Abstract
Occupational and environmental exposures to industrial chemicals are well known to cause hepatotoxicity and liver injury. However, despite extensive evidence showing that exposure can lead to disease, current research approaches and regulatory policies fail to address the possibility that subtle changes caused by low level exposure to chemicals may also enhance preexisting conditions. In recent years, the conceptual understanding of the contribution of environmental chemicals to liver disease has progressed significantly. Mitochondria are often target of toxicity of environmental toxicants resulting in multisystem disorders involving different cells, tissues, and organs. Here, we review persistent maladaptive changes to mitochondria in response to environmental toxicant exposure as a mechanism of hepatotoxicity. With better understanding of the mechanism(s) and risk factors that mediate the initiation and progression of toxicant-induced liver disease, rational targeted therapy can be developed to better predict risk, as well as to treat or prevent this disease.
KEY WORDS: Organochlorines, Metals, Persistent organic pollutants, TASH, Liver disease, Hepatotoxicity, Mitochondrial maladaptation, Mitohormesis
Graphical abstract
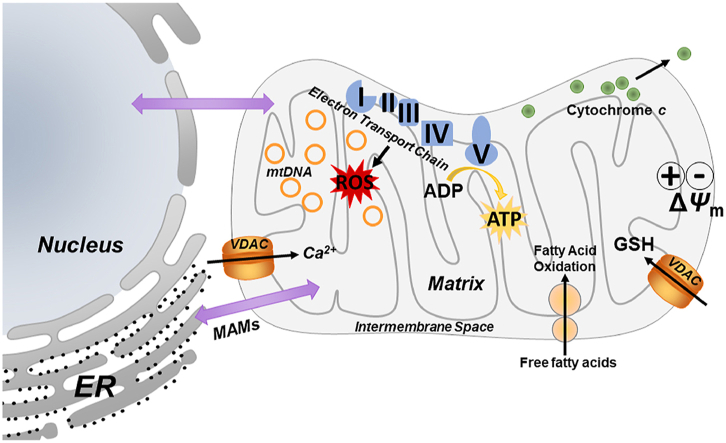
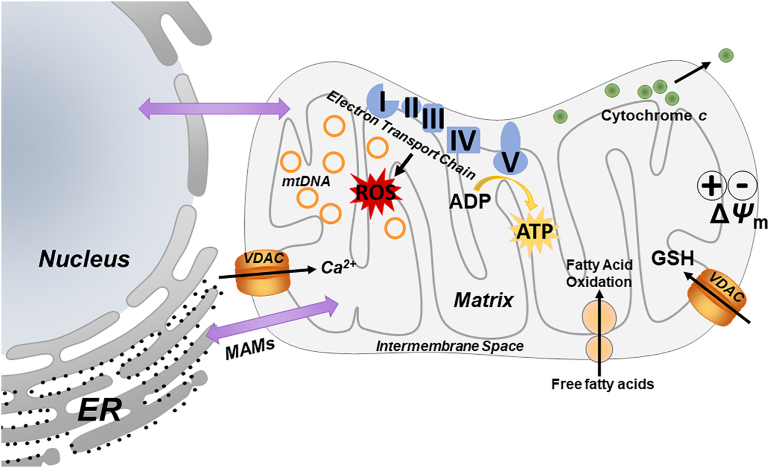
Potential mitochondrial targets include the electron transport chain, mitochondrial membrane potential (ΔΨm), and organ-to-organ communication, via mitochondrial-associated membranes (MAMs). Dysfunction of any or several of these targets may perpetuate injury.
1. Introduction
Fatty liver disease (FLD) is not a single clinical manifestation; but rather a spectrum of pathologies with various stages of severity1, ranging from lipid accumulation (steatosis), to an inflammatory response (steatohepatitis) and subsequently to a more severe phenotype, characterized by accumulation of extracellular matrix proteins resulting in scar formation within the liver tissue (fibrosis and cirrhosis)2. These end-stages of liver disease may ultimately lead to hepatocellular carcinoma (HCC). The progressive nature of FLD is consistent and independent of the etiologic source of the injury. These etiologies include alcohol-related, nonalcoholic, and toxicant-associated fatty liver diseases (ALD, NAFLD and TAFLD)3.
The global burden of FLD has been steadily increasing4,5. ALD ensues as a component of a broader perspective of alcohol use disorders, and is the most frequent cause of morbidity and mortality in patients with alcohol use disorders6,7. NAFLD is not simply a consequence of obesity and metabolic syndrome, but also is a key player in these processes8,9. NAFLD is an independent risk factor for insulin resistance9, 10, 11, hypertension12, dyslipidemia13, and even cardiovascular disease in metabolic syndrome14. The most recently recognized FLD is toxicant-associated fatty liver disease (TAFLD). TAFLD has been associated with high occupational and environmental chemical exposure [e.g., vinyl chloride (VC)]15. However, it is becoming more accepted that low environmental exposures may, at least in part, contribute to the progression of underlying FLD16.
Mitochondria are key regulators of overall metabolic function of eukaryotic organisms. Indeed, inherited disorders of mitochondrial function cause significant functional and pathologic effects17. Moreover, the complex machinery of mitochondria makes them sensitive targets to acquired changes via damage to biomolecules in that machinery. It is known that FLD causes changes in mitochondrial function. The purpose of this review is to discuss these changes, both adaptive and maladaptive. Moreover, this review will discuss the potential of mitochondrial damage caused by environmental exposure to increase the likelihood of a maladaptive response and drive the progression of FLD.
2. Interaction of environmental toxicants with primary risk factors for FLD
Although pure forms of ALD, NAFLD and TAFLD do exist, overlap occurs and has recently led to a more inclusive term for FLDs: metabolic-associated fatty liver disease (MAFLD)18. Key interactions between NAFLD and environmental toxicants have been well-established19, 20, 21. Physiological or biochemical changes to the liver that are pathologically inert can enhance hepatotoxicity of other insults (i.e., “multi-hit” hypothesis)22,23. For example, it is well known that the macrophage-mediated inflammatory response is enhanced in FLD24,25. This phenomenon is called ‘priming’26. In addition to priming inflammatory cells, liver cells appear to be sensitized to inflammatory stimuli by FLD26. Specifically, hepatocytes isolated from fatty livers are more sensitive to stress and to cytotoxic killing27, 28, 29, 30. As a result, hepatocyte death is enhanced in experimental FLD24,31,32. A leitmotif in this review is to discuss potential unifying mechanisms in the interaction of environmental toxicants and underlying FLD.
3. Mitochondrial adaptation or maladaptation as a causal factor for FLD?
The liver is the central organ involved in metabolism, storage and elimination of xenobiotics and postprandial-derived metabolites. It also maintains the energetic demand during fasting conditions to preserve whole-body energy homeostasis. Hepatic mitochondria play a central role in these functions. Mitochondria provide energy by production of adenosine triphosphate (ATP) via oxidative phosphorylation (OXPHOS), regulate other functions such as β-oxidation of fatty acids, flux through the tricarboxylic acid cycle and ketogenesis. Due to this crucial role in energy metabolism in the liver, hepatic mitochondria are not only highly enriched in number and density but also respond to stress signals, nutrient status, and environmental signals33. Therefore, maintenance and regulation of hepatic mitochondrial morphology and functional homeostasis is crucial to the overall health of an organism. Identified key processes in maintaining this balance are detailed below.
3.1. Mitochondrial morphology and dynamics
The traditional view of mitochondria as static organelles has been reassessed. Mitochondria are highly adaptive and dynamic organelles that go through fusion and fission events, leading to a diverse range of mitochondrial morphologies, from fragmented states to continuous networks (i.e., network remodeling)34,35. This leads to a visual morphological spectrum with different stages of elongation and fragmentation. Mitochondrial plasticity ensures the adaptive flexibility to adjust to changing cellular stresses and metabolic demands36. Constant network remodeling also establishes a mechanism for quality control of the mitochondrial population with important consequences for long-term function and health.
Mitochondrial morphology and functionality are strictly correlated, and mitochondrial dynamics are constantly adjusting mitochondrial shape to maintain homeostasis37. The cell also uses mitochondrial fission and fusion to segregate damaged from healthy mitochondria. During this process, damaged mitochondria are degraded while healthy mitochondria fuse, with subsequent redistribution of mitochondrial proteins and replacement of damaged mitochondrial DNA (mtDNA)38. However, these responses can also be dysfunctional, and therefore drive pathogenesis. Indeed, it is an increasingly emerging concept that continuous (mal)adaptation of mitochondrial function and shape play key roles in pathways involved in the progression of metabolic disorders39, 40, 41, 42. Changes in mitochondrial morphology caused by different insults (e.g., hypercaloric diets, alcohol or toxicants), are mediated, at least in part, by hepatic mitochondrial remodeling, including elongation or overall enlargement of the mitochondria43,44, by fusion of mitochondria (i.e., mitochondrial hypertrophy), or by mitochondrial swelling45,46. Mitochondrial hypertrophy is associated with normal cristae, normal matrix density and normal oxidative phosphorylation, whereas mitochondrial swelling is associated with swollen cristae, irregular matrix density and uncoupled oxidative phosphorylation. These morphological differences thereby impact overall mitochondrial function and efficiency.
3.2. Oxidative stress
Oxidative stress plays a major role in FLD47, 48, 49, 50. Reactive oxygen species (ROS) are products of normal cellular metabolism and mediate a variety of cellular signaling pathways. However, an imbalance of ROS and intracellular antioxidant defenses results in oxidative stress51. Mitochondria are also a significant source of endogenous ROS via electron leakage during normal or impaired oxidative respiration52,53. ROS can cause lipid oxidation and generate aldehydes, and phospholipid aldehydes32. Aldehydes can form adducts with reactive residues on proteins or small molecules, and these chemical modifications can alter and/or interfere with normal biologic processes, such as signal transduction, and/or be directly toxic to the cell7. In particular, mtDNA is susceptible to ROS-induced damage, due to its close proximity to the ETC, a lack of histones and few available DNA repair pathways (Fig. 1). It has also been shown that toxic aldehydes induce voltage-dependent anion channel (VDAC) closure, contributing to a decrease in mitochondrial GSH, as GSH influx into the mitochondria is VDAC-dependent (Fig. 1)54. Overall, high levels of ROS contribute to decreased GSH-linked antioxidant defenses. Taken together, oxidative stress observed in FLD is likely caused by both a net increase in ROS formation/leakage, as well as an impairment of antioxidant defenses against these species.
Figure 1.
Mitochondrial targets of environmental toxicants.
3.3. Endoplasmic reticulum (ER) stress
Mitochondrial damage has also been linked to the induction of ER stress, which can indirectly affect cellular function. In particular, the ER regulates fundamental metabolites (e.g., lipids) and messengers (e.g., Ca2+) that control mitochondrial function and the fate of the cell. Therefore mitochondrial stress can be an important consequence of ER stress55. Aldehydes avidly react with proteins, for example by binding to them or by interfering with ER-associated proteins, limiting the protein-folding capacity. The modified proteins accumulate in the ER and cause proteostasis56, leading to ER stress and disruption of normal ER function57,58. Indeed, ER stress is a major player in FLD and has been associated with the transition from NAFLD to NASH59.
3.4. Mitochondrial-associated membranes
Recent work suggests that stress to mitochondria and the ER is not distinct, but rather that mitochondrial/ER crosstalk is critically-involved in normal and altered function in both organelles60, 61, 62. Mitochondria and the ER physically interact via specialized contact sites called mitochondria-associated membranes (MAMs)55,63, 64, 65. These contact sites are sensitive to (patho)physiological conditions, and maladaptive changes to MAM dynamics or dysfunction at either organelle has been linked to pathophysiological states including metabolic diseases and NAFLD61,62. Importantly, MAMs house key components that impact cellular and organelle function by regulating and controlling mitochondrial function, ER stress signaling and autophagy61, making them sensitive targets. It has been demonstrated that ER-mitochondria interactions are decreased in obese mice60, leading to ER-mitochondria miscommunication. VDAC, the major permeability pathway in the mitochondrial outer membrane (see Fig. 1) is located within the MAM domain66. Aldehydes have been shown to cause VDAC closure and therefore alter ER-mitochondria interaction. Importantly, VDAC closure favors Ca2+ flux into the mitochondria, where an accumulation of Ca2+ can act as a signal for cell death67, similar to cytochrome c release68. Combined these events may impair the cell's ability to recover from metabolic stress triggered during FLD, creating a vicious cycle of damage and dysfunction and contribute to disease pathogenesis (Fig. 1)61,69, 70, 71.
3.5. Mitohormesis
An ancient concept termed hormesis is defined as an adaptive response exhibiting a biphasic dose response, suggesting that a mild, sublethal stress can leave the cell less susceptible to subsequent stresses72. In the context of mitochondrial adaptation to stress, called mitohormesis, benign mitochondrial stress that can be produced by a variety of insults, results in functional improvement that can lead to lasting adaptive metabolic and biochemical changes73. Rather than being harmful, these adaptive improvements in mitochondrial function (i.e., mitohormesis) may protect the individual from disease, even if other risk factors are present. This phenomenon is well-known in the response of skeletal muscle mitochondria to intense training stress74. Specifically, training stress improves mitochondrial function which protects the cell from additional stressors74.
Mitohormesis may also be applied conceptually to NAFLD. Specifically, although nutritional overload contributes to obesity and to the susceptibility for NAFLD, it can induce a metabolic response in the mitochondria that protect against this overload and thereby protect the liver and the organism. Interindividual variation in this adaptive response might explain why it is that not all obese individuals developed clinically apparent liver disease75. By extension, individuals that do develop disease76 may not possess an inherited or acquired ability to appropriately adapt to the condition by mitohormesis. We hypothesize that exposure to environmental chemicals may cause an acquired dysfunction in this adaptive response.
3.6. Mitochondrial maladaptation in FLD
Although the pathophysiology of FLD is multifactorial, it has been suggested that mitochondrial maladaptation, leading to mitochondrial dysfunction and altered mitochondrial structure plays a critical role in the development and progression of FLD77. Various hepatotoxic factors have been shown to cause mitochondrial metabolic disruption, resulting in an imbalance of fatty acid synthesis and breakdown78. The subsequent accumulation of lipids caused by these alterations results in more mitochondrial damage by increasing the likelihood of the formation of reactive lipid aldehydes. Mitochondrial dysfunction is also characterized by varying degrees of ultra-structural mitochondrial lesions and respiratory chain dysfunction. These factors lead to ATP depletion, increased permeability of outer and inner membranes, ROS overproduction, and oxidative stress-mediated changes to mtDNA abundance, which are also common aspects of FLD79,80. We hypothesize that mitochondrial maladaptations are a key mechanism by which mitochondria mediate cellular injury, can promote the pathological features of chronic FLD and enhance disease progression (Fig. 1).
4. Volatile organic compounds and other environmental toxicants as contributing factors to FLD
In recent years, the field of environmental toxicology has shifted away from analyses of single, high exposures of chemicals to now examine lower chronic exposures in conjunction with other potential harmful factors. Such “exposure biology” approaches take into consideration more than one factor when analyzing FLD susceptibility. Since the first description of TAFLD in the literature about a decade ago15, considerable progress has been made in the understanding of the underlying mechanisms, particularly in the context of exposure to volatile organic compounds (VOCs)16,81,82. Below, the need for future mechanistic work on environmental exposure studies with not only VOCs, but also other ubiquitous environmental contaminants will be outlined.
VOCs are highly volatile organochlorine chemicals that easily vaporize and often accumulate in enclosed spaces83,84. The typical route for human exposure is via inhalation or dermal absorption85. VOCs have long been used as industrial solvents and occupational exposure has been instrumental in establishing risk assessment safety regulations for many VOCs81. Even though VOCs are known human toxicants, their mechanism of toxicity is still incompletely understood. Importantly, VOCs are directly hepatotoxic at high exposure concentrations, but can also enhance underlying liver injury and therefore contribute to the progression of FLD16,81,82. Recent studies by our laboratory indicate that vinyl chloride (VC), at concentrations that are not hepatotoxic per se, enhances liver disease via sensitization (see Section 2)31,32,86,87. We have shown that canonical mediators of inflammation in FLD are not enhanced by VC exposure in experimental NAFLD32. Instead, VC-exposed cells are more sensitive to cytotoxic killing by proinflammatory cytokines involved in FLD (e.g., TNFα)88. This sensitization appears to be tightly linked with a decrease in the energy reserve capacity of the cell.
Many VOCs are hypothesized to be metabolism-disrupting chemicals (MDCs)16 and their toxicity is mainly attributed to their reactive metabolites. These reactive metabolites, such as aldehydes, are capable of protein carbonylation and oxidation, leading to modified proteins and downstream dysfunction. This carbonyl stress imposed by reactive VOC metabolites damages organelles, therefore contributing to FLD (see also Sections 3, 5). For example, VC and other VOCs such as acrolein, benzene, hexane, toluene, acetaldehyde, formaldehyde and acetone have been shown to damage mitochondria, causing bioenergetic dysfunction and cell damage89, 90, 91, 92, 93. If exposure to VOCs is high enough, this damage and dysfunction can ultimately lead to organ damage.
5. Mitochondrial maladaptation as a unifying mechanism for the enhancement of FLD by environmental toxicants? The example of vinyl chloride exposure
Several VOCs have been demonstrated to impact mitochondrial integrity and function. VC-induced mitochondrial damage represents a canonical example of an environmental exposure that is limiting the capacity of mitochondria to adapt appropriately to the metabolic stress imposed by another factor, such as nutritional overload. Recent studies by our group have shown that VC exposure levels that are not directly hepatotoxic (< 1 ppm), enhanced liver damage caused by experimental NAFLD (high-fat diet feeding) in mice32. This interaction was characterized by altered metabolism, inflammation and oxidative stress. VC exposure also enhanced mitochondrial dysfunction caused by experimental NAFLD32, which is thought to actually drive the other effects observed under these conditions39,94.
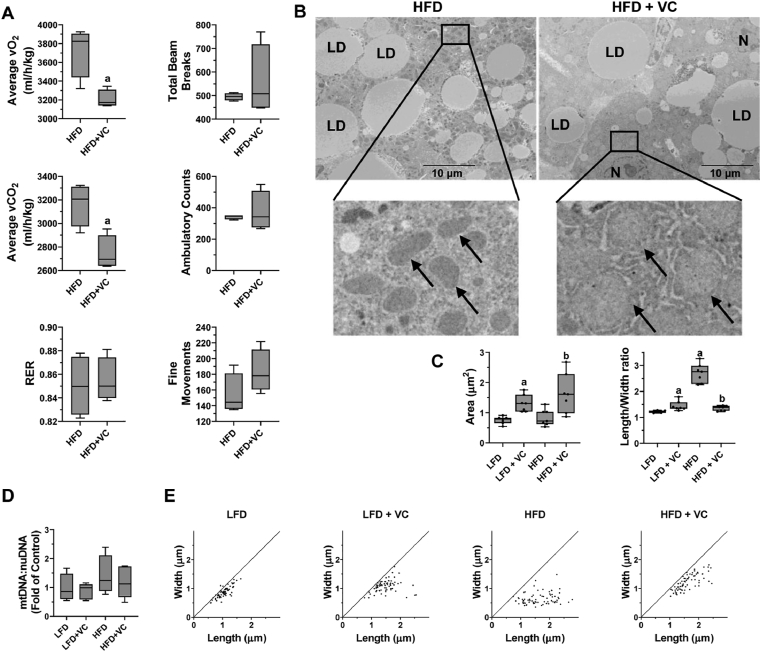
As detailed in Section 3.1, changes in mitochondrial morphology can have significant impacts on function. Indeed, VC exposure significantly changed mitochondrial shape (Fig. 2, unpublished data) towards a more spherical morphology, with an electron-light appearance, suggesting swelling rather than hypertrophy. The structural changes observed under these conditions correlated with altered mitochondrial function. The observation that high-fat diet-induced liver injury enhanced by VC was associated with increases in oxidative stress and energy dysmetabolism32, was different from those observed with NAFLD alone. For example, VC did not affect mtDNA content (Fig. 2, unpublished data), which is known to be increased by experimental NAFLD95,96. The pathologic changes, however, were linked with an overall decrease in external respiration of the animals (i.e. breathing), independent of physical activity (Fig. 2)32. External respiration is directly related to cellular respiration and largely driven by mitochondrial oxidative phosphorylation. We have also demonstrated both in vitro and in vivo that VC and its metabolites directly damage mitochondrial complexes, leading to decreased ATP production, oxygen consumption rate and an uncoupling of the electron transport chain31,32,87,88. This causes the cell to increase flux through anaerobic glycolysis to compensate for this loss of ATP yield32.
Figure 2.
Effect of VC on metabolic phenotype and mitochondrial morphology. (A) Representative parameters of metabolic function are depicted for mice exposed to HFD ± VC32. (B) Representative EM photomicrographs depict elongated organelles in the HFD group and enlarged mitochondria (width, length >1 mm) in the HFD + VC group. Arrows denote mitochondria, LD denote lipid droplet, and N denotes nucleus. (C) Total mitochondrial area (μm2) and mitochondrial length/width ratio are shown. (D) The ratio of hepatic mitochondrial to nuclear DNA (mtDNA:nuDNA) is shown as fold of control compared to LFD control animals. (E) Distribution of the size of 70 mitochondria/group are shown. aP < 0.05 compared to LFD or HFD control. Samples size per group n = 8–10.
Although it is incompletely understood how VC impacts mitochondrial metabolism, recent studies shed light on potential mechanisms. For example, the above described effects of VC on mitochondria were prevented by administration of allosteric ALDH2 activator Alda-187. ALDH2 is not only associated with acetaldehyde metabolism, but is also responsible for the detoxification of most other aldehydes (see Section 3.2), including lipid aldehydes (e.g., 4-HNE) and the VC metabolite, chloroacetaldehyde (CAA)97, 98, 99. Although mitochondria themselves generate ROS, resulting in oxidative stress100, they are also sensitive to ROS damage. ALDH2 serves as a key line of defense against reactive aldehydes. Activation of ALDH2 has been shown to be protective in several models of oxidative stress-induced organ damage, including cardiac ischemia/reperfusion, pulmonary artery hypertension, and hepatic regeneration101, 102, 103. Importantly, it has also been demonstrated previously that ALDH2 activity was decreased in human NASH104. The potential that environmental chemicals impact ALDH2 activity may therefore be a key factor in driving the interaction between toxicant exposure and FLD.
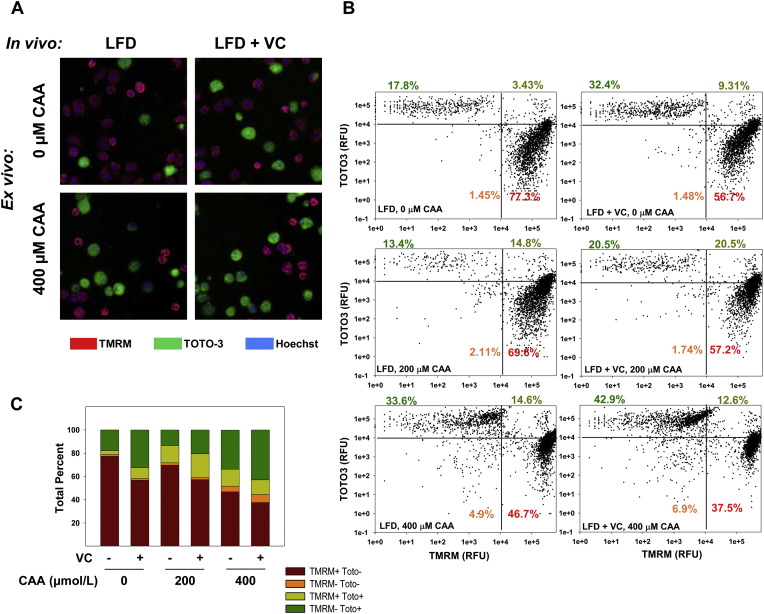
Another key modulator of mitochondrial function and respiration is the mitochondrial membrane potential (ΔΨm, see Fig. 1). The mitochondrial membrane potential is generated and maintained by the proton pumps of the ETC (complexes I, III and IV)105. Previous work by this group has demonstrated that in vitro exposure to VC metabolites renders hepatocytes more sensitive to cell death88. In line with that study, here we demonstrate that in vivo VC exposure decreases mitochondrial membrane potential and sensitizes hepatocytes to ex vivo cytotoxic stimuli resulting in cell death (Fig. 3, unpublished data). We propose that these effects contribute, at least in part, to the overall phenotype caused by VC.
Figure 3.
VC sensitizes hepatocytes to cell death. (A) Representative photomicrographs are shown for hepatocytes isolated from animals exposed in vivo to LFD ± VC for 12 weeks. These hepatocytes were then challenged with ± CAA ex vivo. Cellomics images for TMRM (mitochondrial membrane potential), TOTO-3 (cell death), and Hoechst (nuclear) fluorescent staining are shown. (B) 2D scatter plots of cellomics image analysis for LFD ± VC ± CAA in which TMRM is depicted as a function of TOTO-3. Thresholds were calculated for positive and negative fluorescence of each marker. (C) Relative percentage of cells in each quadrant (of cellomics analysis in B) are shown. Samples size per group n = 8–10.
As outlined in Section 3.2, a key mechanism of FLD is an increased production of ROS, leading to oxidative stress and the generation of aldehydes47, 48, 49, 50. This is increased by VC in mice31,32,86; and in human subjects, VC exposures were associated with antioxidant depletion consistent with oxidative stress, increased lipid peroxidation products, and decreased carnitine/carnitine esters, which were indicative of mitochondrial dysfunction15,106. Moreover, the VC-metabolite CAA selectively depletes glutathione (GSH)88, likely resulting in decreased GSH-linked antioxidant defenses.
VC has recently been demonstrated to cause ER stress at high concentrations107. Moreover, we have observed an enhancement of NAFLD-induced ER stress concomitant with lipid aldehyde (e.g., 4-HNE) adduct formation in response to low concentrations of VC32. This effect of VC could be mediated directly by CAA and therefore form protein adducts, leading to ER stress and proteostasis (see Section 3.3). One hallmark of ER stress is dilation of the ER108, suggestive of proteostasis, which was observed in animals exposed to VC independent of diet32. Given these observations, there is a clear connection between mitochondrial function and ER stress, in the enhanced liver damage caused by the interaction of VC exposure and FLD.
As VC exposure causes ER stress and mitochondrial dysfunction32,87, and these have been shown to be linked events (see Section 3.4), we hypothesize that VC and its metabolites (i.e., CAA) change ER–mitochondria interaction via MAMs, resulting in potential miscommunication, VDAC closure, ER stress and mitochondrial dysfunction (see Fig. 1). Future studies will need to address this hypothesis.
6. Other environmental chemicals
The liver is the first line of defense against potentially harmful xenobiotics, and it is therefore the target organ that is most affected by chemicals and environmental pollutants81,82. In addition to the above described VOCs, multiple additional environmental chemicals have been associated with TAFLD in animal and/or epidemiological studies, such as persistent organic pollutants (POPs), metals, pesticides, particulate matter, and others16,109. While it is unknown how many environmental pollutants cause or contribute to FLD, 33% of the 677 most common workplace chemicals reported in the National Institute of Occupational Safety and Health Pocket Guide are associated with hepatotoxicity110. Additionally, a recent retrospective analysis of comprehensive federal toxicological databases identified 30% of environmental toxicants as hepatotoxicants111.
The potential importance of mitochondrial toxicity as a mode of toxicity for many environmental chemicals has been highlighted for other diseases, such as cancer, neurodegenerative and cardiovascular diseases112. However, their involvement in the initiation and progression of FLD is largely understudied. In contrast, other hepatotoxicants, such as alcohol and drugs, have not only been well-established to cause mitochondrial dysfunction and maladaptation in the liver, but also the underlying mechanisms have been well-characterized54,113. The specific mitotoxic effects of environmental chemicals are only beginning to be understood and demand future studies. Importantly, many environmental pollutants can accumulate in mitochondria, either due to their chemical properties via crossing the phospholipid bilayer (lipophilicity, amphiphilicity) or due to entry via transporters (i.e., VDAC, see also Fig. 1 and Section 6)112. Previous reviews also list a variety of compounds that are not necessarily characterized as directly mitotoxic, but are described to mediate their effects, at least in part, via mitochondrial impairment112,114. A limitation of the studies cited in these reviews is that while chemical exposure is linked to mitochondrial dysfunction, the underlying mechanisms and/or the molecular targets of the compounds are often not identified, leaving the question of ‘the chicken or the egg: is mitochondrial failure cause or consequence of injury?
A major link in multiple studies is that exposure to environmental chemicals can cause the production of ROS and results in oxidative stress. Active VOC metabolites are often extremely electrophilic and therefore highly reactive (see also Sections 3, 5). For example, acrolein is a well-known propagator of oxidative stress by causing lipid peroxidation adducts58,115. Similarly, the major metabolites of trichloroethylene (TCE), trichloroacetic acid (TCA) and dichloroacetic acid (DCA), cause oxidative stress through forming lipid peroxidation adducts in vivo and in vitro116,117. Exposure to dimethylformamide in a human liver cell line caused dose-dependent ROS production118. Similar to VOCs, other chemicals have also been associated with the production of ROS, such as (but not limited to) metals, POPs, and pesticides119, 120, 121, 122, 123. By extension, it is likely that ROS-mediated mitochondrial damage is a shared mechanism of VOC toxicity.
Endocrine-disrupting compounds (EDCs), such as bisphenol A, cadmium, pesticides perfluorinated chemicals etc., interfere with the normal signaling of several hormones related to type 2 diabetes mellitus, obesity, and FLD124. The list of substances classified as EDCs has dramatically increased over the past years124. Moreover, research of their mechanisms-of-action has steadily increased, particularly in chronic metabolic diseases. For example, bisphenol A-induced metabolic dysregulation has been shown to be caused, at least in part, by fatty acid accumulation in hepatocytes leading to their β-oxidation in the mitochondria, resulting in the formation of ROS and mitochondrial dysfunction125. Moreover, bisphenol A, at concentrations below the ‘no observed adverse effect level’ (NOAEL), has been shown to cause structural mitochondrial changes that also correlated with mitochondrial dysfunction126,127.
Many environmental pollutants are chemically reactive. Chemical modifications can alter and/or interfere with normal biologic processes directly via adduct formation (see Section 3.2) or indirectly by altering epigenetic regulators. As a result, environmental pollutant exposure has also been suggested to affect epigenetic reprogramming and transcriptional regulation of key nuclear genes involved in FLD, metabolism and mitochondrial processes128, 129, 130. For example, hydroquinone, a benzene metabolite, mediates demethylation of cytosine (5 mC), resulting in enhanced promoter activity of key genes involved in mitochondrial biogenesis and metabolism131. However, while advances have been made on epigenetic regulation of nuclear encoded mitochondrial proteins, the concept of epigenetic regulation of mtDNA is only beginning to be understood, especially in the context of environmental exposure132,133. While mitochondria lack histones, DNA methyl transferases have been identified in the mitochondria suggesting that methylation occurs within this organelle134. A couple of clinical studies demonstrated that mtDNA methylation was associated with fine particle exposure and altered mtDNA copy number133,135. Similarly, arsenic and the flame retardant BDE-47 (endocrine disruptor) have been associated with hypomethylation of mtDNA in humans and animals, respectively136,137. However, most studies thus far on epigenetic regulation of mtDNA focus on methylation, and other pathways of epigenetic regulation are understudied. Moreover, epitranscriptomics, a relatively new field of study138,139, which includes functionally relevant changes to the genome that do not involve a change in the nucleotide sequence, has hardly been explored in the field of environmental chemicals140.
7. Limitations of published studies and suggested future directions
Critical knowledge gaps remain in the understanding of the impact of environmental chemicals on FLD and require more human and more mechanistic data. Epidemiological studies of the effects of environmental toxicants are limited due to a) analytical difficulties in measuring real exposure concentrations, b) evaluation of personal exposure, and c) delineation of toxicity from multiple compounds. Moreover, while biopsy-based pathological assessment is reliable to diagnose steatohepatitis and fibrosis, this procedure is also associated with risk and often not performed when FLD is asymptomatic or has nonspecific symptoms until it has progressed to final stages of the disease. Indeed, standard serologic biomarkers for liver injury, such as transaminases may be insensitive for the diagnosis of TAFLD15. The lack of human liver tissue paired with exposure assessment data remains a major barrier to the field. Alternatively, exposure assessment in previously biopsied FLD cohorts could be performed. An additional aspect to consider is that FLD often shows regional variability in the incidence, severity and outcomes4,141. Recently, micro-regional (e.g., county-level) analysis of liver disease mortality has indicated that there are local clusters of liver disease142,143. Geospatial analyses more accurately consider local variation in socio-demographics, access-to-care, risk factors and risk modifiers, such as exposure to environmental pollutants144, as local hotspots of environmental chemical exposure often overlap with racial and socioeconomic disparity144.
In addition to the limitations of human studies, there is a substantial lack of basic molecular information on the mechanisms of action of many environmental chemicals. Limitations of in vitro and in vivo studies include the knowledge gaps about key responses or mechanisms of a) lower, not overtly toxic concentrations (including, but not limited to dose-responses for mitochondrial maladaptation), b) toxicant interactions with other factors (i.e., diets, alcohol, genetic predisposition), and c) chemical mixtures, given that environmental exposure to these compounds is usually as a mixture, which may have effects that are more than simply additive.
Mitochondria are important organelles for regulating the balance of redox status (see Section 3.2). An imbalance of the mitochondrial dynamic associated with mitochondrial dysfunction is one major molecular mechanism for oxidative damages. ROS damage the mitochondrial electron transport chain leading to more ROS production and mitochondrial damage, creating a vicious cycle. Although ROS can lead to mitochondrial damage, it is not necessarily valid to universally link these events. This is a common limitation of several studies in that both endpoints were rarely measured. Another basic limitation of studying ROS; due to their inherent reactivity, the parent reactive species is rarely measured. Rather, generally ‘footprints’ indicative of ROS production is measured. It is therefore difficult to separate, cause, proximal causes and effects in the context of ROS and disease145. This issue is especially pertinent in the interaction between ROS and mitochondrial dysfunction. Specifically, ROS not only damage mitochondria, but damaged mitochondria also leak more electrons that perpetuate ROS generation. Although several studies have linked environmental exposure, mitochondrial dysfunction and ROS production, few have addressed the issue of cause versus effect in sufficient detail to yield mechanistic insight. Moreover, few studies have elucidated whether or not the environmental exposure directly damages mitochondria (leading to ROS production) or indirectly (via generating ROS). The specific mitochondrial effects still remain largely unclear for most of environmental chemicals.
In recent years, new approach methodologies (NAMs), referring to in silico methods that improve human relevance and replace or reduce the use of animals, have been established for the assessment of chemical hazards (i.e., computational toxicology)146,147. Such platforms will be crucial in ‘connecting the dots’, and advances in biotechnologies are allowing for improved sensitivity with smaller sample requirements to extract biological data with increased efficiency. Importantly, identification of the etiology of mitochondrial dysfunction caused by environmental chemicals is challenging with laboratory and epidemiologic approaches. Recently, using NAM via the U.S. EPA ToxCast and Tox21 databases, organophosphate and carbamate pesticides, that have previously been demonstrated to alter oxidative phosphorylation complexes and activities, and also disrupt mitochondrial membranes148,149, have been associated with decreased mitochondrial membrane potential in HepG2 cells150. To our knowledge, this finding is the first proof-of-principle of this approach to address the potential role of mitochondria in injury caused by environmental hazards.
8. Conclusions
Understanding the concept of an interaction of environmental chemical exposures with other risk-modifying factors, such as genetics, lifestyle or underlying diseases is gradually increasing in recent years. This knowledge also emphasizes the concerns that individuals suffering from underlying FLD may be at greater risk from exposure to mitochondrial toxicants, or that such exposures may contribute to FLD. However, the underlying mechanisms of FLD caused or enhanced by environmental chemicals, in general and especially mitochondria-related, are still poorly understood. While many studies implicate mitotoxic effects of the compounds, the intricate details of mechanistically involving structurally, functionally and dynamically dysregulated mitochondria and their interactions with other organelles represent a critical knowledge gap and need to be addressed in future studies.
Acknowledgments
This study was funded by awards from the National Institutes of Health: K01 DK096042, R03 DK107912, R21 ES031531, P30DK120531 and P20GM113226, USA.
Author contributions
Research: Regina D. Schnegelberger, Anna L. Lang, and Juliane I. Beier; Article preparation: Regina D. Schnegelberger, Anna L. Lang, Gavin E. Arteel, and Juliane I. Beier. All authors have read and agreed to the published version of the manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
References
- 1.Sayiner M., Koenig A., Henry L., Younossi Z.M. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in the United States and the rest of the world. Clin Liver Dis. 2016;20:205–214. doi: 10.1016/j.cld.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Day C.P. Non-alcoholic fatty liver disease: current concepts and management strategies. Clin Med. 2006;6:19–25. doi: 10.7861/clinmedicine.6-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joshi-Barve S., Kirpich I., Cave M.C., Marsano L.S., McClain C.J. Alcoholic, nonalcoholic, and toxicant-associated steatohepatitis: mechanistic similarities and differences. Cell Mol Gastroenterol Hepatol. 2015;1:356–367. doi: 10.1016/j.jcmgh.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asrani S.K., Devarbhavi H., Eaton J., Kamath P.S. Burden of liver diseases in the world. J Hepatol. 2019;70:151–171. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Mitra S., De A., Chowdhury A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl Gastroenterol Hepatol. 2020;5:16. doi: 10.21037/tgh.2019.09.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beier J.I., Arteel G.E., McClain C.J. Advances in alcoholic liver disease. Curr Gastroenterol Rep. 2011;13:56–64. doi: 10.1007/s11894-010-0157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beier J.I., McClain C.J. Mechanisms and cell signaling in alcoholic liver disease. Biol Chem. 2010;391:1249–1264. doi: 10.1515/BC.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yki-Jarvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2:901–910. doi: 10.1016/S2213-8587(14)70032-4. [DOI] [PubMed] [Google Scholar]
- 9.Anstee Q.M., Targher G., Day C.P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330–344. doi: 10.1038/nrgastro.2013.41. [DOI] [PubMed] [Google Scholar]
- 10.Choi J.H., Rhee E.J., Bae J.C., Park S.E., Park C.Y., Cho Y.K., et al. Increased risk of type 2 diabetes in subjects with both elevated liver enzymes and ultrasonographically diagnosed nonalcoholic fatty liver disease: a 4-year longitudinal study. Arch Med Res. 2013;44:115–120. doi: 10.1016/j.arcmed.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Park S.K., Seo M.H., Shin H.C., Ryoo J.H. Clinical availability of nonalcoholic fatty liver disease as an early predictor of type 2 diabetes mellitus in Korean men: 5-year prospective cohort study. Hepatology. 2013;57:1378–1383. doi: 10.1002/hep.26183. [DOI] [PubMed] [Google Scholar]
- 12.Sung K.C., Wild S.H., Byrne C.D. Development of new fatty liver, or resolution of existing fatty liver, over five years of follow-up, and risk of incident hypertension. J Hepatol. 2014;60:1040–1045. doi: 10.1016/j.jhep.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Adiels M., Olofsson S.O., Taskinen M.R., Boren J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:1225–1236. doi: 10.1161/ATVBAHA.107.160192. [DOI] [PubMed] [Google Scholar]
- 14.Musso G., Gambino R., Cassader M., Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–649. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 15.Cave M., Falkner K.C., Ray M., Joshi-Barve S., Brock G., Khan R., et al. Toxicant-associated steatohepatitis in vinyl chloride workers. Hepatology. 2010;51:474–481. doi: 10.1002/hep.23321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wahlang B., Jin J., Beier J.I., Hardesty J.E., Daly E.F., Schnegelberger R.D., et al. Mechanisms of environmental contributions to fatty liver disease. Curr Environ Health Rep. 2019;6:80–94. doi: 10.1007/s40572-019-00232-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorman G.S., Chinnery P.F., DiMauro S., Hirano M., Koga Y., McFarland R., et al. Mitochondrial diseases. Nat Rev Dis Primers. 2016;2:16080. doi: 10.1038/nrdp.2016.80. [DOI] [PubMed] [Google Scholar]
- 18.Eslam M., Sanyal A.J., George J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 19.Tan M., Schmidt R.H., Beier J.I., Watson W.H., Zhong H., States J.C., et al. Chronic subhepatotoxic exposure to arsenic enhances hepatic injury caused by high fat diet in mice. Toxicol Appl Pharmacol. 2011;257:356–364. doi: 10.1016/j.taap.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wahlang B., Falkner K.C., Gregory B., Ansert D., Young D., Conklin D.J., et al. Polychlorinated biphenyl 153 is a diet-dependent obesogen that worsens nonalcoholic fatty liver disease in male C57BL6/J mice. J Nutr Biochem. 2013;24:1587–1595. doi: 10.1016/j.jnutbio.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cichocki J.A., Furuya S., Konganti K., Luo Y.S., McDonald T.J., Iwata Y., et al. Impact of nonalcoholic fatty liver disease on toxicokinetics of tetrachloroethylene in mice. J Pharmacol Exp Therapeut. 2017;361:17–28. doi: 10.1124/jpet.116.238790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang S.Q., Lin H.Z., Lane M.D., Clemens M., Diehl A.M. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci U S A. 1997;94:2557–2562. doi: 10.1073/pnas.94.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Day C.P., James O.F. Steatohepatitis: a tale of two “hits”?. Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 24.Beier J.I., Luyendyk J.P., Guo L., von Montfort C., Staunton D.E., Arteel G.E. Fibrin accumulation plays a critical role in the sensitization to lipopolysaccharide-induced liver injury caused by ethanol in mice. Hepatology. 2009;49:1545–1553. doi: 10.1002/hep.22847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deaciuc I.V., Nikolova-Karakashian M., Fortunato F., Lee E.Y., Hill D.B., McClain C.J. Apoptosis and dysregulated ceramide metabolism in a murine model of alcohol-enhanced lipopolysaccharide hepatotoxicity. Alcohol Clin Exp Res. 2000;24:1557–1565. [PubMed] [Google Scholar]
- 26.Beier J.I., Arteel G.E. Alcoholic liver disease and the potential role of plasminogen activator inhibitor-1 and fibrin metabolism. Exp Biol Med. 2012;237:1–9. doi: 10.1258/ebm.2011.011255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirsova P., Gores G.J. Death receptor-mediated cell death and proinflammatory signaling in nonalcoholic steatohepatitis. Cell Mol Gastroenterol Hepatol. 2015;1:17–27. doi: 10.1016/j.jcmgh.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ibrahim S.H., Hirsova P., Gores G.J. Non-alcoholic steatohepatitis pathogenesis: sublethal hepatocyte injury as a driver of liver inflammation. Gut. 2018;67:963–972. doi: 10.1136/gutjnl-2017-315691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pastorino J.G., Hoek J.B. Ethanol potentiates tumor necrosis factor-alpha cytotoxicity in hepatoma cells and primary rat hepatocytes by promoting induction of the mitochondrial permeability transition. Hepatology. 2000;31:1141–1152. doi: 10.1053/he.2000.7013. [DOI] [PubMed] [Google Scholar]
- 30.Colell A., Garcia-Ruiz C., Miranda M., Ardite E., Mari M., Morales A., et al. Selective glutathione depletion of mitochondria by ethanol sensitizes hepatocytes to tumor necrosis factor. Gastroenterology. 1998;115:1541–1551. doi: 10.1016/s0016-5085(98)70034-4. [DOI] [PubMed] [Google Scholar]
- 31.Lang A.L., Goldsmith W.T., Schnegelberger R.D., Arteel G.E., Beier J.I. Vinyl chloride and high-fat diet as a model of environment and obesity interaction. J Vis Exp. 2020;(155) doi: 10.3791/60351. :10.3791/60351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lang A.L., Chen L., Poff G.D., Ding W.X., Barnett R.A., Arteel G.E., et al. Vinyl chloride dysregulates metabolic homeostasis and enhances diet-induced liver injury in mice. Hepatol Commun. 2018;2:270–284. doi: 10.1002/hep4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Longo M., Meroni M., Paolini E., Macchi C., Dongiovanni P. Mitochondrial dynamics and nonalcoholic fatty liver disease (NAFLD): new perspectives for a fairy-tale ending?. Metabolism. 2021;117:154708. doi: 10.1016/j.metabol.2021.154708. [DOI] [PubMed] [Google Scholar]
- 34.Hoitzing H., Johnston I.G., Jones N.S. What is the function of mitochondrial networks? A theoretical assessment of hypotheses and proposal for future research. Bioessays. 2015;37:687–700. doi: 10.1002/bies.201400188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams J.A., Ding W.X. Mechanisms, pathophysiological roles and methods for analyzing mitophagy—recent insights. Biol Chem. 2018;399:147–178. doi: 10.1515/hsz-2017-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Bliek A.M., Shen Q., Kawajiri S. Mechanisms of mitochondrial fission and fusion. Cold Spring Harb Perspect Biol. 2013;5:a011072. doi: 10.1101/cshperspect.a011072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyazono Y., Hirashima S., Ishihara N., Kusukawa J., Nakamura K.I., Ohta K. Uncoupled mitochondria quickly shorten along their long axis to form indented spheroids, instead of rings, in a fission-independent manner. Sci Rep. 2018;8:350. doi: 10.1038/s41598-017-18582-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Legros F., Lombes A., Frachon P., Rojo M. Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusins. Mol Biol Cell. 2002;13:4343–4354. doi: 10.1091/mbc.E02-06-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Satapati S., Kucejova B., Duarte J.A., Fletcher J.A., Reynolds L., Sunny N.E., et al. Mitochondrial metabolism mediates oxidative stress and inflammation in fatty liver. J Clin Invest. 2015;125:4447–4462. doi: 10.1172/JCI82204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Satapati S., Sunny N.E., Kucejova B., Fu X., He T.T., Méndez-Lucas A., et al. Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. J Lipid Res. 2012;53:1080–1092. doi: 10.1194/jlr.M023382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patterson R.E., Kalavalapalli S., Williams C.M., Nautiyal M., Mathew J.T., Martinez J., et al. Lipotoxicity in steatohepatitis occurs despite an increase in tricarboxylic acid cycle activity. Am J Physiol Endocrinol Metab. 2016;310:E484–E494. doi: 10.1152/ajpendo.00492.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sunny N.E., Bril F., Cusi K. Mitochondrial adaptation in nonalcoholic fatty liver disease: novel mechanisms and treatment strategies. Trends Endocrinol Metabol. 2017;28:250–260. doi: 10.1016/j.tem.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 43.Palma E., Ma X., Riva A., Iansante V., Dhawan A., Wang S., et al. Dynamin-1-like protein inhibition drives megamitochondria formation as an adaptive response in alcohol-induced hepatotoxicity. Am J Pathol. 2019;189:580–589. doi: 10.1016/j.ajpath.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lionetti L., Mollica M.P., Donizzetti I., Gifuni G., Sica R., Pignalosa A., et al. High-lard and high-fish-oil diets differ in their effects on function and dynamic behaviour of rat hepatic mitochondria. PLoS One. 2014;9 doi: 10.1371/journal.pone.0092753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuhlbrandt W. Structure and function of mitochondrial membrane protein complexes. BMC Biol. 2015;13:89. doi: 10.1186/s12915-015-0201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vincent A.E., Ng Y.S., White K., Davey T., Mannella C., Falkous G., et al. The spectrum of mitochondrial ultrastructural defects in mitochondrial myopathy. Sci Rep. 2016;6:30610. doi: 10.1038/srep30610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dara L., Ji C., Kaplowitz N. The contribution of endoplasmic reticulum stress to liver diseases. Hepatology. 2011;53:1752–1763. doi: 10.1002/hep.24279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu X., Green R.M. Endoplasmic reticulum stress and liver diseases. Liver Res. 2019;3:55–64. doi: 10.1016/j.livres.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delli Bovi A.P., Marciano F., Mandato C., Siano M.A., Savoia M., Vajro P. Oxidative stress in non-alcoholic fatty liver disease. An updated mini review. Front Med. 2021;8:595371. doi: 10.3389/fmed.2021.595371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masarone M., Rosato V., Dallio M., Gravina A.G., Aglitti A., Loguercio C., et al. Role of oxidative stress in pathophysiology of nonalcoholic fatty liver disease. Oxid Med Cell Longev. 2018;2018:9547613. doi: 10.1155/2018/9547613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sies H. In: Oxidative stress. Sies H., editor. Academic Press; London: 1985. Oxidative stress: introductory remarks; pp. 1–8. [Google Scholar]
- 52.Savini I., Catani M.V., Evangelista D., Gasperi V., Avigliano L. Obesity-associated oxidative stress: strategies finalized to improve redox state. Int J Mol Sci. 2013;14:10497–10538. doi: 10.3390/ijms140510497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kozlov A.V., Lancaster J.R., Jr., Meszaros A.T., Weidinger A. Mitochondria-meditated pathways of organ failure upon inflammation. RedoxBiol. 2017;13:170–181. doi: 10.1016/j.redox.2017.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhong Z., Lemasters J.J. A unifying hypothesis linking hepatic adaptations for ethanol metabolism to the proinflammatory and profibrotic events of alcoholic liver disease. Alcohol Clin Exp Res. 2018;42:2072–2089. doi: 10.1111/acer.13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bravo R., Gutierrez T., Paredes F., Gatica D., Rodriguez A.E., Pedrozo Z., et al. Endoplasmic reticulum: ER stress regulates mitochondrial bioenergetics. Int J Biochem Cell Biol. 2012;44:16–20. doi: 10.1016/j.biocel.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plate L., Wiseman R.L. Regulating secretory proteostasis through the unfolded protein response: from function to therapy. Trends Cell Biol. 2017;27:722–737. doi: 10.1016/j.tcb.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vladykovskaya E., Sithu S.D., Haberzettl P., Wickramasinghe N.S., Merchant M.L., Hill B.G., et al. Lipid peroxidation product 4-hydroxy-trans-2-nonenal causes endothelial activation by inducing endoplasmic reticulum stress. J Biol Chem. 2012;287:11398–11409. doi: 10.1074/jbc.M111.320416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mohammad M.K., Avila D., Zhang J., Barve S., Arteel G., McClain C., et al. Acrolein cytotoxicity in hepatocytes involves endoplasmic reticulum stress, mitochondrial dysfunction and oxidative stress. Toxicol Appl Pharmacol. 2012;265:73–82. doi: 10.1016/j.taap.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sozen E., Ozer N.K. Impact of high cholesterol and endoplasmic reticulum stress on metabolic diseases: an updated mini-review. Redox Biol. 2017;12:456–461. doi: 10.1016/j.redox.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tubbs E., Theurey P., Vial G., Bendridi N., Bravard A., Chauvin M.A., et al. Mitochondria-associated endoplasmic reticulum membrane (MAM) integrity is required for insulin signaling and is implicated in hepatic insulin resistance. Diabetes. 2014;63:3279–3294. doi: 10.2337/db13-1751. [DOI] [PubMed] [Google Scholar]
- 61.Rieusset J. The role of endoplasmic reticulum-mitochondria contact sites in the control of glucose homeostasis: an update. Cell Death Dis. 2018;9:388. doi: 10.1038/s41419-018-0416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arruda A.P., Pers B.M., Parlakgul G., Guney E., Inouye K., Hotamisligil G.S. Chronic enrichment of hepatic endoplasmic reticulum–mitochondria contact leads to mitochondrial dysfunction in obesity. Nat Med. 2014;20:1427–1435. doi: 10.1038/nm.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vance J.E. Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem. 1990;265:7248–7256. [PubMed] [Google Scholar]
- 64.Szabadkai G., Bianchi K., Varnai P., De Stefani D., Wieckowski M.R., Cavagna D., et al. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stone S.J., Vance J.E. Phosphatidylserine synthase-1 and -2 are localized to mitochondria-associated membranes. J Biol Chem. 2000;275:34534–34540. doi: 10.1074/jbc.M002865200. [DOI] [PubMed] [Google Scholar]
- 66.Vance J.E. MAM (mitochondria-associated membranes) in mammalian cells: lipids and beyond. Biochim Biophys Acta. 2014;1841:595–609. doi: 10.1016/j.bbalip.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 67.Tan W., Colombini M. VDAC closure increases calcium ion flux. Biochim Biophys Acta. 2007;1768:2510–2515. doi: 10.1016/j.bbamem.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garrido C., Galluzzi L., Brunet M., Puig P.E., Didelot C., Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006;13:1423–1433. doi: 10.1038/sj.cdd.4401950. [DOI] [PubMed] [Google Scholar]
- 69.Rieusset J. Endoplasmic reticulum–mitochondria calcium signaling in hepatic metabolic diseases. Biochim Biophys Acta Mol Cell Res. 2017;1864:865–876. doi: 10.1016/j.bbamcr.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 70.Chen W.Y., Zhang J., Ghare S., Barve S., McClain C., Joshi-Barve S. Acrolein is a pathogenic mediator of alcoholic liver disease and the scavenger hydralazine is protective in mice. Cell Mol Gastroenterol Hepatol. 2016;2:685–700. doi: 10.1016/j.jcmgh.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mitchell T., Chacko B., Ballinger S.W., Bailey S.M., Zhang J., Darley-Usmar V. Convergent mechanisms for dysregulation of mitochondrial quality control in metabolic disease: implications for mitochondrial therapeutics. Biochem Soc Trans. 2013;41:127–133. doi: 10.1042/BST20120231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Calabrese E.J., Baldwin L.A. Defining hormesis. Hum Exp Toxicol. 2002;21:91–97. doi: 10.1191/0960327102ht217oa. [DOI] [PubMed] [Google Scholar]
- 73.Yun J., Finkel T. Mitohormesis. Cell Metabol. 2014;19:757–766. doi: 10.1016/j.cmet.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.MacInnis M.J., Gibala M.J. Physiological adaptations to interval training and the role of exercise intensity. J Physiol. 2017;595:2915–2930. doi: 10.1113/JP273196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kolb H., Eizirik D.L. Resistance to type 2 diabetes mellitus: a matter of hormesis?. Nat Rev Endocrinol. 2011;8:183–192. doi: 10.1038/nrendo.2011.158. [DOI] [PubMed] [Google Scholar]
- 76.Stickel F., Hampe J. Genetic determinants of alcoholic liver disease. Gut. 2012;61:150–159. doi: 10.1136/gutjnl-2011-301239. [DOI] [PubMed] [Google Scholar]
- 77.Li Z., Li Y., Zhang H.X., Guo J.R., Lam C.W.K., Wang C.Y., et al. Mitochondria-mediated pathogenesis and therapeutics for non-alcoholic fatty liver disease. Mol Nutr Food Res. 2019;63 doi: 10.1002/mnfr.201900043. [DOI] [PubMed] [Google Scholar]
- 78.Prasun P., Ginevic I., Oishi K. Mitochondrial dysfunction in nonalcoholic fatty liver disease and alcohol related liver disease. Transl Gastroenterol Hepatol. 2021;6:4. doi: 10.21037/tgh-20-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fromenty B., Pessayre D. Inhibition of mitochondrial beta-oxidation as a mechanism of hepatotoxicity. Pharmacol Ther. 1995;67:101–154. doi: 10.1016/0163-7258(95)00012-6. [DOI] [PubMed] [Google Scholar]
- 80.Larosche I., Lettéron P., Berson A., Fromenty B., Huang T.T., Moreau R., et al. Hepatic mitochondrial DNA depletion after an alcohol binge in mice: probable role of peroxynitrite and modulation by manganese superoxide dismutase. J Pharmacol Exp Therapeut. 2010;332:886–897. doi: 10.1124/jpet.109.160879. [DOI] [PubMed] [Google Scholar]
- 81.Wahlang B., Beier J.I., Clair H.B., Bellis-Jones H.J., Falkner K.C., McClain C.J., et al. Toxicant-associated steatohepatitis. Toxicol Pathol. 2013;41:343–360. doi: 10.1177/0192623312468517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lang A.L., Beier J.I. Interaction of volatile organic compounds and underlying liver disease: a new paradigm for risk. Biol Chem. 2018;399:1237–1248. doi: 10.1515/hsz-2017-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.United States Environmental Protection Agency. Ambient water quality criteria for vinyl; chloride. 2017. Available from: https://www.epa.gov/indoor-air-quality-iaq/volatile-organic-compounds-impact-indoor-air-quality. [DOI] [PubMed]
- 84.Cleary E., Asher M., Olawoyin R., Zhang K. Assessment of indoor air quality exposures and impacts on respiratory outcomes in River Rouge and Dearborn, Michigan. Chemosphere. 2017;187:320–329. doi: 10.1016/j.chemosphere.2017.08.091. [DOI] [PubMed] [Google Scholar]
- 85.United States Environmental ProtectionAgency. Ambient water quality criteria for vinyl; chloride. 2017. Available from: https://www.epa.gov/sites/default/files/2019-03/documents/ambient-wqc-vinylchloride-1980.pdf.
- 86.Wahlang B., Hardesty J.E., Head K.Z., Jin J., Falkner K.C., Prough R.A., et al. Hepatic injury caused by the environmental toxicant vinyl chloride is sex-dependent in mice. Toxicol Sci. 2020;174:79–91. doi: 10.1093/toxsci/kfz236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen L., Lang A.L., Poff G.D., Ding W.X., Beier J.I. Vinyl chloride-induced interaction of nonalcoholic and toxicant-associated steatohepatitis: protection by the ALDH2 activator Alda-1. Redox Biol. 2019;24:101205. doi: 10.1016/j.redox.2019.101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Anders L.C., Lang A.L., Anwar-Mohamed A., Douglas A.N., Bushau A.M., Falkner K.C., et al. Vinyl chloride metabolites potentiate inflammatory liver injury caused by LPS in mice. Toxicol Sci. 2016;151:312–323. doi: 10.1093/toxsci/kfw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Szadkowski A., Myers C.R. Acrolein oxidizes the cytosolic and mitochondrial thioredoxins in human endothelial cells. Toxicology. 2008;243:164–176. doi: 10.1016/j.tox.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Landar A., Zmijewski J.W., Dickinson D.A., Le Goffe C., Johnson M.S., Milne G.L., et al. Interaction of electrophilic lipid oxidation products with mitochondria in endothelial cells and formation of reactive oxygen species. Am J Physiol Heart Circ Physiol. 2006;290:H1777–H1787. doi: 10.1152/ajpheart.01087.2005. [DOI] [PubMed] [Google Scholar]
- 91.Takano T., Miyazaki Y. Effect of chlorinated ethanes and ethylenes on electron transport in rat liver mitochondria. J Toxicol Sci. 1982;7:143–149. doi: 10.2131/jts.7.143. [DOI] [PubMed] [Google Scholar]
- 92.Higdon A.N., Benavides G.A., Chacko B.K., Ouyang X., Johnson M.S., Landar A., et al. Hemin causes mitochondrial dysfunction in endothelial cells through promoting lipid peroxidation: the protective role of autophagy. Am J Physiol Heart Circ Physiol. 2012;302:H1394–H1409. doi: 10.1152/ajpheart.00584.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dezest M., Le Bechec M., Chavatte L., Desauziers V., Chaput B., Grolleau J.L., et al. Oxidative damage and impairment of protein quality control systems in keratinocytes exposed to a volatile organic compounds cocktail. Sci Rep. 2017;7:10707. doi: 10.1038/s41598-017-11088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mansouri A., Gattolliat C.H., Asselah T. Mitochondrial dysfunction and signaling in chronic liver diseases. Gastroenterology. 2018;155:629–647. doi: 10.1053/j.gastro.2018.06.083. [DOI] [PubMed] [Google Scholar]
- 95.Malik A.N., Simoes I.C.M., Rosa H.S., Khan S., Karkucinska-Wieckowska A., Wieckowski M.R. A diet induced maladaptive increase in hepatic mitochondrial DNA precedes OXPHOS defects and may contribute to non-alcoholic fatty liver disease. Cells. 2019;8:1222. doi: 10.3390/cells8101222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Czajka A., Ajaz S., Gnudi L., Parsade C.K., Jones P., Reid F., et al. Altered mitochondrial function, mitochondrial DNA and reduced metabolic flexibility in patients with diabetic nephropathy. EBioMedicine. 2015;2:499–512. doi: 10.1016/j.ebiom.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sampey B.P., Stewart B.J., Petersen D.R. Ethanol-induced modulation of hepatocellular extracellular signal-regulated kinase-1/2 activity via 4-hydroxynonenal. J Biol Chem. 2007;282:1925–1937. doi: 10.1074/jbc.M610602200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Horimoto M., Fulop P., Derdak Z., Wands J.R., Baffy G. Uncoupling protein-2 deficiency promotes oxidant stress and delays liver regeneration in mice. Hepatology. 2004;39:386–392. doi: 10.1002/hep.20047. [DOI] [PubMed] [Google Scholar]
- 99.Bolt H.M. Vinyl chloride-a classical industrial toxicant of new interest. Crit Rev Toxicol. 2005;35:307–323. doi: 10.1080/10408440490915975. [DOI] [PubMed] [Google Scholar]
- 100.Boveris A., Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xu T., Liu S., Ma T., Jia Z., Zhang Z., Wang A. Aldehyde dehydrogenase 2 protects against oxidative stress associated with pulmonary arterial hypertension. Redox biology. 2017;11:286–296. doi: 10.1016/j.redox.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen C.H., Budas G.R., Churchill E.N., Disatnik M.H., Hurley T.D., Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–1495. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ding X., Beier J.I., Baldauf K.J., Jokinen J.D., Zhong H., Arteel G.E. Acute ethanol preexposure promotes liver regeneration after partial hepatectomy in mice by activating ALDH2. Am J Physiol Gastrointest Liver Physiol. 2014;306:G37–G47. doi: 10.1152/ajpgi.00085.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li H., Toth E., Cherrington N.J. Alcohol metabolism in the progression of human nonalcoholic steatohepatitis. Toxicol Sci. 2018;164:428–438. doi: 10.1093/toxsci/kfy106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zorova L.D., Popkov V.A., Plotnikov E.Y., Silachev D.N., Pevzner I.B., Jankauskas S.S., et al. Mitochondrial membrane potential. Anal Biochem. 2018;552:50–59. doi: 10.1016/j.ab.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guardiola J.J., Beier J.I., Falkner K.C., Wheeler B., McClain C.J., Cave M. Occupational exposures at a polyvinyl chloride production facility are associated with significant changes to the plasma metabolome. Toxicol Appl Pharmacol. 2016;313:47–56. doi: 10.1016/j.taap.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang Q., Zhang L., Chen S.Q., Ma W.Y., Guo Y.L., Gao Y., et al. Role of endoplasmic reticulum stress and oxidative stress in vinyl chloride-induced hepatic steatosis in mice. Toxicol Appl Pharmacol. 2019:114730. doi: 10.1016/j.taap.2019.114730. [DOI] [PubMed] [Google Scholar]
- 108.Wang M., Kaufman R.J. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature. 2016;529:326–335. doi: 10.1038/nature17041. [DOI] [PubMed] [Google Scholar]
- 109.Cave M., Falkner K.C., McClain C.J. In: Boyer D.T., Manns M.P., Sanyal A.J., editors. vol. 6. Saunders; Philadelphia: 2012. Occupational and environmental hepatotoxicity; pp. 476–492.https://epdf.pub/zakim-and-boyers-hepatology-a-textbook-of-liver-disease-6th-edition.html (Zakim and Boyer's hepatology). Available from: [Google Scholar]
- 110.Tolman K.G., Sirrine R. Occupational hepatotoxicity. Clin Liver Dis. 1998;2:563–589. [Google Scholar]
- 111.Al-Eryani L., Wahlang B., Falkner K.C., Guardiola J.J., Clair H.B., Prough R.A., et al. Identification of environmental chemicals associated with the development of toxicant-associated fatty liver disease in rodents. Toxicol Pathol. 2015;43:482–497. doi: 10.1177/0192623314549960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Meyer J.N., Leung M.C., Rooney J.P., Sendoel A., Hengartner M.O., Kisby G.E., et al. Mitochondria as a target of environmental toxicants. Toxicol Sci. 2013;134:1–17. doi: 10.1093/toxsci/kft102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ramachandran A., Visschers R.G.J., Duan L., Akakpo J.Y., Jaeschke H. Mitochondrial dysfunction as a mechanism of drug-induced hepatotoxicity: current understanding and future perspectives. J Clin Transl Res. 2018;4:75–100. doi: 10.18053/jctres.04.201801.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kaiser J.P., Lipscomb J.C., Wesselkamper S.C. Putative mechanisms of environmental chemical-induced steatosis. Int J Toxicol. 2012;31:551–563. doi: 10.1177/1091581812466418. [DOI] [PubMed] [Google Scholar]
- 115.Moghe A., Ghare S., Lamoreau B., Mohammad M., Barve S., McClain C., et al. Molecular mechanisms of acrolein toxicity: relevance to human disease. Toxicol Sci. 2015;143:242–255. doi: 10.1093/toxsci/kfu233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hassoun E., Cearfoss J., Mamada S., Al-Hassan N., Brown M., Heimberger K., et al. The effects of mixtures of dichloroacetate and trichloroacetate on induction of oxidative stress in livers of mice after subchronic exposure. J Toxicol Environ Health. 2014;77:313–323. doi: 10.1080/15287394.2013.864576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hassoun E., Mettling C. Dichloroacetate and trichloroacetate toxicity in AML12 cells: role of oxidative stress. J Biochem Mol Toxicol. 2015;29:508–512. doi: 10.1002/jbt.21720. [DOI] [PubMed] [Google Scholar]
- 118.Wang C., Yang J., Lu D., Fan Y., Zhao M., Li Z. Oxidative stress-related DNA damage and homologous recombination repairing induced by N,N-dimethylformamide. J Appl Toxicol. 2016;36:936–945. doi: 10.1002/jat.3226. [DOI] [PubMed] [Google Scholar]
- 119.Chen P., Bornhorst J., Diana Neely M., Avila D.S. Mechanisms and disease pathogenesis underlying metal-induced oxidative stress. Oxid Med Cell Longev. 2018;2018:7612172. doi: 10.1155/2018/7612172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nault R., Doskey C.M., Fader K.A., Rockwell C.E., Zacharewski T. Comparison of hepatic NRF2 and aryl hydrocarbon receptor binding in 2,3,7,8-tetrachlorodibenzo-p-dioxin-treated mice demonstrates NRF2-independent PKM2 induction. Mol Pharmacol. 2018;94:876–884. doi: 10.1124/mol.118.112144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Deng P., Barney J., Petriello M.C., Morris A.J., Wahlang B., Hennig B. Hepatic metabolomics reveals that liver injury increases PCB 126-induced oxidative stress and metabolic dysfunction. Chemosphere. 2019;217:140–149. doi: 10.1016/j.chemosphere.2018.10.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sun Y.J., Cao Q.J., Xu M.Y., Yang L., Wu Y.J. Individual and combined hepatocytotoxicity of DDT and cadmium in vitro. Toxicol Ind Health. 2021;37 doi: 10.1177/07482337211007361. [DOI] [PubMed] [Google Scholar]
- 123.Arteel G.E., Guo L., Schlierf T., Beier J.I., Kaiser J.P., Chen T.S., et al. Subhepatotoxic exposure to arsenic enhances lipopolysaccharide-induced liver injury in mice. Toxicol Appl Pharmacol. 2008;226:128–139. doi: 10.1016/j.taap.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dallio M., Diano N., Masarone M., Gravina A.G., Patanè V., Romeo M., et al. Chemical effect of bisphenol A on non-alcoholic fatty liver disease. Int J Environ Res Publ Health. 2019;16:3134. doi: 10.3390/ijerph16173134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Begriche K., Igoudjil A., Pessayre D., Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 126.Moon M.K., Kim M.J., Jung I.K., Koo Y.D., Ann H.Y., Lee K.J., et al. Bisphenol A impairs mitochondrial function in the liver at doses below the no observed adverse effect level. J Kor Med Sci. 2012;27:644–652. doi: 10.3346/jkms.2012.27.6.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Khan S., Beigh S., Chaudhari B.P., Sharma S., Aliul Hasan Abdi S., Ahmad S., et al. Mitochondrial dysfunction induced by bisphenol A is a factor of its hepatotoxicity in rats. Environ Toxicol. 2016;31:1922–1934. doi: 10.1002/tox.22193. [DOI] [PubMed] [Google Scholar]
- 128.Zhang Y., Li K., Kong A., Zhou Y., Chen D., Gu J., et al. Dysregulation of autophagy acts as a pathogenic mechanism of non-alcoholic fatty liver disease (NAFLD) induced by common environmental pollutants. Ecotoxicol Environ Saf. 2021;217:112256. doi: 10.1016/j.ecoenv.2021.112256. [DOI] [PubMed] [Google Scholar]
- 129.Klaunig J.E., Li X., Wang Z. Role of xenobiotics in the induction and progression of fatty liver disease. Toxicol Res. 2018;7:664–680. doi: 10.1039/c7tx00326a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Byun H.M., Baccarelli A.A. Environmental exposure and mitochondrial epigenetics: study design and analytical challenges. Hum Genet. 2014;133:247–257. doi: 10.1007/s00439-013-1417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tommasi S., Besaratinia A. DNA hydroxymethylation at the interface of the environment and nonalcoholic fatty liver disease. Int J Environ Res Publ Health. 2019;16:2791. doi: 10.3390/ijerph16152791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Meyer J.N., Hartman J.H., Mello D.F. Mitochondrial toxicity. Toxicol Sci. 2018;162:15–23. doi: 10.1093/toxsci/kfy008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Byun H.M., Panni T., Motta V., Hou L., Nordio F., Apostoli P., et al. Effects of airborne pollutants on mitochondrial DNA methylation. Part Fibre Toxicol. 2013;10:18. doi: 10.1186/1743-8977-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sharma N., Pasala M.S., Prakash A. Mitochondrial DNA: epigenetics and environment. Environ Mol Mutagen. 2019;60:668–682. doi: 10.1002/em.22319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhong J., Karlsson O., Wang G., Li J., Guo Y., Lin X., et al. B vitamins attenuate the epigenetic effects of ambient fine particles in a pilot human intervention trial. Proc Natl Acad Sci U S A. 2017;114:3503–3508. doi: 10.1073/pnas.1618545114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Byun H.M., Benachour N., Zalko D., Frisardi M.C., Colicino E., Takser L., et al. Epigenetic effects of low perinatal doses of flame retardant BDE-47 on mitochondrial and nuclear genes in rat offspring. Toxicology. 2015;328:152–159. doi: 10.1016/j.tox.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sanyal T., Bhattacharjee P., Bhattacharjee S., Bhattacharjee P. Hypomethylation of mitochondrial D-loop and ND6 with increased mitochondrial DNA copy number in the arsenic-exposed population. Toxicology. 2018;408:54–61. doi: 10.1016/j.tox.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 138.Frye M., Jaffrey S.R., Pan T., Rechavi G., Suzuki T. RNA modifications: what have we learned and where are we headed?. Nat Rev Genet. 2016;17:365–372. doi: 10.1038/nrg.2016.47. [DOI] [PubMed] [Google Scholar]
- 139.Sarkar A., Gasperi W., Begley U., Nevins S., Huber S.M., Dedon P.C., et al. Wiley Interdiscip Rev RNA; 2021. Detecting the epitranscriptome; p. e1663. [DOI] [PubMed] [Google Scholar]
- 140.Cayir A., Byun H.M., Barrow T.M. Environmental epitranscriptomics. Environ Res. 2020;189:109885. doi: 10.1016/j.envres.2020.109885. [DOI] [PubMed] [Google Scholar]
- 141.Tapper E.B., Parikh N.D. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: observational study. BMJ. 2018;362 doi: 10.1136/bmj.k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dwyer-Lindgren L., Bertozzi-Villa A., Stubbs R.W., Morozoff C., Kutz M.J., Huynh C., et al. US county-level trends in mortality rates for major causes of death, 1980–2014. J Am Med Assoc. 2016;316:2385–2401. doi: 10.1001/jama.2016.13645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Goldberg D., Ross-Driscoll K., Lynch R. County differences in liver mortality in the United States: impact of sociodemographics, disease risk factors, and access to care. Gastroenterology. 2021;160:1140–11450.e1. doi: 10.1053/j.gastro.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Smith A., Laribi O. Environmental justice in the american public health context: trends in the scientific literature at the intersection between health, environment, and social status. J Racial Ethn Health Disparities. 2021 doi: 10.1007/s40615-020-00949-7. Available from: [DOI] [PubMed] [Google Scholar]
- 145.Arteel G.E. Leveraging oxidative stress questions in vivo: implications and limitations. Arch Biochem Biophys. 2016;595:40–45. doi: 10.1016/j.abb.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.European Chemicals Agency (ECHA) Finnland; Helsinki: 2016. New approach methodologies in regulatory science; pp. 1–65.https://echa.europa.eu/documents/10162/21838212/scientific_ws_proceedings_en.pdf/a2087434-0407-4705-9057-95d9c2c2cc57 Available from: [Google Scholar]
- 147.Friedman KP. Duke risk assessment class. Center for Computational Toxicology and Exposure, Office of Research and Development, US EPA. Avialable from: https://www.epa.gov/chemical-research/epa-new-approach-methods-work-plan-reducing-use-animals-chemical-testing.
- 148.Karami-Mohajeri S., Abdollahi M. Mitochondrial dysfunction and organophosphorus compounds. Toxicol Appl Pharmacol. 2013;270:39–44. doi: 10.1016/j.taap.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 149.Karami-Mohajeri S., Ahmadipour A., Rahimi H.R., Abdollahi M. Adverse effects of organophosphorus pesticides on the liver: a brief summary of four decades of research. Arh Hig Rada Toksikol. 2017;68:261–275. doi: 10.1515/aiht-2017-68-2989. [DOI] [PubMed] [Google Scholar]
- 150.Leung M.C.K., Meyer J.N. Mitochondria as a target of organophosphate and carbamate pesticides: revisiting common mechanisms of action with new approach methodologies. Reprod Toxicol. 2019;89:83–92. doi: 10.1016/j.reprotox.2019.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]