Abstract
Recent data suggest that the disease-associated microenvironment, known as the leukemic stem cell (LSC) niche, is substantially involved in drug resistance of LSC in BCR-ABL1+ chronic myeloid leukemia (CML). Attacking the LSC niche in CML may thus be an effective approach to overcome drug resistance. We have recently shown that osteoblasts are a major site of niche-mediated LSC resistance against second- and third-generation tyrosine kinase inhibitors (TKI) in CML. In the present study, we screened for drugs that are capable of suppressing the growth and viability of osteoblasts and/or other niche cells and can thereby overcome TKI resistance of CML LSC. Proliferation was analyzed by determining 3H-thymidine uptake in niche-related cells, and apoptosis was measured by Annexin-V/DAPI-staining and flow cytometry. We found that the dual PI3 kinase (PI3K) and mTOR inhibitor BEZ235 and the selective pan-PI3K inhibitor copanlisib suppress proliferation of primary osteoblasts (BEZ235 IC50: 0.05 μM; copanlisib IC50: 0.05 μM), the osteoblast cell line CAL-72 (BEZ235 IC50: 0.5 μM; copanlisib IC50: 1 μM), primary umbilical vein-derived endothelial cells (BEZ235 IC50: 0.5 μM; copanlisib IC50: 0.5 μM), and the vascular endothelial cell line HMEC-1 (BEZ235 IC50: 1 μM; copanlisib IC50: 1 μM), whereas no comparable effects were seen with the mTOR inhibitor rapamycin. Furthermore, we show that BEZ235 and copanlisib cooperate with nilotinib and ponatinib in suppressing proliferation and survival of osteoblasts and endothelial cells. Finally, BEZ235 and copanlisib were found to overcome osteoblast-mediated resistance against nilotinib and ponatinib in K562 cells, KU812 cells and primary CD34+/CD38- CML LSC. Together, targeting osteoblastic niche cells through PI3K inhibition may be a new effective approach to overcome niche-induced TKI resistance in CML. Whether this approach can be translated into clinical application and can counteract drug resistance of LSC in patients with CML remains to be determined in clinical trials.
Keywords: CML, BCR-ABL1 inhibitors, leukemic stem cells, drug resistance, stem cell niche, PI3 kinase, mTOR, precision medicine
Introduction
Chronic myeloid leukemia (CML) is a hematopoietic stem cell disease characterized by expansion of myelopoietic (progenitor) cells in the bone marrow (BM) and blood, and the reciprocal chromosome translocation t(9;22) [1]. The resulting fusion gene product, BCR-ABL1, is a cytoplasmic 210 kDa oncoprotein that acts as a major driver of chronic phase (CP) CML [1-5]. In particular, BCR-ABL1 augments a number of critical downstream signaling cascades and molecules, including the phosphoinositide-3-kinase (PI3K), AKT, the mechanistic target of rapamycin (mTOR), the RAS-MEK-ERK pathway, and the signal transducer and activator of transcription 5 (STAT-5) [1-6]. These signaling molecules and pathways are considered to act together to trigger oncogenesis and leukemic cell growth in patients with CML.
In the past 20 years, a number of BCR-ABL1-targeting drugs have been developed. The first compound that led to a breakthrough in the treatment of CML was imatinib [7-11]. In fact, therapy with imatinib can induce major cytogenetic and molecular responses in a majority of all patients with CP CML [8-10]. Therefore, imatinib has long been considered as the ‘gold-standard’ in the treatment of this disease [11-13]. However, not all patients with CML respond to imatinib, and others may respond initially but later develop a relapse [10-13]. In addition, dose-limiting side effects may occur during treatment with imatinib [8-11]. More recently, second- and third generation BCR-ABL1 tyrosine kinase inhibitors (TKI) have been developed and have successfully been translated into clinical practice. These drugs include nilotinib, dasatinib, bosutinib, ponatinib, and asciminib [14-26].
Most patients with imatinib-resistant CML respond to these novel BCR-ABL1-targeting agents [11,18-26]. However, even these BCR-ABL1 TKI are not always able to control the disease for unlimited time periods in CML, and toxicities produced by these agents may be substantial [18-27]. In non-responding patients, leukemic stem cells (LSC) are often resistant against most or all of the above mentioned TKI [12,13,28-32].
A number of different mechanisms have been described to contribute to the resistance of LSC against TKI in CML [28-36]. Acquired resistance is a sub-clone-specific phenomenon and is often associated with the occurrence of (secondary) BCR-ABL1 mutations [12,13,33,36-38]. By contrast, intrinsic stem cell resistance is a common mechanism shared by virtually all sub-clones and their LSC [13,30-32]. One important and clinically relevant form of LSC resistance is niche-mediated resistance [32,34,35,39-42]. However, so far, little is known about the molecular mechanisms and cells contributing to this form of drug resistance in CML.
Several previous studies have suggested that stromal cells and mesenchymal stem cells play a role in niche-mediated LSC resistance [39-42]. More recently, we and others have shown that osteoblasts can contribute to drug resistance [43,44]. In particular, osteoblasts can mediate resistance of CML LSC against BCR-ABL1 TKI, including nilotinib and ponatinib [43]. Other studies have shown that angiogenic factors and endothelial cells may contribute to drug resistance in CML [45]. In addition, endothelial cell growth and angiogenesis are known to correlate with disease progression and prognosis in CML [46,47]. All in all, niche-related cells, including osteoblasts and endothelial cells, are considered to contribute to TKI resistance in CML.
Therefore, niche cells have also been discussed as potential therapeutic targets in the context of CML. However, little is known about the effects of various TKI and other drugs on osteoblasts and endothelial cells in CML. It also remains unknown whether targeting of osteoblasts may overcome osteoblast-induced LSC resistance in CML LSC. The aims in our current study were to define mechanisms and signaling pathways contributing to niche-induced resistance of LSC against TKI and to examine the effects of various signal transduction inhibitors and other drugs on growth and survival of niche-related cells and on niche-induced resistance of CML LSC.
Materials and methods
Reagents
Imatinib and nilotinib were purchased from Chemietek (Indianapolis, IN, USA), rapamycin from Calbiochem (San Diego, CA, USA), and BEZ235, copanlisib, ponatinib (AP24534) and asciminib (ABL001) from Selleck Chemicals (Houston, TX, USA). Endothelial cell growth basal medium 2 (EBM-2), endothelial cell growth supplement 2 (EGM-2), RPMI 1640 medium and antibiotics (penicillin, streptomycin) were from Lonza (Basel, Switzerland), amphotericin B from PAN-Biotech (Aidenbach, Germany) and bovine serum albumin (BSA) from Affymetrix/USB (Cleveland, OH, USA). Dulbecco’s Modified Eagle’s medium (DMEM), L-glutamine, MCDB 131 medium, fetal calf serum (FCS), trypsin-EDTA, insulin-transferrin-selenium supplement and non-essential amino acids were purchased from Gibco Life Technologies (Carlsbad, CA, USA), HEPES from Boehringer Ingelheim (Mannheim, Germany), EDTA from Pierce (Rockford, IL, USA), and osteoblast growth medium and supplement from PromoCell (Heidelberg, Germany). 3H-thymidine was purchased from PerkinElmer (Waltham, Massachusetts, USA), trypan blue, 4’,6-diamidino-2-phenylindole (DAPI) and sodium pyruvate from Sigma-Aldrich (St. Louis, Missouri, USA) and Ficoll from Biochrom (Los Altos, CA, USA). Stock solutions of drugs were prepared by dissolving in dimethylsulfoxide (DMSO) (Sigma-Aldrich). When tested as vehicle-control, DMSO showed no inhibitory effects on cell growth. Monoclonal antibodies (mAb) used in this study (for flow cytometry or Western blotting) are shown in Table S1.
Primary cells, cell lines and culture conditions
Primary leukemic cells were obtained during routine diagnostic investigations from 12 patients with BCR-ABL1+ CML. The patients’ characteristics are shown in Table S2. Samples were collected from the BM or peripheral blood (PB) and were stored in a local biobank. All patients gave written informed consent before samples were collected. The study was approved by the ethics committee of the Medical University of Vienna and conducted in accordance with the declaration of Helsinki. BM cells were layered over Ficoll to isolate mononuclear cells (MNC). These cells were stored in a local biobank until used. Normal healthy CD34+ BM stem/progenitor cells were purchased from Lonza and were cultured in StemPro-34 SFM medium supplemented with StemPro-34 nutrient supplement at 37°C and 5% CO2. The human CML cell line KU812 was kindly provided by Dr. Kenji Kishi (Niigata University, Niigata, Japan), and K562 were kindly provided by Dr. Michael Deininger (University of Utah, Salt Lake City, UT). Both cell lines were cultured in RPMI 1640 medium, 10% FCS and antibiotics at 37°C and 5% CO2. The microvascular human endothelial cell line HMEC-1 [48] was cultured in MCDB 131 medium supplemented with 10% FCS and antibiotics at 37°C and 5% CO2. Human umbilical vein-derived endothelial cells (HUVEC) were purchased from PromoCell (Heidelberg, Germany) and cultured in EBM-2 medium supplemented with EGM-2 at 37°C and 5% CO2. Primary human osteoblasts were purchased from PromoCell and were cultured in osteoblast growth medium with osteoblast growth supplement at 37°C and 5% CO2. The human osteoblast-like osteosarcoma cell line CAL-72 and the monocytic cell lines THP-1 and Mono Mac 6 were purchased from the German collection of microorganisms and cell culture (DSMZ, Leibniz, Germany). CAL-72 cells were cultured in DMEM with 10% FCS, 1% L-glutamine, 1% insulin-transferrin-selenium supplement and antibiotics at 37°C and 5% CO2. THP-1 cells were cultured in RPMI 1640 medium supplemented with 20% FCS and antibiotics at 37°C and 5% CO2. Mono Mac 6 cells were cultured in RPMI 1640 medium with 10% FCS, 2 mM L-glutamine, 2 mM non-essential amino acids, 1 mM sodium pyruvate, insulin-transferrin-selenium supplement (10 μg/ml) and antibiotics at 37°C and 5% CO2. Macrophages were differentiated from blood MNC of healthy donors using macrophage colony-stimulating factor (Biolegends, San Diego, CA, USA) and cultured in RPMI 1640 medium with 10% FCS and antibiotics at 37°C and 5% CO2 as reported [49]. All donors (n=3) gave written informed consent before samples were collected. The study was approved by the ethics committee of the Medical University of Vienna (EK number #75/2014) and conducted in accordance with the Declaration of Helsinki.
Measurement of 3H-thymidine uptake
To examine anti-proliferative drug effects, HMEC-1, HUVEC, CAL-72, primary human osteoblasts, Mono Mac 6, THP-1 and primary human macrophages were incubated in control medium or in various concentrations of BEZ235 (0.005-5 μM), copanlisib (0.005-5 μM), rapamycin (0.01-5 μM), nilotinib (0.1-5 μM), ponatinib (0.005-2 μM), asciminib (0.01-5 μM), or imatinib (0.1-10 μM) at 37°C for 48 hours. Thereafter, 0.5 μCi 3H-thymidine was added at 37°C for 16 hours. Then, cells were harvested on filter membranes (Packard Bioscience, Meriden, Connecticut) in a filtermate 196 harvester (Packard Bioscience). Filters were air-dried and the bound radioactivity was measured in a β-counter (TopCount NXT, Packard Bioscience). All experiments were performed in triplicates. In drug combination experiments, HMEC-1 and CAL-72 cells were incubated with BEZ235 and nilotinib, BEZ235 and ponatinib, copanlisib and nilotinib, copanlisib and ponatinib, either as single agents or in combination at a fixed ratio of drug concentrations as reported [50]. Drug combination effects were classified as additive or synergistic regarding growth-inhibition, based on calculations of combination index (CI) values using Calcusyn software (Calcusyn, Biosoft, Ferguson, MO, USA) as described [50]. A CI below 1 indicates a synergistic drug interaction.
Measurement of apoptosis
To analyze drug effects on cell survival and apoptosis, HMEC-1, HUVEC, CAL-72 cells, primary osteoblasts, Mono Mac 6, THP-1 and primary macrophages were incubated in control medium or in various concentrations of BEZ235 (1-5 μM), copanlisib (1-5 μM), rapamycin (1-5 μM), nilotinib (1-5 μM), ponatinib (0.1-1 μM), asciminib (1-5 μM), or imatinib (2.5-10 μM) at 37°C for 48 hours. Then, cells were harvested, washed, and incubated in Annexin-V-FITC (eBioscience, Vienna, Austria) and Annexin binding-buffer containing HEPES (10 mM, pH 7.4), NaCl (140 mM), and CaCl2 (2.5 mM) for 15 minutes. Then, cells were washed and DAPI (0.4 μg/mL) was added. Cells were analyzed by flow cytometry on a FACSCanto II (BD Biosciences, San Jose, CA, USA) as described [51,52]. The total fraction of apoptotic cells was quantified by determining the percentage of Annexin-V-positive (early apoptotic) plus DAPI-positive (late apoptotic). In drug combination experiments, cells were incubated in various concentrations of BEZ235 and nilotinib, BEZ235 and ponatinib, BEZ235 and asciminib, copanlisib and nilotinib, copanlisib and ponatinib, or copanlisib and asciminib, either as single agents or in combination, before measuring apoptosis.
Analysis of AKT and S6 phosphorylation by Western blotting
Prior to Western blotting, HMEC-1 and CAL-72 cells were incubated in control medium or in medium containing nilotinib (10 μM), ponatinib (2 μM), BEZ235 (5 μM), copanlisib (5 μM), or rapamycin (5 μM) at 37°C for 4 hours. Then, Western blotting was performed essentially as described [50]. In brief, cell lysates were separated in 7.5% sodium dodecyl sulfate polyacrylamide gels and transferred to polyvinylidenfluorid membranes (GE Healthcare, Buckinghamshire, UK). Membranes were blocked using 5% BSA for 30 minutes. Then, rabbit mAb D9E against phosphorylated (p) AKT (phosphorylation site: S473), rabbit mAb D57.2.2E against pS6 (S235/236), a polyclonal antibody against total AKT, and a mAb against S6 (all from Cell Signaling Technology, Danvers, MA, USA) and a mouse mAb (2Q1055) against human actin from Santa Cruz Biotechnology (Santa Cruz, CA, USA) (Table S1) were applied according to the manufacturers’ instructions. Equal loading was confirmed by probing for actin expression. Antibody reactivity was made visible with donkey anti-rabbit IgG or sheep anti-mouse IgG (both from GE Healthcare) and ECL Plus Western Blot Substrate (Thermo Scientific, Rockford, IL, USA).
Analysis of expression of pAKT and pS6 in niche-related cells by flow cytometry
To quantify expression of pAKT and pS6 in KU812, K562, HMEC-1, CAL-72, Mono Mac 6, and THP-1 cells an intracellular staining protocol was applied. Before staining with antibodies against pAKT and pS6, HMEC-1, CAL-72, Mono Mac 6, and THP-1 were incubated in control medium or in various concentrations of BEZ235 (0.5-5 μM), copanlisib (0.5-5 μM), or rapamycin (0.5-5 μM) at 37°C for 4 hours. Thereafter, cells were harvested and fixed in 2% formaldehyde (Merck, Darmstadt, Germany). Then, cells were permeabilized by methanol (-20°C, 15 minutes) and incubated with a PE-labeled mouse mAb against pAKT (phosphorylation site: pS473; BD Biosciences) or with an Alexa Fluor 647-labeled mouse mAb against pS6 (phosphorylation site: Y223; BD Bioscience) for 30 minutes. An alexa fluor 647-labeled mouse IgG1 mAb and a PE-labeled mouse IgG1 mAb (BD Biosciences) were used as isotype-matched control antibodies. Expression of pAKT and pS6 was quantified by flow cytometry on a FACSCanto II (BD Biosciences). In a separate set of experiments, co-cultured cells (CAL-72 plus KU812; CAL-72 plus K562) were examined for viability after incubation with BEZ235 (0.5-5 μM) or copanlisib (0.5-5 μM) at 37°C for 48 hours. In these experiments, multi-color flow cytometry was applied: CAL-72 cells were defined as CD45-/CD44+ cells, KU812 cells as CD45+/CD44+ cells, and K562 as CD45+/CD44- cells. Expression of pAKT and pS6 was quantified in both cell types in the same way as in the separately cultured cell lines using a FACSCanto II.
Evaluation of drug effects on survival of CML cells in co-cultures
To analyze drug effects on osteoblast-induced TKI resistance of CML cells, primary CML cells, K562 cells, and KU812 cells were co-cultured with the osteoblast-like cell line CAL-72 essentially as described [43]. In typical experiments, co-cultured cells were incubated in medium containing various concentrations of BEZ235 (0.5-3 μM), copanlisib (0.5-3 μM), nilotinib (100-5000 nM), ponatinib (10-500 nM), or asciminib (20-50 nM), either as single agents or in combination at 37°C for 48 hours. After incubation, cells were stained with Annexin-V-FITC in Annexin binding-buffer containing HEPES (10 mM, pH 7.4), NaCl (140 mM) and CaCl2 (2.5 mM). Afterwards, DAPI (0.4 μg/ml) was added and cells were analyzed by flow cytometry on a FACSCanto II. The percentage of apoptotic CD44- K562 cells and CD45+ KU812 cells was quantified by measuring DAPI- and Annexin-V-positive cells. In primary CML samples, drug effects on survival (apoptosis) of CD34+/CD38- LSC were determined by assessing the percentage of apoptotic (Annexin-V/DAPI-positive) cells by multicolor flow cytometry essentially as described [51,52]. To analyze hematologic toxicity of drug combinations normal CD34+ stem/progenitor cells were co-cultured with the osteoblast-like cell line CAL-72 and incubated in medium containing BEZ235 (5 μM), copanlisib (5 μM), nilotinib (5 μM), ponatinib (0.5 μM), or asciminib (1 μM), either as single agents or in combination at 37°C for 48 hours. In normal CD34+ stem/progenitor cells, drug effects on survival (apoptosis) of CD34+/CD38- cells were determined by assessing the percentage of apoptotic (Annexin-V/DAPI-positive) cells by multicolor flow cytometry.
In a separate set of experiment, CAL-72 cells were incubated in medium containing BEZ235 (3 μM) or copanlisib (3 μM) at 37°C for 24 hours. Then, CAL-72 cells were washed, and KU812 and K562 cells were loaded on drug-pre-incubated layers of CAL-72 cells in co-cultures which were then incubated in medium containing nilotinib (100-250 nM), ponatinib (10-25 nM) or asciminib (20-50 nM) at 37°C for 48 hours. Afterwards, cells were incubated with Annexin-V-FITC and DAPI (0.4 μg/ml), and the percentage of apoptotic CD44- K562 cells and CD45+ KU812 cells (DAPI- and Annexin-V-positive) was quantified on a FACSCanto II.
Quantitative real-time PCR (qPCR)
HMEC-1 and CAL-72 cells were incubated in control medium or in medium containing various concentrations of BEZ235 (0.5-5 μM), copanlisib (0.5-5 μM), or rapamycin (0.5-5 μM) at 37°C for 48 hours. Then, RNA was isolated using the RNeasy MinEluteCleanupKit (Qiagen, Hilden, Germany). cDNA was synthesized using MMLV RT and random primers (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. PCR conditions were: denaturation 15 seconds (95°C) and annealing and extension for 1 minute at 60°C. qPCR was performed on a Quantstudio 3 Real-Time PCR System (Thermofisher Scientific, Waltham, MA, USA) using iTaq Universal SYBR Green Supermix (Bio-Rad, Hercules, CA, USA). Expression of NOXA mRNA, PUMA mRNA and BIM mRNA levels were normalized to ABL1 mRNA expression levels. Primers used in PCR studies are shown in Table S3.
Statistical analysis
Data were calculated as mean values from at least 3 independent experiments with standard deviations (S.D.). To examine the significance in differences observed in drug-exposed and untreated cells, the Student’s t test for independent samples was applied. Results were considered statistically significant when P was <0.05.
Results
The PI3K blockers BEZ235 and copanlisib and the BCR-ABL1-targeting drugs nilotinib and ponatinib inhibit proliferation of niche-related cell lines
We screened for drugs that can substantially suppress the proliferation of niche cells, including endothelial cells, osteoblasts and macrophages. In initial screens we employed the cell lines HMEC-1, CAL-72, Mono Mac 6 and THP-1. We found that the dual PI3K/mTOR blocker BEZ235 and the pan-class-I PI3K blocker copanlisib inhibit proliferation in all 4 cell lines (Figure 1A-D). The effects of both agents were dose-dependent, with IC50 values of 0.5-1 μM obtained in HMEC-1 with both agents, 0.1-0.5 μM of BEZ235 and 0.5-1 μM of copanlisib in CAL-72 cells, 0.1-0.5 μM of BEZ235 and 0.05-0.5 μM of copanlisib in Mono Mac 6 cells, and 0.1-0.5 μM of BEZ235 and 0.05-0.5 μM of copanlisib in THP-1 cells (Figure 1A-D; Table 1). Rapamycin was found to exert only weak or no effects in these cell lines (IC50 in HMEC-1: 3-5 μM; CAL-72: 0.5-1 μM; Mono Mac 6: 2-3 μM; THP-1: >5.0 μM) (Figure 1A-D; Table 1). The BCR-ABL1-targeting TKI nilotinib and ponatinib, both of which are interacting with a broad spectrum of kinase targets [53,54], were also found to inhibit proliferation in niche-related cells, whereas imatinib and asciminib did not show comparable growth-inhibitory effects in these cells (Table 1; Figure S1).
Figure 1.
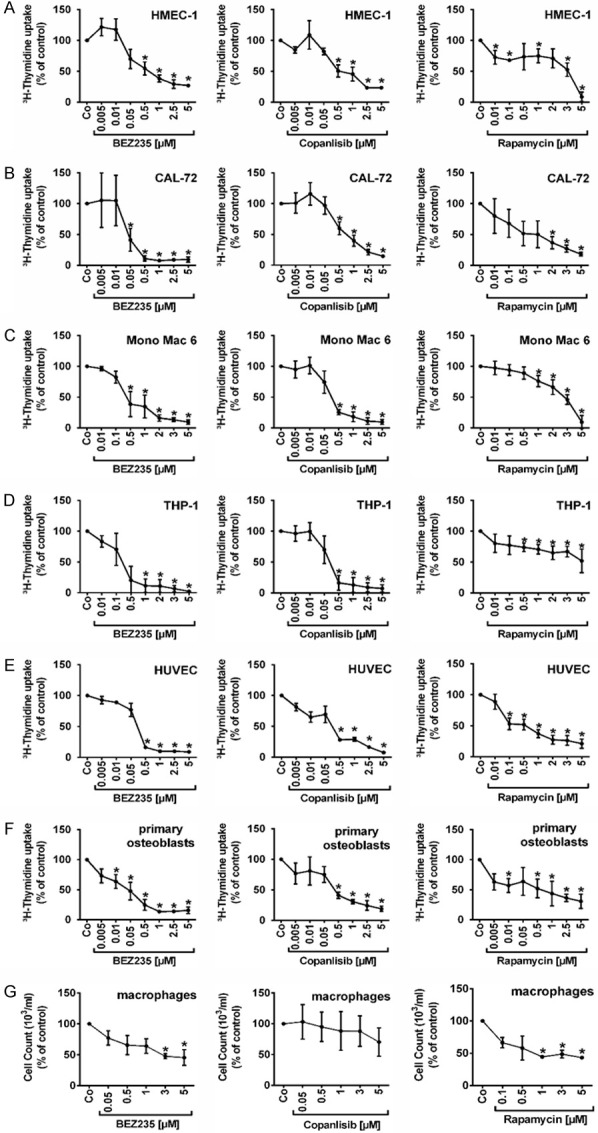
Effects of BEZ235, copanlisib and rapamycin on proliferation of endothelial and osteoblastic niche cells. HMEC-1 cells (A), CAL-72 cells (B), Mono Mac 6 cells (C), THP-1 cells (D), HUVEC cells (E), primary osteoblasts (F) and primary macrophages isolated from heathy donors (G) were incubated in control medium (Co) or in medium containing various concentrations of BEZ235 (0.005-5 μM), copanlisib (0.01-5 μM) and rapamycin (0.01-5 μM) at 37°C for 48 hours. Thereafter, 3H-thymidine uptake was measured. Results show 3H-thymidine uptake as percentage of control (=100%, Co) and represent the mean ± SD of three independent experiments. Asterisk (*): P<0.05 compared to control.
Table 1.
Growth-inhibitory effects of PI3-kinase (PI3K) inhibitors and BCR-ABL1-targeting drugs on endothelial cells, osteoblasts, monocytic cells and CML cell lines
| Cell line | IC50 (μM) Nilotinib | IC50 (μM) Ponatinib | IC50 (μM) Imatinib | IC50 (μM) Asciminib | IC50 (μM) BEZ235 | IC50 (μM) Copanlisib | IC50 (μM) Rapamycin |
|---|---|---|---|---|---|---|---|
| HMEC-1 | 0.1-0.5 | 0.1-0.5 | >10 | >5 | 0.5-1 | 0.5-1 | 3-5 |
| HUVEC | 0.5-1 | 0.1-0.5 | >10 | 2.5-5 | 0.05-0.5 | 0.05-0.5 | 0.5-1 |
| CAL-72 | 0.1-0.5 | 0.1-0.5 | 2.5-5 | >5 | 0.1-0.5 | 0.5-1 | 0.5-1 |
| Primary osteoblasts | 0.01-0.05 | 0.5-1 | 5-10 | >5 | 0.01-0.05 | 0.05-05 | 0.5-1 |
| THP-1 | 1-2 | 0.5-1 | >10 | >5 | 0.1-0.5 | 0.05-0.5 | >5 |
| Mono Mac 6 | 0.5-1 | 0.01-0.05 | >10 | >5 | 0.1-0.5 | 0.05-0.5 | 2-3 |
| KU812 | 0.01-0.015 | 0.00005-0.001 | 0.25-0.3 | 0.0025-0.005 | 0.05-0.5 | 0.05-0.5 | 3-5 |
| K562 | 0.015-0.02 | 0.0001-0.0005 | 0.4-0.5 | 0.02-0.05 | 0.05-0.5 | 0.5-1 | 3-5 |
Niche cells and CML cell lines were incubated in various concentrations of BEZ235, rapamycin, nilotinib, ponatinib, imatinib, asciminib or copanlisib at 37°C for 48 hours. Then, proliferation was measured by determining 3H-thymidine uptake. The table provides the IC50 values (ranges) obtained from at least three independent experiments. Abbreviations: BCR-ABL1; break point cluster region-Abelson murine leukemia viral oncogene homolog1; CML, chronic myeloid leukemia; IC50, half maximal inhibitory concentration; HMEC-1, human microvascular endothelial cell; HUVEC, human umbilical vein endothelial cell.
BEZ235, copanlisib, nilotinib and ponatinib inhibit proliferation of primary niche-related cells
We next examined drug effects on proliferation of primary niche-related cells. In primary endothelial cells (HUVEC) and primary osteoblasts, 3H-thymidine uptake was measured. In primary macrophages, we were unable to determine proliferation by 3H-thymidine uptake for technical reasons. We therefore measured cell counts before and after drug exposure. BEZ235 was found to inhibit the proliferation of HUVEC and primary human osteoblasts in a dose-dependent manner, with IC50 values of 0.05-0.5 μM in HUVEC and 0.01-0.05 μM in primary osteoblasts (Figure 1E and 1F) whereas in primary macrophages, BEZ235 was less potent (Figure 1G). Similar effects were seen with copanlisib, with IC50 values of 0.05-0.5 μM in HUVEC, 0.05-05 μM in primary osteoblasts, and 5 μM in primary macrophages. Overall, drug effects on growth and proliferation were stronger in primary osteoblasts and primary endothelial cells than in the respective cell lines tested. Rapamycin also showed some growth-inhibitory effects in HUVEC and primary osteoblasts, but the effects were weak compared to BEZ235 and copanlisib (Figure 1E and 1F). Finally, we were able to show that nilotinib and ponatinib inhibit the proliferation of HUVEC and primary osteoblasts with IC50 values corresponding to those obtained in our previous studies (Figure S1) [55]. We also tested the effects of the novel BCR-ABL1 TKI asciminib (ABL001) on growth and survival of endothelial cells, osteoblasts and macrophages. However, unlike nilotinib or ponatinib, asciminib did not substantially suppress growth of niche-related cells in this study (Figure S1).
PI3K-targeting drugs induce little if any apoptosis in endothelial cells, osteoblasts and macrophages
To study the mechanism of drug-induced growth inhibition, we examined survival in drug-exposed cells. As assessed by combined Annexin-V/DAPI staining and flow cytometry, BEZ235 and copanlisib were found to induce some apoptosis in HMEC-1, CAL-72, Mono Mac 6 and THP-1 cells, but the concentrations required to induce cell death were rather high (Figure 2A-D). At high concentrations, copanlisib also induced apoptosis in HUVEC, primary osteoblasts, and primary macrophages (Figure 2E-G). By contrast, BEZ235 did not induce apoptosis in primary osteoblasts or primary macrophages (Figure 2F and 2G). Rapamycin induced only little if any apoptosis in endothelial cells (HMEC-1, HUVEC) or macrophages (Figure 2A, 2E and 2G). In CAL-72 cells, rapamycin produced some apoptosis whereas in primary osteoblasts, rapamcyin did not show apoptosis-inducing effects (Figure 2F). The BCR-ABL1-targeting TKI tested (nilotinib, ponatinib, asciminib, imatinib) showed weak apoptosis-inducing effects in primary osteoblasts (Figure S2). We also tested the effects of the PI3K and/or mTOR inhibitors (BEZ235, copanlisib, rapamycin) on BIM, NOXA and PUMA mRNA expression in HMEC-1 and CAL-27 cells. However, no effects of these drugs on BIM, NOXA or PUMA mRNA expression were found (Figure S3). Together, the BCR-ABL1 TKI and the PI3K-targeting drugs induced little if any apoptosis in niche-related cells.
Figure 2.
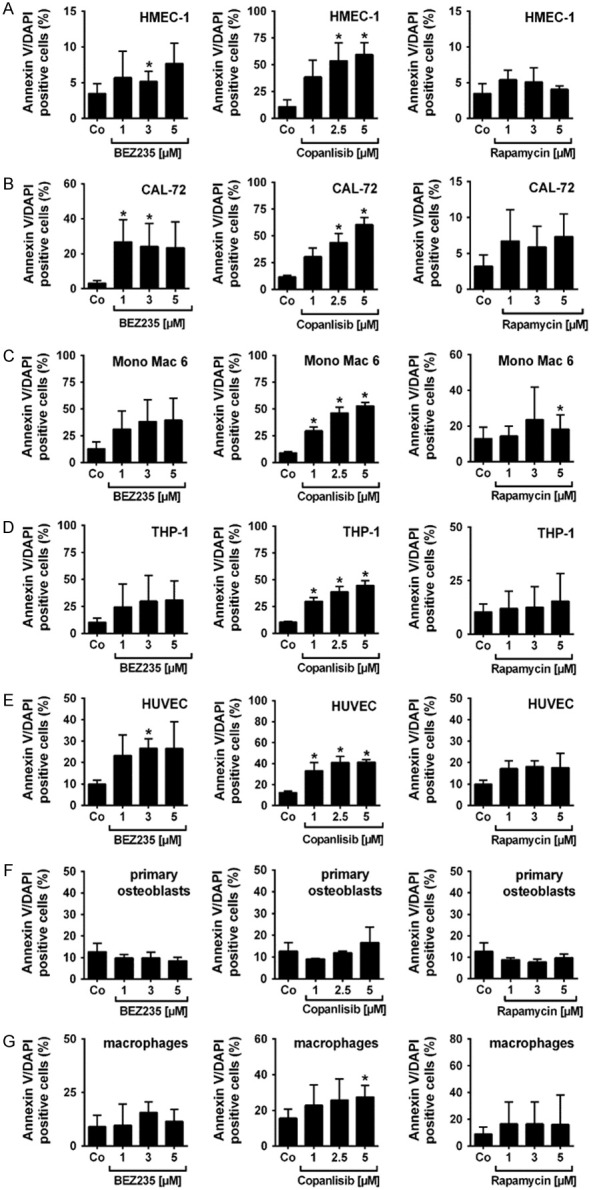
Effects of BEZ235, copanlisib and rapamycin on survival of endothelial and osteoblastic niche cells. HMEC-1 cells (A), CAL-72 cells (B), Mono Mac 6 cells (C), THP-1 cells (D), HUVEC cells (E), primary osteoblasts (F) and macrophages isolated from healthy donors (G) were incubated in control medium (Co) or in medium containing various concentrations of BEZ235 (1-5 μM), copanlisib (1-5 μM) and rapamycin (1-5 μM) at 37°C for 48 hours. Then, cells were examined by flow cytometry to determine the percentage of apoptotic (Annexin-V/DAPI-positive) cells. Results represent the mean ± SD of three independent experiments. Asterisk (*): P<0.05 compared to control.
BEZ235 and copanlisib exert cooperative anti-proliferative and apoptosis-inducing effects on endothelial cells and osteoblasts when combined with BCR-ABL1 TKI
Next, we analyzed the effects of various drug combinations on proliferation of HMEC-1 and CAL-72 cells. We found that the drug combinations ‘nilotinib+BEZ235’ and ‘ponatinib+BEZ235’ produce cooperative growth-inhibitory effects in HMEC-1 and CAL-72 cells, and comparable effects were seen with the other drug combinations tested: ‘nilotinib+copanlisib’ and ‘ponatinib+copanlisib’ (Figure 3). However, most of these cooperative drug effects on cell proliferation were additive in nature. Synergistic growth-inhibitory effects were only seen with the drug combination ‘nilotinib+BEZ235’ in CAL-72 cells (Figure 3C). We also found that the drug combinations ‘nilotinib+BEZ235’, ‘nilotinib+copanlisib’, ‘ponatinib+BEZ235’ and ‘ponatinib+copanlisib’ produced strong cooperative inhibitory effects on cell survival (induction of apoptosis) in HMEC-1 cells and CAL-72 cells (Figure 4).
Figure 3.
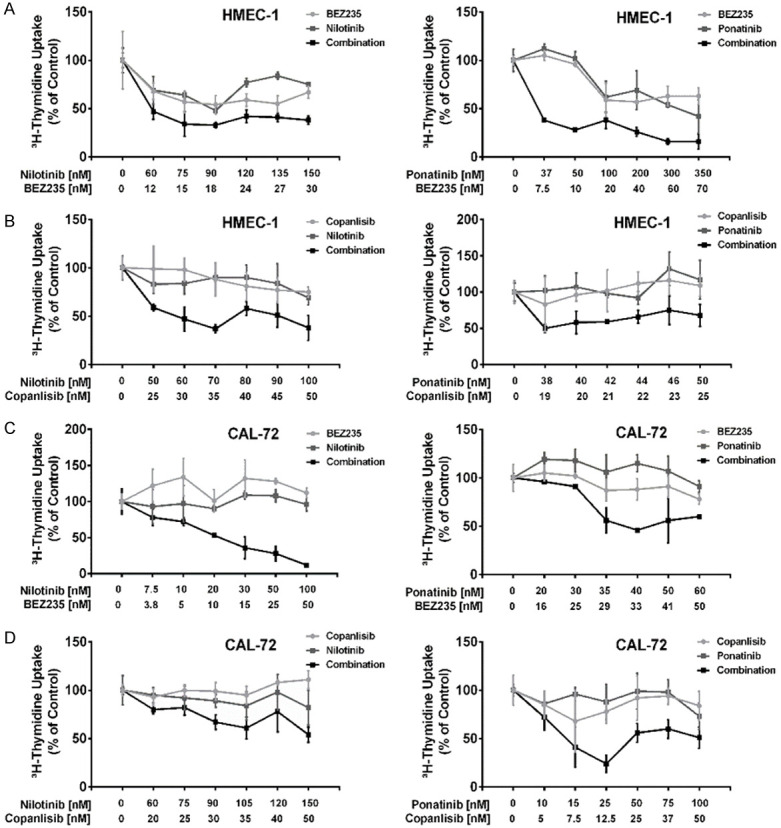
Effects of combinations of BEZ235 or copanlisib with nilotinib or BEZ235 or copanlisib with ponatinib on proliferation of endothelial cells and osteoblastic niche cells. HMEC-1 cells (A, B) and CAL-72 cells (C, D) were incubated in control medium (0), in medium containing various concentrations of BEZ235 or copanlisib (light grey line), nilotinib or ponatinib (dark grey line), or combinations of drugs (BEZ235+nilotinib, BEZ235+ponatinib, copanlisib+nilotinib, copanlisib+ponatinib) (black lines) at fixed ratio of drug concentrations (as indicated) at 37°C for 48 hours. Thereafter, 3H-thymidine uptake was measured. Results show 3H-thymidine uptake as percent of control and represent the mean ± SD of triplicates from one typical experiment.
Figure 4.
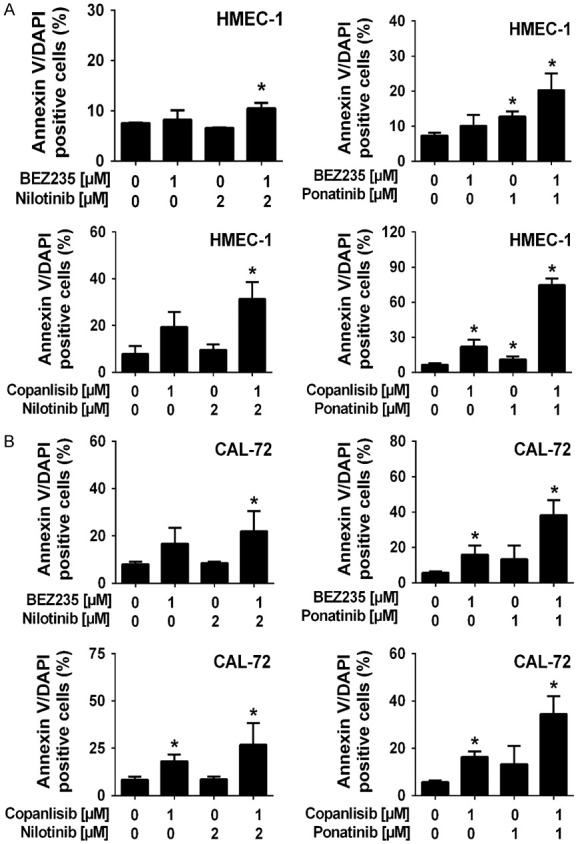
Effects of combinations of BEZ235 or copanlisib with nilotinib or BEZ235 or copanlisib with ponatinib on survival of endothelial and osteoblastic niche cells. HMEC-1 cells (A) and CAL-72 cells (B) were incubated in control medium (0), in medium containing BEZ235, copanlisib, nilotinib or ponatinib, alone or in combinations (BEZ235+nilotinib, copanlisib+nilotinib, BEZ235+ponatinib, copanlisib+ponatinib) as indicated at 37°C for 48 hours. Then, cells were examined by flow cytometry to determine the percentage of Annexin-V-positive cells. Results represent the mean ± SD of three independent experiments. Asterisk (*): P<0.05 compared to control.
PI3K-targeting drugs downregulate phosphorylation of AKT and S6 in niche-related cell lines
As determined by flow cytometry, BEZ235 and copanlisib suppressed the expression of pAKT and pS6 in HMEC-1 cells (Figure 5A). In CAL-72 cells, BEZ235 was found to counteract expression of pAKT and pS6. Copanlisib also suppressed expression of pS6 in these cells but did not suppress expression of pAKT (Figure 5B). Rapamycin was found to downregulate pS6 expression in HMEC-1 and CAL-72 cells, but did not decrease the expression of pAKT in these cells (Figure 5A-D). Corresponding results were obtained for HMEC-1 cells by Western blotting (Figure 5C and 5D). Again, BEZ235 and copanlisib were found to suppress the expression of pAKT and pS6 in these cells. In CAL-72 cells we were not able to detect pAKT because of relatively low levels which was also seen in our flow cytometry experiments. However, pS6 was detected in CAL-72 cells. All three drugs, BEZ235, copanlisib and rapamycin were found to suppress expression of pS6 in CAL-72 cells as determined by Western blotting (Figure 5D). In Mono Mac 6 cells, BEZ235 was found to downregulate the expression of pAKT and pS6 in our flow cytometry experiments, whereas copanlisib and rapamycin were only able to decrease pS6 expression but did not suppress pAKT (Figure S4A). In THP-1 cells, BEZ235, copanlisib and rapamycin failed to modulate phosphorylation of AKT or S6 (Figure S4B).
Figure 5.
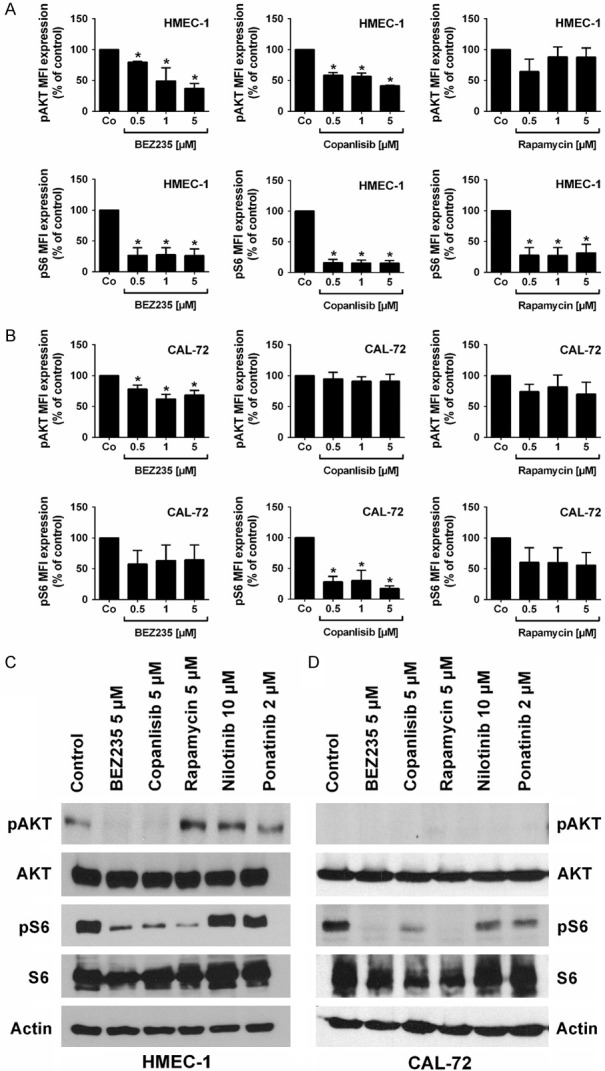
Effects of BEZ235, copanlisib and rapamycin on expression of phosphorylated (p) AKT and S6 in endothelial and osteoblastic niche cells. (A, B) Flow cytometric evaluation of expression of pAKT and pS6: HMEC-1 cells (A) and CAL-72 cells (B) were incubated in control medium (Co) or in medium containing various concentrations of BEZ235 (0.5-5 μM), copanlisib (0.5-5 μM) or rapamycin (0.5-5 μM) at 37°C for 4 hours. Then, cells were permeabilized and stained with antibodies against pAKT (S473) and pS6 (S235/236). Expression of pAKT and pS6 was determined by flow cytometry. Results show median fluorescence intensity (MFI) values expressed as percent of control and represent the mean ± SD from 3 independent experiments. Asterisk (*): P<0.05 compared to control. (C, D) Effects of BEZ235, copanlisib and rapamycin on expression of phosphorylated (p) AKT and S6 in endothelial and osteoblastic niche cells as determined by Western blotting. HMEC-1 cells (C) and CAL-72 cells (D) were incubated in control medium or in medium containing BEZ235 (5 μM), rapamycin (5 μM), copanlisib (5 μM), nilotinib (10 μM), or ponatinib (2 μM) at 37°C for 4 hours. Then, cells were subjected to Western blot analysis as described in text using antibodies directed against pAKT, total AKT, pS6, total S6 or actin. The figure show data from one typical Western blot experiments. Corresponding results were obtained in two other Western blot experiments.
BEZ235 and copanlisib counteract osteoblast-induced resistance of CML cell lines and of primary CML LSC against TKI
We have recently shown that osteoblasts mediate resistance in the BCR-ABL1+ cell lines K562 and KU812 as well as in primary CML LSC [43]. In the current study, we hypothesized that the inhibition of osteoblastic cells by BEZ235 is sufficient to overcome osteoblast-induced resistance of CML LSC against nilotinib, ponatinib or asciminib. To test this hypothesis co-culture experiments were performed using CAL-72, HMEC-1, primary CML cells, and the CML cell lines K562 and KU812. We found that in the presence of CAL-72 cells, the effects of nilotinib, ponatinib and asciminib on survival of K562 and KU812 were much weaker compared to control cultures (Figure 6A and 6B). Addition of BEZ235 (3 μM) or copanlisib (3 μM) in these cultures increased the apoptosis-inducing effects of the TKI in the presence of CAL-72 cells, suggesting that PI3K-inhibition overcomes osteoblast-induced resistance against TKI (Figure 6). The effects of BEZ235 and copanlisib were dose-dependent (Figure S5). Corresponding results were obtained in co-cultures prepared with primary CD34+/CD38- CML LSC (Figure 6C). Again, osteoblasts (CAL-72 cells) induced LSC resistance against BCR-ABL1 TKI, and BEZ235 and copanlisib were found to overcome osteoblast-induced resistance. In normal CD34+/CD38- stem cells, drug combinations consisting of BCR-ABL1 TKI and PI3K inhibitors (nilotinib+copanlisib, ponatinib+BEZ235, ponatinib+copanlisib, asciminib+BEZ235, asciminib+copanlisib) did not induce major inhibitory effects on survival in the presence or absence of CAL-72 cells (Figure S6). To define whether BEZ235 and copanlisib overcome niche-induced resistance of CML cells by acting on niche cells directly or on CML cells, we performed experiments in which CAL-72 cells were first exposed to BEZ235 and copanlisib and washed before added to the co-cultures. In these experiments, both drugs were found to overcome TKI resistance in K562 cells and KU812 cells in the same way as in the co-culture experiments where both cell types were exposed together to these drugs (Figure S7). We also asked whether BEZ235 and copanlisib downregulate pAKT and pS6 levels in osteoblasts (CAL-72), CML cells or both cell types in our co-cultures. To delineate the effects of BEZ235 and copanlisib on CML cells (KU812 and K562 cells) and osteoblasts, we performed multi-color flow cytometry staining experiments using antibodies against CD44, CD45, pAKT and pS6. As expected, both phosphorylated targets were detected in CAL72 cells as well as in KU812 and K562. In both CML cell lines, pS6 expression decreased upon exposure to BEZ235 or copanlisib in the absence of osteoblasts but did not decrease in the presence of osteoblasts, confirming the protective effect of osteoblasts on CML cells (Figure S8). However, in co-cultured CAL-72 cells, BEZ235 and copanlisib suppressed pS6 expression in the absence or presence of CML cells (Figure S8). These data suggest that in our co-culture system (niche-like condition) the primary effects of the PI3-kinase blockers were exerted on osteoblasts rather than CML cells.
Figure 6.
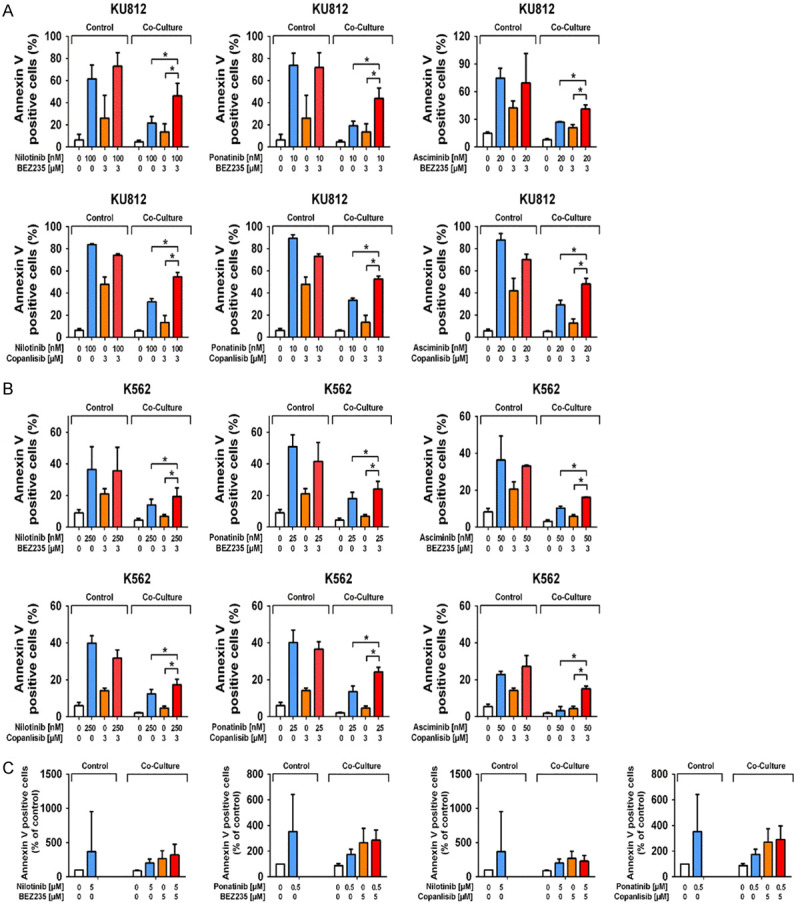
Effects of BEZ235 and copanlisib on niche-induced TKI resistance of CML cells. KU812 cells (A) and K562 cells (B) were incubated in control medium (0) or in medium containing nilotinib (100-250 nM), ponatinib (10-25 nM), asciminib (20-50 nM), BEZ235 (3 μM) and copanlisib (3 μM) or in medium with a combination of drugs (BEZ235+nilotinib, BEZ235+ponatinib, BEZ235+asciminib, copanlisib+nilotinib, copanlisib+ponatinib, copanlisib+asciminib) in the absence or in presence (Co-culture) of CAL-72 cells at 37°C for 48 hours. Then, cells were examined by flow cytometry to determine the percentage of apoptotic (Annexin-V/DAPI-positive) cells. Results represent the mean ± SD of three independent experiments. Asterisk (*): P<0.05. Primary CML MNC (mononuclear cells) (C) were incubated in control medium (0) or in medium containing nilotinib (5 μM), ponatinib (0.5 μM), BEZ235 (5 μM) and copanlisib (5 μM) or in medium with a combination of drugs (BEZ235+nilotinib, BEZ235+ponatinib) or (copanlisib+nilotinib, copanlisib+ponatinib) in the absence or in presence (Co-culture) of CAL-72 cells at 37°C for 48 hours. Then, cells were examined by flow cytometry to determine the percentage of CD34+/CD38-/Annexin-V-positive cells among DAPI-negative cells. Results represent the mean ± SD of twelve independent experiments.
Finally, we asked whether endothelial cells would also introduce TKI resistance in CML cells. However, HMEC-1 did not substantially block TKI effects on growth or viability of K562 and KU812 cells (Figure S9). These data suggest that the primary niche cell introducing TKI resistance in CML cells may be the osteoblast.
Discussion
Recent data suggest that the stem cell niche is involved in the pathogenesis of various hematologic neoplasms and that niche cells can mediate resistance against anti-neoplastic drugs in leukemic cells [34,35,39-47,56-61]. In CML, niche cells reportedly induce resistance against BCR-ABL1-targeting drugs [40-43]. We have recently shown that osteoblasts confer resistance of CML stem cells against nilotinib and ponatinib [43]. Thus, targeting niche cells may be an attractive approach to counteract niche-mediated resistance. In the present study, we found that the dual PI3K/mTOR blocker BEZ235 and the PI3K-targeting drug copanlisib inhibit growth and survival of various niche-related cells, including endothelial cells, osteoblasts and macrophages. Moreover, BEZ235 and copanlisib were found to counteract osteoblast-mediated resistance against nilotinib, ponatinib and asciminib in CML stem cells and in the CML-related cell lines K562 and KU812. These observations may have clinical implications and may pave the way for the development of more effective anti-CML therapies that may achieve disease eradication through combined targeting of both, CML LSC and the protective (resistance-inducing) stem cell niche.
A number of niche cells, including endothelial cells, osteoblasts, stromal cells and fibroblasts, may act together to provide an optimal environment for normal and neoplastic stem cells [34,35,40-43,59-62]. These niche cells regulate survival, self-renewal and redistribution of normal stem cells and protect these cells against various toxic conditions. Unfortunately, niche cells also protect neoplastic (stem) cells against various therapies [34,35,40-43,56-62]. Our initial screen aimed at identifying drugs that can inhibit growth and survival of three important niche cells, endothelial cells, osteoblasts, and macrophages. Most drugs failed to suppress growth of these cells. Likewise, asciminib and imatinib failed to inhibit growth of osteoblasts at pharmacologic concentrations. However, nilotinib, ponatinib and the PI3K-targeting drugs BEZ235 and copanlisib were found to inhibit proliferation and survival in all three types of niche cells, including cell lines and primary niche-related cells. For determining growth of primary macrophages, we counted cell numbers (after drug incubation) instead of performing 3H-thymidine uptake for technical reasons and because macrophages showed only a slow proliferation rate. However, in control experiments using non-adherent monocytic cell lines, we were able to show that cell counting and 3H-thymidine uptake experiments yielded almost the same results (data not shown).
Recent data suggest that mTORC1 is required for the proliferation and differentiation of osteoblasts as well as regulation of osteoblast cell function [63]. It has also been shown that the PI3K-AKT-mTOR pathway plays an important role in the regulation of proliferation, survival and activation of endothelial cells and macrophages [64,65]. In our experiments the PI3K-specific drug copanlisib and the dual inhibitor BEZ235 (blocking PI3K and mTOR) induced growth inhibition, whereas the mTOR-specific drug rapamycin did not show comparable effects on growth of niche cells in our experiments. These data suggest that the PI3K is a major target mediating growth and viability of niche cells, including osteoblasts. Interestingly, the PI3K-targeting drugs showed only strong effects on proliferation of niche cells but weak or no effects on niche cell survival. For example, BEZ235 suppressed proliferation but did not suppress the viability of primary osteoblasts. This may best be explained by the fact that the PI3K and PI3K-downstream signaling molecules, including mTOR, are contributing primarily to mechanisms regulating cell cycle progression, cell division and proliferation rather than survival of niche cells.
We and others have recently shown that nilotinib and ponatinib suppress the in vitro growth of endothelial cells [55,66-69]. In the present study we confirmed these TKI effects. By contrast, imatinib and asciminib, two other BCR-ABL1-targeting drugs showed no substantial effects on proliferation of endothelial cells. These differential effects are best explained by the many ‘vascular’ targets of nilotinib and ponatinib, like KDR or TEK, that are not recognized by imatinib or asciminib [17,53,55,70]. These target-related TKI effects have also been associated with potential vascular toxicities such as arterial occlusive disease [21,25,26,55,67-69]. Thus, our observation may be relevant clinically: in fact, based on our observations, asciminib is not expected to induce vascular adverse events in the same way as ponatinib or nilotinib, which is in line with first reports from clinical trials performed with asciminib [23,70].
As mentioned before, niche cells exert profound effects on CML LSC and can promote the resistance of these cells against nilotinib and ponatinib [34,35,41-44]. Since the PI3K-AKT-mTOR pathway apparently is important for the maintenance and function of niche cells, targeting of this pathway may also alter the ability of these cells to communicate with and to protect CML cells and thus overcomes niche-mediated drug resistance. In addition, several studies have shown that PI3K-AKT-mTOR pathway is a key player in the regulation of normal and neoplastic stem cells and considered to play an important role in mediating survival and resistance of CML LSC [1-3,71-74].
We therefore tested the effects of the PI3K and/or mTOR inhibitors on LSC-niche interactions. In these experiments, we combined BEZ235 or copanlisib with nilotinib or ponatinib and applied these combinations in a co-culture assay containing osteoblasts (or osteoblast-like CAL-72 cells) and CML cells, including LSC or the CML cell lines KU812 and K562. Whereas osteoblasts introduced resistance against TKI in CML cells, the drug combinations applied (BEZ235 or copanlisib with nilotinib or ponatinib) were found to counteract niche-induced resistance of CML LSC and CML cell lines against both TKI. These data suggest that the combination of a PI3K inhibitor (BEZ235 or copanlisib) with a BCR-ABL1 TKI (nilotinib or ponatinib) increase the sensitivity of CML cells and their LSC against BCR-ABL1-targeting drugs. Based on our data we hypothesize that these drug combination effects can be divided into i) a direct combinatorial effect on CML cells and ii) an indirect effect on CML cells by targeting niche cells and by disrupting niche cell-CML cell interactions thereby blocking the protective effects of niche cells on CML cells. With regard to direct effects of the drug combinations on CML cells, it is worth noting that it has been described that PI3K inhibition sensitizes CML cells against the effects of various TKI [74]. With regard to indirect effects via niche cell blockage, we performed a control experiment where osteoblasts were incubated with PI3K-targeting drugs and washed before CML cells and BCR-ABL1 TKI were added. In these experiments niche mediated resistance was suppressed in the same way as in co-cultures where osteoblasts were not washed before CML cells and TKI were added (continuous exposure to PI3K blocker), suggesting that osteoblast inhibition by PI3K-blockage is important and contributes to improved CML cell responsiveness against BCR-ABL1 TKI in these experiments.
So far, only a few concepts are based on attacking niche cells in CML. In fact, whereas a number of attempts have been made to attack angiogenesis and thus the vascular niche in myeloid neoplasms including CML [75,76], no attempts have been made to specifically suppress niche cells and explore niche-mediated resistance of CML (stem) cells against various TKI. In the current study, we asked for drugs that can suppress growth of osteoblasts and thereby can interfere with osteoblast-mediated resistance of LSC in CML. Furthermore, this study is probably the first to ask for drugs directed against osteoblast-induced resistance of CML cells. We have identified such drugs and effective drug combinations targeting niche cells in CML, with the hope that these drugs can also overcome niche-mediated resistance in patients.
During treatment with second- or third-generation TKI in CML a number of recurrent adverse events may cause clinical problems [21,55,67-69]. One major problem is the potential occurrence of vascular (arterial) occlusive events in patients receiving nilotinib or ponatinib [55,67-69]. The mechanisms of TKI-induced arterial events have not entirely been deciphered. However, both TKI were found to exert direct effects on growth and viability of vascular endothelial cells and nilotinib also affects expression of pro-atherogenic molecules in endothelial cells [55,66-69]. One strategy to avoid vascular toxicity would be to switch to other, less toxic drugs, such as asciminib in high-risk patients. Our data would support such strategy as asciminib did not affect endothelial cells. Another question is whether combined targeting of PI3K and various targets detected by the BCR-ABL1 TKI applied could result in major hematologic toxicity. To address this question, we exposed normal CD34+/CD38- stem cells to combinations of BCR-ABL1 TKI and PI3K inhibitors. However, no substantial effects of these drug combinations on cell survival were found.
In summary, our data show that inhibition of the PI3K/mTOR pathway is an effective approach to overcome niche-induced resistance of CML cells against BCR-ABL1 TKI. Whether this concept can be translated into clinical application and can lead to the development of more effective anti-CML treatment or even LSC eradication remains to be determined in forthcoming studies.
Acknowledgements
We would like to thank Christina Schewzik, Anna-Katharina Schruef, Christian Milosits and Gabriele Stefanzl for excellent technical assistance. This study was supported by the Austrian Science Fund (FWF), grants No. F4704-B20 and P30625-B28.
Disclosure of conflict of interest
The authors declare that they have no study-specific conflict of interest (COI) to disclose. COI to disclose outside of this study: K.V.G. received honoraria from Novartis, Incyte, BMS, Pfizer and Abbvie. P.V. received grant support from Celgene/BMS, Pfizer, and Incyte, and consultancy honoraria from Novartis, Pfizer, Incyte, Celgene/BMS, and OAP Orphan Pharmaceuticals.
Supporting Information
References
- 1.Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood. 2000;96:3343–3356. [PubMed] [Google Scholar]
- 2.Arlinghaus R, Sun T. Signal transduction pathways in Bcr-Abl transformed cells. Cancer Treat Res. 2004;119:239–270. doi: 10.1007/1-4020-7847-1_12. [DOI] [PubMed] [Google Scholar]
- 3.Melo JV, Deininger MW. Biology of chronic myelogenous leukemia--signaling pathways of initiation and transformation. Hematol Oncol Clin North Am. 2004;18:545–568. doi: 10.1016/j.hoc.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Faderl S, Talpaz M, Estrov Z, O’Brien S, Kurzrock R, Kantarjian HM. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164–172. doi: 10.1056/NEJM199907153410306. [DOI] [PubMed] [Google Scholar]
- 5.Sattler M, Salgia R, Okuda K, Uemura N, Durstin MA, Pisick E, Xu G, Li JL, Prasad KV, Griffin JD. The proto-oncogene product p120CBL and the adaptor proteins CRKL and c-CRK link c-ABL, p190BCR/ABL and p210BCR/ABL to the phosphatidylinositol-3’ kinase pathway. Oncogene. 1996;12:839–846. [PubMed] [Google Scholar]
- 6.Sillaber C, Gesbert F, Frank DA, Sattler M, Griffin JD. STAT5 activation contributes to growth and viability in Bcr/Abl-transformed cells. Blood. 2000;95:2118–2125. [PubMed] [Google Scholar]
- 7.Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, Zimmermann J, Lydon NB. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 8.Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno-Jones S, Sawyers CL. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 9.Kantarjian H, Sawyers C, Hochhaus A, Guilhot F, Schiffer C, Gambacorti-Passerini C, Niederwieser D, Resta D, Capdeville R, Zoellner U, Talpaz M, Druker B, Goldman J, O’Brien SG, Russell N, Fischer T, Ottmann O, Cony-Makhoul P, Facon T, Stone R, Miller C, Tallman M, Brown R, Schuster M, Loughran T, Gratwohl A, Mandelli F, Saglio G, Lazzarino M, Russo D, Baccarani M, Morra E International STI571 CML Study Group. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346:645–652. doi: 10.1056/NEJMoa011573. [DOI] [PubMed] [Google Scholar]
- 10.Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, Deininger MW, Silver RT, Goldman JM, Stone RM, Cervantes F, Hochhaus A, Powell BL, Gabrilove JL, Rousselot P, Reiffers J, Cornelissen JJ, Hughes T, Agis H, Fischer T, Verhoef G, Shepherd J, Saglio G, Gratwohl A, Nielsen JL, Radich JP, Simonsson B, Taylor K, Baccarani M, So C, Letvak L, Larson RA IRIS Investigators. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 11.Kantarjian H, Cortes J. BCR-ABL tyrosine kinase inhibitors in chronic myeloid leukemia: using guidelines to make rational treatment choices. J Natl Compr Canc Netw. 2008;6(Suppl 2):S37–42. [PubMed] [Google Scholar]
- 12.Deininger M. Resistance and relapse with imatinib in CML: causes and consequences. J Natl Compr Canc Netw. 2008;6(Suppl 2):S11–S21. [PubMed] [Google Scholar]
- 13.Valent P. Emerging stem cell concepts for imatinib-resistant chronic myeloid leukaemia: implications for the biology, management, and therapy of the disease. Br J Haematol. 2008;142:361–378. doi: 10.1111/j.1365-2141.2008.07197.x. [DOI] [PubMed] [Google Scholar]
- 14.Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- 15.Weisberg E, Manley PW, Breitenstein W, Brüggen J, Cowan-Jacob SW, Ray A, Huntly B, Fabbro D, Fendrich G, Hall-Meyers E, Kung AL, Mestan J, Daley GQ, Callahan L, Catley L, Cavazza C, Azam M, Neuberg D, Wright RD, Gilliland DG, Griffin JD. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7:129–141. doi: 10.1016/j.ccr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Weisberg E, Manley PW, Cowan-Jacob SW, Hochhaus A, Griffin JD. Second generation inhibitors of BCR-ABL for the treatment of imatinib-resistant chronic myeloid leukaemia. Nat Rev Cancer. 2007;7:345–356. doi: 10.1038/nrc2126. [DOI] [PubMed] [Google Scholar]
- 17.Wylie AA, Schoepfer J, Jahnke W, Cowan-Jacob SW, Loo A, Furet P, Marzinzik AL, Pelle X, Donovan J, Zhu W, Buonamici S, Hassan AQ, Lombardo F, Iyer V, Palmer M, Berellini G, Dodd S, Thohan S, Bitter H, Branford S, Ross DM, Hughes TP, Petruzzelli L, Vanasse KG, Warmuth M, Hofmann F, Keen NJ, Sellers WR. The allosteric inhibitor ABL001 enables dual targeting of BCR-ABL1. Nature. 2017;543:733–737. doi: 10.1038/nature21702. [DOI] [PubMed] [Google Scholar]
- 18.Kantarjian H, Giles F, Wunderle L, Bhalla K, O’Brien S, Wassmann B, Tanaka C, Manley P, Rae P, Mietlowski W, Bochinski K, Hochhaus A, Griffin JD, Hoelzer D, Albitar M, Dugan M, Cortes J, Alland L, Ottmann OG. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354:2542–2551. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- 19.Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, Cortes J, O’Brien S, Nicaise C, Bleickardt E, Blackwood-Chirchir MA, Iyer V, Chen TT, Huang F, Decillis AP, Sawyers CL. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 20.Jabbour E, Kantarjian H, Cortes J. Use of second- and third-generation tyrosine kinase inhibitors in the treatment of chronic myeloid leukemia: an evolving treatment paradigm. Clin Lymphoma Myeloma Leuk. 2015;15:323–334. doi: 10.1016/j.clml.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre PD, Paquette R, Chuah C, Nicolini FE, Apperley JF, Khoury HJ, Talpaz M, DeAngelo DJ, Abruzzese E, Rea D, Baccarani M, Müller MC, Gambacorti-Passerini C, Lustgarten S, Rivera VM, Haluska FG, Guilhot F, Deininger MW, Hochhaus A, Hughes TP, Shah NP, Kantarjian HM. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 2018;132:393–404. doi: 10.1182/blood-2016-09-739086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2018 update on diagnosis, therapy and monitoring. Am J Hematol. 2018;93:442–459. doi: 10.1002/ajh.25011. [DOI] [PubMed] [Google Scholar]
- 23.Hughes TP, Mauro MJ, Cortes JE, Minami H, Rea D, DeAngelo DJ, Breccia M, Goh YT, Talpaz M, Hochhaus A, le Coutre P, Ottmann O, Heinrich MC, Steegmann JL, Deininger MWN, Janssen J, Mahon FX, Minami Y, Yeung D, Ross DM, Tallman MS, Park JH, Druker BJ, Hynds D, Duan Y, Meille C, Hourcade-Potelleret F, Vanasse KG, Lang F, Kim DW. Asciminib in chronic myeloid leukemia after ABL kinase inhibitor failure. N Engl J Med. 2019;381:2315–2326. doi: 10.1056/NEJMoa1902328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hochhaus A, Breccia M, Saglio G, García-Gutiérrez V, Réa D, Janssen J, Apperley J. Expert opinion-management of chronic myeloid leukemia after resistance to second-generation tyrosine kinase inhibitors. Leukemia. 2020;34:1495–1502. doi: 10.1038/s41375-020-0842-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cortes J, Lang F. Third-line therapy for chronic myeloid leukemia: current status and future directions. J Hematol Oncol. 2021;14:44. doi: 10.1186/s13045-021-01055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osman AEG, Deininger MW. Chronic myeloid leukemia: modern therapies, current challenges and future directions. Blood Rev. 2021;49:100825. doi: 10.1016/j.blre.2021.100825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valent P, Hadzijusufovic E, Schernthaner GH, Wolf D, Rea D, le Coutre P. Vascular safety issues in CML patients treated with BCR/ABL1 kinase inhibitors. Blood. 2015;125:901–906. doi: 10.1182/blood-2014-09-594432. [DOI] [PubMed] [Google Scholar]
- 28.Elrick LJ, Jorgensen HG, Mountford JC, Holyoake TL. Punish the parent not the progeny. Blood. 2005;105:1862–1866. doi: 10.1182/blood-2004-08-3373. [DOI] [PubMed] [Google Scholar]
- 29.Barnes DJ, Melo JV. Primitive, quiescent and difficult to kill: the role of non-proliferating stem cells in chronic myeloid leukemia. Cell Cycle. 2006;5:2862–2866. doi: 10.4161/cc.5.24.3573. [DOI] [PubMed] [Google Scholar]
- 30.Jiang X, Zhao Y, Smith C, Gasparetto M, Turhan A, Eaves A, Eaves C. Chronic myeloid leukemia stem cells possess multiple unique features of resistance to BCR-ABL targeted therapies. Leukemia. 2007;21:926–935. doi: 10.1038/sj.leu.2404609. [DOI] [PubMed] [Google Scholar]
- 31.Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, Druker BJ. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Invest. 2011;121:396–409. doi: 10.1172/JCI35721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valent P. Targeting of leukemia-initiating cells to develop curative drug therapies: straightforward but nontrivial concept. Curr Cancer Drug Targets. 2011;11:56–71. doi: 10.2174/156800911793743655. [DOI] [PubMed] [Google Scholar]
- 33.O’Hare T, Eide CA, Deininger MW. Bcr-Abl kinase domain mutations, drug resistance, and the road to a cure for chronic myeloid leukemia. Blood. 2007;110:2242–2249. doi: 10.1182/blood-2007-03-066936. [DOI] [PubMed] [Google Scholar]
- 34.Nair RR, Tolentino J, Hazlehurst LA. The bone marrow microenvironment as a sanctuary for minimal residual disease in CML. Biochem Pharmacol. 2010;80:602–612. doi: 10.1016/j.bcp.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhatia R. Targeting leukemia stem cell resustance in chronic myelogenous leukemia. Trans Am Clin Climatol Assoc. 2019;130:246–254. [PMC free article] [PubMed] [Google Scholar]
- 36.Branford S, Hughes TP. Mutational analysis in chronic myeloid leukemia: when and what to do? Curr Opin Hematol. 2011;18:111–116. doi: 10.1097/MOH.0b013e32834399ef. [DOI] [PubMed] [Google Scholar]
- 37.Soverini S, de Benedittis C, Mancini M, Martinelli G. Mutations in the BCR-ABL1 kinase domain and elsewhere in chronic myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2015;15(Suppl):S120–128. doi: 10.1016/j.clml.2015.02.035. [DOI] [PubMed] [Google Scholar]
- 38.Patel AB, O’Hare T, Deininger MW. Mechanisms of resistance to ABL kinase inhibition in chronic myeloid leukemia and the development of next generation ABL kinase inhibitors. Hematol Oncol Clin North Am. 2017;31:589–612. doi: 10.1016/j.hoc.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agarwal P, Bhatia R. Influence of bone marrow microenvironment on leukemic stem cells: breaking up an intimate relationship. Adv Cancer Res. 2015;127:227–252. doi: 10.1016/bs.acr.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 40.Arrigoni E, Del Re M, Galimberti S, Restante G, Rofi E, Crucitta S, Baratè C, Petrini M, Danesi R, Di Paolo A. Concise review: chronic myeloid leukemia: stem cell niche and response to pharmacologic treatment. Stem Cells Transl Med. 2018;7:305–314. doi: 10.1002/sctm.17-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agarwal P, Isringhausen S, Li H, Paterson AJ, He J, Gomariz Á, Nagasawa T, Nombela-Arrieta C, Bhatia R. Mesenchymal niche-specific expression of Cxcl12 controls quiescence of treatment-resistant leukemia stem cells. Cell Stem Cell. 2019;24:769–784. e766. doi: 10.1016/j.stem.2019.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Busch C, Wheadon H. Bone marrow niche crosses paths with BMPs: a road to protection and persistence in CML. Biochem Soc Trans. 2019;47:1307–1325. doi: 10.1042/BST20190221. [DOI] [PubMed] [Google Scholar]
- 43.Peter B, Eisenwort G, Keller A, Bauer K, Berger D, Sadovnik I, Stefanzl G, Hoermann G, Wolf D, Racil Z, Mayer J, Zuber J, Sperr WR, Winter GE, Willmann M, Rülicke T, Valent P. BRD4 degradation is a potent approach to block MYC expression and to overcome multiple forms of stem cell resistance in Ph+ CML. Blood. 2018;132:1722. [Google Scholar]
- 44.Sugimoto K, Miyata Y, Nakayama T, Saito S, Suzuki R, Hayakawa F, Nishiwaki S, Mizuno H, Takeshita K, Kato H, Ueda R, Takami A, Naoe T. Fibroblast growth factor-2 facilitates the growth and chemo-resistance of leukemia cells in the bone marrow by modulating osteoblast functions. Sci Rep. 2016;6:30779. doi: 10.1038/srep30779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sillaber C, Mayerhofer M, Aichberger KJ, Krauth MT, Valent P. Expression of angiogenic factors in chronic myeloid leukaemia: role of the bcr/abl oncogene, biochemical mechanisms, and potential clinical implications. Eur J Clin Invest. 2004;34(Suppl 2):2–11. doi: 10.1111/j.0960-135X.2004.01365.x. [DOI] [PubMed] [Google Scholar]
- 46.Aguayo A, Kantarjian H, Manshouri T, Gidel C, Estey E, Thomas D, Koller C, Estrov Z, O’Brien S, Keating M, Freireich E, Albitar M. Angiogenesis in acute and chronic leukemias and myelodysplastic syndromes. Blood. 2000;96:2240–2245. [PubMed] [Google Scholar]
- 47.Korkolopoulou P, Viniou N, Kavantzas N, Patsouris E, Thymara I, Pavlopoulos PM, Terpos E, Stamatopoulos K, Plata E, Anargyrou K, Androulaki A, Davaris P, Yataganas X. Clinicopathologic correlations of bone marrow angiogenesis in chronic myeloid leukemia: a morphometric study. Leukemia. 2003;17:89–97. doi: 10.1038/sj.leu.2402769. [DOI] [PubMed] [Google Scholar]
- 48.Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, Lawley TJ. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol. 1992;99:683–690. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- 49.Hohensinner PJ, Baumgartner J, Kral-Pointner JB, Uhrin P, Ebenbauer B, Thaler B, Doberer K, Stojkovic S, Demyanets S, Fischer MB, Huber K, Schabbauer G, Speidl WS, Wojta J. PAI-1 (plasminogen activator inhibitor-1) expression renders alternatively activated human macrophages proteolytically quiescent. Arterioscler Thromb Vasc Biol. 2017;37:1913–1922. doi: 10.1161/ATVBAHA.117.309383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gleixner KV, Sadovnik I, Schneeweiss M, Eisenwort G, Byrgazov K, Stefanzl G, Berger D, Herrmann H, Hadzijusufovic E, Lion T, Valent P. A kinase profile-adapted drug combination elicits synergistic cooperative effects on leukemic cells carrying BCR-ABL1(T315I) in Ph+ CML. Leuk Res. 2019;78:36–44. doi: 10.1016/j.leukres.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herrmann H, Sadovnik I, Cerny-Reiterer S, Rülicke T, Stefanzl G, Willmann M, Hoermann G, Bilban M, Blatt K, Herndlhofer S, Mayerhofer M, Streubel B, Sperr WR, Holyoake TL, Mannhalter C, Valent P. Dipeptidylpeptidase IV (CD26) defines leukemic stem cells (LSC) in chronic myeloid leukemia. Blood. 2014;123:3951–3962. doi: 10.1182/blood-2013-10-536078. [DOI] [PubMed] [Google Scholar]
- 52.Eisenwort G, Sadovnik I, Schwaab J, Jawhar M, Keller A, Stefanzl G, Berger D, Blatt K, Hoermann G, Bilban M, Willmann M, Winding C, Sperr WR, Arock M, Rülicke T, Reiter A, Valent P. Identification of a leukemia-initiating stem cell in human mast cell leukemia. Leukemia. 2019;33:2673–2684. doi: 10.1038/s41375-019-0460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rix U, Hantschel O, Dürnberger G, Remsing Rix LL, Planyavsky M, Fernbach NV, Kaupe I, Bennett KL, Valent P, Colinge J, Köcher T, Superti-Furga G. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood. 2007;110:4055–4063. doi: 10.1182/blood-2007-07-102061. [DOI] [PubMed] [Google Scholar]
- 54.Huang WS, Metcalf CA, Sundaramoorthi R, Wang Y, Zou D, Thomas RM, Zhu X, Cai L, Wen D, Liu S, Romero J, Qi J, Chen I, Banda G, Lentini SP, Das S, Xu Q, Keats J, Wang F, Wardwell S, Ning Y, Snodgrass JT, Broudy MI, Russian K, Zhou T, Commodore L, Narasimhan NI, Mohemmad QK, Iuliucci J, Rivera VM, Dalgarno DC, Sawyer TK, Clackson T, Shakespeare WC. Discovery of 3-[2-(imidazo[1,2-b]pyridazin-3-yl)ethynyl]-4-methyl-N-{4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl}benzamide (AP24534), a potent, orally active pan-inhibitor of breakpoint cluster region-abelson (BCR-ABL) kinase including the T315I gatekeeper mutant. J Med Chem. 2010;53:4701–4719. doi: 10.1021/jm100395q. [DOI] [PubMed] [Google Scholar]
- 55.Hadzijusufovic E, Albrecht-Schgoer K, Huber K, Hoermann G, Grebien F, Eisenwort G, Schgoer W, Herndlhofer S, Kaun C, Theurl M, Sperr WR, Rix U, Sadovnik I, Jilma B, Schernthaner GH, Wojta J, Wolf D, Superti-Furga G, Kirchmair R, Valent P. Nilotinib-induced vasculopathy: identification of vascular endothelial cells as a primary target site. Leukemia. 2017;31:2388–2397. doi: 10.1038/leu.2017.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Konopleva MY, Jordan CT. Leukemia stem cells and microenvironment: biology and therapeutic targeting. J. Clin. Oncol. 2011;29:591–599. doi: 10.1200/JCO.2010.31.0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou HS, Carter BZ, Andreeff M. Bone marrow niche-mediated survival of leukemia stem cells in acute myeloid leukemia: Yin and Yang. Cancer Biol Med. 2016;13:248–259. doi: 10.20892/j.issn.2095-3941.2016.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Toofan P, Irvine D, Hopcroft L, Copland M, Wheadon H. The role of the bone morphogenetic proteins in leukaemic stem cell persistence. Biochem Soc Trans. 2014;42:809–815. doi: 10.1042/BST20140037. [DOI] [PubMed] [Google Scholar]
- 59.Shah M, Bhatia R. Preservation of quiescent chronic myelogenous leukemia stem cells by the bone marrow microenvironment. Adv Exp Med Biol. 2018;1100:97–110. doi: 10.1007/978-3-319-97746-1_6. [DOI] [PubMed] [Google Scholar]
- 60.Guerrouahen BS, Al-Hijji I, Tabrizi AR. Osteoblastic and vascular endothelial niches, their control on normal hematopoietic stem cells, and their consequences on the development of leukemia. Stem Cells Int. 2011;2011:375857. doi: 10.4061/2011/375857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schepers K, Campbell TB, Passegué E. Normal and leukemic stem cell niches: insights and therapeutic opportunities. Cell Stem Cell. 2015;16:254–267. doi: 10.1016/j.stem.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Valent P, Sadovnik I, Eisenwort G, Herrmann H, Bauer K, Mueller N, Sperr WR, Wicklein D, Schumacher U. Redistribution, homing and organ-invasion of neoplastic stem cells in myeloid neoplasms. Semin Cancer Biol. 2020;60:191–201. doi: 10.1016/j.semcancer.2019.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen J, Long F. mTORC1 signaling promotes osteoblast differentiation from preosteoblasts. PLoS One. 2015;10:e0130627. doi: 10.1371/journal.pone.0130627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karar J, Maity A. PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci. 2011;4:51. doi: 10.3389/fnmol.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Covarrubias AJ, Aksoylar HI, Horng T. Control of macrophage metabolism and activation by mTOR and Akt signaling. Semin Immunol. 2015;27:286–296. doi: 10.1016/j.smim.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gover-Proaktor A, Granot G, Shapira S, Raz O, Pasvolsky O, Nagler A, Lev DL, Inbal A, Lubin I, Raanani P, Leader A. Ponatinib reduces viability, migration, and functionality of human endothelial cells. Leuk Lymphoma. 2017;58:1455–1467. doi: 10.1080/10428194.2016.1239258. [DOI] [PubMed] [Google Scholar]
- 67.Hadzijusufovic E, Kirchmair R, Theurl M, Gamperl S, Lener D, Gutmann C, Stanzl U, Kirsch A, Frank S, Valent P. Ponatinib exerts multiple effects on vascular endothelial cells: possible mechanisms and explanations for the adverse vascular events seen in CML patients treated with ponatinib. Blood. 2016;128:1883. [Google Scholar]
- 68.Haguet H, Bouvy C, Delvigne AS, Modaffari E, Wannez A, Sonveaux P, Dogné JM, Douxfils J. The risk of arterial thrombosis in patients with chronic myeloid leukemia treated with second and third generation BCR-ABL tyrosine kinase inhibitors may be explained by their impact on endothelial cells: an in-vitro study. Front Pharmacol. 2020;11:1007. doi: 10.3389/fphar.2020.01007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Valent P, Hadzijusufovic E, Hoermann G, Füreder W, Schernthaner GH, Sperr WR, Kirchmair R, Wolf D. Risk factors and mechanisms contributing to TKI-induced vascular events in patients with CML. Leuk Res. 2017;59:47–54. doi: 10.1016/j.leukres.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Romero D. Initial results with asciminib in CML. Nat Rev Clin Oncol. 2020;17:135. doi: 10.1038/s41571-019-0324-z. [DOI] [PubMed] [Google Scholar]
- 71.Ghosh J, Kapur R. Regulation of hematopoietic stem cell self-renewal and leukemia maintenance by the PI3K-mTORC1 pathway. Curr Stem Cell Rep. 2016;2:368–378. [Google Scholar]
- 72.Martelli AM, Evangelisti C, Chiarini F, Grimaldi C, Cappellini A, Ognibene A, McCubrey JA. The emerging role of the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin signaling network in normal myelopoiesis and leukemogenesis. Biochim Biophys Acta. 2010;1803:991–1002. doi: 10.1016/j.bbamcr.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 73.Singh P, Kumar V, Gupta SK, Kumari G, Verma M. Combating TKI resistance in CML by inhibiting the PI3K/Akt/mTOR pathway in combination with TKIs: a review. Med Oncol. 2021;38:10. doi: 10.1007/s12032-021-01462-5. [DOI] [PubMed] [Google Scholar]
- 74.Airiau K, Mahon FX, Josselin M, Jeanneteau M, Belloc F. PI3K/mTOR pathway inhibitors sensitize chronic myeloid leukemia stem cells to nilotinib and restore the response of progenitors to nilotinib in the presence of stem cell factor. Cell Death Dis. 2013;4:e827. doi: 10.1038/cddis.2013.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karp JE, Gojo I, Pili R, Gocke CD, Greer J, Guo C, Qian D, Morris L, Tidwell M, Chen H, Zwiebel J. Targeting vascular endothelial growth factor for relapsed and refractory adult acute myelogenous leukemias: therapy with sequential 1-beta-d-arabinofuranosylcytosine, mitoxantrone, and bevacizumab. Clin Cancer Res. 2004;10:3577–3585. doi: 10.1158/1078-0432.CCR-03-0627. [DOI] [PubMed] [Google Scholar]
- 76.Zahiragic L, Schliemann C, Bieker R, Thoennissen NH, Burow K, Kramer C, Zühlsdorf M, Berdel WE, Mesters RM. Bevacizumab reduces VEGF expression in patients with relapsed and refractory acute myeloid leukemia without clinical antileukemic activity. Leukemia. 2007;21:1310–1312. doi: 10.1038/sj.leu.2404632. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


