Abstract
Background
A crucial component to improving patient care is better clinician understanding of patients’ health-related quality of life (HRQoL). In orthopaedic surgery, HRQoL assessment instruments such as the NIH developed Patient Reported Outcomes Measurement Information System (PROMIS), provide surgeons with a framework to assess how a treatment or medical condition is affecting each patient’s HRQoL. PROMIS has been demonstrated as a valuable instrument in many diseases; however, the extent to which orthopaedic surgery subspecialties have used and validated PROMIS measures in peer-reviewed research is unclear.
Methods
Systematic scoping methodology was used to investigate the characteristics of studies using PROMIS to assess HRQoL measures as orthopaedic surgical outcomes as well as studies validating computerized adaptive test (CAT) PROMIS physical health (PH) domains including: Physical Function (PF), Upper Extremity (UE), Lower Extremity (LE).
Results
A systematic search of PubMed identified 391 publications utilizing PROMIS in orthopaedics; 153 (39%) were PROMIS PH CAT validation publications. One-hundred publications were in Hand and Upper Extremity, 69 in Spine, 44 in Adult Reconstruction, 43 in Foot and Ankle, 43 in Sports, 37 in Trauma, 31 in General orthopaedics, and 24 in Tumor. From 2011 through 2020 there was an upward trend in orthopaedic PROMIS publications each year (range, 1–153) and an increase in studies investigating or utilizing PROMIS PH CAT domains (range, 1–105). Eighty-five percent (n = 130) of orthopaedic surgery PROMIS PH CAT validation publications (n = 153) analyzed PF; 30% (n = 46) analyzed UE; 3% (n = 4) analyzed LE.
Conclusions
PROMIS utilization within orthopaedics as a whole has significantly increased within the past decade, particularly within PROMIS CAT domains. The existing literature reviewed in this scoping study demonstrates that PROMIS PH CAT domains (PF, UE, and LE) are reliable, responsive, and interpretable in most contexts of patient care throughout all orthopaedic surgery subspecialties. The expanded use of PROMIS CATs in orthopaedic surgery highlights the potential for improved quality of patient care. While challenges of integrating PROMIS into electronic medical records exist, expanded use of PROMIS CAT measurement instruments throughout orthopaedic surgery should be performed.
Plain english summary In orthopaedic surgery, health-related quality of life tools such as the NIH developed Patient Reported Outcomes Measurement Information System (PROMIS), offer patients an opportunity to better understand their medical condition and be involved in their own care. Additionally, PROMIS provides surgeons with a framework to assess how a treatment or medical condition is affecting each patient’s functional status and quality of life. The efficacy of PROMIS has been demonstrated in many diseases; however, its application throughout orthopaedic care has yet to be depicted. This study sought to identify the extent to which all orthopaedic surgery subspecialties have used and validated PROMIS measures in peer-reviewed research in order to identify its potential as an applicable and valuable tool across specialties. We determined that PROMIS utilization has significantly increased within the past decade. The existing literature reviewed in this scoping study demonstrates that the PROMIS computerized adaptive test domains evaluating physical function status are reliable, responsive, and interpretable in most contexts of patient care throughout all orthopaedic surgery subspecialties. Based on these results, this study recommends the expanded and more uniform use of PROMIS computerized adaptive test measurement instruments in the clinical care of orthopaedic patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s41687-021-00407-w.
Keywords: PROMIS, Orthopaedic patient-reported outcomes, Orthopaedics, Orthopaedic surgery, PROMIS validation, PROMIS use
Introduction
Advances in health information technology have the potential to elevate the quality of patient care, especially by providing clinicians with efficient measures of patient reported outcomes (PROs) that provide insights into health-related quality of life (HRQoL) during treatment. In the field of orthopaedic surgery, HRQoL assessment instruments help elucidate patients’ well-being and functional capabilities beyond visible outcomes [1, 2]. Numerous validated HRQoL assessment instruments exist in orthopaedics, commonly described as legacy measures. These include American Shoulder and Elbow Surgeons Score (ASES), Disabilities of the Arm, Shoulder and Hand (DASH), Foot and Ankle Ability Measure (FAAM), Knee Injury and Osteoarthritis Outcome Score (KOOS), and others [1, 3]. However, most of these instruments are narrow in scope, limited to specific outcomes or mobility constructs [1, 3]. The National Institute of Health (NIH) Patient Reported Outcomes Measurement Information System (PROMIS) was developed to deliver standardized, precise, quantitative values for individual domains of health and well-being [4], and has great potential to improve understandings of PROs in orthopaedic cases. By design, PROMIS outcome instruments report outcomes utilizing standardized T-scores. The computerized adaptive test (CAT) feature, available for many PROMIS instruments, is the most efficient method of collecting useful PROs in a multitude of musculoskeletal conditions by utilizing item response theory [1, 5, 6]. Patients respond to questions and the system is programmed to select subsequent questions based on answers of previous questions, which minimizes the burden on the patient while providing maximally useful information for clinicians [6]. The most important outcome domain in orthopaedic surgery is physical health (PH) [7]. In PROMIS, PH includes Physical Function (PF) and subdomains, such as Pediatric Mobility, Upper Extremity (UE), and Lower Extremity (LE) [8]. PROMIS PF CAT selects from a 124-item bank [6], and requires 12 or fewer questions to identify the most informative PF value [5]. While PROMIS PF CAT was not designed for any particular disease, the range of PRO values available allow it to be tailored for use in specific medical conditions.
Psychometric validation of HRQoL assessment instruments generally requires evaluation of reliability, responsiveness, and validity [9, 10]. However, the use of different terminology for the same measurement properties can complicate the consensus for validity of an assessment instrument [11]. While Sullivan established guidelines for assessing the validity of PROs assessment instruments [12], and the Consensus-based Standards for the selection of health status Measurement Instruments (COSMIN) study developed international agreement on taxonomy, terminology, and definitions of measurement properties [11], the subsequent application of these terms remains to be evaluated.
A scoping study allows us to establish uniform application of terms and definitions for assessing validity of measurement properties as applied in orthopaedic research. This approach is defined by Daudt et al., as an attempt to “map the literature on a particular topic or research area and provide an opportunity to identify key concepts; gaps in the research; and types and sources of evidence to inform practice, policymaking, and research” [13]. A key feature of a scoping study is that the research aims to provide an overview of all existing literature concerning a broad topic [13, 14], whereas the purpose of a systematic review is to provide a summary of the leading existing research on a specific question [15].
HRQoL assessment instruments provide patients an opportunity to better understand their medical condition and be involved in their own care—key steps in reaching an appropriate and successful treatment plan. Given growing recognition of the importance of patients’ involvement in their own care, PROMIS is a measurement system which contains unique measures for improving patient care throughout orthopaedics. The efficacy of PROMIS has been demonstrated in many diseases including rheumatoid arthritis, chronic heart failure, and cancer [16]. However, its application throughout orthopaedic care has yet to be depicted. This scoping study sought to elucidate the extent to which orthopaedic surgery subspecialties have used and validated PROMIS measures in peer-reviewed research in order to identify its potential as an applicable and valuable tool across subspecialties in orthopaedics.
Methods
Approach
This study followed the methodology developed by Arksey and O’Malley [14], and further enhanced by Daudt et al. [13]. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) checklist was followed for reporting the results of the study [17].
Data source
We identified all peer-reviewed publications in the National Library of Medicine (NLM) PubMed database that utilized PROMIS measures within adult orthopaedic surgery using specific search criteria: (PROMIS) AND (orthopaedics OR orthopaedic OR orthopedics OR orthopedic). The NLM PubMed database search was conducted on January 1, 2021. This search identified both assessments of surgical patient outcomes with PROMIS and analyses of the quality of PROMIS as a measurement system within orthopaedic surgery. Pediatric publications, literature reviews, and publications that were unrelated to the care of orthopaedic patients or did not utilize PROMIS in the study were excluded from analysis as identified by individual review of publications. The full text of each publication was independently reviewed by one of two reviewers.
Outcomes and variables collected
We then evaluated and charted each publication for study design, level of evidence, number of patients, and PROMIS domains and instrument format tested. Publications were separated by orthopaedic surgery subspecialties, including Foot and Ankle (FA), Hand and Upper Extremity (HUE), Tumor, Trauma, Adult Reconstruction (AR), Sports, Spine, and General Orthopaedics. We excluded review and editorial publications as well as pediatric orthopaedic surgery publications. Level of evidence was determined following the updated assignments provided by the Journal of Bone and Joint Surgery [18]. PROMIS domains included Global Health (Physical and Mental), PF, UE, LE, Pain Interference, Pain Intensity, Pain Behavior, Depression, Anxiety, Social Satisfaction, and Fatigue. Instrument format of PROMIS domains included CAT or short form. Study design included prospective, retrospective, cross-sectional, randomized controlled trial (RCT), and case report or series.
Assessment of PROMIS validation studies
After initial review, we further assessed each publication to determine whether the performance of PROMIS PH CAT measurement instruments (PF, UE, and LE) use in orthopaedic surgery care was analyzed following the validation guidelines developed by Sullivan [12], with respect to reliability, responsiveness, and validity following terminology defined by COSMIN [11]. In this study, a PROMIS validation study refers to a publication that statistically analyzed a PROMIS domains’ reliability [19], responsiveness [20], or validity [21], using the following statistical tests described [22, 23]. The statistical analysis of a PROMIS domain’s validity relates to the evaluation of content validity, construct validity, or criterion validity [11]. Each validation study was assessed for any recommendations on whether the PROMIS PH CAT domains utilized (PF, UE, and LE) were accurate and useful in orthopaedic patients.
Reliability including internal consistency and inter- and intra-rater reliability, was presented by Cronbach’s alpha, kappa statistics, percentage agreement, or a correlation coefficient [11, 24].
Internal and external responsiveness was assessed using a range of statistical tests including effect size, standardized response mean, relative efficiency statistic, the response statistic, and correlation (using Spearman’s rho) [11, 20, 25]. While evaluating the minimal clinically important difference and floor and ceiling effects of assessment instruments risks spurious change and does not maintain the same statistical integrity as the prior evaluation tests, studies that calculated these values were included as psychometric tests of responsiveness, as these calculations are necessary to measure responsiveness of a given instrument [26].
Modern validity theory from the psychometric perspective requires specific contexts to be evaluated in order to assess the validity of a PROs assessment instruments [27, 28]. Therefore, the types of validity evaluated by this study looked to denote how interpretable PROMIS PH CAT scores are in various contexts of orthopaedic clinical care and research [29]. We included the three types of validity defined by COSMIN when evaluating the performance of PROMIS PH CAT: content validity, construct validity, and criterion validity [11]. Assessment of content validity uses judgements from experts in the field to give a scale of relevance for the construct or the dimensions of the construct evaluated and an average relevance is calculated [30]. Additionally, confirmatory factor analysis, a special form of structural equating modeling, analyzes specific structures and components of the construct through correlations between latent variables: mathematically inferred variables from observed variables [31]. Use of structural equating modeling to confirm specified relationships between PROMIS domains and events of interest in a disease or treatment qualified as measurement of content validity in the publications found [31, 32].
Assuming content validity, construct validity evaluates the consistency of the assessment instrument with different hypotheses [11]. For instance, evaluating construct validity can refer to the ability of PROMIS to discriminate between relevant groups or confirm relationships to known risk factors [11, 21]. Several methods for testing construct validity have been described including correlation calculations such as Pearson’s rho, multivariate analysis, confirmatory factor analysis, and covariance component analysis [33, 34]. Finally, criterion validity refers to the degree to which the assessment instrument correlates to previously validated or gold standard instruments [11], as tested by correlation coefficients [35].
Results
Selection of sources of evidence
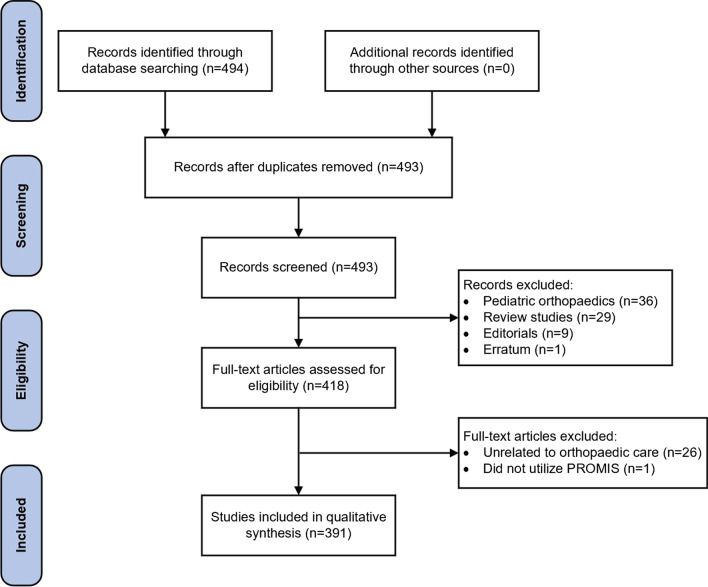
The NLM PubMed database identified 493 non-duplicated publications. Individual review of the search results identified 102 publications that were not related to the primary objective of the search criteria and therefore excluded from further analysis: 36 pediatric orthopaedics publications, 29 review studies, 9 editorials, 1 published erratum, and 27 publications that were unrelated to the care of orthopaedic patients or did not utilize PROMIS in the study. The Preferred Reporting Items for Systematic Reviews of Meta-Analyses (PRISMA) diagram in Fig. 1 illustrates the sequence of review results collected in this study [36].
Fig. 1.
The preferred reporting items for systematic reviews of meta-analyses flow diagram for Patient-Reported Outcomes Information System (PROMIS) publications in adult orthopaedic surgery collected
All orthopaedic surgery PROMIS publications
Of the total 391 publications assessed (Additional File 1), 153 (39%) were PROMIS PH CAT validation publications. From 2011 through 2020 there were increasingly more orthopaedic PROMIS studies published each year in all specialties except for Trauma (Table 1) and an increase in the number of studies investigating or utilizing PROMIS PH CAT domains in all specialties (Fig. 2). PROMIS publications most often reported HUE outcomes (26%, n = 100), followed by Spine (18%, n = 69) (Table 2). More Level I (8%, n = 3) and RCT (11%, n = 4) studies were published in the Trauma subspecialty relative to other subspecialties. UE, Pain Interference, and Depression domains were utilized the most frequently in HUE; PF and Pain Intensity domains were utilized the most in Spine. Six percent (n = 22) of publications were Level I; 33% (n = 129) were Level II; 50% (n = 196) were Level III; 11% (n = 43) were Level IV.
Table 1.
Characteristics of all PROMIS publications by publication year
| Variable | Publication year | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | |
| n | 1 | 2 | 1 | 9 | 12 | 19 | 25 | 52 | 117 | 153 |
| Number of patients, median (IQR) | 865 (865–865) | 334.5 (311–358) | 288 (288–288) | 140 (126–311) | 129 (49–151) | 93 (31–118) | 109 (74–300) | 161 (91–1027) | 172 (94–489) | 166 (86–351) |
| Subspecialty, n (%) | ||||||||||
| Adult reconstruction | – | – | – | – | – | – | – | 9 (17) | 12 (10) | 23 (15) |
| Foot and ankle | – | 2 (100) | 1 (100) | 3 (33) | – | 3 (16) | 1 (4) | 2 (4) | 9 (8) | 22 (14) |
| General orthopaedics | 1 (100) | – | – | – | – | – | 1 (4) | 5 (10) | 13 (11) | 11 (7) |
| Hand and upper extremity | – | – | – | 3 (33) | 7 (58) | 7 (37) | 8 (32) | 12 (23) | 28 (24) | 35 (23) |
| Spine | – | – | – | 1 (11) | 1 (8) | 1 (5) | 4 (16) | 8 (15) | 24 (21) | 30 (20) |
| Sports | – | – | – | 1 (11) | – | 2 (11) | 4 (16) | 7 (14) | 12 (10) | 17 (11) |
| Trauma | – | – | – | 1 (11) | 12 (17) | 3 (16) | 2 (8) | 7 (14) | 11 (9) | 11 (7) |
| Tumor | – | – | – | – | 12 (17) | 3 (16) | 5 (20) | 2 (4) | 8 (7) | 4 (3) |
| Publication type, n (%) | ||||||||||
| PROMIS validation | 1 (100) | 2 (100) | 1 (100) | 8 (89) | 3 (25) | 8 (42) | 16 (64) | 26 (50) | 59 (50) | 53 (35) |
| Patient outcomes | – | – | – | 1 (11) | 9 (75) | 11 (58) | 9 (36) | 26 (50) | 58 (50) | 100 (65) |
| Study design, n (%) | ||||||||||
| Case report or series | – | – | – | 1 (11) | 1 (8) | 2 (11) | – | 2 (4) | 5 (4) | 13 (9) |
| Cross sectional | – | – | – | – | 3 (25) | 3 (16) | 3 (12) | 11 (21) | 11 (9) | 8 (5) |
| Prospective cohort | 1 (100) | 1 (50) | 1 (100) | 6 (67) | 3 (25) | 8 (42) | 12 (48) | 18 (35) | 36 (31) | 33 (22) |
| Retrospective cohort | – | – | – | – | 4 (33) | 6 (32) | 9 (36) | 17 (33) | 62 (53) | 92 (60) |
| Randomized controlled trial | – | 1 (50) | – | 2 (22) | 1 (8) | – | 1 (4) | 4 (8) | 3 (3) | 7 (5) |
| Level of evidence, n (%) | ||||||||||
| I | – | – | – | 3 (33) | 1 (8) | 1 (5) | 1 (4) | 6 (12) | 4 (3) | 6 (4) |
| II | 1 (100) | 1 (50) | – | 3 (33) | 2 (17) | 8 (42) | 12 (50) | 18 (35) | 43 (37) | 40 (26) |
| III | – | 1 (50) | 1 (100) | 2 (22) | 5 (42) | 6 (32) | 10 (42) | 25 (48) | 56 (48) | 91 (59) |
| IV | – | – | – | 1 (11) | 4 (33) | 4 (21) | 1 (4) | 3 (6) | 14 (12) | 16 (11) |
| PROMIS format, n (%) | ||||||||||
| CAT | 1 (100) | 2 (100) | 1 (100) | 9 (100) | 9 (75) | 14 (74) | 20 (80) | 42 (81) | 92 (79) | 111 (73) |
| Short form | – | – | – | – | 3 (25) | 2 (11) | 6 (24) | 12 (23) | 26 (22) | 47 (31) |
| Format unknown | – | – | – | – | – | 3 (16) | – | – | 2 (2) | 1 (1) |
| PROMIS domains, n (% of specified publication year) | ||||||||||
| Global physical and mental health | – | – | – | – | – | 2 (11) | 2 (8) | 7 (14) | 10 (9) | 30 (20) |
| Physical function | 1 (100) | – | 1 (100) | 6 (67) | 7 (58) | 11 (58) | 16 (64) | 37 (71) | 83 (71) | 102 (67) |
| Upper extremity | – | – | – | 1 (11) | 1 (8) | 3 (16) | 11 (44) | 12 (23) | 33 (28) | 28 (18) |
| Lower extremity | – | 2 (100) | – | 2 (22) | – | – | 1 (4) | – | – | – |
| Pain interference | – | – | 1 (100) | 1 (11) | 7 (58) | 12 (63) | 14 (56) | 29 (56) | 65 (56) | 82 (54) |
| Pain intensity | – | – | – | – | 1 (8) | 3 (16) | 4 (16) | 4 (8) | 17 (15) | 18 (12) |
| Pain behavior | – | – | – | 1 (11) | 2 (17) | 1 (5) | 1 (4) | 1 (2) | 5 (4) | 2 (1) |
| Depression | – | – | – | – | 6 (50) | 6 (32) | 7 (28) | 16 (31) | 33 (28) | 51 (33) |
| Anxiety | – | – | – | – | 3 (25) | 1 (5) | 7 (28) | 6 (12) | 13 (11) | 18 (12) |
| Social satisfaction | – | – | – | – | 1 (8) | – | 3 (12) | 3 (6) | 6 (5) | 14 (9) |
| Fatigue | – | – | – | – | 1 (8) | – | 3 (12) | 3 (6) | 6 (5) | 11 (7) |
IQR interquartile range, CAT computerized adaptive test
Fig. 2.
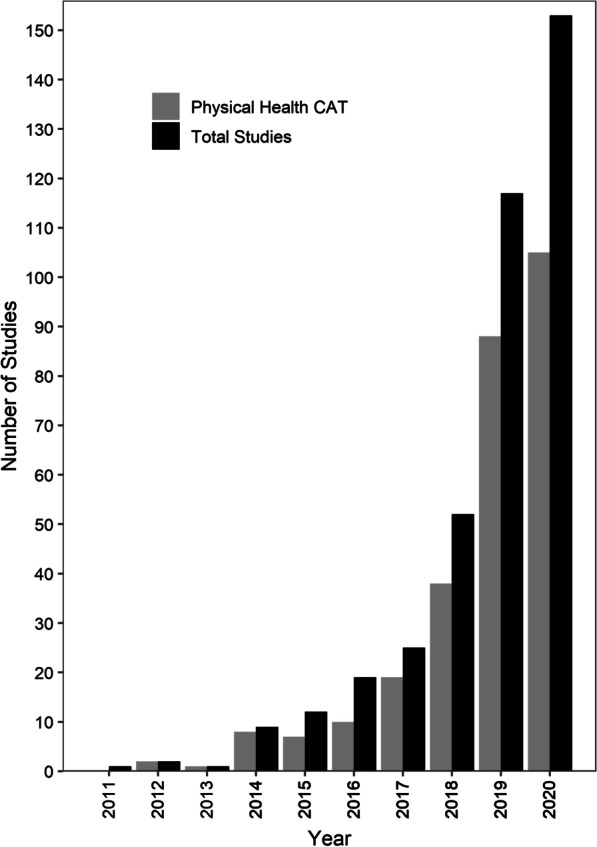
Number of all adult orthopaedic surgery Patient-Reported Outcomes Information System (PROMIS) studies and PROMIS physical health computerized adaptive test (CAT) studies (Physical Function, Upper Extremity, and Lower Extremity) published each year from 2011 through December 31, 2020
Table 2.
Characteristics of all PROMIS publications by orthopaedic subspecialty
| Variable | Orthopaedic subspecialty | |||||||
|---|---|---|---|---|---|---|---|---|
| Adult reconstruction | Foot and ankle | General orthopaedics | Hand and upper extremity | Spine | Sports | Trauma | Tumor | |
| n | 44 | 43 | 31 | 100 | 69 | 43 | 37 | 24 |
| Number of patients, median (IQR) | 186 (96–421) | 148 (73–294) | 435 (256–2566) | 140.5 (88–351) | 167 (98–421) | 145 (58–272) | 134 (63–198) | 91 (33–138) |
| Publication type, n (%) | ||||||||
| PROMIS validation | 18 (41) | 17 (40) | 12 (39) | 47 (47) | 40 (58) | 25 (58) | 11 (30) | 7 (29) |
| Patient outcomes | 26 (59) | 26 (60) | 19 (61) | 53 (53) | 29 (42) | 18 (42) | 26 (70) | 17 (71) |
| Study design, n (%) | ||||||||
| Case report or series | 4 (9) | 3 (7) | – | 8 (8) | 1 (1) | 2 (5) | 4 (11) | 2 (8) |
| Cross sectional | 2 (5) | 1 (2) | 8 (26) | 15 (15) | 2 (3) | 5 (12) | 2 (5) | 4 (17) |
| Prospective cohort | 9 (20) | 15 (35) | 11 (36) | 37 (37) | 15 (22) | 16 (37) | 11 (30) | 5 (21) |
| Retrospective cohort | 27 (61) | 24 (56) | 10 (32) | 36 (36) | 50 (73) | 17 (40) | 16 (43) | 13 (54) |
| Randomized controlled trial | 2 (5) | – | 2 (6) | 4 (4) | 1 (1) | 3 (7) | 4 (11) | – |
| Level of evidence, n (%) | ||||||||
| I | 3 (7) | 3 (7) | 2 (6) | 6 (6) | 2 (3) | 3 (7) | 3 (8) | – |
| II | 8 (18) | 12 (28) | 13 (42) | 41 (41) | 23 (33) | 15 (35) | 12 (32) | 5 (22) |
| III | 26 (59) | 23 (53) | 16 (52) | 43 (43) | 40 (58) | 21 (49) | 15 (41) | 12 (52) |
| IV | 7 (16) | 5 (12) | – | 10 (10) | 4 (6) | 4 (9) | 7 (19) | 6 (26) |
| PROMIS format, n (%) | ||||||||
| CAT | 20 (46) | 37 (86) | 25 (81) | 92 (92) | 55 (80) | 35 (81) | 27 (73) | 9 (38) |
| Short form | 25 (57) | 9 (21) | 6 (19) | 9 (9) | 14 (20) | 8 (19) | 10 (27) | 15 (63) |
| Format unknown | – | 2 (5) | 1 (3) | 1 (1) | – | – | 1 (3) | 1 (4) |
| PROMIS domains, n (% of specified subspecialty) | ||||||||
| Global physical and mental health | 20 (45) | 6 (14) | 3 (10) | 5 (5) | 8 (12) | 4 (9) | 2 (5) | 3 (13) |
| Physical function | 23 (52) | 38 (88) | 21 (68) | 45 (43) | 61 (88) | 33 (77) | 27 (73) | 16 (67) |
| Upper extremity | 1 (2) | – | 3 (10) | 61 (61) | 1 (1) | 10 (23) | 9 (24) | 4 (17) |
| Lower extremity | – | 3 (7) | – | – | – | 1 (2) | – | 1 (4) |
| Pain interference | 12 (27) | 35 (81) | 20 (65) | 56 (56) | 37 (54) | 21 (49) | 15 (41) | 15 (63) |
| Pain intensity | 2 (5) | 8 (19) | 3 (10) | 5 (5) | 15 (22) | 3 (7) | 2 (5) | 9 (38) |
| Pain behavior | 1 (2) | 2 (5) | 1 (3) | 2 (2) | 2 (3) | 2 (5) | 1 (3) | 2 (8) |
| Depression | 7 (16) | 19 (44) | 17 (55) | 35 (35) | 13 (19) | 15 (35) | 2 (5) | 11 (46) |
| Anxiety | 3 (7) | 2 (5) | 13 (42) | 9 (9) | 8 (12) | 4 (9) | – | 9 (38) |
| Social satisfaction | 1 (2) | 1 (2) | 8 (26) | 2 (2) | 7 (10) | 3 (7) | 1 (3) | 4 (17) |
| Fatigue | 2 (5) | 1 (2) | 7 (23) | 2 (2) | 5 (7) | 3 (7) | – | 4 (17) |
IQR interquartile range, CAT computerized adaptive test
PF (I: 50%, n = 11; II: 73%, n = 94; III: 68%, n = 133; IV: 58%, n = 25) and then Pain Interference (I: 36%, n = 8; II: 63%, n = 81; III: 51%, n = 99; IV: 53%, n = 23) were utilized the most within each level of evidence degree, and within each orthopaedic subspecialty with the exception of General Orthopaedics (Table 3).
Table 3.
Characteristics of all PROMIS publications by level of evidence
| Variable | Level of evidence | |||
|---|---|---|---|---|
| I | II | III | IV | |
| n | 22 | 129 | 196 | 43 |
| Number of patients, median (IQR) | 130 (56–207) | 183 (100–472) | 172 (100–373) | 27 (16–66) |
| Year, n (%) | ||||
| 2011 | – | 1 (1) | – | – |
| 2012 | – | 1 (1) | 1 (1) | – |
| 2013 | – | 1 (1) | – | – |
| 2014 | 3 (16) | 3 (3) | 2 (1) | 1 (3) |
| 2015 | 1 (5) | 2 (2) | 5 (3) | 4 (10) |
| 2016 | 1 (5) | 8 (7) | 6 (4) | 4 (10) |
| 2017 | 1 (5) | 12 (10) | 10 (6) | 1 (3) |
| 2018 | 6 (32) | 18 (15) | 25 (15) | 3 (8) |
| 2019 | 4 (21) | 43 (36) | 56 (32) | 14 (35) |
| 2020 | 6 (27) | 40 (31) | 91 (46) | 16 (37) |
| Subspecialty, n (%) | ||||
| Adult reconstruction | 3 (14) | 8 (6) | 26 (13) | 7 (16) |
| Foot and ankle | 3 (14) | 12 (9) | 23 (12) | 5 (12) |
| General orthopaedics | 2 (9) | 13 (10) | 16 (8) | – |
| Hand and upper extremity | 6 (27) | 41 (32) | 43 (22) | 10 (23) |
| Spine | 2 (9) | 23 (18) | 40 (20) | 4 (9) |
| Sports | 3 (14) | 15 (12) | 21 (11) | 4 (9) |
| Trauma | 3 (14) | 12 (9) | 15 (8) | 7 (16) |
| Tumor | – | 5 (4) | 12 (6) | 6 (14) |
| Publication type, n (%) | ||||
| PROMIS validation | 7 (32) | 89 (69) | 76 (39) | 4 (9) |
| Patient outcomes | 15 (68) | 40 (31) | 120 (61) | 39 (91) |
| Study design, n (%) | ||||
| Case report or series | – | – | – | 24 (56) |
| Cross sectional | – | 4 (3) | 33 (17) | 2 (5) |
| Prospective cohort | 7 (32) | 105 (81) | 5 (3) | 1 (2) |
| Retrospective cohort | 1 (4) | 19 (15) | 157 (80) | 16 (37) |
| Randomized controlled trial | 14 (64) | 1 (1) | 1 (1) | – |
| PROMIS format, n (%) | ||||
| CAT | 15 (68) | 112 (87) | 148 (76) | 25 (58) |
| Short form | 8 (36) | 19 (15) | 53 (27) | 15 (35) |
| Format unknown | – | 1 (1) | 2 (1) | 3 (7) |
| PROMIS domains, n (% of specified level of evidence) | ||||
| Global physical and mental health | 3 (14) | 14 (11) | 30 (15) | 4 (9) |
| Physical function | 11 (50) | 94 (73) | 133 (68) | 25 (58) |
| Upper extremity | 5 (23) | 35 (27) | 43 (22) | 5 (12) |
| Lower extremity | 1 (5) | 2 (2) | 2 (1) | – |
| Pain interference | 8 (36) | 81 (63) | 99 (51) | 23 (53) |
| Pain intensity | 3 (14) | 16 (12) | 23 (12) | 5 (12) |
| Pain behavior | 1 (5) | 5 (4) | 3 (2) | 4 (9) |
| Depression | 3 (14) | 39 (30) | 65 (33) | 12 (28) |
| Anxiety | 1 (5) | 18 (14) | 23 (12) | 6 (14) |
| Social satisfaction | 3 (14) | 13 (10) | 9 (5) | 2 (5) |
| Fatigue | 1 (5) | 11 (9) | 8 (4) | 4 (9) |
IQR interquartile range, CAT computerized adaptive test
Orthopaedic surgery physical health PROMIS validation publications
Ninety-five percent (n = 146) of all orthopaedic surgery PROMIS PH CAT validation publications determined that the instruments were responsive, reliable, and valid. Two studies in AR (18%), two in HUE (5%), two in Sports (8%), and one in Trauma (10%) did not find PROMIS PH CAT instruments to be valid instrument within their respective field. Specifically, these studies found problems with PROMIS PH CAT criterion validity and responsiveness.
Eighty-five percent (n = 130) of all orthopaedic surgery PROMIS PH CAT validation publications analyzed PF, 30% (n = 46) analyzed UE, and 3% (n = 4) analyzed LE. More PROMIS PH CAT validation publications were performed in 2019 (35%, n = 53) than any other year (Table 4). PROMIS PH CAT validation publications most often reported HUE outcomes (26%, n = 40), followed by Spine (23%, n = 35) and Sports (16%, n = 24) (Table 5).
Table 4.
Characteristics of publications validating physical health CAT PROMIS domains by publication year (includes physical function, upper extremity, lower extremity)
| Variable | Publication year | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | |
| n | 2 | 1 | 8 | 1 | 7 | 15 | 23 | 53 | 43 |
| Number of patients, median (IQR) | 334.5 (311–358) | 288 (288–288) | 147 (132–343) | 187 (187–187) | 108 (82–204) | 107 (84–726) | 231 (94–1969) | 173 (100–748) | 166 (115–259) |
| Subspecialty, n (%) | |||||||||
| Adult reconstruction | – | – | – | – | – | – | 4 (17) | 1 (2) | 6 (14) |
| Foot and ankle | 2 (100) | 1 (100) | 3 (38) | – | – | 1 (7) | 1 (4) | 5 (9) | 4 (9) |
| General orthopaedics | – | – | – | – | – | – | 4 (17) | 5 (9) | 1 (2) |
| Hand and upper extremity | – | – | 2 (25) | 1 (100) | 2 (29) | 6 (40) | 4 (17) | 13 (25) | 12 (28) |
| Spine | – | – | 1 (13) | – | 1 (14) | 2 (13) | 5 (22) | 15 (28) | 11 (26) |
| Sports | – | – | 1 (13) | – | 1 (14) | 3 (20) | 3 (13) | 8 (15) | 8 (19) |
| Trauma | – | – | 1 (13) | – | 2 (29) | 1 (7) | 2 (9) | 4 (8) | – |
| Tumor | – | – | – | – | 1 (14) | 2 (13) | – | 2 (4) | 1 (2) |
| Study design, n (%) | |||||||||
| Case report or series | – | – | – | – | – | – | 1 (4) | – | 1 (2) |
| Cross sectional | – | – | – | – | 2 (29) | 2 (13) | 5 (22) | 6 (11) | 2 (5) |
| Prospective cohort | 1 (50) | 1 (100) | 6 (75) | – | 4 (57) | 10 (67) | 12 (52) | 29 (55) | 10 (23) |
| Retrospective cohort | 1 (50) | – | 2 (25) | 1 (100) | 1 (14) | 3 (20) | 5 (22) | 18 (34) | 30 (70) |
| Level of evidence, n (%) | |||||||||
| I | – | – | 3 (38) | – | – | – | 2 (9) | 2 (4) | – |
| II | 1 (50) | 1 (100) | 3 (38) | – | 4 (57) | 10 (67) | 12 (52) | 32 (60) | 14 (33) |
| III | 1 (50) | – | 2 (25) | 1 (100) | 3 (43) | 5 (33) | 8 (35) | 18 (34) | 27 (63) |
| IV | – | – | – | – | – | – | 1 (4) | 1 (2) | 2 (5) |
| PROMIS domains, n (% of specified publication year) | |||||||||
| Physical function | – | 1 (100) | 6 (75) | 1 (100) | 6 (86) | 13 (87) | 21 (91) | 50 (94) | 32 (74) |
| Upper extremity | – | – | 1 (13) | – | 2 (29) | 8 (53) | 5 (22) | 15 (28) | 15 (35) |
| Lower extremity | 2 (100) | – | 2 (25) | – | – | – | – | – | – |
| Validation method tested, n (%) | |||||||||
| Reliability | 2 (100) | 1 (100) | 7 (88) | 1 (100) | 3 (43) | 3 (20) | 1 (4) | 8 (15) | 3 (7) |
| Responsiveness | 2 (100) | 1 (100) | 8 (100) | 1 (100) | 2 (29) | 12 (80) | 13 (57) | 41 (77) | 30 (70) |
| Validity | |||||||||
| 1 validity criteria | 1 (50) | – | 2 (25) | – | 6 (86) | 7 (47) | 15 (65) | 28 (53) | 32 (74) |
| > 1 validity criterion | 1 (50) | 1 (100) | 6 (75) | 1 (100) | 1 (14) | 3 (20) | 2 (9) | 9 (17) | 3 (7) |
IQR interquartile range, CAT computerized adaptive test
Table 5.
Characteristics of publications validating physical health CAT PROMIS domains by orthopaedic subspecialty (includes physical function, upper extremity, lower extremity)
| Variable | Orthopaedic subspecialty | |||||||
|---|---|---|---|---|---|---|---|---|
| Adult reconstruction | Foot and ankle | General orthopaedics | Hand and upper extremity | Spine | Sports | Trauma | Tumor | |
| n | 11 | 17 | 10 | 40 | 35 | 24 | 10 | 6 |
| Number of patients, median (IQR) | 172 (105–762) | 288 (126–441) | 2566 (566–12,353) | 169.5 (100–782) | 158 (120–360) | 138.5 (76–255) | 163.5 (123–208) | 100 (98–115) |
| Study design, n (%) | ||||||||
| Case report or series | 1 (9) | – | – | – | – | 1 (4) | – | – |
| Cross sectional | 1 (9) | – | 3 (30) | 5 (13) | 2 (6) | 3 (13) | 2 (20) | 1 (17) |
| Prospective cohort | 4 (36) | 10 (59) | 5 (50) | 21 (53) | 12 (34) | 13 (54) | 5 (50) | 3 (50) |
| Retrospective cohort | 5 (46) | 7 (41) | 2 (20) | 14 (35) | 21 (60) | 7 (29) | 3 (30) | 2 (33) |
| Level of evidence, n (%) | ||||||||
| I | 1 (9) | 3 (18) | – | 2 (5) | 1 (3) | – | – | – |
| II | 3 (27) | 7 (41) | 6 (60) | 22 (55) | 17 (49) | 13 (54) | 6 (60) | 3 (50) |
| III | 6 (55) | 7 (41) | 4 (40) | 16 (40) | 16 (46) | 9 (38) | 4 (40) | 3 (50) |
| IV | 1 (9) | – | – | – | 1 (3) | 2 (8) | – | – |
| PROMIS domains, n (% of specified subspecialty) | ||||||||
| Physical function | 11 (100.0) | 15 (88) | 9 (90) | 22 (55) | 35 (100) | 22 (92) | 10 (100) | 6 (100) |
| Upper extremity | 1 (9) | – | 2 (20) | 32 (80) | 1 (3) | 6 (25) | 3 (30) | 1 (17) |
| Lower extremity | – | 3 (18) | – | – | – | 1 (42) | – | – |
| Validation method tested, n (%) | ||||||||
| Reliability | – | 6 (35) | 1 (10) | 12 (30) | 3 (9) | 1 (4) | 2 (20) | 4 (67) |
| Responsiveness | 7 (64) | 15 (88) | 2 (20) | 33 (83) | 21 (60) | 19 (79) | 7 (70) | 6 (100) |
| Validity | ||||||||
| 1 validity criteria | 8 (73) | 7 (41) | 8 (80) | 21 (53) | 20 (57) | 16 (67) | 8 (80) | 3 (50) |
| > 1 validity criterion | – | 4 (24) | – | 9 (23) | 9 (26) | 2 (8) | 1 (10) | 2 (33) |
IQR interquartile range, CAT computerized adaptive test
Reliability was the least-often analyzed component of PROMIS PH CAT performance throughout each subspecialty (range, 4–67%), as compared to responsiveness or validity. Reliability was analyzed most frequently in HUE validation studies (n = 12), followed by FA validation studies (n = 6). Responsiveness was analyzed most frequently in HUE validation studies (n = 33), followed by Spine (n = 21) and Sports (n = 19) studies. At least one form of validity (criterion, content, or construct) was analyzed in over 65% of all subspecialties and analyzed in over 80% of General Orthopaedics, Spine, Trauma, and Tumor validation studies. More than one form of validity was analyzed in over 20% of FA, HUE, Spine, and Tumor validation studies. Five percent (n = 7) of validation publications were Level I studies; 50% (n = 77) were Level II studies; 42% (n = 65) were Level III; 3% (n = 4) were Level IV (Table 6). The majority of Level I studies were performed in FA (43%, n = 3), Level II studies in HUE (29%, n = 22), and Level III studies in both HUE and Spine (25%, n = 16).
Table 6.
Characteristics of publications validating physical health CAT PROMIS domains by level of evidence (includes physical function, upper extremity, lower extremity)
| Variable | Level of evidence | |||
|---|---|---|---|---|
| I | II | III | IV | |
| n | 7 | 77 | 65 | 4 |
| Number of patients, median (IQR) | 983 (219–2507) | 157 (100–734) | 166 (108–290) | 85.5 (67–135) |
| Year, n (%) | ||||
| 2012 | – | 1 (1) | 1 (2) | – |
| 2013 | – | 1 (1) | – | – |
| 2014 | 3 (43) | 3 (4) | 2 (3) | – |
| 2015 | – | – | 1 (2) | – |
| 2016 | – | 4 (5) | 3 (5) | – |
| 2017 | – | 10 (13) | 5 (8) | – |
| 2018 | 2 (29) | 12 (16) | 8 (12) | 1 (25) |
| 2019 | 2 (29) | 32 (42) | 18 (28) | 1 (25) |
| 2020 | – | 14 (18) | 27 (42) | 2 (50) |
| Subspecialty, n (%) | ||||
| Adult reconstruction | 1 (14) | 3 (4) | 6 (9) | 1 (25) |
| Foot and ankle | 3 (43) | 7 (9) | 7 (11) | – |
| General orthopaedics | – | 6 (8) | 4 (6) | – |
| Hand and upper extremity | 2 (29) | 22 (29) | 16 (25) | – |
| Spine | 1 (14) | 17 (22) | 16 (25) | 1 (25) |
| Sports | – | 13 (17) | 9 (14) | 2 (50) |
| Trauma | – | 6 (8) | 4 (6) | – |
| Tumor | – | 3 (4) | 3 (5) | – |
| Study design, n (%) | ||||
| Case report or series | – | – | – | 2 (50) |
| Cross sectional | – | 1 (1) | 16 (25) | – |
| Prospective cohort | 6 (86) | 65 (84) | 2 (3) | – |
| Retrospective cohort | 1 (14) | 11 (14) | 47 (72) | 2 (50) |
| PROMIS domains, n (% of specified level of evidence) | ||||
| Physical function | 6 (86) | 66 (86) | 54 (83) | 4 (100) |
| Upper extremity | 2 (29) | 27 (35) | 17 (26) | – |
| Lower extremity | 1 (14) | 2 (3) | 1 (2) | – |
| Validation method tested, n (%) | ||||
| Reliability | 4 (57) | 14 (18) | 11 (17) | – |
| Responsiveness | 7 (100) | 57 (74) | 43 (66) | 3 (75) |
| Validity | ||||
| 1 validity criteria | – | 42 (55) | 46 (71) | 3 (75) |
| > 1 validity criterion | 4 (57) | 17 (22) | 6 (9) | – |
IQR interquartile range, CAT computerized adaptive test
PROMIS PF CAT specifically was validated in 130 studies, 50% of which were Level II studies. Since 2011, PROMIS PF CAT was analyzed for reliability 29 times, responsiveness 110 times, and at least one form of validity 118 times.
Discussion
The increased utilization of PROMIS measurement instruments across all types of orthopaedic surgery has enabled surgeons to gain a deeper understanding of patients’ physical and mental health while engaging patients more directly in their care. Compared to legacy measurement instruments (ASES, DASH, FAAM, KOOS) which are generally narrow in scope and can incur patient and administrative burden [1, 3], PROMIS CATs have the capacity to be tuned to orthopaedic diseases and improve patients’ experiences in orthopaedic surgery clinics [37]. These tests are enabling surgeons to interpret the patient’s HRQoL before and after treatment [3]. Additionally, understanding the degree and impact of a patient’s pain provides surgeons with a metric for tailoring treatment to each patient’s specific goals and needs, whether that be surgical or medical management [38, 39]. This scoping study demonstrates that in addition to becoming a more frequent subject of analysis, the PROMIS PH CAT domains (PF, UE, and LE) have repeatedly been shown to be reliable, responsive, and interpretable instruments when utilized in most contexts of orthopaedic surgery.
This scoping study determined that from January 1, 2011, through December 31, 2020, the PROMIS PH CAT was found to be interpretable as analyzed by at least one type of validity in various contexts throughout all orthopaedic surgery subspecialties in a total of 146 studies. In particular, PROMIS PF CAT was interpretable in 130 studies, 50% of which were Level II studies. Specific PROMIS PH CAT subdomains were first proposed in 2011 by Hung et al. [8], and have since been tested for reliability 29 times, responsiveness 110 times, and at least one form of validity 118 times. The extensive analysis of PROMIS PH CAT validity demonstrates the potential of PROMIS to assess PH in orthopaedic surgery patients. More importantly, this establishes an instrument that should effectively depict the patient’s perception of physical function status. As a widely interpretable outcome assessment instrument, PROMIS PH CAT may benefit patient care and advance orthopaedic outcomes research.
While PROMIS CAT is being shown to be interpretable more frequently and in more contexts, several limitations remain. Integrating these measurement instruments into electronic medical records remains a substantial obstacle, predominately due to financial, logistic, and technological barriers [40]. However, large-scale clinical implementation is possible and has valuable potential for improved patient care and experience [41]. Furthermore, while the short form format of PROMIS allows it to be administered as a physical test, the CAT format requires extra technology. The potential benefits outlined above may outweigh these costs in many settings. Additionally, CAT has been shown to have an improved ability to distinguish between two patients with similar health status [42], which can provide valuable insight when distinguishing between small details that can improve capabilities to diagnose and provide care.
Limitations
We note several limitations of this analysis. The evaluation of each publication was performed by two reviewers, which risked reporting bias of selective inclusion of research findings. However, the studies analyzed had clear descriptions of collection variables and followed the terminology and guidelines created for studies validating assessment instruments [11, 12], which contributed to more reliable evaluation of publications. Utilization of specific statistical methods in evaluation of instrument validation reduced potential disagreement of publication type and analyses performed. Additionally, publications were not evaluated on quality of the results; recommendations for PROMIS instruments from validation studies were taken directly from the publication, following common methodology of scoping studies [13, 43].
Our scoping study solely searched the NLM PubMed database, which risked evidence selection bias due to the potential for missed studies published in other databases. However, the relatively high number of 391 publications demonstrated sufficient evidence of PROMIS usage in orthopaedic surgery. At the time of the search, the orthopaedic surgery Tumor subspecialty had only six PH CAT validation publications, which may be an area of further exploration. Finally, given the nature of a scoping study, the results can only be as good as the publications evaluated. Therefore, each publication was evaluated for number of patients studied and publication level of evidence, and validation was evaluated based on statistical methods. Stratification of the publications based on these variables allows readers to observe these differences and make their own inferences. Regardless of these limitations, our scoping study provides an exhaustive overview of the existing literature on the usage of PROMIS in orthopaedic surgery [13].
Conclusions
PROMIS utilization within orthopaedics as a whole has significantly increased within the past decade, particularly within PROMIS CAT domains. The existing literature reviewed in this scoping study demonstrates that PROMIS physical health CAT domains (PF, UE, and LE) are reliable, responsive, and interpretable in most contexts of patient care throughout all orthopaedic surgery subspecialties. PROMIS enables orthopaedic surgeons to gain a deeper understanding of a patient’s physical and mental health directly from the patient, facilitating the potential to improve shared decision-making and quality of care. With numerous validation analyses of PROMIS PH CAT domains and the increasing utilization of PROMIS instruments, this study demonstrates that PROMIS PH CAT measurement instruments have much success in various contexts of orthopaedic clinical care and research. Clinicians and researchers should consider the use of PROMIS instruments within each context specifically, but in many instances, PROMIS PH CAT measures may work well in orthopaedic applications. While challenges of integrating these measurement instruments into electronic medical records exist, large-scale clinical implementation is possible and has valuable potential for improved patient care and experience; this implementation process should be an area of further research and a future healthcare objective.
Supplementary Information
Additional file 1: Supplementary Materials. All manuscripts evaluated.
Acknowledgements
The authors would like to thank Kenneth R. Gundle MD, Marie Kane MS, and Shelly Steward PhD for their insightful recommendations and direction in this study.
Abbreviations
- PRO
Patient reported outcome
- HRQoL
Health-related quality of life
- ASES
American Shoulder and Elbow Surgeons
- DASH
Disabilities of the Arm, Shoulder and Hand
- FAAM
Foot and Ankle Ability Measure
- KOOS
Knee Injury and Osteoarthritis Outcome Score
- NIH
National Institute of Health
- PROMIS
Patient Reported Outcomes Measurement Information System
- CAT
Computerized adaptive test
- PH
Physical health
- PF
Physical Function
- UE
Upper Extremity
- LE
Lower Extremity
- COSMIN
Consensus-based Standards for the selection of health status Measurement Instruments
- PRISMA-ScR
Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews
- FA
Foot and Ankle
- HUE
Hand and Upper Extremity
- AR
Adult Reconstruction
- RCT
Randomized controlled trial
Authors' contributions
Conception of work: JM. Study design, data acquisition: LW, JM. Analysis of data, interpretation of data and manuscript drafting: LW. Critical revision of manuscript: LW, JM. All authors read and approved the final manuscript.
Funding
This research project was undertaken without financial assistance of any kind.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cheung EC, Moore LK, Flores SE, Lansdown DA, Feeley BT, Zhang AL. Correlation of PROMIS with orthopaedic patient-reported outcome measures. JBJS Rev. 2019;7(8):e9. doi: 10.2106/jbjs.Rvw.18.00190. [DOI] [PubMed] [Google Scholar]
- 2.Gundle KR, Cizik AM, Jones RL, Davidson DJ. Quality of life measures in soft tissue sarcoma. Expert Rev Anticancer Ther. 2015;15(1):95–100. doi: 10.1586/14737140.2015.972947. [DOI] [PubMed] [Google Scholar]
- 3.Brodke DJ, Saltzman CL, Brodke DS. PROMIS for orthopaedic outcomes measurement. J Am Acad Orthop Surg. 2016;24(11):744–749. doi: 10.5435/jaaos-d-15-00404. [DOI] [PubMed] [Google Scholar]
- 4.Broderick JE, DeWitt EM, Rothrock N, Crane PK, Forrest CB. Advances in patient-reported outcomes: the NIH PROMIS((R)) measures. EGEMS (Wash DC) 2013;1(1):1015. doi: 10.13063/2327-9214.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, Ader D, Fries JF, Bruce B, Rose M. The patient-reported outcomes measurement information system (PROMIS): progress of an NIH roadmap cooperative group during its first two years. Med Care. 2007;45(5 Suppl 1):S3–s11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rose M, Bjorner JB, Becker J, Fries JF, Ware JE. Evaluation of a preliminary physical function item bank supported the expected advantages of the Patient-Reported Outcomes Measurement Information System (PROMIS) J Clin Epidemiol. 2008;61(1):17–33. doi: 10.1016/j.jclinepi.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 7.Ayers DC, Bozic KJ. The importance of outcome measurement in orthopaedics. Clin Orthop Relat Res. 2013;471(11):3409–3411. doi: 10.1007/s11999-013-3224-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hung M, Clegg DO, Greene T, Saltzman CL. Evaluation of the PROMIS physical function item bank in orthopaedic patients. J Orthop Res. 2011;29(6):947–953. doi: 10.1002/jor.21308. [DOI] [PubMed] [Google Scholar]
- 9.Guyatt G, Walter S, Norman G. Measuring change over time: assessing the usefulness of evaluative instruments. J Chronic Dis. 1987;40(2):171–178. doi: 10.1016/0021-9681(87)90069-5. [DOI] [PubMed] [Google Scholar]
- 10.Deyo RA, Diehr P, Patrick DL. Reproducibility and responsiveness of health status measures. Statistics and strategies for evaluation. Control Clin Trials. 1991;12(4 Suppl):142s–158s. doi: 10.1016/s0197-2456(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 11.Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, Bouter LM, de Vet HC. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol. 2010;63(7):737–745. doi: 10.1016/j.jclinepi.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan GM. A primer on the validity of assessment instruments. J Grad Med Educ. 2011;3(2):119–120. doi: 10.4300/jgme-d-11-00075.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daudt HM, van Mossel C, Scott SJ. Enhancing the scoping study methodology: a large, inter-professional team's experience with Arksey and O'Malley's framework. BMC Med Res Methodol. 2013;13:48. doi: 10.1186/1471-2288-13-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 15.Clarke J. What is a systematic review? Evid Based Nurs. 2011;14(3):64–64. doi: 10.1136/ebn.2011.0049. [DOI] [PubMed] [Google Scholar]
- 16.Schalet BD, Hays RD, Jensen SE, Beaumont JL, Fries JF, Cella D. Validity of PROMIS physical function measured in diverse clinical samples. J Clin Epidemiol. 2016;73:112–118. doi: 10.1016/j.jclinepi.2015.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, Hempel S, Akl EA, Chang C, McGowan J, Stewart L, Hartling L, Aldcroft A, Wilson MG, Garritty C, Lewin S, Godfrey CM, Macdonald MT, Langlois EV, Soares-Weiser K, Moriarty J, Clifford T, Tunçalp Ö, Straus SE. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 18.Marx RG, Wilson SM, Swiontkowski MF. Updating the assignment of levels of evidence. J Bone Joint Surg Am. 2015;97(1):1–2. doi: 10.2106/jbjs.N.01112. [DOI] [PubMed] [Google Scholar]
- 19.Schiphof D, de Klerk BM, Koes BW, Bierma-Zeinstra S. Good reliability, questionable validity of 25 different classification criteria of knee osteoarthritis: a systematic appraisal. J Clin Epidemiol. 2008;61(12):1205–1215.e1202. doi: 10.1016/j.jclinepi.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Husted JA, Cook RJ, Farewell VT, Gladman DD. Methods for assessing responsiveness: a critical review and recommendations. J Clin Epidemiol. 2000;53(5):459–468. doi: 10.1016/s0895-4356(99)00206-1. [DOI] [PubMed] [Google Scholar]
- 21.Cook DA, Beckman TJ. Current concepts in validity and reliability for psychometric instruments: theory and application. Am J Med. 2006;119(2):166.e167–116. doi: 10.1016/j.amjmed.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 22.Dimitrov D. Statistical methods for validation of assessment scale data in counseling and related fields. Appl Psychol Meas. 2012;31:367–387. doi: 10.1177/0146621606295199. [DOI] [Google Scholar]
- 23.Tsang S, Royse CF, Terkawi AS. Guidelines for developing, translating, and validating a questionnaire in perioperative and pain medicine. Saudi J Anaesth. 2017;11(Suppl 1):S80–s89. doi: 10.4103/sja.SJA_203_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 25.Wright JG, Young NL. A comparison of different indices of responsiveness. J Clin Epidemiol. 1997;50(3):239–246. doi: 10.1016/s0895-4356(96)00373-3. [DOI] [PubMed] [Google Scholar]
- 26.Cook CE. Clinimetrics corner: the minimal clinically important change score (MCID): a necessary pretense. J Man Manip Ther. 2008;16(4):E82–83. doi: 10.1179/jmt.2008.16.4.82E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinfurt KP. Constructing arguments for the interpretation and use of patient-reported outcome measures in research: an application of modern validity theory. Qual Life Res. 2021;30(6):1715–1722. doi: 10.1007/s11136-021-02776-7. [DOI] [PubMed] [Google Scholar]
- 28.Edwards MC, Slagle A, Rubright JD, Wirth RJ. Fit for purpose and modern validity theory in clinical outcomes assessment. Qual Life Res. 2018;27(7):1711–1720. doi: 10.1007/s11136-017-1644-z. [DOI] [PubMed] [Google Scholar]
- 29.Coles TM, Hernandez AF, Reeve BB, Cook K, Edwards MC, Boutin M, Bush E, Degboe A, Roessig L, Rudolph A, McNulty P, Patel N, Kay-Mugford T, Vernon M, Woloschak M, Buchele G, Spertus JA, Roe MT, Bury D, Weinfurt K. Enabling patient-reported outcome measures in clinical trials, exemplified by cardiovascular trials. Health Qual Life Outcomes. 2021;19(1):164. doi: 10.1186/s12955-021-01800-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koller I, Levenson MR, Glück J. What do you think you are measuring? A mixed-methods procedure for assessing the content validity of test items and theory-based scaling. Front Psychol. 2017;8:126. doi: 10.3389/fpsyg.2017.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hays R, Revicki D, Coyne K. Application of structural equation modeling to health outcomes research. Eval Health Prof. 2005;28:295–309. doi: 10.1177/0163278705278277. [DOI] [PubMed] [Google Scholar]
- 32.Magasi S, Ryan G, Revicki D, Lenderking W, Hays RD, Brod M, Snyder C, Boers M, Cella D. Content validity of patient-reported outcome measures: perspectives from a PROMIS meeting. Qual Life Res. 2012;21(5):739–746. doi: 10.1007/s11136-011-9990-8. [DOI] [PubMed] [Google Scholar]
- 33.Shrout PE, Fiske ST (1995) Personality research, methods, and theory: A festschrift honoring Donald W. Fiske. Personality research, methods, and theory: A festschrift honoring Donald W. Fiske. Lawrence Erlbaum Associates, Inc, Hillsdale, NJ, US
- 34.Reichardt CS, Coleman SC. The criteria for convergent and discriminant validity in a multitrait-multimethod matrix. Multivar Behav Res. 1995;30(4):513–538. doi: 10.1207/s15327906mbr3004_3. [DOI] [PubMed] [Google Scholar]
- 35.Karras DJ. Statistical methodology: II. Reliability and validity assessment in study design, Part B. Acad Emerg Med. 1997;4(2):144–147. doi: 10.1111/j.1553-2712.1997.tb03723.x. [DOI] [PubMed] [Google Scholar]
- 36.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernstein DN, Fear K, Mesfin A, Hammert WC, Mitten DJ, Rubery PT, Baumhauer JF. Patient-reported outcomes use during orthopaedic surgery clinic visits improves the patient experience. Musculoskelet Care. 2019;17(1):120–125. doi: 10.1002/msc.1379. [DOI] [PubMed] [Google Scholar]
- 38.Ho B, Houck JR, Flemister AS, Ketz J, Oh I, DiGiovanni BF, Baumhauer JF. Preoperative PROMIS scores predict postoperative success in foot and ankle patients. Foot Ankle Int. 2016;37(9):911–918. doi: 10.1177/1071100716665113. [DOI] [PubMed] [Google Scholar]
- 39.Amtmann D, Kim J, Chung H, Askew RL, Park R, Cook KF. Minimally important differences for patient reported outcomes measurement information system pain interference for individuals with back pain. J Pain Res. 2016;9:251–255. doi: 10.2147/jpr.S93391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lavallee DC, Chenok KE, Love RM, Petersen C, Holve E, Segal CD, Franklin PD. Incorporating patient-reported outcomes into health care to engage patients and enhance care. Health Aff. 2016;35(4):575–582. doi: 10.1377/hlthaff.2015.1362. [DOI] [PubMed] [Google Scholar]
- 41.Papuga MO, Dasilva C, McIntyre A, Mitten D, Kates S, Baumhauer JF. Large-scale clinical implementation of PROMIS computer adaptive testing with direct incorporation into the electronic medical record. Health Syst (Basingstoke) 2018;7(1):1–12. doi: 10.1057/s41306-016-0016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Segawa E, Schalet B, Cella D. A comparison of computer adaptive tests (CATs) and short forms in terms of accuracy and number of items administrated using PROMIS profile. Qual Life Res. 2020;29(1):213–221. doi: 10.1007/s11136-019-02312-8. [DOI] [PubMed] [Google Scholar]
- 43.Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5(1):69. doi: 10.1186/1748-5908-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Materials. All manuscripts evaluated.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.