Abstract
Ferroptosis is a recently recognized type of programmed cell death and emerges to play an important role in cancer biology and therapies. This unique form of cell death, characterized by iron-dependent lipid peroxidation, is exquisitely regulated by the cellular metabolic networks such as lipid, iron and amino acid metabolism. The sensitivity to ferroptosis varies among different tumors. Recent evidence reveals that triple-negative breast cancer (TNBC), a highly aggressive disease with limited effective targeted therapies is particularly vulnerable to ferroptosis inducers, suggesting this new form of non-apoptotic cell death as an attractive target for the treatment of the “difficult-to-treat” tumor. Intriguingly, ferroptosis has recently been implicated to be involved in T cell-mediated anti-tumor immunity and affect the efficacy of cancer immunotherapy. Better understanding of this ferroptotic cell death will shed light on the discovery of novel combination therapeutic strategies for cancer treatment. Herein, we provide an overview of the key hallmarks of ferroptosis, use TNBC as a model to characterize the regulation of ferroptosis in cancer, and highlight ferroptosis-modulating combination therapeutic strategies in the context of cancer immunotherapy.
Keywords: Ferroptosis, triple-negative breast cancer, cancer immunotherapy
Introduction
Most living organisms need oxygen to survive. Oxygen acts as a double-edged sword, not only providing “fuel” for energy production, but also generating the “by-products”: reactive oxygen species (ROS). Due to metabolic and signaling aberrations, oxidative stress is a common feature for cancers [1,2]. Excessive ROS damages cellular components such as DNA, proteins and lipids. Unrestrained peroxidation of lipids in membrane bilayers is the hallmark of a unique form of regulated cell death: ferroptosis, which was discovered in the year 2012 [3,4]. Ferroptosis is distinct from other forms of regulated cell death, such as apoptosis and pyroptosis, in morphology and molecular mechanisms [5-7].
The sensitivity of cancer cells to ferroptosis is determined by intracellular metabolic processes including lipid metabolism, iron metabolism and amino acid metabolism [8,9]. Breast cancer is one of the most common cancers in women. Triple-negative breast cancer (TNBC), which lacks the expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), is the most aggressive type of breast cancer characterized by high invasiveness, rapid recurrence, and poor prognosis [10,11]. Due to its special molecular phenotype, TNBC is not sensitive to endocrine therapy or HER2 targeted therapy. Although several therapeutic agents such as immunotherapy have been approved to treat these patients, there is still urgent need to further improve the therapeutic responses. Recent evidence suggests that TNBC is intrinsically susceptible to ferroptosis [12,13], highlights induction of this non-apoptotic cell death as a potential strategy for TNBC treatment.
Immunotherapy represented by PD-1/PD-L1 immune checkpoint blockade has achieved promising clinical outcomes in multiple cancer types including TNBC [14,15]. Although it is encouraging, the relatively low response rate with a single agent and the emergence of resistance leaves large room to improve the clinical benefits. An important and interesting concept emerging from the recent research is that ferroptosis is involved in T cell-mediated anti-tumor immunity and affect the efficacy of cancer immunotherapy [16]. Combination of immunotherapy with ferroptosis-inducing radiotherapy [17] or targeted therapy [18], produces synergistic effect to promote tumor clearance. Therefore, ferroptosis is of great biological significance and clinical relevance.
In this review, we summarize three hallmarks of ferroptosis, namely lipid peroxidation, iron accumulation, and antioxidant vulnerability, characterize the regulatory mechanisms in cancer particularly using TNBC as a model, and highlight the opportunities and challenges of targeting ferroptosis in cancer immunotherapy.
The molecular mechanisms of ferroptosis
Ferroptosis is a modality of regulated cell death driven by iron-dependent lipid peroxidation. Three key hallmarks of ferroptosis have been deciphered: the peroxidation of membrane lipids, the availability of intracellular iron, and the loss of antioxidant defense [3].
Lipid peroxidation
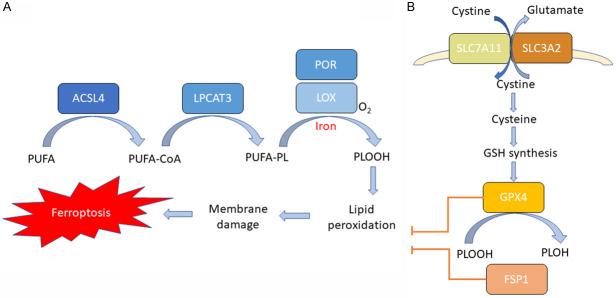
Lipid peroxidation causes the destruction of the lipid bilayer and the damage of membranes, subsequently leading to cell death [19] (Figure 1A). The cellular membranes are rich in phospholipids (PLs) containing polyunsaturated fatty acids (PUFAs), which are highly vulnerable to ROS-induced peroxidation. The availability of membrane PUFAs competent to undergo peroxidation is essential for the execution of ferroptosis [20]. PUFAs need to be synthesized, activated and incorporated into membrane PLs to participate in this lethal process, which requires two key enzymes, acyl-CoA synthetase long-chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3). ACSL4 is able to catalyze the ligation of long-chain PUFAs with coenzyme A (CoA) [12], and LPCAT3 promotes the esterification and incorporation of these products into membrane phospholipids [21,22].
Figure 1.
Molecular mechanisms of ferroptosis. A. Lipid peroxidation pathways. ACSL4 catalyzes the ligation of long-chain PUFAs with CoA, and LPCAT3 promotes the esterification and incorporation of these products into membrane phospholipids (PL). PUFA-containing PL is oxidized by iron-dependent enzymes LOX or POR, resulting in lipid peroxidation, membrane damage and subsequent ferroptosis. B. Antioxidant pathways. Cysteine is imported into the cell by SLC7A11/SLC3A2 complex for the synthesis of GSH. GPX4 uses GSH as a substrate and reduces the membrane phospholipid hydroperoxide to harmless lipid alcohols, thereby preventing the accumulation of lethal lipid ROS and suppressing ferroptosis. Alternatively, cells utilize FSP1 axis to suppress lipid peroxidation and prevent ferroptosis.
Certain lipoxygenases (LOXs) are considered to be the major enzymes that can directly oxygenate PUFA-containing lipids in membrane bilayers [23]. However, the mechanisms underlying LOX-mediated ferroptosis induction remain to be further dissected [24]. Another enzyme, cytochrome P450 oxidoreductase (POR), has recently been implicated to be involved in initiating the peroxidation of lipid [25].
Iron accumulation
As the name “ferroptosis” implies, iron is essential for the execution of ferroptotic cell death. Iron is indispensable for Fenton reaction, which generates free radicals and mediates lipid peroxidation [26]. In addition, iron is required for the activation of iron-containing enzymes LOXs and POR, which are responsible for oxidizing membrane PUFAs. Moreover, iron is important for redox-based metabolic processes involved in the production of cellular ROS.
Due to the key role of iron in the execution of ferroptosis, cellular iron pool is intricately controlled via the regulation of genes involved in intracellular iron storage, release, import and export [27,28]. Change in cellular labile iron affects the sensitivity of cells to ferroptosis. For example, the increase of iron importer transferrin [29] or the degradation of iron-storage protein ferritin [30], has been reported to increase cellular iron availability and sensitizes cells to ferroptosis.
Antioxidant vulnerability
Under normal conditions, iron-mediated lipid oxidation is tightly controlled by cellular anti-antioxidant defense systems (Figure 1B). Glutathione peroxidase 4 (GPX4) is thought to be the key antioxidant enzyme directly acting to eliminate the hydroperoxides in lipid bilayers and prevent the accumulation of lethal lipid ROS [31]. GPX4 uses glutathione (GSH) as a substrate and reduces the membrane phospholipid hydroperoxide to harmless lipid alcohols. The synthesis of GSH, which is essential for the activity of GPX4, requires three amino acids: cysteine, glycine and glutamic acid. Cysteine, as an essential cellular building block of GSH, is the rate-limiting substrate of GSH synthesis. The abundance of cysteine within mammalian cells, is mainly regulated by system xc, which consists of two subunits, solute carrier family 7 member 11 (SLC7A11) and SLC3A2 [32]. System xc plays a major role in importing cystine (the oxidized form of cysteine) into cells, and its expression and activity is exquisitely regulated by oncogenes and tumor suppressors in cancer cells through a variety of mechanisms [33-35]. Small molecule inhibitors such as erastin [36], which suppresses SLC7A11-mediated cystine import, can induce ferroptosis in multiple cancers.
An alternative GXP4-independent ferroptosis-suppressing mechanism has been recently uncovered [37,38]. Two independent genetic screens revealed that the ferroptosis suppressor protein 1 (FSP1)-CoQ system is capable of protecting cells against ferroptosis induced by GPX4 inhibition [37]. FSP1 could prevent lipid peroxidation via reduction of lipid radicals. Therefore, cells utilize two pathways, cyst(e)ine-GSH-GPX4 and FSP1-CoQ axis, to suppress lipid peroxidation and prevent ferroptosis. Ferroptosis occurs when these antioxidant defense systems are overwhelmed by iron-dependent lipid ROS accumulation.
Ferroptosis in TNBC
Ferroptosis sensitivity varies widely among different cancers. Since ferroptosis is linked to tumor suppression, a key question in cancer therapy is which tumor types would be more likely to benefit from the ferroptosis inducers (FINs). Recent evidence suggests the expression of genes involved in ferroptosis-associated metabolic pathways [9], such as lipid, iron and amino acid metabolism, are altered in TNBC, rendering this difficult-to-treat tumor intrinsically susceptible to ferroptosis. The particular sensitivity of TNBC to ferroptosis highlights this non-apoptotic death pathway as an attractive TNBC druggable target. Herein, we use TNBC as a model to summarize the regulation of ferroptosis in cancer, and similar mechanisms may also exist in other cancer types.
Lipid metabolism
Deregulated lipid metabolism can lead to lipid peroxidation and induce ferroptosis. In an effort to identify the key factors that determine ferroptosis sensitivity, two independent screening approaches were applied and uncovered ACSL4 as an essential component for ferroptosis execution [12]. Interestingly, it was found that ACSL4 was preferentially expressed in TNBC compared with other types of breast cancer, and its expression predicted their sensitivity to ferroptosis. Significant high expression of ACSL4 in TNBC tumors and cell lines was also observed in a recent study [13]. Given that ACSL4 is responsible for enriching cellular membranes with long PUFAs, these findings suggest that TNBC is prone to be PUFA-rich and thus particularly sensitive to ferroptosis.
Iron metabolism
Sufficient intracellular iron is essential for the execution of ferroptosis. Compared with normal cells, cancer cells exhibits higher reliance on iron to enable growth [39]. By analysis of clinical datasets and breast cancer specimens, a recent study revealed that the genes that regulate intracellular iron levels were distinctly expressed in TNBC versus non-TNBC tumors and cell lines [13]. In particular, a substantial low level of the iron exporter ferroportin was observed in TNBC, concomitant with the high expression level of the iron importer transferrin receptor [13,40]. These alterations in the expression of genes involved in iron metabolism regulation may contribute to an increase in cellular labile iron pool and facilitate iron-dependent lipid peroxidation, rendering TNBC to be iron-rich tumor and susceptible to ferroptosis.
Amino acid metabolism
Amino acid metabolism is essential for the anti-oxidation defense system composed by SLC7A11-mediated cystine uptake, GSH biosynthesis, and GPX4 activity. Cancer cells may exhibit altered dependency on specific amino acid metabolic pathways, and targeting these dependencies will be a promising therapeutic strategy. An earlier study applying functional metabolic analyses found that compared with other types of breast cancer, TNBC exhibited a marked reliance on glutamine metabolism necessary to fuel SLC7A11 [41], hinting a potential link of TNBC to ferroptosis and unveiling SLC7A11 as a promising therapeutic target for TNBC.
As a non-enzymatic antioxidant molecule, GSH plays an important role in maintaining redox homeostasis. The expression of GSH synthetase (GSS), one of the critical enzymes for GSH synthesis was found to be decreased in TNBC versus non-TNBC tumors [13]. Indeed, an earlier study used mass spectrometry-based metabolomic analysis and revealed that levels of the cellular GSH were lower in TNBC cell lines compared to controls [42]. Moreover, the expression of GPX4 was also lower in TNBC compared with other types of breast cancer [13]. Low intracellular GSH and reduced GPX4 expression may weaken the anti-oxidation defense capacity and increase the probability of lipid peroxidation, rendering TNBC particularly responsive to agents that promote ferroptosis.
Ferroptosis in cancer immunotherapy
The role of ferroptosis in cancer immunity and immunotherapy has been a topic of substantial interest for years. Ferroptosis has recently been revealed to contribute to the anti-tumor effect of CD8+ T cells and affect the efficacy of anti-PD-1/PD-L1 immunotherapy. Combination of immunotherapy with ferroptosis-promoting modalities, such as radiotherapy and targeted therapy, produces synergistic effects through ferroptosis to promote tumor control.
Combination of immunotherapy with cystine restriction
In cancer immunotherapy, CD8+ T cells are thought to kill cancer cells by activating FAS death receptor pathway and the granzyme-mediated tumoral apoptosis or pyroptosis [43]. Intriguingly, an alternative tumoral cell death, ferroptosis, has recently been reported to be involved in the anti-tumor activity of CD8+ T cells [16]. CD8+ T cells activated by anti-PD-L1 immunotherapy were found to promote ferroptosis in tumor cells by secreting interferon gamma (IFNγ) upon PD-L1 blockade. Secreted IFNγ significantly downregulated the expression of SLC3A2 and SLC7A11 in tumor cells, which resulted in reduced cystine uptake, enhanced lipid peroxidation and subsequent ferroptosis. Cyst(e)ine deprivation by cyst(e)inase synergized with anti-PD-L1 to induce potent anti-tumor immunity by inducing ferroptosis. This study characterizes T cell-promoted tumoral ferroptosis as a novel anti-tumor mechanism and reveals targeting this pathway in combination with immunotherapy is a promising therapeutic approach.
Combination of immunotherapy with targeted therapy
Given that ferroptosis contributes to the anti-tumor efficacy of anti-PD-L1 therapy, it is interesting to ask whether the resistant mechanisms of PD-L1 blockade might involve the inhibition of ferroptosis. Indeed, a recent study indicated that the resistance of anti-PD-L1 therapy was overcome by the combination with a TYRO3 receptor tyrosine kinase (RTK) inhibitor that promoted ferroptosis [18]. The expression of TYRO3 was found to be increased in the anti-PD-1 resistant tumors. Mechanically, TYRO3 signaling pathway upregulated the expression of key ferroptosis genes such as SLC3A2, thereby suppressing tumoral ferroptosis. Inhibiting TYRO3 promoted ferroptosis and sensitized the tumors to anti-PD-1 therapy in a TNBC syngeneic mouse model. The study revealed ferroptosis suppression as a novel mechanism contributing to anti-PD-L1 resistance, and uncovered derepressing ferroptosis with a TYRO3 inhibitor as a useful strategy to overcome the resistance to immunotherapy.
Combination of immunotherapy with radiotherapy
Radiotherapy has been used in clinic to improve the efficacy of immunotherapy for cancer patients, however, the underlying mechanisms remain to be further investigated [44,45]. Recent evidence indicates that increased sensitivity to ferroptosis is involved in this synergistic effect. Radiation has been shown to induce ferroptosis, and genetic and biochemical hallmarks of ferroptosis were observed in radiation-treated cancer cells. The mechanisms involve radiation-induced generation of ROS and upregulation of ACSL4, which result in enhanced lipid synthesis, increased lipid peroxidation, and subsequent membrane damage [46-48]. Thus, the anti-tumor effect of radiotherapy is not only attributable to DNA-damage induced cell death, but also to the induction of ferroptosis. Radiotherapy synergized with immunotherapy in downregulating SLC7A11, mediated by DNA damage-activated kinase ATM and IFNγ [16,17], leading to reduced cystine uptake, increased ferroptosis, and enhanced tumor control. These studies unveil ferroptosis as a novel mechanism underlying the synergy of immunotherapy and radiotherapy.
Combination of immunotherapy with T cell ferroptosis inhibitor
For years, extensive studies have been focused on the ferroptosis in tumor cells, however, ferroptosis in T cells has remained rarely explored. In addition to inducing tumoral ferroptosis, T cell themselves can also undergo ferroptosis [49,50], which may attenuate their immune response. T cells lacking GPX4 rapidly accumulates membrane lipid peroxides, concomitantly undergoes ferroptosis and prevents the immunity to infection, unveiling an essential role of GPX4 for T cell immunity [51]. Similar to cancer cells, ACSL4 is also essential for the ferroptosis of CD8+ T cells and their immune functions [52].
Recently, a fatty acid transporter CD36, has been implicated in promoting T cell ferroptosis. Two studies showed that the expression of CD36 was increased in CD8+ tumor infiltrating lymphocytes (TILs) [53,54]. T cell-intrinsic CD36 promoted the uptake of oxidized lipids and induced lipid peroxidation, thereby resulting in CD8+ T cell dysfunction. These findings uncover CD8+ T cell ferroptosis as a novel mode of immunosuppression in tumors and underscore the therapeutic potential of blocking CD36 to boost anti-tumor immunity. Notably, the study also suggested a role for GPX4 in the regulation of anti-tumor functions of CD8+ TILs. Therefore, therapeutic induction of ferroptosis by a GPX4 inhibitor in cancer cells may cause unwanted off-target effects on T cells and exert undesirable toxicities [50].
Conclusions and perspectives
Ferroptosis is driven by the oxidation of PUFA-containing lipids, the accumulation of intracellular iron and the loss of anti-oxidant defense. The regulation of ferroptosis has been characterized in a variety of cancer types including TNBC. TNBC exhibits a unique pattern of expression of the ferroptosis-related genes, rendering it prone to have sufficient PUFAs and iron, and decreased anti-oxidation capacity, thus particularly vulnerable to ferroptosis inducers. Therefore, targeting ferroptosis is likely to be a promising therapeutic strategy for this difficult-to-treat tumor.
Ferroptosis plays an important role in T cell mediated anti-tumor immunity and affects the efficacy of immunotherapy. Inducing ferroptosis with direct or indirect FINs, such as radiotherapy and targeted therapy, emerges to be promising combination modalities to improve anti-PD-1/PD-L1 immunotherapy. Further exploration of the ferroptosis-modulating modalities in combination with immunotherapy is warranted. These latest findings have broadened and deepened our understanding of this new form of cell death, exerted significant impacts on the development of ferroptosis-associated cancer therapies, and provided new opportunities for future research directions.
Acknowledgements
This work was funded in part by the National Natural Science Foundation of China (82172687 and 81972186 to L.S); Natural Science Foundation of Tianjin (20JCYBJC00360 and 16JCYBJC24400 to L.S); Key Project of Tianjin Lung Cancer Institute (TJLCZD2021-02 to L.S); Ministry of Science and Technology in Taiwan (110-2639-B-039-001-ASP to MCH).
Disclosure of conflict of interest
None.
References
- 1.Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44:479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martínez-Reyes I, Chandel NS. Cancer metabolism: looking forward. Nat Rev Cancer. 2021;21:669–680. doi: 10.1038/s41568-021-00378-6. [DOI] [PubMed] [Google Scholar]
- 3.Dixon SJ, Stockwell BR. The hallmarks of ferroptosis. Annu Rev Cancer Biol. 2019;3:35–54. [Google Scholar]
- 4.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22:266–282. doi: 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, Kagan VE, Noel K, Jiang X, Linkermann A, Murphy ME, Overholtzer M, Oyagi A, Pagnussat GC, Park J, Ran Q, Rosenfeld CS, Salnikow K, Tang D, Torti FM, Torti SV, Toyokuni S, Woerpel KA, Zhang DD. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hou J, Hsu JM, Hung MC. Molecular mechanisms and functions of pyroptosis in inflammation and antitumor immunity. Mol Cell. 2021;81:4579–4590. doi: 10.1016/j.molcel.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stockwell BR, Jiang X. The chemistry and biology of ferroptosis. Cell Chem Biol. 2020;27:365–375. doi: 10.1016/j.chembiol.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng J, Conrad M. The metabolic underpinnings of ferroptosis. Cell Metab. 2020;32:920–937. doi: 10.1016/j.cmet.2020.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13:674–690. doi: 10.1038/nrclinonc.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weigelt B, Reis-Filho JS. Histological and molecular types of breast cancer: is there a unifying taxonomy? Nat Rev Clin Oncol. 2009;6:718–730. doi: 10.1038/nrclinonc.2009.166. [DOI] [PubMed] [Google Scholar]
- 12.Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A, Prokisch H, Trümbach D, Mao G, Qu F, Bayir H, Füllekrug J, Scheel CH, Wurst W, Schick JA, Kagan VE, Angeli JP, Conrad M. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verma N, Vinik Y, Saroha A, Nair NU, Ruppin E, Mills G, Karn T, Dubey V, Khera L, Raj H, Maina F, Lev S. Synthetic lethal combination targeting BET uncovered intrinsic susceptibility of TNBC to ferroptosis. Sci Adv. 2020;6:eaba8968. doi: 10.1126/sciadv.aba8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters R, Yanase Y. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2018;97:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams S, Loi S, Toppmeyer D, Cescon DW, De Laurentiis M, Nanda R, Winer EP, Mukai H, Tamura K, Armstrong A, Liu MC, Iwata H, Ryvo L, Wimberger P, Rugo HS, Tan AR, Jia L, Ding Y, Karantza V, Schmid P. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: Cohort B of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30:405–411. doi: 10.1093/annonc/mdy518. [DOI] [PubMed] [Google Scholar]
- 16.Wang W, Green M, Choi JE, Gijón M, Kennedy PD, Johnson JK, Liao P, Lang X, Kryczek I, Sell A, Xia H, Zhou J, Li G, Li J, Li W, Wei S, Vatan L, Zhang H, Szeliga W, Gu W, Liu R, Lawrence TS, Lamb C, Tanno Y, Cieslik M, Stone E, Georgiou G, Chan TA, Chinnaiyan A, Zou W. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270–274. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lang X, Green MD, Wang W, Yu J, Choi JE, Jiang L, Liao P, Zhou J, Zhang Q, Dow A, Saripalli AL, Kryczek I, Wei S, Szeliga W, Vatan L, Stone EM, Georgiou G, Cieslik M, Wahl DR, Morgan MA, Chinnaiyan AM, Lawrence TS, Zou W. Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discov. 2019;9:1673–1685. doi: 10.1158/2159-8290.CD-19-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang Z, Lim SO, Yan M, Hsu JL, Yao J, Wei Y, Chang SS, Yamaguchi H, Lee HH, Ke B, Hsu JM, Chan LC, Hortobagyi GN, Yang L, Lin C, Yu D, Hung MC. TYRO3 induces anti-PD-1/PD-L1 therapy resistance by limiting innate immunity and tumoral ferroptosis. J Clin Invest. 2021;131:e139434. doi: 10.1172/JCI139434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, Kang R, Tang D. Ferroptosis: process and function. Cell Death Differ. 2016;23:369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A. 2016;113:E4966–E4975. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan H, Li X, Zhang X, Kang R, Tang D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun. 2016;478:1338–1343. doi: 10.1016/j.bbrc.2016.08.124. [DOI] [PubMed] [Google Scholar]
- 22.Dixon SJ, Winter GE, Musavi LS, Lee ED, Snijder B, Rebsamen M, Superti-Furga G, Stockwell BR. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem Biol. 2015;10:1604–1609. doi: 10.1021/acschembio.5b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah R, Shchepinov MS, Pratt DA. Resolving the role of lipoxygenases in the initiation and execution of ferroptosis. ACS Cent Sci. 2018;4:387–396. doi: 10.1021/acscentsci.7b00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, Basavarajappa D, Rådmark O, Kobayashi S, Seibt T, Beck H, Neff F, Esposito I, Wanke R, Förster H, Yefremova O, Heinrichmeyer M, Bornkamm GW, Geissler EK, Thomas SB, Stockwell BR, Odonnell VB, Kagan VE, Schick JA, Conrad M. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–1191. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bunney PE, Zink AN, Holm AA, Billington CJ, Kotz CM. Cytochrome P450 oxidoreductase contributes to phospholipid peroxidation in ferroptosis. Physiol Behav. 2017;176:139–148. [Google Scholar]
- 26.Porter NA, Caldwell SE, Mills KA. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 1995;30:277–290. doi: 10.1007/BF02536034. [DOI] [PubMed] [Google Scholar]
- 27.Andrews NC, Schmidt PJ. Iron homeostasis. Annu Rev Physiol. 2007;69:69–85. doi: 10.1146/annurev.physiol.69.031905.164337. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021;18:280–296. doi: 10.1038/s41571-020-00462-0. [DOI] [PubMed] [Google Scholar]
- 29.Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell. 2015;59:298–308. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ, Kang R, Tang D. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425–1428. doi: 10.1080/15548627.2016.1187366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang WS, Sriramaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem. 1999;274:11455–11458. doi: 10.1074/jbc.274.17.11455. [DOI] [PubMed] [Google Scholar]
- 33.Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, Baer R, Gu W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koppula P, Zhuang L, Gan B. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell. 2021;12:599–620. doi: 10.1007/s13238-020-00789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang LC, Chiang SK, Chen SE, Yu YL, Chou RH, Chang WC. Heme oxygenase-1 mediates BAY 11-7085 induced ferroptosis. Cancer Lett. 2018;416:124–137. doi: 10.1016/j.canlet.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 36.Dolma S, Lessnick SL, Hahn WC, Stockwell BR. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3:285–296. doi: 10.1016/s1535-6108(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 37.Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, Bassik MC, Nomura DK, Dixon SJ, Olzmann JA. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, Grocin AG, Xavier da Silva TN, Panzilius E, Scheel CH, Mourão A, Buday K, Sato M, Wanninger J, Vignane T, Mohana V, Rehberg M, Flatley A, Schepers A, Kurz A, White D, Sauer M, Sattler M, Tate EW, Schmitz W, Schulze A, O’Donnell V, Proneth B, Popowicz GM, Pratt DA, Angeli JP, Conrad M. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693–698. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 39.Hassannia B, Vandenabeele P, Vanden Berghe T. Targeting ferroptosis to iron out cancer. Cancer Cell. 2019;35:830–849. doi: 10.1016/j.ccell.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Feng H, Schorpp K, Jin J, Yozwiak CE, Hoffstrom BG, Decker AM, Rajbhandari P, Stokes ME, Bender HG, Csuka JM, Upadhyayula PS, Canoll P, Uchida K, Soni RK, Hadian K, Stockwell BR. Transferrin receptor is a specific ferroptosis marker. Cell Rep. 2020;30:3411–3423. e7. doi: 10.1016/j.celrep.2020.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Timmerman LA, Holton T, Yuneva M, Louie RJ, Padró M, Daemen A, Hu M, Chan DA, Ethier SP, van’t Veer LJ, Polyak K, McCormick F, Gray JW. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple-negative breast tumor therapeutic target. Cancer Cell. 2013;24:450–465. doi: 10.1016/j.ccr.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beatty A, Fink LS, Singh T, Strigun A, Peter E, Ferrer CM, Nicolas E, Cai KQ, Moran TP, Reginato MJ, Rennefahrt U, Peterson JR. Metabolite profiling reveals the glutathione biosynthetic pathway as a therapeutic target in triple-negative breast cancer. Mol Cancer Ther. 2018;17:264–275. doi: 10.1158/1535-7163.MCT-17-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Z, He H, Wang K, Shi X, Wang Y, Su Y, Wang Y, Li D, Liu W, Zhang Y, Shen L, Han W, Shen L, Ding J, Shao F. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science. 2020;368:eaaz7548. doi: 10.1126/science.aaz7548. [DOI] [PubMed] [Google Scholar]
- 44.Weichselbaum RR, Liang H, Deng L, Fu YX. Radiotherapy and immunotherapy: a beneficial liaison? Nat Rev Clin Oncol. 2017;14:365–379. doi: 10.1038/nrclinonc.2016.211. [DOI] [PubMed] [Google Scholar]
- 45.Sun LL, Yang RY, Li CW, Chen MK, Shao B, Hsu JM, Chan LC, Yang Y, Hsu JL, Lai YJ, Hung MC. Inhibition of ATR downregulates PD-L1 and sensitizes tumor cells to T cell-mediated killing. Am J Cancer Res. 2018;8:1307–1316. [PMC free article] [PubMed] [Google Scholar]
- 46.Lei G, Zhang Y, Koppula P, Liu X, Zhang J, Lin SH, Ajani JA, Xiao Q, Liao Z, Wang H, Gan B. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 2020;30:146–162. doi: 10.1038/s41422-019-0263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ye LF, Chaudhary KR, Zandkarimi F, Harken AD, Kinslow CJ, Upadhyayula PS, Dovas A, Higgins DM, Tan H, Zhang Y, Buonanno M, Wang TJC, Hei TK, Bruce JN, Canoll PD, Cheng SK, Stockwell BR. Radiation-induced lipid peroxidation triggers ferroptosis and synergizes with ferroptosis inducers. ACS Chem Biol. 2020;15:469–484. doi: 10.1021/acschembio.9b00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lei G, Mao C, Yan Y, Zhuang L, Gan B. Ferroptosis, radiotherapy, and combination therapeutic strategies. Protein Cell. 2021;12:836–857. doi: 10.1007/s13238-021-00841-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu S, Min J, Wang F. Ferroptosis: an emerging player in immune cells. Sci Bull. 2021 doi: 10.1016/j.scib.2021.02.026. [DOI] [PubMed] [Google Scholar]
- 50.Xu H, Ye D, Ren M, Zhang H, Bi F. Ferroptosis in the tumor microenvironment: perspectives for immunotherapy. Trends Mol Med. 2021;27:856–867. doi: 10.1016/j.molmed.2021.06.014. [DOI] [PubMed] [Google Scholar]
- 51.Matsushita M, Freigang S, Schneider C, Conrad M, Bornkamm GW, Kopf M. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J Exp Med. 2015;212:555–568. doi: 10.1084/jem.20140857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Drijvers JM, Gillis JE, Muijlwijk T, Nguyen TH, Gaudiano EF, Harris IS, LaFleur MW, Ringel AE, Yao CH, Kurmi K, Juneja VR, Trombley JD, Haigis MC, Sharpe AH. Pharmacologic screening identifies metabolic vulnerabilities of CD8+ T cells. Cancer Immunol Res. 2021;9:184–199. doi: 10.1158/2326-6066.CIR-20-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu S, Chaudhary O, Rodríguez-Morales P, Sun X, Chen D, Zappasodi R, Xu Z, Pinto AFM, Williams A, Schulze I, Farsakoglu Y, Varanasi SK, Low JS, Tang W, Wang H, McDonald B, Tripple V, Downes M, Evans RM, Abumrad NA, Merghoub T, Wolchok JD, Shokhirev MN, Ho PC, Witztum JL, Emu B, Cui G, Kaech SM. Uptake of oxidized lipids by the scavenger receptor CD36 promotes lipid peroxidation and dysfunction in CD8+ T cells in tumors. Immunity. 2021;54:1561–1577. e7. doi: 10.1016/j.immuni.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma X, Xiao L, Liu L, Ye L, Su P, Bi E, Wang Q, Yang M, Qian J, Yi Q. CD36-mediated ferroptosis dampens intratumoral CD8+ T cell effector function and impairs their antitumor ability. Cell Metab. 2021;33:1001–1012. e5. doi: 10.1016/j.cmet.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]