Abstract
Current evidence suggests that intrauterine bisphenol A (BPA) exposure increases the risk of developing cardiovascular diseases in later stages of life. The beneficial effect of resveratrol (Rsv) on developmental programming of atherosclerosis lesions formation in offspring is seldom reported. Hence, we sought to study the effect of maternal Rsv in ameliorating perinatal BPA exposure-induced atherosclerosis lesions formation in adult offspring using the apolipoprotein E-deficient (ApoE−/−) mice model. The pregnant ApoE−/− mice were allocated into three groups: control, BPA, BPA + resveratrol (BPA + Rsv). The BPA group mice received BPA in their drinking water (1 μg/ml). BPA + Rsv group mice received BPA in their drinking water (1 μg/ml) and were treated orally with Rsv (20 mg kg−1 day−1). All the treatments were continued throughout the gestation and lactation period. Quantitative analysis of Sudan IV-stained aorta revealed a significantly increased area of atherosclerotic lesions in both female (p < 0.01) and male adult offspring mice (p < 0.01) in the BPA group. Supplementation with Rsv significantly reduced the BPA-induced atherosclerotic lesion development in the female offspring mice (p < 0.05). Transmission electron microscopy revealed the presence of a significantly high incidence of autophagic endothelial, smooth muscle, and macrophage cells in the aorta of BPA-exposed mice. Rsv treatment reduced the incidence of autophagic cells in BPA-exposed mice. In conclusion, maternal Rsv supplementation significantly prevents the BPA-induced atherosclerotic lesions formation in a sex-dependent manner potentially by acting as an autophagy modulator.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-021-03078-y.
Keywords: Bisphenol A, Resveratrol, Pregnancy, Atherosclerosis, Autophagy
Introduction
Bisphenol A (BPA) is a chief endocrine-disrupting compound structurally resembling diethylstilbestrol (Dodds and Lawson 1936). Its presence has been detected in breast milk, neonatal blood, amniotic fluid, cord blood, and placenta (Rochester 2013). BPA exposure can cause various health problems in humans including reproductive toxicity, endocrine disorders, altered immune function, metabolic diseases, cardiovascular diseases (CVD), and alteration of epigenetic markers and gene expression (Rochester 2013). Exposure to BPA is positively linked with coronary arterial atherosclerosis (Melzer et al. 2012), carotid atherosclerosis (Lind and Lind 2011), and peripheral arterial disease (Shankar et al. 2012). Experimental studies have demonstrated that BPA exposure in adults increases the susceptibility to atherosclerosis (Fang et al. 2015, 2014; Sui et al. 2014; Kim et al. 2014).
Evidence from the “Developmental Origins of Health and Disease” theory suggests that exposure to environmental insults during sensitive periods of development promotes the risk of developing diseases in later stages of life (Gluckman et al. 2005). Further, exposure during these critical periods may cause epigenetic modifications and promote the chances of the development of disease in future generations (Wallack and Thornburg 2016; Baird et al. 2017). It has been found that BPA can cross the placenta and reach the developing fetuses (Takahashi and Oishi 2000). BPA exposure during the early stages of development can augment the development of metabolic diseases like diabetes mellitus and obesity (Wei et al. 2011; Ma et al. 2013; Alonso-Magdalena et al. 2010; Angle et al. 2013; Miyawaki et al. 2007). Although atherosclerotic lesions are more prevalent in adulthood, their occurrence is also identified in fetuses and children (Napoli et al. 1997, 1999). In a recent study, maternal BPA exposure increased atherosclerosis development in adult offspring mice (Sui et al. 2018). Autophagy is an intracellular process that degrades the dysfunctional organelles. This process involves the formation of autophagosomes, autophagosome-lysosome fusion, substrate degradation, and autophagic lysosome formation. In autophagy research, transmission electron microscopy (TEM) examination is considered the gold standard technique (Klionsky et al. 2007). Defective autophagy is implicated in many diseases including lipid accumulation and atherosclerosis (Mizushima and Komatsu 2011). In vitro and in vivo studies have found an association between BPA exposure and autophagy (Quan et al. 2017; Song et al. 2019).
Currently, there is a critical need for developing a new treatment strategy to prevent and/or ameliorate the adverse fetal programming effects. Resveratrol (Rsv, 3,4,5-trans-trihydroxystilbene), is a polyphenol compound found in red grape skin, peanuts, mulberries, peanuts, rhubarb, and many other plants. In plants, it protects against environmental stress (Diaz-Gerevini et al. 2016). The biological role of Rsv in various health-promoting benefits have been demonstrated in both clinical and experimental studies. It is known to have anti-inflammatory (Oliveira et al. 2017), antioxidant (Truong et al. 2018), antiobesogenic (Martel et al. 2017), antiatherosclerotic (Fan et al. 2008), and antidiabetic (Zhu et al. 2017) properties. It is known to cross the placenta (Bourque et al. 2012) and its intake during gestation is found to be safe (Williams et al. 2009). Maternal Rsv exposure has been reported to improve lipid metabolism (Liu et al. 2020; Zou et al. 2017) and cardiovascular health in offspring (Shah et al. 2016). Its intake at doses up to 5 g/day is reported to be safe for humans (Boocock et al. 2007). Apolipoprotein E-deficient (ApoE−/−) mice are the most widely used model of experimental atherosclerosis as it exhibits severe hypercholesterolemia and atherosclerotic lesions (Paigen et al. 1994). This study was performed to observe the effect of BPA exposure during pregnancy and lactation period on the atherosclerosis development in the adult offspring mice and investigate whether Rsv treatment could ameliorate BPA-induced atherosclerotic lesions formation.
Methods
Animals
Male and female ApoE−/− mice (C57BL/6 background; model # B6.129P2-Apoetm1Unc N11) purchased from Taconic Biosciences, Inc. (Rensselaer, NY, USA) were used in the present study. Mice were housed in polypropylene cages and provided a standard laboratory chow diet (Oman Flour mills, Oman) and were placed in the small animal house facility of Sultan Qaboos University. Proper ventilation with an ambient temperature of 22 ± 2 °C, humidity (60%), and a 12 h light: dark cycle was maintained throughout the experiment. All the animal experimental procedures of the present study were conducted as per the international laws and were performed after obtaining approval from the Sultan Qaboos University Animal Ethical Committee (SQU/AEC/2016–17/12).
Treatment
After two weeks of acclimatization, 10 weeks old female and male mice were kept for mating at a 2:1 ratio. Detection of vaginal plug was used to confirm the pregnancy. The pregnant mice were then randomly allocated into three groups: control (n = 8); BPA (n = 8); BPA + Rsv (n = 8). The BPA group mice received BPA (Sigma-Aldrich, St Louis, MO, USA) in their drinking water at a concentration of 1 μg/ml. BPA was first dissolved in ethanol and then diluted to make the final concentration of ethanol to 0.1% (v/v). BPA + Rsv group mice were treated orally with Rsv (Sigma-Aldrich, St Louis, MO, USA) at a dose of 20 mg kg−1 day−1 and received BPA in their drinking water (1 μg/ml). Rsv was first dissolved in ethanol, then diluted such that ethanol final concentration was 0.5% (v/v). The doses of BPA and Rsv were chosen based on previous studies (Juan et al. 2002; Penumathsa et al. 2007; Miyawaki et al. 2007). The control group were exposed to 0.1% ethanol in drinking water and 0.5% of ethanol orally. All the treatments were started from the 1st day of gestation and continued throughout the gestation and lactation period (till post-natal day 21). Based on the water intake, the estimated levels of BPA consumed daily by each pregnant mouse on 7th day and 11th day of gestation were found to be 159.6 ± 16.58 and 160.81 ± 13.56 µg kg−1 respectively, in the control group and 156.3 ± 17.9 and 155.99 ± 22.58 µg kg−1 respectively, in BPA group and 157.7 ± 12.8 and 152 ± 19.9 µg kg−1 respectively, in the BPA + Rsv group. After weaning (PND21 day), offspring were separated from their mother and housed in separate cages. The bodyweight of offspring was measured at regular intervals. At the end of the 20th week, offspring mice in all the groups were used for two different sets of analysis. In set I, a total of 60 mice with 20 mice (n = 10 female; n = 10 male) from each group were sacrificed and the whole aorta was used for the Sudan IV preparation. In set II, a total of 60 mice with 20 mice (n = 10 female; n = 10 male) from each group were sacrificed and the proximal segment of the aorta was collected for TEM.
Sudan IV preparation and quantification of atherosclerotic lesions
In each mouse, the whole aorta was subjected to Sudan IV preparation for gross atherosclerotic lesions quantification (Mohanta et al. 2016). Briefly, in each mouse after careful dissection, the aorta was exposed and perfused with ice-cold phosphate buffer saline. Then, aortic trees were dissected out carefully and fixed in paraformaldehyde-sucrose solution. Fixed tissues were stained with Sudan IV solution at room temperature. The digital images of the en-face stained total aorta were captured with a known scale. Quantification of the atherosclerotic lesions was performed using the Image J program (National Institutes of Health). Using quantification features, the total vessel area and stained lesion areas in the aortic surface were measured manually. All the measurements were exported to an excel file to calculate the percentage of a lesion to the total aortic area. Additional care was taken to exclude the Sudan IV-stained adipose tissues that were located outside the adventitia.
Transmission electron microscopy
After careful dissection, tissues were fixed in a glutaraldehyde (2.5%) solution. Fixed tissues were washed in sodium cacodylate buffer (Ph. 7.4) and kept for 1 h in osmium tetroxide at room temperature. Then, samples were subjected to dehydration in serial of gradually increasing acetone solutions and preceded for embedding and sectioning. Remarkably very thin Sects. (90 nm) were stained first by uranyl acetate and then with lead citrate. The stained ultrathin sections of the proximal segment of the aortic arch were screened to quantify the incidence of autophagy in the three major cell types of the aorta (Perrotta 2013). Briefly, in each specimen, autophagic cells were identified by the presence of double-membrane bound vacuoles/vesicles containing amorphous materials of cytoplasm (autophagosomes) and intact nuclear envelope and nucleolar components. Ultrastructural examination and identification of autophagic endothelial, smooth muscle, and macrophage cells were performed as described previously (Perrotta 2013). The presence of autophagic cells among the total cells counted in 3 different grids was noted. The incidence of autophagic cells was calculated using the following formula. Percentage of autophagic cells = number of autophagic cells/total cells counted × 100 (Perrotta 2013).
Statistical analysis
The statistical analysis was performed using IBM Statistical Package for the Social Sciences (SPSS) Statistics for Windows [version 23.0, Professional] (IBM Corp., Armonk, New York). Data are presented as mean ± standard error of the mean. One-way ANOVA followed by Tukey's multiple comparison test was used to evaluate differences between groups. A P value of < 0.05 was considered to indicate statistical significance.
Results
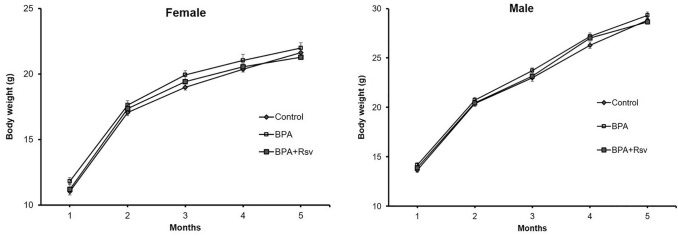
We determined the effects of maternal BPA or BPA + Rsv exposure on weight gain in the offspring at regular intervals and growth curves have been plotted for the experimental groups (Fig. 1). No statistically significant differences were observed between the groups, indicating that maternal BPA or BPA + Rsv exposure didn’t interfere in the body weight gain in the adult offspring.
Fig. 1.
Effect of maternal bisphenol A or bisphenol A and resveratrol exposure on female and male offspring body weight. Growth curves of control, bisphenol A (BPA) or bisphenol A and resveratrol (BPA + Rsv) groups (n = 10). No statistically significant differences were observed between the groups
Effect of Rsv on BPA-induced atherosclerotic lesions formation in adult offspring mice
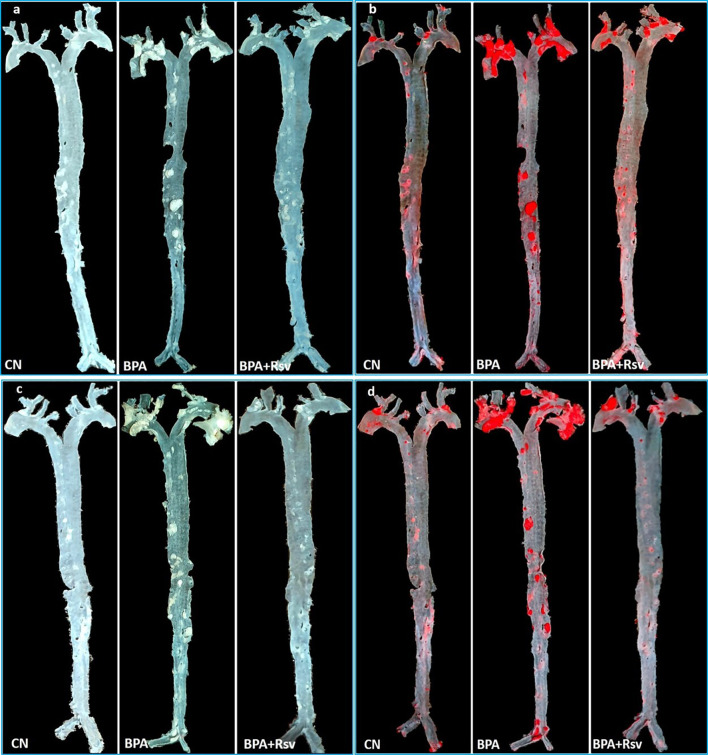
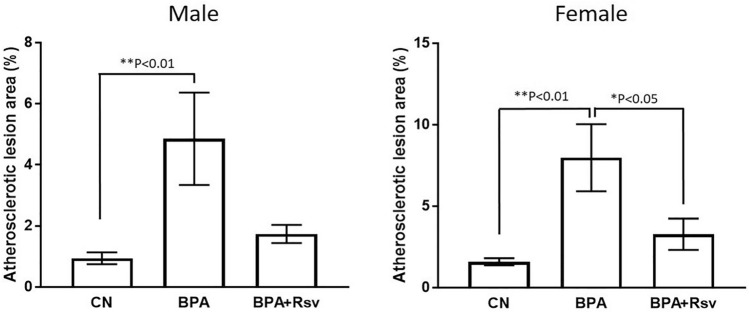
Effect of Rsv on BPA-induced atherosclerotic lesions formation in adult female (Fig. 2a, b) and male offspring (Fig. 2c, d) mice was determined by quantitative analysis of Sudan IV-stained aorta. A significantly increased area of atherosclerotic lesions was observed in the BPA group of female offspring mice (p < 0.01) and male offspring mice (p < 0.01) when compared to control group mice respectively, suggesting that intrauterine exposure to BPA induces the development of atherosclerotic lesions in the adult offspring (Fig. 5). Maternal Rsv treatment significantly reduced the BPA-induced atherosclerotic lesions formation in the female offspring mice (p < 0.05), but not in the male offspring (p > 0.05) (Fig. 3).
Fig. 2.
Effect of maternal resveratrol on bisphenol A-induced atherosclerotic lesions formation in adult offspring. Representative images of the whole unstained aorta and Sudan IV-stained aorta in female (a, b) and male (c, d) offspring. Note the increased area of atherosclerotic lesions in bisphenol A (BPA) exposed mice compared to control (CN) mice. Treatment with resveratrol reduced the atherosclerotic lesions in BPA-exposed mice (BPA + Rsv)
Fig. 3.
Quantitative analysis of atherosclerotic lesion areas in the Sudan IV-stained aorta of both male and female offspring mice from control (CN) or bisphenol A (BPA) or bisphenol A and resveratrol (BPA + Rsv) groups (n = 10). One-way ANOVA followed by Tukey's post hoc test
Detection of autophagy in the aorta of adult offspring mice
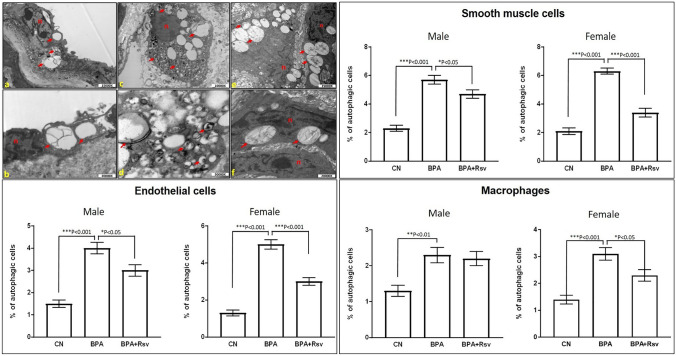
The incidence of autophagy in the aorta was determined using TEM examination. The autophagic cells were identified in all three major cell types of the aorta including endothelial, macrophage, and smooth muscle cells, in the offspring of all the three study groups (Fig. 4). The percentage of autophagic endothelial, macrophage and smooth muscle cells among three different groups were plotted (Fig. 4). The incidence of autophagic cells was significantly more in BPA-exposed group when compared to the control group, in both male and female offspring mice. The increased number of autophagic cells along with increased atherosclerotic lesions in the BPA-exposed group indicates the defective autophagy machinery in the aorta. Maternal Rsv treatment significantly reduced the incidence of autophagic endothelial and smooth muscle cells when compared to BPA group, in both female and male offspring mice. However, Rsv treatment significantly reduced the incidence of autophagic macrophages only in the female offspring mice but not in the male offspring. This result indicates that Rsv treatment prevented the atherosclerosis progression, particularly in female offspring, potentially by modulating the BPA-induced defective autophagy.
Fig. 4.
Representative transmission electron micrographs of cells of aorta showing the autophagosome formation and vacuolization in endothelial (a, b), macrophage (c, d), and smooth muscle (e, f) cells in ApoE−/− mice. The red arrows indicate the double-membrane autophagic vacuoles or autophagosomes (n-nucleus). Quantitative analysis of the incidence of autophagic endothelial, smooth muscle and macrophages in both male and female offspring mice from control (CN) or bisphenol A (BPA) or bisphenol A and resveratrol (BPA + Rsv) groups (n = 10) is also preseneted. One-way ANOVA followed by Tukey's post hoc test
Discussion
Although the effect of Rsv on atherosclerosis development is very well investigated in both animals and humans in adulthood, the beneficial effect of Rsv on developmental programming of atherosclerosis lesions formation in offspring has not been studied (Hsu et al. 2021; Zheng et al. 2018). Further, the effect of maternal Rsv supplemenation on perinatal BPA exposure-induced atherosclerosis in the adult offspring has not been explored. To the best of our knowledge, this is the first study to report augmenting the role of maternal Rsv in ameliorating BPA-induced atherosclerosis lesions formation in adult offspring using the ApoE−/− mice model.
The evidence from epidemiological and experimental studies demonstrates that intrauterine BPA exposure significantly affects global health problems including the risk of developing CVD. BPA exposure during pregnancy induces obesity and hypertension (Rasdi et al. 2020; Batista et al. 2012; Han and Hong 2016). Although underlying mechanisms of associations between BPA and CVD are still unclear, several factors viz. epigenetic changes, endocrine modulation, oxidative stress, and inflammation induction, etc. have been implicated (Rasdi et al. 2020). Recently, Sui et al. have demonstrated that intrauterine and early perinatal BPA exposure increases atherosclerosis development in later life, in the PXR-humanized apolipoprotein E-deficient mouse model (Sui et al. 2018). Findings of this study showed that maternal BPA exposure increases the aortic atherosclerotic lesions through the PXR-dependent epigenetic regulation of CD36 expression in the adult offspring without any alterations in the plasma lipid levels (Sui et al. 2018). Similar to the previous study, in the present study maternal BPA exposure significantly increased the atherosclerotic lesion area when compared to the control littermates.
In our study, TEM examination results revealed the presence of a significantly more autophagic cells in the aorta of the BPA-exposed mice. Basal autophagy is an evolutionarily conserved dynamic process, by recycling the biomolecules, performs the cellular homeostatic function and stress adaptation. Autophagy is activated/triggered when cells are exposed to intracellular and extracellular stress stimuli as a part of the cell survival mechanism (Mizushima and Komatsu 2011). Earlier incidence of autophagy in atherosclerosis has been demonstrated (Perrotta 2013; Liu et al. 2015; Martinet and De Meyer 2009). Previous studies have shown that in atherosclerosis, autophagy can occur in all major cell types of the arterial wall (Perrotta 2013; Liu et al. 2015; Martinet and De Meyer 2009). Similarly, in the present study autophagy has been observed in all the major cell types of the aorta including endothelial, macrophage, and smooth muscle cells. Generally, defects in autophagy are known to occur in two main stages of the autophagy machinery. However, in atherosclerosis defects in autophagy are reported to be mainly due to the impaired autophagosome-lysosome fusion or the lysosomal-mediated degradation (Mitchinson 1982). In advanced plaques, the accumulated ceroids in lysosomes inhibit their participation in active autolysosomes formation which are the end products of autophagosome-lysosome fusion (Kurz et al. 2007). The accumulated cholesterol crystals in advanced plaques are also known to damage the lysosomal membrane and prevent autophagy completion (Duewell et al. 2010). These findings demonstrate that in response to autophagy inducers of the plaque such as reactive oxygen species (ROS), oxidized lipids, and ER stress, etc., the initial step of autophagy is still activated in advanced lesions resulting in more number of autophagic cells, but the second stage of process becomes dysfunctional (Grootaert et al. 2018). Hence, BPA-induced stress conditions could be one of the reasons for the presence of an increased number of autophagic cells in the aorta of the BPA-exposed mice that were observed in the present study.
It has been demonstrated that BPA exposure is associated with autophagy dysregulation in the pathogenesis of various diseases (Meng et al. 2020; Lin et al. 2019). However, to date, the role of BPA on autophagy modulation in atherosclerosis has not been studied. Increased number of autophagic cells in BPA-exposed mice indicates that the BPA did not interfere with the autophagosome formation. In an in vitro study, BPA exposure caused excessive lipid droplet and ROS accumulation by inhibiting the autophagogome-lysosome fusion. However, BPA did not interfere with autophagic cell activation and autophagosome formation (Song et al. 2019). The soluble N-ethylmaleimide-sensitive factor activating protein receptors (SNAREs) play an important role in autophagosome-lysosome fusion. Song et al. (2019) demonstrated that BPA inhibits the autophagogome-lysosome fusion by decreasing the translocation of Syntaxin-17 (SNAREs family) to lysosome (Song et al. 2019). Based on these findings, it can be hypothesized that maternal BPA exposure contributes to the epigenetic modifications that are responsible for the maladaptive autophagic pathway particularly defective autophagogome-lysosome fusion and autolysosome formation, resulting in increased atherosclerotic plaques in the BPA-exposed adult mice.
Interestingly, we observed that the use of maternal Rsv prevented BPA-induced atherosclerotic changes of the aorta in the adult offspring mice. The beneficial role of Rsv against CVD is found to be due to its antioxidative and anti-inflammatory effects, modulatory effect on lipoproteins, inhibition of oxidative stress/reactive oxygen species generation, prevention of endothelial dysfunction, and/or endothelial inflammation (Fan et al. 2008). In previous studies, epigenetic modifications were linked to maternal nutrition intervention and offspring improved cardiometabolic health (Estampador and Franks 2014; Zheng et al. 2017). Hence, the beneficial effects of Rsv against the BPA-induced atherosclerosis development in offspring could be through epigenetic modification. Pharmacological approaches have been developed to prevent atherosclerosis progression by modulating autophagy (Pattison et al. 2012; Hassanpour et al. 2019). Rsv is known to promote autophagy by inhibiting mammalian target of rapamycin kinase (mTOR) (Sanches-Silva et al. 2020), activating 5’-adenosine monophosphate-activated protein kinase (AMPK) (Szkudelski and Szkudelska 2015), and sirtuin family members (SIRT1) (Takeda-Watanabe et al. 2012). The beneficial effect of Rsv against BPA-induced defective autophagy flux would be attributed to its enhancing role of autophagosome-lysosome fusion. In support of these views, in an in vitro study, Rsv prevented the oxidized low-density lipoprotein-induced autophagy dysfunction of human umbilical vein endothelial cells by restoring the lysosomal function (Zhang et al. 2016). In addition, Rsv treatment significantly increased the SNAREs in adipose tissue of diabetic rats (Rezaei Farimani et al. 2015). In the present study, we have noted a significantly low number of autophagic cells in the BPA + Rsv treated mice indicating that Rsv which is a known autophagy modulator would have upregulated the BPA-induced impaired autophagic flux, thereby reducing the atherosclerosis progression and/or burden in the BPA + Rsv treated mice. Based on these observations, the beneficial role of Rsv observed in the present study could be attributed to its autophagy modulatory properties. However, more molecular investigations are required to confirm these inferences. Further, in the present study Rsv supplementation showed anti-atherogenic effects in a sex-dependent manner. Rsv significantly prevented the BPA-induced atherosclerosis progression only in female offspring but not in the male offspring. Evidence suggests that early nutritional intervention and hormonal manipulations can show effects differently in males and females (Aiken and Ozanne 2012; Guo et al. 2012; Mela et al. 2012; Argente-Arizon et al. 2016). Earlier, the sexual dimorphic impact of Rsv has been reported in its modulatory effects on metabolic health in the adult offspring (Ros et al. 2018). More studies need to be conducted to address the mechanisms of sexual dimorphic effects of Rsv on offspring health. This study has the following limitations. We could not perform the assay of autophagosome-lysosome fusion and its subsequent degradation. Immunoblotting assay of autophagic substrate p62 in the study samples would have perhaps helped to monitor the complete autophagy flux.
Conclusion
To summarize, the study results support the previous findings of maternal BPA exposure inducing atherosclerotic lesion formation in the adult offspring. Maternal Rsv supplementation significantly prevents the BPA-induced atherosclerotic changes potentially by acting as an autophagy modulator and/or by causing epigenetic modifications. However, more investigations exploring the molecular mechanisms involved in the autophagy modulatory effects of Rsv against BPA-induced atherosclerosis are warranted.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Kawther Al Adawi from the department of Pathology, Sultan Qaboos University for her help in TEM. We also thank Taconic Biosciences, Inc. for providing ApoE−/− mice.
Author contributions
SRS, IAH, and MAM contributed to the study conception and design. SRS, IAH, and MAM conducted the animal experiments. SRS, IAH, NA and FA performed tissue processing, data collection and results analysis. SRS, IAH and MAM drafted the manuscript. NA and FA gave vital inputs to the manuscript. All authors commented on previous versions of the manuscript.
Funding
This study was funded by Internal Grant no. IG/MED/ANAT/18/01 from Sultan Qaboos University.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval
All the animal experimental procedures of the present study were conducted in accordance with the international laws and policies and were performed after obtaining the approval from the Sultan Qaboos University Animal Ethical Committee (SQU/AEC/2016–17/12).
Consent to participate
not applicable.
Consent for publication
All authors have read and approved the final draft of the manuscript for the publication.
References
- Aiken CE, Ozanne SE. Sex differences in developmental programming models. Reproduction. 2012;145:R1–R13. doi: 10.1530/REP-11-0489. [DOI] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Vieira E, Soriano S, et al. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ Health Perspect. 2010;118:1243–1250. doi: 10.1289/ehp.1001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angle BM, Do RP, Ponzi D, et al. Metabolic disruption in male mice due to fetal exposure to low but not high doses of bisphenol A (BPA): evidence for effects on body weight, food intake, adipocytes, leptin, adiponectin, insulin and glucose regulation. Reprod Toxicol. 2013;42:256–268. doi: 10.1016/j.reprotox.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argente-Arizon P, Ros P, Diaz F, et al. Age and sex dependent effects of early overnutrition on metabolic parameters and the role of neonatal androgens. Biol Sex Differ. 2016;7:26. doi: 10.1186/s13293-016-0079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird J, Jacob C, Barker M, et al. Developmental origins of health and disease: a lifecourse approach to the prevention of non-communicable diseases. Healthcare. 2017;5:14. doi: 10.3390/healthcare5010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista TM, Alonso-Magdalena P, Vieira E, et al. Short-term treatment with bisphenol-A leads to metabolic abnormalities in adult male mice. PLoS ONE. 2012;7:e33814. doi: 10.1371/journal.pone.0033814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boocock DJ, Faust GE, Patel KR, et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomark Prev. 2007;16:1246–1252. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- Bourque SL, Dolinsky VW, Dyck JR, Davidge ST. Maternal resveratrol treatment during pregnancy improves adverse fetal outcomes in a rat model of severe hypoxia. Placenta. 2012;33:449–452. doi: 10.1016/j.placenta.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Diaz-Gerevini GT, Repossi G, Dain A, Tarres MC, Das UN, Eynard AR. Beneficial action of resveratrol: how and why? Nutrition. 2016;32:174–178. doi: 10.1016/j.nut.2015.08.017. [DOI] [PubMed] [Google Scholar]
- Dodds EC, Lawson W. Synthetic estrogenic agents without the phenanthrene nucleus. Nature. 1936;137:996. [Google Scholar]
- Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estampador AC, Franks PW. Genetic and epigenetic catalysts in early-life programming of adult cardiometabolic disorders. Diabetes Metab Syndr Obes. 2014;7:575–586. doi: 10.2147/DMSO.S51433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan E, Zhang L, Jiang S, Bai Y. Beneficial effects of resveratrol on atherosclerosis. J Med Food. 2008;11:610–614. doi: 10.1089/jmf.2007.0091. [DOI] [PubMed] [Google Scholar]
- Fang C, Ning B, Waqar AB, et al. Bisphenol A exposure enhances atherosclerosis in WHHL rabbits. PLoS ONE. 2014;9:e110977. doi: 10.1371/journal.pone.0110977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang C, Ning B, Waqar AB, et al. Bisphenol A exposure induces metabolic disorders and enhances atherosclerosis in hyperlipidemic rabbits. J Appl Toxicol. 2015;35:1058–1070. doi: 10.1002/jat.3103. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Pinal C. The developmental origins of adult disease. Matern Child Nutr. 2005;3:130–141. doi: 10.1111/j.1740-8709.2005.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootaert MOJ, Roth L, Schrijvers DM, De Meyer GRY, Martinet W. Defective autophagy in atherosclerosis: to die or to senesce? Oxid Med Cell Longev. 2018;2018:7687083. doi: 10.1155/2018/7687083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Li C, Myatt L, Nathanielsz PW, Sun K. Sexually dimorphic effects of maternal nutrient reduction on expression of genes regulating cortisol metabolism in fetal baboon adipose and liver tissues. Diabetes. 2012;62:1175–1185. doi: 10.2337/db12-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Hong YC. Bisphenol A, hypertension, and cardiovascular diseases: epidemiological, laboratory, and clinical trial evidence. Curr Hypertens Rep. 2016;18:11. doi: 10.1007/s11906-015-0617-2. [DOI] [PubMed] [Google Scholar]
- Hassanpour M, Rahbarghazi R, Nouri M, Aghamohammadzadeh N, Safaei N, Ahmadi M. Role of autophagy in atherosclerosis: foe or friend? J Inflamm (lond) 2019;16:8. doi: 10.1186/s12950-019-0212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CN, Hou CY, Tain YL. Preventive aspects of early resveratrol supplementation in cardiovascular and kidney disease of developmental origins. Int J Mol Sci. 2021;22:4210. doi: 10.3390/ijms22084210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan ME, Vinardell MP, Planas JM. The daily oral administration of high doses of trans-resveratrol to rats for 28 days is not harmful. J Nutr. 2002;132:257–260. doi: 10.1093/jn/132.2.257. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Moon MK, Kang GH, et al. Chronic exposure to bisphenol A can accelerate atherosclerosis in high-fat-fed apolipoprotein E knockout mice. Cardiovas Toxicol. 2014;14:120–128. doi: 10.1007/s12012-013-9235-x. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Cuervo AM, Seglen PO. Methods for monitoring autophagy from yeast to human. Autophagy. 2007;3:181–206. doi: 10.4161/auto.3678. [DOI] [PubMed] [Google Scholar]
- Kurz T, Terman A, Brunk UT. Autophagy, ageing and apoptosis: the role of oxidative stress and lysosomal iron. Arch Biochem Biophys. 2007;462:220–230. doi: 10.1016/j.abb.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Lin R, Wu D, Wu FJ, et al. Non-alcoholic fatty liver disease induced by perinatal exposure to bisphenol a is associated with activated mTOR and TLR4/NF-κB signaling pathways in offspring rats. Front Endocrinol. 2019;10(10):620. doi: 10.3389/fendo.2019.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind PM, Lind L. Circulating levels of bisphenol A and phthalates are related to carotid atherosclerosis in the elderly. Atherosclerosis. 2011;218:207–213. doi: 10.1016/j.atherosclerosis.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Liu H, Cao Y, Tong T, et al. Autophagy in atherosclerosis: a phenomenon found in human carotid atherosclerotic plaques. Chin Med J (engl) 2015;128:69–74. doi: 10.4103/0366-6999.147815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TY, Yu HR, Tsai CC, et al. Resveratrol intake during pregnancy and lactation re-programs adiposity and ameliorates leptin resistance in male progeny induced by maternal high-fat/high sucrose plus postnatal high-fat/high sucrose diets via fat metabolism regulation. Lip Health Dis. 2020;19:174. doi: 10.1186/s12944-020-01349-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Xia W, Wang DQ, et al. Hepatic DNA methylation modifications in early development of rats resulting from perinatal BPA exposure contribute to insulin resistance in adulthood. Diabetologia. 2013;56:2059–2067. doi: 10.1007/s00125-013-2944-7. [DOI] [PubMed] [Google Scholar]
- Martel J, Ojcius DM, Chang CJ, et al. Anti-obesogenic and antidiabetic effects of plants and mushrooms. Nat Rev Endocrinol. 2017;13:149–160. doi: 10.1038/nrendo.2016.142. [DOI] [PubMed] [Google Scholar]
- Martinet W, De Meyer GR. Autophagy in atherosclerosis: a cell survival and death phenomenon with therapeutic potential. Circ Res. 2009;104:304–317. doi: 10.1161/CIRCRESAHA.108.188318. [DOI] [PubMed] [Google Scholar]
- Mela V, Llorente-Berzal A, Díaz F, Argente J, Viveros MP, Chowen JA. Maternal deprivation exacerbates the response to a high fat diet in a sexually dimorphic manner. PLoS ONE. 2012;7(11):e48915. doi: 10.1371/journal.pone.0048915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer D, Gates P, Osborne NJ, et al. Urinary bisphenol A concentration and angiography-defined coronary artery stenosis. PLoS ONE. 2012;7:e43378. doi: 10.1371/journal.pone.0043378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Yannan Z, Ren L, Qi S, Wei W, Lihong J. Adverse reproductive function induced by maternal BPA exposure is associated with abnormal autophagy and activating inflamation via mTOR and TLR4/NF-κB signaling pathways in female offspring rats. Reprod Toxicol. 2020;96:185–194. doi: 10.1016/j.reprotox.2020.07.001. [DOI] [PubMed] [Google Scholar]
- Mitchinson MJ. Insoluble lipids in human atherosclerotic plaques. Atherosclerosis. 1982;45:11–15. doi: 10.1016/0021-9150(82)90167-8. [DOI] [PubMed] [Google Scholar]
- Miyawaki J, Sakayama K, Kato H, Yamamoto H, Masuno H. Perinatal and postnatal exposure to bisphenol a increases adipose tissue mass and serum cholesterol level in mice. J Atheroscler Thromb. 2007;14:245–252. doi: 10.5551/jat.e486. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- Mohanta S, Yin C, Weber C, Hu D, Habenicht AJ. Aorta atherosclerosis lesion analysis in hyperlipidemic mice. Bio Protoc. 2016;6:e1833. doi: 10.21769/bioprotoc.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C, D’ Armiento FP, Mancini FP, Postiglione A, Witztum JL, Palumbo G, et al. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J Clin Invest. 1997;100:2680–2690. doi: 10.1172/JCI119813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C, Glass CK, Witztum JL, Deutsch R, D'Armiento FP, Palinski W. Influence of maternal hypercholesterolaemia during pregnancy on progression of early atherosclerotic lesions in childhood: fate of early lesions in children (FELIC) STUDY. Lancet. 1999;9(354):1234–1241. doi: 10.1016/S0140-6736(99)02131-5. [DOI] [PubMed] [Google Scholar]
- Oliveira ALB, Monteiro VVS, Navegantes-Lima KC, et al. Resveratrol role in autoimmune disease-a mini-review. Nutrients. 2017;9:1306. doi: 10.3390/nu9121306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paigen B, Plump AS, Rubin EM. The mouse as a model for human cardiovascular disease and hyperlipidemia. Curr Opin Lipidol. 1994;5:258–264. doi: 10.1097/00041433-199408000-00003. [DOI] [PubMed] [Google Scholar]
- Pattison JS, Robbins J, Martinez J, Tabas I. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab. 2012;15:545–553. doi: 10.1016/j.cmet.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penumathsa SV, Thirunavukkarasu M, Koneru S, et al. Statin and resveratrol in combination induces cardioprotection against myocardial infarction in hypercholesterolemic rat. J Mol Cell Cardiol. 2007;42:508–516. doi: 10.1016/j.yjmcc.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotta I. The use of electron microscopy for the detection of autophagy in human atherosclerosis. Micron. 2013;50:7–13. doi: 10.1016/j.micron.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Quan C, Wang C, Duan P, et al. Bisphenol a induces autophagy and apoptosis concurrently involving the Akt/mTOR pathway in testes of pubertal SD rats. Environ Toxicol. 2017;32:1977–1989. doi: 10.1002/tox.22339. [DOI] [PubMed] [Google Scholar]
- Rasdi Z, Kamaludin R, Ab Rahim S, et al. The impacts of intrauterine bisphenol A exposure on pregnancy and expression of miRNAs related to heart development and diseases in animal model. Sci Rep. 2020;10:5882. doi: 10.1038/s41598-020-62420-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaei Farimani A, Saidijam M, Goodarzi MT, et al. Effect of resveratrol supplementation on the SNARE proteins expression in adipose tissue of stroptozotocin-nicotinamide induced type 2 diabetic rats. Iran J Med Sci. 2015;40:248–55. [PMC free article] [PubMed] [Google Scholar]
- Rochester JR. Bisphenol A and human health: a review of the literature. Reprod Toxicol. 2013;42:132–155. doi: 10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Ros P, Diaz F, Freire-Regatillo A, et al. Resveratrol intake during pregnancy and lactation modulates the early metabolic effects of maternal nutrition differently in male and female offspring. Endocrinology. 2018;159:810–825. doi: 10.1210/en.2017-00610. [DOI] [PubMed] [Google Scholar]
- Sanches-Silva A, Testai L, Nabavi SF, et al. Therapeutic potential of polyphenols in cardiovascular diseases: regulation of mTOR signaling pathway. Pharmacol Res. 2020;152:104626. doi: 10.1016/j.phrs.2019.104626. [DOI] [PubMed] [Google Scholar]
- Shah A, Reyes LM, Morton JS, Fung D, Schneider J, Davidge ST. Effect of resveratrol on metabolic and cardiovascular function in male and female adult offspring exposed to prenatal hypoxia and a high-fat diet. J Physiol. 2016;594:1465–1482. doi: 10.1113/JP271133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar A, Teppala S, Sabanayagam C. Bisphenol A and peripheral arterial disease: results from the NHANES. Environ Health Perspect. 2012;120:1297–1300. doi: 10.1289/ehp.1104114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D, Chen Y, Wang B, et al. Bisphenol A inhibits autophagosome-lysosome fusion and lipid droplet degradation. Ecotoxicol Environ Saf. 2019;15(183):109492. doi: 10.1016/j.ecoenv.2019.109492. [DOI] [PubMed] [Google Scholar]
- Sui Y, Park SH, Helsley RN, et al. Bisphenol A increases atherosclerosis in pregnane X receptor-humanized ApoE deficient mice. J Am Heart Assoc. 2014;3:e000492. doi: 10.1161/JAHA.113.000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Y, Park SH, Wang F, Zhou C. Perinatal bisphenol A exposure increases atherosclerosis in adult male PXR-humanized mice. Endocrinology. 2018;159:1595–1608. doi: 10.1210/en.2017-03250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szkudelski T, Szkudelska K. Resveratrol and diabetes: from animal to human studies. Biochim Biophys Acta. 2015;1852:1145–1154. doi: 10.1016/j.bbadis.2014.10.013. [DOI] [PubMed] [Google Scholar]
- Takahashi O, Oishi S. Disposition of orally administered 2, 2-Bis (4-hydroxyphenyl) propane (Bisphenol A) in pregnant rats and the placental transfer to fetuses. Environ Health Perspect. 2000;108:931–935. doi: 10.1289/ehp.00108931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda-Watanabe A, Kitada M, Kanasaki K, Koya D. SIRT1 inactivation induces inflammation through the dysregulation of autophagy in human THP-1 cells. Biochem Biophys Res Commun. 2012;427:191–196. doi: 10.1016/j.bbrc.2012.09.042. [DOI] [PubMed] [Google Scholar]
- Truong VL, Jun M, Jeong WS. Role of resveratrol in regulation of cellular defense systems against oxidative stress. BioFactors. 2018;44:36–49. doi: 10.1002/biof.1399. [DOI] [PubMed] [Google Scholar]
- Wallack L, Thornburg K. Developmental origins, epigenetics, and equity: moving upstream. Matern Child Health J. 2016;20:935–940. doi: 10.1007/s10995-016-1970-8. [DOI] [PubMed] [Google Scholar]
- Wei J, Lin Y, Li Y, et al. Perinatal exposure to bisphenol A at reference dose predisposes offspring to metabolic syndrome in adult rats on a high-fat diet. Endocrinology. 2011;152:3049–3061. doi: 10.1210/en.2011-0045. [DOI] [PubMed] [Google Scholar]
- Williams LD, Burdock GA, Edwards JA, Beck M, Bausch J. Safety studies conducted on high-purity trans-resveratrol in experimental animals. Food Chem Toxicol. 2009;47:2170–2182. doi: 10.1016/j.fct.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cao X, Zhu W, et al. Resveratrol enhances autophagic flux and promotes Ox-LDL degradation in HUVECs via upregulation of SIRT1. Oxid Med Cell Longev. 2016;2016:7589813. doi: 10.1155/2016/7589813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Xiao X, Zhang Q, Wang T, Yu M, Xu J. Maternal low-protein diet modulates glucose metabolism and hepatic micrornas expression in the early life of offspring dagger. Nutrients. 2017;9:1–14. doi: 10.3390/nu9030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S, Feng Q, Cheng J, Zheng J. Maternal resveratrol consumption and its programming effects on metabolic health in offspring mechanisms and potential implications. Biosci Rep. 2018;38:BSR20171741. doi: 10.1042/BSR20171741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Wu C, Qiu S, Yuan X, Li L. Effects of resveratrol on glucose control and insulin sensitivity in subjects with type 2 diabetes: systematic review and meta-analysis. Nutr Metab (lond) 2017;14:60. doi: 10.1186/s12986-017-0217-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou T, Chen D, Yang Q, et al. Resveratrol supplementation of high-fat diet-fed pregnant mice promotes brown and beige adipocyte development and prevents obesity in male offspring. J Physiol. 2017;595:1547–1562. doi: 10.1113/JP273478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.
Not applicable.