Abstract
Combined immune checkpoint inhibitors (ICIs) along with tyrosine kinase inhibitors (TKIs) and locoregional therapies have been used increasingly to treat hepatocellular carcinoma (HCC). Biomarkers are required to predict the treatment efficacy of ICIs with or without combination therapies in patients with unresectable HCC. This study enrolled 95 consecutive patients with unresectable HCC from May 2017 to June 2021 from two hospitals retrospectively. Of the 95 patients, 15 and 80 had Barcelona Clinic Liver Cancer stages B and C, respectively. The median ICI treatment duration was 3.43 (1.87-7.87) months, and 77 patients received combination therapies. Radiological imaging was not performed in 13 patients. Objective response and disease control rates were 27.4% and 53.7%, respectively. The duration of progression-free survival (PFS) and overall survival (OS) was 4.07 (1.59-6.54) months and 14.53 (6.93-22.14) months, respectively. Alpha-fetoprotein (AFP) response was defined as a decline of >15% in the serum AFP level within the initial 3 months of ICI therapy according to Youden’s index. AFP response was determined to be a predictor of disease control (odds ratio: 11.657, 95% confidence interval [CI]: 2.834-47.941, P=.001). Macrovascular invasion (MVI), AFP response (hazard ratio [HR]: 0.488, 95% CI: 0.255-0.934, P=.030), combination therapy, and disease control were predictors of PFS, and MVI, AFP response (HR: 0.344, 95% CI: 0.160-0.737, P=.006), and disease control were predictors of OS. AFP response was a predictor of disease control, PFS, and OS. These findings indicate that AFP response can serve as a biomarker to predict treatment outcomes in patients with unresectable HCC receiving ICIs with or without TKIs or locoregional therapies.
Keywords: AFP response, hepatocellular carcinoma, immune checkpoint inhibitor, tyrosine kinase inhibitor, locoregional therapy, survival
Introduction
Immune checkpoint inhibitors (ICIs) are an emerging treatment option for hepatocellular carcinoma (HCC) [1,2]. However, the outcomes of second-line ICI monotherapy are unsatisfactory, and hence, combination therapies with ICIs have become a trend for treating HCC [3]. In the nivolumab plus ipilimumab cohort of the CheckMate 040 study, patients with advanced HCC who were previously treated with sorafenib and administered a combination of nivolumab (1 mg/kg) and ipilimumab (3 mg/kg) every 3 weeks for a total of four doses followed by nivoluimab (240 mg) every 2 weeks exhibited a longer median overal survival (OS) duration of 22.8 months (95% confidence interval [CI]: 9.4-not reached) [4]. In the IMbrave150 trial, patients with unresectable HCC who received a combination of atezolizumab and bevacizumab had longer progression-free survival (PFS; hazard ratio [HR]: 0.59) and OS (HR: 0.58) than did those who received sorafenib [5]. Thus, the combination of atezolizumab and bevacizumab has become the benchmark for first-line systemic HCC therapy. In addition, other combinations, lenvatinib plus pembrolizumab [6] and tremelimumab plus duralumab [7], have exhibited promising results.
The cost of combination therapy with ICIs may not be affordable for some patients because of tight finances or their insurance reimbursement policy. Therefore, tyrosine kinase inhibitors (TKIs) and locoregional therapies for HCC, including radiofrequency ablation (RFA), transarterial chemoembolization (TACE), and stereotactic body radiotherapy (SBRT), have been used in concurrent or sequential combination with ICIs in real-world practice.
Biomarkers are required to predict the treatment efficacy of ICIs with or without combination therapy in patients with unresectable HCC. Serum α-fetoprotein (AFP), a secreted glycoprotein by HCC cells, could indirectly reflect tumor burden in patients with HCC under treatment [8,9]. Previous studies have demonstrated an association between a >10% or >20% decline in the serum AFP level within the first 4 or 12 weeks of ICI treatment and more favorable treatment efficacy [10-12]. However, whether a decline in the AFP level can predict treatment efficacy in patients with unresectable HCC receiving ICIs with or without TKIs or locoregional therapies remains unclear. Hence, this study investigated whether a decline in the AFP level can predict treatment response in patients with unresectable HCC and identified other potential predictors of disease control, PFS, and OS in this patient population.
Patients and methods
Patients
This retrospective study enrolled 128 consecutive patients with unresectable HCC who received at least one dose of nivolumab or pembrolizumab at China Medical University Hospital and Asia University Hospital in central Taiwan between May 2017 and June 2021. Patients who had early or terminal stage HCC, a malignancy other than HCC, no record of a decline in AFP level, undergone liver transplantation, or human immunodeficiency virus infection were excluded. Of the 95 patients included in the final analysis, 82 had evaluable radiological imaging; 10 died and 3 were lost to follow-up before the first radiological assessment (Supplementary Figure 1).
Hematologic and biochemical values, virological features, comorbidities, and tumoral characteristics were recorded at baseline. The AFP level was recorded at baseline and 4, 8, and 12 weeks and then every 2 to 3 months after the initiation of ICI therapy. The AFP kinetics in the first 3 months of ICI therapy was determined by the maximal difference between AFP at baseline and 4, 8, or 12 weeks after the initiation of ICI therapy. In addition, information regarding combination therapies with ICIs including TKIs and locoregional therapies (RFA, TACE, and SBRT) was recorded. This study was performed in accordance with the 1975 Declaration of Helsinki. This study was approved by the Research Ethics Committee of China Medical University Hospital, Taichung, Taiwan (CMUH108-REC3-140). Each patient’s identification number was encrypted to protect their privacy; thus, the need for informed consent was waived.
ICI and TKI doses, locoregional therapies, tumor assessment, and safety
Per the protocols of previous studies, the doses of ICIs were administered (2-3 mg/kg every 2 weeks for nivolumab and every 3 weeks for pembrolizumab). The doses of sorafenib and lenvatinib were 400-800 mg and 8-12 mg per day, respectively, and the dose of regorafenib was 80 mg per day or 120-160 mg per day for the first 21 days of each 28-day cycle. The patients receiving a combination of an ICI and a TKI for more than 7 days were considered to be receiving combined TKI therapy. One patient received real-time ultrasound-guided RFA (Covidien, Dublin, Ireland) for three tumors (1.3-2.0 cm in size) 10 days after initiating nivolumab therapy. The patients with HCC with Child-Pugh classification A or B and main portal vein patency or main portal vein thrombosis with cavernous transformation were considered to be eligible for TACE. Combined radiotherapy was defined as overlapping ICI therapy with SBRT for HCC. The detailed procedures of TACE [13] and SBRT [14] have been described previously.
Tumor response was evaluated by performing dynamic computed tomography per 8 to 12 weeks according to the Modified Response Evaluation Criteria in Solid Tumors (mRECIST) [15]. Patients with objective response were defined as patients with complete response (CR) or partial response (PR), and patients with disease control were defined as patients with CR, PR, or stable disease (SD). Safety was evaluated following the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03).
Laboratory tests
Blood biochemistry tests (Beckman Coulter, CA, USA) and complete blood count analyses (Sysmex HST series, Kanagawa, Japan) were performed in the central laboratory of the hospitals. The presence of the serum hepatitis B surface antigen for more than 6 months defined hepatitis B virus (HBV) infection, and the presence of the serum anti-HCV antibody for more than 6 months and detectable HCV RNA defined hepatitis C virus (HCV) infection. Liver cirrhosis was defined based on unequivocal clinical, ultrasonographic, or histological analysis.
Statistical analysis
Continuous variables are presented as the median (interquartile range), PFS and OS are presented as the median (95% CI), and categorical variables are presented as the frequency (percentage). Between-group comparisons of continuous variables were performed using the Mann-Whitney U test. The predictive performance of serum AFP kinetics for disease control was examined by performing area under the receiver operating characteristic (AUROC) curve analysis. Youden’s index was used to identify the optimal cutoff point for a decrease in the serum AFP level within the initial 3 months of ICI therapy. Logistic regression analysis was performed to identify factors associated with disease control, and Cox regression analysis was performed to identify factors associated with PFS or OS. Variables with P<.20 in the univariate analysis were subjected to multivariate logistic or Cox regression analysis to determine their association with disease control, PFS, or OS. Kaplan-Meier analysis with the log-rank test was used to compare PFS and OS between patient subgroups. The formula of total tumor volume (TTV) was (4/3) × 3.14 × (radius of the tumor in cm)3 [16]. The software for statistical analyses was SPSS (IBM SPSS 25.0, NY, USA). Statistical significance was defined as a P value of <.05 (two-sided).
Results
Baseline characteristics
The median age of the 95 patients was 63.8 (55.6-70.4) years, and 84 (88.4%) of the 95 patients were men. Furthermore, 15 (15.8%) and 80 (84.2%) patients had Barcelona Clinic Liver Cancer (BCLC) stages B and C, respectively. In total, 48 (50.5%), 25 (26.3%), 25 (26.3%), and 32 (33.7%) patients reported having HBV infection, having HCV infection, drinking alcohol, and having diabetes mellitus (DM), respectively. The median neutrophil-to-lymphocyte ratio (NLR), aspartate aminotransferase (AST) level, alanine aminotransferase (ALT) level, total bilirubin level, albumin level, international normalized ratio, and AFP level were 4.52 (2.87-7.78), 55 (34-94) U/L, 41 (26-60) U/L, 1.1 (0.7-1.6) mg/dL, 3.7 (3.2-4.0) g/dL, 1.08 (1.03-1.16), and 114.53 (10.15-7601.00) ng/mL, respectively. The median Child-Pugh score was 6 (5-7), and the median Cancer of the Liver Italian Program score was 2 (1-3). The maximum tumor size was 4.7 (2.5-8.6) cm. The TTV was 767.2 (127.0-3689.0) cm3. Extrahepatic metastasis (EHM) and macrovascular invasion (MVI) were observed in 58 (61.1%) and 54 (56.8%) patients, respectively. A small proportion of patients (n=20, 21.1%) received ICIs as the first-line systemic therapy. Most of the patients received combination therapies (n=77, 81.1%), and 63 (66.3%) patients received a combination of ICIs and TKIs. The most commonly administered combined TKI was sorafenib (n=34, 35.8%), followed by lenvatinib (n=33, 34.7%). A total of 21 (22.1%) and 22 (23.2%) patients received combined ICIs with TACE and SBRT for HCC, respectively (Table 1).
Table 1.
Patient demographics, baseline characteristics, and therapeutic responses
| Character | All (n=95) | With AFP response (n=46) | Without AFP response (n=49) | P value |
|---|---|---|---|---|
| Age (years) | 63.8 (55.6-70.4) | 64.3 (56.6-72.0) | 61.3 (51.9-68.6) | .107 |
| Sex (male), n (%) | 84 (88.4) | 38 (82.6) | 46 (93.9) | .088 |
| Body mass index (kg/m2) | 24.34 (21.28-27.02) | 25.41 (21.57-28.06) | 23.13 (21.12-25.77) | .041 |
| NLR | 4.52 (2.87-7.78) | 3.53 (2.21-6.22) | 5.23 (3.35-7.85) | .028 |
| Platelet count (× 109/L) | 156 (103-233) | 155 (102-228) | 157 (103-238) | .961 |
| AST (U/L) | 55 (34-94) | 43 (31-74) | 69 (41-110) | .007 |
| ALT (U/L) | 41 (26-60) | 34 (24-57) | 51 (29-70) | .032 |
| Total bilirubin (mg/dL) | 1.1 (0.7-1.6) | 0.9 (0.6-1.3) | 1.2 (0.7-1.7) | .063 |
| Albumin (g/dL) | 3.7 (3.2-4.0) | 3.8 (3.2-4.1) | 3.5 (3.1-3.9) | .190 |
| INR | 1.08 (1.03-1.16) | 1.07 (1.02-1.15) | 1.09 (1.04-1.18) | .363 |
| Etiology | ||||
| Alcohol | 25 (26.3) | 10 (21.7) | 15 (30.6) | .329 |
| HBV | 48 (50.5) | 19 (41.3) | 29 (59.2) | .083 |
| HCV | 25 (26.3) | 15 (32.6) | 10 (20.4) | .179 |
| Diabetes mellitus | 32 (33.7) | 21 (45.7) | 11 (22.4) | .017 |
| Liver cirrhosis | 66 (69.5) | 30 (65.2) | 36 (73.5) | .385 |
| Child-Pugh score | 6 (5-7) | 6 (5-7) | 6 (5-7) | .145 |
| Class A/B | 62 (66.0)/32 (34.0) | 32 (69.6)/14 (30.4) | 30 (62.5)/18 (37.5) | .472 |
| ALBI grade 1/2/3 | 24 (25.8)/59 (63.4)/10 (10.8) | 15 (32.6)/28 (60.9)/3 (6.5) | 9 (19.1)/31 (66.0)/7 (14.9) | .076 |
| AFP (ng/mL) | 114.53 (10.15-7601.00) | 79.68 (11.80-1387.42) | 343.5 (8.85-13740.00) | .260 |
| AFP ≥400 ng/mL | 38 (40.0) | 14 (30.4) | 24 (49.0) | .067 |
| BCLC stage B/C | 15 (15.8)/80 (84.2) | 7 (15.2)/39 (84.8) | 8 (16.3)/41 (83.7) | .883 |
| CLIP score | 2 (1-3) | 2 (1-3) | 3 (2-4) | .080 |
| Max. tumor size (cm) | 4.7 (2.5-8.6) | 4.4 (2.2-8.7) | 5.0 (2.5-9.14) | .726 |
| Total tumor volume (cm3) | 767.2 (127.0-3689.0) | 462.3 (82.4-3711.9) | 986.9 (214.2-3766.9) | .308 |
| MVIa | 54 (56.8) | 24 (52.2) | 30 (61.2) | .376 |
| VP3/VP4/hepatic vein | 19 (20.0)/32 (33.7)/3 (3.2) | 7 (15.2)/16 (34.8)/1 (2.2) | 12 (24.5)/16 (32.7)/2 (4.1) | |
| EHMa | 58 (61.1) | 25 (54.3) | 33 (67.3) | .196 |
| Prior therapy | ||||
| Sorafenib | 61 (64.2) | 25 (54.3) | 36 (73.5) | |
| Lenvatinib | 16 (16.8) | 9 (19.6) | 7 (14.3) | |
| Surgery | 20 (21.1) | 8 (17.4) | 12 (24.5) | |
| PEI/RFA | 5 (5.3)/13 (13.7) | 3 (6.5)/6 (13.0) | 2 (4.1)/7 (14.3) | |
| TACEb/TARE | 60 (63.2)/2 (2.1) | 28 (60.9)/1 (2.2) | 32 (65.3)/1 (2.0) | |
| Radiotherapy | 63 (66.3) | 31 (67.4) | 32 (65.3) | |
| ICI duration (months) | 3.43 (1.87-7.87) | 6.45 (2.91-11.59) | 2.40 (1.52-3.97) | <.001 |
| Nivolumabc | 83 (87.4) | 37 (80.4) | 46 (93.9) | |
| Pembrolizumabc | 14 (14.7) | 10 (21.7) | 4 (8.2) | |
| Reduction >25% | 34 (35.8) | 23 (50.0) | 11 (22.4) | |
| As 1st/2nd/3rd/4th-line systemic therapy | 20 (21.1)/57 (60.0)/12 (12.6)/6 (6.3) | 13 (28.3)/25 (54.3)/5 (10.9)/3 (6.5) | 7 (14.3)/32 (65.3)/7 (14.3)/3 (6.1) | |
| Combination therapy | 77 (81.1) | 39 (84.8) | 38 (77.6) | .371 |
| Sorafenibd | 34 (35.8) | 16 (34.8) | 18 (36.7) | |
| Lenvatinibd | 33 (34.7) | 20 (43.5) | 13 (26.5) | |
| Regorafenibd | 8 (8.4) | 2 (4.3) | 3 (6.1) | |
| Chemotherapy | 7 (7.4) | 4 (8.7) | 3 (6.1) | |
| RFA | 1 (1.1) | 0 (0) | 1 (2.0) | |
| TACE | 21 (22.1) | 15 (32.6) | 6 (12.2) | |
| SBRT for HCC | 22 (23.2) | 13 (28.3) | 9 (18.4) | |
| Therapeutic response | ||||
| Best Response | ||||
| Complete response | 7 (7.4) | 7 (15.2) | 0 (0) | |
| Partial response | 19 (20.0) | 14 (30.4) | 5 (10.2) | |
| Stable disease | 25 (26.3) | 16 (34.8) | 9 (18.4) | |
| Progressive disease | 31 (32.6) | 6 (13.0) | 25 (51.0) | |
| Not evaluable | ||||
| Death before evaluation | 10 (10.5) | 2 (4.3) | 8 (16.2) | |
| Lost to follow-upe | 3 (3.2) | 1 (2.2) | 2 (4.1) | |
| Objective response | 26 (27.4) | 21 (45.7) | 5 (10.2) | <.001 |
| Disease control | 51 (53.7) | 37 (80.4) | 14 (28.6) | <.001 |
| Progression-free survival (months)* | 4.07 (1.59-6.54) | 7.47 (4.57-10.6) | 2.33 (2.04-2.63) | <.001 |
| Overall survival (months)* | 14.53 (6.93-22.14) | 21.87 (11.35-32.39) | 5.60 (3.22-7.98) | <.001 |
Data are presented as the median (first quartile-third quartile).
Data are presented as the median (95% confidence interval).
AFP, α-fetoprotein; ALBI, albumin-bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BCLC, Barcelona Clinic Liver Cancer; EHM, extrahepatic metastasis; CLIP, Cancer of the Liver Italian Program; HBV, hepatitis B virus; HCV, hepatitis C virus; ICI, immune checkpoint inhibitor; IQR, interquartile range; MVI, macrovascular invasion; NLR, neutrophil-to-lymphocyte ratio; SBRT, stereotactic body radiotherapy; TACE, transarterial chemoembolization; TARE, transarterial radioembolization; TKI, tyrosine kinase inhibitor; PEI, percutaneous ethanol injection; INR, international normalized ratio; RFA, radiofrequency ablation.
A total of 32 patients with HCC had both macrovascular invasion and extrahepatic metastasis.
The median number of TACE sessions was 3 (2-6).
A total of four patients received sequential ICI therapy because of progressive disease: nivolumab→atezolizumab plus bevacizumab (2) and nivolumab→pembrolizumab (2).
A total of 14 patients received sequential TKI therapy because of progressive disease: sorafenib→regorafenib (4), sorafenib→lenvatinib (3), sorafenib→regorafenib→lenvatinib (2), lenvatinib→sorafenib (1), and lenvatinib→cabozantinib (4).
A total of three patients were lost to follow-up because of immune-related adverse events (n=2) and financial reasons (n=1).
Therapeutic response
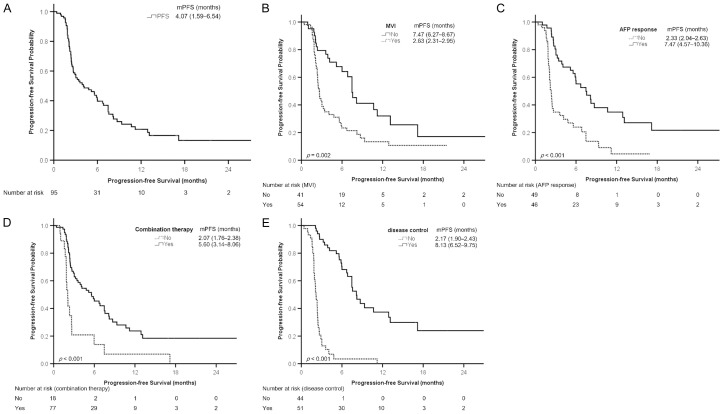
The median treatment duration of ICIs was 3.43 (1.87-7.87) months. A total of 13 patients did not undergo radiological imaging; among them, 10 (10.5%) died before the evaluation, and 3 (3.2%) were lost to follow-up because of treatment-related adverse events (TRAEs; n=2) and financial reasons (n=1). The numbers of the patients with CR, PR, SD, and progressive disease (PD) were 7 (7.4%), 19 (20.0%), 25 (26.3%), and 31 (32.6%), respectively. The objective response (CR+PR) and disease control (CR+PR+SD) rates were 27.4% (26/95) and 53.7% (51/95), respectively. The durations of PFS and OS were 4.07 (1.59-6.54) months and 14.53 (6.93-22.14) months, respectively (Figures 1A and 2A; Table 1).
Figure 1.
Kaplan-Meier analysis of progression-free survival. A. All patients. B. Patients with or without macrovascular invasion (MVI). C. Patients with or without AFP response. D. Patients with or without combination therapy. E. Patients with or without disease control. Survival is presented as the median (95% confidence interval). AFP, α-fetoprotein protein; mPFS, median progression-free survival.
Figure 2.
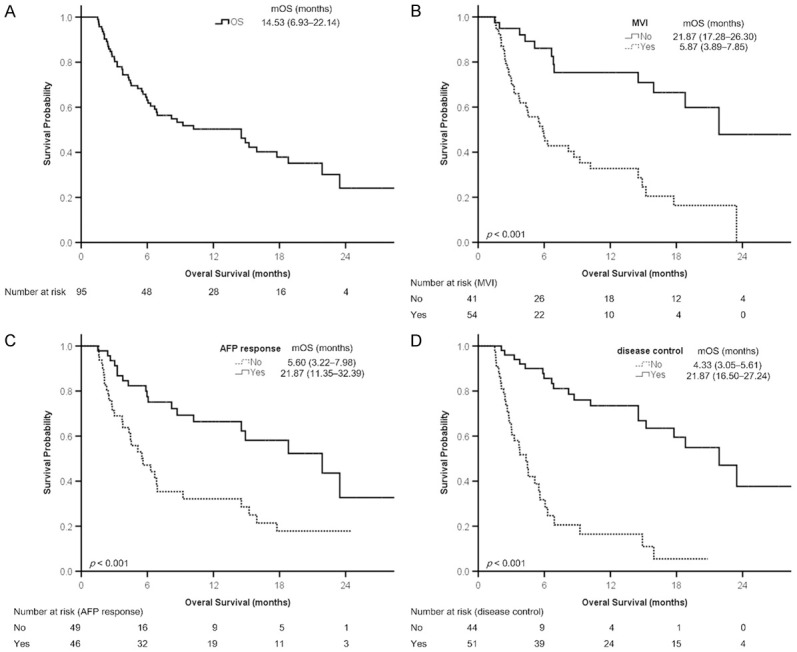
Kaplan-Meier analysis of overall survival. A. All patients. B. Patients with or without macrovascular invasion (MVI). C. Patients with or without AFP response. D. Patients with or without disease control. Survival is presented as the median (95% confidence interval). AFP, α-fetoprotein protein; mOS, median overall survival.
More than half patients (n=66, 69.5%) patients experienced at least one TRAE of any grade. A total of 16 patients experienced ≥ grade 3 TRAEs, namely hepatitis (n=7), dermatitis (n=4), pneumonitis (n=4), colitis (n=2), hand-foot syndrome (n=2), fatigue (n=1), fever (n=1), and gastric necrosis (n=1). Three and two patients died from severe hepatitis and pneumonitis, respectively (Supplementary Table 1).
Defining the cutoff value for AFP response
We investigated the effects of serum AFP kinetics on disease control. The AUROC curve was 0.771. According to Youden’s index, AFP response was defined as a decline of >15% in the AFP level within the initial 3 months of ICI therapy. Previous studies have reported that declines of >10% [11] and >20% [10,12] in the AFP level were associated with treatment outcomes, and the sensitivity, specificity, and Youden’s index of the three cutoff values (>10%, >15%, and >20%) were similar (Supplementary Table 2). Therefore, AFP response was defined as a decline of >15% in the AFP level according to the highest Youden’s index. In addition, declines of >10% and >20% in the AFP level were analyzed in separate logistic and Cox regression analyses.
Compared with the patients without AFP response (n=49), the patients with AFP response (n=46) had a higher body mass index; lower levels of NLR, AST, and ALT; a higher proportion of DM, objective response, and disease control; and longer ICI treatment duration, PFS, and OS (Table 1).
AFP response was the only independent predictor of disease control (CR+PR+SD)
Among the 82 patients who underwent radiological imaging, univariate logistic regression analysis identified age, grade 1-2 TRAEs, MVI, AFP levels at baseline (≥400 vs. <400 ng/mL), NLR (>3.0 vs. ≤3.0), and AFP response as significantly associated with disease control. The findings of multivariate logistic regression analysis indicated that AFP response (OR: 11.657, 95% CI: 2.834-47.941, P=.001) was the only independent predictor of disease control (Table 2). Declines of >10% (Supplementary Table 3) and >20% (Supplementary Table 4) in the AFP level were independent predictors of disease control in separate analyses.
Table 2.
Factors associated with disease control in 82 patients with HCC who underwent radiological imaging
| Character | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
|
|
|
||||
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age (year) | 1.064 (1.016-1.113) | .008 | |||
| Sex | Male vs. female | 0.371 (0.073-1.872) | .230 | ||
| Alcohol | Yes vs. no | 0.752 (0.274-2.065) | .580 | ||
| HBV | Yes vs. no | 0.452 (0.180-1.133) | .090 | ||
| HCV | Yes vs. no | 2.600 (0.848-7.971) | .095 | ||
| DM | Yes vs. no | 1.247 (0.486-3.202) | .647 | ||
| Grade 1-2 TRAEs | Yes vs. no | 2.769 (1.103-6.954) | .030 | ||
| Grade ≥3 TRAEs | Yes vs. no | 1.256 (0.345-4.576) | .730 | ||
| TTV (cm3) | >1000 vs. ≤1000 | 0.633 (0.256-1.565) | .322 | ||
| MVI | Yes vs. no | 0.336 (0.130-0.870) | .025 | ||
| EHM | Yes vs. no | 0.498 (0.192-1.290) | .151 | ||
| AFP (ng/mL) | ≥400 vs. <400 | 0.273 (0.107-0.701) | .007 | ||
| AST (U/L) | >40 vs. ≤40 | 0.412 (0.142-1.197) | .103 | ||
| ALT (U/L) | >40 vs. ≤40 | 0.452 (0.180-1.133) | .090 | ||
| NLR | >3.0 vs. ≤3.0 | 0.205 (0.062-0.673) | .009 | ||
| Child-Pugh class | B vs. A | 0.757 (0.285-2.010) | .576 | ||
| ALBI grade | 2/3 vs. 1 | 0.571 (0.195-1.673) | .307 | ||
| AFP decline >15% | Yes vs. no | 11.012 (3.730-32.512) | <.001 | 11.657 (2.834-47.941) | .001 |
| Combination therapy* | Yes vs. no | 2.683 (0.769-9.359) | .121 | ||
Combination therapy includes tyrosine kinase inhibitors, radiofrequency ablation, transarterial chemoembolization, and stereotactic body radiotherapy for hepatocellular carcinoma.
AFP, α-fetoprotein; ALBI, albumin-bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DM, diabetes mellitus; EHM, extrahepatic metastasis; HBV, hepatitis B virus; HCV, hepatitis C virus; TRAEs, treatment-related adverse events; MVI, macroscopic vascular invasion; NLR, neutrophil-to-lymphocyte ratio; TTV, total tumor volume.
AFP response is a predictor of PFS
The results of univariate Cox regression analysis revealed that age, alcohol consumption, grade 1-2 TRAEs, TTV (>1000 vs. ≤1000 cm3), MVI, AFP levels at baseline (≥400 vs. <400 ng/mL), AST and ALT levels (>40 vs. ≤40 U/L), NLR (>3.0 vs. ≤3.0), Child-Pugh class (B vs. A), AFP response, combination therapy (including combined ICI therapy with TKIs, RFA, TACE, or SBRT for HCC vs. ICI monotherapy), and disease control were significantly associated with PFS among the 95 enrolled patients. The findings of multivariate Cox regression analysis indicated that MVI (HR: 3.182, 95% CI: 1.584-6.390, P=.001), AFP response (HR: 0.488, 95% CI 0.255-0.934, P=.030), combination therapy (HR: 0.250, 95% CI: 0.113-0.552, P=.001), and disease control (HR: 0.131, 95% CI: 0.056-0.303, P<.001) were independent predictors of PFS (Table 3). In addition, declines of >10% (Supplementary Table 5) and >20% (Supplementary Table 6) in the AFP level were independent predictors of PFS in separate analyses.
Table 3.
Factors associated with progression-free survival in 95 patients with unresectable hepatocellular carcinoma
| Character | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
|
|
|
||||
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (year) | 0.976 (0.955-0.998) | .034 | |||
| Sex | Male vs. female | 1.270 (0.607-2.659) | .526 | ||
| Alcohol | Yes vs. no | 2.004 (1.211-3.316) | .007 | ||
| HBV | Yes vs. no | 1.107 (0.690-1.777) | .673 | ||
| HCV | Yes vs. no | 0.641 (0.365-1.124) | .120 | ||
| DM | Yes vs. no | 0.814 (0.495-1.340) | .419 | ||
| Grade 1-2 TRAEs | Yes vs. no | 0.572 (0.356-0.919) | .021 | ||
| Grade ≥3 TRAEs | Yes vs. no | 1.455 (0.799-2.647) | .220 | ||
| TTV (cm3) | >1000 vs. ≤1000 | 1.641 (1.021-2.635) | .041 | ||
| MVI | Yes vs. no | 2.193 (1.323-3.635) | .002 | 3.182 (1.584-6.390) | .001 |
| EHM | Yes vs. no | 1.375 (0.837-2.260) | .209 | ||
| AFP (ng/mL) | <400 vs. ≥400 | 2.021 (1.246-3.278) | .004 | ||
| AST (U/L) | >40 vs. ≤40 | 1.939 (1.094-3.436) | .023 | ||
| ALT (U/L) | >40 vs. ≤40 | 1.644 (1.011-2.672) | .045 | ||
| NLR | >3.0 vs. ≤3.0 | 2.181 (1.221-3.895) | .008 | ||
| Child-Pugh class | B vs. A | 1.659 (1.018-2.704) | .042 | ||
| ALBI grade | 2/3 vs. 1 | 1.649 (0.936-2.905) | .084 | ||
| AFP decline >15% | Yes vs. no | 0.338 (0.206-0.556) | <.001 | 0.488 (0.255-0.934) | .030 |
| Combination therapy* | Yes vs. no | 0.363 (0.206-0.641) | <.001 | 0.250 (0.113-0.552) | .001 |
| Best response | CR+PR+SD vs. none | 0.112 (0.064-0.198) | <.001 | 0.131 (0.056-0.303) | <.001 |
Combination therapy includes tyrosine kinase inhibitors, radiofrequency ablation, transarterial chemoembolization, and stereotactic body radiotherapy for hepatocellular carcinoma.
AFP, α-fetoprotein; ALBI, albumin-bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DM, diabetes mellitus; EHM, extrahepatic metastasis; HBV, hepatitis B virus; HCV, hepatitis C virus; TRAEs, treatment-related adverse events; MVI, macroscopic vascular invasion; NLR, neutrophil-to-lymphocyte ratio; TTV, total tumor volume.
BCLC stage was not analyzed as a variable because mainly enrolled patients had BCLC stage C (n=80, 84.2%) confounded with MVI and EHM. The patients with objective response and disease control overlapped, and objective response did not reach statistical significance in multivariate analysis (data not shown). Therefore, we used disease control instead of objective response to prevent collinearity.
The results of Kaplan-Meier analysis revealed that the probability of PFS significantly differed between the patients with and without MVI (Figure 1B), those with and without AFP response (Figure 1C), those with and without combination therapy (Figure 1D), and those with and without disease control (Figure 1E).
AFP response is a predictor of OS
The results of univariate Cox regression analysis indicated that grade 1-2 TRAEs, TTV (>1000 vs. ≤1000 cm3), MVI, AFP levels at baseline (≥400 vs. <400 ng/mL), AST and ALT levels (>40 vs. ≤40 U/L), NLR (>3.0 vs. ≤3.0), Child-Pugh class (B vs. A), albumin-bilirubin grade (2/3 vs. 1), AFP response, combination therapy, and disease control were significantly associated with OS. The findings of multivariate Cox regression analysis indicated that MVI (HR: 4.313, 95% CI: 1.747-10.646, P=.002), AFP response (HR: 0.344, 95% CI 0.160-0.737, P=.006), and disease control (HR: 0.460, 95% CI: 0.216-0.981, P=.044) were independent predictors of OS (Table 4). In addition, declines of >10% (Supplementary Table 7) and >20% (Supplementary Table 8) in the AFP level were determined to be independent predictors of OS in separate analyses.
Table 4.
Factors associated with overall survival in 95 patients with unresectable hepatocellular carcinoma
| Character | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
|
|
|
||||
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (year) | 0.985 (0.959-1.012) | .266 | |||
| Sex | Male vs. female | 2.416 (0.751-7.771) | .139 | ||
| Alcohol | Yes vs. no | 1.447 (0.777-2.695) | .244 | ||
| HBV | Yes vs. no | 1.134 (0.645-1.994) | .663 | ||
| HCV | Yes vs. no | 0.727 (0.363-1.455) | .368 | ||
| DM | Yes vs. no | 0.646 (0.348-1.199) | .166 | ||
| Grade 1-2 TRAEs | Yes vs. no | 0.431 (0.243-0.763) | .004 | ||
| Grade ≥3 TRAEs | Yes vs. no | 1.755 (0.894-3.446) | .102 | ||
| TTV (cm3) | >1000 vs. ≤1000 | 2.247 (1.278-3.950) | .005 | ||
| MVI | Yes vs. no | 3.803 (1.961-7.375) | <.001 | 4.313 (1.747-10.646) | .002 |
| EHM | Yes vs. no | 1.310 (0.733-2.339) | .362 | ||
| AFP (ng/mL) | <400 vs. ≥400 | 3.113 (1.744-5.557) | <.001 | ||
| AST (U/L) | >40 vs. ≤40 | 3.510 (1.564-7.876) | .002 | ||
| ALT (U/L) | >40 vs. ≤40 | 2.163 (1.208-3.872) | .009 | ||
| NLR | >3.0 vs. ≤3.0 | 3.704 (1.657-8.280) | .001 | ||
| Child-Pugh class | B vs. A | 2.492 (1.417-4.381) | .002 | ||
| ALBI grade | 2/3 vs. 1 | 3.499 (1.553-7.883) | .003 | ||
| AFP decline >15% | Yes vs. no | 0.371 (0.208-0.662) | .001 | 0.344 (0.160-0.737) | .006 |
| Combination therapy* | Yes vs. no | 0.441 (0.218-0.892) | .023 | ||
| Best response | CR+PR+SD vs. none | 0.165 (0.088-0.308) | <.001 | 0.460 (0.216-0.981) | .044 |
Combination therapy includes tyrosine kinase inhibitors, radiofrequency ablation, transarterial chemoembolization, and stereotactic body radiotherapy for hepatocellular carcinoma.
AFP, α-fetoprotein; ALBI, albumin-bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DM, diabetes mellitus; EHM, extrahepatic metastasis; HBV, hepatitis B virus; HCV, hepatitis C virus; TRAEs, treatment-related adverse events; MVI, macroscopic vascular invasion; NLR, neutrophil-to-lymphocyte ratio; TTV, total tumor volume.
The results of Kaplan-Meier analysis revealed that the probability of survival significantly differed between the patients with or without MVI (Figure 2B), those with and without AFP response (Figure 2C), and those with and without disease control (Figure 2D). In addition, the probability of survival significantly differed between the patients with and without AFP response in a subgroup of patients receiving combined ICI and TKI therapies (n=63, Supplementary Figure 2A), as indicated by the findings of Kaplan-Meier analysis. In another subgroup of the patients receiving combined ICI and SBRT for HCC (n=22), AFP response tended to be a predictor of OS (P=.085, Supplementary Figure 2B).
Discussion
The results of this real-world study revealed that AFP response was a predictor of disease control, PFS, and OS in patients with unresectable HCC receiving ICIs with or without TKIs or locoregional therapies. In addition to AFP response (a decline of >15% in the AFP level within the initial 3 months of ICI therapy), we observed that declines of >10% and >20% in the AFP level could predict disease control, PFS, and OS. AFP response was a predictor of OS in a subgroup of patients receiving combined ICI and TKI therapies (n=63). In addition, AFP response tended to be a predictor of OS in another subgroup of patients receiving combined ICI and SBRT for HCC (n=22, P=.085).
Early biomarkers after initial ICI therapy can help physicians select therapies for patients with unresectable HCC. Lee et al. reported that the 10-10 rule (patients with an AFP level of ≥10 ng/mL at baseline and an early decline of >10% in the AFP level within the initial 4 weeks of ICI therapy) could predict objective response and OS in patients with unresectable HCC [11]. Shao et al. and our previous study have demonstrated that an early decline of >20% in the AFP level within the initial 4 or 12 weeks of ICI therapy was associated with more favorable treatment outcomes [10,12]. Teng et al. reported that a decline of >50% in the AFP level at week 4 and >10% at week 12 of nivolumab monotherapy were predictors of objective response, PFS, and OS in patients with unresectable HCC [17]. Kim et al. demonstrated that a decline of >20% in the AFP level at 6-10 or 14-18 weeks of ICI therapy in patients with unresectable HCC and baseline AFP ≥20 ng/mL, defined as AFP response, was a predictor of OS. AFP responders also had a high rate of objective response (41.7%) and disease control (95.8%) at 6-10 weeks of ICI therapy [18].
However, only a portion of patients received combination therapies with ICIs in previous studies [11,12]. In this study, we observed that a decline of >15% in the AFP level in the initial 3 months of ICI therapy, defined according to the highest Youden’s index, was a predictor of disease control, PFS, and OS in patients receiving ICI monotherapy or combination therapies. The patients with AFP response had a higher rate of objective response and disease control (Table 1), which was consistent with previous findings [17,18]. AFP response was a predictor of disease control, PFS, and OS. Therefore, AFP response could be used as an early predictor for justifying continuation or modification of ongoing therapeutic modalities.
In the present study, 77 (81.1%), 54 (56.8%), and 58 (61.1%) patients received ICI combination therapies, had MVI, and had EHM, respectively, and 26 (27.4%) and 51 (53.7%) patients had objective response and disease control, respectively. We identified disease control instead of objective response as a predictor of PFS and OS in this study. Zhong et al. reported that 496 patients with unresectable HCC receiving combined TACE and sorafenib who achieved disease control had longer OS than did those without disease control [19]. Another study reported that disease control was a significant predictor of OS [20]. Therefore, compared with objective response, disease control might be a more relevant predictor of PFS and OS in the patients with HCC who received combination therapy such as TACE or molecular targeted therapy in addition to ICIs. The patients with objective response and disease control overlapped; thus, we did not include objective response in statistical analyses to prevent collinearity.
Besides using serum AFP kinetics to predict the ICI-based therapeutic efficacy in patients with unresectable HCC, other predictors of immunotherapy were proposed [21-25]. Microsatellite instability (MSI) and tumor mutational burden (TMB) analysis are helpful predictors of immunotherapy. The accumulation of DNA mutations leads to increased neoantigens formation [21]. However, the prevalence of MSI-high or TMB-high in HCC is rare (<3%) [22]. Harding et al. implemented next-generation sequencing of tumoral DNA in 127 patients with HCC. They found that WNT/β-catenin pathway alternations were associated with lower disease control and shorter PFS and OS in patients receiving ICI therapy [23]. Dai et al. used The Cancer Genome Atlas, GSE14520 cohort, and Immunology Database and Analysis Portal database to develop an immune-related gene-based prognostic index (IRGPI). The index could predict the survival and efficacy of immunotherapy in patients with HCCs [24]. The real-world application of IRGPI is still unknown, and tumor specimens may not be accessible in some patients with HCC. Therefore, liquid biopsy to identify circulating tumor cells, circulating tumor DNA, and extracellular vesicles, might be an alternative approach to the prediction of efficacy of immunotherapy [25].
ICIs are an emergent treatment modality against HCC. However, second-line monotherapy with nivolumab (CheckMate 040) [26] or pembrolizumab (KEYNOTE-240) [27] resulted in only suboptimal treatment outcomes in patients with advanced HCC. Combination therapies including two ICIs or ICIs with TKIs or locoregional therapies have been receiving increasing attention. The combination of nivolumab plus ipilimumab resulted in longer median OS in patients with advanced HCC following first-line sorafenib therapy [4]. Wong et al. reported that patients with advanced HCC refractory to prior ICIs who received ipilimumab (1 mg/kg) with nivolumab (3 mg/kg) or pembrolizumab (2 mg/kg) every 3 weeks had an acceptable median OS duration of 10.9 months and an objective response rate of 16% [28]. The combination of atezolizumab and bevacizumab has become a new standard first-line systemic treatment in patients with unresectable HCC [5], and the combinations of lenvatinib plus pembrolizumab [6] and tremelimumab plus durvalumab [7] have demonstrated promising results. Furthermore, combined ICIs with locoregional therapies are frequently used in real-world practice [29].
Multidisciplinary and integral locoregional interventions for HCC are named liver-directed therapies, which are used for local disease control or as as bridge to curative treatment. Locoregional therapies can promote antitumor immunity through local inflammation and by releasing tumor-associated antigens. The released antigens are taken up by antigen-presenting cells to increase host innate and adaptive immunity [30,31]. Duffy et al. designed a pilot study using tremelimumab monotherapy (n=5) or tremelimumab in combination with RFA (n=12) or TACE (n=11) to investigate the role of immunotherapy in combination with locoregional therapies in patients with advanced HCC. The overall median OS duration was 12.3 months, and OS was not compared between the groups. The authors concluded that combination therapy can be a potential treatment modality for patients with advanced HCC [32]. Preliminary results of a phase Ib study investigating the efficiacy of pembrolizumab following TACE in patients with intermediate-stage HCC revealed a tolerable safety profile and preliminary efficacy data (three and one of four radiologically evaluable patients had SD and PD, respectively) [33]. Clinical trials evaluating the combination of TACE and ICIs are ongoing [34,35]. SBRT is a promising noninvasive ablative modality for unresectable HCC that demonstrated a high local control rate of 83.9% three years after SBRT [36]. Several trials are evaluating the combination of SBRT and ICIs for patients with HCC [37].
This study has several limitations. Firstly, only 95 patients were enrolled in this retrospective study. Secondly, mRECIST [15] instead of RECIST version 1.1 [38] was used to evaluate the radiological response. Because 60 (63.2%) and 63 (66.3%) patients received prior TACE and radiotherapy, respectively, for which mRECIST is a more suitable assessment tool [39]. Furthermore, the radiological response evaluated using mRECIST has been demonstrated to be an independent predictor of survival in patients with advanced HCC [39,40]. Third, the expression of programmed cell death ligand 1 in tumors or adjacent tissues was not examined.
In conclusion, this study demonstrated that AFP response was a predictor of disease control, PFS, and OS. Thus, AFP response can serve as a biomarker for predicting treatment outcomes in patients with unresectable HCC receiving ICIs with or without TKIs or locoregional therapies.
Acknowledgements
This study was supported by a grant (DMR-111-030) from China Medical University Hospital, Taichung, Taiwan.
Disclosure of conflict of interest
Cheng-Yuan Peng has served as an advisory committee member for AbbVie, Bristol-Myers Squibb, Gilead, and Merck Sharp & Dohme. All other coauthors have any conflicts of interest to declare.
Supporting Information
References
- 1.Federico P, Petrillo A, Giordano P, Bosso D, Fabbrocini A, Ottaviano M, Rosanova M, Silvestri A, Tufo A, Cozzolino A, Daniele B. Immune checkpoint inhibitors in hepatocellular carcinoma: current status and novel perspectives. Cancers (Basel) 2020;12:3025. doi: 10.3390/cancers12103025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bangaru S, Marrero JA, Singal AG. Review article: new therapeutic interventions for advanced hepatocellular carcinoma. Aliment Pharmacol Ther. 2020;51:78–89. doi: 10.1111/apt.15573. [DOI] [PubMed] [Google Scholar]
- 3.Cheng AL, Hsu C, Chan SL, Choo SP, Kudo M. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J Hepatol. 2020;72:307–319. doi: 10.1016/j.jhep.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 4.Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, Tovoli F, Knox JJ, Ruth He A, El-Rayes BF, Acosta-Rivera M, Lim HY, Neely J, Shen Y, Wisniewski T, Anderson J, Hsu C. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 2020;6:e204564. doi: 10.1001/jamaoncol.2020.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL IMbrave150 Investigators. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 6.Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, Kaneko S, Pracht M, Mamontov K, Meyer T, Kubota T, Dutcus CE, Saito K, Siegel AB, Dubrovsky L, Mody K, Llovet JM. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J. Clin. Oncol. 2020;38:2960–2970. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelley RK, Sangro B, Harris W, Ikeda M, Okusaka T, Kang YK, Qin S, Tai DW, Lim HY, Yau T, Yong WP, Cheng AL, Gasbarrini A, Damian S, Bruix J, Borad M, Bendell J, Kim TY, Standifer N, He P, Makowsky M, Negro A, Kudo M, Abou-Alfa GK. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: randomized expansion of a phase I/II study. J. Clin. Oncol. 2021;39:2991–3001. doi: 10.1200/JCO.20.03555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 9.European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Shao YY, Liu TH, Hsu C, Lu LC, Shen YC, Lin ZZ, Cheng AL, Hsu CH. Early alpha-foetoprotein response associated with treatment efficacy of immune checkpoint inhibitors for advanced hepatocellular carcinoma. Liver Int. 2019;39:2184–2189. doi: 10.1111/liv.14210. [DOI] [PubMed] [Google Scholar]
- 11.Lee PC, Chao Y, Chen MH, Lan KH, Lee CJ, Lee IC, Chen SC, Hou MC, Huang YH. Predictors of response and survival in immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. Cancers (Basel) 2020;12:182. doi: 10.3390/cancers12010182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu WF, Chuang PH, Chen CK, Wang HW, Tsai MH, Su WP, Chen HY, Yang CY, Lin CC, Huang GT, Lin JT, Lai HC, Peng CY. Predictors of response and survival in patients with unresectable hepatocellular carcinoma treated with nivolumab: real-world experience. Am J Cancer Res. 2020;10:4547–4560. [PMC free article] [PubMed] [Google Scholar]
- 13.Hsiao WD, Peng CY, Chuang PH, Lai HC, Cheng KS, Chou JW, Chen YY, Yu CJ, Feng CL, Su WP, Chen SH, Kao JT. Evaluation of dose-efficacy of sorafenib and effect of transarterial chemoembolization in hepatocellular carcinoma patients: a retrospective study. BMC Gastroenterol. 2016;16:50. doi: 10.1186/s12876-016-0464-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiang CL, Chan ACY, Chiu KWH, Kong FS. Combined stereotactic body radiotherapy and checkpoint inhibition in unresectable hepatocellular carcinoma: a potential synergistic treatment strategy. Front Oncol. 2019;9:1157. doi: 10.3389/fonc.2019.01157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 16.Hsu CY, Huang YH, Hsia CY, Su CW, Lin HC, Loong CC, Chiou YY, Chiang JH, Lee PC, Huo TI, Lee SD. A new prognostic model for hepatocellular carcinoma based on total tumor volume: the Taipei integrated scoring system. J Hepatol. 2010;53:108–117. doi: 10.1016/j.jhep.2010.01.038. [DOI] [PubMed] [Google Scholar]
- 17.Teng W, Lin CC, Ho MM, Lui KW, Wang SF, Hsu CW, Lin SM. Alpha-fetoprotein response at different time-points is associated with efficacy of nivolumab monotherapy for unresectable hepatocellular carcinoma. Am J Cancer Res. 2021;11:2319–2330. [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HI, Lim J, Shim JH. Role of the alpha-fetoprotein response in immune checkpoint inhibitor-based treatment of patients with hepatocellular carcinoma. J Cancer Res Clin Oncol. 2021 doi: 10.1007/s00432-021-03727-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Zhong BY, Yan ZP, Sun JH, Zhang L, Hou ZH, Zhu XL, Wen L, Ni CF. Random survival forests to predict disease control for hepatocellular carcinoma treated with transarterial chemoembolization combined with sorafenib. Front Mol Biosci. 2021;8:618050. doi: 10.3389/fmolb.2021.618050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuo YH, Yen YH, Chen YY, Kee KM, Hung CH, Lu SN, Hu TH, Chen CH, Wang JH. Nivolumab versus regorafenib in patients with hepatocellular carcinoma after sorafenib failure. Front Oncol. 2021;11:683341. doi: 10.3389/fonc.2021.683341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA Jr. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ang C, Klempner SJ, Ali SM, Madison R, Ross JS, Severson EA, Fabrizio D, Goodman A, Kurzrock R, Suh J, Millis SZ. Prevalence of established and emerging biomarkers of immune checkpoint inhibitor response in advanced hepatocellular carcinoma. Oncotarget. 2019;10:4018–4025. doi: 10.18632/oncotarget.26998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harding JJ, Nandakumar S, Armenia J, Khalil DN, Albano M, Ly M, Shia J, Hechtman JF, Kundra R, El Dika I, Do RK, Sun Y, Kingham TP, D’Angelica MI, Berger MF, Hyman DM, Jarnagin W, Klimstra DS, Janjigian YY, Solit DB, Schultz N, Abou-Alfa GK. Prospective genotyping of hepatocellular carcinoma: clinical implications of next-generation sequencing for matching patients to targeted and immune therapies. Clin Cancer Res. 2019;25:2116–2126. doi: 10.1158/1078-0432.CCR-18-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai Y, Qiang W, Lin K, Gui Y, Lan X, Wang D. An immune-related gene signature for predicting survival and immunotherapy efficacy in hepatocellular carcinoma. Cancer Immunol Immunother. 2021;70:967–979. doi: 10.1007/s00262-020-02743-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Felden J, Garcia-Lezana T, Schulze K, Losic B, Villanueva A. Liquid biopsy in the clinical management of hepatocellular carcinoma. Gut. 2020;69:2025–2034. doi: 10.1136/gutjnl-2019-320282. [DOI] [PubMed] [Google Scholar]
- 26.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL KEYNOTE-240 investigators. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J. Clin. Oncol. 2020;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 28.Wong JSL, Kwok GGW, Tang V, Li BCW, Leung R, Chiu J, Ma KW, She WH, Tsang J, Lo CM, Cheung TT, Yau T. Ipilimumab and nivolumab/pembrolizumab in advanced hepatocellular carcinoma refractory to prior immune checkpoint inhibitors. J Immunother Cancer. 2021;9:e001945. doi: 10.1136/jitc-2020-001945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viveiros P, Riaz A, Lewandowski RJ, Mahalingam D. Current state of liver-directed therapies and combinatory approaches with systemic therapy in hepatocellular carcinoma (HCC) Cancers (Basel) 2019;11:1085. doi: 10.3390/cancers11081085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jagodinsky JC, Harari PM, Morris ZS. The promise of combining radiation therapy with immunotherapy. Int J Radiat Oncol Biol Phys. 2020;108:6–16. doi: 10.1016/j.ijrobp.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leone P, Solimando AG, Fasano R, Argentiero A, Malerba E, Buonavoglia A, Lupo LG, De Re V, Silvestris N, Racanelli V. The evolving role of immune checkpoint inhibitors in hepatocellular carcinoma treatment. Vaccines (Basel) 2021;9:532. doi: 10.3390/vaccines9050532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T, ElGindi M, Uppala A, Korangy F, Kleiner DE, Figg WD, Venzon D, Steinberg SM, Venkatesan AM, Krishnasamy V, Abi-Jaoudeh N, Levy E, Wood BJ, Greten TF. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545–551. doi: 10.1016/j.jhep.2016.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinato DJ, Cole T, Bengsch B, Tait P, Sayed AA, Abomeli F, Gramenitskaya D, Allara E, Thomas R, Ward C, Wong CN, Akarca AU, Miguens Blanco J, Marafioti T, Marchesi J, Sharma R. A phase Ib study of pembrolizumab following trans-arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): PETAL. ESMO. 2019;30:v288. [Google Scholar]
- 34.Harding JJ, Erinjeri JP, Tan BR, Reiss KA, Mody K, Khalil D, Yarmohammadi H, Nadolski G, Giardina JD, Capanu M, Do RKG, Bradley M, Boussayoud C, Valentino E, Merghoub T, Jarnagin WR, Soulen MC, Brown KT, Abou-Alfa GK. A multicenter pilot study of nivolumab (NIVO) with drug eluting bead transarterial chemoembolization (deb-TACE) in patients (pts) with liver limited hepatocellular carcinoma (HCC) J. Clin. Oncol. 2018;36:TPS4146–TPS4146. [Google Scholar]
- 35.Sangro B, Kudo M, Qin S, Ren Z, Chan S, Joseph E, Arai Y, Mann H, Morgan S, Cohen G, Lencioni R. P-347 A phase 3, randomized, double-blind, placebo-controlled study of transarterial chemoembolization combined with durvalumab or durvalumab plus bevacizumab therapy in patients with locoregional hepatocellular carcinoma: EMERALD-1. Ann Oncol. 2020;31:S202–S203. [Google Scholar]
- 36.Rim CH, Kim HJ, Seong J. Clinical feasibility and efficacy of stereotactic body radiotherapy for hepatocellular carcinoma: a systematic review and meta-analysis of observational studies. Radiother Oncol. 2019;131:135–144. doi: 10.1016/j.radonc.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Perez-Romasanta LA, Gonzalez-Del Portillo E, Rodriguez-Gutierrez A, Matias-Perez A. Stereotactic radiotherapy for hepatocellular carcinoma, radiosensitization strategies and radiation-immunotherapy combination. Cancers (Basel) 2021;13:192. doi: 10.3390/cancers13020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 39.Choi MH, Park GE, Oh SN, Park MY, Rha SE, Lee YJ, Jung SE, Choi JI. Reproducibility of mRECIST in measurement and response assessment for hepatocellular carcinoma treated by transarterial chemoembolization. Acad Radiol. 2018;25:1363–1373. doi: 10.1016/j.acra.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Scheiner B, Kirstein MM, Hucke F, Finkelmeier F, Schulze K, von Felden J, Koch S, Schwabl P, Hinrichs JB, Waneck F, Waidmann O, Reiberger T, Muller C, Sieghart W, Trauner M, Weinmann A, Wege H, Trojan J, Peck-Radosavljevic M, Vogel A, Pinter M. Programmed cell death protein-1 (PD-1)-targeted immunotherapy in advanced hepatocellular carcinoma: efficacy and safety data from an international multicentre real-world cohort. Aliment Pharmacol Ther. 2019;49:1323–1333. doi: 10.1111/apt.15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.