Abstract
Background:
Patients experiencing acute neurological injury often receive hourly neurological assessments (“neurochecks”) to capture signs of deterioration. While commonly utilized in the intensive care unit (ICU) setting, little is known regarding practices (i.e., variations by age and ordering services) and patterns (i.e., duration and post-discontinuation plans) of hourly neurochecks. To inform future quality improvement intervention efforts, we performed an analysis of hourly neurochecks using an electronic health record-based dataset.
Study Design and Methods:
Our 75-month retrospective dataset consisted of all health system ICU patients who received hourly neurochecks. Variables included age, admission diagnosis category, ordering provider, post-discontinuation order, and discharge destination. Multivariable Cox regression was used to evaluate factors associated with hourly neurocheck duration.
Results:
We evaluated 9,513 first admission hourly neurocheck orders in 8,936 patients. The trauma, neurosurgery, and neurocritical care services were responsible for 4,067 (43%), 2,071 (22%) and 1,697 (18%) hourly neurocheck orders, respectively. Median (interquartile range) hourly neurocheck duration was 1.09 (0.69, 2.35) days, and was greater than 3 and 7 days, respectively, for 1,773 (19%) and 640 (7%) patients. Median hourly neurocheck duration ranged from 0.87 (0.65, 1.68) to 1.60 (0.83, 2.97) days for neurosurgical and non-neurological ICU services, respectively. Upon discontinuation, 2,225 (23%) of hourly neurochecks were transitioned to no neurochecks.
Conclusion:
Substantial differences exist between ICU services and practice patterns surrounding hourly neurochecks. Understanding these differences will help inform intervention efforts aimed at streamlining hourly neurocheck practices and outcomes for patients with acute neurological injury.
Keywords: neurological examination, intensive care unit, acute brain injury, neuroassessment, neurocheck
Introduction
In the intensive care unit (ICU) setting, patients with acute neurological injury often undergo frequent serial neurological examinations (“neurochecks”) to monitor for signs of neurological deterioration. These neurochecks are often ordered hourly and are vital during the acute phase of injury to monitor for neurological deterioration1,2; however, they may also persist even after clinical stabilization. Prolonged hourly neurochecks may have deleterious effects for patients,3 including sleep disruption and delirium, while also contributing to staff burnout.4–6
Despite their potential deleterious effects, multiple guidelines and society recommendations recommend “frequentneurological examinations” or “repeated neurological assessments” as standard of care following acute brain injury, including ischemic stroke,7–9 intracerebral hemorrhage,10 and subarachnoid hemorrhage.11,12 As a result, “hourly neurochecks” have become standard of care in many institutions despite a lack of strong supporting evidence.9 More specifically, little knowledge exists regarding practices(i.e., variations by age and ordering service) or patterns (i.e., duration and post-discontinuation plans) surrounding frequent neurological assessments, in particular hourly neurochecks. As such, to inform future research and improvement efforts, we used an electronic health record (EHR)-based dataset from a tertiary care academic setting to evaluate practices and patterns of hourly neurochecks along with associated clinical outcomes (e.g., hospital length of stay, mortality).
Methods
Study Design and Data Source
As part of a quality improvement (QI) initiative, we teamed with our institutional Health System analytics core to perform this retrospective EHR-based evaluation of hourly neurocheck practices and patterns within our 110 ICU-bed Health System. Our EHR-based dataset encompassed a 75-month period from January 1, 2012 through March 31, 2018 and included all hourly neurocheck orders for hospitalized adult patients during that timeframe. Variables extracted for each hourly neurocheck order included start and stop time, age, admission diagnosis category, ordering provider, replacement order at the time of discontinuation (e.g., “every 2 hours”), and associated admission/discharge dates and discharge destinations. As we aimed to use administrative data to learn about hourly neurocheck practices and patterns across the Health System, our deidentified dataset included only basic patient-specific demographic (i.e., age) and clinical (i.e., admission diagnosis) variables.
Of note, over our dataset timeframe, all Health System admissions necessitating hourly neurochecks required ICU level of care. These admissions included patients with acute brain injury, spinal cord injury, or those requiring post-operative monitoring following intradural manipulation (e.g., tumor resection) or dural compromise, and those at risk for cerebral hemodynamic changes (e.g., following carotid endarterectomy). While hourly neurochecks could be performed in any of the ICUs within our Health System, most patients requiring close neurological monitoring were admitted to or consulted on by services with expertise in management of acute neurological injury. Neurochecks were performed by trained nurses who completed mandatory neurological education, and included an assessment of the patient’s mental status, cranial nerves, and motor function. More specifically, a standard ICU-level neurocheck included a Glasgow coma score, level of arousal assessment, and evaluations of orientation, attention, language, pupillary size/position/reactivity, facial symmetry, cough reflex (in intubated patients), pronator drift, motor response (to command or noxious stimulation), and, as indicated, sensation and coordination. All Health System ICUs have a nurse-to-patient ratio of at least 1:2, with day and night shift nurses working 7 am to 7 pm and 7 pm to 7 am, respectively.
In accordance with Standards for Quality Improvement Reporting Excellence (SQUIRE) guidelines, before performing efforts at a Health System level, we performed this hourly neurocheck analysis to (1) describe the problem at a local level; (2) provide context necessary to engage key stakeholders; and (3) establish quantitative process measures and outcomes that could be used for to inform the future intervention effort.13 Hence, this quality improvement initiative was deemed exempt from full review by the UCSD Institutional Review Board. In interpreting the results of this project, please note that this dataset represents the descriptive stage of a QI project, and places it within the context of available knowledge while assessing hypotheses that may drive future interventions.
Data Handling
Our analysis focused on inpatient adult admissions involving hourly neurochecks, excluding readmissions. For each admission, we used the earliest start and latest order stop times to calculate total hourly neurocheck duration. For admissions involving multiple hourly neurocheck orders, we collapsed orders separated by ≤8 hours and treated orders as unique if separated by >8 hours (8 hours is the maximum time that can elapse between morning and afternoon ICU rounds, when patient orders are typically reviewed).
Hourly neurocheck ordering services were categorized as neurocritical care, neurosurgery (including otolaryngology-skull base surgery), trauma/acute surgical care, non-neurocritical ICU (including pulmonary/critical care medicine, anesthesia critical care, and cardiac intensive care), and other/non-ICU (including neurology, hospital medicine, other surgery specialties). Orders placed by emergency medicine providers were reassigned to admitting services.
Regarding clinical variables, we recategorized the continuous age variable into young (<65 years), old (65–79 years), and oldest-old (≥80 years), based on ranges used previously (e.g., by Medicare and the US Census Bureau).14–16 Patients <18 years old and those with missing age data on admission (i.e., trauma, comatose) comprised <1% of our sample and were excluded. Additionally, ICD-10-code based admission diagnoses included 1,631 unique categories and were therefore recategorized (e.g., ischemic stroke, traumatic brain injury).
Statistical Analysis
Descriptive statistics included median and interquartile range to summarize right-skewed hourly neurocheck durations. Univariable and multivariable Cox regression was used to evaluate the association of ordering services and temporal factors (year, quarter) with hourly neurocheck duration, while univariable logistic regression was used to evaluate between-service differences in neurocheck orders following hourly neurocheck discontinuation. All analyses were performed using STATA 16.1 (College Station, TX). A P < 0.05 denoted statistical significance, with Tukey corrections for multiple comparisons if indicated.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.
Results
Our dataset included 8,936 patients who ever received hourly neurochecks, spanning 9,982 hospital admissions (1,046 readmissions). Across 8,936 initial hospitalizations, the mean (SD) age of patients was 56 (20), with 22% (n = 1,944) and 14% (n = 1,208) aged 65–79 and ≥80 years old, respectively. The most common admission diagnosis categories included non-traumatic coma or increased intracranial pressure (n = 1,209, 14%), trauma without mention of brain injury (n = 1,180, 13%), and ischemic stroke (n = 907, 10%); due to ICD-10 code unavailability, 24% (n = 2,149) of admission diagnoses were missing (Table 1).
Table 1.
Summary of Hourly Neurochecks Across 8,936 Hospitalizations and 9,513 Orders.
| Order duration (days) | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | n, % | Median (IQR) | Maximum | hospital LOS, median % (IQR)a |
|
| ||||
| Ageb | ||||
| 18 to 64 years old | 5,784 (65%) | 1.10 (0.68, 2.43) | 81 | 31% (16%, 55%) |
| 65 to 79 years old | 1,944 (22%) | 1.28 (0.76, 2.76) | 66 | 32% (17%, 57%) |
| 80 years and older | 1,208 (14%) | 1.14 (0.74, 2.05) | 69 | 30% (17%, 52%) |
| Ordering service | ||||
| Neurocritical care | 1,697 (18%) | 1.54 (0.80, 3.59) | 69 | 33% (17%, 57%) |
| Neurosurgery | 2,071 (22%) | 0.87 (0.65, 1.68) | 65 | 29% (18%, 55%) |
| Trauma | 4,067 (43%) | 1.11 (0.67, 2.08) | 37 | 34% (18%, 54%) |
| Non-neurological ICUc | 639 (7%) | 1.60 (0.83, 2.97) | 81 | 21% (9%, 45%) |
| Non-ICU / other | 1,039 (11%) | 1.08 (0.67, 2.31) | 38 | 28% (12%, 53%) |
| Post-discontinuation orderd | ||||
| Every 2 to 3 hours | 4,251 (45%) | 1.20 (0.71, 2.53) | 65 | 27% (16%, 43%) |
| Every 4 hours | 2,708 (28%) | 1.02 (0.67, 2.24) | 45 | 28% (15%, 46%) |
| Every 6 to 24 hours | 324 (3%) | 1.06 (0.66, 2.18) | 37 | 28% (15%, 50%) |
| No more neurochecks | 2,225 (23%) | 1.03 (0.69, 2.05) | 81 | 63% (26%, 94%) |
| Admission diagnosisb,e | ||||
| Coma and increased ICP | 1,209 (14%) | 1.38 (0.84, 2.73) | 37 | 38% (24%, 57%) |
| Traumatic non-TBI | 1,180 (13%) | 1.06 (0.57, 2.01) | 19 | 24% (12%, 43%) |
| Ischemic stroke | 907 (10%) | 1.45 (0.86, 3.01) | 60 | 36% (21%, 69%) |
| Non-neurological | 846 (9%) | 1.26 (0.66, 2.66) | 81 | 21% (8%, 47%) |
| CNS tumor | 783 (9%) | 0.82 (0.64, 1.56) | 27 | 22% (14%, 34%) |
| Traumatic brain injury | 702 (8%) | 1.12 (0.67, 2.49) | 32 | 37% (24%, 59%) |
| Other Neurologicalf | 320 (4%) | 0.80 (0.61, 1.42) | 38 | 24% (14%, 41%) |
| Elective vascular | 221 (2%) | 0.93 (0.77, 1.13) | 66 | 67% (31%, 83%) |
| Spine and spinal cord injury | 211 (2%) | 1.58 (0.73, 2.85) | 17 | 22% (12%, 38%) |
| Seizure | 164 (2%) | 0.94 (0.61, 1.92) | 15 | 27% (14%, 51%) |
| Intracranial hemorrhage | 152 (2%) | 1.81 (0.88, 4.56) | 22 | 33% (16%, 52%) |
| Subarachnoid hemorrhage | 92 (1%) | 4.68 (1.54, 11.90) | 55 | 56% (31%, 80%) |
| None listed | 2,149 (24%) | 1.16 (0.73, 2.64) | 69 | 37% (19%, 64%) |
| Discharge destinationb | ||||
| Home | 5,190 (58%) | 0.92 (0.64, 1.63) | 81 | 31% (17%, 52%) |
| Non-homeg | 2,881 (32%) | 1.67 (0.86, 3.95) | 69 | 29% (14%, 51%) |
| Died in hospital | 865 (10%) | 2.38 (0.99, 5.02) | 33 | 58% (22%, 91%) |
| Year | ||||
| 2012 | 1,186 (12%) | 1.21 (0.75, 2.74) | 30 | 39% (19%, 69%) |
| 2013 | 1,380 (15%) | 1.05 (0.67, 2.24) | 45 | 32% (17%, 57%) |
| 2014 | 1,522 (16%) | 1.09 (0.65, 2.56) | 46 | 30% (17%, 52%) |
| 2015 | 1,565 (16%) | 1.04 (0.67, 2.28) | 65 | 30% (16%, 52%) |
| 2016 | 1,659 (17%) | 1.10 (0.70, 2.26) | 81 | 29% (16%, 52%) |
| 2017 | 1,807 (19%) | 1.10 (0.69, 2.23) | 69 | 30% (16%, 51%) |
| 2018 | 394 (4%) | 0.95 (0.70, 1.71) | 21 | 29% (18%, 47%) |
| Quarter | ||||
| January to March | 2,577 (27%) | 1.04 (0.68, 2.11) | 69 | 31% (17%, 54%) |
| April to June | 2,210 (23%) | 1.05 (0.66, 2.47) | 81 | 31% (17%, 55%) |
| July to September | 2,366 (25%) | 1.14 (0.72, 2.39) | 45 | 32% (17%, 54%) |
| October to December | 2,360 (25%) | 1.10 (0.68, 2.37) | 38 | 31% (16%, 54%) |
| All hospitalizationsb | 8,936 | 1.13 (0.71, 2.46) | 81 | 31% (17%, 55%) |
| All orders | 9,513 | 1.09 (0.69, 2.35) | 81 | 31% (17%, 54%) |
Abbreviations: CNS, central nervous system; ICP, intracranial pressure; ICU, intensive care unit; IQR, interquartile range; LOS, length of stay; TBI, traumatic brain injury.
N = 8,933 (9,510 hourly neurocheck orders) due to missing length of stay data for 3 hospitalizations.
Cumulative neurocheck duration over the entire admission.
Includes pulmonary critical care, anesthesia and cardiology critical care.
N = 9,508 hourly neurocheck orders; 5 assessments (not listed) changed to every 30 minutes.
Based on ICD-10 code; unless otherwise specified, diagnoses are non-traumatic.
Includes, for example, migraine, myasthenia gravis, CNS infection, multiple sclerosis, dementia.
Includes rehabilitation facility, skilled nursing facility, long-term care facility, hospital-to-hospital transfer, jail, against medical advice, psychiatric facility.
Among these admissions, we identified 9,513 hourly neurocheck orders, which were ordered by the trauma (n = 4,067, 43%), neurosurgery (2,071, 22%), neurocritical care (1,697, 18%), non-ICU (1,039, 11%), and non-neurological ICU (639, 7%) services (Table 1). At hospital discharge, 5,190 patients (58%) were discharged home, 2,881 (32%) to a non-home location, and 865 (10%) died in the hospital.
Hourly Neurocheck Duration
Across the 9,513 hourly neurocheck orders, the median (IQR) order duration was 1.09 (0.69, 2.35) days, with a maximum of 81 days (Table 1, Figure 1). One-half and one-quarter of patients spent at least 31% and 55% of their hospitalizations receiving hourly neurochecks, respectively, with no substantial differences across age groups (Table 1). Additionally, 1,773 (19%) of hourly neurocheck orders lasted ≥3 days and 640 (7%) lasted ≥7 days; among these 640 orders, the median (IQR) duration was 10.6 (8.7, 14.7, maximum 81) days, with coma/increased ICP (n = 102), ischemic stroke (n = 79), and TBI (n = 70) representing the most common corresponding admission diagnoses. Finally, among these ≥7-day orders, 147 (23%) and 35 (5%) occurred in patients aged 65–79 and ≥80 years old, respectively, with median (IQR) durations of 10.5 (8.6, 13.7) and 9.6 (8.3, 13.4) days.
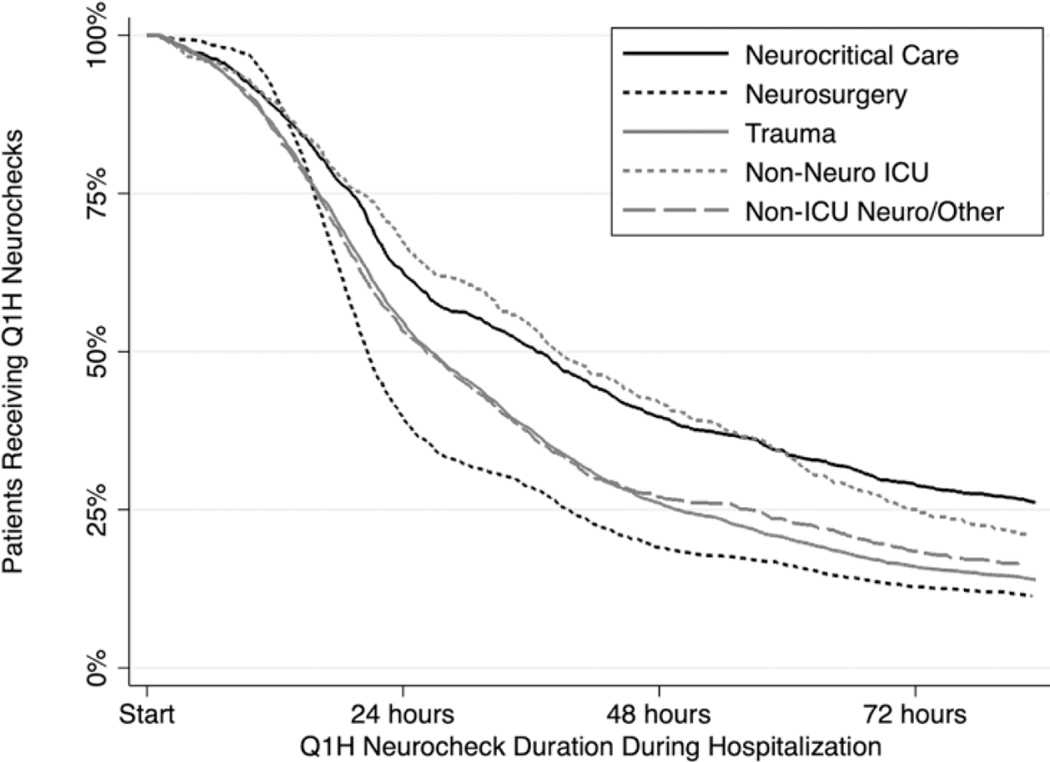
Figure 1.
Hourly neurocheck duration. Hourly neurocheck order duration stratified by ordering service. Neuro indicates neurological; ICU, intensive care unit.
By service line, median (IQR) duration of hourly neurochecks was shortest for neurosurgery (0.87 [0.65, 1.68] days) and longest for neurocritical care (1.54 [0.80, 3.59] days) and non-neurological ICU (1.60 [0.83, 2.97] days) services (Table 1, Figure 1). Hourly neurocheck duration differed significantly between ordering services (P < 0.05 for all pairwise comparisons), with the exception of neurocritical care versus non-neuro ICU and trauma versus neurosurgery.
Discontinuation of Hourly Neurochecks
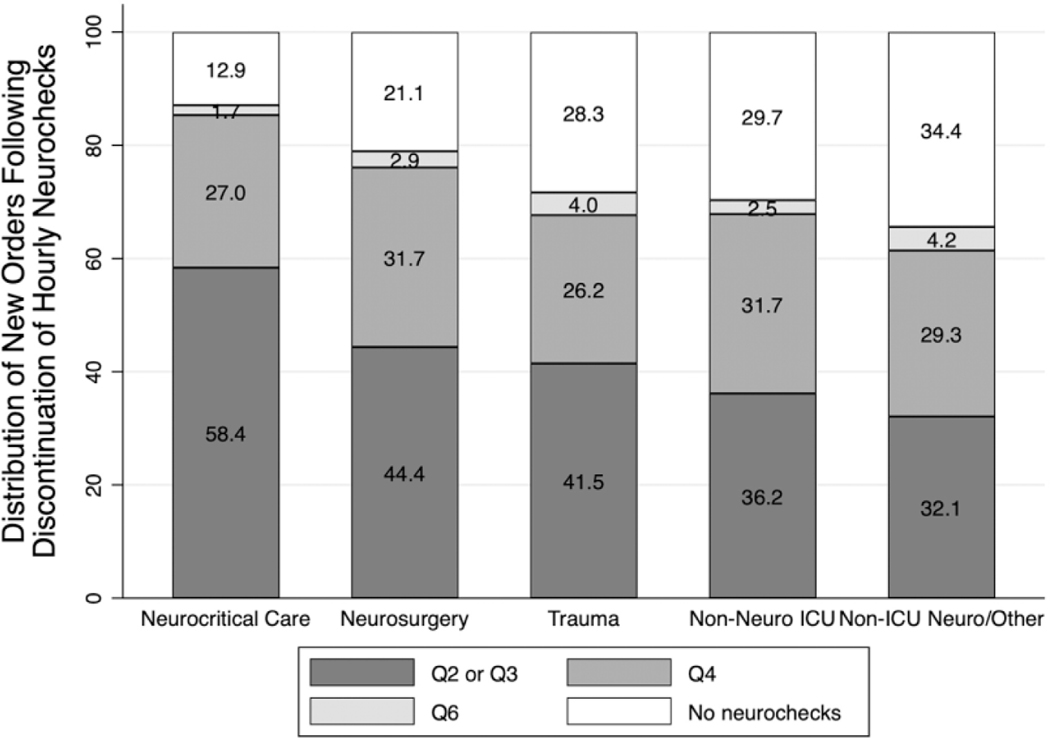
Hourly neurocheck discontinuation was commonly followed by an order for neurochecks every 2 to 3 hours (n = 4,251, 45%) or every 4 hours (n = 2,708, 28%). Additionally, 23% (n = 2,225) of hourly neurocheck orders were followed by complete neurocheck discontinuation; in these cases, hourly neurocheck orders constituted a median (IQR) of 63% (26%, 94%) of patients’ hospital stay (Table 1), after which 1,186 (53%) patients were discharged home and 511 (23%) died in the hospital. When stratified by ordering service, significant order variability was observed following hourly neurocheck discontinuation (unadjusted P < 0.001, Figure 2). However, switching to every 2-to-3-hour neurochecks remained the most common new order for all services except non-ICU neuro/other, which most commonly transitioned to no neurochecks (Figure 2). Significant between-service differences were observed in ordering every 2-to-3-hour neurochecks and discontinuing all neurochecks (P < 0.05 for all pairwise comparisons except for the differences between trauma versus non-neurological ICU services, and the differences between non-ICU neurological versus non-neurological ICU services).
Figure 2.
Distribution of new orders following discontinuation of hourly neurochecks. Stacked bar graph showing subsequent neurocheck order following discontinuation of hourly neurochecks, stratified by ordering service. Significant differences were observed between ordering services regarding every 2-to-3-hour neurochecks and complete neurocheck discontinuation (P < 0.05 for all pairwise comparisons except for trauma versus non-neuro ICU and non-ICU neuro versus non-neuro ICU). Neuro indicates neurological; ICU, intensive care unit.
Age and Admission Diagnosis Categories
The median (IQR) cumulative duration of hourly neurochecks was 1.10 (0.68, 2.43), 1.28 (0.76, 2.76), and 1.14 (0.74, 2.05) days in patients aged 18 to 64, 65 to 79, and ≥80 years old, respectively (Table 1). In adjusted models, patients older than 80 had significantly shorter hourly neurocheck duration than other age groups (P < 0.001, Table 2). Notably, hourly neurocheck duration did not differ by age group when evaluated as a proportion of hospital stay (Table 1) but was lower in older adults across all discharge destination categories.
Table 2.
Multivariable Regression of Hourly Neurocheck Order Discontinuation.
| Variable | Multivariable hazard ratio (95% CI)a | P valueb |
|---|---|---|
|
| ||
| Age | ||
| 18 to 64 years old | REF | |
| 65 to 79 years old | 0.97 (0.92, 1.02) | 0.193 |
| 80 years or older | 1.19 (1.11, 1.26) | <0.001 |
| Admission diagnosis category | ||
| None listed | REF | |
| Coma and increased ICP | 0.76 (0.66, 0.88) | <0.001 |
| Traumatic non-TBI | 1.03 (0.90, 1.20) | 0.617 |
| Ischemic stroke | 1.01 (0.87, 1.17) | 0.931 |
| Non-neurological | 1.04 (0.90, 1.21) | 0.565 |
| CNS tumor | 1.64 (1.41, 1.91) | <0.001 |
| TBI | 0.91 (0.78, 1.05) | 0.211 |
| Other neurological | 1.58 (1.32, 1.87) | <0.001 |
| Elective vascular | 1.59 (1.30, 1.92) | <0.001 |
| Spine and SCI | 0.81 (0.67, 0.98) | 0.032 |
| Seizure | 1.47 (1.19, 1.80) | <0.001 |
| Intracranial hemorrhage | 0.89 (0.73, 1.10) | 0.285 |
| Subarachnoid hemorrhage | 0.54 (0.42, 0.69) | <0.001 |
| Ordering service | ||
| Neurocritical care | REF | |
| Neurosurgery | 1.49 (1.37, 1.61) | <0.001 |
| Trauma | 1.52 (1.42, 1.65) | <0.001 |
| Non-neuro ICU | 1.03 (0.93, 1.14) | 0.536 |
| Non-ICU/other | 1.39 (1.27, 1.51) | <0.001 |
| Year | ||
| 2012 | REF | |
| 2013 | 1.15 (1.06, 1.25) | 0.001 |
| 2014 | 1.19 (1.02, 1.39) | 0.027 |
| 2015 | 1.20 (0.03, 1.40) | 0.022 |
| 2016 | 1.17 (0.99, 1.36) | 0.052 |
| 2017 | 1.20 (1.03, 1.40) | 0.018 |
| 2018 | 1.44 (1.20, 1.73) | <0.001 |
| Quarter | ||
| January to March | REF | |
| April to June | 1.00 (0.94, 1.07) | 0.900 |
| July to September | 1.00 (0.94, 1.06) | 0.981 |
| October to December | 1.02 (0.95, 1.08) | 0.628 |
Higher hazard ratio (HR) represents faster hourly neurocheck discontinuation.
Calculated using multivariable Cox regression.
Regarding admission diagnosis categories, median (IQR) cumulative duration of hourly neurochecks ranged from 0.80 (0.61, 1.42) to 4.68 days (1.54, 11.90) days for other neurological and subarachnoid hemorrhage, respectively (Table 1). In multivariable analysis, median hourly neurocheck duration was significantly longer for coma/increased ICP, spine and spinal cord injury, and subarachnoid hemorrhage, and shorter for CNS tumor, elective vascular procedures, and seizure (versus no diagnosis, P < 0.05, Table 2). Hourly neurocheck orders comprised the highest proportion of hospital LOS for patients undergoing elective vascular procedures (median [IQR] proportion 67% [31%, 83%]) and with subarachnoid hemorrhage (56% [31%, 80%]) (Table 1).
Temporal Changes in Hourly Neurochecks
The duration of hourly neurochecks remained unchanged or declined each calendar year (Table 1). In adjusted models, discontinuation occurred sooner in all years as compared to 2012 (P < 0.001, Table 2), yielding a 2.1% year-to-year absolute reduction in hourly neurocheck duration (P < 0.001). We did not observe an association with quarter and hourly neurocheck order duration (i.e., no “July effect”).
Hourly Neurochecks and Outcomes
Median (IQR) hospital length of stay (LOS) for patients who underwent hourly neurochecks was 4.3 (2.3, 9.6) days, with a range of 2.3 hours to 830 days. During admissions lasting ≤24 hours, hourly neurochecks comprised a median (IQR) of 88% (61%, 100%) of the hospitalization as compared to 21% (11%, 41%) for patients with LOS ≥96 hr. Each additional day of hourly neurochecks was associated with a 1.21 day increase in hospital stay (P < 0.001). There was no association between calendar year or quarter with LOS.
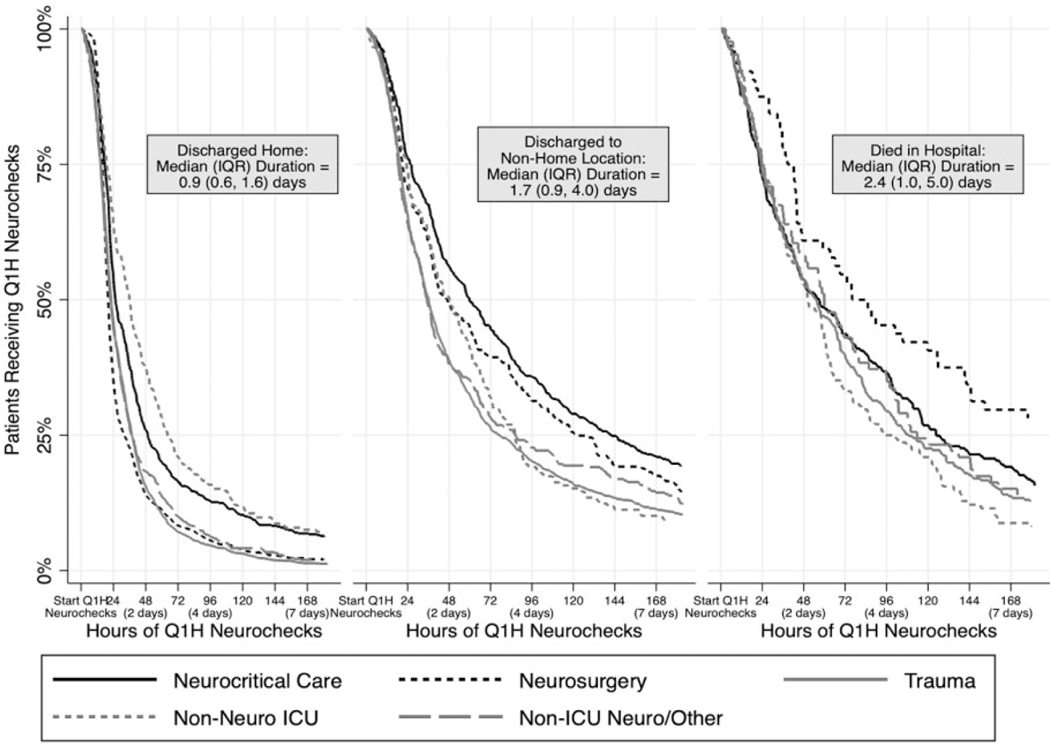
The median (IQR) duration of neurochecks was 0.92 days (IQR 0.64, 1.63; median [IQR] proportion of hospital LOS 31% [17%, 52%]) for patients discharged home, 1.67 (IQR 0.86, 3.95; 29% [14%, 51%]) for those discharged to non-home location, and 2.38 days (IQR 0.99, 5.02; 58% [22%, 91%]) for those who died in the hospital (Table 1, Figure 3). In multivariable models, there were significant between-group differences in hourly neurocheck duration when comparing all 3 discharge destinations (adjusted P < 0.05).
Figure 3.
Proportion of patients remaining on hourly neurochecks over time. Proportion of patients remaining on hourly neurochecks over time, stratified by discharge destination and ordering service. Neuro indicates neurological; ICU, intensive care unit.
Discussion
In this retrospective analysis of 8,936 patients hospitalized with acute brain injury, we observed that frequent and prolonged neurochecks were common, occupying nearly one-third of the hospital stay for 50% of patients, with 20% of patients experiencing hourly neurochecks for ≥3 consecutive days and 7% experiencing them for ≥7 days. Patients admitted with coma, increased ICP, and subarachnoid hemorrhage tended to experience hourly neurochecks for the longest duration. Additionally, upon discontinuation, nearly 25% of hourly neurocheck orders were transitioned to no neurochecks.
Despite being a cornerstone of care provided to patients with neurologic injuries, neurochecks interestingly remain an understudied monitoring technique. To our knowledge, this analysis represents the first EHR-based evaluation of hourly neurochecks in patients at risk for neurological deterioration, providing quantitative data regarding 9,513 hourly neurocheck orders. Though a single-center study, the duration of hourly neurochecks we observed were consistent with previous literature,17 suggesting generalizability of our findings that could inform future efforts, including those to evaluate how abnormal hourly neurocheck findings impact clinical decision making and to define the optimal duration for neurochecks following specific neurological injuries.
More specifically, we found that neurosurgical patients experienced the shortest neurocheck duration, likely due to only requiring brief periods of close monitoring during otherwise uneventful post-operative courses. Conversely, neurocritical care and non-neurological ICU patients experienced hourly neurochecks for the longest duration, potentially exposing them to unnecessary care interactions and negative associated consequences such as sleep fragmentation and delirium.18,19 While some of these prolonged neurochecks may have been necessary (i.e., for neurocritical care patients experiencing vasospasm and/or cerebral edema as part of acute brain injury evolution), the majority were likely the byproduct of an order (e.g., 1 placed in the emergency department or based on a consultant recommendation) that unintentionally went unnoticed and/or lacked a corresponding discontinuation order or recommendation, a theory supported by the large proportion of such orders that transitioned directly from hourly to no neurochecks. As observed in our analysis, the duration of neurochecks improved yearly, demonstrating the potential for practices to improve over time. Though likely driven by Health System initiatives over time—including opening of a neurocritical care-focused ICU and expansion of neurocritical care coverage (i.e., hiring new physician and midlevel providers)—future analyses must evaluate these trends, in particular a deeper evaluation of at-risk populations, neurocheck operations, and the role of EHR alerting systems on hourly neurocheck duration.
Surprisingly, in unadjusted and adjusted models, we observed that the oldest patients (age ≥80 years old) experienced shorter hourly neurocheck durations as compared to their younger counterparts. Although delirium data were not available for this analysis, the presence of real or perceived delirium in this at-risk population20,21 may have motivated early hourly neurocheck discontinuation as a delirium prevention measure. Other factors may also have been involved, including survivor treatment bias22 or the tendency for providers to perform fewer cognitive evaluations in older adults.23 Prospective studies could evaluate these factors further, including a qualitative evaluation of provider perceptions.
Regarding diagnosis categories, we observed significantly longer hourly neurocheck duration for both coma and increased ICP and subarachnoid hemorrhage (SAH), with the latter occupying nearly two-thirds of hospitalization time. As SAH accounts for *10% of neurocritical volume at our institution and 6%−16% worldwide,24 we surmise that our observed 1% SAH proportion was quite low, likely due to recategorization of these patients into the coma and increased ICP category (as these are common presenting signs secondary to SAH). Additionally, because of changes in ICD-10 coding, it is possible that aneurysmal SAH patients were also misclassified into the unknown diagnosis category. Future investigations distinguishing between admission signs and diagnoses could address this limitation.
Next, we observed significant between-service heterogeneity in hourly neurocheck durations and post-discontinuation orders, which may reflect differences in patient populations or established ordering service practices. The variability seen in this large dataset may provide useful information when designing interventions to reduce neurocheck frequency or duration. In fact, these interesting observations lend themselves to easy interventions; for example, quality improvement efforts involving educational interventions, or EHR-based alerts to heighten awareness and prompt order reconciliation for patients receiving prolonged neurochecks.
Last, we observed that longer hospitalizations and those culminating in death were associated with longer durations of hourly neurochecks. Causal relationships could not be drawn between death and neurochecks from this dataset, as unmeasured confounders (e.g., severity of illness) likely influenced this observation; these confounders will be important to consider in future investigations given the potential link between mortality and hourly neurochecks. Additionally, other outcomes that may be mediated by neurocheck frequency, such as delirium incidence, are of particular importance.
A review of the literature reveals that there is minimal guidance on what should constitute an ICU-level neurocheck. Our institutional practice for neurochecks—like many other comprehensive stroke centers—is biased to detect deficits in motor, language, and brainstem systems, and may miss new deficits in other areas (e.g., nondominant parietal lobe), so the utility of frequent basic monitoring depends substantially on which part of the neurological exam the healthcare team is interested in monitoring. Current evidence suggests that neurological deterioration most often occurs within the first 24 hours of admission.3 Imaging characteristics such as infarct volume25 or diffusion-weighted signal on MRI26 may help identify patients requiring ICU admission and having a higher likelihood of neurological deterioration. Hence, knowing the temporal profile of neurological deterioration1,3 may be useful in determining how soon hourly neurochecks could be safely discontinued. Future clinical practice guidelines must consider a more personalized approach, outlining specific content, duration, and frequency of assessments based on a patient’s diagnosis and clinical trajectory, while weighing available hospital resources.
Despite our study’s strengths, we acknowledge several limitations. First, as a retrospective evaluation, we were unable to draw specific causal inferences regarding hourly neurochecks. However, our analysis met the goal of providing valuable information for hypothesis generation and future intervention development. Second, our analysis was limited by a lack of comprehensive, demographic, clinical (e.g., specific neurological diagnoses, Hunt-Hess/Glasgow Coma scores) and outcomes (e.g., Modified Rankin Scores) data, along with unavailability of nearly one-quarter of ICD-10 admission diagnosis codes. While such data could aid further in intervention development and identifying populations at risk for prolonged neurochecks, we feel the analysis presented provides ample foundation for prospective studies aimed at better understanding and improving neurocheck practices. Third, because we sought to learn about practices and patterns, we did not evaluate the impact of hourly neurochecks on clinically important and patient-reported outcomes, such as sleep quality and delirium. Future efforts should include comprehensive patient-oriented and system-level measures as a method to inform and improve clinical practices.
In conclusion, hourly neurochecks are frequently used to monitor patients with acute neurological injury but vary considerably by ordering service. While hourly neurochecks are often utilized to compensate for inherent limitations of other neurological monitoring modalities, the content, utility, duration and frequency of these detailed neurological assessments deserve careful study going forward. Our data suggest that hourly neurochecks may continue longer than necessary, an observation that can inform future interventions to improve outcomes for patients with neurological injury.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Kamdar is supported by a Paul B. Beeson Career Development Award through the National Institutes of Health/National Institute on Aging (K76 AG059936).
Abbreviations
- EHR
Electronic Health Record
- ICD
International Classification of Diseases
- ICU
intensive care unit
- IQR
interquartile range
- LOS
length of stay
- MRI
magnetic resonance imaging
- QI
quality improvement
Footnotes
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Drs Malhotra, Owens, and Kamdar are funded by the NIH. Dr Malhotra reports income from Merck and Livanova related to medical education. ResMed Medication Company provided a philanthropic donation to UC San Diego.
References
- 1.Maas MB, Berman MD, Guth JC, Liotta EM, Prabhakaran S, Naidech AM. Neurochecks as a biomarker of the temporal profile and clinical impact of neurologic changes after intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2015;24(9):2026–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maas MB, Rosenberg NF, Kosteva AR, et al. Surveillance neuroimaging and neurologic examinations affect care for intracerebral hemorrhage. Neurology. 2013;81(2):107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McLaughlin DC, Hartjes TM, Freeman WD. Sleep deprivation in neurointensive care unit patients from serial neurological checks: how much is too much? J Neurosci Nurs. 2018;50(4):205–210. [DOI] [PubMed] [Google Scholar]
- 4.Chang VA, Owens RL, LaBuzetta JN. Impact of sleep deprivation in the neurological intensive care unit: a narrative review. Neurocrit Care. 2020;32(2):596–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finan PH, Quartana PJ, Smith MT. The effects of sleep continuity disruption on positive mood and sleep architecture in healthy adults. Sleep. 2015;38(11):1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matthews EE. Sleep disturbances and fatigue in critically ill patients. AACN Adv Crit Care. 2011;22(3):204–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–e418. [DOI] [PubMed] [Google Scholar]
- 8.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3): e46–e110. [DOI] [PubMed] [Google Scholar]
- 9.Wijdicks EF, Sheth KN, Carter BS, et al. Recommendations for the management of cerebral and cerebellar infarction with swelling: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(4):1222–1238. [DOI] [PubMed] [Google Scholar]
- 10.Hemphill JC III, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(7): 2032–2060. [DOI] [PubMed] [Google Scholar]
- 11.Diringer MN, Bleck TP, Claude Hemphill J III, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society’s Multidisciplinary Consensus Conference. Neurocrit Care. 2011;15(2):211–240. [DOI] [PubMed] [Google Scholar]
- 12.Rudd AG, Bowen A, Young GR, James MA. The latest national clinical guideline for stroke. Clin Med (Lond). 2017;17(2): 154–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogrinc G, Armstrong GE, Dolansky MA, Singh MK, Davies L.SQUIRE-EDU (Standards for quality improvement reporting excellence in education): publication guidelines for educational improvement. Acad Med. 2019;94(10):1461–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen-Ranberg K, Petersen I, Robine J-M, Christensen K. Who are the oldest-old? In: Borsch-Supan A, Brugiavini A, Jurges H, Mackenbach J, Siegrist J, Weber G, eds. Health, Ageing and Retirement in Europe: First Results From the Survey of Health, Ageing and Retirement in Europe. Mannheim Research Institute for the Economics of Aging; 2005:35–40. [Google Scholar]
- 15.Lai X, Zhu H, Huo X, Li Z. Polypharmacy in the oldest old(>/=80 years of age) patients in China: a cross-sectional study. BMC Geriatr. 2018;18(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Census USBot. Current Population Reports, Special Studies. P23–190, 65+ in the United States. U.S. Government Printing Office; 1996. [Google Scholar]
- 17.Stone JJ, Childs S, Smith LE, Battin M, Papadakos PJ, Huang JH. Hourly neurologic assessments for traumatic brain injury in the ICU. Neurol Res. 2014;36(2):164–169. [DOI] [PubMed] [Google Scholar]
- 18.Trompeo AC, Vidi Y, Locane MD, et al. Sleep disturbances in the critically ill patients: role of delirium and sedative agents. Minerva Anestesiol. 2011;77(6):604–612. [PubMed] [Google Scholar]
- 19.Watson PL, Ceriana P, Fanfulla F. Delirium: is sleep important? Best Pract Res Clin Anaesthesiol. 2012;26(3):355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckstein C, Burkhardt H. Multicomponent, nonpharmacological delirium interventions for older inpatients: a scoping review. Z Gerontol Geriatr. 2019;52(suppl 4):229–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou Y, Dan X, Babbar M, et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15(10):565–581. [DOI] [PubMed] [Google Scholar]
- 22.Austin PC, Mamdani MM, van Walraven C, Tu JV. Quantifying the impact of survivor treatment bias in observational studies. J Eval Clin Pract. 2006;12(6):601–612. [DOI] [PubMed] [Google Scholar]
- 23.Neuman MD, Speck RM, Karlawish JH, Schwartz JS, Shea JA. Hospital protocols for the inpatient care of olderadults: results from a statewide survey. J Am Geriatr Soc. 2010;58(10):1959–1964. [DOI] [PubMed] [Google Scholar]
- 24.Venkatasubba Rao CP, Suarez JI, Martin RH, et al. Global survey of outcomes of neurocritical care patients: analysis of the PRINCE study part 2. Neurocrit Care. 2020;32(1):88–103. [DOI] [PubMed] [Google Scholar]
- 25.Faigle R, Wozniak AW, Marsh EB, Llinas RH, Urrutia VC. Infarct volume predicts critical care needs in stroke patients treated with intravenous thrombolysis. Neuroradiology. 2015;57(2):171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faigle R, Marsh EB, Llinas RH, Urrutia VC. Critical care needs in patients with diffusion-weighted imaging negative MRI after tPA—does one size fit all? PLoS One. 2015;10(10):e0141204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.