Abstract
Background and Objectives:
Watch-and-wait is variably adopted by surgeons and the impact of this on outcomes is unknown. We compare the disease-free survival and organ preservation rates of locally advanced rectal cancer patients treated by expert colorectal surgeons at a comprehensive cancer center.
Methods:
This study included retrospective data on patients diagnosed with stage II/III rectal adenocarcinoma from January 2013 to June 2017 who initiated neoadjuvant therapy (either with chemoradiation, chemotherapy, or a combination of both) and were treated by an expert colorectal surgeon.
Results:
444 locally advanced rectal cancer patients managed by 5 surgeons were included. Tumor distance from the anal verge, type of neoadjuvant therapy, and organ preservation rates varied by treating surgeon. There was no difference in disease-free survival after stratifying by the treating surgeon (p=0.2). On multivariable analysis, neither the type of neoadjuvant therapy nor the treating surgeon was associated with disease-free survival.
Conclusions:
While neoadjuvant therapy type and organ preservation rates varied among surgeons, there were no meaningful differences in disease-free survival. These data suggest that amongst expert colorectal surgeons, differing thresholds for selecting patients for watch-and-wait do not affect survival.
Keywords: Rectal cancer, watch-and-wait, nonoperative management, survival, organ preservation
Introduction:
As neoadjuvant therapy has become the standard of care in treating locally advanced rectal cancer (LARC), various neoadjuvant therapy regimens have been developed in an attempt to maximize tumor response and improve survival.1–7 Complete tumor response after neoadjuvant therapy is clinically important as it can prognosticate survival and determine potential candidates fit for watch-and-wait (WW) approaches. The goal of WW is organ preservation, which results in a superior quality of life and limits morbidity associated with total mesorectal excision (TME).8–10 Also, WW has been shown to be oncologically safe. Several studies evaluating patients who achieved a complete clinical response (cCR) and were followed nonoperatively had similar survival outcomes compared to those with a pathological complete response (pCR) that underwent total mesorectal excision (TME).11–13 While the rates of local tumor regrowth during WW are around 25%, the majority of these cases can be safely salvaged.8,12,14–16 These results have driven the movement to adopt WW protocols to nonoperatively manage patients with a good clinical response after neoadjuvant therapy.14,17
Despite the plethora of data supporting the safety and efficacy of WW, this approach has yet to be widely adopted.18 One of the barriers is the need for experienced physicians to accurately identify response and then evaluate these patients long-term.19 The diverse neoadjuvant treatment modalities can result in different spectrums of response rates, which may inherently limit the proportion of patients eligible for WW.4,20 Another challenge is that the assessment of clinical response is currently qualitative and subject to user-dependent accuracy.21–24 WW protocols published by various leading groups are quite heterogeneous which make it difficult for practitioners to decide on the optimal protocol.8,11,12,25–27 Most importantly, as the majority of studies on WW are retrospective in nature, there is a concern for patient selection bias, and a need for prospective data to further elucidate if patients managed with WW versus TME truly have non-inferior long term survival outcomes.2,11,12,28,29 For the reasons above, the implementation of WW is quite heterogeneous in the clinical setting.
In this study, we explored whether surgeon variability in the adoption of WW impacts patient outcomes. We evaluated the utilization rates of WW amongst expert colorectal surgeons at a high-volume comprehensive cancer center and investigated its association with disease-free survival and organ preservation rates. Our hypothesis was that variations in practice of WW between the colorectal surgeons would not impact disease free survival; however, organ preservation rates would be higher for colorectal surgeons that placed more patients into the WW protocol.
Materials and Methods:
Patients
After approval by the institutional review board at Memorial Sloan Kettering Cancer Center (MSK), consecutive patients diagnosed with rectal adenocarcinoma from January 1, 2013 to June 30, 2017 treated at MSK were retrospectively collected. All these patients had American Joint Committee on Cancer (AJCC) clinical stage II (T3–4, N0) or III (any T, N1–2) rectal adenocarcinoma with a distal tumor border within 12 cm of the anal verge.30 We excluded patients who did not receive neoadjuvant therapy, patients who started neoadjuvant therapy prior to being evaluated by the treating surgeon, patients who underwent pelvic exenteration, and patients with synchronous tumors. As the purpose of this study was to study the variation in the management of LARC patients by surgeons in regards to WW and assess the oncologic outcome by disease-free survival, we excluded patients that were treated by surgeons who treated less than 10 patients during this study period in order to collect mature follow-up and outcome data. The five surgeons evaluated in this study were all clinical faculty in the colorectal surgery division at MSK who maintained active practices throughout the study period (Figure S1A, S1B). These surgeons shared the same resources at our institution and were considered to be experts in their field.
Treatment and response assessment
Patients received either chemoradiation, systemic chemotherapy, or the combination of both as total neoadjuvant therapy (TNT) depending upon recommendations from their treating physician. Chemoradiation was provided according to NCCN guidelines.31 Neoadjuvant systemic chemotherapy consisted of mFOLFOX for 8 cycles or CAPOX for 5 cycles.20 TNT is commonly utilized at MSK, and the regimen of neoadjuvant systemic chemotherapy given either before or after chemoradiation has been previously published.20 While not all LARC patients underwent TNT (due to comorbidities and preferences), TNT was the standard of care at MSK during the study period (Figure S1C). TNT was utilized to increase compliance to chemotherapy, treat possible micro-metastases early, and increase the incidence of complete response.20
After the completion of neoadjuvant therapy, tumor regression was assessed by digital rectal exam, endoscopic evaluation, and MRI.12,17,25,29 We took the treating surgeon’s first endoscopic examination date after the completion of neoadjuvant therapy as the response assessment date. The decision to pursue WW protocol or TME was typically based on the colorectal surgeon’s recommendation along with the patient’s preference. Some challenging cases may have been discussed within our multidisciplinary tumor board to reach a joint consensus, but in general, the workflow was dependent on the provider. The criteria used for the assessment of clinical response at MSK has been standardized.32 Patients with an incomplete response were recommended to undergo TME. Patients with good or complete tumor regression were eligible for organ preservation based on the discretion of their surgeon. Due to the heterogenous practice of WW and its evolution over the study period, this assessment was not always available. We defined patients following WW as those that did not undergo TME within the 3 months following reassessment. Patients following the WW protocol subsequently underwent surveillance as previously described.8,29,32 For those who underwent immediate TME, we defined pathological complete response (pCR) as the absence of tumor cells in the surgical specimen as previously described.33,34
Outcomes:
Organ preservation was defined as rectum preservation at the end of follow-up without TME. Patients who underwent a local excision for either diagnostic or therapeutic purposes were considered to have organ preservation. Survival was measured from the diagnosis date. Disease-free survival included local recurrence (defined as pelvic recurrence after TME), non-salvageable regrowth in WW patients (defined as tumor regrowth that was unable to be removed with negative margins), distant metastasis, or death as events.
Statistics
Continuous variables were compared using the Kruskal-Wallis test. Categorical variables were compared using Chi-square test. For the Kaplan-Meier survival analysis, log-rank test was used for statistical comparison. Univariable and multivariable Cox regression analysis were performed for disease-free survival. Baseline characteristics that were significantly different (Table 1) and the patient’s age were entered as variables in the model. P-values of <0.05 were considered to be statistically significant.
Table 1:
Demographic and treatment information of patients by treating surgeon
| S1 (N=126) | S2 (N=92) | S3 (N=30) | S4 (N=79) | S5 (N=117) | p-value | |
|---|---|---|---|---|---|---|
| Age (yrs) | 58.5 (13.4) | 55.3 (12.6) | 55.7 (11.5) | 56.3 (13.0) | 53.3 (13.3) | 0.072 |
| Male sex | 75 (60%) | 55 (60%) | 15 (50%) | 42 (53%) | 68 (58%) | 0.8 |
| Tumor distance from anal verge (cm) | 6.4 (2.8) | 6.8 (2.4) | 6.8 (3.4) | 7.4 (2.9) | 7.4 (3.2) | 0.0072 |
| cT | ||||||
| 1 or 2 | 14 (11%) | 7 (8%) | 1 (3%) | 5 (6%) | 4 (3%) | 0.088 |
| 3 | 99 (79%) | 75 (82%) | 25 (83%) | 73 (92%) | 99 (85%) | |
| 4 | 13 (10%) | 9 (10%) | 4 (13%) | 1 (1%) | 13 (11%) | |
| X | 0 | 1 | 0 | 0 | 1 | |
| cN | ||||||
| Neg | 25 (20%) | 24 (26%) | 5 (17%) | 25 (32%) | 28 (24%) | 0.3 |
| Pos | 101 (80%) | 68 (74%) | 25 (83%) | 54 (68%) | 89 (76%) | |
| Neoadjuvant Therapy (NAT) | ||||||
| Chemoradiation only | 14 (11%) | 17 (18%) | 0 (0%) | 4 (5%) | 13 (11%) | <0.0001 |
| TNT | 103 (82%) | 70 (76%) | 28 (93%) | 55 (70%) | 78 (67%) | |
| Chemotherapy only | 9 (7%) | 5 (5%) | 2 (7%) | 20 (25%) | 26 (22%) |
Data represents n (%) or mean (stdev). P-values provided by Kruskal-Wallis tests or Chi-square tests.
TNT= total neoadjuvant therapy
Results:
Patient Demographics
A total of 444 LARC patients met our study criteria (Figure 1). These patients were managed by 5 different surgeons (surgeons 1–5) at MSK. The patient demographic and treatment information were categorized according to the treating surgeon (Table 1). Most patients were male, and there was no difference in sex when grouping patients by the treating surgeon. The mean age at diagnosis ranged from 53.3 to 58.5 years, but the differences were not statistically different between groups (p=0.072). The mean tumor distance from the anal verge ranged from 6.4cm to 7.4cm among the groups (p=0.0072). There appeared to be no difference in tumor characteristics based on the cT and cN classification (p=0.088 and p=0.3, respectively). Most patients had cT3 tumors or had positive nodes at diagnosis.
Figure 1.
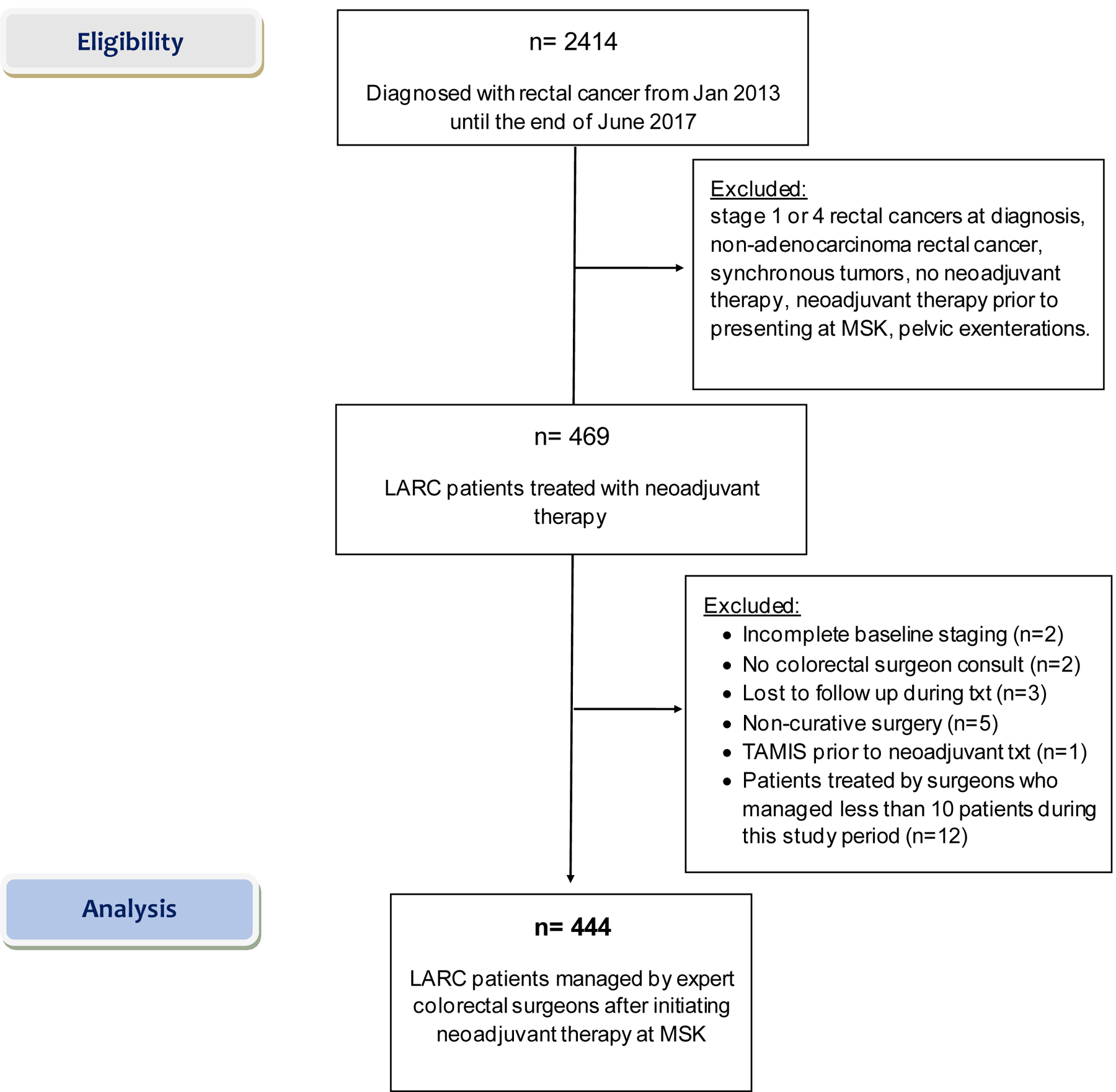
Consort diagram
Treatment
LARC patients underwent either chemoradiation, systemic chemotherapy only, or TNT. Interestingly, the distribution of patients treated by these therapies was different when stratifying the groups by the treating surgeon (p<0.0001). TNT was the most common treatment approach (range 67% to 93% of patients). Additionally, 0% to 18% of patients received chemoradiation and 5% to 25% of patients only received systemic chemotherapy. Despite the variation in the prescribed neoadjuvant treatment modality, all surgeons initially assessed for their patient’s tumor response at approximately 5–6 weeks after treatment.
The proportion of patients followed by WW compared to those who underwent immediate TME within 3 months from reassessment varied significantly among surgeons (Table 2, p<0.0001). Notably, surgeons S2 and S5 offered WW only to a small subset of their patients (11% and 27% of patients, respectively). On the contrary, surgeons S1, S3, and S4 managed nearly half of their patients with WW. To understand whether this difference was due to variability in the individual surgeon’s threshold to select candidates for WW, we examined the pathological complete response (pCR) rates of the patients who underwent immediate TME (Table 2). Surgeons S2 and S5, who offered WW less frequently, had the highest proportion of patients with pCR (19% each) that underwent immediate TME. In contrast, surgeon S3, who offered WW to the highest proportion of his patients (50%), had only 7% of patients with pCR. Surgeons S1 and S4 who also generously practiced WW had less patients with pCR compared to surgeons S2 and S5 (Table 2). While the comparisons were not statistically significant, these trends suggest that the variations in the proportion of WW patients in these groups may be related to the differences in the individual surgeon’s threshold to offer WW. Additionally, we found a trend towards higher usage of WW in the latter years of the study (Figure S1D), but the proportion of patients managed by each of the five surgeons remained relatively constant over the study period (Figure S1A, S1B). This indicates that the trends in WW over time is not driven by the practice pattern of one particular surgeon. In general, the threshold to offer WW decreased with more experience.
Table 2:
Operative management after reassessment
| S1 (N=124) | S2 (N=87) | S3 (N=30) | S4 (N=78) | S5 (N=115) | p-value | |
|---|---|---|---|---|---|---|
| Time to reassessment from end of NAT (weeks) | 6.4 (2.6) | 5.3 (2.1) | 6.0 (2.4) | 5.8 (2.7) | 5.7 (3.2) | 0.045 |
| Management decision after reassessment | ||||||
| WW* | 60 (48%) | 10 (11%) | 15 (50%) | 36 (46%) | 31 (27%) | <0.0001 |
| Immediate TME | 64 (52%) | 77 (89%) | 15 (50%) | 42 (54%) | 84 (73%) | |
| Pathological response of immediate TME patients | ||||||
| pCR | 10 (16%) | 15 (19%) | 1 (7%) | 5 (12%) | 16 (19%) | 0.6 |
| non-pCR | 54 (84%) | 62 (81%) | 14 (93%) | 37 (88%) | 68 (81%) | |
| Rectum preserved at last follow up | ||||||
| Organ preservation | 48 (39%) | 6 (7%) | 10 (33%) | 30 (38%) | 19 (17%) | <0.0001 |
Data represents n (%) or mean (stdev) of patients that had documented reassessments after neoadjuvant therapy. P-values provided by Kruskal-Wallis tests or Chi-square tests.
operative candidates who did not undergo total mesorectal excision (TME) within 3 months after reassessment were assumed to be in watch-and-wait (WW) protocol
Immediate TME: TME within 3 months from reassessment
pCR: pathological complete response
As another measure to assess the practice of WW, we compared the organ preservation rates (proportion of patients who preserved their rectum up to last follow-up date) by the treating surgeon. Patients that received definitive local excision (n=9) or brachytherapy (n=1) following neoadjuvant therapy were considered to have organ preservation. 4 patients who declined TME despite the persistent recommendation by the treating surgeon were not considered to have organ preservation as these patients did not follow the typical WW protocol. The organ preservation rates by the treating surgeons varied from 7–39% (p<0.0001) (Table 2). Surgeons S2 and S5 had the lowest organ preservation rates (7% and 17%, respectively). These patterns are consistent with the trends observed using TME at 3 months as the cut-off to assess the practice of WW by surgeon. These results collectively indicate the variable adoption of WW among surgeons at MSK.
Variables associated with Disease-Free Survival
To examine whether this variability in the treatment and adoption of WW would correlate with survival, we compared the disease-free survival of the patients stratified by the treating surgeon utilizing Kaplan-Meier estimates. A total of 440 patients were included in this analysis after excluding the 4 patients that declined curative TME. There was a total of 102 disease-free survival events in this group with a median follow-up of 4.1 years. We did not observe meaningful differences in disease-free survival upon stratifying the patients by the treating surgeon (p=0.2) (Figure 2). We performed a univariable analysis followed by a multivariable analysis to investigate this further after adjusting for potential confounding factors. In the univariable analysis, we explored possible associations with disease-free survival using the patient’s age group, tumor distance from the anal verge, neoadjuvant treatment regiment, and the treating surgeon as the independent variables. We found that the patient age group was associated with disease-free survival (age 51–60, HR=0.57, 95% CI 0.32–1.00; p=0.049) (Table 3). However, tumor distance from the anal verge, neoadjuvant treatment regimen, and the treating surgeon were not associated with disease-free survival (Table 3). On a multivariable analysis model using the same variables, we observed similar findings (Table 4). These results indicate that the variability in the management of LARC does not impact disease-free survival.
Figure 2. Disease-free survival of rectal cancer patients by treating surgeon.
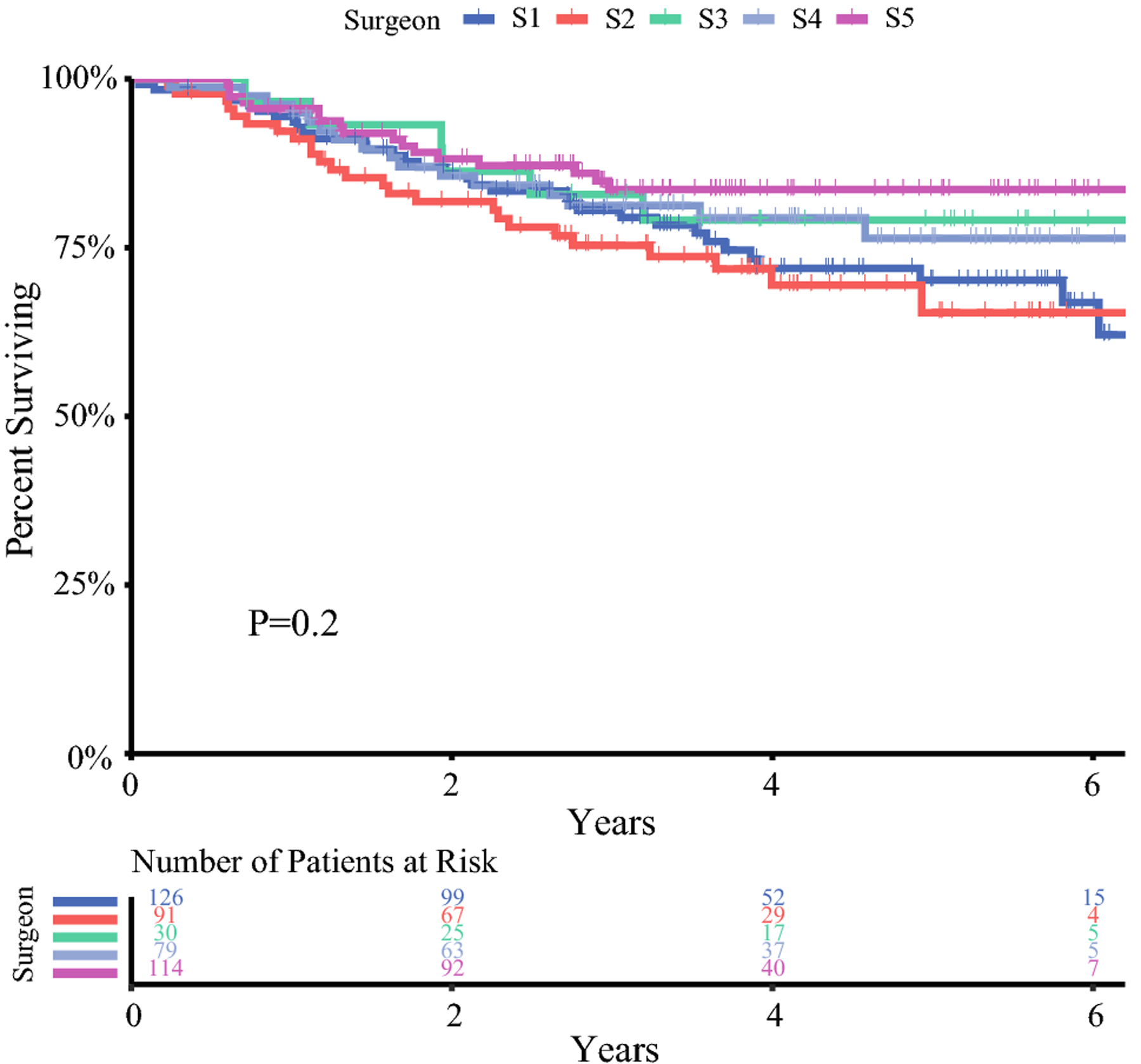
Disease free survival by treating surgeon
Kaplan-Meier curve of disease-free survival is shown for 440 patients treated by 5 expert colorectal surgeons (S1-S5). The number of patients at risk at each time point is shown below the curves.
Table 3:
Univariable associations with disease-free survival
| HR | 95% CI | p-value | |
|---|---|---|---|
| Age (yrs) | |||
| ≤50 | Ref | ||
| 51–60 | 0.57 | 0.32–1.00 | 0.049 |
| >60 | 1.07 | 0.69–1.66 | 0.8 |
| Tumor distance from anal verge (cm) | |||
| 8+ | 0.79 | 0.45–1.38 | 0.4 |
| 4.1–8 | 0.85 | 0.49–1.45 | 0.5 |
| ≤4 | Ref | ||
| Neoadjuvant Therapy | |||
| Chemoradiation only | Ref | ||
| TNT | 1.46 | 0.68–3.17 | 0.3 |
| Chemotherapy only | 0.99 | 0.38–2.60 | 0.9 |
| Surgeon | |||
| S1 | 1.29 | 0.71–2.34 | 0.4 |
| S2 | 1.48 | 0.78–2.78 | 0.2 |
| S3 | 1.19 | 0.51–2.78 | 0.7 |
| S4 | Ref | ||
| S5 | 0.74 | 0.38–1.48 | 0.4 |
HR= hazard ratio, CI= confidence interval, TNT= total neoadjuvant therapy.
p-values by Wald test
Table 4:
Associations with disease-free survival by multivariable cox regression model
| HR | 95% CI | p-value | |
|---|---|---|---|
| Age (yrs) | |||
| ≤50 | Ref | ||
| 51–60 | 0.53 | 0.30–0.94 | 0.031 |
| >60 | 0.99 | 0.64–1.56 | 0.9 |
| Tumor distance from anal verge (cm) | |||
| 8+ | 0.93 | 0.52, 1.64 | 0.8 |
| 4.1–8 | 0.89 | 0.52, 1.53 | 0.7 |
| ≤4 | Ref | ||
| Neoadjuvant Therapy | |||
| Chemoradiation only | Ref | ||
| TNT | 1.54 | 0.70–3.37 | 0.2 |
| Chemotherapy only | 1.10 | 0.40–2.97 | 0.9 |
| Surgeon | |||
| S1 | 1.22 | 0.66–2.23 | 0.5 |
| S2 | 1.41 | 0.74–2.68 | 0.3 |
| S3 | 1.05 | 0.44–2.51 | 0.9 |
| S4 | Ref | ||
| S5 | 0.73 | 0.36–1.47 | 0.9 |
HR= hazard ratio, CI= confidence interval, TNT= total neoadjuvant therapy.
p-values by Wald test
Discussion:
WW for patients with LARC who have a good response following neoadjuvant therapy remains controversial and has not been adopted widely. Within our institution, we found that the management of these patients is heterogenous. Patients underwent various neoadjuvant treatments, and the practice of WW varied depending upon the treating surgeon. Avid WW practitioners resulted in the highest proportion of patients with organ preservation, and the lowest rate of pCR. However, we did not observe differences in disease-free survival when stratifying the patients by the treating surgeon. Our study suggests that variable adoption of WW does not compromise patient survival; however, it may have other advantages, such as organ preservation.
Since the standardization of neoadjuvant chemoradiation, neoadjuvant therapy has evolved to optimize outcomes in LARC.17 Providing systemic chemotherapy along with chemoradiation in the neoadjuvant setting further has shown to improve response rates.3,20,35 The time at which response is assessed after neoadjuvant therapy is also associated with the response rate. Several studies have shown that lengthening the time to reassessment after chemoradiation is safe and associated with higher response rates.36–39 Optimizing the response rate after neoadjuvant therapy may increase the number of patients eligible for WW.
Along with the variability in response associated with neoadjuvant therapies, several other factors make WW complex. It has been shown that variability exists in clinically assessing response following neoadjuvant therapy. The surgeon assessment of response is currently qualitative with inaccuracies noted in intra-observer and inter-observer assessment of response, as well as in the accuracy of other diagnostic modalities that are utilized.21,23,40 Therefore, it is challenging to clinically identify complete responders. Moreover, the selection of candidates appropriate for WW is not clearly established. WW was initially introduced to patients with a cCR after neoadjuvant therapy.17 However, some practitioners have now extended WW to near-complete responders (patients who have a good response, but not all the features of a cCR) without compromising oncologic outcomes.41
Our study did not find differences in disease-free survival depending on surgeon practice preferences. However, we did find that experienced surgeons who more frequently offered WW demonstrated a trend towards lower pCR rates. These data suggest the potential to maximize neoadjuvant therapies for the individual patient based on stated goals of WW. These surgeons also had increased organ preservation rates which has potential positive implications on quality of life. Multiple studies have shown that patients who successfully preserved their rectums through WW had improved urinary and bowel function compared to those who underwent sphincter preserving surgery.9,10 Furthermore, a study by Meyer et al found that the patients comfortability was not undermined by delaying surgery under a WW protocol.42
Our retrospective study has several limitations. We used a 3-month cut off from the first surgeon assessment as a surrogate because the documentation of WW was not standardized. Furthermore, while our institution had established guidelines to assess clinical response, the elements of the criteria are subjective. Apart from the potential differences in the surgeon’s interpretation of response and comfortability in offering WW, the patient’s preference could have also contributed to this variation. Adjuvant therapy regimens were incomplete, and thus not analyzed. While there is controversy regarding the role of adjuvant therapy relative to survival for rectal cancer patients, this treatment may have had an impact on survival.43 Due to limited numbers, we chose to categorize patients who underwent local excisions or brachytherapy as candidates for organ preservation; however, the functional outcomes of these patients may actually be worse than the patients who completely avoided surgery.27,44
Conclusion:
The heterogeneous implementation of WW by surgeons at a tertiary cancer center was not associated with disease-free survival differences in locally advanced rectal cancer patients. Wide adoption of WW may result in a greater proportion of patients achieving organ preservation which has positive implications on the patient’s quality of life.
Supplementary Material
Figure S1. Trends of patient volume and treatment over time
S1A) Number of patients treated by each surgeon over the study period. S1B) Proportion of patients treated by each surgeon over the study period. S1C) Proportion of neoadjuvant therapy regimens that were prescribed over the study period. S1D) Trends of patients entered in Watch and Wait versus surgery over the study period.
Synopsis for Table of Contents:
Watch-and-wait is variably adopted by surgeons and the impact of this on outcomes is unknown. While neoadjuvant therapy type and organ preservation rates varied among surgeons in this study, there were no meaningful differences in disease-free survival.
Disclosures and Funding Sources:
Dr. Kim was supported in part by NIH Grant T32CA009501.
Dr. Smith has received travel support from Intuitive Surgical Inc. for fellow education (2015) and has served as a clinical advisor for Guardant Health, Inc (2019).
Dr. Garcia-Aguilar has received honorarium for being a consultant with the following: Medtronics, Ethicon J&J, Da Vinci Intuitive Surgical.
Data Availability Statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request
References
- 1.Appelt AL, Ploen J, Harling H, et al. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol. 2015;16(8):919–927. [DOI] [PubMed] [Google Scholar]
- 2.Habr-Gama A, Sao Juliao GP, Vailati BB, et al. Organ Preservation in cT2N0 Rectal Cancer After Neoadjuvant Chemoradiation Therapy: The Impact of Radiation Therapy Dose-escalation and Consolidation Chemotherapy. Ann Surg. 2019;269(1):102–107. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Aguilar J, Chow OS, Smith DD, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16(8):957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahadoer RR, Dijkstra EA, van Etten B, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):29–42. [DOI] [PubMed] [Google Scholar]
- 5.George TJ, Yothers G, Hong TS, et al. NRG-GI002: A phase II clinical trial platform using total neoadjuvant therapy (TNT) in locally advanced rectal cancer (LARC)—First experimental arm (EA) initial results. Journal of Clinical Oncology. 2019;37(15_suppl):3505–3505. [Google Scholar]
- 6.Conroy T, Lamfichekh N, Etienne P-L, et al. Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiation in patients with locally advanced rectal cancer: Final results of PRODIGE 23 phase III trial, a UNICANCER GI trial. Journal of Clinical Oncology. 2020;38(15_suppl):4007–4007. [Google Scholar]
- 7.Garcia-Aguilar J, Patil S, Kim JK, et al. Preliminary results of the organ preservation of rectal adenocarcinoma (OPRA) trial. Journal of Clinical Oncology. 2020;38(15_suppl):4008–4008. [Google Scholar]
- 8.Maas M, Beets-Tan RG, Lambregts DM, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. 2011;29(35):4633–4640. [DOI] [PubMed] [Google Scholar]
- 9.Quezada-Diaz FF, Smith JJ, Jimenez-Rodriguez RM, et al. Patient-Reported Bowel Function in Patients With Rectal Cancer Managed by a Watch-and-Wait Strategy After Neoadjuvant Therapy: A Case-Control Study. Dis Colon Rectum. 2020;63(7):897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hupkens BJP, Martens MH, Stoot JH, et al. Quality of Life in Rectal Cancer Patients After Chemoradiation: Watch-and-Wait Policy Versus Standard Resection - A Matched-Controlled Study. Dis Colon Rectum. 2017;60(10):1032–1040. [DOI] [PubMed] [Google Scholar]
- 11.Renehan AG, Malcomson L, Emsley R, et al. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol. 2016;17(2):174–183. [DOI] [PubMed] [Google Scholar]
- 12.Smith JD, Ruby JA, Goodman KA, et al. Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann Surg. 2012;256(6):965–972. [DOI] [PubMed] [Google Scholar]
- 13.Dossa F, Chesney TR, Acuna SA, Baxter NN. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2(7):501–513. [DOI] [PubMed] [Google Scholar]
- 14.van der Valk MJM, Hilling DE, Bastiaannet E, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018;391(10139):2537–2545. [DOI] [PubMed] [Google Scholar]
- 15.Habr-Gama A, Gama-Rodrigues J, Sao Juliao GP, et al. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys. 2014;88(4):822–828. [DOI] [PubMed] [Google Scholar]
- 16.Dattani M, Heald RJ, Goussous G, et al. Oncological and Survival Outcomes in Watch and Wait Patients With a Clinical Complete Response After Neoadjuvant Chemoradiotherapy for Rectal Cancer: A Systematic Review and Pooled Analysis. Ann Surg. 2018;268(6):955–967. [DOI] [PubMed] [Google Scholar]
- 17.Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240(4):711–717; discussion 717–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caycedo-Marulanda A, Patel SV, Verschoor CP, et al. A Snapshot of the International Views of the Treatment of Rectal Cancer Patients, a Multi-regional Survey: International Tendencies in Rectal Cancer. World J Surg. 2021;45(1):302–312. [DOI] [PubMed] [Google Scholar]
- 19.Smith JJ, Paty PB, Garcia-Aguilar J. Watch and Wait in Rectal Cancer or More Wait and See? JAMA Surg. 2020;155(7):657–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cercek A, Roxburgh CSD, Strombom P, et al. Adoption of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer. JAMA Oncol. 2018;4(6):e180071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Sande ME, Maas M, Melenhorst J, Breukink SO, van Leerdam ME, Beets GL. Predictive Value of Endoscopic Features for a Complete Response After Chemoradiotherapy for Rectal Cancer. Ann Surg. 2019. [DOI] [PubMed] [Google Scholar]
- 22.Kawai K, Ishihara S, Nozawa H, et al. Prediction of Pathological Complete Response Using Endoscopic Findings and Outcomes of Patients Who Underwent Watchful Waiting After Chemoradiotherapy for Rectal Cancer. Dis Colon Rectum. 2017;60(4):368–375. [DOI] [PubMed] [Google Scholar]
- 23.Maas M, Lambregts DM, Nelemans PJ, et al. Assessment of Clinical Complete Response After Chemoradiation for Rectal Cancer with Digital Rectal Examination, Endoscopy, and MRI: Selection for Organ-Saving Treatment. Ann Surg Oncol. 2015;22(12):3873–3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Paardt MP, Zagers MB, Beets-Tan RG, Stoker J, Bipat S. Patients who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic MR imaging: a systematic review and meta-analysis. Radiology. 2013;269(1):101–112. [DOI] [PubMed] [Google Scholar]
- 25.Beets-Tan RGH, Lambregts DMJ, Maas M, et al. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2018;28(4):1465–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perez RO, Habr-Gama A, Gama-Rodrigues J, et al. Accuracy of positron emission tomography/computed tomography and clinical assessment in the detection of complete rectal tumor regression after neoadjuvant chemoradiation: long-term results of a prospective trial (National Clinical Trial 00254683). Cancer. 2012;118(14):3501–3511. [DOI] [PubMed] [Google Scholar]
- 27.Martens MH, Maas M, Heijnen LA, et al. Long-term Outcome of an Organ Preservation Program After Neoadjuvant Treatment for Rectal Cancer. J Natl Cancer Inst. 2016;108(12). [DOI] [PubMed] [Google Scholar]
- 28.Lopez-Campos F, Martin-Martin M, Fornell-Perez R, et al. Watch and wait approach in rectal cancer: Current controversies and future directions. World J Gastroenterol. 2020;26(29):4218–4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Habr-Gama A, Perez RO, Wynn G, Marks J, Kessler H, Gama-Rodrigues J. Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: characterization of clinical and endoscopic findings for standardization. Dis Colon Rectum. 2010;53(12):1692–1698. [DOI] [PubMed] [Google Scholar]
- 30.Amin MB, American Joint Committee on Cancer., American Cancer Society. AJCC cancer staging manual. Eight edition / editor-in-chief, Amin, MD, FCAP Mahul B.; , Edge, MD, FACS Stephen B. and 16 others; Gress, RHIT, CTR Donna M. - Technical editor; Meyer, CAPM Laura R. - Managing editor. ed. Chicago IL: American Joint Committee on Cancer, Springer; 2017. [Google Scholar]
- 31.Benson AB, Venook AP, Al-Hawary MM, et al. NCCN Guidelines Insights: Rectal Cancer, Version 6.2020. J Natl Compr Canc Netw. 2020;18(7):806–815. [DOI] [PubMed] [Google Scholar]
- 32.Smith JJ, Chow OS, Gollub MJ, et al. Organ Preservation in Rectal Adenocarcinoma: a phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer. 2015;15:767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trakarnsanga A, Gonen M, Shia J, et al. Comparison of tumor regression grade systems for locally advanced rectal cancer after multimodality treatment. J Natl Cancer Inst. 2014;106(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Washington MK, Berlin J, Branton P, et al. Protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum. Arch Pathol Lab Med. 2009;133(10):1539–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Habr-Gama A, Perez RO, Sao Juliao GP, et al. Consolidation chemotherapy during neoadjuvant chemoradiation (CRT) for distal rectal cancer leads to sustained decrease in tumor metabolism when compared to standard CRT regimen. Radiat Oncol. 2016;11:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tulchinsky H, Shmueli E, Figer A, Klausner JM, Rabau M. An interval >7 weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer. Ann Surg Oncol. 2008;15(10):2661–2667. [DOI] [PubMed] [Google Scholar]
- 37.Moore HG, Gittleman AE, Minsky BD, et al. Rate of pathologic complete response with increased interval between preoperative combined modality therapy and rectal cancer resection. Dis Colon Rectum. 2004;47(3):279–286. [DOI] [PubMed] [Google Scholar]
- 38.Sloothaak DA, Geijsen DE, van Leersum NJ, et al. Optimal time interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg. 2013;100(7):933–939. [DOI] [PubMed] [Google Scholar]
- 39.Petrelli F, Sgroi G, Sarti E, Barni S. Increasing the Interval Between Neoadjuvant Chemoradiotherapy and Surgery in Rectal Cancer: A Meta-analysis of Published Studies. Ann Surg. 2016;263(3):458–464. [DOI] [PubMed] [Google Scholar]
- 40.Felder SI, Patil S, Kennedy E, Garcia-Aguilar J. Endoscopic Feature and Response Reproducibility in Tumor Assessment after Neoadjuvant Therapy for Rectal Adenocarcinoma. Ann Surg Oncol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hupkens BJP, Maas M, Martens MH, et al. Organ Preservation in Rectal Cancer After Chemoradiation: Should We Extend the Observation Period in Patients with a Clinical Near-Complete Response? Ann Surg Oncol. 2018;25(1):197–203. [DOI] [PubMed] [Google Scholar]
- 42.Meyer VM, Meuzelaar RR, Schoenaker Y, et al. Delayed Surgery after Neoadjuvant Treatment for Rectal Cancer Does Not Lead to Impaired Quality of Life, Worry for Cancer, or Regret. Cancers (Basel). 2021;13(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spiegel DY, Boyer MJ, Hong JC, et al. Survival Advantage With Adjuvant Chemotherapy for Locoregionally Advanced Rectal Cancer: A Veterans Health Administration Analysis. J Natl Compr Canc Netw. 2020;18(1):52–58. [DOI] [PubMed] [Google Scholar]
- 44.Jones HJS, Al-Najami I, Cunningham C. Quality of life after rectal-preserving treatment of rectal cancer. Eur J Surg Oncol. 2020;46(11):2050–2056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Trends of patient volume and treatment over time
S1A) Number of patients treated by each surgeon over the study period. S1B) Proportion of patients treated by each surgeon over the study period. S1C) Proportion of neoadjuvant therapy regimens that were prescribed over the study period. S1D) Trends of patients entered in Watch and Wait versus surgery over the study period.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request


