Abstract
Plastic recombination in Drosophila melanogaster has been associated with a variety of extrinsic and intrinsic factors such as temperature, starvation, and parasite infection. The bacterial endosymbiont Wolbachia pipientis has also been associated with plastic recombination in D. melanogaster. Wolbachia infection is pervasive in arthropods and this infection induces a variety of phenotypes in its hosts, the strength of which can depend on bacterial titer. Here, we test the hypothesis that the magnitude of Wolbachia-associated plastic recombination in D. melanogaster depends on titer. To manipulate titer, we raised Wolbachia-infected and uninfected flies on diets that have previously been shown to increase or decrease Wolbachia titer relative to controls. We measured recombination in treated and control individuals using a standard backcrossing scheme with two X-linked visible markers. Our results recapitulate previous findings that Wolbachia infection is associated with increased recombination rate across the yellow-vermillion interval of the X chromosome. Our data show no significant effect of diet or diet by Wolbachia interactions on recombination, suggesting that diet-induced changes in Wolbachia titer have no effect on the magnitude of plastic recombination. These findings represent one of the first steps toward investigating Wolbachia-associated plastic recombination and demonstrate that the phenotype is a discrete response rather than a continuous one.
Keywords: Drosophila melanogaster, Wolbachia pipientis, plastic recombination, bacterial titer, host diet
Introduction
Phenotypic plasticity is the phenomenon by which a single genotype may produce multiple phenotypes in response to variable environmental stimuli. Plasticity is pervasive in nature, affecting a range of phenotypes like morphology, development, behavior, and reproduction in bacteria, plants, and animals (Fusco and Minelli 2010; Forsman 2015; Fox et al. 2019). Meiotic recombination has also been shown to be phenotypically plastic, where the proportion of recombinant offspring increases in response to environmental stimuli. Plastic recombination has been observed in a number of taxa and in response to different stimuli: yeast experience elevated recombination rates under nutrient stress (Abdullah and Borts 2001), Arabidopsis displays recombination plasticity when exposed to extreme temperatures (Francis et al. 2007; Saini et al. 2017; Lloyd et al. 2018; Modliszewski et al. 2018), infection causes increased recombination in mosquitoes (Zilio et al. 2018) and plants (Chiriac et al. 2006; Andronic 2012), and social stress is associated with plastic recombination in male mice (Belyaev and Borodin 1982).
Plastic recombination also has a rich history of study in the fruit fly, Drosophila melanogaster. Temperature was the first condition associated with plastic recombination in D. melanogaster, a phenomenon which has been well-characterized over the last century (Plough 1917, 1921; Stern 1926; Hayman and Parsons 1962; Grell 1978; Kohl and Singh 2018). Several other factors have been identified which induce plastic recombination in D. melanogaster, including maternal age (Bridges 1927; Priest et al. 2007; Hunter et al. 2016a), starvation (Neel 1941), heat shock (Zhong and Priest 2011; Jackson et al. 2015), and parasite infection (Singh et al. 2015).
More recently, infection with the bacteria Wolbachia pipientis has been associated with plastic recombination in D. melanogaster (Singh 2019). Wolbachia is a gram-negative endosymbiont that infects approximately 40% of terrestrial arthropod species including insects, spiders, and mites (Zug and Hammerstein 2012). Though Wolbachia is found throughout the somatic and germline tissues of its hosts (for review see Pietri et al. 2016), it is particularly abundant in germ cells and is maternally inherited through the oocyte (Dobson et al. 1999; Clark et al. 2002). Different Drosophila species are infected with unique strains of Wolbachia, each with varied effects on host biology (for review see Serbus et al. 2008; Werren et al. 2008; Correa and Ballard 2016; Kaur et al. 2021). One of the most well-studied Wolbachia-associated phenotypes is cytoplasmic incompatibility, which causes certain mating pairings between infected and uninfected flies to produce nonviable embryos (Turelli and Hoffmann 1995). Other strains of Wolbachia can cause phenotypes like male offspring killing or decreased lifespan in Drosophila (Hurst et al. 2000; Chrostek and Teixeira 2015). The native Wolbachia strain in D. melanogaster, wMel, has been shown to provide protection against viral pathogens (Hedges et al. 2008; Teixeira et al. 2008), increase host fecundity (Fry et al. 2004; Fast et al. 2011), and now is associated with plastic increases in recombination rate (Singh 2019; Bryant and Newton 2020).
Since Wolbachia’s role in plastic recombination is a recent discovery, there remains a large gap in our understanding of this interaction. One of the first papers to identify this phenomenon observed a correlation between Wolbachia infection and increased recombination across an interval of the X chromosome, but not on chromosome 3 (Hunter et al. 2016b). This finding was experimentally validated and expanded upon to demonstrate that Wolbachia’s effect on recombination was plastic and occurred in multiple strains of D. melanogaster (Singh 2019). Yet the scope, magnitude, and mechanisms behind this phenomenon are unclear.
Of particular interest is the potential effect of magnitude in Wolbachia-associated plastic recombination. Plastic phenotypes can often be described as either categorical, where the phenotype exists in discrete forms, or continuous, where the phenotype may display dose-dependency and scale with the magnitude of extrinsic or intrinsic factors (Scheiner and Levis 2021). Plastic recombination in D. melanogaster has displayed dose-dependency in response to temperature changes, where increased exposure time to heat shock continuously increased the magnitude of plastic recombination (Jackson et al. 2015). This raises an interesting question of how plastic recombination may be influenced by the strength of Wolbachia infection.
An obvious candidate for testing this question of magnitude is the number of bacteria present within a cell, referred to as titer. Wolbachia-associated phenotypes can vary according to bacterial titer, including cytoplasmic incompatibility (Calvitti et al. 2015), lifespan reduction (Chrostek and Teixeira 2015), and viral pathogen protection (Chrostek et al. 2013; Ye et al. 2016). These phenotypes are considered dose-dependent because the strength of the phenotype continuously scales with the number of Wolbachia cells present within the host. However, some Wolbachia-associated phenotypes may display both categorical and continuous responses; at low bacterial titer, the phenotype exists in discrete forms which are not expressed until a certain Wolbachia titer has been reached, after which the response scales continuously with increasing bacterial titer. This has been observed in both male-killing (Hurst et al. 2000) and lifespan reduction (Reynolds et al. 2003), where the phenotypes display both discrete and continuous responses.
If both plastic recombination and Wolbachia-associated phenotypes can display dose-dependency, this suggests that Wolbachia-associated plastic recombination may also follow the same pattern. Recently, Bryant and Newton (2020) tested this by using flies infected with two Wolbachia strains that maintain different titers and found that flies infected with a higher titer of Wolbachia also had a higher recombination rate. Though these results are consistent with the idea that Wolbachia-associated plastic recombination responds continuously to bacterial titer, it is difficult to determine since different Wolbachia strains were used. Because titer and Wolbachia strain were conflated, the distinct contributions of Wolbachia genotype and titer cannot be determined. Thus, additional research is needed to discern whether plastic recombination responds continuously to Wolbachia titer. It is also certainly possible that the response is continuous under some environmental conditions and discrete under others.
To address this question, we tested the effect of Wolbachia titer on plastic recombination in D. melanogaster. We used host diet to manipulate Wolbachia titer in fly ovaries under control, yeast-enriched, and sucrose-enriched conditions to evaluate the effect of titer on plastic recombination. Recombination rate was measured using classic genetic approaches in Wolbachia-infected and uninfected flies across a genomic interval on the X chromosome. Our data recapitulate that Wolbachia infection is associated with increased recombination rate and find that diet-induced changes in titer had no effect on the magnitude of plastic recombination. These findings demonstrate that Wolbachia-associated plastic recombination displays discrete phenotypes in response to diet-induced changes in Wolbachia titer in D. melanogaster.
Materials and methods
Fly strain and rearing
The D. melanogaster strain used in this experiment was RAL306, which comes from the Drosophila genetics reference panel (DGRP; Mackay et al. 2012; Huang et al. 2014). We used the RAL306 strain because it is naturally infected with Wolbachia and exhibits Wolbachia-associated plastic recombination (Hunter et al. 2016b; Singh 2019). To generate uninfected controls, we raised flies on tetracycline-containing media for two generations to remove Wolbachia. Tetracycline-containing media was created using standard cornmeal/molasses media mixed with ethanol-dissolved tetracycline at a final concentration of 0.25 mg/ml media (Holden et al. 1993). Following two generations of tetracycline treatment, flies were raised on standard media for over 10 generations to allow passive recolonization of the gut microbiome via the fly’s external microbiome.
We used PCR to confirm Wolbachia infection status prior to the start of the experiment. Briefly, single females were collected from stock vials of Wolbachia-infected and uninfected RAL306 flies. DNA was extracted with a standard squish protocol (Gloor and Engels 1992) and used in PCR with primers for the Wolbachia gene, Wolbachia surface protein (wsp), to identify the presence of Wolbachia (Jeyaprakash and Hoy 2000; Singh 2019; Supplementary Table S1).
Diet treatments
For both Wolbachia-infected and uninfected groups, F1 virgin females were raised on one of three diet treatments: control, yeast-enriched, or sucrose-enriched. After 3 days, males were added to diet treatment vials with virgin females for crossing. We set up 10 replicate vials for each experimental group in a single block, repeated for four total blocks (Supplementary Figure S1).
To produce the sucrose-enriched diets, we made a 40% sucrose mixture following Serbus et al. (2015). Initially, we crossed flies in pure sucrose-enriched vials, but larvae raised on sucrose media showed increased mortality and slower development (unpublished observations). Therefore, we devised a strategy to allow adult flies to feed on the sucrose-enriched media while also promoting normal larval development by using “sucrose patties.” Sucrose-enriched mixture was poured into vials and allowed to cool before being sliced into 1 cm patties, which were placed on top of control diet vials. This strategy allowed adult flies to feed on the sucrose-enriched media while larvae could burrow down to feed on control media after hatching.
To make the yeast-enriched diets, we made a standard yeast paste by mixing dry active yeast and deionized water (Serbus et al. 2015). Approximately 2 ml of paste was added to control diet vials for the yeast-enriched treatments. Similar to the sucrose-enriched patties, this allowed adult flies to feed on yeast-enriched media while larvae could develop on control media.
Experimental crosses
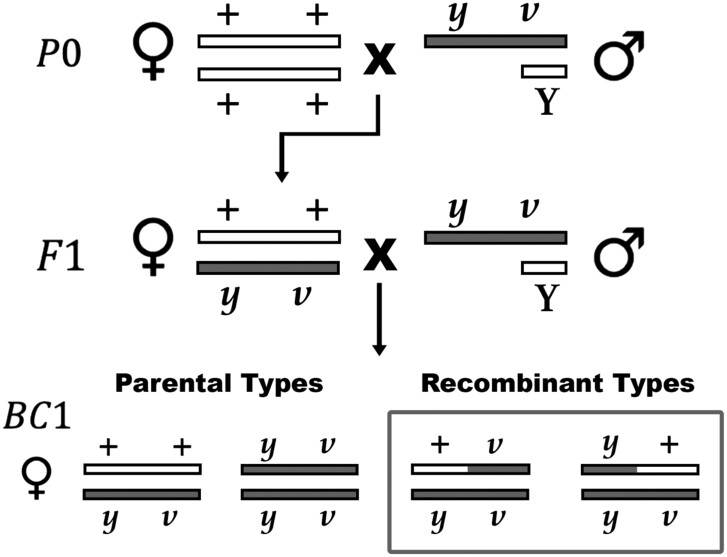
Since Wolbachia have been shown to increase recombination on the X chromosome (Singh 2019), we measured recombination with a standard two-step backcrossing scheme using the markers yellow (y) and vermillion (v) (33 centimorgans (cM) apart) (Figure 1). In the first cross, roughly 20 RAL306 females and 20 yv males were crossed in 8 oz bottles. Heterozygous F1 virgin female offspring were collected from these bottles. For the second cross, 5 F1 females were backcrossed to 5 yv males in a vial, with approximately 10 vials per diet treatment per block, repeated for a total of 4 blocks (Supplementary Figure S1). BC1 offspring (Figure 1) were counted to estimate recombination rate in F1 females by calculating the recombinant fraction (cM/100), which is the proportion of recombinant types to the total number of offspring. For these crosses, recombinant types were heterozygous (female BC1) or hemizygous (male BC1) for either the y or v allele (Figure 1).
Figure 1.
A two-step crossing scheme to measure recombination. Recombination rate can be estimated on the X chromosome using the recessive visible markers yellow (y) and vermillion (v) (33 cM). Males with the y v markers are crossed to wildtype (+ +) females. Heterozygous F1 females are backcrossed to the same male strain to produce BC1 progeny. Progeny which display either the yellow or vermillion phenotype are considered recombinant. Male BC1 genotypes are not shown, but males are heterogametic and require only one copy of the yellow or vermillion marker to display a phenotype.
All crosses were conducted at 25°C with a 12:12 h light: dark cycle. Virgins were age-matched at approximately 48 h before crossing. In each cross, flies were allowed to mate and lay eggs for 4 days before being removed.
Measuring Wolbachia titer
We collected and froze F1 females at −20°C after egg-laying for ovary dissections and DNA extraction. Flies from blocks 1–3 were dissected in 1x PBS for a total of 22 ovaries per experimental group. DNA was extracted from ovary samples using the DNeasy® Blood & Tissue Kit (Qiagen) following insect and Gram-negative bacteria protocols. Quantitative PCR (qPCR) was conducted to amplify the Wolbachia gene, wsp, and estimate the average ovarian titer within each experimental group relative to two host genes, αTub84B and CG15365 (Supplementary Table S1). Each experimental group consisted of seven technical replicates for wsp and four technical replicates for each of the host genes, along with nontemplate controls to assess qPCR reaction efficacy. We used the SYBR Green Mastermix and standard manufacturer’s protocols for qPCR on a QuantStudio3 Real-Time PCR System (Life Technologies). We also estimated primer efficiencies using a 1:5 serial dilution standard curve with five dilutions using DNA extracted from Wolbachia-infected flies.
Statistical analyses
Recombination rate between groups was compared using a logistic regression model to evaluate statistical significance of the effect of Wolbachia infection (), diet (), or Wolbachia by diet interaction effects (). The full model is as follows, where Y refers to observed recombination data, refers to overall mean recombination rate, and refers to random variation:, (for ). We used the statistical software JMP Pro (v16.0.0) for logistic regression modeling, using a general linear model with binomial distribution and link logit function.
All other statistical analyses were carried out in RStudio (v1.2.5033). Mutant markers were tested for viability defects using G-tests for goodness of fit. A one-way ANOVA and Tukey’s multiple comparisons test were used to analyze differences in fly fecundity between experimental groups. A post-hoc analysis of recombination rate variance was conducted using a modified robust Brown-Forsythe Levene-type test and Tukey’s multiple comparisons test. For qPCR, raw Cq scores were analyzed using the Livak and Pfaffl methods (Livak and Schmittgen 2001; Pfaffl 2001) and differences between groups were tested using a one-way ANOVA and Tukey’s multiple comparisons test. The significance threshold for all statistical tests was set at 0.05.
Power analyses for recombination rate comparisons were conducted using the R package “SIMR” to validate experimental results (Green and Macleod 2016). Simulated data were generated in R to produce a range of differences in mean recombination rate between groups, which were tested using repeated simulations in SIMR to calculate statistical power, where 80% power or greater is considered ideal.
Results
Fly fecundity
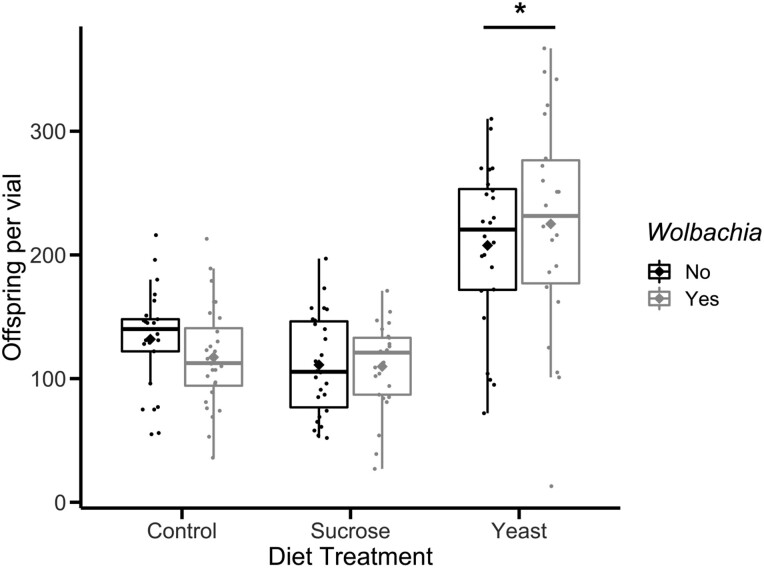
To assess the effect of Wolbachia titer on plastic recombination, we set up crosses for Wolbachia-infected and uninfected flies on three diet treatments and measured recombination between the yellow and vermillion interval on the X chromosome. In total, 22,228 BC1 flies were scored for recombination (Table 1). For flies fed a control diet, the number of progeny per vial for Wolbachia-infected flies averaged 110 flies/vial, while uninfected flies averaged 111 flies/vial. On a sucrose-enriched diet, Wolbachia-infected flies produced an average of 117 flies/vial, compared to uninfected flies, which produced an average of 132 flies/vial. Finally, the number of progeny per vial for flies fed a yeast-enriched diet averaged 225 flies/vial, while uninfected flies averaged 208 flies/vial (Figure 2). Results from a one-way ANOVA test demonstrated that diet treatment [P < 2e−16, ANOVA (N = 150, df = 2)], but not Wolbachia infection [P = 0.942, ANOVA (N = 150, df = 1)] significantly affected fly fecundity. Further analysis with a Tukey’s multiple comparisons test found that the yeast-enriched diet increased fecundity significantly compared to the control (P < 1.3e−13) and sucrose diet treatments (P < 3.1e−14).
Table 1.
Offspring counts for experimental groups
| Diet treatment | Wolbachia-infected | Uninfected | Total |
|---|---|---|---|
| Control | 3,284 | 3,296 | 6,580 |
| Sucrose | 2,824 | 2,888 | 5,712 |
| Yeast | 4,952 | 4,984 | 9,936 |
| Total | 11,060 | 11,168 | 22,228 |
Figure 2.
Fecundity, or number of offspring per vial, of experimental groups. Wolbachia-infected flies are shown in gray, while uninfected flies are shown in black. Each point corresponds to the total number of offspring in a single vial. Boxplots present summary statistics, where the top and bottom edges encompass the first to third quartiles and the middle bar represents the median for each group. Boxplot whiskers extend to the smallest and largest nonoutliers. The diamond in each boxplot represents the mean fecundity for each group. Statistically significant groups (P < 0.05) are denoted with an asterisk (*).
Viability effects of mutant markers
To determine whether the viability of the mutant markers affected the ratios of offspring phenotypes, we performed G-tests for goodness of fit within each vial for the following ratios: males versus females, wildtype (wt) flies versus yv flies, and yellow flies versus vermillion flies. The null hypothesis is a 1:1 ratio for all phenotypic classes compared. Significant deviations from expected ratios would indicate that the markers affected the viability of certain phenotype combinations, which would negatively impact recombination rate estimates.
Similar to previous work (Hunter et al. 2016b; Singh 2019), we find small but nonsignificant viability defects associated with these markers. Out of 151 crosses, seven showed significant deviation with regards to the male-female ratio, 11 deviated from expected wildtype to yv ratios, and nine deviated from the expected ratio of yellow to vermillion flies. However, after using the Bonferroni correction for multiple tests, only one of the deviant crosses remained significant (P = 1.14 E-12, G-test). This specific cross had a ratio of 9.8 wildtype flies to yv flies and a recombinant fraction of 0.05. This likely stems from mating contamination and we discarded this cross from further analyses.
Host diet and quantitative PCR
To compare Wolbachia titer between diet treatment groups, we ran qPCR with DNA extracted from frozen female F1 flies collected after egg-laying. Results are shown in Table 2, where gene expression of wsp relative to host genes in Wolbachia-infected flies was calculated using the Livak and Pfaffl methods (Livak and Schmittgen 2001; Pfaffl 2001). Analysis of qPCR data using either the Livak or Pfaffl method produced similar results, where flies fed a sucrose-enriched diet had the highest relative gene expression of wsp compared to control group flies and flies on the yeast-enriched diet (Table 2; Supplementary Figure S2). Since wsp expression is correlated with Wolbachia titer, this corresponds to a 3% increase in Wolbachia titer in flies on the sucrose diet treatment and a 23% decrease in Wolbachia titer in flies on the yeast diet treatment. Relative gene expression of wsp was significantly affected by diet treatment for both the Livak [P = 0.0019, ANOVA (N = 21, df = 2)] and Pfaffl analysis methods [P = 0.005, ANOVA (N = 21, df = 2)]. A Tukey’s multiple comparisons test indicated that Wolbachia titer was significantly reduced in the yeast-enriched diet treatment compared to flies in the control (P = 0.008 Livak, P = 0.018 Pfaffl) and sucrose-enriched diets (P = 0.003 Livak, P = 0.007 Pfaffl).
Table 2.
Relative gene expression of WSP in fly ovaries
P < 0.05, Tukey’s multiple comparisons.
The effect of infection and diet on recombination
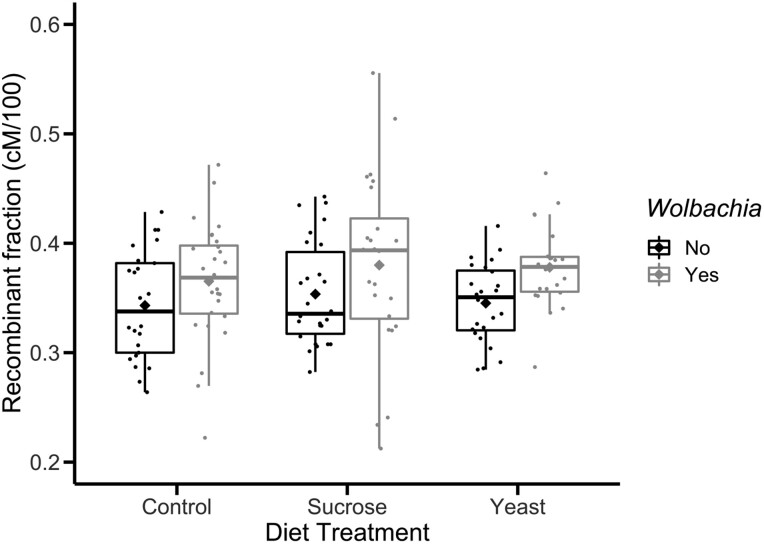
We used logistic regression modeling to identify variables which significantly contributed to differences in mean recombination rate between experimental groups. Results are shown in Table 3, where Wolbachia infection [P = 0.0008, X2 test (N = 150, df = 1)] and experimental block [P = 0.0001, X2 test (N = 150, df = 3)] were significantly associated with differences in recombination rate. We measured recombination rate across the y—v interval (33 cM) of the X chromosome, with an expected recombination rate of 30–35 cM. The effect of Wolbachia infection can be seen clearly in Figure 3, where Wolbachia-infected flies display an average recombination rate of 37.1 cM while uninfected flies display an average rate of 34.7 cM, resulting in an average increase of 2.4 cM in recombination rate across all diet treatments. Neither host diet [P = 0.42, X2 test (N = 150, df = 2)] nor infection by diet interaction effects [P = 0.43, X2 test (N = 150, df = 2)] was significant. Based on the power of our tests, we would have been able to detect a difference of 5.8% or greater between group means, which corresponds to a difference in recombination rate of approximately 2 cM (Supplementary Figure S3). This indicates that the effect of diet or Wolbachia titer, if present, was weaker than the effect of Wolbachia infection alone.
Table 3.
Results of logistic regression modeling on recombinant fraction
| Source | df | L-R χ2 | Prob χ2 |
|---|---|---|---|
| Wolbachia | 1 | 11.315 | 0.0008* |
| Diet | 2 | 1.723 | 0.42 |
| Wolbachia *Diet | 2 | 1.686 | 0.43 |
| Block | 3 | 20.435 | 0.0001* |
P < 0.05, general linear model.
Figure 3.
Recombination rate, reported as recombinant fraction, of experimental groups. The recombinant fraction is the proportion of recombinant progeny compared to the total number of progeny produced for each cross, which is equivalent to cM divided by 100. Wolbachia-infected flies are shown in gray, while uninfected flies are shown in black. Each point corresponds to the recombinant fraction of a single vial. Boxplots present summary statistics, where the top and bottom edges encompass the first to third quartiles and the middle bar represents the median for each group. Boxplot whiskers extend to the smallest and largest nonoutliers. The diamond in each boxplot represents the mean recombination rate for each group. Statistical significance was tested using a general linear model, where Wolbachia and experimental block significantly affected recombination rate (P < 0.05), while diet and Wolbachia-diet interactions were not significant.
Since the sucrose diet treatment did not significantly increase Wolbachia titer relative to the control diet, we performed additional logistic regression modeling on the control and yeast diet treatment groups. Wolbachia infection [P = 0.0001, X2 test (N = 99, df = 1)] and experimental block [P = 0.038, X2 test (N = 99, df = 3)] were significantly associated with recombination rate differences, while host diet [P = 0.26, X2 test (N = 99, df = 1)] and infection by diet interaction effects [P = 0.97, X2 test (N = 99, df = 1)] were not significant (Supplementary Table S2).
We also tested for the effect of Wolbachia infection, titer, and diet on recombination rate variance, which was calculated as absolute residuals. Uninfected flies showed no significant difference in recombination rate variance between diet treatment groups [P = 0.25, Levene’s test (N = 75, df = 2)], and a comparison between uninfected and Wolbachia-infected flies was also nonsignificant [P = 0.11, Levene’s test (N = 150, df = 1)]. However, Wolbachia-infected flies displayed significant differences in variance between diet treatment groups [P = 0.007, Levene’s test (N = 75, df = 2)] and a Tukey’s multiple comparisons test found that infected flies on a sucrose-enriched diet were significantly different from flies on a control (P = 0.03) and yeast-enriched diet (P = 0.003).
Discussion
Effect of Wolbachia infection and diet on recombination
The goal of this experiment was to assess whether Wolbachia-associated plastic recombination in D. melanogaster is continuous or discrete in response to changes in bacterial titer. Wolbachia cannot currently be transgenically modified, making it impossible to use genetic engineering to test for differences in titer. Several other factors have been shown to alter Wolbachia titer, including temperature (Hurst et al. 2000; Moghadam et al. 2018), bacterial genotype (Chrostek and Teixeira 2015), and host diet (Serbus et al. 2015). However, both temperature (e.g., Plough 1917; Grell 1978; Jackson et al. 2015) and bacterial genotype (Singh et al. 2015; Bryant and Newton 2020) also affect recombination rate in D. melanogaster. Host diet can alter Wolbachia titer within fly ovaries, specifically that a yeast-enriched diet decreases titer while a sucrose-enriched diet increases titer (Serbus et al. 2015; Camacho et al. 2017; Christensen et al. 2019). Therefore, we used host diet to manipulate Wolbachia titer and tested the effects of Wolbachia infection, host diet, and Wolbachia titer on recombination rate. We find that Wolbachia infection is associated with a significant increase in recombination rate across the y—v interval on the X chromosome (Table 3). Our data indicate that the Wolbachia-associated increase in recombination is robust with regards to variation in host diet, as Wolbachia-infected flies displayed a higher recombination rate than their uninfected counterparts in each diet treatment (Figure 3). This finding adds to a growing body of literature which supports Wolbachia as an inducer of plastic recombination in D. melanogaster (Hunter et al. 2016b; Singh 2019; Bryant and Newton 2020).
Since we used host diet to manipulate Wolbachia titer, we also assessed whether diet treatments had an impact on plastic recombination. We find that our diet treatments did not significantly affect recombination rate in Wolbachia-uninfected flies (Table 3, Figure 3). The effect of diet on plastic recombination in flies has been severely understudied, where one previous study reported that starvation in larvae was associated with increased recombination rate (Neel 1941). Differences between our study and the previous one may indicate that only severe changes in diet such as starvation are sufficient to induce plastic recombination in D. melanogaster. However, it should also be noted that Neel’s study was carried out using markers on chromosome 3 (1941) while our study assessed recombination on the X chromosome. This may suggest that diet-associated plastic recombination is variable across the genome, as is the case for other conditions associated with plastic recombination such as temperature and Wolbachia infection (Grell 1978; Singh 2019). Outside of the present study, no recent investigations have been made into how starvation or diet affects recombination in flies, highlighting a need for additional research into the role diet may play in plastic recombination. Our study only uses three diet treatments, while a more rigorous investigation of the effects of varying levels of carbohydrates, proteins, and caloric content is needed to definitively assess the effect of diet on plastic recombination in flies.
Effect of Wolbachia infection and diet on fecundity
Though our diet treatments did not affect recombination rate, there was an effect of diet on fecundity. We observed that the average number of offspring per vial was significantly different between diet treatments, with yeast-fed flies displaying the highest average fecundity (Figure 2). The influence of diet on lifespan and fecundity in D. melanogaster has been well-characterized, especially regarding sucrose and yeast content (Drummond-Barbosa and Spradling 2001; Bass et al. 2007). Specifically concerning fecundity, yeast-enriched diets greatly increase female fecundity, while sucrose-enriched diets decrease female fecundity (Bass et al. 2007).
Wolbachia are often associated with increased fecundity in host fly species (Weeks et al. 2007; Mazzetto et al. 2015; Singh 2019), yet we found no significant effect of Wolbachia infection on fecundity. However, this may reflect a strain-specific response, rather than the effect of Wolbachia infection on D. melanogaster as a whole. Differences in fly fecundity depend on Wolbachia genotype (Gruntenko et al. 2019), host genotype (Fry et al. 2004), and bacterial-host interactions (Singh 2019). For instance, the strain used in this experiment, RAL306, was also used in a study which reported an overall effect of Wolbachia infection on fecundity across multiple strains (Singh 2019). However, when examined individually, Wolbachia-infected RAL306 flies had a lower mean fecundity than uninfected RAL306 flies (Singh 2019). This suggests that Wolbachia broadly impacts fecundity, but this effect may vary with host genotype.
Effect of Wolbachia titer on recombination rate
By using host diet to manipulate Wolbachia titer, we tested the effect of titer on the magnitude of Wolbachia-associated plastic recombination. We measured wsp gene expression relative to host genes to measure Wolbachia titer in infected fly ovaries for each diet treatment group. Our results agree with other studies which find that yeast-enriched diets decrease Wolbachia titer and sucrose-enriched diets increase Wolbachia titer in fly ovaries (Table 2; Serbus et al. 2015; Christensen et al. 2019). Our yeast diet treatment had a much stronger effect on Wolbachia titer than our sucrose diet treatment, resulting in a 23% decrease in titer compared to control flies, while flies on a sucrose diet showed a 3% increase in titer compared to control flies.
Combined with the recombination analysis which found no effect of infection by diet interactions (Table 3), these results suggest that changes in Wolbachia titer did not induce a continuous response in plastic recombination. This is, perhaps, not surprising in the case of the sucrose diet treatment, since a small increase in Wolbachia titer may not be enough to significantly affect the host fly’s biological processes. However, reanalysis of the data using only the control and yeast diet treatment groups still finds that Wolbachia infection significantly impacted recombination rate, but Wolbachia titer did not (Supplementary Table S2). So, while the yeast diet treatment significantly decreased Wolbachia titer, this decrease did not lower recombination rate relative to Wolbachia-infected control flies, yet still resulted in an increase in recombination rate relative to uninfected flies. These results provide us with several new pieces of information about Wolbachia-associated plastic recombination, which are discussed in more detail below.
Though Wolbachia titer did not affect the magnitude of recombination, it did influence recombination rate variance. Wolbachia-infected flies fed a sucrose-enriched diet, to promote high Wolbachia titer, had significantly greater variance than Wolbachia-infected flies on either a control or yeast-enriched diet. This finding suggests that increased Wolbachia titer may increase recombination rate variation, rather than increase the average rate of recombination beyond that caused by standard Wolbachia infection. Changes in variance have not previously been reported for other inducers of plastic recombination in D. melanogaster, nor for other Wolbachia-associated host phenotypes, suggesting that this may be a unique feature of Wolbachia-associated plastic recombination. This finding inspires multiple questions for future research, including why low Wolbachia titer did not result in decreased variance and whether this phenomenon is robust in response to other modifiers of Wolbachia titer.
Discrete phenotypic responses
Based on our results, there are several new pieces of information we can conclude about Wolbachia-associated plastic recombination. First, the phenotype must require relatively large changes in bacterial titer to elicit a corresponding change in response. Neither the sucrose diet treatment group nor the yeast diet treatment group significantly affected recombination rate relative to controls. This suggests that changes in titer need to be more dramatic than what we observed (3%–23%) to potentially affect recombination rates. It is possible that these changes in Wolbachia titer caused small, nonsignificant changes in recombination rate; if so, these changes are smaller than the effect of Wolbachia infection alone. This suggests that this phenotype displays discrete rather than continuous responses, where large changes in Wolbachia titer are required to cause the magnitude of plastic recombination to increase. It is also interesting that the yeast diet treatment decreased Wolbachia titer, but not enough to eliminate the Wolbachia-associated plastic recombination phenotype. Logic would suggest that there must be some minimum threshold of bacteria below which plastic recombination would not be induced in flies, but we did not reach that minimum in this experiment. Future work exploring even lower ranges in Wolbachia titer may be able to locate this threshold level.
If Wolbachia-associated plastic recombination displays discrete phenotypic responses, this follows the same trend as male-killing, another Wolbachia-driven trait in Drosophila. In D. bifasciata, Wolbachia infection causes increased mortality of male offspring, leading to modified sex ratios (Hurst et al. 2000). However, Wolbachia titer decreases in flies exposed to elevated temperatures, which causes male mortality rates to decrease and offspring sex ratios to return to normal (Hurst et al. 2000). These findings suggested that this phenotype requires a threshold level of Wolbachia to be expressed and displays discrete responses at low titers and continuous responses at high titers. The same may be true for Wolbachia-associated plastic recombination, where recombination is modified in discrete amounts in infected flies. It may also be true that Wolbachia-associated plastic recombination is continuous and dose-dependent, but only at titer levels more extreme than could be achieved through manipulations in host diet.
Another study looked at the effect of bacterial titer on plastic recombination using different strains of Wolbachia (Bryant and Newton 2020). They find that D. melanogaster infected with the Wolbachia strain wMelPop display a higher recombination rate across the yellow-vermillion interval of the X chromosome when compared to flies infected with a different Wolbachia strain, wMel (Bryant and Newton 2020). The wMelPop strain maintains a much higher titer in flies, which could suggest that the magnitude of recombination corresponded with Wolbachia titer and indicates a dose-dependent relationship. Yet, as noted above, this study cannot separate the effect of titer from Wolbachia strain since two different strains were used in the experiment. Though wMel is the native Wolbachia strain in D. melanogaster, wMelPop is considered pathogenic because it maintains a high titer and significantly decreases host lifespan (Strunov et al. 2013; Chrostek and Teixeira 2015). Other pathogenic bacteria have been shown to plastically increase recombination rate in D. melanogaster (Singh et al. 2015), making it difficult to say whether an increase in recombination rate in wMelPop-infected flies is due to bacterial titer, its pathogenic nature, additional genetic differences between the two bacterial strains, or a combination of factors.
Our data are consistent with the plastic recombinational response to Wolbachia infection being discrete, with even low bacterial titers inducing the response. It is certainly possible that larger changes in Wolbachia titer can induce different magnitudes of plastic recombination in the host. Future experiments which test a large range of Wolbachia titers are necessary to fully understand the nature of titer effects on Wolbachia-associated plastic recombination.
The Drosophila microbiome
It may also be true that Wolbachia-associated plastic recombination is continuous and dose-dependent, but that this effect is masked in our study due to complex interactions between diet, host, and the microbiome. Diet is known to have a significant impact on microbiome composition in several species (Turnbaugh et al. 2008; Read and Holmes 2017; Erkosar et al. 2018). In D. melanogaster, diets rich in either yeast or sucrose caused significant changes in abundance of certain members of the gut microbiome (Chandler et al. 2011). These changes in microbiome composition can have drastic impacts on host biology including hormone production, metabolism, and nutrient acquisition (Leulier et al. 2017). Specific members of the D. melanogaster microbiome have been shown to support larval feeding under starvation conditions (Consuegra et al. 2020), suggesting that diet-induced changes in the microbiome can significantly impact host development. Though our results suggest that diet had no significant effect on recombination rate, as uninfected flies showed similar mean recombination rate for each diet treatment (Figure 3), it is difficult to rule out without directly measuring changes in microbiome composition.
In addition to the gut microbiome, which comes in direct contact with nutritional elements, host diet also affects Wolbachia. One finding our study takes advantage of is that increased sucrose or yeast in D. melanogaster diets can manipulate Wolbachia titer (Serbus et al. 2015). Wolbachia rely on their host to acquire nutrients, so changes in diet can affect microbe behavior and replication and may ultimately impact host biology. Yeast diets have been shown to affect Wolbachia cell physiology, which could influence the growth and behavior of the bacteria (Serbus et al. 2015). However, Wolbachia in flies fed the yeast diet treatment still produced the same increase in recombination rate as control flies, suggesting that changes in cell physiology did not impact plastic recombination. In addition, Wolbachia-infected D. melanogaster have been shown to alter behavior and diet preference, potentially as a strategy to reduce negative effects on lifespan and fecundity (Ponton et al. 2015; Truitt et al. 2018). Though flies may alter their behavior under these conditions, Wolbachia have been shown to have no effect on emergence time or host nutrition under starvation conditions (Harcombe and Hoffmann 2004). We saw no significant differences in fecundity or viability related to infection status in this study, but it is unclear whether Wolbachia-infected flies fed experimental diets experienced changes in behavior that may have impacted recombination estimates.
Finally, the gut microbiome and Wolbachia have been shown to influence one another. Wolbachia infection can alter relative abundances of members of the gut microbiome compared to uninfected flies (Simhadri et al. 2017). Conversely, ingestion of certain species of gut bacteria has been shown to influence Wolbachia abundance (Rudman et al. 2019). Taken together, these findings present a complex web of interactions between host, diet, the gut microbiome, and Wolbachia. Though it is difficult to estimate the impact of these interactions on our results, it remains clear that Wolbachia-associated plastic recombination is robust in response to both measured changes in diet and unmeasured changes in microbiome composition. Future work may focus on studying Wolbachia-only experimental flies, where germ-free flies are reinfected with Wolbachia, to remove potentially confounding variables caused by these complex interactions. However, there is also value in studying these systems in their natural state in order to gain a more complete understanding of native host-microbe associations.
Conclusions
Our current inability to transgenically modify Wolbachia makes it impossible to assess the effect of titer alone on Wolbachia-associated phenotypes. Though differences in titer can be assessed through manipulation of host diet (Serbus et al. 2015), temperature (Hurst et al. 2000; Moghadam et al. 2018), or Wolbachia strain (Chrostek and Teixeira 2015), these methods include confounding variables which make it difficult to definitively assign Wolbachia titer as the causative agent in phenotypes of interest. Our present study controls for host and microbe genotype and finds that Wolbachia-associated plastic recombination is a phenotype with discrete responses, while acknowledging the ways in which changes in host diet may influence that finding. Future advances toward making genetic manipulation possible in Wolbachia would allow the role of titer to be more definitively tested without confounding effects.
Data availability
Fly strains are available upon request. All raw data are available on Dryad: https://doi.org/10.5061/dryad.866t1g1qj.
Supplementary material is available at G3 online.
Supplementary Material
Acknowledgments
The authors would like to thank the Singh lab group for help with fly counting and feedback on the manuscript. They are also grateful for comments from an associate editor and two anonymous reviewers which improved this manuscript.
Funding
This work was supported by funding from the University of Oregon and the National Institute of General Medical Sciences of the N.I.H. (award number T32GM007413-43) to S.L.M.
Conflicts of interest
The authors declare that there is no conflict of interest.
Literature cited
- Abdullah MFF, Borts RH.. 2001. Meiotic recombination frequencies are affected by nutritional states in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 98:14524–14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andronic L. 2012. Viruses as triggers of DNA rearrangements in host plants. Can J Plant Sci. 92:1083–1091. [Google Scholar]
- Bass TM, Grandison RC, Wong R, Martinez P, Partridge L, et al. 2007. Optimization of dietary restriction protocols in Drosophila. J Gerontol A Biol Sci Med Sci. 62:1071–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyaev DK, Borodin PM.. 1982. The influence of stress on variation and its role in evolution. Biologisches Zentralblatt. 101:705–714. [Google Scholar]
- Bridges CB. 1927. The relation of the age of the female to crossing over in the third chromosome of Drosophila melanogaster. J Gen Physiol. 8:689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant KN, Newton ILG.. 2020. The intracellular symbiont Wolbachia pipientis enhances recombination in a dose-dependent manner. Insects. 11:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvitti M, Marini F, Desiderio A, Puggioli A, Moretti R.. 2015. Wolbachia density and cytoplasmic incompatibility in Aedes albopictus: concerns with using artificial Wolbachia infection as a vector suppression tool. PLoS One. 10:e0121813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho M, Oliva M, Serbus LR.. 2017. Dietary saccharides and sweet tastants have differential effects on colonization of Drosophila oocytes by Wolbachia endosymbionts. Biol Open. 6:1074–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler JA, Lang J, Bhatnagar S, Eisen JA, Kopp A.. 2011. Bacterial communities of diverse Drosophila species: ecological context of a host-microbe model system. PLoS Genet. 7:e1002272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiriac GI, Andronic LI, Bujoreanu VV, Marii LI.. 2006. Features of crossing-over in virus-infected tomato. Central Eur J Biol. 1:386–398. [Google Scholar]
- Christensen S, Camacho M, Sharmin Z, Momtaz AJMZ, Perez L, et al. 2019. Quantitative methods for assessing local and bodywide contributions to Wolbachia titer in maternal germline cells of Drosophila. BMC Microbiol. 19:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrostek E, Marialva MSP, Esteves SS, Weinert LA, Martinez J, et al. 2013. Wolbachia variants induce differential protection to viruses in Drosophila melanogaster: a phenotypic and phylogenomic analysis. PLoS Genet. 9:e1003896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrostek E, Teixeira L.. 2015. Mutualism breakdown by amplification of Wolbachia genes. PLoS Biol. 13:e1002065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark ME, Veneti Z, Bourtzis K, Karr TL.. 2002. The distribution and proliferation of the intracellular bacteria Wolbachia during spermatogenesis in Drosophila. Mech Dev. 111:3–15. [DOI] [PubMed] [Google Scholar]
- Consuegra J, Grenier T, Baa-Puyoulet P, Rahioui I, Akherraz H, et al. 2020. Drosophila-associated bacteria differentially shape the nutritional requirements of their host during juvenile growth. PLoS Biol. 18:e3000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa CC, Ballard JWO.. 2016. Wolbachia associations with insects: winning or losing against a master manipulator. Front Ecol Evol. 3:153. [Google Scholar]
- Dobson SL, Bourtzis K, Braig HR, Jones BF, Zhou W, et al. 1999. Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochem Mol Biol. 29:153–160. [DOI] [PubMed] [Google Scholar]
- Drummond-Barbosa D, Spradling AC.. 2001. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev Biol. 231:265–278. [DOI] [PubMed] [Google Scholar]
- Erkosar B, Yashiro E, Zajitschek F, Friberg U, Maklakov AA, et al. 2018. Host diet mediates a negative relationship between abundance and diversity of Drosophila gut microbiota. Ecol Evol. 8:9491–9502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fast EM, Toomey ME, Panaram K, Desjardins D, Kolaczyk ED, et al. 2011. Wolbachia enhance Drosophila stem cell proliferation and target the germline stem cell niche. Science. 334:990–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsman A. 2015. Rethinking phenotypic plasticity and its consequences for individuals, populations, and species. Heredity (Edinb). 115:276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox RJ, Donelson JM, Schunter C, Ravasi T, Gaitán-Espitia JD.. 2019. Beyond buying time: the role of plasticity in phenotypic adaptation to rapid environmental change. Philos Trans R Soc Lond B Biol Sci. 374:20180174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis KE, Lam SY, Harrison BD, Bey AL, Berchowitz LE, et al. 2007. Pollen tetrad-based visual assay for meiotic recombination in Arabidopsis. Proc Natl Acad Sci USA. 104:3913–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry AJ, Palmer MR, Rand DM.. 2004. Variable fitness effects of Wolbachia infection in Drosophila melanogaster. Heredity (Edinb). 93:379–389. [DOI] [PubMed] [Google Scholar]
- Fusco G, Minelli A.. 2010. Phenotypic plasticity in development and evolution: facts and concepts. Philos Trans R Soc Lond B Biol Sci. 365:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloor G, Engels WR.. 1992. Gloor: single-fly DNA preps for PCR. Dros Info Ser. 71:148–149. [Google Scholar]
- Green P, Macleod CJ.. 2016. SIMR: an R package for power analysis of generalized linear mixed models by simulation. Methods Ecol Evol. 7:493–498. [Google Scholar]
- Grell RF. 1978. A comparison of heat and interchromosomal effects on recombination and interference in Drosophila melanogaster. Genetics. 89:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruntenko NE, Karpova EK, Adonyeva NV, Andreenkova OV, Burdina EV, et al. 2019. Drosophila female fertility and juvenile hormone metabolism depends on the type of Wolbachia infection. J Exp Biol. 222:jeb195347. [DOI] [PubMed] [Google Scholar]
- Harcombe W, Hoffmann AA.. 2004. Wolbachia effects in Drosophila melanogaster: in search of fitness benefits. J Invertebr Pathol. 87:45–50. [DOI] [PubMed] [Google Scholar]
- Hayman DL, Parsons PA.. 1962. The effect of temperature, age and an inversion on recombination values and interference in the X-chromosome of Drosophila melanogaster. Genetica. 32:74–88. [DOI] [PubMed] [Google Scholar]
- Hedges LM, Brownlie JC, O’Neill SL, Johnson KN.. 2008. Wolbachia and virus protection in insects. Science. 322:702. [DOI] [PubMed] [Google Scholar]
- Holden PR, Jones P, Brookfield JFY.. 1993. Evidence for a Wolbachia symbiont in Drosophila melanogaster. Genet Res. 62:23–29. [DOI] [PubMed] [Google Scholar]
- Huang W, Massouras A, Inoue Y, Peiffer J, Ràmia M, et al. 2014. Natural variation in genome architecture among 205 Drosophila melanogaster genetic reference panel lines. Genome Res. 24:1193–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter CM, Huang W, Mackay TFC, Singh ND.. 2016b. The genetic architecture of natural variation in recombination rate in Drosophila melanogaster. PLoS Genet. 12:e1005951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter CM, Robinson MC, Aylor DL, Singh ND.. 2016a. Genetic background, maternal age, and interaction effects mediate rates of crossing over in Drosophila melanogaster females. G3 (Bethesda). 6:1409–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst GDD, Johnson AP, Hinrich J, Schulenburg GVD, Fuyama Y.. 2000. Male-killing Wolbachia in Drosophila: a temperature-sensitive trait with a threshold bacterial density. Genetics. 156:699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S, Nielsen DM, Singh ND.. 2015. Increased exposure to acute thermal stress is associated with a non-linear increase in recombination frequency and an independent linear decrease in fitness in Drosophila. BMC Evol Biol. 15:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaprakash A, Hoy MA.. 2000. Long PCR improves Wolbachia DNA amplification: wsp sequences found in 76% of sixty-three arthropod species. Insect Mol Biol. 9:393–405. [DOI] [PubMed] [Google Scholar]
- Kaur R, Shropshire JD, Cross KL, Leigh B, Mansueto AJ, et al. 2021. Living in the endosymbiotic world of Wolbachia: a centennial review. Cell Host Microbe. 29:879–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl KP, Singh ND.. 2018. Experimental evolution across different thermal regimes yields genetic divergence in recombination fraction but no divergence in temperature associated plastic recombination. Evolution. 72:989–999. [DOI] [PubMed] [Google Scholar]
- Leulier F, MacNeil LT, Lee W, Rawls JF, Cani PD, et al. 2017. Integrative physiology: at the crossroads of nutrition, microbiota, animal physiology, and human health. Cell Metab. 25:522–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, SchmittgenTD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif.). 25:402–8. Doi: 10.1006/meth.2001.1262. 11846609. [DOI] [PubMed] [Google Scholar]
- Lloyd A, Morgan C, Franklin FCH, Bomblies K.. 2018. Plasticity of meiotic recombination rates in response to temperature in Arabidopsis. Genetics. 208:1409–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay TFC, Richards S, Stone EA, Barbadilla A, Ayroles JF, et al. 2012. The Drosophila melanogaster genetic reference panel. Nature. 482:173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzetto F, Gonella E, Alma A.. 2015. Wolbachia infection affects female fecundity in Drosophila suzukii. Bull Insectol. 68:153–157. [Google Scholar]
- Modliszewski JL, Wang H, Albright AR, Lewis SM, Bennett AR, et al. 2018. Elevated temperature increases meiotic crossover frequency via the interfering (Type I) pathway in Arabidopsis thaliana. PLoS Genet. 14:e1007384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadam NN, Thorshauge PM, Kristensen TN, de Jonge N, Bahrndorff S, et al. 2018. Strong responses of Drosophila melanogaster microbiota to developmental temperature. Fly (Austin). 12:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel JV. 1941. A relation between larval nutrition and the frequency of crossing over in the third chromosome of Drosophila melanogaster. Genetics. 26:506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietri JE, DeBruhl H, Sullivan W.. 2016. The rich somatic life of Wolbachia. Microbiologyopen. 5:923–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plough HH. 1917. The effect of temperature on crossingover in Drosophila. J Exp Zool. 24:147–189. [Google Scholar]
- Plough HH. 1921. Further studies on the effect of temperature on crossing over. J Exp Zool. 32:187–202. [Google Scholar]
- Ponton F, Wilson K, Holmes A, Raubenheimer D, Robinson KL, et al. 2015. Macronutrients mediate the functional relationship between Drosophila and Wolbachia. Proc Biol Sci. 282:20142029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest NK, Roach DA, Galloway LF.. 2007. Mating-induced recombination in fruit flies. Evolution. 61:160–167. [DOI] [PubMed] [Google Scholar]
- Read MN, Holmes AJ.. 2017. Towards an integrative understanding of diet-host. Gut microbiome interactions . Front Immunol. 8:538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds KT, Thomson LJ, Hoffmann AA.. 2003. The effects of host age, host nuclear background and temperature on phenotypic effects of the virulent Wolbachia strain popcorn in Drosophila melanogaster. Genetics. 164:1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudman SM, Greenblum S, Hughes RC, Rajpurohit S, Kiratli O, et al. 2019. Microbiome composition shapes rapid genomic adaptation of Drosophila melanogaster. Proc Natl Acad Sci USA. 116:20025–20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini R, Singh AK, Dhanapal S, Saeed TH, Hyde GJ, et al. 2017. Brief temperature stress during reproductive stages alters meiotic recombination and somatic mutation rates in the progeny of Arabidopsis. BMC Plant Biol. 17:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiner SM, Levis NA.. 2021. The loss of phenotypic plasticity via natural selection: genetic assimilation. In: Pfennig DW, editor. Phenotypic Plasticity & Evolution: Causes, Consequences, Controversies. 1st ed.Boca Raton, FL: CRC Press. p. 161–177. [Google Scholar]
- Serbus LR, Casper-Lindley C, Landmann F, Sullivan W.. 2008. The genetics and cell biology of Wolbachia-host interactions. Annu Rev Genet. 42:683–707. [DOI] [PubMed] [Google Scholar]
- Serbus LR, White PM, Silva JP, Rabe A, Teixeira L, et al. 2015. The impact of host diet on Wolbachia titer in Drosophila. PLoS Pathog. 11:e1004777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simhadri RK, Fast EM, Guo R, Schultz MJ, Vaisman N, et al. 2017. The gut commensal microbiome of Drosophila melanogaster is modified by the endosymbiont Wolbachia. mSphere. 2:e00287–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh ND. 2019. Wolbachia infection associated with increased recombination in Drosophila. G3 (Bethesda). 9:229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh ND, Criscoe DR, Skolfield S, Kohl KP, Keebaugh ES, et al. 2015. Fruit flies diversify their offspring in response to parasite infection. Science. 349:747–750. [DOI] [PubMed] [Google Scholar]
- Stern C. 1926. An effect of temperature and age on crossing-over in the first chromosome of Drosophila melanogaster. Proc Natl Acad Sci USA. 12:530–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunov A, Kiseleva E, Gottlieb Y.. 2013. Spatial and temporal distribution of pathogenic Wolbachia strain wMelPop in Drosophila melanogaster central nervous system under different temperature conditions. J Invertebr Pathol. 114:22–30. [DOI] [PubMed] [Google Scholar]
- Teixeira L, Ferreira Á, Ashburner M.. 2008. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 6:e1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truitt AM, , KapunM, , KaurR, , MillerWJ. 2018. Wolbachia modifies thermal preference in Drosophila melanogaster. Environmental microbiology. Doi: 10.1111/1462-2920.14347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli M, Hoffmann AA.. 1995. Cytoplasmic incompatibility in Drosophila simulans: dynamics and parameter estimates from natural populations. Genetics. 140:1319–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI.. 2008. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 3:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks AR, Turelli M, Harcombe WR, Reynolds KT, Hoffmann AA.. 2007. From parasite to mutualist: rapid evolution of Wolbachia in natural populations of Drosophila. PLoS Biol. 5:e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren JH, Baldo L, Clark ME.. 2008. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol. 6:741–751. [DOI] [PubMed] [Google Scholar]
- Ye YH, Carrasco AM, Dong Y, Sgrò CM, Mcgraw EA.. 2016. The effect of temperature on Wolbachia-mediated dengue virus blocking in Aedes aegypti. Am J Trop Med Hyg. 94:812–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W, Priest NK.. 2011. Stress-induced recombination and the mechanism of evolvability. Behav Ecol Sociobiol. 65:493–502. [Google Scholar]
- Zilio G, Moesch L, Bovet N, Sarr A, Koella JC.. 2018. The effect of parasite infection on the recombination rate of the mosquito Aedes aegypti. PLoS One. 13:e0203481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zug R, Hammerstein P.. 2012. Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS One. 7:e38544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Fly strains are available upon request. All raw data are available on Dryad: https://doi.org/10.5061/dryad.866t1g1qj.
Supplementary material is available at G3 online.