Abstract
Over the past decade, immune checkpoint inhibitors (ICI) have dramatically improved the prognosis of many cancer patients, but many immune-related adverse cardiovascular events (ACEs) have been observed. We aimed to investigate the occurrence of ACEs in the real world after receiving ICI and provide clinical reference for how to evaluate it. The study retrospectively included 204 patients who received ICI from October 2019 to November 2020 and 205 patients who only received traditional chemotherapy. The mean duration of follow-up for ICI group was 4.88 months, and the control group was 4.79 months. Patients in the control group did not develop myocarditis, only 2 cases of new-onset pericardial effusion occurred. In contrast, among ICI group, there were 3 cases of ICI-associated myocarditis, accounting for 1.47% (3/204), 6 cases of pericardial effusion. The incidence of new-onset ECG abnormalities in the ICI group was significantly higher than that of the control group (38/180 VS 16/178, HR 2.71, 95% CI: 1.449-5.067, P=0.001). In the ICI group, after receiving ICI treatment, cardiac biomarkers including average cardiac troponin T and N terminal pro B type natriuretic peptide increased significantly, peak in about 1 month, and then gradually decreasing. After the third or fourth month, the cardiac biomarkers gradually increased again. In conclusion, ICI may lead to various ACEs, and its incidence is higher than that of patients who only receive traditional chemotherapy. The changing trend of cardiac biomarkers reflects that ICI may cause acute and chronic myocardial damage. Regularly performing ECG, echocardiogram and cardiac biomarker examinations are helpful for early detection of ACEs caused by ICI and providing timely treatment.
Keywords: Immune checkpoint inhibitors (ICI), immunotherapy, adverse cardiovascular events, myocarditis, cardiac biomarker
Introduction
Immune checkpoints are immunosuppressive molecules that maintain self-tolerance by regulating the immune response, thereby protecting human tissues and organs. Immune checkpoint inhibitors (ICI) are monoclonal antibodies that can block these molecules to unleash the immune system and kill tumor cells [1]. In recent years, ICI has been widely used in the treatment of many kinds of tumors, especially metastatic and advanced cancer [2], which have improved the prognosis of many patients and have become a new promising method [3]. However, over-augmented immune response may result in a wide spectrum of immune-related adverse events (irAEs), including cardiovascular toxicity which may be severe and have a poor prognosis [4]. The incidence of adverse cardiovascular events (ACEs) caused by ICI is low. For example, the incidence of ICI-associated myocarditis is about 1% [5], but once it happens, its prognosis is poor. Previous studies have shown that the mortality rate of myocarditis caused by ICI is very high, ranging from 35% to 50% [6,7]. ICI may cause a variety of ACEs, such as myocarditis, pericardial disease [8], vasculitis [9], Tako Tsubo Syndrome [10,11], non-inflammatory left ventricular dysfunction [12], and myocardial infarction [13]. Early diagnosis and timely treatment are the main methods to reduce ACEs caused by ICI. However, in the real world, the understanding of cardiotoxicity caused by ICI is insufficient. Due to the relatively low incidence of serious ACEs caused by ICI, many oncologists are not concerned about this. Most published reports on ICI have underestimated its cardiotoxicity. On the other hand, there is still a lack of consensus on how to detect cardiotoxicity caused by ICI as early as possible.
In recent years, some methods of monitoring cardiotoxicity have been used in some traditional chemotherapy. These methods have been proved be able to predict the subsequent ACEs of tumor patients. Among them, electrocardiogram (ECG) [14], echocardiogram [15], cardiac biomarkers including cardiac troponin T (cTnT) and N terminal pro B type natriuretic peptide (NT-proBNP) are widely used to monitor the cardiotoxicity of tumor treatment drugs [16]. These methods are noninvasive and easy to operate, and can be used to monitor cardiotoxicity caused by ICI. At present, there are many case reports of cardiotoxicity caused by ICI, but there are few reports about the occurrence of ACEs caused by ICI in the real world and how to detect them early. The purpose of this study is to evaluate the incidence of ACEs in cancer patients receiving ICI in the real world, explore the early diagnosis value of ECG and cardiac biomarkers, and provide reference for how to monitor and early diagnose cardiotoxicity caused by ICI.
Methods
Patients
ICI is a new type of drug for the treatment of tumors, and many cancer patients in our hospital have their treatment indications. In the past two years, many cancer patients at the hospital have received ICI treatment. According to the electronic medical record database, the names of the ICI were used as the search key to select patients who have received ICI. We searched for all cancer patients in our center who received ICI from October 2019 to November 2020. Patients who received known cardiovascular toxic drugs such as anthracyclines or targeted HER-2 drugs at the same time or before receiving ICI were excluded. Eligible patients received at least one of ECG, cardiac biomarkers, and echocardiography before and after ICI treatment. Patients who met the above criteria were included in the ICI group.
During the same period, we searched all cancer patients who received traditional chemotherapy, and selected 250 of them through a random data table. Patients who received anthracyclines or targeted drugs or other drugs that may cause cardiotoxicity were excluded. Eligible patients also need to receive at least one of ECG, cardiac biomarkers, and echocardiography before and after receiving traditional chemotherapy. Patients met the above criteria were included in the control group.
Clinical data collection
Data of the study were extracted retrospectively from electronic medical records of the First Affiliated Hospital of Chongqing Medical University. This hospital is a large general hospital, and the patients receiving tumor treatment in the hospital are representative. We have recorded the patient’s diagnosis according to the IC-10 code on the first page of the electronic medical record. According to the electronic medical records, basic demographic data about gender, age, body mass index (BMI), nicotine/tobacco use, alcohol consumption, etc. as well as the patient’s cardiovascular disease-related medical history and risk factors including hypertension, diabetes, coronary heart disease were recorded.
We collected information about the characteristics of the patient’s cancer, including the diagnosis, whether to undergo surgery, radiotherapy, and details of chemotherapy drugs or ICIs. In order to investigate the impact of tumor treatment on the cardiovascular system, we followed up the patient’s heart rate, blood pressure, ECG, cardiac markers including cTnT and NT-proBNP, and echocardiogram or chest CT for pericardial effusion detection after receiving traditional chemotherapy or ICI. The diagnosis of ICI-associated myocarditis was confirmed after the discussion of the oncologist and cardiologist team of our hospital.
This study was a retrospective controlled study which has received funding from the Science Commission and the Health Department of Chongqing city (Fund number, 2018ZDXM010). This study was conducted in accordance with the principles of the Declaration of Helsinki, and the study protocol was approved by the ethics committee of the first affiliated hospital of Chongqing medical university (ethics number, 2018-10-2). As the information was obtained retrospectively, the patient’s name and privacy were not involved.
Primary and secondary endpoints
The primary endpoint of the study was the incidence of ACEs (including cardiac death, acute heart failure, pericardial effusion, abnormal ECG, and acute myocarditis) in patients receiving ICI only or combined with traditional chemotherapy, and comparison with patients who only received traditional chemotherapy. The secondary endpoints were the changes in cardiac biomarkers and heart rate before and after ICI treatment in ICI group and comparison with patients who only received traditional chemotherapy.
Statistical analysis and definitions
ACEs (including cardiac death, acute heart failure, pericardial effusion and acute myocarditis) and ECG abnormalities in the ICI group or control group were recorded and counted in detail. The changes of serum cardiac biomarkers including NT-proBNP and cTnT before and after treatment in the two groups were analyzed descriptively, and a mixed linear model was used to compare the differences in cardiac biomarkers before and after treatment. Continuous variables were described as mean ± standard deviation, and their normality was evaluated by K-S test. According to their normality, Student’s t-tests or Wilcoxon rank sum test was used to compare continuous variables. Categorical variables were presented as percentage and were compared using the Pearson chi-square test. Statistical tests were 2 sided, and 5% was set as the level of significance. Statistical analyses were performed using IBM SPSS Statistics version 21 (IBM, Armonk, New York).
Results
Basic demographic characteristics
A total of 232 people in our cancer treatment center received ICI treatment from October 2019 to November 2020. After screening, a total of 204 people were included in the ICI group. In the same period, there were 2121 patients who received only traditional chemotherapy. We randomly selected 250 cancer patients as the control group. After screening, 205 patients were eligible for inclusion (Figure 1). The average follow-up time of the ICI group was 4.88 months, and the control group was 4.79 months. Baseline characteristics of patients included in our study were summarized in Table 1. Among them, the mean age of patients in the ICI group was 59.9±8.2 year (30-81 years old) and is slightly higher than the control group (61.7±8.1 year, 28-77 years old, P=0.033). There was no significant difference between ICI group and control group in gender, height, and weight. The proportion of male patients in the ICI group was 78.4% (160/204), while the control group was 78.5% (161/205). In comparison with ICI group, control group had a higher body mass index (23.1±2.9 VS 22.5±2.6, P=0.02).
Figure 1.
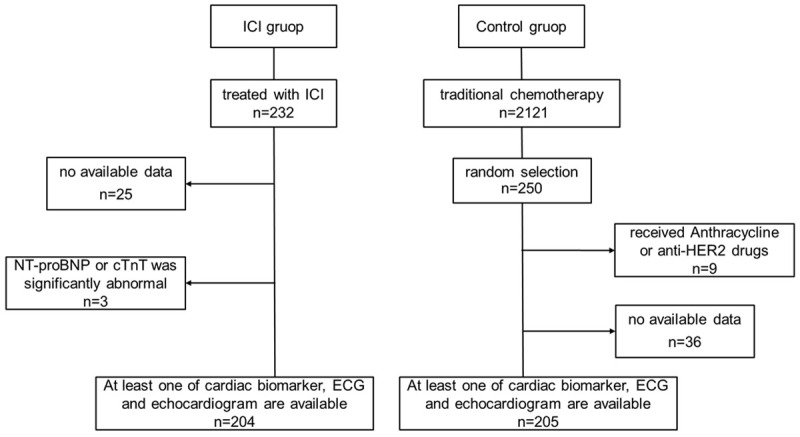
A consort flow diagram of cohort. A total of 232 patients received ICI treatment, and a total of 204 patients met the inclusion criteria and were included in the ICI group. After screening, a total of 205 patients who received traditional chemotherapy at the same time were included in the control group.
Table 1.
Baseline patient characteristics
| Variables | All patients N=409 | Control N=205 | ICI group N=204 | P value |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Mean age, years | 60.78±8.2 | 59.9±8.2 | 61.7±8.1 | 0.033 |
| Gender, male, % | 321 (78.5) | 161 (78.9) | 160 (78.0) | 0.830 |
| Height, cm | 164.2±6.7 | 164.0±6.7 | 164.4±6.7 | 0.555 |
| Weight, kg | 61.6±9.3 | 62.3±9.7 | 60.8±8.7 | 0.108 |
| Body mass index, kg/m2 | 22.8±2.9 | 23.1±2.9 | 22.5±2.6 | 0.020 |
| Hemodynamics | ||||
| HR, beats per minute | 81.4±8.0 | 80.8±7.8 | 82.0±8.3 | 0.555 |
| BP systolic, mmHg | 123.7±18.1 | 123.8±16.8 | 123.6±19.5 | 0.908 |
| BP diastolic, mmHg | 76.48±11.5 | 77.6±10.2 | 75.3±12.6 | 0.040 |
| Cancer category | ||||
| Lung cancer | 267 | 153 | 114 | <0.001 |
| Esophageal cancer | 104 | 31 | 73 | <0.001 |
| other | 38 | 21 | 17 | 0.721 |
| Cardiovascular disease or risk factor | ||||
| Diabetes | 17 | 10 | 17 | 0.167 |
| Hypertension | 86 | 42 | 44 | 0.828 |
| Coronary Heart Disease | 12 | 7 | 5 | 0.552 |
| Smoking | 171 | 90 | 81 | 0.345 |
| Drinking | 118 | 61 | 57 | 0.640 |
| Baseline examination | ||||
| NT-proBNP, μg/ml | 85.78±129.4 | 69.1±60.6 | 104.0±174.6 | 0.011 |
| cTnT, μg/ml | 0.0079±0.0039 | 0.0077±0.0047 | 0.0081±0.0103 | 0.571 |
| Abnormal ECG | 75 | 37/152 | 38/153 | 0.938 |
| Pericardial effusion | 1 | 0 | 1 | NA |
Abbreviations: HR, heart rate; BP, blood pressure; NT-proBNP, N terminal pro B type natriuretic peptide; cTnT, cardiac troponin T; ECG, electrocardiogram.
Cancer and treatment
The most common tumors were lung cancer and esophageal cancer in this study. The proportion of lung cancer in the control group was higher than that of the ICI group (153/205 VS 114/204, P<0.001), while the proportion of esophageal cancer was lower (31/205 VS 73/204, P<0.001). Some patients underwent surgery before receiving ICI or chemotherapy. The control group received only traditional chemotherapy, and the most commonly used drugs were docetaxel and paclitaxel-albumin. The most commonly used ICI include Carrelizumab, Pembrolizumab, and Tislelizumab. In addition to ICI treatment, most patients in the ICI group also received traditional chemotherapy drugs similar to those in the control group (Supplementary Tables 1 and 2).
Adverse cardiovascular events
There were 3 cases (1.47%, 3/204) of acute immune myocarditis in the ICI group, but none in the control group. The 3 patients with ICI-associated myocarditis included in this article were treated with Camrelizumab. The main clinical manifestations are dyspnea, fatigue, abnormal ECG, and elevated cardiac biomarkers. The occurrence time of 3 cases of acute immune myocarditis was 21 days, 28 days and 31 days, respectively. All 3 patients received methylprednisolone to suppress the immune system. Two of the patients gradually improved after treatment and eventually recovered and were discharged from the hospital. However, the heart failure of one of the cancer patients did not improve and died of ventricular fibrillation. The figures showed cTnT changes in 3 patients, ECG changes in case 1, and NT-proBNP changes in case 2 (Figures 2, 3 and 4). 39 days after receiving Camrelizumab, case 3 underwent a CMR examination, and the results showed that the T2 value of the ventricular septum increased to 68.76 ms (Figure 5).
Figure 2.
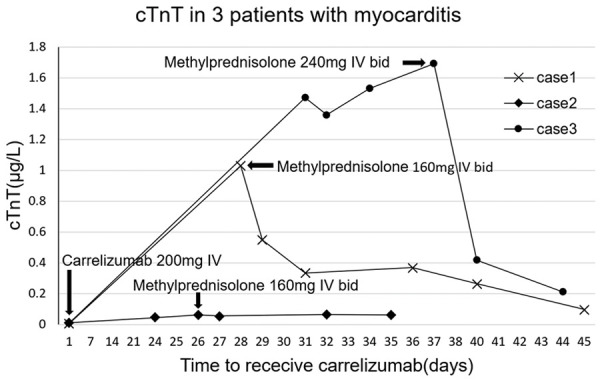
Changes of cTnT in 3 patients with myocarditis before and after receiving carrelizumab and glucocorticoid. After receiving carrelizumab treatment, the cTnT of case 2 increased slightly, the cTnT of case 1 and case 3 both increased significantly within 1 month, and the troponin decreased significantly after methylprednisolone treatment.
Figure 3.
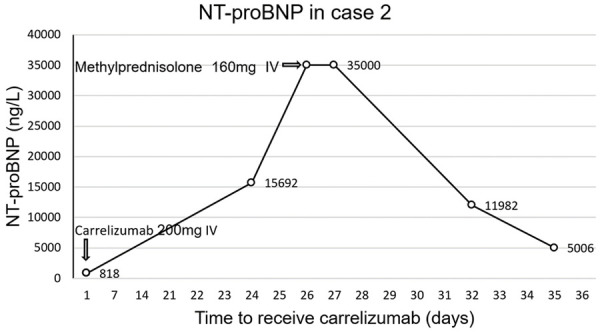
Changes of NT-proBNP before and after treatment with carrelizumab and glucocorticoid in case 2. NT-proBNP increased sharply 24 days after receiving carrelizumab treatment, and decreased after receiving methylprednisolone treatment.
Figure 4.
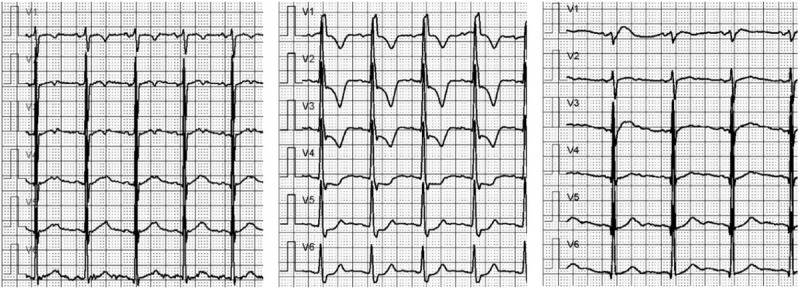
The ECG changes before and after ICI treatment in case 1. Left: before receiving Carrelizumab; Middle: day 24 after receiving Carrelizumab, ECG examination showed abnormal ST-T; Right: day 29 after receiving carrelizumab (5 days after receiving glucocorticoid), ECG shows that ST-T abnormality has returned to normal.
Figure 5.
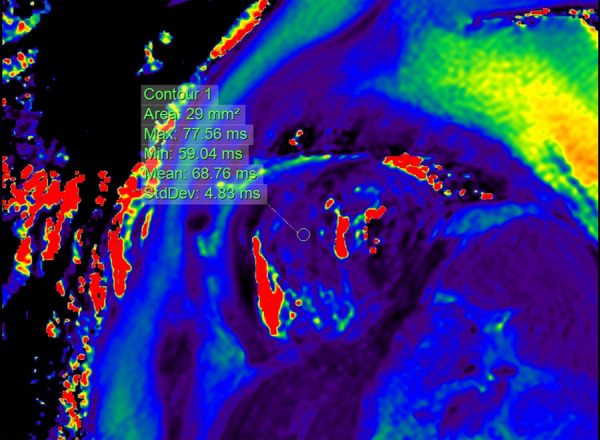
Myocardial T2 mapping by CMR in case 3. 39 days after receiving Carrelizumab, CMR showed an increase in the T2 value of the ventricular septum, which indicated local edema of the myocardium.
There were 2 cases of new-onset pericardial effusion in the control group and 6 cases of new-onset pericardial effusion in the ICI group. Abnormal ECG was the most common ACEs in cancer patients after receiving ICI, a total of 38 patients developed new ECG abnormalities, including sinus bradycardia, sinus tachycardia, bundle branch block, atrioventricular block, premature atrial or ventricular contraction, ST segment or T Wave changes. The incidence of new-onset ECG abnormalities in the ICI group was significantly higher than that of the control group (38/180 VS 16/178, HR 2.71, 95% CI: 1.449-5.067, P=0.001). In the ICI group, the NT-proBNP of 10 patients with a normal baseline significantly increased (more than 2 times the upper limit of normal) after receiving ICI, while 5 patients in the control group increased, but there was no statistical difference between the two groups (P=0.162). In addition, 9 patients in the ICI group had elevated cTnT (At least 2 times higher than the upper limit of normal), which was higher than 5 patients in the control group (P=0.028) (Table 2).
Table 2.
Adverse cardiovascular events after tumor treatment
| Control (N=205) | ICI group (N=204) | P value | |||
|---|---|---|---|---|---|
| Abnormal ECG | 178 (miss27) | 16 | 180 (miss24) | 38 | 0.001 |
| Pericardial effusion | 202 (miss 3) | 2 | 203 (miss 1) | 6 | 0.135 |
| NT-ProBNP abnormal | 201 (miss 4) | 4 | 187 (miss 17) | 10 | 0.076 |
| Sinus tachycardia | 202 (miss 3) | 6 | 203 (miss 1) | 15 | 0.045 |
| Acute myocarditis | 205 | 0 | 204 | 3 | 0.083 |
| cTnT abnormal | 201 (miss 4) | 2 | 203 (miss 1) | 9 | 0.028 |
Abbreviations: ECG, electrocardiogram; NT-proBNP, N terminal pro B type natriuretic peptide; cTnT, cardiac troponin T.
Carrelizumab (n=154), pembrolizumab (n=20), and tislelizumab (n=11) were the most commonly used ICIs in this study. After receiving different ICIs, there were differences in the incidence of various ACEs (Supplementary Table 3). Among them, the incidence of abnormal cTnT caused by carrelizumab was relatively high, and all 3 cases of myocarditis occurred after the use of carrelizumab. The incidence of abnormal ECG caused by Pembrolizumab was higher, and the incidence of pericardial effusion and abnormal NT-proBNP was higher after the use of Navulumab. However, due to the low incidence of ACEs and there were few users of some ICI drugs, it was difficult to statistically determine whether there were differences between various drugs. The incidence of ACEs in different cancer stages (stages 1-4) after ICI treatment was analyzed. Cancer patients receiving ICI treatment were more in stage 3 and stage 4. There was no statistical difference in the incidence of abnormal ECG among patients in each stage. Acute myocarditis and abnormal cTnT only occurred in patients with stage 3-4 cancer. Pericardial effusion and abnormal NT-ProBNP occurred in patients with stage 2-4 cancer, but there was no statistical difference (Supplementary Table 4).
Cardiac biomarkers
Most of the patients included in the study underwent regular testing of cardiac biomarkers including cTnT and NT-proBNP. Before receiving ICI or traditional chemotherapy, the average cTnT of the ICI group was not different from the control (0.0081±0.0103, 0.0077±0.0047, respectively, P=0.571). We used a linear mixed model to analyze the troponin levels of the two groups after receiving tumor treatment. The results showed that compared with the control group, after receiving ICI (with or without traditional chemotherapy), the ICI group has a higher cTnT (P<0.001). In the ICI group, about 1 month after receiving ICI, cTnT was significantly higher than the baseline (0.0267±0.0102 VS 0.0081±0.0103, P=0.039) and it gradually decreased in the second to fourth months. In the 5th and 6th months after ICI treatment, the average cTnT gradually increased compared with the 4th month (Figure 6).
Figure 6.
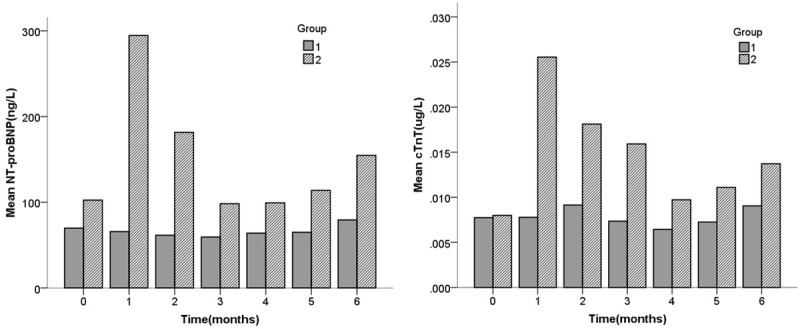
Changes of cTnT and NT-proBNP at baseline and after receiving anti-tumor drug treatment in control group (group 1) and ICI group (group 2).
NT-proBNP was examined in 187 (93.5%) patients in the ICI group and 201 (98.0%) patients in the control group. Compared with the control group, before receiving ICI or traditional chemotherapy, the average NT-proBNP of the ICI group was slightly higher than the control (85.78±129.4 μg/L VS 69.1±60.6 μg/L, P=0.011). We conducted a mixed linear model analysis on the NT-proBNP of the two groups. The results showed that the average NT-proBNP of the ICI group was significantly higher than that of the control group after receiving cancer treatment (P=0.005). The average NT-proBNP of the ICI group increased drastically from 104.0±174.6 ug/L at baseline to 310.86±2730.58 ug/L in the first month after receiving ICI. It declined in the second and third months, but gradually increased in the fourth to sixth months. No similar changes were observed in the control group, and compared with baseline, there were no significant changes in cardiac biomarkers after receiving traditional chemotherapy (Figure 6).
Discussion
This study was a retrospective controlled study focusing on cardiotoxicity caused by ICI in the real world. We found that some patients receiving ICI developed ACEs, such as acute myocarditis (n=3), pericardial effusion (n=6) and abnormal ECG (n=38), and its incidence were significantly higher than that of patients who only received traditional chemotherapy. In addition, we found that after cancer patients received ICI, the average cTnT and NT-proBNP were higher than the baseline and the control group, indicating that ICI-induced myocardial immune damage and decreased cardiac function.
The human immune system controls the immune response by regulating the surface molecules of immune cells to protect the human body from threats and not respond to self-antigens [17]. Cytotoxic T lymphocyte associate protein-4 (CTLA-4) and programmed cell death protein 1 (PD-1) or its ligands, programmed cell death 1 ligand 1 (PD-L1) are negative immunomodulatory molecules which are called immune checkpoints [18]. ICI is a monoclonal antibody that can block one of these immune checkpoints to enhance the body’s immune function to kill tumor cells [1].
PD-1 is located on the surface of T cells, and PD-L1 is its ligand and can be expressed in tumor cells or cardiomyocytes. The activation of T cells can be suppressed after PD-1 and PD-L1 binding. ICIs such as pembrolizumab, nivolumab, and carrelizumab are antibodies that target PD-1, while atezolizumab, durvalumab, and avelumab are antibodies that target PD-L1. These drugs can block the binding of PD-1 and PD-L1, thus enhancing the activation of T cells against tumors. PD-L1 can be expressed on the cardiomyocytes of adult animals and plays an important role in maintaining the immune tolerance of the heart [19]. After PD-1 or PDL-1 is inhibited by ICI, the immune tolerance of the heart is reduced, and the activated immune system can lead to autoimmunity of the heart [20]. CTLA-4 is another negative regulator of T cell activation, which is very important for maintaining the stability of lymphocytes. CTLA-4-deficient mice have suffered autoimmune damage, with particularly severe myocarditis and pancreatitis [21,22]. CTLA-4 antibody such as ipilimumab, the first drug approved for the treatment of advanced melanoma, which can inhibit CTLA-4 to relieve its inhibitory effect on T cell activation. The activation of T cells can kill tumor cells, but at the same time, over-activated T cells can cause damage to organs and tissues such as myocardium. Cardiomyocytes may have shared antigen target with tumor cells, and autoimmunity occurs after recognition by the activated immune system [23]. ICI can also increase the level of cytokines and promote the formation of autoantibodies against target tissues, resulting in immune damage [24].
In recent years, many ICIs have been approved to treat a variety of malignant tumors and improve the prognosis of many cancer patients. However, the suppression of immune checkpoints by ICI may lead to excessive activation of the immune system and cause inflammatory side effects, which are called irAEs [25]. Among them, ACEs caused by ICI have been continuously reported in recent years [26,27]. Previous studies have shown that the cardiotoxicity of ICI may lead to acute myocarditis, pericardial disease, arrhythmia and other diseases [28].
ICI-associated autoimmune myocarditis is an acute cardiovascular complication with a poor prognosis. A meta-analysis showed that the incidence of ICI-associated myocarditis was between 0.27% and 1.14% [5,23], and the mortality rate was as high as 30%-50%. The onset time of ICI-associated myocarditis was mostly within 2 months after receiving ICI treatment, with a median time of 30 days [29]. In this study, 3 patients developed ICI-associated myocarditis (1.46%), and the onset time was within 30 days. All 3 patients were treated with steroids, but one of them died of heart failure. The basic condition of the heart may be one of the risk factors for death after the occurrence of myocarditis. The patient who died of myocarditis had signs of cardiac dysfunction before using ICI. The echocardiogram showed that the motion of the left ventricle wall was uncoordinated, and NT-proBNP (818 ng/L) was high. Therefore, it is necessary to comprehensively assess the patient’s baseline cardiovascular status and carry out risk stratification.
Arrhythmia and pericardial diseases are also common complications related to tumor treatment. ECG abnormalities caused by ICI may present atrioventricular block, bundle branch block, atrial and ventricular arrhythmia, and severe arrhythmia may lead to syncope and sudden death [30]. In this study, 38 patients with normal baseline ECG developed abnormal ECG after receiving ICI. Among them, 18 patients developed atrial or ventricular arrhythmia, and 10 patients developed tachycardia. The abnormal ECG may reflect the early immune damage of the myocardium. Many oncology drugs or chest radiotherapy may cause pericardial disease. Echocardiogram and chest CT can effectively detect pericardial diseases. 204 patients in the ICI group and 205 patients in the control group underwent chest CT or/and echocardiogram during tumor treatment. Among them, 6 patients in the ICI group developed pericardial effusion (2.9%), while only 2 patients in the control group. Heart rate can reflect the state of heart function and may be related to the prognosis of cardiovascular disease. After receiving ICI, the heart rate of cancer patients was significantly higher than the baseline.
Cardiac biomarkers are commonly used indicators to detect myocardial damage and cardiac dysfunction, which are related to the prognosis of cardiovascular system and are often used to monitor cardiotoxicity caused by tumor treatment drugs [31,32]. In this study, the average cTnT and NT-proBNP in patients were significantly increased about 1 month after receiving ICI, which reflected early myocardial immune damage. In the second month, the average cTnT and NT-proBNP decreased, indicated that myocardial inflammatory injury has been partially recovered. As time gone by, the average cTnT and NT-proBNP in the ICI group gradually increased. This reflected chronic accumulation and delayed immune damage in myocardium.
There have been many reports of acute ACEs caused by ICI, and its main mechanism is acute autoimmune injury. ICI leads to excessive activation of T cells, promoting inflammatory cytokines, and other possible mechanisms lead to acute myocardial immune injury. However, there were few research reports on chronic ACEs caused by ICI. A retrospective study carried out by Dolladille C showed that 19 patients had late (≥90 days) ICI-related ACEs after receiving ICI treatment, accounting for 50% of the total ACEs [33], and none of them had previous acute (<90 days) ACEs. The mechanisms of chronic ACEs caused by ICI were not fully understood. The continuous activation of T cells caused by periodic use of ICI may cause chronic and cumulative injury to the myocardium, which gradually lead to the remodeling of myocardial structure. Some clinical cases have reported the presence of myocardial fibrosis detected by MRI or endomyocardial biopsy in patients with ICI-related dilated cardiomyopathy, which was the clinical evidence of ICI leads to chronic injuries of myocardium [11]. In addition, the production of autoantibodies may also be one of the mechanisms of chronic myocardial injury. Taku Okazaki found that autoantibody against cardiac troponin I in PD-1 deficient mice was a possible mechanism for the development of dilated cardiomyopathy in mice [34]. This study found that after accepting ICI 3-4 months, the average cardiac biomarkers gradually increase, which reflected the chronic myocardial injury caused by ICI.
For patients suspected of acute myocarditis, CMR is a very important examination method. The elevated T1 and T2 values reflect the inflammation and edema of the myocardium. A recent study showed that T1 and T2 values were abnormal in 78% and 43% of the patients with ICI-associated myocarditis, respectively [35]. Case 2 with myocarditis in this study had an elevated T2 value in the ventricular septum, which indicated edema of the myocardium (Figure 5). The value of CMR in the detection of cardiovascular toxicity in cancer patients treated with ICI deserves further study.
With the increasing use of ICI in clinical practice, reports of cardiovascular complications continue to increase. However, in general, insufficient attention has been paid to the cardiotoxicity of ICI. It is very important to actively monitor the cardiotoxicity of ICI and treat it. The study showed that ECG, cardiac biomarkers and echocardiogram are common methods that can be used to assess cardiotoxicity caused by ICI. For patients with suspected acute myocarditis or heart failure, radionuclide myocardial imaging, angiography, CMR and endocardial biopsy can also be selected according to the condition. Cardiotoxicity caused by ICI should be brought to the attention of clinicians. The key to prevention and treatment is to assess the cardiovascular condition before using ICI, monitor closely, find and diagnose it as early as possible, and provide timely treatment after cardiovascular complications occur.
Conclusions
Compared with patients who only received traditional chemotherapy, patients who received ICI had a higher incidence of ACEs. The cardiovascular toxicity of ICI may lead to various ACEs such as acute myocarditis, pericardial effusion, arrhythmia, and cardiac dysfunction. Among patients receiving ICI, the average NT-proBNP and cTnT reached the highest around the first month, decreased in the second month, and gradually increased from the third or fourth month. This indicates that cTnT and NT-proBNP can detect acute and chronic myocardial injury caused by ICI. The combined use of ECG, echocardiography and cardiac biomarkers can help early detection of cardiovascular events caused by ICI.
Study limitations
Due to the low incidence of ICI-associated myocarditis, pericarditis and heart failure, cancer patients with no cardiovascular clinical symptoms undergoing ICI treatment have not received enough attention from oncologists to receive regular cardiovascular assessments. For example, whether a patient receives echocardiogram or CMR usually depends on the doctor, and this results in a relatively small number of cancer patients undergoing echocardiogram or CMR, and difficult to conduct statistical analysis. This may cause some data loss and statistical bias in the assessment of ICI cardiotoxicity.
Acknowledgements
This work was supported by Science Commission and Health department of Chongqing city (grant no. 2018ZDXM010).
Disclosure of conflict of interest
None.
Abbreviations
- ICI
immune checkpoint inhibitors
- ECG
electrocardiogram
- irAEs
immune-related adverse events
- ACEs
adverse cardiovascular events
- cTnT
cardiac troponin T
- NT-proBNP
N terminal pro B type natriuretic peptide
- BMI
body mass index
- CTLA-4
Cytotoxic T lymphocyte associate protein-4
- PD-1
programmed cell death protein 1
- PD-L1
Programmed cell death 1 ligand 1
- CMR
cardiac magnetic resonance
- HR
heart rate
- BP
blood pressure
Supporting Information
References
- 1.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2:e192535. doi: 10.1001/jamanetworkopen.2019.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mobini N, Dhillon R, Dickey J, Spoon J, Sadrolashrafi K. Exclusive cutaneous and subcutaneous sarcoidal granulomatous inflammation due to immune checkpoint inhibitors: report of two cases with unusual manifestations and review of the literature. Case Rep Dermatol Med. 2019;2019:6702870. doi: 10.1155/2019/6702870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L, Jones-O’Connor M, Awadalla M, Zlotoff DA, Thavendiranathan P, Groarke JD, Villani AC, Lyon AR, Neilan TG. Cardiotoxicity of immune checkpoint inhibitors. Curr Treat Options Cardiovasc Med. 2019;21:32. doi: 10.1007/s11936-019-0731-6. [DOI] [PubMed] [Google Scholar]
- 5.Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, Sullivan RJ, Damrongwatanasuk R, Chen CL, Gupta D, Kirchberger MC, Awadalla M, Hassan MZO, Moslehi JJ, Shah SP, Ganatra S, Thavendiranathan P, Lawrence DP, Groarke JD, Neilan TG. Myocarditis in patients treated with immune checkpoint Inhibitors. J Am Coll Cardiol. 2018;71:1755–1764. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Awadalla M, Golden DLA, Mahmood SS, Alvi RM, Mercaldo ND, Hassan MZO, Banerji D, Rokicki A, Mulligan C, Murphy SPT, Jones-O’Connor M, Cohen JV, Heinzerling LM, Armanious M, Sullivan RJ, Damrongwatanasuk R, Chen CL, Gupta D, Kirchberger MC, Moslehi JJ, Shah SP, Ganatra S, Thavendiranathan P, Rizvi MA, Sahni G, Lyon AR, Tocchetti CG, Mercurio V, Thuny F, Ederhy S, Mahmoudi M, Lawrence DP, Groarke JD, Nohria A, Fradley MG, Reynolds KL, Neilan TG. Influenza vaccination and myocarditis among patients receiving immune checkpoint inhibitors. J Immunother Cancer. 2019;7:53. doi: 10.1186/s40425-019-0535-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neilan TG, Rothenberg ML, Amiri-Kordestani L, Sullivan RJ, Steingart RM, Gregory W, Hariharan S, Hammad TA, Lindenfeld J, Murphy MJ, Moslehi JJ Checkpoint Inhibitor Safety Working Group. Myocarditis associated with immune checkpoint inhibitors: an expert consensus on data gaps and a call to action. Oncologist. 2018;23:874–878. doi: 10.1634/theoncologist.2018-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Almeida DVP, Gomes JR, Haddad FJ, Buzaid AC. Immune-mediated pericarditis with pericardial tamponade during nivolumab therapy. J Immunother. 2018;41:329–331. doi: 10.1097/CJI.0000000000000217. [DOI] [PubMed] [Google Scholar]
- 9.Daxini A, Cronin K, Sreih AG. Vasculitis associated with immune checkpoint inhibitors-a systematic review. Clin Rheumatol. 2018;37:2579–2584. doi: 10.1007/s10067-018-4177-0. [DOI] [PubMed] [Google Scholar]
- 10.Anderson RD, Brooks M. Apical takotsubo syndrome in a patient with metastatic breast carcinoma on novel immunotherapy. Int J Cardiol. 2016;222:760–761. doi: 10.1016/j.ijcard.2016.07.291. [DOI] [PubMed] [Google Scholar]
- 11.Heinzerling L, Ott PA, Hodi FS, Husain AN, Tajmir-Riahi A, Tawbi H, Pauschinger M, Gajewski TF, Lipson EJ, Luke JJ. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer. 2016;4:50. doi: 10.1186/s40425-016-0152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roth ME, Muluneh B, Jensen BC, Madamanchi C, Lee CB. Left ventricular dysfunction after treatment with ipilimumab for metastatic melanoma. Am J Ther. 2016;23:e1925–e1928. doi: 10.1097/MJT.0000000000000430. [DOI] [PubMed] [Google Scholar]
- 13.Weinstock C, Khozin S, Suzman D, Zhang L, Tang S, Wahby S, Goldberg KB, Kim G, Pazdur R. U.S. Food and drug administration approval summary: atezolizumab for metastatic non-small cell lung cancer. Clin Cancer Res. 2017;23:4534–4539. doi: 10.1158/1078-0432.CCR-17-0540. [DOI] [PubMed] [Google Scholar]
- 14.Dyhl-Polk A, Schou M, Vistisen KK, Sillesen AS, Serup-Hansen E, Faber J, Klausen TW, Bojesen SE, Vaage-Nilsen M, Nielsen DL. Myocardial ischemia induced by 5-fluorouracil: a prospective electrocardiographic and cardiac biomarker study. Oncologist. 2021;26:e403–e413. doi: 10.1002/onco.13536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambert J, Lamacie M, Thampinathan B, Altaha MA, Esmaeilzadeh M, Nolan M, Fresno CU, Somerset E, Amir E, Marwick TH, Wintersperger BJ, Thavendiranathan P. Variability in echocardiography and MRI for detection of cancer therapy cardiotoxicity. Heart. 2020;106:817–823. doi: 10.1136/heartjnl-2019-316297. [DOI] [PubMed] [Google Scholar]
- 16.Michel L, Mincu RI, Mahabadi AA, Settelmeier S, Al-Rashid F, Rassaf T, Totzeck M. Troponins and brain natriuretic peptides for the prediction of cardiotoxicity in cancer patients: a meta-analysis. Eur J Heart Fail. 2020;22:350–361. doi: 10.1002/ejhf.1631. [DOI] [PubMed] [Google Scholar]
- 17.Ceeraz S, Nowak EC, Burns CM, Noelle RJ. Immune checkpoint receptors in regulating immune reactivity in rheumatic disease. Arthritis Res Ther. 2014;16:469. doi: 10.1186/s13075-014-0469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tarrio ML, Grabie N, Bu DX, Sharpe AH, Lichtman AH. PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J Immunol. 2012;188:4876–4884. doi: 10.4049/jimmunol.1200389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Okazaki IM, Yoshida T, Chikuma S, Kato Y, Nakaki F, Hiai H, Honjo T, Okazaki T. PD-1 deficiency results in the development of fatal myocarditis in MRL mice. Int Immunol. 2010;22:443–452. doi: 10.1093/intimm/dxq026. [DOI] [PubMed] [Google Scholar]
- 21.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealin g a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 22.Klocke K, Sakaguchi S, Holmdahl R, Wing K. Induction of autoimmune disease by deletion of CTLA-4 in mice in adulthood. Proc Natl Acad Sci U S A. 2016;113:E2383–2392. doi: 10.1073/pnas.1603892113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, Becker JR, Slosky DA, Phillips EJ, Pilkinton MA, Craig-Owens L, Kola N, Plautz G, Reshef DS, Deutsch JS, Deering RP, Olenchock BA, Lichtman AH, Roden DM, Seidman CE, Koralnik IJ, Seidman JG, Hoffman RD, Taube JM, Diaz LA Jr, Anders RA, Sosman JA, Moslehi JJ. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375:1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sury K, Perazella MA, Shirali AC. Cardiorenal complications of immune checkpoint inhibitors. Nat Rev Nephrol. 2018;14:571–588. doi: 10.1038/s41581-018-0035-1. [DOI] [PubMed] [Google Scholar]
- 25.Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, Jordan K ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(Suppl 4):iv119–iv142. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 26.Matsui H, Kawai T, Sato Y, Ishida J, Kadowaki H, Akiyama Y, Yamada Y, Nakamura M, Yamada D, Akazawa H, Suzuki M, Komuro I, Kume H. A fatal case of myocarditis following myositis induced by pembrolizumab treatment for metastatic upper urinary tract urothelial carcinoma. Int Heart J. 2020;61:1070–1074. doi: 10.1536/ihj.20-162. [DOI] [PubMed] [Google Scholar]
- 27.Xie X, Wang F, Qin Y, Lin X, Xie Z, Liu M, Ouyang M, Luo B, Gu Y, Li S, Gu D, Chen R, Zhou C. Case report: fatal multiorgan failure and heterochronous pneumonitis following pembrolizumab treatment in a patient with large-cell neuroendocrine carcinoma of lung. Front Pharmacol. 2020;11:569466. doi: 10.3389/fphar.2020.569466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Behravesh S, Shomali N, Danbaran GR, Aslani S, Hemmatzadeh M, Hosseinzadeh R, Gowhari-Shabgah A, Mohammadi H. Cardiotoxicity of immune checkpoint inhibitors: an updated review. Biotechnol Appl Biochem. 2020 doi: 10.1002/bab.2081. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, Gobert A, Spano JP, Balko JM, Bonaca MP, Roden DM, Johnson DB, Moslehi JJ. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–1589. doi: 10.1016/S1470-2045(18)30608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Heliö T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. 2648a–2648d. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 31.Spallarossa P, Tini G, Sarocchi M, Arboscello E, Grossi F, Queirolo P, Zoppoli G, Ameri P. Identification and management of immune checkpoint inhibitor-related myocarditis: use troponin wisely. J. Clin. Oncol. 2019;37:2201–2205. doi: 10.1200/JCO.18.02464. [DOI] [PubMed] [Google Scholar]
- 32.Skovgaard D, Hasbak P, Kjaer A. BNP predicts chemotherapy-related cardiotoxicity and death: comparison with gated equilibrium radionuclide ventriculography. PLoS One. 2014;9:e96736. doi: 10.1371/journal.pone.0096736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dolladille C, Ederhy S, Allouche S, Dupas Q, Gervais R, Madelaine J, Sassier M, Plane AF, Comoz F, Cohen AA, Thuny FR, Cautela J, Alexandre J. Late cardiac adverse events in patients with cancer treated with immune checkpoint inhibitors. J Immunother Cancer. 2020;8:e000261. doi: 10.1136/jitc-2019-000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, Ishida M, Hiai H, Matsumori A, Minato N, Honjo T. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med. 2003;9:1477–1483. doi: 10.1038/nm955. [DOI] [PubMed] [Google Scholar]
- 35.Thavendiranathan P, Zhang L, Zafar A, Drobni ZD, Mahmood SS, Cabral M, Awadalla M, Nohria A, Zlotoff DA, Thuny F, Heinzerling LM, Barac A, Sullivan RJ, Chen CL, Gupta D, Kirchberger MC, Hartmann SE, Weinsaft JW, Gilman HK, Rizvi MA, Kovacina B, Michel C, Sahni G, Gonzalez-Mansilla A, Calles A, Fernandez-Aviles F, Mahmoudi M, Reynolds KL, Ganatra S, Gavira JJ, Gonzalez NS, Garcia de Yebenes Castro M, Kwong RY, Jerosch-Herold M, Coelho-Filho OR, Afilalo J, Zatarain-Nicolas E, Baksi AJ, Wintersperger BJ, Calvillo-Arguelles O, Ederhy S, Yang EH, Lyon AR, Fradley MG, Neilan TG. Myocardial T1 and T2 mapping by magnetic resonance in patients with immune checkpoint inhibitor-associated myocarditis. J Am Coll Cardiol. 2021;77:1503–1516. doi: 10.1016/j.jacc.2021.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


