Abstract
Recently, a prospective randomized study suggested that transcatheter arterial chemoembolization (TACE) plus lenvatinib, as opposed to TACE plus sorafenib, was an effective and promising treatment for patients with advanced hepatocellular carcinoma (HCC) having portal vein thrombus (PVTT) and large tumor burden. However, no propensity score matching retrospective studies on TACE with drug-eluting beads (DEB-TACE) plus lenvatinib (DEB-TACE+LEN) versus DEB-TACE plus sorafenib (DEB-TACE+SOR) for advanced HCC has been reported to date. The medical records of consecutive patients with advanced HCC who underwent DEB-TACE+LEN or DEB-TACE+SOR between January 2017 and December 2020 were retrospectively reviewed. Mutation genes (VEGF, ANG2, FGF19, FGF21, and FGF23) were measured by whole-exome sequencing (WES). Adverse events (AEs), objective response rate (ORR), disease control rate (DCR), overall survival (OS) and time to progression (TTP) were compared between patients who underwent DEB-TACE+LEN and DEB-TACE+SOR. In total, 150 patients were enrolled in this study. The DEB-TACE+LEN group (n=50) showed significantly better ORR (64.0% vs. 33.3%; P=0.008), OS (hazard ratio [HR]=0.63, 95% confidence interval (CI): 0.41-0.98; P=0.043), and TTP (HR=0.65, 95% CI: 0.45-0.94; P=0.023) than that in the DEB-TACE+SOR group (n=100). Subgroup analyses showed that in patients with portal vein tumor thrombus (PVTT), OS and TTP were significantly longer in the DEB-TACE+LEN group than in the DEB-TACE+SOR group (HR=0.59, 95% CI: 0.36-0.98; P=0.043; HR=0.89, 95% CI: 0.35-2.29; P=0.035). In patients with FGF21 amplification, OS was also significantly longer in the DEB-TACE+LEN group than that in the DEB-TACE+SOR group (HR=0.19, 95% CI: 0.06-0.66; P=0.003). The patients in DEB-TACE+LEN group had a significantly lower incidence of hand-foot skin reaction (32.0% vs. 49.0%; P=0.048), but a higher incidence of proteinuria (26.0% vs. 10.0%; P=0.010) than that in the DEB-TACE+SOR group. In conclusion, DEB-TACE+LEN conferred better ORR, OS and TTP than did DEB-TACE+SOR in patients with advanced HCC, especially those with PVTT and FGF21 amplification, with acceptable AEs; thus making it a superior treatment modality for these patients.
Keywords: Lenvatinib, sorafenib, hepatocellular carcinoma, DEB-TACE, tumor response, FGF21
Implications for practice
Patients in the DEB-TACE+LEN group achieved better ORR, OS and TTP than those in the DEB-TACE+SOR group, especially those with PVTT and FGF21 amplification. AEs were also within the acceptable frequency in both groups. Thus, DEB-TACE plus lenvatinib is a superior treatment modality for these patients.
Introduction
Hepatocellular carcinoma (HCC) is the most frequent primary liver malignancies. More than 70% of patients are unsuitable for curative therapy, such as hepatectomy, transplantation or liver ablation [1]. In 2007, sorafenib was the first oral tyrosine kinase inhibitor (TKI) as a standard treatment in patients with unresectable HCC [2]. In 2018, the REFLECT trial showed that lenvatinib was non-inferior to sorafenib in overall survival (OS), for patients with unresectable HCC [3]. Then, both the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL) recommended the first-line administration of lenvatinib or sorafenib as standard of care for HCC patients with Barcelona Clinic Liver Cancer Stage (BCLC) stage C [4,5]. Up until 2020, atezolizumab plus bevacizumab was recommended as the first-line treatment in patients with unresectable HCC by the Food and Drug Administration (FDA), based on data in the IMbrave 150 trial, which widened the treatment landscape for unresectable HCC [6,7]. However, there were 48% of patients receiving prior local therapy in the atezolizumab-bevacizumab group, which indicated the importance of local plus systemic therapy.
For patients with unresectable HCC, China Liver cancer staging (CNLC) IIIa or IIIb, Chinese practice guidelines for transcatheter arterial chemoembolization (TACE) consider it to be an effective modality [8]. There were many studies on TACE plus sorafenib for unresectable HCC. Most studies on TACE plus sorafenib versus TACE alone for unresectable HCC, such as the SPACE, post-TACE and TACE 2 trials [9-11], did not show any clinical benefit when sorafenib was added to TACE. In contrast, the TACTICS trial was the first to demonstrate that TACE plus sorafenib achieves significantly better progression-free survival (PFS) compared to TACE alone in unresectable HCC, especially BCLC stage C. The benefit is primarily due to longer treatment duration of sorafenib and more reasonable experimental design [12]. Victor et al. also found that the addition of TACE to sorafenib improved OS compared with sorafenib alone for patients with HCC, BCLC stage C [13]. These results suggest that TACE plus sorafenib is a effective and promising treatment for patients with unresectable HCC, especially BCLC stage C.
Recently, a prospective randomized single-center study showed that TACE plus lenvatinib had more favorable efficacy compared to TACE plus sorafenib in advanced HCC [14]. However, the reasons on the superiority of TACE plus lenvatinib versus TACE plus sorafenib as first-line therapy in advanced HCC remains unknown. Richard SF et al. firstly reported that vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF) 19, FGF21, FGF23, and angiopoietin (ANG) 2 may be related to OS treated with lenvatinib or sorafenib in patients with unresectable HCC. Higher baseline FGF21 had better OS with lenvatinib compared to sorafenib [15]. However, this needs to be verified on whether mutation genes (VEGF, ANG2, FGF19, FGF23, and FGF21) were among reasons why TACE plus lenvatinib was superior to TACE plus sorafenib in advanced HCC.
Drug-eluting beads (DEB) can be loaded with chemotherapy drugs to lower peripheral blood drug concentration, delivering longer concentrations to the target than conventional lipiodol [16-18]. Pawlik et al. firstly reported that TACE with DEB (DEB-TACE) plus sorafenib was tolerable and safe in unresectable HCC, 64% of whom were BCLC stage C [19]. In this 1:2 propensity score matching retrospective study, we aimed to investigate the safety and efficacy of DEB-TACE plus lenvatinib (DEB-TACE+LEN) in comparison with that of DEB-TACE plus sorafenib (DEB-TACE+SOR) for advanced HCC and confirm the correlation between mutation genes (VEGF, ANG2, FGF19, FGF21, and FGF23) and the clinical outcome in both groups.
Materials and methods
Study design and patients
This was a retrospective controlled study of 774 consecutive patients with advanced HCC who underwent DEB-TACE+LEN or DEB-TACE+SOR between January 2017 and December 2020 at the First Affiliated Hospital of Sun Yat-Sen University. The inclusion criteria were as follows: (a) Diagnosed via biopsy, cytology or diagnostic imaging (e.g., computed tomography (CT) or magnetic resonance (MR)) according to the criteria of AASLD [4]; (b) Age ≥18 years; (c) Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1 [2]; (d) Child-Pugh A or B and adequate organ function; (e) BCLC Stage C tumors. The exclusion criteria were as follows: (a) Diffuse tumor lesions or main portal vein tumor thrombus (PVTT); (b) Child-Pugh C; (c) Another previous or current malignant tumor; (d) Cardiac disease or serious and active infection, except for hepatitis B virus (HBV); (e) Hepatic encephalopathy, uncontrolled ascites, or pleural effusion; (f) Underwent surgery, liver transplantation, or other local-regional therapies (hepatic artery infusion chemotherapy, liver ablation, or iodine 125 particles implantation); (g) Underwent previous anti-tumor treatments.
After propensity score matching, safety and efficacy outcomes were compared between 50 DEB-TACE+LEN patients and 100 DEB-TACE+SOR patients. This study was approved by the Institutional Review Board. The requirement to obtain informed consent was waived owing to the retrospective nature of the study.
Treatment protocol
The choice of DEB-TACE+LEN or DEB-TACE+SOR was recommended by interventional physicians (L.J.P., W.Y., F.W.Z.). If the patient agreed with the physicians’ suggestion, lenvatinib or sorafenib was administered 2-3 weeks prior to the first DEB-TACE session. All patients were started on sorafenib (800 mg/day, Nexavar® [Bayer Co., Ltd., Leverkusen, Germany]) or lenvatinib (8 mg/day (<60 kg) or 12 mg/day (≥60 kg), Lenvima® [Eisai Co., Ltd., Tokyo, Japan]). Lenvatinib or sorafenib was terminated for 2 days before and 2 days after each DEB-TACE session. For patients who experienced sorafenib- or lenvatinib-related adverse events (AEs), the dose was reduced based on the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE), version 3.0 [16].
TACE was performed with microcatheter (Renegade, Boston Scientific, Natick, Mass; Progreat, Terumo, Tokyo, Japan) through liver arteries, depending on tumor blood supply distribution. Initially, an emulsion of 100-300 µm Callisphere DEB (Hengrui, Jiangsu, China) and 40-80 mg doxorubicin (Pfizer, New York, USA) was injected into the feeding arteries. The dosage of Callisphere and doxorubicin was decided according to tumor number, tumor size, blood supply, presence of arterioportal shunt and underlying liver function. The first image was taken 4 weeks after the first DEB-TACE procedure. Repeat TACE was recommended when blood supply range was >50% that of the baseline tumor or new intrahepatic lesions measuring >10 mm was found by using CT or MR.
Propensity score matching
The propensity score was estimated to fit the following 11 variables: age, sex, the presence of HBV, cirrhosis, ECOG PS score, alpha-fetoprotein (AFP) level, Child-Pugh class, intrahepatic tumor number, tumor size, PVTT and extrahepatic metastasis. To create a propensity-matched set of patients treated with DEB-TACE+LEN or DEB-TACE+SOR (1:2 match), a nearest neighbor matching algorithm with a greedy heuristic was used [21].
Whole-exome sequencing
Samples obtained from the tumor biopsy were stored in liquid nitrogen at -80°C before the extraction. There were 43 samples collected for whole-exome sequencing (WES) in the 150 advanced HCC patients after propensity score matching. There were about 27,000 encoded exons sequenced and compared to obtain specific mutation information about the tumors using SureSelect Human All Exome Kit V5 (Agilent Technologies, Santa Clara, CA, USA). Exome shotgun libraries were sequenced on Illumina Xten platforms, generating 150 bp paired reads at each end. Sequencing adapters and low-quality reads were removed for high quality reads. Then, mutation genes (VEGF, ANG2, FGF19, FGF21, and FGF23) were detected in the 43 patients based on WES.
Follow-up
The first follow-up was performed 4 weeks after the first DEB-TACE and involved clinical, laboratory, and radiologic assessments. Radiologic evaluations included CT or MR imaging. Laboratory examination included numbers of blood cells, AFP, prothrombin time (PT), aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TBil) and serum albumin (ALB), etc. Follow-up of all patients was performed at a 4-6 weeks interval after oral TKI or DEB-TACE.
Assessments
AEs associated with DEB-TACE, TKI were reported according to the NCI-CTCAE version 3.0. DEB-TACE related AEs, including abdominal pain, fever, nausea, and vomiting, were recorded until 4 weeks after DEB-TACE. Lenvatinib-related or sorafenib-related AEs, including hand-foot syndrome, hypertension, and proteinuria were observed until discontinuation of lenvatinib or sorafenib.
Treatment response was assessed using CT or MR imaging based on the modified Response Evaluation Criteria in Solid Tumors (mRECIST) [22]. Treatment response was accordingly classified as complete response (CR), partial response (PR), stable response (SD), and progressive disease (PD). Tumor response was performed every 4-8 weeks. We compared the objective response rate (ORR) (CR+PR) and disease control rate (DCR) (CR+PR+SD) between the DEB-TACE+LEN and DEB-TACE+SOR groups.
We also compared OS and time to progression (TTP) between the two groups. OS was defined as the time from the start of sorafenib or lenvatinib to death from any cause. TTP was defined as the time from the start of sorafenib or lenvatinib to progression from any cause. Progression in the study was defined as radiological progression as determined by mRECIST, transient deterioration of liver function to Child-Pugh C after the combination therapy or any cause of death. We compared the AEs, ORR, DCR, OS and TTP between the DEB-TACE+LEN and DEB-TACE+SOR groups.
Statistical analyses
Between-group comparisons were performed using Pearson χ2, Fisher’s exact tests, and continuity correction and independent samples t. A logistic regression model was used to perform propensity score matching and univariate and multivariate analyses. Survival curves were calculated using the Kaplan-Meier method. Confidence interval (CI) and hazard ratio [HR] were estimated using Cox proportional hazards models. All statistical analyses were performed using SPSS (version 23.0; SPSS, Chicago, IL, USA). All tests were two-tailed, and P<0.05 was considered significant.
Results
Patient characteristics
Before matching, the study included 176 patients (DEB-TACE+LEN, n=54; DEB-TACE+SOR, n=199) (Figure 1; Table 1). After matching, there were 150 patients included in the study (DEB-TACE+LEN, n=50; DEB-TACE+SOR, n=100). The baseline patient characteristics, including median age, serum AFP level, incidence of HBV, PVTT and extrahepatic metastasis, were not significantly different between the two groups (Table 1).
Figure 1.
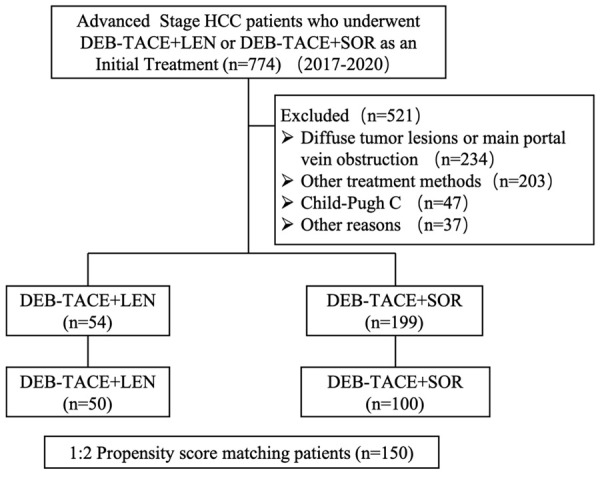
Patient inclusion flowchart. A total of 774 patients received DEB-TACE+LEN or DEB-TACE+SOR as an initial treatment for advanced HCC between 2017 and 2020. Of these, 54 patients in the DEB-TACE+LEN group and 199 in the DEB-TACE+SOR group met the eligibility criteria of this study. After propensity score matching, safety and efficacy was compared between 50 patients in the DEB-TACE+LEN group and 100 in the DEB-TACE+SOR group.
Table 1.
Patient baseline characteristics at the time of study entry before and after propensity score matching
| Characteristicsa | Before Matching | After Matching | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| DEB-TACE+LEN (n=54) | DEB-TACE+SOR (n=199) | P value | DEB-TACE+LEN (n=50) | DEB-TACE+SOR (n=100) | P value | |
| Age (y) | 54 (50-62) | 51 (43-60) | 0.017 | 54 (49-61) | 54 (49-63) | 0.863 |
| Sex | 0.497 | 0.171 | ||||
| Male | 50 (92.6%) | 189 (95.0%) | 46 (92.0%) | 97 (97.0%) | ||
| Female | 4 (7.4%) | 10 (5.0%) | 4 (8.0%) | 3 (3.0%) | ||
| HBV | 0.043 | 0.171 | ||||
| Absence | 6 (11.1%) | 8 (4.0%) | 46 (92.0%) | 97 (97.0%) | ||
| Presence | 48 (88.9%) | 191 (96.0%) | 4 (8.0%) | 3 (3.0%) | ||
| Cirrhosis | 0.784 | 1.000 | ||||
| Absence | 14 (25.9%) | 48 (24.1%) | 12 (24.0%) | 24 (24.0%) | ||
| Presence | 40 (74.1%) | 151 (75.9%) | 38 (76.0%) | 76 (76.0%) | ||
| ECOG score | 0.095 | 0.144 | ||||
| 0 | 39 (72.2%) | 164 (82.4%) | 37 (74.0%) | 84 (84.0%) | ||
| 1 | 15 (27.8%) | 35 (17.6%) | 13 (26.0%) | 16 (16.0%) | ||
| AFP (ng/ml) | 1329 (10-9471) | 3170 (234-39513) | 0.033 | 1329 (18-10462) | 264 (28-4234) | 0.164 |
| ≤200 | 23 (42.6%) | 46 (23.1%) | 0.004 | 21 (42.0%) | 45 (45.0%) | 0.727 |
| >200 | 31 (57.4%) | 153 (76.9%) | 29 (58.0%) | 55 (55.0%) | ||
| Child-Pugh class | 0.476 | 0.757 | ||||
| A | 44 (81.5%) | 170 (85.4%) | 41 (82.0%) | 84 (84.0%) | ||
| B | 10 (18.5%) | 29 (14.6%) | 9 (18.0%) | 16 (16.0%) | ||
| Intrahepatic tumors number | 0.095 | 0.581 | ||||
| ≤3 | 12 (22.2%) | 26 (13.1%) | 10 (20.0%) | 24 (24.0%) | ||
| >3 | 42 (77.8%) | 173 (86.9%) | 40 (40.0%) | 76 (76.0%) | ||
| Tumor sizeb (cm) | 8.7 (4.6-12.1) | 10.0 (6.7-12.6) | 0.498 | 8.7 (4.6-12.1) | 9.0 (5.6-11.5) | 0.658 |
| ≤5 | 14 (25.9%) | 30 (15.1%) | 0.062 | 13 (26.0%) | 19 (19.0%) | 0.324 |
| <5 | 40 (74.1%) | 169 (84.9%) | 37 (74.0%) | 81 (81.0%) | ||
| PVTT | <0.001 | 0.210 | ||||
| Absence | 17 (31.5%) | 19 (9.5%) | 14 (28.0%) | 19 (19.0%) | ||
| Presence | 37 (68.5%) | 180 (90.5%) | 36 (72.0%) | 81 (81.0%) | ||
| Extrahepatic metastasis | 0.003 | 0.298 | ||||
| Absence | 24 (44.4%) | 133 (66.8%) | 23 (46.0%) | 55 (55.0%) | ||
| Presence | 30 (55.6%) | 66 (33.2%) | 27 (54.0%) | 45 (45.0%) | ||
Median with interquartile range is shown for quantitative variables, whereas counts with proportions are shown for categorical variables.
Tumor size, size of the largest tumor.
AFP, alpha-fetoprotein; HBV, hepatitis B virus; PVTT, portal vein tumor thrombus.
Tumor response
In total, 28.0% (14/50) and 36.0% (18/50) of patients in the DEB-TACE+LEN group and 15.0% (15/100) and 26.0% (26/100) of patients in the DEB-TACE+SOR group achieved CR and PR, respectively. The DEB-TACE+LEN group showed significantly better ORR than did the DEB-TACE+SOR group (64.0% vs. 33.3%; P=0.008). However, DCR was not significantly different between the two groups (76.0% vs. 68.0%; P=0.310) (Table 2).
Table 2.
Tumor response in patients with advanced HCC between the two groups (after propensity score matching)
| Response Category | DEB-TACE+LEN (N=50) (%) | DEB-TACE+SOR (N=100) (%) | P value |
|---|---|---|---|
| CR | 14 (28.0%) | 15 (15.0%) | 0.057 |
| PR | 18 (36.0%) | 26 (26.0%) | 0.205 |
| ORR (CR+PR) | 32 (64.0%) | 41 (41.0%) | 0.008 |
| DCR (CR+PR+SD) | 38 (76.0%) | 68 (68.0%) | 0.310 |
OS and TTP between the two groups
The median OS was 14.9 (95% CI: 11.2-18.6) and 12.3 (95% CI: 9.9-14.7) months for the DEB-TACE+LEN and DEB-TACE+SOR groups, respectively. OS was significantly longer in the DEB-TACE+LEN group than in the DEB-TACE+SOR group (HR=0.63, 95% CI: 0.41-0.98; P=0.043) (Figure 2). The median TTP was 8.4 (95% CI: 6.0-10.8) and 6.0 (95% CI: 5.5-6.5) months for the DEB-TACE+LEN and DEB-TACE+SOR groups, respectively. TTP was significantly better in the DEB-TACE+LEN group than the DEB-TACE+SOR group (HR=0.65, 95% CI: 0.45-0.94; P=0.023) (Figure 2).
Figure 2.
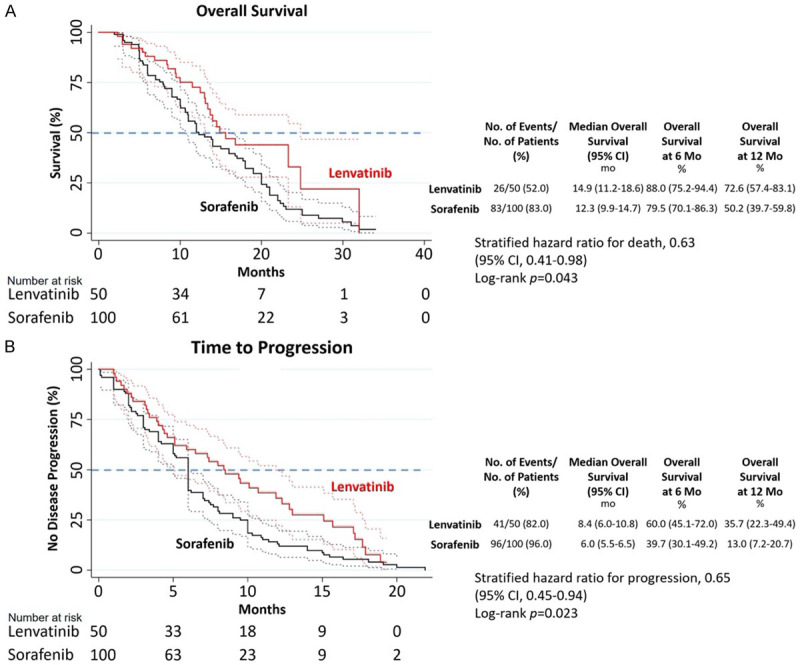
Kaplan-Meier curves in the DEB-TACE+LEN and DEB-TACE+SOR groups. A. Overall Survival (OS). B. Time to Progression (TTP).
In patients with PVTT, subgroup analyses showed that median OS was 10.8 and 7.5 months in the DEB-TACE+LEN and DEB-TACE+SOR groups, respectively (HR=0.59, 95% CI: 0.36-0.98; P=0.043). Median TTP was 5.1 and 3.2 months in the DEB-TACE+LEN and DEB-TACE+SOR groups, respectively (HR=0.89, 95% CI: 0.35-2.29; P=0.035). However, in patients without PVTT, subgroup analyses showed that there was no significant difference in OS and TTP between the two groups. Other subgroup analyses showed that OS and TTP were longer, and no significant difference was observed between the two groups (Figure 3).
Figure 3.
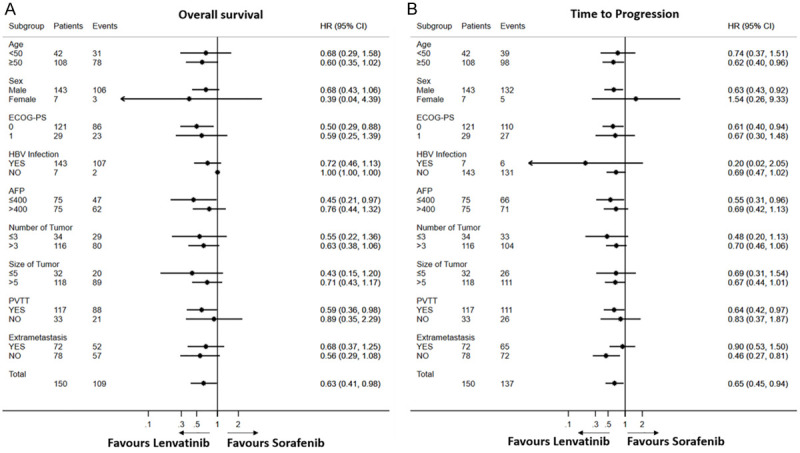
Forest plot of OS and TTP in subgroups of patients treated with DEB-TACE+LEN and DEB-TACE+SOR. OS, overall survival; TTP, time to progression. A. OS. B. TTP.
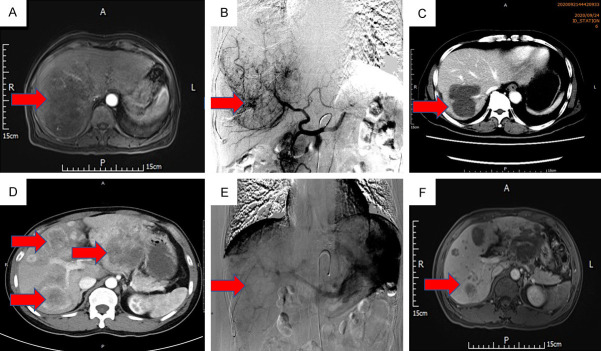
As was shown in the Figure 4, case 1 who underwent DEB-TACE+LEN was evaluated for PR 1 month after DEB-TACE+LEN; OS and TTP were 23 and 7 months, respectively. Case 2 who underwent DEB-TACE+SOR was evaluated for PD 1 month after DEB-TACE+SOR; OS and TTP were 10 and 1 months, respectively.
Figure 4.
Two cases with advanced HCC who underwent DEB-TACE+LEN or DEB-TACE+SOR. A. A case of HCC with a massive tumor in the right lobe with tumor thrombus in the right portal vein. B. DEB-TACE was performed after 1 week of oral lenvatinib. C. One month after the first DEB-TACE, most tumor blood supplies disappeared. D. A case of HCC with multiple nodules on the left and right sides of the liver. E. DEB-TACE was performed after 1 week of oral sorafenib. F. One month after the first DEB-TACE, diffuse recurrent nodules appeared around the original lesion.
Association between mutation genes and overall survival
Of the 43 advanced HCC patients who had undergone WES, 21 patients were in the DEB-TACE+LEN group and 22 were in the DEB-TACE+SOR group. Among them, VEGF, ANG2, FGF19, FGF23, and FGF21 mutation were observed in 17, 15, 20, 22, 17 patients detected, respectively. In patients with VEGF, ANG2, FGF19, and FGF23 mutation, there was not significantly difference in OS between the two groups (Figure 5). However, in patients with FGF21 amplification, median OS was 10.4 (95% CI: 5.4-15.4) and 5.7 (95% CI: 5.4-5.9) months for the DEB-TACE+LEN and DEB-TACE+SOR groups, respectively. OS was significantly longer in the DEB-TACE+LEN group than in the DEB-TACE+SOR group (HR=0.19, 95% CI: 0.06-0.66; P=0.003) (Figure 5). Additionally, in patients without FGF21 amplification, there was not significantly difference in OS between the two groups (Figure 5).
Figure 5.
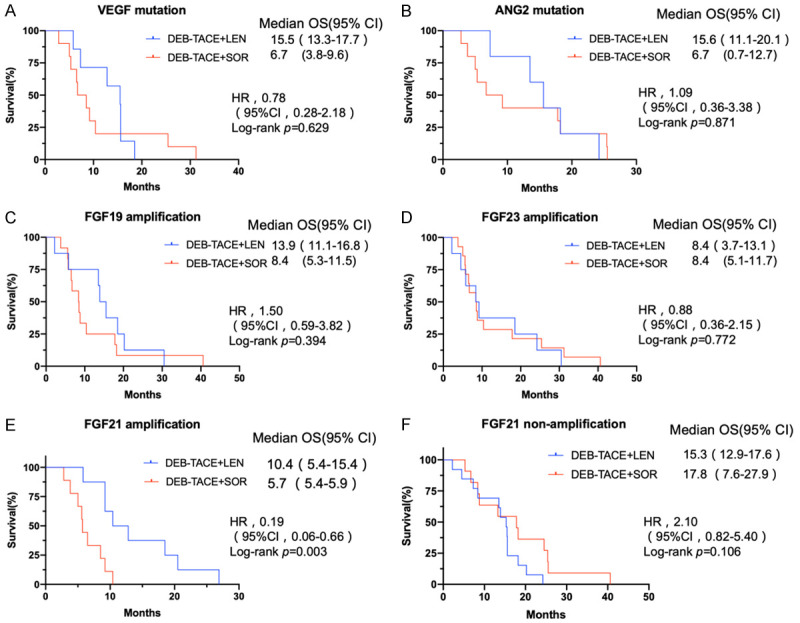
Kaplan-Meier curves for OS between the DEB-TACE+LEN and DEB-TACE+SOR groups under different mutation status. A. VEGF mutation. B. ANG2 mutation. C. FGF19 amplification. D. FGF23 amplification. E. FGF21 amplification. F. FGF21 non-amplification.
Safety
The treatment-emergent AEs that occurred in ≥10% of patients after DEB-TACE+LEN or DEB-TACE+SOR are shown in Table 3. In both groups, the most common treatment-emergent AEs were abdominal pain (125/150, 83.3%), fever (121/150, 80.7%), fatigue (107/150, 71.3%), nausea and vomiting (86/150, 57.3%), decreased appetite (54/150, 36.0%), liver dysfunction (61/150, 40.7%), hypertension (49/150, 32.7%) and hand-foot skin reaction (65/150, 43.3%). The DEB-TACE+LEN group had a significantly lower incidence of hand-foot skin reaction (32.0% vs. 49.0%; P=0.048) and higher incidence of proteinuria (26.0% vs. 10.0%; P=0.010) than did the DEB-TACE+SOR group. Grade 3-4 AEs occurred in 24 (48%) and 42 (42%) patients in the DEB-TACE+LEN and DEB-TACE+SOR groups, respectively (P=0.485).
Table 3.
All-grade AEs within 4 weeks after first DEB-TACE with frequency ≥10% in either group and corresponding Grades 3 and 4 AEs
| Adverse Event | DEB-TACE+LEN Group (N=50) | DEB-TACE+SOR Group (N=100) | P value | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| All grades | Grades 1-2 | Grade 3-4 | All grades | Grades 1-2 | Grades 3-4 | ||
| Abdominal pain | 42 (84) | 32 (64) | 10 (20) | 83 (83) | 68 (68) | 15 (15) | 0.877 |
| Fever | 37 (74) | 30 (60) | 7 (14) | 84 (84) | 72 (72) | 12 (12) | 0.144 |
| Fatigue | 31 (62) | 29 (58) | 2 (4) | 76 (76) | 69 (69) | 7 (7) | 0.074 |
| Nausea and vomiting | 26 (52) | 19 (38) | 7 (14) | 60 (60) | 52 (52) | 8 (8) | 0.350 |
| Decreased appetite | 22 (44) | 18 (36) | 4 (8) | 32 (32) | 28 (28) | 4 (4) | 0.149 |
| Liver dysfunction | 20 (40) | 16 (32) | 4 (8) | 41 (41) | 35 (35) | 6 (6) | 0.906 |
| Hypertension | 19 (38) | 15 (30) | 4 (8) | 30 (30) | 28 (28) | 2 (2) | 0.325 |
| Hand-foot skin reaction | 16 (32) | 11 (22) | 5 (10) | 49 (49) | 36 (36) | 13 (13) | 0.048 |
| Diarrhea | 15 (30) | 12 (24) | 3 (6) | 25 (25) | 20 (20) | 5 (5) | 0.514 |
| Proteinuria | 13 (26) | 9 (18) | 4 (8) | 10 (10) | 7 (7) | 3 (3) | 0.010 |
| Thrombocytopenia | 12 (24) | 8 (16) | 4 (8) | 22 (22) | 15 (15) | 7 (7) | 0.783 |
| Neutropenia | 10 (20) | 7 (14) | 3 (6) | 18 (18) | 14 (14) | 4 (4) | 0.767 |
| Ascites | 8 (16) | 5 (10) | 3 (6) | 14 (14) | 12 (12) | 2 (2) | 0.744 |
| Weight loss | 7 (14) | 7 (14) | 0 | 10 (10) | 9 (9) | 1 (1) | 0.466 |
| Rash | 6 (12) | 5 (10) | 1 (2) | 12 (12) | 8 (8) | 4 (4) | 1.000 |
Note: Data are numbers of events. Data in parentheses are percentages.
Discussion
In our study, DEB-TACE+LEN conferred better ORR, TTP, and OS than DEB-TACE+SOR, especially in patients with PVTT. These results were in accordance with a recent study by Ding et al., who firstly reported that TACE plus lenvatinib had favorable efficacy (TTP and ORR) compared to TACE plus sorafenib for the treatment of advanced HCC with PVTT and large tumor burden. In this study, 64 patients were each randomized in a TACE plus lenvatinib arm (arm L) and a TACE plus sorafenib arm (arm S) (1:1). Median TTP were 4.7 and 3.1 months in arm L and S, respectively (HR, 0.55; 95% CI, 0.32-0.95; P=0.029) and ORR were 53.1% and 25.0% in arm L and S, respectively (P=0.039) [14]. However, different from ours, in the study by Ding et al., there was not significantly difference in OS between the two groups. The reason may be attributed to the choice of embolic agent, larger sample size and propensity score matching in our study.
Interestingly, OS was also significantly longer in the DEB-TACE+LEN group than in the DEB-TACE+SOR group in patients with FGF21 amplification based on WES data in our study. The result was in accordance with the study by Richard S. Finn et al. They found that higher baseline FGF21 may be related to longer OS with lenvatinib compared to sorafenib [15]. The reasons may be as follows: First, there are different drug targets between lenvatinib and sorafenib. In contrast to sorafenib, lenvatinib has a higher affinity for VEGFR2 [23]. Further, in addition to VEGF, lenvatinib also inhibits FGF and other targets, thereby limiting tumor angiogenesis and tumor cell proliferation [24,25]. FGF21 is an important effect molecule of endoplasmic mesh stress in the liver, which can alleviate the fatty degeneration of liver cells induced by endoplasmic mesh stress and play a protective role of liver [26]. FGF21 receptor inhibition was possibly one of the key points for the difference between lenvatinib and sorafenib when combined with DEB-TACE treatment in advanced HCC. Second, TACE plus TKI could achieve longer survival compared to TKI alone in advanced HCC [13,19]. The potential mechanism is that TACE causes tumor hypoxia, inducing an increase in VEGF and basic FGF (bFGF) levels by upregulating hypoxia-inducible factor-1-α (HIF-1-α) [27,28]. TKIs can improve the efficacy of TACE treatment by inhibiting drug targets, including VEGF and bFGF. FGF21 amplification may be the most important one in the FGF family, which are related to the efficacy of DEB-TACE+LEN. Finally, lenvatinib treatment could restrain cell proliferation by blocking RET receptor, which is connected to several signaling pathways, including RAS/MAPK and PI3K/AKT pathways [29,30]. Taisuke et al. reported that lenvatinib induces HCC cell apoptosis by activating FGF signaling pathway through the inhibition of the FGFR-MAPK pathway [31]. FGF21 amplification accelerates the development of HCC through TGF-β signaling pathways [32]. Thus, DEB-TACE+LEN achieve better therapeutic efficacy than DEB-TACE+SOR by inhibiting VEGF, FGF and RET receptors, including FGF21 receptor. FGF21 amplification was predictive for longer OS with DEB-TACE+LEN compared to DEB-TACE+SOR.
Finally, the superiority of DEB-TACE+LEN to DEB-TACE+SOR in advanced HCC may be explained by the better synergistic effect in the DEB-TACE+LEN group. Digital subtraction angiography imaging showed that lenvatinib had a high effect on the shrinkage of hepatic blood vessels, thereby optimizing the embolism effect and resulting in survival benefit. Compared to sorafenib, there is a better preservation of liver function for the more selective and radical DEB-TACE 2-3 weeks after lenvatinib treatment [33]. Besides, lenvatinib exhibits antitumor activity by synergistically modulating effector T cell function in the tumor microenvironment (TME) and by mutually regulating tumor vessel normalization. The lenvatinib-induced regulation of TME may improve the efficacy of DEB-TACE treatment [34].
In our study, the addition of TKI did not result in differences in DEB-TACE-related AEs (e.g., abdominal pain, fever, fatigue, nausea and vomiting, decreased appetite, and liver dysfunction) between the two groups. However, TKI-related AEs differed between the two groups. The DEB-TACE+LEN group showed a significantly higher incidence of proteinuria and a significantly lower incidence of hand-foot skin reaction than did the DEB-TACE+SOR group. These results are in accordance with the REFLECT trial [3]. The difference may be related to the structural characteristics and different drug targets of sorafenib and lenvatinib.
Our study had some limitations. First, this was retrospective in design, and thus our findings need to be verified in a powerful prospective randomized trial of DEB-TACE+LEN versus DEB-TACE+SOR in advanced HCC. In addition, when stratified according to different mutation status based on the WES data, the number of patients in each group was too small, resulting in the likelihood of error. Second, therapeutic choice (DEB-TACE+LEN vs. DEB-TACE+SOR) in patients with advanced HCC was decided based on the interventional physician’s preference. Finally, the comparison of the safety and efficacy was based on combination therapy, without a single drug control group set. Thus, we performed propensity score matching to reduce selection bias between the two groups. Future research should explore the mechanisms of action of these two drugs to validate the findings of this study.
In conclusion, DEB-TACE combined with lenvatinib achieves better ORR, TTP and OS than DEB-TACE combined with sorafenib for advanced HCC, especially in patients with PVTT and FGF21 amplification. These findings indicate the superiority of DEB-TACE combined with lenvatinib to DEB-TACE combined with sorafenib for patients with advanced HCC.
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel R, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150:835–853. doi: 10.1053/j.gastro.2015.12.041. [DOI] [PubMed] [Google Scholar]
- 3.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TR, Lopez C, Dutcus CO, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomized phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 4.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the study of liver diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 5.European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Casak SJ, Donoghue M, Fashoyin-Aje L, Jiang X, Rodriguez L, Shen YL, Xu Y, Jiang X, Liu J, Zhao H, Pierce WF, Mehta S, Goldberg KB, Theoret MR, Kluetz PG, Pazdur R, Lemery SJ. FDA approval summary: atezolizumab plus bevacizumab for the treatment of patients with advanced unresectable or metastatic hepatocellular carcinoma. Clin Cancer Res. 2021;27:1836–1841. doi: 10.1158/1078-0432.CCR-20-3407. [DOI] [PubMed] [Google Scholar]
- 7.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL IMbrave150 Investigators. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 8.Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9:452–463. doi: 10.21037/hbsn-20-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Guglielmi A, Paik SW, Reig M, Kim DY, Chau GY, Luca A, Del AL, Leberre MA, Niu W, Nicholson K, Meinhardt G, Bruix J. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J Hepatol. 2016;64:1090–1098. doi: 10.1016/j.jhep.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Kudo M, Imanaka K, Chida N, Nakachi K, Tak WY, Takayama T, Yoon JH, Hori T, Kumada H, Hayashi N, Kaneko S, Tsubouchi H, Suh DJ, Furuse J, Okusaka T, Tanaka K, Matsui O, Wada M, Yamaguchi I, Ohya T, Meinhardt G, Okita K. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117–2127. doi: 10.1016/j.ejca.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Meyer T, Fox R, Ma YT, Ross PJ, James MW, Sturgess R, Stubbs C, Stocken DD, Wall L, Watkinson A, Hacking N, Evans TR, Collins P, Hubner RA, Cunningham D, Primrose JN, Johnson PJ, Palmer DH. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2:565–575. doi: 10.1016/S2468-1253(17)30156-5. [DOI] [PubMed] [Google Scholar]
- 12.Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Kuzuya T, Isoda N, Yasui K, Aino H, Ido A, Kawabe N, Nakao K, Wada Y, Yokosuka O, Yoshimura K, Okusaka T, Furuse J, Kokudo N, Okita K, Johnson PJ, Arai Y TACTICS study group. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2021;69:1492–1501. doi: 10.1136/gutjnl-2019-318934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kok VC, Chen YC, Chen YY, Su YC, Ku MC, Kuo JT, Yoshida GJ. Sorafenib with transarterial chemoembolization achieves improved survival vs. Sorafenib alone in advanced hepatocellular carcinoma: a nationwide population-based cohort study. Cancers (Basel) 2019;11:985. doi: 10.3390/cancers11070985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding X, Sun W, Li W, Shen Y, Guo X, Teng Y, Liu X, Zheng L, Li W, Chen J. Transarterial chemoembolization plus lenvatinib versus transarterial chemoembolization plus sorafenib as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: a prospective randomized study. Cancer. 2021;127:3782–3793. doi: 10.1002/cncr.33677. [DOI] [PubMed] [Google Scholar]
- 15.Finn RS, Kudo M, Cheng AL, Wyrwicz L, Ngan R, Blanc JF, Baron AD, Vogel A, Ikeda M, Piscaglia F, Han KH, Qin S, Minoshima Y, Kanekiyo M, Ren M, Dairiki R, Tamai T, Dutcus CE, Ikezawa H, Funahashi Y, Evans TR. Pharmacodynamic biomarkers predictive of survival benefit with lenvatinib in unresectable hepatocellular carcinoma: from the phase III REFLECT study. Clin Cancer Res. 2021;27:4848–4858. doi: 10.1158/1078-0432.CCR-20-4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 17.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon R, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 18.Song MJ, Chun HJ, Song DS, Kim HY, Yoo SH, Park CH, Bae SH, Choi JY, Chang U, Yang JM, Lee HG, Yoon SK. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol. 2012;57:1244–1250. doi: 10.1016/j.jhep.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 19.Pawlik TM, Reyes DK, Cosgrove D, Kamel IR, Bhagat N, Geschwind JF. Phase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J. Clin. Oncol. 2011;29:3960–3967. doi: 10.1200/JCO.2011.37.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basch E, Iasonos A, McDonough T, Barz A, Culkin A, Kris MG, Scher HI, Schrag D. Patient versus clinician symptom reporting using the National Cancer Institute common terminology criteria for adverse events: results of a questionnaire-based study. Lancet Oncol. 2006;7:903–909. doi: 10.1016/S1470-2045(06)70910-X. [DOI] [PubMed] [Google Scholar]
- 21.Austin PC, Mamdani MM. A comparison of propensity score methods: a case-study estimating the effectiveness of post-AMI statin use. Stat Med. 2006;25:2084–2106. doi: 10.1002/sim.2328. [DOI] [PubMed] [Google Scholar]
- 22.Llovet JM, Lencioni R. mRECIST for HCC: performance and novel refinements. J Hepatol. 2020;72:288–306. doi: 10.1016/j.jhep.2019.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okamoto K, Ikemori-Kawada M, Jestel AK, Funahashi Y, Matsushima T, Tsuruoka A, Inoue A, Matsui J. Distinct binding mode of multikinase inhibitor lenvatinib revealed by biochemical characterization. ACS Med Chem Lett. 2015;6:89–94. doi: 10.1021/ml500394m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamamoto Y, Matsui J, Matsushima T, Obaishi H, Miyazaki K, Nakamura K, Tohyama O, Semba T, Yamaguchi A, Hoshi SS, Mimura F, Haneda T, Fukuda Y, Kamata JI, Takahashi K, Matsukura M, Wakabayashi T, Asada M, Nomoto KI, Watanabe T, Dezso Z, Yoshimatsu K, Funahashi Y, Tsuruoka A. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell. 2014;6:18. doi: 10.1186/2045-824X-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuki M, Hoshi T, Yamamoto Y, Ikemori-Kawada M, Minoshima Y, Funahashi Y, Matsui J. Lenvatinib inhibits angiogenesis and tumor fibroblast growth factor signaling pathways in human hepatocellular carcinoma models. Cancer Med. 2018;7:2641–2653. doi: 10.1002/cam4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tillman EJ, Rolph T. FGF21: an emerging therapeutic target for non-alcoholic steatohepatitis and related metabolic diseases. Front Endocrinol (Lausanne) 2020;11:601290. doi: 10.3389/fendo.2020.601290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrillo M, Patella F, Pesapane F, Suter MB, Ierardi AM, Angileri SA, Floridi C, de Filippo M, Carrafiello G. Hypoxia and tumor angiogenesis in the era of hepatocellular carcinoma transarterial loco-regional treatments. Future Oncol. 2018;14:2957–2967. doi: 10.2217/fon-2017-0739. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Feng GS, Zheng CS, Zhuo CK, Liu Xi. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol. 2004;10:2878–2882. doi: 10.3748/wjg.v10.i19.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tohyama O, Matsui J, Kodama K, Hata-Sugi N, Kimura T, Okamoto K, Minoshima Y, Iwata M, Funahashi Y. Antitumor activity of lenvatinib (e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res. 2014;2014:638747. doi: 10.1155/2014/638747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romei C, Ciampi R, Elisei R. A comprehensive overview of the role of the RET proto-oncogene in thyroid carcinoma. Nat Rev Endocrinol. 2016;12:192–202. doi: 10.1038/nrendo.2016.11. [DOI] [PubMed] [Google Scholar]
- 31.Hoshi T, Watanabe MS, Watanabe H, Sonobe R, Seki Y, Ohta E, Nomoto K, Matsui J, Funahashi Y. Lenvatinib induces death of human hepatocellular carcinoma cells harboring an activated FGF signaling pathway through inhibition of FGFR-MAPK cascades. Biochem Biophys Res Commun. 2019;513:1–7. doi: 10.1016/j.bbrc.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 32.Xu P, Zhang Y, Liu Y, Yuan Q, Song L, Liu M, Liu Z, Yang Y, Li J, Li D, Ren G. Fibroblast growth factor 21 attenuates hepatic fibrogenesis through TGF-β/smad2/3 and NF-κB signaling pathways. Toxicol Appl Pharmacol. 2016;290:43–53. doi: 10.1016/j.taap.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Kudo M, Ueshima K, Chan S, Minami T, Chishina H, Aoki T, Takita M, Hagiwara S, Minami Y, Ida H, Takenaka M, Sakurai T, Watanabe T, Morita M, Ogawa C, Wada Y, Ikeda M, Ishii H, Izumi N, Nishida N. Lenvatinib as an initial treatment in patients with intermediate-stage hepatocellular carcinoma beyond up-to-seven criteria and child-pugh a liver function: a proof-of-concept study. Cancers (Basel) 2019;11:1084. doi: 10.3390/cancers11081084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng H, Kan A, Lyu N, Mu L, Han Y, Liu L, Zhang Y, Duan Y, Liao S, Li S, Xie Q, Gao T, Li Y, Zhang Z, Zhao M. Dual vascular endothelial growth factor receptor and fibroblast growth factor receptor inhibition elicits antitumor immunity and enhances programmed cell death-1 checkpoint blockade in hepatocellular carcinoma. Liver Cancer. 2020;9:338–357. doi: 10.1159/000505695. [DOI] [PMC free article] [PubMed] [Google Scholar]