Abstract
Background
T cell immunoglobulin and mucin domain-containing protein 3 (TIM3) is a crucial immune checkpoint and is considered as an emerging target for cancer treatment. However, the clinical significance and immune-related role of TIM3+ cells in gastric cancer remain unknown. This study aimed to investigate the clinical significance of tumour-infiltrating TIM3+ cells and their association with immune contexture in gastric cancer.
Methods
This study enrolled three cohorts, including 436 tumour tissue microarray specimens and 58 fresh tumour tissues of gastric cancer patients from Zhongshan Hospital, and 330 transcriptional data from The Cancer Genome Atlas. TIM3+ cells and their association with CD8+ T cells were evaluated by immunohistochemistry and flow cytometry analyses. Kaplan–Meier curves, Cox model and interaction test were performed to assess clinical outcomes.
Results
Tumour-infiltrating TIM3+ cells’ high subgroups experienced poorer overall survival and disease-free survival and predicted inferior therapeutic responsiveness to fluorouracil-based adjuvant chemotherapy. TIM3 indicated CD8+ T cell dysfunction, which impeded chemotherapeutic responsiveness. Besides, HAVCR2 messenger RNA expression was associated with specific molecular characteristics.
Conclusions
The abundance of tumour-infiltrating TIM3+ cells could identify an immunoevasive subtype gastric cancer with CD8+ T cell dysfunction, suggesting that TIM3 might serve as a promising target for immunotherapy in gastric cancer.
Subject terms: Gastric cancer, Cancer microenvironment, Tumour immunology
Introduction
Gastric cancer is a significant factor of morbidity and mortality worldwide, ranking the fifth most frequently diagnosed cancer and the third leading cause of cancer-related death [1]. Although radical gastrectomy is the most effective treatment [2], advanced gastric cancer patients suffer a high disease relapse rate. Accordingly, fluorouracil-based adjuvant chemotherapy (ACT) is applied as a common first-line postoperative adjuvant therapy for advanced gastric cancer patients [3, 4]. However, patient survival benefit is still limited due to acquired chemoresistance [5, 6]. Therefore, a more accurate and comprehensive classification of gastric cancer patients for survival benefits and chemotherapeutic responsiveness is needed.
Recent advances in immunotherapy, such as immune checkpoint (ICP) inhibitors, have provided novel opportunities for cancer treatment by targeting the tumour microenvironment (TME) to reinvigorate antitumor immunity [7]. As shown in clinical trials KEYNOTE-059 [8], ATTRACTION-2 [9] and CheckMate-032 [10], ICP inhibitors targeting programmed cell death protein-1 (PD-1)/programmed cell death protein-1 ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) have been used for advanced or metastatic gastric cancer patients. Nevertheless, current ICP inhibitors provided survival benefits for only 10–20% of gastric cancer patients, and the underlying mechanisms that potentially improve ICP inhibitor response rates are still obscure. Thus, investigation of other coinhibitory receptors and immunoevasive mechanisms in gastric cancer is of urgent need.
T cell immunoglobulin and mucin domain-containing protein 3 (TIM3), encoded by HAVCR2 [11], is identified as an emerging target for cancer immunotherapy [12]. TIM3 is a type I membrane protein, which was originally found to be expressed in CD4+ and CD8+ T cells [13]. Recently, many studies have revealed the expression of TIM3 on multiple immune cells, including T helper type 1 (Th1) cells, Th17 cells, regulatory T (Treg) cells and innate immune cells [12, 14]. As an immunoregulatory molecule, TIM3 mediates T cell exhaustion by binding to its ligands, galectin-9 [15], carcinoembryonic antigen-related cell adhesion molecule 1 [16] and high-mobility group protein B1 [17]. Recently, several studies have reported the significant roles of TIM3 in various malignancies, including colon carcinoma, prostate cancer and clear cell renal cell carcinoma [11, 15, 18]. However, the clinical significance of TIM3 in gastric cancer still remains unknown.
In this study, we found that TIM3 could predict poor prognosis and inferior responsiveness to ACT in gastric cancer. Meanwhile, TIM3 indicated T cell dysfunction. Furthermore, TIM3-associated CD8+ T cell dysfunction yielded inferior chemotherapeutic responsiveness. In addition, we also investigated the relationship between HAVCR2 expression and molecular subtypes of gastric cancer. These results indicated the rationale that TIM3 might be a potential immunotherapeutic target for gastric cancer.
Methods
Study cohort
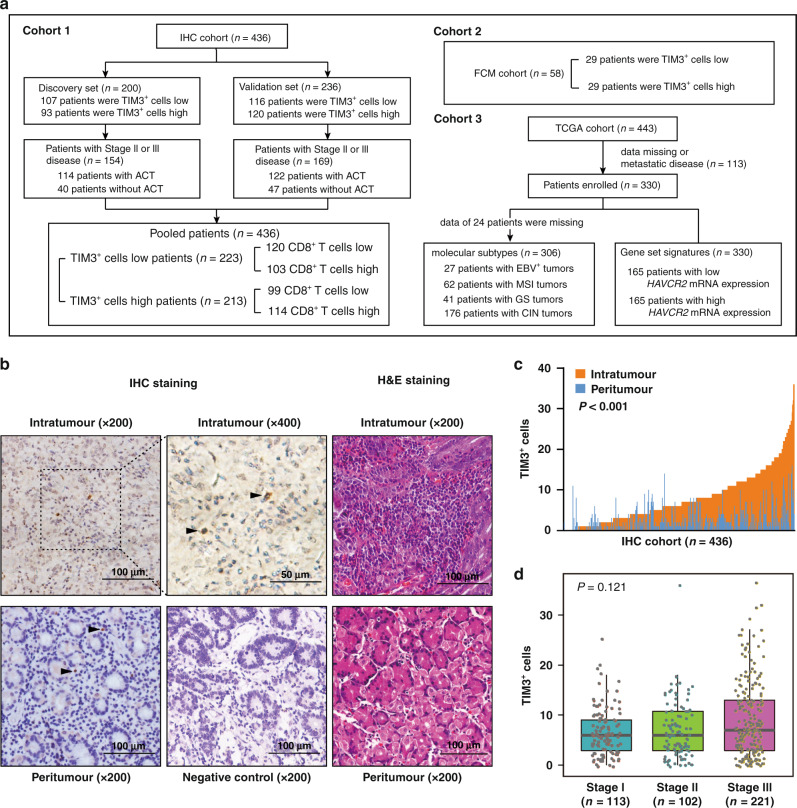
As the study design shown in Fig. 1a, three independent cohorts, immunohistochemistry (IHC) cohort, flow cytometry (FCM) cohort and The Cancer Genome Atlas (TCGA) cohort, were included in this study. IHC cohort was derived from 496 gastric cancer patients from Zhongshan Hospital, Fudan University (Shanghai, China), all of whom underwent gastrectomy between August 2007 and December 2008. After gastrectomy, patients with advanced tumours (stage II/III) routinely received fluorouracil-based ACT. No radiotherapy was performed on any of the patients. The tissue samples of IHC cohort were formalin-fixed, paraffin-embedded (FFPE) and constructed into tissue microarrays (TMAs) as we described previously [19]. A total of 60 patients were excluded owing to metastatic diseases, data missing or dot loss. The remaining 436 patients were randomly divided into two independent data sets, the Discovery set (n = 200) and the Validation set (n = 236). Clinicopathological characteristics were summarised in Supplementary Table S1. The pathological tumour stage was assessed according to the 7th edition of the American Joint Committee on Cancer Staging System. The endpoint of interest was overall survival (OS) and disease-free survival (DFS). OS was calculated from the date of gastrectomy to the date of death or the last follow-up, while DFS was calculated from the date of gastrectomy to the date of first recurrence or the last follow-up. Patients were observed until April 2014. FCM cohort recruited 60 gastric cancer patients from Zhongshan Hospital, Fudan University. These patients underwent gastrectomy between August 2018 and November 2018. During surgery, fresh tissue samples were obtained for FCM analysis. Corresponding 60 FFPE tissues were constructed as an independent TMA for TIM3+ cells evaluation, and two of them were excluded for dot loss. The third cohort was derived from RNA-sequencing data of 443 gastric cancer patients in the TCGA database, and 330 gastric cancer patients with available clinical information were included. This cohort also enrolled 306 gastric cancer patients with the data of molecular subtypes [20]. Four patients with polymerase epsilon subtype were integrated into the microsatellite instability (MSI) group characterised by hypermutated tumours. The cut-off values for TIM3+ cells and HAVCR2 expression were the median value (TIM3+ cells in IHC cohort: 6 cells, with ×200 magnification; TIM3+ cells in FCM cohort: 16 cells, with ×200 magnification; HAVCR2 expression in TCGA cohort: 260). This study was approved by the Clinical Research Ethics Committee of Zhongshan Hospital, Fudan University. Written informed consent has been obtained from all patients enrolled.
Fig. 1. TIM3+ cells are densely infiltrated in gastric cancer tissues and associated with disease progression.
a Flow diagram for characterisation of three cohorts involved in this study. b Representative images of immunohistochemistry (IHC) for TIM3+ cells (left and median panel) and haematoxylin–eosin (H&E) staining (right panel) in gastric tissues. Arrowheads show TIM3+ cells. c Comparison of TIM3+ cell infiltration between intratumoural (n = 436) and peritumoural (n = 436) gastric tissues based on IHC evaluation. Paired t test. d Boxplots display the association between intratumoural TIM3+ cell infiltration and TNM stage. Kruskal–Wallis test. P values less than 0.05 were shown in bold.
Immunohistochemistry
For IHC staining, all TMAs were constructed by Shanghai Outdo Biotech Co. TMA slides were baked at 60 °C for at least 6 h. Next, the slides were deparaffinized in xylene (three times, 15 min each, room temperature) and rehydrated in graded alcohol. Endogenous peroxidase was blocked with 3% H2O2 for 30 min. The slides were then immersed in a suitable buffer solution (according to the manufacturer’s instructions), heated for antigen retrieval and incubated with 10% goat serum at 37 °C for 2 h. Subsequently, rabbit anti-human TIM3 (1:500 dilution, ab185703, Abcam) and mouse anti-human CD8 (Ready-to-use, Clone C8/144B, Dako) primary antibodies were applied to incubate the slides overnight at 4 °C. After rinsing three times with 0.01 M phosphate buffer (pH = 8.0), the slides were incubated with the secondary antibody at 37 °C for 20 min and performed diaminobenzidine-H2O2 chromogenic reaction. Ultimately, the slides were counterstained with hematoxylin, dehydrated, and mounted with neutral resins.
Evaluation of TIM3+ cell density in IHC specimens
After IHC staining, the TMAs were scanned at high magnification (×200) under Nikon eclipse Ti-s microscope (Nikon, Tokyo, Japan). TIM3+ cells were stained with brown colour, which could be easily distinguished from purple TIM3− cells (Fig. 1b). We utilised NIS-Elements F3.2 to capture the three most representative microscopic fields for each intratumoural and peritumoural region. Two pathologists who were blinded to clinicopathological data scored all samples using Image J separately and variations exceeding five cells in the enumeration would be re-evaluated to reach a consensus. Ultimately, the average count of their evaluation was adopted as the final score.
Flow cytometry
Fresh tumour tissues were digested into single-cell suspension with collagenase IV at 37 °C for 2 h and filtered through a 70-μm cell strainer. The single-cell suspension was incubated with RBC lysis buffer (BD Biosciences) on ice for 10 min and then incubated with FcR-blocking reagent (BioLegend) for 15 min. Subsequently, cells were stained with fluorochrome-conjugated antibodies against membrane markers at 4 °C in the dark for 30 min. If necessary, cells were fixed with Fixation/Permeabilization Solution Kit (BD Biosciences) for 20 min and stained with intracellular fluorochrome-conjugated antibodies for 30 min. Stained cells were re-suspended in cell staining buffer and performed FCM analysis on BD FACSCelesta flow cytometer. Collected data were analysed by FlowJo v10.0 (Treestar). Dead cells were excluded according to the scatter profile. Antibodies involved were demonstrated in Supplementary Table 2.
Statistical analysis
Data were visualised as scatter plots and boxplots in this study. Each box of boxplots indicated the median and interquartile range of the data. Categorical variables were evaluated with χ2 test or Fisher’s exact test. For continuous variables, t test or Wilcoxon’s rank-sum test was performed. Kruskal–Wallis test followed by Dunn’s multiple comparisons test was applied for the correlation between HAVCR2 messenger RNA (mRNA) expression and TCGA gastric cancer subtypes. Kaplan–Meier curves were constructed to compare OS and DFS and detected by log-rank test. Cox proportional hazard regression model was performed to estimate effects on survival and interactions between covariates. Spearman’s rank correlation was utilised to evaluate the correlation between different variables. Gene Set Enrichment Analysis (GSEA) was used in the TCGA cohort to identify the pathways that were significantly enriched between high and low TIM3/HACVR2 tumour samples. The statistical analysis was performed by IBM SPSS Statistics v20.0, MedCalc v15.6, GSEA v4.1.0 and GraphPad Prism v8.3. All statistical tests were two-tailed and P < 0.05 was judged as statistically significant.
Results
Tumour-infiltrating TIM3+ cells are accumulated in gastric cancer
The distribution of TIM3+ cells was evaluated in 436 gastric cancer tissues by IHC; meanwhile, haematoxylin–eosin staining was performed for histological verification (Fig. 1b). The tumour tissue was derived from the tumour centre area, while the peritumoural tissue was obtained from the areas ≥2 cm from the tumour margin. Compared with peritumoural tissues, there were more TIM3+ cells in tumour tissues (P < 0.001; Fig. 1c). Consequently, we focused on TIM3+ cells in tumour tissues in our following study. The clinicopathological characteristics of gastric cancer patients with high or low TIM3+ cells in both the Discovery set and Validation set were summarised (Supplementary Table S1). Moreover, we did not observe a statistically significant association between the density of TIM3+ cells and TNM stages (P = 0.121; Fig. 1d).
Tumour-infiltrating TIM3+ cells predict poor prognosis in gastric cancer
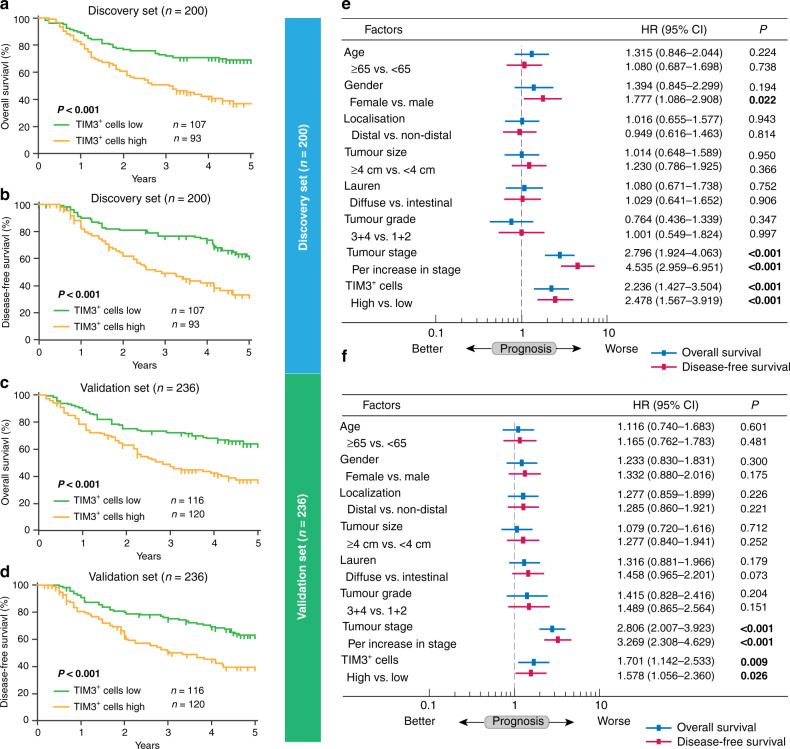
Then, we explored the potential prognostic value of TIM3+ cell infiltration. We found that in both Discovery set and Validation set, TIM3+ cells’ high subgroup experienced significantly poorer OS (P < 0.001 and P < 0.001; Fig. 2a, b) and DFS (P < 0.001 and P < 0.001; Fig. 2c, d). Multivariate Cox analysis identified TIM3+ cell infiltration as an independent risk factor for OS (hazard ratio (HR): 2.236, 95% confidence interval (CI): 1.427–3.504, P < 0.001 and HR: 1.701, 95% CI: 1.142–2.533, P = 0.009; Fig. 2e, f) and DFS (HR: 2.478, 95% CI: 1.567–3.919, P < 0.001 and HR: 1.578, 95% CI: 1.056–2.360, P = 0.026; Fig. 2e, f). Besides, TIM3+ cell density showed no association with TNM stage (Fig. 1d). Together, these data indicate that tumour-infiltrating TIM3+ cells could be an independent prognosticator for gastric cancer.
Fig. 2. Tumour-infiltrating TIM3+ cells predict poor prognosis in gastric cancer patients.
a–d Kaplan–Meier curves of overall survival (OS) and disease-free survival (DFS) based on IHC evaluation of tumour-infiltrating TIM3+ cells in the Discovery set (a, b) and Validation set (c, d). Log-rank test was utilised for the analysis. e, f Multivariate Cox analysis of OS and DFS was performed based on tumour-infiltrating TIM3+ cells and clinicopathological factors in the Discovery set (e) and Validation set (f). HR hazard ratio, CI confidence interval. P values less than 0.05 were shown in bold.
Tumour-infiltrating TIM3+ cells impede responsiveness to ACT in gastric cancer patients
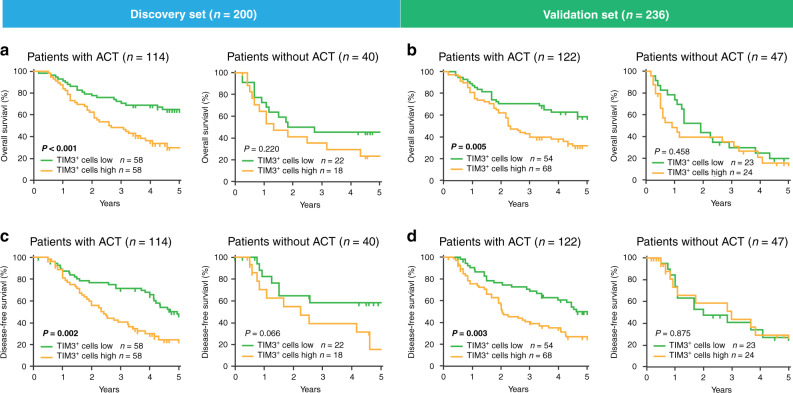
We further investigated the effect of TIM3+ cell infiltration on responsiveness to ACT in gastric cancer patients with stage II or III disease. In patients with fluorouracil-based ACT, TIM3+ cells high subgroup significantly experienced poor OS (P < 0.001 and P = 0.005; Fig. 3a, b, left panel) and DFS (P = 0.002 and P = 0.003; Fig. 3c, d, left panel). However, in patients without ACT treatment, high/low infiltration of TIM3+ cells could not predict survival benefits (Fig. 3a–d, right panel). Conclusively, these results suggest that tumour-infiltrating TIM3+ cells might indicate inferior responsiveness to ACT in gastric cancer patients.
Fig. 3. Relationship between tumour-infiltrating TIM3+ cells and responsiveness to ACT.
a Kaplan–Meier curves of overall survival (OS) between patients with high and low TIM3+ cell infiltration in ACT/Non-ACT subgroups in the Discovery set. b Kaplan–Meier curves of OS between patients with high and low TIM3+ cell infiltration in ACT/Non-ACT subgroups in the Validation set. c Kaplan–Meier curves of disease-free survival (DFS) between patients with high and low TIM3+ cell infiltration in ACT/Non-ACT subgroups in the Discovery set. d Kaplan–Meier curves of DFS between patients with high and low TIM3+ cell infiltration in ACT/Non-ACT subgroups in the Validation set. Log-rank test was utilised for the analysis. P values less than 0.05 were shown in bold.
Tumour-infiltrating TIM3+ cells are associated with dysfunctional CD8+ T cell phenotype
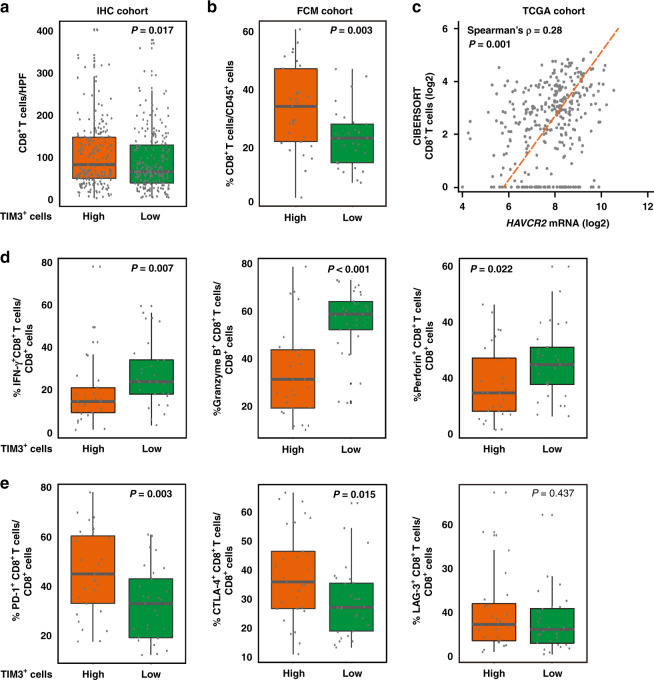
Because of the critical role of tumour immune microenvironment in survival outcomes and chemotherapy responsiveness, IHC analysis was applied for multiple immune cells in the TME of gastric cancer [21]. We found that in IHC cohort, infiltration of CD4+ T cells did not display significant difference between TIM3+ cells low and high subgroups (P = 0.197; Supplementary Fig. S1A), while CD8+ T cells, Foxp3+ Treg cells and CD56+ NK cells were enriched in TIM3+ cells high subgroup (P = 0.017, P = 0.002 and P = 0.055; Fig. 4a and Supplementary Fig. S1B, C). CD68+ macrophages showed more infiltration in neither TIM3+ cells low/high subgroups (P = 0.229; Supplementary Fig. S1D), whereas TIM3+ cells’ high subgroup possessed more CD163+ M2 macrophage infiltration and higher M2/M1 ratio (P < 0.001 and P = 0.009; Supplementary Fig. S1E, F), suggesting that TIM3 might be related to M2 polarisation of macrophage. Generally, TIM3 indicated an immunoevasive TME.
Fig. 4. Tumour-infiltrating TIM3+ cells are associated with dysfunctional CD8+ T cells.
a–c Association between TIM3+ cells and CD8+ T cells in IHC cohort (a) and FCM cohort (b), and Spearman’s correlation analysis of HAVCR2 mRNA expression and CD8+ T cells in TCGA cohort (c). Student’s t test for IHC cohort and Mann–Whitney U test for FCM cohort. CIBERSORT analysis was performed to detect CD8+ T cells. d, e Flow cytometry analysis was performed for evaluation of effector molecules (IFN-γ, Granzyme B and perforin) (d) and coinhibitory receptors (PD-1, CTLA-4 and LAG-3) (e) in CD8+ T cells between tumour-infiltrating TIM3+ cells’ low and high subgroups. Mann–Whitney U test for (b) and LAG-3 in (c); Student’s t test for PD-1 and CTLA-4 in (c). IFN-γ interferon-γ, PD-1 programmed death-1, CTLA-4 cytotoxic T-lymphocyte-associated protein-4, LAG-3 lymphocyte-activation gene-3. P values less than 0.05 were shown in bold.
Considering CD8+ T cells are essential to adaptive immune resistance associated with TIM3 expression in malignancies, we further evaluated the relationship between TIM3 and CD8+ T cell functional phenotype. Interestingly, TIM3+ cells high subgroup contained higher numbers of CD8+ T cells in FCM cohorts as well (P = 0.003; Fig. 4b). Correlation analysis in the TCGA cohort confirmed a positive association between TIM3 (HAVCR2) and CD8+ T cells (P < 0.001; Fig. 4c). We next investigated the phenotypes of CD8+ T cells in TIM3+ cells’ low/high subgroups. As shown in Fig. 4d, e, CD8+ T cells in TIM3 high subgroup exhibited an exhausted phenotype, featured by decreased interferon-γ (IFN-γ), granzyme B (GzmB) and perforin expression (P = 0.007, P < 0.001 and P = 0.022; Fig. 4d), yet elevated PD-1 and CTLA-4 expression (P = 0.003 and P = 0.015; Fig. 4e). Furthermore, by means of GSEA, EXHAUSTED_VS_MEMORY_CD8_TCELL_UP gene set was found significantly enriched in HAVCR2 high tumours in gastric cancer patients (Supplementary Fig. S2), revealing the relationship between TIM3/HAVCR2 high expression and exhausted CD8+ T cells. Together, these findings suggest that TIM3 might potentially facilitate CD8+ T cell dysfunction in gastric cancer.
TIM3-associated CD8+ T cell dysfunction indicates inferior chemotherapeutic responsiveness
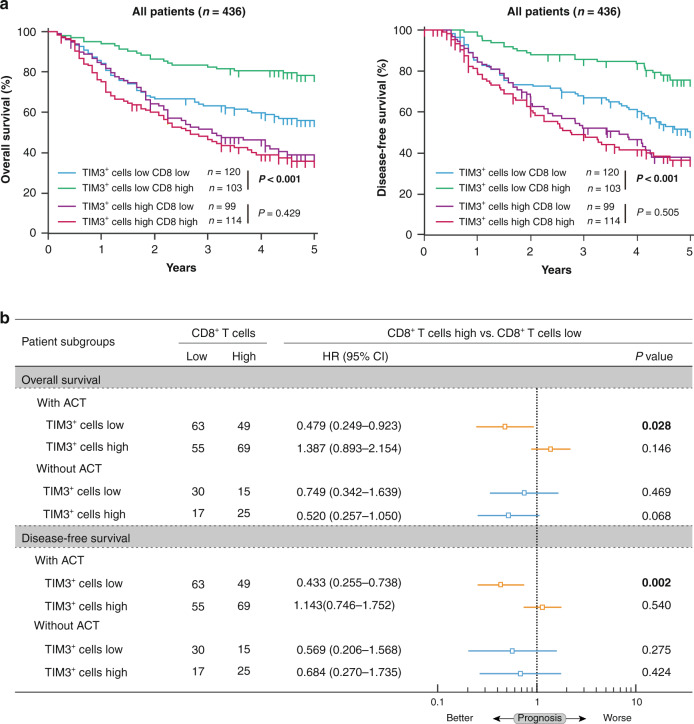
To further explore whether TIM3 affects the prognostic value of CD8+ T cells in gastric cancer, we divided all patients into four subgroups based on TIM3 and CD8+ T cells for survival analysis (Supplementary Fig. S3A, B). Interestingly, we observed that CD8+ T cells could only predict better OS and DFS in TIM3+ cells’ low subgroup (P < 0.001 and P < 0.001; Fig. 5a, left panel) compared with that in TIM3+ cells’ high subgroup (P = 0.429 and P = 0.505; Fig. 5a, right panel). These results support our observation that TIM3+ cells might be related to CD8+ T cell dysfunction in gastric cancer. To further investigate whether TIM3-associated CD8+ T cell dysfunction impacts chemotherapeutic responsiveness, we divided stage II or III patients into four subgroups based on TIM3+ cells and CD8+ T cell infiltration. We observed that only in the patients who received ACT and with low TIM3+ cell infiltration, CD8+ T cells could predict better OS and DFS (HR: 0.479, 95% CI: 0.249-0.923, P = 0.028 and HR: 0.443, 95% CI: 0.255-0.738, P = 0.002; Fig. 5b). However, in the patients who received ACT and with high TIM3+ cell infiltration, or within the patients who did not receive ACT, CD8+ T cells could not predict survival benefits (Fig. 5b). Conclusively, these data indicate that inferior chemotherapeutic responsiveness in tumour-infiltrating TIM3+ cells’ high subgroup might be attributed to TIM3-associated CD8+ T cell dysfunction.
Fig. 5. TIM3-associated CD8+ T cell dysfunction indicates inferior chemotherapeutic responsiveness in gastric cancer.
a Kaplan–Meier curves of overall survival (left panel) and disease-free survival (right panel) based on the combination of CD8+ T cells and tumour-infiltrating TIM3+ cells in all patients. Log-rank test was utilised for the analysis. b Multivariate Cox analysis of overall survival and disease-free survival was performed based on CD8+ T cell infiltration in TIM3 low and high subgroups, with ACT treatment or not. HR hazard ratio, CI confidence interval. P values less than 0.05 were shown in bold.
Association between HAVCR2 expression and TCGA subtypes in gastric cancer
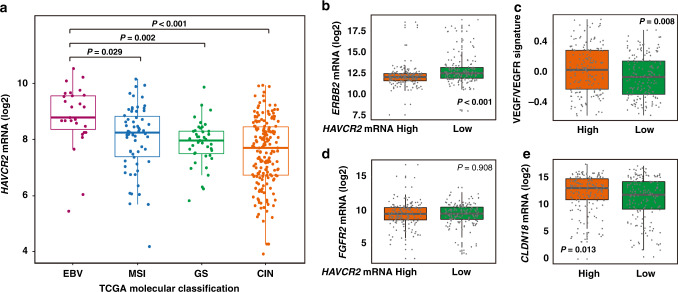
Growing studies have revealed that molecular subtypes of gastric cancer opened new perspectives for patient stratification and individualised therapy [22]. Therefore, we also analysed the relationship between HAVCR2 mRNA expression and molecular subtypes in the TCGA cohort. Interestingly, although intensive in most gastric cancer subtypes, HAVCR2 mRNA expression was significantly enriched in Epstein–Barr virus (EBV)-positive subtype (Fig. 6a).
Fig. 6. Characterisation of molecular subtypes based on HAVCR2 mRNA expression.
a Comparison of HAVCR2 mRNA expression in TCGA database across 2018 TCGA molecular classification. Kruskal–Wallis test followed by Dunn’s multiple comparisons. b, e Comparison of ERBB2 mRNA expression (b), VEGF/VEGFR signature (c), FGFR2 mRNA expression (d), CLDN18 mRNA expression (e) between low and high HAVCR2 mRNA expression subgroups in TCGA database. Mann–Whitney U test for (b); Student’s t test for (c–e). EBV Epstein–Barr virus, MSI microsatellite instability, GS genomically stable, CIN chromosomal instability. P values less than 0.05 were shown in bold.
Since we have observed that TIM3 could predict inferior chemotherapeutic responsiveness (Fig. 3), we sought to discover the potential association between TIM3 and responsiveness to other treatment strategies in gastric cancer [22]. The addition of some targeted therapies like Trastuzumab and Ramucirumab have added a modest benefit, but only in human epidermal growth factor receptor 2 (ERBB2 or HER2)-positive patients and in the second-line setting, respectively [23]. Notably, we found that HAVCR2 high tumours showed significantly lower ERBB2 mRNA expression, indicating that HAVCR2/TIM3 high tumours might be less responsive to Trastuzumab-targeted therapy (P < 0.001; Fig. 6b). However, the HAVCR2 high tumours showed higher VEGF/VEGFR signature (P = 0.008; Fig. 6c and Supplementary Table S3), suggesting that HAVCR2/TIM3 high tumours might be more sensitive to Ramucirumab-based treatment. Bemarituzumab and Zolbetuximab were emerging targeted therapies for gastric cancer, having brought benefit to FGFR-2b-positive patients and Claudin 18.2 (CLDN18)-positive patients, respectively [24, 25]. No obvious difference was observed regarding FGFR2 expression in HAVCR2 low/high subgroups (P = 0.908; Fig. 6d), but HAVCR2 high tumours showed higher expression of CLDN18 (P = 0.013; Fig. 6e), indicating superior responsiveness to Zolbetuximab-targeted therapy. Cumulatively, our results indicate that HAVCR2/TIM3 expression was associated with molecular subtypes and might indicate responsiveness to targeted therapies in gastric cancer.
Discussion
Recently, an immunotherapy that targets the ICP pathway has provided novel strategies for the treatment of malignant tumours [26]. Nevertheless, the overall response rates are unsatisfying and many advanced cancer patients develop adaptive resistance [27]. To overcome the immunotherapy resistance and improve clinical outcomes, further studies found that upregulation of other ICPs, notably TIM3, might be associated with adaptive resistance to anti-PD-1 blockade [28, 29], attracting researchers’ attention to another coinhibitory receptor, TIM3. Monoclonal antibodies targeting TIM3 were discovered to display antitumor effects in preclinical models of hepatocellular carcinoma [30, 31]. In melanoma, prostate tumour and colon carcinoma, the combined blocking of PD-1 and TIM3 pathways demonstrated synergistic antitumor effects compared with blocking either pathway alone [32, 33]. A comprehensive profiling of gastric adenocarcinoma with peritoneal metastasis also proposed that TIM3 could be a novel target with potential therapeutic value [34]. Therefore, further studies on the clinical significance of TIM3 in gastric cancer is crucial. In this study, we found that tumour-infiltrating TIM3+ cells could serve as an independent adverse prognosticator for survival and chemotherapeutic resistance in gastric cancer, indicating that TIM3 might be a predictive biomarker and a therapeutic target for gastric cancer.
Adaptive immune responses triggered by adaptive immune cells within TME critically contribute to the antigen-specific immune response against tumours, affecting survival outcomes and progression, and CD8+ T cells are regarded as the main force in antitumor immune responses [35]. Current studies revealed that TIM3 expressed on CD8+ T cells exhibited an exhausted phenotype in several kinds of cancers, including melanoma, hepatocellular carcinoma and ovarian cancer [14, 36, 37]. Our results consistently found that tumour-infiltrating TIM3+ cells correlated with dysfunctional CD8+ T cells characterised by decreased IFN-γ, perforin and GzmB, yet elevated PD-1 and CTLA-4 in gastric cancer. In addition, TIM3-associated CD8+ T cell dysfunction was also found to attenuate chemotherapeutic responsiveness of gastric cancer patients, providing a new treatment strategy to improve the efficiency of chemotherapies.
Through comprehensive analyses of genomic and epigenomic data, the molecular classification of gastric cancer proposed by TCGA has expanded our understanding of the characteristics of gastric cancer, which could unmask tumour biological properties and guide therapeutic options for gastric cancer [38]. Here, we found that compared with chromosomal instability (CIN) subtype, HAVCR2 mRNA expression was higher in EBV-positive and MSI subtypes of gastric cancer, which were characterised by increased immune signatures and superior responsiveness to immunotherapy [39]. Thus, we inferred ICP blockade therapies may potentially take effect in these tumours. Meanwhile, HAVCR2 high tumours also indicated decreased ERBB2 mRNA expression, yet enhanced angiogenesis. Targeting ERBB2/HER2 has added a modest benefit in gastric cancer, but only in ERBB2/HER2-positive patients [23]. As a result, detecting TIM3 in gastric cancer might identify the patients who might show inferior responsiveness to Trastuzumab, and targeting TIM3 and ERBB2/HER2 might have a synergistic effect in gastric cancer. However, the relationship between HAVCR2 mRNA expression and certain molecular characterisations in gastric cancer and the detailed mechanisms underlying the predictive value of HAVCR2 mRNA expression in patient stratification and personalised therapeutic strategies still required further investigation in our following studies.
Conclusively, we found that tumour-infiltrating TIM3+ cells could serve as an independent adverse prognosticator for survival and chemotherapeutic resistance in gastric cancer. Moreover, TIM3+ cell infiltration was associated with exhausted CD8+ T cell phenotype and TIM3-associated CD8+ T cell dysfunction might attenuate chemotherapeutic responsiveness. Therefore, our study indicated that TIM3 might be a promising target for immunotherapy in gastric cancer.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We thank Dr. Lingli Chen (Department of Pathology, Zhongshan Hospital, Fudan University, Shanghai, China) and Dr. Yunyi Kong (Department of Pathology, Shanghai Cancer Centre, Fudan University, Shanghai, China) for their excellent pathological technology help.
Author contributions
K.C., Y.G. and Y.C.: acquisition of data, analysis and interpretation of data, statistical analysis and drafting of the manuscript; H.F., K.L., X.L., X.H., J.W., C.L., H.L., H.Z. and H.H.: technical and material support; J.X., H.L. and R.L.: study concept and design, analysis and interpretation of data, drafting of the manuscript and obtained funding and study supervision. All authors read and approved the final manuscript.
Funding information
This study was funded by grants from the National Natural Science Foundation of China (31770851, 81871930, 81902402, 81902901, 81972219, 82003019 and 82103313) and Shanghai Sailing Programme (18YF1404600, 19YF1407500 and 21YF1407600). All the sponsors have no roles in the study design, in the collection, analysis and interpretation of data.
Data availability
All data generated that are relevant to the results presented in this article are included in this article. Other data that were not relevant for the results presented here are available from the corresponding author Dr. Xu upon reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was approved by the Clinical Research Ethics Committee of Zhongshan Hospital, Fudan University. Written informed consent was obtained from each patient included and this study was performed in accordance with the Declaration of Helsinki.
Consent to publish
All authors provided their consent for the publication of the manuscript.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ke Chen, Yun Gu, Yifan Cao.
Contributor Information
Jiejie Xu, Email: jjxufdu@fudan.edu.cn.
He Li, Email: li.he@zs-hospital.sh.cn.
Ruochen Li, Email: rcli12@fudan.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01607-3.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–49. doi: 10.1016/s1470-2045(10)70070-x. [DOI] [PubMed] [Google Scholar]
- 3.De Vita F, Orditura M, Matano E, Bianco R, Carlomagno C, Infusino S, et al. A phase II study of biweekly oxaliplatin plus infusional 5-fluorouracil and folinic acid (FOLFOX-4) as first-line treatment of advanced gastric cancer patients. Br J Cancer. 2005;92:1644–9. doi: 10.1038/sj.bjc.6602573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishida T. Adjuvant therapy for gastric cancer after D2 gastrectomy. Lancet. 2012;379:291–2. doi: 10.1016/s0140-6736(11)61928-4. [DOI] [PubMed] [Google Scholar]
- 5.Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389–96. doi: 10.1016/s1470-2045(14)70473-5. [DOI] [PubMed] [Google Scholar]
- 6.Smyth EC, Fassan M, Cunningham D, Allum WH, Okines AF, Lampis A, et al. Effect of pathologic tumor response and nodal status on survival in the Medical Research Council Adjuvant Gastric Infusional Chemotherapy Trial. J Clin Oncol. 2016;34:2721–7. doi: 10.1200/jco.2015.65.7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smyth MJ, Ngiow SF, Ribas A, Teng MW. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol. 2016;13:143–58. doi: 10.1038/nrclinonc.2015.209. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018;4:e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang Y-K, Boku N, Satoh T, Ryu M-H, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–71. doi: 10.1016/s0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 10.Janjigian YY, Bendell J, Calvo E, Kim JW, Ascierto PA, Sharma P, et al. CheckMate-032 Study: efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol. 2018;36:2836–44. doi: 10.1200/jco.2017.76.6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolf Y, Anderson AC, Kuchroo VK. TIM3 comes of age as an inhibitory receptor. Nat Rev Immunol. 2020;20:173–85. doi: 10.1038/s41577-019-0224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedlaender A, Addeo A, Banna G. New emerging targets in cancer immunotherapy: the role of TIM3. ESMO Open. 2019;4:e000497. doi: 10.1136/esmoopen-2019-000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao X, Zhu Y, Li G, Huang H, Zhang G, Wang F, et al. TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PLoS ONE. 2012;7. 10.1371/journal.pone.0030676. [DOI] [PMC free article] [PubMed]
- 14.Du W, Yang M, Turner A, Xu C, Ferris RL, Huang J, et al. TIM-3 as a target for cancer immunotherapy and mechanisms of action. Int J Mol Sci. 2017;18. 10.3390/ijms18030645. [DOI] [PMC free article] [PubMed]
- 15.Das M, Zhu C, Kuchroo VK. Tim-3 and its role in regulating anti-tumor immunity. Immunol Rev. 2017;276:97–111. doi: 10.1111/imr.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang YH, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature. 2015;517:386–90. doi: 10.1038/nature13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang D, Lotze MT. Tumor immunity times out: TIM-3 and HMGB1. Nat Immunol. 2012;13:808–10. doi: 10.1038/ni.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He Y, Cao J, Zhao C, Li X, Zhou C, Hirsch FR. TIM-3, a promising target for cancer immunotherapy. Onco Targets Ther. 2018;11:7005–9. doi: 10.2147/ott.S170385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao Y, Liu H, Li H, Lin C, Li R, Wu S, et al. Association of O6-methylguanine-DNA methyltransferase protein expression with postoperative prognosis and adjuvant chemotherapeutic benefits among patients with stage II or III gastric cancer. JAMA Surg. 2017;152:e173120. doi: 10.1001/jamasurg.2017.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Sethi NS, Hinoue T, Schneider BG, Cherniack AD, Sanchez-Vega F, et al. Comparative molecular analysis of gastrointestinal adenocarcinomas. Cancer Cell. 2018;33:721–35 e8. doi: 10.1016/j.ccell.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Cao Y, Li R, Gu Y, Chen Y, Qi Y, et al. Poor clinical outcomes of intratumoral dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin-positive macrophages associated with immune evasion in gastric cancer. Eur J Cancer. 2020;128:27–37. doi: 10.1016/j.ejca.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Zeng H, Zhou Q, Wang Z, Zhang H, Liu Z, Huang Q, et al. Stromal LAG-3(+) cells infiltration defines poor prognosis subtype muscle-invasive bladder cancer with immunoevasive contexture. J Immunother Cancer. 2020;8. 10.1136/jitc-2020-000651. [DOI] [PMC free article] [PubMed]
- 23.Alsina M, Moehler M, Hierro C, Guardeño R, Tabernero J. Immunotherapy for gastric cancer: a focus on immune checkpoints. Target Oncol. 2016;11:469–77. doi: 10.1007/s11523-016-0421-1. [DOI] [PubMed] [Google Scholar]
- 24.FGFR inhibitor stymies gastric cancer. Cancer Discov. 2021. 10.1158/2159-8290.Cd-nb2021-0312. [DOI] [PubMed]
- 25.Sahin U, Türeci Ö, Manikhas G, Lordick F, Rusyn A, Vynnychenko I, et al. FAST: A randomised phase II study of zolbetuximab (IMAB362) plus EOX vs EOX alone for first-line treatment of advanced CLDN18.2 positive gastric and gastro-oesophageal adenocarcinoma. Ann Oncol. 2021. 10.1016/j.annonc.2021.02.005. [DOI] [PubMed]
- 26.Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. 2020;367. 10.1126/science.aax0182. [DOI] [PMC free article] [PubMed]
- 27.He X, Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020;30:660–9. doi: 10.1038/s41422-020-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shayan G, Srivastava R, Li J, Schmitt N, Kane LP, Ferris RL. Adaptive resistance to anti-PD1 therapy by Tim-3 upregulation is mediated by the PI3K-Akt pathway in head and neck cancer. Oncoimmunology. 2017;6:e1261779. doi: 10.1080/2162402x.2016.1261779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou G, Sprengers D, Boor PPC, Doukas M, Schutz H, Mancham S, et al. Antibodies against immune checkpoint molecules restore functions of tumor-infiltrating T cells in hepatocellular carcinomas. Gastroenterology. 2017;153:1107–19.e10. doi: 10.1053/j.gastro.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 31.Liu F, Zeng G, Zhou S, He X, Sun N, Zhu X, et al. Blocking Tim-3 or/and PD-1 reverses dysfunction of tumor-infiltrating lymphocytes in HBV-related hepatocellular carcinoma. Bull Cancer. 2018;105:493–501. doi: 10.1016/j.bulcan.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 32.Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-γ-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71:3540–51. doi: 10.1158/0008-5472.Can-11-0096. [DOI] [PubMed] [Google Scholar]
- 33.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–94. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang R, Song S, Harada K, Ghazanfari Amlashi F, Badgwell B, Pizzi MP, et al. Multiplex profiling of peritoneal metastases from gastric adenocarcinoma identified novel targets and molecular subtypes that predict treatment response. Gut. 2020;69:18–31. doi: 10.1136/gutjnl-2018-318070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reiser J, Banerjee A. Effector, memory, and dysfunctional CD8(+) T cell fates in the antitumor immune response. J Immunol Res. 2016;2016:8941260. doi: 10.1155/2016/8941260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu F, Liu Y, Chen Z. Tim-3 expression and its role in hepatocellular carcinoma. J Hematol Oncol. 2018;11:126. doi: 10.1186/s13045-018-0667-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fucikova J, Rakova J, Hensler M, Kasikova L, Belicova L, Hladikova K, et al. TIM-3 dictates functional orientation of the immune infiltrate in ovarian cancer. Clin Cancer Res. 2019;25:4820–31. doi: 10.1158/1078-0432.Ccr-18-4175. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Sethi NS, Hinoue T, Schneider BG, Cherniack AD, Sanchez-Vega F, et al. Comparative molecular analysis of gastrointestinal adenocarcinomas. Cancer Cell. 2018;33:721-+. doi: 10.1016/j.ccell.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim ST, Cristescu R, Bass AJ, Kim K-M, Odegaard JI, Kim K, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24:1449–+. doi: 10.1038/s41591-018-0101-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated that are relevant to the results presented in this article are included in this article. Other data that were not relevant for the results presented here are available from the corresponding author Dr. Xu upon reasonable request.