Abstract
Objective: We aimed to study the effects of anaesthetics on bladder function using repeated urodynamic investigation (UDI) including external urethral sphincter (EUS) electromyography (EMG) in awake restrained mice. Materials and Methods: Female C57Bl/6J mice underwent either bladder catheter (n=6) or bladder catheter plus electrodes (n=10) implantation next to the EUS. A control group (n=3) was included for histological analysis. Following awake UDI, the effects of midazolam (5 mg/kg) and opioids (fentanyl (50 μg/kg) and hydromorphine (250 μg/kg)) on bladder function were studied. Mice were allowed to recover from drug application for at least one day before being subjected to the next drug and UDI. Bladder weight was assessed and fibrotic changes were analysed by Masson’s trichrome staining. Results: EUS-EMG activity during voiding was reduced compared to before and after voiding in baseline measurements. Threshold and maximal detrusor pressure were significantly increased in both midazolam and the opioids. The opioids lead to either a significantly increased bladder filling volume and micturition cycle duration (hydromorphine) or a complete loss of the voiding phase leading to overflow incontinence (fentanyl). Bladder-to bodyweight ratio was significantly increased in both groups with an implanted catheter compared to controls. No differences were observed between the groups with- or without implanted electrodes regarding bladder-to bodyweight ratio, bladder fibrosis and urodynamic parameters. Conclusions: Repeated UDIs combined with EUS-EMG are feasible in the awake mouse model. The presence of electrodes next to the EUS does not obstruct the bladder outlet. Opioids and benzodiazepines severely interfere with physiological bladder function: fentanyl and hydromorphine disrupted the voiding phase evidenced by the reduced coordination of EUS activity with detrusor contraction, while bladder emptying under midazolam was achieved by EUS relaxation only.
Keywords: Urodynamic investigation (UDI), external urethral sphincter (EUS), midazolam, fentanyl, hydromorphine, awake mice, electromyography (EMG)
Introduction
Lower urinary tract dysfunction (LUTD) has various causes, including benign prostatic obstruction or neurological diseases such as spinal cord injury, Multiple Sclerosis and Parkinson’s disease. Neurogenic LUTD often leads to detrusor-sphincter dyssynergia (DSD), a dangerous and potentially life threatening condition, where the detrusor and external urethral sphincter (EUS) muscle contractions are uncoordinated, leading to high-pressure voiding, detrusor overactivity and development of residual urine. The only objective method to assess LUTD in humans is an urodynamic investigation (UDI). Several animal models for UDI exist, where the bladder pressure is measured during a micturition cycle through an implanted catheter and voided volumes are recorded. To date, reported UDI studies incorporating EUS-electromyography (EUS-EMG) measurements in mice were performed at only one time point, usually immediately after surgical insertion of the electrodes into the EUS followed by a short recovery phase from anaesthesia [1,2]. To avoid the side effects of acute electrode insertion, which might lead to local inflammation and possible afferent nerve stimulation, we adapted the methodology, recently developed in rats [3], to mice. We implanted electrodes next to the EUS, allowed the mice to completely recover post-operation, and subjected them to repeated UDIs with EUS-EMG measurements.
Anaesthetics or analgesics affect bladder function often leading to urinary retention in humans [4]. This is observed both postoperatively and under chronic use of opioids [5]. Bladder catheterization during and after surgeries employing anaesthetics acting on μ-receptors (fentanyl, morphine) is of great importance to prevent bladder over-distention and urinary retention (reviewed in [6]). The exact mechanism behind this observation however is not well understood. A great number of UDI studies are performed in anaesthetized animals even though the effects of anaesthetics on bladder function including decreased detrusor pressure, changed bladder capacity or suppression of the micturition reflex are well-documented (reviewed in [7-9]) and despite the fact that human UDI is performed without anaesthesia.
Here we employed repeated UDI with simultaneous EUS-EMG to study the effects of anaesthetic agents midazolam (acting on gamma-aminobutyric acid (GABA) [10]), fentanyl and hydromorphine (µ-receptor agonists) on the bladder function in the same mouse. No mouse- or rat-model LUT studies using fentanyl or midazolam have been published to date [11].
Materials and methods
Animals
Female C57Bl/6J mice (n=19) were obtained from Charles River Laboratories (France) and housed at a 12/12 light-dark cycle. Food and water was available ad libitum. One week after arrival, mice were handled and acclimatized to the experimenter and measurement setup three times during one week. The following week, at the age of 11 weeks, the mice were randomly divided into the three study groups. The catheter only (cath., n=6) and catheter plus electrodes (Cath. + El., n=10) mice were implanted as described below (see section “Catheter and Electrodes Implantation”). Following implantation single housing was necessary throughout the whole experiment to avoid implant damage by cage-mate biting. Cages with inserted dividers (green line, Tecniplast, Buguggiate, Italy) were employed to allow two mice per cage, thus reducing the animal’s stress caused by social deprivation by allowing interaction by sound and smell. The control mice (ctrl, n=3) were housed together in a conventional cage.
Study design
One week after implantation surgery the baseline UDI was measured. All mice were subjected to UDI under subsequent applications of midazolam, fentanyl and hydromorphine, always with at least one day in between to allow complete drug metabolisation before exposure to the next drug. On each experimental day a baseline measurement of at least 3 established micturition cycles was performed before each drug application. At least one day after the last measurement and three weeks post-surgery mice were euthanized and bladders excised for histological analysis.
Animal grouping
Catheter group
Mice (n=6) were handled for one week, then underwent catheter implantation. One week later, mice underwent baseline UDI. Thereafter, mice underwent 3 sessions of UDI under 3 different drugs (midazolam, hydromorphine and fentanyl). There was always at least one day between these 3 sessions, and a baseline measurement was carried out before each drug application to ensure the functional recovery. At the end of the experiment, mice were sacrificed and bladders were harvested for histological analysis.
Catheter + electrodes group
Mice (n=10) were handled for one week, then underwent catheter and electrode implantation. One week later, mice underwent baseline UDI. Thereafter, mice underwent 3 sessions of UDI under 3 different anesthetic drugs (midazolam, hydromorphine and fentanyl). There was always at least one day between these 3 sessions, and a baseline measurement was carried out before each drug application to ensure the functional recovery. At the end of the experiment, mice were sacrificed, electrode placement was checked and bladders were harvested for histological analysis.
Control group
Mice (n=3) did not undergo any surgery or UDI. These mice lived together in a cage and were sacrificed at the end of the experiment. Bladders were harvested for histological analysis.
Study approval
The animal experiments were performed in accordance with the relevant Swiss laws and approved by the Veterinary Commission for Animal Research of the Canton of Berne, Switzerland (Licence Nr BE 53/18).
Catheter and electrodes implantation
Mice were anaesthetized with a s.c. injection of medetomidine (0.5 mg/kg, Domitor, Orion Corporation, Espoo, Finland) midazolam (5 mg/kg, Dormicum® Midazolamum 5 mg/ml, Roche Pharma (Schweiz) AG, Reinach, Switzerland) and fentanyl (50 µg/kg, Fentanyl Sintetica, Sintetica S.A., Mendrisio, Switzerland) and placed onto a heating pad. Upon loss of pedal reflex the lower abdomen and neck were shaved and disinfected (Betadine®, Mundipharma Medical Company, Basel, Switzerland) and eye ointment (Viscotears® Carbomerum 980 2 mg/g, Bausch + Lomb, Rochester, New York, USA) applied. A median laparotomy was performed, the bladder exposed and a small incision made into the dome of the bladder. A catheter with a flared end (Intravascular PE-10 tubing, SAI Infusion Technologies, Lake Villa, Illinois, USA) was inserted and fixed in place with a purse string suture (6-0 PROLENE®, 8807H, Ethicon, Somerville, New Jersey, USA). Thereafter the catheter was tunneled subcutaneously to the back of the neck and exteriorized. The abdominal muscle was sutured (6-0 coated Vicryl, V926H, Ethicon, Somerville, New Jersey, USA), as well as the skin (6-0 coated Vicryl, V926H, Ethicon, Somerville, New Jersey, USA) in the Cath. group. In mice of the Cath. + El. group the urethra was exposed below the abdominal muscle. A self-made silvertip electrode (AGW1030, World Precision Instruments, Sarasota, Florida, USA) with a Teflon coated stainless steel wire (AS631, Cooner Wire, Chatsworth, California, USA) was placed on either side of the EUS and fixed to the lateral fat tissue. A third electrode serving as ground was sutured rostral to the EUS-electrodes to the abdominal muscle. All three wires were tunnelled together subcutaneously to the back of the neck and exteriorized. Then the abdominal skin was sutured (6-0 coated Vicryl, V926H, Ethicon, Somerville, New Jersey, USA). Animals of both groups were fitted into an infusion harness (SMH, SAI Infusion Technologies, Lake Villa, Illinois, USA) and the catheter plugged to the harness to prevent leakage. The electrode-wires were soldered to a connector (850-10-050-10-001101, Preci-Dip, Delémont, Switzerland) and affixed to the harness where applicable. Prior to recovery by s.c. injections of Flumazenil-Mepha® (0.5 mg/kg, Mepha Pharma AG, Basel, Switzerland) and Antisedan (2.5 mg/kg, Atipamezoli hydrochloridum, Orion Pharma, Espoo, Finland), the analgesics Temgesic® (0.1 mg/kg, Buprenorphinum, Indivior Schweiz AG, Baar, Switzerland) and carprofen (5 mg/kg, Norocarp®, Ufamed AG, Sursee, Switzerland) and the antibiotic Borgal® 24% (15 mg/kg Sulfadoxinum and Trimethoprimum 1:5, MSD Animal Health GmbH, Lucerne, Switzerland) were s.c. injected. During the first night post-surgery, mice were kept in warmed cages. Daily s.c. injections of carprofen (5 mg/kg, Norocarp®, Ufamed AG, Sursee, Switzerland) and Borgal® 24% (15 mg/kg, Sulfadoxinum and Trimethoprimum 1:5, MSD Animal Health GmbH, Lucerne, Switzerland) were given for 5 days and thereafter Borgal® 24% (10 mg/ml, Sulfadoxinum and Trimethoprimum 1:5, MSD Animal Health GmbH, Lucerne, Switzerland) was provided throughout the experiment in drinking water to prevent urinary tract infections. The animal’s health was monitored daily by assessing bodyweight, physical appearance (mobility, bite wounds) and signs of pain throughout the experiment.
UDI setup
UDI was performed as described by Schneider et al. [3] and is briefly summarized here. Mice were placed into an adapted restrainer (KN-326-4, Natsume Seisakusho Co., Ltd., Tokyo, Japan) with a hole at the bottom to allow urine collection on an analytical balance (ML303E/03, Mettler Toledo, Columbus, Ohio, USA) underneath the mouse. The restrainer was made of metal rods, adjusted in a way that mice were not able to turn around or walk forwards or backwards, but otherwise were in an awake and fully conscious state during UDI measurements, before the specified drugs were applied. The catheter was connected to a computer-controlled syringe pump (R-100EC, Razel Scientific Instruments, Saint Albans, Vermont, USA) with an in-line pressure transducer (TDR-100-1, Med Associates Inc., Fairfax, Vermont, USA) that measures the intravesical pressure during infusion. The pressure signal was then amplified (CSG-6080, Catamount R & D Inc., St. Albans, Vermont, USA). Where applicable, the EMG plug was connected to an amplifier (Model 1700, Science Products GmbH, Hofheim, Germany), where the EMG signal was band-pass filtered between 10-10,000 Hz and the gain was set to ×1,000. A notch filter was also included to reduce the power line interference. All signals were sampled through a data acquisition board (NI USB-6211, National Instruments, Austin, Texas, USA) at a sampling frequency of 500 Hz and recorded on the computer with a self-programmed LabVIEW v2012 (National Instruments, Austin, Texas, USA) application.
UDI measurements
Mice were connected to the UDI station and bladder was infused with NaCl 0.9% (Grosse Apotheke Dr. G. Bichsel AG, Interlaken, Switzerland) at a rate of 20 µl/min. After stable micturition cycles were established, the UDI was continued for four to five cycles in baseline (no drug) condition. After baseline recording, mice were subjected to the respective drugs. The following dosages were applied: 5 mg/kg (i.p.) midazolam (Dormicum® Midazolamum 5 mg/ml, Roche Pharma (Schweiz) AG, Reinach, Switzerland), 50 µg/kg (s.c.) fentanyl (Fentanyl Sintetica, Sintetica S.A., Mendrisio, Switzerland) and 250 µg/kg (s.c.) hydromorphone (Palladon® Inject, Hydromorphoni hydrochloridum 2 mg/ml, Mundipharma. Basel, Switzerland). Only one drug was applied at a time and the mice were allowed to recover for at least one day between the UDI measurements.
UDI analysis
All urodynamic data was analysed using MATLAB® (The MathWorks Inc., Massachusetts, USA). Per mouse and measurement condition, three micturition cycles (where applicable) were analyzed and mean values calculated. Intravesical pressure measurements were normalized to the lowest value per micturition cycle. The maximum detrusor pressure (Pmax) was the highest intravesical pressure recorded within one micturition cycle. The threshold detrusor pressure (Pthresh) was defined as the intravesical pressure when the derivative of the smoothed pressure signal (moving average of 50) for the first time reached 65% of the maximal derivative within 20 s prior to Pmax. The micturition cycle duration (Tmic) was the time from the beginning of the micturition cycle till the end of the cycle. Filling volume (Vfilling) was defined as the volume being infused into the bladder from the cycle beginning till Pthresh was reached.
EUS-EMG analysis
The time-frequency analysis of the EUS-EMG signal was performed by implementing the Hilbert-Huang transform [12]. The total energy at low frequencies (0-20 Hz) and high frequencies (21-250 Hz) per time point were calculated respectively. We also summed up total energy across all frequencies (0-250 Hz). Then the time from Pthresh to Pmax was divided into equal fifths. The middle (third) fifth was considered the section when voiding happens, thus the EUS-EMG energy content of this section was compared to the energy content of the sections before (first two fifths) and after (last two fifths) voiding. The energy content was normalized to the duration of the respective section and is shown as a percentage between these three sections (before, during and after voiding).
Euthanasia & tissue processing
At least one day after the last UDI and three weeks after the implantation surgery, mice were deeply anesthetized with an overdose i.p. injection of Esconarkon® ad. us. vet. (150 mg/kg, Streuli Pharma AG, Uznach, Switzerland) diluted 1:3 in NaCl 0.9% (Grosse Apotheke Dr. G. Bichsel AG, Interlaken, Switzerland). As soon as the respiratory reflex arrested, mice were transcardially perfused (7.6 ml/min) with chilled (4°C) NaCl 0.9% (Grosse Apotheke Dr. G. Bichsel AG, Interlaken, Switzerland) for two min followed by chilled (4°C) 4% paraformaldehyde (Sigma-Aldrich Chemie GmbH, Buchs, Switzerland) diluted in 0.1 M phosphate buffer (pH 7.3) for five minutes. The urethra was exposed below the abdominal muscle and the placing of electrodes controlled post-mortem. Subsequently, bladders were excised, weighted and covered in the same fixative overnight, then stored in PBS with 30% sucrose (84097-250G, Sigma-Aldrich Chemie GmbH, Buchs, Switzerland) at 4°C. Thereafter, bladders were enclosed in TissueTek® O.C.T.TM compound (Sakura, Staufen, Germany) and cryosectioned at five µm thickness. A Masson’s trichrome stain (ab150686, Abcam, Cambridge, England) was performed according to the user manual. Images were taken with a slide scanner (Pannoramic 250 Flash II, 3DHistech, Budapest, Hungary). One section per mouse was analysed using ImageJ [13] to assess bladder fibrosis by calculating the ratio of collagen tissue to the whole bladder tissue. The analysed region of interest (ROI) was selected to include a homogeneous selection throughout all the bladder layers, excluding folded or broken tissue areas. The analysis was run by two experimenters blinded for the animal groups, then mean values were used for the statistical analysis. Additionally, bladder- to bodyweight ratio was calculated by dividing the bladder weight by the mean of the animal’s bodyweight measured on the last three consecutive days.
Statistical analysis
Body weight and bladder- to bodyweight ratio as well as collagen content analysis of unrelated samples (Ctrl., Cath. + El. and Cath. groups) were compared using a one-way ANOVA followed by Tukey’s multiple comparison testing. UDI parameters of unrelated samples (Cath. + El. and Cath. groups) were tested for statistical significance using an unpaired t-test. A one-way repeated-measures ANOVA followed by Bonferroni’s post hoc testing was applied to each UDI parameter within a group, comparing the baseline measurement to the three drug conditions. Differences were considered significant at P<0.05. Statistical analyses were performed using the Stata software (version 14.2, StataCorp, TX, USA) and GraphPad Prism (version 7.04, GraphPad Software, Inc., CA, USA).
Results
Repeated UDI including EUS-EMG measurements reveal effects of anaesthetics on bladder function
Implantation of catheter and electrodes was generally well tolerated. One mouse in the Cath. + El. group was excluded and euthanized two days after surgery due to unrecoverable dehydration. Another three mice from the same group destroyed the wires from their implanted electrodes (one prior to baseline UDI, two prior to UDI under midazolam), and were included in the UDI analysis only.
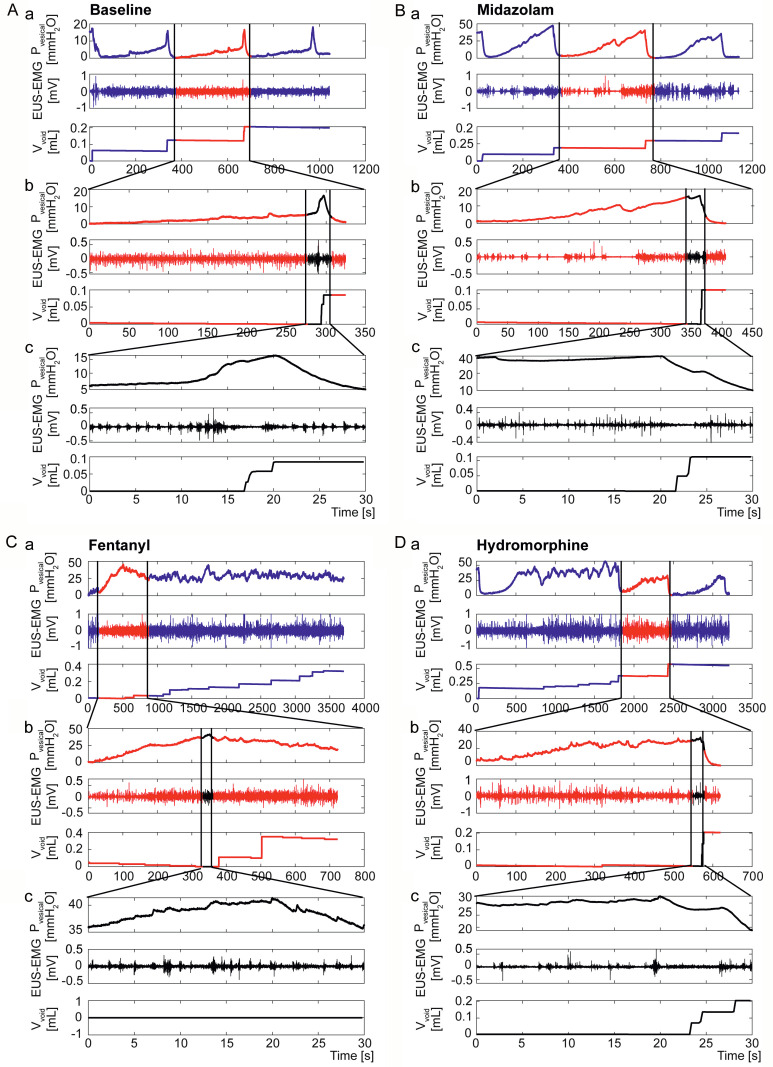
Baseline UDI including EUS-EMG was recorded prior to drug application. Representative traces of intravesical pressure under all three drug conditions compared to baseline are shown in Figure 1. On the day of experiment, after stable micturition cycles were established, the UDI was performed for four to five cycles to record baseline (no drug) condition. Baseline traces showed a slow increase in intravesical pressure during the filling phase followed by a sharp pressure increase, concomitant with bladder emptying (Figure 1A). After baseline recording, mice were subjected to the respective drugs. Under midazolam, intravesical pressure rose constantly during the filling phase at a higher rate than in the baseline condition followed by a sudden pressure decrease with bladder emptying followed by relaxation (Figure 1B). With fentanyl intravesical pressure rose continuously during the filling phase, Pmax reached a much higher level than baseline and remained at that level (Figure 1C). Under hydromorphine, intravesical pressure during the filling phase increased at a rate comparable to baseline, however Pmax reached a higher level and oscillated for varying periods of time, before a rapid decrease occurred (Figure 1D). Such high pressure oscillations were observed in all animals under fentanyl (100%), in 60% of animals (67% in the Cath. and 56% in the Cath. + El. group) under hydromorphine and in 20% of animals (33% in the Cath. group and 16% in the Cath. + El. group) under midazolam. Analyses on Vfilling, and Tmic were excluded in these cases. The entire UDI recording of one mouse (Cath. group) under midazolam was excluded from the analysis as the catheter was partially blocked and saline infusion into the bladder was unreliable.
Figure 1.
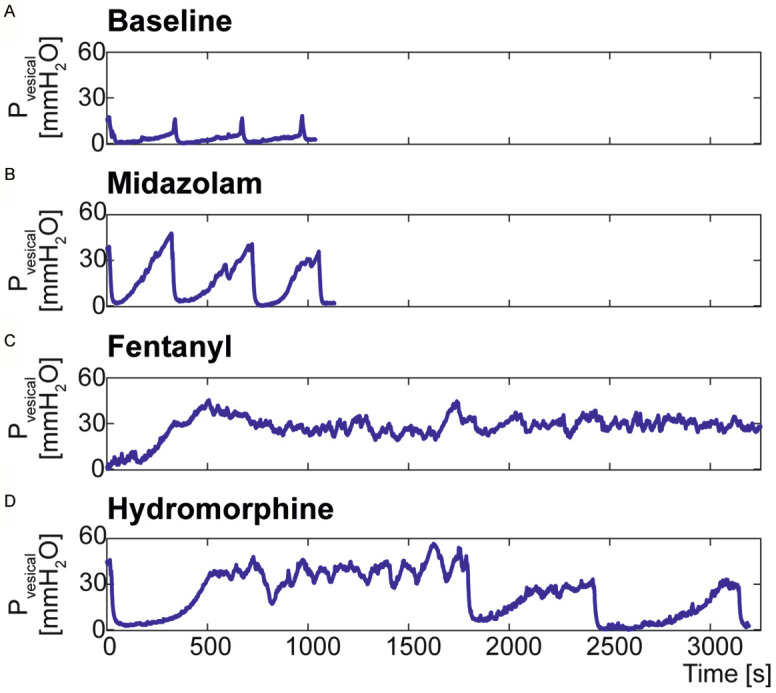
Representative UDI traces of a Cath. + El. mouse under all measurement conditions. Shown is the intravesical pressure (Pvesical) during three micturition cycles under baseline (A), midazolam (B), fentanyl (C) and hydromorphine (D) conditions.
Simultaneous UDI and EMG recordings with and without anaesthesia
Detailed UDI/EMG recordings with a zoom window into one micturition cycle and a further zoom into 30 s around the peak of intravesical pressure (20 s before and 10 s after) are shown in Figure 2 for baseline, midazolam, fentanyl and hydromorphine. In the baseline UDI (Figure 2A), a clear drop in EUS-EMG activity was observed in the midsection of the steep intravesical pressure increase, concomitant with voiding recorded on the scale or visualized. Under midazolam (Figure 2B), no steep intravesical pressure increase was observed, although bladder emptying was achieved efficiently. Here, the EUS-EMG activity was reduced after Pmax was reached, indicating that voiding depended solely on EUS relaxation. Three animals (two Cath. and one Cath. + El.) showed a delayed bladder emptying (20 to 27 min) in one micturition cycle, indicating a temporal loss of bladder control. Under fentanyl (Figure 2C), only one animal (Cath. group) recovered within 45 min from the high intravesical pressure oscillations and returned to the characteristic intravesical pressure cycling of the micturition cycles, while none of other mice could regain bladder control within this time span. There was no correlation between the EUS-EMG pattern and the intravesical pressure in all mice with a continuous high intravesical pressure and dribbling while EUS-EMG activity remained unchanged. Under hydromorphine (Figure 2D), nine mice (four in the Cath. Group and five in the Cath. + El. Group) temporally lost bladder control, lasting between 25 min and up to the end of the recording. When intravesical pressure oscillated at a high level (between 25-50 mmH2O) urine dribbling was observed with no coordination between intravesical pressure and EUS-EMG (Figure 2D). Animals that were able to empty the bladder achieved this by EUS relaxation alone, without detrusor contraction (Figure 2D).
Figure 2.
Representative UDI traces of a mouse with implanted catheter and electrodes under all four conditions. Baseline UDI trace without drug exposure (A). UDI trace after the administration of midazolam (B), fentanyl (C) and hydromorphine (D). Represented are three micturition cycles (a), a zoom to one cycle (b) and a zoom to the region around the maximal detrusor pressure (c). The intravesical pressure (Pvesical) is shown in the upper panel, the EUS-EMG in the middle panel, and the voided volume (Vvoid) in the lower panel.
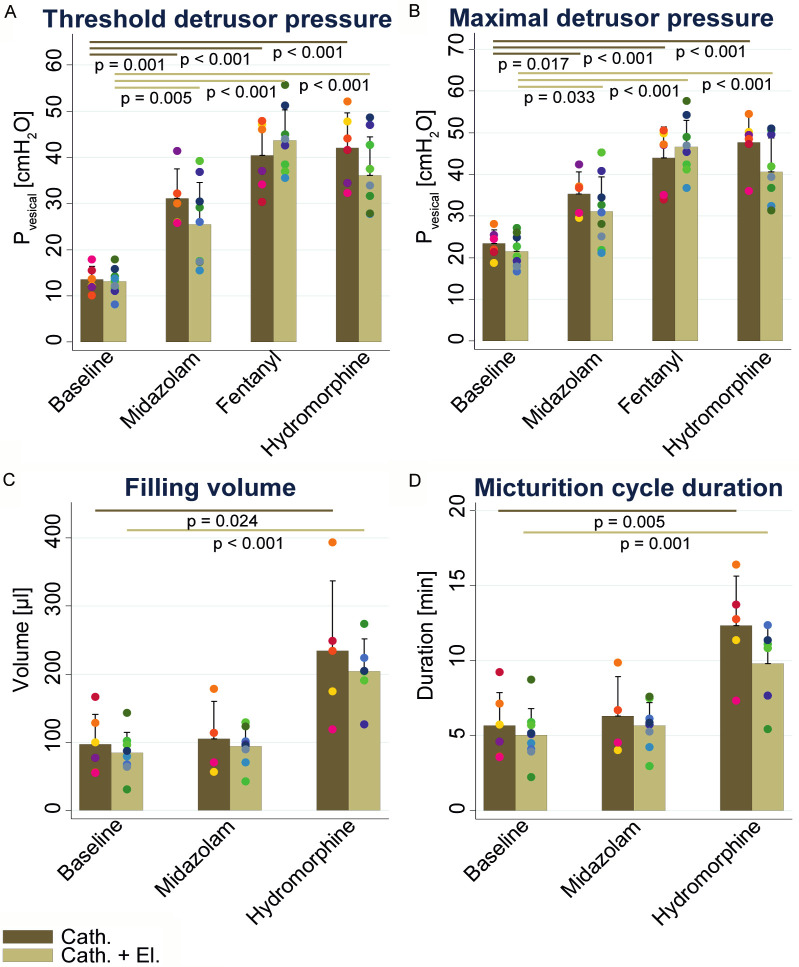
The analysed UDI parameters are summarized in Figure 3, and the group mean ± SD and P-values (compared to baseline condition) for each UDI parameter are listed in Table 1. Significant increases in Pthresh and Pmax were measured in all three drug conditions compared to baseline (Cath. group: P=0.001 & P=0.017 for midazolam; P<0.001 & P<0.001 for fentanyl and P<0.001 & P<0.001 for hydromorphine; Cath. + El. group: P=0.005 & P=0.033 for midazolam; P<0.001 & P<0.001 for fentanyl and P<0.001 & P<0.001 for hydromorphine; Figure 3A, 3B). Vfilling and Tmic were analysed for baseline, midazolam and hydromorphine conditions only, as under fentanyl administration, no micturition cycles were observed in any animal. Vfilling and Tmic were significantly increased upon hydromorphine (Cath. group: P=0.024 & P=0.005; Cath. + El. group: P<0.001 & P=0.001) administration compared to baseline while midazolam had no influence (Figure 3C, 3D). No differences in the parameters Pthresh, Pmax, Vfilling and Tmic across the measurement conditions were found between the Cath. and Cath. + El. groups (Figure 3).
Figure 3.
UDI parameters of the Cath. and Cath. + El. group at baseline and under the influence of midazolam, fentanyl and hydromorphine. Threshold detrusor pressure [mmH2O] (A). Maximal detrusor pressure [mmH2O] (B). Bladder filling volume [µl] (C). Micturition cycle duration [s] (D). Data are presented as means ± SD with color-coded dots representing the value of each animal and statistical significance (P<0.05) are indicated.
Table 1.
UDI outcome of the Cath. and Cath. + El. Group
| Baseline | Midazolam | Fentanyl | Hydromorphine | ||
|---|---|---|---|---|---|
| Pthresh | Cath. | 13.57±2.8 | 31.11±6.4 (P=0.001) | 40.46±7.56 (P<0.001) | 42.06±7.61 (P<0.001) |
| Cath. + El. | 13.14±2.84 | 25.54±9.01 (P=0.005) | 43.72±6.54 (P<0.001) | 36.12±8.32 (P<0.001) | |
| Pmax | Cath. | 23.38±3.31 | 35.33±5.22 (P=0.017) | 43.95±7.43 (P<0.001) | 47.67±6.2 (P<0.001) |
| Cath. + El. | 21.47±3.85 | 31.17±8.22 (P=0.033) | 46.57±6.44 (P<0.001) | 40.53±8.23 (P<0.001) | |
| Vfilling | Cath. | 97.29±43.96 | 104.94±54.89 (P=1) | NA | 234.01±102.93 (P=0.024) |
| Cath. + El. | 84.3±30.63 | 93.9±27.66 (P=1) | NA | 203.84±47.71 (P<0.001) | |
| Tmic | Cath. | 5.66±2.22 | 6.3±2.66 (P=1) | NA | 12.33±3.33 (P=0.005) |
| Cath. + El. | 5.05±1.77 | 5.67±1.55 (P=1) | NA | 9.81±2.66 (P=0.001) |
Shown are mean values ± SD and P-values (in brackets) comparing midazolam, fentanyl and hydromorphine to the respective baseline parameter. Vfilling: filling volume, corresponding to bladder capacity, Pmax: maximal detrusor pressure, Pthresh: threshold detrusor pressure, Tmic: micturition cycle duration.
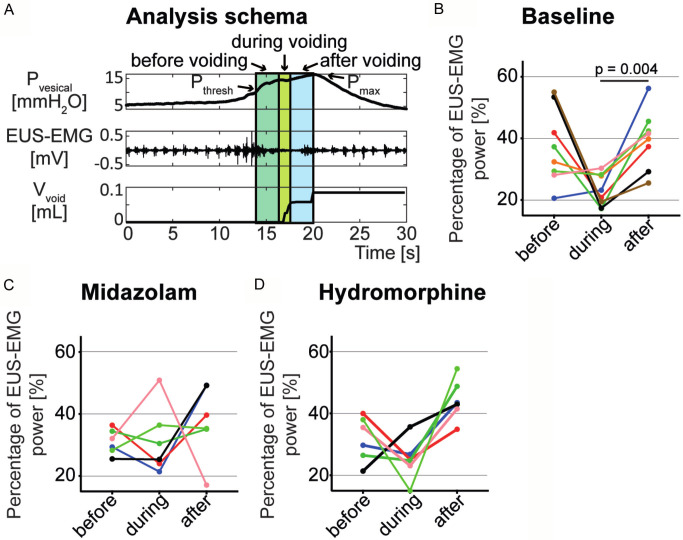
Quantification of the EMG results
In order to analyse EUS-EMG recordings, the power (energy per unit time) of the EUS-EMG signal during voiding was compared to the power before and after voiding. Figure 4A shows the three analysed time sections in a standard micturition cycle in relation to Pthresh and Pmax. The frequencies from 0-20 Hz (mainly corresponding to smooth muscle activity [14]) and 21-250 Hz (mainly corresponding to striated muscle activity [15]) were analysed independently, however the activity pattern was very similar (data not shown). Thus, all frequencies from 0-250 Hz were summed up and are shown in Figure 4B-D. An increased EMG activity was observed before voiding (compared to during voiding) in six out of eight mice. EMG activity was significantly reduced during voiding compared to after voiding in non-anaesthetized mice (P=0.004; Figure 4B). Under the influence of midazolam and hydromorphine, the EUS-EMG activity showed a less uniform activity pattern between Pthresh and Pmax compared to baseline, although a tendency towards increased activity was observed after voiding (Figure 4C, 4D).
Figure 4.
The activity pattern of the EUS measured by EMG before, during and after voiding. The three analysed time periods, with the middle one corresponding to the time of voiding are illustrated on the intravesical pressure (Pvesical), EUS-EMG and voided volume (Vvoid) graphs of the baseline condition, in relation to threshold detrusor pressure (Pthresh) and maximal detrusor pressure (Pmax) (A). The percentage of EMG power for baseline (B), midazolam (C) and hydromorphine (D) conditions are shown for each mouse with a unique color and statistical significance (P<0.05) is indicated.
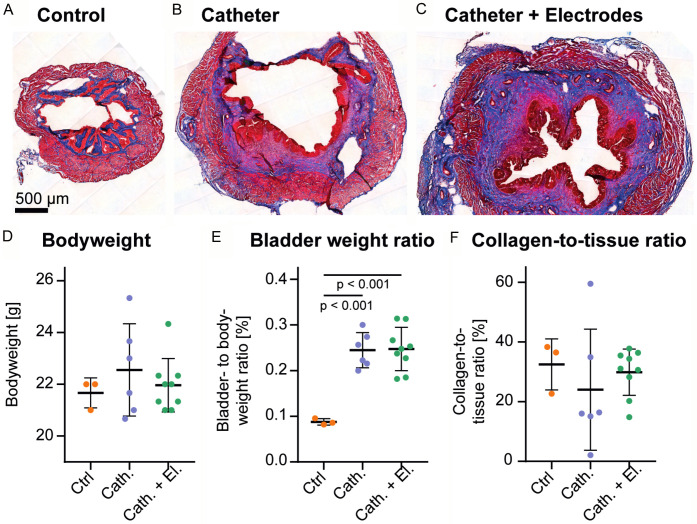
Histology
Masson’s trichrome stained bladder tissue of all three animal groups was analysed to assess fibrotic organ remodeling following catheter and electrode implantation (Figure 5A-C). There was no significant difference in bodyweight at the end of the experiment between groups (Figure 5D). However, the bladder- to bodyweight ratio was significantly increased in animals that underwent surgery (Cath. group: P<0.001; Cath. + El. group; P<0.001; Figure 5E), indicating an increase in bladder weight. Tissue collagenation level showed no difference three weeks post-surgery, thus the bladder weight increase was not due to fibrotic remodeling (Figure 5F).
Figure 5.
Histological examination of bladder tissue by Masson’s trichrome staining. A representative image of a bladder from the Ctrl (A), Cath. (B) and Cath. + El. (C) groups are shown. The animal’s bodyweight (D), the bladder- to bodyweight ratio (E) and the percentage of collagen- to the whole tissue ratio (F) are represented as group means ± SD, showing single animals as dots and statistical significance (P<0.05) is indicated.
Discussion
We performed repeated UDI and EUS-EMG activity measurements in the same mouse over a period of several days. Under baseline conditions, the UDI recordings reproducibly showed characteristic intravesical pressure tracings with a continuous but slight pressure increase during the filling phase (high Vfilling), followed by a sharp rise and fall in intravesical pressure concomitant with urine expulsion during the voiding phase of the micturition cycle.
The EUS-EMG methodology described by Schneider et al. [3] in the rat model was successfully applied in mice. We observed a high overall activity (0-250 Hz) before and after voiding, with a lower energy phase during voiding corresponding to a coordinated EUS relaxation during bladder contraction. No differences in EUS activity were observed in lower (0-20 Hz, representing mainly smooth muscle activity [14]) and higher (21-250 Hz, representing mainly striated muscle activity [15]) frequencies. In contrast, rats showed specific high frequency EUS activity before and after voiding and low frequency activity during voiding [3,16].
While awake restrained cystometry in mice has been reported previously [17], this is the first time that repeated EUS-EMG was performed in mice following implantation of electrodes on both sides of the EUS. In prior publications EUS-EMG in restrained awake mice were carried out immediately after electrode placement in the sphincter muscle after which the mice were euthanized [1,2]. In our study, the electrodes were placed next to the EUS and fixated to the lateral fat tissue to avoid muscle trauma. We cannot exclude EUS-EMG measurements of other pelvic floor muscles contributed to recording EUS activity. Although in our setting the electrode positioning could not be confirmed between repeated measurements, it was monitored post-mortem at the end of the study; our results show that the presence of electrodes next to the EUS did not obstruct the bladder outlet, as no differences in functional parameters were detected between the Cath. and Cath. + El. groups. Furthermore, neither bladder- to bodyweight ratio nor fibrosis was increased three weeks after electrode implantation compared to the Cath. group. Similar to the observation made earlier in rats [3], catheter implantation might have caused urothelial and sub-urothelial thickening due to oedema as a result of irritation by the surgery and/or the catheter. Despite these changes, this procedure does not impart the micturition, as it has been shown that LUT function in animals with catheter and electrode implantation was indistinguishable from assessments done in the metabolic cage [18].
Fentanyl and hydromorphine administration lead to a significant increase of Pthresh and Pmax, and disrupted the voiding phase evidenced by the reduced coordination of EUS activity with detrusor contraction, which resulted in urine dribbling. A similar loss of voiding phase was observed in rats subjected to various anaesthetic agents [3,19,20]. In accordance, systemic administration of fentanyl or morphine is known to cause urinary retention in human patients [6], and our observations point to the urine retention and overflow incontinence in fentanyl-treated mice. Inhumans, opioids decrease the force of detrusor contraction, decrease the sensation of fullness, but probably do not increase sphincter tone. Typical μ-opioid agonists such as morphine and fentanyl produce these effects, and naloxone and methylnaltrexone can reverse them [21], suggesting that a significant component of the effect is owing to actions on the brain and spinal cord, although peripheral effects at the bladder may also play a role. Anaesthesia with urethane is preferably used in animal UDI studies since the voiding reflex is considered to be unaffected, however the preservation of normal intravesical pressures is controversial and bladder capacity was found to be reduced [8,9,22,23].
UDI following midazolam exposure demonstrated bladder emptying, albeit with a threefold increase of Pthresh and an increase of Pmax by around 50% (Table 1). We hypothesize that bladder emptying was achieved by EUS relaxation only, as no sharp increase in intravesical pressure resulting from detrusor contraction could be observed. These results are in accordance with previous observations of a continuous leakage of urine after fentanyl-fluanisone-midazolam application in rodents, suggestive of EUS relaxation [11]. Overall, GABA receptors agonists are thought to induce detrusor relaxation, though the exact rationale behind this is still unclear [7,24]. One could argue that administration of GABA receptors agonists inhibits voiding mainly due to the effect on the central nervous system [25]. Our results confirm this hypothesis in mice but are in contradiction to findings in rats, where UDI parameters remains similar to baseline under 25 mg/kg i.p. of midazolam [26].
Using combined UDI and EUS-EMG measurements, the effect of drugs on both the bladder and the bladder outlet can be assessed in awake restrained mice, thus increasing the translational value of this model. Further advantages of this method are its increased statistical power (the same mice were used in different measurement conditions, so samples are paired) and conformity with the 3R principle of animal research because it allows long-term repeated measurements, reducing the number of experimental animals needed.
In summary, we showed that simultaneous UDI and EUS-EMG measurements could be repeatedly performed in the same mouse in awake conditions. This method is suitable for long-term studies, where UDI may be carried out frequently during the progression of LUTD in order to identify the critical changes in bladder and EUS function. Furthermore, we showed that similar to humans, in mice the anaesthetic agents acting on GABA (midazolam) or μ-receptors (fentanyl and hydromorphine) relevantly disrupted bladder and EUS function. The possibility to study bladder function and EUS activity in awake animals highly increases translational values of mouse models of LUTD, including the role of DSD in neurogenic LUTD.
Acknowledgements
The authors thank PD Dr. Francesco Clavica for his scientific support in UDI and EMG data analysis and Andrea Mathys for histological processing of bladder tissue. This study was funded by the Swiss National Science Foundation (SNF Grant 310030_175773/1).
Disclosure of conflict of interest
None.
References
- 1.Kadekawa K, Yoshimura N, Majima T, Wada N, Shimizu T, Birder LA, Kanai AJ, de Groat WC, Sugaya K, Yoshiyama M. Characterization of bladder and external urethral activity in mice with or without spinal cord injury--a comparison study with rats. Am J Physiol Regul Integr Comp Physiol. 2016;310:R752–758. doi: 10.1152/ajpregu.00450.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DePaul MA, Lin CY, Silver J, Lee YS. Peripheral nerve transplantation combined with acidic fibroblast growth factor and chondroitinase induces regeneration and improves urinary function in complete spinal cord transected adult mice. PLoS One. 2015;10:e0139335. doi: 10.1371/journal.pone.0139335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schneider MP, Hughes FM Jr, Engmann AK, Purves JT, Kasper H, Tedaldi M, Spruill LS, Gullo M, Schwab ME, Kessler TM. A novel urodynamic model for lower urinary tract assessment in awake rats. BJU Int. 2015;115(Suppl 6):8–15. doi: 10.1111/bju.13039. [DOI] [PubMed] [Google Scholar]
- 4.Verhamme KM, Sturkenboom MC, Stricker BH, Bosch R. Drug-induced urinary retention: incidence, management and prevention. Drug Saf. 2008;31:373–388. doi: 10.2165/00002018-200831050-00002. [DOI] [PubMed] [Google Scholar]
- 5.Herman RM, Wainberg MC, delGiudice PF, Willscher MK. The effect of a low dose of intrathecal morphine on impaired micturition reflexes in human subjects with spinal cord lesions. Anesthesiology. 1988;69:313–318. doi: 10.1097/00000542-198809000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Wuethrich PY, Burkhard FC. Thoracic epidural analgesia: what about the urinary bladder? Trends in Anaesthesia and Critical Care. 2012;2:138–144. [Google Scholar]
- 7.Baldini G, Bagry H, Aprikian A, Carli F. Postoperative urinary retention: anesthetic and perioperative considerations. Anesthesiology. 2009;110:1139–1157. doi: 10.1097/ALN.0b013e31819f7aea. [DOI] [PubMed] [Google Scholar]
- 8.Andersson KE, Soler R, Fullhase C. Rodent models for urodynamic investigation. Neurourol Urodyn. 2011;30:636–646. doi: 10.1002/nau.21108. [DOI] [PubMed] [Google Scholar]
- 9.Ito H, Pickering AE, Igawa Y, Kanai AJ, Fry CH, Drake MJ. Muro-neuro-urodynamics; a review of the functional assessment of mouse lower urinary tract function. Front Physiol. 2017;8:49. doi: 10.3389/fphys.2017.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olkkola KT, Ahonen J. Midazolam and other benzodiazepines. In: Schüttler J, Schwilden H, editors. Handbook of experimental pharmacology. Berlin, Heidelberg: Springer; 2008. pp. 335–360. [DOI] [PubMed] [Google Scholar]
- 11.Abdelkhalek AS, Youssef HA, Saleh AS, Bollen P, Zvara P. Anesthetic protocols for urodynamic studies of the lower urinary tract in small rodents-A systematic review. PLoS One. 2021;16:e0253192. doi: 10.1371/journal.pone.0253192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang NE, Shen S. Hilbert-Huang transform and its applications. Interdisciplinary Mathematical Sciences. 2014 [Google Scholar]
- 13.Schindelin J, Rueden CT, Hiner MC, Eliceiri KW. The ImageJ ecosystem: an open platform for biomedical image analysis. Mol Reprod Dev. 2015;82:518–529. doi: 10.1002/mrd.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domino M, Pawlinski B, Gajewski Z. Biomathematical pattern of EMG signal propagation in smooth muscle of the non-pregnant porcine uterus. PLoS One. 2017;12:e0173452. doi: 10.1371/journal.pone.0173452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solomonow M, Baten C, Smit J, Baratta R, Hermens H, D’Ambrosia R, Shoji H. Electromyogram power spectra frequencies associated with motor unit recruitment strategies. J Appl Physiol (1985) 1990;68:1177–1185. doi: 10.1152/jappl.1990.68.3.1177. [DOI] [PubMed] [Google Scholar]
- 16.Schneider MP, Sartori AM, Ineichen BV, Moors S, Engmann AK, Hofer AS, Weinmann O, Kessler TM, Schwab ME. Anti-nogo-A antibodies as a potential causal therapy for lower urinary tract dysfunction after spinal cord injury. J Neurosci. 2019;39:4066–4076. doi: 10.1523/JNEUROSCI.3155-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inouye BM, Hughes FM Jr, Jin H, Lütolf R, Potnis KC, Routh JC, Rouse DC, Foo WC, Purves JT. Diabetic bladder dysfunction is associated with bladder inflammation triggered through hyperglycemia, not polyuria. Res Rep Urol. 2018;10:219–225. doi: 10.2147/RRU.S177633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider MP, Sartori AM, Tampé J, Moors S, Engmann AK, Ineichen BV, Hofer AS, Schwab ME, Kessler TM. Urodynamic measurements reflect physiological bladder function in rats. Neurourol Urodyn. 2018;37:1266–1271. doi: 10.1002/nau.23455. [DOI] [PubMed] [Google Scholar]
- 19.Yaksh TL, Durant PA, Brent CR. Micturition in rats: a chronic model for study of bladder function and effect of anesthetics. Am J Physiol. 1986;251:R1177–1185. doi: 10.1152/ajpregu.1986.251.6.R1177. [DOI] [PubMed] [Google Scholar]
- 20.Matsuura S, Downie JW. Effect of anesthetics on reflex micturition in the chronic cannula-implanted rat. Neurourol Urodyn. 2000;19:87–99. doi: 10.1002/(sici)1520-6777(2000)19:1<87::aid-nau9>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 21.Rosow CE, Gomery P, Chen TY, Stefanovich P, Stambler N, Israel R. Reversal of opioid-induced bladder dysfunction by intravenous naloxone and methylnaltrexone. Clin Pharmacol Ther. 2007;82:48–53. doi: 10.1038/sj.clpt.6100164. [DOI] [PubMed] [Google Scholar]
- 22.Cannon TW, Damaser MS. Effects of anesthesia on cystometry and leak point pressure of the female rat. Life Sci. 2001;69:1193–1202. doi: 10.1016/s0024-3205(01)01182-1. [DOI] [PubMed] [Google Scholar]
- 23.Smith PP, Kuchel GA. Continuous uroflow cystometry in the urethane-anesthetized mouse. Neurourol Urodyn. 2010;29:1344–1349. doi: 10.1002/nau.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyazato M, Sasatomi K, Hiragata S, Sugaya K, Chancellor MB, de Groat WC, Yoshimura N. GABA receptor activation in the lumbosacral spinal cord decreases detrusor overactivity in spinal cord injured rats. J Urol. 2008;179:1178–1183. doi: 10.1016/j.juro.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pehrson R, Lehmann A, Andersson KE. Effects of gamma-aminobutyrate B receptor modulation on normal micturition and oxyhemoglobin induced detrusor overactivity in female rats. J Urol. 2002;168:2700–2705. doi: 10.1016/S0022-5347(05)64247-4. [DOI] [PubMed] [Google Scholar]
- 26.Ozkurkcugil C, Ozkan L. Effects of anesthetics on cystometric parameters in female rats. Int Urol Nephrol. 2010;42:909–913. doi: 10.1007/s11255-010-9745-4. [DOI] [PubMed] [Google Scholar]