Abstract
Recent studies identified that low levels of tumor suppressor microRNAs in plasma/serum relate to tumor progression and poor outcomes in cancers. This study explored decreased tumor suppressor microRNA (miRNA) plasma levels in gastric cancer (GC) patients to clarify their potential as novel biomarkers and therapeutic targets. We focused on five candidates (miR-148a, miR-101, miR-129, miR-145 and miR-206) of tumor suppressor miRNAs in GC by a systematic review of NCBI database. Of these, miR-148a levels were significantly down-regulated in plasma of GC patients compared to healthy volunteers by test- and validation-scale analyses (P<0.0001). A Low level of plasma miR-148a was significantly associated with venous invasion, lymph node metastasis, advanced stage and peritoneal recurrence, and was an independent poor prognostic factor (P=0.0296, Hazard ratio 4.2). Overexpression of miR-148a in GC cells inhibited cell proliferation, migration, invasion and epithelial-mesenchymal transition. In vivo, the restoration and maintenance of miR-148a in plasma significantly inhibited tumor growth in mice with peritoneal metastasis (P=0.0050). In conclusions, depletion of the tumor suppressor miRNA-148a in plasma relates to tumor progression and poor outcomes. The restoration of the blood miR-148a level might be a novel nucleic acid anticancer therapy for GC.
Keywords: Tumor suppressor microRNA, biomarker, liquid biopsy, oligonucleotide therapy, gastric cancer
Introductions
Gastric cancer is considered to be the fifth most common cancer and the third leading cause of death worldwide [1]. While improved perioperative management and diagnostic techniques have decreased mortality while boosting early detection in recent years, GC continues to constitute a global health problem as a prevalent form of cancer [2,3]. Until now, only a few biomarker molecules have been employed for the early diagnosis of GC in clinical settings, and researchers have validated only a scant number of molecules as therapeutic targets [4-6]. Therefore, identifying novel clinical biomarkers and molecular targets for GC remain pivotal clinical issues.
MicroRNAs (miRNAs), which are small non-coding RNAs, regulate the translation of specific protein-coding genes. Since their discovery in 1993 [7], numerous studies have identified alterations in miRNA expression that are correlated with the progression of various diseases, including several cancer types [8-11]. In recent decades, several studies have elucidated that miRNAs are detectable in plasma/serum and are present in a remarkably stable form [10,12-15]. Plasma/serum miRNAs are resistant to endogenous ribonuclease activity by binding to specific plasma proteins [16,17] or are packaged by various types of secretory vesicles, including apoptotic bodies and exosomes in plasma/serum [14,18-20]. Furthermore, some extracellular miRNAs occur through active secretion [21-23] as intercellular transmitters [15,21,24,25]. Thus, various up-regulated and blood-based miRNAs have been identified and can be used for cancer detection, monitoring tumor dynamics, and predicting prognosis and chemoresistance [26-38].
Previously, Kosaka and Ochiya et al. suggested a novel mechanism that miRNAs could facilitate the system of maintenance and surveillance against cancer progression. Tumor suppressor miRNAs are normally secreted from neighboring healthy cells to cancer cells to inhibit cancer progression [39]. During the initial stage of tumourigenesis, the down-regulation of tumor suppressor miRNAs in cancer cells may be compensated for by the surrounding healthy cells, which supply exosomes containing tumor suppressor miRNAs. However, once the surrounding cells can no longer meet this demand, cancer cells progress to an advanced stage. In our previous study, we identified that some tumor suppressor miRNAs in plasma were significantly down-regulated in cancer patients compared with healthy volunteers [26,28,30]. As circulating miRNAs are considered to be released from cancer tissues as well as normal tissues, most of these miRNAs are expected to have originated from normal tissues; thus, we hypothesized that tumor suppressor miRNAs might become depleted from healthy cells in accordance with cancer progression. Indeed, we demonstrated that the low plasma level of some tumor suppressor miRNAs in cancer patients were associated with tumor progression and poor prognostic outcomes in miR-375 and miR-655 of esophageal cancer [28,40] and miR-107 of pancreatic cancer [41]. Specifically, the expression of miR-107 in specific normal organs of liver and kidney was reduced in mice bearing pancreatic cancer tumor [41]. Moreover, we demonstrated that the depletion of these tumor suppressor miRNAs could be targets of oligonucleotide therapy [40,41].
In this study, we focused on tumor suppressor miRNAs that are down-regulated in GC patient plasma, and we demonstrated the potential utility of the restoration of these tumor suppressor miRNAs as a therapeutic strategy for this lethal disease. We selected six down-regulated tumor suppressor miRNAs (miR-148a, miR-101, miR-129, miR-145 and miR-206) and finally validated that depleted tumor suppressor miRNA-148a plasma levels are related to tumor progression and poor outcomes. The restoration and maintenance of the miR-148a plasma level significantly inhibited peritoneal metastasis in vivo. Our results and concepts provide evidence that the restoration and maintenance of the tumor suppressor miR-148a plasma level could be a novel treatment strategy for GC patients using nucleic acid medicine.
Materials and methods
Patients and samples
The study was approved by the institutional review board of Kyoto Prefectural University of Medicine, and each subject provided written informed consent. Between January 2010 and April 2014, a total of 132 plasma samples from GC patients consisted of 10 small-scale samples and 122 validation samples from the Kyoto Prefectural University of Medicine were collected. The sixty samples from healthy volunteers included those from medical personnel and patients with benign disease, such as cholecystolithiasis and inguinal herniation. These patients underwent medical examinations and were shown not to have any pancreatic or cancerous diseases. Tumor stages were assessed according to the Union for International Cancer Control classification system [42].
Peripheral blood (7 ml) was obtained from each patient at the time of diagnosis or before surgery and from the healthy volunteers. The blood was transferred into sodium heparin tubes (BD Vacutainer, Franklin Lakes, NJ) and immediately subjected to the three-spin protocol (1500 r.p.m. for 30 min, 3000 r.p.m. for 5 min, and 4500 r.p.m. for 5 min) to prevent contamination by cellular nucleic acids. Plasma was collected and then stored at -80°C until further processing. Histological evaluations were performed for tissues adjacent to specimens, according to the criteria of the World Health Organization. In all cases, two pathologists agreed with the pathological observations and confirmed the diagnosis.
RNA extraction
Total RNA was extracted from 400 μl of plasma using a mirVana PARIS Kit (Ambion, Austin, TX) and finally eluted into 100 μl of preheated (95°C) Elution Solution according to the manufacturer’s protocol. Total RNA was also extracted from four 15-μm-thick slices of formalin-fixed and paraffin-embedded tissue using a RecoverAll Total Nucleic Acid Isolation Kit (Ambion) and then eluted into 60 μl of Elution Solution according to the manufacturer’s protocol.
Selection of plasma miRNA candidates based on a systematic review of the NCBI database
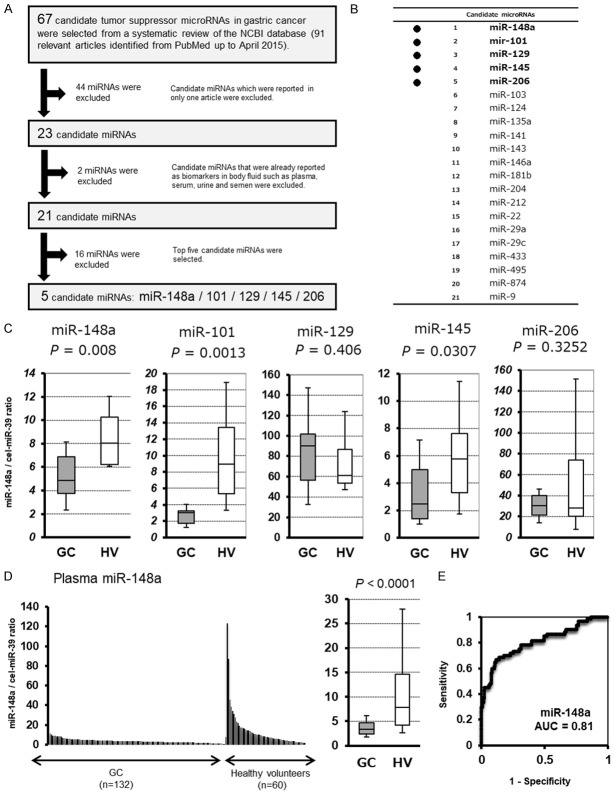
We searched for all studies related to GC miRNAs in PubMed up to April 2015 and 91 relevant articles identified. From a systematic review of these articles, 67 candidate tumor suppressor microRNAs in gastric cancer were selected as shown in Table S1. From these candidates, as shown in Figure 1A, 44 candidate miRNAs which were reported in only one article were excluded, and 2 candidate miRNAs that were already reported as biomarkers in body fluid such as plasma, serum, urine and semen were excluded (Figure 1B). After a series of these exclusion criteria were applied, we selected 21 candidates and consequently selected top five candidate miRNAs: miR-148a [43], miR-101 [44], miR-129 [45], miR-145 [46] and miR-206 [47].
Figure 1.
Selection of plasma miRNA candidates, and test- and large-scale analysis of the candidate plasma level in GC patients and healthy volunteers. A. Study design to find novel candidate miRNAs that decreased in patient plasma as a therapeutic target for GG. B. Twenty-one candidate miRNAs. C. Small-scale analysis of the plasma levels of five miRNAs in GC patients and healthy volunteers by qRT-PCR. The change in the expression of miR-148a, miR-101 and miR-145 were found to be the significant. D. Large-scale analysis indicated that the plasma level of miR-148a was significantly lower in GC patients than in healthy volunteers (P<0.0001). E. ROC analysis indicated the AUC value to be 0.81 with a sensitivity of 88.6% and a specificity of 66.7%.
Quantification of miRNA by qRT-PCR
The amounts of miRNAs were quantified by qRT-PCR using a Human TaqMan MicroRNA Assay Kit (Applied Biosystems, Foster City, CA). The reverse transcription reaction was conducted with a TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems) and gene-specific primers (hsa-miR-148a, Assay ID: 000470; hsa-miR-101, Assay ID: 002143; hsa- miR-129, Assay ID: 000590; hsa-miR-145, Assay ID: 002278; hsa-miR-206, Assay ID: 000510; cel-miR-39, Assay ID: 000200; and RNU6B, Assay ID: 001093). qPCR was run on a StepOnePlus PCR system (Applied Biosystems), and Cycle threshold (Ct) values were calculated with StepOne Software v2.0 (Applied Biosystems).
As previously reported [14], we normalized the data across samples using the 2-ΔΔCt method relative to cel-miR-39. However, the expression of miRNAs from tissue samples and cultured cells was normalized using the 2-ΔΔCt method relative to U6 small nuclear RNA (RNU6B).
Small-scale analysis of the plasma levels of five miRNAs in GC patients and healthy volunteers
First, we investigated the plasma levels of the selected five miRNAs in 10 GC patients and 10 healthy volunteers by qRT-PCR using a small-scale analysis. As shown by the results of the miRNA array-based approach, the levels of three candidate miRNAs tended to be lower in the plasma of GC patients than in that of the healthy volunteers, and the difference in the expression level of miR-148a (P=0.008), miR-101 (P=0.001) and miR-145 (P=0.031) were determined to be the significant (Figure 1C). Regarding the miR-101, we subsequently demonstrated as a biomarker that depletion of the tumor suppressor miRNA-101 in plasma is related to tumor progression and poor outcomes [48]. Therefore, in this study, we selected most significant miR-148a for further analyses.
Culture of GC cell lines
The GC cell lines NUGC4 and MKN45 were purchased from RIKEN Cell Bank (Tsukuba, Japan) and cultured in Roswell Park Memorial Institute 1640 medium (Sigma, St. Louis, MO) or Dulbecco’s Modified Eagle Medium (Nacalai, Japan) supplemented with 10% fetal bovine serum (Trace Scientific, Melbourne, Australia). All cells were cultured in 5% carbon dioxide at 37°C in a humidified chamber.
Transfection of PCa cells with miRNA mimics
For the overexpression of miR-148a, the miR-148a mimic (Assay ID: MC10263) or negative control mimic miRNA (mirVana miRNA mimic Negative Control #1), both of which were selected from the mirVana miRNA mimic panel (Ambion), was used to transfect the cells at a final concentration of 12 μM by using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer’s instructions. After 72 h, the overexpression of miR-148a was confirmed by qRT-PCR.
Proliferation assay and cell cycle analysis
To measure cell growth, the number of viable cells at various time points after transfection was assessed by the colorimetric water-soluble tetrazolium salt assay (Cell Counting Kit 8; Dojindo Laboratories, Kumamoto, Japan). Cell viability was determined by reading the optical density at 450 nm. The cell cycle was evaluated 72 h after transfection by fluorescence-activated cell sorting (FACS).
Transwell migration and invasion assays
Transwell migration and invasion assays were conducted in 24-well modified Boyden chambers (Transwell chambers, BD Transduction, Franklin Lakes, NJ). The upper surface of 6.4-mm-diameter filters with 8-µm pores was precoated with (invasion assay) or without (migration assay) Matrigel (BD Transduction). The miRNA mimic transfectants (5×105 cells per well) were transferred into the upper chamber. Following 22 h of incubation, the migrated or invasive cells on the lower surface of the filters were fixed and stained with Diff-Quik stain (Sysmex, Kobe, Japan), and stained cell nuclei were counted directly in triplicate.
Western blot analysis
Anti-Vimentin and anti-E-cadherin, anti-ACTB antibodies were purchased from Cell Signaling Technology (Cell Signaling Technology, USA). Cells were lysed, and their proteins were extracted using M-PER Mammalian® Protein Extraction Reagent (Thermo Scientific, USA).
Animal experimental protocol
For the in vivo model, GFP-labeled MKN45 cells (5×106) were injected in the peritoneal cavity of SCID mice. Treatment began at 3 days after tumor cell injection. Either 1 nmol of the control miRNA mimic or 1 nmol of the miR-148a mimic with AteloGene Local Use Quick gelation (Koken, Co., Tokyo, Japan) was injected in the peritoneal cavity every three days, according to the manufacturer’s protocol. The tumor volume was calculated according to the formula V = A×B2/2 (mm3), where A is the largest diameter (mm), and B is the smallest diameter (mm). At 10 days after tumor cell implantation, the mice were sacrificed and blood samples were collected for further analysis.
Statistical analysis
The Mann-Whitney U test and the t-test for unpaired data were performed for comparing plasma or tissue sample data. The Wilcoxon test was used to compare the paired data. The Chi-square test or Fisher’s exact probability test was used to evaluate correlations between the plasma miRNA levels and clinicopathological factors. A P-value<0.05 was considered statistically significant.
For the survival rate analysis, Kaplan-Meier survival curves were constructed for groups based on univariate predictors, and differences between the groups were analyzed with the log-rank test or the Wilcoxon test. Univariate and multivariate survival analyses were performed using the likelihood ratio test of the stratified Cox proportional hazards model. A P-value<0.05 was considered statistically significant.
Results
Large-scale analysis of the miR-148a plasma level in GC patients
We next validated our observations in a large-scale setting. Plasma miR-148a was detectable in all samples from 132 GC patients and 60 healthy volunteers. We observed that the miR-148a plasma level was significantly lower in the GC patients than in the healthy volunteers (P<0.0001) (Figure 1D). Furthermore, to detect cut-off points that could differentiate cancer patients from healthy volunteers, we utilized the AUC value and the Youden index and found that the AUC value was 0.81. The optimal relative expression cut-off point was indicated to be 20.5, with a sensitivity of 88.6% and a specificity of 66.7%. Our results provide evidence that the miR-148a plasma level can be used to distinguish GC patients from healthy volunteers to a clinically satisfactory degree in comparison with conventional tumor markers.
Correlation between the miR-148a plasma level and clinicopathological factors in GC patients
We analyzed the correlation between the miR-148a plasma level and clinicopathological factors in 132 GC patients undergoing curative gastrectomy (Table 1). A low miR-148a plasma level was significantly correlated with the presence of venous invasion (P=0.0279) and lymph node metastasis (P=0.0267), and advanced stage (P=0.0040) and recurrences (P=0.0194). Also, a low miR-148a plasma level tended to be associated with advanced T stages (P=0.0810). Regarding the types of recurrence, patients with a low miR-148a plasma level more frequently developed the peritoneal recurrence (P=0.0010) (Table 2).
Table 1.
Correlation between plasma miR-148a levels and clinicopathological characteristics in GC patients with gastrectomy
| n | Plasma miR-148a concentration | Univariatea | ||
|---|---|---|---|---|
|
|
|
|||
| High | Low | p-value | ||
| Total | 132 | 59 (45%) | 73 (55%) | |
| Age | 0.5693 | |||
| <65 | 37 | 18 (31%) | 19 (26%) | |
| ≥65 | 95 | 41 (69%) | 54 (74%) | |
| Sex | 0.9308 | |||
| male | 80 | 36 (61%) | 44 (60%) | |
| female | 52 | 23 (39%) | 29 (40%) | |
| Histological type | 0.5187 | |||
| Differentiated | 63 | 30 (51%) | 33 (45%) | |
| Un-differentiated | 69 | 29 (49%) | 40 (55%) | |
| Lymphatic invasion | 0.3018 | |||
| Present | 67 | 27 (46%) | 40 (55%) | |
| Absent | 65 | 32 (54%) | 33 (45%) | |
| Venous invasion | 0.0279 | |||
| Present | 54 | 18 (31%) | 36 (49%) | |
| Absent | 78 | 41 (69%) | 37 (51%) | |
| pT (TNM) | 0.0810 | |||
| T1/T2 | 88 | 44 (75%) | 44 (60%) | |
| T3/T4 | 44 | 15 (25%) | 29 (40%) | |
| pN (TNM) | 0.0267 | |||
| NO | 85 | 44 (75%) | 41 (56%) | |
| N1 | 47 | 15 (25%) | 32 (44%) | |
| pStage (TNM) | 0.0040 | |||
| I/II | 104 | 53 (90%) | 51 (70%) | |
| III | 28 | 6 (10%) | 22 (30%) | |
| Recurrence | 0.0194 | |||
| absent | 110 | 54 (92%) | 56 (77%) | |
| present | 22 | 5 (8%) | 17 (23%) | |
Chi-square test.
NOTE: Significant values are in bold.
Table 2.
Correlation between plasma miR-148a levels and recurrences in GC patients following curative gastrectomy
| n | Plasma miR-148a concentration | Univariatea | ||
|---|---|---|---|---|
|
|
|
|||
| High | Low | p-value | ||
| 132 | 59 | 73 | ||
| Recurrence | 22 | 5 (8%) | 17 (23%) | 0.0194 |
| Hematogenous recurrence | 4 | 2 (3%) | 2 (3%) | 0.8290 |
| Lymphatic recurrence | 5 | 3 (5%) | 2 (3%) | 0.4841 |
| Peritoneal recurrence | 14 | 1 (2%) | 13 (18%) | 0.0010 |
Chi-square test.
NOTE: Significant values are in bold.
Potential utility of miR-148a as a prognostic biomarker in GC patient plasma
Moreover, a prognostic analysis revealed that a low miR-148a plasma level was significantly associated with a worse overall survival rate in all GC patients (P=0.0034) (Figure 2). Multivariate analyses revealed that a low miR-148a level (P=0.0228; hazard ratio, 4.41; 95% confidence interval [CI]: 1.13-27.20) as well as advanced stage III were independent factors predicting poor prognosis in GC patients (Table 3).
Figure 2.
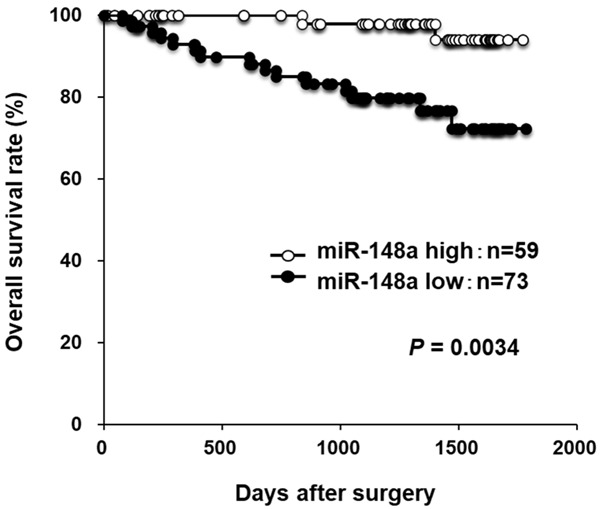
Overall survival curves according to the plasma status of miR-148a in CG patients. A low miR-148a plasma level was significantly associated with a worse overall survival rate in 132 consecutive GC patients.
Table 3.
Univariate and multivariate analyses for survival using the Cox’s proportional hazard model in GC patients following curative gastrectomy
| Variable | Univariatea | Multivariateb | |||
|---|---|---|---|---|---|
|
|
|
||||
| p-value | HR | 95% Cl | p-value | ||
| Sex | male vs. female | 0.7403 | 0.79 | 0.43-3.21 | 0.7894 |
| Age | ≥65 vs. 65< | 0.5041 | 1.41 | 0.48-5.11 | 0.5476 |
| Stage (TNM) | Stage III vs. Stage I/II | <0.0001 | 15.38 | 5.30-55.67 | <0.0001 |
| Plasma miR-148a expression | Low vs. High | 0.0034 | 4.20 | 1.13-27.20 | 0.0296 |
Kaplan-Meiyer method; significance was determined by log-rank test.
Multivariate survival analysis was performed using Cox’s proportional hazard model.
HR: Hazard ratio; Cl: confidence interval. NOTE: Significant values are in bold.
Investigation of the tumor suppressor function of miR-148a in GC cells
We first performed a cell proliferation assay using miRNA mimics to investigate whether miR-148a overexpression would suppress GC cell proliferation (Figure 3A). Proliferation was significantly suppressed in NUGC4 and MKN45 cell lines after miR-148a mimic transfection compared with negative control mimic transfection. The FACS analysis revealed that transfecting GC cells with the miR-148a mimic induced the accumulation of G2/M phase cells compared with negative control mimic transfection in both NUGC4 and MKN45 cell lines.
Figure 3.
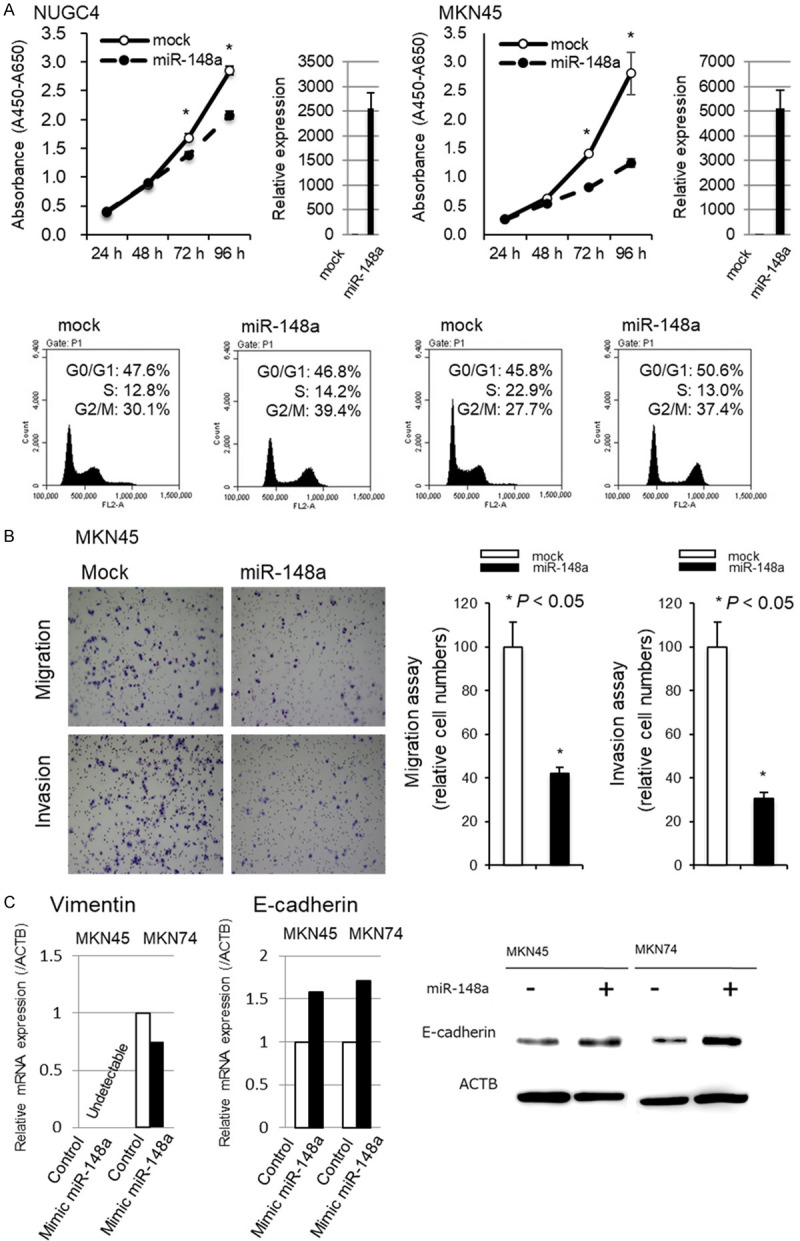
Investigation of the tumor suppressor function of miR-148a in GC cells. A. Cell proliferation was significantly suppressed in both NUGC4 and MKN45 cells transfected with the miR-148a mimic compared with cells transfected with the negative control mimic. The FACS analysis demonstrated that transfecting NUGC4 and MKN45 cells with the miR-148a mimic resulted in an accumulation of cells in the G2/M phase compared with transfection with the control miRNA mimic. B. The transwell migration and invasion assays demonstrated that overexpression of miR-148a in GC cells inhibited cell migration and invasion. C. The expression levels of EMT-associated mRNA, such as E-cadherin and Vimentin, in all GC cell lines. After the overexpression of miR-148a mimic in MKN 45 and MKN74 cells, E-cadherin mRNA and protein level were increased, whereas Vimentin mRNA was decreased in MKN45 at 72 h after transfection of miR-148a mimic.
Next, transwell migration and invasion assays were performed to examine the ability of MKN45 cells transfected with miR-148a mimics to move through pores under different conditions. An uncoated membrane was used for migration assays, whereas a Matrigel-coated membrane was used for invasion assays. As shown in Figure 3B, the number of MKN45 cells that migrated into the lower chamber was significantly lower for miR-148a mimic-transfected cells than for mock-transfected cells under both conditions, suggesting that miR-148a suppressed the ability of gastric cancer cells to migrate and invade.
Furthermore, we investigated whether tumor suppressor miR-148a would suppress endothelial-mesenchymal transition (EMT). To select the most appropriate GC cell line for this assay, we confirmed the expression levels of EMT-associated mRNA, such as E-cadherin and Vimentin, in all GC cell lines. After the overexpression of miR-148a mimic in MKN 45 and MKN74 cells, E-cadherin mRNA and protein level were increased, whereas Vimentin mRNA was decreased in MKN45 at 72 h after transfection of miR-148a mimic (Figure 3C). As previously reported in hepatoma cells and gastric cancer cells [49,50], we confirmed that miR-148a could suppress EMT in GC cells.
Investigation whether the tumor suppressor miR-148a could suppress tumor peritoneal dissemination and restore plasma miR-148a in vivo
Next, we examined the possible tumor suppressor function of miR-148a in vivo using miRNA mimics and SCID mice with peritoneal tumors. The miR-148a or negative control mimic with atelocollagen was injected in the peritoneal cavity every three day for 10 days after the initial treatment (Figure 4A). Compared with the negative control mimic, the miR-148a mimic more significantly suppressed peritoneal dissemination (P=0.0050) (Figure 4B and 4C). The miR-148a plasma level was significantly lower in control mice treated with the control mimic than in mice treated with the miR-148a mimic (P=0.0020). In contrast, the miR-148a plasma level in mice treated with the miR-148a mimic was significantly higher than that in non-treated mice without tumors (P=0.0059) (Figure 4D). These findings strongly suggested that the recovery and restoration of miR-148a in plasma could significantly inhibit GC tumor growth.
Figure 4.
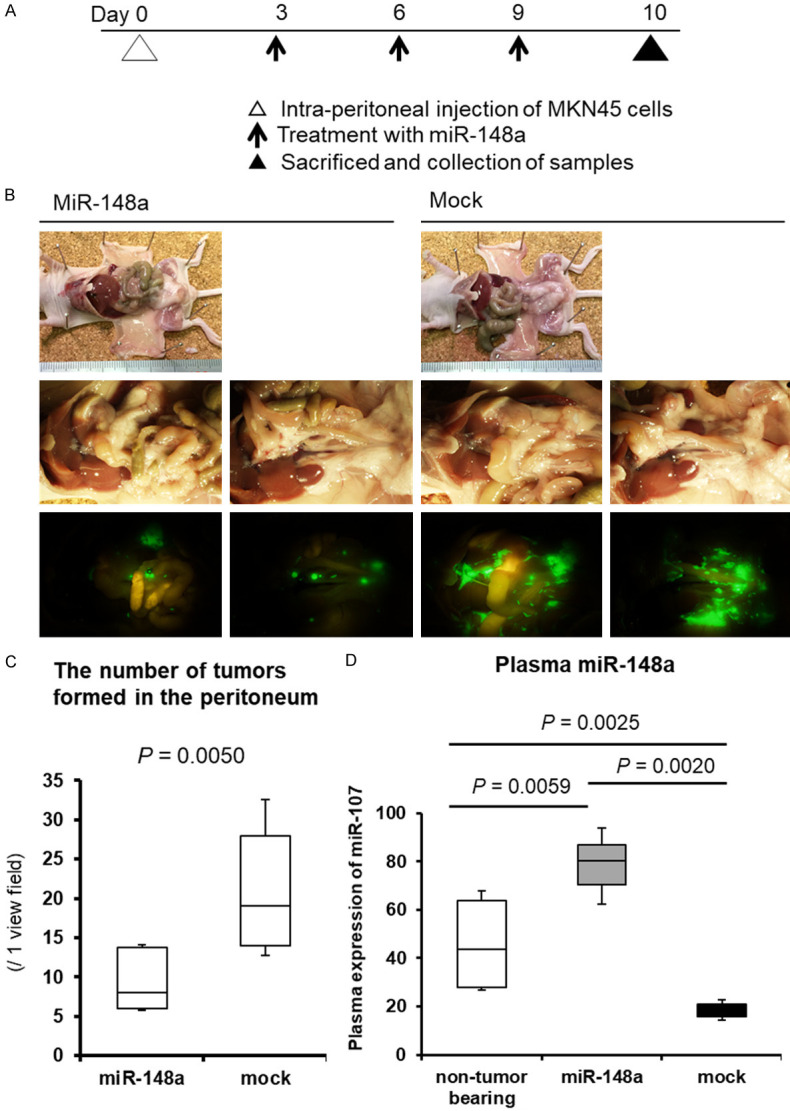
Restoration and maintenance of the miR-148a plasma level could suppress tumor peritoneal dissemination in vivo. A. The miR-148a or negative control mimic with atelocollagen was injected in the peritoneal cavity every three day for 10 days after the initial treatment. B. The miR-148a mimic suppressed peritoneal dissemination compared with the negative control mimic. C. Compared with the negative control mimic, the miR-148a mimic more significantly suppressed peritoneal dissemination (P=0.0050). D. Plasma miR-148a level was significantly down-regulated in control mice with peritoneal metastasis. Whereas, plasma miR-148a level in mice treated with mimic miR-148a was maintained more than normal level. The restoration of miR-148a in plasma significantly inhibited tumor growth associated with peritoneal dissemination.
Discussion
Through the selection of five candidate tumor suppressor miRNAs (miR-148a, miR-101, miR-129, miR-145 and miR-206) by a systematic review of the NCBI database, we finally validated a novel tumor suppressor miR-148a as a plasma biomarker and treatment target in GC patients. A low level of miR-148a in plasma was significantly related to peritoneal metastasis and poor outcomes in GC. Moreover, the overexpression of miR-148a in GC cells inhibited cell proliferation, migration, and invasion thorough the G2/M arrest and MET in vitro. Furthermore, in vivo analysis showed that the peritoneal injection of miR-148a enabled the recovery and restoration of miR-148a in plasma and significantly inhibited tumor progression compared with the controls. These results provide evidence that the tumor suppressor miR-148a could facilitate a novel microRNA-based therapy for GC, particularly by inhibiting peritoneal metastasis in GC.
Concerning the molecular functions of miR-148a in cancer, reduced miR-148a expression has been reported to contribute to carcinogenesis and tumor development in various types of cancer such as pancreatic cancer [51], hepatocellular carcinoma [50], nasopharyngeal carcinoma [52], lung cancer [53], breast cancer [54], bladder cancer [55], osteosarcoma [56] including gastric cancer [43,49,57]. Previous reports have demonstrated that miR-148a could suppress the epithelial-mesenchymal transition in several cancers [49,50]. In this study, we also demonstrated in GC. These previous results support the tumor suppressor role of miR-148a in GC demonstrated in our study.
Most striking finding in this study, the miR-148a mimic suppressed peritoneal dissemination in compared to control in vivo analysis, recovering the plasma level of miR-148a. In our study, low level of plasma miR-148a was significantly associated with peritoneal recurrence (P=0.0010). This finding strongly suggested that the restoration of miR-148a level in body fluid might be a novel oligonucleotide therapy for GC peritoneal recurrences. Moreover, recent studies identified that miR-148a improved the response to chemotherapy such as cisplatin [58], paclitaxel [59], and improved the immunosuppression in DNA mismatch repair-deficient cancer by targeting PD-L1 [60]. Therefore, chemotherapy plus the oligonucleotide therapy using miR-148a might be a novel therapy, enhancing the response to chemotherapy such as anticancer drugs and PD-1 blockade.
Regarding the clinical application of miRNAs, two promising clinical studies have been performed. The first study focused on the therapeutic silencing of disease-associated miRNAs using miRNA inhibitors. Miravirsen (Santaris Pharma) is one of several promising miRNA inhibitors; it can bind to miR-122 and inhibit its biogenesis. Miravirsen was developed for the treatment for hepatitis C and is currently under evaluation in clinical trials [61,62]. The second study focused on therapeutic miRNA-based drugs using synthetic miRNA mimics. MIRX34 (Mirna Therapeutics, Inc.) is a synthetic miRNA mimic of the tumor suppressor miR-34, and a phase I clinical trial using MIRX34 was performed [63]. In this trial, MIRX34 was administered to patients with primary or metastatic liver cancer. Unfortunately, this trial was ended because of serious adverse immune-related effects caused by the ability of miRNAs to target and regulate multiple genes affecting various functions. These studies strongly suggested that attention should be paid using miRNA-based medicine.
In our study, in vivo oligonucleotide treatment using mimic miR-148a in GC in mice did not cause any clinical adverse events. Therefore, we believe that the restoration of tumor suppressor miR-148a, which is abundantly detected in the plasma of healthy human individuals, may be a safe treatment for minimizing various physiological risks in clinical applications. Indeed, in previous our studies, in vivo oligonucleotide treatment using miR-107 in pancreatic cancer and miR-655 in esophageal cancer in mice did not only cause any clinical adverse events [40,41]. Indeed, there were no side effects in blood-based parameters reflecting organ disorders using miR-655 in esophageal cancer [40].
This is the first report to demonstrate that the tumor suppressor miR-148a, which was depleted in the plasma of GC patients, could be a plasma biomarker as well as a therapeutic target for GC. However, many issues must still be addressed before these findings can be translated into a clinically useful biomarker and treatment agent for GC patients. Detailed examinations of the physiological effects of miR-148a are needed for its safe clinical utilization. Moreover, further studies are needed on the cellular uptake or secretion systems of tumor suppressor miRNAs, leading to the development of miRNA delivery systems for future therapeutic and diagnostic applications [64-67]. These studies are currently under evaluation and are expected to be reported upon in the near future.
Acknowledgements
This study was partially supported by the research grant from the Foundation for Okinaka Memorial Institute for Medical Research (2016), Japan. Patient’s plasma and data were collected with written informed consent, approved by the Hospital Ethical Committee of the Kyoto Prefectural University of Medicine.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654–2664. doi: 10.1016/S0140-6736(16)30354-3. [DOI] [PubMed] [Google Scholar]
- 3.Komatsu S, Otsuji E. Essential updates 2017/2018: recent topics in the treatment and research of gastric cancer in Japan. Ann Gastroenterol Surg. 2019;3:581–591. doi: 10.1002/ags3.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Ruschoff J, Kang YK ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry DR, Melichar B, Tehfe M, Topuzov E, Zalcberg JR, Chau I, Campbell W, Sivanandan C, Pikiel J, Koshiji M, Hsu Y, Liepa AM, Gao L, Schwartz JD, Tabernero J. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 6.Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Chen LT. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–2471. doi: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 7.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 8.He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, Hammond SM. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 10.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 11.He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, Jackson AL, Linsley PS, Chen C, Lowe SW, Cleary MA, Hannon GJ. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, Li Q, Li X, Wang W, Zhang Y, Wang J, Jiang X, Xiang Y, Xu C, Zheng P, Zhang J, Li R, Zhang H, Shang X, Gong T, Ning G, Wang J, Zen K, Zhang J, Zhang CY. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 13.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ichikawa D, Komatsu S, Konishi H, Otsuji E. Circulating microRNA in digestive tract cancers. Gastroenterology. 2012;142:1074–1078. e1071. doi: 10.1053/j.gastro.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasselmann DO, Rappl G, Tilgen W, Reinhold U. Extracellular tyrosinase mRNA within apoptotic bodies is protected from degradation in human serum. Clin Chem. 2001;47:1488–1489. [PubMed] [Google Scholar]
- 19.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Kosaka N, Iguchi H, Ochiya T. Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010;101:2087–2092. doi: 10.1111/j.1349-7006.2010.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 22.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11:426–437. doi: 10.1038/nrc3066. [DOI] [PubMed] [Google Scholar]
- 24.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rechavi O, Erlich Y, Amram H, Flomenblit L, Karginov FV, Goldstein I, Hannon GJ, Kloog Y. Cell contact-dependent acquisition of cellular and viral nonautonomously encoded small RNAs. Genes Dev. 2009;23:1971–1979. doi: 10.1101/gad.1789609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsujiura M, Ichikawa D, Komatsu S, Shiozaki A, Takeshita H, Kosuga T, Konishi H, Morimura R, Deguchi K, Fujiwara H, Okamoto K, Otsuji E. Circulating microRNAs in plasma of patients with gastric cancers. Br J Cancer. 2010;102:1174–1179. doi: 10.1038/sj.bjc.6605608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morimura R, Komatsu S, Ichikawa D, Takeshita H, Tsujiura M, Nagata H, Konishi H, Shiozaki A, Ikoma H, Okamoto K, Ochiai T, Taniguchi H, Otsuji E. Novel diagnostic value of circulating miR-18a in plasma of patients with pancreatic cancer. Br J Cancer. 2011;105:1733–1740. doi: 10.1038/bjc.2011.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Komatsu S, Ichikawa D, Takeshita H, Konishi H, Nagata H, Hirajima S, Kawaguchi T, Arita T, Shiozaki A, Fujiwara H, Okamoto K, Otsuji E. Prognostic impact of circulating miR-21 and miR-375 in plasma of patients with esophageal squamous cell carcinoma. Expert Opin Biol Ther. 2012;12(Suppl 1):S53–59. doi: 10.1517/14712598.2012.681373. [DOI] [PubMed] [Google Scholar]
- 29.Hirajima S, Komatsu S, Ichikawa D, Takeshita H, Konishi H, Shiozaki A, Morimura R, Tsujiura M, Nagata H, Kawaguchi T, Arita T, Kubota T, Fujiwara H, Okamoto K, Otsuji E. Clinical impact of circulating miR-18a in plasma of patients with oesophageal squamous cell carcinoma. Br J Cancer. 2013;108:1822–1829. doi: 10.1038/bjc.2013.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawaguchi T, Komatsu S, Ichikawa D, Morimura R, Tsujiura M, Konishi H, Takeshita H, Nagata H, Arita T, Hirajima S, Shiozaki A, Ikoma H, Okamoto K, Ochiai T, Taniguchi H, Otsuji E. Clinical impact of circulating miR-221 in plasma of patients with pancreatic cancer. Br J Cancer. 2013;108:361–369. doi: 10.1038/bjc.2012.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Komatsu S, Ichikawa D, Hirajima S, Kawaguchi T, Miyamae M, Okajima W, Ohashi T, Arita T, Konishi H, Shiozaki A, Fujiwara H, Okamoto K, Yagi N, Otsuji E. Plasma microRNA profiles: identification of miR-25 as a novel diagnostic and monitoring biomarker in oesophageal squamous cell carcinoma. Br J Cancer. 2014;111:1614–1624. doi: 10.1038/bjc.2014.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Komatsu S, Ichikawa D, Miyamae M, Kawaguchi T, Morimura R, Hirajima S, Okajima W, Ohashi T, Imamura T, Konishi H, Shiozaki A, Ikoma H, Okamoto K, Taniguchi H, Otsuji E. Malignant potential in pancreatic neoplasm; new insights provided by circulating miR-223 in plasma. Expert Opin Biol Ther. 2015;15:773–785. doi: 10.1517/14712598.2015.1029914. [DOI] [PubMed] [Google Scholar]
- 33.Miyamae M, Komatsu S, Ichikawa D, Kawaguchi T, Hirajima S, Okajima W, Ohashi T, Imamura T, Konishi H, Shiozaki A, Morimura R, Ikoma H, Ochiai T, Okamoto K, Taniguchi H, Otsuji E. Plasma microRNA profiles: identification of miR-744 as a novel diagnostic and prognostic biomarker in pancreatic cancer. Br J Cancer. 2015;113:1467–1476. doi: 10.1038/bjc.2015.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsujiura M, Komatsu S, Ichikawa D, Shiozaki A, Konishi H, Takeshita H, Moriumura R, Nagata H, Kawaguchi T, Hirajima S, Arita T, Fujiwara H, Okamoto K, Otsuji E. Circulating miR-18a in plasma contributes to cancer detection and monitoring in patients with gastric cancer. Gastric Cancer. 2015;18:271–279. doi: 10.1007/s10120-014-0363-1. [DOI] [PubMed] [Google Scholar]
- 35.Kawaguchi T, Komatsu S, Ichikawa D, Tsujiura M, Takeshita H, Hirajima S, Miyamae M, Okajima W, Ohashi T, Imamura T, Kiuchi J, Konishi H, Shiozaki A, Okamoto K, Otsuji E. Circulating MicroRNAs: a next-generation clinical biomarker for digestive system cancers. Int J Mol Sci. 2016;17:1459. doi: 10.3390/ijms17091459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Komatsu S, Ichikawa D, Kawaguchi T, Miyamae M, Okajima W, Ohashi T, Imamura T, Kiuchi J, Konishi H, Shiozaki A, Fujiwara H, Okamoto K, Otsuji E. Circulating miR-21 as an independent predictive biomarker for chemoresistance in esophageal squamous cell carcinoma. Am J Cancer Res. 2016;6:1511–1523. [PMC free article] [PubMed] [Google Scholar]
- 37.Komatsu S, Ichikawa D, Kawaguchi T, Takeshita H, Miyamae M, Ohashi T, Okajima W, Imamura T, Kiuchi J, Arita T, Konishi H, Shiozaki A, Fujiwara H, Okamoto K, Otsuji E. Plasma microRNA profiles: identification of miR-23a as a novel biomarker for chemoresistance in esophageal squamous cell carcinoma. Oncotarget. 2016;7:62034–62048. doi: 10.18632/oncotarget.11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okajima W, Komatsu S, Ichikawa D, Miyamae M, Kawaguchi T, Hirajima S, Ohashi T, Imamura T, Kiuchi J, Arita T, Konishi H, Shiozaki A, Moriumura R, Ikoma H, Okamoto K, Taniguchi H, Itoh Y, Otsuji E. Circulating microRNA profiles in plasma: identification of miR-224 as a novel diagnostic biomarker in hepatocellular carcinoma independent of hepatic function. Oncotarget. 2016;7:53820–53836. doi: 10.18632/oncotarget.10781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kosaka N, Iguchi H, Yoshioka Y, Hagiwara K, Takeshita F, Ochiya T. Competitive interactions of cancer cells and normal cells via secretory microRNAs. J Biol Chem. 2012;287:1397–1405. doi: 10.1074/jbc.M111.288662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiuchi J, Komatsu S, Imamura T, Nishibeppu K, Shoda K, Arita T, Kosuga T, Konishi H, Shiozaki A, Okamoto K, Fujiwara H, Ichikawa D, Otsuji E. Low levels of tumour suppressor miR-655 in plasma contribute to lymphatic progression and poor outcomes in oesophageal squamous cell carcinoma. Mol Cancer. 2019;18:2. doi: 10.1186/s12943-018-0929-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imamura T, Komatsu S, Ichikawa D, Miyamae M, Okajima W, Ohashi T, Kiuchi J, Nishibeppu K, Konishi H, Shiozaki A, Morimura R, Ikoma H, Ochiai T, Okamoto K, Taniguchi H, Otsuji E. Depleted tumor suppressor miR-107 in plasma relates to tumor progression and is a novel therapeutic target in pancreatic cancer. Sci Rep. 2017;7:5708. doi: 10.1038/s41598-017-06137-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sobin LH, Compton CC. TNM seventh edition: what’s new, what’s changed: communication from the International Union Against Cancer and the American Joint Committee on Cancer. Cancer. 2010;116:5336–5339. doi: 10.1002/cncr.25537. [DOI] [PubMed] [Google Scholar]
- 43.Zheng B, Liang L, Wang C, Huang S, Cao X, Zha R, Liu L, Jia D, Tian Q, Wu J, Ye Y, Wang Q, Long Z, Zhou Y, Du C, He X, Shi Y. MicroRNA-148a suppresses tumor cell invasion and metastasis by downregulating ROCK1 in gastric cancer. Clin Cancer Res. 2011;17:7574–7583. doi: 10.1158/1078-0432.CCR-11-1714. [DOI] [PubMed] [Google Scholar]
- 44.Wang HJ, Ruan HJ, He XJ, Ma YY, Jiang XT, Xia YJ, Ye ZY, Tao HQ. MicroRNA-101 is down-regulated in gastric cancer and involved in cell migration and invasion. Eur J Cancer. 2010;46:2295–2303. doi: 10.1016/j.ejca.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 45.Tsai KW, Wu CW, Hu LY, Li SC, Liao YL, Lai CH, Kao HW, Fang WL, Huang KH, Chan WC, Lin WC. Epigenetic regulation of miR-34b and miR-129 expression in gastric cancer. Int J Cancer. 2011;129:2600–2610. doi: 10.1002/ijc.25919. [DOI] [PubMed] [Google Scholar]
- 46.Gao P, Xing AY, Zhou GY, Zhang TG, Zhang JP, Gao C, Li H, Shi DB. The molecular mechanism of microRNA-145 to suppress invasion-metastasis cascade in gastric cancer. Oncogene. 2013;32:491–501. doi: 10.1038/onc.2012.61. [DOI] [PubMed] [Google Scholar]
- 47.Zhang L, Liu X, Jin H, Guo X, Xia L, Chen Z, Bai M, Liu J, Shang X, Wu K, Pan Y, Fan D. miR-206 inhibits gastric cancer proliferation in part by repressing cyclinD2. Cancer Lett. 2013;332:94–101. doi: 10.1016/j.canlet.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 48.Imamura T, Komatsu S, Ichikawa D, Miyamae M, Okajima W, Ohashi T, Kiuchi J, Nishibeppu K, Kosuga T, Konishi H, Shiozaki A, Okamoto K, Fujiwara H, Otsuji E. Low plasma levels of miR-101 are associated with tumor progression in gastric cancer. Oncotarget. 2017;8:106538–106550. doi: 10.18632/oncotarget.20860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang SH, Li X, Zhou LS, Cao ZW, Shi C, Zhou CZ, Wen YG, Shen Y, Li JK. microRNA-148a suppresses human gastric cancer cell metastasis by reversing epithelial-to-mesenchymal transition. Tumour Biol. 2013;34:3705–3712. doi: 10.1007/s13277-013-0954-1. [DOI] [PubMed] [Google Scholar]
- 50.Zhang JP, Zeng C, Xu L, Gong J, Fang JH, Zhuang SM. MicroRNA-148a suppresses the epithelial-mesenchymal transition and metastasis of hepatoma cells by targeting Met/Snail signaling. Oncogene. 2014;33:4069–4076. doi: 10.1038/onc.2013.369. [DOI] [PubMed] [Google Scholar]
- 51.Liffers ST, Munding JB, Vogt M, Kuhlmann JD, Verdoodt B, Nambiar S, Maghnouj A, Mirmohammadsadegh A, Hahn SA, Tannapfel A. MicroRNA-148a is down-regulated in human pancreatic ductal adenocarcinomas and regulates cell survival by targeting CDC25B. Lab Invest. 2011;91:1472–1479. doi: 10.1038/labinvest.2011.99. [DOI] [PubMed] [Google Scholar]
- 52.Li HP, Huang HY, Lai YR, Huang JX, Chang KP, Hsueh C, Chang YS. Silencing of miRNA-148a by hypermethylation activates the integrin-mediated signaling pathway in nasopharyngeal carcinoma. Oncotarget. 2014;5:7610–7624. doi: 10.18632/oncotarget.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joshi P, Jeon YJ, Laganà A, Middleton J, Secchiero P, Garofalo M, Croce CM. MicroRNA-148a reduces tumorigenesis and increases TRAIL-induced apoptosis in NSCLC. Proc Natl Acad Sci U S A. 2015;112:8650–8655. doi: 10.1073/pnas.1500886112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xue J, Chen Z, Gu X, Zhang Y, Zhang W. MicroRNA-148a inhibits migration of breast cancer cells by targeting MMP-13. Tumour Biol. 2016;37:1581–1590. doi: 10.1007/s13277-015-3926-9. [DOI] [PubMed] [Google Scholar]
- 55.Ma L, Xu Z, Xu C, Jiang X. MicroRNA-148a represents an independent prognostic marker in bladder cancer. Tumour Biol. 2016;37:7915–7920. doi: 10.1007/s13277-015-4688-0. [DOI] [PubMed] [Google Scholar]
- 56.Bhattacharya S, Chalk AM, Ng AJ, Martin TJ, Zannettino AC, Purton LE, Lu J, Baker EK, Walkley CR. Increased miR-155-5p and reduced miR-148a-3p contribute to the suppression of osteosarcoma cell death. Oncogene. 2016;35:5282–5294. doi: 10.1038/onc.2016.68. [DOI] [PubMed] [Google Scholar]
- 57.Sakamoto N, Naito Y, Oue N, Sentani K, Uraoka N, Oo HZ, Yanagihara K, Aoyagi K, Sasaki H, Yasui W. MicroRNA-148a is downregulated in gastric cancer, targets MMP7, and indicates tumor invasiveness and poor prognosis. Cancer Sci. 2014;105:236–243. doi: 10.1111/cas.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hummel R, Watson DI, Smith C, Kist J, Michael MZ, Haier J, Hussey DJ. Mir-148a improves response to chemotherapy in sensitive and resistant oesophageal adenocarcinoma and squamous cell carcinoma cells. J Gastrointest Surg. 2011;15:429–438. doi: 10.1007/s11605-011-1418-9. [DOI] [PubMed] [Google Scholar]
- 59.Fujita Y, Kojima K, Ohhashi R, Hamada N, Nozawa Y, Kitamoto A, Sato A, Kondo S, Kojima T, Deguchi T, Ito M. MiR-148a attenuates paclitaxel resistance of hormone-refractory, drug-resistant prostate cancer PC3 cells by regulating MSK1 expression. J Biol Chem. 2010;285:19076–19084. doi: 10.1074/jbc.M109.079525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ashizawa M, Okayama H, Ishigame T, Thar Min AK, Saito K, Ujiie D, Murakami Y, Kikuchi T, Nakayama Y, Noda M, Tada T, Endo H, Fujita S, Sakamoto W, Saito M, Saze Z, Momma T, Ohki S, Mimura K, Kono K. miRNA-148a-3p regulates immunosuppression in DNA mismatch repair-deficient colorectal cancer by targeting PD-L1. Mol Cancer Res. 2019;17:1403–1413. doi: 10.1158/1541-7786.MCR-18-0831. [DOI] [PubMed] [Google Scholar]
- 61.Lanford RE, Hildebrandt-Eriksen ES, Petri A, Persson R, Lindow M, Munk ME, Kauppinen S, Orum H. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327:198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gebert LF, Rebhan MA, Crivelli SE, Denzler R, Stoffel M, Hall J. Miravirsen (SPC3649) can inhibit the biogenesis of miR-122. Nucleic Acids Res. 2014;42:609–621. doi: 10.1093/nar/gkt852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bouchie A. First microRNA mimic enters clinic. Nat Biotechnol. 2013;31:577. doi: 10.1038/nbt0713-577. [DOI] [PubMed] [Google Scholar]
- 64.Chen Y, Zhu X, Zhang X, Liu B, Huang L. Nanoparticles modified with tumor-targeting scFv deliver siRNA and miRNA for cancer therapy. Mol Ther. 2010;18:1650–1656. doi: 10.1038/mt.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hatakeyama H, Murata M, Sato Y, Takahashi M, Minakawa N, Matsuda A, Harashima H. The systemic administration of an anti-miRNA oligonucleotide encapsulated pH-sensitive liposome results in reduced level of hepatic microRNA-122 in mice. J Control Release. 2013;173:43–50. [PubMed] [Google Scholar]
- 66.Takahashi M, Yamada N, Hatakeyama H, Murata M, Sato Y, Minakawa N, Harashima H, Matsuda A. In vitro optimization of 2’-OMe-4’-thioribonucleoside-modified anti-microRNA oligonucleotides and its targeting delivery to mouse liver using a liposomal nanoparticle. Nucleic Acids Res. 2013;41:10659–10667. doi: 10.1093/nar/gkt823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakase I, Noguchi K, Fujii I, Futaki S. Vectorization of biomacromolecules into cells using extracellular vesicles with enhanced internalization induced by macropinocytosis. Sci Rep. 2016;6:34937. doi: 10.1038/srep34937. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.