rheumatology/kew017 [DOI] [PubMed] [Google Scholar]
- 31.Denton CP, Bickerstaff MCM, Shiwen X, Carulli MT, Haskard DO, Dubois RM, et al. Serial circulating adhesion molecule levels reflect disease severity in systemic sclerosis. Rheumatology. (1995) 34:1048–54. 10.1093/rheumatology/34.11.1048 [DOI] [PubMed] [Google Scholar]
- 32.Gruschwitz MS, Hornstein OP, Driesch PVD. Correlation of soluble adhesion molecules in the peripheral blood of scleroderma patients with their in situ expression and with disease activity. Arthrit Rheumat. (1995) 38:184–9. 10.1002/art.1780380206 [DOI] [PubMed] [Google Scholar]
- 33.Hasegawa M. Biomarkers in systemic sclerosis: their potential to predict clinical courses. J Dermatol. (2016) 43:29–38. 10.1111/1346-8138.13156 [DOI] [PubMed] [Google Scholar]
- 34.Ihn H, Sato S, Fujimoto M, Igarashi A, Yazawa N, Kubo M, et al. Characterization of autoantibodies to endothelial cells in systemic sclerosis (SSc): association with pulmonary fibrosis. Clin Exp Immunol. (2000) 119:203–9. 10.1046/j.1365-2249.2000.01115.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iversen LV, Ullman S, Østergaard O, Nielsen CT, Halberg P, Karlsmark T, et al. Cross-sectional study of soluble selectins, fractions of circulating microparticles and their relationship to lung and skin involvement in systemic sclerosis. BMC Musculoskelet Disord. (2015) 16:191. 10.1186/s12891-015-0653-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wodok-Wieczorek K, Salwowska N, Syguła E, Wodok A, Wcisło-Dziadecka D, Bebenek K, et al. The correlation between serum E-selectin levels and soluble interleukin-2 receptors with relation to disease activity in localized scleroderma. pdia. (2018) 35:614–9. 10.5114/ada.2018.77613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerlicz Z, Dziankowska-Bartkowiak B, Dziankowska-Zaborszczyk E, Sysa-Jedrzejowska A. Disturbed balance between serum levels of receptor tyrosine kinases Tie-1, Tie-2 and angiopoietins in systemic sclerosis. Dermatology. (2014) 228:233–9. 10.1159/000357806 [DOI] [PubMed] [Google Scholar]
- 38.Ichimura Y, Asano Y, Akamata K, Aozasa N, Noda S, Taniguchi T, et al. Serum angiopoietin-like protein 3 levels: possible correlation with progressive skin sclerosis, digital ulcers and pulmonary vascular involvement in patients with systemic sclerosis. Acta Derm Venereol. (2014) 94:157–62. 10.2340/00015555-1680 [DOI] [PubMed] [Google Scholar]
- 39.Michalska-Jakubus M, Kowal-Bielecka O, Chodorowska G, Bielecki M, Krasowska D. Angiopoietins-1 and−2 are differentially expressed in the sera of patients with systemic sclerosis: high angiopoietin-2 levels are associated with greater severity and higher activity of the disease. Rheumatology. (2011) 50:746–55. 10.1093/rheumatology/keq392 [DOI] [PubMed] [Google Scholar]
- 40.Michalska-Jakubus M, Cutolo M, Smith V, Krasowska D. Imbalanced serum levels of Ang1, Ang2 and VEGF in systemic sclerosis: integrated effects on microvascular reactivity. Microvasc Res. (2019) 125:103881. 10.1016/j.mvr.2019.103881 [DOI] [PubMed] [Google Scholar]
- 41.Noda S, Asano Y, Aozasa N, Akamata K, Yamada D, Masui Y, et al. Serum Tie2 levels: clinical association with microangiopathies in patients with systemic sclerosis: serum Tie2 levels in systemic sclerosis. J Eur Acad Dermatol Venereol. (2011) 25:1476–9. 10.1111/j.1468-3083.2011.04012.x [DOI] [PubMed] [Google Scholar]
- 42.Noda S, Asano Y, Aozasa N, Akamata K, Taniguchi T, Takahashi T, et al. Clinical significance of serum soluble Tie1 levels in patients with systemic sclerosis. Arch Dermatol Res. (2013) 305:325–31. 10.1007/s00403-012-1307-4 [DOI] [PubMed] [Google Scholar]
- 43.Sulli A, Ruaro B, Smith V, Pizzorni C, Zampogna G, Gallo M, et al. Progression of nailfold microvascular damage and antinuclear antibody pattern in systemic sclerosis. J Rheumatol. (2013) 40:634–9. 10.3899/jrheum.121089 [DOI] [PubMed] [Google Scholar]
- 44.Steen VD, Lucas M, Fertig N, Medsger TA Jr. Pulmonary arterial hypertension and severe pulmonary fibrosis in systemic sclerosis patients with a nucleolar antibody. J Rheumatol. (2007) 34:2230–5. [PubMed] [Google Scholar]
- 45.Kampolis C, Plastiras S, Vlachoyiannopoulos P, Moyssakis I, Tzelepis G. The presence of anti-centromere antibodies may predict progression of estimated pulmonary arterial systolic pressure in systemic sclerosis. Scand J Rheumatol. (2008) 37:278–83. 10.1080/03009740801978871 [DOI] [PubMed] [Google Scholar]
- 46.Becker MO, Kill A, Kutsche M, Guenther J, Rose A, Tabeling C, et al. Vascular receptor autoantibodies in pulmonary arterial hypertension associated with systemic sclerosis. Am J Respir Crit Care Med. (2014) 190:808–17. 10.1164/rccm.201403-0442OC [DOI] [PubMed] [Google Scholar]
- 47.Bhavsar SV, Carmona R. Anti-RNA polymerase III antibodies in the diagnosis of scleroderma renal crisis in the absence of skin disease. J Clin Rheumatol. (2014) 20:379–82. 10.1097/RHU.0000000000000167 [DOI] [PubMed] [Google Scholar]
- 48.Cavazzana I, Angela C, Paolo A, Stefania Z, Angela T, Franco F. Anti-RNA polymerase III antibodies: a marker of systemic sclerosis with rapid onset and skin thickening progression. Autoimmun Rev. (2009) 8:580–4. 10.1016/j.autrev.2009.02.002 [DOI] [PubMed] [Google Scholar]
- 49.Ceribelli A, Cavazzana I, Airò P, Franceschini F. Anti-RNA polymerase III antibodies as a risk marker for early gastric antral vascular ectasia (GAVE) in systemic sclerosis. J Rheumatol. (2010) 37:1544. 10.3899/jrheum.100124 [DOI] [PubMed] [Google Scholar]
- 50.Emilie S, Goulvestre C, Bérezné A, Pagnoux C, Guillevin L, Mouthon L. Anti-RNA polymerase III antibodies are associated with scleroderma renal crisis in a French cohort. Scand J Rheumatol. (2011) 40:404–6. 10.3109/03009742.2011.569753 [DOI] [PubMed] [Google Scholar]
- 51.Hamaguchi Y, Kodera M, Matsushita T, Hasegawa M, Inaba Y, Usuda T, et al. Clinical and immunologic predictors of scleroderma renal crisis in Japanese systemic sclerosis patients with anti-RNA polymerase III autoantibodies: anti-RNAP I/II/III antibodies and renal crisis in SSc. Arthrit Rheumatol. (2015) 67:1045–52. 10.1002/art.38994 [DOI] [PubMed] [Google Scholar]
- 52.Hesselstrand R, Scheja A, Wuttge D. Scleroderma renal crisis in a Swedish systemic sclerosis cohort: survival, renal outcome, and RNA polymerase III antibodies as a risk factor. Scand J Rheumatol. (2012) 41:39–43. 10.3109/03009742.2011.610032 [DOI] [PubMed] [Google Scholar]
- 53.Hoffmann-Vold A-M, Midtvedt Ø, Tennøe AH, Garen T, Lund MB, Aaløkken TM, et al. Cardiopulmonary disease development in anti-RNA polymerase III-positive systemic sclerosis: comparative analyses from an unselected, prospective patient cohort. J Rheumatol. (2017) 44:459–65. 10.3899/jrheum.160867 [DOI] [PubMed] [Google Scholar]
- 54.Nikpour M, Hissaria P, Byron J, Sahhar J, Micallef M, Paspaliaris W, et al. Prevalence, correlates and clinical usefulness of antibodies to RNA polymerase III in systemic sclerosis: a cross-sectional analysis of data from an Australian cohort. Arthritis Res Ther. (2011) 13:R211. 10.1186/ar3544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Serling-Boyd N, Chung MP-S, Li S, Becker L, Fernandez-Becker N, Clarke J, et al. Gastric antral vascular ectasia in systemic sclerosis: association with anti-RNA polymerase III and negative anti-nuclear antibodies. Semin Arthrit Rheum. (2020) 50:938–42. 10.1016/j.semarthrit.2020.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Terras S, Hartenstein H, Höxtermann S, Gambichler T, Kreuter A. RNA polymerase III autoantibodies may indicate renal and more severe skin involvement in systemic sclerosis. Int J Dermatol. (2016) 55:882–5. 10.1111/ijd.13032 [DOI] [PubMed] [Google Scholar]
- 57.Denton CP, Krieg T, Guillevin L, Schwierin B, Rosenberg D, Silkey M, et al. Demographic, clinical and antibody characteristics of patients with digital ulcers in systemic sclerosis: data from the DUO registry. Ann Rheum Dis. (2012) 71:718–21. 10.1136/annrheumdis-2011-200631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wipff J, Avouac J, Borderie D, Zerkak D, Lemarechal H, Kahan A, et al. Disturbed angiogenesis in systemic sclerosis: high levels of soluble endoglin. Rheumatology. (2008) 47:972–5. 10.1093/rheumatology/ken100 [DOI] [PubMed] [Google Scholar]
- 59.Abraham DJ, Vancheeswaran R, Dashwood MR, Rajkumar VS, Pantelides P, Shi-wen X, et al. Increased levels of endothelin-1 and differential endothelin type A and B receptor expression in scleroderma-associated fibrotic lung disease. Am J Pathol. (1997) 151:831–41. [PMC free article] [PubMed] [Google Scholar]
- 60.Cambrey AD, Harrison NK, Dawes KE, Southcott AM, Black CM, du Bois RM, et al. Increased levels of endothelin-1 in bronchoalveolar lavage fluid from patients with systemic sclerosis contribute to fibroblast mitogenic activity in vitro. Am J Respir Cell Mol Biol. (1994) 11:439–45. 10.1165/ajrcmb.11.4.7917311 [DOI] [PubMed] [Google Scholar]
- 61.Ciurzyński M, Bienias P, Irzyk K, Kostrubiec M, Bartoszewicz Z, Siwicka M, et al. Serum endothelin-1 and NT-proBNP, but not ADMA, endoglin and TIMP-1 levels, reflect impaired right ventricular function in patients with systemic sclerosis. Clin Rheumatol. (2014) 33:83–9. 10.1007/s10067-013-2354-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vancheeswaran R, Magoulas T, Efrat G, Wheeler-Jones C, Olsen I, Penny R, et al. Circulating endothelin-1 levels in systemic sclerosis subsets–a marker of fibrosis or vascular dysfunction? J Rheumatol. (1994) 21:1838–44. [PubMed] [Google Scholar]
- 63.Yamane K, Miyauchi T, Suzuki N, Yuhara T, Akama T, Suzuki H, et al. Significance of plasma endothelin-1 levels in patients with systemic sclerosis. J Rheumatol. (1992) 19:1566–71. [PubMed] [Google Scholar]
- 64.Farouk HM, Hamza SH, El Bakry SA, Youssef SS, Aly IM, Moustafa AA, et al. Dysregulation of angiogenic homeostasis in systemic sclerosis. Int J Rheum Dis. (2013) 16:448–54. 10.1111/1756-185X.12130 [DOI] [PubMed] [Google Scholar]
- 65.Gigante A, Navarini L, Margiotta D, Amoroso A, Barbano B, Cianci R, et al. Angiogenic and angiostatic factors in renal scleroderma-associated vasculopathy. Microvasc Res. (2017) 114:41–5. 10.1016/j.mvr.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 66.Stratton RJ, Pompon L, Coghlan JG, Pearson JD, Black CM. Soluble thrombomodulin concentration is raised in scleroderma associated pulmonary hypertension. Ann Rheum Dis. (2000) 59:132–4. 10.1136/ard.59.2.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suárez-Calvet X, Alonso-Pérez J, Castellví I, Carrasco-Rozas A, Fernández-Simón E, Zamora C, et al. Thrombospondin-1 mediates muscle damage in brachio-cervical inflammatory myopathy and systemic sclerosis. Neurol Neuroimmunol Neuroinflamm. (2020) 7:e694. 10.1212/NXI.0000000000000694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Avouac J, Vallucci M, Smith V, Senet P, Ruiz B, Sulli A, et al. Correlations between angiogenic factors and capillaroscopic patterns in systemic sclerosis. Arthritis Res Ther. (2013) 15:R55. 10.1186/ar4217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Davies CA, Jeziorska M, Freemont AJ, Herrick AL. The differential expression of VEGF, VEGFR-2, and GLUT-1 proteins in disease subtypes of systemic sclerosis. Hum Pathol. (2006) 37:190–7. 10.1016/j.humpath.2005.10.007 [DOI] [PubMed] [Google Scholar]
- 70.Distler O, Del Rosso A, Giacomelli R, Cipriani P, Conforti ML, Guiducci S, et al. Angiogenic and angiostatic factors in systemic sclerosis: increased levels of vascular endothelial growth factor are a feature of the earliest disease stages and are associated with the absence of fingertip ulcers. Arthritis Res. (2002) 4:R11. 10.1186/ar596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Manetti M, Guiducci S, Romano E, Rosa I, Ceccarelli C, Mello T, et al. Differential expression of junctional adhesion molecules in different stages of systemic sclerosis. Arthrit Rheum. (2013) 65:247–57. 10.1002/art.37712 [DOI] [PubMed] [Google Scholar]
- 72.Papaioannou AI, Zakynthinos E, Kostikas K, Kiropoulos T, Koutsokera A, Ziogas A, et al. Serum VEGF levels are related to the presence of pulmonary arterial hypertension in systemic sclerosis. BMC Pulm Med. (2009) 9:18. 10.1186/1471-2466-9-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De Santis M, Ceribelli A, Cavaciocchi F, Crotti C, Massarotti M, Belloli L, et al. Nailfold videocapillaroscopy and serum VEGF levels in scleroderma are associated with internal organ involvement. Autoimmun Highlights. (2016) 7:5. 10.1007/s13317-016-0077-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Katsumoto TR, Whitfield ML, Connolly MK. The pathogenesis of systemic sclerosis. Annu Rev Pathol Mech Dis. (2011) 6:509–37. 10.1146/annurev-pathol-011110-130312 [DOI] [PubMed] [Google Scholar]
- 75.Avouac J, Fransen J, Walker UA, Riccieri V, Smith V, Muller C, et al. EUSTAR Group. Preliminary criteria for the very early diagnosis of systemic sclerosis: results of a Delphi Consensus Study from EULAR Scleroderma Trials and Research Group. Ann Rheum Dis. (2011) 70:476–81. 10.1136/ard.2010.136929 [DOI] [PubMed] [Google Scholar]
- 76.Pokeerbux MR, Giovannelli J, Dauchet L, Mouthon L, Agard C, Lega JC, et al. Survival and prognosis factors in systemic sclerosis: data of a French multicenter cohort, systematic review, and meta-analysis of the literature. Arthritis Res Ther. (2019) 21:86. 10.1186/s13075-019-1867-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arnaud L, Huart A, Plaisier E, Francois H, Mougenot B, Tiev K, et al. ANCA-related crescentic glomerulonephritis in systemic sclerosis: revisiting the “normotensive scleroderma renal crisis.” Clin Nephrol. (2007) 68:165–70. 10.5414/CNP68165 [DOI] [PubMed] [Google Scholar]
- 78.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis. Ann Rheum Dis. (2007) 66:940–4. 10.1136/ard.2006.066068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Teixeira L, Mouthon L, Mahr A, Berezné A, Agard C, Mehrenberger M, et al. Group Français de Recherche sur le Sclérodermie (GFRS). Mortality and risk factors of scleroderma renal crisis: a French retrospective study of 50 patients. Ann Rheum Dis. (2008) 67:110–6. 10.1136/ard.2006.066985 [DOI] [PubMed] [Google Scholar]
- 80.Tyndall AJ, Bannert B, Vonk M, Airò P, Cozzi F, Carreira PE, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. (2010) 69:1809–15. 10.1136/ard.2009.114264 [DOI] [PubMed] [Google Scholar]
- 81.Elhai M, Meune C, Avouac J, Kahan A, Allanore Y. Trends in mortality in patients with systemic sclerosis over 40 years: a systematic review and meta-analysis of cohort studies. Rheumatology. (2012) 51:1017–26. 10.1093/rheumatology/ker269 [DOI] [PubMed] [Google Scholar]
- 82.Nihtyanova SI, Schreiber BE, Ong VH, Rosenberg D, Moinzadeh P, Coghlan JG, et al. Prediction of pulmonary complications and long-term survival in systemic sclerosis. Arthritis Rheumatol. (2014) 66:1625–35. 10.1002/art.38390 [DOI] [PubMed] [Google Scholar]
- 83.Avouac J, Meune C, Chenevier-Gobeaux C, Borderie D, Lefevre G, Kahan A, et al. Cardiac biomarkers in systemic sclerosis: contribution of high-sensitivity cardiac troponin in addition to N-terminal pro-brain natriuretic peptide. Arthritis Care Res. (2015) 67:1022–30. 10.1002/acr.22547 [DOI] [PubMed] [Google Scholar]
- 84.Jaafar S, Lescoat A, Huang S, Gordon J, Hinchcliff M, Shah AA, et al. Clinical characteristics, visceral involvement, and mortality in at-risk or early diffuse systemic sclerosis: a longitudinal analysis of an observational prospective multicenter US cohort. Arthritis Res Ther. (2021) 23:170. 10.1186/s13075-021-02548-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hung EW, Mayes MD, Sharif R, Assassi S, Machicao VI, Hosing C, et al. Gastric Antral vascular ectasia and its clinical correlates in patients with early diffuse systemic sclerosis in the SCOT trial. J Rheumatol. (2013) 40:455–60. 10.3899/jrheum.121087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ghrénassia E, Avouac J, Khanna D, Derk CT, Distler O, Suliman YA, et al. Prevalence, correlates and outcomes of gastric antral vascular ectasia in systemic sclerosis: a EUSTAR case-control study. J Rheumatol. (2014) 41:99–105. 10.3899/jrheum.130386 [DOI] [PubMed] [Google Scholar]
- 87.Kavian N, Batteux F. Macro- and microvascular disease in systemic sclerosis. Vasc Pharmacol. (2015) 71:16–23. 10.1016/j.vph.2015.05.015 [DOI] [PubMed] [Google Scholar]
- 88.Kowal-Bielecka O, Fransen J, Avouac J, Becker M, Kulak A, Allanore Y, et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis. (2017) 76:1327–39. 10.1136/annrheumdis-2016-209909 [DOI] [PubMed] [Google Scholar]
- 89.Zhao M, Wu J, Wu H, Sawalha AH, Lu Q. Clinical treatment options in scleroderma: recommendations and comprehensive review. Clinic Rev Allerg Immunol. (2021). 10.1007/s12016-020-08831-4. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 90.Thompson AE, Shea B, Welch V, Fenlon D, Pope JE. Calcium-channel blockers for Raynaud's phenomenon in systemic sclerosis. Arthrit Rheum. (2001) 44:1841–47. [DOI] [PubMed] [Google Scholar]
- 91.Allanore Y, Borderie D, Lemaréchal H, Ekindjian OG, Kahan A. Acute and sustained effects of dihydropyridine-type calcium channel antagonists on oxidative stress in systemic sclerosis. Am J Med. (2004) 116:595–600. 10.1016/j.amjmed.2003.11.022 [DOI] [PubMed] [Google Scholar]
- 92.Mak IT, Zhang J, Weglicki WB. Protective effects of dihydropyridine Ca-blockers against endothelial cell oxidative injury due to combined nitric oxide and superoxide. Pharmacol Res. (2002) 45:27–33. 10.1006/phrs.2001.0903 [DOI] [PubMed] [Google Scholar]
- 93.Allanore Y, Borderie D, Meune C, Cabanes L, Weber S, Ekindjian OG, et al. N-terminal pro-brain natriuretic peptide as a diagnostic marker of early pulmonary artery hypertension in patients with systemic sclerosis and effects of calcium-channel blockers. Arthritis Rheum. (2003) 48:3503–8. 10.1002/art.11345 [DOI] [PubMed] [Google Scholar]
- 94.Kahan A, Allanore Y. Primary myocardial involvement in systemic sclerosis. Rheumatology. (2006) 45:iv14–7. 10.1093/rheumatology/kel312 [DOI] [PubMed] [Google Scholar]
- 95.Korn JH, Mayes M, Matucci Cerinic M, Rainisio M, Pope J, Hachulla E, et al. Digital ulcers in systemic sclerosis: prevention by treatment with bosentan, an oral endothelin receptor antagonist. Arthritis Rheum. (2004) 50:3985–9. 10.1002/art.20676 [DOI] [PubMed] [Google Scholar]
- 96.Matucci-Cerinic M, Denton CP, Furst DE, Mayes MD, Hsu VM, Carpentier P, et al. Bosentan treatment of digital ulcers related to systemic sclerosis: results from the RAPIDS-2 randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. (2011) 70:32–8. 10.1136/ard.2010.130658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Parisi S, Peroni CL, Laganà A, Scarati M, Ambrogio F, Bruzzone M, et al. Efficacy of ambrisentan in the treatment of digital ulcers in patients with systemic sclerosis: a preliminary study. Rheumatology. (2013) 52:1142–4. 10.1093/rheumatology/ket019 [DOI] [PubMed] [Google Scholar]
- 98.Bose N, Bena J, Chatterjee S. Evaluation of the effect of ambrisentan on digital microvascular flow in patients with systemic sclerosis using laser Doppler perfusion imaging: a 12-week randomized double-blind placebo controlled trial. Arthritis Res Ther. (2015) 17:44. 10.1186/s13075-015-0558-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Launay D, Sitbon O, Le Pavec J, Savale L, Tchérakian C, Yaïci A, et al. Long-term outcome of systemic sclerosis-associated pulmonary arterial hypertension treated with bosentan as first-line monotherapy followed or not by the addition of prostanoids or sildenafil. Rheumatology. (2010) 49:490–500. 10.1093/rheumatology/kep398 [DOI] [PubMed] [Google Scholar]
- 100.Pan Z, Marra AM, Benjamin N, Eichstaedt CA, Blank N, Bossone E, et al. Early treatment with ambrisentan of mildly elevated mean pulmonary arterial pressure associated with systemic sclerosis: a randomized, controlled, double-blind, parallel group study (EDITA study). Arthritis Res Ther. (2019) 21:217. 10.1186/s13075-019-1981-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Iannone F, Riccardi MT, Guiducci S, Bizzoca R, Cinelli M, Matucci-Cerinic M, et al. Bosentan regulates the expression of adhesion molecules on circulating T cells and serum soluble adhesion molecules in systemic sclerosis-associated pulmonary arterial hypertension. Ann Rheum Dis. (2008) 67:1121–6. 10.1136/ard.2007.080424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Corallo C, Cutolo M, Kahaleh B, Pecetti G, Montella A, Chirico C, et al. Bosentan and macitentan prevent the endothelial-to-mesenchymal transition (EndoMT) in systemic sclerosis: in vitro study. Arthritis Res Ther. (2016) 18:228. 10.1186/s13075-016-1122-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tantini B, Manes A, Fiumana E, Pignatti C, Guarnieri C, Zannoli R, et al. Antiproliferative effect of sildenafil on human pulmonary artery smooth muscle cells. Basic Res Cardiol. (2005) 100:131–8. 10.1007/s00395-004-0504-5 [DOI] [PubMed] [Google Scholar]
- 104.Tingey T, Shu J, Smuczek J, Pope J. Meta-analysis of healing and prevention of digital ulcers in systemic sclerosis. Arthritis Care Res. (2013) 65:1460–71. 10.1002/acr.22018 [DOI] [PubMed] [Google Scholar]
- 105.Roustit M, Blaise S, Allanore Y, Carpentier PH, Caglayan E, Cracowski JL. Phosphodiesterase-5 inhibitors for the treatment of secondary Raynaud's phenomenon: systematic review and meta-analysis of randomised trials. Ann Rheum Dis. (2013) 72:1696–9. 10.1136/annrheumdis-2012-202836 [DOI] [PubMed] [Google Scholar]
- 106.Kumar U, Sankalp G, Sreenivas V, Kaur S, Misra D. Prospective, open-label, uncontrolled pilot study to study safety and efficacy of sildenafil in systemic sclerosis-related pulmonary artery hypertension and cutaneous vascular complications. Rheumatol Int. (2013) 33:1047–52. 10.1007/s00296-012-2466-5 [DOI] [PubMed] [Google Scholar]
- 107.Coghlan JG, Galiè N, Barberà JA, Frost AE, Ghofrani HA, Hoeper MM, et al. AMBITION investigators. Initial combination therapy with ambrisentan and tadalafil in connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH): subgroup analysis from the AMBITION trial. Ann Rheum Dis. (2017) 76:1219–27. 10.1136/annrheumdis-2016-210236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shenoy PD, Kumar S, Jha LK, Choudhary SK, Singh U, Misra R, et al. Efficacy of tadalafil in secondary Raynaud's phenomenon resistant to vasodilator therapy: a double-blind randomized cross-over trial. Rheumatology. (2010) 49:2420–8. 10.1093/rheumatology/keq291 [DOI] [PubMed] [Google Scholar]
- 109.Herrick AL, van den Hoogen F, Gabrielli A, Tamimi N, Reid C, O'Connell D, et al. Modified-release sildenafil reduces Raynaud's phenomenon attack frequency in limited cutaneous systemic sclerosis. Arthritis Rheum. (2011) 63:775–82. 10.1002/art.30195 [DOI] [PubMed] [Google Scholar]
- 110.Andrigueti FV, Ebbing PCC, Arismendi MI, Kayser C. Evaluation of the effect of sildenafil on the microvascular blood flow in patients with systemic sclerosis: a randomised, double-blind, placebo-controlled study. Clin Exp Rheumatol. (2017) 35 (Suppl. 106):151–8. [PubMed] [Google Scholar]
- 111.Proietti M, Aversa A, Letizia C, Rossi C, Menghi G, Bruzziches R, et al. Erectile dysfunction in systemic sclerosis: effects of longterm inhibition of phosphodiesterase type-5 on erectile function and plasma endothelin-1 levels. J Rheumatol. (2007) 34:1712–7. [PubMed] [Google Scholar]
- 112.Stubbe B, Opitz CF, Halank M, Habedank D, Ewert R. Intravenous prostacyclin-analogue therapy in pulmonary arterial hypertension - a review of the past, present and future. Respir Med. (2021) 179:106336. 10.1016/j.rmed.2021.106336 [DOI] [PubMed] [Google Scholar]
- 113.Scorza R, Caronni M, Mascagni B, Berruti V, Bazzi S. Effects of long-term cyclic iloprost therapy in systemic sclerosis with Raynaud' s phenomenon. A randomized, controlled study. Clin Exp Rheumatol. (2001) 19:503–8. [PubMed] [Google Scholar]
- 114.Milio G, Corrado E, Genova C, Amato C, Raimondi F, Almasio PL, et al. Iloprost treatment in patients with Raynaud's phenomenon secondary to systemic sclerosis and the quality of life: a new therapeutic protocol. Rheumatology. (2006) 45:999–1004. 10.1093/rheumatology/kel038 [DOI] [PubMed] [Google Scholar]
- 115.Kawald A, Burmester GR, Huscher D, Sunderkötter C, Riemekasten G. Low versus high-dose iloprost therapy over 21 days in patients with secondary Raynaud's phenomenon and systemic sclerosis: a randomized, open, single-center study. J Rheumatol. (2008) 35:1830. [PubMed] [Google Scholar]
- 116.Rehberger P, Beckheinrich-Mrowka P, Haustein UF, Sticherling M. Prostacyclin analogue iloprost influences endothelial cell-associated soluble adhesion molecules and growth factors in patients with systemic sclerosis: a time course study of serum concentrations. Acta Derm Venereol. (2009) 89:245–9. 10.2340/00015555-0632 [DOI] [PubMed] [Google Scholar]
- 117.Caravita S, Wu SC, Secchi MB, Dadone V, Bencini C, Pierini S. Long-term effects of intermittent Iloprost infusion on pulmonary arterial pressure in connective tissue disease. Eur J Intern Med. (2011) 22:518–21. 10.1016/j.ejim.2011.02.005 [DOI] [PubMed] [Google Scholar]
- 118.Negrini S, Magnani O, Matucci-Cerinic M, Carignola R, Data V, Montabone E, et al. Iloprost use and medical management of systemic sclerosis-related vasculopathy in Italian tertiary referral centers: results from the PROSIT study. Clin Exp Med. (2019) 19:357–66. 10.1007/s10238-019-00553-y [DOI] [PubMed] [Google Scholar]
- 119.Cutolo M, Zampogna G, Vremis L, Smith V, Pizzorni C, Sulli A. Longterm effects of endothelin receptor antagonism on microvascular damage evaluated by nailfold capillaroscopic analysis in systemic sclerosis. J Rheumatol. (2013) 40:40–5. 10.3899/jrheum.120416 [DOI] [PubMed] [Google Scholar]
- 120.Vayssairat M. Preventive effect of an oral prostacyclin analog, beraprost sodium, on digital necrosis in systemic sclerosis. French Microcirculation Society Multicenter Group for the Study of Vascular Acrosyndromes. J Rheumatol. (1999) 26:2173–8. [PubMed] [Google Scholar]
- 121.Pussadhamma B, Tatsanavivat P, Nanagara R, Mahakkanukrauh A, Kiatchoosakun S. Hemodynamic efficacy of beraprost therapy in systemic sclerosis-related pulmonary artery hypertension. Int J Med Pharm Sci. (2012) 2:1–8. [Google Scholar]
- 122.Klings ES, Hill NS, Ieong MH, Simms RW, Korn JH, Farber HW. Systemic sclerosis-associated pulmonary hypertension: short- and long-term effects of epoprostenol (prostacyclin). Arthritis Rheum. (1999) 42:2638–45. [DOI] [PubMed] [Google Scholar]
- 123.Badesch DB, Tapson VF, McGoon MD, Brundage BH, Rubin LJ, Wigley FM et al. Continuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease: a randomized, controlled trial. Ann Intern Med. (2000) 132:425–34. 10.7326/0003-4819-132-6-200003210-00002 [DOI] [PubMed] [Google Scholar]
- 124.Stochmal A, Czuwara J, Zaremba M, Rudnicka L. Epoprostenol up-regulates serum adiponectin level in patients with systemic sclerosis: therapeutic implications. Arch Dermatol Res. (2021) 313:783–91. 10.1007/s00403-020-02172-0 [DOI] [PubMed] [Google Scholar]
- 125.Lee YH, Song GG. Meta-analysis of circulating adiponectin, leptin, and resistin levels in systemic sclerosis. Z Rheumatol. (2017) 76:789–97. 10.1007/s00393-016-0172-5 [DOI] [PubMed] [Google Scholar]
- 126.Shah AA, Schiopu E, Hummers LK, Wade M, Phillips K, Anderson C, et al. Open label study of escalating doses of oral treprostinil diethanolamine in patients with systemic sclerosis and digital ischemia: pharmacokinetics and correlation with digital perfusion. Arthritis Res Ther. (2013) 15:R54. 10.1186/ar4216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gaillard-Bigot F, Roustit M, Blaise S, Cracowski C, Seinturier C, Imbert B, et al. Treprostinil iontophoresis improves digital blood flow during local cooling in systemic sclerosis. Microcirculation. (2016) 23:266–70. 10.1111/micc.12272 [DOI] [PubMed] [Google Scholar]
- 128.Chung L, Fiorentino D. A pilot trial of treprostinil for the treatment and prevention of digital ulcers in patients with systemic sclerosis. J Am Acad Dermatol. (2006) 54:880–2. 10.1016/j.jaad.2006.02.004 [DOI] [PubMed] [Google Scholar]
- 129.Mecoli CA, Shah AA, Boin F, Wigley FM, Hummers LK. Vascular complications in systemic sclerosis: a prospective cohort study. Clin Rheumatol. (2018) 37:2429–37. 10.1007/s10067-018-4148-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stitham J, Arehart E, Gleim SR, Li N, Douville K, Hwa J. New insights into human prostacyclin receptor structure and function through natural and synthetic mutations of transmembrane charged residues. Br J Pharmacol. (2007) 152:513–22. 10.1038/sj.bjp.0707413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gaine S, Chin K, Coghlan G, Channick R, Di Scala L, Galiè N, et al. Selexipag for the treatment of connective tissue disease-associated pulmonary arterial hypertension. Eur Respir J. (2017) 50:1602493. 10.1183/13993003.02493-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Denton CP, Hachulla É, Riemekasten G, Schwarting A, Frenoux JM, Frey A, et al. Raynaud study investigators. Efficacy and safety of selexipag in adults with Raynaud's Phenomenon secondary to systemic sclerosis: a randomized, placebo-controlled, phase II study. Arthrit Rheumatol. (2017) 69:2370–2379. 10.1002/art.40242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Del Papa N, Vitali C, Bilia S, Giannini D, Pignataro F, Minniti A, et al. Selexipag may be effective in inducing digital ulcers healing in patients with systemic sclerosis. Clin Exp Rheumatol. (2020) 125:181–2. [PubMed] [Google Scholar]
- 134.HM, de Gasparo M, El-Kersh M, Carey RM. Angiotensin-converting enzyme inhibition potentiates angiotensin II type 1 receptor effects on renal bradykinin and cGMP. Hypertension. (2001) 38:183–6. 10.1161/01.HYP.38.2.183 [DOI] [PubMed] [Google Scholar]
- 135.Steen VD. Scleroderma renal crisis. Rheum Dis Clin North Am. (2003) 29:315–33. 10.1016/S0889-857X(03)00016-4 [DOI] [PubMed] [Google Scholar]
- 136.Hudson M, Baron M, Tatibouet S, Furst DE, Khanna D, International Scleroderma Renal Crisis Study Investigators. Exposure to ACE inhibitors prior to the onset of scleroderma renal crisis-results from the International Scleroderma Renal Crisis Survey. Semin Arthritis Rheum. (2014) 43:666–72. 10.1016/j.semarthrit.2013.09.008 [DOI] [PubMed] [Google Scholar]
- 137.Bütikofer L, Varisco PA, Distler O, Kowal-Bielecka O, Allanore Y, Riemekasten G, et al. ACE inhibitors in SSc patients display a risk factor for scleroderma renal crisis-a EUSTAR analysis. Arthritis Res Ther. (2020) 22:59. 10.1186/s13075-020-2141-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Belik J. Riociguat, an oral soluble guanylate cyclase stimulator for the treatment of pulmonary hypertension. Curr Opin Investig Drugs. (2009) 10:971–9. [PubMed] [Google Scholar]
- 139.Khaybullina D, Patel A, Zerilli T. Riociguat (Adempas): a novel agent for the treatment of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. P T. (2014) 39:749–58. [PMC free article] [PubMed] [Google Scholar]
- 140.Galiè N, Müller K, Scalise AV, Grünig E. PATENT PLUS: a blinded, randomised and extension study of riociguat plus sildenafil in pulmonary arterial hypertension. Eur Respir J. (2015) 45:1314–22. 10.1183/09031936.00105914 [DOI] [PubMed] [Google Scholar]
- 141.Toxvig AK, Wehland M, Grimm D, Infanger M, Krüger M. A focus on riociguat in the treatment of pulmonary arterial hypertension. Basic Clin Pharmacol Toxicol. (2019) 125:202–14. 10.1111/bcpt.13272 [DOI] [PubMed] [Google Scholar]
- 142.Bruni C, Praino E, Allanore Y, Distler O, Gabrielli A, Iannone F, et al. Use of biologics and other novel therapies for the treatment of systemic sclerosis. Expert Rev Clin Immunol. (2017) 13:469–82. 10.1080/1744666X.2017.1263153 [DOI] [PubMed] [Google Scholar]
- 143.Chamorro V, Morales-Cano D, Milara J, Barreira B, Moreno L, Callejo M, et al. Riociguat versus sildenafil on hypoxic pulmonary vasoconstriction and ventilation/perfusion matching. PLoS ONE. (2018) 13:e0191239. 10.1371/journal.pone.0191239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xiao S, Li Q, Hu L, Yu Z, Yang J, Chang Q et al. Soluble Guanylate cyclase stimulators and activators: where are we and where to go? Mini Rev Med Chem. (2019) 19:1544–57. 10.2174/1389557519666190730110600 [DOI] [PubMed] [Google Scholar]
- 145.Klinger JR, Chakinala MM, Langleben D, Rosenkranz S, Sitbon O. Riociguat: clinical research and evolving role in therapy. Br J Clin Pharmacol. (2021) 87:2645–62. 10.1111/bcp.14676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Humbert M, Coghlan JG, Ghofrani HA, Grimminger F, He JG, Riemekasten G, et al. Riociguat for the treatment of pulmonary arterial hypertension associated with connective tissue disease: results from PATENT-1 and PATENT-2. Ann Rheum Dis. (2017) 76:422–6. 10.1136/annrheumdis-2015-209087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nagaraja V, Spino C, Bush E, Tsou PS, Domsic RT, Lafyatis R, et al. A multicenter randomized, double-blind, placebo-controlled pilot study to assess the efficacy and safety of riociguat in systemic sclerosis-associated digital ulcers. Arthritis Res Ther. (2019) 21:202. 10.1186/s13075-019-1979-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Khanna D, Allanore Y, Denton CP, Kuwana M, Matucci-Cerinic M, Pope J E, et al. Riociguat in patients with early diffuse cutaneous systemic sclerosis (RISE-SSc): randomised, double-blind, placebo-controlled multicentre trial. Ann Rheum Dis. (2020) 79:618. 10.1136/annrheumdis-2019-216823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee JJ, Pope JE. Emerging drugs and therapeutics for systemic sclerosis. Expert Opin Emerg Drugs. (2016) 21:421–30. 10.1080/14728214.2016.1257607 [DOI] [PubMed] [Google Scholar]
- 150.Jain S, Dhir V. Riociguat in systemic sclerosis: a potential for disease modification. Ann Rheum Dis. (2020). 10.1136/annrheumdis-2020-218180. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 151.Sakkas LI, Chikanza IC, Platsoucas CD. Mechanisms of disease: the role of immune cells in the pathogenesis of systemic sclerosis. Nat Clin Pract Rheumatol. (2006) 2:679–85. 10.1038/ncprheum0346 [DOI] [PubMed] [Google Scholar]
- 152.Caramaschi P, Volpe A, Pieropan S, Tinazzi I, Mahamid H, Bambara LM, et al. Cyclophosphamide treatment improves microvessel damage in systemic sclerosis. Clin Rheumatol. (2009) 28:391–5. 10.1007/s10067-008-1058-y [DOI] [PubMed] [Google Scholar]
- 153.Apras S, Ertenli I, Ozbalkan Z, Kiraz S, Ozturk MA, Haznedaroglu IC, et al. Effects of oral cyclophosphamide and prednisolone therapy on the endothelial functions and clinical findings in patients with early diffuse systemic sclerosis. Arthritis Rheum. (2003) 48:2256–61. 10.1002/art.11081 [DOI] [PubMed] [Google Scholar]
- 154.Furuya Y, Okazaki Y, Kaji K, Sato S, Takehara K, Kuwana M. Mobilization of endothelial progenitor cells by intravenous cyclophosphamide in patients with systemic sclerosis. Rheumatology. (2010) 49:2375–80. 10.1093/rheumatology/keq259 [DOI] [PubMed] [Google Scholar]
- 155.Strieter RM, Mehrad B. New mechanisms of pulmonary fibrosis. Chest. (2009) 136:1364–70. 10.1378/chest.09-0510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Borghini A, Manetti M, Nacci F, Bellando-Randone S, Guiducci S, Matucci-Cerinic, et al. Systemic sclerosis sera impair angiogenic performance of dermal microvascular endothelial cells: therapeutic implications of cyclophosphamide. PLoS ONE. (2015) 10:e0130166. 10.1371/journal.pone.0130166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Halpern A, Kuhn PH, Shaftel HE, Samuels SS, Shaftel N, Selman D, et al. Raynaud's disease, Raynaud's phenomenon, and serotonin. Angiology. (1960) 11:151–67. 10.1177/000331976001100301 [DOI] [PubMed] [Google Scholar]
- 158.Seibold JR. Serotonin and Raynaud's phenomenon. J Cardiovasc Pharm. (1985) 7:S95–8. 10.1097/00005344-198500077-00027 [DOI] [PubMed] [Google Scholar]
- 159.Abou-Raya A, Abou-Raya S, Helmii M. Statins: potentially useful in therapy of systemic sclerosis-related Raynaud's phenomenon and digital ulcers. J Rheumatol. (2008) 35:1801–8. [PubMed] [Google Scholar]
- 160.Watts SW, Morrison SF, Davis RP, Barman SM. Serotonin and blood pressure regulation. Daws LC, editor. Pharmacol Rev. (2012) 64:359–88. 10.1124/pr.111.004697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ungvari Z, Pacher P, Kecskeméti V, Koller A. Fluoxetine dilates isolated small cerebral arteries of rats and attenuates constrictions to serotonin, norepinephrine, and a voltage-dependent Ca 2+ channel opener. Stroke. (1999) 30:1949–54. 10.1161/01.STR.30.9.1949 [DOI] [PubMed] [Google Scholar]
- 162.Pereira CA, Ferreira NS, Mestriner FL, Antunes-Rodrigues J, Evora PRB, Resstel LBM, et al. Chronic fluoxetine treatment increases NO bioavailability and calcium-sensitive potassium channels activation in rat mesenteric resistance arteries. Eur J Pharmacol. (2015) 765:375–83. 10.1016/j.ejphar.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 163.Gupta M, Neavin D, Liu D, Biernacka J, Hall-Flavin D, Bobo WV, et al. TSPAN5, ERICH3 and selective serotonin reuptake inhibitors in major depressive disorder: pharmacometabolomics-informed pharmacogenomics. Mol Psychiatry. (2016) 21:1717–25. 10.1038/mp.2016.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Coleiro B, Marshall SE, Denton CP, Howell K, Blann A, Welsh KI, et al. Treatment of Raynaud's phenomenon with the selective serotonin reuptake inhibitor fluoxetine. Rheumatology. (2001) 40:1038–43. 10.1093/rheumatology/40.9.1038 [DOI] [PubMed] [Google Scholar]
- 165.Bellosta S, Paoletti R, Corsini A. Safety of statins: focus on clinical pharmacokinetics and drug interactions. Circulation. (2004) 109:III50–7. 10.1161/01.CIR.0000131519.15067.1f [DOI] [PubMed] [Google Scholar]
- 166.Ladak K, Pope J. A review of the effects of statins in systemic sclerosis. Semin Arthrit Rheum. (2015) 45:698–705. 10.1016/j.semarthrit.2015.10.013 [DOI] [PubMed] [Google Scholar]
- 167.Gonçalves RSG, Dantas AT, Pereira MC, de Almeida AR, Rego MJBM, da Rocha Pitta I, et al. Statins inhibit cytokines in a dose-dependent response in patients with systemic sclerosis. Inflammation. (2019) 42:407–11. 10.1007/s10753-018-0907-3 [DOI] [PubMed] [Google Scholar]
- 168.Timár O, Szekanecz Z, Kerekes G, Végh J, Oláh AV, Nagy G, et al. Rosuvastatin improves impaired endothelial function, lowers high sensitivity CRP, complement and immuncomplex production in patients with systemic sclerosis–a prospective case-series study. Arthritis Res Ther. (2013) 15:R105. 10.1186/ar4285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kuwana M, Okazaki Y, Kaburaki J. Long-term beneficial effects of statins on vascular manifestations in patients with systemic sclerosis. Mod Rheumatol. (2009) 19:530–5. 10.3109/s10165-009-0199-4 [DOI] [PubMed] [Google Scholar]
- 170.Kuwana M, Kaburaki J, Okazaki Y, Yasuoka H, Kawakami Y, Ikeda Y. Increase in circulating endothelial precursors by atorvastatin in patients with systemic sclerosis. Arthritis Rheum. (2006) 54:1946–51. 10.1002/art.21899 [DOI] [PubMed] [Google Scholar]
- 171.Curtiss P, Schwager Z, Cobos G, Lo Sicco K, Franks AG Jr. A systematic review and meta-analysis of the effects of topical nitrates in the treatment of primary and secondary Raynaud's phenomenon. J Am Acad Dermatol. (2018) 78:1110–8.e3. 10.1016/j.jaad.2018.01.043 [DOI] [PubMed] [Google Scholar]
- 172.Kan C, Akimoto S, Abe M, Okada K, Ishikawa O. Preliminary thermographic evaluation of new nitroglycerine tape on the peripheral circulatory disturbance in systemic sclerosis. Ann Rheum Dis. (2002) 61:177–9. 10.1136/ard.61.2.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hummers LK, Dugowson CE, Dechow FJ, Wise RA, Gregory J, Michalek J, et al. A multi-centre, blinded, randomised, placebo-controlled, laboratory-based study of MQX-503, a novel topical gel formulation of nitroglycerine, in patients with Raynaud phenomenon. Ann Rheum Dis. (2013) 72:1962–7. 10.1136/annrheumdis-2012-201536 [DOI] [PubMed] [Google Scholar]
- 174.Hughes M, Moore T, Manning J, Wilkinson J, Dinsdale G, Roberts C, et al. Reduced perfusion in systemic sclerosis digital ulcers (both fingertip and extensor) can be increased by topical application of glyceryl trinitrate. Microvasc Res. (2017) 111:32–6. 10.1016/j.mvr.2016.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Acharya N, Sharma SK, Mishra D, Dhooria S, Dhir V, Jain S. Efficacy and safety of pirfenidone in systemic sclerosis-related interstitial lung disease-a randomised controlled trial. Rheumatol Int. (2020) 40:703–10. 10.1007/s00296-020-04565-w [DOI] [PubMed] [Google Scholar]
- 176.Beck L, Pinilla E, Arcanjo DDR, Hernanz R, Prat-Duran J, Petersen AG, et al. Pirfenidone is a vasodilator: involvement of KV7 channels in the effect on endothelium-dependent vasodilatation in type-2 diabetic mice. Front Pharmacol. (2021) 11:619152. 10.3389/fphar.2020.619152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lima-Posada I, Fontana F, Pérez-Villalva R, Berman-Parks N, Bobadilla NA. Pirfenidone prevents acute kidney injury in the rat. BMC Nephrol. (2019) 20:158. 10.1186/s12882-019-1364-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Segreto F, Marangi GF, Cerbone V, Persichetti P. The role of botulinum toxin A in the treatment of Raynaud phenomenon. Ann Plast Surg. (2016) 77:318–23. 10.1097/SAP.0000000000000715 [DOI] [PubMed] [Google Scholar]
- 179.Bello RJ, Cooney CM, Melamed E, Follmar K, Yenokyan G, Leatherman G, et al. The therapeutic efficacy of botulinum toxin in treating scleroderma-associated Raynaud's phenomenon: a randomized, double-blind, placebo-controlled clinical trial: botulinum toxin for scleroderma-associated raynaud's phenomenon. Arthrit Rheumatol. (2017) 69:1661–9. 10.1002/art.40123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Motegi S, Sekiguchi A, Saito S, Ishibuchi H, Kishi C, Yasuda M, et al. Successful treatment of Raynaud's phenomenon and digital ulcers in systemic sclerosis patients with botulinum toxin B injection: assessment of peripheral vascular disorder by angiography and dermoscopic image of nail fold capillary. J Dermatol. (2018) 45:349–52. 10.1111/1346-8138.14140 [DOI] [PubMed] [Google Scholar]
- 181.Lautenbach G, Dobrota R, Mihai C, Distler O, Calcagni M, Maurer B. Evaluation of botulinum toxin A injections for the treatment of refractory chronic digital ulcers in patients with systemic sclerosis. Clin Exp Rheumatol. (2020) 38:154–60. [PubMed] [Google Scholar]
- 182.Hughes M, Moore T, Manning J, Wilkinson J, Watson S, Samraj P, et al. A feasibility study of a novel low-level light therapy for digital ulcers in systemic sclerosis. J Dermatol Treat. (2019) 30:251–7. 10.1080/09546634.2018.1484875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dinsdale G, Murray A, Moore T, Ferguson J, Wilkinson J, Richards H, et al. A comparison of intense pulsed light and laser treatment of telangiectases in patients with systemic sclerosis: a within-subject randomized trial. Rheumatology. (2014) 53:1422–30. 10.1093/rheumatology/keu006 [DOI] [PubMed] [Google Scholar]
- 184.Bogoch ER, Gross DK. Surgery of the hand in patients with systemic sclerosis: outcomes and considerations. J Rheumatol. (2005) 32:642–8. [PubMed] [Google Scholar]
- 185.Rosa I, Romano E, Fioretto BS, Matucci-Cerinic M, Manetti M. Adipose-derived stem cells: pathophysiologic implications vs therapeutic potential in systemic sclerosis. World J Stem Cells. (2021) 13:30–48. 10.4252/wjsc.v13.i1.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sterodimas A, de Faria J, Nicaretta B, Pitanguy I. Tissue engineering with adipose-derived stem cells (ADSCs): current and future applications. J Plast Reconstr Aesthet Surg. (2010) 63:1886–92. 10.1016/j.bjps.2009.10.028 [DOI] [PubMed] [Google Scholar]
- 187.Minteer DM, Marra KG, Rubin JP. Adipose stem cells: biology, safety, regulation, and regenerative potential. Clin Plast Surg. (2015) 42:169–79. 10.1016/j.cps.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 188.Bank J, Fuller SM, Henry GI, Zachary LS. Fat grafting to the hand in patients with Raynaud phenomenon: a novel therapeutic modality. Plast Reconstr Surg. (2014) 133:1109–18. 10.1097/PRS.0000000000000104 [DOI] [PubMed] [Google Scholar]
- 189.Bene MD, Pozzi MR, Rovati L, Mazzola I, Erba G, Bonomi S. Autologous fat grafting for scleroderma-induced digital ulcers. An effective technique in patients with systemic sclerosis. Handchir Mikrochir Plast Chir. (2014) 46:242–7. 10.1055/s-0034-1376970 [DOI] [PubMed] [Google Scholar]
- 190.Granel B, Daumas A, Jouve E, Harlé JR, Nguyen PS, Chabannon C, et al. Safety, tolerability and potential efficacy of injection of autologous adipose-derived stromal vascular fraction in the fingers of patients with systemic sclerosis: an open-label phase I trial. Ann Rheum Dis. (2015) 74:2175–82. 10.1136/annrheumdis-2014-205681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Del Papa N, Caviggioli F, Sambataro D, Zaccara E, Vinci V, Di Luca G, et al. Autologous fat grafting in the treatment of fibrotic perioral changes in patients with systemic sclerosis. Cell Transplant. (2015) 24:63–72. 10.3727/096368914X674062 [DOI] [PubMed] [Google Scholar]
- 192.Guillaume-Jugnot P, Daumas A, Magalon J, Jouve E, Nguyen PS, Truillet R, et al. Autologous adipose-derived stromal vascular fraction in patients with systemic sclerosis: 12-month follow-up. Rheumatology. (2016) 55:301–6. 10.1093/rheumatology/kev323 [DOI] [PubMed] [Google Scholar]
- 193.Del Papa N, Di Luca G, Andracco R, Zaccara E, Maglione W, Pignataro F, et al. Regional grafting of autologous adipose tissue is effective in inducing prompt healing of indolent digital ulcers in patients with systemic sclerosis: results of a monocentric randomized controlled study. Arthritis Res Ther. (2019) 21:7. 10.1186/s13075-018-1792-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Virzì F, Bianca P, Giammona A, Apuzzo T, Di Franco S, Mangiapane LR, et al. Combined platelet-rich plasma and lipofilling treatment provides great improvement in facial skin-induced lesion regeneration for scleroderma patients. Stem Cell Res Ther. (2017) 8:236. 10.1186/s13287-017-0690-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pignatti M, Spinella A, Cocchiara E, Boscaini G, Lusetti IL, Citriniti G, et al. Autologous fat grafting for the oral and digital complications of systemic sclerosis: results of a prospective study. Aesthetic Plast Surg. (2020) 44:1820–1832. 10.1007/s00266-020-01848-2 [DOI] [PubMed] [Google Scholar]
- 196.Daumas A, Magalon J, Delaunay F, Abellan M, Philandrianos C, Sabatier F, et al. Fat grafting for treatment of facial scleroderma. Clin Plast Surg. (2020) 47:155–63. 10.1016/j.cps.2019.08.016 [DOI] [PubMed] [Google Scholar]
- 197.Suga H, Glotzbach JP, Sorkin M, Longaker MT, Gurtner GC. Paracrine mechanism of angiogenesis in adipose-derived stem cell transplantation. Ann Plast Surg. (2014) 72:234–41. 10.1097/SAP.0b013e318264fd6a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. (2004) 109:1292–8. 10.1161/01.CIR.0000121425.42966.F1 [DOI] [PubMed] [Google Scholar]
- 199.Griffin M, Ryan CM, Pathan O, Abraham D, Denton CP, Butler PE. Characteristics of human adipose derived stem cells in scleroderma in comparison to sex and age matched normal controls: implications for regenerative medicine. Stem Cell Res Ther. (2017) 8:23. 10.1186/s13287-016-0444-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lee R, Del Papa N, Introna M, Reese CF, Zemskova M, Bonner M, et al. Adipose-derived mesenchymal stromal/stem cells in systemic sclerosis: alterations in function and beneficial effect on lung fibrosis are regulated by caveolin-1. J Scleroderma Relat Disord. (2019) 4:127–36. 10.1177/2397198318821510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kuca-Warnawin E, Skalska U, Janicka I, Musiałowicz U, Bonek K, Głuszko P, et al. The phenotype and secretory activity of adipose-derived mesenchymal stem cells (ASCs) of patients with rheumatic diseases. Cells. (2019) 8:1659. 10.3390/cells8121659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Snowden JA, Badoglio M, Labopin M, Giebel S, McGrath E, Marjanovic Z, et al. Evolution, trends, outcomes, and economics of hematopoietic stem cell transplantation in severe autoimmune diseases. Blood Adv. (2017) 1:2742–55. 10.1182/bloodadvances.2017010041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Burt RK, Shah SJ, Dill K, Grant T, Gheorghiade M, Schroeder J, et al. Autologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): an open-label, randomised phase 2 trial. Lancet. (2011) 378:498–506. 10.1016/S0140-6736(11)60982-3 [DOI] [PubMed] [Google Scholar]
- 204.van Laar JM, Farge D, Sont JK, Naraghi K, Marjanovic Z, Larghero J, et al. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA. (2014) 311:2490. 10.1001/jama.2014.6368 [DOI] [PubMed] [Google Scholar]
- 205.Del Papa N, Pignataro F, Zaccara E, Maglione W, Minniti A. Autologous hematopoietic stem cell transplantation for treatment of systemic sclerosis. Front Immunol. (2018) 9:1–12. 10.3389/fimmu.2018.02390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sullivan KM, Goldmuntz EA, Keyes-Elstein L, McSweeney PA, Pinckney A, Welch B, et al. Myeloablative autologous stem-cell transplantation for severe scleroderma. N Engl J Med. (2018) 378:35–47. 10.1056/NEJMoa1703327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Farge D, Burt RK, Oliveira M-C, Mousseaux E, Rovira M, Marjanovic Z, et al. Cardiopulmonary assessment of patients with systemic sclerosis for hematopoietic stem cell transplantation: recommendations from the European Society for Blood and Marrow Transplantation Autoimmune Diseases Working Party and collaborating partners. Bone Marrow Transplant. (2017) 52:1495–503. 10.1038/bmt.2017.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Arruda LCM, Clave E, Moins-Teisserenc H, Douay C, Farge D, Toubert A. Resetting the immune response after autologous hematopoietic stem cell transplantation for autoimmune diseases. Curr Res Trans Med. (2016) 64:107–13. 10.1016/j.retram.2016.03.004 [DOI] [PubMed] [Google Scholar]
- 209.Snowden JA, Sharrack B, Akil M, Kiely DG, Lobo A, Kazmi M, et al. Autologous haematopoietic stem cell transplantation (aHSCT) for severe resistant autoimmune and inflammatory diseases - a guide for the generalist. Clin Med. (2018) 18:329–34. 10.7861/clinmedicine.18-4-329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Daikeler T, Kump E, Stern M, Hügle T, Hij A, Haeuserman P, et al. Autologous hematopoietic stem cell transplantation reverses skin fibrosis but does not change skin vessel density in patients with systemic sclerosis. Pathol Biol. (2015) 63:164–8. 10.1016/j.patbio.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 211.Miniati I, Guiducci S, Conforti ML, Rogai V, Fiori G, Cinelli M, et al. Autologous stem cell transplantation improves microcirculation in systemic sclerosis. Ann Rheum Dis. (2009) 68:94–8. 10.1136/ard.2007.082495 [DOI] [PubMed] [Google Scholar]
- 212.Fleming JN, Nash RA, McLeod DO, Fiorentino DF, Shulman HM, Connolly MK, et al. Capillary regeneration in scleroderma: stem cell therapy reverses phenotype? PLoS ONE. (2008) 3:e1452. 10.1371/annotation/6b021f46-17bd-4ffe-a378-a1b8d24a1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Singh RK, Gutman M, Bucana CD, Sanchez R, Llansa N, Fidler IJ. Interferons alpha and beta down-regulate the expression of basic fibroblast growth factor in human carcinomas. Proc Natl Acad Sci USA. (1995) 92:4562–6. 10.1073/pnas.92.10.4562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Michel L, Farge D, Baraut J, Marjanovic Z, Jean-Louis F, Porcher R, et al. Evolution of serum cytokine profile after hematopoietic stem cell transplantation in systemic sclerosis patients. Bone Marrow Transplant. (2016) 51:1146–9. 10.1038/bmt.2016.77 [DOI] [PubMed] [Google Scholar]
- 215.Escobar-Soto CH, Mejia-Romero R, Aguilera N, Alzate-Granados JP, Mendoza-Pinto C, Munguía-Realpozo P, et al. Human mesenchymal stem cells for the management of systemic sclerosis. Systematic review. Autoimmun Rev. (2021) 20:102831. 10.1016/j.autrev.2021.102831 [DOI] [PubMed] [Google Scholar]
- 216.Wang M, Yuan Q, Xie L. Mesenchymal stem cell-based immunomodulation: properties and clinical application. Stem Cells Int. (2018) 2018:3057624. 10.1155/2018/3057624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal stem cells for regenerative medicine. Cells. (2019) 8:886. 10.3390/cells8080886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Cipriani P, Guiducci S, Miniati I, Cinelli M, Urbani S, Marrelli A, et al. Impairment of endothelial cell differentiation from bone marrow-derived mesenchymal stem cells: new insight into the pathogenesis of systemic sclerosis. Arthritis Rheum. (2007) 56:1994–2004. 10.1002/art.22698 [DOI] [PubMed] [Google Scholar]
- 219.Guiducci S, Porta F, Saccardi R, Guidi S, Ibba-Manneschi L, Manetti M, et al. Autologous mesenchymal stem cells foster revascularization of ischemic limbs in systemic sclerosis: a case report. Ann Intern Med. (2010) 153:650–4. 10.7326/0003-4819-153-10-201011160-00007 [DOI] [PubMed] [Google Scholar]
- 220.Christopeit M, Schendel M, Föll J, Müller LP, Keysser G, Behre G. Marked improvement of severe progressive systemic sclerosis after transplantation of mesenchymal stem cells from an allogeneic haploidentical-related donor mediated by ligation of CD137L. Leukemia. (2008) 22:1062–4. 10.1038/sj.leu.2404996 [DOI] [PubMed] [Google Scholar]
- 221.Keyszer G, Christopeit M, Fick S, Schendel M, Taute BM, Behre G, et al. Treatment of severe progressive systemic sclerosis with transplantation of mesenchymal stromal cells from allogeneic related donors: report of five cases. Arthritis Rheum. (2011) 63:2540–2. 10.1002/art.30431 [DOI] [PubMed] [Google Scholar]
- 222.van Rhijn-Brouwer FCC, Gremmels H, Fledderus JO, Schuurman AH, Bonte-Mineur F, Vonk MC, et al. MANUS Study Group. A randomised placebo-controlled double-blind trial to assess the safety of intramuscular administration of allogeneic mesenchymal stromal cells for digital ulcers in systemic sclerosis: the MANUS Trial protocol. BMJ Open. (2018) 8:e020479. 10.1136/bmjopen-2017-020479 [DOI] [PMC free article] [PubMed] [Google Scholar]