Abstract
Artificial intelligence (AI) is concretely reshaping the landscape and horizons of oncology, opening new important opportunities for improving the management of cancer patients. Analysing the AI-based devices that have already obtained the official approval by the Federal Drug Administration (FDA), here we show that cancer diagnostics is the oncology-related area in which AI is already entered with the largest impact into clinical practice. Furthermore, breast, lung and prostate cancers represent the specific cancer types that now are experiencing more advantages from AI-based devices. The future perspectives of AI in oncology are discussed: the creation of multidisciplinary platforms, the comprehension of the importance of all neoplasms, including rare tumours and the continuous support for guaranteeing its growth represent in this time the most important challenges for finalising the ‘AI-revolution’ in oncology.
Subject terms: Cancer, Developing world
Introduction
Artificial intelligence (AI) is concretely reshaping our lives and it is time to understand its evolution and achievements to model future development strategies. This is true also for oncology and related fields, where AI is now opening new important opportunities for improving the management of cancer patients, as will be highlighted in this perspective paper.
In 1950, Alan Turing was the first that conceives the idea of using computers to mimic intelligent behaviour and critical thinking [1]. In 1956, John McCarthy coined the term ‘artificial intelligence’ as ‘the science and engineering of making intelligent machines’ [1, 2]. AI began as a simple series of ‘if, then rules’, and has advanced in subsequent years for encompassing multifaceted and composite algorithms that perform similarly to the human brain [1].
Nowadays, AI represents an emerging and rapidly evolving model that regards different scientific fields, also those devoted to the management of cancer patients [2–5]. It can be seen as a general concept indicating the ability of a machine to learn and recognise patterns and interactions from a sufficient number of representative models, and to use this information for improving the current approach towards the process of decision-making in a specific field [3–5].
In precision oncology, AI is reshaping the existing scenario, aiming at integrating the large amount of data derived from multi-omics analyses with current advances in high-performance computing and groundbreaking deep-learning strategies [3]. Notably, the applications of AI are expanding and include new approaches for cancer detection, screening, diagnosis and classification, the characterisation of cancer genomics, the analysis of tumour microenvironment, the assessment of biomarkers with prognostic and predictive purposes and of strategies for follow-up and drug discovery [3–6].
For better understanding current roles and future perspectives of AI, two important terms/definitions, which are strictly associated with AI, should be enlightened: machine learning and deep learning. Machine learning is a general concept indicating the ability of a machine in learning and thus improving patterns and models of analysis, whereas deep learning indicates a machine-learning method that utilises complex and deep networks to finalise a highly predictive performance [3, 4]. Of note, these two concepts are central also in the AI revolution in the management of cancer patients.
Through a systematic review-based approach, we aim to clarify which are the current applications of AI in oncology-related fields, with a specific focus on already-approved devices. This approach will allow to better understand roles and potentialities of AI in the management of cancer patients, representing also a reliable point of start for discussing the most important future perspectives of AI in this field.
Methods
The systematic review-based approach adhered to the PRISMA statement preset protocol [7]. For providing a comprehensive portrait of the current situation of the roles played by AI in the management of cancer patients, a systematic review was performed, investigating the AI-based devices that have already obtained an official approval for entering into clinical practice in oncology and its related fields. To this aim, two authors (C.L. and A.P.) retrieved all AI-based devices that have obtained the Federal Drug Administration (FDA) approval in oncology-related fields, extracting all potential data by searching FDA official databases (https://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm514737.pdf; https://www.fda.gov/media/145022/download; https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/denovo.cfm; https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm; https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm. Last access for all documents: 05/31/2021. Such data were also integrated with all previous related reviews or commentaries. All data were organised to be separately presented by the specific oncologic areas in the text, as well as in a summary figure (Fig. 1).
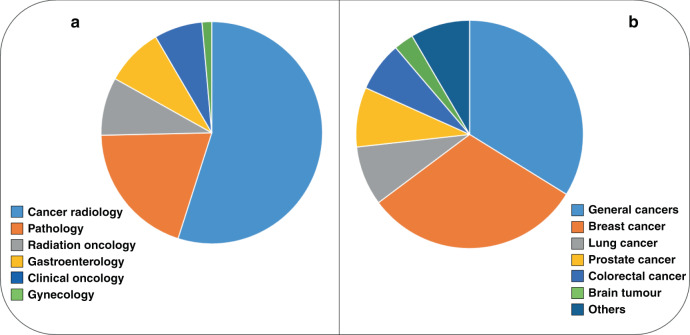
Fig. 1. Current status of Artificial intelligence in oncology and related fields.
Summarising representations of the artificial intelligence-based devices, FDA-approved, expressed by oncology-related specialties (a: cancer radiology 54.9%, pathology 19.7%, radiation oncology 8.5%, gastroenterology 8.5%, clinical oncology 7.0% and gynaecology 1.4%) and by tumour types (b: general cancers 33.8%, breast cancer 31.0%, lung cancer 8.5%, prostate cancer 8.5%, colorectal cancer 7.0% and brain tumours 2.8%, others: 6 tumour types, 1.4% each).
Results
Altogether, the search documented the presence of 71 AI-associated or AI-associable devices that have already received an official FDA approval (Table 1), matching data also from previous related reviews [2, 8–10]. The oncology-related field that counts for the largest number of AI devices is cancer radiology, with the majority of approved devices (54.9%). It is followed by pathology (19.7%), radiation oncology (8.5%), gastroenterology (8.5%), clinical oncology (7.0%) and gynaecology 1 (1.4%) (Table 1, Fig. 1a). The vast majority of the approved devices (>80%) regarded the complex area of cancer diagnostics.
Table 1.
List of AI-associated/associable equipped medical devices approved by the US FDA specifically for oncology-related fields.
| N° | Month of approval | Name of the device | Description of the device and its role | Specific area of interest |
|---|---|---|---|---|
| 1 | February 2015 | ER APP, Breast Cancer (Visiopharm A/S) | Determination of oestrogen receptor positivity and negativity in breast cancer | Pathology |
| 2 | February 2015 | PR APP, Breast Cancer (Visiopharm A/S) | Determination of progesterone receptor positivity and negativity in breast cancer | Pathology |
| 3 | August 2015 | Kaiku Health (Kaiku Oy) | Outcome monitoring and symptom tracking for cancer patients | Clinical Oncology |
| 4 | November 2015 | ClearRead CT (Riverain Technologies LLC.) | Assistance in review of multi-slice computed tomography exams of the chest and detection of potential nodules that radiologist should review | Cancer Radiology |
| 5 | December 2015 | Transpara (ScreenPoint Medical BV) | Reading aid for physicians interpreting screening mammograms to identify regions suspicious for breast cancer | Cancer Radiology |
| 6 | June 2016 | SmartTarget (SmartTarget Ltd.) | Image-guided interventional and diagnostic procedures involving the prostate gland | Cancer Radiology |
| 7 | September 2016 | Eclipse Treatment Planning System V15.6 (arian Medical Systems Inc.) | Radiotherapy treatment planning for patients with malignant or benign diseases | Radiation Oncology |
| 8 | August 2016 | LungQ (Thirona Corp.) | Support in diagnosis and documentation of pulmonary tissues images (eg, abnormalities) from CT thoracic datasets | Cancer Radiology |
| 9 | March 2017 | ColonFlag (Medial EarlySign Inc.) | High risk colorectal cancer detection support for pre-symptomatic patients | Gastroenterology |
| 10 | May 2017 | AmCAD-US (AmCad BioMed Corporation) | A software to visualise and quantify ultrasound image data with backscattered signals. | Cancer Radiology |
| 11 | June 2017 | C the Signs (C the Signs Ltd.) | Assessment of symptoms to support cancer diagnosis | Clinical Oncology |
| 12 | July 2017 | QuantX (Quantitative Insights) | An AI-equipped diagnosis system to aid in accurate diagnosis of breast cancer. | Cancer Radiology |
| 13 | December 2017 | Veye Chest (Aidence BV) | Pulmonary nodule detection support from CT scans | Cancer Radiology |
| 14 | January 2018 | Arterys Oncology DL (Arterys) | An AI-based, cloud-based medical imaging software that automatically measures and tracks lesions and nodules in MRI and CT. | Cancer Radiology |
| 15 | January 2018 | GI Genius (Medtronic Inc. (parent company: Medtronic plc.)) | Colorectal cancer detection support | Gastroenterology |
| 16 | January 2018 | QVCAD (QView Medical Inc.) | Aid to detect mammography-occult lesions in regions not known to have suspicious findings | Cancer Radiology |
| 17 | February 2018 | DLCExpert (Mirada Medical Ltd.) | Contouring assistance for radiation therapy from CT scans | Radiation Oncology |
| 18 | May 2018 | HealthMammo (Zebra Medical Vision Inc.) | Mammograms processed and analysed for suspected breast cancer lesions | Cancer Radiology |
| 19 | July 2018 | Arterys Oncology DL (Arterys Inc.) | Support of oncological workflow by helping user confirm absence or presence of lesions; application supports anatomical datasets, such as CT or MR | Cancer Radiology |
| 20 | October 2018 | Hot Spot APP (Visiopharm A/S) | Hotspot scoring method for various cancer applications | Pathology |
| 21 | October 2018 | Invasive Tumour Detection APP (Visiopharm A/S) | Cytokeratin and p63 marker assessment for invasive and non-invasive tumour distinguishment | Pathology |
| 22 | October 2018 | AmCAD-UT (AmCad BioMed Corporation) | Assistance in analysis of thyroid ultrasound images | Cancer Radiology |
| 23 | October 2018 | Mia -Mammography Intelligent Assessment (KheironMedical Technologies Ltd.) | Breast cancer detection support from mammograms | Cancer Radiology |
| 24 | October 2018 | Arterys MICA (Arterys) | An AI-based platform for analysing medical images such as MRI and CT. | Cancer Radiology |
| 25 | November 2018 | SubtlePET (Subtle Medical) | An AI-powered technology that enables centers to deliver a faster and safer patient scanning experience, while enhancing exam throughput and provider profitability. | Cancer Radiology |
| 26 | February 2019 | DERM (Skin Analytics Ltd.) | Skin cancer diagnosis support | Clinical Oncology |
| 27 | February 2019 | ART-Plan.annotate (heraPanacea SAS) | Contouring of tumour and surrounding organs for radiotherapy | Radiation Oncology |
| 28 | March 2019 | cmTriage (CureMetrix) | An AI-based triage software for mammography. | Cancer Radiology |
| 29 | April 2019 | Deep Learning Image Reconstruction (GE Medical Systems) | A deep-learning-based CT image reconstruction technology. | Cancer Radiology |
| 30 | April 2019 | Auto Lung Nodule Detection (Samsung Electronics Co. Ltd. (parent company: Samsung Group) | Lung nodule detection for diagnostic support from X-ray images | Cancer Radiology |
| 31 | May 2019 | JPC-01K (JLK Inspection Inc.) | Prostate cancer detection for diagnostic support from MRI images | Cancer Radiology |
| 32 | May 2019 | syngo.Breast Care (Siemens Healthcare GmbH (parent company: Siemens AG)) | Reading and reporting for diagnostic support from mammograms | Cancer Radiology |
| 33 | June 2019 | Aquilion ONE (TSX-305A/6) V8.9 with AiCE (Canon MedicalSystems Corporation) | A device to acquire and display cross-sectional volumes of the whole body, including the head, with the capability of imaging whole organs in a single rotation. | Cancer Radiology |
| 34 | July 2019 | ProFound AI for 2D Mammography (iCAD Inc.) | Breast cancer detection assistance and workflow solution from 2D mammograms | Cancer Radiology |
| 35 | July 2019 | ProFound AI for Digital Breast Tomosynthesis (iCAD Inc.) | Computer-assisted detection and diagnosis (CAD) software device intended to be used while reading digital breast tomosynthesis (DBT) exams | Cancer Radiology |
| 36 | July 2019 | RayCare 2.3 (RaySearch Laboratories) | An oncology information system used to support workflows, scheduling and clinical information management for oncology care and follow-up. | Cancer Radiology |
| 37 | August 2019 | Ethos Radiotherapy Treatment (Varian Medical Systems Inc.) | Managing and monitoring radiation therapy treatment plans and sessions | Radiation Oncology |
| 38 | September 2019 | AVEC (Automated Visual Evaluation of the Cervix) (MobileODT Ltd.) | Cervical cancer screening support for diagnostic support | Gynecology |
| 39 | September 2019 | Breast-SlimView (Hera-MI SAS) | Breast cancer detection for diagnostic support from mammograms | Cancer Radiology |
| 40 | September 2019 | Vara (Merantix Healthcare GmbH) | Breast cancer screening support and triaging from mammograms | Cancer Radiology |
| 41 | October 2019 | ProFound AI Software V2.1 (iCAD) | A CAD software device intended to be used concurrently by interpreting physicians while reading DBT | Cancer Radiology |
| 42 | October 2019 | DeepDx-Prostate Connect (Deep Bio Inc.) | Recognition of acinar adenocarcinoma of the prostate | Pathology |
| 43 | November 2019 | Paige Prostate (Paige Inc.) | Cancer detection in prostate needle biopsies | Pathology |
| 44 | November 2019 | Paige Insight (Paige Inc.) | Digital pathology viewer for diagnostic support | Pathology |
| 45 | December 2019 | Transpara (ScreenPoint Medical) | A device for use as a concurrent reading aid for physicians interpreting screening mammograms from compatible FFDM systems to identify regions suspicious for breast cancer and assess their likelihood of malignancy. | Cancer Radiology |
| 46 | December 2019 | QyScore software (Qynapse SAS) | Automatic labelling, visualisation and volumetric quantification of segmentable brain structures and lesions from MR images | Cancer Radiology |
| 47 | December 2019 | Discovery AI (Pentax Medical GmbH (parent company: Pentax Corporation)) | Polyp detection support during a colorectal examination | Gastroenterology |
| 48 | December 2019 | RayStation (RaySearch Laboratories AB) | Treatment planning and analysis of radiation therapy | Radiation Oncology |
| 49 | December 2019 | RayCare 2.3 (RaySearch Laboratories AB) | Support of workflows, scheduling and clinical information management for oncology care and follow-up | Clinical Oncology |
| 50 | January 2020 | JBD-01K (JLK Inspection Inc.) | Breast cancer detection for diagnostic support from mammograms | Cancer Radiology |
| 51 | January 2020 | AI-Pathway Companion Prostate Cancer (Siemens Healthcare GmbH (parent company: Siemens AG) | Prostate cancer detection for diagnostic support | Clinical Oncology |
| 52 | January 2020 | MRCAT Brain (Philips Medical Systems MR Finland (parent company: Philips NV)) | Radiation therapy planning through automated image segmentation for brain tumour patients | Radiation Oncology |
| 53 | February 2020 | InferRead CT Lung (Beijing Infervision Technology Co. Ltd.) | Lung cancer screening and management tool from CT scans | Cancer Radiology |
| 54 | February 2020 | b-box (b-rayZ GmbH) | Assessment of mammography image quality and breast density from mammograms | Cancer Radiology |
| 55 | February 2020 | Metastasis Detection App (Visiopharm A/S) | Metastasis detection in lymph nodes for colorectal and breast adenocarcinoma | Pathology |
| 56 | February 2020 | Galen Prostate (Ibex Medical Analytics Ltd) | Identification of suspected cancer on prostate core needle biopsies | Pathology |
| 57 | February 2020 | densitasAI (Densitas Inc.) | Breast density assessment support from mammograms | Cancer Radiology |
| 58 | March 2020 | Broncholab (Fluidda Inc) | Support in diagnosis and documentation of pulmonary tissue images(eg, abnormalities) from CT thoracic datasets | Cancer Radiology |
| 59 | March 2020 | Syngo.CT Lung CAD (Siemens Medical Solutions Inc. (parent company: Siemens AG)) | Assistance in detection of solid pulmonary nodules during review of multi-detector computed tomography examinations of the chest | Cancer Radiology |
| 60 | March 2020 | MammoScreen (Therapixel SA) | Help to identify findings on screening FFDM acquired with compatible mammography systems and assess level of suspicion | Cancer Radiology |
| 61 | March 2020 | CAD EYE (FUJIFILM Europe GmbH) | Colonic polyps detection and characterisation support during a colonoscopy | Gastroenterology |
| 62 | May 2020 | NaviCam Capsule Endoscope System with NaviCam Stomach Capsule (AnX Robotica, Inc.) | A magnetically maneuvered capsule endoscopy system consists of an ingestible capsule and magnetic controller and is used for visualisation of the stomach and duodenum. The magnetic controller is used outside of the patient and is magnetically coupled with the capsule to control its location and viewing direction. | Gastroenterology |
| 63 | June 2020 | Cobas® EZH2 Mutation Test (Roche Molecular System, Inc.) | The test is intended for the identification of follicular lymphoma patients with an EZH2 mutation for treatment with TAZVERIK (tazemetostat); coupled with the cobas z 480 analyzer. | Pathology |
| 64 | July 2020 | Her2 dual ish dna probe cocktail | It is intended to determineHER2 gene amplification status by enumeration of the ratio of the HER2 gene to Chromosome 17 by light microscopy. | Pathology |
| 65 | October 2020 | Cintec plus cytology (Ventana Medical Systems, Inc.) | Qualitative immunocytochemical assay for the simultaneous detection of the p16INK4a and Ki-67 proteins in cervical specimens, intended for the diagnosis of cervical cancer. | Pathology |
| 66 | November 2020 | Genius AI Detection (Hologic, Inc.) | Software device intended to identify potential abnormalities in breast tomosynthesis images | Cancer Radiology |
| 67 | November 2020 | FoundationOne Liquid CDx (Foundation Medicine, Inc.) | It is a qualitative NGS-based test interrogating 311 genes. It utilises circulating cell-free DNA (cfDNA) isolated from plasma of cancer patients, and is intended to be used as a companion diagnostic to identify patients who may benefit from treatment with targeted therapies (targets identified with NGS) | Pathology |
| 68 | January 2021 | Visage Breast Density (Visage Imaging) | The software application is intended for use with compatible full-field digital mammography to aid radiologists in the assessment of breast tissue composition | Cancer Radiology |
| 69 | January 2021 | Imagio Breast Imaging System (Seno Medical Instruments, Inc.) | Allows an improved classification of breast masses compared to ultrasound alone; includes an AI-based software. | Cancer Radiology |
| 70 | April 2021 | VENTANA MMR RxDx Panel (Ventana Medical Systems, Inc.) | CDx for identifying patients with endometrial cancer with dMMR status who may benefit from treatment with Jemperli (dostarlimab-gxly). | Pathology |
| 71 | April 2021 | GI Genius (Cosmo Artificial Intelligence—AI, LTD) | It is a computer-assisted reading tool designed to aid endoscopists in detecting colonic mucosal lesions (such as polyps and adenomas) in real time during standard white-light endoscopy. | Gastroenterology |
Summary of the different oncology-related medical areas of all AI-associated devices approved by FDA: 39 cancer radiology (54.9%); 14 pathology (19.7%); 6 radiation oncology (8.5%); 6 gastroenterology (8.5%); 5 clinical oncology (7.0%), gynecology 1 (1.4%).
Summary of the different tumour types investigated by the presented devices: 24 general cancers (33.8%); 22 breast cancer (31.0%); 6 lung cancer (8.5%); 6 prostate cancer (8.5%); 5 colorectal cancer (7.0%); 2 brain tumours (2.8%); 6 others (6 types): 1.4% each.
AI artificial intelligence, US FDA United States Food and Drug Administration, CT computed tomography, MRI magnetic resonance imaging, ECG electrocardiogram, CAD computer-aided detection/diagnosis, DBT digital breast tomosynthesis, FFDM full-field digital mammography.
Regarding the different tumour types that can be investigated by adopting such devices, the majority of them has been conceived for being applied to a wide spectrum of solid malignancies (cancer in general, 33.8%). The specific tumour that counts for the largest number of AI devices is breast cancer (31.0%), followed by lung and prostate cancer (8.5% each), colorectal cancer (7.0%), brain tumours (2.8%) and others (6 types, 1.4% each) (Table 1, Fig. 1b).
Discussion and future perspectives
In this paper, a comprehensive overview on current applications of AI in oncology-related areas is provided, specifically describing the AI-based devices that have already obtained the official approval to enter into clinical practice. Starting from its birth, AI demonstrated its cross-cutting importance in all scientific branches, showing an impressive growth potential for the future. As highlighted in this study, this growth has interested also oncology and related specialties.
In general, the application of the FDA-approved devices has not been conceived as a substitute of classical analysis/diagnostic workflow, but is intended as an integrative tool, to be used in selected cases, potentially representing the decisive step for improving the management of cancer patients. Currently, in this field, the branches where AI is gaining a larger impact are represented by the diagnostic areas, which count for the vast majority of the approved devices (>80%), and in particular radiology and pathology.
Cancer diagnostics classically represents the necessary point of start for designing appropriate therapeutic approaches and clinical management, and its AI-based refining represents a very important achievement. Furthermore, this indicates that future developments of AI should also consider unexplored but pivotal horizons in this landscape, including drug discovery, therapy administration and follow-up strategies. In our opinion, for determining a decisive improvement in the management of cancer patients, indeed, the growth of AI should follow comprehensive and multidisciplinary patterns. This represents one of the most important opportunities provided by AI, which will allow the correct interactions and integration of oncology-related areas on a specific patient, rendering possible the challenging purposes of personalised medicine.
The specific cancer types that now are experiencing more advantages from AI-based devices in clinical practice are first of all breast cancer, lung cancer and prostate cancer. This should be seen as the direct reflection of their higher incidence compared with other tumour types, but in the future, additional tumour types should be taken into account, including rare tumours that still suffer from the lack of standardised approaches. Since AI is based on the collection and analysis of large datasets of cases, however, the improvement in the treatment of rare neoplasms will likely represent a late achievement. Notably, if together considered, rare tumours are one of the most important category in precision oncology [11]. Thus, in our opinion, ongoing strategies of AI development cannot ignore this tumour group; although the potential benefits seem far away, it is already time to start collecting data on rare neoplasms.
One of the most promising expectancy for AI is the possibility to integrate different and composite data derived from multi-omics approaches to oncologic patients. The promising tools of AI could be the only able to manage the big amount of data from different types of analysis, including information derived from DNA and RNA sequencing. Along this line, the recent release of American College of Medical Genetics standards and guidelines for the interpretation of the sequence variants [12] has fostered a new wave of AI development, with innovative opportunities in precision oncology (https://www.businesswire.com/news/home/20190401005976/en/Fabric-Genomics-Announces-AI-based-ACMG-Classification-Solution-for-Genetic-Testing-with-Hereditary-Panels; last access 09/21/2021). In our opinion, however, the lack of ground-truth information derived from protected health- data repositories still represents a bottleneck in evaluating the accuracy of AI applications for clinical decision-making.
Overall considered, AI is providing a growing impact to all scientific branches, including oncology and its related fields, as highlighted in this study. For designing new development strategies with concrete impacts, the first steps are representing by knowing its historical background and understanding its current achievements. As here highlighted, AI is already entered into the oncologic clinical practice, but continuous and increasing efforts should be warranted to allow AI expressing its entire potential. In our opinion, the creation of multidisciplinary/integrative developmental views, the immediate comprehension of the importance of all neoplasms, including rare tumours and the continuous support for guaranteeing its growth represent in this time the most important challenges for finalising the ‘AI-revolution’ in oncology.
Acknowledgements
Not applicable.
Author contributions
CL and AS: study conception and design; CL, AP and AS: systematic review; CL and AS: writing, original draft; CL, AP and AS: final editing and approval of the present version.
Funding information
This study is supported by Associazione Italiana Ricerca sul Cancro (AIRC 5x1000 n. 12182); Fondazione Cariverona: Oncology Biobank Project “Antonio Schiavi” (prot. 203885/2017); Fondazione Italiana Malattie Pancreas (FIMP-Ministero Salute J38D19000690001); Italian Ministry of Health (RF CO-2019-12369662: CUP: B39C21000370001).
Data availability
All data are available in the paper.
Ethics approval and consent to participate
Not applicable (review—personal perspective).
Consent to publish
Not applicable (review—personal perspective).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Claudio Luchini, Email: claudio.luchini@univr.it.
Aldo Scarpa, Email: aldo.scarpa@univr.it.
References
- 1.Kaul V, Enslin S, Gross SA. History of artificial intelligence in medicine. Gastrointest Endosc. 2020;92:807–12. doi: 10.1016/j.gie.2020.06.040. [DOI] [PubMed] [Google Scholar]
- 2.Hamamoto R, Suvarna K, Yamada M, Kobayashi K, Shinkai N, Miyake M, et al. Application of artificial intelligence technology in oncology: towards the establishment of precision medicine. Cancers (Basel) 2020;12:3532. doi: 10.3390/cancers12123532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhinder B, Gilvary C, Madhukar NS, Elemento O. Artificial intelligence in cancer research and precision medicine. Cancer Disco. 2021;11:900–15. doi: 10.1158/2159-8290.CD-21-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kann BH, Hosny A, Aerts HJWL. Artificial intelligence for clinical oncology. Cancer Cell. 2021;39:916–27. doi: 10.1016/j.ccell.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huynh E, Hosny A, Guthier C, Bitterman DS, Petit SF, Haas-Kogan DA, et al. Artificial intelligence in radiation oncology. Nat Rev Clin Oncol. 2020;17:771–81. doi: 10.1038/s41571-020-0417-8. [DOI] [PubMed] [Google Scholar]
- 6.Benzekry S. Artificial intelligence and mechanistic modeling for clinical decision making in oncology. Clin Pharmacol Ther. 2020;108:471–86. doi: 10.1002/cpt.1951. [DOI] [PubMed] [Google Scholar]
- 7.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu E, Wu K, Daneshjou R, Ouyang D, Ho DE, Zou J. How medical AI devices are evaluated: limitations and recommendations from an analysis of FDA approvals. Nat Med. 2021;27:582–4. doi: 10.1038/s41591-021-01312-x. [DOI] [PubMed] [Google Scholar]
- 9.Muehlematter UJ, Daniore P, Vokinger KN. Approval of artificial intelligence and machine learning-based medical devices in the USA and Europe (2015–20): a comparative analysis. Lancet Digit Health 2021;3:e195–e203. [DOI] [PubMed]
- 10.Benjamens S, Dhunnoo P, Meskó, B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database. NPJ Digit Med. 2020;3:118. [DOI] [PMC free article] [PubMed]
- 11.Luchini C, Lawlor RT, Milella M, Scarpa A. Molecular tumor boards in clinical practice. Trends Cancer. 2020;6:738–44. doi: 10.1016/j.trecan.2020.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available in the paper.