Abstract
The fusion gene AML1-ETO is the product of t(8;21)(q22;q22), one of the most common chromosomal translocations associated with acute myeloid leukemia. To investigate the impact of AML1-ETO on hematopoiesis, tetracycline-inducible AML1-ETO-expressing cell lines were generated using myeloid cells. AML1-ETO is tightly and strongly induced upon tetracycline withdrawal. The proliferation of AML1-ETO+ cells was markedly reduced, and most of the cells eventually underwent apoptosis. RNase protection assays revealed that the amount of Bcl-2 mRNA was decreased after AML1-ETO induction. Enforced expression of Bcl-2 was able to significantly delay, but not completely overcome, AML1-ETO-induced apoptosis. Prior to the onset of apoptosis, we also studied the ability of AML1-ETO to modulate differentiation. AML1-ETO expression altered granulocytic differentiation of U937T-A/E cells. More significantly, this change of differentiation was associated with the down-regulation of CCAAT/enhancer binding protein α (C/EBPα), a key regulator of granulocytic differentiation. These observations suggest a dichotomy in the functions of AML1-ETO: (i) reduction of granulocytic differentiation correlated with decreased expression of C/EBPα and (ii) growth arrest leading to apoptosis with decreased expression of CDK4, c-myc, and Bcl-2. We predict that the preleukemic AML1-ETO+ cells must overcome AML1-ETO-induced growth arrest and apoptosis prior to fulfilling their leukemogenic potential.
Acute myeloid leukemia (AML) is a common hematopoietic malignancy characterized by the abnormal proliferation and differentiation of myeloid progenitor cells. The fusion gene AML1-ETO is the product of the t(8;21)(q22;q22) chromosomal translocation, which is associated with approximately 40% of cases with the M2 subtype of AML (8, 10, 30, 44). The chimeric protein (AML1-ETO) contains the N terminus of the AML1 (RUNX1, CBFα, PEBP2αB) transcription factor and nearly the full-length ETO (MTG8) (39, 40). The AML1 moiety of the AML1-ETO fusion protein has the Drosophila melanogaster protein runt homology domain, which is required for DNA binding and interaction with its heterodimerization partner, CBFβ, but which lacks the transcriptional activation domain of AML1 (35, 46, 61, 68, 70). AML1 is a common target for chromosomal translocations and is involved in several other hematopoietic malignancies, such as the TEL-AML1 fusion observed in t(12;21), which is involved in approximately 25% of childhood pre-B-cell acute lymphoblastic leukemias (14, 56, 59); AML1-MTG16 from t(16;21) with AML1 fused to ETO-related gene MTG16 (11); and AML1-EVI1 from t(3;21) in lymphoblast crises evolving from chronic myelogenous leukemia and in therapy-related AML (37, 42, 43). These translocations point to a fundamental role of AML1 in hematopoiesis. Furthermore, analysis of AML1 knockout mice demonstrates that AML1 is a crucial factor for definitive hematopoiesis (48, 67). The ETO moiety of AML1-ETO is able to recruit the nuclear receptor corepressor (N-CoR)–mammalian Sin3 (mSin3)–histone deacetylase-1 (HDAC1) complex (13, 32, 65). ETO recruitment of the N-CoR–mSin3–HDAC1 complex leads to lower levels of histone acetylation and less-accessible chromatin. Inhibition of HDAC has been shown to decrease ETO-mediated repression, inducing partial differentiation of AML1-ETO-positive Kasumi-1 cells (66). Understanding how AML1 and its fusion proteins are involved in hematopoiesis and leukemogenesis will provide valuable insight into cell differentiation and cancer development.
Various attempts to develop a mouse model with AML1-ETO have so far failed to generate AML in adult mice. AML1-ETO heterozygous knockin mice died at the embryonic stage and displayed a block in definitive hematopoiesis (47, 75). Inducible AML1-ETO-expressing transgenic mice were generated with a tetracycline-inducible system (55). Unlike the knockin model mice, these mice are viable but fail to develop any malignancies despite strong expression of AML1-ETO in the bone marrow upon withdrawal of tetracycline. More recently, a promising murine model that mimics the t(8;21) chromosomal translocation through Cre/loxP-mediated recombination has been generated (5). However, hematopoietic studies have yet to reveal any malignancies. These results suggested that AML1-ETO expression at a particular stage of hematopoietic cell development may be critical for its leukemogenic potential and that AML1-ETO-associated leukemogenesis may also require a secondary event or “hit” for AML1-ETO-positive cells to adopt leukemogenic behavior, including block of differentiation, dysregulation of proliferation, and/or reduction of apoptotic potential. Various cell lines have been established to study AML1 and AML1-ETO proteins. AML1 has been shown to promote cell cycle progression of myeloid progenitor cell line 32Dcl3 and to transform NIH 3T3 cells (25, 62). Ectopic expression of AML1-ETO inhibits granulocytic differentiation of 32Dcl3 and L-G myeloid cell lines, monocytic differentiation of U937 cells, and erythroid differentiation of K562 and TF-1 cells (1, 13, 22, 24, 26, 71). In addition, it has been reported that AML1-ETO expression decreases proliferation by up to threefold in interleukin-3-dependent L-G cells and induces cell-cycle arrest in 32Dcl3 and Ba/F3 cell lines (22, 29). Furthermore, ectopic expression of AML1-ETO in NIH 3T3 cells has induced cell transformation (9).
Here, we report the generation of inducible AML1-ETO-expressing cell line U937T-A/E using human myeloid cells to investigate the effect of AML1-ETO on hematopoiesis. Unlike most previously reported stable cell lines that continuously express AML1-ETO (1, 24, 58), these cell lines present the advantage of not having been directly selected for AML1-ETO expression during their establishment. As such, these cell lines have not been preselected with particular features such as reduced apoptosic potential or enhanced proliferation prior to the analysis of AML1-ETO expression. In U937T-A/E cells, AML1-ETO is tightly regulated and strongly induced only upon tetracycline withdrawal at both mRNA and protein levels. Upon induction, the proliferation of AML1-ETO+ cells was severely reduced as the cells arrest in G1 phase of the cell cycle. Eventually, most of these cells underwent apoptosis and exhibited decreased Bcl-2 expression. Enforced expression of Bcl-2 using retrovirus infection was able to significantly delay AML1-ETO-induced apoptosis, while the growth arrest remained. Furthermore, we also studied the effect of AML1-ETO on U937T cell differentiation. Induced expression of AML1-ETO in U937T-A/E cells significantly decreased the expression of CCAAT/enhancer binding protein α (C/EBPα) and altered the neutrophilic differentiation of U937T cells. These observations suggest a dichotomy of AML1-ETO properties in that the protein is able not only to block cell differentiation but also to induce cell cycle arrest, which eventually leads to apoptosis. We predict that the preleukemic AML1-ETO+ cell needs to overcome the growth arrest and/or the apoptotic potential of AML1-ETO prior to fulfilling its leukemogenic potential.
MATERIALS AND METHODS
Cell culture
Myeloid U937 and AML1-ETO-expressing Kasumi-1 cells were cultured in RPMI 1640 medium (GIBCO/BRL, Rockville, Md.) supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, Utah) and 2 mM l-glutamine (GIBCO/BRL). Phoenix amphotropic cells (21) were maintained in Dulbecco's modified Eagle's medium (GIBCO/BRL) supplemented with 10% FBS, 2 mM l-glutamine, and 5 μM β-mercaptoethanol (Fisher Scientific, Fair Lawn, N.J.). All cells were maintained in a 37°C incubator with 5% CO2. U937T cells are U937 cells stably transfected with a tet-VP16 fusion gene under the control of a tetracycline-inducible promoter; the cells were kindly provided by Gerald Grossveld (3, 15). U937T cells were cultured in RPMI 1640 medium supplemented with 10% FBS, 2 mM l-glutamine, 1 μg of tetracycline (Sigma, St. Louis, Mo.)/ml, and 0.5 μg of puromycin (Sigma)/ml.
Plasmid construction.
The construction of pUHD-AML1/ETO was described previously (55). The cDNAs for Bcl-2 (GenBank accession no. GI4557356) and for CDK4 (GenBank accession no. GI6753379) were subcloned into the EcoRI site of the MSCV-internal ribosome entry signal (IRES)-enhanced green fluorescent protein (EGFP) coding sequence (16) to form murine stem cell virus (MSCV)-Bcl-2-IRES-EGFP and MSCV-CDK4-IRES-EGFP (17). The cDNA for c-myc (GenBank accession no. GI34820) was subcloned into HpaI of MSCV-IRES-EGFP to form MSCV-cMyc-IRES-EGFP. The BamHI and XhoI cDNA fragment for C/EBPα (GenBank accession no. GI4757971) was subcloned into BglII and XhoI of MSCV-IRES-EGFP to form MSCV-C/EBPα-IRES-EGFP.
Establishment of U937T-A/E stable transformants.
U937T cells were washed in RPMI 1640 medium without FBS and resuspended at 2 × 107 cells/ml in 500 μl of RPMI 1640 medium without FBS. Fifty micrograms of linearized pUHD-AML1/ETO (55) and 1 μg of linearized pGK-Neo in 45 μl of TE (10 mM Tris-HCl, 1 mM EDTA, pH 7.5) were mixed with the cells, and the mixture was transferred to electroporation cuvettes with a 0.4-cm gap (Bio-Rad, Hercules, Calif.). Electroporations were performed using a Gene-Pulser II (Bio-Rad) at 300 V and 960 μF. The samples were then transferred to complete RPMI 1640 medium containing 1 μg of tetracycline and 0.5 μg of puromycin/ml and incubated at 37°C in 5% CO2. Twenty-four hours later, G418 (GIBCO/BRL) was added at a concentration of 1 mg/ml. Positive polyclonal populations (pools) were identified based on Southern blot hybridization and on the induction of AML1-ETO expression following tetracycline withdrawal and were named U937T-A/E. Individual U937T-A/E clones were eventually isolated by limiting dilution and assayed for AML1-ETO inducibility. U937T-A/E cells were maintained in RPMI 1640 medium supplemented with 10% FBS, 2 mM l-glutamine, and 1 μg of tetracycline, 0.5 μg of puromycin, and 1 mg of G418/ml.
Retrovirus infection of cell lines.
For the production of retrovirus, Phoenix amphotropic cells were transfected with MSCV–Bcl-2–IRES–EGFP, MSCV-CDK4-IRES-EGFP, MSCV-cMyc-IRES-EGFP, MSCV-C/EBPα-IRES-EGFP, or MSCV-IRES-EGFP vector by calcium phosphate precipitation (21). Retrovirus supernatants were harvested 48 h after transfection, filtered through 0.45-μm-pore-size filters, and stored at −80°C. Filtered supernatant was used to infect U937T and U937T-A/E cells in the presence of 8 μg of Polybrene (Sigma)/ml. The infected cells were then centrifuged at 700 × g for 1 h at room temperature and incubated at 37°C in 5% CO2. The medium was changed 24 h after virus infection. Cells infected with the various MSCV-IRES-EGFP constructs were identified on the basis of green fluorescence.
Northern blot analysis.
Total RNA was isolated from the U937T-A/E cell lines by extraction with guanidine isothiocyanate and centrifugation through cesium chloride, and Northern blot analysis was performed as described previously (55, 76). Blots were exposed to MR X-ray films (Kodak, Rochester, N.Y.).
RPA.
The RNA samples were hybridized with the hAPO-2c (BD Pharmingen, La Jolla, Calif.) set of probes against the main members of the Bcl-2 family. The RNase protection assay (RPA) was performed using the RiboQuant RPA starter package according to the manufacturer's instructions (BD Pharmingen). The level of modulation of Bcl-2 was determined by using a Storm phosphorimager (Molecular Dynamics, Sunnyvale, Calif.) and Image software (Scion Corporation, Frederick, Md.).
Transient transfection assays.
Electroporation was performed with a Bio-Rad Gene-Pulser II (230 V, 975 μF). U937T cells were transfected with 25 μg of total DNA, including 13 μg of pXP1 (GenBank accession no. GI3929277) or 20 μg of pBcl2-luc (equimolarity) or 20 μg of MDR-1-luc (63) or 1.4 μg of pCMV5 (empty expression vector; GenBank accession no. GI7542546) or 2 or 4 μg of pCMV5-AML1-ETO and 100 ng of pRL-Null (GenBank accession no. GI7638455) to normalize transfection efficiency. Alternatively, U937T and U937T-A/E cells were induced for 12 h prior to transfection. Transfected cells were harvested 24 h after electroporation, and luciferase activity was measured using the dual-luciferase reporter assay system (Promega).
Western blot analysis.
Cells were washed in phosphate-buffered saline (PBS) and resuspended in Laemmli buffer (1% sodium dodecyl sulfate [SDS], 0.1275 M Tris-HCl [pH 6.8], 1% β-mercaptoethanol). The protein concentration of the supernatant was assessed using the Bio-Rad protein assay according to the manufacturer's instructions. Following SDS-polyacrylamide gel electrophoresis (PAGE), proteins were transferred to polyvinylidene difluoride membranes (Bio-Rad) using a Mini-Trans-Blot cell (Bio-Rad). The blot was then blocked in 5% dry milk in Tris-buffered saline with 0.1% Tween 20. The blot was then incubated with a 1:1,000 dilution of rabbit anti-ETO antibody (a gift from Scott Hiebert), a 1:1,000 dilution of rabbit anti-human Bcl-2 antibody (a gift from John Reed), and a 1:5,000 dilution of mouse anti-human β-actin (AC15) (Sigma). The blot was then incubated with appropriate secondary antibodies conjugated to horseradish peroxidase (Amersham Pharmacia Biotech, Piscataway, N.J.). The blot was developed using a chemiluminescent substrate (Renaissance; NEN, Boston, Mass.) and exposed to MR X-ray film (Kodak). In all Western blot analyses, β-actin or Coomassie staining of the SDS-PAGE gel after transfer was used as a loading control.
Analysis of cell proliferation, differentiation, morphology, and cell cycle.
For the induction of tetracycline-controlled AML1-ETO, the cells were washed three times in 50 ml of PBS and seeded at 105 cells/ml in the maintenance medium in the presence of the desired tetracycline concentration. The viability of the cells was assessed daily by trypan blue exclusion. The cells were induced to differentiate by adding 65 nM phorbol 12-myristate 13-acetate (TPA) (Sigma) to the culture medium 24 h after tetracycline withdrawal. Morphology was determined by Wright-Giemsa staining of air-dried cytospin preparations. Cell cycle distribution was evaluated by flow cytometry analysis of propidium iodide-stained nuclei (41). A minimum of 104 cells were analyzed using a FACSCalibur (BD Immunocytometry, San Jose, Calif.). The cell cycle distribution was assessed using ModFit LT software (Verity Software House, Topsham, Maine)
Detection of apoptosis.
Apoptotic cells were identified by double staining with acridine orange (100 μg/ml; Sigma) and ethidium bromide (100 μg/ml; Sigma) (36). The level of apoptosis was also assessed by flow cytometry of cells stained with annexin V-phycoerythrin (PE) and 7-aminoactinomycin D (7-AAD; BD Pharmingen). The cells (105) were washed twice in PBS and resuspended in annexin V binding buffer (0.01 M HEPES [pH 7.4], 0.14 M NaCl, 2.5 mM CaCl2) and then double stained with annexin V-PE and 7-AAD. A minimum of 104 cells were analyzed.
Differentiation marker analysis.
For each flow cytometry analysis, 106 cells were washed twice in washing buffer (PBS, 0.1% [wt/vol]NaN3, 1% FBS) and resuspended in 100 μl of washing buffer with 2 μl of anti-human CD11b-PE (clone ICRF44), CD18-fluorescein isothiocyanate (FITC) (clone 6.7), or immunoglobulin G1(κ)–PE-FITC (clone MOPC-21; BD Pharmingen) as an isotype control. Incubation was performed at room temperature for 30 min. A minimum of 104 cells were analyzed by flow cytometry.
RESULTS
Establishment of myeloid cell lines with inducible expression of AML1-ETO.
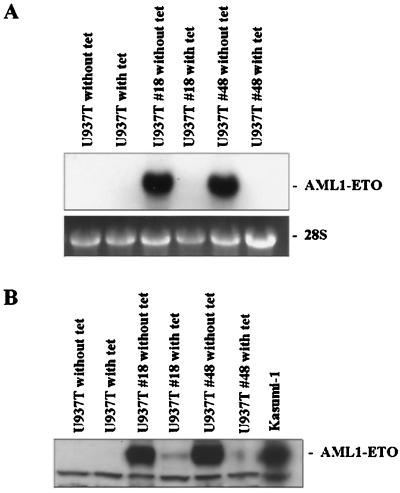
To study the effect of AML1-ETO on the growth, survival, and differentiation of myeloid cells, we made inducible AML1-ETO-expressing U937T cell lines. The AML1-ETO cDNA was inserted into tetracycline-responsive expression vector pUHD10–3 to form pUHD-AML1/ETO (55). pUHD-AML1/ETO was introduced into the U937T cells, which contain stably transfected pUHD-tTA (a tetracycline-responsive transcription activator [tTA], whose expression is under the control of tetracycline) to establish the inducible cell lines (3). In principle, in the presence of tetracycline, the expression of tTA and AML1-ETO should be extremely low; in the absence of tetracycline, tTA activates its own promoter to produce more tTA and also activates AML1-ETO expression. Forty-eight pools of U937T cells stably transfected with pUHD-AML1/ETO were confirmed by Southern blot hybridization. Cells were cultured in the presence or absence of tetracycline to test the inducibility of AML1-ETO expression. Northern blot hybridization using RNA harvested from these cultures demonstrated that only two pools (pools 18 and 48) of U937T-A/E cells were inducible for AML1-ETO expression upon withdrawal of tetracycline (Fig. 1A). All the other pools did not show AML1-ETO expression in either the presence or absence of tetracycline (data not shown). Pools 18 and 48 showed very tight control of AML1-ETO expression. Furthermore, the inducible up-regulation of AML1-ETO was also demonstrated at the protein level in Western blot analysis (Fig. 1B).
FIG. 1.
Inducible AML1-ETO expression in U937T cells stably transfected with pUHD-AML1-ETO. (A) Northern blot analyses showing the expression of AML1-ETO in the presence or absence of tetracycline (with tet or without tet, respectively) in U937T parental cells and stably transfected cells. Total RNA (10 μg) for each sample was electrophoresed, blotted, and hybridized with α-32P-labeled ETO cDNA. The 28S rRNA is shown for the relative amounts of loaded RNA. (B) Western blot analyses showing the expression of AML1-ETO protein in U937T-A/E pools 18 and 48 following tetracycline withdrawal for 72 h. Proteins from U937T and U937T-A/E cell lines were analyzed by immunodetection using an anti-human ETO antibody. Protein prepared from t(8;21) Kasumi-1 cells was used as a positive control.
Inducible expression of AML1-ETO reduces proliferation and affects the viability of U937T-A/E cells.
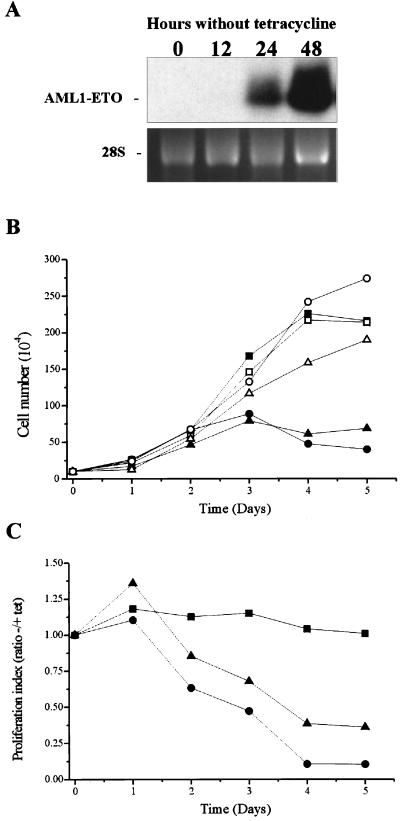
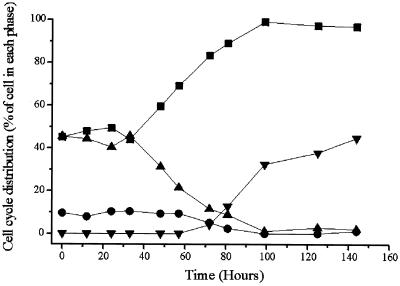
We first tested the effect of AML1-ETO expression on cell proliferation. U937T-A/E pool 18 and 48 cells and their parental cell line, U937T, were cultured in the presence of tetracycline (no induction of AML-ETO expression) and in the absence of tetracycline (induction of AML1-ETO expression). AML1-ETO transcripts were clearly detectable 24 h after the induction and reached a maximum at 48 h postinduction (Fig. 2A). The growth curve showed a significant reduction of cell growth when AML1-ETO was expressed. This delay was initiated 2 days after induction and continued, with the proliferation being substantially reduced compared to that for uninduced cells (Fig. 2B and C). The proliferation of the parental U937T cells was the same in the presence and the absence of tetracycline, indicating that the effect was due solely to the expression of AML1-ETO. Besides the reduction of the proliferative potential of the U937T-A/E pool 18 and 48 cells, a clear reduction of the viability of AML1-ETO-expressing cells was observed 3 days after induction. To determine how the expression of AML1-ETO affected the cell cycle, we analyzed by flow cytometry the DNA content of propidium iodide-stained nuclei from induced cells using pool 48 cells and clones U937T-A/E 48–5 and 48–9 (no significant differences between the different populations were detected; data from pool 48 are represented in Fig. 3). After 57 h of AML1-ETO induction, the proportion of cells in S phase decreased from 45 to 22% and the percentage of cells in G0/G1 phase increased from 45 to 69% (Fig. 3), indicating the blockage of the G1-to-S phase transition. At this time point, no cells with a sub-G1 DNA content were detected. However, by 81 h, 13% of cells displayed a sub-G1 DNA content, and the proportion rose to 33% after 100 h. Nicoletti et al. (41) have shown that a cell with hypoploid content is an indication of the cell becoming apoptotic. As shown in Fig. 4, dual acridine orange and ethidium bromide staining of the AML1-ETO-induced U937T-A/E pool 48 cells showed an accumulation of cells with chromatin condensation and fragmentation, which confirmed that AML1-ETO-induced cell death was caused by apoptosis. Furthermore, terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling assays to detect double-strand DNA breaks also demonstrated apoptotic events in AML1-ETO-expressing cells (data not shown) (12).
FIG. 2.
Induction of AML1-ETO expression inhibits the proliferation of U937T-A/E cells. (A) Northern blot analyses showing the time course of AML1-ETO induction following tetracycline removal in U937T-A/E pool 48 cells. Total RNA (10 μg) was prepared from U937T-A/E pool 48 cells cultured for 0, 12, 24, and 48 h after withdrawal of tetracycline, electrophoresed, blotted, and hybridized with α-32P-labeled ETO cDNA. The 28S rRNA is shown to demonstrate relative loading. (B and C) Growth curves for induced U937T-A/E cells. Cells were maintained in the medium either with or without tetracycline (1 μg/ml). The viability was measured daily by trypan blue exclusion. This experiment is representative of three independent experiments that gave similar results. (B) Only viable cells with the ability to exclude trypan blue are represented. Results for U937T cells (square), U937T-A/E pool 18 cells (triangle), and U937T-A/E pool 48 cells (circle) are shown. Solid symbols, absence of tetracycline; open symbols, presence of tetracycline. (C) The ratio of viable cells in the presence or the absence of tetracycline is shown for each cell line. Cells unaffected by the withdrawal of tetracycline exhibit a ratio close to 1. Results for U937T cells (square), U937T-A/E pool 18 cells (triangle), and U937T-A/E pool 48 cells (circle) are shown.
FIG. 3.
Cell cycle analysis of U937T-A/E pool 48 cells upon tetracycline withdrawal. The cell cycle distribution was determined by propidium iodide staining of the cell nuclei at different time points as indicated. The cells were analyzed by flow cytometry. Results for cells in G0/G1 phase (square), G2/M phase (circle), S phase (triangle), and sub-G1 phase (inverted triangle) are shown.
FIG. 4.
Inducible expression of AML1-ETO leads to apoptosis in U937T-A/E cells. Detection of apoptotic cells was performed by acridine orange and ethidium bromide double staining. U937T-A/E cells were maintained for 72 h in the medium either containing 1 μg of tetracycline/ml (A) or not containing tetracycline (B). Both live and dead cells take up acridine orange. Acridine orange intercalates into DNA and RNA, making the former appear green while the latter stains red. Thus a viable cell has bright green chromatin in its nucleus and red-orange cytoplasm. Ethidium bromide is only taken up by nonviable cells. Ethidium bromide intercalates into DNA, making it appear orange, but binds only weakly to RNA, which may appear slightly red. Thus a dead cell has bright orange chromatin (the ethidium overwhelms the acridine) and its cytoplasm, if it has any contents remaining, appears dark red. Cells that have undergone necrosis have the fluorescent features of nonviable cells but do not have apoptotic nuclear morphology. VN, viable cells with normal nuclei (bright green chromatin with organized structure); VA, viable cells with apoptotic nuclei (bright green chromatin which is highly condensed or fragmented); NVA, nonviable cells with apoptotic nuclei (bright orange chromatin which is highly condensed or fragmented).
AML1-ETO-induced apoptosis is associated with the decrease of Bcl-2 expression.
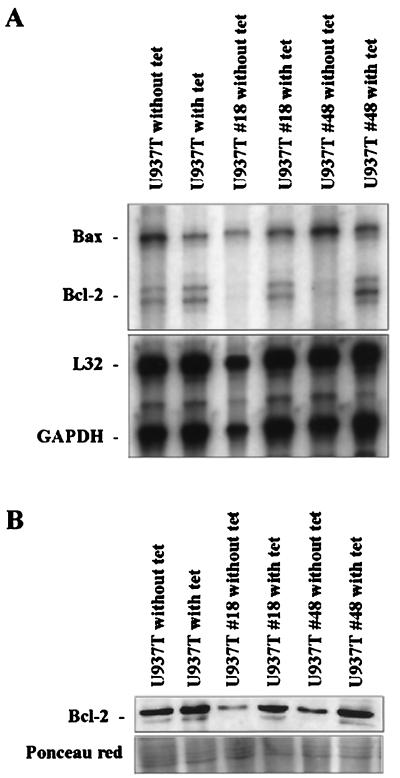
To further analyze the molecular components involved in the induction of apoptosis in U937T-A/E cells, we examined the RNA expression pattern of the members of the Bcl-2 family by RPA (Fig. 5A). Three days after withdrawal of tetracycline to induce AML1-ETO expression, the amounts of Bcl-2 transcripts were significantly reduced in both U937T-A/E pool 18 and 48 cells. Densitometry analysis showed a 10-fold difference in Bcl-2 RNA levels between AML1-ETO-induced cells and uninduced cells, compared to the difference for housekeeping gene transcripts. Some of the other members of antiapoptosis or proapoptosis genes of the Bcl-2 gene family also showed different levels of expression, but the difference was not as dramatic as that for the Bcl-2 gene. Furthermore, a reduction of Bcl-2 at the protein level was also observed by Western blotting (Fig. 5B).
FIG. 5.
The Bcl-2 apoptotic pathway is involved in apoptotic response of induced U937T-A/E cells. (A) RPA analyses showing the reduced expression of Bcl-2 mRNA. Total RNA (10 μg) was prepared from U937T and derived clones cultured for 72 h after withdrawal of tetracycline and hybridized with α-32P-labeled in vitro-transcribed probes from the Bcl-2 family (only Bcl-2 and Bax are represented along with L32 and GAPDH [glyceraldehyde-3-phosphate dehydrogenase] as the internal control) and resolved on a denaturing polyacrylamide gel. (B) Western blot analyses showing the expression of Bcl-2 proteins in U937T cells and U937T-A/E pool 18 and 48 cells following tetracycline withdrawal for 72 h. Ponceau red staining is shown to indicate the relative amount of protein in each lane.
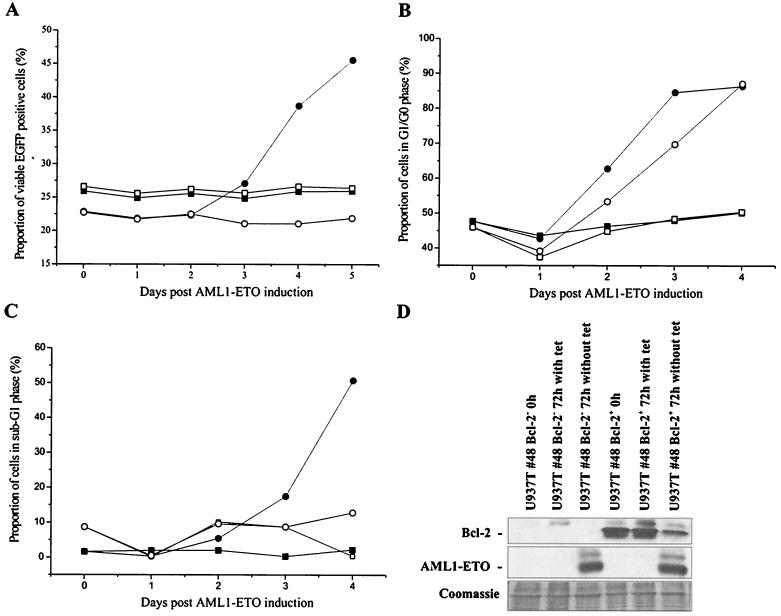
Bcl-2 is an important antiapoptosis factor (7, 54). Bcl-2 expression has also been reported to affect cell cycle progression (4, 33, 49). Therefore, to determine whether the suppression of Bcl-2 expression significantly contributed to the alteration of cell proliferation and apoptosis in induced U937T-A/E cells, Bcl-2 was ectopically expressed in U937T and U937T-A/E cells using an MSCV-Bcl2-IRES-EGFP retrovirus construct. Viral particles prepared from Phoenix amphotropic cells were used to infect U937T and U937T-A/E cells. EGFP expressed through the IRES was used to monitor ectopic Bcl-2-expressing cells. Following retrovirus infection, the proportion of EGFP-positive cells infected with control virus (MSCV-IRES-EGFP) and Bcl-2-expressing virus (MSCV–Bcl-2–IRES–EGFP) remained stable at approximately 20% for more than 1 month in both U937T and U937T-A/E pool 48 cells in the presence of tetracycline as determined by flow cytometry (data not shown). Upon induction of AML1-ETO expression, the proportion of viable EGFP+ U937T-A/E cells remained steady at 22% for 2 days before progressively increasing to 47% 5 days after induction in U937T-A/E cells infected with MSCV–Bcl-2–IRES–EGFP, whereas the proportion of viable EGFP+ cells remained constant in U937T cells and U937T-A/E cells infected with vector control MSCV-IRES-EGFP (Fig. 6A). Although the proportion of EGFP+ Bcl-2+ cells increased to up to one-half of the viable U937T-A/E cells, the overexpression of Bcl-2 was unable to restore the proliferation of those cells and to suppress apoptosis, as all cells eventually underwent apoptosis. However, the increase of Bcl-2-positive cells in the culture indicated that Bcl-2 expression was able to delay apoptosis. To better understand the effect of Bcl-2, the cells infected with MSCV–Bcl-2–IRES–EGFP were sorted by flow cytometry on the basis of the EGFP expression. Later, the cell cycle distributions of both EGFP+ and EGFP− cells were analyzed upon AML1-ETO induction. As seen previously in the mix population, the ectopic expression of Bcl-2 dramatically reduced apoptosis over a period of 96 h (Fig. 6C). However, the ectopic expression of Bcl-2 was unable to prevent the AML1-ETO+ cells from arresting in G0/G1 phase (Fig. 6B). We also confirmed that the level of ectopic Bcl-2 expression was essentially unchanged for the length of experiments, whereas AML1-ETO was significantly up-regulated by 72 h in the absence of tetracycline (Fig. 6D).
FIG. 6.
Ectopic expression of Bcl-2 delays apoptosis in U937T-A/E cells. (A) U937T-A/E cells were infected with either MSCV–Bcl-2–IRES–EGFP (circle) or MSCV-IRES-EGFP (square) and cultured in the presence (open symbol) or the absence (closed symbol) of tetracycline (1 μg/ml). Retrovirus-infected cells were confirmed based on EGFP fluorescence. Apoptotic and necrotic cells were discriminated from viable cells by double annexin V-PE and 7-AAD staining. (B and C) Cell cycle analysis of U937T-A/E pool 48 cells infected with MSCV–Bcl-2–IRES–EGFP upon tetracycline withdrawal. The cell cycle distribution was determined by propidium iodide staining of the cell nuclei at different time points as indicated. The cells were initially sorted on the basis of EGFP expression. Open symbols, EGFP+ cells; solid symbols, EGFP− cells. The cells were cultured in the presence (square) or absence (circle) of tetracycline and were analyzed by flow cytometry. (B) Cells in G0/G1 phase; the percentage does not reflect the proportion of cells in sub-G1 phase. (C) Proportion of cells in sub-G1 phase (apoptotic) compared to the total population. (D) Western blot analyses showing the expression of Bcl-2 and AML1-ETO proteins in EGFP+ and EGFP− U937T-A/E pool 48 cells infected with MSCV–Bcl-2–IRES–EGFP following tetracycline withdrawal for 72 h. Coomassie staining is shown to indicate the relative amount of protein in each lane.
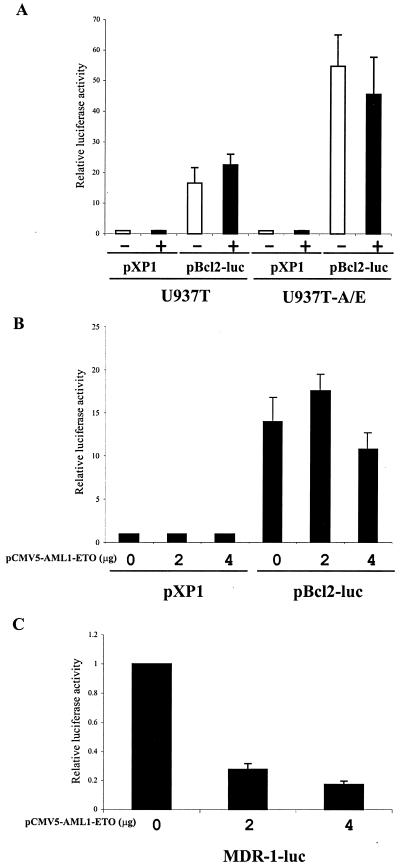
The effect of AML1-ETO on Bcl-2 expression has been studied previously. Klampfler et al. reported the transactivation of the Bcl-2 promoter by AML1-ETO via an AML1 binding consensus sequence (23). Two cell lines derived from t(8;21) leukemia patient cells showed higher expression of Bcl-2 (23, 24). However, studies using 29 (57) and 17 (2) primary t(8;21) leukemia patient samples indicated that Bcl-2 expression was generally down-regulated compared to that for nonleukemic or non-t(8;21) AML samples. Therefore, we performed studies to determine whether the down-regulation of Bcl-2 was the direct effect of AML1-ETO on a 3.7-kb Bcl-2 promoter in U937T and U937T-A/E cells using transient transfection assays (Fig. 7A and B). The data indicated that AML1-ETO down-regulation of Bcl-2 expression in this particular cell line was not a direct effect of AML1-ETO on the 3.7-kb Bcl-2 gene upstream regulatory element. To ensure the accuracy of the data presented, as the current results did not agree with previous studies (23), we assessed whether AML1-ETO was also able to repress the expression of the (−137 to +30) multidrug resistance 1 (MDR-1) promoter as previously reported (31). The data confirmed that AML1-ETO represses transcription of the MDR-1 promoter in U937T (Fig. 7C).
FIG. 7.
Transactivation analysis of the Bcl-2 promoter with AML1-ETO. (A) U937T and U937T-A/E cells were transfected with either promoterless firefly luciferase reporter pXP1 or the 3.7-kb Bcl-2 gene upstream sequence–firefly luciferase reporter pBcl2-luc after they had been cultured in the absence or presence of tetracycline for 12 h. The pRL-Null Renilla luciferase construct was cotransfected to normalize transfection efficiency. (B) U937T cells were transfected with pXP1 or pBcl2-luc and various amounts of pCMV5 or pCMV5-AML1-ETO, along with pRL-Null to normalize transfection efficiency. The activity of the promoter was calculated as the ratio of the firefly luciferase activity and the Renilla luciferase activity. The transactivation was calculated as the ratio between pXP1 and pBcl2-luc with 0, 2, and 4 μg of pCMV5-AML1-ETO, assuming a value of 1 for each in the presence of pXP1. (C) U937T cells were transfected with MDR-1-luc and various amounts of pCMV5 or pCMV5-AML1-ETO, along with pRL- Null to normalize transfection efficiency. The activity of the promoter was calculated as the ratio of the firefly luciferase activity and the Renilla luciferase activity. The transactivation was calculated as the ratio between 0 μg and 2 or 4 μg of pCMV5-AML1-ETO. The results are the means of three independent experiments (standard errors of the means are shown when measurable).
AML1-ETO alters myeloid differentiation.
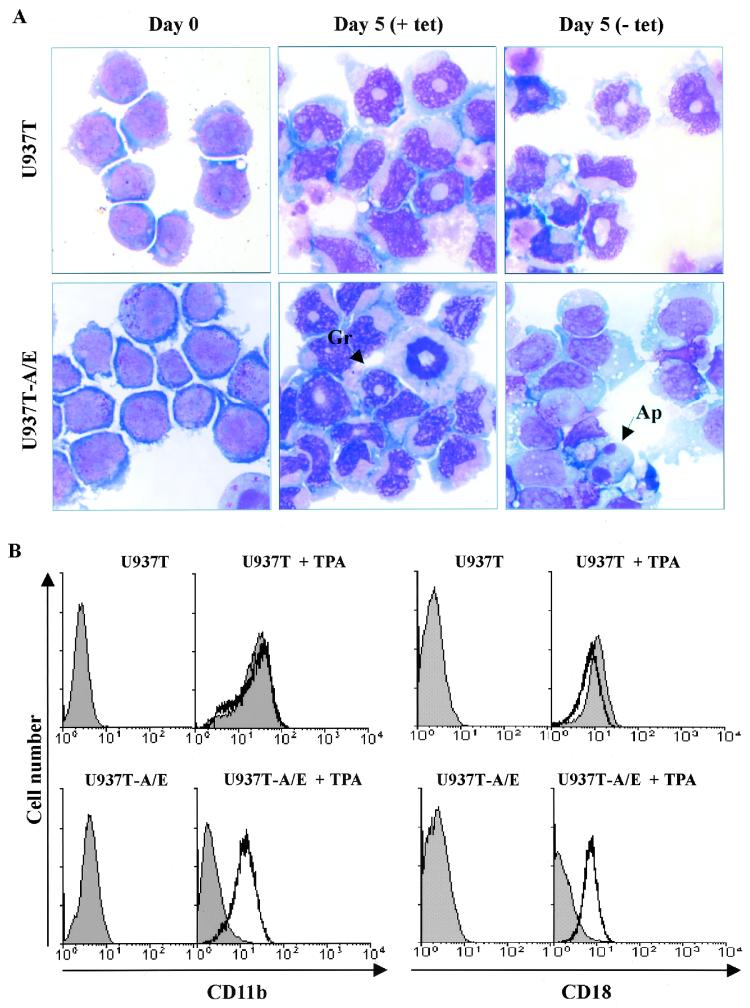
The effect of AML1-ETO on U937T cell differentiation was also studied with these inducible AML1-ETO-expressing cells. Parental U937T cells and U937T-A/E cells were treated with TPA. Interestingly the differentiation patterns of U937 and U937T cells were different, as U937 cells treated with TPA rapidly assumed (24 h) a macrophage-like morphology (data not shown) whereas U937T and uninduced U937T-A/E pool 48 cells slowly displayed “doughnut-shaped” nuclei characteristic of granulocytes upon TPA treatment (Fig. 8A). In contrast, the surviving fraction of induced U937T-A/E cells treated with TPA did not exhibit doughnut-shaped nuclei. Nonetheless, the cells displayed some signs of maturation, with cytoplasm larger than that of untreated samples, which displayed lymphoblast-like morphologies. As in previous studies, the effects of AML1-ETO on differentiation were further analyzed by flow cytometry (13, 28), which revealed a clear increase of the cell surface differentiation markers CD11b and CD18 upon TPA treatment (Fig. 8B; from CD11blow and CD18low to CD11bhigh and CD18high). The increase in the CD11b and CD18 cell surface markers precedes the occurrence of the doughnut-shaped nuclei mentioned previously. However, the induction of AML1-ETO significantly reduced the differentiation of U937T-A/E cells. These cells lost their original lymphoblast-like morphology without displaying granulocytic morphology (Fig. 8A) and exhibited a block of differentiation at an early stage (Fig. 8B; CD11blow and CD18low).
FIG. 8.
AML1-ETO blocks granulocytic differentiation of U937T-A/E cells. TPA (65 nM) was added to the cell culture medium after U937T and U937T-A/E cells were cultured in the presence or absence of tetracycline (1 μg/ml) for 24 h. Cell culture was continued in the same condition after the addition of TPA. (A) Wright-Giemsa staining of cytospin preparation of U937T and U937T-A/E cells without (+ tet) or with (− tet) AML1-ETO expression upon TPA treatment. Gr, granulocyte; Ap, apoptotic cell. (B) Flow cytometry profile of U937T and U937T-A/E cells treated for 48 h with 65 nM TPA 24 h after AML1-ETO induction. The cells were stained with anti-human CD11b-PE or anti-human CD18-FITC. Profiles of cells in the presence (unshaded) or the absence (shaded) of tetracycline are presented. Undifferentiated cells appeared as CD11blow and CD18low. Differentiated cells appeared as CD11bhigh and CD18high.
C/EBPα is down-regulated upon AML1-ETO expression.
The alteration of granulocytic differentiation suggested that AML1-ETO expression might affect certain critical factors involved in myeloid lineage commitment. Since C/EBPα and C/EBPɛ are important transcription factors for granulocytic cell differentiation, we hypothesized that down-regulation of their expression may be involved in the block of granulocytic differentiation (53, 72–74, 78). Therefore, Northern blot analysis was used to study the expression of C/EBPα and C/EBPɛ. C/EBPɛ expression is barely detectable in uninduced U937T-A/E cells. Upon induction, no obvious change of C/EBPɛ was detected (data not shown). C/EBPα was expressed at high levels in U937 and U937T parental cells and uninduced U937T-A/E cells (Fig. 9A). Upon induction of AML1-ETO expression, C/EBPα was considerably down-regulated (Fig. 9A). In a time course analysis, the decrease of C/EBPα transcripts showed a tight correlation with the induced expression of AML1-ETO (Fig. 9B). Furthermore, the expression of two cell cycle-related genes, cdk4 and c-myc, were also down-regulated (Fig. 9B). The effects of TPA treatment on the level of C/EBPα were also assayed (Fig. 9C). Treatment with TPA did not alter the down-regulation of C/EBPα by AML1-ETO.
FIG. 9.
AML1-ETO down-regulates the expression of C/EBPα, CDK4, and c-myc. (A) Total RNA (10 μg) was prepared from Kasumi-1 cells and U937T cells and their derived clones after they had been cultured in the presence or the absence of tetracycline for 72 h, electrophoresed, blotted, and hybridized with α-32P-labeled C/EBPα cDNA. (B) Time course of the expression of C/EBPα, CDK4, and c-myc upon tetracycline withdrawal. (C) Effects of TPA on the level of C/EBPα mRNA. The cells were treated with 65 nM TPA for 48 h starting 24 h after induction of AML1-ETO. The 28S rRNA is shown to show relative RNA loading in the gel.
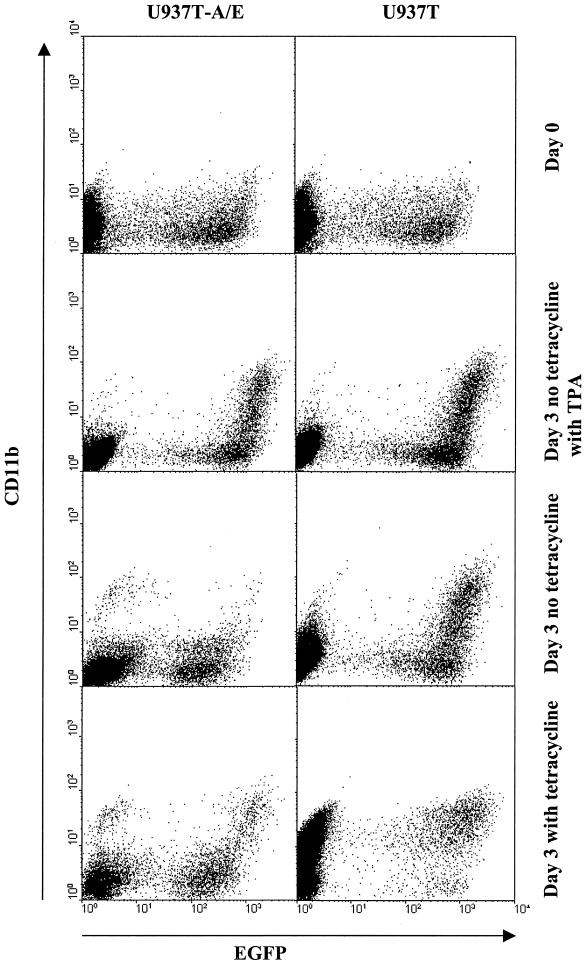
To determine whether the down-regulation of C/EBPα was a central part of the inhibition of differentiation by AML1-ETO, C/EBPα was ectopically expressed in U937T and U937T-A/E using an MSCV-C/EBPα-IRES-EGFP retrovirus construct. Following retrovirus infection, the proportion of EGFP-positive cells decreased rapidly from 30 to 10% within 5 days after the infection (data not shown). The reduction is the result of a reduced proliferation caused by the spontaneous differentiation of both U937T and uninduced U937T-A/E due to C/EBPα expression as determined by CD11b staining (Fig. 10). Interestingly, the induction of AML1-ETO halted the differentiation induced by ectopic C/EBPα as determined by CD11b staining (Fig. 10). The addition of TPA in conjunction with the ectopic expression of C/EBPα was only able to cause a small increase of CD11b-positive cells. Even in the presence of TPA, the ectopic expression of C/EBPα was unable to antagonize the effect of AML1-ETO.
FIG. 10.
AML1-ETO inhibits spontaneous U937T differentiation caused by ectopic expression of C/EBPα. Flow cytometry profile of U937T and U937T-A/E cells 2 days (day 0 for tetracycline withdrawal) and 5 days (3 days after tetracycline withdrawal) after infection with MSCV-C/EBPα-IRES-EGFP. The cells were also treated with 65 nM TPA 24 h after AML1-ETO induction. The cells were stained with anti-human CD11b-PE. Profiles of cells in the presence (unshaded) or absence (shaded) of tetracycline are presented. Undifferentiated cells appeared as CD11blow. Differentiated cells appeared as CD11bhigh.
DISCUSSION
To investigate the role of AML1-ETO in leukemogenesis, we examined the biological properties of the AML1-ETO fusion protein by inducible expression in U937T cells. The most interesting finding from this study is that AML1-ETO plays antagonistic roles regarding cancer development. AML1-ETO expression favors leukemogenesis by blocking cell differentiation associated with the decrease of C/EBPα protein level and opposes leukemogenesis by blocking cell proliferation, leading to apoptosis.
AML1-ETO blocks granulocytic differentiation and down-regulates C/EBPα expression.
The treatment of U937T and uninduced U937T-A/E cells with TPA leads to progressive granulocytic maturation associated with an increased expression of cell surface markers CD11b and CD18. This differentiation was significantly reduced in AML1-ETO+ cells. This altered differentiation potential is correlated with the down-regulation of C/EBPα upon induction of AML1-ETO (Fig. 9). C/EBPα is the founding member of the C/EBP transcription factor family, regulating many genes involved in myeloid lineage commitment and differentiation (6, 19, 20, 27, 45, 52, 60, 69, 77; A. Khanna-Gupta, T. Zibello, C. Simkevich, A. G. Rosmarin, and N. Berliner, Abstr. Third Int. Meet. Mol. Aspects Myeloid Stem Cell Dev., abstr. 10, 1999). C/EBPα is up-regulated during granulocytic differentiation, and overexpression of C/EBPα in U937 cells leads to granulocytic differentiation (53). Furthermore, C/EBPα-deficient mice lack mature granulocytes, indicating a critical role in granulocytic differentiation (78). Interestingly, C/EBPα is consistently expressed at much lower level in AML M2 subtype patients with t(8;21) translocation than in patients with other subtypes of AML (50). Moreover, dominant-negative mutations of C/EBPα leading to a failure to induce granulocytic differentiation have been detected in 16% of AML M2 patients without t(8;21) translocation, emphasizing the important contribution of C/EBPα to AML leukemogenesis (51).
Although U937 cells constitutively express C/EBPα, U937T and uninduced U937T-A/E cells express markedly higher levels of C/EBPα mRNA than the parental U937 cells. This difference may be reflected in the higher propensity for granulocytic differentiation of the U937T cells.
The ectopic expression of C/EBPα causes a rapid up-regulation of the CD11b cell surface marker in both U937T and uninduced U937T-A/E cells. However, the induction of AML1-ETO prevents the CD11b up-regulation induced by C/EBPα in U937T-A/E cells, suggesting that the down-regulation of C/EBPα probably contributes to the inhibition of differentiation but is not enough to fully explain this inhibition. In contrast, ectopic expression of C/EBPα in Kasumi-1 cells leads to granulocytic differentiation (50). One explanation could be that the cell cycle arrest caused by the induction of AML1-ETO blocks the differentiation despite the presence of C/EBPα, in contrast to what is found for Kasumi-1 cells. Alternatively, Westendorf et al. have previously demonstrated that AML1-ETO physically interacts with C/EBPα, abrogates C/EBPα-dependent transcription, and ultimately blocks granulocytic differentiation (71). C/EBPα activates its own promoter through an upstream stimulatory factor (USF) binding site (63). It has been shown that AML1-ETO decreases C/EBPα promoter activity also via a USF binding site (50). Therefore, it is possible that AML1-ETO prevents the autoactivation of the C/EBPα promoter by USF through protein-protein interaction with C/EBPα
Shimizu et al. (58) reported recently that AML1-ETO up-regulates C/EBPɛ expression without identifying an AML1 binding site in the C/EBPɛ promoter, suggesting the existence of additional steps between the expression of AML1-ETO and the up-regulation of C/EBPɛ. Interestingly, the expression of C/EBPɛ was also reported in cell lines derived from AML M2 patients but not in other hematopoietic cell lines such as U937. In the present studies, we did not observe any significant modulation of C/EBPɛ expression. The difference in C/EBPɛ expression between AML M2-derived cell lines and the present model could be reconciled by the presence or absence of a yet to be identified factor that would cooperate with AML1-ETO to induce the expression of C/EBPɛ.
AML1-ETO blocks cell proliferation by inducing cell arrest in G0/G1.
The induction of AML1-ETO in U937T-A/E cells causes a progressive cell cycle arrest in G0/G1 phase. This growth arrest coincides with the down-regulation of c-myc and CDK4 (Fig. 9B). Lou et al. have recently reported that the tamoxifen-inducible fusion protein KRAB-AML1-ER prolongs G1 phase (29), possibly through the down-regulation of CDK4. Furthermore, Hermeking et al. have also identified CDK4 as a transcriptional target of c-myc (18). These authors suggest that the ability of c-myc to promote cell cycle reentry is in part due to its ability to directly induce the transcription of CDK4. We assume that the down-regulation of c-myc causes the down-regulation of CDK4, thereby contributing to blocking the cell cycle. Upon induction of AML1-ETO, the expression pattern of CDK4 closely matches that of c-myc expression, as transcription culminates 12 h after induction before decreasing to its lowest level by 48 h (highest level of AML1-ETO; Fig. 9B). The overexpression of CDK4 or c-myc in U937T-A/E cells using the MSCV-CDK4-IRES-EGFP or MSCV-cMyc-IRES-EGFP retrovirus construct was not able to prevent the growth arrest and apoptosis caused by the conditional expression of AML1-ETO in U937T-A/E cells (data not shown). Interestingly, the down-regulation of C/EBPα may actually antagonize growth arrest, as C/EBPα has been reported in hepatocytes to increase the level of p21/WAF-1, thereby causing growth arrest (64). Therefore, we hypothesize that AML1-ETO deregulates the expression of a yet to be identified factor that may be responsible for the down-regulation of both c-myc and CDK4. However, c-myc and CDK4 may not be the only targets of this putative factor to provoke cell cycle arrest of AML1-ETO+ cells. If such a factor were identified, we predict that the separation of its function with those of AML1-ETO would potentially unlock the growth arrest and contribute significantly to the leukemogenic process. Another interesting question raised by these results is whether DNA binding is required for AML1-ETO to induce cell cycle arrest or whether the growth arrest occurs through protein-protein interaction possibly mediated through the ETO moiety as reported recently by Melnick et al. (34).
AML1-ETO induces apoptosis and reduces Bcl-2 level.
Eventually, the induction of AML1-ETO results in the onset of apoptosis in U937T-A/E cells. We also report the down-regulation of Bcl-2 at the RNA and protein levels following induction of AML1-ETO, suggesting that down-regulation of Bcl-2 contributed to the reduced survival of U937T-A/E cells. However, ectopic expression of Bcl-2 delays apoptosis without preventing AML1-ETO-induced G1/G0 arrest. Moreover, the inability of Bcl-2, which acts essentially downstream of the cell cycle, to prevent cell cycle arrest despite delaying apoptosis suggests that Bcl-2 is not a direct target of AML1-ETO (Fig. 6). Therefore, the AML1-ETO+ cells may initiate apoptosis through their inability to overcome cell cycle arrest.
Two groups have reported that t(8;21)-bearing de novo leukemia cells express, in general, lower levels of Bcl-2 protein than normal bone marrow, other non-t(8;21) AML samples, and Kasumi-1 and SKNO-1 cells (2, 23, 24, 57). Klampfer et al. report that AML1-ETO is able to transactivate the Bcl-2 promoter via a consensus AML1 binding sequence (23). As our results indicate that the induction of AML1-ETO results in the down-regulation of Bcl-2, we reexamined the effects of transient expression or induction of AML1-ETO in the specific context of U937T and U937T-A/E cells using the same Bcl-2 promoter fragment (Fig. 7) (23, 38). AML1-ETO does not transactivate the Bcl-2 promoter in U937T cells, indicating a cell type-specific effect of AML1-ETO on the regulation of the Bcl-2 promoter. Furthermore, the delay in the onset of apoptosis also reinforces the idea that Bcl-2 is not a target of AML1-ETO in U937T-A/E cells.
In summary, our studies with inducible cells indicate that expression of AML1-ETO blocks cell differentiation and proliferation and consequently induces apoptosis. The reduced granulocytic maturation in AML1-ETO+ cells correlates with the down-regulation of C/EBPα. Modulation of C/EBPα is critical for the commitment to the granulocytic lineage (53, 78). The low level of C/EBPα reported in t(8;21) patients also suggests that the reduction of C/EBPα is central for blocking AML cells at the M2 differentiation stage (50). In view of the effects of AML1-ETO on the regulation of C/EBPα function, further studies are required to clarify whether overexpression of C/EBPα in t(8;21) patients is enough to induce the maturation of the leukemic cells and improve their clinical outcome.
In addition to blocking cell differentiation, AML1-ETO also blocks proliferation by inducing cell cycle arrest. This finding is further supported by the data from AML1-ETO knockin mice and the tetracycline-inducible mouse model. In previous studies, the effect of AML1-ETO on the cell cycle or apoptosis in the bone marrow of tetracycline-inducible AML1-ETO mice could not be determined. However, the new perspective offered by our findings now supports the negative effect of AML1-ETO on cell proliferation and survival observed in primary bone marrow and CFU–granulocyte-macrophage (GM) assays. First, AML1-ETO knockin mice died in midgestation without the development of definitive hematopoietic cells, raising the possibility of AML1-ETO expression blocking the proliferation of hematopoietic precursor cells (47, 75). Second, the infection of adult murine bone marrow with MSCV-AML1-ETOneo leads to a lower number of colonies than with the retrovirus vector control (75). Last, AML1-ETO-inducible transgenic mice, although viable, do not develop leukemia, but induction of AML1-ETO in the bone marrow of those mice exerts a detrimental effect on CFU GM number and size as well as blocking cell differentiation (55). The homeostasis in vivo and the high concentration of growth factors used in in vitro CFU assays may contribute to a decrease of AML1-ETO effects compared to those reported in U937T-A/E cells. Both Yergeau et al. and Okuda et al. followed the same experimental procedure for the CFU assay, including the use of fetal liver cells from the same developmental stage, for assessing the outcome of AML1-ETO expression. Nonetheless, the different origins of cells used to establish the AML1-ETO knockin mice could be another source of discrepancies between both models. While Okuda et al. reports the presence of dysplastic hematopoietic colonies with high replating efficiency, Yergeau et al. did not detect any dysplastic replating colonies (47, 75). In fact, no colonies were detected in the fetal liver culture. Additional mutations could reconcile the observed differences.
If growth arrest and apoptosis are general features associated with the expression of AML1-ETO, we predict that the putative link that unites them to AML1-ETO must also be severed prior to the occurrence of the t(8;21) translocation to allow the survival and expansion of the preleukemic cells. Inhibition of proliferation or apoptosis would not be favorable to the propagation of cells harboring the t(8;21) translocation. Therefore, we favor the hypothesis that other oncogenic events promoting proliferation or cell survival are necessary for the development of t(8;21) AML.
ACKNOWLEDGMENTS
We thank John Reed, Stephen Nimer, Nancy Speck, Beatrice Muller, and Alan Friedman for valuable discussion and Gerald Grosveld, Scott Hiebert, Warren Pear, Gary Nolan, Jonathan Licht, Stanley Korsmeyer, Philip Koeffler, Kimiko Shimizu, Scott Kogan, and John Schuetz for DNA constructs, cell lines, and antibodies. We thank Ernest Beutler and Kenneth Ritchie for editing the manuscript.
This work was supported by National Institutes of Health grant CA72009 and American Cancer Society grant LBC-99438. D.E.Z. is a Leukemia and Lymphoma Society Scholar. S.A.B. was supported by a fellowship from the Lady Tata Memorial Trust. The Stein Endowment Fund partially supported the Departmental Molecular Biology Service Laboratory for DNA Sequencing and Oligonucleotide Synthesis.
Footnotes
Paper 13690-MEM from The Scripps Research Institute.
REFERENCES
- 1.Ahn M Y, Huang G, Bae S C, Wee H J, Kim W Y, Ito Y. Negative regulation of granulocytic differentiation in the myeloid precursor cell line 32Dcl3 by ear-2, a mammalian homolog of Drosophila seven-up, and a chimeric leukemogenic gene, AML1/ETO. Proc Natl Acad Sci USA. 1998;95:1812–1817. doi: 10.1073/pnas.95.4.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banker D E, Radich J, Becker A, Kerkof K, Norwood T, Willman C, Appelbaum F R. The t(8;21) translocation is not consistently associated with high Bcl-2 expression in de novo acute myeloid leukemias of adults. Clin Cancer Res. 1998;4:3051–3062. [PubMed] [Google Scholar]
- 3.Boer J, Bonten-Surtel J, Grosveld G. Overexpression of the nucleoporin CAN/NUP214 induces growth arrest, nucleocytoplasmic transport defects, and apoptosis. Mol Cell Biol. 1998;18:1236–1247. doi: 10.1128/mcb.18.3.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borner C. Diminished cell proliferation associated with the death-protective activity of Bcl-2. J Biol Chem. 1996;271:12695–12698. doi: 10.1074/jbc.271.22.12695. [DOI] [PubMed] [Google Scholar]
- 5.Buchholz F, Refaeli Y, Trumpp A, Bishop J M. Inducible chromosomal translocation of AML1 and ETO genes through Cre/loxP-mediated recombination in the mouse. EMBO Rep. 2000;1:133–139. doi: 10.1093/embo-reports/kvd027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao Z, Umek R M, McKnight S L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3–L1 cells. Genes Dev. 1991;5:1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- 7.Chao D T, Korsmeyer S J. BCL-2 family: regulators of cell death. Annu Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 8.Downing J R. The AML1-ETO chimaeric transcription factor in acute myeloid leukaemia: biology and clinical significance. Br J Haematol. 1999;106:296–308. doi: 10.1046/j.1365-2141.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 9.Frank R C, Sun X, Berguido F J, Jakubowiak A, Nimer S D. The t(8;21) fusion protein, AML1/ETO, transforms NIH3T3 cells and activates AP-1. Oncogene. 1999;18:1701–1710. doi: 10.1038/sj.onc.1202459. [DOI] [PubMed] [Google Scholar]
- 10.Friedman A D. Leukemogenesis by CBF oncoproteins. Leukemia. 1999;13:1932–1942. doi: 10.1038/sj.leu.2401590. [DOI] [PubMed] [Google Scholar]
- 11.Gamou T, Kitamura E, Hosoda F, Shimizu K, Shinohara K, Hayashi Y, Nagase T, Yokoyama Y, Ohki M. The partner gene of AML1 in t(16;21) myeloid malignancies is a novel member of the MTG8(ETO) family. Blood. 1998;91:4028–4037. [PubMed] [Google Scholar]
- 12.Gavrieli Y, Sherman Y, Ben Sasson S A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gelmetti V, Zhang J, Fanelli M, Minucci S, Pelicci P G, Lazar M A. Aberrant recruitment of the nuclear receptor corepressor-histone deacetylase complex by the acute myeloid leukemia fusion partner ETO. Mol Cell Biol. 1998;18:7185–7191. doi: 10.1128/mcb.18.12.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golub T R, Barker G F, Bohlander S K, Hiebert S W, Ward D C, Bray-Ward P, Morgan E, Raimondi S C, Rowley J D, Gilliland D G. Fusion of the TEL gene on 12p13 to the AML1 gene on 21q22 in acute lymphoblastic leukemia. Proc Natl Acad Sci USA. 1995;92:4917–4921. doi: 10.1073/pnas.92.11.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawley R G, Hawley T S, Fong A Z, Quinto C, Collins M, Leonard J P, Goldman S J. Thrombopoietic potential and serial repopulating ability of murine hematopoietic stem cells constitutively expressing interleukin 11. Proc Natl Acad Sci USA. 1996;93:10297–10302. doi: 10.1073/pnas.93.19.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawley R G, Lieu F H, Fong A Z, Hawley T S. Versatile retroviral vectors for potential use in gene therapy. Gene Ther. 1994;1:136–138. [PubMed] [Google Scholar]
- 18.Hermeking H, Rago C, Schuhmacher M, Li Q, Barrett J F, Obaya A J, O'Connell B C, Mateyak M K, Tam W, Kohlhuber F, Dang C V, Sedivy J M, Eick D, Vogelstein B, Kinzler K W. Identification of CDK4 as a target of c-MYC. Proc Natl Acad Sci USA. 2000;97:2229–2234. doi: 10.1073/pnas.050586197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hohaus S, Petrovick M S, Voso M T, Sun Z, Zhang D-E, Tenen D G. PU.1 (Spi-1) and C/EBP alpha regulate expression of the granulocyte-macrophage colony-stimulating factor receptor alpha gene. Mol Cell Biol. 1995;15:5830–5845. doi: 10.1128/mcb.15.10.5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwama A, Zhang P, Darlington G J, McKercher S R, Maki R, Tenen D G. Use of RDA analysis of knockout mice to identify myeloid genes regulated in vivo by PU.1 and C/EBPalpha. Nucleic Acids Res. 1998;26:3034–3043. doi: 10.1093/nar/26.12.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinsella T M, Nolan G P. Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum Gene Ther. 1996;7:1405–1413. doi: 10.1089/hum.1996.7.12-1405. [DOI] [PubMed] [Google Scholar]
- 22.Kitabayashi I, Ida K, Morohoshi F, Yokoyama A, Mitsuhashi N, Shimizu K, Nomura N, Hayashi Y, Ohki M. The AML1-MTG8 leukemic fusion protein forms a complex with a novel member of the MTG8(ETO/CDR) family, MTGR1. Mol Cell Biol. 1998;18:846–858. doi: 10.1128/mcb.18.2.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klampfer L, Zhang J, Zelenetz A O, Uchida H, Nimer S D. The AML1/ETO fusion protein activates transcription of BCL-2. Proc Natl Acad Sci USA. 1996;93:14059–14064. doi: 10.1073/pnas.93.24.14059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohzaki H, Ito K, Huang G, Wee H J, Murakami Y, Ito Y. Block of granulocytic differentiation of 32Dcl3 cells by AML1/ETO(MTG8) but not by highly expressed Bcl-2. Oncogene. 1999;18:4055–4062. doi: 10.1038/sj.onc.1202735. [DOI] [PubMed] [Google Scholar]
- 25.Kurokawa M, Tanaka T, Tanaka K, Ogawa S, Mitani K, Yazaki Y, Hirai H. Overexpression of the AML1 proto-oncoprotein in NIH3T3 cells leads to neoplastic transformation depending on the DNA-binding and transactivational potencies. Oncogene. 1996;12:883–892. [PubMed] [Google Scholar]
- 26.Le X F, Claxton D, Kornblau S, Fan Y H, Mu Z M, Chang K S. Characterization of the ETO and AML1-ETO proteins involved in 8;21 translocation in acute myelogenous leukemia. Eur J Haematol. 1998;60:217–225. doi: 10.1111/j.1600-0609.1998.tb01027.x. [DOI] [PubMed] [Google Scholar]
- 27.Lekstrom-Himes J, Xanthopoulos K G. Biological role of the CCAAT/Enhancer-binding protein family of transcription factors. J Biol Chem. 1998;273:28545–28548. doi: 10.1074/jbc.273.44.28545. [DOI] [PubMed] [Google Scholar]
- 28.Liu L-Q, Ilaria R, Jr, Kingsley P D, Iwama A, van Etten R, Palis J, Zhang D-E. A novel ubiquitin-specific protease, UBP43, cloned from leukemia fusion protein AML1-ETO-expressing mice, functions in hematopoietic cell differentiation. Mol Cell Biol. 1999;19:3029–3038. doi: 10.1128/mcb.19.4.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lou J, Cao W, Bernardin F, Ayyanathan K, Rauscher III F J, Friedman A D. Exogenous cdk4 overcomes reduced cdk4 RNA and inhibition of G1 progression in hematopoietic cells expressing a dominant-negative CBF—a model for overcoming inhibition of proliferation by CBF oncoproteins. Oncogene. 2000;19:2695–2703. doi: 10.1038/sj.onc.1203588. [DOI] [PubMed] [Google Scholar]
- 30.Lutterbach B, Hiebert S W. Role of the transcription factor AML-1 in acute leukemia and hematopoietic differentiation. Gene. 2000;245:223–235. doi: 10.1016/s0378-1119(00)00014-7. [DOI] [PubMed] [Google Scholar]
- 31.Lutterbach B, Sun D, Schuetz J, Hiebert S W. The MYND motif is required for repression of basal transcription from the multidrug resistance 1 promoter by the t(8;21) fusion protein. Mol Cell Biol. 1998;18:3604–3611. doi: 10.1128/mcb.18.6.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lutterbach B, Westendorf J J, Linggi B, Patten A, Moniwa M, Davie J R, Huynh K D, Bardwell V J, Lavinsky R M, Rosenfeld M G, Glass C, Seto E, Hiebert S W. ETO, a target of t(8;21) in acute leukemia, interacts with the N-CoR and mSin3 corepressors. Mol Cell Biol. 1998;18:7176–7184. doi: 10.1128/mcb.18.12.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazel S, Burtrum D, Petrie H T. Regulation of cell division cycle progression by bcl-2 expression: a potential mechanism for inhibition of programmed cell death. J Exp Med. 1996;183:2219–2226. doi: 10.1084/jem.183.5.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melnick A M, Westendorf J J, Polinger A, Carlile G W, Arai S, Ball H J, Lutterbach B, Hiebert S W, Licht J D. The ETO protein disrupted in t(8;21)-associated acute myeloid leukemia is a corepressor for the promyelocytic leukemia zinc finger protein. Mol Cell Biol. 2000;20:2075–2086. doi: 10.1128/mcb.20.6.2075-2086.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyers S, Downing J R, Hiebert S W. Identification of AML-1 and the (8;21) translocation protein (AML-1/ETO) as sequence-specific DNA-binding proteins: the runt homology domain is required for DNA binding and protein-protein interactions. Mol Cell Biol. 1993;13:6336–6345. doi: 10.1128/mcb.13.10.6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mishell B B, Shiigi S M, Henry C, Chan E L, North J, Gallily R, Slomich M, Miller K, Marbrook J, Parks D, Good A H. Preparation of mouse cell suspensions. In: Mishell B B, Shiigi S M, editors. Selected methods in cellular immunology. W. H. New York, N.Y: Freeman; 1980. pp. 21–22. [Google Scholar]
- 37.Mitani K, Ogawa S, Tanaka T, Miyoshi H, Kurokawa M, Mano H, Yazaki Y, Ohki M, Hirai H. Generation of the AML1-EVI-1 fusion gene in the t(3;21)(q26;q22) causes blastic crisis in chronic myelocytic leukemia. EMBO J. 1994;13:504–510. doi: 10.1002/j.1460-2075.1994.tb06288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyashita T, Harigai M, Hanada M, Reed J C. Identification of a p53-dependent negative response element in the bcl-2 gene. Cancer Res. 1994;54:3131–3135. [PubMed] [Google Scholar]
- 39.Miyoshi H, Kozu T, Shimizu K, Enomoto K, Maseki N, Kaneko Y, Kamada N, Ohki M. The t(8;21) translocation in acute myeloid leukemia results in production of an AML1-MTG8 fusion transcript. EMBO J. 1993;12:2715–2721. doi: 10.1002/j.1460-2075.1993.tb05933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyoshi H, Shimizu K, Kozu T, Maseki N, Kaneko Y, Ohki M. t(8;21) breakpoints on chromosome 21 in acute myeloid leukemia are clustered within a limited region of a single gene, AML1. Proc Natl Acad Sci USA. 1991;88:10431–10434. doi: 10.1073/pnas.88.23.10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicoletti I, Migliorati G, Pagliacci M C, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 42.Nucifora G, Begy C R, Erickson P, Drabkin H A, Rowley J D. The 3;21 translocation in myelodysplasia results in a fusion transcript between the AML1 gene and the gene for EAP, a highly conserved protein associated with the Epstein-Barr virus small RNA EBER 1. Proc Natl Acad Sci USA. 1993;90:7784–7788. doi: 10.1073/pnas.90.16.7784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nucifora G, Birn D J, Espinosa R, III, Erickson P, LeBeau M M, Roulston D, McKeithan T W, Drabkin H, Rowley J D. Involvement of the AML1 gene in the t(3;21) in therapy-related leukemia and in chronic myeloid leukemia in blast crisis. Blood. 1993;81:2728–2734. [PubMed] [Google Scholar]
- 44.Nucifora G, Rowley J D. AML1 and the 8;21 and 3;21 translocations in acute and chronic myeloid leukemia. Blood. 1995;86:1–14. [PubMed] [Google Scholar]
- 45.Oelgeschlager M, Nuchprayoon I, Luscher B, Friedman A D. C/EBP, c-Myb, and PU.1 cooperate to regulate the neutrophil elastase promoter. Mol Cell Biol. 1996;16:4717–4725. doi: 10.1128/mcb.16.9.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogawa E, Inuzuka M, Maruyama M, Satake M, Naito-Fujimoto M, Ito Y, Shigesada K. Molecular cloning and characterization of PEBP2 beta, the heterodimeric partner of a novel Drosophila runt-related DNA binding protein PEBP2 alpha. Virology. 1993;194:314–331. doi: 10.1006/viro.1993.1262. [DOI] [PubMed] [Google Scholar]
- 47.Okuda T, Cai Z, Yang S, Lenny N, Lyu C J, van Deursen J M, Harada H, Downing J R. Expression of a knocked-in AML1-ETO leukemia gene inhibits the establishment of normal definitive hematopoiesis and directly generates dysplastic hematopoietic progenitors. Blood. 1998;91:3134–3143. [PubMed] [Google Scholar]
- 48.Okuda T, van Deursen J, Hiebert S W, Grosveld G, Downing J R. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84:321–330. doi: 10.1016/s0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- 49.O'Reilly L A, Huang D C, Strasser A. The cell death inhibitor Bcl-2 and its homologues influence control of cell cycle entry. EMBO J. 1996;15:6979–6990. [PMC free article] [PubMed] [Google Scholar]
- 50.Pabst T, Mueller B U, Harakawa N, Schoch T, Haferlach G, Behre G, Hiddemann W, Zhang D-E, Tenen D G. AML1–ETO downregulates the granulocytic differentiation factor C/EBP-α in t(8;21) myeloid leukemia. Nat Med. 2001;7:444–451. doi: 10.1038/86515. [DOI] [PubMed] [Google Scholar]
- 51.Pabst T, Mueller B U, Zhang P, Radomska H S, Narravula S, Schnittger S, Behre G, Hiddemann W, Tenen D G. Dominant-negative mutations of CEBPA, encoding CCAAT/enhancer binding protein-alpha (C/EBPalpha), in acute myeloid leukemia. Nat Genet. 2001;27:263–270. doi: 10.1038/85820. [DOI] [PubMed] [Google Scholar]
- 52.Petrovick M S, Hiebert S W, Friedman A D, Hetherington C J, Tenen D G, Zhang D-E. Multiple functional domains of AML1: PU.1 and C/EBPα synergize with different regions of AML1. Mol Cell Biol. 1998;18:3915–3925. doi: 10.1128/mcb.18.7.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Radomska H S, Huettner C S, Zhang P, Cheng T, Scadden D T, Tenen D G. CCAAT/enhancer binding protein alpha is a regulatory switch sufficient for induction of granulocytic development from bipotential myeloid progenitors. Mol Cell Biol. 1998;18:4301–4314. doi: 10.1128/mcb.18.7.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reed J C. Bcl-2 family proteins. Oncogene. 1998;17:3225–3236. doi: 10.1038/sj.onc.1202591. [DOI] [PubMed] [Google Scholar]
- 55.Rhoades K L, Hetherington C J, Harakawa N, Yergeau D A, Zhou L, Liu L Q, Little M T, Tenen D G, Zhang D-E. Analysis of the role of AML1-ETO in leukemogenesis, using an inducible transgenic mouse model. Blood. 2000;96:2108–2115. [PubMed] [Google Scholar]
- 56.Romana S P, Mauchauffe M, Le Coniat M, Chumakov I, Le Paslier D, Berger R, Bernard O A. The t(12;21) of acute lymphoblastic leukemia results in a tel-AML1 gene fusion. Blood. 1995;85:3662–3670. [PubMed] [Google Scholar]
- 57.Shikami M, Miwa H, Nishii K, Takahashi T, Sekine T, Mahmud N, Nishikawa M, Shiku H, Kamada N, Kita K. Low BCL-2 expression in acute leukemia with t(8;21) chromosomal abnormality. Leukemia. 1999;13:358–368. doi: 10.1038/sj.leu.2401343. [DOI] [PubMed] [Google Scholar]
- 58.Shimizu K, Kitabayashi I, Kamada N, Abe T, Maseki N, Suzukawa K, Ohki M. AML1-MTG8 leukemic protein induces the expression of granulocyte colony-stimulating factor (G-CSF) receptor through the up-regulation of CCAAT/enhancer binding protein epsilon. Blood. 2000;96:288–296. [PubMed] [Google Scholar]
- 59.Shurtleff S A, Buijs A, Behm F G, Rubnitz J E, Raimondi S C, Hancock M L, Chan G C, Pui C H, Grosveld G, Downing J R. TEL/AML1 fusion resulting from a cryptic t(12;21) is the most common genetic lesion in pediatric ALL and defines a subgroup of patients with an excellent prognosis. Leukemia. 1995;9:1985–1989. [PubMed] [Google Scholar]
- 60.Smith L T, Hohaus S, Gonzalez D A, Dziennis S E, Tenen D G. PU.1 (Spi-1) and C/EBP alpha regulate the granulocyte colony-stimulating factor receptor promoter in myeloid cells. Blood. 1996;88:1234–1247. [PubMed] [Google Scholar]
- 61.Speck N A, Stacy T, Wang Q, North T, Gu T L, Miller J, Binder M, Marin-Padilla M. Core-binding factor: a central player in hematopoiesis and leukemia. Cancer Res. 1999;59:1789s–1793s. [PubMed] [Google Scholar]
- 62.Strom D K, Nip J, Westendorf J J, Linggi B, Lutterbach B, Downing J R, Lenny N, Hiebert S W. Expression of the AML-1 oncogene shortens the G(1) phase of the cell cycle. J Biol Chem. 2000;275:3438–3445. doi: 10.1074/jbc.275.5.3438. [DOI] [PubMed] [Google Scholar]
- 63.Thottassery J V, Zambetti G P, Arimori K, Schuetz E G, Schuetz J D. p53-dependent regulation of MDR1 gene expression causes selective resistance to chemotherapeutic agents. Proc Natl Acad Sci USA. 1997;94:11037–11042. doi: 10.1073/pnas.94.20.11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Timchenko N A, Wilde M, Nakanishi M, Smith J R, Darlington G J. CCAAT/enhancer-binding protein alpha (C/EBP alpha) inhibits cell proliferation through the p21 (WAF-1/CIP-1/SDI-1) protein. Genes Dev. 1996;10:804–815. doi: 10.1101/gad.10.7.804. [DOI] [PubMed] [Google Scholar]
- 65.Wang J, Hoshino T, Redner R L, Kajigaya S, Liu J M. ETO, fusion partner in t(8;21) acute myeloid leukemia, represses transcription by interaction with the human N-CoR/mSin3/HDAC1 complex. Proc Natl Acad Sci USA. 1998;95:10860–10865. doi: 10.1073/pnas.95.18.10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang J, Saunthararajah Y, Redner R L, Liu J M. Inhibitors of histone deacetylase relieve ETO-mediated repression and induce differentiation of AML1-ETO leukemia cells. Cancer Res. 1999;59:2766–2769. [PubMed] [Google Scholar]
- 67.Wang Q, Stacy T, Binder M, Marin-Padilla M, Sharpe A H, Speck N A. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc Natl Acad Sci USA. 1996;93:3444–3449. doi: 10.1073/pnas.93.8.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang S W, Speck N A. Purification of core-binding factor, a protein that binds the conserved core site in murine leukemia virus enhancers. Mol Cell Biol. 1992;12:89–102. doi: 10.1128/mcb.12.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang X, Scott E, Sawyers C L, Friedman A D. C/EBPalpha bypasses granulocyte colony-stimulating factor signals to rapidly induce PU.1 gene expression, stimulate granulocytic differentiation, and limit proliferation in 32D cl3 myeloblasts. Blood. 1999;94:560–571. [PubMed] [Google Scholar]
- 70.Westendorf J J, Hiebert S W. Mammalian runt-domain proteins and their roles in hematopoiesis, osteogenesis, and leukemia. J Cell Biochem. 1999;32–33(Suppl.):51–58. doi: 10.1002/(sici)1097-4644(1999)75:32+<51::aid-jcb7>3.3.co;2-j. [DOI] [PubMed] [Google Scholar]
- 71.Westendorf J J, Yamamoto C M, Lenny N, Downing J R, Selsted M E, Hiebert S W. The t(8;21) fusion product, AML-1-ETO, associates with C/EBP-α, inhibits C/EBP-α-dependent transcription, and blocks granulocytic differentiation. Mol Cell Biol. 1998;18:322–333. doi: 10.1128/mcb.18.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Williams S C, Du Y, Schwartz R C, Weiler S R, Ortiz M, Keller J R, Johnson P F. C/EBPepsilon is a myeloid-specific activator of cytokine, chemokine, and macrophage-colony-stimulating factor receptor genes. J Biol Chem. 1998;273:13493–13501. doi: 10.1074/jbc.273.22.13493. [DOI] [PubMed] [Google Scholar]
- 73.Yamanaka R, Barlow C, Lekstrom-Himes J, Castilla L H, Liu P P, Eckhaus M, Decker T, Wynshaw-Boris A, Xanthopoulos K G. Impaired granulopoiesis, myelodysplasia, and early lethality in CCAAT/enhancer binding protein epsilon-deficient mice. Proc Natl Acad Sci USA. 1997;94:13187–13192. doi: 10.1073/pnas.94.24.13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamanaka R, Kim G D, Radomska H S, Lekstrom-Himes J, Smith L T, Antonson P, Tenen D G, Xanthopoulos K G. CCAAT/enhancer binding protein epsilon is preferentially up-regulated during granulocytic differentiation and its functional versatility is determined by alternative use of promoters and differential splicing. Proc Natl Acad Sci USA. 1997;94:6462–6467. doi: 10.1073/pnas.94.12.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yergeau D A, Hetherington C J, Wang Q, Zhang P, Sharpe A H, Binder M, Marin-Padilla M, Tenen D G, Speck N A, Zhang D-E. Embryonic lethality and impairment of haematopoiesis in mice heterozygous for an AML1-ETO fusion gene. Nat Genet. 1997;15:303–306. doi: 10.1038/ng0397-303. [DOI] [PubMed] [Google Scholar]
- 76.Zhang D-E, Hetherington C J, Gonzalez D A, Chen H M, Tenen D G. Regulation of CD14 expression during monocytic differentiation induced with 1 alpha,25-dihydroxyvitamin D3. J Immunol. 1994;153:3276–3284. [PubMed] [Google Scholar]
- 77.Zhang D-E, Hetherington C J, Meyers S, Rhoades K L, Larson C J, Chen H-M, Hiebert S W, Tenen D G. CCAAT enhancer-binding protein (C/EBP) and AML1 (CBFα2) synergistically activate the macrophage colony-stimulating factor receptor promoter. Mol Cell Biol. 1996;16:1231–1240. doi: 10.1128/mcb.16.3.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang D-E, Zhang P, Wang N D, Hetherington C J, Darlington G J, Tenen D G. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein alpha-deficient mice. Proc Natl Acad Sci USA. 1997;94:569–574. doi: 10.1073/pnas.94.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]