Abstract
Venous thromboembolism is the most common complication and the secondary cause of death in pancreatic cancer. Moreover, the hypercoagulable state induces microcirculation dysfunction, acidosis and hypoxia, and further enhances tumor immune evasion, tumor growth and metastasis. Numerous studies have revealed that patients with malignant tumors have high levels of IL-6, which stimulates hepatocytes to synthesize thrombopoietin, causing an increase in platelets. This study found that the concentration of IL-6 in pancreatic cancer patient sera was higher than that in healthy donors, while pancreatic cancer cells had low expression levels of IL-6, which was different from other types of cancer. This contradictory result prompted us to uncover the underlying mechanism. Our data revealed that pancreatic cancer enhanced IL-6 production in fibroblasts via the Jagged/Notch axis, while IL-6 further elevated Jagged-1/2 expression in a paracrine positive feedback loop in pancreatic cancer. Inhibition experiments and RNAi studies demonstrated that IL-6-induced Jagged-1/2 production in pancreatic cancer depended on STAT3 and that Jagged-1/2 enhanced IL-6 mRNA expression in HSFs through the NF-κB pathway. Finally, the animal study showed that knockdown of Jagged-1/2 or blockade of the Jagged/Notch pathway by Nirogacestat could alleviate pancreatic cancer-induced hypercoagulability. Accordingly, our findings clarified the key role of the Jagged/Notch/IL-6/STAT3 feedback loop in the development of a hypercoagulable state in pancreatic cancer, which also provides new therapeutic strategies for pancreatic cancer patients who suffer from hypercoagulability.
Keywords: Pancreatic cancer, IL-6, hypercoagulability, jagged/notch, fibroblast, feedback loop
Introduction
Pancreatic cancer is hidden at onset and progresses rapidly to malignancy of the digestive system with a poor prognosis [1]. The median survival time of pancreatic cancer patients is less than 12 months after standard therapy, and the five-year survival rate is also less than 3% [2-5], which is why pancreatic cancer named the “emperor of cancer” [6]. Thousands of studies clarified that malignant cancer patients had disorders of the coagulation and fibrinolysis system [7-9]. Dysfunction of these two systems not only causes thrombosis, but also plays critical roles in tumor growth, progression and prognosis [10,11]. Pancreatic cancer tends to result in thrombosis, and venous thromboembolism (VTE) is the secondary cause of death of pancreatic cancer patients [12,13]. Even worse, conventional anticoagulation treatment tends to cause several adverse effects in the patients.
Interleukin-6 (IL-6) is a well-known pro-inflammatory cytokine involved in both immune defense and cancer progression [14,15]. Moreover, several types of cancers had high levels of IL-6 in patient sera [16-18]. Naina et al. showed that the high level of IL-6 produced by ovarian cancer cells could promote TPO expression in hepatocytes and elevate the abundance of platelets in peripheral blood, resulting in a hypercoagulable or prothrombotic state in patients [19]. In addition, Gao H et al. revealed that IL-6 regulated the expression of tissue factor (TF) in human umbilical vein endothelial cells [20]. Accordingly, IL-6 exerts vital roles in VTE by inducing TF and TPO. Most cancer patients suffer from a hypercoagulable state due to high levels of IL-6 in peripheral blood [21]. However, some types of cancer cells cannot produce high levels of IL-6, while the concentration of IL-6 in patient sera is still higher than that in healthy volunteers [22], indicating that IL-6 in these types of cancer is mainly secreted by other cancer-associated cells, including immune cells, fibroblasts, endothelium, myocytes, adipocytes, a variety of endocrine cells, and PC cells [22-24]. It remains unclear which type of cancer-associated cells are the main producers and how tumor cells promote the secretion of IL-6 by cells in the tumor microenvironment.
Pancreatic cancer and breast cancer cells have high levels of Jagged-1/2, the ligands of the Notch pathway [25,26]. A recent study clarified that myeloma cells enhanced IL-6 expression in hematopoietic stem cells through the Notch pathway [27]. Among these, activation of the Notch pathway elevated IL-6 promoter activity to enhance IL-6 transcriptional levels in macrophages [28]. More importantly, IL-6 acted on Jagged-1 and angiopoietin 2 expression in pericytes [29,30]. Thus, we hypothesized that a high level of Jagged-1/2 in pancreatic cancer cells could induce IL-6 secretion in tumor stromal cells, such as fibroblasts, followed by a high level of IL-6-induced Jagged-1/2 expression in cancer cells to exacerbate the positive feedback loop and hypercoagulable state in the peripheral blood of cancer patients.
In the present study, we found that both Jagged-1 and Jagged-2 upregulated IL-6 production in fibroblasts, while knockdown of Jagged-1/2 in cancer cells blocked IL-6 induction triggered by cancer cells. Inhibition experiments revealed that STAT3 was involved in Jagged-1/2 expression in cancer cells after IL-6 treatment. Animal studies also demonstrated that blockade of the Jagged/Notch/IL-6/STAT3 axis could attenuate the hypercoagulable state in pancreatic cancer burden mice.
Materials and methods
Patients
The pancreatic cancer blood samples and blood samples from healthy controls used in this study were obtained from the First Affiliated Hospital of Wenzhou Medical University from 2018 to 2020. Written informed consent was obtained from all patients in accordance with institutional guidelines before sample collection. The study was approved by the committee for ethical review of research at the First Affiliated Hospital of Wenzhou Medical University.
Cell lines
Human pancreatic cancer cell lines (PaTu-8988, PANC-1) and fibroblast cell lines (HSFs) were obtained from the American Type Culture Collection. HSFs were purchased from the Kunming Cell Bank of China Infrastructure of Cell Line Resources (Kunming, China). PaTu-8988 and PANC-1 cell lines were chosen because of their low expression of IL-6. We established stable JAG1/2-silenced PANC-1/PaTu-8988 transfectants by infecting the cells with a lentivirus encoding JAG1/2 shRNA, and IL-6-silenced HSFs in the same way. The target sequences for constructing shRNA were shown as followed: 5’-AACCTGTAACATAGCCCGAAA-3’ for Jagged-1, 5’-CAAGGTGGAGACGGTTGTTAC-3’ for Jagged-2, 5’-AAGAATCTAGATGCAATAA-3’ for IL-6, 5’-GCACAATCTACGAAGAATCAA-3’ for STAT3 and 5’-CACCATCAACTATGATGAGTT-3’ for NF-κBp65 (p65). Lentiviruses were purchased from Genechem (Shanghai, China).
All cell lines were maintained in complete growth medium as recommended by the manufacturer. The cultured cells were maintained in a humidified 5% CO2 atmosphere at 37°C. The genetic identity of all cell lines was confirmed by short tandem repeat profiling.
Antibodies and reagents
Jagged-1 (D4Y1R) XP Rabbit mAb (cat. #70109) and Jagged-2 (C23D2) Rabbit mAb (cat. #2210), ERK1/2 (137F5) Rabbit mAb (cat. #4695), ERK1/2pT202/Y204 (cat. #9101), AKT1pS473 (587F11) Mouse mAb (cat. #4051), AKT (11E7) Rabbit mAb (cat. #4685), STAT3pY705 (D3A7) Rabbit mAb (cat. #9145), STAT3 (124H6) Mouse mAb (cat. #9139) and β-actin (8H10D10) Mouse mAb (cat. #3700) were purchased from Cell Signaling Technology (Beverly, MA, USA). Anti-IL-6 antibody (cat. #ab6672) was purchased from Abcam (Cambridge, MA, USA). Recombinant Human IL-6 (cat. #200-06) was purchased from Proteintech (Chicago, IL, USA). Nirogacestat (cat. #HY-15185) was bought from MedChemExpress (NJ, USA). Recombinant Human Jagged 1 Fc Chimera (cat. #1277-JG-050), Recombinant Human Jagged 2 Fc Chimera (cat. #1726-JG-050), Human Thrombopoietin Immunoassay (cat. #DTP00B), Human Coagulation Factor III/Tissue Factor Immunoassay (cat. #DCF300) and Human IL-6 Immunoassay (cat. #D6050) were purchased from R&D Systems (Minneapolis, USA). U0126 (cat. #S1102), MK2206 (cat. #S1078), STATTIC (cat. #S7024) and PDTC (cat. #S3633) were purchased from Selleckchem (Houston, TX).
Western blot analysis
The cells were harvested, and total protein was extracted from the stable cell lines. Briefly, cells were washed with PBS, and protein was isolated according to the manufacturer’s instructions (M-PERTM Mammalian Protein Extraction Reagent, Thermo Scientific, USA) with protease and phosphatase inhibitor cocktail (Cell Signaling Technology, USA). The protein concentration was examined with a BCA protein concentration assay kit (Beyotime, China) according to the manufacturer’s instructions. Equal amounts of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% SDS-PAGE) and then transferred onto a polyvinylidene difluoride (PVDF) membrane (Millipore, USA). The immunoblots were incubated in 5% (w/v) skim milk powder dissolved in TBST (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, and 0.05% Tween-20) for 1 h at room temperature. Next, the blots were probed first with specific antibodies and then with the appropriate secondary antibodies. β-Actin was used as control. Signals were quantified using ImageQuant TL software (GE).
RNA isolation and RT-qPCR analysis
Total RNA was extracted from cells using TriPure Isolation Reagent (Roche, USA) according to the manufacturer’s instructions. RNA was reverse transcribed to generate cDNA using a RevertAid First Strand cDNA Synthesis kit (Thermofisher, USA). mRNA expression levels were detected by qPCR using FastStart Universal SYBR® Green Master Mix (Rox) (Roche, USA) following the manufacturer’s instructions. The primer sequences are provided in Table 1.
Table 1.
The primer sequences for qPCR
| Primers | Forward | Reverse |
|---|---|---|
| IL-6 | GGTGTTGCCTGCTGCCTTCC | GTTCTGAAGAGGTGAGTGGCTGTC |
| Jagged-1 | TGTGGCTTGGATCTGTTGCTTGG | ACGTTGTTGGTGGTGTTGTCCTC |
| Jagged-2 | GCGTGGTCCTGTGCGTGTG | GGCGGCGGCGTGAAGTTC |
| Notch-1 | TGCGAGACCAACATCAACGAGTG | TCAGGCAGAAGCAGAGGTAGGC |
| Notch-2 | ACCACTGCTTCAAGAACACGGATG | GGATTCACTGACGACAGACACAAGAG |
| Notch-3 | CTGTGAGACCGATGTCAACGAGTG | GGTTCCTGTGAAGCCTGCCATAC |
| Notch-4 | CTCAACACTCCTGGCTCCTTCAAC | AGTAGGTCCAGACAGGTGCTTCC |
Murine models of pancreatic cancer
BALB/C nude mice were acquired from the Laboratory Animal Center of Wenzhou Medical University, and all mice were female and 5-6 weeks of age. Mice were maintained under specific pathogen-free conditions in laminar-flow benches and were allowed to adapt to the environment for 1 or 2 weeks before experiments.
A total of 5×106 PaTu-8988, PaTu-8988 jag1-/- and PaTu-8988 jag2-/- cells were injected hypodermically (i.H.) into 20 g nude mice. After approximately 45 days, the volume of tumors reached approximately 400 to 500 mm3. The PaTu-8988 nude mouse group was randomly separated into 2 groups (n=6), named the PaTu-8988 group and Nigrogacestat-treated group. Nude mice in the Nigrogacestat-treated group were administered Nigrogacestat orally at 2 mg/20 g/2 days in 200 μL of normal saline. Mice in the PaTu-8988 group were administered 200 μL of normal saline orally every 2 days. Blood was collected from periocular venous blood of nude mice. Mice were observed daily for tumor growth and killed if peritoneal swelling reached UK Home Office limits (20% increase in abdominal girth).
Statistical analysis
All statistical analyses were performed by GraphPad Prism version 8.0 software (GraphPad Software, USA). All data are reported as the mean ± SD of triplicate experiments, and the differences between two groups were compared by two-tailed Student’s t-test. *P<0.05 was considered statistically significant.
Results
Abnormal expression of IL-6, TF and TPO in the peripheral blood of pancreatic cancer patients
Pancreatic cancer patients had a relatively high rate of deep vein thrombosis or pulmonary thromboembolism. Both TPO and TF are involved in the formation of a hypercoagulable state in patients, which leads to these two fatal complications. We examined TPO and TF expression levels in peripheral blood, and the results showed that both TPO and TF levels were elevated in the pancreatic cancer patients compared with the healthy donors (Figure 1A and 1B). More importantly, IL-6, a key factor for TPO and TF induction in patient sera, showed higher levels in the pancreatic cancer patients than in the controls (Figure 1C).
Figure 1.
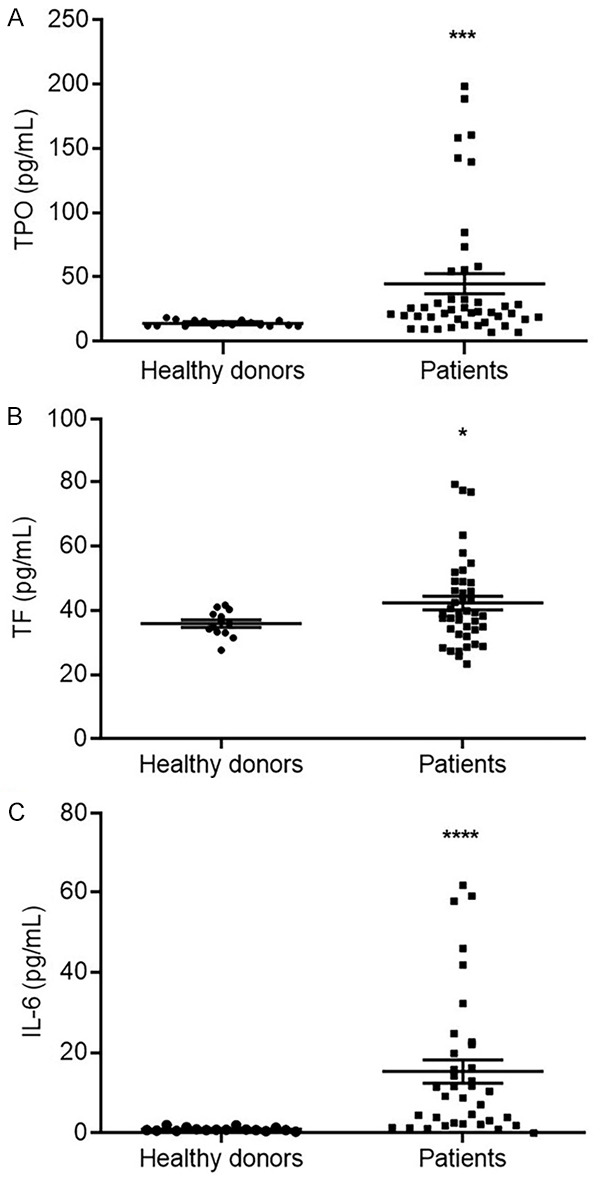
The expression levels of TPO, TF and IL-6 in the peripheral blood. The sera of 39 pancreatic cancer patients and 17 healthy donors were collected, and the expression level of TPO (A), TF (B) and IL-6 (C) were detected by ELISA method. (*P<0.05, **P<0.01 versus the healthy donors).
Pancreatic cancer cells were not the major producers of IL-6, but could induce IL-6 expression in fibroblasts via direct interaction
Previous studies validated that ovarian cancer, liver cancer and colorectal cancer could induce the liver to secrete TPO by producing IL-6 [19,31,32]. In contrast, our results showed that IL-6 was undetectable in the culture media of the pancreatic cancer cell lines PaTu-8988, BxPc-3 and MIA PaCa-2, and only PANC-1 culture medium contained 2.71 pg/ml IL-6 (Table 2). Due to the features of the tumor microenvironment, fibroblasts are the major stromal cells that exist in the microenvironment, and we hypothesized that cancer cells trigger IL-6 production in fibroblasts. Thus, we co-cultured the pancreatic cancer cells and HSFs directly and found that the IL-6 production level was higher in the co-culture system than that of HSF alone (Figure 2). Interestingly, the expression levels of IL-6 in the Transwell system were increased slightly, which separated pancreatic cancer cells and HSFs by chamber (Figure 2), indicating that IL-6 induction in fibroblasts were majorly regulated by direct contact of HSF and pancreatic cancer cells.
Table 2.
The expression levels of IL-6 in the culture media of pancreatic cancer cell lines
| Cell lines | IL-6 (pg/ml) |
|---|---|
| PaTu-8988 | 0 |
| PANC-1 | 2.71±0.12 |
| BXPC-3 | 0 |
| MIA PaCa-2 | 0 |
Note: The pancreatic cancer cells were seeded into the 6 cm dish and cultured for 24 hours, then the culture media were collected for determining IL-6 secretion by ELISA. Data are shown as mean ± SD.
Figure 2.
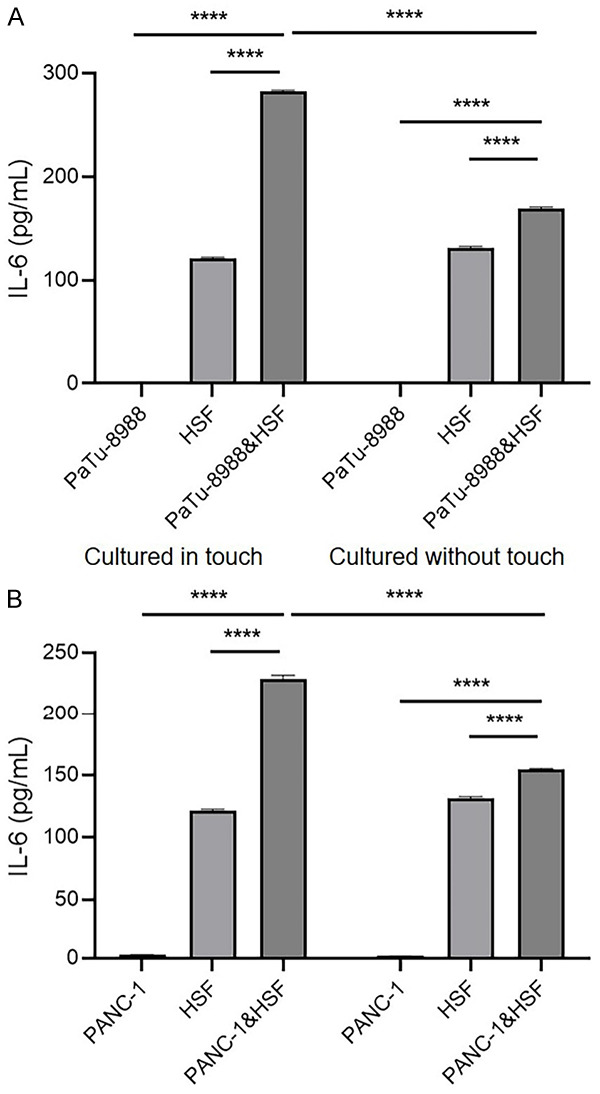
Pancreatic cancer cells could induce IL-6 expression in fibroblasts via direct interaction. The secretion of IL-6 with cultured in touch or cultured without touch of PaTu-8988 cell line or PANC-1 cell line with HSF after 24 hours. Cultured in touch (co-culture system): 1×105 PaTu-8988/PANC-1 cells or (and) HSF cells were seeded into the 6 cm dish and cultured for 24 hours, then the media were collected for detecting IL-6 production; cultured without touch (Transwell system): 1×105 PaTu-8988/PANC-1 cells were seeded in the sublayer of transwell apparatus, or (and) 1*105 HSFs were seeded in the chamber of transwell apparatus, then the media were collected for detecting IL-6 production. (****P<0.0001 versus corresponding group).
Pancreatic cancer cell-stimulated IL-6 production in HSFs was Jagged/Notch axis dependent
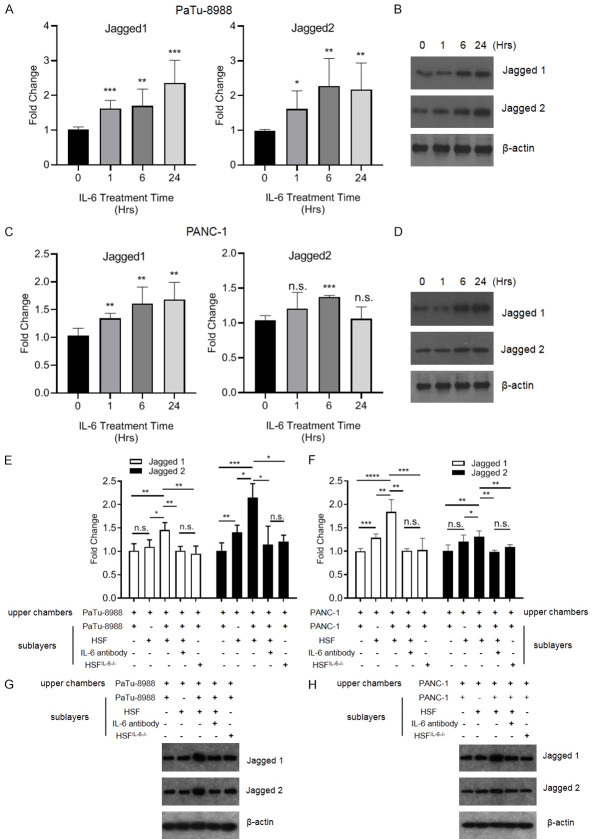
High expression levels of Jagged in pancreatic cancer and breast cancer were demonstrated by Buchler, P et al. Jagged/Notch is one of most important pathways involved in direct contact interactions between cancer cells and stromal cells in the tumor environment [25]. To determine whether activation of the Jagged/Notch pathway could alter IL-6 in fibroblasts, we treated HSFs with Jagged-1/2 and found that IL-6 expression was greatly enhanced after Jagged-1/2 treatment (Figure 3A); moreover, Notch1-3 expression was increased after Jagged-1/2 treatment (Figure 3B). Notch-4 expression level was undetectable in HSFs before or after IL-6 treatment (data not shown). Notably, pancreatic cancer cells increased IL-6 expression in the HSFs, and the expression was partially attenuated by Jagged-1 or 2 shRNA expression in pancreatic cancer cells (Figure 3C and 3D). Moreover, pretreatment of the HSFs & PaTu-8988 or PANC-1 co-culture system with the Notch pathway inhibitor Nirogacestat alleviated IL-6 production in the medium (Figure 3E).
Figure 3.
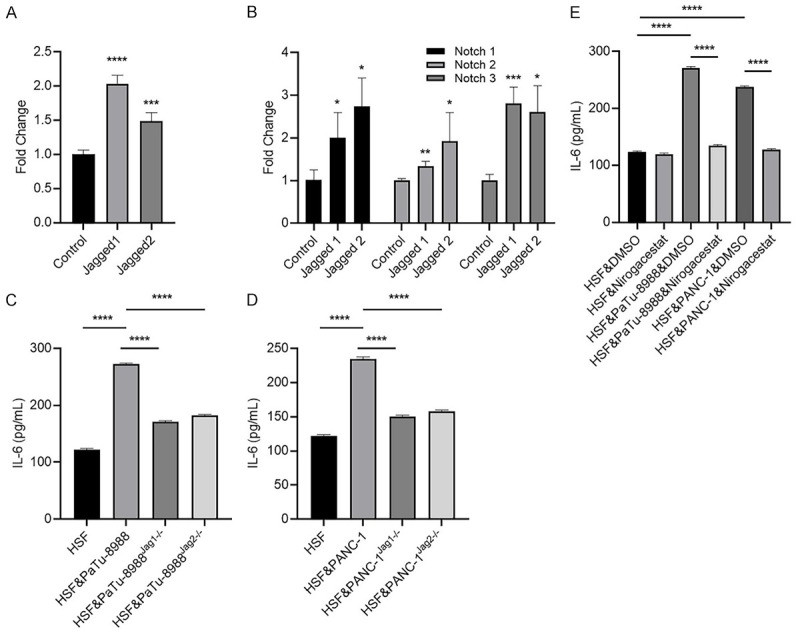
Jagged-1/2 could stimulate tumor-associated stromal cells (HSF) to secrete IL-6 by up-regulating the notch signaling pathway. (A, B) HSF cells were collected after treated with 4 μg/ml Jagged-1 or 5 μg/ml Jagged-2 for 24 hours, for measuring mRNA expression levels of IL-6 (A), Notch-1/2/3 (B) by RT-qPCR. (C, D) HSF cells were cultured with/without wild type, Jagged-1 or Jagged-2 knockdown PaTu-8988 (C) or PANC-1 cells (D), then culture media were collected and subjected to IL-6 quantification by ELISA after 24 hours co-incubation. (E) HSF cells were pretreated with Nirogacestat or DMSO for half an hour, then the HSF cells were co-cultured with or without PaTu-8988/PANC-1 cells, after that, culture media were collected and subjected to IL-6 quantification by ELISA after 24 hours co-incubation. (*P<0.05, **P<0.01, ***P<0.001 versus control group).
Paracrine production of IL-6 enhanced Jagged-1 and Jagged-2 expression in pancreatic cancer cells
IL-6 is a pluripotent cytokine that participates in multiple processes of cancer progression. Thus, we treated pancreatic cancer cells with recombinant IL-6 (rhIL-6) and found that both Jagged-1 and Jagged-2 transcript levels were increased in PaTu-8988 cells and PANC-1 cells (Figure 4A and 4C), In addition, immunoblotting showed similar expression patterns of Jagged-1 and 2 in pancreatic cancer cells after IL-6 treatment (Figures 4B, 4D and S1). We showed that pancreatic cancer cells could enhance IL-6 expression in HSFs via the Jagged/Notch axis, but whether IL-6 produced by HSFs could alter Jagged-1 and 2 levels in pancreatic cancer cells was elusive. We found that the co-culture of HSFs and PaTu-8988 cells in the sublayers of Transwells stimulated Jagged-1 and Jagged-2 expression in PaTu-8988 cells cultured in the upper layers of the chamber (Figure 4E and 4G). Additionally, both Jagged-1 and Jagged-2 expression levels were upregulated in PANC-1 cells cultured in the upper chamber, which were co-cultured with PANC-1 cells & HSFs in the sublayers (Figure 4F and 4H). Neutralization of IL-6 in the Transwell system attenuated the upregulation of Jagged-1/2 expression in upper layer pancreatic cancer cells which cultured with pancreatic cancer cells & HSFs in the sublayers. Furthermore, knockdown of IL-6 in HSFs co-cultured with pancreatic cancer in sublayers also blocked Jagged-1/2 expression in upper layer pancreatic cancer cells (Figure 4E-H).
Figure 4.
IL-6 could upregulate the Jagged-1/2 expression level in pancreatic cancer cells. (A-D) The PaTu-8988 (A, B) and PANC-1 (C, D) were treated with recombinant human IL-6 (rhIL-6, 100 ng/ml) for different times, and collected the cells for analyzing Jagged-1/2 expression level by RT-qPCR or immunoblotting. (E-H) PaTu-8988 or PANC-1 were co-culture with (or without) wild type or IL-6 knockdown HSF, or added IL-6 antibody in the co-culture medium, then the Patu-8988 or PANC-1 cells in the upper chambers were collected for analyzing Jagged-1 and Jagged-2 expression level by RT-qPCR or immunoblotting. (*P<0.05, **P<0.01, ***P<0.001 versus the control group).
STAT3 and NF-κB signaling were involved in IL-6-promoted Jagged-1/2 expression in pancreatic cancer cells and Jagged-1/2-enhanced HSF IL-6 production, respectively
IL-6 plays multiple roles in cancer progression mainly through the ERK, AKT and STAT3 pathways. Therefore, PaTu-8988 cells were pretreated with the ERK1/2 inhibitor U0126, AKT inhibitor MK2206 or STAT3 inhibitor STATTIC, and the results showed that STATTIC blocked IL-6-induced Jagged-1/2 expression in PaTu-8988 cells (Figure 5A). Additionally, knockdown of STAT3 by siRNA alleviated upregulation of Jagged-1/2 expression induced by IL-6 (Figure 5B). The NF-κB pathway is known to be involved in Jagged/Notch signaling and exerts its effects on the transcription of a set of genes. Figure 5C shows that the NF-κB inhibitor PDTC suppressed Jagged1- or 2-mediated upregulation of IL-6 expression. Furthermore, we confirmed that NF-κB subunit p65 siRNA blocked IL-6 production in HSFs after Jagged-1 or Jagged-2 treatment (Figure 5D).
Figure 5.
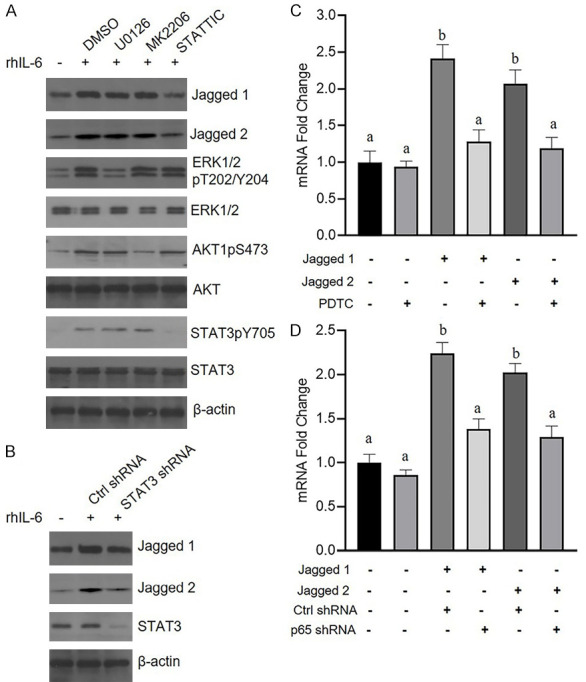
STAT3 and NF-κB signaling were involved in IL-6-promoted Jagged-1/2 expression in pancreatic cancer cells and Jagged1/2-enhanced HSF production of IL-6, respectively. A. The PaTu-8988 cells were pretreated with (or with) DMSO, U0126, MK2206 or STATTIC for half an hour, then the cells were challenged with rhIL-6 for 12 hours, after that, the cell were subjected to immunoblotting with corresponding antibodies. B. PaTu-8988 were transfected with control shRNA or STAT3 shRNA lentiviruses for 48 hours, then the cells were challenged with rhIL-6 for 12 hours, after that, the cell were subjected to immunoblotting with corresponding antibodies. C. HSF cells were pretreated with (or without) PDTC for half an hour, then the cells were treated with 4 μg/ml Jagged-1 or 5 μg/ml Jagged-2 for 24 hours, after that, the cell were collected for analyzing IL-6 mRNA level by RT-qPCR. D. HSF cells were transfected with control shRNA or STAT3 shRNA lentiviruses for 48 hours, then the HSF cell challenged with 4 μg/ml Jagged-1 or 5 μg/ml Jagged-2 for 24 hours, after that, the cells were collected for analyzing IL-6 mRNA level by RT-qPCR. (*P<0.05, **P<0.01, ***P<0.001 versus the control group; Different alphabets denote a significant difference at P<0.05).
Blockade of Jagged/Notch signaling alleviated VTE by downregulating IL-6, TPO and TF expression in a pancreatic cancer mouse model
We examined the role of the Jagged/Notch axis in VTE in a pancreatic cancer mouse model. We subcutaneously injected WT PaTu-8988 and Jagged-1 or Jagged-2 knockdown PaTu-8988 cells in the mice. Peripheral blood was collected after 45 days, and our study showed that knockdown of Jagged-1 or Jagged-2 in PaTu-8988 cells could reduce IL-6, TF and TPO production in mice compared with that of the control group (Figure 6A-C). We further implanted PaTu-8988 cells in nude mice, and the mice were divided into two groups: the control group and the Notch inhibitor (Nirogacestat) group. Then, mouse peripheral blood was collected at days 0, 2, 4, and 6 after drug administration, and ELISAs were performed to detect the IL-6, TPO and TF levels in each group. The results showed that these three markers were dramatically decreased at days 2-4 after Nirogacestat administration (Figure 6D-F).
Figure 6.
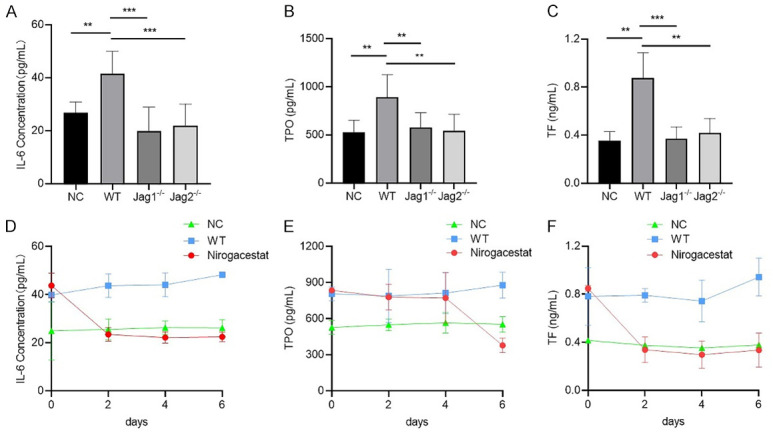
Blockade of Jagged/Notch signaling alleviated VTE by downregulating IL-6, TPO and TF expression in a pancreatic cancer mouse model. (A-C) The sera were collected from mice, then the level of IL-6 (A), TPO (B) and TF (C) were determined by ELISA. NC: the mice without any treatment; WT: the mice were implanted with PaTu-8988 cells stably express control shRNA; Jagged1-/- or Jagged2-/-: the mice were implanted with PaTu-8988 which stably express Jagged-1 or Jagged-2 shRNA. (D-F) The sera were collected from mice, then the level of IL-6 (D), TPO (E) and TF (F) were determined by ELISA. NC: the mice without any treatment; WT: the mice were implanted with PaTu-8988 cells; Nirogacestat: the mice were implanted with PaTu-8988 cells and treated with Nirogacestat as described above. (*P<0.05, **P<0.01, ***P<0.001 versus corresponding group).
Discussion
Dysfunction of the coagulation and fibrinolysis system leads to VTE and tumor progression in patients. VTE is the most common complication of malignant cancer and one of the major causes of death in cancer patients. The occurrence rate of deep vein thrombosis or pulmonary thromboembolism in cancer patients, especially pancreatic cancer patients, is higher than that in the healthy population [33]. Thus, elucidation of the relationship between cancer cells and the hypercoagulable state could enrich our knowledge of VTE, but also provide new clues to prevent VTE in cancer patients.
Although the involvement of IL-6 in the occurrence of VTE has been well illustrated, IL-6 expression in pancreatic cancer cells is very low. In this study, we showed that fibroblasts mingled with cancer cells had relatively high levels of IL-6. Moreover, we found that TPO and TF expression levels in pancreatic cancer patients were higher than those in healthy volunteers. Numerous studies have demonstrated that IL-6 could stimulate TPO and TF production in hepatocytes. Accordingly, our study suggested that IL-6, which is mainly produced by fibroblasts, is the key driving factor involved in the hypercoagulation state of pancreatic cancer.
We next explored the mechanism of high production of IL-6 in fibroblasts. Surprisingly, our results revealed that fibroblasts that directly contact cancer cells produce abundant IL-6, indicating that the pathway that depends on direct contact is involved in cancer cell-regulated IL-6 expression in fibroblasts. The activation of the Notch pathway depends on two membranous proteins those are expressed at nearby cells: delta-like ligands or Jagged ligands. Furthermore, pancreatic cancer cells typically have high levels of Jagged-1/2. Thus, we wondered whether high expression of Jagged-1/2 in pancreatic cancer cells could enhance IL-6 production in fibroblasts. As shown in Figure 3C-E, both the inhibition experiment and depletion of Jagged-1/2 in pancreatic cancer showed that cancer cell-induced IL-6 expression in fibroblasts was dependent on the Jagged/Notch pathway.
To explore the involvement of IL-6 in the positive feedback loop of the Jagged/Notch/IL-6/STAT3 axis in the tumor microenvironment, we assessed Jagged-1/2 in pancreatic cancer cells after IL-6 treatment in vitro. Additionally, a research model was established in our study, in which cancer cells cultured in the upper chamber and fibroblasts & cancer cells were cultured in the sublayer of the Transwell apparatus. Knockdown of IL-6 in the HSFs or neutralization of IL-6 in this model blocked Jagged-1/2 expression in cancer cells induced by a sublayer of fibroblasts & cancer cells. Accordingly, these two results demonstrated that the positive feedback loop of Jagged/IL-6 existed in the pancreatic cancer microenvironment, and the schematic diagram was shown in Figure 7. Furthermore, we confirmed that STAT3 and NF-κB signaling were involved in this positive feedback loop.
Figure 7.
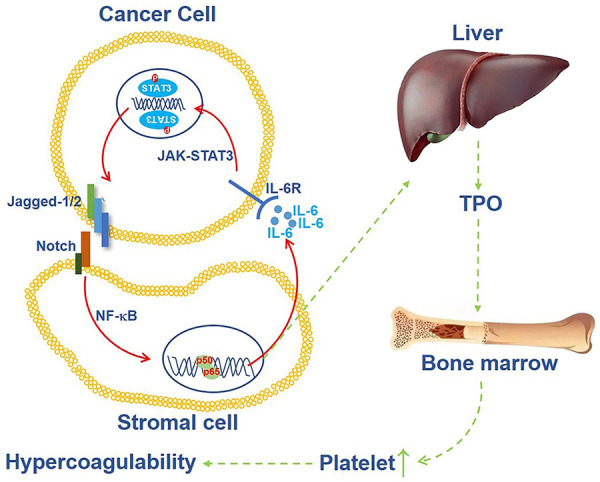
A mechanism of hypercoagulability of pancreatic cancer patients which induced by Jagged-Notch/NF-κB/IL-6/JAK-STAT3 feedback loop. Cancer cells induce IL-6 expression in HSF cells by Jagged/Notch/NF-κB axis, then high level of IL-6 promotes Jagged expression through JAK-STAT3 pathway, meanwhile, IL-6 enhances TPO production in liver, followed by TPO enhances hypercoagulable state in the patients.
Although aspirin and low-molecular-weight heparin have been widely used as first-line drugs for VTE in pancreatic cancer, the side effects remain an issue [12]. Exploring an effective therapeutic strategy for pancreatic cancer is hanging over our head. Nirogacestat is an inhibitor of γ-secretase and has been applied to treat desmoid tumors in phase III clinical trials. Consequently, Nirogacestat was used in the pancreatic cancer mouse model to verify its efficacy in treating VTE. The animal studies revealed that knockdown of Jagged-1/2 or treatment of the pancreatic cancer mouse model with Nirogacestat suppressed TPO and TF production in mouse peripheral blood. Moreover, in the Nirogacestat treatment group, the subcutaneous tumor volume was decreased compared with that in the other groups (data not shown), suggesting that treatment with Nigrogacestat is a good strategy to block pancreatic cancer growth and relieve VTE complications in cancer patients.
Taken together, we demonstrated that IL-6, which is mainly produced by fibroblasts that directly contact cancer cells, contributes to the hypercoagulation state in pancreatic cancer. We proposed the existence of a Jagged/IL-6 positive feedback loop in the tumor microenvironment, which was strongly supported by our co-culture model. To explore a new therapeutic method for VTE in pancreatic cancer, we used a pancreatic cancer animal model, and the results revealed that treatment with Nigrogacestat is a promising way to deal with this complication.
Acknowledgements
This work was supported by grants from the Natural Science Foundation Grant of Zhejiang Province (LY18H200004) to Lihong Yang, and from the Zhejiang Province Public Welfare Technology Application Research Project (LGF20H160018) and Wenzhou Science & Technology Bureau Foundation Grant (Y20190750) to Silu Wang.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008–2020. doi: 10.1016/S0140-6736(20)30974-0. [DOI] [PubMed] [Google Scholar]
- 2.Leinwand J, Miller G. Regulation and modulation of antitumor immunity in pancreatic cancer. Nat Immunol. 2020;21:1152–1159. doi: 10.1038/s41590-020-0761-y. [DOI] [PubMed] [Google Scholar]
- 3.Buscail L, Bournet B, Cordelier P. Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2020;17:153–168. doi: 10.1038/s41575-019-0245-4. [DOI] [PubMed] [Google Scholar]
- 4.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039–1049. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- 5.Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, Palmer DH. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol. 2018;15:333–348. doi: 10.1038/s41575-018-0005-x. [DOI] [PubMed] [Google Scholar]
- 6.Tempero MA. Introduction: pancreatic adenocarcinoma: the emperor of all cancer maladies. Cancer J. 2017;23:309. doi: 10.1097/PPO.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 7.Gabazza EC, Taguchi O, Yamakami T, Machishi M, Ibata H, Tsutsui K, Suzuki S. Coagulation-fibrinolysis system and markers of collagen metabolism in lung cancer. Cancer. 1992;70:2631–2636. doi: 10.1002/1097-0142(19921201)70:11<2631::aid-cncr2820701111>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Vossen CY, Hoffmeister M, Chang-Claude JC, Rosendaal FR, Brenner H. Clotting factor gene polymorphisms and colorectal cancer risk. J. Clin. Oncol. 2011;29:1722–1727. doi: 10.1200/JCO.2010.31.8873. [DOI] [PubMed] [Google Scholar]
- 9.Matsuyama W, Hashiguchi T, Mizoguchi A, Iwami F, Kawabata M, Arimura K, Osame M. Serum levels of vascular endothelial growth factor dependent on the stage progression of lung cancer. Chest. 2000;118:948–951. doi: 10.1378/chest.118.4.948. [DOI] [PubMed] [Google Scholar]
- 10.Caine GJ, Stonelake PS, Lip GY, Kehoe ST. The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia. 2002;4:465–473. doi: 10.1038/sj.neo.7900263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nash GF, Turner LF, Scully MF, Kakkar AK. Platelets and cancer. Lancet Oncol. 2002;3:425–430. doi: 10.1016/s1470-2045(02)00789-1. [DOI] [PubMed] [Google Scholar]
- 12.Frere C, Bournet B, Gourgou S, Fraisse J, Canivet C, Connors JM, Buscail L, Farge D, Consortium B. Incidence of venous thromboembolism in patients with newly diagnosed pancreatic cancer and factors associated with outcomes. Gastroenterology. 2020;158:1346–1358. e4. doi: 10.1053/j.gastro.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Farge D, Bournet B, Conroy T, Vicaut E, Rak J, Zogoulous G, Barkun J, Ouaissi M, Buscail L, Frere C. Primary thromboprophylaxis in pancreatic cancer patients: why clinical practice guidelines should be implemented. Cancers (Basel) 2020;12:618. doi: 10.3390/cancers12030618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol. 2014;26:54–74. doi: 10.1016/j.smim.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Hirano T. Interleukin 6 and its receptor: ten years later. Int Rev Immunol. 1998;16:249–284. doi: 10.3109/08830189809042997. [DOI] [PubMed] [Google Scholar]
- 16.Yokoigawa N, Takeuchi N, Toda M, Inoue M, Kaibori M, Yanagida H, Tanaka H, Ogura T, Takada H, Okumura T, Kwon AH, Kamiyama Y, Nakada H. Enhanced production of interleukin 6 in peripheral blood monocytes stimulated with mucins secreted into the bloodstream. Clin Cancer Res. 2005;11:6127–6132. doi: 10.1158/1078-0432.CCR-05-0292. [DOI] [PubMed] [Google Scholar]
- 17.Bharti R, Dey G, Das AK, Mandal M. Differential expression of IL-6/IL-6R and MAO-A regulates invasion/angiogenesis in breast cancer. Br J Cancer. 2018;118:1442–1452. doi: 10.1038/s41416-018-0078-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wouters M, Dijkgraaf EM, Kuijjer ML, Jordanova ES, Hollema H, Welters M, van der Hoeven J, Daemen T, Kroep JR, Nijman HW, van der Burg SH. Interleukin-6 receptor and its ligand interleukin-6 are opposite markers for survival and infiltration with mature myeloid cells in ovarian cancer. Oncoimmunology. 2014;3:e962397. doi: 10.4161/21624011.2014.962397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naina HV, Harris S. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366:1840. doi: 10.1056/NEJMc1203095. [DOI] [PubMed] [Google Scholar]
- 20.Gao H, Zhang Q, Chen J, Cooper DKC, Hara H, Chen P, Wei L, Zhao Y, Xu J, Li Z, Cai Z, Luan S, Mou L. Porcine IL-6, IL-1beta, and TNF-alpha regulate the expression of pro-inflammatory-related genes and tissue factor in human umbilical vein endothelial cells. Xenotransplantation. 2018;25:e12408. doi: 10.1111/xen.12408. [DOI] [PubMed] [Google Scholar]
- 21.Wada H, Tanigawa M, Wakita Y, Nakase T, Minamikawa K, Kaneko T, Ohiwa M, Kageyama S, Kobayashi T, Noguchi T, et al. Increased plasma level of interleukin-6 in disseminated intravascular coagulation. Blood Coagul Fibrinolysis. 1993;4:583–590. doi: 10.1097/00001721-199308000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Martignoni ME, Kunze P, Hildebrandt W, Kunzli B, Berberat P, Giese T, Kloters O, Hammer J, Buchler MW, Giese NA, Friess H. Role of mononuclear cells and inflammatory cytokines in pancreatic cancer-related cachexia. Clin Cancer Res. 2005;11:5802–5808. doi: 10.1158/1078-0432.CCR-05-0185. [DOI] [PubMed] [Google Scholar]
- 23.Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- 24.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 25.Buchler P, Gazdhar A, Schubert M, Giese N, Reber HA, Hines OJ, Giese T, Ceyhan GO, Muller M, Buchler MW, Friess H. The notch signaling pathway is related to neurovascular progression of pancreatic cancer. Ann Surg. 2005;242:791–800. doi: 10.1097/01.sla.0000189115.94847.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geng Y, Fan J, Chen L, Zhang C, Qu C, Qian L, Chen K, Meng Z, Chen Z, Wang P. A notch-dependent inflammatory feedback circuit between macrophages and cancer cells regulates pancreatic cancer metastasis. Cancer Res. 2021;81:64–76. doi: 10.1158/0008-5472.CAN-20-0256. [DOI] [PubMed] [Google Scholar]
- 27.De Vos J, Couderc G, Tarte K, Jourdan M, Requirand G, Delteil MC, Rossi JF, Mechti N, Klein B. Identifying intercellular signaling genes expressed in malignant plasma cells by using complementary DNA arrays. Blood. 2001;98:771–780. doi: 10.1182/blood.v98.3.771. [DOI] [PubMed] [Google Scholar]
- 28.Wongchana W, Palaga T. Direct regulation of interleukin-6 expression by Notch signaling in macrophages. Cell Mol Immunol. 2012;9:155–162. doi: 10.1038/cmi.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kugathasan L, Ray JB, Deng Y, Rezaei E, Dumont DJ, Stewart DJ. The angiopietin-1-Tie2 pathway prevents rather than promotes pulmonary arterial hypertension in transgenic mice. J Exp Med. 2009;206:2221–2234. doi: 10.1084/jem.20090389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gopinathan G, Milagre C, Pearce OM, Reynolds LE, Hodivala-Dilke K, Leinster DA, Zhong H, Hollingsworth RE, Thompson R, Whiteford JR, Balkwill F. Interleukin-6 stimulates defective angiogenesis. Cancer Res. 2015;75:3098–3107. doi: 10.1158/0008-5472.CAN-15-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaser A, Brandacher G, Steurer W, Kaser S, Offner FA, Zoller H, Theurl I, Widder W, Molnar C, Ludwiczek O, Atkins MB, Mier JW, Tilg H. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood. 2001;98:2720–2725. doi: 10.1182/blood.v98.9.2720. [DOI] [PubMed] [Google Scholar]
- 32.Josa V, Ferenczi S, Szalai R, Fuder E, Kuti D, Horvath K, Hegedus N, Kovacs T, Bagamery G, Juhasz B, Winkler Z, Veres DS, Zrubka Z, Mathe D, Baranyai Z. Thrombocytosis and effects of IL-6 knock-out in a colitis-associated cancer model. Int J Mol Sci. 2020;21:6218. doi: 10.3390/ijms21176218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khorana AA, Fine RL. Pancreatic cancer and thromboembolic disease. Lancet Oncol. 2004;5:655–663. doi: 10.1016/S1470-2045(04)01606-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.