Abstract
Liver diseases are considered to predominantly possess an inherited or xenobiotic etiology. However, inheritance drives the ability to appropriately adapt to environmental stressors, and disease is the culmination of a maladaptive response. Thus “pure” genetic and “pure” xenobiotic liver diseases are modified by each other and other factors, identified or unknown. The purpose of this review is to highlight the knowledgebase of environmental exposure as a potential risk modifying agent for the development of liver disease by other causes. This exercise is not to argue that all liver diseases have an environmental component, but to challenge the assumption that the current state of our knowledge is sufficient in all cases to conclusively dismiss this as a possibility. This review also discusses key new tools and approaches that will likely be critical to address this question in the future. Taken together, identifying the key gaps in our understanding is critical for the field to move forward, or at the very least to “know what we don't know.”
KEY WORDS: Hepatic injury, Exposomics, Liver disease, Drug-induced liver injury, Alcoholic liver disease, Non-alcoholic liver disease, Inherited liver disease, Autoimmune liver disease
Graphical abstract
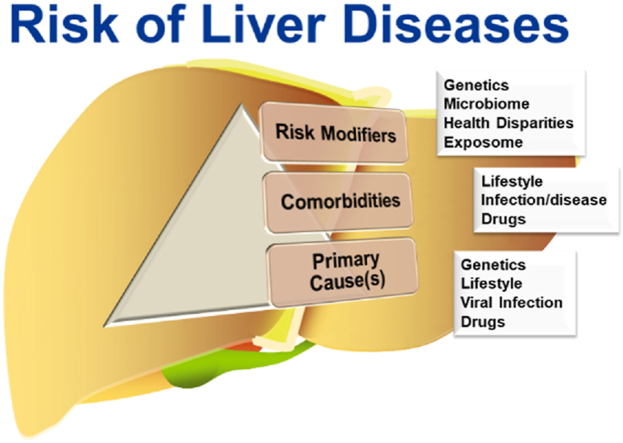
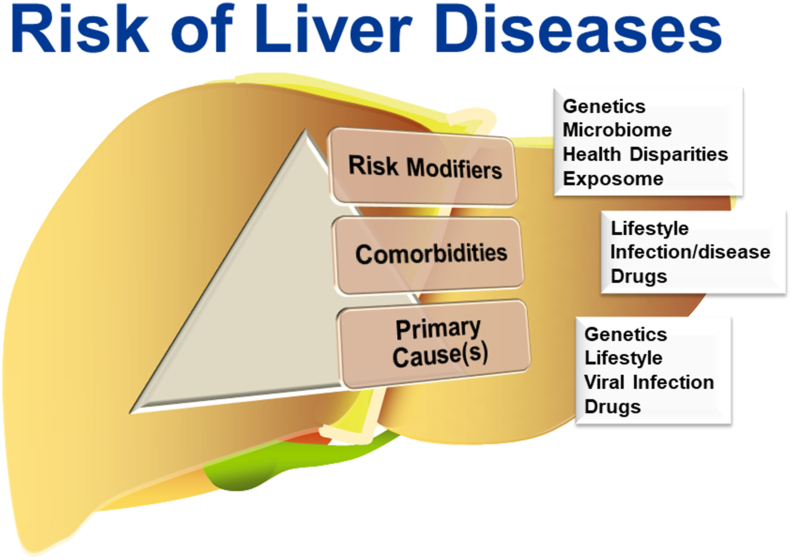
Liver injury and disease have primary underlying causes. Liver disease is also impacted by comorbities that influence the severity and outcome. Moreover, risk modifiers (e.g., “exposome”) influence interindividual susceptibility.
1. Introduction
…informed ignorance provides the natural state of mind for research scientists at the ever-shifting frontiers of knowledge. People who believe themselves ignorant of nothing have neither looked for, nor stumbled upon, the boundary between what is known and unknown in the cosmos1.
This famous quote by the astrophysicist Neil deGrasse Tyson highlights the difficulty in making conclusions on what is known when all data are not available. This review will highlight the knowledgebase of environmental exposure as a potential risk-modifying agent for the development of liver disease by other causes (Table 12, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55). We will focus on what is known, as well as prospects for better understanding (i.e., unknowns).
Table 1.
Factors that influence the development and severity of liver diseases.
| Liver disease | Geneticsa | Lifestyle/comorbiditiesb | Environmental toxicantsc | Microbiomed |
|---|---|---|---|---|
| Inherited liver diseases | +++2,3 | +4 | – | +3 |
| Biliary atresia | ++5,6 | +(+)5 | ++7 | +8 |
| Primary biliary cholangitis | ++9 | – | ++10, 11, 12, 13, 14, 15 | +16 |
| Primary sclerosing cholangitis | ++17,18 | – | – | +19,20 |
| Autoimmune hepatitis | ++21, 22, 23, 24 | ++21, 22, 23, 24 | +23, 24, 25 | ++26 |
| Direct DILI | +27, 28, 29 | ++30, 31, 32 | +33, 34, 35 | ++36 |
| Idiosyncratic DILI | +37 | +38 | – | – |
| Viral hepatitis | ++39,40 | ++41,42 | +(+)43,44 | – |
| NAFLD/MAFLD | ++45 | +++45 | ++(+)46, 47, 48, 49 | ++(+)50,51 |
| ALD | ++52 | +++52 | – | +++51,53 |
| TAFLD/TASH | – | – | +++46,54 | +55 |
–, no known association or not classifiable. Evidence inadequate in humans and limited in experimental models.
+, possible association. Limited evidence in humans and in experimental models.
++, probable association. Limited evidence in humans and sufficient in experimental models.
+++, known association and/or primary cause. Clear cause and effect association is known.
Sexual dimorphism, familial associations, linkage disequilibrium, etc.
Viral infections, metabolic syndrome, alcohol, underlying liver disease, etc.
Exposure to environmental/occupational chemicals or toxins, natural or anthropogenic, that was not self-administered.
Altered microbiome/dysbiosis in diseased patients or in animal models.
1.1. The liver as a target organ of toxicity
The liver plays multiple roles in the intact organism. It is critical for maintaining systemic metabolic homeostasis and for synthesis of lipids and carbohydrates56. The liver is also the site of synthesis of the majority of plasma proteins (e.g., albumin and clotting factors), and it synthesizes and excretes bile acids, which are critical for normal uptake of vitamins and lipids, as well as for and excretion of xenobiotics57. The liver also has a unique immune function, as it serves as a key regulator of tolerance, immunity and crosstalk between the innate and adaptive immune responses58.
The liver is a key organ for xenobiotic metabolism. The strategic location of the liver between the intestinal tract and the rest of the body makes it a critical physical and biochemical barrier between absorbed xenobiotics and the systemic circulation. It is therefore not surprising that the liver has a very high capacity for phase I and II metabolic processes, which is predominantly responsible for the well-known ‘1st pass effect’ in xenobiotic metabolism. As the main detoxifying organ in the body, the liver has a high likelihood of toxic injury. Indeed, liver injury is a common reason for cessation of preclinical screening of new drugs59, as well as removal of approved drugs from the market38,60. Moreover, ∼30% of common workplace chemicals are associated with hepatotoxicity61.
The death of injured hepatic cells may be viewed an adaptive response that prevents the accumulation of irreversibly-damaged cells in the organ; as such, this process may protect against organ aging and cancer62,63. Indeed, the high potential of hepatic injury by xenobiotics is offset by the tremendous regenerative capacity of the organ64. This capacity distinguishes the liver from other encapsulated organs (e.g., the brain, heart, and lungs) that are far less able to replace functional tissue once it has been destroyed. In experimental animals, the liver can fully regenerate after surgical removal of 2/3 of the organ within 7–10 days65. Although hepatocytes rarely proliferate in the healthy adult liver, virtually all surviving hepatocytes replicate at least once after partial hepatectomy or acute toxic liver injury. Residual hepatocytes upregulate both proliferative and liver-specific gene expression to preserve tissue specific function. During liver regeneration, a complex network of cytokines, growth factors, kinases, and transcription factors drive hepatocytes out of the G0 phase to enter and progress through replication66. In addition to hepatocyte proliferation, there is a tightly coordinated response to complement the regenerative process (e.g., angiogenesis, extracellular matrix metabolism).
The complex and synchronized regenerative response in liver can be perturbed and thereby can impact normal tissue recovery from injury or damage. Indeed, it is now clear that impaired or altered regeneration and/or restitution is critical to the chronicity of numerous hepatic diseases67. Chronic liver injury, coupled with impaired/incomplete regeneration and restitution of said injury, leads to an almost universal endpoint of collagenous scarring of the liver (i.e., fibrosis), regardless of etiology68. It is hypothesized that hepatic fibrosis is initiated predominantly by a failure of the liver to sufficiently restitute itself after chronic injury62. Given the clinical implications of fibrosis/cirrhosis, and the relative difficulty of reversing this pathology, research in liver disease has focused on better means to identify and prevent fibrosis progression69.
1.2. Liver disease at the crossroads of environment and genetics
The broader definition of environmental exposure includes any non-inherited influences. For the context of this review, we will define environmental exposure more narrowly to exposure to environmental chemicals or toxins, natural or anthropogenic, that was not self-administered (e.g., alcohol) or the result of a pathogen infection (e.g., hepatitis viruses). Although they are not synonymous, we will also not distinguish occupational from true environmental exposures.
Liver diseases are considered to predominantly possess an inherited (e.g., Wilson's disease)70 or xenobiotic (e.g., alcohol-related liver disease; ALD)52 etiology. However, the risk of both genetic- and xenobiotic-derived liver diseases are modified by other factors (see Fig. 1). For example, the penetrance of Wilson's disease is lower than expectations based on allele frequency, and it can be prevented by diet modification and/or pharmacotherapy70. Likewise, only a fraction of even the heaviest drinkers develops clinically relevant ALD52. Judith Stern is quoted as stating, “Genetics loads the gun, but the environment pulls the trigger”71. This statement aptly emphasizes the point that inheritance drives the ability to appropriately adapt to environmental stressors, and that disease is the culmination of a maladaptive response. This is certainly true for liver diseases, in which even “pure” genetic and “pure” xenobiotic diseases are modified by each other and other factors, known or unknown (Fig. 1).
Figure 1.
The totality of risk of liver diseases. Liver diseases have primary underlying causes, be they genetic (e.g., inborn errors of metabolism), lifestyle (e.g., metabolic syndrome, alcohol consumption), viral infection (e.g., HBV/HCV infection) and drugs (e.g., acetaminophen overdose). However, the interindividual risk for the development of liver disease is also impacted by other factors that influence the severity and outcome driven by the primary cause(s). Comorbidities influenced by lifestyle, coinfections or other underlying diseases, and drug exposure may all positively and negative influence disease severity and progression. Moreover, risk modifiers, such as genetic susceptibility, alterations in the microbiome, as well as biologic (e.g., age) and sociodemographic health disparities influence interindividual susceptibility. Lastly, the totality of exposure to environmental chemicals (i.e., the “exposome”) may influence disease progression.
2. Inherited liver diseases and environmental exposure
Congenital variations in genes critical to metabolic pathways can lead to deficiencies and/or accumulation of toxic intermediates that damage target organs. As mentioned above, the liver plays a key role in maintaining overall metabolic function of the organism. As such, inherited variations in metabolic pathways often manifest in hepatic or liver-dependent extra-hepatic phenotypes. These can often be severe and subsequently present very early in the lifespan. Indeed, inborn errors of metabolism are a common reason for pediatric liver transplantation72.
In general, the impact of environmental exposure on the penetrance and severity of inherited liver diseases is not well-known. These liver diseases are very rare and possess a clear, and often known, genetic component. Nevertheless, a role of environmental exposure in modifying risk cannot be ruled out in all cases. For example, Alagille syndrome is an inherited disease that causes bile duct paucity and cholestasis (see Section 3, below). Key autosomal dominant variants in the Notch signaling pathway have been established as causal for the cholestatic liver disease associated with Alagille syndrome4. However, despite the autosomal dominance of the disease, penetrance and severity has been variable, even within the same family4. Some case reports of discordant disease and clinical phenotypes in monozygotic twins with Alagille syndrome has led to the speculation that environmental factors may contribute to disease severity73,74. However, these speculations have yet to translate to identifying potential environmental factors that drive disease phenotype4.
Some inherited liver diseases are well-known to have environmentally modifiable responses, at least in other target organs. For example, α1-antitrypsin (AAT) deficiency is caused by an inherited deficit in the generation of AAT by the liver. Although liver injury often presents in AAT deficiency, the most common complication is lung disease, where AAT is required to prevent localized damage by neutrophil elastase. Lung disease associated with AAT-deficiency is modifiable by environmental exposures75. Less is known about the interaction between AAT-deficiency and environmental exposure and hepatic disease. However, recent studies suggest that this deficiency may be also an independent risk factor for cirrhosis by other causes, which suggests a genetics/environment interaction is at least possible76. Likewise, lung complications associated with polymorphisms in the cystic fibrosis transmembrane receptor (CFTR) pathway are known to be heavily influenced by environmental exposures77. Although less is known about hepatic complications of CFTR polymorphisms78,79, some environmental chemicals that bioaccumulate in the liver are known to interact with the CFTR pathway (e.g., fungicides and perfluoroalkyl compounds80,81), which could exacerbate inherited CFTR dysfunction in the liver. Recent studies have indicated that several environmental chemicals may alter metal metabolism and transport within the cell, including dioxins and poly-chlorinated biphenyls82,83. Environmental exposures that alter metal transport/metabolism could also potentially impact inherited diseases of metal dyshomeostasis (e.g., Wilson's disease, and hereditary hemochromatosis), although this needs to pursued experimentally. In general, however, the potential for such interactions between inherited diseases and environmental exposures has not been extensively addressed and mechanistic insight is subsequently lacking.
3. Cholestatic liver diseases
Cholestasis refers to disruption of proper bile acid and bilirubin metabolism, resulting in their increased levels in blood and peripheral tissues. Cholestasis generally falls into two categories: intrahepatic and extrahepatic (obstructive) cholestasis. Intrahepatic cholestasis can be primary or secondary to other liver diseases (e.g., drug-induced liver injury; DILI, and ALD). These diseases can also be developmental or acquired.
3.1. Biliary atresia
Biliary atresia (BA) is a developmental disorder that impacts the extrahepatic bile ducts of neonates. It is the most common cause of pediatric liver transplantation, as the progressive damage and fibrosis of the bile ducts lead to complete obstruction. The underlying etiology is incompletely understood but appears to involve dyshomeostasis between the innate and adaptive immune responses, leading to destruction of the bile duct epithelial cells5. In contrast to inherited diseases of extrahepatic cholestasis (e.g., Alagille syndrome, see above), there is not a clear single genetic susceptibility factor driving BA. There are several polymorphisms that are associated with BA susceptibility5, but the allele frequency and penetrance cannot explain the entirety of the disease6.
BA is well-known to have regional variation in the disease incidence worldwide6. This and other factors led to speculation that BA and other pediatric cholangiopathies may have a xenobiotic component7. There are data supporting the hypothesis that in utero exposure to viral infections (e.g., rotavirus) may be a triggering factor in BA5. Building on observations of naturally-occurring outbreaks of BA in Australian livestock84, researchers isolated a plant toxin that was demonstrated to cause experimental BA in zebrafish, mice and in human biliary epithelial cells85,86. Although it is unlikely that humans will be exposed to this plant toxin and local outbreaks of human BA have not been identified, these results support the notion that environmental exposure can cause BA. To date, no anthropogenic product has been linked to BA.
3.2. Primary biliary cholangitis
Primary biliary cholangitis (PBC) is a rare chronic autoimmune disease in which the biliary epithelial cells of the small to medium intrahepatic bile ducts are progressively destroyed9. PBC can manifest at any time in the lifespan but is generally an adult-onset disease. PBC is also sexually dimorphic, with a much higher prevalence in women. Although PBC can respond to interventive strategies (e.g., immune suppression, disease modifying agents and antifibrotics), it often progresses to biliary cirrhosis87.
Although the initiation of PBC is incompletely understood, it is thought that the breaking of self-tolerance to the biliary epithelium is the key step; the presence of antimitochondrial antibodies is a dominant phenotype of active autoimmunity associated PBC9. As with the case of most autoimmune diseases, there appears to be a genetic contribution to susceptibility. There is a strong familial association and a relatively high concordance rate among monozygotic twins88,89. Large-scale sequencing studies have identified variants in the human leucocyte antigen (HLA) axis as a dominant risk factor90, there are also risk associations with non-HLA variants related to recognition-of-self9.
PBC has long been thought to have an environmental trigger that leads to the breakage of self-tolerance91. Viral or bacterial infections and GI tract dysbiosis have all been associated with PBC92, 93, 94. There are also relatively strong data supporting exposure to anthropogenic xenobiotics may serve as a trigger for PBC. For example, frequent use of nail polish was identified as a risk factor in an analysis of the US National Health and Nutrition Examination Study10. There is also enrichment of PBC cases near toxic waste sites11,12. A recent study indicated that PBC is also geospatially associated with areas in the United Kingdom with high potential of exposure to cadmium13. The mechanism of breakage of self-tolerance is generally suspected to be via presentation of biomimetic neoepitopes and/or via inappropriate activation of the adaptive immune response. Some extensive structure–activity relationships have identified that some anthropogenic chemicals (e.g., 2-octynoic acid) may form adducts with native biomolecules; these hybrid compounds may become molecular mimics that induce expansion of the canonical anti-mitochondrial antibody response found in PBC14,15. These chemicals are ubiquitous in middle- and high-income countries and may represent a direct mechanistic cause and effect between environmental exposure and PBC.
3.3. Primary sclerosing cholangitis
Primary sclerosing cholangitis (PSC) is also a rare cholestatic liver disease that is characterized by inflammatory destruction of the bile ducts. In contrast to PBC, the medium to large intra- and extra-hepatic bile ducts are targeted. The natural history of the disease is characterized by progressive evolution to biliary cirrhosis, coupled with a high risk of malignancy (e.g., cholangiocarcinoma)95. PSC is also predominantly an adult-onset disease and is sexually dimorphic, with a higher prevalence in men and is frequently preceded by inflammatory bowel disease17.
The etiology if PSC is unclear, but also shares some similarities with PBC. It is also a familial-associated disease18, and several studies have identified variants in the HLA genes that associate with the risk of developing PSC17. By extension, PSC is considered an autoimmune disease and most patients possess non-specific and biliary epithelium autoantibodies96,97. The incidence of PSC is higher in middle-to high-income countries and has shown geospatial variability within defined areas13,98. In contrast to PBC, there have been no clear cause and effect linkages between environmental exposure and PSC have been identified. Likewise, potential mechanisms by which environmental exposures could enhance PSC are unclear.
4. Autoimmune hepatitis
Autoimmune hepatitis (AIH) is a broad family of disorders characterized generally by a self-perpetuating loss of self-tolerance against hepatocytes in the liver. AIH is usually defined as type I and type II, based on the types of autoantibodies found in the patient99. The age of presentation and clinical course differs between types I and II, but both types are generally characterized by progressive interface hepatitis that often leads to fibrosis/cirrhosis. As is the case with PBC and PSC, there are genetic predispositions that increase the risk of developing AIH with predominance in variants in the HLA genes21,22. Also similar to PBC and PSC, there is assumed to be an external trigger that causes the breakage of self-tolerance and initiates disease progression99.
In addition to infections, it is thought that xenobiotic chemicals may be a trigger in AIH23,24. Indeed, DILI (see below) often has components of AIH100. Beyond viral and specific drug-induced cases of AIH, there have been few examples of environmental exposure potentially causing or contributing to AIH. There is a relatively strong linkage to the development of AIH and other autoimmune diseases to trichloroethylene exposure101. Based on studies in animal models, it is hypothesized that trichloroethylene may trigger AIH by preventing activation-induced apoptosis of CD4+ T cells, while simultaneously creating protein adducts that can serve as neoantigens for this expanded population of activated T cells25. Whether this mechanism is unifying for all environmental exposures that may contribute to AIH is unclear.
5. Drug-induced liver injury
DILI is defined as an acute hepatotoxic event that can be directly linked to the ingestion of drugs. DILI is the most common cause of acute liver failure in middle- and high-income countries and a common cause for liver transplantation102. The diagnosis of DILI relies not only on the exclusion of other causes, but also on timing of injury after drug administration has been started, recovery after the drug has been discontinued, and relapse of injury after reintroduction of the drug103. DILI is further categorized into two main groups, direct and idiosyncratic103.
5.1. Direct DILI (dDILI)
dDILI is characterized by predictable, dose-dependent liver injury by compounds known to be intrinsically hepatotoxic in humans and in preclinical models103. The pattern of injury is usually specific to the compound ingested103, but is most frequently characterized by a rapid onset and acute hepatic necrosis coupled with elevations of serum liver enzymes (e.g., AST and ALT). Less frequently, dDILI may show a cholestatic or mixed phenotype. The most common and well-characterized example of dDILI is acetaminophen (APAP) toxicity104, but is also common for other classes of drugs (e.g., chemotherapeutics)103.
The dose-dependence of dDILI implies that it is commonly the result of accidental or intentional overdose102. However, there are nevertheless cases in which patients develop dDILI within the normal therapeutic range of the compound. These factors have fueled discussion as to whether other factors (genetic or lifestyle) modify interindividual risk for the development of dDILI105. There a are few examples in which genetic predispositions for dDILI have been identified, most of which are centered on polymorphisms associated with drug metabolism27, 28, 29. Several studies have also indicated that other host factors influence the development of dDILI, especially when toxicity occurs in the normal drug therapeutic range105. For example, the hepatotoxicity of therapeutic doses APAP is associated with chronic alcohol misuse, malnutrition, age, and underlying liver disease (e.g., NAFLD and hepatitis C virus; HCV)30, 31, 32. There are few human studies suggesting that environmental exposure influences the incidence or severity of dDILI. In contrast, several studies in preclinical models support at least the notion of such a potential interaction. For example, APAP toxicity is enhanced by coexposure to several environmental chemicals in animal models33, 34, 35. Interestingly, the finding that respiratory ozone exposure enhances experimental APAP-induced liver injury suggests the potential for organ–organ interactions in this process33. The mechanisms by which environmental chemicals may enhance dDILI focus largely on increased metabolic intoxication (e.g., the interaction between alcohol exposure and acetaminophen), enhanced response to injury (e.g., inflammation), as well as impaired regeneration/recovery (see Section 9).
5.2. Idiosyncratic DILI (iDILI)
As point of comparison to dDILI, iDILI is unpredictable, does not demonstrate an apparent dose-dependence and usually cannot be recapitulated in preclinical models. The latter point is a major cause for concern in that the hepatotoxicity generally was not predicted in toxicity screens for drugs already approved by the U.S. Food and Drug Administration, and is only discovered once the compound reaches the market102. Importantly, iDILI also is increasingly associated with over the counter and herbal supplements that are not regulated prospectively by the US Food and Drug Administration106. The idiosyncratic nature of this disorder implies that other factors beyond drug exposure contribute to the susceptibility and severity of iDILI. Genetic risk factors have been identified for iDILI, many of which overlap with AIH (e.g., HLA axis polymorphisms; see Section 4)37. Indeed, iDILI cases are often suspected to be AIH in which drug exposure is the triggering factor that breaks self-tolerance37.
The scarcity, complexity, and unpredictability of this multifactorial family of disorders hamper development of clear models that predict the risks of iDILI. Although some genetic linkages have been identified, it is generally assumed that genetic variance alone does not completely explain interindividual risk of iDILI development. Indeed, several host-factors have been suggested to increase the risk of iDILI, including sex, age, lifestyle (e.g., alcohol consumption, nutrition), co-medications and underlying diseases that may drive differential pharmacokinetic/pharmacodynamic responses to drugs38. However, many of these linkages also have contradictory findings in the literature (e.g., Ref.107). There are few linkages between environmental exposure and the risk of iDILI, although several environmental compounds can contribute to altered pharmacokinetic/pharmacodynamic responses in hosts108,109. For example, several environmental chemicals have been demonstrated to interact with drug transporters110. Given the critical importance that altered drug metabolism and transport is hypothesized to play in iDILI111,112, such an interaction could lead to intoxication. This area should be explored in future research.
6. Viral hepatitis
Several viruses are hepatotrophic and can cause liver injury. Most hepatitis viruses only cause acute infections that are usually subclinical and self-limiting in immunocompetent adults (e.g., hepatitis A and E)113,114. In contrast, hepatitis B virus (HBV) and HCV often transition to a chronic infection and liver disease. The natural history of liver disease from chronic HBV/HCV infection is similar to fatty liver disease (see Section 7). Despite the existence of an effective vaccine (HBV) and antiviral therapies (HBV and HCV), chronic viral liver disease remains a leading cause of cirrhosis, hepatocellular carcinoma (HCC), liver transplantation and death worldwide115. There are genetic factors that influence host susceptibility to HBV and HCV infection and liver injury39,40. There are also some data indicating an interaction between other factors and viral pathogenesis, the most notable is coexposure to aflatoxin, a regional food-born contaminant (see Section 8)43,44. For example, alcohol consumption is a known risk factor severe liver disease during HBV/HCV infection41,42. However, beyond these findings, few interactions between environmental exposure and viral liver disease have been identified.
7. Fatty liver diseases (ALD, NAFLD/MAFLD, TAFLD)
Chronic fatty liver diseases are a spectrum of diseases ranging from simple steatosis, to active inflammation (steatohepatitis) to fibrosis/cirrhosis, and potentially HCC (see Section 8, below). The principle primary causes of fatty liver diseases are alcohol consumption (i.e., ALD) and obesity/metabolic syndrome, which was originally coined nonalcoholic fatty liver disease (NAFLD)52,116,117. The natural history and pathologic progression of both diseases are similar, and the underlying causes are usually only determined by medical history questionnaires. Although there are clear cases of “pure” ALD (i.e., liver disease in lean heavy drinkers) and NAFLD (i.e., liver disease in obese non-drinkers), it is also clear that risk factors and lifestyles often overlap118. Moreover, as reviewed elsewhere in this issue (Schnegelberger et al.), there are now data indicating that environmental toxicant exposure can lead to fatty liver disease53,119. This understanding has led to the recent reframing of fatty liver diseases under the inclusive umbrella term of metabolic-associated fatty liver disease (MAFLD)120.
It is estimated that up to 1/3 of the global population has fatty liver disease and it is considered to be a looming health burden121. Regardless of primary causes, the risk of developing severe fatty liver disease is well-known to be modified by other factors both genetic and lifestyle45,52,122. In contrast to the other liver diseases discussed in this review, there are several lines of evidence that indicate that environmental toxicants may be a risk factor for the development and progression of fatty liver diseases, especially due to obesity/insulin resistance46, 47, 48, 49,123, 124, 125. There are relatively strong data linking exposure to persistent organic pollutants (e.g., dioxin), volatile organic compounds (e.g., vinyl chloride; VC), metals (e.g., arsenic) as well as some herbicides/fungicides with the development of experimental and human fatty liver disease46,124. Although the underlying mechanisms of potential interactions between environmental exposure and fatty liver diseases are still being elucidated, the environmental agents can generally be classed as endocrine-, metabolism- and signaling-disrupting chemicals that potentially exacerbate the metabolic dysfunction and/or disease progression in fatty liver diseases46,126,127.
Environmental exposure in some cases has been identified as a primary cause in the development of fatty liver diseases, coined toxicant-associated fatty liver disease (TAFLD)128. It is likely that one of the clearest examples of an environmental liver disease is VC exposure. VC is chiefly employed in the rubber manufacturing industry as it is an intermediary monomer used in the production of the polymer polyvinyl chloride129. One of the first cases to report hepatic injury caused by VC exposure dated back to 1974 when 3 polyvinyl chloride factory workers were diagnosed with hepatic hemangiosarcoma, a rare form of liver cancer130, 131, 132. More recently, the prevalence of TAFLD was identified in VC workers119. TAFLD is a progressive form of fatty liver disease that bears some resemblance to NAFLD53. While its histological characteristics are similar to ALD/NAFLD, TAFLD has been associated with normal liver enzyme levels, making it more challenging to diagnose53,119. It is nevertheless clear that at high enough exposure levels, VC is sufficient to cause significant liver disease119. At least in animal models, exposure to several environmental chemicals is sufficient to also cause fatty liver disease133,134.
8. Environmental exposure and primary liver cancers
Primary liver cancer, predominantly HCC, is the 6th most common cancer worldwide135. Treatment options for non-resectable HCC and other liver cancers are limited and the 5-year survival rate for HCC remains and is the 2nd most lethal solid cancer136. In regions in which HBV infection and exposure to aflatoxin is not endemic, HCC occurs almost exclusively on the background on severe cirrhosis, which is the primary risk factor for HCC development137. The incidence of HCC has been increasing in recent years, which may be a reflection of an increase in underlying liver disease in the population (e.g., MAFLD; see Section 7)135. Moreover, as the risk of HCC increases with the length of cirrhosis, improvements in the clinical management of compensated (i.e., ‘stable’) cirrhosis may also contribute to this increasing incidence of HCC138.
As HCC and other liver cancers are generally dependent upon preexisting chronic liver disease, environmental exposures discussed in previous sections that contribute to that liver disease by extension may contribute to the risk of HCC. For example, coexposure to the mutagen aflatoxin greatly increases the risk of the development of HCC by HBV (and possibly HCV) infection43,44. Moreover, exposure to genotoxic chemicals may be an independent risk factor for HCC occurrence. Exposure to a myriad of such chemicals has been linked with at least an increase in HCC incidence, including polyhalogenated biphenyls, volatile organic compounds (e.g., VC) benzo[α]pyrene and arsenic139, 140, 141, 142. It is hypothesized that clonal expansion of cells mutated by these agents during cirrhosis accelerates the risk of progression to HCC.
9. Altered liver regeneration as a unifying mechanism?
As mentioned in Section 1.1, the ability of the liver to regenerate after injury affords a unique protective mechanism for this organ against damage. The development of chronic liver diseases is almost universally present on the background of genetic/acquired impairment of regeneration and restitution from injury, which allows damage to accumulate67. Even acute liver injury, such as dDILI caused by APAP overdose (see Section 5.1) is driven, at least in part, by the rate of hepatic regeneration after injury143. Thus, impaired regeneration may be a unifying mechanism that influences liver injury and liver disease, regardless of underlying causes.
As described in Section 1.1, the regenerative response is a complex and tightly coordinated process that by extension is sensitive to perturbation. Some environmental chemicals have been described to impair liver regeneration, at least in experimental models, including heavy metals144, and dioxin-like chemicals145, but information on other chemicals is limited. Conversely, some compounds (e.g., organochlorine pesticides, phthalates and perfluoroalkyl chemicals) have been demonstrated to enhance liver proliferation and cause hepatomegaly146, 147, 148. The interplay between altered proliferation has implications not only for liver disease, but also for hepatocarcinogenesis (see Section 8, above).
10. Environmental exposure and liver disease: Prospects ‘omics’ and big data
The lack of information regarding a potential role of environmental exposure in some liver diseases is understandable. Some of these diseases (e.g., developmental liver diseases) are extremely rare and the prevalence even in large institutional catchments may be too low for any reasonably-powered assessment of risk-modifying agents. This limitation has been partially addressed by collaborative cohorting and biobanking across institutions; these cohorts exist for several liver diseases, including DILI149, developmental/pediatric liver diseases150, NAFLD151 and others. These advances have led to other issues, namely that it is difficult to manage and analyze large, heterogeneous multiplatform datasets to achieve meaningful results. These issues have lessened with continued development of machine learning to agnostically analyze ‘Big Data’ and metadata152,153. These approaches offer the opportunity to generate new hypotheses that explain interindividual risk of disease development (i.e., precision medicine). Some promising platforms relevant to questions regarding the role of environmental exposure in liver diseases are discussed below.
10.1. Microbiome
The microbiome, especially the gut microbiome, is now established as a dynamic and diverse compartment that plays key roles in mediating health and disease. Given the proximal location of the liver immediately downstream of the gastrointestinal tract, it is not surprising that the role of the gut microbiome in liver disease has been extensively studied154. It can be argued that our understanding of the bidirectional ‘gut–liver axis’ has coevolved with our understanding of liver disease for at least the last half century155. As summarized in Table 1, almost every liver disease covered in this review has been associated with an altered gut microbiome156. These associations have the exciting potential for the development of non-invasive biomarkers of disease phenotype50. Whether these changes are causally involved in disease development also has larger implications for precision medicine and targeted interventive strategies50. The latter point is incompletely understood in the context of most liver diseases, with some exciting exceptions. For example, recent work indicates that bacteriophage delivery to selectively target specific bacterial species is protective in experimental and human ALD53. Another area of microbiome research that has been heavily studied is the influence of environmental chemicals on gut microbiome alpha- and beta-diversity, given the high level of potential exposure of this compartment157, 158, 159. Integrative incorporation of these knowledgebases could yield new information and insight into the potential interaction between environmental exposure and liver diseases.
10.2. Geospatial studies
Health disparities are a complicated subject, driven by potential or established differences in genetics, culture and socioeconomic status160. Liver diseases, like most diseases, are well-known to show categorical health disparities within a given population160, 161, 162. It is also understood that liver diseases often show inter- and intra-regional variability in the incidence, severity, and outcomes162,163. More recently, micro-regional (e.g., county- or even neighborhood-level) analysis of liver disease mortality has indicated that there are ‘hot-spots’ of liver disease164,165. This level of geospatial analysis takes better into account local variation in sociodemographics, risk factors and access-to-care. One of the components of consideration in local health disparities is differential exposure to environmental chemicals that may impact health166. It may not be surprising that local hotspots of environmental exposure often overlap with racial and socioeconomic disparity166. As mentioned above, some geospatial analyses have linked specific liver diseases to local environmental exposures (e.g., Refs.11, 12, 13), but this is an area that should be further developed.
10.3. Exposomics
As mentioned above, disparities in exposure to environmental hazards often overlaps with other sociodemographic disparities167. As such, geospatial analysis has limited value to inform on underlying causes without being integrated with other analyses (e.g., exposure assessment). Moreover, single exposure analysis does not factor in the dynamics of mixtures in environmental exposure and by design is restricted to hypothesis-driven approaches168. These limitations have led to the development of the concept of the “exposome”, in which the totality of exposure would be measured, analogous to other ‘omic’ approaches169,170. This field is rapidly developing in conjunction with machine learning. There is high potential for exposomics to integrate with other ‘omics’ (e.g., genomics, transcriptomics, etc.) in a multiomic platform to yield new information in disease research171, 172, 173, including liver diseases174.
11. Summary and conclusions. Do we know what we don't know?
Exposure to environmental chemicals is ubiquitous and even neonates have detectable levels of anthropogenic environmental chemicals in their blood and this exposure may be a key factor in the developmental origins of disease175,176. Importantly, several of these chemicals are known hepatotoxicants (e.g., heavy metals)177. As outlined above (see Table 1), there are some examples in which the weight-of-evidence strongly supports at least a modifying role of environmental exposure and the development and severity of liver diseases (e.g., PBC and NAFLD/MAFLD).
As a field, it is critical to direct research to address the “unknowns”, i.e., liver diseases for which little is known regarding the impact of environmental exposure (Table 1). These would include inherited liver diseases, PSC, iDILI and ALD (Table 1). This gap in our understanding is especially apparent for liver diseases in which the natural history is similar, but there is differential understanding of this potential interaction. For example, few studies have investigated the possible influence of environmental exposure in ALD, although it is acknowledged that the pathogenesis of ALD shares many similarities to NAFLD/MAFLD, where this issue has been studied extensively46, 47, 48, 49. Likewise, although much is known about PBC and environmental exposure, there is little information for PSC, which shares mechanisms of disease of at least initiation (e.g., breakage of self-tolerance). These gaps should be filled.
The purpose of this review is not to argue that all liver diseases have an environmental component, but to challenge the assumption that the current state of our knowledge is sufficient in all cases to conclusively dismiss this as a possibility. This review identified key new tools and approaches that will likely be critical in addressing these questions in the future. Taken together, identifying the key gaps in our understanding is critical for the field to move forward, or at the very least to “know what we don't know.”
Acknowledgments
Supported, in part, by grants from NIH (R01 AA021978, P30 DK120531, and R21 ES031531, USA).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Juliane I. Beier, Email: jibeier@pitt.edu.
Gavin E. Arteel, Email: gearteel@pitt.edu.
Author contributions
Juliane I Beier: drafting of the manuscript, and critical revision. Gavin E. Arteel: drafting of the manuscript, and critical revision. All authors: final approval of manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Tyson Nd, Goldsmith D. 6th ed. WW Norton & Co.; New York: 2004. Origins: fourteen billion years of cosmic evolution. [Google Scholar]
- 2.Clayton P.T. Inborn errors presenting with liver dysfunction. Semin Neonatol. 2002;7:49–63. doi: 10.1053/siny.2001.0086. [DOI] [PubMed] [Google Scholar]
- 3.Fabris L., Fiorotto R., Spirli C., Cadamuro M., Mariotti V., Perugorria M.J., et al. Pathobiology of inherited biliary diseases: a roadmap to understand acquired liver diseases. Nat Rev Gastroenterol Hepatol. 2019;16:497–511. doi: 10.1038/s41575-019-0156-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell E., Gilbert M., Loomes K.M. Alagille syndrome. Clin Liver Dis. 2018;22:625–641. doi: 10.1016/j.cld.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Kilgore A., Mack C.L. Update on investigations pertaining to the pathogenesis of biliary atresia. Pediatr Surg Int. 2017;33:1233–1241. doi: 10.1007/s00383-017-4172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lakshminarayanan B., Davenport M. Biliary atresia: a comprehensive review. J Autoimmun. 2016;73:1–9. doi: 10.1016/j.jaut.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Landing B.H. Considerations of the pathogenesis of neonatal hepatitis, biliary atresia and choledochal cyst-the concept of infantile obstructive cholangiopathy. Prog Pediatr Surg. 1974;6:113–139. [PubMed] [Google Scholar]
- 8.Jain V., Alexander E.C., Burford C., Verma A., Dhawan A. Gut microbiome: a potential modifiable risk factor in biliary atresia. J Pediatr Gastroenterol Nutr. 2021;72:184–193. doi: 10.1097/MPG.0000000000002973. [DOI] [PubMed] [Google Scholar]
- 9.Lleo A., Leung P.S.C., Hirschfield G.M., Gershwin E.M. The pathogenesis of primary biliary cholangitis: a comprehensive review. Semin Liver Dis. 2020;40:34–48. doi: 10.1055/s-0039-1697617. [DOI] [PubMed] [Google Scholar]
- 10.Gershwin M.E., Selmi C., Worman H.J., Gold E.B., Watnik M., Utts J., et al. Risk factors and comorbidities in primary biliary cirrhosis: a controlled interview-based study of 1032 patients. Hepatology. 2005;42:1194–1202. doi: 10.1002/hep.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ala A., Stanca C.M., Bu-Ghanim M., Ahmado I., Branch A.D., Schiano T.D., et al. Increased prevalence of primary biliary cirrhosis near Superfund toxic waste sites. Hepatology. 2006;43:525–531. doi: 10.1002/hep.21076. [DOI] [PubMed] [Google Scholar]
- 12.Prince M.I., Chetwynd A., Diggle P., Jarner M., Metcalf J.V., James O.F. The geographical distribution of primary biliary cirrhosis in a well-defined cohort. Hepatology. 2001;34:1083–1088. doi: 10.1053/jhep.2001.29760. [DOI] [PubMed] [Google Scholar]
- 13.Dyson J.K., Blain A., Foster Shirley M.D., Hudson M., Rushton S., Jeffreys Jones D.E. Geo-epidemiology and environmental co-variate mapping of primary biliary cholangitis and primary sclerosing cholangitis. JHEP Rep. 2021;3:100202. doi: 10.1016/j.jhepr.2020.100202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burroughs A.K., Butler P., Sternberg M.J., Baum H. Molecular mimicry in liver disease. Nature. 1992;358:377–378. doi: 10.1038/358377a0. [DOI] [PubMed] [Google Scholar]
- 15.Amano K., Leung P.S., Rieger R., Quan C., Wang X., Marik J., et al. Chemical xenobiotics and mitochondrial autoantigens in primary biliary cirrhosis: identification of antibodies against a common environmental, cosmetic, and food additive, 2-octynoic acid. J Immunol. 2005;174:5874–5883. doi: 10.4049/jimmunol.174.9.5874. [DOI] [PubMed] [Google Scholar]
- 16.Quigley E.M. Primary biliary cirrhosis and the microbiome. Semin Liver Dis. 2016;36:349–353. doi: 10.1055/s-0036-1594006. [DOI] [PubMed] [Google Scholar]
- 17.Karlsen T.H., Folseraas T., Thorburn D., Vesterhus M. Primary sclerosing cholangitis — a comprehensive review. J Hepatol. 2017;67:1298–1323. doi: 10.1016/j.jhep.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 18.Bergquist A., Lindberg G., Saarinen S., Broomé U. Increased prevalence of primary sclerosing cholangitis among first-degree relatives. J Hepatol. 2005;42:252–256. doi: 10.1016/j.jhep.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Hov J.R., Karlsen T.H. The microbiome in primary sclerosing cholangitis: current evidence and potential concepts. Semin Liver Dis. 2017;37:314–331. doi: 10.1055/s-0037-1608801. [DOI] [PubMed] [Google Scholar]
- 20.Liwinski T., Zenouzi R., John C., Ehlken H., Rühlemann M.C., Bang C., et al. Alterations of the bile microbiome in primary sclerosing cholangitis. Gut. 2020;69:665–672. doi: 10.1136/gutjnl-2019-318416. [DOI] [PubMed] [Google Scholar]
- 21.Béland K., Lapierre P., Alvarez F. Influence of genes, sex, age and environment on the onset of autoimmune hepatitis. World J Gastroenterol. 2009;15:1025–1034. doi: 10.3748/wjg.15.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higuchi T., Oka S., Furukawa H., Tohma S., Yatsuhashi H., Migita K. Genetic risk factors for autoimmune hepatitis: implications for phenotypic heterogeneity and biomarkers for drug response. Hum Genom. 2021;15:6. doi: 10.1186/s40246-020-00301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Francque S., Vonghia L., Ramon A., Michielsen P. Epidemiology and treatment of autoimmune hepatitis. Hepat Med. 2012;4:1–10. doi: 10.2147/HMER.S16321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lammert C. Genetic and environmental risk factors for autoimmune hepatitis. Clin Liver Dis (Hoboken) 2019;14:29–32. doi: 10.1002/cld.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilbert K.M. Xenobiotic exposure and autoimmune hepatitis. Hepat Res Treat. 2010;2010:248157. doi: 10.1155/2010/248157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai W., Ran Y., Li Y., Wang B., Zhou L. Intestinal microbiome and permeability in patients with autoimmune hepatitis. Best Pract Res Clin Gastroenterol. 2017;31:669–673. doi: 10.1016/j.bpg.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Yang S., Hwang S.J., Park J.Y., Chung E.K., Lee J.I. Association of genetic polymorphisms of CYP2E1, NAT2, GST and SLCO1B1 with the risk of anti-tuberculosis drug-induced liver injury: a systematic review and meta-analysis. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-027940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heruth D.P., Shortt K., Zhang N., Li D.Y., Zhang L.Q., Qing Ye S. Genetic association of single nucleotide polymorphisms with acetaminophen-induced hepatotoxicity. J Pharmacol Exp Therapeut. 2018;367:95–100. doi: 10.1124/jpet.118.248583. [DOI] [PubMed] [Google Scholar]
- 29.Russmann S., Jetter A., Kullak-Ublick G.A. Pharmacogenetics of drug-induced liver injury. Hepatology. 2010;52:748–761. doi: 10.1002/hep.23720. [DOI] [PubMed] [Google Scholar]
- 30.Louvet A., Ntandja Wandji L.C., Lemaître E., Khaldi M., Lafforgue C., Artru F., et al. Acute liver injury with therapeutic doses of acetaminophen: a prospective study. Hepatology. 2021;73:1945–1955. doi: 10.1002/hep.31678. [DOI] [PubMed] [Google Scholar]
- 31.Myers R.P., Shaheen A.A. Hepatitis C, alcohol abuse, and unintentional overdoses are risk factors for acetaminophen-related hepatotoxicity. Hepatology. 2009;49:1399–1400. doi: 10.1002/hep.22798. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen G.C., Sam J., Thuluvath P.J. Hepatitis C is a predictor of acute liver injury among hospitalizations for acetaminophen overdose in the United States: a nationwide analysis. Hepatology. 2008;48:1336–1341. doi: 10.1002/hep.22536. [DOI] [PubMed] [Google Scholar]
- 33.Aibo D.I., Birmingham N.P., Lewandowski R., Maddox J.F., Roth R.A., Ganey P.E., et al. Acute exposure to ozone exacerbates acetaminophen-induced liver injury in mice. Toxicol Sci. 2010;115:267–285. doi: 10.1093/toxsci/kfq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guéguen Y., Grandcolas L., Baudelin C., Grison S., Tissandié E., Jourdain J.R., et al. Effect of acetaminophen administration to rats chronically exposed to depleted uranium. Toxicology. 2007;229:62–72. doi: 10.1016/j.tox.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Majhi C.R., Khan S., Leo M.D., Prawez S., Kumar A., Sankar P., et al. Acetaminophen increases the risk of arsenic-mediated development of hepatic damage in rats by enhancing redox-signaling mechanism. Environ Toxicol. 2014;29:187–198. doi: 10.1002/tox.20785. [DOI] [PubMed] [Google Scholar]
- 36.Gong S., Lan T., Zeng L., Luo H., Yang X., Li N., et al. Gut microbiota mediates diurnal variation of acetaminophen induced acute liver injury in mice. J Hepatol. 2018;69:51–59. doi: 10.1016/j.jhep.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stephens C., Lucena M.I., Andrade R.J. Genetic risk factors in the development of idiosyncratic drug-induced liver injury. Expert Opin Drug Metab Toxicol. 2021;17:153–169. doi: 10.1080/17425255.2021.1854726. [DOI] [PubMed] [Google Scholar]
- 38.Chen M., Suzuki A., Borlak J., Andrade R.J., Lucena M.I. Drug-induced liver injury: interactions between drug properties and host factors. J Hepatol. 2015;63:503–514. doi: 10.1016/j.jhep.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 39.O’Brien T.R., Yang H.I., Groover S., Jeng W.J. Genetic factors that affect spontaneous clearance of hepatitis C or B virus, response to treatment, and disease progression. Gastroenterology. 2019;156:400–417. doi: 10.1053/j.gastro.2018.09.052. [DOI] [PubMed] [Google Scholar]
- 40.Xu J., Zhan Q., Fan Y., Yu Y., Zeng Z. Human genetic susceptibility to hepatitis B virus infection. Infect Genet Evol. 2021;87:104663. doi: 10.1016/j.meegid.2020.104663. [DOI] [PubMed] [Google Scholar]
- 41.Ganesan M., Eikenberry A., Poluektova L.Y., Kharbanda K.K., Osna N.A. Role of alcohol in pathogenesis of hepatitis B virus infection. World J Gastroenterol. 2020;26:883–903. doi: 10.3748/wjg.v26.i9.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pawlotsky J.M. Pathophysiology of hepatitis C virus infection and related liver disease. Trends Microbiol. 2004;12:96–102. doi: 10.1016/j.tim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 43.Kew M.C. Synergistic interaction between aflatoxin B1 and hepatitis B virus in hepatocarcinogenesis. Liver Int. 2003;23:405–409. doi: 10.1111/j.1478-3231.2003.00869.x. [DOI] [PubMed] [Google Scholar]
- 44.Moudgil V., Redhu D., Dhanda S., Singh J. A review of molecular mechanisms in the development of hepatocellular carcinoma by aflatoxin and hepatitis B and C viruses. J Environ Pathol Toxicol Oncol. 2013;32:165–175. doi: 10.1615/jenvironpatholtoxicoloncol.2013007166. [DOI] [PubMed] [Google Scholar]
- 45.Eslam M., Valenti L., Romeo S. Genetics and epigenetics of NAFLD and NASH: clinical impact. J Hepatol. 2018;68:268–279. doi: 10.1016/j.jhep.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 46.Wahlang B., Jin J., Beier J.I., Hardesty J.E., Daly E.F., Schnegelberger R.D., et al. Mechanisms of environmental contributions to fatty liver disease. Curr Env Health Rep. 2019;6:80–94. doi: 10.1007/s40572-019-00232-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun T., Kang Y., Liu J., Zhang Y., Ou L., Liu X., et al. Nanomaterials and hepatic disease: toxicokinetics, disease types, intrinsic mechanisms, liver susceptibility, and influencing factors. J Nanobiotechnol. 2021;19:108. doi: 10.1186/s12951-021-00843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng S., Yang Y., Wen C., Liu W., Cao L., Feng X., et al. Effects of environmental contaminants in water resources on nonalcoholic fatty liver disease. Environ Int. 2021;154:106555. doi: 10.1016/j.envint.2021.106555. [DOI] [PubMed] [Google Scholar]
- 49.Cave M.C., Clair H.B., Hardesty J.E., Falkner K.C., Feng W., Clark B.J., et al. Nuclear receptors and nonalcoholic fatty liver disease. Biochim Biophys Acta. 2016;1859:1083–1099. doi: 10.1016/j.bbagrm.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharpton S.R., Schnabl B., Knight R., Loomba R. Current concepts, opportunities, and challenges of gut microbiome-based personalized medicine in nonalcoholic fatty liver disease. Cell Metabol. 2021;33:21–32. doi: 10.1016/j.cmet.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lang S., Schnabl B. Microbiota and fatty liver disease-the known, the unknown, and the future. Cell Host Microbe. 2020;28:233–244. doi: 10.1016/j.chom.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seitz H.K., Bataller R., Cortez-Pinto H., Gao B., Gual A., Lackner C., et al. Alcoholic liver disease. Nature Rev Dis primers. 2018;4:16. doi: 10.1038/s41572-018-0014-7. [DOI] [PubMed] [Google Scholar]
- 53.Duan Y., Llorente C., Lang S., Brandl K., Chu H., Jiang L., et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature. 2019;575:505–511. doi: 10.1038/s41586-019-1742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wahlang B., Beier J.I., Clair H.B., Bellis-Jones H.J., Falkner K.C., McClain C.J., et al. Toxicant-associated steatohepatitis. Toxicol Pathol. 2013;41:343–360. doi: 10.1177/0192623312468517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wahlang B., Alexander N.C., 2nd, Li X., Rouchka E.C., Kirpich I.A., Cave M.C. Polychlorinated biphenyls altered gut microbiome in CAR and PXR knockout mice exhibiting toxicant-associated steatohepatitis. Toxicol Rep. 2021;8:536–547. doi: 10.1016/j.toxrep.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones J.G. Hepatic glucose and lipid metabolism. Diabetologia. 2016;59:1098–1103. doi: 10.1007/s00125-016-3940-5. [DOI] [PubMed] [Google Scholar]
- 57.Corless J.K., Middleton H.M., 3rd Normal liver function. A basis for understanding hepatic disease. Arch Intern Med. 1983;143:2291–2294. doi: 10.1001/archinte.143.12.2291. [DOI] [PubMed] [Google Scholar]
- 58.Racanelli V., Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54–S62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 59.Seeff L.B. Drug-induced liver injury is a major risk for new drugs. Dig Dis. 2015;33:458–463. doi: 10.1159/000374089. [DOI] [PubMed] [Google Scholar]
- 60.Regev A. Drug-induced liver injury and drug development: industry perspective. Semin Liver Dis. 2014;34:227–239. doi: 10.1055/s-0034-1375962. [DOI] [PubMed] [Google Scholar]
- 61.Tolman K.G., Sirrine R.W. Occupational hepatotoxicity. Clin Liv Dis. 1998;2:563–589. [Google Scholar]
- 62.Schwabe R.F., Luedde T. Apoptosis and necroptosis in the liver: a matter of life and death. Nat Rev Gastroenterol Hepatol. 2018;15:738–752. doi: 10.1038/s41575-018-0065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Calcinotto A., Kohli J., Zagato E., Pellegrini L., Demaria M., Alimonti A. Cellular senescence: aging, cancer, and injury. Physiol Rev. 2019;99:1047–1078. doi: 10.1152/physrev.00020.2018. [DOI] [PubMed] [Google Scholar]
- 64.Preziosi M.E., Monga S.P. Update on the mechanisms of liver regeneration. Semin Liver Dis. 2017;37:141–151. doi: 10.1055/s-0037-1601351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 66.Schwabe R.F., Seki E., Brenner D.A. Toll-like receptor signaling in the liver. Gastroenterology. 2006;130:1886–1900. doi: 10.1053/j.gastro.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 67.Michalopoulos G.K., Bhushan B. Liver regeneration: biological and pathological mechanisms and implications. Nat Rev Gastroenterol Hepatol. 2021;18:40–55. doi: 10.1038/s41575-020-0342-4. [DOI] [PubMed] [Google Scholar]
- 68.Seki E., Brenner D.A. Recent advancement of molecular mechanisms of liver fibrosis. J Hepatobiliary Pancreat Sci. 2015;22:512–518. doi: 10.1002/jhbp.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arteel G.E., Naba A. The liver matrisome—looking beyond collagens. JHEP Rep. 2020;2:100115. doi: 10.1016/j.jhepr.2020.100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Poujois A., Woimant F. Wilson's disease: a 2017 update. Clin Res Hepatol Gastroenterol. 2018;42:512–520. doi: 10.1016/j.clinre.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 71.Ramos R.G., Olden K. Gene-environment interactions in the development of complex disease phenotypes. Int J Environ Res Public Health. 2008;5:4–11. doi: 10.3390/ijerph5010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rawal N., Yazigi N. Pediatric liver transplantation. Pediatr Clin North Am. 2017;64:677–684. doi: 10.1016/j.pcl.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 73.Izumi K., Hayashi D., Grochowski C.M., Kubota N., Nishi E., Arakawa M., et al. Discordant clinical phenotype in monozygotic twins with Alagille syndrome: possible influence of non-genetic factors. Am J Med Genet. 2016;170a:471–475. doi: 10.1002/ajmg.a.37429. [DOI] [PubMed] [Google Scholar]
- 74.Kamath B.M., Krantz I.D., Spinner N.B., Heubi J.E., Piccoli D.A. Monozygotic twins with a severe form of Alagille syndrome and phenotypic discordance. Am J Med Genet. 2002;112:194–197. doi: 10.1002/ajmg.10610. [DOI] [PubMed] [Google Scholar]
- 75.Senn O., Russi E.W., Imboden M., Probst-Hensch N.M. α1-Antitrypsin deficiency and lung disease: risk modification by occupational and environmental inhalants. Eur Respir J. 2005;26:909–917. doi: 10.1183/09031936.05.00021605. [DOI] [PubMed] [Google Scholar]
- 76.Strnad P., Buch S., Hamesch K., Fischer J., Rosendahl J., Schmelz R., et al. Heterozygous carriage of the alpha1-antitrypsin Pi∗Z variant increases the risk to develop liver cirrhosis. Gut. 2019;68:1099–1107. doi: 10.1136/gutjnl-2018-316228. [DOI] [PubMed] [Google Scholar]
- 77.Turton K.B., Ingram R.J., Valvano M.A. Macrophage dysfunction in cystic fibrosis: nature or nurture?. J Leukoc Biol. 2021;109:573–582. doi: 10.1002/JLB.4RU0620-245R. [DOI] [PubMed] [Google Scholar]
- 78.Sontag M.K., Accurso F.J. Gene modifiers in pediatrics: application to cystic fibrosis. Adv Pediatr. 2004;51:5–36. [PubMed] [Google Scholar]
- 79.Staufer K., Halilbasic E., Trauner M., Kazemi-Shirazi L. Cystic fibrosis related liver disease—another black box in hepatology. Int J Mol Sci. 2014;15:13529–13549. doi: 10.3390/ijms150813529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang C., Zhang Y., Deng M., Wang X., Tu W., Fu Z., et al. Bioaccumulation in the gut and liver causes gut barrier dysfunction and hepatic metabolism disorder in mice after exposure to low doses of OBS. Environ Int. 2019;129:279–290. doi: 10.1016/j.envint.2019.05.056. [DOI] [PubMed] [Google Scholar]
- 81.Wang Y., Jin C., Wang D., Zhou J., Yang G., Shao K., et al. Effects of chlorothalonil, prochloraz and the combination on intestinal barrier function and glucolipid metabolism in the liver of mice. J Hazard Mater. 2021;410:124639. doi: 10.1016/j.jhazmat.2020.124639. [DOI] [PubMed] [Google Scholar]
- 82.Kim J.H., Matsubara T., Lee J., Fenollar-Ferrer C., Han K., Kim D., et al. Lysosomal SLC46A3 modulates hepatic cytosolic copper homeostasis. Nature Commun. 2021;12:290. doi: 10.1038/s41467-020-20461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jin J., Wahlang B., Shi H., Hardesty J.E., Falkner K.C., Head K.Z., et al. Dioxin-like and non-dioxin-like PCBs differentially regulate the hepatic proteome and modify diet-induced nonalcoholic fatty liver disease severity. Med Chem Res. 2020;29:1247–1263. doi: 10.1007/s00044-020-02581-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harper P., Plant J.W., Unger D.B. Congenital biliary atresia and jaundice in lambs and calves. Aust Vet J. 1990;67:18–22. doi: 10.1111/j.1751-0813.1990.tb07385.x. [DOI] [PubMed] [Google Scholar]
- 85.Lorent K., Gong W., Koo K.A., Waisbourd-Zinman O., Karjoo S., Zhao X., et al. Identification of a plant isoflavonoid that causes biliary atresia. Sci Transl Med. 2015;7:286ra67. doi: 10.1126/scitranslmed.aaa1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Waisbourd-Zinman O., Koh H., Tsai S., Lavrut P.M., Dang C., Zhao X., et al. The toxin biliatresone causes mouse extrahepatic cholangiocyte damage and fibrosis through decreased glutathione and SOX17. Hepatology. 2016;64:880–893. doi: 10.1002/hep.28599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shah R.A., Kowdley K.V. Current and potential treatments for primary biliary cholangitis. Lancet Gastroenterol Hepatol. 2020;5:306–315. doi: 10.1016/S2468-1253(19)30343-7. [DOI] [PubMed] [Google Scholar]
- 88.Selmi C., Mayo M.J., Bach N., Ishibashi H., Invernizzi P., Gish R.G., et al. Primary biliary cirrhosis in monozygotic and dizygotic twins: genetics, epigenetics, and environment. Gastroenterology. 2004;127:485–492. doi: 10.1053/j.gastro.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 89.Boonstra K., Beuers U., Ponsioen C.Y. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56:1181–1188. doi: 10.1016/j.jhep.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 90.Invernizzi P., Ransom M., Raychaudhuri S., Kosoy R., Lleo A., Shigeta R., et al. Classical HLA-DRB1 and DPB1 alleles account for HLA associations with primary biliary cirrhosis. Genes Immun. 2012;13:461–468. doi: 10.1038/gene.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tanaka A., Leung P.S., Gershwin M.E. Environmental basis of primary biliary cholangitis. Exp Biol Med. 2018;243:184–189. doi: 10.1177/1535370217748893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Selmi C., Balkwill D.L., Invernizzi P., Ansari A.A., Coppel R.L., Podda M., et al. Patients with primary biliary cirrhosis react against a ubiquitous xenobiotic-metabolizing bacterium. Hepatology. 2003;38:1250–1257. doi: 10.1053/jhep.2003.50446. [DOI] [PubMed] [Google Scholar]
- 93.Vergani D., Bogdanos D.P., Baum H. Unusual suspects in primary biliary cirrhosis. Hepatology. 2004;39:38–41. doi: 10.1002/hep.20028. [DOI] [PubMed] [Google Scholar]
- 94.Tang R., Wei Y., Li Y., Chen W., Chen H., Wang Q., et al. Gut microbial profile is altered in primary biliary cholangitis and partially restored after UDCA therapy. Gut. 2018;67:534–541. doi: 10.1136/gutjnl-2016-313332. [DOI] [PubMed] [Google Scholar]
- 95.Bergquist A., Said K., Broomé U. Changes over a 20-year period in the clinical presentation of primary sclerosing cholangitis in Sweden. Scand J Gastroenterol. 2007;42:88–93. doi: 10.1080/00365520600787994. [DOI] [PubMed] [Google Scholar]
- 96.Angulo P., Peter J.B., Gershwin M.E., DeSotel C.K., Shoenfeld Y., Ahmed A.E., et al. Serum autoantibodies in patients with primary sclerosing cholangitis. J Hepatol. 2000;32:182–187. doi: 10.1016/s0168-8278(00)80061-6. [DOI] [PubMed] [Google Scholar]
- 97.Xu B., Broome U., Ericzon B.G., Sumitran-Holgersson S. High frequency of autoantibodies in patients with primary sclerosing cholangitis that bind biliary epithelial cells and induce expression of CD44 and production of interleukin 6. Gut. 2002;51:120–127. doi: 10.1136/gut.51.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Eaton J.E., Talwalkar J.A., Lazaridis K.N., Gores G.J., Lindor K.D. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology. 2013;145:521–536. doi: 10.1053/j.gastro.2013.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Manns M.P., Lohse A.W., Vergani D. Autoimmune hepatitis–update 2015. J Hepatol. 2015;62:S100–S111. doi: 10.1016/j.jhep.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 100.Lucena M.I., Kaplowitz N., Hallal H., Castiella A., García-Bengoechea M., Otazua P., et al. Recurrent drug-induced liver injury (DILI) with different drugs in the Spanish Registry: the dilemma of the relationship to autoimmune hepatitis. J Hepatol. 2011;55:820–827. doi: 10.1016/j.jhep.2010.12.041. [DOI] [PubMed] [Google Scholar]
- 101.Cooper G.S., Makris S.L., Nietert P.J., Jinot J. Evidence of autoimmune-related effects of trichloroethylene exposure from studies in mice and humans. Environ Health Perspect. 2009;117:696–702. doi: 10.1289/ehp.11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Katarey D., Verma S. Drug-induced liver injury. Clinical Med (Lond) 2016;16:s104–s109. doi: 10.7861/clinmedicine.16-6s-s104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hoofnagle J.H., Björnsson E.S. Drug-induced liver injury—types and phenotypes. N Engl J Med. 2019;381:264–273. doi: 10.1056/NEJMra1816149. [DOI] [PubMed] [Google Scholar]
- 104.Ramachandran A., Jaeschke H. Acetaminophen hepatotoxicity. Semin Liver Dis. 2019;39:221–234. doi: 10.1055/s-0039-1679919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Andrade R.J., Chalasani N., Björnsson E.S., Suzuki A., Kullak-Ublick G.A., Watkins P.B., et al. Drug-induced liver injury. Nature Rev Dis Primers. 2019;5:58. doi: 10.1038/s41572-019-0105-0. [DOI] [PubMed] [Google Scholar]
- 106.Navarro V.J., Khan I., Bjornsson E., Seeff L.B., Serrano J., Hoofnagle J.H. Liver injury from herbal and dietary supplements. Hepatology. 2017;65:363–373. doi: 10.1002/hep.28813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dakhoul L., Ghabril M., Gu J., Navarro V., Chalasani N., Serrano J. Heavy consumption of alcohol is not associated with worse outcomes in patients with idiosyncratic drug-induced liver injury compared to non-drinkers. Clin Gastroenterol Hepatol. 2018;16:722–729. doi: 10.1016/j.cgh.2017.12.036. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dragnev K.H., Nims R.W., Fox S.D., Lindahl R., Lubet R.A. Relative potencies of induction of hepatic drug-metabolizing enzyme genes by individual PCB congeners. Toxicol Appl Pharmacol. 1995;132:334–342. doi: 10.1006/taap.1995.1115. [DOI] [PubMed] [Google Scholar]
- 109.Suzuki T., Hidaka T., Kumagai Y., Yamamoto M. Environmental pollutants and the immune response. Nat Immunol. 2020;21:1486–1495. doi: 10.1038/s41590-020-0802-6. [DOI] [PubMed] [Google Scholar]
- 110.Fardel O., Kolasa E., Le Vee M. Environmental chemicals as substrates, inhibitors or inducers of drug transporters: implication for toxicokinetics, toxicity and pharmacokinetics. Expert Opin Drug Metab Toxicol. 2012;8:29–46. doi: 10.1517/17425255.2012.637918. [DOI] [PubMed] [Google Scholar]
- 111.Corsini A., Bortolini M. Drug-induced liver injury: the role of drug metabolism and transport. J Clin Pharmacol. 2013;53:463–474. doi: 10.1002/jcph.23. [DOI] [PubMed] [Google Scholar]
- 112.Real M., Barnhill M.S., Higley C., Rosenberg J., Lewis J.H. Drug-induced liver injury: highlights of the recent literature. Drug Saf. 2019;42:365–387. doi: 10.1007/s40264-018-0743-2. [DOI] [PubMed] [Google Scholar]
- 113.Pintó R.M., Pérez-Rodríguez F.J., Costafreda M.I., Chavarria-Miró G., Guix S., Ribes E., et al. Pathogenicity and virulence of hepatitis A virus. Virulence. 2021;12:1174–1185. doi: 10.1080/21505594.2021.1910442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Goel A., Aggarwal R. Hepatitis E: epidemiology, clinical course, prevention, and treatment. Gastroenterol Clin North Am. 2020;49:315–330. doi: 10.1016/j.gtc.2020.01.011. [DOI] [PubMed] [Google Scholar]
- 115.Ringelhan M., McKeating J.A., Protzer U. Viral hepatitis and liver cancer. Philos Trans R Soc Lond B Biol Sci. 2017;372:20160274. doi: 10.1098/rstb.2016.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pellicoro A., Ramachandran P., Iredale J.P., Fallowfield J.A. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14:181–194. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- 117.Ludwig J., Viggiano T.R., McGill D.B., Oh B.J. Nonalcoholic steatohepatitis: mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–438. [PubMed] [Google Scholar]
- 118.Mahli A., Hellerbrand C. Alcohol and obesity: a dangerous association for fatty liver disease. Dig Dis. 2016;34 Suppl 1:32–39. doi: 10.1159/000447279. [DOI] [PubMed] [Google Scholar]
- 119.Cave M., Falkner K.C., Ray M., Joshi-Barve S., Brock G., Khan R., et al. Toxicant-associated steatohepatitis in vinyl chloride workers. Hepatology. 2010;51:474–481. doi: 10.1002/hep.23321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Eslam M., Sanyal A.J., George J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999–2014. doi: 10.1053/j.gastro.2019.11.312. e1. [DOI] [PubMed] [Google Scholar]
- 121.Loomba R., Sanyal A.J. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 122.Younossi Z., Anstee Q.M., Marietti M., Hardy T., Henry L., Eslam M., et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 123.Lang A.L., Chen L., Poff G.D., Ding W.X., Barnett R.A., Arteel G.E., et al. Vinyl chloride dysregulates metabolic homeostasis and enhances diet-induced liver injury in mice. Hepatol Commun. 2018;2:270–284. doi: 10.1002/hep4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lang A.L., Beier J.I. Interaction of volatile organic compounds and underlying liver disease: a new paradigm for risk. Biol Chem. 2018;399:1237–1248. doi: 10.1515/hsz-2017-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Angrish M.M., Kaiser J.P., McQueen C.A., Chorley B.N. Tipping the balance: hepatotoxicity and the 4 apical key events of hepatic steatosis. Toxicol Sci. 2016;150:261–268. doi: 10.1093/toxsci/kfw018. [DOI] [PubMed] [Google Scholar]
- 126.Heindel J.J., Blumberg B., Cave M., Machtinger R., Mantovani A., Mendez M.A., et al. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol. 2017;68:3–33. doi: 10.1016/j.reprotox.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang Y., Li K., Kong A., Zhou Y., Chen D., Gu J., et al. Dysregulation of autophagy acts as a pathogenic mechanism of non-alcoholic fatty liver disease (NAFLD) induced by common environmental pollutants. Ecotoxicol Environ Saf. 2021;217:112256. doi: 10.1016/j.ecoenv.2021.112256. [DOI] [PubMed] [Google Scholar]
- 128.Joshi-Barve S., Kirpich I., Cave M.C., Marsano L.S., McClain C.J. Alcoholic, nonalcoholic, and toxicant-associated steatohepatitis: mechanistic similarities and differences. Cell Mol Gastroenterol Hepatol. 2015;1:356–367. doi: 10.1016/j.jcmgh.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.International Agency for Research on Cancer IARC monographs on the evaluation of carcinogenic risks to humans. Volume 97. 1,3-Butadiene, ethylene oxide and vinyl halides (vinyl fluoride, vinyl chloride and vinyl bromide) IARC Monogr Eval Carcinog Risks Hum. 2008;97:3–471. [PMC free article] [PubMed] [Google Scholar]
- 130.Makk L., Creech J.L., Whelan J.G., Jr., Johnson M.N. Liver damage and angiosarcoma in vinyl chloride workers. a systematic detection program. JAMA. 1974;230:64–68. [PubMed] [Google Scholar]
- 131.Tamburro C.H., Makk L., Popper H. Early hepatic histologic alterations among chemical (vinyl monomer) workers. Hepatology. 1984;4:413–418. doi: 10.1002/hep.1840040310. [DOI] [PubMed] [Google Scholar]
- 132.Block J.B. Angiosarcoma of the liver following vinyl chloride exposure. JAMA. 1974;229:53–54. [PubMed] [Google Scholar]
- 133.Al-Eryani L., Wahlang B., Falkner K.C., Guardiola J.J., Clair H.B., Prough R.A., et al. Identification of environmental chemicals associated with the development of toxicant-associated fatty liver disease in rodents. Toxicol Pathol. 2015;43:482–497. doi: 10.1177/0192623314549960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Armstrong L.E., Guo G.L. Understanding environmental contaminants' direct effects on non-alcoholic fatty liver disease progression. Current Environ Health Rep. 2019;6:95–104. doi: 10.1007/s40572-019-00231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380:1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 136.Jemal A., Ward E.M., Johnson C.J., Cronin K.A., Ma J., Ryerson B., et al. Annual report to the nation on the status of cancer, 1975–2014, featuring survival. J Natl Cancer Inst. 2017;109 doi: 10.1093/jnci/djx030. djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.La Vecchia C., Negri E., Cavalieri d'Oro L., Franceschi S. Liver cirrhosis and the risk of primary liver cancer. Eur J Cancer Prev. 1998;7:315–320. doi: 10.1097/00008469-199808000-00007. [DOI] [PubMed] [Google Scholar]
- 138.Fattovich G., Stroffolini T., Zagni I., Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 139.Ludewig G., Robertson L.W. Polychlorinated biphenyls (PCBs) as initiating agents in hepatocellular carcinoma. Cancer Lett. 2013;334:46–55. doi: 10.1016/j.canlet.2012.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sherman M. Vinyl chloride and the liver. J Hepatol. 2009;51:1074–1081. doi: 10.1016/j.jhep.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 141.Liu J., Waalkes M.P. Liver is a target of arsenic carcinogenesis. Toxicol Sci. 2008;105:24–32. doi: 10.1093/toxsci/kfn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tian M., Zhao B., Zhang J., Martin F.L., Huang Q., Liu L., et al. Association of environmental benzo[a]pyrene exposure and DNA methylation alterations in hepatocellular carcinoma: a Chinese case-control study. Sci Tot Environ. 2016;541:1243–1252. doi: 10.1016/j.scitotenv.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 143.Bhushan B., Apte U. Liver regeneration after acetaminophen hepatotoxicity: mechanisms and therapeutic opportunities. Am J Pathol. 2019;189:719–729. doi: 10.1016/j.ajpath.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tzirogiannis K.N., Panoutsopoulos G.I., Demonakou M.D., Hereti R.I., Alexandropoulou K.N., Basayannis A.C., et al. Time-course of cadmium-induced acute hepatotoxicity in the rat liver: the role of apoptosis. Arch Toxicol. 2003;77:694–701. doi: 10.1007/s00204-003-0499-y. [DOI] [PubMed] [Google Scholar]
- 145.Bauman J.W., Goldsworthy T.L., Dunn C.S., Fox T.R. Inhibitory effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on rat hepatocyte proliferation induced by 2/3 partial hepatectomy. Cell Prolif. 1995;28:437–451. doi: 10.1111/j.1365-2184.1995.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 146.Beggs K.M., McGreal S.R., McCarthy A., Gunewardena S., Lampe J.N., Lau C., et al. The role of hepatocyte nuclear factor 4-alpha in perfluorooctanoic acid- and perfluorooctanesulfonic acid-induced hepatocellular dysfunction. Toxicol Appl Pharmacol. 2016;304:18–29. doi: 10.1016/j.taap.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Abdo W., Hirata A., Sakai H., El-Sawak A., Nikami H., Yanai T. Combined effects of organochlorine pesticides heptachlor and hexachlorobenzene on the promotion stage of hepatocarcinogenesis in rats. Food Chem Toxicol. 2013;55:578–585. doi: 10.1016/j.fct.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 148.Gonzalez F.J., Peters J.M., Cattley R.C. Mechanism of action of the nongenotoxic peroxisome proliferators: role of the peroxisome proliferator-activator receptor alpha. J Natl Cancer Inst. 1998;90:1702–1709. doi: 10.1093/jnci/90.22.1702. [DOI] [PubMed] [Google Scholar]
- 149.Chalasani N., Bonkovsky H.L., Fontana R., Lee W., Stolz A., Talwalkar J., et al. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology. 2015;148:1340–1352. doi: 10.1053/j.gastro.2015.03.006. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sokol R.J. Reloading against rare liver diseases. J Pediatr Gastroenterol Nutr. 2010;50:9–10. doi: 10.1097/MPG.0b013e3181b47b49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Brunt E.M., Kleiner D.E., Wilson L.A., Sanyal A.J., Neuschwander-Tetri B.A. Improvements in histologic features and diagnosis associated with improvement in fibrosis in nonalcoholic steatohepatitis: results from the Nonalcoholic Steatohepatitis Clinical Research Network Treatment trials. Hepatology. 2019;70:522–531. doi: 10.1002/hep.30418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ping P., Hermjakob H., Polson J.S., Benos P.V., Wang W. Biomedical Informatics on the cloud: a treasure hunt for advancing cardiovascular medicine. Circ Res. 2018;122:1290–1301. doi: 10.1161/CIRCRESAHA.117.310967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mooney S.J., Pejaver V. Big data in public health: terminology, machine learning, and privacy. Annu Rev Public Health. 2018;39:95–112. doi: 10.1146/annurev-publhealth-040617-014208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tripathi A., Debelius J., Brenner D.A., Karin M., Loomba R., Schnabl B., et al. The gut–liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15:397–411. doi: 10.1038/s41575-018-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Broitman S.A., Gottlieb L.S., Zamcheck N. Influence of neomycin and ingested endotoxin in the pathogenesis of choline deficiency cirrhosis in the adult rat. J Exp Med. 1964;119:633–642. doi: 10.1084/jem.119.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lynch S.V., Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375:2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 157.Rosenfeld C.S. Gut dysbiosis in animals due to environmental chemical exposures. Front Cell Infect Microbiol. 2017;7:396. doi: 10.3389/fcimb.2017.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rodricks J., Huang Y., Mantus E., Shubat P. Do interactions between environmental chemicals and the human microbiome need to be considered in risk assessments?. Risk Anal. 2019;39:2353–2358. doi: 10.1111/risa.13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Peters A., Nawrot T.S., Baccarelli A.A. Hallmarks of environmental insults. Cell. 2021;184:1455–1468. doi: 10.1016/j.cell.2021.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nguyen G.C., Thuluvath P.J. Racial disparity in liver disease: biological, cultural, or socioeconomic factors. Hepatology. 2008;47:1058–1066. doi: 10.1002/hep.22223. [DOI] [PubMed] [Google Scholar]
- 161.Flores Y.N., Yee H.F., Jr., Leng M., Escarce J.J., Bastani R., Salmerón J., et al. Risk factors for chronic liver disease in Blacks, Mexican Americans, and Whites in the United States: results from NHANES IV, 1999–2004. Am J Gastroenterol. 2008;103:2231–2238. doi: 10.1111/j.1572-0241.2008.02022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tapper E.B., Parikh N.D. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: observational study. BMJ. 2018;362 doi: 10.1136/bmj.k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Asrani S.K., Devarbhavi H., Eaton J., Kamath P.S. Burden of liver diseases in the world. J Hepatol. 2019;70:151–171. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 164.Dwyer-Lindgren L., Bertozzi-Villa A., Stubbs R.W., Morozoff C., Kutz M.J., Huynh C., et al. US county-level trends in mortality rates for major causes of death, 1980–2014. JAMA. 2016;316:2385–2401. doi: 10.1001/jama.2016.13645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Goldberg D., Ross-Driscoll K., Lynch R. County differences in liver mortality in the United States: impact of sociodemographics, disease risk factors, and access to care. Gastroenterology. 2021;160:1140–11450. doi: 10.1053/j.gastro.2020.11.016. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Smith A., Laribi O. Environmental justice in the American public health context: trends in the scientific literature at the intersection between health, environment, and social status. J Racial Ethn Health Disparities. 2021 doi: 10.1007/s40615-020-00949-7. Available from: [DOI] [PubMed] [Google Scholar]
- 167.Johnston J., Cushing L. Chemical exposures, health, and environmental justice in communities living on the fenceline of industry. Curr Environ Health Rep. 2020;7:48–57. doi: 10.1007/s40572-020-00263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Birnbaum L.S., Jung P. From endocrine disruptors to nanomaterials: advancing our understanding of environmental health to protect public health. Health Aff (Millwood) 2011;30:814–822. doi: 10.1377/hlthaff.2010.1225. [DOI] [PubMed] [Google Scholar]
- 169.Wild C.P. Complementing the genome with an "exposome": the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14:1847–1850. doi: 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]
- 170.Jones D.P. Sequencing the exposome: a call to action. Toxicol Rep. 2016;3:29–45. doi: 10.1016/j.toxrep.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Papaioannou N., Distel E., de Oliveira E., Gabriel C., Frydas I.S., Anesti O., et al. Multi-omics analysis reveals that co-exposure to phthalates and metals disturbs urea cycle and choline metabolism. Environ Res. 2021;192:110041. doi: 10.1016/j.envres.2020.110041. [DOI] [PubMed] [Google Scholar]
- 172.Orešič M., McGlinchey A., Wheelock C.E., Hyötyläinen T. Metabolic signatures of the exposome-quantifying the impact of exposure to environmental chemicals on human health. Metabolites. 2020;10:454. doi: 10.3390/metabo10110454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ghosh D., Bernstein J.A., Khurana Hershey G.K., Rothenberg M.E., Mersha T.B. Leveraging multilayered "omics" data for atopic dermatitis: a road map to precision medicine. Front Immunol. 2018;9:2727. doi: 10.3389/fimmu.2018.02727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cheung A.C., Walker D.I., Juran B.D., Miller G.W., Lazaridis K.N. Studying the Exposome to understand the environmental determinants of complex liver diseases. Hepatology. 2020;71:352–362. doi: 10.1002/hep.31028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gómez-Roig M.D., Pascal R., Cahuana M.J., García-Algar O., Sebastiani G., Andreu-Fernández V., et al. Environmental exposure during pregnancy: influence on prenatal development and early life: a comprehensive review. Fetal Diagn Ther. 2021;48:245–257. doi: 10.1159/000514884. [DOI] [PubMed] [Google Scholar]
- 176.Heindel J.J., Balbus J., Birnbaum L., Brune-Drisse M.N., Grandjean P., Gray K., et al. Developmental origins of health and disease: integrating environmental influences. Endocrinology. 2015;156:3416–3421. doi: 10.1210/EN.2015-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Renu K., Chakraborty R., Myakala H., Koti R., Famurewa A.C., Madhyastha H., et al. Molecular mechanism of heavy metals (lead, chromium, arsenic, mercury, nickel and cadmium)-induced hepatotoxicity—a review. Chemosphere. 2021;271:129735. doi: 10.1016/j.chemosphere.2021.129735. [DOI] [PubMed] [Google Scholar]