Abstract
The therapeutic strategy is determined by protein expression using immunohistochemistry of estrogen receptor (ER), progesterone receptor, and human epidermal growth factor receptor 2 (HER2) in formalin-fixed paraffin-embedded (FFPE) breast cancer tissues. However, few proteins function independently, and many of them functions due to protein-protein interactions (PPIs) with other proteins. Therefore, it is important to focus on PPIs. This review summarizes the PPIs of ER and HER2 in breast cancer, especially those using a proximity ligation assay that can visualize PPIs in FFPE tissues. In particular, assessing the interaction of CEACAM6 with HER2 may serve as a surrogate marker for the efficacy of trastuzumab in patients with breast cancer. Therefore, in this review, the technique used to detect the interaction of CEACAM6 and HER2 in routinely processed pathological specimens will be applied to the clinical practice of drug selection. We showed the possibility as a novel pathological examination method using PPIs.
Keywords: protein-protein interaction, breast cancer, estrogen receptor, human epidermal growth factor receptor 2, proximity ligation assay
I. Introduction
Over 80% of proteins function by forming complexes rather than staying as a single unit [7]. The function of many proteins (signal transduction, transport, and metabolism) depends on structural changes and reactions caused by interactions with other proteins. Therefore, protein-protein interactions (PPIs) are crucial to protein functions, and elucidating PPIs helps in understanding the biological characteristics. It has been reported that aberrant PPIs are implicated in the development of cancer cells. Therefore, clarifying the significance of PPIs is an essential strategy to treat diseases [28]. Similarly, we have previously reported on PPIs in hormone-dependent cancers [32]. Immunoreactivity with the estrogen receptor α (ERα), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) helps in assessing the prognosis, predictors, and indication of therapeutics in breast cancer [2]. For instance, ERα-positive and/or PR-positive breast cancer patients are treated with endocrine therapy; meanwhile, HER2-positive breast cancer patients are treated with HER2 inhibitor [2]. Endocrine therapy (drugs include tamoxifen and aromatase inhibitors) targets estrogen action, is used in ER and/or PR-positive breast cancer, and has clinical benefit. However, their efficacy is limited by intrinsic and acquired therapeutic resistance [33–35]. HER2 inhibitors (HER2i), such as trastuzumab, enhances the clinical benefit of first-line chemotherapy in patients with HER2-positive breast cancer. Although the effect of trastuzumab in combination with chemotherapy is known, the therapeutic effect of trastuzumab monotherapy is not always clinically sufficient [5, 15, 41, 46]. Therefore, we sought to examine PPIs that contribute to providing appropriate medical care to patients, by providing personalized medicine. Studies using gene analysis are being conducted for the implementation of personalized medicine, but we focused on PPIs using histopathological specimens, that is, protein expression analysis performed by immunohistochemistry using formalin-fixed paraffin-embedded (FFPE) tissues [2]. Therefore, a new pathological examination method can be proposed by PPI analysis using FFPE tissue and immunohistochemistry (Fig. 1). Proximity ligation assay (PLA) analysis was developed to visualize PPIs and can be applied to human tissues [43]. This review summarizes the effects of these biomarkers on PPIs and breast cancer cells, and to use PLA analysis in breast cancer cells.
Fig. 1.
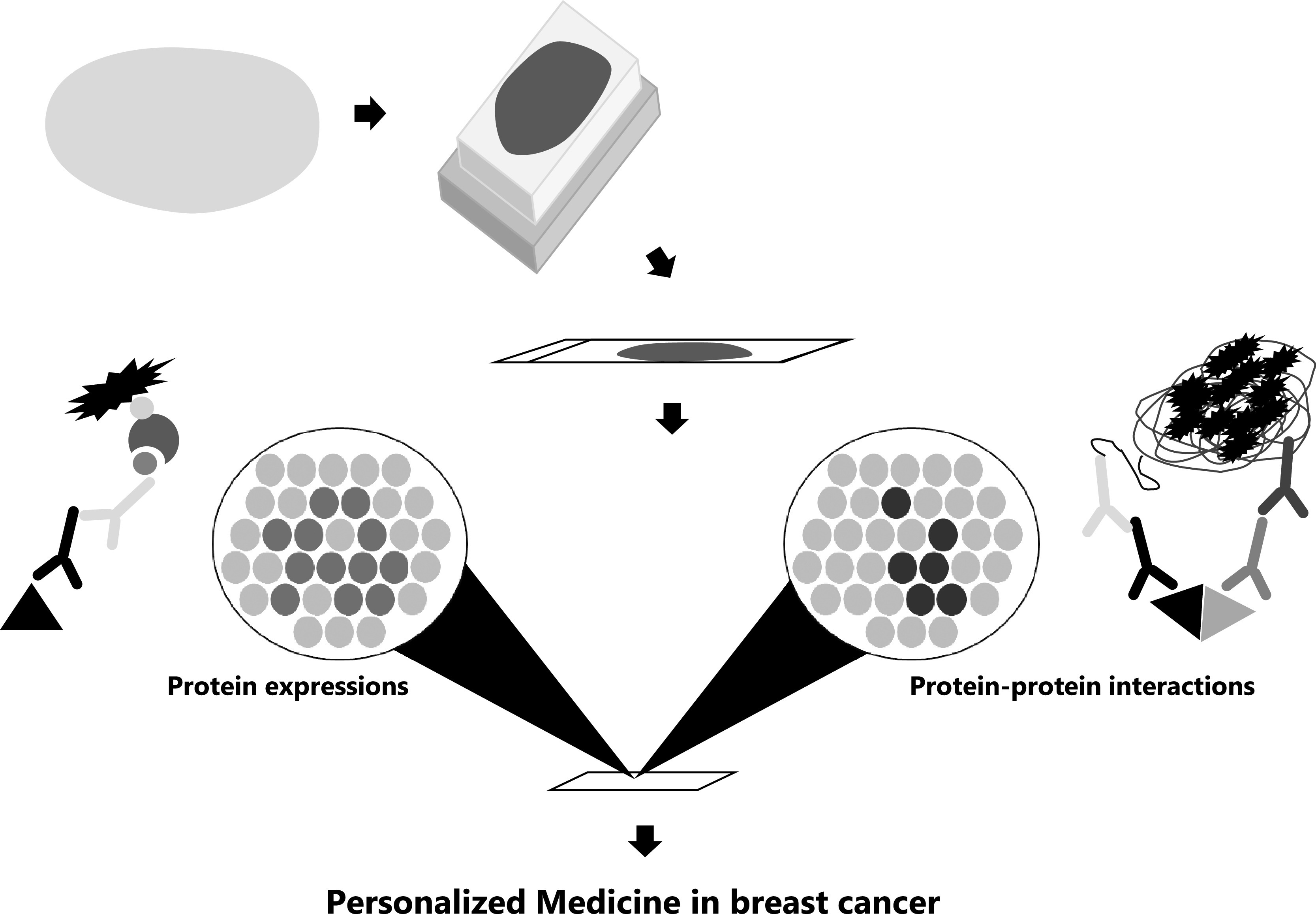
Application of protein-protein interactions in pathological examination. Diagram showing the implementation of personalized medicine utilizing visualization of protein expression and protein-protein interactions. A specimen is submitted as a surgical resection, a thinly cut sample is mounted in a paraffin block, a thinly cut section is mounted on a glass slide, and then the protein expression and protein-protein interaction analysis was examined.
II. Dimerization of Estrogen Receptors
Approximately 70% of patients with breast cancer expressed the ER [33]. ERs have two isoforms, ERα and ERβ, which are well known to form homodimers (α/α, β/β) or heterodimers (α/β) [25]. In ERα-positive breast cancer, cancer cells are promoted by estrogen binding to the ERα. However, ERβ inhibited the cell proliferation induced via ERα [29]. Therefore, it is also known that their dimerization has different effects on the intracellular signal transduction mechanism. Estrogen-induced cell proliferation was promoted by the ERα homodimer but inhibited by the ERβ homodimer [29]. ERβ inhibited ERα-mediated cell proliferation in co-expression with ERα and ERβ, and thus the ER heterodimer inhibited cell proliferation [29]. Therefore, it is necessary to assess the status of ER dimerization in patients with breast cancer and consider the potential effects of estrogen on cancer cell proliferation. By using PLA and structured illumination microscopy (SIM), we have previously reported the presence of ERα homodimers and ER heterodimers in breast cancer cells and tissues [16, 17]. Due to the diffraction limit of light, the resolution of conventional fluorescence microscopes including the laser scanning confocal microscope is 200 nm [47]. Therefore, many proteins may appear to be colocalized, hindering detailed analysis of spatial relationships. Also, the SIM summarized in this study has a resolution of 100 nm, and may be used to evaluate its close proximity, such as PPIs [47]. In both PLA and SIM, the ERα homodimer signal was higher in MCF-7 (ERα-positive) cells than in T-47D cells (ERα-positive) and was not detected in MDA-MB-231 cells (ERα-negative) [16, 17]. In addition, in MCF-7, E2 induced-ERα homodimers increased by minute 15 and 45; in both PLA and N-SIM analysis, the peak of the ERα homodimer occurred at 45 min of E2 treatment [16, 17]. ER heterodimers were detected by E2 treatment at 15 min in MCF-7 cells. The expression of ERα was similar in MCF-7 cells and T-47D cells, and the expression of ERβ was higher in T-47D cells than in MCF-7 cells [17]. Therefore, compared to the ER heterodimer, the ERα homodimer may be predominantly induced by E2 [17]. In T-47D, the ERα homodimer did not increase after 15–90 min of E2 treatment. Therefore, the E2-induced ERβ homodimer is detected in breast cancer cells with high ERβ expression. The dimerization pattern of ER may depend on the expression profile and the protein expression of the ER isoform in breast cancer cells. Both the ERα homodimer and ER heterodimer were detected using PLA in breast cancer tissues [17]. The ERα homodimer in breast cancer cells was positively associated with the status of ERα and PR, which is an ER target gene considered to be derived from the activation of ER. ER heterodimer in breast cancer cells was also positively associated with the status of ERα, but not associated to both PR and ERβ [17]. These results indicated that ERα activity in breast cancer tissues could be evaluated by detecting the ERα homodimer using PLA.
There are several studies on the detection of ER dimers or PPIs with ER using PLA. Majumdar et al. found that membrane ERα/ERβ2 heterodimers activated MAPK (Mitogen-activated protein kinase) and AKT pathways in prostate stem and progenitor cells [30]. Jehanno et al. showed changes in the interaction between ERα and coactivators or corepressors using PLA when myocardin-related transcription factor A accumulates in the nucleus, and enhances MAPK and AKT activites in MCF-7 cells [21]. Gandhi et al. also reported that interaction of p53 with ERα and AMPK was determined by PLA, indicating that ERα-p53 crosstalk could contribute to mTOR pathway [12]. In addition, the visualization of the interaction between ER and PR was seen. Concerning the interactions between ER and PR isoform, PR-B frequency was an independent predictive factor for relapse, whereas PR immunoreactivity was not. Snell et al. concluded that ER and PgR-B interactions could be used in predicting patient response to adjuvant aromatase inhibitor therapy [42]. Konan et al. showed that ERα-36 interacts constitutively with PR in the nucleus of tumor cells. ERα-36 binds to PgR, and regulates signal transduction, and interferes with its transcriptional activity, progesterone-induced antiproliferative effects, and migratory capacity [23]. Heterogeneous nuclear ribonucleoprotein (hnRNPK), is involved in chromatin remodeling, transcription, splicing, and translation processes, and as reported as a binding protein of ERα. It directly interacted with ERα and was involved in the ER-mediated signaling pathway in breast cancer [20]. The interaction between hnRNPK and ERα could stabilize ERα and enhance the patient’s therapeutic response to endocrine therapy. Furthermore, we reported that Fe65, which is a binding protein of amyloid precursor protein (APP), translocates into the nucleus by phosphorylation of APP and is involved in promoting the cancer cell proliferation and cancer cell migration in breast cancer cells [48]. In addition, Fe65 binds to ER, therefore further investigations are required to clarify effects of proteins that bind to ER on estrogen signaling.
There are various studies on the mechanism of endocrine therapy resistance, such as ER mutation, the presence of ERβ, and crosstalk with growth factor receptors [33–35]. Moreover, the alternative components of the NFκB signal transduction cascade could directly interact with ER or ER coactivators [38]. The effects of endocrine therapy could be visualized by using PPI analysis in combination with ER, as well as the formation of ER homodimers and heterodimers.
III. Dimerization of HER Family
Human epidermal growth factor receptor 2 (HER2) belongs to the HER family of receptor tyrosine kinases and is overexpressed in approximately 10–34% of breast cancers [37]. Ligand binding and/or receptor overexpression form homodimer or heterodimer, transphosphorylation of the kinase domains, and subsequent activation of downstream signaling pathways, such as the phosphoinositide 3-kinase (PI3K)–AKT and MAPK pathways [10, 31]. The HER family has four members: EGFR (epidermal growth factor receptor), HER2, HER3, and HER4 [9]. Binding of ligands leads to the homodimer and heterodimer formation of the receptor tyrosine kinase [49]. There are several reports on the visualization of HER family dimers. The HER2 homodimer and HER2/HER3 heterodimer has been reported in breast cancer tissues using PLA. Both high levels of HER2 homodimers and HER2/HER3 heterodimers were significantly associated with reduced relapse-free and overall survival [44]. Multiple protein complexes involving EGFR, HER2, and HER3 homodimers and heterodimers have been reported in breast cancer tissues [26]. Barros et al. reported that 74%, 66%, and 58% of HER2-positive cases showed heterodimers with EGFR, HER3, and HER4, respectively. HER2 heterodimers were associated with aggressive clinicopathological features and poor outcome [4]. In situ PLA also allowed the detection of HER family dimers in non-small cell lung cancer (NSCLC) tissue, and quantitative assessment of EGFR homodimers and heterodimers could contribute to predict the response of patients with NSCLC to EGFR-tyrosine kinase inhibitor treatment [27].
IV. Resistance to HER2 Inhibitors
HER2 inhibitors such as trastuzumab are used therapeutically in HER2-positive breast cancer. The antitumor effects of trastuzumab involve the inhibition of HER2-mediated signaling and activation of antibody-dependent cellular cytotoxicity (ADCC) [45]. PPIs between HER2 and other proteins, except proteins in the HER family, have been reported to affect HER2 signaling and subsequent therapeutic efficacy of HER2 inhibitors. Trastuzumab resistance in breast cancer is through HER2 and CUB domain-containing protein 1 (CDCP1) cooverexpression. This leads to increased transformation ability, cell migration, and tumor formation, enhanced HER2 activation, and downstream signaling [1]. Also, interaction between HER2 and CEACAM6 could be related to trastuzumab sensitivity [18]. CEACAM6 belongs to the CEA family and plays an important role as an intracellular adhesion molecule in many normal tissues including the lung, mammary gland, and colon [6]. In our study, we used HER2i-sensitive BT-474 and resistant MDA-MB-361, which express the CEACAM6 protein [18]. CEACAM6 knockdown by siRNA decreased the sensitivity of BT-474 cells to trastuzumab by both HER2 signaling and ADCC activity, suggesting that CEACAM6 is involved in trastuzumab sensitivity in breast cancer cell [18]. In addition, an interaction between HER2 and CEACAM6 was detected by PLA, immunoprecipitation, and super-resolution imaging analysis in BT-474 cells [18]. CEACAM6 knockdown inhibited HER2 internalization, thus PPIs between HER2 and CEACAM6 contribute to antitumor effects of trastuzumab via HER2 signaling and ADCC activity, by suppressing HER2 internalization [18]. In addition, HER2 and CEACAM6 interactions were detected using PLA in CEACAM6 and HER2 positive breast cancer tissues, and their PLA score was significantly associated with trastuzumab treatment efficacy [18].
Several cell adhesion molecules are involved in HER2 signaling and the subsequent therapeutic effect of HER2 inhibitors. MUC4, a membrane-associated mucin, has been reported to mask HER2 and reduce trastuzumab binding to HER2-positive breast cancer cells. In pancreatic cancer, MUC4 interacted with HER2 and helped promote cancer cell growth and metastasis [39]. PPIs between CD44 and HER2 on the cell surface are involved in stimulating CD44-associated HER2 tyrosine kinase activity by hyaluronic acid and promoted ovarian cancer proliferation [3]. These studies could be applied to pathological examinations by visualizing PPIs in human tissues. In addition, previously reported PPIs such as CD44 and HER2 can be analyzed in tissues by using PLA, which may contribute to proper drug selection by PPI analysis.
V. Heterodimerization of CEACAM6 and CEACAM8
Similar to CEACAM6, CEACAM8 also belongs to the CEA family and is involved in cell adhesion, and intracellular and intercellular signaling [6]. The CEA family has also been reported to form homodimers and heterodimers via the N-terminal IgV domain [8, 24, 36]. CEACAM1 and 5 have been reported to form homodimers with strong affinity. CEACAM6 and 8 form homodimers with very weak affinity, however, they form heterodimers with stronger affinity [40]. Heterodimerization of CEACAM6 and CEACAM8 has been reported in neutrophils, which increase cell adhesion to endothelial cells through CD11/CD18 activation [40]. However, the effects of CEACAM6 and CEACAM8 heterodimers in cancer cells are still unclear. Therefore, MCF-7 cells that were stably transfected with CEACAM8 in a previous study demonstrated CEACAM6 and CEACAM8 interaction using PLA [19]. Coexpression of CEACAM6 and CEACAM8 in MCF-7 cells suppressed the proliferative capacity and transendothelial invasion compared to either CEACAM6 or CEACAM8 expression [19]. Furthermore, immunohistochemical analysis using breast cancer tissues also indicate that CEACAM6 and CEACAM8 double-positive carcinoma cells were of a low histological grade and stage than single-positive or double-negative cells [19]. Therefore, CEACAM6 or CEACAM8 alone contributed to promote cell proliferation and transendothelial invasion in breast cancer cells, but PPIs between CEACAM6 and CEACAM8 in cancer cells could inhibit the adhesion to vascular endothelial cells and subsequent collapse of endothelial junctions. Some studies showed the involvement of adhesion molecules in specific organs in cancer metastasis. High expression of activated ALCAM has been reported in the skin metastases of breast cancer cells [14]. Increased expression of E-selectin has also been reported in inflammation-related lung metastases of mouse breast cancer [22]. CEACAM6 expression in our study was higher in bone than in lung metastases [19]. CEACAM6 expression of pancreatic adenocarcinoma cells interact with αvβ3 integrin, and subsequent increased adhesion of ECM components, and can result in cell invasion and metastasis [11]. Therefore, we believe the PPIs between CEACAM6 and CEACAM8 may differ in the metastatic microenvironment of the bone and lung. In addition, some studies showed that the CEA family interacted with other proteins. CEA-SLex interactions have been reported as aggressive tumor features in gastric cancer, and detection of their PPIs using PLA was useful as a biomarker for the prognosis of patients with gastric cancer in theranostic applications [13]. The CEA family dimer pattern and/or PPIs with other proteins, and its function are not well known and therefore further studies are needed. On the other hand, there was a difference in the affinity to form homodimer and/or heterodimer in the CEA family [40]. The PLA method can also be used to perform a multiplex proximity ligation assay to simultaneously visualize multiple protein complexes in situ [26]. Therefore, in tumors with multiple CEA family proteins, the simultaneous detection of multiple PPIs may reveal differences in dimer formation affinity and the function of dimer patterns.
VI. Conclusion
Assessment of CEACAM6 and HER2 interaction may be a surrogate marker for the efficacy of trastuzumab in patients with breast cancer. Therefore, this review showed that the techniques used to detect CEACAM6 and HER2 interactions in routinely processed pathological specimens, in addition to assessing the HER2 status, are useful methods to select drugs in clinical practice. PPIs with the ER and CEA family were also successfully detected in both breast cancer cells and human breast cancer tissues, but further studies are needed to clarify the possibility of application of visualization of PPIs to pathological examination. PLA can be analyzed using FFPE tissues and immunohistochemistry, and is suitable for examination using a relatively large number of cases on pathological diagnosis. In addition, PPI analysis by PLA can be observed with an optical microscope using nova red and Texas red for a fluorescence microscope. This can be performed even in a facility without a fluorescence microscope (Fig. 2). We showed the possibility of using PPIs as a novel pathological examination method. PPI analysis can lead to proper use during endocrine or anti-HER2 therapy.
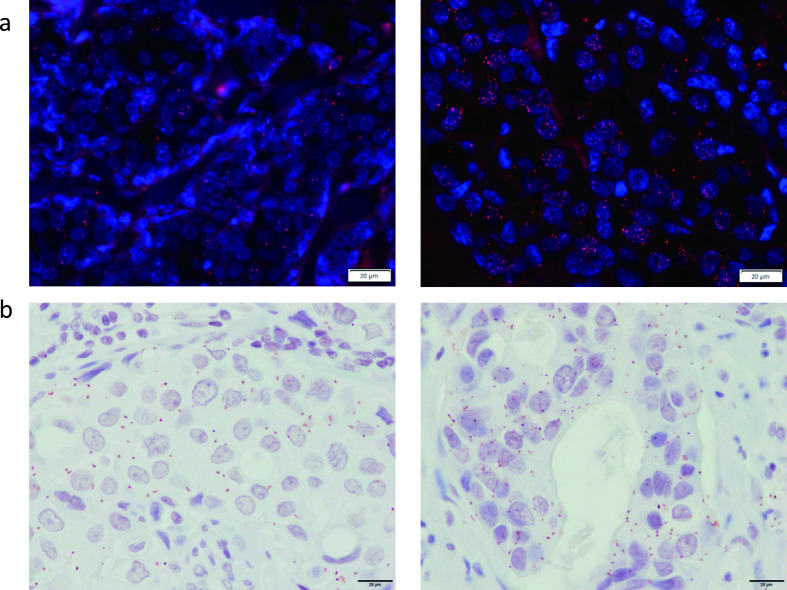
Fig. 2.
Detection of PPIs in breast cancer tissues. (a) Detection of ERα homodimers using Texas red in ERα-positive breast cancer issues. Right shows a high PLA score. Left shows the middle PLA score. (b) Detection of PPIs between HER2 and CEACAM6 using Nova red in both HER2 and CEACAM6 positive breast cancer issues. Right shows a high PLA score. Left shows a middle PLA score. Bars = 20 μm.
VII. Conflicts of Interest
All authors declare that they have no conflict of interest.
VIII. References
- 1.Alajati, A., Guccini, I., Pinton, S., Garcia-Escudero, R., Bernasocchi, T., Sarti, M., et al. (2015) Interaction of CDCP1 with HER2 enhances HER2-driven tumorigenesis and promotes trastuzumab resistance in breast cancer. Cell Rep. 11; 564–576. [DOI] [PubMed] [Google Scholar]
- 2.Allred, D. C. (2010) Issues and updates: evaluating estrogen receptor-alpha, progesterone receptor, and HER2 in breast cancer. Mod. Pathol. 23 Suppl 2; S52–S59. [DOI] [PubMed] [Google Scholar]
- 3.Bao, W., Fu, H. J., Xie, Q. S., Wang, L., Zhang, R., Guo, Z. Y., et al. (2011) HER2 interacts with CD44 to up-regulate CXCR4 via epigenetic silencing of microRNA-139 in gastric cancer cells. Gastroenterology 141; 2076–2087. [DOI] [PubMed] [Google Scholar]
- 4.Barros, F. F., Abdel-Fatah, T. M., Moseley, P., Nolan, C. C., Durham, A. C., Rakha, E. A., et al. (2014) Characterisation of HER heterodimers in breast cancer using in situ proximity ligation assay. Breast Cancer Res. Treat. 144; 273–285. [DOI] [PubMed] [Google Scholar]
- 5.Baselga, J., Carbonell, X., Castaneda-Soto, N. J., Clemens, M., Green, M., Harvey, V., et al. (2005) Phase II study of efficacy, safety, and pharmacokinetics of trastuzumab monotherapy administered on a 3-weekly schedule. J. Clin. Oncol. 23; 2162–2171. [DOI] [PubMed] [Google Scholar]
- 6.Beauchemin, N. and Arabzadeh, A. (2013) Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev. 32; 643–671. [DOI] [PubMed] [Google Scholar]
- 7.Berggard, T., Linse, S. and James, P. (2007) Methods for the detection and analysis of protein-protein interactions. Proteomics 7; 2833–2842. [DOI] [PubMed] [Google Scholar]
- 8.Bonsor, D. A., Gunther, S., Beadenkopf, R., Beckett, D. and Sundberg, E. J. (2015) Diverse oligomeric states of CEACAM IgV domains. Proc. Natl. Acad. Sci. U S A 112; 13561–13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burden, S. and Yarden, Y. (1997) Neuregulins and their receptors: a versatile signaling module in organogenesis and oncogenesis. Neuron 18; 847–855. [DOI] [PubMed] [Google Scholar]
- 10.Dankort, D., Maslikowski, B., Warner, N., Kanno, N., Kim, H., Wang, Z., et al. (2001) Grb2 and Shc adapter proteins play distinct roles in Neu (ErbB-2)-induced mammary tumorigenesis: implications for human breast cancer. Mol. Cell. Biol. 21; 1540–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duxbury, M. S., Ito, H., Ashley, S. W. and Whang, E. E. (2004) c-Src-dependent cross-talk between CEACAM6 and alphavbeta3 integrin enhances pancreatic adenocarcinoma cell adhesion to extracellular matrix components. Biochem. Biophys. Res. Commun. 317; 133–141. [DOI] [PubMed] [Google Scholar]
- 12.Gandhi, N., Oturkar, C. C. and Das, G. M. (2021) Estrogen Receptor-Alpha and p53 Status as Regulators of AMPK and mTOR in Luminal Breast Cancer. Cancers (Basel) 13; 3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes, C., Almeida, A., Barreira, A., Calheiros, J., Pinto, F., Abrantes, R., et al. (2019) Carcinoembryonic antigen carrying SLe(X) as a new biomarker of more aggressive gastric carcinomas. Theranostics 9; 7431–7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ihnen, M., Kilic, E., Kohler, N., Loning, T., Witzel, I., Hagel, C., et al. (2011) Protein expression analysis of ALCAM and CEACAM6 in breast cancer metastases reveals significantly increased ALCAM expression in metastases of the skin. J. Clin. Pathol. 64; 146–152. [DOI] [PubMed] [Google Scholar]
- 15.Inoue, K., Nakagami, K., Mizutani, M., Hozumi, Y., Fujiwara, Y., Masuda, N., et al. (2010) Randomized phase III trial of trastuzumab monotherapy followed by trastuzumab plus docetaxel versus trastuzumab plus docetaxel as first-line therapy in patients with HER2-positive metastatic breast cancer: the JO17360 Trial Group. Breast Cancer Res. Treat. 119; 127–136. [DOI] [PubMed] [Google Scholar]
- 16.Iwabuchi, E., Miki, Y., Ono, K., Onodera, Y. and Sasano, H. (2017) In Situ Evaluation of Estrogen Receptor Dimers in Breast Carcinoma Cells: Visualization of Protein-Protein Interactions. Acta Histochem. Cytochem. 50; 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwabuchi, E., Miki, Y., Ono, K., Onodera, Y., Suzuki, T., Hirakawa, H., et al. (2017) In situ detection of estrogen receptor dimers in breast carcinoma cells in archival materials using proximity ligation assay (PLA). J. Steroid Biochem. Mol. Biol. 165; 159–169. [DOI] [PubMed] [Google Scholar]
- 18.Iwabuchi, E., Miki, Y., Kanai, A., Miyashita, M., Kijima, G., Hirakawa, H., et al. (2018) The interaction between carcinoembryonic antigen-related cell adhesion molecule 6 and human epidermal growth factor receptor 2 is associated with therapeutic efficacy of trastuzumab in breast cancer. J. Pathol. 246; 379–389. [DOI] [PubMed] [Google Scholar]
- 19.Iwabuchi, E., Miki, Y., Onodera, Y., Shibahara, Y., Takagi, K., Suzuki, T., et al. (2019) Co-expression of carcinoembryonic antigen-related cell adhesion molecule 6 and 8 inhibits proliferation and invasiveness of breast carcinoma cells. Clin. Exp. Metastasis 36; 423–432. [DOI] [PubMed] [Google Scholar]
- 20.Iwabuchi, E., Miki, Y., Suzuki, T., Hirakawa, H., Ishida, T. and Sasano, H. (2021) Heterogeneous Nuclear Ribonucleoprotein K Is Involved in the Estrogen-Signaling Pathway in Breast Cancer. Int. J. Mol. Sci. 22; 2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jehanno, C., Percevault, F., Boujrad, N., Le Goff, P., Fontaine, C., Arnal, J. F., et al. (2021) Nuclear translocation of MRTFA in MCF7 breast cancer cells shifts ERalpha nuclear/genomic to extra-nuclear/non genomic actions. Mol. Cell. Endocrinol. 530; 111282. [DOI] [PubMed] [Google Scholar]
- 22.Jiang, M., Xu, X., Bi, Y., Xu, J., Qin, C. and Han, M. (2014) Systemic inflammation promotes lung metastasis via E-selectin upregulation in mouse breast cancer model. Cancer Biol. Ther. 15; 789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konan, H. P., Kassem, L., Omarjee, S., Surmieliova-Garnes, A., Jacquemetton, J., Cascales, E., et al. (2020) ERalpha-36 regulates progesterone receptor activity in breast cancer. Breast Cancer Res. 22; 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korotkova, N., Yang, Y., Le Trong, I., Cota, E., Demeler, B., Marchant, J., et al. (2008) Binding of Dr adhesins of Escherichia coli to carcinoembryonic antigen triggers receptor dissociation. Mol. Microbiol. 67; 420–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar, R., Zakharov, M. N., Khan, S. H., Miki, R., Jang, H., Toraldo, G., et al. (2011) The dynamic structure of the estrogen receptor. J. Amino Acids 2011; 812540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leuchowius, K. J., Clausson, C. M., Grannas, K., Erbilgin, Y., Botling, J., Zieba, A., et al. (2013) Parallel visualization of multiple protein complexes in individual cells in tumor tissue. Mol. Cell. Proteomics 12; 1563–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, R., Ota, K., Iwama, E., Yoneshima, Y., Tanaka, K., Inoue, H., et al. (2021) Quantification of HER family dimers by proximity ligation assay and its clinical evaluation in non-small cell lung cancer patients treated with osimertinib. Lung Cancer 158; 156–161. [DOI] [PubMed] [Google Scholar]
- 28.Lu, H., Zhou, Q., He, J., Jiang, Z., Peng, C., Tong, R., et al. (2020) Recent advances in the development of protein-protein interactions modulators: mechanisms and clinical trials. Signal Transduct. Target. Ther. 5; 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madak-Erdogan, Z., Charn, T. H., Jiang, Y., Liu, E. T., Katzenellenbogen, J. A. and Katzenellenbogen, B. S. (2013) Integrative genomics of gene and metabolic regulation by estrogen receptors alpha and beta, and their coregulators. Mol. Syst. Biol. 9; 676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Majumdar, S., Rinaldi, J. C., Malhotra, N. R., Xie, L., Hu, D. P., Gauntner, T. D., et al. (2019) Differential Actions of Estrogen Receptor alpha and beta via Nongenomic Signaling in Human Prostate Stem and Progenitor Cells. Endocrinology 160; 2692–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merkhofer, E. C., Cogswell, P. and Baldwin, A. S. (2010) Her2 activates NF-kappaB and induces invasion through the canonical pathway involving IKKalpha. Oncogene 29; 1238–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miki, Y., Iwabuchi, E., Ono, K., Sasano, H. and Ito, K. (2018) Exploring Protein–Protein Interaction in the Study of Hormone-Dependent Cancers. Int. J. Mol. Sci. 19; 3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy, C. G. and Dickler, M. N. (2016) Endocrine resistance in hormone-responsive breast cancer: mechanisms and therapeutic strategies. Endocr. Relat. Cancer 23; R337–R352. [DOI] [PubMed] [Google Scholar]
- 34.Musgrove, E. A. and Sutherland, R. L. (2009) Biological determinants of endocrine resistance in breast cancer. Nat. Rev. Cancer 9; 631–643. [DOI] [PubMed] [Google Scholar]
- 35.Normanno, N., Di Maio, M., De Maio, E., De Luca, A., de Matteis, A., Giordano, A., et al. (2005) Mechanisms of endocrine resistance and novel therapeutic strategies in breast cancer. Endocr. Relat. Cancer 12; 721–747. [DOI] [PubMed] [Google Scholar]
- 36.Oikawa, S., Inuzuka, C., Kuroki, M., Arakawa, F., Matsuoka, Y., Kosaki, G., et al. (1991) A specific heterotypic cell adhesion activity between members of carcinoembryonic antigen family, W272 and NCA, is mediated by N-domains. J. Biol. Chem. 266; 7995–8001. [PubMed] [Google Scholar]
- 37.Ross, J. S. and Fletcher, J. A. (1998) The HER-2 neu oncogene in breast cancer prognostic factor, predictive factor, and target for therapy. Stem Cells 16; 413–428. [DOI] [PubMed] [Google Scholar]
- 38.Sas, L., Lardon, F., Vermeulen, P. B., Hauspy, J., Van Dam, P., Pauwels, P., et al. (2012) The interaction between ER and NFκB in resistance to endocrine therapy. Breast Cancer Res. 14; 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh, A. P., Moniaux, N., Chauhan, S. C., Meza, J. L. and Batra, S. K. (2004) Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Res. 64; 622–630. [DOI] [PubMed] [Google Scholar]
- 40.Skubitz, K. M. and Skubitz, A. P. (2008) Interdependency of CEACAM-1, -3, -6, and -8 induced human neutrophil adhesion to endothelial cells. J. Transl. Med. 6; 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slamon, D. J., Leyland-Jones, B., Shak, S., Fuchs, H., Paton, V., Bajamonde, A., et al. (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344; 783–792. [DOI] [PubMed] [Google Scholar]
- 42.Snell, C. E., Gough, M., Liu, C., Middleton, K., Pyke, C., Shannon, C., et al. (2018) Improved relapse-free survival on aromatase inhibitors in breast cancer is associated with interaction between oestrogen receptor-alpha and progesterone receptor-b. Br. J. Cancer 119; 1316–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soderberg, O., Gullberg, M., Jarvius, M., Ridderstrale, K., Leuchowius, K. J., Jarvius, J., et al. (2006) Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods 3; 995–1000. [DOI] [PubMed] [Google Scholar]
- 44.Spears, M., Taylor, K. J., Munro, A. F., Cunningham, C. A., Mallon, E. A., Twelves, C. J., et al. (2012) In situ detection of HER2:HER2 and HER2:HER3 protein-protein interactions demonstrates prognostic significance in early breast cancer. Breast Cancer Res. Treat. 132; 463–470. [DOI] [PubMed] [Google Scholar]
- 45.Valabrega, G., Montemurro, F. and Aglietta, M. (2007) Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann. Oncol. 18; 977–984. [DOI] [PubMed] [Google Scholar]
- 46.Vogel, C. L., Cobleigh, M. A., Tripathy, D., Gutheil, J. C., Harris, L. N., Fehrenbacher, L., et al. (2002) Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J. Clin. Oncol. 20; 719–726. [DOI] [PubMed] [Google Scholar]
- 47.Ward, E. N. and Pal, R. (2017) Image scanning microscopy: an overview. J. Microsc. 266; 221–228. [DOI] [PubMed] [Google Scholar]
- 48.Xu, J., Iwabuchi, E., Miki, Y., Kanai, A., Ishida, T. and Sasano, H. (2021) FE65 in breast cancer and its clinicopathological significance. Breast Cancer. doi: 10.1007/s12282-021-01291-4. [DOI] [PubMed] [Google Scholar]
- 49.Yarden, Y. and Sliwkowski, M. X. (2001) Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2; 127–137. [DOI] [PubMed] [Google Scholar]