Abstract
Background:
Acute exacerbations of interstitial lung diseases (AE-ILD) have a high mortality rate with no effective medical therapies. Lung transplantation is a potentially life-saving option for patients with AE-ILD, but its role is not well-established. The aim of this study is to determine if this therapy during AE-ILD significantly affects post-transplant outcomes in comparison to those transplanted with stable disease.
Methods:
We conducted a retrospective study of consecutive patients with AE-ILD admitted to our institution from 2015 to 2018. The comparison group included patients with stable ILD listed for lung transplant during the same period. The primary end-points were in-hospital mortality for patients admitted with AE-ILD and one-year survival for the transplanted patients.
Results:
Of 53 patients admitted for AE-ILD, 28 were treated with medical therapy alone and 25 underwent transplantation. All patients with AE-ILD who underwent transplantation survived to hospital discharge whereas only 43% of the AE-ILD medically treated did. During the same period, 67 patients with stable ILD underwent transplantation. Survival at one-year for the transplanted patients was not different for the AE-ILD group versus stable ILD group (96 % vs 92.5%). The rates of primary graft dysfunction, post-transplant hospital length-of-stay, and ACR were similar between the groups.
Conclusion:
ILD patients transplanted during AE-ILD had no meaningful difference in overall survival, rate of primary graft dysfunction, or acute rejection compared to those transplanted with stable disease. Our results suggest that lung transplantation can be considered as a therapeutic option for selected AE-ILD patients.
Keywords: Interstitial lung disease, acute exacerbation of interstitial lung disease, lung transplantation
Introduction
Interstitial lung diseases (ILDs) comprise a group of parenchymal lung diseases characterized histopathologically by chronic inflammation and fibrosis. Due to the heterogeneous nature of these diseases, the clinical course of patients is variable and unpredictable (1, 2). A subset of patients will present with acute exacerbations of ILD (AE-ILD) defined as a respiratory deterioration over less than one month associated with new radiographic infiltrates not fully explained by volume overload. The annual incidence of AE-ILD is 7–20% per patient (3–7). In-hospital mortality for AE-ILD patients ranges from 30–50% and can be as high as 90% in those requiring mechanical ventilatory support (6, 7). Post-hospitalization survival is also impacted, with survivors having a median survival time of 2.2 months (3). No treatment has been shown to affect the outcome of this condition; although published guidelines (2) have made a weak recommendation for high-dose corticosteroid administration in these cases, recent evidence suggests that this may in fact contribute to reduced survival (8).
In the absence of effective medical treatment at the current time, lung transplantation remains a potential therapy for eligible patients, but the evidence for support of this treatment is limited to single-center retrospective studies. Three small series, presented as meeting abstracts but not published in manuscript form, reported similar post-transplant outcomes in patients with an acute exacerbation of idiopathic pulmonary fibrosis (AE-IPF) in comparison to stable IPF (9–11), but the largest study to-date reported a substantially higher mortality after transplantation in AE-IPF patients as compared to stable IPF, with significantly reduced one- and three-year survival (12). To address whether lung transplant is a viable option for therapy in AE-ILD, we examined outcomes amongst consecutive patients with AE-ILD who were or were not listed for transplantation and compared them to stable ILD patients undergoing this procedure during the same time period.
Material & Methods
Study Population
A single-center retrospective analysis was performed of all patients with a diagnosis of ILD admitted with exacerbation to the University of Florida Health Shands Hospital (UF) from January 01, 2015 to December 31, 2018. All adult patients with a diagnosis of ILD with stable disease who were listed for lung transplantation during the same interval were included as well. The end of the study period was set at March 31, 2020 to ensure that all transplanted patients had at least one year of post-transplant follow-up data to be included in the final analysis. This study was approved by the University of Florida Institutional Review Board (IRB #201900323).
Diagnostic Definitions
The diagnosis of ILD was made by a multidisciplinary committee of pulmonary physicians based on published guidelines (1). Briefly, a diagnosis of idiopathic pulmonary fibrosis (IPF) was made based on either computed tomography (CT) of the chest demonstrating typical or probable usual interstitial pneumonia (UIP) pattern or a surgical lung biopsy demonstrating UIP pattern, as well as a negative work-up for secondary causes such as connective tissue disease, hypersensitivity pneumonitis, pneumoconiosis, vasculitis, or drug toxicity (13). The diagnosis of interstitial pneumonitis with autoimmune features (IPAF) or connective-tissue disease (CTD) ILD was made based on an official ERS / ATS research statement and American College of Rheumatology classification criteria, respectively (14). The diagnosis of hypersensitivity pneumonitis was made based on positive exposure (per history or serological studies), CT chest showing mosaic attenuation with air trapping and/or centrilobular nodules, a lymphocytic-predominant bronchoalveolar lavage, and /or lung biopsy demonstrating airway-centered inflammation and /or fibrosis. A diagnosis of “unclassifiable” ILD was assigned to patients when: 1) clinical and radiological data were insufficient for accurate diagnosis; 2) there were discrepant or overlapping histopathological findings; 3) there were discrepant features between clinical, radiological and pathological findings; or 4) there were significant overlapping clinical or radiological features that precluded diagnosis.
We used the revised definition for acute exacerbations in patients with IPF (6) for all ILD patients, if they met the following criteria: 1) diagnosis of ILD; 2) acute worsening or development of dyspnea <1 month in duration; 3) high-resolution CT chest imaging demonstrating new bilateral ground-glass opacities and/or consolidation superimposed on a background pattern consistent with fibrotic lung disease; and 4) deterioration not explained by a reversible cause (e.g. fluid overload, thromboembolic disease) (6, 7).
Patients admitted for AE-ILD who met the following criteria were evaluated by a lung transplant physician to determine candidacy: 1) hospitalization due to respiratory decline, or acute exacerbation, 2) age no older than 75 years, 3) no significant functional status impairment prior to admission, 4) body mass index less than 30kg/m2, 5) no major or relative contraindication for listing per the International Society of Heart and Lung Transplantation (ISHLT) consensus statements (15).
Standard care of lung transplant recipients
For stable patients with ILD, anti-fibrotic agents were continued up until the day of transplantation. If these patients were on corticosteroids, the dose was weaned to less than or equal to 5 mg per day. Post transplant, per our center’s protocol, for induction therapy, patients received basiliximab (20 mg) on the day of transplantation and post-operative day four. Recipients were maintained on a triple-drug immunosuppressive regimen (prednisone, tacrolimus, and mycophenolate) and calcineurin inhibitor doses were adjusted based on trough levels. In the first year, recipients typically underwent surveillance bronchoscopy examinations performed at 1, 2, 3, 6, 9, and 12 months post-transplant, as well as with any significant change in pulmonary status (e.g. fever, cough, shortness of breath, new radiographic infiltrates, >10% decline from baseline FEV1, or hypoxemia). Tissue and bronchoalevolar lavage fluid (BAL) from all procedures were analyzed for evidence of acute rejection and infection.
Primary graft dysfunction (PGD) and acute cellular rejection (ACR) were diagnosed and graded per ISHLT guidelines (16, 17). PGD was assessed at 0, 24, 48, and 72 hours post-transplantation. ACR was calculated by composite rejection standardized score which was calculated by summing the vascular (A) grades on any biopsy divided by the total number of biopsies received up to the end of follow-up as previously defined. The date of acute rejection was defined as the date of biopsy, and time to acute rejection was calculated as the time from transplantation to the first episode of acute rejection. ACR was defined by the 2006 International Society of Heart and Lung Transplantation criteria (17).
Outcomes
The primary outcome of the study was survival. For AE-ILD non-transplanted patients, survival time was calculated from the date of hospitalization to the date of death or censure (end of study observation period). For transplanted patients, survival time was calculated from the date of transplant to date of death or censure (which included a minimum of one year of follow-up for all post-transplant patients). Secondary outcomes in transplanted patients included incidence of primary graft dysfunction at 0, 24, 48, and 72 hours after transplantation, re-intubation rate, tracheostomy rate, hospital length-of-stay, airway dehiscence, and incidence and severity of ACR.
Statistical analysis
Data were analyzed using GraphPad Prism (Version 8, GraphPad, San Diego, CA, USA). Continuous variables were presented as the median with interquartile range (IQR). Chi-square test was used to compare categorical data, Fisher’s exact test was used for simple between-group comparisons, unpaired Student’s t test was used for continuous data, and the Mann-Whitney U test was used for non-parametric data.
Survival time for the transplanted group was calculated from date of transplant to the end of the study period or death. Survival time for the AE-ILD group was calculated from the date of hospital admission to the end of study period, last date of known follow-up, or death. Survival times were censured on March 31, 2020. Survival data was expressed using a Kaplan-Meier curve; curves were compared with a log-rank test. This statistical analysis was performed using IBM SPSS, Version 23.0 (IBM Corp, Armonk, NY).
Results
Between January 01, 2015 and December 31, 2018, 55 patients with AE-ILD were hospitalized at our institution. Of these, 27 patients were listed and 25 ultimately underwent lung transplantation. Two patients died while on the wait list (one on extracorporeal membrane oxygenation (ECMO) of sepsis and multi-organ dysfunction and one who presented with acute-on-chronic respiratory failure and concomitant renal failure). Twenty-eight patients with AE-ILD were not listed for lung transplantation. Among these, 15 patients were assessed for candidacy, but they were deemed ineligible due advanced age (>75 years), obesity (BMI>32 kg/m2), severe medical comorbidities (predominantly chronic kidney disease), or substance abuse. The 13 remaining non-transplanted AE-ILD patients either declined transplant evaluation or were deemed unsuitable transplant candidates by the treating intensive care unit physicians (lung transplant physicians were not consulted) due to multi-organ dysfunction at presentation and/or similar comorbidities as described above.
During this same study period, 132 ILD patients were referred for evaluation for lung transplant candidacy. Sixty-three patients were deemed not to be transplant candidates due to social issues or specific comorbidities (Supplemental Table 1). Sixty-nine patients with ILD without exacerbation (stable ILD) were listed and 67 underwent lung transplantation (Figure 1). Two patients in this group were delisted (one due to a new diagnosis of myelofibrosis, one due to severe deconditioning).
Figure 1. Flow diagram of study participants.
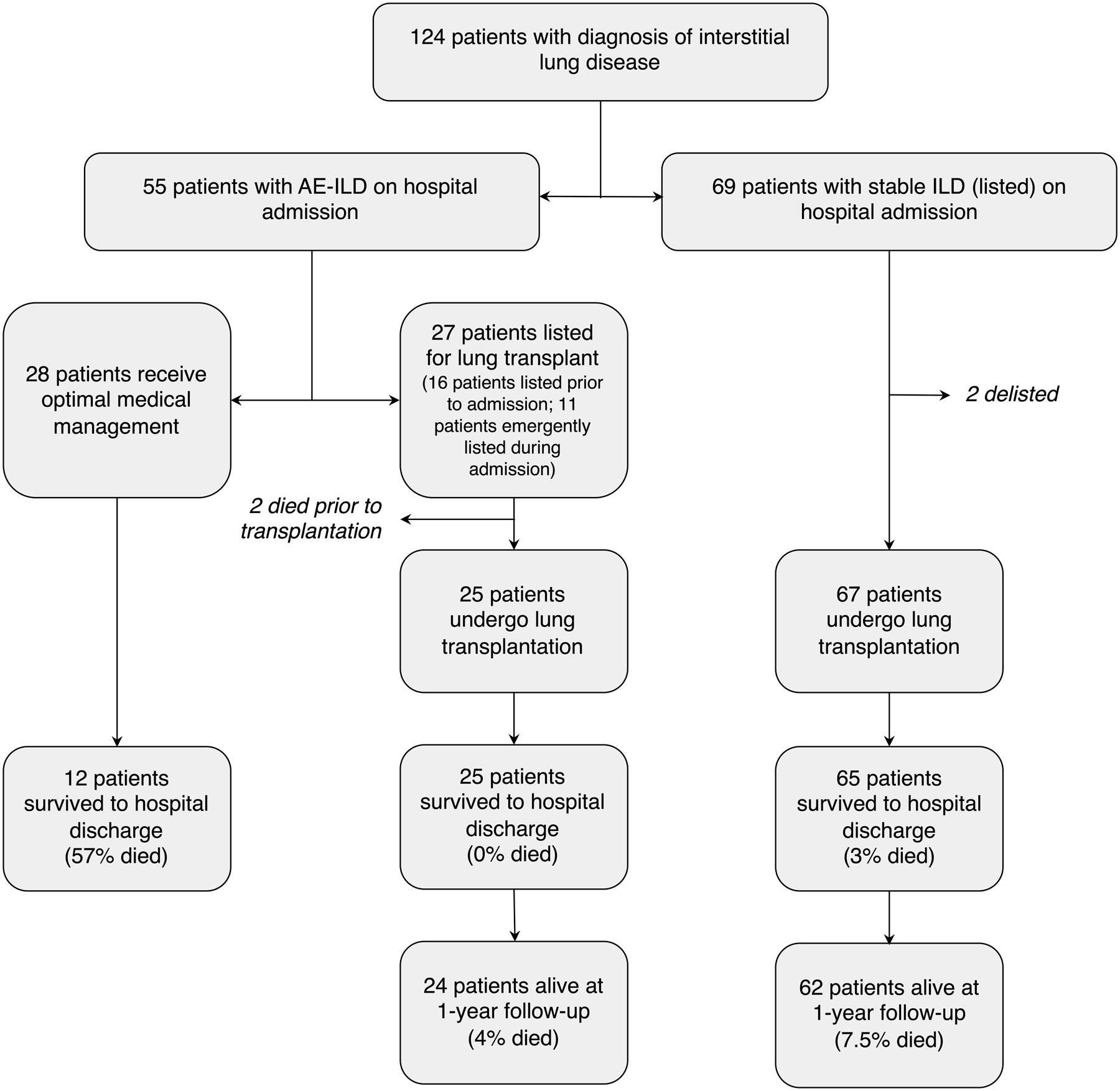
Patients who met a clinical/radiographic diagnosis of interstitial lung disease with acute exacerbation (AE-ILD) were considered for this study. Patients with stable interstitial lung disease (stable ILD) who underwent lung transplantation in the absence of acute exacerbation were used as the comparison control group.
There was no significant difference between the three groups in terms of patient age and gender, however, IPF and HP were diagnosed more commonly in the stable ILD group. A higher rate of pulmonary hypertension on right heart catheterization was also found in patients that eventually underwent transplantation (Table 1).
Table 1.
Clinical Characteristics of Study Subjects
| AE-ILD; No Transplant (n = 28) | AE-ILD; Transplant (n=25) | Stable ILD; Transplant (n=67) | p value | ||
|---|---|---|---|---|---|
| Median age/years (IQR) | 63 (58–68) | 62 (56–65) | 61 (55–66) | 0.40 | |
| Male sex | 21 (75%) | 15 (60%) | 52 (78%) | 0.23 | |
| Diagnosis | 0.04 | ||||
| Idiopathic pulmonary fibrosis | 0 (0%) | 2 (8%) | 13 (19%) | ||
| IPAF / CTD | 9 (32%) | 8 (32%) | 19 (28%) | ||
| Hypersensitivity pneumonitis | 0 (0%) | 3 (12%) | 11 (16%) | ||
| Unclassifiable | 17 (60%) | 10 (40%) | 22 (33%) | ||
| Other a | 2 (8%) | 2 (8%) | 2 (3%) | ||
| Smoking history (%) | 0.65 | ||||
| Ever | 18 (64%) | 17 (68%) | 39 (58%) | ||
| Never | 10 (36%) | 8 (32%) | 28 (42%) | ||
| Lung Function (median & IQR) | |||||
| Percent predicted FVC (IQR) | 57 (37–68) | 47 (39–63) | 44 (37–53) | 0.199 | |
| Percent predicted DLCO (IQR) | 32 (23–43) | 24 (18–28) | 27 (20–32) | 0.11 | |
| Comorbidities (%) | |||||
| Pulmonary hypertension | 3 (11%) | 14 (56%) | 37 (55%) | 0.0002 | |
| Sleep apnoea | 3 (11%) | 1 (4%) | 7 (10%) | 0.60 | |
| Coronary artery disease | 7 (25%) | 3 (12%) | 10 (15%) | 0.38 | |
| Gastroesphageal reflux disease | 3 (11%) | 7 (28%) | 17 (25%) | 0.22 | |
| BMI (kg/m2; median and IQR) | 28 (25–32) | 25 (23–28) | 28 (26–30) | 0.0027 | |
| Outpatient immunosuppression prior to transplantation | |||||
| Prednisone | 12 (43%) | 9 (36%) | 24 (36%) | 0.8 | |
| Mycophenolate | 6 (21%) | 3 (12%) | 21 (31%) | 0.14 | |
| Azathioprine | 2 (7%) | 0 (0%) | 6 (9%) | 0.31 | |
| Outpatient antifibrotic therapy | |||||
| Pirfenidone | 2 (7%) | 0 (0%) | 11 (16%) | 0.06 | |
| Nintedanib | 4 (14%) | 4 (16%) | 7 (10%) | 0.73 | |
| Median GAP score (IQR) | 4 (4–6) | 5 (4–6) | 5 (5–6) | 0.047 | |
Abbreviations: AE-ILD, acute exacerbation of interstitial lung disease; IPAF/CTD, interstitial pneumonia with autoimmune features/connective tissue disease associated lung disease; FVC, forced vital capacity; DLCO, diffusing capacity of the lung for carbon monoxide; BMI, body mass index; GAP (gender/age/physiology) Score.
Other diagnoses included inflammatory bowel disease-related ILD (1), non-specific interstitial pneumonia (1), Hermansky-Pudlak syndrome (2), pneumoconiosis (1), nitrofurantoin-induced lung disease (1), desquamative interstitial pneumonia (1)
All patients hospitalized with AE-ILD required advanced respiratory support which included high-flow nasal cannula (HFNC), mechanical ventilation (non-invasive or invasive), administration of inhaled pulmonary vasodilators, and/or ECMO. Among AE-ILD patients, those who were not transplanted received more corticosteroids, whereas those who were transplanted received more inhaled pulmonary vasodilators (Supplemental Table 2). Of the 27 listed AE-ILD patients, 11 patients had been evaluated and listed prior to admission. Six patients had completed evaluation prior to admission but were only listed after their admission for AE-ILD. Seven patients underwent an urgent de-novo lung transplant evaluation and three completed evaluations initiated prior during their hospitalization.
Of the 92 patients undergoing lung transplantation, the AE-ILD patients had a significantly higher lung allocation score (LAS; 81.14 versus 42.25, p < 0.0001) and a lower body mass index (BMI; 25 versus 28, p = 0.0027) compared to stable ILD patients. Additional demographics were otherwise similar (Tables 1 and 2). The median waiting list time from listing to transplantation for stable ILD patients was 22 days (IQR 10 to 44 days) and for AE-ILD patients was 10 days (IQR 4 to 23 days). Of the 25 patients who underwent transplant for AE-ILD, nine were listed before their acute exacerbation and their median waiting time from listing to transplantation was 13 days (IQR 4 to 29 days). The remaining 16 patients had their evaluation completed and / or were listed during AE-ILD and their waiting time from listing to transplantation was 9 days (IQR 4 to 20 days). There was no significant difference in wait-list time when comparing stable ILD versus AE-ILD patients (either those evaluated prior to or during their exacerbation; p = 0.2). There was no statistical difference in type of lung transplant performed (single versus bilateral) or in the preference for intraoperative mechanical circulatory support.
Table 2.
Transplant and Post-Transplant Outcomes Data
| AE-ILD; Transplant (n = 25) | Stable ILD; Transplant (n = 67) | p value | ||
|---|---|---|---|---|
| Median LAS (IQR) | 82 (61–87) | 42 (38–50) | <0.0001 | |
| Median time on waiting list (IQR) | 10 (4–23) | 22 (10–44) | 0.0008 | |
| Type of Transplant (%) | 0.10 | |||
| Single | 0 (0%) | 9 (13%) | ||
| Bilateral | 25 (100%) | 58 (87%) | ||
| Intra-operative ECMO or CPB | 24 (96%) | 62 (93%) | >0.99 | |
| Post-operative ECMO at 24hrs | 5 (20%) | 6 (9%) | 0.16 | |
| Reintubation after surgery (%) | 3 (12%) | 8 (12%) | >0.99 | |
| Tracheostomy | 4 (16%) | 10 (15%) | >0.99 | |
| Median duration of mechanical ventilation/days (IQR) | 2 (1–7.75) | 1 (1–2) | 0.16 | |
| Median hospital stay after transplantation/days (IQR) | 24.5 (16–29) | 18 (12–30) | 0.125 | |
| Any PGD (%) at 0 hrs * | 21 (88%) | 38 (59%) | 0.01 | |
| Any PGD (%) at 24 hrs * | 16 (67%) | 32 (49%) | 0.15 | |
| Any PGD (%) at 48 hrs * | 12 (50%) | 27 (41%) | 0.47 | |
| Any PGD (%) at 72 hrs | 4 (16%) | 13 (19%) | >0.99 | |
| Median time to first Episode of ACR/days (IQR) | 56 (41–214) | 31 (30–76) | 0.026 | |
| Number of Patients with Any ISHLT Grade Rejection (%) | 12 (48%) | 36 (54%) | 0.65 | |
| Median cumulative A-grade Rejection Score at 1 year (IQR) | 3 (2–5) | 3 (1–5) | 0.74 | |
| Incomplete Surveillance Bronchoscopy Protocol | 8 (32%) | 28 (42%) | 0.47 | |
| Airway dehiscence within 30 days of Transplant (%) | 1 (4%) | 3 (4%) | >0.99 | |
| Median follow-up/months (IQR) | 22 (18–29) | 30 (19–41) | 0.16 | |
Abbreviations: ILD, interstitial lung disease; LAS, lung allocation score; ECMO, extracorporeal membrane oxygenation; CPB, cardiopulmonary bypass; ACR, acute cellular rejection; CLAD, chronic lung allograft dysfunction
At T 0hrs, one patient was ungradable in the AE-ILD group and 3 were ungradable in the stable group. At T 24hrs and 48 hrs, there was one was ungradable patient in each group.
Post-transplantation, the rate of any grade of PGD at T0 hours was statically higher in the AE-ILD group (88% for AE-ILD vs 59% for stable ILD, p = 0.01), but the rate of Grade 3 PGD was not statistically significant between the two groups (4% vs 3%, p = 0.63). The rate of PGD at T24, T48 and T72 hours was also not significantly different between the two groups. In addition, the groups had similar lengths of mechanical ventilation, rates of re-intubation, airway dehiscence, and tracheostomy placement (Table 2). There was no difference in hospital length-of-stay or 90-day readmission rate (Supplemental Table 3). Histopathology from the explanted lungs was reviewed; findings of end-stage lung disease (predominately honeycombing) were present in most patients. Diffuse alveolar damage was not a common feature in our AE-ILD group, whereas organizing pneumonia was (Supplemental Table 4).
Survival to hospital discharge also did not differ between the two groups of patients that underwent lung transplant (100% for AE-ILD, 97% for stable ILD). The median follow-up for both transplanted groups was 28 months (IQR 18.5–40.5 months). Eight percent of patients transplanted for AE-ILD died in a mean follow-up of 27±13 (SD) months whereas 15% of patients transplanted during stable disease died in a mean follow-up of 31±15 months. In contrast, only 43% of non-listed AE-ILD patients survived to hospital discharge; 9 of 12 patients subsequently died within one month of discharge, one more died within six months, and the remaining two patients were lost to follow-up. The 1-year survival of patients transplanted with AE-ILD (96.0%) was comparable to patients with stable ILD (92.5%), and greatly exceeded AE-ILD patients who were not listed for transplant (7.1%) (Figure 2 and Figure 3).
Figure 2.
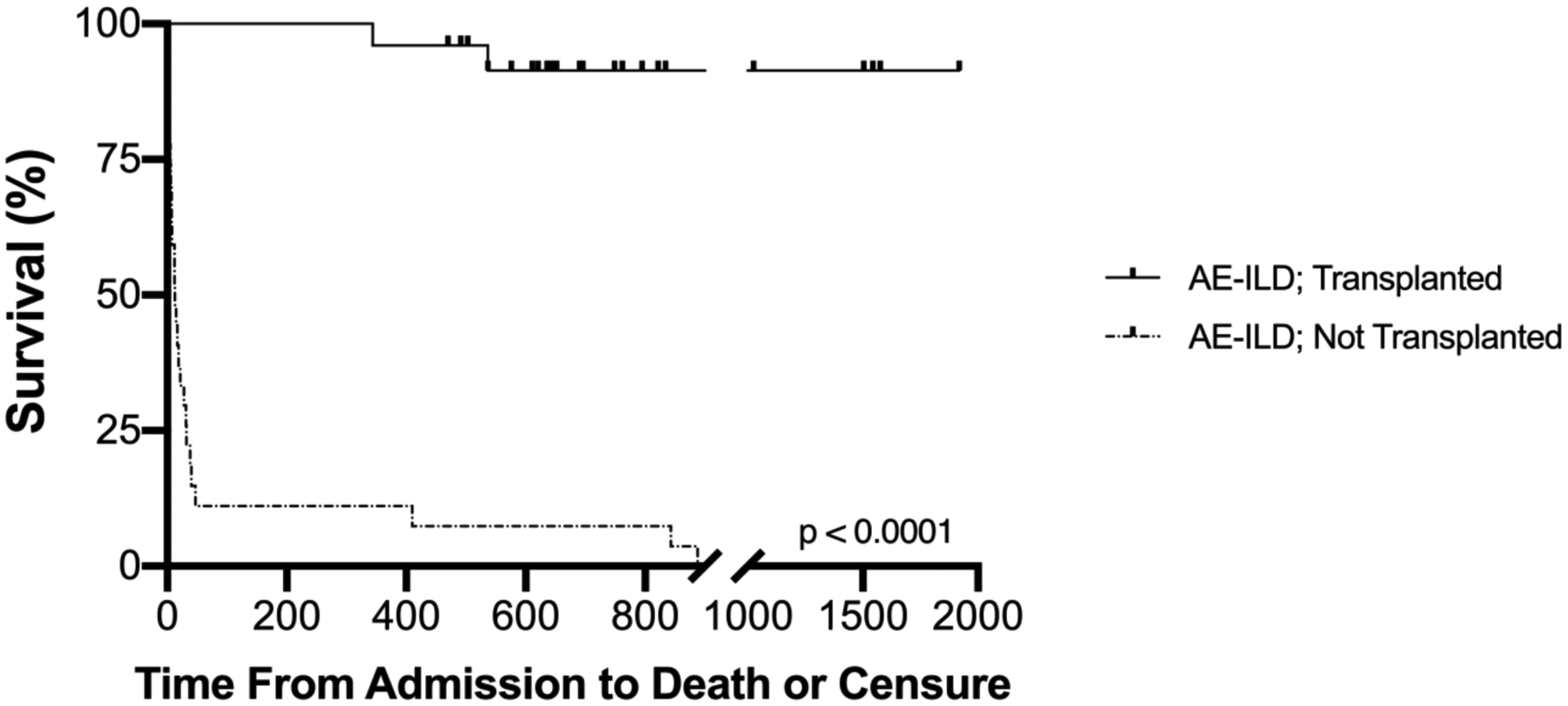
Survival time for patients admitted with acute ILD exacerbation (AE-ILD) for those that underwent lung transplantation versus those managed medically. Cox regression proportional hazard survival analysis was performed adjusting for age; survival for those treated with medical therapy alone was significantly worse (p<0.0001).
Figure 3.
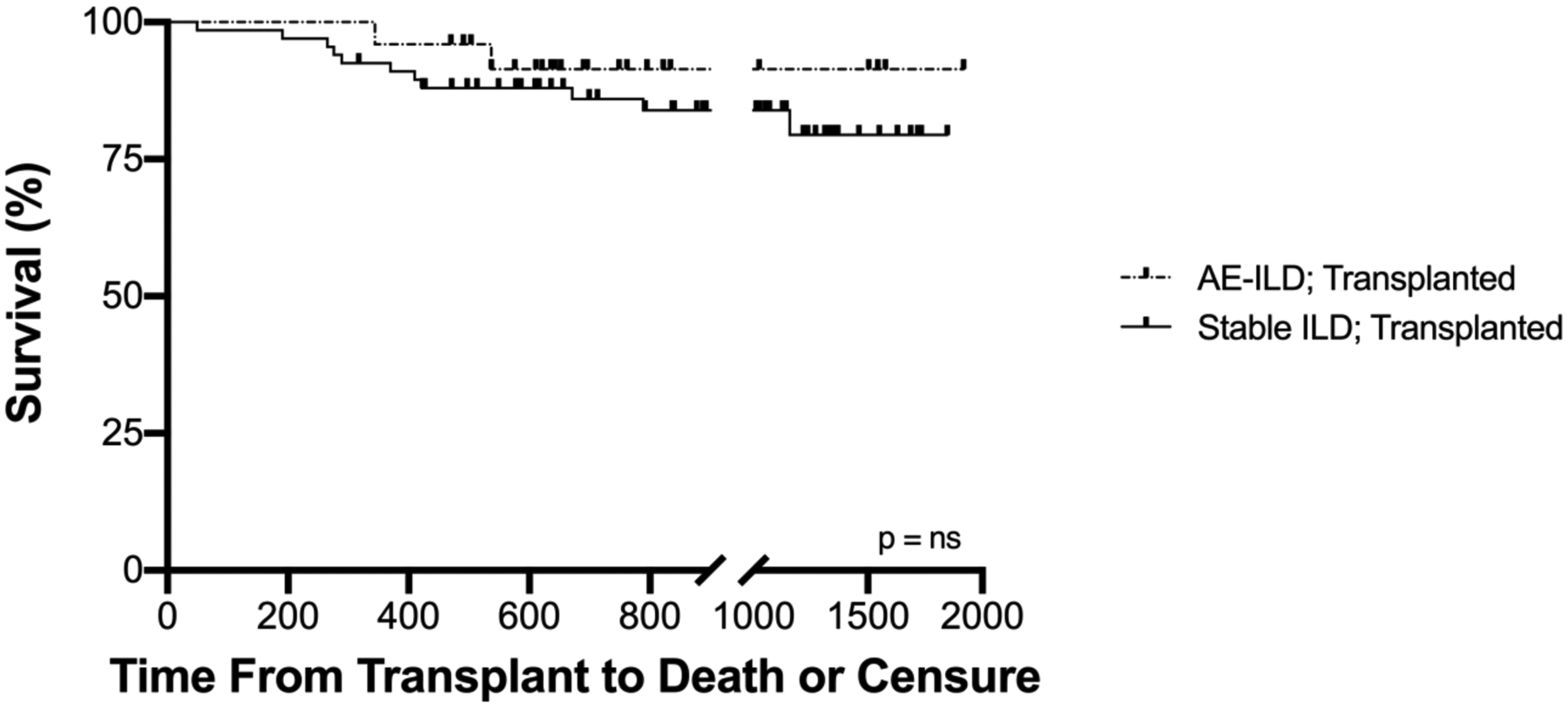
Survival time for patients transplanted during AE-ILD versus those with stable ILD. Cox regression proportional hazard survival analysis was performed adjusting for age, LAS, and type of transplant (single versus bilateral); there was no significant difference in survival between the two groups (p = 0.37, ns)
Lastly, the majority of acute cellular rejection episodes during this time were mild (ISHLT Grade A1) and the composite A-grade rejection score at one year was not significantly different (3.0 vs 2.5, p=0.74) (Table 2).
Discussion
To our knowledge, the current report is the first published analysis assessing outcomes of lung transplantation in a cohort of patients with AE-ILD not limited to IPF. Our data reinforces the high short-term mortality of patients with AE-ILD that are not candidates for lung transplantation. However, it also indicates that in appropriately selected patients, transplantation resulted in excellent and equivalent one-year survival to those transplanted with stable disease, despite the high acuity of this patient population.
Four retrospective studies (three abstracts, one published) have examined lung transplant outcomes in ILD patients, but these have been limited to those with IPF. Mudambi et al (11) and Nair et al (10) noted no difference in 30-day, 90-day, or one year post-transplant survival between stable IPF patients and those transplanted with AE-IPF (1-year survival 76.0% vs 84.6% p = 0.73, and 77.8%vs 78.6% p = 0.86, respectively). Tomic et al (9) found no significant difference in PGD (30.8% in AE-ILD patients versus 14.4% in stable patients (p = 0.273)); however, 1-year survival was not reported. In the largest published study to-date, Dotan et al (12) described 28 patients with IPF transplanted during an acute exacerbation had significantly worse one-year and three-year survival in comparison to those transplanted with stable disease (94% and 90% versus 71% and 60%, respectively), suggesting that lung transplantation in patients with LAS greater than 80 may not confer a survival benefit for these patients. Nine listed patients died prior to transplantation in the AE-IPF group, resulting in a survival since listing of 54%.
Several factors could explain the outcomes in the current study as compared to prior reports. First, the majority of our patients was supported with high flow nasal cannula (HFNC), unlike previous reports where a significant number received mechanical ventilation, extracorporeal life support, or both. Forty percent of patients in the study from Dotan et al. were bridged with mechanical ventilation and/or ECMO; which may be reflective of severe critical illness not accounted for in the LAS score. In contrast, in our cohort, only one patient was bridged with veno-venous ECMO prior to transplantation. The use of HFNC has been shown to be of value in patients with acute hypoxic respiratory failure mostly in the setting of bacterial pneumonia (18), but may be beneficial in the setting of AE-ILD. Second, the time spent on the waiting list for our AE-ILD patients was significantly less (10 days versus 40 days) compared to the patients in the study from Dotan et al. This could have potentially mitigated further deterioration in these critically ill patients and improved post-transplant outcomes.
Operative considerations may also have contributed: for instance, patients in our cohort predominately underwent bilateral lung transplantation whereas a significant proportion of those in prior reports underwent single lung transplantation. Moreover, the majority of our patients were supported intra-operatively with veno-arterial ECMO; whereas Dotan et al. reported 68% of bypass use in AE-IPF patients. There is growing evidence supporting utilization of intra-operative ECMO over both cardiopulmonary bypass (19–24) or no support at all (25, 26).
Lastly, differences between our findings and prior experience may be due to recipient factors. For example, the patients in our study were younger than those described in previous reports (60 ± 3 versus 67 ± 5 years). We also included all forms of ILD (not limited to IPF) which may portend different clinical trajectories post-transplantation.
Overall, our findings support the use of a multidisciplinary approach to the care of these complex patients. For example, combined rounds with the lung transplant physicians and thoracic surgeons occur at least on a daily basis. Medical interventions, such as aggressive physical therapy, pre-operative right ventricular optimization in patients with pulmonary hypertension, and consideration for ideal timing for use of extracorporeal life support as a bridge to transplantation are discussed and implemented per protocol. In addition, close relationship between the interstitial lung disease and lung transplant physicians facilitates early referral of AE-ILD patients for transplant evaluation, even when patients are initially encountered in the critical care setting.
In terms of post-transplant outcomes, although there was an increased frequency of PGD at T0 hours in the AE-ILD group, there was no significant difference in severe PGD at T48 hours or T72 hours, which are time points that are thought to portend worse long-term prognosis (16). The length of initial hospital length-of-stay, re-intubation rate, and tracheostomy rate did not differ between the two groups, nor did the two groups show any difference in subsequent rate of episodes of hospital readmission or episodes of acute cellular rejection as defined by the composite A rejection score. Although it is difficult to directly compare our results with prior studies given the established differences in patient population and medical therapy described above, this study contrasts the results by Dotan et al, where patients with AE-IPF and very high LAS did not experience the survival advantage expected from lung transplantation. Since fibrotic lung disease is the most common indication for lung transplantation in North America (27) and AE-ILD carries significant mortality (3), we found that expeditious referral and evaluation by lung transplant specialists of these patients has the potential to significantly improve outcomes. Moreover, the excellent survival outcomes and the minimal waitlist mortality (4.1%) reinforce the potential value of this therapy in this critical population with scarce therapeutic options.
Our study has a number of limitations. First, although our study has a larger cohort of patients than prior studies, the sample sizes for the groups in our study remain small. Thus, our study may be underpowered to adjust the results for potential confounders in a multivariate analysis and detect small differences in some of the outcomes described. In addition, it is a retrospective single-center study, and thus the results may not be generalizable to transplant centers that employ different surgical techniques, post-operative care, and alternative immunosuppressive regimens.
In summary, we report favorable early outcomes after lung transplantation in patients with AE-ILD that is comparable to those with stable ILD, despite a significantly higher LAS score in AE-ILD patients at the time of transplantation. We also confirmed that AE-ILD patients to have a high mortality when options are limited to medical management, reinforcing the survival benefit of lung transplantation in carefully selected patients in this setting.
Supplementary Material
Key Messages.
What is the key question?
Is lung transplantation a safe therapeutic option in appropriate candidates with interstitial lung disease (ILD) who present with an acute exacerbation (AE-ILD)?
What is the bottom line?
In patients with AE-ILD, lung transplantation offered a significant survival benefit in comparison to medical therapy and resulted in comparable short- and long-term outcomes in comparison to patients who underwent lung transplantation for stable ILD.
Why read on?
ILD patients presenting with an acute exacerbation of their disease have a high morbidity and mortality, and no medical therapies have been shown to be effective in this condition. Clinicians should recognize that lung transplantation is a potentially life-saving option for appropriate candidates with AE-ILD.
Funding Sources:
B.M NIH U01EB024501, NIH R01AI135128, American Heart Association 18TPA34170486
Abbreviation List
- ACR
acute cellular rejection
- AE-ILD
acute exacerbation of interstitial lung disease
- ATS
American Thoracic Society
- BMI
body mass index
- CAD
coronary artery disease
- CLAD
chronic lung allograft dysfunction
- CTD-ILD
connective tissue disease associated interstitial lung disease
- COP
cryptogenic organizing pneumonia
- CPB
cardiopulmonary bypass
- CTA
computed tomography angiography
- DAD
diffuse alveolar damage
- DIP
desquamative interstitial pneumonia
- DLCO
diffusing capacity of the lung for carbon monoxide
- SLT/DLT
single/double lung transplantation
- ECMO
extra corporal membrane oxygenation
- ERS
European Respiratory Society
- FVC
forced vital capacity
- GERD
gastroesophageal reflux disease
- HP
hypersensitivity pneumonitis
- HRCT
high resolution computerized tomography
- ILD
interstitial lung disease
- IPF
idiopathic pulmonary fibrosis
- ISHLT
International Society for Heart and Lung Transplantation
- LAS
lung allocation score
- LOS
length of stay
- MV
mechanical ventilation
- OSA
obstructive sleep apnea
- PH
pulmonary hypertension
- UIPL
usual interstitial pneumonia
- UF
University of Florida Shands Hospital
- UNOS
United Network for Organ Sharing
- VQ
ventilation-perfusion
References
- 1.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. American journal of respiratory and critical care medicine 2013;188(6):733–48. doi: 10.1164/rccm.201308-1483ST [published Online First: 2013/09/17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. American journal of respiratory and critical care medicine 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL [published Online First: 2011/04/08] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song JW, Hong SB, Lim CM, et al. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology 2011;37(2):356–63. doi: 10.1183/09031936.00159709 [published Online First: 2010/07/03] [DOI] [PubMed] [Google Scholar]
- 4.Park IN, Kim DS, Shim TS, et al. Acute exacerbation of interstitial pneumonia other than idiopathic pulmonary fibrosis. Chest 2007;132(1):214–20. doi: 10.1378/chest.07-0323 [published Online First: 2007/04/03] [DOI] [PubMed] [Google Scholar]
- 5.Suda T, Kaida Y, Nakamura Y, et al. Acute exacerbation of interstitial pneumonia associated with collagen vascular diseases. Respiratory medicine 2009;103(6):846–53. doi: 10.1016/j.rmed.2008.12.019 [published Online First: 2009/02/03] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collard HR, Ryerson CJ, Corte TJ, et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. American journal of respiratory and critical care medicine 2016;194(3):265–75. doi: 10.1164/rccm.201604-0801CI [published Online First: 2016/06/15] [DOI] [PubMed] [Google Scholar]
- 7.Leuschner G, Behr J. Acute Exacerbation in Interstitial Lung Disease. Front Med (Lausanne) 2017;4:176. doi: 10.3389/fmed.2017.00176 [published Online First: 2017/11/08] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farrand E, Vittinghoff E, Ley B, et al. Corticosteroid use is not associated with improved outcomes in acute exacerbation of IPF. Respirology 2020;25(6):629–35. doi: 10.1111/resp.13753 [published Online First: 2019/12/18] [DOI] [PubMed] [Google Scholar]
- 9.Tomic R, Keenan A, Dincer E, et al. Impact of Acute Exacerbation of Idiopathic Pulmonary Fibrosis on Outcomes after Lung Transplantation. J Heart Lung Transpl 2016;35(4):S232–S32. doi: DOI 10.1016/j.healun.2016.01.659 [DOI] [Google Scholar]
- 10.Nair KD, Kesavan R, Barrios R, et al. Clinical Outcomes In Patients With Acute Exacerbation Of Idiopathic Pulmonary Fibrosis Undergoing Lung Transplantation. American journal of respiratory and critical care medicine 2011;183 [Google Scholar]
- 11.Mudambi R, Narula R, Santangelo GD. Special issue of Industry and Innovation on “Location, Collocation and Innovation across National Borders: Connecting the International Business, Economic Geography and Innovation Communities”. Ind Innov 2015;22(5):443–44. doi: 10.1080/13662716.2015.1076636 [DOI] [Google Scholar]
- 12.Dotan Y, Vaidy A, Shapiro WB, et al. Effect of Acute Exacerbation of Idiopathic Pulmonary Fibrosis on Lung Transplantation Outcome. Chest 2018;154(4):818–26. doi: 10.1016/j.chest.2018.06.027 [published Online First: 2018/07/04] [DOI] [PubMed] [Google Scholar]
- 13.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. American journal of respiratory and critical care medicine 2018;198(5):e44–e68. doi: 10.1164/rccm.201807-1255ST [published Online First: 2018/09/01] [DOI] [PubMed] [Google Scholar]
- 14.Fischer A, Antoniou KM, Brown KK, et al. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology 2015;46(4):976–87. doi: 10.1183/13993003.00150-2015 [published Online First: 2015/07/15] [DOI] [PubMed] [Google Scholar]
- 15.Driessen AJ. Secondary transport of amino acids by membrane vesicles derived from lactic acid bacteria. Antonie Van Leeuwenhoek 1989;56(2):139–60. doi: 10.1007/BF00399978 [published Online First: 1989/08/01] [DOI] [PubMed] [Google Scholar]
- 16.Snell GI, Yusen RD, Weill D, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, part I: Definition and grading-A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation 2017;36(10):1097–103. doi: 10.1016/j.healun.2017.07.021 [published Online First: 2017/09/26] [DOI] [PubMed] [Google Scholar]
- 17.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation 2007;26(12):1229–42. doi: 10.1016/j.healun.2007.10.017 [published Online First: 2007/12/22] [DOI] [PubMed] [Google Scholar]
- 18.Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. The New England journal of medicine 2015;372(23):2185–96. doi: 10.1056/NEJMoa1503326 [published Online First: 2015/05/20] [DOI] [PubMed] [Google Scholar]
- 19.Biscotti M, Yang J, Sonett J, et al. Comparison of extracorporeal membrane oxygenation versus cardiopulmonary bypass for lung transplantation. The Journal of thoracic and cardiovascular surgery 2014;148(5):2410–5. doi: 10.1016/j.jtcvs.2014.07.061 [published Online First: 2014/12/03] [DOI] [PubMed] [Google Scholar]
- 20.Hoechter DJ, Shen YM, Kammerer T, et al. Extracorporeal Circulation During Lung Transplantation Procedures: A Meta-Analysis. ASAIO J 2017;63(5):551–61. doi: 10.1097/MAT.0000000000000549 [published Online First: 2017/03/04] [DOI] [PubMed] [Google Scholar]
- 21.Ius F, Kuehn C, Tudorache I, et al. Lung transplantation on cardiopulmonary support: venoarterial extracorporeal membrane oxygenation outperformed cardiopulmonary bypass. The Journal of thoracic and cardiovascular surgery 2012;144(6):1510–6. doi: 10.1016/j.jtcvs.2012.07.095 [published Online First: 2012/09/05] [DOI] [PubMed] [Google Scholar]
- 22.Machuca TN, Collaud S, Mercier O, et al. Outcomes of intraoperative extracorporeal membrane oxygenation versus cardiopulmonary bypass for lung transplantation. The Journal of thoracic and cardiovascular surgery 2015;149(4):1152–7. doi: 10.1016/j.jtcvs.2014.11.039 [published Online First: 2015/01/15] [DOI] [PubMed] [Google Scholar]
- 23.Bermudez CA, Shiose A, Esper SA, et al. Outcomes of intraoperative venoarterial extracorporeal membrane oxygenation versus cardiopulmonary bypass during lung transplantation. The Annals of thoracic surgery 2014;98(6):1936–42; discussion 42–3. doi: 10.1016/j.athoracsur.2014.06.072 [published Online First: 2014/12/03] [DOI] [PubMed] [Google Scholar]
- 24.Hoetzenecker K, Benazzo A, Stork T, et al. Bilateral lung transplantation on intraoperative extracorporeal membrane oxygenator: An observational study. The Journal of thoracic and cardiovascular surgery 2020;160(1):320–27 e1. doi: 10.1016/j.jtcvs.2019.10.155 [published Online First: 2020/01/15] [DOI] [PubMed] [Google Scholar]
- 25.Kiziltug H, Falter F. Circulatory support during lung transplantation. Curr Opin Anaesthesiol 2020;33(1):37–42. doi: 10.1097/ACO.0000000000000806 [published Online First: 2019/11/13] [DOI] [PubMed] [Google Scholar]
- 26.Diamond JM, Lee JC, Kawut SM, et al. Clinical risk factors for primary graft dysfunction after lung transplantation. American journal of respiratory and critical care medicine 2013;187(5):527–34. doi: 10.1164/rccm.201210-1865OC [published Online First: 2013/01/12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2018 Annual Data Report: Lung. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2020;20 Suppl s1:427–508. doi: 10.1111/ajt.15677 [published Online First: 2020/01/04] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


