Abstract
Introduction
Randomised controlled trials provide evidence that a treatment works. Real world evidence is required to assess if proven treatments are effective in practice.
Method
Retrospective data collection on patients given aflibercept for diabetic macular oedema over 3 years from 21 UK hospitals: visual acuity (VA); Index of multiple deprivation score (IMD); injection numbers; protocols used, compared as a cohort and between sites.
Results
Complete data: 1742 patients (from 2196 eligible) at 1 year, 860 (from 1270) at 2, 305 (from 506) at 3 years. The median VA improved from 65 to 71, 70, 70 (ETDRS letters) at 1, 2 and 3 years with 6, 9 and 12 injections, respectively. Loss to follow-up: 10% 1 year, 28.8% at 3. Centres varied: baseline: mean age 61–71 years (p < 0.0001); mean IMD score 15–37 (p < 0.0001); mean VA 49–68 (p < 0.0001). Only four centres provided a loading course of five injections at monthly intervals and one 6. This did not alter VA outcome at 1 year. Higher IMD was associated with younger age (p = 0.0023) and worse VA at baseline (p < 0.0001) not total number of injections or change in VA. Lower starting VA, higher IMD and older age were associated with lower adherence (p = 0.0010).
Conclusions
The data showed significant variation between treatment centres for starting age, VA and IMD which influenced adherence and chances of good VA. Once treatment was started IMD did not alter likelihood of improvement. Loading dose intensity did not alter outcome at one year.
Subject terms: Eye diseases, Scientific community
Introduction
Randomised controlled trials provide evidence of the efficacy of a treatment. Real world evidence (RWE) assesses the effectiveness of a treatment when introduced into routine clinical practice [1]. For anti-vascular endothelial growth factor (anti-VEGF) treatment, RWE can be used for measuring outcomes such as numbers of injections given over time and adherence to follow-up. In addition, RWE can be used to compare centres for patient population characteristics and treatment provision in order to provide evidence enabling service improvements [2]. On the contrary, comparing VA outcomes for different treatment regimes, using multi-centre data collection might not be as robust due to influences such as service capacity, clinician choice, patient adherence and payer requirements.
The VIVID and VISTA studies showed that treatment with aflibercept, an anti-VEGF, in a clinical setting resulted in a mean visual acuity (VA) gain of 10 ETDRS (Early Treatment Diabetic Retinopathy Study) letters, from a mean baseline of 60 letters, at 1 year, in eyes with DME [3]. This was similar to that seen in the RISE and RIDE studies for ranibizumab [4]. In the VIVID and VISTA trials, five injections were given a month apart then 2 monthly for a year, forming the basis of aflibercept’s posology in DME. In Protocol T of DRCR.net, initially four injections were given 4 weeks apart supplemented by two more injections 4 weeks apart if DME was still present [5], leading to some clinicians promoting a protocol of 6 injections at 4 weekly intervals [5]. Previously published RWE in the UK for ranibizumab found that, when compared to VIVID and VISTA, the mean starting VA was lower at 51.2 letters and VA gains modest, increasing to 54.2 and 52.5 letters at 1 and 2 years respectively, but an average of only 3.3 injections were actually given during the first year [6].
Ranibizumab was approved as a first-line treatment in the UK for centre involving DME over 400 microns in 2012 and aflibercept followed in 2015. Aflibercept has replaced ranibizumab as first-line DME treatment in many centres. The aim of this study was to assess the effectiveness of aflibercept in improving and preserving vision in patients with DME in clinical practice over three years in the UK.
We assessed the number of injections given, initial treatment protocols used, changes in VA and adherence to treatment in a multicentre cohort of patients and specifically examined differences between centres. To assess potential differences between patient populations in participating centres we correlated this with the index of multiple deprivation score (IMD), mean age, mean duration of diabetes, proportion of diabetes type, and mean baseline VA. In addition, we used survival analysis as a means of assessing outcomes which is less affected by annual time points and loss to follow-up [7].
Methods
Relevant anonymised data of DME patients undergoing aflibercept injections from 21 UK hospitals that used a dedicated ophthalmology electronic patient record system (Medisoft Ophthalmology, Medisoft Limited, Leeds, UK) was exported in December 2019. If both eyes were treated, only data from the first eye to be treated were included; for those in whom treatment was commenced simultaneously in both eyes, the eye with better VA was used. Cases were excluded from analysis If additional pharmaceutical therapy had been given. All data from patients who had a baseline VA measurement and the potential for 1, 2 or 3 years of follow-up was accounted for. Included in the 1, 2 and 3-year VA outcome analysis were those who had, both baseline VA and a VA measurement within 8 weeks of the specified year point and a further measurement beyond that year point.
The lead clinician and Caldicott Guardian (responsible nominee for data protection) at each NHS hospital gave written approval for anonymised data extraction. Anonymized database analyses of this type do not require ethical permission as they are viewed as service evaluation. This study was conducted in accordance with the Declaration of Helsinki 2013 and the United Kingdom’s Data Protection Act 2018.
VA records are entered as ETDRS letter scores at 2 m in the EMR system. At each visit, the best-measured VA value was used in the analysis, usually with a habitual correction rather than refraction, as this is routine clinical practice. VA of count fingers, hand movements, perception of light (PL) and no PL were substituted with a value of 0 letters. Both the mean and median values are reported in this study as the data are unlikely to be normally distributed, and the median is less influenced by outliers, such as very low VA in patients with vitreous haemorrhage.
Linear regression was used to relate VA change over 12 months from the first injection with age (grouped <65 years, 65–74, 75 and above), baseline letter score (<50 letters, 50–59, 60–69, 70–79, 80 or more), and number of injections. The Cox proportional hazards model was used for survival analysis of time to VA improvement in relation to injection numbers and time to lost to follow-up.
The number of patients completing an initial course of 3, 5 and 6 monthly injections was analysed and total number of injections per treatment regime used was compared between centres.
In the UK deprivation is measured using the IMD [8]. The relationship between IMD and the age of presentation, VA, number of injections given and adherence to follow-up was assessed.
All statistical analyses were carried out using SAS 9.4. Descriptive statistics for baseline characteristics were obtained using PROC MEANS and PROC FREQ and plots done with PROC GPLOT. Time to event data was analysed using PROC LIFETEST and PROC PHREG.
Results
In total 2196 patients at the 21 sites had an aflibercept injection for DME at least 1 year prior to data extraction and had a baseline VA measurement, being eligible for 1-year follow-up. Of these, 226 had no data at or beyond 1 year (10%). An additional 228 had no data within 8 weeks of the anniversary. Altogether 1742 were analysed for the 1-year outcomes. Corresponding numbers for year 2 and 3 are given in Table 1.
Table 1.
Numbers of patients with potential data and actual data over three years.
| Year 1 | Year 2 | Year 3 | |
|---|---|---|---|
| Total number of patients | 2196 | 1270 | 506 |
| Excluded from annual time point: no data within 8 weeks but has data at a further time point | 228 | 163 | 58 |
| No data at or beyond annual time point | 226 (10%) | 241 (19.5%) | 146 (28.8%) |
| Total analysed for annual time points | 1742 | 860 | 305 |
There was no significant heterogeneity between centres in duration of diabetes (mean 15–19 years, p = 0.68). The majority had type 2 diabetes (86%) and 62% were male (Table 2).
Table 2.
Demographics and visual acuity (VA) changes at 1, 2 and 3 years.
| One-year cohort | Two-year cohort | Three-year cohort | |
|---|---|---|---|
| N | 1742 | 860 | 305 |
| Age at first injection (years) (Median 25th to 75th centile) | 64 (56–73) | 64 (56–72) | 63 (55–72) |
| Gender (M/F) | 61%/39 % | 62%/38% | 63%/37% |
| Type of diabetes (T1DM/T2DM) | 14%/86% | 14%/86% | 15%/85% |
| Time since diagnosis of diabetes (years) | 15 (10–21) | 15 (9–21) | 14 (8–21) |
| Injections (n) | 6 (5–8) | 9 (6–11) | 12 (7–16) |
| VA at start of period (letters) (Median ETDRS letters) | 64 (55–74) | 65 (54–72) | 65 (55–72) |
| VA at anniversary (letters) (Median EDTRS letters) | 71 (60–78) | 70 (60–78) | 70 (60–77) |
| VA gain (letters) | 5 (−1 to 12) | 5 (−2 to 12) | 5 (−2 to 13) |
There was significant heterogeneity between centres for baseline age (61–71 (p < 0.0001)), IMD score (mean 15–37 (p < 0.0001), and VA (49–68 letters p < 0.0001). Deaths were recorded in 119 cases, varying from 0% to 7.5% between centres.
The 1742 patients in the 1-year cohort had a median age of 64 years (56–73 years; 25th to 75th centile), mean baseline VA 62 (ETDRS) letters (median 65; (Inter Quartile Range IQR 55 to 74), improving by 5 letters (IQR-1 to 12) to a mean of 67, median of 71 (IQR 60–78), after a median of 6 (IQR 5 to 8) injections. Worse baseline VA was associated with the greatest improvement in VA (p < 0.001), those with fewer than 50 letters at baseline having improved 13.9 letters (19.3 mean standard deviation (SD)) and those with 80 letters or more losing 3.2 (7.0 SD) letters.
The 860 patients with 2-year data had a median baseline VA of 65 letters (IQR 54-72) and median VA at 2 years of 70 (IQR 60–78) after a median of 9 (6–11) injections.
For the 305 patients with 3 years of follow-up, the median VA at baseline was 65 (IQR 55 to 72) and at 3 years 70 (IQR 60–77), after a median of 12 (IQR 7–16) injections. The median OCT thickness reduced from 441(IQR 394–506) to 283 μm at 3 years, (IQR 241 to 316).
Older patients had a smaller VA improvement (p = 0.0002), >75 years and above gaining 4.2 (14.1 SD) letters and <55 years gaining 6.6 (14.2 SD) letters. After adjustment for age group and baseline VA each additional injection gave an improvement of 0.56(0.14) letters (p < 0.0001).
An alternative parameterisation of the data uses survival analysis to look at time to sustained gain of ten letters, using number of injections as a time-dependent co-variate in a Cox proportional hazards model. This model shows that those with better VA and those who are older are less likely to gain ten letters and also that at each time point having had one more injection increased the probability of achieving a ten-letter gain, hazard ratio 1.10 (95% CI 1.06–1.15).
The proportion of patients with different levels of vision is given in Fig. 1. Patients with >70 letters increased from 39% at baseline to 56% at 1 year, 55% at 2 and 51% at 3 years, for those still under follow-up.
Fig. 1. ETDRS letter score colour bands at each year time point.
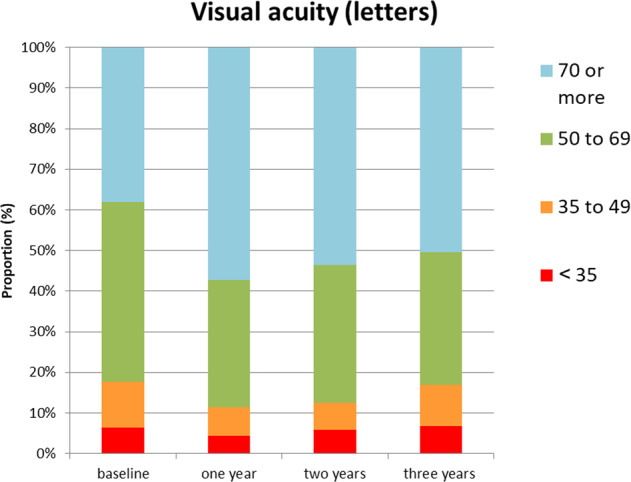
Proportion of patients, who had data, with different levels of visual acuity (ETDRS letter scores) at 1, 2 and 3 years.
We analysed which sites used a 3, 5 or 6 initial monthly injection protocol and whether that influenced the total number of injections given over time or the VA outcomes. (Table 3) A three-injection loading dose protocol defined as giving three injections 28 days apart was given in 1393/1742 (80%), with substantial difference between centres (35–100%). Altogether 256 patients had a five-injection loading protocol, the majority in four centres who completed this in 75–79% of cases. Only 86 patients received a six injection loading phase, the majority of whom were from one centre, which used this protocol in 80% of their patients.
Table 3.
Proportion of Patients given three, five or six injection initial treatment protocols in the different centres and the relation to visual acuity (VA) over 1 year.
| Variable | N (3) | Mean (s.d.) | Median (25th to 75th centile) | N (5) | Mean (s.d.) | Median (25th to 75th centile) | N (6) | Mean (s.d.) | Median (25th to 75th centile) | P value anova | P value non param |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at first injection | 1393 | 63.9 (12.8) | 64 (56–73) | 256 | 63.5 (13.2) | 64 (55– 72.5) | 86 | 63.5 (13.2) | 65 (56–76) | 0.7 | 0.6 |
| years since diagnosis of diabetes | 838 | 16.4 (10.2) | 15 (10–21) | 147 | 16 (10.3) | 15 (9–20) | 50 | 16 (10.3) | 14.5 (6– 19) | 0.9 | 0.6 |
| VA (letters) at first injection | 1393 | 61.3 (16.1) | 65 (54–73) | 256 | 64.8 (14.4) | 68 (58–75) | 86 | 64.8 (14.4) | 70 (55–75) | 0.003 | <0.001 |
| VA (letters) at one year | 1393 | 66.7 (15.9) | 70 (60–78) | 256 | 71.3 (14.1) | 75 (67.5– 80) | 86 | 71.3 (14.1) | 70 (56–79) | <0.001 | <0.001 |
| Gain (letters) | 1393 | 5.4 (13.4) | 5 (−1 to 11) | 256 | 6.5 (13.2) | 6 (0–12) | 86 | 6.5 (13.2) | 4 (−5 to 12) | 0.076 | 0.10 |
| Number of injections in first year | 1393 | 5.9 (2) | 6 (5–7) | 256 | 6.8 (1.9) | 7 (5–8) | 86 | 6.8 (1.9) | 7 (6–7) | <0.001 | <0.001 |
N number of patients with data available; (3)(5)(6) protocol used.
We found no difference in the VA at 1 year regardless of the initial treatment protocol used and the average number of injections was the same, although there was an association with more improvement with more injections. There was no difference between the patients given the different initial treatment protocols in age or duration of diabetes at first injection, however, those on the five and six injection loading protocols had better VA at the start (three or four more letters), so whilst there was no difference in the gain, the VA at 1 year was better.
The number of injections during the 3 years of treatment varied slightly depending on the starting loading strategy, but overall, there was no statistically significant difference in the final number of injections regardless of which loading protocol was used. Of those who were lost to follow-up 42% had an injection at their last visit.
Additional focal laser was administered to 5.3% in the 1st year, 10.3% in the 2nd and 13.4% in the 3rd year. An additional 259 patients, not included in our analysis as we only included those given aflibercept alone, had been started on aflibercept but subsequently switched to Ozurdex (241) or Iluvien (18).
Using survival modelling with loss to follow-up as the event of interest we found that those with better baseline VA are more likely to continue to attend (70 or more EDTRS letter compared to <50, Hazard ratio 0.68 (0.55–0.84) and those in the oldest age group are least likely to attend (>75 compared to <55 years, Hazard ratio 1.28 (1.02–1.61).
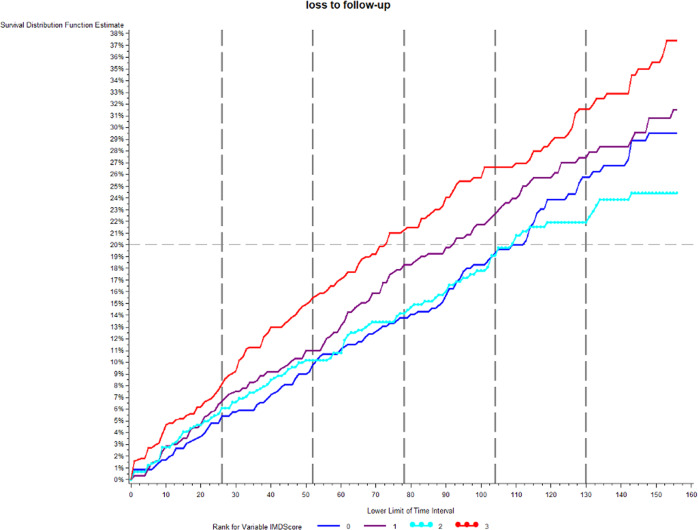
The IMD was correlated with age at first injection and VA at first injection. The most deprived were younger (p = 0.0017) and had worse VA at first injection (p = 0.0001). The number of injections was not related to deprivation (p = 0.91). There was no correlation between IMD and change in VA Hence the most deprived started from a lower baseline VA and had worse vision at 1 year (p = 0.0001) Those in the most deprived group were more likely to be lost to follow-up, 15% of the most deprived versus 10% in the less deprived groups by end of 1 year (Log rank test p = 0.0010 (Fig. 2).
Fig. 2. Percentage lost to follow-up over time in months in relation to the index of multiple deprivation score (IMD).
The IMD has been grouped into four bands with least deprived being 0 and most 3 for the purpose of this analysis.
Discussion
Our study found that mean VA at which treatment is started has risen from 51 letters to 62 (Median 65) since the last RWE in DME data were published from the UK [6]. This is in parallel with a starting VA rise seen in clinical trials, such as 57 in RISE and RIDE and 60 in VIVID and VISTA [3, 4].
The DRCR.net has found that eyes with lower VA at baseline were more likely to achieve larger gains in VA but less likely to reach near-normal VA with only 17% of eyes with 25–50 letters reaching 72 letters or better with treatment [9]. Good starting VA is therefore crucial for good outcomes, however, recent data support that for those >72 ETDRS letters delaying treatment over 2 years may not actually lead to a worse outcome [10].
We found a five mean letter gain after a relatively low number of injections compared to clinical trials in the first year (mean no = 6). However, this was nearly double that of a previous UK RWE report in which the mean number of injections was 3.3 [6]. This was however reported using ranibizumab before aflibercept was available. A five letter mean gain is about half of that observed in VIVID and VISTA. The baseline VA of our patient cohort was marginally higher at 62 (Median 65) compared to the baseline VA of 60 in the pivotal trials but the mean VA achieved was a lower at 67 (Median 71) vs 70 suggesting there is room for improvement if more injections were given. Indeed, we found that the likelihood of gaining ten letters increased with each additional injection. The VA in our study was tested with habitual correction rather than refraction each time so was likely to have underestimated VA. In a UK prospective data collection study, which used refracted VA, the baseline VA was 69.5 letters [11].
Retention of DME patients is problematic in most clinical settings. In one RWE reporting on 117 patients, a gain of 4.8 and 9.6 letters were seen over 1 and 2 years, starting at 60 letters, with 5.5 injections at 1 year and a total of 8.7 at 2, however, only 31 patients had data at 2 years [12]. In the APOLLON study, a prospective 1-year follow-up RWE study in France 147 patients were included in the analysis but the cohort started at 402 (77 treatment naive). The baseline VA was 62.7 letters increasing by +7.8 letters after a mean of 7.6 injections at 1 year [13].
The Fight Retinal Blindness registry reported visual gains of +1.4 letters for 217 eyes receiving aflibercept with 70 or better VA at baseline and +10.6 with those <65, using a mean of 8 aflibercept injections at 12 months, 21% were lost to follow-up, compared to 10% at 1 year in our study [14].
A larger multicentre study from Japan of 2049 eyes measured a mean gain of 2.2 letters using a mixture of treatments. For patients just given anti-VEGF injections it was +4.5 but only 4.3 injections were given over 2 years. This study was unable to account for patients who did not have data at 2 years [15].
RWE reported from America from the Vestrum Health Retina Database had 15,608 DME patient eyes in the analysis. In the 12-month cohort, of 1379 eyes initially treated with aflibercept, the mean 12-month improvement was +5.5 letters (95% confidence interval [CI] +4.5 to +6.6 letters, P < 0.001) from a baseline of 57.9, after 7.5 injections on average [16].
Recommendations by a UK expert panel on the use of aflibercept in DME, published in a 2020 report that, “most panel members use six initial 4 weekly doses, as in Protocol T, rather than five initial monthly doses as recommended in the Summary of product characteristics (SmPC)” [17]. Our data show that only a small percentage of patients received 5 or 6 four weekly injections, either because this was not possible or not felt necessary by the clinician or patients.
The use of additional focal laser rose from 5.3% at the end of the first year to 13.4% at 3 years which was a lot lower than in the DRCR.net protocol T study in which laser varied from 30–40% [5].
Loss to follow-up is a weakness of our study for assessing VA outcomes, especially by 3 years, however, loss to follow-up rates is an important outcome in itself as this is a very important issue for the effectiveness of the treatment. This is a large study from 21 centres showing this is a widespread problem. Some missing data were due to incomplete EMR system use in some centres. Missing annual data were partly due to patients not attending regularly or not needing to be seen within the 8-week anniversary window. We found that attrition was not random with an increased rate for those starting with lower vision and being of older age also patients were more likely to attend if they were actively having injections. By 3 years a proportion of patients may have been discharged back to diabetic eye screening if they were stable. Death was recorded in 119 (4%) but this may have been an underestimate due to variation in how the EMR links to other records. Full data on comorbidities were not available, and these, especially strokes and heart attacks, could considerably change attendance and treatment frequency.
We found that those in the most deprived areas were also more likely to be lost to follow-up, be younger and have worse vision at the first injection. On the positive side, the number of injections and the change in VA were not related to deprivation suggesting that once aflibercept treatment was started, patient care was not compromised as a consequence of deprivation status. A previous large study of 79,775 patients in the UK also found an association between later presentation with worse VA for patients with diabetic retinopathy who were from more deprived areas [8]. Our data show that part of the differences in VA outcomes amongst the centres included in this study can be explained by the variations in the age profile of patients, their initial VA and the deprivation levels. Further work is needed to understand reasons for non-adherence to visit schedules and to find solutions [18, 19].
Summary
What was known before
Randomised clinical trials of aflibercept for DME show it can improve vision. In clinical practice, the same results may not be achieved
What this study adds
There was variation between centres for Index of multiple deprivation, age and baseline visual acuity. These factors affected the visual acuity outcomes. The provision of Initial injection protocols of 3–6 monthly injections varied greatly between centres but did not affect the mean Visual acuity at 1 year.
Acknowledgements
Bayer provided funding for data extraction and for support with analysis but did not have a role in the analysis or writing of the manuscript.
Author contributions
SJT: designed study analysis plan, wrote paper; IS: statistics and writing; TP: analysis ideas, editing; AL: analysis ideas, editing; UC: editing; HE: editing; SI: editing, ND: editing; PS: project lead.
Competing interests
SJT: Research and Advisory boards for Bayer; Novartis; Roche; Allergan; Alimera; IS: Consultant for Novo Nordisk; TP: Speaker and advisory board Bayer, Novartis, Roche; AL: None; UC: Chair of International trials sponsored by Bayer; HE: Research and Travel Bayer; SI: Research Bayer; travel Bayer and Novartis; ND: Research Novartis; Travel Novartis; Allergan; Bayer; Speaker Novartis; Advisory Board Novartis; PS: Research and audit grants from Allergan, Boehringer, Novartis, Bayer; Advisory Boards for Pfizer; Allergan; Roche; Boehringer and Bayer.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
S. J. Talks, Email: james.talks@nhs.net
the UK Aflibercept Users’ Group:
James Talks, Peter Scanlon, Quresh Mohamed, Andrew Lotery, Sharam Kashani, Nasos Georgas, Colin Jones, Abdisattar Gashut, Cynthia Santiago, Romi Chhabra, Richard Antcliff, Naren Dhingra, Clare Bailey, Usha Chakravarthy, Tunde Peto, Faruque Ghanchi, Linda Mcinerney, Salim Natha, Rehna Khan, Indra Dias, Raj Mukhrejee, Shahrnaz Izadi, Irfan Tahir, and Haralabos Eleftheriadis
References
- 1.Talks J, Daien V, Finger RP, Eldem B, Sakamoto T, Cardillo JA, et al. The use of real-world evidence for evaluating anti–vascular endothelial growth factor treatment of neovascular age-related macular degeneration. Surv Ophthalmol. 2019;64:707–19. doi: 10.1016/j.survophthal.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Talks JS, James P, Sivaprasad S, Johnston RL, McKibbin M, UK Aflibercept Users Group. Appropriateness of quality standards for meaningful intercentre comparisons of aflibercept service provision for neovascular age-related macular degeneration. Eye. 2017;31:1613–20. doi: 10.1038/eye.2017.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heier JS, Korobelnik JF, Brown DM, Schmidt-Erfurth U, Do DV, Midena E, et al. Intravitreal aflibercept for diabetic macular edema: 148-week results from the VISTA and VIVID studies. Ophthalmology. 2016;123:2376–85. doi: 10.1016/j.ophtha.2016.07.032. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Ranibizumab for diabetic macular edema: results from 2 phase iii randomized trials: RISE and RIDE. Ophthalmology. 2012;123:2376–85. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 5.Diabetic retinopathy research network. Aflibercept,bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1192–203. doi: 10.1056/NEJMoa1414264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egan C, Zhu H, Lee A, Sim D, Mitry D, Bailey C, et al. The United Kingdom Diabetic Retinopathy Electronic Medical Record Users Group, Report 1: baseline characteristics and visual acuity outcomes in eyes treated with intravitreal injections of ranibizumab for diabetic macular oedema. Br J Ophthalmol. 2017;101:75–80. doi: 10.1136/bjophthalmol-2016-309313. [DOI] [PubMed] [Google Scholar]
- 7.Fu DJ, Keenan TD, Faes L, Lim E, Wagner SK, Moraes G, et al. Insights from survival analyses during 12 years of anti–vascular endothelial growth factor therapy for neovascular age-related macular degeneration. JAMA Ophthalmol. 2021;139:57–67. doi: 10.1001/jamaophthalmol.2020.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denniston AK, Lee AY, Lee CS, Crabb DP, Bailey C, Lip PL, et al. United Kingdom diabetic retinopathy electronic medical record (UK DR EMR) users group: report 4, real-world data on the impact of deprivation on the presentation of diabetic eye disease at hospital services. Br J Ophthalmol. 2019;103:837–43. doi: 10.1136/bjophthalmol-2018-312568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bressler SB, Qin H, Beck RW, Chalam KV, Kim JE, Melia M, et al. Factors associated with changes in visual acuity and central subfield thickness at 1 year after treatment for diabetic macular edema with ranibizumab. Arch Ophthalmol. 2012;30:1153–61. doi: 10.1001/archophthalmol.2012.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker CW, Glassman AR, Beaulieu WT, Antoszyk AN, Browning DJ, Chalam KV, et al. Effect of Initial management with aflibercept vs laser photocoagulation vs observation on vision loss among patients with diabetic macular edema involving the center of the macula and good visual acuity: a randomized clinical trial. J Am Med Assoc. 2019;321:1880–94. doi: 10.1001/jama.2019.5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sivaprasad S, Ghanchi F, Kelly S, Kotagiri A, Talks J, Scanlon P, et al. Real-life study assessing the effectiveness of intravitreal aflibercept injection (IAI) treatment of diabetic macular edema (DME) in UK routine clinical practice (DRAKO): baseline characteristics. Investig Ophthalmol Vis Sci. 2019;60:3650. [Google Scholar]
- 12.Kern C, Schiefelbein J, Fu DJ, Schworm B, Sim D, Herold T, et al. Two year visual acuity and structural outcomes in patients with diabetic macular oedema treated with intravitreal aflibercept - a retrospective cohort study. Clin Ophthalmol. 2020;14:533–41. doi: 10.2147/OPTH.S237586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korobelnik JF, Daien V, Faure C, Tadayoni R, Giocanti-Auregan A, Dot C, et al. Real-world outcomes following 12 months of intravitreal aflibercept monotherapy in patients with diabetic macular edema in France: results from the APOLLON study. Graefe’s Arch Clin Exp Ophthalmol. 2020;258:521–8. doi: 10.1007/s00417-019-04592-9. [DOI] [PubMed] [Google Scholar]
- 14.Bhandari S, Nguyen V, Fraser-Bell S, Mehta H, Viola F, Baudin F, et al. Ranibizumab or aflibercept for diabetic macular edema: comparison of 1-year outcomes from the fight retinal blindness! registry. Ophthalmology. 2020;127:608–15. doi: 10.1016/j.ophtha.2019.11.018. [DOI] [PubMed] [Google Scholar]
- 15.Shimura M, Kitano S, Muramatsu D, Fukushima H, Takamura Y, Matsumoto M, et al. Real-world management of treatment-naïve diabetic macular oedema in Japan: Two-year visual outcomes with and without anti-VEGF therapy in the STREAT-DME study. Br J Ophthalmol. 2019;104:1209–15. doi: 10.1136/bjophthalmol-2019-315199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciulla TA, Bracha P, Pollack J, Williams DF. Real-world outcomes of anti-vascular endothelial growth factor therapy in diabetic macular edema in the United States. Ophthalmol Retin. 2018;2:1179–87. doi: 10.1016/j.oret.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Pearce I, Bailey C, Fletcher E, Ghanchi F, Rennie C, Santiago C, et al. Translating evidence into practice: recommendations by a UK expert panel on the use of aflibercept in diabetic macular oedema. Eye. 2020;34:969–81. doi: 10.1038/s41433-019-0615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiss M, Sim DA, Herold T, Schumann RG, Liegl R, Kern C, et al. Compliance and adherence of patients with diabetic macular edema to Iintravitreal anti-vascular endothelial growth factor therapy in daily practice. Retina. 2018;38:2293–2300. doi: 10.1097/IAE.0000000000001892. [DOI] [PubMed] [Google Scholar]
- 19.Okada M, Mitchell P, Finger RP, Eldem B, Talks SJ, Hirst C, et al. Nonadherence or nonpersistence to intravitreal injection therapy for neovascular age-related macular degeneration: a mixed-methods systematic review. Ophthalmology. 2020; 10.1016/j.ophtha.2020.07.060. [DOI] [PMC free article] [PubMed]