Abstract
Background
The accurate assessment of total body and regional body circumferences, volumes, and compositions are critical to monitor physical activity and dietary interventions, as well as accurate disease classifications including obesity, metabolic syndrome, sarcopenia, and lymphedema. We assessed body composition and anthropometry estimates provided by a commercial 3-dimensional optical (3DO) imaging system compared to criterion measures.
Methods
Participants of the Shape Up! Adults study were recruited for similar sized stratifications by sex, age (18 – 40, 40-60, >60 years), BMI (under, normal, overweight, obese), and across five ethnicities (non-Hispanic [NH] Black, NH White, Hispanic, Asian, Native Hawaiian/Pacific Islander). All participants received manual anthropometry assessments, duplicate whole-body 3DO (Styku S100), and dual-energy X-ray absorptiometry (DXA) scans. 3DO estimates provided by the manufacturer for anthropometry and body composition were compared to the criterion measures using concordance correlation coefficient (CCC) and Bland-Altman analysis. Test-retest precision was assessed by root mean square error (RMSE) and coefficient of variation.
Results
A total of 188 (102 female) participants were included. The overall fat free mass (FFM) as measured by DXA (54.1 ± 15.2 kg) and 3DO (55.3 ± 15.0 kg) showed a small mean difference of 1.2 ± 3.4 kg (95% limits of agreement −7.0 to +5.6) and the CCC was 0.97 (95% CI: 0.96-0.98). The CCC for FM was 0.95 (95% CI: 0.94-0.97) and the mean difference of 1.3 ± 3.4 kg (95% CI: −5.5 to +8.1) reflected the difference in FFM measures. 3DO anthropometry and body composition measurements showed high test-retest precision for whole body volume (1.1 L), fat mass (0.41 kg), percent fat (0.60 %), arm and leg volumes, (0.11 and 0.21 L, respectively), and waist and hip circumferences (all < 0.60 cm). No group differences were observed when stratified by body mass index, sex, or race/ethnicity.
Conclusions
The anthropometric and body composition estimates provided by the 3DO scanner are precise and accurate to criterion methods if offsets are considered. This method offers a rapid, broadly available, and automated method of body composition assessment regardless of body size. Further studies are recommended to examine the relationship between measurements obtained by 3DO scans and metabolic health in healthy and clinical populations.
Keywords: 3-dimensional optical, digital anthropometry, DXA, fat free mass, body fat percent, validation
Introduction
Excess adiposity is associated with metabolic changes that significantly increase the risk of developing cardiovascular disease (CVD) and 13 types of cancer, with obesity being the cause of 20% of cancer cases and 13% of CVD mortality (1–3). The assessment of body composition serves as a key indicator of nutrition and functional status, where management of body compartments can aid in the prevention of modifiable disease risks including type 2 diabetes (T2D), cardiometabolic diseases including metabolic syndrome (MetS), malnutrition, sarcopenia, and cancer (2, 4–9). Further, assessments of regional body composition have been shown to be more predictive than whole body adiposity measures for a variety of diseases and conditions such as trunk fat for insulin resistance and dyslipidemia, trunk to leg volume ratio for diabetes and mortality, and appendicular lean mass index for sarcopenia (10–13). Regional body composition has, to date, been quantified using criterion imaging methods such as dual energy X-ray absorptiometry (DXA), magnetic resonance imaging, and computed tomography; however, training, cost and portability limit their utilization as a part of routine clinical screening for these conditions (14–17). Bioelectrical impedance, another candidate technology for nutritional assessment, may have limited accuracy when applied outside of the subject population for which it was derived (18). As such, the Global Leadership Initiative on Malnutrition recommends measures of anthropometry as alternative measures of risk assessment (9, 19–22). Because anthropometric measurements may not be culturally or socially acceptable and have lower reliability in overweight subjects, there is a need for the identification of cost-effective and accessible technologies capable of providing accurate and precise total and regional body composition estimates (9, 23, 24).
Digital anthropometry by three-dimensional optical (3DO) imaging systems has recently been shown to provide noninvasive and cost-effective anthropometry and body composition assessments (7, 25–29). These systems do not require human contact as does manual anthropometry (30). Multiple 3DO scanning systems capable of reporting body composition are marketed to the sports, fitness, and clothes fitting markets; the candidate 3DO system, for example, is available in over 1,000 locations across 30 countries. However, these measures have primarily been evaluated in young, healthy adult populations and not in populations representative of the US in terms of age, sex, race/ethnicity, and obesity status. The purpose of this study was to examine the accuracy and repeatability of total and regional body measures of anthropometry and composition on a commercially available 3DO body composition scanner. We compared this scanner to criterion measures of DXA body composition and manual anthropometry in a diverse population of adult men and women.
Methods
This analysis was a cross-sectional study of healthy adult volunteers as part of the ongoing Shape Up! Adults study (NIH R01 DK109008, clinicaltrials.gov ID NCT03637855). The Shape Up! Adults study aimed to recruit a diverse sample of 720 adults with equal stratifications by sex, age (18-40 y, 40-60 y, >60 y), ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, Asian, and native Hawaiian or Pacific Islander), body mass index (BMI in kg/m2; <18, 18-25, 25-30, >30), and geographic location (San Francisco, CA; Baton Rouge, LA; or Honolulu, HI). For this study, only the San Francisco and Honolulu samples were used since the manufacturer had access to the Baton Rouge data for previous studies. All participants were recruited between October 2016 and January 2020 using methods including web advertisements, posted flyers, and word of mouth. Of note, body composition assessments were performed using the same 3DO device at both sites as the equipment was transferred to the University of Hawaii in April 2018 following the change in University by the Co-PI (JAS). Participants completed a pre-evaluation screening questionnaire during the recruitment process to ensure good health in order to participate. Prospective participants were excluded if they could not stand for 2 min without aid, lie supine for 10 min without movement, had metal implants, or had significant body shape-altering procedures (e.g., liposuction, amputations, breast augmentation or reduction). Female participants were also excluded if pregnant or breastfeeding. Participants included in the dataset were scanned at the UCSF Clinical and Translational Science Institute or UHCC Body Composition Laboratory. The study protocols were approved by institutional review boards at all sites and all participants provided written informed consent.
Anthropometric measurements
Weight and height were collected in duplicate and triplicate waist and hip circumference measures were recorded. Following the standard protocol from the US National Health and Nutrition Examination Survey (NHANES), flexible measuring tapes were used to collect waist and hip circumferences (31). Measurements were recorded in triplicate to the nearest 0.1 cm; results were averaged. Waist circumference measurements were taken using marks placed on the top of the iliac crest as reference while the participant stood up straight with their arms crossed. Participants remained in the same position for hip circumference measurements, which were made at the most protruding part of the buttocks (31). For height measurement, participants stood barefoot with their backs against the stadiometer with heels together touching the wall-plate. Participants stood erect with back and buttocks touching the measurement surface and oriented their head to look straight ahead, with orbitale-tragion line forming a horizontal line. Height and mass were measured to the nearest 0.1 cm and 0.1 kg respectively with a stadiometer/digital scale combination (Seca 264, Chino, CA).
Three dimensional optical (3DO) scans
Duplicate 3DO surface scans were obtained using the Styku S100 scanner (Styku LLC, Los Angeles, CA, software version 4.1) beginning in April 2017. Participants wore form-fitting shorts, a sports bra for females, and a swim cap. Height and weight were entered into the 3DO device, then participants were measured on the scanning platform using the standardized positioning protocol provided by the manufacturer. The 3DO scanner is comprised of a Microsoft Kinect V2 camera (Microsoft, Redmond, WA), a rotating platform, and the analysis software. Participants stood on the turntable with legs separated, arms away from the body and within the image guide provided on the 3DO software screen, and hands closed into fists. During the scans, the platform rotates 360-degrees over a period of 30-40 seconds, with the camera system emitting a light and reflections being recorded by the Kinect camera. The device extracts whole-body and segmental surface areas and volumes as well as circumference measures to estimate body composition. The timeframe to prepare a participant, complete a scan, and obtain results in the provided software is 5 minutes. The software generates body composition estimates from two models, “Advanced” that is calibrated to DXA measures and “Basic” that is calibrated to bioelectrical impedance measures (personal communication with manufacturer). All results are reported for the Advanced DXA calibration option. A sample 3DO scan and measurements of interest are shown in Fig 1.
Fig 1.
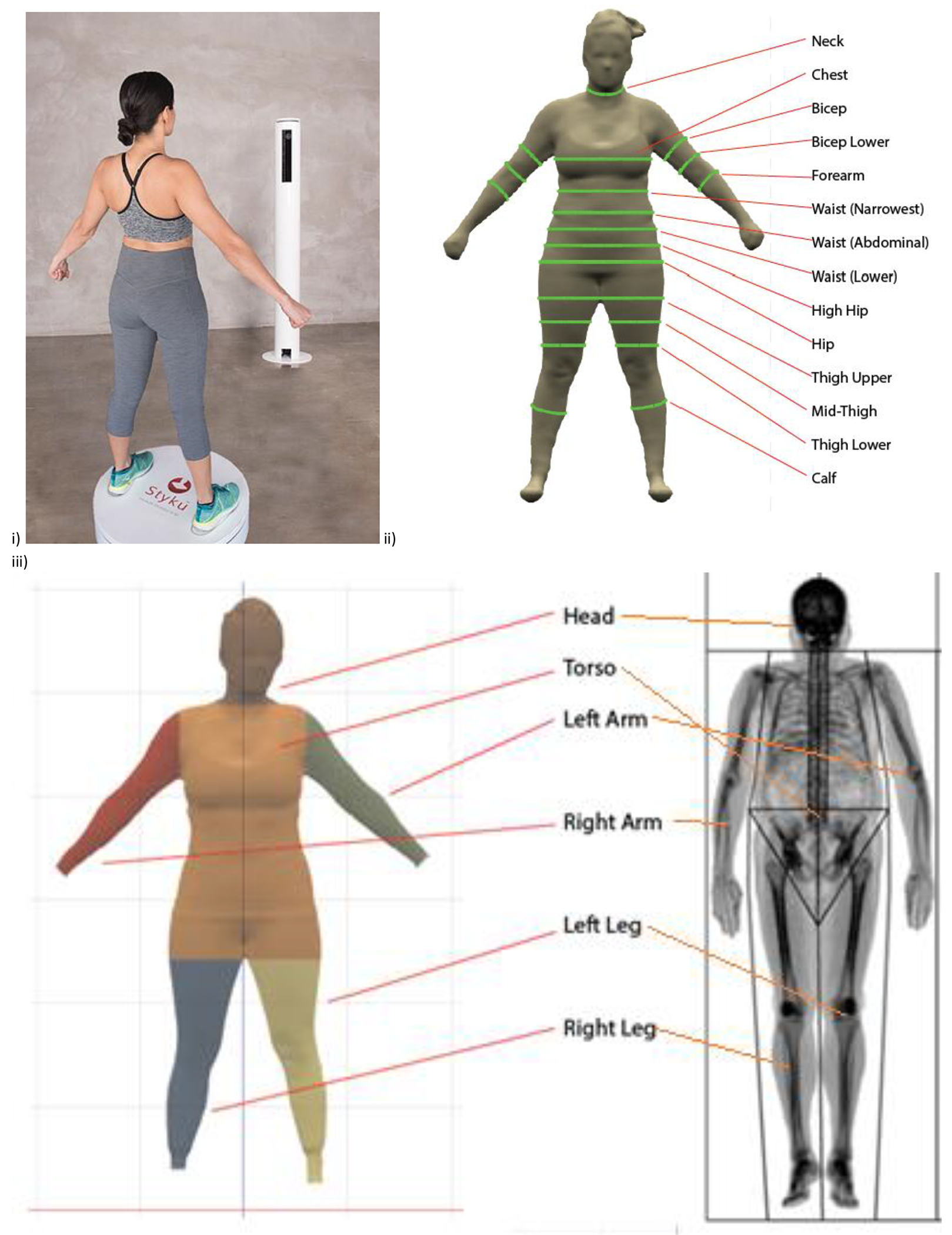
Image of 3DO scan (i) as provided on device software. Regional measures for 3DO (ii) and DXA (iii) as outlined on device software.
Dual energy X-ray Absorptiometry (DXA) scans
Each participant completed duplicate whole-body DXA scans on a Hologic Discovery/A system (Hologic Inc., Marlborough, MA) by a certified DXA radiology technician. DXA systems were compared between institutions using phantoms and showed no significant differences (p>0.05). Inter-site variations were not removed to ensure that comparisons between DXA and 3DO properly reflect variation between devices in the field. DXA scans were analyzed at UHCC by a trained technologist using Hologic Apex version 5.6 with the National Health and Nutrition Examination Survey Body Composition Analysis calibration option disabled. Participants wore the same form-fitting clothes as used in the 3DO scans. The timeframe to prepare a participant, complete a scan, and analyze results in the DXA software is 20 minutes. As per International Society for Clinical Densitometry guidelines, offset scanning was performed for subjects too wide to fit in the DXA scan field. DXA systems were calibrated according to standard Hologic procedures (32, 33).. Body composition measurements from DXA included total body mass, total and regional (trunk, arms, legs) fat mass (FM), percent body fat (PBF), bone mineral content (BMC), and fat free mass (FFM). DXA-derived volume measurements were obtained from the attenuation of tissue; previous research has shown the accuracy (r2 = 0.99) of DXA measures of body volume to the clinical method of air displacement plethysmography (34). A sample image showing standardized manufacturer and software-defined sub regional divisions in a DXA scan is shown in Fig 1.
Statistical Methods
Data are expressed as means ± standard deviation (SD). Coefficient of variation (%CV) and root mean square error (RMSE) were calculated for the matched test-retest measurements when available (35). Technical error of measurement (TEM), relative TEM (%TEM), and coefficient of reliability (R) were used to assess intra-observer reliability of 3DO anthropometry and the first two collected tape anthropometry measures (36). Only right limb comparisons are included due to size limitations of the DXA scanning table, where subjects with too large body size had their left arm or leg cut off and data mirrored from the right. However, on samples that contained both left and right arms, right arm relationships were indistinguishable from the average of left and right arms (data not shown). Test-retest analysis was performed for the left-to-right volume ratios on the 3DO but not compared to DXA due to the arm measurement issue noted above.
Lin’s concordance correlation coefficients (CCC) were calculated to assess accuracy and precision in body composition estimates by 3DO compared to the criterion for body composition (DXA) and circumference (anthropometry) measures (37). Measurements were assumed to be normally distributed. Bland-Altman analyses were performed to evaluate the agreement and potential bias between both methods for all measures of body composition (38). Univariate linear regression analysis was performed to examine the relationship between body composition measures with 3DO to body composition and volume measures by DXA and circumference measures by manual anthropometry for the entire dataset. Significant differences based on sex, race/ethnicity, age (18-40y, 40-60y, >60y) and BMI were assessed via Least Square MEANS; variables that differed statistically by sex were analyzed separately to provide corrections of 3DO to the criterion. Statistical analysis was performed using SAS version 9.4 for Windows (SAS Institute, Cary, NC).
Results
A total of 619 adults were recruited through February 2020. Of this sample, 345 participants from the PBRC site were excluded due to data access by the manufacturer, noted above. 3DO scans were missing on 81 participants, resulting in data being available for 193 adults. Participants were removed if the DXA scan data was invalid due to metal implant (n=1), breast implant (n=1), or the participant was wearing a wire bra (n=1) or if a physiologically abnormal (negative) body fat was reported by 3DO (n=2). After these exclusions, 188 (102 female; age = 44.5 ± 15.4 years, height = 166.7 ± 10.3 cm, weight = 76.2 ± 23.2 kg, BMI = 27.3 ± 7.4 kg/m2) participants were included in the analysis. For test-retest precision, 185 participants had valid duplicate scans for 3DO, where duplicate measures failed for 3 subjects. Summary characteristics and subject counts by ethnicity are presented in Table 1.
Table 1.
Subject Characteristics (n = 188 [102 female])
| Variable | Mean (SD) | Min | Max | |
|---|---|---|---|---|
|
| ||||
| Age (years) | 44.5 (15.4) | 18.0 | 89.0 | |
| Height (cm) | 166.7 (10.3) | 144.2 | 202.0 | |
| Weight (kg) | 76.2 (23.2) | 35.4 | 142.7 | |
| BMI (kg/m2) | 27.3 (7.4) | 14.2 | 51.3 | |
| DXA FFM (kg) | 54.1 (15.2) | 28.6 | 102.2 | |
| DXA FM (kg) | 22.2 (12.3) | 5.1 | 65.7 | |
| DXA PBF (%) | 28.2 (9.1) | 9.0 | 48.1 | |
| DXA VAT (kg) | 0.4 (0.3) | 0.1 | 1.5 | |
| DXA Body Volume (L) | 77.2 (24.1) | 35.3 | 148.6 | |
| DXA Trunk Volume (L) | 37.3 (12.9) | 16.0 | 79.4 | |
| DXA Arm Volume (L) | 9.4 (3.4) | 3.9 | 19.0 | |
| DXA Leg Volume (L) | 25.6 (7.9) | 11.7 | 49.1 | |
| DXA R/L Arm Volume Ratio | 1.0 (0.0) | 0.9 | 1.2 | |
| DXA R/L Leg Volume Ratio | 1.0 (0.0) | 0.9 | 1.1 | |
| Waist Circumference (cm)† | 94.2 (17.8) | 59.4 | 157.1 | |
| Hip Circumference (cm)† | 102.3 (14.1) | 74.3 | 155.9 | |
|
| ||||
| Count | % | |||
|
| ||||
| Ethnicity | ||||
| Asian | 72 | 38.3 | ||
| NH Black | 12 | 6.4 | ||
| Hispanic | 36 | 19.2 | ||
| NHOPI1 | 43 | 22.9 | ||
| NH White | 25 | 13.3 | ||
| AGE (years) | ||||
| 18-40 | 193 | 38.5 | ||
| 40-60 | 176 | 35.1 | ||
| >60 | 132 | 26.3 | ||
| BMI (kg/m2) | ||||
| <18.5 | 28 | 5.6 | ||
| 18.5-25.0 | 169 | 33.7 | ||
| 25.0-30.0 | 160 | 31.9 | ||
| >30.0 | 144 | 28.7 | ||
Abbreviations: SD: standard deviation, BMI: body mass index, DXA: dual energy X-ray absorptiometry, FFM: fat free mass, FM: fat mass, PBF: percent body fat, VAT: visceral adipose tissue, NH: non-Hispanic
Test-retest precision results are presented in Table 2. Precision error for 3DO was low for whole body volume (RMSE = 1.10 L) as well as the regional volume measures for arm (RMSE = 0.11 L) and leg (RMSE = 0.21 L). Circumference measures showed low precision error for waist (RMSE = 0.60 cm) and hip (RMSE = 0.53 cm) circumferences. Body composition precision for FFM (RMSE = 0.40 kg), FM (RMSE = 0.41 kg), and PBF (RMSE = 0.60 %) were comparable to DXA.
Table 2:
Test-retest precision of 3DO and DXA measures (n=185)
| Variable | 3DO | DXA | ||
|---|---|---|---|---|
|
| ||||
| %CV | RMSE | %CV | RMSE | |
| FFM (kg) | 0.75 | 0.40 | 0.67 | 0.33 |
| FM (kg) | 1.94 | 0.41 | 1.42 | 0.31 |
| PBF (%) | 0.60 | 0.35 | ||
| VAT (kg) | 3.67 | 0.02 | 8.61 | 0.04 |
| Whole Body Volume (L) | 1.45 | 1.10 | 0.47 | 0.36 |
| Right Arm Volume (L) | 4.58 | 0.11 | 3.16 | 0.15 |
| Right Leg Volume (L) | 3.14 | 0.21 | 2.27 | 0.29 |
| L/R Arm volume ratio | 5.28 | 0.05 | 4.27 | 0.04 |
| L/R Leg volume ratio | 2.81 | 0.03 | 1.51 | 0.02 |
| Waist circumference (cm) | 0.63 | 0.60 | ||
| Hip circumference (cm) | 0.51 | 0.53 | ||
Abbreviations: CV: coefficient of variation; RMSE: root mean square error; FFM: fat free mass, FM: fat mass, PBF: percent body fat, VAT: visceral adipose tissue; L/R: left to right
Table 1Table 3 and Figure 2 show the agreement between the criterion and 3DO for the measured variables. The overall FFM as measured by DXA (54.1 ± 15.2 kg) and 3DO (55.3 ± 15.0 kg) showed a small mean difference of 1.2 ± 3.4 kg (95% limits of agreement −7.0 to +5.6) and the CCC was 0.97 (95% CI: 0.96-0.98). The CCC for FM was 0.95 (95% CI: 0.94-0.97) and the mean difference of 1.3 ± 3.4 kg 95% limits of agreement −5.5 to +8.1) reflected the difference in FFM measures. Though the mean differences for body composition measurements were small, the wider limits of agreement (LOA) observed are in line with other 3DO validations to DXA (29). Though these results limit the interchangeability of DXA and 3DO in clinical practice, they reflect the LOAs found in studies of other body composition technologies such as bioimpedance in comparison to DXA (39). The differences were also evaluated by sex, race/ethnicity, and BMI. Arm volume, leg volume, waist and hip circumferences statistically differed (p<0.05) by sex, however no differences were observed in different race/ethnicity, age, or BMI groups (all p>0.05). The sex-specific plots and equations are shown in the supplement where appropriate.
Table 3:
Assessment of mean difference and agreement between criterion and 3DO by variable
| Measurement | Difference | Concordance correlation | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Population | Variable | DXA (mean ± SD) | 3DO (mean ± SD) | (mean ± SD) | 95% Limits of Agreement | Coefficient (95% CI) | ||
|
| ||||||||
| Total (n=188) | FFM (kg)1 | 54.1 ± 15.2 | 55.3 ± 15.0 | −1.2 ± 3.4 | −7.0 to 5.6 | 0.97 (0.96, 0.98) | ||
| FM (kg)1 | 22.2 ± 12.3 | 20.9 ± 11.8 | 1.3 ± 3.4 | −5.5 to 8.1 | 0.95 (0.94, 0.97) | |||
| PBF (%)1 | 28.2 ± 9.1 | 26.3 ± 9.2 | 1.9 ± 4.5 | −7.1 to 10.9 | 0.86 (0.82, 0.90) | |||
| VAT mass (kg)1 | 0.4 ± 0.3 | 0.5 ± 0.3 | 0.1 ± 0.2 | −0.3 to 0.5 | 0.81 (0.76, 0.85) | |||
| Whole body volume (L)1 | 77.2 ± 24.1 | 76.7 ± 27.1 | 0.6 ± 4.2 | −7.8 to 9.0 | 0.99 (0.98, 0.99) | |||
| Trunk volume (L)1 | 37.3 ± 12.9 | 58.0 ± 27.1 | −20.6 ± 14.6 | −49.8 to −8.6 | 0.52 (0.47, 0.57) | |||
| Female (n=102) | ||||||||
| Right arm volume (L)1 | 4.2 ± 1.7 | 2.0 ± 0.7 | 2.1 ± 1.1 | −0.1 to 4.3 | 0.32 (0.27, 0.37) | |||
| Right leg volume (L)1 | 12.1 ± 4.2 | 6.5 ± 2.6 | 5.6 ± 1.8 | 2.0 to 9.2 | 0.33 (0.28, 0.37) | |||
| Waist circumference (cm)2 | 93.6 ± 19.1 | 95.7 ± 17.5 | −2.1 ± 4.5 | −11.1 to 6.9 | 0.97 (0.96, 0.98) | |||
| Hip circumference (cm)2 | 102.9 ± 16.4 | 103.8 ± 15.7 | −1.0 ± 2.7 | −6.4 to 4.4 | 0.98 (0.98, 0.99) | |||
| Male (n=86) | ||||||||
| Right arm volume (L)1 | 5.5 ± 1.5 | 3.0 ± 0.9 | 2.5 ± 0.7 | 1.1 to 3.9 | 0.32 (0.27, 0.37) | |||
| Right leg volume (L)1 | 13.9 ± 3.6 | 7.2 ± 2.3 | 6.6 ± 1.4 | 3.8 to 9.4 | 0.33 (0.28, 0.37) | |||
| Waist circumference (cm)2 | 95.0 ± 16.2 | 94.1 ± 15.1 | 0.9 ± 2.6 | −4.3 to 6.1 | 0.97 (0.96, 0.98) | |||
| Hip circumference (cm)2 | 101.6 ± 11.0 | 101.7 ± 11.2 | −0.1 ± 2.1 | −4.3 to 4.2 | 0.98 (0.98, 0.99) | |||
Abbreviations: FFM: fat free mass, FM: fat mass, PBF: percent body fat, VAT: visceral adipose tissue, kg: kilograms, L: liters, cm: centimeters
Females and males evaluated separately when significant sex differences (p<. 05) were observed
Criterion variable is DXA
anthropometric tape measurement
Figure 2:
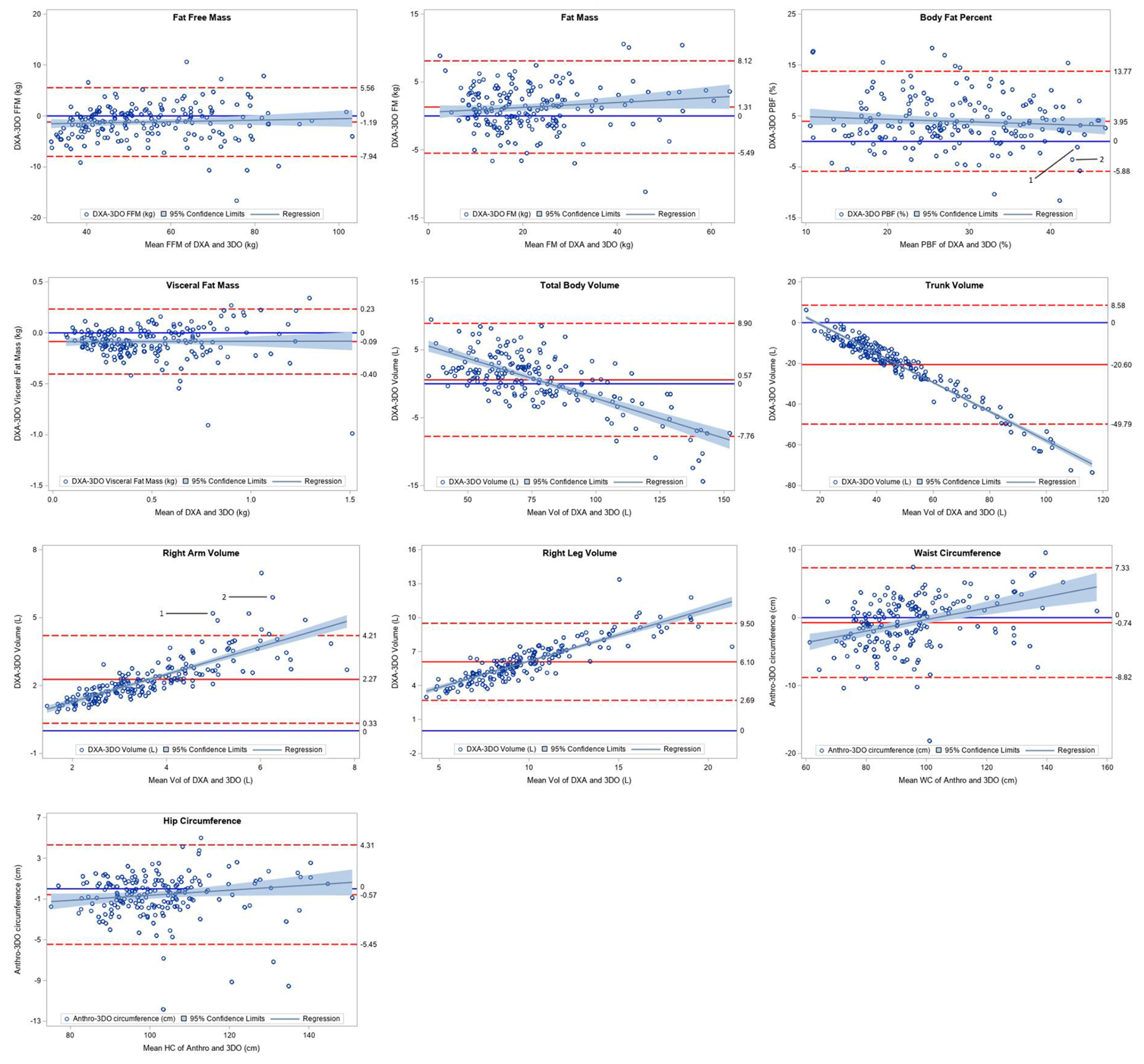
Bland-Altman Plots for Body Composition and Circumference Estimates
As seen in Fig 1, partition placement for defining regional measures differed between 3DO and DXA, resulting in systematic differences in regional volume measurements. Placement of the DXA trunk/leg partition runs diagonally from the crotch to the hips, placing the femur in the leg compartment (40). This differs from the horizontal partition on the 3DO scanner that places a portion of the leg into the trunk region. With the shoulder partition of the DXA scan intersecting the acromion process, arm volume in DXA was greater in comparison to the 3DO volume set by the vertical partition at the armpit or in women, at the end of the breast tissue. These differences explain 3DO’s significant overestimation of trunk (20.6 ± 14.6 L, p<0.0001) and significant underestimation of right arm (−2.3 ± 1.0 L, p<0.0001) and right leg (−6.1 ±1.7 L, p<0.0001) volumes. Examining overall body volume differences, a non-significant underestimation by 3DO was reported (−0.6 ± 4.2 L, p=.06).
Additional examination was performed on the arm volume by sex. Females generally had a lower precision error between arm volume (r2=0.81, p<0.01; RMSE = 0.67 L) volume when compared to males (r2=0.85, p<0.01; RMSE = 0.55 L). This may be the result of compression and overlapping of breast tissue during supine DXA measurements in addition to the difficulty in achieving arm separation during routine 3DO measures. As seen in the supplemental material, the tissue in the DXA scan appears to be partially subdivided into the arm region in larger subjects. The phenomenon, in addition to the segmenting differences noted above, explain the large overestimation (>5 L) of arm volume in these participants. Despite this difference, the overall body composition measures were within the limits of agreement. The sample data presented in the supplement are labeled 1 and 2 in the arm volume and PBF analysis presented in Figure 2. These issues result in a lower CCC for arm and leg volumes, however the high correlation and provided regression equations (Table 4) allow for correction of these variables by sex.
Intra-observer error of waist and hip circumferences are reported in the supplement (Table 5). The TEM of waist circumference was slightly larger for 3DO (0.46 cm) than tape anthropometry (0.36 cm), while the hip circumference showed better precision for 3DO (0.39 cm) compared to tape anthropometry (0.57 cm). The excellent reliability (both R>0.99) supports 3DO for longitudinal assessment, reducing subjective error observed even in trained anthropometrists (41). Though both waist and hip circumference showed a bias at different sizes and genders, we could not identify the cause of this phenomenon due to the possibility of different definitions utilized by the 3DO system compared to the NHANES anthropometric protocol used in this study. That said, the sex-specific correction factors for these variables are presented in Table 4.
Discussion
This study found excellent correlations for volume and circumference measures that reflect the results of previous studies using other 3DO systems (40, 42). Due to the differences in cut points for regions of interest, volume measurements differed but in a systematic fashion, meaning corrections can be applied to ensure agreement between methods. These results provide direct corrections of 3DO to criterion measures and supports the widespread use of 3DO systems in clinical practice.
In regards to body composition, strong CCC relationships were observed for FFM and FM between 3DO and DXA. These findings were consistent when assessed by age, sex, race/ethnicity, and BMI class. The agreement for PBF was lower, consistent with other studies examining the accuracy of PBF using 3DO (43). The findings of the current study show a clear improvement over validations reported on prior versions of the 3DO system, with precision for FFM and FM measures remaining consistent even at increased adiposity (44).
These findings are in line with other studies supporting the accuracy of 3DO systems for the assessment of FFM and FM when compared to DXA in adults and children (40, 45). Using DXA and the Fit3D 3DO system to develop body composition regression equations, Ng et al. (2016) reported similar RMSE for FFM (2.2 kg) (40). In children, RMSE for FFM was reported at 2.8 kg with similar r2 values for body composition as seen in our study (45). The results support the use of 3DO systems for the assessment of regional and whole-body composition in a broad range of patient populations.
Tinsley et al. (2020) noted a significant difference in percent body fat (−4.2%) between the Styku 3DO scanner and a simplified four compartment (4C) model (46). The above findings are in agreement with Cabre et al. (2020), who reported a significant difference between a Styku 3DO and the 4C model (−2.26%) (29). However, no difference was found in this study when 3DO was compared to DXA (1.65%) (29). The 4C models in these studies use BIA, a validated assessment of total body water that has previously shown an error of 2.21 L with limits of agreement from −4.50 to 4.31 L in comparison to the criterion deuterium dilution. Using the error in total body water estimation via BIA and the average subject data from the current study, the limits of agreement are responsible for ±7% error in PBF. This error in total body water estimation may explain the disagreement between 4C and 3DO (47). Nevertheless, our study reports small mean differences when using DXA, a common clinical assessment tool.
Test-retest precision measures in the following study are in line with DXA values as well as previous studies. Bourgeois et al. (2017) showed that the Styku 3DO scanner coefficients of variation are generally < 1% in circumference measurements and <3% in volume measurements, where we observed < 0.60 % for circumference measurements and 1.45 % for whole body volume (28). Least significant change to have 95% confidence that a difference between two measures is greater than zero for evaluating change in anthropometric measures has been reported for waist (3.8 cm), hip (1.3 cm), and arm circumference (2.1 cm) (25). Finally, Silver et al. (2020) reported minimal detectable change (MDC) in PBF of 1.1 % for within-day repeated measures, where we report an RMSE of 0.60 (27). While a software upgrade by the manufacturer may have caused results to differ, our precision likely also outperformed these studies due to the larger sample size of our study (n=188) compared to Bourgeois et al. (n = 113) and Silver et al. (n = 33) (35). Test precision is an essential asset of medical technologies, ensuring medical professionals can confidently identify changes in body composition over repeated measures (23). The high precision observed in this study further support the ability and practicality of 3DO to quantify body composition change in clinical practice.
Accurate and precise measures from the 3DO scanning system provide key metrics associated with increased disease risk. Elevated VAT mass has been shown to have an association with cardiovascular disease risk as well as diabetes (4, 48). Alongside visceral measures, waist circumference and waist-to-hip ratios are independent predictors of T2D. Using a 3DO system, studies have found associations of waist-to-thigh ratio (WTR) with T2D (49–51). With the low inter-individual error and high test-retest precision of 3DO measures, these metrics could simplify the clinical assessment of metabolic disease risk.
Volume estimates have been suggested using methods of assessment other than the criteria of air displacement plethysmography or underwater weighing. DXA has been validated for its use in multicompartment models of body composition, a criterion measure of body composition in vivo (52–55). Our study shows the accuracy of 3DO total body volume measures in relation to DXA. In addition to providing highly reproducible results, applying corrections to the body volumes derived from the 3DO scanner could allow for a simplified field method of regional and whole-body multicompartment composition assessment.
The high reproducibility of regional volume measurements further supports the utility of 3DO scans in clinical populations where circumference and volume measures diagnose and assess disease progression (56). Lymphedema, characterized by excess lymph volume primarily in the limbs resulting from a variety of causes including cancer treatment, utilizes circumference measures to assess treatment outcomes (57). Additionally, limb volume via 3-dimensional optical perometry or total body water comparison via bioimpedance are widely accepted measures of lymphedema through comparisons of affected to unaffected limb or to measures of the same limb prior to diagnosis (11, 58). Because DXA systems rely on image mirroring in large individuals and lymphedema is associated with overweight/obesity, the ability to utilize DXA volume in lymphedema assessment may be limited (59). This highlights another clinical advantage to 3DO systems, of which have been previously validated for use in lymphedema assessment, to provide objective measures of circumference and volume measures at annual physicals that serve as reference data for comparison at disease diagnosis (60).
A strength of this study was that it utilized a diverse sample of adults of varying age and ethnicity to validate the measures provided by the 3DO scanner. The wide range of BMIs examined (14.8-50.2 kg/m2) serves as the widest range of body masses assessed to date, addressing the need for validation in populations with varying regional tissue distribution (61). This study also had a few limitations. Findings were assessed from a healthy population that did not include patients with conditions such as myopenia or malnutrition. While 28 participants were classified as underweight and thus at “high risk” for malnutrition, we did not collect additional information to assess for clinical malnutrition (62). Though our sample included a wide range of large body mass subjects, we did not analyze the impact of thighs or arms touching on the accuracy specifically in larger participants beyond the presence of regional cut points discussed previously. As noted, region partitions differ between different DXA systems as well as 3DO, which may limit the comparison of results across studies(63). Similarly, caution must be observed in applying this information across other 3DO systems to ensure equivalence of measures provided by the candidate device. Further analysis of reliability and accuracy is also recommended in a population of adults with obesity.
Our study highlighted the advantages 3DO measurement provides for research and clinical practice. The 3DO system evaluated in this study provides measurements that agree strongly to a valid reference method. With no need for additional training or analysis, this method is a viable alternative to DXA measurements that may be inaccessible or time consuming during routine physical assessments. The high test-retest precision reduces the technical errors associated with repeated anthropometric measurements, while the measurement of volumes and circumferences affords medical professionals metrics useful in prevention and identification of obesity and T2D risk as well as clinical conditions including lymphedema. Future work is warranted to evaluate the ability for these devices to quantify skeletal muscle mass as well as the role of body composition variables in accurately predicting disease risk (64). Additionally, future work should assess the ability of 3DO systems to improve the detection of FFM change related to malnutrition and the ability for body shape and body composition to aid in the diagnosis of MetS, providing further evidence of the clinical utility of this technology.
Supplementary Material
Acknowledgements:
The authors would like to thank the subjects for their participation and Jack Reich for his assistance formatting figures.
Funding:
Funding for this study was part of the Shape Up! Adults grant (NIH R01 DK109008)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: All authors report no conflict of interest.
Contributor Information
Jonathan P. Bennett, Graduate Program in Human Nutrition, University of Hawai’i Manoa, Agricultural Science Building, 1955 East-West Rd, Honolulu, Hawaii, 96822, USA; Department of Epidemiology, University of Hawai’i Cancer Center, 701 Ilalo St, Honolulu, Hawaii, 96813 USA
Yong En Liu, Department of Epidemiology, University of Hawai’i Cancer Center, 701 Ilalo St, Honolulu, Hawaii, 96813 USA.
Brandon K. Quon, Department of Epidemiology, University of Hawai’i Cancer Center, 701 Ilalo St, Honolulu, Hawaii, 96813 USA
Nisa N. Kelly, Department of Epidemiology, University of Hawai’i Cancer Center, 701 Ilalo St, Honolulu, Hawaii, 96813 USA
Michael C. Wong, Graduate Program in Human Nutrition, University of Hawai’i Manoa, Agricultural Science Building, 1955 East-West Rd, Honolulu, Hawaii, 96822, USA; Department of Epidemiology, University of Hawai’i Cancer Center, 701 Ilalo St, Honolulu, Hawaii, 96813 USA
Samantha F. Kennedy, Pennington Biomedical Research Center, Louisiana State University, 6400 Perkins Rd, Baton Rouge, Louisiana, 70808 USA
Dominic C. Chow, John A. Burns School of Medicine, University of Hawaii, 651 Ilalo St, Honolulu, Hawaii 96813 USA
Andrea K. Garber, Division of Adolescent & Young Adult Medicine, University of California, San Francisco, 3333 California Street, Suite 245, San Francisco, California, 94118 USA
Ethan J. Weiss, University of California School of Medicine, 555 Mission Bay Blvd South, San Francisco, California, 94158 USA
Steven B. Heymsfield, Pennington Biomedical Research Center, Louisiana State University, 6400 Perkins Rd, Baton Rouge, Louisiana, 70808 USA
John A. Shepherd, Graduate Program in Human Nutrition, University of Hawai’i Manoa, Agricultural Science Building, 1955 East-West Rd, Honolulu, Hawaii, 96822, USA; Department of Epidemiology, University of Hawai’i Cancer Center, 701 Ilalo St, Honolulu, Hawaii, 96813 USA
References
- 1.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-Specific Excess Deaths Associated With Underweight, Overweight, and Obesity. JAMA. 2007;298(17):2028. [DOI] [PubMed] [Google Scholar]
- 2.Wolin KY, Carson K, Colditz GA. Obesity and Cancer. The Oncologist. 2010;15(6):556–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body Fatness and Cancer — Viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Després J-P. Body Fat Distribution and Risk of Cardiovascular Disease. Circulation. 2012; 126(10):1301–13. [DOI] [PubMed] [Google Scholar]
- 5.Abdullah A, Stoelwinder J, Shortreed S, Wolfe R, Stevenson C, Walls H, et al. The duration of obesity and the risk of type 2 diabetes. Public Health Nutr. 2011;14(1):119–26. [DOI] [PubMed] [Google Scholar]
- 6.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of comorbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health. 2009;9(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward LC. Human body composition: yesterday, today, and tomorrow. Eur J Clin Nutr. 2018;72(9):1201–7. [DOI] [PubMed] [Google Scholar]
- 8.Batsis JA, Mackenzie TA, Barre LK, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr. 2014;68(9):1001–7. [DOI] [PubMed] [Google Scholar]
- 9.Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition – A consensus report from the global clinical nutrition community. Journal of Cachexia, Sarcopenia and Muscle. 2019;10(1):207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med. 2001;137(4):231–43. [DOI] [PubMed] [Google Scholar]
- 11.Taylor R, Jayasinghe UW, Koelmeyer L, Ung O, Boyages J. Reliability and Validity of Arm Volume Measurements for Assessment of Lymphedema. Phys Ther. 2006;86(2):205–14. [PubMed] [Google Scholar]
- 12.Van Pelt R, Evans E, Schechtman K, Ehsani A, Kohrt W. Contributions of total and regional fat mass to risk for cardiovascular disease in older women. American Journal of Physiology-Endocrinology and Metabolism. 2002. [DOI] [PubMed] [Google Scholar]
- 13.Zhang C, Rexrode KM, Van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality. Circulation. 2008;117(13):1658–67. [DOI] [PubMed] [Google Scholar]
- 14.Shepherd JA, Ng BK, Sommer MJ, Heymsfield SB. Body composition by DXA. Bone. 2017;104:101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells JCK. Measuring body composition. Arch Dis Child. 2005;91(7):612–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross R, Goodpaster B, Kelley D, Boada F. Magnetic resonance imaging in human body composition research: from quantitative to qualitative tissue measurement. Ann N Y Acad Sci. 2000;904(1):12–7. [DOI] [PubMed] [Google Scholar]
- 17.Goodpaster BH, Thaete FL, Kelley DE. Composition of skeletal muscle evaluated with computed tomography. Ann N Y Acad Sci. 2000;904(1):18–24. [DOI] [PubMed] [Google Scholar]
- 18.Mialich M, Sicchieri JMF, Junior AAJ. Analysis of Body Composition: A Critical Review of the Use of Bioelectrical Impedance Analysis. International Journal of Clinical Nutrition. 2014;2(1):1–10. [Google Scholar]
- 19.Frisancho A Anthropometric Standards: An Interactive Nutritional Reference of Body Size and Body Composition for Children and Adults. 2 ed. Press UoM, editor. Ann Arbor: University of Michigan Press; 2008 2008. 352 p. [Google Scholar]
- 20.LaForgia J, Gunn S, Withers R. Body composition: validity of segmental bioelectrical impedance analysis. Asia Pac J Clin Nutr. 2008;17(4):586–91. [PubMed] [Google Scholar]
- 21.Leahy S, O’Neill C, Sohun R, Jakeman P. A comparison of dual energy X-ray absorptiometry and bioelectrical impedance analysis to measure total and segmental body composition in healthy young adults. European Journal of Applied Physiology. 2012;112(2):589–95. [DOI] [PubMed] [Google Scholar]
- 22.Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Manuel Gómez J, et al. Bioelectrical impedance analysis—part II: utilization in clinical practice. Clinical Nutrition. 2004;23(6):1430–53. [DOI] [PubMed] [Google Scholar]
- 23.Eaton-Evans J Nutritional Assessment: Anthropometry. In: B C, editor. Encyclopedia of Human Nutrition. 4. Amsterdam, The Netherlands: Elsevier; 2005. p. 311–8. [Google Scholar]
- 24.Nordhamn K, Södergren E, Olsson E, Karlström B, Vessby B, Berglund L. Reliability of anthropometric measurements in overweight and lean subjects: consequences for correlations between anthropometric and other variables. Int J Obes. 2000;24(5):652–7. [DOI] [PubMed] [Google Scholar]
- 25.Tinsley GM, Moore ML, Dellinger JR, Adamson BT, Benavides ML. Digital anthropometry via three-dimensional optical scanning: evaluation of four commercially available systems. Eur J Clin Nutr. 2020;74(7):1054–64. [DOI] [PubMed] [Google Scholar]
- 26.Derouchey JD, Tomkinson GR, Rhoades JL, Fitzgerald JS. Reliability of the Styku 3D Whole-Body Scanner for the Assessment of Body Size in Athletes. Measurement in Physical Education and Exercise Science. 2020;24(3):228–34. [Google Scholar]
- 27.Silver B, Wilson PB. Reliability and Minimal Detectable Change of the Styku 3D Body Scanner. Measurement in Physical Education and Exercise Science. 2020:1–7. [Google Scholar]
- 28.Bourgeois B, Ng B, Latimer D, Stannard C, Romeo L, Li X, et al. Clinically applicable optical imaging technology for body size and shape analysis: comparison of systems differing in design. Eur J Clin Nutr. 2017;71(11):1329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cabre HE, Blue MN, Hirsch KR, Brewer GJ, Gould LM, Nelson AG, et al. Validity of a Three-Dimensional Body Scanner: Comparison Against a 4-Compartment Model and Dual Energy X-Ray Absorptiometry. Applied Physiology, Nutrition, and Metabolism. 2020(ja). [DOI] [PubMed] [Google Scholar]
- 30.Heymsfield SB, Bourgeois B, Ng BK, Sommer MJ, Li X, Shepherd JA. Digital anthropometry: a critical review. Eur J Clin Nutr. 2018;72(5):680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prevention CfDCa. National Health and Nutrition Examination Survey (NHANES) Anthropometry Procedures Manual. Available from: https://wwwn.cdc.gov/nchs/data/nhanes/2017-2018/manuals/2017_Anthropometry_Procedures_Manual.pdf.
- 32.Lu Y, Mathur AK, Blunt BA, Glüer CC, Will AS, Fuerst TP, et al. Dual X-ray absorptiometry quality control: Comparison of visual examination and process-control charts. J Bone Miner Res. 1996;11(5):626–37. [DOI] [PubMed] [Google Scholar]
- 33.Hangartner TN, Warner S, Braillon P, Jankowski L, Shepherd J. The Official Positions of the International Society for Clinical Densitometry: Acquisition of Dual-Energy X-Ray Absorptiometry Body Composition and Considerations Regarding Analysis and Repeatability of Measures. J Clin Densitom. 2013;16(4):520–36. [DOI] [PubMed] [Google Scholar]
- 34.Wilson JP, Mulligan K, Fan B, Sherman JL, Murphy EJ, Tai VW, et al. Dual-energy X-ray absorptiometry–based body volume measurement for 4-compartment body composition. The American Journal of Clinical Nutrition. 2012;95(1):25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glüer C-C, Blake G, Lu Y, Blunt B, Jergas M, Genant H. Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporos Int. 1995;5(4):262–70. [DOI] [PubMed] [Google Scholar]
- 36.Ulijaszek SJ, Kerr DA. Anthropometric measurement error and the assessment of nutritional status. Br J Nutr. 1999;82(3):165–77. [DOI] [PubMed] [Google Scholar]
- 37.Lawrence I, Lin K. A Concordance Correlation Coefficient to Evaluate Reproducibility. Biometrics. 1989;45:255–68. [PubMed] [Google Scholar]
- 38.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Int J Nurs Stud. 2010;47(8):931–6. [PubMed] [Google Scholar]
- 39.Day K, Kwok A, Evans A, Mata F, Verdejo-Garcia A, Hart K, et al. Comparison of a bioelectrical impedance device against the reference method dual energy X-ray absorptiometry and anthropometry for the evaluation of body composition in adults. Nutrients. 2018;10(10):1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ng BK, Hinton BJ, Fan B, Kanaya AM, Shepherd JA. Clinical anthropometrics and body composition from 3D whole-body surface scans. Eur J Clin Nutr. 2016;70(11):1265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goto R, Mascie-Taylor CGN. Precision of Measurement as a Component of Human Variation. J Physiol Anthropol. 2007;26(2):253–6. [DOI] [PubMed] [Google Scholar]
- 42.Pepper MR, RD JH, RD WY, Stanforth PR, Cahill JM, Mahometa M, et al. Validation of a 3-Dimensional Laser Body Scanner for Assessment of Waist and Hip Circumference. J Am Coll Nutr. 2010;29(3):179–88. [DOI] [PubMed] [Google Scholar]
- 43.Ng BK, Sommer MJ, Wong MC, Pagano I, Nie Y, Fan B, et al. Detailed 3-dimensional body shape features predict body composition, blood metabolites, and functional strength: the Shape Up! studies. The American Journal of Clinical Nutrition. 2019;110(6):1316–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harbin MM, Kasak A, Ostrem JD, Dengel DR. Validation of a three-dimensional body scanner for body composition measures. Eur J Clin Nutr. 2018;72(8):1191–4. [DOI] [PubMed] [Google Scholar]
- 45.Wong MC, Ng BK, Kennedy SF, Hwaung P, Liu EY, Kelly NN, et al. Children and Adolescents’ Anthropometrics Body Composition from 3-D Optical Surface Scans. Obesity. 2019;27(11):1738–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tinsley GM, Moore ML, Benavides ML, Dellinger JR, Adamson BT. 3-Dimensional optical scanning for body composition assessment: A 4-component model comparison of four commercially available scanners. Clin Nutr. 2020. [DOI] [PubMed] [Google Scholar]
- 47.Moon JR, Tobkin SE, Roberts MD, Dalbo VJ, Kerksick CM, Bemben MG, et al. Total body water estimations in healthy men and women using bioimpedance spectroscopy: a deuterium oxide comparison. Nutr Metab (Lond). 2008;5(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jung SH, Ha KH, Kim DJ. Visceral Fat Mass Has Stronger Associations with Diabetes and Prediabetes than Other Anthropometric Obesity Indicators among Korean Adults. Yonsei Med J. 2016;57(3):674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chuang Y, Hsu K, Hwang C, Hu P, Lin T, Chiou W. Waist-to-Thigh Ratio Can also be a Better Indicator Associated with Type 2 Diabetes Than Traditional Anthropometrical Measurements in Taiwan Population. 2006;16(5):321–31. [DOI] [PubMed] [Google Scholar]
- 50.Qiao Q, Nyamdorj R. Is the association of type II diabetes with waist circumference or waist-to-hip ratio stronger than that with body mass index? Eur J Clin Nutr. 2010;64(1):30–4. [DOI] [PubMed] [Google Scholar]
- 51.Lu B, Zhou J, Waring ME, Parker DR, Eaton CB. Abdominal obesity and peripheral vascular disease in men and women: A comparison of waist-to-thigh ratio and waist circumference as measures of abdominal obesity. Atherosclerosis. 2010;208(1):253–7. [DOI] [PubMed] [Google Scholar]
- 52.Ng BK, Liu YE, Wang W, Kelly TL, Wilson KE, Schoeller DA, et al. Validation of rapid 4-component body composition assessment with the use of dual-energy X-ray absorptiometry and bioelectrical impedance analysis. The American Journal of Clinical Nutrition. 2018;108(4):708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith-Ryan AE, Mock MG, Ryan ED, Gerstner GR, Trexler ET, Hirsch KR. Validity and reliability of a 4-compartment body composition model using dual energy x-ray absorptiometry-derived body volume. Clin Nutr. 2017;36(3):825–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tinsley GM. Reliability and agreement between DXA-derived body volumes and their usage in 4-compartment body composition models produced from DXA and BIA values. J Sports Sci. 2018;36(11):1235–40. [DOI] [PubMed] [Google Scholar]
- 55.Wang Z, Pi-Sunyer FX, Kotler DP, Wielopolski L, Withers RT, Pierson RN, et al. Multicomponent methods: evaluation of new and traditional soft tissue mineral models by in vivo neutron activation analysis. The American Journal of Clinical Nutrition. 2002;76(5):968–74. [DOI] [PubMed] [Google Scholar]
- 56.Yahathugoda C, Weiler MJ, Rao R, De Silva L, Dixon JB, Weerasooriya MV, et al. Use of a Novel Portable Three-Dimensional Imaging System to Measure Limb Volume and Circumference in Patients with Filarial Lymphedema. The American Journal of Tropical Medicine and Hygiene. 2017;97(6):1836–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carl H, Walia G, Bello R, Clarke-Pearson E, Hassanein A, Cho B, et al. Systematic Review of the Surgical Treatment of Extremity Lymphedema. J Reconstr Microsurg. 2017;33(06):412–25. [DOI] [PubMed] [Google Scholar]
- 58.Stanton A, Northfield J, Holroyd B, Mortimer P, Levick J. Validation of an optoelectronic limb volumeter (Perometer®). Lymphology. 1997;30(2):77–97. [PubMed] [Google Scholar]
- 59.Mehrara BJ, Greene AK. Lymphedema and obesity: is there a link? Plast Reconstr Surg. 2014;134(1):154e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mastick J, Smoot BJ, Paul SM, Kober KM, Cooper BA, Madden LK, et al. Assessment of Arm Volume Using a Tape Measure Versus a 3D Optical Scanner in Survivors with Breast Cancer-Related Lymphedema. Lymphat Res Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wells JC. Three-dimensional optical scanning for clinical body shape assessment comes of age. The American Journal of Clinical Nutrition. 2019;110(6):1272–4. [DOI] [PubMed] [Google Scholar]
- 62.Stratton RJ, Hackston A, Longmore D, Dixon R, Price S, Stroud M, et al. Malnutrition in hospital outpatients and inpatients: prevalence, concurrent validity and ease of use of the ‘malnutrition universal screening tool’ (‘MUST’) for adults. Br J Nutr. 2004;92(5):799–808. [DOI] [PubMed] [Google Scholar]
- 63.Lohman TC Z Dual energy X-ray absorptiometry. In: Heymsfield S, Lohman TG, Wang Z, and Going SB, editor. In Human body composition. 2. Champaign, IL: Human Kinetics; 2005. p. 63–77. [Google Scholar]
- 64.Sager R, Güsewell S, Rühli F, Bender N, Staub K. Multiple measures derived from 3D photonic body scans improve predictions of fat and muscle mass in young Swiss men. PLoS One. 2020;15(6):e0234552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


