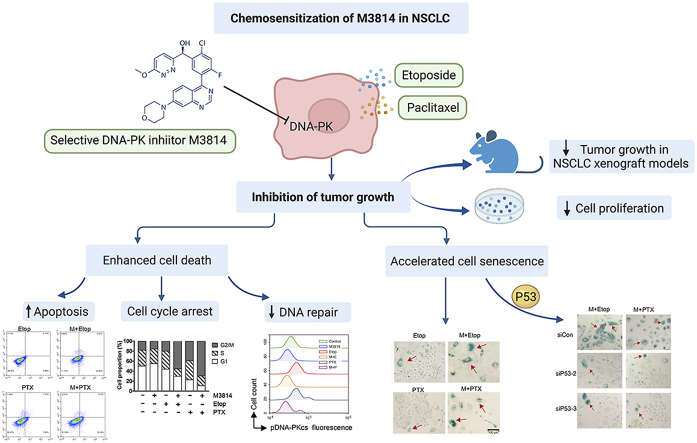
Abstract
A significant proportion of non-small cell lung cancer (NSCLC) patients experience accumulating chemotherapy-related adverse events, motivating the design of chemosensitizating strategies. The main cytotoxic damage induced by chemotherapeutic agents is DNA double-strand breaks (DSB). It is thus conceivable that DNA-dependent protein kinase (DNA-PK) inhibitors which attenuate DNA repair would enhance the anti-tumor effect of chemotherapy. The present study aims to systematically evaluate the efficacy and safety of a novel DNA-PK inhibitor M3814 in synergy with chemotherapies on NSCLC. We identified increased expression of DNA-PK in human NSCLC tissues which was associated with poor prognosis. M3814 potentiated the anti-tumor effect of paclitaxel and etoposide in A549, H460 and H1703 NSCLC cell lines. In the four combinations based on two NSCLC xenograft models and two chemotherapy, we also observed tumor regression at tolerated doses in vivo. Moreover, we identified a P53-dependent accelerated senescence response by M3814 following treatment with paclitaxel/etoposide. The present study provides a theoretical basis for the use of M3814 in combination with paclitaxel and etoposide in clinical practice, with hope to aid the optimization of NSCLC treatment.
KEY WORDS: M3814, Paclitaxel, Etoposide, DNA-dependent protein kinase, Non-small cell lung cancer, DNA repair, Cell senescence, Chemosensitization
Abbreviations: DDR, DNA damage response; DNA-PK, DNA-dependent protein kinase; DNA-PKcs, DNA-dependent protein kinase catalytic subunit; DSB, DNA double-strand breaks; dsDNA, double strand DNA; HR, homologous recombination; IHC, immunohistochemistry; LADC, lung adenocarcinoma; LCLC, large-cell carcinoma; LSCC, lung squamous cell carcinoma; NHEJ, non homologous end joining; NSCLC, non-small cell lung cancer
Graphical abstract
This study elucidates the chemosensitization effect of M3814, a new-generation DNA-PK inhibitor on non-small cell lung cancer, providing theoretical bases for the clinical application of M3814-chemotherapy synergy.
1. Introduction
Non-small cell lung cancer (NSCLC) is a worldwide malignancy and the leading cause of cancer-related mortality1. Surgical resection remains the primary treatment for NSCLC at early stage2. On one hand, a large proportion of patients are diagnosed at advanced stage and are thus ineligible for operations3. On the other hand, only a small number of patients benefit from the postoperative adjuvant chemotherapy4.
DNA double-strand breaks (DSBs) are considered as the most lethal form of cytotoxic damage5, 6, 7. DSB can be caused by endogenous or exogenous toxins, and if left unrepaired, can lead to cell cycle arrest and cell deaths. The potent DNA damage repair system in tumor cells includes non homologous end joining (NHEJ) and homologous recombination (HR) pathway, contributing to the therapeutic resistance of tumor cells8. HR repair pathway is mostly active during S phase, whereas NHEJ pathway can occur in all cell cycle phases, suggesting that HR mainly repairs DSBs that occur during DNA replication9. It also has to be addressed that the DNA repair kinetics of NHEJ pathway is faster than that of HR pathway10,11. NHEJ is thus characterized as the major DSB repair pathway in mammalian cells.
Previous reports have emphasized DNA-dependent protein kinase (DNA-PK) as an essential component of the NHEJ pathway12. DNA-PK is a serine/threonine protein kinase consisting of the catalytic subunit of DNA-PK (DNA-PKcs), and a heterodimer composed of the Ku70 and Ku80 subunits13. In the light of the crucial function of DNA-PK in DNA damage response (DDR), DNA-PK targeted therapy potentially works in synergy with DNA-damaging agents14. Once DSBs are induced, the Ku70/80 heterodimers rapidly recognize and flock to the DNA damage sites15, 16, 17, and form a cyclic structure which stabilizes the broken ends of double strand DNA (dsDNA). Furthermore, Ku heterodimers recruit DNA-PKcs to form a synaptic complex at the DNA termini which holds the broken ends of dsDNA together18, 19, 20, 21. The binding of DNA-PKcs to the DSB sites then leads to the translocation of the Ku70/80 and the subsequent activation of DNA-PKcs22,23.
Substantial literature has identified relatively higher expression of DNA-PK in cancer tissues. For example, compared with adjacent normal mucosa, esophageal cancer tissues exhibit increased DNA-PK expression by 49%. The death risk of NSCLC patients with high DNA-PKcs expression is 2.13 times higher than that with normal expression24. On the other hand, the expression of DNA-PK in tumor cells is significantly correlated with tumor response to DNA-damaging agents25. In the residual cervical cancer cells that were resistant to radiotherapy, the expression of DNA-PKcs was markedly elevated, indicating a positive correlation between radiation resistance and DNA-PK expression levels26. Likewise, the down-regulation of DNA-PKcs was correlated with the increased radiosensitivity of lymphoblastic cell lines27, whereas the increased DNA-PKcs expression limited the response to standard radiotherapy in prostate cancer patients28.
Based on these findings, it is conceivable that the inhibition of DNA-PK is an attractive strategy to overcome resistance to DSB-inducing treatments in cancer cells. Old-generation DNA-PK inhibitors have been proved effective but are limited in terms of selectivity due to structural similarities with PI3Ks29. M3814 represents the new-generation of selective DNA-PK protein kinase inhibitors30. Recent preclinical studies mainly focused on M3814 activity in combination with chemotherapies in ovarian cancer31 and acute myeloid leukemia (AML)32, or with ionizing radiation in lung cancer33. The aim of the present study is to evaluate the efficacy and safety of M3814 in synergy with paclitaxel and etoposide to treat NSCLC, and to explore the possible mechanism of action, so as to provide theoretical basis for the use of M3814 in combination with paclitaxel and etoposide in clinical practice.
2. Materials and methods
2.1. Patient cohorts and tissue specimens
Tumor specimens and clinical data of patients were provided by Shanghai Outdo Biotech (National Engineering Centre for Biochip at Shanghai, China). The informed consent was obtained from patients and the study was approved by the Ethics Committee of National Human Genetic Resources Sharing Service Platform (permit number: 2005DKA21300). Tumor tissues and their adjacent normal tissue specimens were obtained from 174 patients diagnosed with non-small cell lung cancer who underwent surgical resections between July 2004 and June 2006. The median survival time was defined as the time duration between initial diagnosis of NSCLC to the final follow-up visit. Tissue specimens were immersed in 4% paraformaldehyde, embedded in paraffin, and then stained with anti-DNA-PKcs antibody (CST, #12311S). The paraffin-embedded tissues were independently graded by two board-certified pathologists. Based on the percentage of cells positive for DNA-PKcs nuclear staining in the whole field, the positive staining rate of tissues were scored as follows: 0 (negative), 1 (1%–25%), 2 (26%–50%), 3 (51%–75%), 4 (76%–100%), whereas the staining intensity was scored as: 0 (negative), 1 (weak), 2 (medium), and 3 (strong). Final expression scores were recorded as positive staining rates × staining intensity.
2.2. Reagents and antibodies
M3814 was purchased from Medchemexpress (MCE, HY-101570). Paclitaxel and etoposide phosphate were purchased form Selleck Chemicals (S1150) and Meilunbio (MB1102), respectively. For in vitro studies, M3814 and etoposide was initially dissolved in dimethyl sulfoxide (DMSO; Sigma) as 100 mmol/L stock solution and stored at −20 °C. The stock solution was diluted to reach corresponding concentrations and added to cell cultures. Treatment controls were set as the 0.5% DMSO, the same volume as the other groups. For in vivo experiments, M3814 were orderly added with the following solvent: 10% DMSO, 40% PEG400 (Sigma–Aldrich), 5% Tween-80 (Sigma–Aldrich) and 45% saline. Anti-phospho-histone γ-H2AX (Ser139) was purchased from Abcam (ab11174; Cambridge, UK). Anti-P53 antibody was purchased from HUABIO (ET1601-13; Hangzhou, China). Anti-P21 (2947S), anti-DNA-PKcs (12311S) and anti-p-DNA-PK (Ser2056, 68716S) antibodies were purchased from Cell Signaling Technology (CST, MA, USA). Anti-GAPDH antibody was purchased from Santa Cruz Biotechnology (sc-32233; Santa Cruz, CA, USA). Fluorescent secondary antibodies were obtained from Invitrogen.
2.3. Cell lines and culture
The human NSCLC cell lines A549, H460 and H1703 were provided by the American Type Culture Collection (ATCC, Rockville, MD, USA). Cell lines were cultured in RPMI 1640 containing 10% fetal bovine serum (FBS; Gibco) and 1% antibiotics (penicillin and streptomycin, Gibco) at 37 °C with 5% CO2.
2.4. Cell viability assay
Cell viability of NSCLC cell lines treated with either M3814 alone, or in combination with etoposide was evaluated by the Counting Kit-8 (CCK-8) provided by MCE, according to the kit instructions. In brief, 100 μL of A549 and H460 cells were seeded in 96-well plates (1000–4000 cells/well) and pre-incubated for 24 h in a humidified incubator at 37 °C, 5% CO2. Cells were treated with paclitaxel and etoposide (concentration gradient from 1 nmol/L to 100 μmol/L), in the absence or presence of M3814 (5 μmol/L). After incubation for an appropriate length of time (24, 48 and 72 h), 10 μL of CCK-8 solution and 90 μL fresh medium were added to each well and followed by 1–4 h of incubation at 37 °C. The absorbance of plates was measured at 450 nm using a microplate reader, and the half inhibitory concentration (IC50) was calculated using Prism 7 (GraphPad software). The combination index (CI) of M3814 and chemotherapy was calculated with CalculSyn software using Chou Talalay method. Among them, synergistic effect is CI < 1, cumulative effect is CI = 1, antagonistic effect is CI > 134.
2.5. Colony formation assays
A549 and H460 cells were treated with paclitaxel and etoposide in the absence or in the presence of M3814 (5 μmol/L) for 24 h, and then cultured in 6-well plates for another 14 days. Rinse wells with PBS for 3 times and stain cell colonies with crystal violet 0.5% solution for 10 min. After air drying at room temperature, plates were photographed under light microscopy for analyzing the number of colonies.
2.6. EdU-based proliferation assay
EdU (5-ethynyl-2′-deoxyuridine) is a thymine nucleoside analogue which can be inserted into replicating DNA when tumor cells proliferate, thus directly reflecting DNA replication activity. Click-iT EdU Assay Kit (RiBo Bio, Guangzhou, China) was used to label proliferating human NSCLC cells following the manufacturer's recommendation. In brief, A549 and H460 cells were seeded in 96-well plates overnight, and treated with paclitaxel (5 nmol/L) and etoposide (100 nmol/L) with or without M3814 (5 μmol/L) for 18 h. We next added EdU solutions to the plates and incubated for 2–3 h in the dark at 37 °C to enable the incorporation of EdU into the DNA. We then washed the plates and observed cells under the inverted fluorescent microscope (Carl Zeiss, Germany). EdU-positive cells (3 random fields at 10 ×) were quantified using ImageJ software. The EdU flow cytometry assay was performed according to manufacturer's instructions.
2.7. Cell apoptosis assays by flow cytometry
A549 and H460 cells were seeded in 6-well plates at 1 × 105 per well. Paclitaxel (5 nmol/L) and etoposide (100 nmol/L) with or without M3814 (5 μmol/L) was added to each well. The DMSO-treated group was used as the control. After incubation for 48 h, the cells were performed with Annexin V-FITC apoptosis detection kit. Briefly, the cells were harvested and washed twice with cold PBS and then resuspended. Each sample was stained with 5 μL of Annexin V-FITC for 10 min and subsequently stained with 5 μL of PI for 5 min in the dark at room temperature. All samples were acquired by flow cytometry (ACEA NovoCyte) and analysed with NovoExpress software. The Annexin V-FITC/PI apoptosis detection kit (556,547) was purchased from BD Biosciences (Oxford, UK).
2.8. Cell apoptosis assay by TUNEL staining
Grow A549 and H460 cells on 96-well plates and incubate cells with paclitaxel (5 nmol/L), etoposide (100 nmol/L) with or without M3814 (5 μmol/L) for 48 h, wash the slides twice with PBS and process directly according to manufacturer's instructions (Promega corporation G3250). Briefly, permeabilize cells by immersing the slides in 0.2% Triton® X-100 solution for 5 min, and equilibrate at room temperature for 5–10 min. Add 50 μL of rTdT incubation buffer to the cells on a 5 cm2 area. Cell nuclei were stained with DAPI to assess gross cell morphology. Immediately analyze TUNEL fluorescence under a fluorescence microscope using fluorescein at 520 nm and DAPI at 460 nm.
2.9. Cell cycle analyses by flow cytometry
Exponentially growing A549 and H460 cells were seeded in 6-well plates and grown for 24 h. Cells were treated with 5 μmol/L M3814, 5 nmol/L paclitaxel, 100 nmol/L etoposide, or their combinations, and incubated for 12 h. Cells were then washed twice with PBS solution, fixed with 75% pre-cooling ethanol, and placed at −20 °C for at least 24 h. Wash and resuspend cells with PBS and incubate cells with 20 μL 1 mg/mL RNase A (Beyotime) for 30 min at 37 °C. Stain cells with 25 μg/mL propidium iodide (Sigma–Aldrich) for 30 min at room temperature. The cell cycle distribution was measured with flow cytometry (ACEA NovoCyte) and analyzed with NovoExpress software.
2.10. γ-H2AX focus assay by immunofluorescence
Cellular DSBs were quantified by the detection of γ-H2AX (Ser139) focus formation. A549 and H460 cells were grown on round 24 mm climbing sheets in 6-well plates and incubated in the presence or absence of 5 μmol/L M3814 for 1 h before adding 100 nmol/L paclitaxel or 1 μmol/L etoposide. Following extensive washing in drug-free medium, cells were incubated with or without 5 μmol/L M3814 for another 4 h, and then fixed in 4% paraformaldehyde, permeabilized in 0.5% Triton X-100, and blocked with 5% bovine serum albumin. Next, cells were incubated with anti-γ-H2AX (Ser139) antibodies overnight at 4 °C, washed with PBS and incubated with fluorescent secondary antibodies for 1 h at 37 °C away from light. Cell nuclei were stained with DAPI to assess gross cell morphology. The percentage of γ-H2AX (Ser139) (red) was determined at each time point, with data presented as mean ± standard deviation (SD).
2.11. Western blot analysis
After indicated treatment for designed time duration, cells were harvested and washed twice with cold PBS and lysed through RIPA Lysis Buffer (Beyotime) mixed with 1 × Protease Inhibitor Cocktail (Millipore). Cells then underwent grinding 30–60 s (Homogenizer, Servicebio KZ-II), and the supernatant was collected after centrifugation. With the use of the Pierce™ Rapid Gold BCA Protein Assay Kit (23,225; Thermo Fisher, USA), the protein quantification assay was performed to determine the protein concentration for each cell lysate. Next, the cell lysates were mix with loading buffer (Beyotime) and denatured in 100 °C metal bath for 10 min. Equal amounts of protein and molecular weight marker were loaded into the proper of sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) gels of appropriate concentration, and transferred onto polyvinylidene difluoride (PVDF) membranes. Next we blocked the membrane for 1 h at room temperature with 5% milk dissolved in Tris-buffered saline and 0.1% Tween-20 (TBS-T) followed by incubation with corresponding primary antibodies overnight at 4 °C. Membranes were washed 3 times with TBS-T, and incubated with secondary antibodies for 1 h at room temperature. Band images were acquired using darkroom development techniques for chemiluminescence (Bio-Rad Laboratories).
2.12. Senescence-associated β-galactosidase assay
Senescence β-Galactosidase Staining Kit was purchased from Cell Signaling Technology (9860S) and staining was performed according to manufacturer's guide. In brief, cells were treated with paclitaxel (5 nmol/L), etoposide (100 nmol/L) with or without M3814 (5 μmol/L) for 24 h and then incubated for another 72 h. Cells were then washed with PBS and fixed in fixative solution to for 10–15 min at room temperature. Next, we added β-galactosidase staining solution to plates and incubated the plates at 37 °C overnight in a non-CO2, dry incubator. Images were obtained with a Leica inverted microscope at 200 × magnification.
2.13. Gene silencing by P53-targeting siRNA
The control double-stranded small RNA siRNA-Control and siRNA-P53 were designed and chemically synthesized based on sequences shown in Table 1 (GeneChem, Shanghai, China). Control scrambled siRNA had no bio-informatically predicted sequence target in the human genome and was used as a negative control. Lipofectamine™ 3000 Transfection Reagent (Thermo Fisher Scientific, Waltham, MA, USA) and Opti-MEM I (Thermo Fisher Scientific, Waltham, MA, USA) were used to perform the transfection, according to the manufacturer's protocol. Cells were incubated for 48 h in a 5% CO2 incubator at 37 °C and then lysed for Western blot assays to examine the knockdown effect of siRNA.
Table 1.
siRNAs used for P53 knockdown.
| Gene target | Sequence |
|
|---|---|---|
| Sense(5′–3′) | Antisense(5′–3′) | |
| Negative control | UUCUCCGAACGUGUCACGUTT | ACGUGACACGUUCGGAGAATT |
| TP53-Homo-136 | CCCGGACGAUAUUGAACAATT | UUGUUCAAUAUCGUCCGGGTT |
| TP53-Homo-846 | GCACAGAGGAAGAGAAUCUTT | AGAUUCUCUUCCUCUGUGCTT |
| TP53-Homo-687 | CCACCAUCCACUACAACUATT | UAGUUGUAGUGGAUGGUGGTT |
2.14. In vivo anti-tumor efficacy study
Animal studies were conducted under Institutional Animal Care and Use Committee of Sichuan University approved guidelines for animal welfare. Human NSCLC cell lines A549 cells (1 × 107 cells/100 μL) and H460 cells (5 × 106 cells/100 μL) were subcutaneously injected into the right posterior flanks of female NOD-SCID (nod-obese diabetic/severe combined immunodeficient) mice (6 weeks old). When NSCLC xenografts s.c. reached 5 mm in diameter (7 days after implantation, n = 7 per group), mice were treated with 1) solvent (control); 2) single agent M3814 (50 mg/kg) i.g. daily; 3) paclitaxel (10 mg/kg) i.v. once 3 days; 4) etoposide (10 mg/kg) i.p. once 3 days; 5) M3814+paclitaxel combination; 6) M3814+etoposide combination. For combination treatment group, M3814 was given immediately before chemotherapy. M3814 and paclitaxel were dissolved in each solvent one by one: 10% DMSO, 40% PEG400, 5% Tween-80 and 45% saline, and etoposide was dissolved in 10% DMSO and 90% saline. Tumor volume was measured with two-dimensional electronic caliper and calculated as Eq. (1):
| (1) |
where a is the smallest diameter measurement, and b is the diameter largest measurement. Data were presented as median relative tumor volume (RTV), which was calculated as tumor volume divided by tumor volume on the initial day of treatment (Day 0).
2.15. Statistical analysis
Data were presented as mean ± SD of three separated experiments. We analyzed data with GraphPad Prism 7.0. The significance of differences between two groups were determined by Student's t test (parametric) and ANOVA multiple comparison tests. The expression of DNA-PKcs between NSCLC tissues and paired non-cancerous tissues was determined by χ2 test. Statistically significant P values were labelled as: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001. Kaplan–Meier survival analyses and log-rank tests were performed to evaluate the correlation between clinicopathological characteristics and patient prognosis.
3. Results
3.1. Expression of DNA-PKcs is upregulated in human NSCLC tissues and correlates with poor prognosis
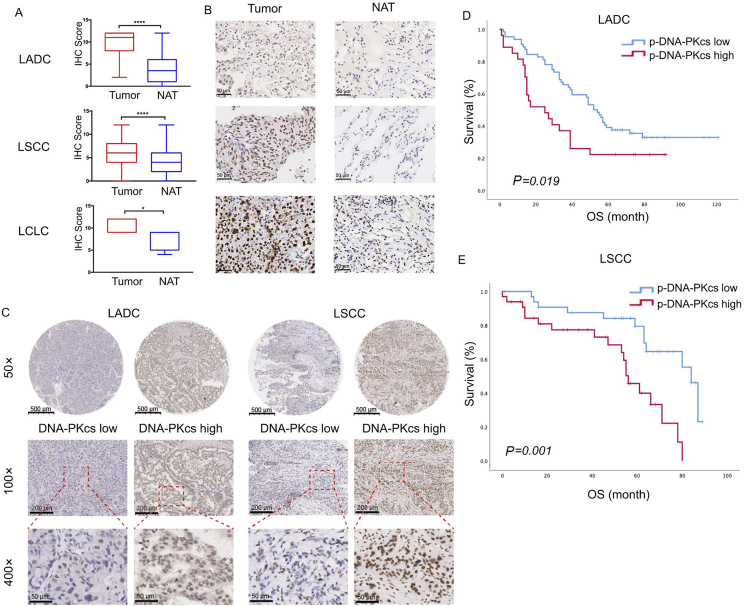
Clinicopathological characteristics of 174 NSCLC patients, including 91 with lung adenocarcinoma (LADC), 78 with lung squamous cell carcinoma (LSCC) and 5 with large-cell carcinoma (LCLC), are presented in Table 2. Among all patients, 10 patients with lung adenocarcinoma were excluded from assessment of DNA-PKcs expression, due to lack of paired adjacent noncancerous tissues. The five LCLC patients were excluded from survival analyses due to small sample size. Finally, DNA-PKcs expression levels of a total number of 164 human NSCLC tissues (81 LADC, 78 LSCC, and 5 LCLC) and their paired adjacent noncancerous tissues were detection of by immunohistochemistry (IHC) assays. The calculated IHC scores revealed an elevated expression level of DNA-PKcs in 164 paraffin-embedded NSCLC tissue samples compared with their adjacent noncancerous tissues (Fig. 1A and B). DNA-PKcs expressions were divided as high and low according to the optimal cut-off values calculated from ROC analyses (Fig. 1C). Based on follow-up outcomes between November 2005 and August 2018, we further assessed the correlation between tumor DNA-PKcs levels and patient prognoses. The overall survival (OS) time of patients with low and high DNA-PKcs expression was 54.00 ± 27.69 and 38.5 ± 25.74 months, respectively. Kaplan–Meier analyses demonstrated that higher expression of DNA-PKcs was significantly associated with poorer OS in LADC patients (Fig. 1D, log-rank test, χ2 = 5.53, P = 0.019). Similar trends were observed in patients with LSCC, where patients with higher DNA-PKcs tumor expression presented shorter survival time (Fig. 1E, log-rank test, χ2 = 10.76, P = 0.001).
Table 2.
Clinicopathological information of NSCLC patients.
| Variable | n (%) |
|---|---|
| Gender | |
| Male | 130(74.7) |
| Female | 44 (25.3) |
| Tumor size | |
| < 5 cm | 119(68.4) |
| ≥ 5 cm | 55 (31.6) |
| Grade | |
| I | 4 (2.3 ) |
| II | 120(69.0) |
| III | 50 (28.7) |
| Histologic type | |
| Adenocarcinoma | 91 (52.3) |
| Squamous cell carcinoma | 78 (44.8) |
| Large cell lung carcinoma | 5 (2.9) |
| T stage | |
| 1 | 29 (16.7) |
| 2 | 101(58.0) |
| 3 | 35 (20.1) |
| 4 | 9 (5.2 ) |
| N stage | |
| x | 22 (12.6) |
| 0 | 92 (52.9) |
| 1 | 32 (18.4) |
| 2 | 24 (13.8) |
| 3 | 4 (2.3 ) |
| M stage | |
| 0 | 172(98.9) |
| 1 | 2(1.1 ) |
| Tumor stage | |
| I–II | 99 (56.9) |
| III–IV | 75 (43.1) |
| DNA-Pkcs expression | |
| High | 71 (40.8) |
| Low | 103(59.2) |
Figure 1.
The expression of the catalytic kinase subunit of DNA-dependent protein kinase (DNA-PKcs) in human non-small cell lung cancer (NSCLC) tumor specimens and its correlation with prognosis. (A) and (B) Expression levels of DNA-PKcs in 164 paired NSCLC tumor specimens and their normal tissue adjacent to the tumor (NAT), independently interpreted by two researchers. Data presented as means ± SD [n = 81 for lung adenocarcinoma (LADC), n = 78 for lung squamous cell carcinoma (LSCC), n = 5 for large-cell carcinoma (LCLC), ∗∗∗∗P < 0.0001, ∗P < 0.05], scale bar = 50 μm. (C) Representative immunohistochemical staining for DNA-PKcs in human NSCLC tissues, scale bar = 500 μm (magnification, 50 ×), 100 μm (magnification, 100 ×) and 20 μm (magnification, 400 ×); High/low indicates the expression level of DNA-PKcs in NSCLC tumor microarray. (D) and (E) Kaplan–Meier survival curves of 169 NSCLC (91 LADC and 78 LSCC) patients grouped by nuclear DNA-PKcs expression.
3.2. M3814 potentiates the inhibitory effect of chemotherapy on NSCLC cell proliferation in vitro
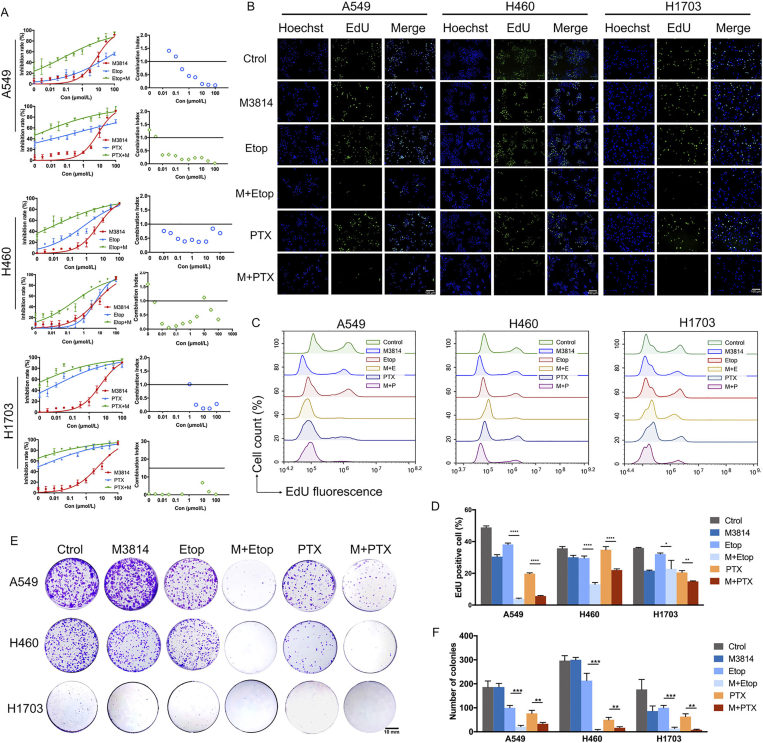
Initial experiments sought to determine the cytotoxic effects of chemotherapy alone (0–100 mmol/L), and in combination with DNA-PK inhibitor M3814 in NSCLC cell lines. Following 24, 48 and 72 h of treatment, cell viability was assessed with CCK-8 cytotoxicity assays. Both paclitaxel and etoposide exhibited a more potent inhibitory effect on cell proliferation when combined with 5 μmol/L M3814 (Supporting Information Fig. S1). The IC50 values of paclitaxel on A549 cells when used alone at 24, 48 and 72 h were 645.5, 386.1, and 11.14 nmol/L, respectively. With concomitant exposure to 5 μmol/L M3814, the IC50 of paclitaxel decreased to 6.19, 0.24 and 0.15 nmol/L respectively at 24, 48 and 72 h (Fig. S1). In H460 cell line, the IC50 of paclitaxel were 1.40 μmol/L, 15.15 nmol/L and 0.85 nmol/L, at 24, 48 and 72 h, which decreased to 2.88, 0.27 and 0.004 nmol/L, respectively, when combined with M3814 (Fig. S1). In H1703 cell line, the IC50 of paclitaxel were 1.80 μmol/L, 0.98 nmol/L and 0.42 nmol/L, at 24, 48 and 72 h, which decreased to 2.16, 0.14 and 0.01 nmol/L, respectively, when combined with M3814 (Fig. S1). Similar trends could be observed in cells treated with etoposide suggesting the chemo-sensitization effect of M3814 on NSCLC cells. Moreover, the inhibition on cell proliferation was dose- and time-dependent, with maximal inhibition observed in cells treated with 100 μmol/L of paclitaxel/etoposide for 72 h. To determine whether the enhancement of cell death induced by M3814–etoposide combination was synergistic, additive, or antagonistic, cells were treated with chemotherapy, M3814 or their combination for 48 h (Fig. 2A). The calculated CI values indicated that M3814 acted in a synergistic manner with paclitaxel/etoposide on A549 and H460 NSCLC cells (CI < 1), and with etoposide at doses higher than 1 μmol/L on H1703 cells (Fig. 2A).
Figure 2.
M3814 potentiates the inhibitory effect of chemotherapy on NSCLC cell proliferation in vitro. (A) Cell viability curves of NSCLC cell lines A549, H460 and H1703 treated with paclitaxel (PTX) and etoposide (Etop) (concentration gradient from 1 nmol/L to 100 μmol/L), in the absence or presence of M3814 (5 μmol/L) for 48 h. Data are presented as mean ± SD (n = 3). The combination index (CI) of the drug combination determined with Chou-Talalay Method. CI < 1 indicates the synergistic effect between M3814 and chemotherapy. (B) Representative images of EdU-DNA incorporation assay in NSCLC cells treated with 5 μmol/L M3814 and 100 nmol/L etoposide for 18 h. EdU staining (green fluorescence), Hoechst staining (blue fluorescence), scale bar = 100 μm (magnification, 100 ×). (C) Flow cytometric analysis of EdU fluorescence. (D) Quantitative data of the EdU-positive fraction. Data presented as mean ± SD (n = 3, ∗∗∗∗P < 0.0001, ∗∗P < 0.01, ∗P < 0.05). (E) and (F) Representative images of colonies (E) and quantitative analyses (F) formation tests in A549, H460 and H1703 cells were treated with paclitaxel (5 nmol/L) and etoposide (100 nmol/L) in the absence or in the presence of M3814 (5 μmol/L) for 18 h, and incubated in 6-well plates for another 14 days. Colonies observed by inverted microscope. Data presented as mean ± SD (n = 3, ∗∗∗P < 0.001, ∗∗P < 0.01, ∗P < 0.05).
We next performed EdU–DNA incorporation assays to investigate the inhibitory effect of M3814 and etoposide on newly synthesized DNA. As shown in Fig. 2B, A549, H460 and H1703 cells undergoing 18 h of combinational treatment displayed a significant decrease in nuclear EdU fluorescence staining (green), compared with cells treated with monotherapy. The reduced nuclear incorporation of EdU was further verified by flow cytometry assays (Fig. 2C and D), with EdU-positive A549 cell counts decreased from 19.8 ± 0.49% to 5.67 ± 0.31% when M3814 was added to paclitaxel treatment, and 38.21 ± 0.89% to 3.96 ± 0.39% when added to etoposide. Similar trends were observed in H460 and H1703 cell lines. These results suggest the synergistic inhibition on cell proliferation by M3814–paclitaxel/etoposide combination through the impairment of DNA synthesis. Colony formation tests further demonstrated the chemo-sensitization effect of M3814 on NSCLC cells, as evidenced by the decrease of colonies formed by cells treated with combination therapy. While paclitaxel/etoposide monotherapy modestly reduced colony formation, the addition of M3814 markedly enhanced the inhibitory effect of chemotherapy on NSCLC cell lines (Fig. 2E and F).
3.3. M3814 promotes cell apoptosis and G2/M cell cycle arrest induced by chemotherapy in vitro
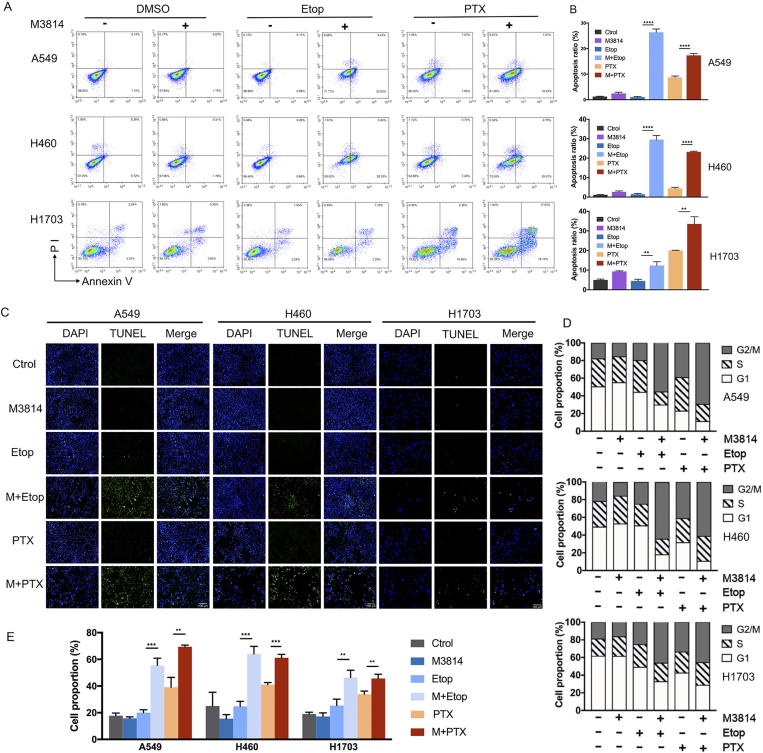
To elucidate potential mechanisms underlying chemo-potentiation by M3814 on cell death, NSCLC cells labelled with Annexin V/PI were analyzed by FACS. As shown in Fig. 3A and B, either etoposide or M3814 alone induced limited cell apoptosis following 48 h of indicated treatments. Moreover, the most prominent increase in cell apoptotic fractions was observed in the etoposide + M3814 combinational treatment group (26.40 ± 1.29% in A549, 29.54 ± 2.09% in H460, and 12.34 ± 1.94% in H1703), and paclitaxel + M3814 group (17.36 ± 0.73% in A549, 23.23 ± 0.37% in H460, and 33.59 ± 3.64% in H1703) as compared with etoposide (1.06 ± 0.24% in A549, 1.42 ± 0.38% in H460, and 4.52 ± 0.86% in H1703) and paclitaxel monotherapy group (7.52 ± 1.17% in A549, 4.53 ± 0.51% in H460, and 20.05 ± 0.10% in H1703). To provide additional evaluation of cell apoptosis, we conducted TUNEL assay on these three NSCLC cell lines. Results confirm the enhanced apoptosis by M3814 on chemotherapy-treated cells, with higher TUNEL-positive fractions in combination treatment groups (Fig. 3C).
Figure 3.
M3814 promotes cell apoptosis and G2/M cell cycle arrest induced by chemotherapy in vitro. (A) NSCLC cell lines A549, H460 and H1703 were treated with paclitaxel (5 nmol/L) and etoposide (100 nmol/L) with or without M3814 (5 μmol/L) for 48 h, stained with Annexin V/PI and evaluated through flow cytometry. (B) Quantitative analysis of apoptotic cell proportion. Apoptotic cells included those with Annexin V+/PI− and Annexin V+/PI+ labelling. Data presented as means ± SD (n = 3, ∗∗∗∗P < 0.0001, ∗∗P < 0.01). (C) Representative immunofluorescent images of TUNEL staining at 48 h following indicated treatment. TUNEL staining (green), DAPI staining (blue), scale bar = 100 μm (magnification, 100 ×). (D) Cell cycle distribution detected by flow cytometry analyses on A549 and H460 cells. Cells were treated with paclitaxel (5 nmol/L) and etoposide (100 nmol/L) with or without M3814 (5 μmol/L), and stained with propidium iodide (PI) at 12 h post-treatment. (E) Quantitative analysis of G2/M fraction of NSCLC cells with indicated treatments. Data are presented as mean ± SD (n = 3, ∗∗∗P < 0.001, ∗∗P < 0.01).
In response to DNA-damaging chemotherapies, sustained G2/M phase growth arrest is often stimulated as a classic cellular response, which allows for cells to repair DNA damage35. To provide a potential mechanism for cell growth inhibition induced by the short-term treatments, H460 and A549 cells with indicated treatments were stained with propidium iodide (PI) and analyzed by flow cytometry. Noteworthy, co-therapy with M3814 significantly enhanced the G2/M phase accumulation induced by etoposide in H460 (24.80 ± 3.76% to 64.13 ± 5.64%, P < 0.001), A549 cells (20.03 ± 2.16% to 55.5 ± 5.41%, P < 0.001), and H1703 cells (25.39 ± 4.84% to 46.40 ± 5.44%, P < 0.01) (Fig. 3D and E). Similar trends were observed in paclitaxel treatment, with G2/M fraction rising from 39.13 ± 7.34% to 69.43 ± 13.05% (P < 0.01) in A549 cells, 41.10 ± 1.51% to 61.20 ± 2.56% (P < 0.001) in H460 cells, and 33.88 ± 2.33% to 45.69 ± 3.15% (P < 0.01) in H1703 cells (Fig. 3D and E). Given that persistent G2/M cell cycle arrest was related to a mode of cell death, this finding accorded with our earlier results that short-term combination treatment could induce a prolonged decrease in colony formation. Taken together, these results suggest an irreversible growth arrest in NSCLC cells by M3814–paclitaxel or M3814–etoposide combination by promoting apoptosis and G2/M cell cycle arrest.
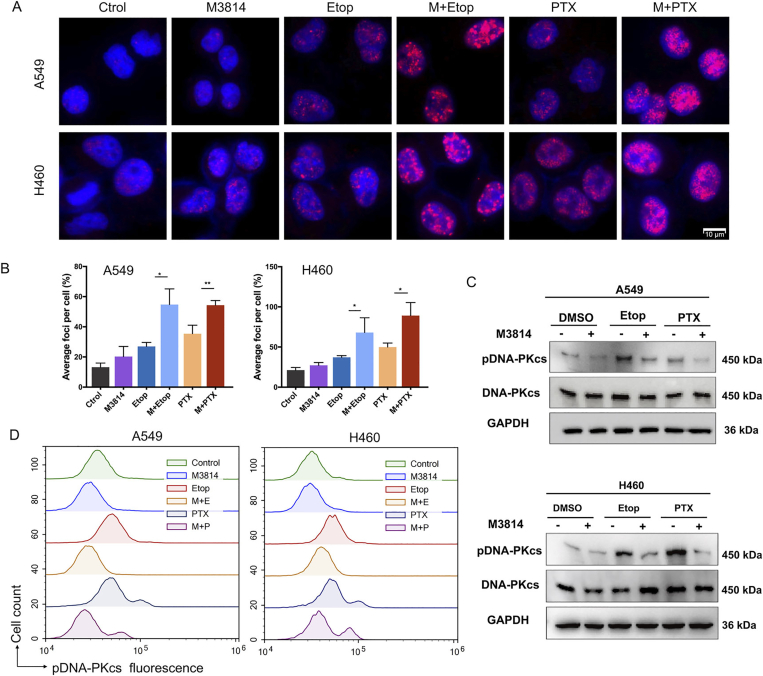
3.4. M3814 impairs chemotherapy-induced DSB repair by inhibiting DNA-PKcs activation in vitro
γ-H2AX (Ser139) is formed by the phosphorylation of the Ser139 residue of the histone variant H2AX, and is a reliable DSB marker36. Given that nearly 20 DSBs are required to achieve the activating threshold of G2/M checkpoint37, we conducted γ-H2AX (Ser139) immunofluorescence staining to quantify γ-H2AX foci in NSCLC cells. Cells were treated with 100 nmol/L paclitaxel or 1 μmol/L etoposide for 1 h, followed by extensive washing in drug-free medium, and incubation with or without 5 μmol/L M3814 for another 4 h. We noticed a rapid induction of DNA damage by either 100 nmol/L paclitaxel or 1 μmol/L etoposide, with a more striking increase in the number of γ-H2AX foci observed in the combination group (Fig. 4A). Compared with etoposide or paclitaxel monotherapy, the addition of M3814 significantly increased the number of γ-H2AX foci per cell following paclitaxel (P = 0.007 in A549 cells and P = 0.016 in H460 cells) and etoposide treatment (P = 0.011 in A549 cells and P = 0.044 in H460 cells) (Fig. 4B).
Figure 4.
M3814 impairs chemotherapy-induced double-strand breaks (DSBs) repair by inhibiting DNA-PKcs activation in vitro. (A) Representative images of NSCLC cells incubated in the presence or absence of 5 μmol/L M3814 for 1 h before adding 100 nmol/L paclitaxel or 1 μmol/L etoposide. Following extensive washing in drug-free medium, cells were incubated with or without 5 μmol/L M3814 for another 4 h, and immunostained for γ-H2AX (red) foci. DAPI staining (blue fluorescence), scale bar = 10 μm (magnification, 1000 ×). (B) Quantitative analysis of γ-H2AX foci per cells. Data are presented as mean ± SD (n = 3, ∗∗P < 0.01, ∗P < 0.05). (C) The phosphorylation of DNA-PKcs and the expression of total-DNA-PKcs were determined by Western blot analysis on NSCLC cells with indicated treatment. (D) Flow cytometric analysis of phospho-DNA-PKcs fluorescence.
The efficiency of DSB induction and its subsequent repair substantially determines the efficacy of chemotherapy in tumor cells. DNA-PKcs activation can be evaluated by the phosphorylation of catalytic subunit DNA-PKcs at Ser2056 site. Notably, Western blot results reveal no significant change in protein expression of total-DNA-PKcs, but suggest a rapid up-regulation of p-DNA-PK (Ser2056) expression in cells treated with chemotherapy (Fig. 4C). However, compared with paclitaxel or etoposide monotherapy, the addition of M3814 significantly inhibited the autophosphorylation of DNA-PKcs induced by chemotherapy, as evidenced by the relatively lower protein expression of p-DNA-PKcs in cells with concomitant treatment, which was further solidified by p-DNA-PKcs flow cytometry assay where M3814 prevented the increase of p-DNA-PKcs (Ser2056) fluorescence in chemotherapy-treated cells (Fig. 4D). These results collectively suggest that chemotherapy-induced DNA damage led to DNA-PKcs autophosphorylation so as to initiate DSBs repair in tumor cells, which was nevertheless suppressed by M3814. Thus, DNA-PK inhibitor M3814 impaired the DSB-repair machinery in NSCLC cells through the down-regulation of p-DNA-PKcs (Ser2056).
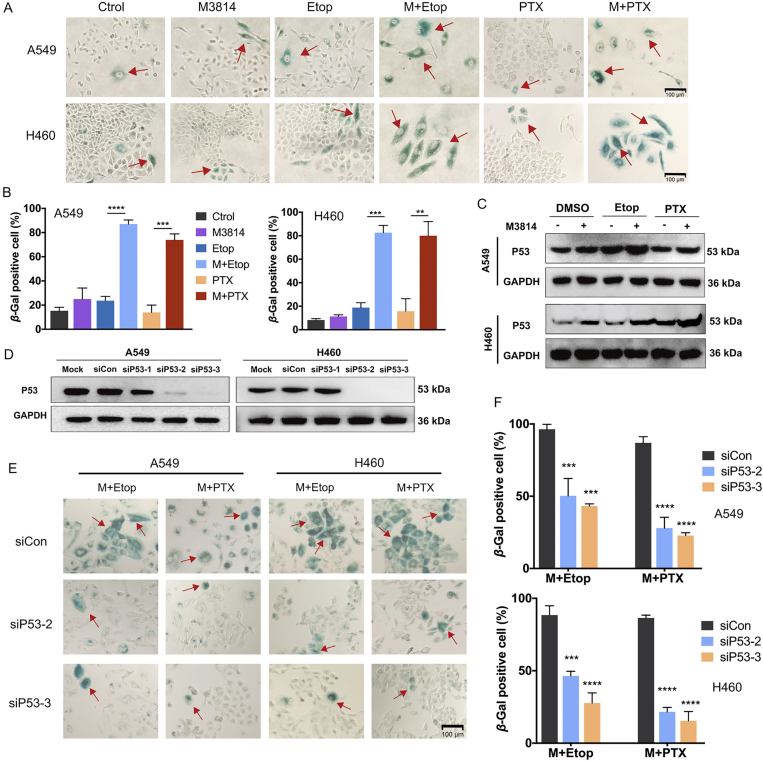
3.5. P53-dependent accelerated senescence response by M3814 following chemotherapy treatment
On the basis of early knowledge that delayed DSB repair contributes to cellular senescence, which has historically been characterized as a predominant change during irreversible cell-cycle arrest38, we subjected post-treatment NSCLC cells to senescence-associated β-galactosidase (SA-βGal) staining assays. In both H460 and A549 cell lines, single treatment with M3814 or chemotherapy failed to induce substantial senescence response relative to untreated controls (Fig. 5A). On the other hand, the concomitant treatment with M3814 and chemotherapy significantly increased the SA-βGal-positive fraction (P < 0.001 in paclitaxel group, and P < 0.0001 in etoposide group), indicating a synergistic promotion of senescence by M3814 and chemotherapy (Fig. 5B). Noteworthy, SA-βGal-positive cells exhibited characteristic morphologic features of senescence, including cell enlargement, elongation, and multinucleation (Fig. 5A). Cellular senescence induced by DNA damage response in human cells is typically characterized by the upregulation of P5339, 40, 41, 42. We identified a significant upregulation of P53 protein expression in combination treatment groups by Western blot analyses (Fig. 5C).
Figure 5.
P53-dependent accelerated senescence response by M3814 following chemotherapy treatment. (A) Representative images of senescence-associated beta-galactosidase (SA-βgal) activity in NSCLC cells after 24 h of indicated treatment followed by another 72 h incubation. Scale bar = 100 μm (magnification, 100 ×). (B) Quantitative analysis of SA-βGal positive cells. Data are presented as mean ± SD (n = 3, ∗∗∗∗P < 0.0001, ∗∗∗P < 0.001, ∗∗P < 0.01). (C) Western blot assays on P53 expressions in H460 and A549 cells at 12 h post-treatment. (D) Expression levels of P53 determined by Western blot analyses at 72 h post-transfection for the selection of candidate siRNA-P53. (E) Representative images of SA-βGal activity in NSCLC cells transfected with siRNA-control and two siRNA-P53. Scale bar = 100 μm (magnification, 100 ×). (F) Quantitative analysis of SA-βGal positive cells. Data are presented as mean ± SD (n = 3, ∗∗∗∗P < 0.0001, ∗∗∗P < 0.001).
As both A549 and H460 cells are P53-wild-type43, we investigated whether the accelerated senescence induced by M3814 in chemotherapy-treated cells is P53 dependent. Expression levels of P53 were analyzed 72 h post-transfection by Western blot and two candidate siRNA-P53 were selected (Fig. 5D). Although both M3814–paclitaxel and M3814–etoposide combination led to notable SA-βGal staining in P53 wild-type A549 and H460 cells, the accelerated senescence was significantly weakened by siRNA knock-down of P53 (Fig. 5E and F). Together, these findings collectively suggest that the accelerated senescence response of NSCLC cells following M3814+chemotherapy combination is P53-dependent.
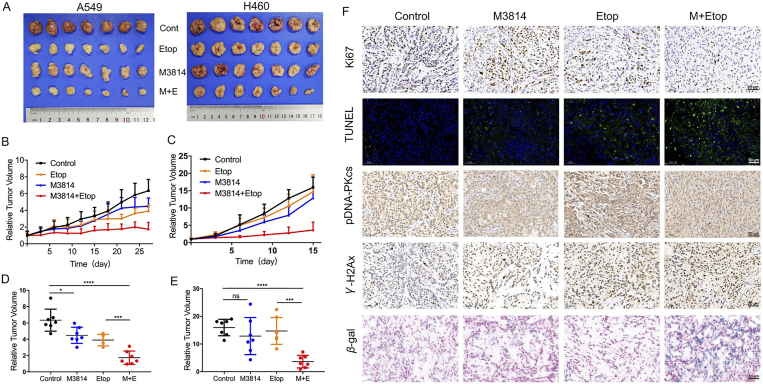
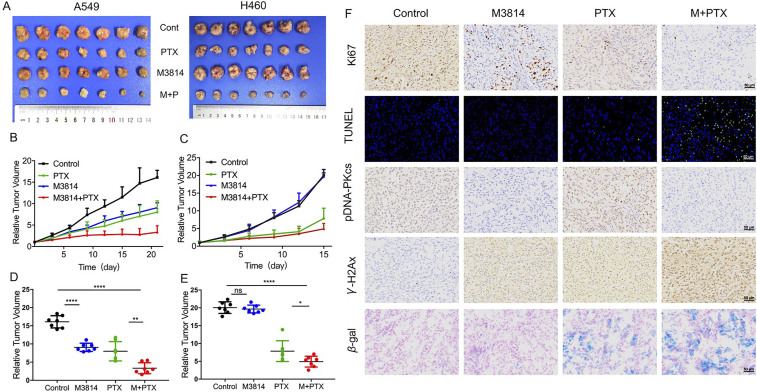
3.6. M3814 potentiates the antitumor activities of chemotherapy in NSCLC xenograft models
The preliminary in vitro success motivated further research on the chemosensitization effect of M3814 in vivo. We evaluated the anti-tumor efficacy of M3814 and chemotherapy in two subcutaneous NSCLC xenograft models (Figure 6, Figure 7). To elicit a measurable antitumor activity without inducing significant toxicity, treatment regimens include a suggested dose of etoposide (10 mg/kg i.p.) every three days44,45 and a tolerated dose of M3814 (40 mg/kg) orally administrated once daily. At the end of experiment, tumors from control groups of A549 and H460 models reached 6.34 ± 1.35 and 15.95 ± 2.95 times their starting volume (RTV6.34 and RTV15.95), respectively (Fig. 6A). As shown in Fig. 6B–E, combinational treatment with M3814 and etoposide substantially delayed tumor growth compared with either treatment alone. As for the anti-tumor effect of M3814+paclitaxel in vivo, we found that M3814 administered orally at a tolerated dose of 40 mg/kg once daily further enhanced the treatment response to paclitaxel (10 mg/kg i.p. once every three days) in mice implanted with A549 and H460 xenografts (Fig. 7A). Whereas M3814 monotherapy induced tumor growth inhibition in A549 xenograft model, H460 tumors were insensitive to M3814 single treatment (Fig. 7B–E).
Figure 6.
The synergistic anti-tumor effect of M3814 and etoposide in NSCLC xenografts. NOD-SCID mice implanted with A549 and H460 tumor xenografts were treated with M3814 (50 mg/kg, i.g. daily) and etoposide (10 mg/kg, i.p. once 3 days). (A) Subcutaneous A549 and H460 tumors at the end of this experiment. (B) and (C) Growth of A549 and H460 xenografts presented as mean RTV (relative tumor volume). Error bars denote SD. (D) and (E) The final RTV of xenograft tumors measured in each group. Data are presented as mean ± SD (n = 7, ∗∗∗∗P < 0.0001, ∗∗∗P < 0.001, ∗P < 0.05; ns, P > 0.05). (F) Representative images of immunohistochemistry staining of Ki67, γ-H2AX, pDNA-PKcs and immunofluorescence staining of TUNEL evaluated on paraffin-embedded A549 tumor sections, scale bar = 50 μm (magnification, 200 ×). Representative images of SA-βGal staining of frozen sections of A549 tumors, scale bar = 50 μm (magnification, 200 ×).
Figure 7.
The synergistic anti-tumor effect of M3814 and paclitaxel in NSCLC xenografts. NOD-SCID mice implanted with A549 and H460 tumor xenografts were treated with M3814 (50 mg/kg, i.g. daily) and paclitaxel (10 mg/kg, i.v. once 3 days). (A) Subcutaneous A549 and H460 tumors at the end of this experiment. (B) and (C) Growth of A549 and H460 xenografts presented as mean RTV (relative tumor volume). Error bars denote SD. (D) and (E) The final RTV of xenograft tumors measured in each group. Data are presented as mean ± SD (n = 7, ∗∗∗∗P < 0.0001, ∗∗∗P < 0.001, ∗∗P < 0.01, ∗P < 0.05; ns, P > 0.05). (F) Representative images of immunohistochemistry staining of Ki67, γ-H2AX, pDNA-PKcs and immunofluorescence staining of TUNEL evaluated on paraffin-embedded A549 tumor sections, scale bar = 50 μm (magnification, 200 ×). Representative images of SA-βGal staining of frozen sections of A549 tumors, scale bar = 50 μm (magnification, 200 ×).
Immunohistochemical analyses were used to evaluate tumor cell proliferation in vivo, characterized by Ki67-positive rates in xenografts. As shown in Figure 6, Figure 7F, concomitant use of M3814 and chemotherapy decreased Ki67 expression in tumor tissues relative to controls. Tumor tissues were also subjected to TUNEL staining assays for the detection of apoptotic DNA fragmentation. Though chemotherapy treatment alone upregulated TUNEL staining in tumor tissues, the effect was most pronounced in the combination group, which was in accordance with our in vitro results that M3814 worked synergistically with chemotherapy to induce apoptosis. Comparable with in vitro results, the addition of M3814 elevated γ-H2AX (Ser139) levels relative to chemotherapy treatment alone. Moreover, we observed an increase in p-DNA-PK (Ser2056) in xenografts treated with chemotherapy, which was abolished by the additional treatment with M3814. We then examined SA-βGal activities in A549 xenografts to validate the senescence-inducing effect of the drug combination in vitro. Tumors treated with M3814 monotherapy displayed minimal SA-βGal staining whereas tumors from combination treatment groups demonstrated significantly higher SA-βGal positivity (Figure 6, Figure 7F). This evidence collectively reveals the chemosensitization efficacy of M3814 in NSCLC xenograft models, through the suppression of tumor cell proliferation, and the induction of apoptosis, DNA damage and cell senescence.
3.7. Toxicity evaluation
In the four combinations based on two NSCLC xenograft models and two chemotherapy, single use of M3814 did not induce unacceptable toxicity. Though A549 xenograft mice treated with etoposide monotherapy experienced body weight loss compared with control group mice (maximum body weight loss <20%, final body weight >18 g; Supporting Information Fig. S2), co-therapy with M3814 did not induce further weight loss. It is thus interesting to speculate whether the body weight loss caused by combinational treatment was attributed to the potential toxicity of etoposide. To further assess the in vivo safety of the treatment, we performed blood biochemical analyses, which revealed no significant differences in biochemical indexes between groups (Fig. S2).
4. Discussion
A large proportion of NSCLC patients are ineligible for operations due to late diagnosis. Recent attempts have thus converged on systemic treatments based on chemotherapies and target therapies46. Currently etoposide-cisplatin (EP) and carboplatin-paclitaxel (PC) regimens remain the two most commonly-used concurrent regimens for advanced NSCLC patients47,48. Although these two regimes have been extensively applied in clinical practice, accumulating side effects due to large doses and frequently-developed chemoresistance have made the treatment results suboptimal. Old-generation DNA-PK inhibitors have been proved effective but are limited in terms of selectivity due to structural similarities with PI3Ks. M3814 represents the new-generation of selective DNA-PK protein kinase inhibitors. To the best of our knowledge, this is the first study to systematically evaluate the chemosensitization effect of M3814 on paclitaxel and etoposide in NSCLC models, both in vitro and in vivo. The present study provides a theoretical basis for the use of M3814 in combination with chemotherapy in clinical practice, with which we hope to aid the optimization of NSCLC treatment.
We identified enhanced cytotoxic effect by M3814 in NSCLC cell lines treated with paclitaxel and etoposide, both in vitro and in vivo. CCK8 assay results reveal a synergistic inhibition (CI < 1) of M3814 combined with low, noncytotoxic concentrations of etoposide and paclitaxel. The direct detection of tumor cell DNA synthesis is the most accurate way to evaluate cell proliferation49. In recent years, the EdU assay is more often used than the BrdU (bromo deoxyuridine) method for the detection of DNA synthesis, due to the fact that EdU method does not require antibody reaction and demonstrates higher detection sensitivity. EdU is a thymine nucleoside analogue and can be inserted into the replicating DNA when tumor cells proliferate, thus directly reflecting DNA replication activity. Compared with monotherapy, we observed a significantly smaller number of EdU positive cells in the combination treatment group, suggesting that concomitant treatments with chemotherapies and M3814 could inhibit NSCLC cell proliferation by disrupting the DNA synthesis. Based on the promising inhibition synergy between M3814 and chemotherapeutic agents in vitro, animal studies using A549 and H460 human NSCLC xenograft systems were conducted. In the four combinations based on two NSCLC xenograft models and two chemotherapies, we also observed tumor regression at tolerated doses in vivo.
DNA double-strand breaks (DSBs) are considered the most lethal form of DNA lesions, and can be induced by genotoxic agents such as chemotherapeutic agents used in cancer treatments50. γ-H2AX (Ser139) is formed by the phosphorylation of the Ser139 residue of the histone variant H2AX, and is commonly used to measure early cellular response to DSB stimulants51. Recently, growing evidence suggests that paclitaxel could induce cellular DNA damage52, 53, 54, 55. In line with these results, we noticed a rapid induction of DNA damage by short-term treatment (1 h) of high concentration of paclitaxel (100 nmol/L), with more striking DNA-damage induction observed in the combination group. Moreover, the induction of DNA damage by paclitaxel was further proved by the phosphorylation of DNA-PKcs, suggesting that DNA damage caused by paclitaxel initiated the DNA repair process in tumor cells. The efficiency of DSB induction and its subsequent repair substantially determines the efficacy of chemotherapy in tumor cells. A pivotal mechanism for DNA repair in mammals is the activation of DNA-PKcs at DSB sites56. Multiple phosphorylation sites of DNA-PKcs have continuously been identified including serine 2056 (Ser2056), the phosphorylation of which is essential to NHEJ pathway by preventing DNA end processing57,58. Our results reveal no significant change in protein expression of total-DNA-PKcs after treatment with M3814+etoposide for 4 h. On the other hand, the expression of p-DNA-PK (Ser2056) was down-regulated by combined treatment relative to chemotherapy alone, suggesting a potential mechanism for this drug synergy that M3814 impaired the DSB-repair machinery of NSCLC cells by suppressing the phosphorylation of DNA-PKcs.
Conventional anti-cancer treatments such as certain types of chemotherapy are built on the premise of enhanced DNA damage-related cell death, which may lead to serious side effects or commonly-developed chemoresistance59. Instead of directly causing cytotoxic cell death, some treatment modalities aim to permanently inhibit cancer cell proliferation by stimulating growth arrest. In this study, we found that both paclitaxel and etoposide work synergistically with M3814 to induce cellular senescence so as to inhibit tumor growth. Persistent G2/M cell cycle arrest is a classic cellular response to DNA damage35, allowing the repair of DSBs before cell enter mitosis and thus maintaining genomic stability of cells60, 61, 62, 63. In the present study, we found that the chemosensitization effect of M3814 was accompanied by an increased level of G2–M cell cycle arrest. Accumulating evidence suggests that cell cycle arrest contributes to tumor cell senescence64, which can be triggered by the delayed repair of DSBs65,66 and is thus considered as a potential mechanism for tumor inhibition. The senescent cells were large in size and expressed high activity β-galactosidase at pH 6.0. We observed higher levels of β-galactosidase staining in cells treated with chemotherapy–M3814 combination than with either monotherapy. Similar findings have previously been addressed that the selective knockout of DNA-PK could induce accelerated senescence of cancer cells after radiotherapy39. Cell senescence induced by DNA damage response in human cells is typically characterized by the upregulation of P5339, 40, 41, 42. We found that the accelerated cell senescence induced by combination treatments was significantly weakened by siRNA knock-down of P53. These findings collectively reveal a P53-dependent accelerated senescence response by M3814 following treatment with paclitaxel/etoposide.
The results of this study highlight the therapeutic potential of combining DNA-PK blockade with chemotherapy in human NSCLC. To the best of our knowledge, this is the first study to systematically evaluate the chemosensitization effect of M3814, a new-generation selective DNA-PK inhibitors in NSCLC models, both in vitro and in vivo. Importantly, multiple anti-tumor modalities of M3814–chemotherapy combination were identified including direct cytotoxic cell death, and permanent growth arrest.
5. Conclusions
The new-generation DNA-PK selective inhibitor M3814 potentiates the anti-tumor effect of paclitaxel and etoposide in A549 and H460 human NSCLC cell lines. Tumor regression was also observed at tolerated doses in vivo. The accelerated senescence of NSCLC cells induced by M3814–chemotherapy combination was P53-dependent, indicating a potential explanation for the anti-tumor effect of this drug synergy. The present study provides a theoretical basis for the use of M3814 in combination with paclitaxel and etoposide in clinical practice, which warrants further evaluation and would hopefully aid the optimization of NSCLC treatment.
Acknowledgments
This work is supported by the National Natural Science Foundation Regional Innovation and Development (U19A2003, China) and by the Excellent Youth Foundation of Sichuan Scientific Committee Grant in China (No. 2019JDJQ008).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2021.07.029.
Author contributions
Manni Wang and Siyuan Chen conducted experiments, analyzed data, and wrote the manuscript. Yuquan Wei and Xiawei Wei provided conceptual idea and cost, guided the project and revised the manuscript. The authors read and approved the final manuscript.
Conflicts of interest
The authors declare no competing interests.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Gerard C., Debruyne C. Immunotherapy in the landscape of new targeted treatments for non-small cell lung cancer. Mol Oncol. 2009;3:409–424. doi: 10.1016/j.molonc.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandler A.B. Chemotherapy for small cell lung cancer. Semin Oncol. 2003;30:9–25. doi: 10.1053/sonc.2003.50012. [DOI] [PubMed] [Google Scholar]
- 3.Lackey A., Donington J.S. Surgical management of lung cancer. Semin Intervent Radiol. 2013;30:133–140. doi: 10.1055/s-0033-1342954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mountain C.F., Dresler C.M. Regional lymph node classification for lung cancer staging. Chest. 1997;111:1718–1723. doi: 10.1378/chest.111.6.1718. [DOI] [PubMed] [Google Scholar]
- 5.Johnston S.R. Ovarian cancer: review of the national institute for clinical excellence (NICE) guidance recommendations. Cancer Invest. 2004;22:730–742. doi: 10.1081/cnv-200032761. [DOI] [PubMed] [Google Scholar]
- 6.Ward J.F. The yield of DNA double-strand breaks produced intracellularly by ionizing radiation: a review. Int J Radiat Biol. 1990;57:1141–1150. doi: 10.1080/09553009014551251. [DOI] [PubMed] [Google Scholar]
- 7.Iliakis G. The role of DNA double strand breaks in ionizing radiation-induced killing of eukaryotic cells. Bioessays. 1991;13:641–648. doi: 10.1002/bies.950131204. [DOI] [PubMed] [Google Scholar]
- 8.Zhuang W., Li B., Long L., Chen L., Huang Q., Liang Z. Induction of autophagy promotes differentiation of glioma-initiating cells and their radiosensitivity. Int J Cancer. 2011;129:2720–2731. doi: 10.1002/ijc.25975. [DOI] [PubMed] [Google Scholar]
- 9.Mao Z., Bozzella M., Seluanov A., Gorbunova V. DNA repair by nonhomologous end joining and homologous recombination during cell cycle in human cells. Cell Cycle. 2008;7:2902–2906. doi: 10.4161/cc.7.18.6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahaney B.L., Meek K., Lees-Miller S.P. Repair of ionizing radiation-induced DNA double-strand breaks by non-homologous end-joining. Biochem J. 2009;417:639–650. doi: 10.1042/BJ20080413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lieber M.R. The mechanism of human nonhomologous DNA end joining. J Biol Chem. 2008;283:1–5. doi: 10.1074/jbc.R700039200. [DOI] [PubMed] [Google Scholar]
- 12.Chan D.W., Chen B.P., Prithivirajsingh S., Kurimasa A., Story M.D., Qin J., et al. Autophosphorylation of the DNA-dependent protein kinase catalytic subunit is required for rejoining of DNA double-strand breaks. Genes Dev. 2002;16:2333–2338. doi: 10.1101/gad.1015202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gottlieb T.M., Jackson S.P. The DNA-dependent protein kinase: requirement for DNA ends and association with Ku antigen. Cell. 1993;72:131–142. doi: 10.1016/0092-8674(93)90057-w. [DOI] [PubMed] [Google Scholar]
- 14.Davidson D., Amrein L., Panasci L., Aloyz R. Small molecules, inhibitors of DNA-PK, targeting DNA repair, and beyond. Front Pharmacol. 2013;4:5. doi: 10.3389/fphar.2013.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mari P.O., Florea B.I., Persengiev S.P., Verkaik N.S., Bruggenwirth H.T., Modesti M., et al. Dynamic assembly of end-joining complexes requires interaction between Ku70/80 and XRCC4. Proc Natl Acad Sci U S A. 2006;103:18597–18602. doi: 10.1073/pnas.0609061103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Britton S., Coates J., Jackson S.P. A new method for high-resolution imaging of Ku foci to decipher mechanisms of DNA double-strand break repair. J Cell Biol. 2013;202:579–595. doi: 10.1083/jcb.201303073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shao Z., Davis A.J., Fattah K.R., So S., Sun J., Lee K.J., et al. Persistently bound Ku at DNA ends attenuates DNA end resection and homologous recombination. DNA Repair. 2012;11:310–316. doi: 10.1016/j.dnarep.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uematsu N., Weterings E., Yano K., Morotomi-Yano K., Jakob B., Taucher-Scholz G., et al. Autophosphorylation of DNA-PKCS regulates its dynamics at DNA double-strand breaks. J Cell Biol. 2007;177:219–229. doi: 10.1083/jcb.200608077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cary R.B., Peterson S.R., Wang J., Bear D.G., Bradbury E.M., Chen D.J. DNA looping by Ku and the DNA-dependent protein kinase. Proc Natl Acad Sci U S A. 1997;94:4267–4272. doi: 10.1073/pnas.94.9.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeFazio L.G., Stansel R.M., Griffith J.D., Chu G. Synapsis of DNA ends by DNA-dependent protein kinase. EMBO J. 2002;21:3192–3200. doi: 10.1093/emboj/cdf299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weterings E., van Gent D.C. The mechanism of non-homologous end-joining: a synopsis of synapsis. DNA Repair. 2004;3:1425–1435. doi: 10.1016/j.dnarep.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Yoo S., Dynan W.S. Geometry of a complex formed by double strand break repair proteins at a single DNA end: recruitment of DNA-PKcs induces inward translocation of Ku protein. Nucleic Acids Res. 1999;27:4679–4686. doi: 10.1093/nar/27.24.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calsou P., Frit P., Humbert O., Muller C., Chen D.J., Salles B. The DNA-dependent protein kinase catalytic activity regulates DNA end processing by means of Ku entry into DNA. J Biol Chem. 1999;274:7848–7856. doi: 10.1074/jbc.274.12.7848. [DOI] [PubMed] [Google Scholar]
- 24.Xing J., Wu X., Vaporciyan A.A., Spitz M.R., Gu J. Prognostic significance of ataxia-telangiectasia mutated, DNA-dependent protein kinase catalytic subunit, and Ku heterodimeric regulatory complex 86-kD subunit expression in patients with nonsmall cell lung cancer. Cancer. 2008;112:2756–2764. doi: 10.1002/cncr.23533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shintani S., Mihara M., Li C., Nakahara Y., Hino S., Nakashiro K., et al. Up-regulation of DNA-dependent protein kinase correlates with radiation resistance in oral squamous cell carcinoma. Cancer Sci. 2003;94:894–900. doi: 10.1111/j.1349-7006.2003.tb01372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian X., Chen G., Xing H., Weng D., Guo Y., Ma D. The relationship between the down-regulation of DNA-PKcs or Ku70 and the chemosensitization in human cervical carcinoma cell line HeLa. Oncol Rep. 2007;18:927–932. [PubMed] [Google Scholar]
- 27.Beskow C., Skikuniene J., Holgersson A., Nilsson B., Lewensohn R., Kanter L., et al. Radioresistant cervical cancer shows upregulation of the NHEJ proteins DNA-PKcs, Ku70 and Ku86. Br J Cancer. 2009;101:816–821. doi: 10.1038/sj.bjc.6605201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouchaert P., Guerif S., Debiais C., Irani J., Fromont G. DNA-PKcs expression predicts response to radiotherapy in prostate cancer. Int J Radiat Oncol Biol Phys. 2012;84:1179–1185. doi: 10.1016/j.ijrobp.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 29.Fok J.H.L., Ramos-Montoya A., Vazquez-Chantada M., Wijnhoven P.W.G., Follia V., James N., et al. AZD7648 is a potent and selective DNA-PK inhibitor that enhances radiation, chemotherapy and olaparib activity. Nat Commun. 2019;10:5065. doi: 10.1038/s41467-019-12836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuchss T., Mederski W.W., Emde U., Buchstallter H.-P., Zenke F., Zimmermann A., et al. Abstract 4198: highly potent and selective DNA-PK inhibitor M3814 with sustainable anti-tumor activity in combination with radiotherapy. Cancer Res. 2017;77:4198. [Google Scholar]
- 31.Wise H.C., Iyer G.V., Moore K., Temkin S.M., Gordon S., Aghajanian C., et al. Activity of M3814, an oral DNA-PK inhibitor, in combination with topoisomerase II inhibitors in ovarian cancer models. Sci Rep. 2019;9:18882. doi: 10.1038/s41598-019-54796-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zimmermann A., Carr M., Zenke F.T., Blaukat A., Vassilev L.T. Abstract 269: DNA-PK inhibitor, M3814, as a new combination partner of mylotarg in the treatment of acute myeloid leukemia. Cancer Res. 2019;79:269. doi: 10.3389/fonc.2020.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein C., Dokic I., Mairani A., Mein S., Brons S., Häring P., et al. Overcoming hypoxia-induced tumor radioresistance in non-small cell lung cancer by targeting DNA-dependent protein kinase in combination with carbon ion irradiation. Radiat Oncol. 2017;12:208. doi: 10.1186/s13014-017-0939-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chou T.C., Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzym Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 35.Bunz F., Dutriaux A., Lengauer C., Waldman T., Zhou S., Brown J.P., et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 36.Mah L.J., El-Osta A., Karagiannis T.C. gammaH2AX: a sensitive molecular marker of DNA damage and repair. Leukemia. 2010;24:679–686. doi: 10.1038/leu.2010.6. [DOI] [PubMed] [Google Scholar]
- 37.Deckbar D., Birraux J., Krempler A., Tchouandong L., Beucher A., Walker S., et al. Chromosome breakage after G2 checkpoint release. J Cell Biol. 2007;176:749–755. doi: 10.1083/jcb.200612047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopez-Otin C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azad A., Jackson S., Cullinane C., Natoli A., Neilsen P.M., Callen D.F., et al. Inhibition of DNA-dependent protein kinase induces accelerated senescence in irradiated human cancer cells. Mol Cancer Res. 2011;9:1696–1707. doi: 10.1158/1541-7786.MCR-11-0312. [DOI] [PubMed] [Google Scholar]
- 40.Campisi J., d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 41.Shay J.W., Roninson I.B. Hallmarks of senescence in carcinogenesis and cancer therapy. Oncogene. 2004;23:2919–2933. doi: 10.1038/sj.onc.1207518. [DOI] [PubMed] [Google Scholar]
- 42.Aliouat-Denis C.M., Dendouga N., Van den Wyngaert I., Goehlmann H., Steller U., van de Weyer I., et al. p53-independent regulation of p21Waf1/Cip 1 expression and senescence by Chk2. Mol Cancer Res. 2005;3:627–634. doi: 10.1158/1541-7786.MCR-05-0121. [DOI] [PubMed] [Google Scholar]
- 43.Senoo M., Tsuchiya I., Matsumura Y., Mori T., Saito Y., Kato H., et al. Transcriptional dysregulation of the p73L/p63/p51/p40/KET gene in human squamous cell carcinomas: expression of Delta Np73L, a novel dominant-negative isoform, and loss of expression of the potential tumour suppressor p51. Br J Cancer. 2001;84:1235–1241. doi: 10.1054/bjoc.2000.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drapkin B.J., George J., Christensen C.L., Mino-Kenudson M., Dries R., Sundaresan T., et al. Genomic and functional fidelity of small cell lung cancer patient-derived xenografts. Cancer Discov. 2018;8:600–615. doi: 10.1158/2159-8290.CD-17-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao Y., Thomas H.D., Batey M.A., Cowell I.G., Richardson C.J., Griffin R.J., et al. Preclinical evaluation of a potent novel DNA-dependent protein kinase inhibitor NU7441. Cancer Res. 2006;66:5354–5362. doi: 10.1158/0008-5472.CAN-05-4275. [DOI] [PubMed] [Google Scholar]
- 46.Qin S., Yu H., Wu X., Luo Z., Wang H., Sun S., et al. Weekly albumin-bound paclitaxel/cisplatin versus gemcitabine/cisplatin as first-line therapy for patients with advanced non-small-cell lung cancer: a phase II open-label clinical study. Chin J Cancer Res. 2019;31:339–348. doi: 10.21147/j.issn.1000-9604.2019.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liang J., Bi N., Wu S., Chen M., Lv C., Zhao L., et al. Etoposide and cisplatin versus paclitaxel and carboplatin with concurrent thoracic radiotherapy in unresectable stage III non-small cell lung cancer: a multicenter randomized phase III trial. Ann Oncol. 2017;28:777–783. doi: 10.1093/annonc/mdx009. [DOI] [PubMed] [Google Scholar]
- 48.Eberhardt W.E. Concurrent chemoradiotherapy in stage III non-small-cell lung cancer: what is the best regimen?. J Clin Oncol. 2015;33:532–533. doi: 10.1200/JCO.2014.58.9812. [DOI] [PubMed] [Google Scholar]
- 49.Madhavan H. Simple laboratory methods to measure cell proliferation using DNA synthesis property. J Stem Cells Regen Med. 2007;3:12–14. doi: 10.46582/jsrm.0301003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lama-Sherpa T.D., Shevde L.A. An Emerging regulatory role for the tumor microenvironment in the DNA damage response to double-strand breaks. Mol Cancer Res. 2020;18:185–193. doi: 10.1158/1541-7786.MCR-19-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Redon C., Pilch D., Rogakou E., Sedelnikova O., Newrock K., Bonner W. Histone H2A variants H2AX and H2AZ. Curr Opin Genet Dev. 2002;12:162–169. doi: 10.1016/s0959-437x(02)00282-4. [DOI] [PubMed] [Google Scholar]
- 52.Branham M.T., Nadin S.B., Vargas-Roig L.M., Ciocca D.R. DNA damage induced by paclitaxel and DNA repair capability of peripheral blood lymphocytes as evaluated by the alkaline comet assay. Mutat Res. 2004;560:11–17. doi: 10.1016/j.mrgentox.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 53.Mikula-Pietrasik J., Witucka A., Pakula M., Uruski P., Begier-Krasinska B., Niklas A., et al. Comprehensive review on how platinum- and taxane-based chemotherapy of ovarian cancer affects biology of normal cells. Cell Mol Life Sci. 2019;76:681–697. doi: 10.1007/s00018-018-2954-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poruchynsky M.S., Komlodi-Pasztor E., Trostel S., Wilkerson J., Regairaz M., Pommier Y., et al. Microtubule-targeting agents augment the toxicity of DNA-damaging agents by disrupting intracellular trafficking of DNA repair proteins. Proc Natl Acad Sci U S A. 2015;112:1571–1576. doi: 10.1073/pnas.1416418112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Birinci H., Şen B., Sayğılı S., Ölmez E., Uluer E.T., Özbilgin K. The effect of pycnogenol and paclitaxel on DNA damage in human breast cancer cell line. Proceedings. 2017;1:1023. [Google Scholar]
- 56.Davis A.J., Chen B.P., Chen D.J. DNA-PK: a dynamic enzyme in a versatile DSB repair pathway. DNA Repair. 2014;17:21–29. doi: 10.1016/j.dnarep.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cui X., Yu Y., Gupta S., Cho Y.M., Lees-Miller S.P., Meek K. Autophosphorylation of DNA-dependent protein kinase regulates DNA end processing and may also alter double-strand break repair pathway choice. Mol Cell Biol. 2005;25:10842–10852. doi: 10.1128/MCB.25.24.10842-10852.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meek K., Douglas P., Cui X., Ding Q., Lees-Miller S.P. Trans Autophosphorylation at DNA-dependent protein kinase's two major autophosphorylation site clusters facilitates end processing but not end joining. Mol Cell Biol. 2007;27:3881–3890. doi: 10.1128/MCB.02366-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Faget D.V., Ren Q., Stewart S.A. Unmasking senescence: context-dependent effects of SASP in cancer. Nat Rev Cancer. 2019;19:439–453. doi: 10.1038/s41568-019-0156-2. [DOI] [PubMed] [Google Scholar]
- 60.Boohaker R.J., Xu B. The versatile functions of ATM kinase. Biomed J. 2014;37:3–9. doi: 10.4103/2319-4170.125655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yan H.Q., Huang X.B., Ke S.Z., Jiang Y.N., Zhang Y.H., Wang Y.N., et al. Interleukin 6 augments lung cancer chemotherapeutic resistance via ataxia-telangiectasia mutated/NF-kappaB pathway activation. Cancer Sci. 2014;105:1220–1227. doi: 10.1111/cas.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hsia S.M., Yu C.C., Shih Y.H., Yuanchien Chen M., Wang T.H., Huang Y.T., et al. Isoliquiritigenin as a cause of DNA damage and inhibitor of ataxia-telangiectasia mutated expression leading to G2/M phase arrest and apoptosis in oral squamous cell carcinoma. Head Neck. 2016;38 Suppl 1:E360–E371. doi: 10.1002/hed.24001. [DOI] [PubMed] [Google Scholar]
- 63.Wang H., Zhang X., Teng L., Legerski R.J. DNA damage checkpoint recovery and cancer development. Exp Cell Res. 2015;334:350–358. doi: 10.1016/j.yexcr.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 64.Blagosklonny M.V. Cell cycle arrest is not senescence. Aging. 2011;3:94–101. doi: 10.18632/aging.100281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.d'Adda di Fagagna F. Living on a break: cellular senescence as a DNA-damage response. Nat Rev Cancer. 2008;8:512–522. doi: 10.1038/nrc2440. [DOI] [PubMed] [Google Scholar]
- 66.Gewirtz D.A., Holt S.E., Elmore L.W. Accelerated senescence: an emerging role in tumor cell response to chemotherapy and radiation. Biochem Pharmacol. 2008;76:947–957. doi: 10.1016/j.bcp.2008.06.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.