Abstract
Antibody–drug conjugates (ADCs) are gradually revolutionizing clinical cancer therapy. The antibody–drug conjugate linker molecule determines both the efficacy and the adverse effects, and so has a major influence on the fate of ADCs. An ideal linker should be stable in the circulatory system and release the cytotoxic payload specifically in the tumor. However, existing linkers often release payloads nonspecifically and inevitably lead to off-target toxicity. This defect is becoming an increasingly important factor that restricts the development of ADCs. The pursuit of ADCs with optimal therapeutic windows has resulted in remarkable progress in the discovery and development of novel linkers. The present review summarizes the advance of the chemical trigger, linker‒antibody attachment and linker‒payload attachment over the last 5 years, and describes the ADMET properties of ADCs. This work also helps clarify future developmental directions for the linkers.
KEY WORDS: Antibody–drug conjugate, Linker, Chemical trigger, Linker‒antibody attachment, Linker‒payload attachment
Graphical abstract
Antibody–drug conjugates (ADCs) are revolutionizing cancer therapy. Linkers, determining both efficacy and off-target toxicity, are the core components of ADCs. The advances of linkers over the past 5 years are reviewed.
1. Introduction
Antibody–drug conjugate (ADC), comprising a monoclonal antibody (mAb), the cytotoxic payload and the linker, has developed rapidly in recent years and is gradually revolutionizing clinical cancer therapy. This technology appeared a century ago1, but it is becoming mature in the past 5 years. Currently, 10 ADCs have been approved and more than 80 ADCs are at different phases of clinical trials2, 3, 4, 5, 6, 7, 8.
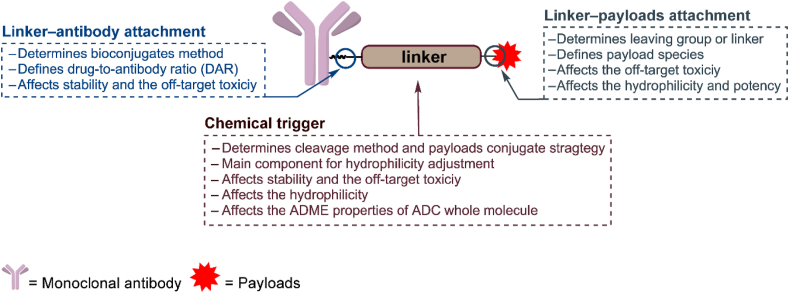
The linker connects the antibody and the cytotoxic payload and is a key component in the function of ADCs. The linker imparts the following characteristics to ADCs: (1) high stability in the circulation, and (2) specific release of payload in the target tissue. These seemingly contradictory requirements of stability and release lead to the major challenge in the development of linkers. To achieve the above requirement, various linkers have been developed and can be divided into two types according to their cleavage method. The first type is the cleavable linker, which has a chemical trigger in its structure that can be efficiently cleaved to release the cytotoxic payload in the tumor. More than 80% of the clinically approved ADCs employ cleavable linkers9, such as inotuzumab ozogamicin (Besponsa) and brentuximab vedotin (Adcetris)10,11. The other type of linker is noncleavable. In contrast to the cleavable linker, there are no chemical triggers in this structure, and the linker is part of the payload. This type of linker has been employed only in ado-trastuzumab emtansine (Kadcyla, T-DM1) among the approved ADCs12.
Although ADCs have achieved great success, future development is increasingly constrained by the linkers. The defects of the classical linkers employed in the marketed ADCs include the following aspects: (1) the nonspecific release of payloads in non-tumorous tissues, leading to off-target toxicity and a limited therapeutic window. The most notable case is that of gemtuzumab ozogamicin (Mylotarg) developed by Pfizer. It was withdrawn in 2010 for causing severe liver toxicity due to an unstable N-acylhydrazone linker13. Although Mylotarg was approved again in 2017 after redesign, the U.S. Food and Drug Administration (FDA) required a black-box warning for the potential of liver toxicity. (2) The retro-Michael elimination reaction of the commonly-used maleimide attachment leads to reduced efficacy of ADCs. For instance, the classic succinimidyl 4-(N-maleimidomethyl) cyclohexane-1-1carboxylate (SMCC) linker degrades to 38% after 120 h in mice plasma, and Kadcyla containing an SMCC linker exhibited a 29% drug-to-antibody ratio (DAR) decrease in mice after 7 days14, 15, 16. (3) The limited linker‒payload attachment is insufficient for the rapid expansion of payloads. Novel ADCs have developed rapidly to treat cancer, microbial infection17, and immune modulation18. Many newly designed payloads are awaiting appropriate linker‒payload attachments.
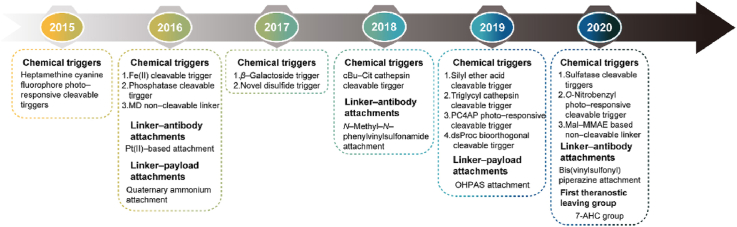
To solve the above problems, there have been important developments in linker design in the past 5 years, which are as follows (Fig. 1): (1) the optimization of the existing chemical triggers and development of novel chemical triggers to generate highly selective linkers; (2) the development of novel linker‒antibody attachments to produce stable and homogeneous ADCs; (3) the development of additional linker‒payload attachments to allow the expansion of payloads; (4) the optimization of linkers to improve the absorption, distribution, metabolism, and excretion (ADME) of ADCs. Therefore, a literature review initially based on the 3 components of linkers was carried out to provide a comprehensive overview of the developments over the past 5 years with regard to the above four aspects.
Figure 1.
The general structure of an ADC and the roles of the chemical trigger, the linker‒antibody attachment and the linker‒payload attachment.
2. Chemical triggers of the linker
The chemical triggers, which control the release of the payloads, are the most important part of linkers. The most serious challenge in their development is the undesired nonspecific release of payloads in normal tissues, which can lead to off-target toxicity. For instance, the dipeptide triggers, which are classical chemical triggers and employed in more than 40 ADCs in clinical use, can be cleaved by cathepsin. Cathepsin is nonspecifically expressed in all tissues, so when the ADCs are taken up into normal tissue with the targeted antigen expression, the dipeptide triggers are activated to release the toxic payloads, leading to adverse effects. To obtain higher selectivity, many novel triggers have been developed over the past 5 years. With current cleavage strategies the chemical triggers can be divided into cathepsin-cleavable triggers, acid-cleavable triggers, GSH-cleavable triggers, Fe(II)-cleavable trigger, novel enzyme-cleavable triggers, photo-responsive-cleavable triggers, and bioorthogonal cleavable triggers (Table 1). Among them, cathepsin-cleavable triggers, GSH-cleavable triggers, and acid-cleavable triggers have been well studied and employed in approved ADCs. Other novel cleavable triggers are designed for selective cleavage in cancer cells to decrease their off-target toxicity from nonspecific uptake. In addition, novel noncleavable linkers also have been introduced.
Table 1.
Chemical triggers described in this review.
| Chemical trigger | Structure | Mechanism | Payload | Ref. |
|---|---|---|---|---|
| Acid cleavable triggers | Hydrazone trigger | Linkers cleavage by low pH of tumor acidic microenvironment or lysosomes | Calicheamicin | 10 |
| Carbonate trigger | SN-38 | 27 | ||
| Silyl ether trigger | MMAE | 28 | ||
| GSH cleavable trigger | Disulfide trigger | Linkers cleavage by high level of GSH in cytoplasm | DM1, DM3, MMAE | 30 |
| PBD | 31 | |||
| Fe(II) cleavable trigger | 1,2,4-Trioxolane trigger | Linker cleavage by elevating levels of ferrous iron | MMAE | 34 |
| Cathepsin cleavable triggers | Dipeptide trigger | Linkers cleavage by cathepsin in lysosomes | MMAE, DM1 | 21 |
| Triglycyl (CX) trigger | DM1 | 22 | ||
| cBu-Cit trigger | MMAE, PBD | 20 | ||
| Glycosidase cleavable triggers | β-Glucuronide trigger | Linkers cleavage by β-glucuronidase in lysosomes | MMAE | 35 |
| β-Galactoside trigger | Linkers cleavage by β-galactosidase in lysosomes | MMAE | 36 | |
| Phosphatase cleavable triggers | Pyrophosphate trigger | Linkers cleavage by phosphatase and pyrophosphates in lysosomes | Budesonide | 18 |
| Sulfatase cleavable trigger | Arylsulfate trigger | Linkers cleavage by sulfatase in lysosomes | MMAE | 37 |
| Photo-responsive cleavable triggers | Heptamethine cyanine fluorophore trigger | Linkers cleavage by irradiation with NIR light (λ = 650–900 nm) | CA-4 | 46 |
| O-Nitrobenzyl trigger | Linkers cleavage by irradiation with UV light (λ = 365 nm) | MMAE | 47 | |
| PC4AP trigger | Linkers cleavage both by irradiation with near-infrared (NIR) light (λ = 365 nm) and intramolecular addition reaction with nearby amine | DOX | 48 | |
| Bioorthogonal cleavable trigger | dsProc trigger | Linkers cleavage by the bioorthogonal cleavage pair: Cu(I)-BTTAA/dsProc | DOX | 55 |
| Non-cleavable linkers | MD linker | No linker cleavage, ADCs metabolizes amino acid appendage, a linker and molecule cytotoxicity upon entry lysosome | TRMRA | 14 |
| PEG linkers with intermediates of alkyne, triazole and piperazine | PBD Dimer | 58 | ||
| Mal-PAB linker | MMAE | 59 |
2.1. Cathepsin cleavable triggers
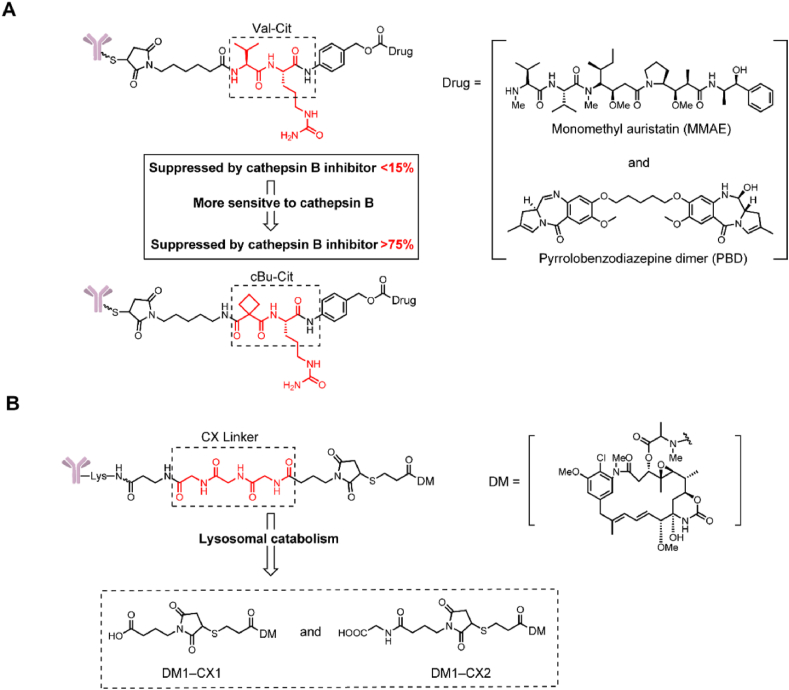
In 2017 Caculitan et al.19 discovered that the valine-citrulline (Val-Cit) linker exhibited widespread sensitivity to a variety of cathepsins, including cathepsin B, cathepsin K, cathepsin L, etc. This could be detrimental, as only cathepsin B is thought to be highly expressed in cancer cells, and the widespread sensitivity to other cathepsins could induce off-target toxicity in normal cells. Wei et al.20 designed a linker that used a cyclobutane-1,1-dicarboxamide (cBu) structure that was predominantly dependent on cathepsin B (Fig. 2A). In intracellular cleavage studies, drug release from cBu-Cit-containing linkers was efficiently suppressed by a cathepsin B inhibitor (over 75%), while a cathepsin K inhibitor did not have a significant effect. Conversely, the traditional Val-Cit-containing linker appeared strongly resistant to all single-protease inhibitors (inhibitors of cathepsins B, L, and K, all less than 15%). Meanwhile the cBu-Cit-containing linkers exhibited a maximum velocity/Michaelis constant (Vmax/Km) like that of the Val-Cit containing linker. Compared with Val-Cit linker-containing ADCs, cBu-Cit linker-containing ADCs exhibited equally potent antiproliferation effects in vitro, and both ADCs were efficacious in inhibiting tumor growth at the dose of 3 mg/kg, but the cBu-Cit linker-containing ADCs exhibited greater tumor suppression.
Figure 2.
Structures of cathepsin-cleavable triggers. (A) The structure of the cBu-Cit-PABC-containing ADCs. Adapted with modification from Ref. 19 © 2017 American Association for Cancer Research. (B) The structure of CX-containing ADCs and catabolites expected from lysosomal proteolysis. Adapted with modification from Ref. 21 © 2016 American Association for Cancer Research.
In 2016 Dorywalska et al.21 confirmed that carboxylesterase 1C (Ces1C) is the enzyme that leads to the instability of Val-containing peptide linkers in mouse plasma. The Val-Cit-containing ADC was highly stable in Ces1C-knockout mice. Based on the versatility and importance of xenograft mouse models in ADC preclinical research, it is important to design peptide linkers that are stable in mouse models. Singh et al. designed a triglycyl peptide linker (CX) for ADCs with maytansinoid (DM1) as the payload (Fig. 2B)22. This linker consisted of three glycyl residues and showed extremely high stability in mouse plasma. In a pharmacokinetics study, the CX-DM1-containing ADCs exhibited stability comparable to that of SMCC-DM1-containing ADCs; for instance, half-life (t1/2) values of 9.9 vs. 10.4 days, clearance (CL) values of 0.7 vs. 0.7 mL/h/kg, and area under the curve (AUC0→∞) values of 15,225 vs. 14,370 h·mg/mL, respectively. Furthermore, the in vitro cytotoxicity of the CX linker-containing ADCs was significantly improved compared with that of the SMCC-DM1-based ADCs. Similar data were obtained in the in vivo experiments; the CX-DM1-containing ADCs, even at 3 mg/kg, were more active than a 15 mg/kg dose of the SMCC-DM1 ADCs. Surprisingly, the CX-DM1-containing ADCs possessed higher in vivo activity and a 50-fold higher preclinical therapeutic index [maximum tolerated dose/minimum effective dose (MTD/MED) ratio] than SMCC-DM1-containing ADCs in both the EGFR and EpCAM xenograft mouse models.
The optimization of peptide linkers is not limited to developing novel structures. Peptide linkers can be optimized by minimal structural changes, including the types and stereochemistry of the amino acids. Our group demonstrated that valine-alanine (Val-Ala) has better hydrophilicity and stability than Val-Cit23. While the two ADCs had an average DAR of approximately 7, the Val-Ala-based ADCs had no obvious increase in the dimeric peak. However, the aggregation of Val-Cit-based ADCs increased to 1.80%. In stereochemical studies, Reid et al.24 and Salomon et al.25 both indicated that ADCs containing an (l,l) dipeptide linker showed higher antitumor activity in vitro and in vivo than other amino acid configurations.
2.2. Acid-cleavable triggers
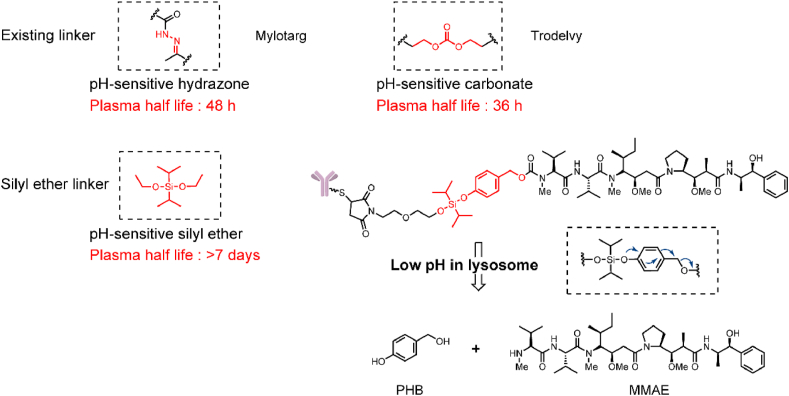
Acid-cleavable linkers utilize the pH difference between tumor tissue (4.0–5.0) and plasma (∼7.4) to selectively release payloads into tumor tissues26. This strategy yielded the earliest clinical success with Mylotarg and was later employed in Besponsa2,10. However, the insufficient stability of acid-cleavable linkers severely limits their application in ADCs, and a phenylketone-derived hydrazone linker was hydrolyzed with a t1/2 = 2 days in human and mouse plasma. The serum stability of the Sacituzumab govitecan (Trodelvy), which contains acid-cleavable carbonate linkers, was also unsatisfactory with a t1/2 = 36 h27. Accordingly, acid-cleavable ADCs require more stable linkers or must employ only moderately cytotoxic payloads. In 2019, our group developed a novel silyl ether-based acid-cleavable ADC carrying highly cytotoxic monomethyl auristatin E (MMAE, Fig. 3)28. This design greatly improved the stability of the acid-cleavable linker and should be sufficient to support acid-cleavable ADCs containing highly cytotoxic payloads. Compared with the traditional hydrazine linker (t1/2 = 2 days) and carbonate linker (t1/2 = 36 h), the t1/2 of the novel silyl ether linker-MMAE conjugate was more than 7 days in human plasma. This novel ADC containing a silyl ether linker possessed strong cell inhibitory activity (HER2+ cell lines, IC50 = 0.028–0.170 nmol/L) and exhibited a better therapeutic effect than monoclonal antibodies in a mouse xenograft model.
Figure 3.
Approved acid-cleavable linker-containing ADCs and the structure of silyl ether-containing ADC. Adapted with modification from Ref. 28 © 2019 Multidisciplinary Digital Publishing Institute.
2.3. Glutathione (GSH)-cleavable triggers
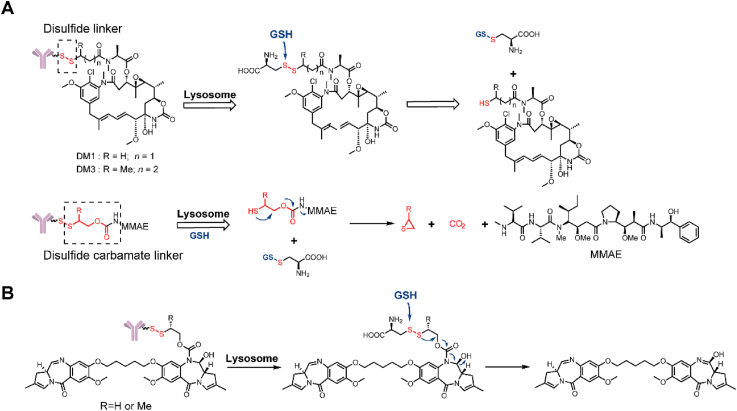
Glutathione (GSH)-cleavable triggers rely on the higher level of glutathione in the cytoplasm (1–10 mmol/L) compared to the blood plasma (∼5 μmol/L)29. Disulfide bonds are most commonly used in these triggers. However, the present disulfide bond constructs cannot achieve a perfect combination of high circulatory stability and efficient intracellular release. In 2017, Thomas et al.30 tried to solve this problem by attaching the small molecule drug directly to engineered cysteines in a THIOMAB antibody (Fig. 4A). By connecting directly to the antibody, a steric protection from the antibody would increase the circulatory stability. Firstly, by screening sites for conjugation, they identified that LC-K149C as a stable conjugation site for disulfide. In vivo stability study showed that when DM1 was attached through a disulfide to K149C, more than 50% of the drug remained attached even after seven days. An in vivo efficacy study showed that this novel anti-CD22-DM1-ADC could induce tumor regression at a single dose of 3 mg/kg in a human lymphoma tumor xenograft mouse model. Furthermore, this novel Cys-linked disulfide-conjugation strategy could also be applied to non-thiol payloads, such as MMAE, by inserting a self-cleaving disulfide linker. In the same year, this novel strategy was used to prepare ADCs armed with a PBD as a payload (Fig. 4B)31. Compared with the maleimide peptide (Val-Cit)-PBD-ADC, the novel disulfide ADC exhibited similar activity at several doses in a human non-Hodgkin lymphoma tumor xenograft mouse model. At the same time, this novel disulfide-ADC had a higher MTD than that of a Val-Cit-ADC (10 vs. 2.5 mg/kg). In conclusion, these results demonstrate the potential for novel linkers to improve the biophysical properties and increase the therapeutic index of ADCs.
Figure 4.
Structures of GSH-cleavable triggers. (A) The structure and release mechanism of an ADC containing a disulfide linker and a disulfide-carbamate linker. Adapted with modification from Ref. 30 © 2017 Royal Society of Chemistry. (B) The structure and release mechanism of an ADC containing a disulfide-carbamate linker with a PBD-dimer. Adapted with modification from Ref. 31 © 2017 American Association for Cancer Research.
2.4. Fe(II) cleavable trigger
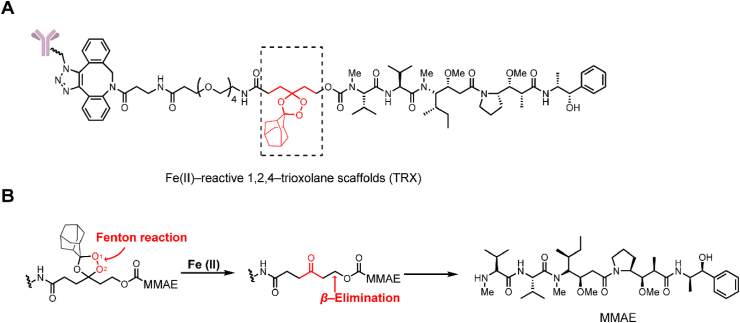
Abnormal iron metabolism can elevate the levels of unbound ferrous iron32. Based on this strategy, increasing the unbound ferrous iron concentration has been utilized in prodrug design33. In 2018, Spangler et al.34 reported an Fe(II)-reactive 1,2,4-trioxolane scaffold (TRX) linker and initially employed this cleavage method with ADCs (Fig. 5A). The linker was cleaved by a Fenton reaction between the O–O bond of TRX and Fe(II), affording a carbonyl intermediate and releasing the payload via β-elimination (Fig. 5B). In an in vitro cytotoxicity study, the TRX linker-containing ADCs demonstrated activity in antigen positive cells (EC50 = 0.07 nmol/L) similar to that of classic Val-Cit linker-containing ADCs. However, the TRX linker-containing ADCs still showed significant toxicity (EC50 = 0.61 nmol/L) in the antigen-negative MDA-MB-468 cell lines. This instability was caused by the nonspecific interaction between the adamantane moiety and the nearby sites on the antibody, resulting in trioxolane heterolytic ring cleavage. The researchers intended to avoid this reaction in the ADCs by the addition of inert polyethylene glycol (PEG) spacers between the antibody and adamantane.
Figure 5.
Structures of Fe(Ⅱ)-cleavable trigger. (A) The structure of the Fe(II)-reactive (TRX) linker-containing ADC. Adapted with modification from Ref. 34 © 2018 American Chemical Society. (B) Release mechanism of Fe(II)-cleavable linker-containing ADCs.
2.5. Novel enzyme-cleavable triggers
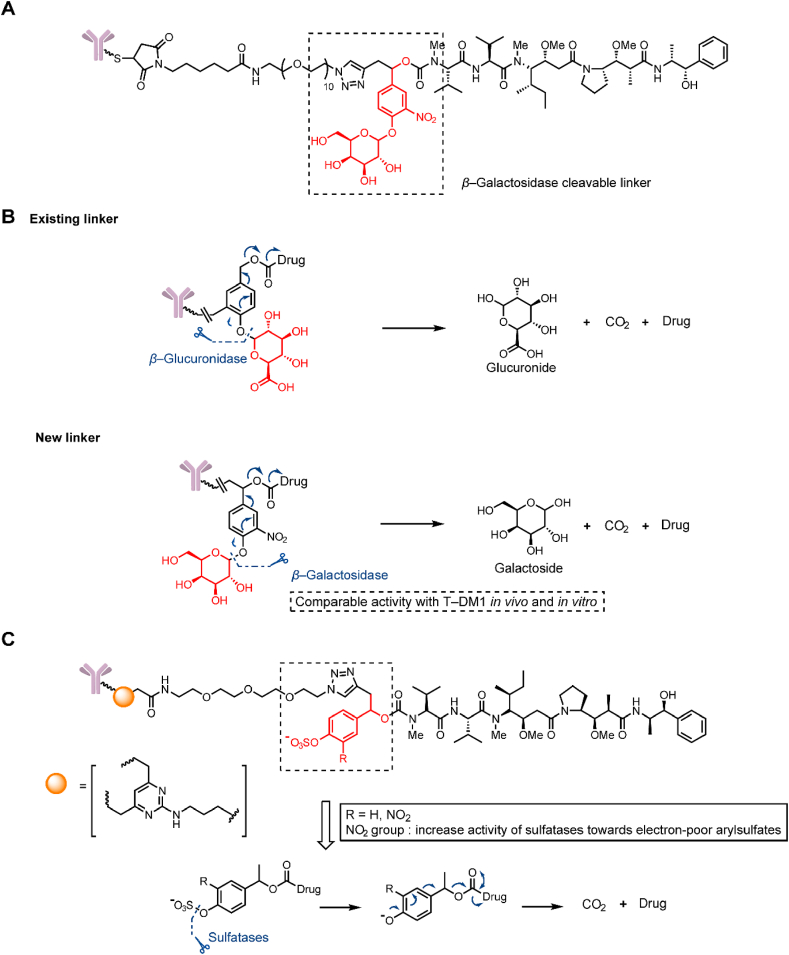
In addition to the classic β-glucuronidase-cleavable linkers that were developed for ADCs in 200635, β-galactosidase was discovered to be overexpressed in tumor cells and possess hydrolytic activity. In 2017, Kolodych et al.36 described a β-galactosidase-cleavable linker-containing ADCs (Fig. 6A). The ADCs containing this β-galactosidase-cleavable linker was rapidly hydrolyzed in vitro at 10 U/mL β-galactosidase (Fig. 6B). The ADC comprising trastuzumab and MMAE via this linker exhibited a lower IC50 (8.8 pmol/L) than that of ADC containing a Val-Cit linker (14.3 pmol/L) and Kadcyla (33 pmol/L). Equivalent results were obtained with in vivo experiments; ADCs containing the β-galactosidase-cleavable linker exhibited a 57% and 58% reduction in tumor volumes in a xenograft mouse model at a single dose of 1 mg/kg, but the efficiency of Kadcyla was not statistically significant at the same dose. In addition, the novel ADCs showed a higher reduction of tumor growth than Kadcyla through a Principal Components Analysis (PCA).
Figure 6.
Structures of glycosidase- and sulfatase-cleavable triggers. (A) The structure of a β-glucuronidase-cleavable, linker-containing ADC. Adapted with modification from Ref. 36 © 2017 Elsevier. (B) Release mechanism of β-glucuronidase and β-glucuronidase-cleavable linker-containing ADCs. (C) The structure and release mechanism of sulfatase-cleavable linker-containing ADCs. Adapted with modification from Ref. 37 © 2020 The Royal Society of Chemistry.
In 2020, a sulfatase-cleavable linker was described by Bargh et al.37 (Fig. 6C). Sulfatase, which is analogous to β-galactosidase, is a hydrolytic enzyme overexpressed in tumor cells. The sulfatase-cleavable linker exhibited definite susceptibility to sulfatase enzymes (t1/2 = 24 min) in a release study. In mouse plasma, compared with Val-Ala and Val-Cit linker conjugates hydrolyzed within 1 h, sulfatase-cleavable linker conjugates demonstrated high plasma stability (over 7 days). Compared with the in vitro cytotoxicity of noncleavable ADCs (IC50 = 609 pmol/L) and Val-Ala containing ADCs (IC50 = 92 pmol/L), sulfatase-linker-containing ADCs exhibited higher cytotoxicity (IC50 = 61 and 111 pmol/L) and a superior selectivity in HER2+ cells.
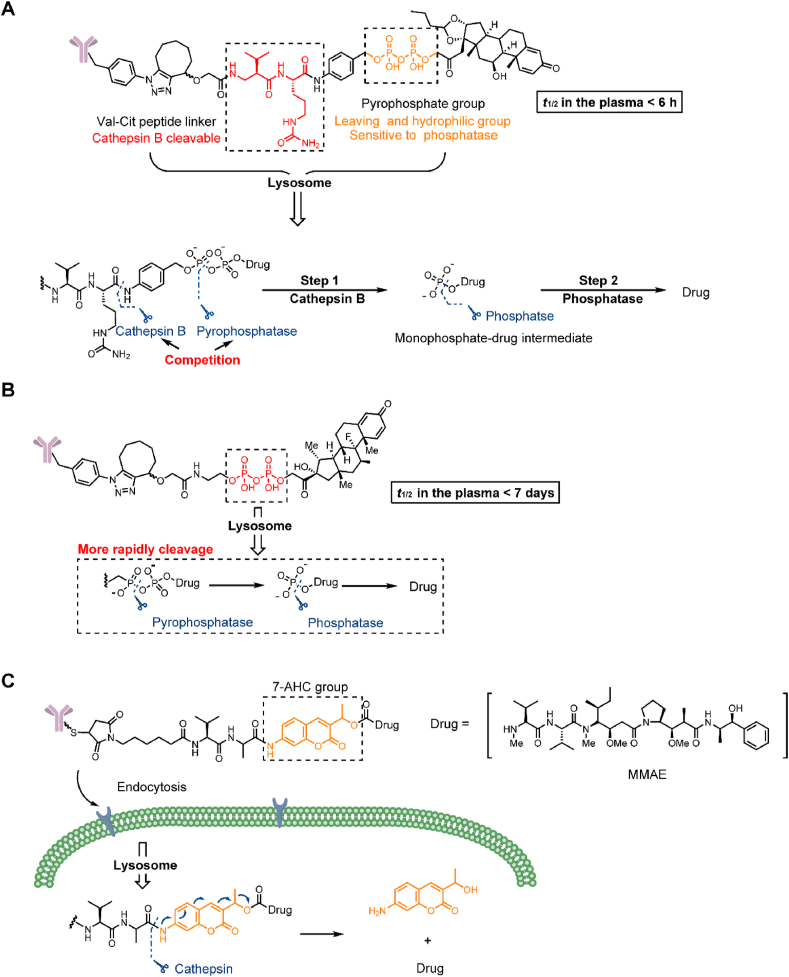
Phosphate and pyrophosphate groups can markedly improve the hydrophilicity of linkers and can be employed to load lipotropic payloads. In 2016, Kern et al.38 employed a terminal phosphate/pyrophosphate as both a leaving group and hydrophilic group for the Val-Cit-p-aminobenzyloxycarbonyl (PAB) linker with the highly lipophilic glucocorticoid budesonide (Fig. 7A). However, the t1/2 in the plasma was less than 6 h, which failed to meet the stability requirement of ADCs. Subsequently, budesonide phosphate could be detected, suggesting that payload release may occur in two steps by cathepsin and phosphatase. The double sensitivity to cathepsin and phosphatase may be responsible for the instability of the linker. While the specific hydrolysis mechanism was not confirmed, it was proven that the phosphate/pyrophosphate structure had the potential to be a novel linker. In the same year, Kern et al.18 replaced the traditional Val-Cit-PAB linker with a phosphate diester structure and synthesized a series of linkers based on the structure of monophosphate, pyrophosphate and triphosphate diesters (Fig. 7B). Compared with the previous linker, the pyrophosphate linker showed extremely high stability over 7 days in mouse and human plasma stabilization experiments. Moreover, the high hydrophilicity of the linker was retained and was able to mitigate the aggregation potential of the ADC with other lipophilic payloads (the solubility of the pyrophosphate diester linker drug is greater than 5 mg/mL). In an in vitro evaluation, ADCs containing pyrophosphate and triphosphate diester linkers are cleaved much more rapidly than monophosphate diesters. According to the analysis of the metabolites of pyrophosphate diester linkers, the ADCs were first hydrolyzed into a monophosphate-payload metabolite and then rapidly produced a prototype drug. The combination of two enzymes may lead to rapid payload release.
Figure 7.
Structures of a pyrophosphate-cleavable trigger and novel leaving group. (A) The structure and release mechanism of Val-Cit-PAB-pyrophosphate linker-containing ADCs. Adapted with modification from Ref. 38 © 2016 American Chemical Society. (B) The structure and release mechanism of pyrophosphate linker-containing ADCs. Adapted with modification from Ref. 39 © 2016 American Chemical Society. (C) The structure and release mechanism of Val-Ala-AHC cleavable linker-containing ADCs. Adapted with modification from Ref. 42 © 2020 Ivyspring International.
Additionally, the cleavable chemical trigger and payload are connected via a self-eliminating leaving group, with the PAB group as the current structure39,40. More recently, our group developed a 7-amino-3-hydroxyethyl-coumarin (7-AHC) group as a potent alternative to PAB, which has been employed in a dipeptide linker (Fig. 7C)41. 7-AHC, as a bifunctional fluorescent group, can allow self-elimination cleavage in the presence of cathepsin B for payload release and fluorophore activation. Rapid payload release was observed within 1 h by the 48-fold enhancement in fluorescence. Importantly, the 7-AHC group retained the traditional advantages of a self-eliminating leaving group. ADCs containing a 7-AHC-based dipeptide linker exhibited good stability (t1/2 > 7 days) and high activity in vitro (IC50 = 0.09–3.74 nmol/L). In a classic breast cancer model, an ADC containing the novel linker induced tumor regression at a dose of 1.5 mg/kg and exhibited antitumor efficacy equivalent to that of the marketed Kadcyla.
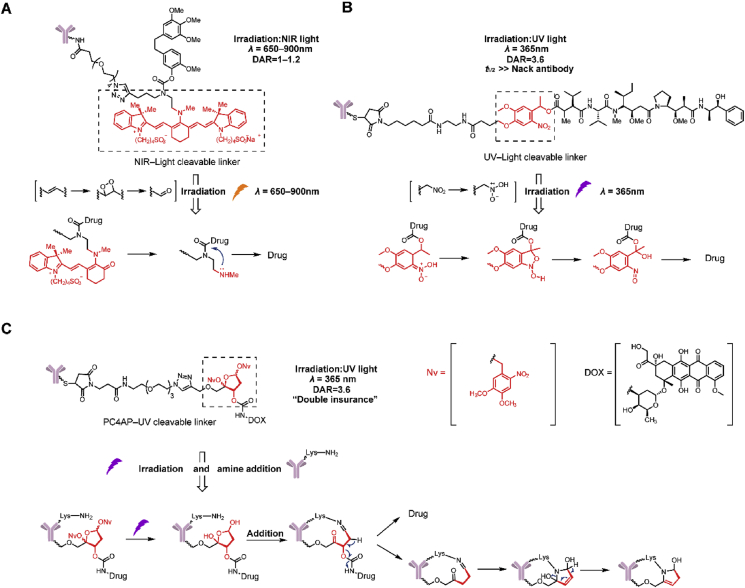
2.6. Photo-responsive cleavable triggers
The strategy of payload release based on photo-responsive cleavable triggers has gradually emerged in recent years42, 43, 44, 45. Photo-responsive cleavable triggers have the following advantages: (1) controlled drug release and effective reduction in off-target toxicity and (2) determination of the cleavage mechanism and no limitation on the intracellular release. Photo-responsive cleavable triggers enjoy the following advantages, including low toxicity, a rapid response, and high sensitivity and specificity.
In 2015, Nani et al.46 firstly employed a near-infrared (NIR) light photo-caging strategy for ADCs (Fig. 8A). The photo-responsive cleavable trigger was based on a heptamethine cyanine fluorophore scaffold. Upon irradiation with NIR light (λ = 650–900 nm), the ADCs effectively released the small molecule cytotoxin CA-4 in the irradiated tumor areas in a site-specific manner. In a stability study, the linker without NIR light in human plasma at 37 °C led to minimal release of CA-4 (<1%) after 72 h. In an in vitro cytotoxicity experiment, ADCs containing the NIR light-cleavable linker exhibited activity (IC50 = 16 nmol/L) equivalent to CA-4 in an EGFR+ cell line upon irradiation and low activity (IC50 = 1.1 μmol/L) without irradiation. However, the self-aggregation and photo-unstable characteristics of this linker limit its applications in biological research and further development as a drug.
Figure 8.
Structures of photo-responsive cleavable triggers. (A) The structure and release of NIR-cleavable linker-containing ADCs. Adapted with modification from Ref. 47 © 2015 WILEY. (B) The structure and release mechanism of UV-cleavable linker-containing ADCs. Adapted with modification from Ref. 48 © 2020 Elsevier. (C) The structure and release mechanism of PC4AP-UV cleavable linker-containing ADCs. Adapted with modification from Ref. 49 © 2019 The Royal Society of Chemistry.
More recently, our group initially reported a novel ultraviolet (UV) light-controlled ADC (Fig. 8B)47. The linkers introduced a UV light-controlled O-nitrobenzyl group as a chemical trigger. In stability and release studies, this linker containing MMAE released <1% under natural light over 6 days and showed the rapid release of MMAE that reached the highest plateau within 10 min upon irradiation. In an in vitro cytotoxicity experiment, after irradiation with 365 nm (40 W) UV light, the activities of the O-nitrobenzyl linker-containing ADCs were greatly increased (both EC50 = 0.04 nmol/L), and were 50-fold higher than that of unirradiated ADCs. From in vivo imaging experiments, it was observed that the O-nitrobenzyl linker-containing ADCs have half-lives almost equal to naked antibodies, maintaining the advantage of a long half-life.
In 2019, Zang et al.48 reported a photo-responsive, self-cleaving linker employing a photo-caged C40-oxidized abasic site (PC4AP). Compared with the 2 ADCs mentioned, this linker has “double insurance” within a single chemical trigger by design. Upon irradiation at 365 nm, the hydroxyl group of PC4AP undergoes an intramolecular addition reaction with a nearby amine on its own antibody, and a subsequent elimination reaction leads to cleavage and payload release (Fig. 8C). In the release study, it was confirmed that payloads can be completely released after incubation for 1 h in the presence of both N-terminal amines and light, and could not be released under a single light condition. Furthermore, Mal-PC4AP-doxorubicin (DOX) exhibited no cytotoxicity under light conditions in the SK-BR-3 cell line, which coincided with the results of the release study. The peptide-PC4AP-DOX-containing ADC showed toxicity equivalent to the payload DOX in positive cells and no cytotoxicity without irradiation.
Near infrared light (λ = 650–900 nm)-controlled cleavage of ADCs faces the problems of complex structure, self-aggregation, photo-instability, and unfavorable pharmacokinetics in vivo49,50. For UV (λ = 365 nm)-controlled ADCs, a high dosage of UV is toxic and can lead to oxidative stress, photoaging and immunosuppression51,52. Moreover, UV blue light cannot penetrate patient skin to reach deeply into the tumor area. Only depths of about 100 μm can be reached51,52.
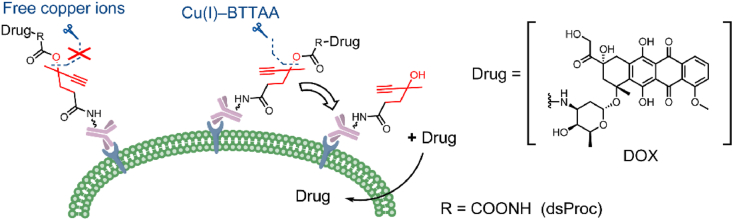
2.7. Bioorthogonal cleavable triggers
Bioorthogonal chemistry refers to a chemical reaction that can occur in the body without interfering with normal biological processes, featuring high selectivity, fast and simple processing and nontoxic byproducts. Therefore, bioorthogonal cleavage pairs are suitable as cleavable triggers53,54. In 2019, Wang et al.55 developed a bioorthogonal cleavable linker that employed the classical bioorthogonal cleavage pairs, Cu(I)-2-[4-[[bis[(1-tert-butyltriazol-4-yl)methyl]amino]methyl]triazol-1-yl]acetic acid (BTTAA) and dual-substituted propargyloxycarbonyl (dsProc). The bioorthogonal cleavable trigger-containing ADCs release payloads at the cancer cell surface (Fig. 9). Although free copper ions are widely distributed, it has been confirmed that dsProc has high selectivity and cleavage reactivity only to Cu(I)-BTTAA. In the in vitro toxicity experiments, it was shown that the addition of 50 μmol/L Cu(I)-BTTAA decreased the IC50 of DOX-dsProc-containing ADCs by 120-fold. While these linkers expanded the cleavage mechanism, they may not be an optimal linker for payloads with poor membrane permeability due to extracellular release.
Figure 9.
The structures and release mechanism of biorthogonal-cleavable linker-containing ADCs. Adapted with modification from Ref. 56 © 2019 American Chemical Society.
The study of bioorthogonal-cleavage triggers developed recently and has focused on in vitro exploration. Problems remain regarding reaction efficiency, reaction rate, substrate stability, biocompatibility and operation convenience. At present, they cannot be applied in vivo, and are far from clinical application.
2.8. Novel noncleavable linkers
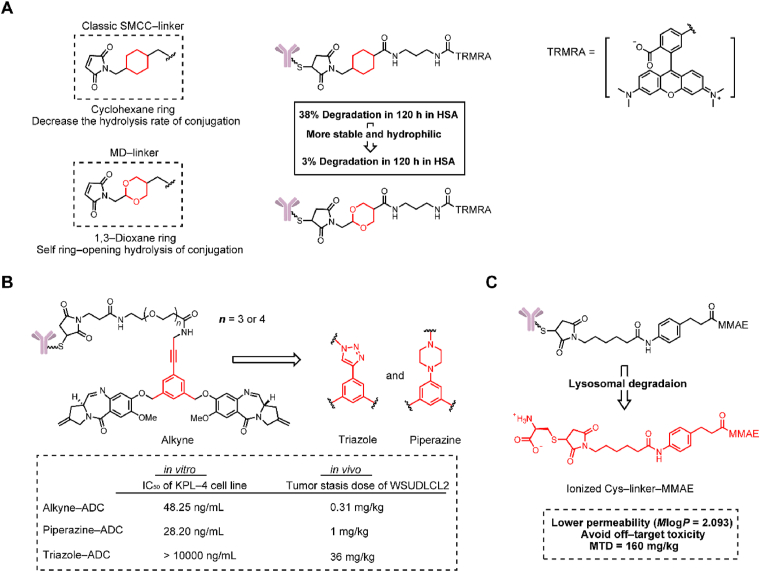
Differing from cleavable linkers, noncleavable linkers have no structural chemical trigger for payload release. Therefore, the active part of a noncleavable ADC is comprised of an amino acid appendage, a linker and a cytotoxic payload56. A fundamental requirement for noncleavable linkers is that they can not reduce the activity of the payload.
SMCC is a classic noncleavable linker that has been employed in Kadcyla. However, SMCC-based conjugates are still limited due to their instability in the circulatory system and hydrophobic properties12. In 2016, Igor Dovgan et al.14 presented 2-(maleimidomethyl)-1,3-dioxane (MD) as a potent alternative to the classical SMCC linker (Fig. 10A). Replacing the cyclohexane ring with 1,3-dioxane, the two intracyclic oxygen atoms increased the hydrophilicity of the novel noncleavable linker. Despite the presence of an acetal moiety, the MD-based linker was remarkably stable even at pH = 0. In a human plasma stability study, MD-linker fluorescence conjugates exhibited four times lower fluorescence than the SMCC linker fluorescence conjugates at 72 h. Different from the SMCC linker, the succinimidyl ring in the MD linkers underwent fast self-stabilization by ring-opening hydrolysis to avoid a retro-Michael reaction. In another stability study, the MD linker-containing ADCs showed only 3% degradation in 120 h compared to 38% degradation of the same SMCC-containing ADCs. Subsequently, Tobaldi et al.57 further explored this novel strategy. By changing the size of the acetal ring and the length of the carbon chain between the acetal and succinimidyl moieties, it was proven that increasing the size of the acetal ring and the length of the carbon chain exhibited better succinimidyl ring-opening kinetics. In general, the length of the carbon chain was the major determinant for achieving self-stabilization.
Figure 10.
Structures of non-cleavable linkers. (A) The structure of MD non-cleavable linker-containing ADCs. Adapted with modification from Ref. 14 © 2016 Nature Publishing Group. (B) The structure of noncleavable ADCs containing an alkyne, a triazole and a piperazine group, respectively. Adapted with modification from Ref. 59 © 2017 American Chemical Society. (C) The structure of noncleavable linker-MMAE-containing ADCs. Adapted with modification from Ref. 60 © 2020 Multidisciplinary Digital Publishing Institute.
In 2017, Gregson et al.58 demonstrated three highly hydrophilic noncleavable linkers synthesized by iodoaryl intermediates to connect pyrrolobenzodiazepine (PBD) dimers (Fig. 10B). This noncleavable linker was constructed with hydrophilic PEG chains including an alkyne, a triazole and a piperazine coupled to the PBD dimer through aryl groups. In contrast to the other two linkers, ADCs containing a triazole were completely inactive in the HER2/3+ KPL-4 cell line in vitro. Equivalent results were also reflected in the in vivo experiment. ADCs containing an alkyne and a piperazine achieved tumor suppression in the WSUDLCL2 lymphoma xenograft model at similar doses (0.31 and 1 mg/kg, respectively), while ADCs containing a triazole needed a dose of 36 mg/kg. Interestingly, these linkers were only distinguishable by the three slightly different functional groups, but their activity was very different. This research suggested that PEG linkers containing alkynes or piperazine would be noncleavable linkers with potential applications.
Without bystander effects, an advantage of the noncleavable linkers is to lower off-target toxicity in normal tissues. Based on this, our group developed a noncleavable ADC with MMAE as the payload, which could broaden the therapeutic window of MMAE-based ADCs (Fig. 10C)59. The active part of the noncleavable ADC (l-cysteine (Cys)-linker-MMAE) not only exhibited similar cytotoxicity to that of MMAE (IC50: 10−11 mol/L), but also reduced toxicity in the bystander effect test. We thought that the low permeability (MlogP = 2.093) helped to avoid off-target toxicity. In the xenograft mouse model, the noncleavable ADC showed significant antitumor activity at a dose of 2.5 mg/kg with a MTD reaching 160 mg/kg, which is almost twofold that of the Val-Ala linker containing ADC.
3. Linker‒antibody attachments of the linker
Linker‒antibody attachments act as a bridge connecting the linker and antibody. At present, two main challenges remain for the linker‒antibody attachment. The first one is the retro-Michael elimination of the classical maleimide attachment, which can eventually lead to off-target toxicity. The optimization in the chemical structures over the last 5 years are reviewed here. The second challenge is the heterogeneous DAR values. Essentially, the approved ADCs are mixtures of different DAR values. Developing a homogeneous ADC has always been the goal. Currently there are 2 strategies: site-specific conjugation technology and chemical structural modification of the linker‒antibody attachment. Site-specific conjugation through antibody engineering is extensively reviewed elsewhere60, 61, 62.
3.1. Maleimide attachment
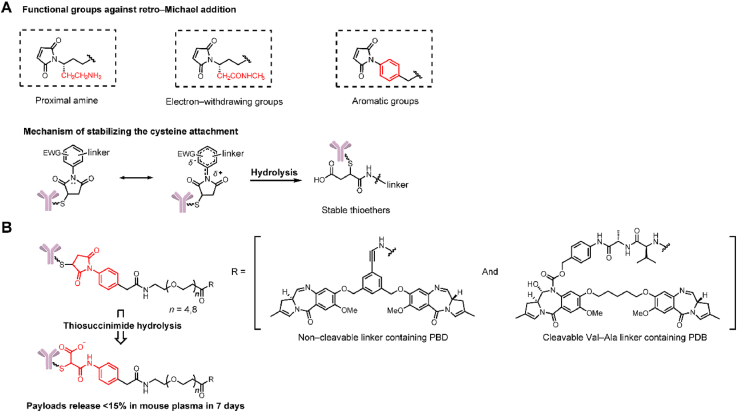
Maleimide structure conjugation has been widely used in ADCs and exhibits the inherent advantages of fast reaction kinetics and excellent thiol specificity. However, thiol-maleimide coupling is susceptible to retro-Michael reaction, which leads to the instability of ADCs in the circulatory system and eventually a low therapeutic index63.
As early as 2014, Lyon et al.64 found that the problem of retro-Michael elimination could be chemically solved by the self-hydrolysis of thiosuccinimide. In 2015, Christie et al.65 and Fontaine et al.66 developed several modified functional groups attached to the maleimide ring to stabilize cysteine conjugation, such as proximal amines and electron-withdrawing groups (Fig. 11A). But we think the real breakthrough work might belong to Christie et al.67 In 2017, their group described a N-phenyl maleimide attachment containing linkers (noncleavable and Val-Ala linkers, Fig. 11B), which could stabilize thiol conjugation through rapid thiosuccinimide hydrolysis. Compared with N-alkyl maleimide attachment employing the same linkers, the noncleavable ADCs containing N-phenyl maleimide exhibited higher conjugation stability in mouse plasma over 7 days (payload release 15% vs. 45%). Similarly for the N-phenyl maleimide-Val-Cit linker, ADCs containing N-phenyl maleimide retain over 90% conjugation. In contrast, ADCs containing N-alkyl maleimide retain only 65% conjugation. N-phenyl maleimide-containing ADCs exhibited toxicities equivalent to those of N-alkyl ADCs in antigen positive cell lines in in vitro cytotoxicity analysis. In the xenograft mouse model, ADCs containing a noncleavable linker with N-phenyl maleimide attachment had better antitumor activity in vivo than ADCs containing N-alkyl maleimide. The former arrested tumor growth at a dose of 1 mg/kg, while the latter exhibited no antitumor activity at the same dose67.
Figure 11.
Structures of the optimized maleimide attachment. (A) The structure and mechanism of optimized maleimide attachment by proximal amines and electron-withdrawing groups.(B) The structure and mechanism of optimized maleimide attachment by N-phenyl maleimide. Adapted with modification from Ref. 68 © 2017 Multidisciplinary Digital Publishing Institute.
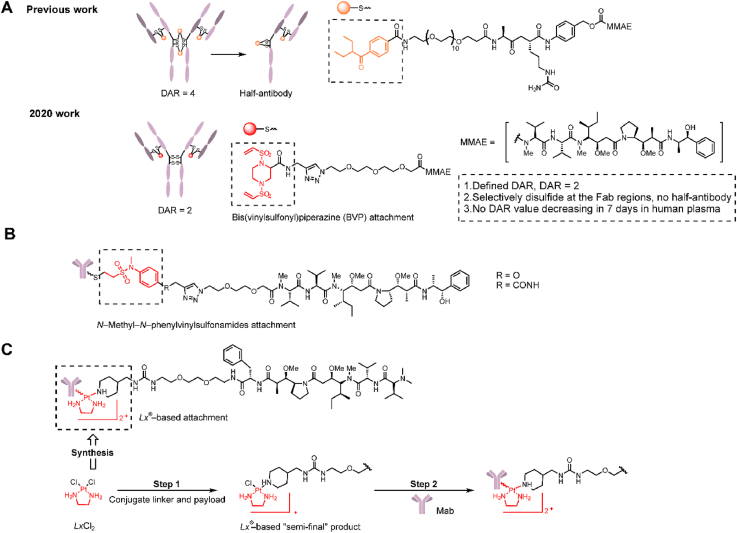
3.2. Bis(vinylsulfonyl)piperazine attachment
As early as 2006, Shaunak et al.68 developed a 3-carbon bridge through bisulfones to cross-link two cysteine residues. Subsequently in 2014, Badescu et al.69 successfully applied this attachment to design and synthesize a homogeneous and stable ADC with a DAR = 4. However, this method commonly led to the production of half-antibodies by conjugation in hinge regions of the monoclonal antibody (Fig. 12A). In 2020, Huang et al.70 continued to develop a novel bis(vinylsulfonyl)piperazine (BVP) linker for the selective conjugation of disulfides mostly in the Fab regions (Fig. 12A). Compared with previous work, this structure could efficiently avoid the formation of a half-antibody71 and facilitate the construction of highly homogeneous ADCs with a DAR = 2. In a stability experiment the BVP conjugates maintained high stability without a decrease in DAR after 7 days at 37 °C in human plasma. In an in vitro cytotoxicity study, a BVP attachment-containing ADC in HER2 negative MDA-MB-231 cells exhibited far lower toxicity than Kadcyla (>500 vs. 51.5 ± 15.7 nmol/L), and these differences in toxicity in antigen-negative cells suggested that the ADC containing BVP conjugation had a lower off-target toxicity.
Figure 12.
Structures of novel linker‒antibody attachments. (A) “Re-bridge” strategy and the structure of BVP conjugation-containing ADCs. Adapted with modification from Ref. 71 © 2020 Elsevier. (B) The structure of N-methyl-N-phenylvinylsulfonamides attachment-containing ADCs. Adapted with modification from Ref. 73 © 2018 American Chemical Society. (C) The structure and synthesis of Lx® attachment-containing ADCs. Adapted with modification from Ref. 75 © 2016 American Association for Cancer Research.
3.3. N-methyl-N-phenylvinylsulfonamide attachment
In addition to modifying the existing maleimide conjugation, Huang et al.72 introduced N-methyl-N-phenylvinylsulfonamide for cysteine-selective conjugation to prevent the retro-Michael reaction (Fig. 12B). N-Methyl-N-phenylvinylsulfonamide conjugation exhibited high stability after 72 h in the presence of the thiol nucleophile glutathione. ADCs containing this conjugation could be defined as a DAR = 8.
3.4. Pt(Ⅱ)-based attachment
Waalboer et al.73 first proposed the conjugation of histidine onto trastuzumab with platinum(II) in 2015 and explored the preliminary stability of platinum(II) conjugates. Subsequently, in 2016, Sijbrandi et al.74 developed a metal-organic [ethylenediamine platinum(II)]2+ linker, termed Lx®, and further constructed trastuzumab-Lx-desferoxamine (DFO)/monomethyl auristatin F (MMAF) ADCs (Fig. 12C). Because histidine is ubiquitous in antibodies and the simple two-step operation, Lx® conjugation has versatile applicability in ADCs. The advantages of this type of conjugation are mainly exhibited in good manufacturability and high safety. The metabolite Lx-MMAF was supposed to be the main candidate for the off-target toxicity for this ADC. Its toxicity was 103- to 104-fold lower than the corresponding ADC and was 102- to 103-fold lower than Mal-MMAF in an in vitro toxicity evaluation. Low off-target toxicity is also verified in in vivo experiments, and trastuzumab-Lx-MMAF at a high dose of 60 mg/kg exhibited good tolerance. Furthermore, trastuzumab-Lx-MMAF at a dose of 15 mg/kg exhibited a better therapeutic effect than Kadcyla in a mouse xenograft tumor model. While the conjugation efficiency with this metal-organic linker to histidines was strongly improved recently75, the same linker can also be used for site-specific coupling to cysteines76.
4. Linker‒payload attachments of the linker
Only limited linker‒payload attachments are employed in traditional ADCs, such as carbamate attachment and carbonate attachment8,11. However, with the rapid expansion of payload arsenal, the existing linker‒payload attachments can not meet the requirements. There are two strategies that have been proposed to solve this problem: (1) modification of the linker to adapt to the payload and (2) adjustment of the payload to the linker. The first strategy seems more reasonable. On the one hand, modification of the payload runs the risk of decreasing activity. For instance, transformation of the hydroxyl of tubulysin to carboxylic acid was found to decrease the activity by 5‒23-fold77. On the other hand, a modified linker has more versatile applicability.
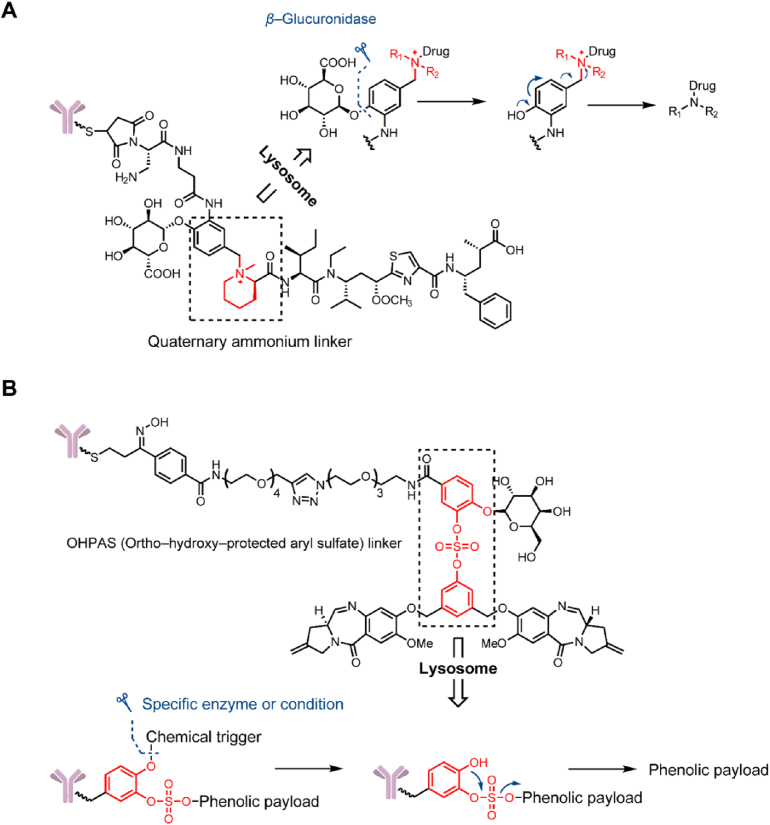
In 2016, Burke et al.78 proposed a quaternary ammonium attachment for connecting payloads with a tertiary amine structure and applied this linker to glucuronide-auristatin E (glucQ-AE, Fig. 13A). In a stability study, the quaternary ammonium-based glucQ-AE linker remained stable in mouse plasma for 10 days, which is equivalent to carbamate-based gluc-MMAE. In an in vitro cell proliferation inhibition experiment, the glucQ-AE conjugate had 1.5‒4-fold higher activity than the gluc-MMAE conjugate. Therefore, the application of quaternary ammonium attachment could effectively connect payloads with a tertiary amine.
Figure 13.
Structures of novel linker‒payload attachments. (A) Structures of tertiary amine attachments. Adapted with modification from Ref. 79 © 2016 American Association for Cancer Research. (B) Structures of OHPAS attachments. Adapted with modification from Ref. 83 © 2019 American Chemical Society.
Hydroxyls, particularly phenols, are far more prevalent in known payloads than amine moieties, such as α-amanitin, tubulysin B, and PNU-15968279, 80, 81. In addition to the conversion of a phenol functional group into an amine or other structure, there have been developments in novel stable linker‒payload connecting strategies. In 2019, Park et al.82 developed an ortho-hydroxy-protected aryl sulfate (OHPAS) attachment for payloads containing an aromatic-OH based on highly stable diaryl sulfate structures (Fig. 13B). Structurally, one aryl was loaded with the payload, and the other was equipped with a chemical trigger. In stability studies, the linker employing a chemical trigger of β-galactoside showed stability for 7 days in both PBS and plasma. To further confirm the versatile applications of the OHPAS attachment-containing linker, the researchers synthesized β-galactoside-OHPAS attachment-containing ADCs loaded with 8 payloads, and these ADCs were tested for their activity in vitro. The ADCs exhibited IC50 values that were equivalent to or even higher than that of Kadcyla in antigen-positive cell lines. Similar results were also observed in in vivo studies; in the amanitin-containing ADC treatment group, the tumor completely resolved from Day 10 to Day 60 after administration (2 and 0.5 mg/kg). In addition to containing different payloads, researchers have tried to employ multiple triggering parts, such as β-galactosidase, β-glucuronide, and levulinate esters, in OHPAS linkers to identify the practical value of this attachment.
5. Absorption, distribution, metabolism, and excretion (ADME) optimization of the linker
With the development of ADCs, hydrophilicity must be considered83. Low hydrophilicity of the linker shows the following disadvantages: (1) low conjugate efficiency and DAR, (2) polymerization and sedimentation in human plasma, (3) off-target toxicity by nonspecific uptake, and (4) undesirable pharmacokinetics by rapid elimination from plasma84,85. Therefore, the hydrophilicity of ADCs is crucial. Currently, strategies for improving the hydrophilicity of ADCs are mainly divided into two categories: (1) incorporating PEG or sulfonate moieties into the linker of the ADC86, 87, 88, 89; or (2) developing highly hydrophilic linkers, such as phosphate-based linkers or charged linkers like [ethylene diamine platinum II]2+ linkers mentioned at the beginning of this review. In addition to linker hydrophilicity affecting the plasma kinetics and off-target toxicity of ADCs, a series of studies by Zhang et al. showed that linkers will also affect payload kinetics in tumors, and thereby determine the in vivo efficacy of ADCs.
5.1. Hydrophilicity optimization
There have been many studies on strategies to increase hydrophilicity with PEG, and studies on the relationship between hydrophilicity and the off-target effects of ADCs have also been conducted in recent years. In 2020, Simmons et al.90 described a family of MMAE-based ADCs with linkers containing PEG chains of 0, 4, 8 or 12 units. To investigate the relationship between off-target toxicity and hydrophilicity, the ADCs in this study were linked with nontargeted antibodies. In the tolerance experiments, all mice died in the PEG0 ADC group on the 5th day at the 20 mg/kg dose, but the survival rate of the PEG8 and PEG12 groups was 100% after 28 days. After immunohistochemical (IHC) staining of the mouse livers, PEG0-containing ADCs showed nonspecific uptake and released a large quantity of MMAE after 2 h at a dose of 10 mg/kg. It was therefore proven that the low dose tolerance of ADCs with low hydrophilicity was caused by nonspecific liver absorption. Meanwhile the ADCs containing PEG12 showed slower plasma clearance (7.3 mL·kg/day) and longer plasma exposure than those containing PEG0 (>46.3 mL·kg/day). In summary, ADCs with relatively high hydrophilicity could improve the pharmacokinetic parameters of ADCs, and significantly decrease the nonspecific uptake and off-target toxicity.
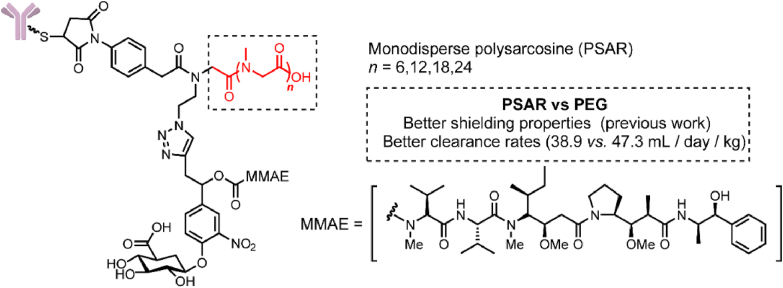
In addition to incorporating short PEG chains into the linker, numerous hydrophilic fragments have been tested to improve linker properties. In 2019, Viricel et al.91 developed monodisperse polysarcosine (PSAR) as a hydrophobic masking entity to construct highly loaded (DAR = 8) β-glucuronidase-responsive ADCs (Fig. 14). PSAR is a polypeptoid composed of endogenous sarcosine amino acids that is employed as a hydrophilic block. In previous work, it was confirmed that PSAR provides slightly better shielding properties than PEG at equal lengths. In this pharmacokinetic study, PSAR more efficiently reduced clearance rates than PEG (38.9 vs. 47.3 mL/day/kg). In a xenograft mouse model, a single dose of the ADC containing PSAR12 at 3 mg/kg induced complete tumor regression. At the same dose, Kadcyla was only able to promote tumor growth delay.
Figure 14.
The structure of PSAR-β-glucuronidase cleavable linker-containing ADCs. Adapted with modification from Ref. 92 © 2019 Royal Society of Chemistry.
5.2. Payload kinetics in tumors
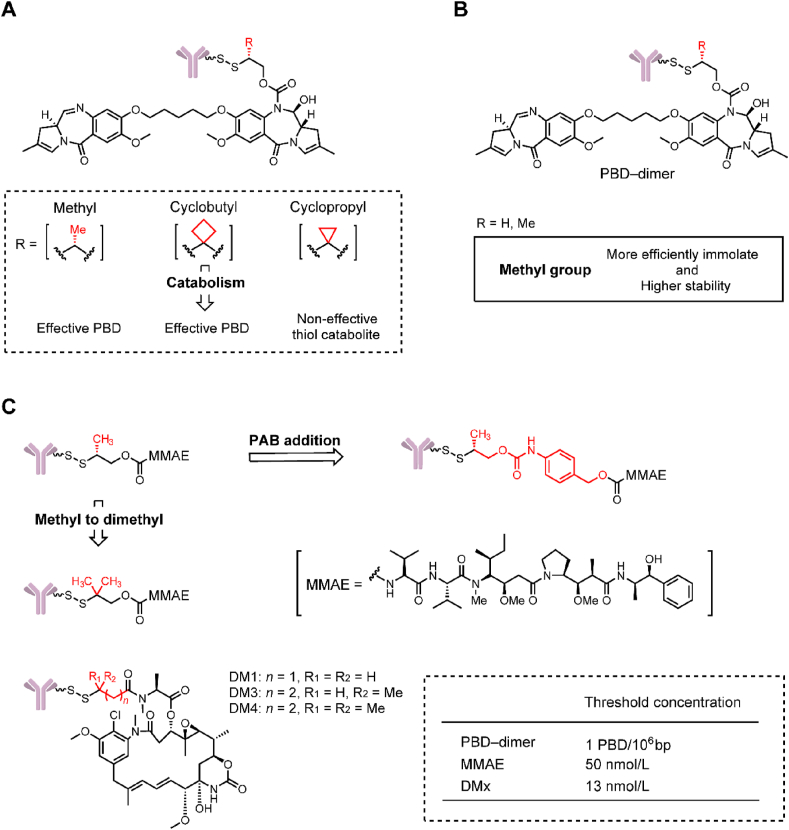
In 2016, Zhang et al.92 found that anti-CD22 disulfide-PBD-ADC containing methyl- and cyclobutyl-substituted disulfide linkers exhibited strong efficacy in a WSU-DLCL2 xenograft mouse model, whereas an ADC with a cyclopropyl linker was inactive (Fig. 15A). This finding was very interesting because the cyclobutyl and cyclopropyl substitutions lead to a large difference in the efficacy of ADCs. Further in vivo pharmacokinetic studies showed that cyclobutyl-containing ADCs could effectively delivered the PBD dimer (1.0–2.0 nmol/L) in tumors at both 24 and 96 h after dosing. In contrast, cyclopropyl-containing ADC could only release the ineffective cyclopropyl thiol catabolite in tumor (4.3–7.5 nmol/L). In 2018, they carried out a further research on the methyl-substituted disulfide linker (Fig. 15B)93. Results suggested that the methyl-containing linker self-cleaved much more efficiently and exhibited higher stability than the non-methyl-containing linker. Payload release in tumor is a net result of disulfide cleavage and subsequent self-cleavage.
Figure 15.
Effects on payload kinetics in tumors of linkers. (A) The structure of PBD-dimer-containing ADCs. Adapted with modification from Ref. 93 © 2016 American Society for Pharmacology and Experimental Therapeutics. (B) The structure and in vivo evaluation of PBD-dimer-containing ADCs. Adapted with modification from Ref. 94 © 2018 American Association for Cancer Research. (C) The structural optimization of MMAE-containing ADCs and the structure of DMx-containing ADCs. Adapted with modification from Ref. 95 © 2019 American Society for Pharmacology and Experimental Therapeutics.
In 2019, the delivery of other commonly used payloads, such as maytansinoids (e.g., DMx), auristatins (e.g., MMAE) by different linkers was studied (Fig. 15C)94. This study further verified that intratumoral payload exposures directly related to the structure of linker. Similar to PBD, MMAE could also be conjugated to the antibody through a self-cleavage disulfide linker. The double-methyl-disulfide (DiMe-SS)-MMAE-ADC showed higher tumor delivery (42.1 vs. 19.1 nmol/L) and tumor growth inhibition (69% vs. 50%) than the methyl-disulfide (Me-SS)-MMAE-ADC. Addition of PAB group to the Me-SS-MMAE-ADC could further improve the MMAE delivery (87.1 nmol/L) in tumor, giving a corresponding 30% tumor regression. The maytansinoids could be directly conjugated to the THIOMAB antibody through the disulfide bond. Direct conjugation of DM4 to light chain at K149C resulted in a very stable ADC with no DAR loss at day 10 in vivo. Their studies also suggested a threshold concentration and a plateau effect for an ADC. A threshold concentration of intratumor payload was required to support sustained efficacy, which was approximately 1 PBD/106 bp for PBD–ADCs and 50 nmol/L for MMAE–ADCs, and 13 nmol/L for DMx–ADCs. A plateau effect means that an ADC can deliver an excessive level of payload to tumors that does not enhance efficacy.
6. Conclusions
There are numerous developments on the structural optimization and mechanism expansion of ADCs over the past five years. Firstly, and most importantly, novel chemical triggers have been developed to obtain higher selectivity in delivery to tumors. For instance, the cBu trigger, the silyl ether trigger, the TRX trigger are valuable approaches. Particularly, novel photo-responsive cleavable triggers and bioorthogonal cleavable triggers could break the intracellular drug release restrictions for traditional ADCs, and provide an opportunity for the use of nonendocytic antibodies, but might not work for low membrane permeability payloads, such as MMAF. Secondly, for linker‒antibody attachment, there are two main problems that remain to be solved. The first problem is the retro-Michael elimination of the classical maleimide attachment. Fortunately, N-phenyl maleimide attachment could significantly improve the stability of ADCs with minor structural alterations, with promising prospects. The second problem is the heterogeneity of the DAR values. Although site-specific conjugation could solve this problem by modifying the antibodies, developing new linker‒antibody attachments might be another effective way. For instance, the BVP attachment could help to produce highly homogeneous ADCs with a DAR = 2. Thirdly, the linker plays an important role in the pharmacokinetics of ADCs. It can not only affect the plasma kinetics by adding hydrophilic fragments, but also affect the kinetics of payload release in tumors by structural optimization. Finally, with the rapid expansion of the payload arsenal, more and more linker‒payload attachments have been developed in recent years. In particular, the quaternary ammonium attachment could connect all payloads with a tertiary amine, such as Carfilzomib, Vinblastine, Duocarmycin, Rifabutin, etc. In conclusion, additional studies are needed to confirm the real effects of the novel linkers, despite their encouraging initial data.
7. Challenges and outlook
An ADC is a precise drug delivery system formed by the combination of a highly targeted antibody, a well-designed linker and a highly active payload. The complex composition of ADCs leads to following challenges at present: (1) toxicity problems remain to be solved. Although ADCs have greatly improved the targeting efficiency (over 100-fold95) compared to traditional chemotherapeutics, research has shown that less than 1% of the dosed ADCs accumulate in the tumors96. The danger is that the payloads, commonly 100-fold more toxic than conventional chemotherapeutic drugs, could be non-specifically released in normal tissues by the linkers. This eventually leads to systemic adverse effects and low MTD of ADCs. For instance, the MTD of Adcetris and Kadcyla is only 1.8 and 3.6 mg/kg respectively97,98. The limited MTD greatly limits the therapeutic potential of ADCs. In the future, more selective linkers can not only release payload rapidly in tumor tissues, but also effectively reduce the off-target toxicity in normal tissues. This important scientific assumption is the guide for our present work, and our unpublished data have initially supported this idea. (2) Drug resistance is another challenge of ADCs. Although the mechanisms of drug resistance for ADCs have not been determined, research has shown that down-regulation of target antigen, reduction of internalization of ADCs and efflux of the active payload are possible causes. For instance, the classic payloads, such as MMAE and DM1, are easily transported by adenosine triphosphate (ATP) binding proteins and lead to drug resistance. Therefore, SN-38, PBD and other novel payloads have been employed in ADCs, such as the Trodelvy. The discovery of new payloads requires the development of new linkers. (3) Full-length antibodies employed in ADCs inevitably face the problem of limited penetration of solid tumors and limited endocytotic efficiency. Along with employing smaller nano-antibodies to improve efficiency, this limitation may also be solved by using linkers with extracellular release capacity. Our group has made an initial attempt to design and synthesize photo-responsive linkers for extracellular release, but the reliability and validity needs further in vivo studies. (4) The relatively complex structure of linkers leads to difficulties in preclinical studies and in clinical applications. Therefore, the development of linkers with simplified structures and integrated functions may be another research direction. Our group attempted to integrate therapy and imaging in a theranostic ADC, which would help promote the preclinical study of ADCs. Thomas et al.30 simplified the linker by directly connecting the payloads to the antibody through a disulfide bond. We believe that with the development of antibody technology, linker technology and novel payloads, ideal ADCs with disruptive efficacy will be finally developed to promote the development of personalized medicines.
Acknowledgments
This work was funded by the Chinese National Natural Science Foundation (Grant Nos. 81872736 and 81903451), and the China Postdoctoral Science Foundation (Grant No. 2019M664015).
Author contributions
Zheng Su and Dian Xiao contributed equally to this work. Zheng Su and Dian Xiao: writing – review & editing, data curation. Fei Xie, Lianqi Liu and Yanming Wang: investigation, data curation, validation. Shiyong Fan, Xinbo Zhou and Song Li: conceptualization, project administration, funding acquisition.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Shiyong Fan, Email: fsyn1996@163.com.
Xinbo Zhou, Email: zhouxinbo@bmi.ac.cn.
References
- 1.Strebhardt K., Ullrich A. Paul Ehrlich's magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008;8:473–480. doi: 10.1038/nrc2394. [DOI] [PubMed] [Google Scholar]
- 2.Lamb Y.N. Inotuzumab ozogamicin: first global approval. Drugs. 2017;77:1603–1610. doi: 10.1007/s40265-017-0802-5. [DOI] [PubMed] [Google Scholar]
- 3.Dhillon S. Moxetumomab pasudotox: first global approval. Drugs. 2018;78:1763–1767. doi: 10.1007/s40265-018-1000-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deeks E.D. Correction to: polatuzumab vedotin: first global approval. Drugs. 2019;79:1829. doi: 10.1007/s40265-019-01214-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang E., Weinstock C., Zhang L., Charlab R., Dorff S.E., Gong Y., et al. FDA approval summary: enfortumab vedotin for locally advanced or metastatic urothelial carcinoma. Clin Cancer Res. 2020;27:922–927. doi: 10.1158/1078-0432.CCR-20-2275. [DOI] [PubMed] [Google Scholar]
- 6.Greenblatt K., Khaddour K. StatPearls Publishing LLC; Dec 19, 2020. Trastuzumab.https://www.ncbi.nlm.nih.gov/books/NBK532246/ Available from: [PubMed] [Google Scholar]
- 7.Markham A. Belantamab mafodotin: first approval. Drugs. 2020;80:1607–1613. doi: 10.1007/s40265-020-01404-x. [DOI] [PubMed] [Google Scholar]
- 8.Wahby S., Fashoyin-Aje L., Osgood C.L., Cheng J., Fiero M.H., Zhang L., et al. FDA approval summary: accelerated approval of sacituzumab govitecan-hziy for third line treatment of metastatic triple-negative breast cancer (mTNBC) Clin Cancer Res. 2021;27:1850–1854. doi: 10.1158/1078-0432.CCR-20-3119. [DOI] [PubMed] [Google Scholar]
- 9.Lyon R. Drawing lessons from the clinical development of antibody‒drug conjugates. Drug Discov Today Technol. 2018;30:105–109. doi: 10.1016/j.ddtec.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Hamann P.R., Hinman L.M., Hollander I., Beyer C.F., Lindh D., Holcomb R., et al. Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody-calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjug Chem. 2002;13:47–58. doi: 10.1021/bc010021y. [DOI] [PubMed] [Google Scholar]
- 11.Perini G.F., Pro B. Brentuximab vedotin in CD30+ lymphomas. Biol Ther. 2013;3:15–23. doi: 10.1007/s13554-013-0008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambert J.M., Chari R.V. Ado-trastuzumab emtansine (T-DM1): an antibody-drug conjugate (ADC) for HER2− positive breast cancer. J Med Chem. 2014;57:6949–6964. doi: 10.1021/jm500766w. [DOI] [PubMed] [Google Scholar]
- 13.Othus M., Appelbaum F.R., Petersdorf S.H., Kopecky K.J., Slovak M., Nevill T., et al. Fate of patients with newly diagnosed acute myeloid leukemia who fail primary induction therapy. Biol Blood Marrow Transplant. 2015;21:559–564. doi: 10.1016/j.bbmt.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dovgan I., Kolodych S., Koniev O., Wagner A. 2-(Maleimidomethyl)-1,3-dioxanes (MD): a serum-stable self-hydrolysable hydrophilic alternative to classical maleimide conjugation. Sci Rep. 2016;6:30835. doi: 10.1038/srep30835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ponte J.F., Sun X., Yoder N.C., Fishkin N., Laleau R., Coccia J., et al. Understanding how the stability of the thiol-maleimide linkage impacts the pharmacokinetics of lysine-linked antibody-maytansinoid conjugates. Bioconjug Chem. 2016;27:1588–1598. doi: 10.1021/acs.bioconjchem.6b00117. [DOI] [PubMed] [Google Scholar]
- 16.Pillow T.H., Tien J., Parsons-Reponte K.L., Bhakta S., Li H., Staben L.R., et al. Site-specific trastuzumab maytansinoid antibody‒drug conjugates with improved therapeutic activity through linker and antibody engineering. J Med Chem. 2014;57:7890–7899. doi: 10.1021/jm500552c. [DOI] [PubMed] [Google Scholar]
- 17.Lehar S.M., Pillow T., Xu M., Staben L., Kajihara K.K., Vandlen R., et al. Novel antibody‒antibiotic conjugate eliminates intracellular S. aureus. Nature. 2015;527:323–328. doi: 10.1038/nature16057. [DOI] [PubMed] [Google Scholar]
- 18.Kern J.C., Cancilla M., Dooney D., Kwasnjuk K., Zhang R., Beaumont M., et al. Discovery of pyrophosphate diesters as tunable, soluble, and bioorthogonal linkers for site-specific antibody–drug conjugates. J Am Chem Soc. 2016;138:1430–1445. doi: 10.1021/jacs.5b12547. [DOI] [PubMed] [Google Scholar]
- 19.Caculitan N.G., Dela Cruz Chuh J., Ma Y., Zhang D., Kozak K.R., Liu Y., et al. Cathepsin B is dispensable for cellular processing of cathepsin B-cleavable antibody‒drug conjugates. Cancer Res. 2017;77:7027–7037. doi: 10.1158/0008-5472.CAN-17-2391. [DOI] [PubMed] [Google Scholar]
- 20.Wei B., Gunzner-Toste J., Yao H., Wang T., Wang J., Xu Z., et al. Discovery of peptidomimetic antibody‒drug conjugate linkers with enhanced protease specificity. J Med Chem. 2018;61:989–1000. doi: 10.1021/acs.jmedchem.7b01430. [DOI] [PubMed] [Google Scholar]
- 21.Dorywalska M., Dushin R., Moine L., Farias S.E., Zhou D., Navaratnam T., et al. Molecular basis of valine-citrulline-PABC linker instability in site-specific ADCs and its mitigation by linker design. Mol Cancer Ther. 2016;15:958–970. doi: 10.1158/1535-7163.MCT-15-1004. [DOI] [PubMed] [Google Scholar]
- 22.Singh R., Setiady Y.Y., Ponte J., Kovtun Y.V., Lai K.C., Hong E.E., et al. A new triglycyl peptide linker for antibody‒drug conjugates (ADCs) with improved targeted killing of cancer cells. Mol Cancer Ther. 2016;15:1311–1320. doi: 10.1158/1535-7163.MCT-16-0021. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y., Fan S., Zhong W., Zhou X., Li S. Development and properties of valine-alanine based antibody‒drug conjugates with monomethyl auristatin E as the potent payload. Int J Mol Sci. 2017;18:1860. doi: 10.3390/ijms18091860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reid E.E., Archer K.E., Shizuka M., Wilhelm A., Yoder N.C., Bai C., et al. Effect of linker stereochemistry on the activity of indolinobenzodiazepine containing antibody‒drug conjugates (ADCs) ACS Med Chem Lett. 2019;10:1193–1197. doi: 10.1021/acsmedchemlett.9b00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salomon P.L., Reid E.E., Archer K.E., Harris L., Maloney E.K., Wilhelm A.J., et al. Optimizing lysosomal activation of antibody-drug conjugates (ADCs) by incorporation of novel cleavable dipeptide linkers. Mol Pharm. 2019;16:4817–4825. doi: 10.1021/acs.molpharmaceut.9b00696. [DOI] [PubMed] [Google Scholar]
- 26.Casey J.R., Grinstein S., Orlowski J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol. 2010;11:50–61. doi: 10.1038/nrm2820. [DOI] [PubMed] [Google Scholar]
- 27.Govindan S.V., Cardillo T.M., Sharkey R.M., Tat F., Gold D.V., Goldenberg D.M. Milatuzumab-SN-38 conjugates for the treatment of CD74+ cancers. Mol Cancer Ther. 2013;12:968–978. doi: 10.1158/1535-7163.MCT-12-1170. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y., Fan S., Xiao D., Xie F., Li W., Zhong W., et al. Novel silyl ether-based acid-cleavable antibody‒MMAE conjugates with appropriate stability and efficacy. Cancers (Basel) 2019;11:957. doi: 10.3390/cancers11070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mills B.J., Lang C.A. Differential distribution of free and bound glutathione and cyst(e)ine in human blood. Biochem Pharmacol. 1996;52:401–406. doi: 10.1016/0006-2952(96)00241-9. [DOI] [PubMed] [Google Scholar]
- 30.Pillow T.H., Sadowsky J.D., Zhang D., Yu S.F., Del Rosario G., Xu K., et al. Decoupling stability and release in disulfide bonds with antibody-small molecule conjugates. Chem Sci. 2017;8:366–370. doi: 10.1039/c6sc01831a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pillow T.H., Schutten M., Yu S.F., Ohri R., Sadowsky J., Poon K.A., et al. Modulating therapeutic activity and toxicity of pyrrolobenzodiazepine antibody‒drug conjugates with self-immolative disulfide linkers. Mol Cancer Ther. 2017;16:871–878. doi: 10.1158/1535-7163.MCT-16-0641. [DOI] [PubMed] [Google Scholar]
- 32.Torti S.V., Torti F.M. Iron and cancer: more ore to be mined. Nat Rev Cancer. 2013;13:342–355. doi: 10.1038/nrc3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spangler B., Fontaine S.D., Shi Y., Sambucetti L., Mattis A.N., Hann B., et al. A novel tumor-activated prodrug strategy targeting ferrous iron is effective in multiple preclinical cancer models. J Med Chem. 2016;59:11161–11170. doi: 10.1021/acs.jmedchem.6b01470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spangler B., Kline T., Hanson J., Li X., Zhou S., Wells J.A., et al. Toward a ferrous iron-cleavable linker for antibody‒drug conjugates. Mol Pharm. 2018;15:2054–2059. doi: 10.1021/acs.molpharmaceut.8b00242. [DOI] [PubMed] [Google Scholar]
- 35.Jeffrey S.C., Andreyka J.B., Bernhardt S.X., Kissler K.M., Kline T., Lenox J.S., et al. Development and properties of beta-glucuronide linkers for monoclonal antibody‒drug conjugates. Bioconjug Chem. 2006;17:831–840. doi: 10.1021/bc0600214. [DOI] [PubMed] [Google Scholar]
- 36.Kolodych S., Michel C., Delacroix S., Koniev O., Ehkirch A., Eberova J., et al. Development and evaluation of beta-galactosidase-sensitive antibody‒drug conjugates. Eur J Med Chem. 2017;142:376–382. doi: 10.1016/j.ejmech.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Bargh J.D., Walsh S.J., Isidro-Llobet A., Omarjee S., Carroll J.S., Spring D.R. Sulfatase-cleavable linkers for antibody‒drug conjugates. Chem Sci. 2020;11:2375–2380. doi: 10.1039/c9sc06410a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kern J.C., Dooney D., Zhang R., Liang L., Brandish P.E., Cheng M., et al. Novel phosphate modified cathepsin B linkers: improving aqueous solubility and enhancing payload scope of ADCs. Bioconjug Chem. 2016;27:2081–2088. doi: 10.1021/acs.bioconjchem.6b00337. [DOI] [PubMed] [Google Scholar]
- 39.Carl P.L., Chakravarty P.K., Katzenellenbogen J.A. A novel connector linkage applicable in prodrug design. J Med Chem. 1981;24:479–480. doi: 10.1021/jm00137a001. [DOI] [PubMed] [Google Scholar]
- 40.Erez R., Shabat D. The azaquinone-methide elimination: comparison study of 1,6- and 1,4-eliminations under physiological conditions. Org Biomol Chem. 2008;6:2669–2672. doi: 10.1039/b808198k. [DOI] [PubMed] [Google Scholar]
- 41.Xiao D., Zhao L., Xie F., Fan S., Liu L., Li W., et al. A bifunctional molecule-based strategy for the development of theranostic antibody‒drug conjugate. Theranostics. 2021;11:2550–2563. doi: 10.7150/thno.51232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fodor S.P., Read J.L., Pirrung M.C., Stryer L., Lu A.T., Solas D. Light-directed, spatially addressable parallel chemical synthesis. Science. 1991;251:767–773. doi: 10.1126/science.1990438. [DOI] [PubMed] [Google Scholar]
- 43.Pelliccioli A.P., Wirz J. Photoremovable protecting groups: reaction mechanisms and applications. Photochem Photobiol Sci. 2002;1:441–458. doi: 10.1039/b200777k. [DOI] [PubMed] [Google Scholar]
- 44.Bryden F., Maruani A., Rodrigues J.M.M., Cheng M.H.Y., Savoie H., Beeby A., et al. Assembly of high-potency photosensitizer-antibody conjugates through application of dendron multiplier technology. Bioconjug Chem. 2018;29:176–181. doi: 10.1021/acs.bioconjchem.7b00678. [DOI] [PubMed] [Google Scholar]
- 45.Ito K., Mitsunaga M., Nishimura T., Saruta M., Iwamoto T., Kobayashi H., et al. Near-infrared photochemoimmunotherapy by photoactivatable bifunctional antibody‒drug conjugates targeting human epidermal growth factor receptor 2 positive cancer. Bioconjug Chem. 2017;28:1458–1469. doi: 10.1021/acs.bioconjchem.7b00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nani R.R., Gorka A.P., Nagaya T., Kobayashi H., Schnermann M.J. Near-IR light-mediated cleavage of antibody‒drug conjugates using cyanine photocages. Angew Chem Int Ed Engl. 2015;54:13635–13638. doi: 10.1002/anie.201507391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li J., Xiao D., Xie F., Li W., Zhao L., Sun W., et al. Novel antibody‒drug conjugate with UV-controlled cleavage mechanism for cytotoxin release. Bioorg Chem. 2021;111:104475. doi: 10.1016/j.bioorg.2020.104475. [DOI] [PubMed] [Google Scholar]
- 48.Zang C., Wang H., Li T., Zhang Y., Li J., Shang M., et al. A light-responsive, self-immolative linker for controlled drug delivery via peptide‒ and protein‒drug conjugates. Chem Sci. 2019;10:8973–8980. doi: 10.1039/c9sc03016f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu Y., Li Z., Malkovskiy A., Sun S., Pang Y. Aggregation control of squaraines and their use as near-infrared fluorescent sensors for protein. J Phys Chem B. 2010;114:8574–8580. doi: 10.1021/jp1029536. [DOI] [PubMed] [Google Scholar]
- 50.McRae E.G., Kasha M. Enhancement of phosphorescence ability upon aggregation of dye molecules. J Chem Phys. 1958;28:721–722. [Google Scholar]
- 51.Matsumura Y., Ananthaswamy H.N. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol. 2004;195:298–308. doi: 10.1016/j.taap.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 52.D'Orazio J., Jarrett S., Amaro-Ortiz A., Scott T. UV radiation and the skin. Int J Mol Sci. 2013;14:12222–12248. doi: 10.3390/ijms140612222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hapuarachchige S., Huang C.T., Donnelly M.C., Barinka C., Lupold S.E., Pomper M.G., et al. Cellular delivery of bioorthogonal pretargeting therapeutics in PSMA-positive prostate cancer. Mol Pharm. 2020;17:98–108. doi: 10.1021/acs.molpharmaceut.9b00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin F., Chen L., Zhang H., Ching Ngai W.S., Zeng X., Lin J., et al. Bioorthogonal prodrug–antibody conjugates for on-target and on-demand chemotherapy. CCS Chemistry. 2019;1:226–236. [Google Scholar]
- 55.Wang X., Liu Y., Fan X., Wang J., Ngai W.S.C., Zhang H., et al. Copper-triggered bioorthogonal cleavage reactions for reversible protein and cell surface modifications. J Am Chem Soc. 2019;141:17133–17141. doi: 10.1021/jacs.9b05833. [DOI] [PubMed] [Google Scholar]
- 56.Doronina S.O., Toki B.E., Torgov M.Y., Mendelsohn B.A., Cerveny C.G., Chace D.F., et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol. 2003;21:778–784. doi: 10.1038/nbt832. [DOI] [PubMed] [Google Scholar]
- 57.Tobaldi E., Dovgan I., Mosser M., Becht J.M., Wagner A. Structural investigation of cyclo-dioxo maleimide cross-linkers for acid and serum stability. Org Biomol Chem. 2017;15:9305–9310. doi: 10.1039/c7ob01757j. [DOI] [PubMed] [Google Scholar]
- 58.Gregson S.J., Masterson L.A., Wei B., Pillow T.H., Spencer S.D., Kang G.D., et al. Pyrrolobenzodiazepine dimer antibody‒drug conjugates: synthesis and evaluation of noncleavable drug-linkers. J Med Chem. 2017;60:9490–9507. doi: 10.1021/acs.jmedchem.7b00736. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y., Liu L., Fan S., Xiao D., Xie F., Li W., et al. Antibody‒drug conjugate using ionized cys-linker-MMAE as the potent payload shows optimal therapeutic safety. Cancers (Basel) 2020;12:744. doi: 10.3390/cancers12030744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li X., Patterson J.T., Sarkar M., Pedzisa L., Kodadek T., Roush W.R., et al. Site-specific dual antibody conjugation via engineered cysteine and selenocysteine residues. Bioconjug Chem. 2015;26:2243–2248. doi: 10.1021/acs.bioconjchem.5b00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lyu Z., Kang L., Buuh Z.Y., Jiang D., McGuth J.C., Du J., et al. A switchable site-specific antibody conjugate. ACS Chem Biol. 2018;13:958–964. doi: 10.1021/acschembio.8b00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou Q. Site-specific antibody conjugation for ADC and beyond. Biomedicines. 2017;5:64. doi: 10.3390/biomedicines5040064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hamblett K.J., Senter P.D., Chace D.F., Sun M.M., Lenox J., Cerveny C.G., et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin Cancer Res. 2004;10:7063–7070. doi: 10.1158/1078-0432.CCR-04-0789. [DOI] [PubMed] [Google Scholar]
- 64.Lyon R.P., Setter J.R., Bovee T.D., Doronina S.O., Hunter J.H., Anderson M.E., et al. Self-hydrolyzing maleimides improve the stability and pharmacological properties of antibody‒drug conjugates. Nat Biotechnol. 2014;32:1059–1062. doi: 10.1038/nbt.2968. [DOI] [PubMed] [Google Scholar]
- 65.Christie R.J., Fleming R., Bezabeh B., Woods R., Mao S., Harper J., et al. Stabilization of cysteine-linked antibody drug conjugates with N-aryl maleimides. J Control Release. 2015;220:660–670. doi: 10.1016/j.jconrel.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 66.Fontaine S.D., Reid R., Robinson L., Ashley G.W., Santi D.V. Long-term stabilization of maleimide-thiol conjugates. Bioconjug Chem. 2015;26:145–152. doi: 10.1021/bc5005262. [DOI] [PubMed] [Google Scholar]
- 67.Christie R.J., Tiberghien A.C., Du Q., Bezabeh B., Fleming R., Shannon A., et al. Pyrrolobenzodiazepine antibody‒drug conjugates designed for stable thiol conjugation. Antibodies (Basel) 2017;6:20. doi: 10.3390/antib6040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shaunak S., Godwin A., Choi J.W., Balan S., Pedone E., Vijayarangam D., et al. Site-specific PEGylation of native disulfide bonds in therapeutic proteins. Nat Chem Biol. 2006;2:312–313. doi: 10.1038/nchembio786. [DOI] [PubMed] [Google Scholar]
- 69.Badescu G., Bryant P., Bird M., Henseleit K., Swierkosz J., Parekh V., et al. Bridging disulfides for stable and defined antibody drug conjugates. Bioconjug Chem. 2014;25:1124–1136. doi: 10.1021/bc500148x. [DOI] [PubMed] [Google Scholar]
- 70.Huang R., Sheng Y., Wei D., Yu J., Chen H., Jiang B. Bis(vinylsulfonyl)piperazines as efficient linkers for highly homogeneous antibody‒drug conjugates. Eur J Med Chem. 2020;190:112080. doi: 10.1016/j.ejmech.2020.112080. [DOI] [PubMed] [Google Scholar]
- 71.Sun S., Akkapeddi P., Marques M.C., Martinez-Saez N., Torres V.M., Cordeiro C., et al. One-pot stapling of interchain disulfides of antibodies using an isobutylene motif. Org Biomol Chem. 2019;17:2005–2012. doi: 10.1039/c8ob02877j. [DOI] [PubMed] [Google Scholar]
- 72.Huang R., Li Z., Sheng Y., Yu J., Wu Y., Zhan Y., et al. N-Methyl-N-phenylvinylsulfonamides for cysteine-selective conjugation. Org Lett. 2018;20:6526–6529. doi: 10.1021/acs.orglett.8b02849. [DOI] [PubMed] [Google Scholar]
- 73.Waalboer D.C., Muns J.A., Sijbrandi N.J., Schasfoort R.B., Haselberg R., Somsen G.W., et al. Platinum(II) as bifunctional linker in antibody‒drug conjugate formation: coupling of a 4-nitrobenzo-2-oxa-1,3-diazole fluorophore to trastuzumab as a model. ChemMedChem. 2015;10:797–803. doi: 10.1002/cmdc.201402496. [DOI] [PubMed] [Google Scholar]
- 74.Sijbrandi N.J., Merkul E., Muns J.A., Waalboer D.C., Adamzek K., Bolijn M., et al. A novel platinum(II)-based bifunctional ADC linker benchmarked using 89Zr-desferal and auristatin F-conjugated trastuzumab. Cancer Res. 2017;77:257–267. doi: 10.1158/0008-5472.CAN-16-1900. [DOI] [PubMed] [Google Scholar]
- 75.Merkul E., Muns J.A., Sijbrandi N.J., Houthoff H.J., Nijmeijer B., van Rheenen G., et al. An efficient conjugation approach for coupling drugs to native antibodies via the Pt(II) linker Lx for improved manufacturability of antibody‒drug conjugates. Angew Chem Int Ed Engl. 2020;60:3008–3015. doi: 10.1002/anie.202011593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Merkul E., Sijbrandi N.J., Muns J.A., Aydin I., Adamzek K., Houthoff H.J., et al. First platinum(II)-based metal-organic linker technology (Lx®) for a plug-and-play development of antibody‒drug conjugates (ADCs) Expert Opin Drug Deliv. 2019;16:783–793. doi: 10.1080/17425247.2019.1645118. [DOI] [PubMed] [Google Scholar]
- 77.Maderna A., Leverett C.A. Recent advances in the development of new auristatins: structural modifications and application in antibody drug conjugates. Mol Pharm. 2015;12:1798–1812. doi: 10.1021/mp500762u. [DOI] [PubMed] [Google Scholar]
- 78.Burke P.J., Hamilton J.Z., Pires T.A., Setter J.R., Hunter J.H., Cochran J.H., et al. Development of novel quaternary ammonium linkers for antibody‒drug conjugates. Mol Cancer Ther. 2016;15:938–945. doi: 10.1158/1535-7163.MCT-16-0038. [DOI] [PubMed] [Google Scholar]
- 79.Block S.S., Stephens R.L., Barreto A., Murrill W.A. Chemical identification of the Amanita toxin in mushrooms. Science. 1955;121:505–506. doi: 10.1126/science.121.3145.505. [DOI] [PubMed] [Google Scholar]
- 80.Pando O., Dörner S., Preusentanz R., Denkert A., Porzel A., Richter W., et al. First total synthesis of tubulysin B. Org Lett. 2009;11:5567–5569. doi: 10.1021/ol902320w. [DOI] [PubMed] [Google Scholar]
- 81.Quintieri L., Geroni C., Fantin M., Battaglia R., Rosato A., Speed W., et al. Formation and antitumor activity of PNU-159682, a major metabolite of nemorubicin in human liver microsomes. Clin Cancer Res. 2005;11:1608–1617. doi: 10.1158/1078-0432.CCR-04-1845. [DOI] [PubMed] [Google Scholar]
- 82.Park S., Kim S.Y., Cho J., Jung D., Seo D., Lee J., et al. Aryl sulfate is a useful motif for conjugating and releasing phenolic molecules: sulfur fluorine exchange click chemistry enables discovery of ortho-hydroxy-protected aryl sulfate linker. Bioconjug Chem. 2019;30:1957–1968. doi: 10.1021/acs.bioconjchem.9b00340. [DOI] [PubMed] [Google Scholar]
- 83.Han T.H., Zhao B. Absorption, distribution, metabolism, and excretion considerations for the development of antibody‒drug conjugates. Drug Metab Dispos. 2014;42:1914–1920. doi: 10.1124/dmd.114.058586. [DOI] [PubMed] [Google Scholar]
- 84.Lyon R.P., Bovee T.D., Doronina S.O., Burke P.J., Hunter J.H., Neff-LaFord H.D., et al. Reducing hydrophobicity of homogeneous antibody‒drug conjugates improves pharmacokinetics and therapeutic index. Nat Biotechnol. 2015;33:733–735. doi: 10.1038/nbt.3212. [DOI] [PubMed] [Google Scholar]
- 85.Sun X., Ponte J.F., Yoder N.C., Laleau R., Coccia J., Lanieri L., et al. Effects of drug-antibody ratio on pharmacokinetics, biodistribution, efficacy, and tolerability of antibody-maytansinoid conjugates. Bioconjug Chem. 2017;28:1371–1381. doi: 10.1021/acs.bioconjchem.7b00062. [DOI] [PubMed] [Google Scholar]
- 86.Abrahams C.L., Li X., Embry M., Yu A., Krimm S., Krueger S., et al. Targeting CD74 in multiple myeloma with the novel, site-specific antibody‒drug conjugate STRO-001. Oncotarget. 2018;9:37700–37714. doi: 10.18632/oncotarget.26491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shao S., Tsai M.H., Lu J., Yu T., Jin J., Xiao D., et al. Site-specific and hydrophilic ADCs through disulfide-bridged linker and branched PEG. Bioorg Med Chem Lett. 2018;28:1363–1370. doi: 10.1016/j.bmcl.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 88.Zhao R.Y., Wilhelm S.D., Audette C., Jones G., Leece B.A., Lazar A.C., et al. Synthesis and evaluation of hydrophilic linkers for antibody-maytansinoid conjugates. J Med Chem. 2011;54:3606–3623. doi: 10.1021/jm2002958. [DOI] [PubMed] [Google Scholar]
- 89.Walker J.A., Sorkin M.R., Ledesma F., Kabaria S.R., Barfield R.M., Rabuka D., et al. Hydrophilic sequence-defined cross-linkers for antibody‒drug conjugates. Bioconjug Chem. 2019;30:2982–2988. doi: 10.1021/acs.bioconjchem.9b00713. [DOI] [PubMed] [Google Scholar]
- 90.Simmons J.K., Burke P.J., Cochran J.H., Pittman P.G., Lyon R.P. Reducing the antigen-independent toxicity of antibody‒drug conjugates by minimizing their non-specific clearance through PEGylation. Toxicol Appl Pharmacol. 2020;392:114932. doi: 10.1016/j.taap.2020.114932. [DOI] [PubMed] [Google Scholar]
- 91.Viricel W., Fournet G., Beaumel S., Perrial E., Papot S., Dumontet C., et al. Monodisperse polysarcosine-based highly-loaded antibody‒drug conjugates. Chem Sci. 2019;10:4048–4053. doi: 10.1039/c9sc00285e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang D., Yu S.F., Ma Y., Xu K., Dragovich P.S., Pillow T.H., et al. Chemical structure and concentration of intratumor catabolites determine efficacy of antibody drug conjugates. Drug Metab Dispos. 2016;44:1517–1523. doi: 10.1124/dmd.116.070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang D., Yu S.F., Khojasteh S.C., Ma Y., Pillow T.H., Sadowsky J.D., et al. Intratumoral payload concentration correlates with the activity of antibody‒drug conjugates. Mol Cancer Ther. 2018;17:677–685. doi: 10.1158/1535-7163.MCT-17-0697. [DOI] [PubMed] [Google Scholar]
- 94.Zhang D., Dragovich P.S., Yu S.F., Ma Y., Pillow T.H., Sadowsky J.D., et al. Exposure-efficacy analysis of antibody‒drug conjugates delivering an excessive level of payload to tissues. Drug Metab Dispos. 2019;47:1146–1155. doi: 10.1124/dmd.119.087023. [DOI] [PubMed] [Google Scholar]
- 95.Sharkey R.M., McBride W.J., Cardillo T.M., Govindan S.V., Wang Y., Rossi E.A., et al. Enhanced delivery of SN-38 to human tumor xenografts with an anti-Trop-2-SN-38 antibody conjugate (sacituzumab govitecan) Clin Cancer Res. 2015;21:5131–5138. doi: 10.1158/1078-0432.CCR-15-0670. [DOI] [PubMed] [Google Scholar]
- 96.Mullard A. Maturing antibody‒drug conjugate pipeline hits 30. Nat Rev Drug Discov. 2013;12:329–332. doi: 10.1038/nrd4009. [DOI] [PubMed] [Google Scholar]
- 97.Younes A., Bartlett N.L., Leonard J.P., Kennedy D.A., Lynch C.M., Sievers E.L., et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363:1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 98.Krop I.E., Beeram M., Modi S., Jones S.F., Holden S.N., Yu W., et al. Phase I study of trastuzumab-DM1, an HER2 antibody‒drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol. 2010;28:2698–2704. doi: 10.1200/JCO.2009.26.2071. [DOI] [PubMed] [Google Scholar]