Abstract
Background:
Burden of atrial fibrillation (AF), as a continuous measure, is an emerging alternative classification often assumed to increase linearly with progression of disease. Yet there are no descriptions of AF burden distributions across populations.
Methods:
We examined patterns of AF burden (% time in AF) across 3 different cohorts: outpatients with AF undergoing Holter monitoring in a national registry (ORBIT-AF II), routine outpatients undergoing Holter monitoring in a tertiary healthcare system (UHealth), and patients >=65 years with cardiac implantable electronic devices (Merlin.net™ linked to Medicare).
Results:
We included 2,058 ORBIT-AF II patients, 4,537 UHealth patients, and 39,710 from Merlin.net™. Mean age ranged from 56 to 77 years, sex ranged from 40% to 61% male, and mean CHA2DS2-VASc scores ranged from 2.2 to 4.9. Across all cohorts, AF burden demonstrated skewed frequency towards the extremes, with the vast majority of patients having either very low or very high AF burden. This bimodal distribution was consistent across cohorts, across clinically-documented AF types (paroxysmal vs. persistent), patients with or without a known AF diagnosis, and among patients with different types of cardiac implantable electronic devices.
Conclusions:
Across 3 broad, diverse cohorts with continuous monitoring, distribution of AF burden was consistently skewed towards the extremes without an even, linear distribution or progression. As AF burden is increasingly recognized as a descriptor and potential risk-stratifier, these findings have important implications for future research and patient care.
Keywords: atrial fibrillation, burden, distribution, ambulatory electrocardiography
Atrial fibrillation (AF), the most common adult arrhythmia, is typically described to occur in two primary phenotypes: paroxysmal (spontaneously limited to 7 days) or persistent (requiring intervention to restore sinus rhythm).1, 2 Differences in clinical characteristics and outcomes between patients with paroxysmal versus persistent AF have been well-described.3 More recently it has been recognized that this categorization of AF is relatively rudimentary in an age of continuous electrocardiographic monitoring and cardiac implantable electronic devices (CIEDs). Moreover, there is accumulating evidence that supports an exposure-response relationship between AF ‘burden’ (using a variety of definitions) and clinical outcomes.4, 5
A more granular, nuanced measure of AF burden could yield improved risk stratification and outcome prediction for patients with AF. Percent of all time in AF, as a continuous measure of AF burden, has emerged as a potentially more powerful alternative. Thus far, researchers and clinicians commonly approach this measure as having a relatively linear progression of increasing AF burden with increasing progression of disease. However, this has not been demonstrated in the literature to date and a more in-depth understanding of population distribution of AF burden is necessary. The aim of our analysis was to assess the distribution of AF burden, measured as the percent of time in AF during continuous electrocardiography to be the overall percent of time in AF, across several broad and distinct patient populations and using both ambulatory electrocardiograms (AECG; e.g., Holter monitoring) and CIEDs. These included (1) a national outpatient registry of AF patients, (2) a broad, tertiary care population of patients with and without AF, and (3) a national remote monitoring dataset from patients with AF and CIEDs.
Methods
We describe the clinical characteristics and distributions of AF burden, as expressed by percent of time in AF, across three cohorts described as follows.
Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II (ORBIT-AF II)
First, we used data from The Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II (ORBIT-AF II), an outpatient registry that enrolled patients aged 21 years or older with ECG-confirmed AF that was not due to a reversible cause. The rationale and design of this study has been previously reported in detail.6 As part of the case report form for ORBIT-AF, enrolling sites were queried as to the availability of AECGs for patients (primarily Holter monitor tests). If available and performed as part of routine clinical care, results of this monitoring were reported in the study. AF burden was recorded as percent of total time in AF, calculated automatically by the AECG device. Additional captured data collected in the registry included demographics, past medical history, treatment for AF, medications, laboratory studies, and cardiac imaging.
University of Utah Health (UHealth)
The second cohort analyzed was a real-world, clinical cohort of patients from University of Utah Health (UHealth), who underwent AECG monitoring for any indication from 2010 to 2020. AF burden in this cohort was categorized as total percent time in AF, as automatically recorded by the AECG monitor. Additional data for these patients were derived from the UHealth enterprise data warehouse, which included detailed institutional data on demographics, administrative billing (diagnosis) codes, procedural coding, medication orders, laboratory studies, and cardiac imaging, as previously described.7 In brief, medical history was derived from administrative billing codes, using previously validated algorithms.9,10 These patients were also categorized according to whether they had any diagnosis of AF at any time during follow-up, before or after AECG monitoring. Diagnosis of outpatients with AF was defined consistent with similar cohorts, as (1) presence of AF on a 12-lead ECG or (2) ≥2 encounters in our health system (and ≥1 as an outpatient) with an International Classification of Disease code for AF (427.31 [9th revision] or I48.0, I48.1, I48.2, I48.91 [10th revision]).
Merlin.net™
Lastly, we analyzed AF burden data from The Merlin.net™ registry, a large, remote monitoring dataset of CIEDs that included permanent pacemakers, implantable cardioverter-defibrillators (ICDs), and cardiac resynchronization devices, in the Merlin.net™ database (Abbott, Chicago, IL, USA). In this analysis, we included patients with de novo device implantations between 4/1/2010 and 12/31/2016 and follow-up through 12/31/2017. The Merlin.net™ database contained patient-level data and records of daily or weekly averaged atrial arrhythmia burden derived from remote device monitoring.
In order to capture patient demographics and medical history, this cohort was linked to Medicare claims from 2009 to 2017 accessed through the Centers for Medicare and Medicaid Services (CMS) Virtual Research Data Center (VRDC). This included inpatient, outpatient, and carrier claims, Part D prescription drug fill records, and the corresponding Master Beneficiary Summary Files (MBSF). Based on Medicare records, we included only patients over 65 and with a clinical diagnosis of AF based on ICD-9-CM 427.31 or ICD-10-CM I48.0*-I48.2*, I48.91 in any position on an inpatient, outpatient or carrier claim in the 12 months prior to CIED implant date, had continuous fee-for-service Medicare enrollment during the 12 months prior to index date, and had at least 1 qualifying 30-day period of AF burden records for cohort inclusion. Linking to Merlin.net™ was based on Medicare claims for a CIED implant and matching patient date of birth, sex, and implantation date. The CIED implantation date was defined as the index date (for purposes of prior medical history). We then allowed for a 30-day blanking period to exclude perioperative influences on AF burden. AF burden was defined as the total daily atrial tachycardia/atrial fibrillation duration (seconds per week) in which the atrial rate exceeded 180 bpm. For some periods, only weekly burden was available, and was divided by 7 for average daily AF burden. The distribution of AF burden in the overall Merlin.net™ cohort, including following up period, is used. This cohort was also stratified by device type, pacemaker versus implantable cardioverter-defibrillator.
Statistical Methods
Baseline data are presented as means (standard deviation) or count (percentage). Univariate comparison statistics are not presented, as these cohorts are not expected to be comparable. AF burdens are described based on histogram distributions and percentiles.
Study Oversight
The ORBIT-AF II Registry was approved by the Duke University Institutional Review Board (IRB), and individual sites received IRB approval pursuant to local regulations. All patients in ORBIT-AF II provided written, informed consent. Analyses of UHealth AECG data has been approved by the University of Utah IRB, with a waiver of consent granted due to the retrospective, minimal-risk nature of the study. Analyses of Merlin.net™ and CMS data was approved by the Duke University IRB, also with a waiver of consent. The primary author had access to all the data herein; owing to the diverse sources of data analyzed, the authors cannot confirm data availability but will evaluate in good faith any formal requests for data availability. Beyond supported effort (see Disclosures), no project-specific extramural funding was used to support this work. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Results
Baseline characteristics for each of the cohorts are shown in Table 1. The Merlin.net cohort linked to Medicare represents the oldest group, with a mean age of 77 years old (SD 11), followed by the ORBIT-AF II (71 years, SD 8.7) cohort, followed by UHealth (56, SD 19) cohort. Proportion male varied from 44% (UHealth) to 61% (Merlin.net), and mean CHA2DS2-VASc scores ranged from 2.22 (UHealth) to 4.9 (Merlin.net).
Table 1.
Baseline Patient Characteristics Across Cohorts
| ORBIT-AF II (n=2,058) | UHealth (n=4,537) | Merlin.net linked to Medicare (n=39,710) | |
|---|---|---|---|
| Age, mean years (SD) | 71 (11) | 56 (19) | 77 (8.7) |
| Male | 1146 (56) | 1995 (44.0) | 24,119 (61) |
| Race* | |||
| White | 1805 (88) | 3867 (85.9) | 36,710 (92.5) |
| Black/African American | 77 (3.7) | 58 (1.3) | 1,997 (5.0) |
| American Indian/Alaska Native | 1 (0.05) | 41 (0.9) | 129 (0.3) |
| Asian | 48 (2.3) | 82 (1.8) | 191 (0.5) |
| Hypertension | 1656 (80) | 1670 (36.8) | 35,940 (91) |
| Diabetes mellitus | 524 (25) | 906 (20.0) | 15,366 (39) |
| Prior myocardial infarction | 204 (9.9) | 507 (11.2) | 12,405 (31) |
| Heart failure | 328 (16) | 733 (16.2) | 25,054 (63) |
| Prior Stroke/TIA | 251 (12) | 348 (7.7) | 3,820 (9.6) |
| CHA2DS2-VASc score, mean (SD) | 3.42 (1.72) | 2.22 (1.79) | 4.9 (1.3) |
| CHA2DS2-VASc Score | |||
| 0 | 71 (3.5) | 617 (13.6) | 0 |
| 1 | 205 (10) | 1462 (32.2) | 167 (0.4) |
| 2-9 | 1782 (87) | 2458 (54.2) | 39,543 (99.6) |
| Cardiac implanted electronic device | |||
| Permanent pacemaker | 175 (8.5) | 89 (2.0) | 20,156 (51) |
| Implantable cardioverter-defibrillator | 28 (1.4) | 68 (1.5) | 19,554 (49) |
Baseline characteristics across cohorts.
Values are presented as n (%), unless noted otherwise.
Subgroups may not sum to 100% due to differences in cohort definition and/or categorization across cohorts.
SD: standard deviation; TIA: cerebrovascular accident/transient ischemic attack.
ORBIT-AF II
The ORBIT-AF II cohort included 2,058 patients with ambulatory monitoring results available. They had a mean age of 71 years, and 56% were male; 87% had CHA2DS2-VASc scores >=2, and nearly 10% had a CIED.
Distributions of AF burdens among ORBIT-AF II patients are shown in Figure 1, overall and according to AF type (new onset, paroxysmal, persistent).
Figure 1.
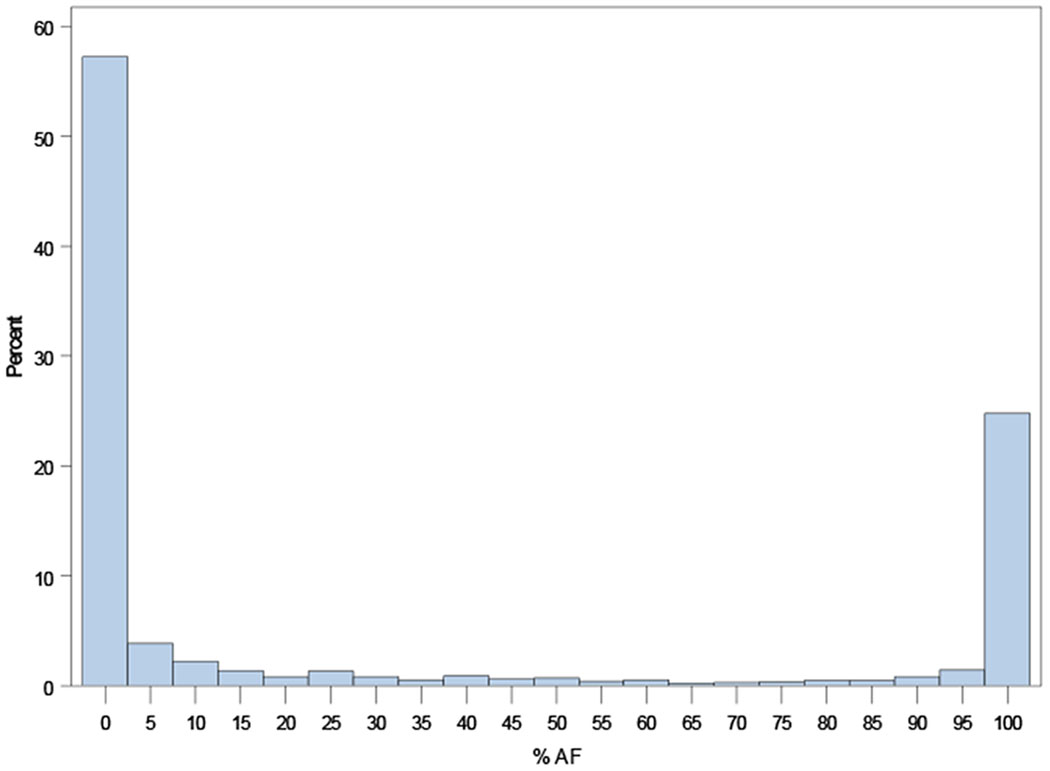
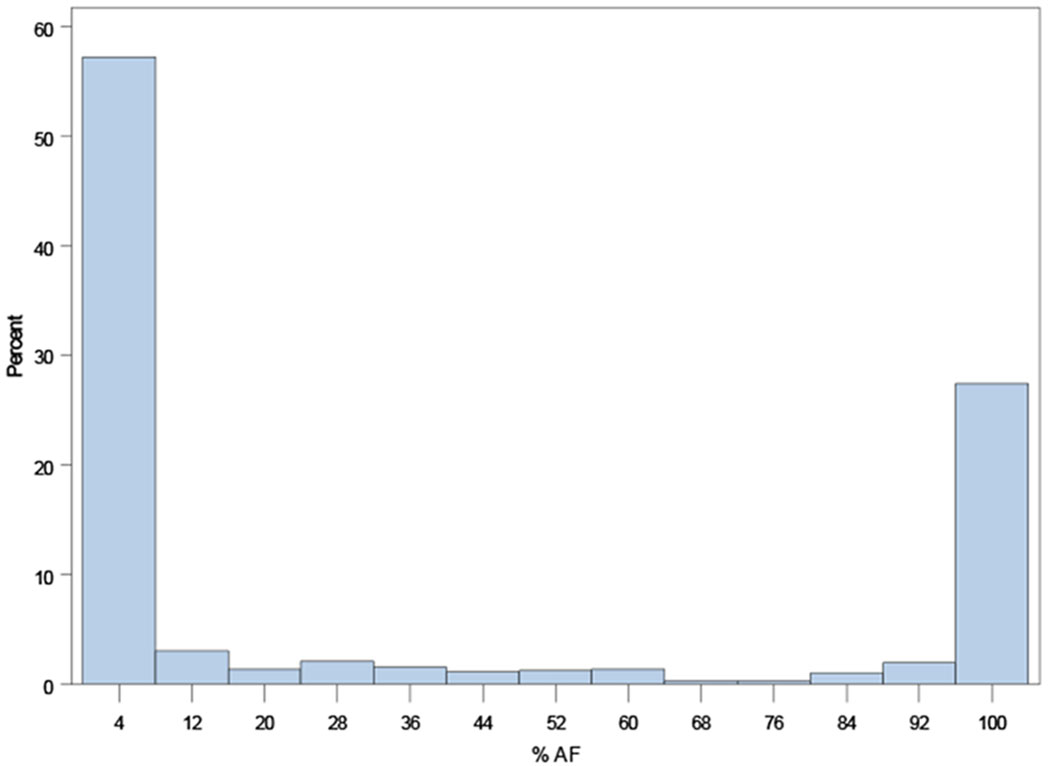
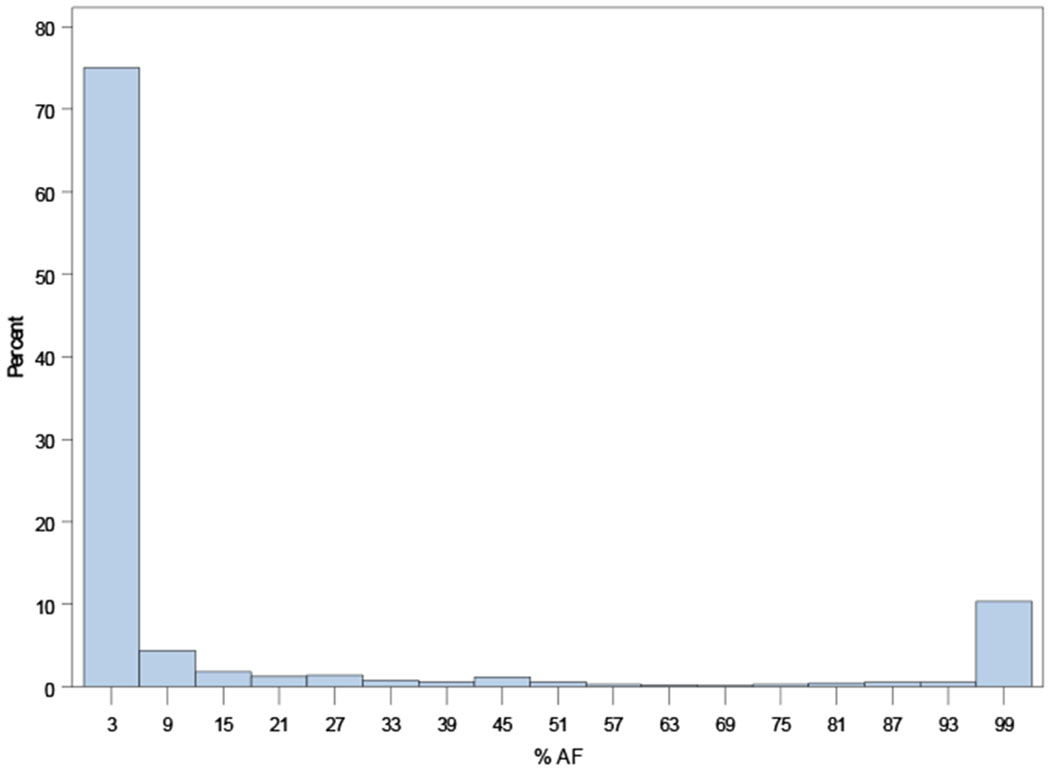
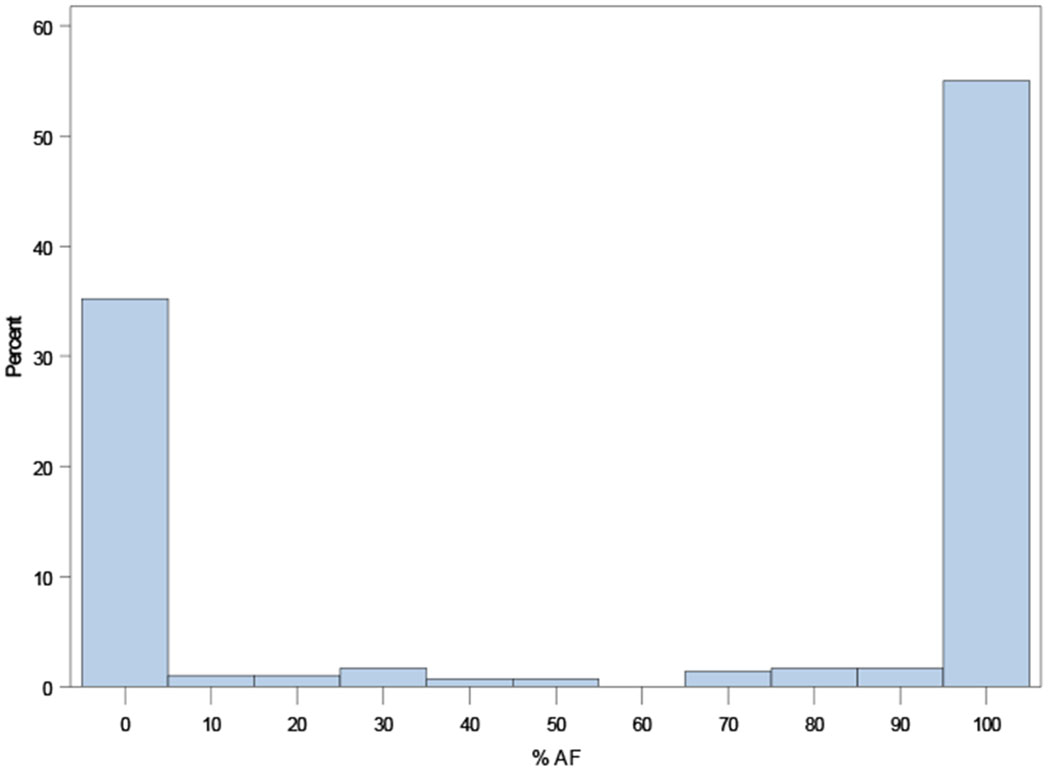
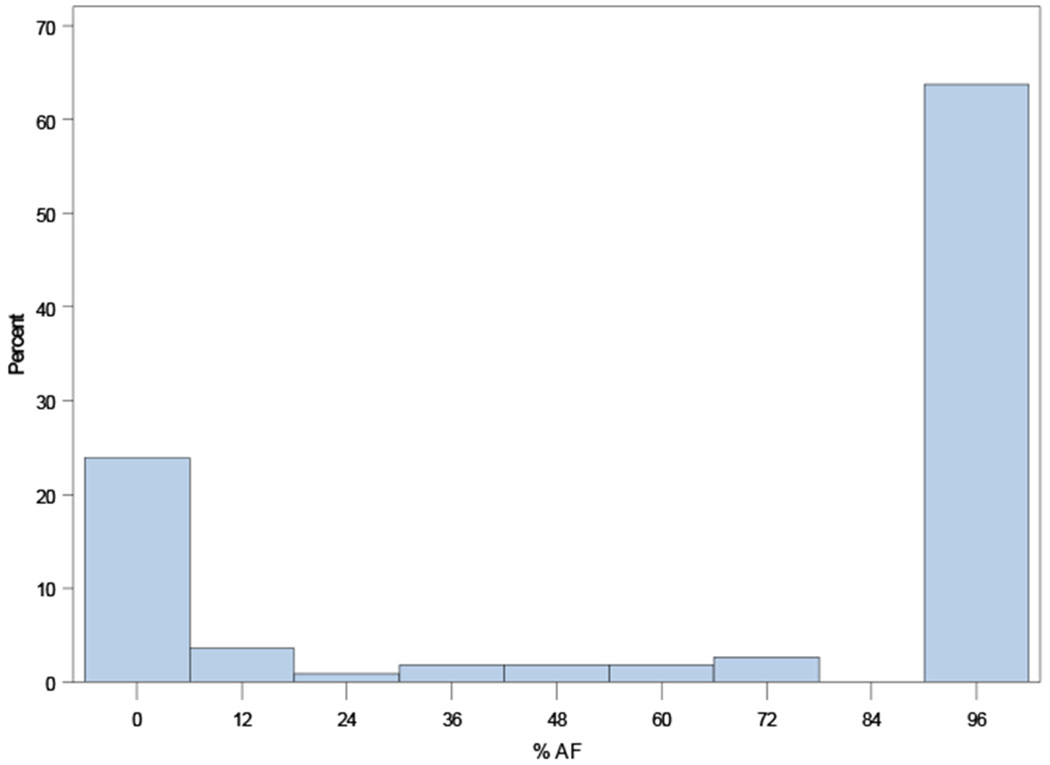
Distribution of AF burden across the ORBIT-AF II population overall (Panel A), and by AF type: new-onset AF (N=718, Panel B); paroxysmal AF (N=929, Panel C); persistent AF (N=298, Panel D); and permanent AF (N=113, Panel E).
A. Overall Distribution of AF Burden (N=2,058).
B: Distribution of AF duration among new-onset AF (N=718)
C. Distribution of AF duration among paroxysmal AF (N=929)
D. Distribution of AF duration among persistent AF (N=298)
E. Distribution of AF duration among permanent AF (N=113)
UHealth
The UHealth cohort included patients who underwent AECG monitoring for any indication from July 8, 2010 through June 28, 2020. This yielded 4,537 patients with AF burden measured in 6,023 AECG studies. The patients in this cohort had a mean age of 56 years, 44% were male, 23% (n=1044) had an AF diagnosis at any time during follow-up, and 7.7% had a history of prior stroke or transient ischemic attach. Approximately 3.5% of these patients had a CIED.
Among the 6,023 AECG studies, the mean monitoring duration was 56 hours (SD 13), with 91.2% recording 2 or 3 days, and 38% (n=2269) were among patients with an AF diagnosis at any time during follow-up. The overall AF burden was 14.9% (SD 33.75) across all studies, and 20% (n=1182) had AF burden >0%.
Distributions of AF burdens in the UHealth Holter monitoring cohort are shown in Figure 2, overall, excluding studies without any AF (0% burden), and among only patients with any AF diagnosis during all follow-up.
Figure 2.
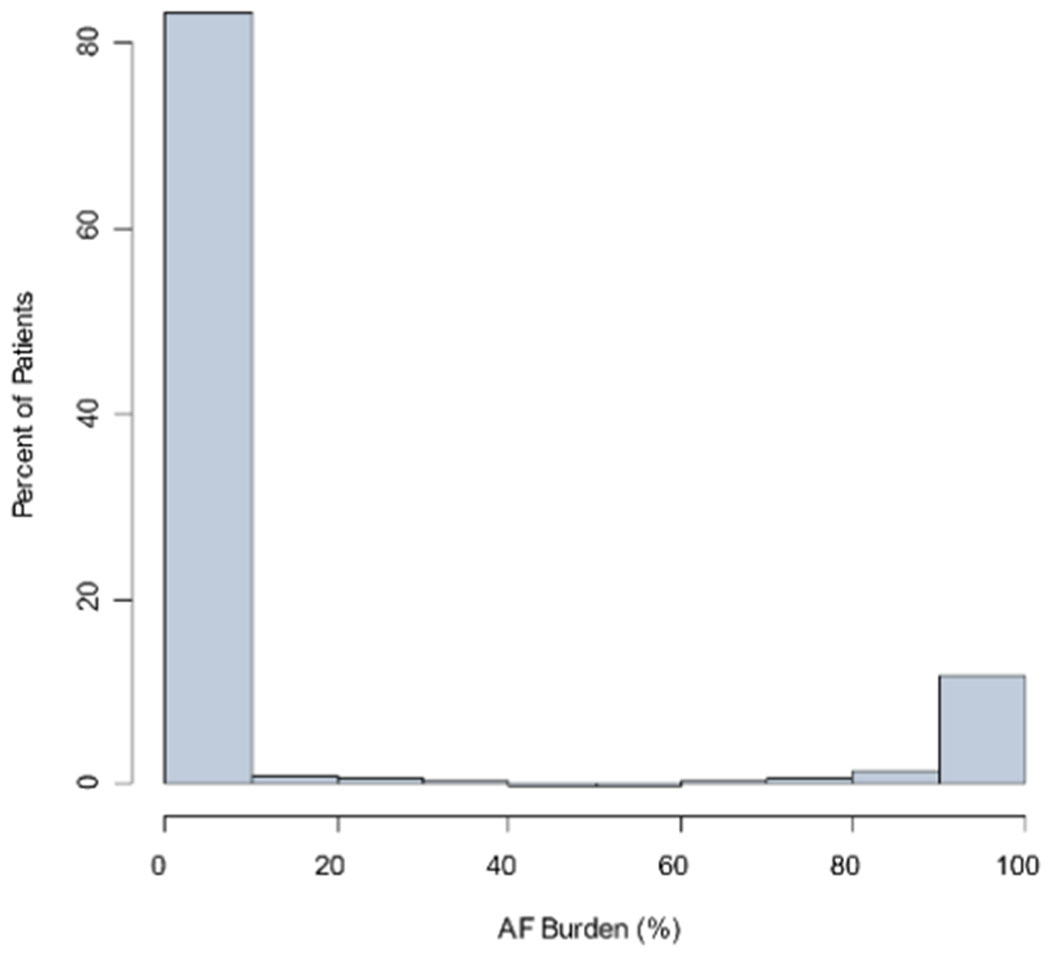
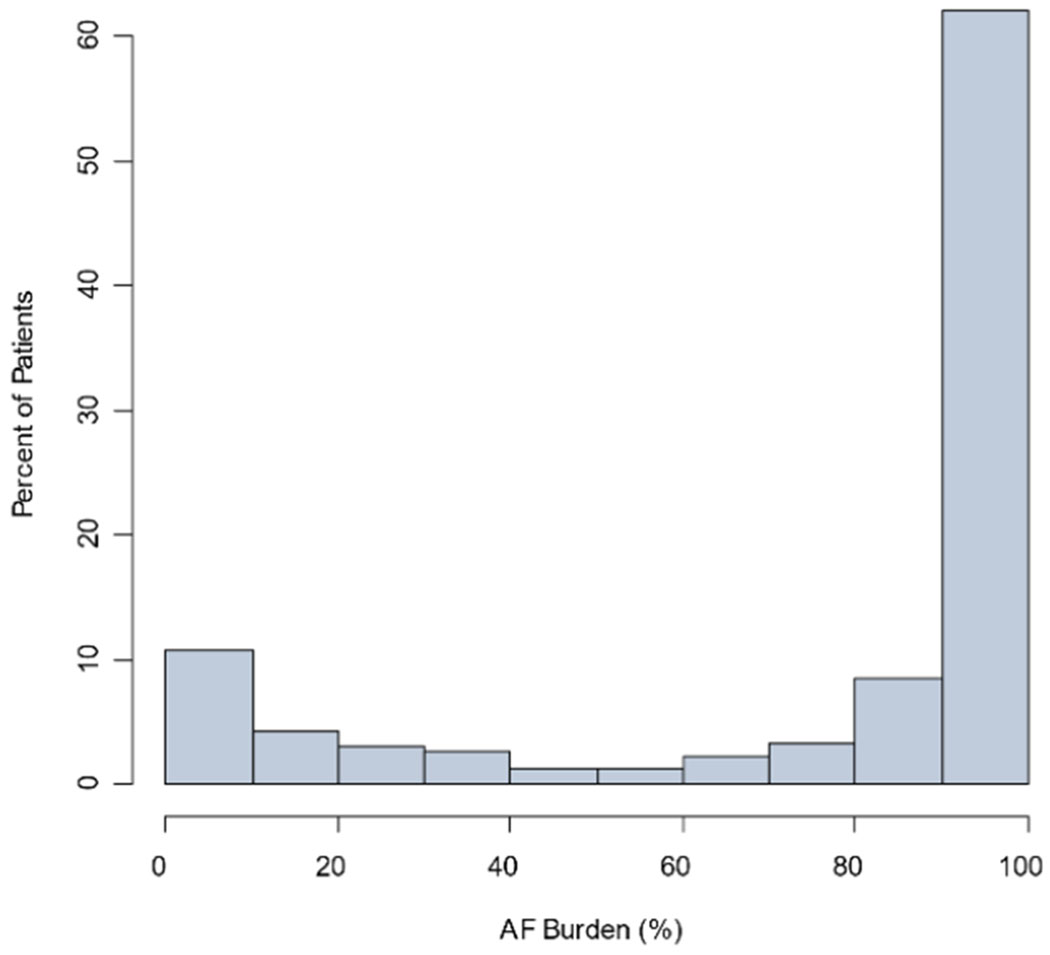
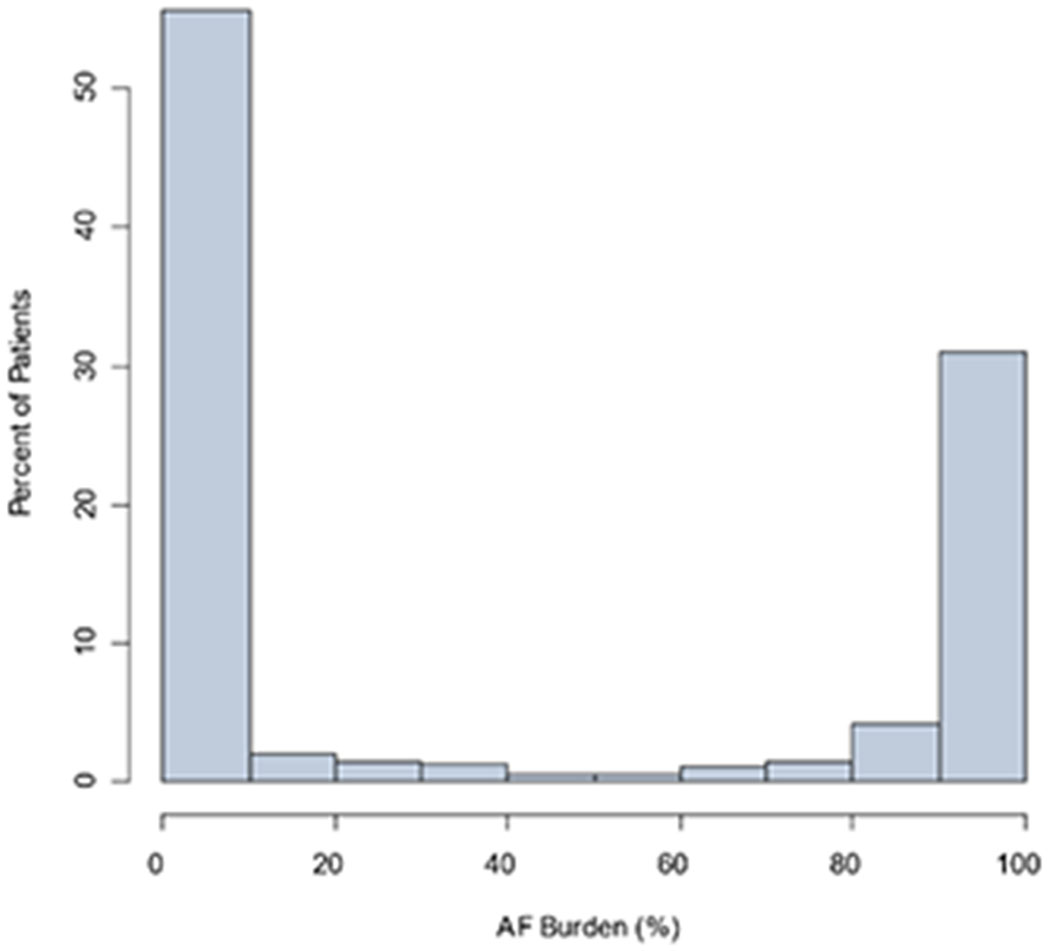
Distribution of AF burden among all UHealth Holter monitoring (Panel A, n=6023 studies), among studies with >0% AF burden (n=1182 studies; Panel B), and among patients with AF diagnosed any time during follow-up (n=2269 studies; Panel C).
A. Distribution of AF burden among all UHealth Holter monitoring (n=6023 studies)
B. Distribution of AF burden among UHealth Holter monitoring among studies with >0% AF burden (n=1182 studies)
C. Distribution of AF burden among all UHealth Holter monitoring among patients with AF diagnosed any time during follow-up (n=2269 studies)
Merlin.net™
The Merlin.net™ cohort included 39,710 patients with CIEDs, a prior diagnosis of AF and no persistent AF at baseline. Each patient in this cohort had at least 30 days of AF burden measured. They had a mean age was of 77 years, and 61% were male, and the type of CIED was evenly split between pacemakers and ICDs (Table 1).
Distribution of AF burden in the overall Merlin.net™ cohort is shown in Figure 3, overall and stratified by device type – pacemaker or implantable cardioverter-defibrillator.
Figure 3.
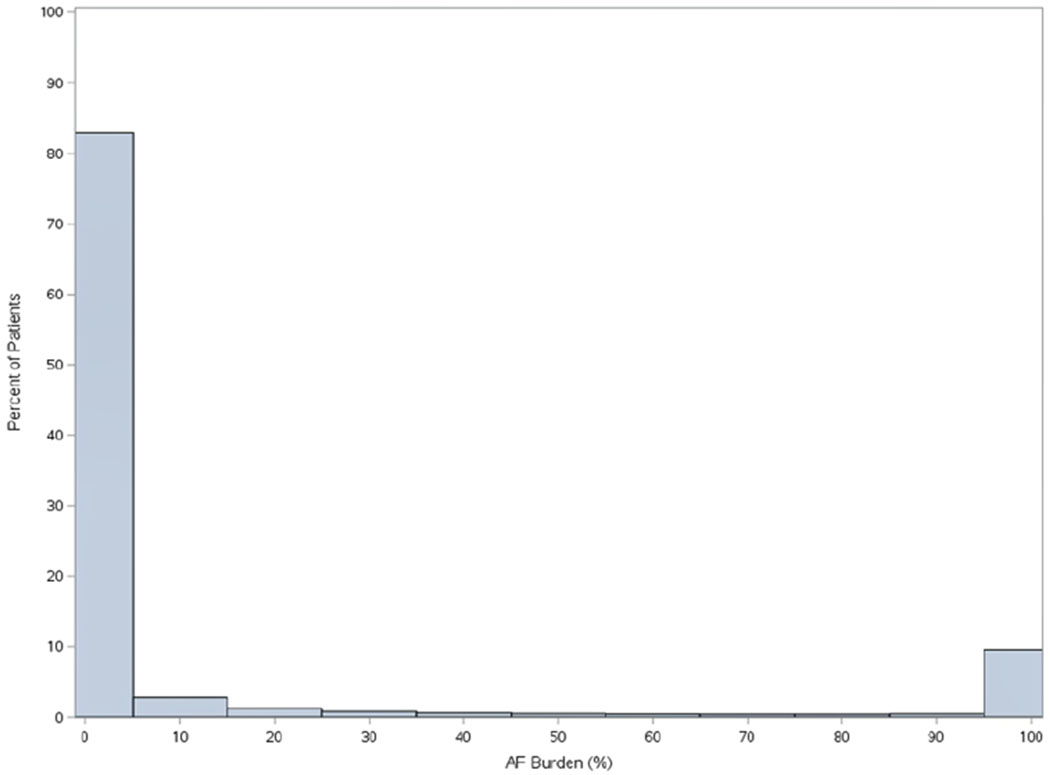
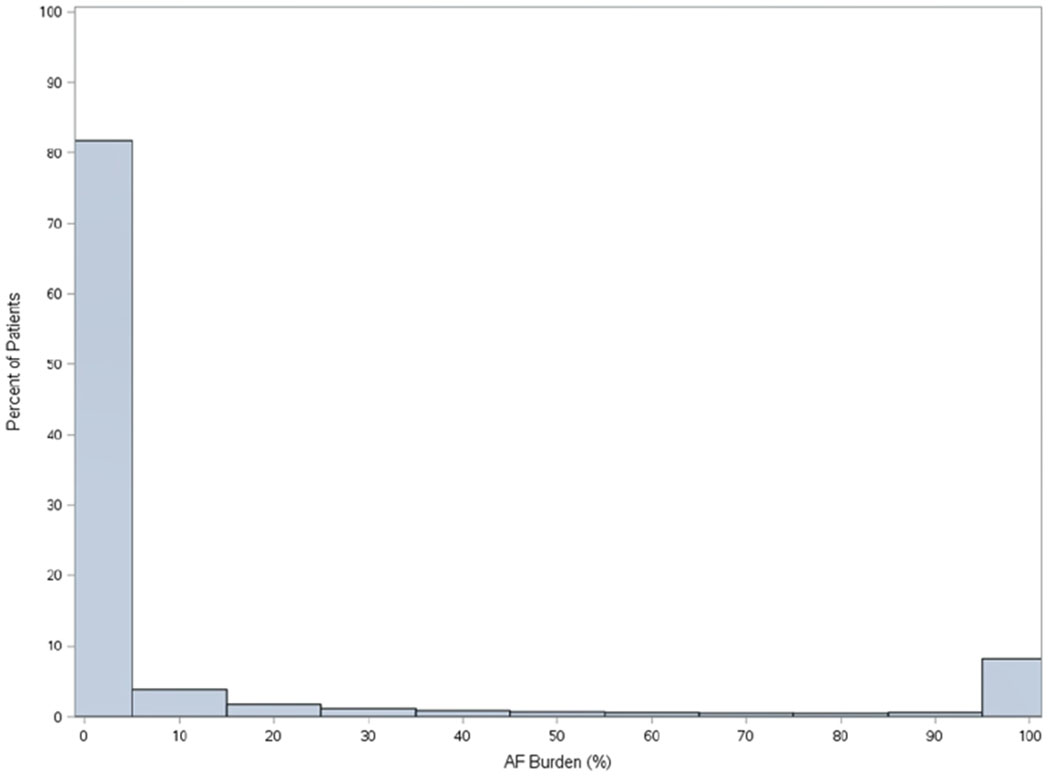
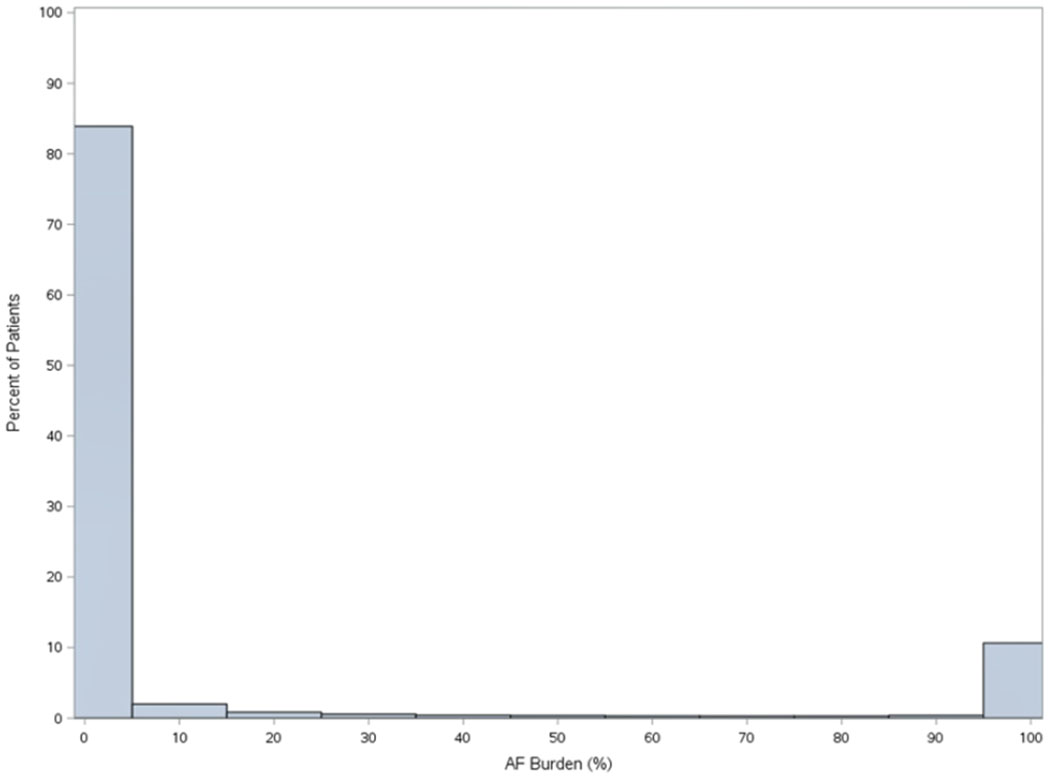
Distribution of AF burden in among patients with non-permanent AF undergoing CIED implant, overall (n=3,9710, Panel A), among patients with pacemakers (n=20,156, Panel B), and among patients with implantable cardioverter defibrillators (n=19,554, Panel C).
A. Overall Distribution of AF Burden (N=3,9710).
B. Overall Distribution of AF Burden in patients with pacemaker (N=20,156)
C. Overall Distribution of AF Burden in Patients with implantable cardioverter-defibrillators (N=19,554)
Discussion
Up to this point both clinicians and researchers alike have generally treated AF burden, a continuous measure of overall time in AF, as a biomarker that is well distributed from 0% to 100% – that is, we expect find relatively similar proportions of patients with AF burdens across this spectrum. However, our data demonstrate that the vast majority of patients either experience AF during a small proportion of the time, or are in AF nearly constantly (i.e., persistent AF). Across 3 different broad populations of patients with continuous electrocardiographic monitoring, including patients with and without a history of AF, and patients with and without CIEDs, we found similar distributions of AF burden. Burden of AF, expressed as a percent of overall monitored time, is highly skewed towards the extremes. These findings have significant implications for both clinical care of AF patients, as well as clinical research in AF.
Clinical Implications
While there are exhaustive descriptions of AF propagation in situ, AF progression within cohorts, and incidence/prevalence at the population level, there are almost no descriptions of AF burden at the population level. The observed AF distributions in these diverse cohorts run contrary to general perception of frequency of AF arrhythmia overtime. Clinicians intuit that patients start initially with low AF burden and then progress to high AF burden, with significant time in between. Yet, this relatively ‘linear’ progression appears to be more the exception than the rule – very few patients in our 3 cohorts, across several phenotypes, had AF burdens between the extremes. While patients may transition between low and high burden, it seems they do not spend a long time in transition. Clinically, this may have significant implications for rhythm control decisions in the context of contemporary research8 – patients with a very low burden may no longer be perceived as a ‘long way from persistent’ and conversely patients with near continuous AF burden may not have such ‘advanced’ disease as perceived. To the extent that AF burden has been used as a surrogate for chronicity of AF, it may be less useful given these data.
These findings lay the foundation for a better understanding of the progression of AF disease. Prior reports have been published regarding progression of AF type from paroxysmal to persistent subtypes.9 However, that approach is a rudimentary assessment of change in disease state and it may be more informative to understand changes in AF burden over time, and rates of progression of burden from very low to intermediate to very high. In our ORBIT-AF II cohort stratified by clinician-defined AF type (paroxysmal, persistent), we found substantial proportions of paroxysmal patients with very high burden and many patients with persistent AF but with much lower percent burden. There may be several explanations for these differences, but the possibility of dynamic burden and change in ‘type’ should be considered (both progression and/or regression with or without intervention).10 The dynamic nature of other cardiovascular markers, such as left ventricular ejection fraction, is now well-described.11 Understanding which patients are at risk for such changes, and at what rate, could have a profound impact on management, including approaches to both stroke prevention and rhythm/symptom control.
Research Implications
The present study can also shed light on prior research exploring the relationship between AF burden and clinical outcomes. While the crude labels of AF type (paroxysmal versus persistent), cannot capture all nuances that define AF phenotype, our findings suggests that dichotomizing AF burden may be reasonable in the context of analyzing the relationship between AF type and outcome. Nevertheless, there may remain many patients in clinical practice who span the spectrum of AF burden, even if they are relatively less common in comparison to those at the extremes. Furthermore, it is possible that those patients with an intermediate AF burden may experience significant impact to their lifestyle and quality of life – the arrhythmia is frequent enough to be bothersome, but not so frequent so as to develop tolerance (e.g., hedonic adaptation). In one study, patient daily physical activity decreased starting at 500 minutes of AF daily, and plummeted at 1000 minutes.12 As a consequence, such patients could consume an out-sized share of healthcare resources and clinician attention. Lastly, it is unlikely that the entire AF phenotype can be captured by either a single number or a broad category. More granular data may help shed light on important differences among groups across this AF burden spectrum – for example, AF burden combined with left atrial size and/or comorbidity burden likely yield better phenotypic and prognostic information compared with any single measure.13
More complete AF phenotyping could include lifestyle history (e.g., extreme exercise or alcohol use), biomarkers, cardiac imaging characteristics, hemodynamics, symptom status and patient reported outcomes, and other comorbidities. Understanding the relationships among these other characteristics, and the AF burden profile, is vital to personalizing our approach to AF treatment. Cluster-type analyses have started to get at these questions, but there remains much to be learned.14
Lastly, this skew of AF burden to the extremes poses analytic challenges to research into these relationships. Analyses that attempt to understand an ‘exposure-response’ relationship between ‘amount’ of AF and outcomes are inherently handicapped by this distribution, particularly for smaller datasets than the ones we used – fewer data-points between the extremes make it difficult to assess a potential ‘graded’ relationship, and even more difficult to clinically-interpret such analyses that often require significant transformation. Nevertheless, some of these obstacles can be overcome through sufficient sample size.15 Moving forward, clinical researchers treating AF burden as a continuous exposure need to be aware of these inherent characteristics.
Limitations
The three cohorts presented herein are from different populations and locales, with unique potential for selection biases in each. This represents perhaps the most challenging limitation for the 2 cohorts of ambulatory monitoring (ORBIT-AF II and UHealth), as patients with intermediate AF burden may not have been tested. However, a similar distribution was observed in patients with uniformly-continuous monitoring from implanted cardiac devices. Furthermore, the distributions across all 3 different cohorts, with noted differences in age, sex, race, and monitoring approach, highlight the consistency in different samples. It is also important to note that AF burden measurements from different automated algorithms may lead to minor differences in burden assessments, and conclusions are limited from the cohorts with significantly shorter monitoring duration (ORBIT-AF II and UHealth). There was no manual review of data for AF burden calculation and there may be some variability among algorithms. However, we believe this represents a strength, as the distribution of burden again appears consistent across patients, devices, and monitoring technologies and durations. Lastly, characteristics of substrate (e.g., left atrial size, left ventricular ejection fraction) and interval interventions, such as catheter ablation, are not available and could impact interpretation of the distribution, particularly across AF types in ORBIT-AF II.
Conclusions
These data from several diverse cohorts, including patients with and without a diagnosis of AF, demonstrate that the distribution of AF burden is heavily skewed towards the extremes. Contrary to widely-held perceptions, relatively few patients have an AF burden between 10 and 90% – the majority of patients have either very little or nearly-continuous AF. While there is ongoing emphasis on overall arrhythmia burden as a more granular outcome measure among AF patients, these data suggest classic categorization of paroxysmal or persistent could maintain most of the discriminatory value of AF burden from an analytic standpoint. Further data are needed to better-define the relationship between burden or density of arrhythmia and clinical outcomes, as well as the potential dynamic nature of AF burden.
Disclosure Information
Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K23HL143156 (to BAS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. BAS reports research support from Abbott, Boston Scientific, and Janssen; and consulting to Janssen, AltaThera, Merit Medical, Bayer, and Crowley Fleck, LLP; and speaking for NACCME (funded by Sanofi). TJB receives grants from Boston Scientific, AltaThera, and Boehringer Ingelheim, JPP receives grants for clinical research from Abbott, American Heart Association, Association for the Advancement of Medical Instrumentation, Bayer, Boston Scientific, and Philips and serves as a consultant to Abbott, Abbvie, Altathera, ARCA Biopharma, Biotronik, Boston Scientific, LivaNova, Medtronic, Milestone, Myokardia, ElectroPhysiology Frontiers, Pfizer, Sanofi, Philips, and Up-to-Date.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr., et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Journal of the American College of Cardiology 2014;64(21):e1–76. [DOI] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr., et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019;74(1):104–132. [DOI] [PubMed] [Google Scholar]
- 3.Chiang CE, Naditch-Brule L, Murin J, Goethals M, Inoue H, O’Neill J, et al. Distribution and risk profile of paroxysmal, persistent, and permanent atrial fibrillation in routine clinical practice: insight from the real-life global survey evaluating patients with atrial fibrillation international registry. Circ Arrhythm Electrophysiol 2012;5(4):632–9. [DOI] [PubMed] [Google Scholar]
- 4.Jansson V, Bergfeldt L, Schwieler J, Kennebäck G, Rubulis A, Jensen SM, et al. Atrial fibrillation burden, episode duration and frequency in relation to quality of life in patients with implantable cardiac monitor. Int J Cardiol Heart Vasc 2021;34:100791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samuel M, Khairy P, Champagne J, Deyell MW, Macle L, Leong-Sit P, et al. Association of Atrial Fibrillation Burden With Health-Related Quality of Life After Atrial Fibrillation Ablation: Substudy of the Cryoballoon vs Contact-Force Atrial Fibrillation Ablation (CIRCA-DOSE) Randomized Clinical Trial. JAMA cardiology 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinberg BA, Blanco RG, Ollis D, Kim S, Holmes DN, Kowey PR, et al. Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II: rationale and design of the ORBIT-AF II registry. Am Heart J 2014;168(2):160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinberg BA, Turner J, Lyons A, Biber J, Chelu MG, Fang JC, et al. Systematic collection of patient-reported outcomes in atrial fibrillation: feasibility and initial results of the Utah mEVAL AF programme. Europace 2020;22(3):368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N Engl J Med 2020;383(14):1305–1316. [DOI] [PubMed] [Google Scholar]
- 9.Holmqvist F, Kim S, Steinberg BA, Reiffel JA, Mahaffey KW, Gersh BJ, et al. Heart rate is associated with progression of atrial fibrillation, independent of rhythm. Heart 2015;101(11):894–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De With RR, Erküner Ö, Rienstra M, Nguyen BO, Körver FWJ, Linz D, et al. Temporal patterns and short-term progression of paroxysmal atrial fibrillation: data from RACE V. Europace 2020;22(8):1162–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savarese G, Vedin O, D’Amario D, Uijl A, Dahlström U, Rosano G, et al. Prevalence and Prognostic Implications of Longitudinal Ejection Fraction Change in Heart Failure. JACC Heart failure 2019;7(4):306–317. [DOI] [PubMed] [Google Scholar]
- 12.Proietti R, Birnie D, Ziegler PD, Wells GA, Verma A. Postablation Atrial Fibrillation Burden and Patient Activity Level: Insights From the DISCERN AF Study. J Am Heart Assoc 2018;7(23):e010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vitolo M, Proietti M, Shantsila A, Boriani G, Lip GYH. Clinical Phenotype Classification of Atrial Fibrillation Patients Using Cluster Analysis and Associations with Trial-Adjudicated Outcomes. Biomedicines 2021;9(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inohara T, Shrader P, Pieper K, Blanco RG, Thomas L, Singer DE, et al. Atrial Fibrillation Clinical Phenotypes: A Cluster Analysis. In: Circulation; 2017. p. Suppl. [Google Scholar]
- 15.Steinberg BA, Li Z, O’Brien EC, Pritchard J, Chew DS, Bunch TJ, et al. Atrial fibrillation burden and heart failure: Data from 39,710 individuals with cardiac implanted electronic devices. Heart Rhythm 2021;18(5):709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]


