Abstract
Aims
Platelet inhibition induced by P2Y12 receptor antagonists in patients with ST-elevation myocardial infarction (STEMI) can be affected by concomitant use of opioids. The aim of this trial was to examine the effect of intravenous (iv) acetaminophen compared with iv fentanyl on P2Y12 receptor inhibition in patients with STEMI.
Methods and results
The Opioids aNd crushed Ticagrelor In Myocardial infarction Evaluation (ON-TIME 3) trial randomized 195 STEMI patients who were scheduled to undergo primary percutaneous coronary intervention (PCI) and were pre-treated with crushed ticagrelor to iv acetaminophen (N = 98) or iv fentanyl (N = 97) in the ambulance. The primary endpoint, consisting of the level of platelet reactivity units (PRU) measured immediately after primary PCI, was not significantly different between the study arms [median PRU 104 (IQR 37–215) vs. 175 (63–228), P = 0.18]. However, systemic levels of ticagrelor were significantly higher in the acetaminophen arm at the start of primary PCI [151 ng/mL (32–509) vs. 60 ng/mL (13–206), P = 0.007], immediately after primary PCI [326 ng/mL (94–791) vs. 115 ng/mL (38–326), P = 0.002], and at 1 h after primary PCI [488 ng/mL (281–974) vs. 372 ng/mL (95–635), P = 0.002]. Acetaminophen resulted in the same extent of pain relief when compared with fentanyl [reduction of 3 points on 10-step-pain scale before primary PCI (IQR 1–5)] in both study arms (P = 0.67) and immediately after PCI [reduction of 5 points (3–7); P = 0.96].
Conclusion
The iv acetaminophen in comparison with iv fentanyl was not associated with significantly lower platelet reactivity in STEMI patients but resulted in significantly higher ticagrelor plasma levels and was effective in pain relief.
Keywords: ST-elevation myocardial infarction, Acetaminophen, Fentanyl, Acetaminophen, Primary coronary intervention, Ticagrelor
Introduction
Optimal platelet inhibition is one of the most important goals in the acute treatment of ST-elevation myocardial infarction (STEMI) patients.1 Opioids are widely used in daily practice but delay the intestinal drug absorption of P2Y12 inhibitors.2 Moreover, nausea and vomiting are more frequently seen in patients receiving opioids,2–4 which further reduce the uptake of oral platelet inhibitors. Also, STEMI patients who undergo primary percutaneous coronary intervention (PCI) and receive morphine more often have high platelet reactivity, which is associated with ischaemic events like stent thrombosis.5,6 Opioids, like morphine and fentanyl, are still recommended in the European and American guidelines on the management of STEMI,7,8 but their class of recommendation has been reduced from Classes I to IIa (level of evidence C) in the European guideline, as increasing knowledge about the adverse effects of opioids became available.
Other analgesics may be an alternative for opioid use in STEMI patients. While non-steroidal anti-inflammatory drugs are known to increase cardiovascular events,9–11 acetaminophen (paracetamol) might be a suitable alternative. Intravenously (iv) administered acetaminophen is more quickly effective than its oral form.12 However, so far no evidence exists about acetaminophen and its effects on platelet inhibition in STEMI patients. Moreover, the effectiveness of opioids and acetaminophen on pain reduction in STEMI patients is unclear.
Alternative routes of administration of P2Y12 receptor inhibitors, like pre-hospital administration of oral platelet inhibitors, crushed or chewed ticagrelor, and intravenous administration of platelet inhibitors,13–15 have been investigated to achieve earlier platelet inhibition. Crushed ticagrelor administration in STEMI patients provided faster platelet inhibition compared with standard integral tablets.15
The Opioids aNd crushed Ticagrelor In Myocardial infarction Evaluation (ON-TIME 3) trial searched for effective pain relief and fast and optimal platelet inhibition by investigating an alternative analgesic, iv acetaminophen, when compared with iv fentanyl in STEMI patients with ongoing chest pain who all received crushed ticagrelor in a pre-hospital setting.
Methods
Study design and patients
The ON-TIME 3 trial (NCT03400267) was an investigator-initiated, prospective, open-label, trial, of which the primary objective was to assess the level of platelet inhibition after primary PCI in STEMI patients who were randomized in the ambulance to either treatment with iv acetaminophen or iv fentanyl for the relief of chest pain. The study was performed in collaboration with the ambulance services of two hospitals: Ambulance service IJsselland and Witte Kruis connected to Isala Hospital Zwolle (The Netherlands) and GGD Zuid Limburg, connected to Zuyderland Medical Centre Heerlen (The Netherlands).
This study was conducted in accordance with the principles of the Declaration of Helsinki, the Medicinal Research Involving Human Subjects Act (Dutch abbreviation: WMO), and Good Clinical Practice. The trial protocol and informed consent was approved by the local ethics committee of both participating centres.
The trial design and rationale of this study have been published previously.16 In brief, STEMI patients (defined as on-going chest pain >30 min and <12 h duration and ST-segment elevation >0.1 mV in at least two contiguous leads) as diagnosed by the paramedic team with a pain score of 4 or higher at a 10-step numeric rating pain score, were included. After verbal informed consent patients were randomized in a 1:1 fashion to either iv acetaminophen or iv fentanyl using an app-based randomization. Written informed consent was obtained during hospitalization.
Study procedures
All patients underwent coronary angiography and primary PCI when indicated. All patients were pre-loaded in the ambulance with unfractionated heparin 5000 IU and intravenous aspirin 500 mg according to local standard of care and 180 mg crushed oral ticagrelor. Ticagrelor was crushed using a pill tool crusher at the patient’s site by the paramedic team in the ambulance. Data on intensity of pain and data on platelet inhibition, including pharmacokinetics and pharmacodynamics, were collected before (T1) and immediately after primary PCI or 1-h post-angiography (T2) at the catheterization laboratory, and at 1-h post-primary PCI or 2 h post-angiography (T3) and 6 h post-primary PCI or 7 h post-angiography (T4) at the coronary care unit. As only a minority of our patients underwent coronary angiography only, we will refer to the time points with regard to PCI in this article.
Pharmacodynamic effects were assessed by a VerifyNow P2Y12 point of care test (Accriva Diagnostics, San Diego, USA, distributed by Werfen, Breda, The Netherlands) for measurement of platelet reactivity units (PRU) of blood samples collected in sodium citrate (3.2%) tubes. Pharmacokinetic effects were determined by the concentration of ticagrelor and its active metabolite, AR-C124910XX, using liquid chromatography-mass spectrometry at the clinical pharmacy laboratory in Zwolle. A 30-day post-randomization follow-up was performed by telephone interview.
Study endpoints
The primary endpoint of the study was the level of PRU measured immediately post-primary PCI (T2). For the assessment of the primary endpoint, blood was obtained just before sheath removal in case of a primary PCI. Secondary endpoints included pain reduction on a 10-step numeric rating pain scale between the level of pain at arrival of the ambulance at the patient site and the level of pain before or immediately post-primary PCI, the level of PRU at other time points, high on-treatment platelet reactivity (HPR) defined as PRU >208 immediately post-primary PCI,5 the concentrations of ticagrelor, its active metabolite and the cumulative concentrations of ticagrelor and its active metabolite at all time points.
Statistical analysis
The sample-size calculation was based on a superiority assumption of the primary endpoint of PRU. Since the effects of acetaminophen on PRU were unknown and comparable studies were lacking, an assumption of the sample size was necessary. We partly based our sample size calculation on data from the Influence of Morphine on Pharmacodynamics and Pharmacokinetics of Ticagrelor in patients With Acute Myocardial Infarction (IMPRESSION) trial2 and Platelet Aggregation With Ticagrelor Inhibition and Fentanyl (PACIFY) trial.17 Assuming the presence of a 60 PRU mean difference (with a standard deviation of 120 PRU) between the two arms immediately after primary PCI, and 20% rate of invalid results due to haemolysis or technical problems, 200 patients were needed with 90% power and a two-sided alpha of 0.05.
The main statistical analysis was based on an intention-to-treat population, but an as-treated population analysis was also performed. Categorical variables were expressed as frequencies and percentages. Comparisons between categorical variables were performed with a Pearson χ2 or Fisher’s exact test in case the proportion of cells with an expected count of <5 exceeded 20%. Continuous variables were presented as mean ± SD or median with interquartile range (IQR), depending on the data distribution which was determined by the Kolmogorov–Smirnov test and Shapiro–Wilk test. The Student’s t test and Mann–Whitney U test were used to compare continuous variables, when appropriate. The Spearman’s correlation test was used to calculate the correlation between PRU and the ticagrelor concentration. As a sensitivity analysis, multiple imputation was used for missing values of the PRU variable. The variables selected as predictors for imputation were age, sex, vomiting, use of anti-emetics in ambulance, renal function, Thrombolysis In Myocardial Infarction (TIMI) flow pre-PCI, TIMI flow post-PCI, myocardial blush grade, and ST-resolution 1 h after primary PCI. In addition, inter- and extrapolation for missing values was used as a second sensitivity analysis. Moreover, the difference (delta) and ratio of PRU between T1 and T2, and T2 and T3 were calculated. Also, PRU-values and ticagrelor concentration levels were compared within a Subgroup of patients without vomiting. Exploratory endpoints were underpowered and therefore were only described. A two-sided alpha <0.05 was considered statistically significant. Statistical analyses were performed with SPSS version 26.0 and R version 1.1.456.
Results
Patient characteristics
From February 2018 till October 2019, a total of 210 STEMI patients were enrolled. Fifteen patients (7%) were excluded due to protocol deviations or withdrawn informed consent (Figure 1). Thus, a total of 195 patients remained eligible for analysis. Demographic, clinical, and procedural characteristics were balanced between the acetaminophen and fentanyl arm (Table 1).
Figure 1.
Patient flow diagram. iv, intravenous.
Table 1.
Baseline and angiographic characteristics
| Acetaminophen, N = 98 | Fentanyl, N = 97 | P-value | |
|---|---|---|---|
| General baseline characteristics | |||
| Age, mean (SD) | 62.7 (12.0) | 64.9 (10.6) | 0.17 |
| Female (%) | 25 (25.5) | 33 (34.0) | 0.25 |
| Diabetes mellitus (%) | 19 (19.4) | 15 (15.5) | 0.59 |
| Hypertension (%) | 37 (37.8) | 40 (41.2) | 0.73 |
| Hypercholesterolaemia (%) | 31 (31.6) | 26 (26.8) | 0.56 |
| Smoking | 0.89 | ||
| Non-smoker (%) | 36 (37.8) | 33 (35.9) | |
| In the past (%) | 19 (20) | 17 (18.5) | |
| Current (%) | 40 (42.1) | 42 (45.7) | |
| Family history of CAD (%) | 43 (43.8) | 49 (50.5) | 0.36 |
| Peripheral artery disease (%) | 2 (2.0) | 2 (2.1) | 1.00 |
| Prior myocardial infarction (%) | 10 (10.2) | 9 (9.3) | 1.00 |
| Prior PCI (%) | 12 (12.2) | 10 (10.3) | 0.82 |
| Prior CABG (%) | 1 (1) | 0 (0) | 1.00 |
| Renal function based on creatinine (median [IQR] | 81 (70–92) | 81 (69–97) | 0.50 |
| Killip class I (%) | 93 (94.9) | 96 (99.0) | 0.21 |
| Vomiting (%) | 3 (3.1) | 14 (14.4) | 0.01 |
| Time from randomization to T1 (min), median (IQR) | 65 (53–78) | 65 (52–79) | 0.48 |
| Time from randomization to T2 (min), median (IQR) | 102 (84–118) | 101 (84–122) | 0.73 |
| Time from randomization to T3 (min), median (IQR) | 185 (163–204) | 176 (146–196) | 0.26 |
| Time from randomization to T4 (min), median (IQR) | 490 (456–514) | 486 (453–520) | 0.92 |
| Angiographic characteristics | |||
| Radial access site (%) | 89 (90.8) | 93 (95.9) | 0.26 |
| Type of procedure | 0.48 | ||
| CAG only (%) | 12 (12.2) | 7 (7.2) | |
| POBA only (%) | 5 (5.1) | 6 (6.2) | |
| Primary PCI (%) | 81 (82.7) | 84 (86.6) | |
| Culprit | 0.58 | ||
| LAD (%) | 32 (32.7) | 32 (33.0) | |
| RCA (%) | 49 (50.0) | 50 (51.5) | |
| RCx (%) | 9 (9.2) | 12 (12.4) | |
| LM (%) | 2 (2.0) | 0 (0) | |
| Arterial graft (%) | 0 (0) | 0 (0) | |
| Venous graft (%) | 0 (0) | 0 (0) | |
| Other/no culprit (%) | 6 (6.1) | 3 (3.1) | |
| Thrombus aspiration (%) | 19 (19.4) | 21 (21.6) | 0.83 |
| TIMI flow grade pre-PCI (%) | 0.35 | ||
| 0 | 48 (55.8) | 45 (50.0) | |
| 1 | 6 (7.0) | 12 (13.3) | |
| 2 | 13 (15.1) | 18 (20.0) | |
| 3 | 19 (22.1) | 15 (16.7) | |
| Glycoprotein IIb/IIIa inhibitor (%) | 0.88 | ||
| None | 80 (81.6) | 79 (81.4) | |
| 6 h infusion | 11 (11.1) | 12 (12.4) | |
| 12 h infusion | 4 (4.1) | 5 (5.2) | |
| 24 h infusion | 3 (3.1) | 1 (1.0) |
CABG, coronary artery bypass grafting; CAD, coronary artery disease; IQR, interquartile range; LAD, left anterior descending artery; PCI, percutaneous coronary intervention; POBA, plain old balloon angiography; RCA, right coronary artery; RCx, ramus circumflex artery; SD, standard deviation, T1, before primary PCI; T2, immediately after primary PCI; T3, 1 h after primary PCI; T4, 6 h after primary PCI; TIMI, thrombolysis in myocardial infarction.
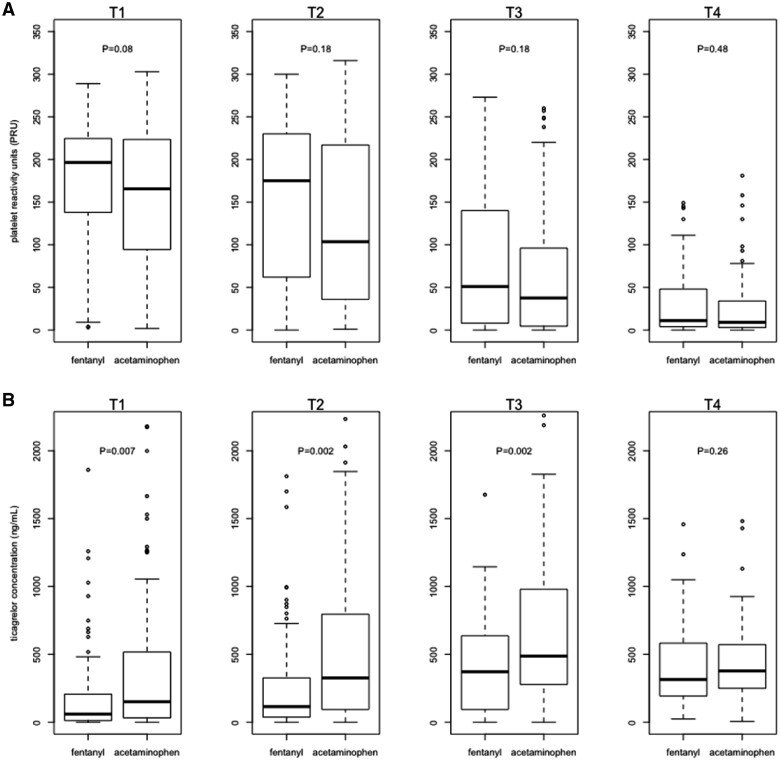
Figure 2.
(A) The platelet reactivity units are shown at different time points for fentanyl intravenously and acetaminophen intravenously. No statistically significant differences between both study arms were seen in platelet reactivity unit at T1 (pre-primary percutaneous coronary intervention; P = 0.08), at T2 (immediately after primary percutaneous coronary intervention; P = 0.18), at T3 (1 h after primary percutaneous coronary intervention; P = 0.18), and at T4 (6 h after primary percutaneous coronary intervention; P = 0.48). (B) The ticagrelor concentrations are shown at different time points for fentanyl iv and acetaminophen iv. Significant differences were seen at T1 (P = 0.007), T2 (P = 0.002), and T3 (P = 0.002), but not for T4 (P = 0.26).
The mean age was 64 years, 29.7% of the patients were female, 17.4% had diabetes mellitus, and the median pain score at randomization was 7 (IQR 6–8; out of a 10-step pain score). Vomiting occurred significantly more often in the fentanyl arm (3.1% in the acetaminophen arm vs. 14.4% in the fentanyl arm, P = 0.01).
The median times from arrival of the ambulance at the patient’s site to arrival at the cathlab [65 (IQR 53–78) vs. 65 min (IQR 52–79), P = 0.48] and end of primary PCI [102 (IQR 84–118) vs. 101 min (IQR 84–122), P = 0.73] were similar in both study arms. Also, TIMI flow grades pre-primary PCI (TIMI flow grade 3: 22.1% vs. 16.7%, P = 0.35), the use of thrombus aspiration (19.4% vs. 21.6%, P = 0.83) and use of glycoprotein IIb/IIIa inhibitors (GPI; 18.3% vs. 18.6%, P = 0.88) during primary PCI were balanced between both arms.
Pharmacodynamics and pharmocokinetics
Table 2 shows the outcomes of the most important primary and secondary outcomes. The primary endpoint, consisting of the PRU-value immediately after primary PCI, was available in 84% of patients. Reasons for missing values were GPI use due to interaction with the VerifyNow assay and logistic measurement errors. The primary endpoint was not significantly different between the study arms [median 104 (IQR 37–215) vs. 175 (IQR 63–228), P = 0.18], Hodges–Lehmann estimator 20 (95% confidence interval −6.0 to 55.0). No significant differences in HPR measured immediately after primary PCI were observed between the arms (26.7% vs. 37.2%, P = 0.21). These effects were also seen in the as-treated population analysis. Sensitivity analyses were performed for the primary endpoint using multiple imputation for missing values, using inter- and extrapolation (Supplementary material online, Table S1) and using the difference (delta) and ratio in PRU between T1 and T2 or T2 and T3. Multiple imputation showed a pooled mean PRU at T2 of 126 (SE 9.7) in the acetaminophen arm and 152 (SE 10.2) in the fentanyl arm (P = 0.07). Inter- and extrapolation showed a median PRU at T2 of 117 (IQR 46–192) in the acetaminophen arm and median PRU of 172 (IQR 96–217) in the fentanyl arm (P = 0.01). The delta and ratio of PRU at T1 and T2 were not significantly different between both arms (P = 0.31 for delta and P = 0.81 for ratio of T1 and T2; P = 0.87 for delta and P = 0.80 for ratio of T2 and T3; Supplementary material online, Table S2).
Table 2.
Primary and secondary outcomes on pharmacodynamics and -kinetics
| Main outcomes | |||
|---|---|---|---|
| PRU at T2, median (IQR) | 104 (37–215); n = 86 | 175 (63–228); n = 78 | 0.18 |
| PRU, median (IQR) | |||
| T1 | 166 (95–223); n = 92 | 197 (138–224); n = 88 | 0.08 |
| T3 | 38 (5–92); n = 76 | 51 (8–136); n = 72 | 0.18 |
| T4 | 9 (3–34); n = 77 | 11 (4–48); n = 73 | 0.48 |
| High platelet reactivity at T2 (%) | 23 (26.7); n = 86 | 29 (37.2); n = 78 | 0.21 |
| Ticagrelor concentration, median (IQR) | |||
| T1 | 151 (32–509); n = 94 | 60 (13–206); n = 96 | 0.007 |
| T2 | 326 (94–791); n = 94 | 115 (38–326); n = 95 | 0.002 |
| T3 | 488 (281–974); n = 86 | 372 (95–635); n = 90 | 0.002 |
| T4 | 378 (252–571); n = 90 | 315 (194–583); n = 91 | 0.26 |
| Ticagrelor active metabolite concentration, median (IQR) | |||
| T1 | 10 (0–47); n = 93 | 4 (0–20); n = 96 | 0.04 |
| T2 | 35 (4–98); n = 93 | 14 (0–54); n = 95 | 0.03 |
| T3 | 114 (41–196); n = 86 | 74 (13–120); n = 90 | 0.005 |
| T4 | 102 (74–157); n = 90 | 97 (50–162); n = 91 | 0.32 |
| Ticagrelor concentration total, median (IQR) | |||
| T1 | 166 (33–587) | 63 (13–222) | 0.007 |
| T2 | 366 (101–918) | 121 (39–391) | 0.003 |
| T3 | 559 (339–1175) | 465 (108–800) | 0.002 |
| T4 | 510 (338–736) | 445 (258–731) | 0.23 |
IQR, interquartile range; PRU, platelet reactivity units; T1, before primary PCI; T2, immediately after primary PCI; T3, 1 h after primary PCI; T4, 6 h after primary PCI.
The ticagrelor concentration at T2 was available in 97% of patients. The ticagrelor concentration was higher in the acetaminophen arm at the start of primary PCI [151 (IQR 32–509) vs. 60 ng/mL (IQR 13–206), P = 0.007], immediately after primary PCI [326 (IQR 94–791) vs. 115 ng/mL (IQR 38–326), P = 0.002] and at 1 h after primary PCI [488 (IQR 281–974) vs. 372 ng/mL (IQR 95–635), P = 0.002]. Similar significant results were seen up to 1 h after primary PCI for the active metabolite concentration and the cumulative concentration of ticagrelor and its active metabolite in favour of acetaminophen (Table 2). These results were consistent in the as-treated population analysis.
Moreover, the results of PRU and ticagrelor concentration measurements were also consistent in patients who did not vomit (Supplementary material online, Table S3).
Relationship pharmacodynamic and pharmacokinetic measurements
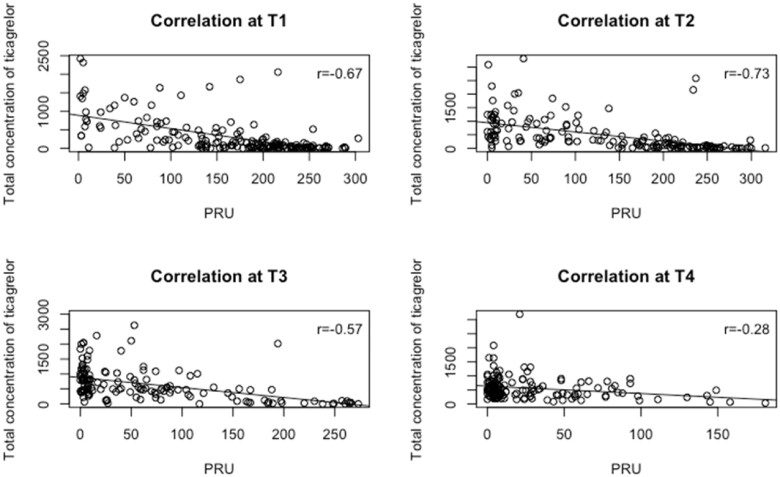
Platelet reactivity unit values were significantly related to ticagrelor concentrations and its active metabolite at all 4 time points (T1: r = −0.67, T2: r = −0.73, T3: r = −0.57, and T4: r = −0.28, Figure 3).
Figure 3.
Correlation figures between the median platelet reactivity units and ticagrelor concentrations at different time points. T1, before primary PCI; T2, immediately after primary PCI; T3, 1 h after primary PCI; T4, 6 h after primary PCI.
Analgesic effects
Acetaminophen resulted in the same extent of pain relief when compared with fentanyl between the moment of randomization and start of primary PCI [reduction of 3 points (IQR 1–5), P = 0.67] and moment of randomization and end of primary PCI [reduction of 5 points (IQR 3–7), P = 0.96] (Table 3). Stratification for TIMI flow grade 0 pre-PCI and TIMI flow grade 1 or higher did not show statistically significant differences in pain reduction between the both arms (Supplementary material online, Table S4). The results on analgesic effects in the intention-to-treat population were comparable to the results in the as-treated population.
Table 3.
Effects on pain reduction
| Pain reduction | Acetaminophen, N = 98 | Fentanyl, N = 97 | P-Value |
|---|---|---|---|
| Pain score at randomization, median (IQR) | 7 (6–8) | 7 (6–8) | 0.45 |
| Pain reduction at T1, median (IQR) | 3 (1–5); n = 95 | 3 (1–5); n = 97 | 0.67 |
| Pain reduction at T2, median (IQR) | 5 (3–7); n = 94 | 5 (3–7); n = 94 | 0.96 |
IQR, interquartile range; T1, before primary PCI; T2, immediately after primary PCI.
Exploratory endpoints
Analysis of the exploratory endpoints showed four MACE in the fentanyl arm, which included one stent thrombosis (15 min post-primary PCI), two re-infarctions (10 h and 6 days post-primary PCI), and one BARC type 3 bleeding event (7 days post-primary PCI), and two MACE in the acetaminophen arm, which included one re-infarction (3 h post-primary PCI) and one bleeding BARC type 3 event (5 days post-primary PCI).
Discussion
The results of this ON-TIME 3 trial showed that iv acetaminophen, compared with iv fentanyl, did not result in significantly lower platelet reactivity but was associated with higher plasma concentrations of crushed ticagrelor and resulted in effective pain relief. These findings overall support the use of iv acetaminophen for pain relief in STEMI patients and suggest the negative impact of fentanyl, and possibly other opioids, on platelet inhibition after pre-loading with crushed ticagrelor and aspirin in the ambulance.
Although opioids are recommended in international guidelines to reduce pain-associated sympathetic activation (which increases vasoconstriction, blood pressure, and heart rate3), their pain-relieving effects in STEMI patients remained unclear. Due to reduced gastric perfusion and impaired gastric emptying, even the absorption of the more potent P2Y12 receptor inhibitors (ticagrelor and prasugrel) is delayed in STEMI patients18 and can be further reduced by using opioids.2 Moreover, nausea and vomiting are more frequently seen in patients receiving opioids, as these are known side effects of opioids.3,4 These adverse effects associated with opioid use formed the main incentive for the ON-TIME 3 trial to search for an alternative analgesic in STEMI patients. This trial showed that patients experienced effective pain relief with both iv acetaminophen and iv fentanyl. Also, vomiting was more frequently observed in patients receiving fentanyl in this trial. However, the results on PRU and ticagrelor concentrations in patients who did not vomit were consistent with the results of the total study population, which may suggest that the observed lower plasma concentrations of ticagrelor in fentanyl treated patients was not solely related to vomiting.
Previous studies also emphasized the adverse effects of opioids. Morphine use was analysed in the MORPHINE-ATLANTIC trial, in which ticagrelor treated STEMI patients with concomitant use of morphine were associated with increased GPI use, less TIMI 3 flow pre-PCI and more often TIMI major bleeding.19 The PRIVATE-ATLANTIC trial showed that morphine administration was associated with delayed onset of platelet inhibition.20 Also, ST-resolution before primary PCI was significantly improved in patients not receiving morphine in the main analysis of the Ambulance for New ST elevation myocardial Infarction to open the Coronary artery (ATLANTIC) trial.21 Moreover, in a registry of 300 STEMI patients, morphine use was associated with less spontaneous ST-resolution, less TIMI 2 or 3 flow and higher peak troponin levels.22 However, an analysis of STEMI patients from the large French Registry of Acute ST-elevation and non-ST-elevation Myocardial Infarction (FAST-MI) did not find an association between pre-hospital morphine use and in-hospital outcome and 1-year mortality.23
The IMPRESSION trial was a randomized double-blind trial comparing morphine to placebo in 70 STEMI and NSTEMI patients treated with in-hospital ticagrelor and showed that morphine delays and attenuates ticagrelor absorption and platelet inhibition.2 However, in the IMPRESSION trial, morphine was given before the loading dose of oral ticagrelor. Furthermore, the study population consisted of a heterogenous group of STEMI and NSTEMI patients and the time interval between morphine and ticagrelor loading dose differed from the interval between placebo and ticagrelor loading dose. Another trial, the PACIFY trial,17 compared fentanyl to placebo in patients undergoing elective coronary angiography and found lower ticagrelor concentrations and delayed platelet inhibition in patients receiving fentanyl. PRU-values and HPR rates of fentanyl treated patients in our trial were comparable with the results of the IMPRESSION2 and PACIFY trial.17
These two trials, however, compared morphine or fentanyl to placebo. Our trial is unique since it compares an opioid drug to a non-opioid analgesic drug for pain relief in STEMI patients in a pre-hospital setting and confirms the adverse effects of fentanyl on the absorption of ticagrelor, even when tablets were crushed, and its delayed and reduced effects on platelet inhibition.
Platelet function testing may provide useful prognostic data for cardiovascular risk prediction and clinical decision making after primary PCI.24 High platelet reactivity is associated with ischaemic events like stent thrombosis5,6 and should be prevented. A number of strategies have been investigated to accelerate the onset of action of P2Y12 inhibitors with various success.25–27 Indeed, the use of intravenous antiplatelet therapies, including cangrelor and GPI, have shown to bridge the gap in platelet inhibition in STEMI patients,13,28 though are associated with a higher rate of bleeding. The ON-TIME 3 trial was a study in which randomization and administration of the study medication occurred in the pre-hospital phase. Therefore, its results are applicable to our daily practice. Moreover, this trial showed that crushing of ticagrelor was feasible by the paramedic team, but did not prevent reduced absorption of the drug by the opioid analgesic.
Future research might focus on optimizing antiplatelet therapy by studying the effect of different strategies with crushed or intravenous platelet inhibitors on angiographic, electrographic, and clinical outcomes. The FABOLUS-FASTER study and COMPARE-CRUSH trial may provide us with more insights on this topic.29,30 Moreover, our study showed no significant differences in TIMI flow grade pre-PCI between the acetaminophen and fentanyl arm, but our study lacks power to analyse such an effect. Future research might focus on the effect of acetaminophen and fentanyl on angiographic and clinical endpoints in STEMI patients, since large randomized trials studying these effects are currently lacking.
Limitations
Several limitations of our study need to be acknowledged. First, the administration of the study medication was open-label and not blinded. Second, patients treated with fentanyl had numerically higher PRU-values up to 1-h post-primary PCI compared with patients treated with acetaminophen, however this difference was not statistically significant (P = 0.18). This result might be related to low PRU-values achieved by crushed ticagrelor in both arms, which requires more statistical power to detect differences, and to the availability of the primary endpoint in 84% of patients. Conversely, results of ticagrelor concentrations were available in 97% of patients and showed significant differences in favour of the acetaminophen group. There was a strong and significant relationship between the PRU values and ticagrelor concentration measurements and these results as well as the results of the sensitivity analysis using inter- and extrapolation, which showed a significant difference in PRU-value immediately after primary PCI in favour of acetaminophen, support the principal finding of the study.
Furthermore, our trial data cannot be extrapolated to patients in cardiogenic shock and/or requiring a nasogastric tube. These patients, although theoretically attractive for the use of crushed P2Y12 receptor inhibitors, were excluded from our study as they would have introduced heterogeneity to our study population and potentially interfered with our pharmacodynamic and -kinetic data. Moreover, measurements of PRU in patients who received GPI failed due to interference with the VerifyNow assay. However, GPI use was balanced between both study arms and therefore it was less likely to affect our results.
Conclusion
Intravenous acetaminophen, compared with iv fentanyl, was not associated with lower platelet reactivity but was associated with significantly higher concentrations of ticagrelor and the active metabolite up to 1 h after primary PCI and resulted in effective pain relief.
Supplementary material
Supplementary material is available at European Heart Journal – Cardiovascular Pharmacotherapy online.
Supplementary Material
Acknowledgements
The authors would like to explicitly thank all participating ambulance services, Ambulancedienst IJsselland, RAV Witte Kruis and GGD Zuid-Limburg, and the participating departments of Isala Hospital and Zuyderland Medical Center for their contribution to this trial.
Contributor Information
Renicus S Hermanides, Department of Cardiology, Isala Hospital, dr. van Heesweg 2, 8025 AB Zwolle, The Netherlands.
Jan Paul Ottervanger, Department of Cardiology, Isala Hospital, dr. van Heesweg 2, 8025 AB Zwolle, The Netherlands.
Rudolf Tolsma, Ambulancedienst IJsselland, Voltastraat 3-A, 8013 PM Zwolle, The Netherlands.
Antony van Beurden, GGD Zuid-Limburg, Heerlen, The Netherlands.
Robbert Jan Slingerland, Department of Clinical Chemistry, Isala, Zwolle, The Netherlands.
Peter G J ter Horst, Department of Clinical Pharmacy, Isala, Zwolle, The Netherlands.
A T Marcel Gosselink, Department of Cardiology, Isala Hospital, dr. van Heesweg 2, 8025 AB Zwolle, The Netherlands.
Jan-Henk E Dambrink, Department of Cardiology, Isala Hospital, dr. van Heesweg 2, 8025 AB Zwolle, The Netherlands.
Maarten A H van Leeuwen, Department of Cardiology, Isala Hospital, dr. van Heesweg 2, 8025 AB Zwolle, The Netherlands.
Vincent Roolvink, Department of Cardiology, Isala Hospital, dr. van Heesweg 2, 8025 AB Zwolle, The Netherlands.
Elvin Kedhi, Department of Cardiology, AZ Sint-Jan, Brugge, Belgium.
Olaf H Klungel, Department of Pharmacoepidemiology, University of Utrecht, Utrecht, The Netherlands.
Svetlana V Belitser, Department of Pharmacoepidemiology, University of Utrecht, Utrecht, The Netherlands.
Dominick J Angiolillo, Division of Cardiology, University of Florida College of Medicine, Jacksonville, FL, USA.
Tobias Pustjens, Department of Cardiology, Maastricht University Medical Centre, Maastricht, The Netherlands; Department of Cardiology, Zuyderland Medical Centre, Heerlen, The Netherlands.
Saman Rasoul, Department of Cardiology, Maastricht University Medical Centre, Maastricht, The Netherlands; Department of Cardiology, Zuyderland Medical Centre, Heerlen, The Netherlands.
Ben Gho, Department of Cardiology, Zuyderland Medical Centre, Heerlen, The Netherlands.
Mera Stein, Department of Cardiology, Zuyderland Medical Centre, Heerlen, The Netherlands.
Lex Ruiters, Department of Cardiology, Zuyderland Medical Centre, Heerlen, The Netherlands.
Arnoud W J van ‘t Hof, Department of Cardiology, Isala Hospital, dr. van Heesweg 2, 8025 AB Zwolle, The Netherlands; Department of Cardiology, Maastricht University Medical Centre, Maastricht, The Netherlands; Department of Cardiology, Zuyderland Medical Centre, Heerlen, The Netherlands.
Funding
The study was conducted with an unrestricted grant from AstraZeneca and an unrestricted grant from the Isala Academy. AstraZeneca and the Isala Academy reviewed the study protocol and manuscript and were allowed to make suggestions. However, the final content was determined by the authors.
Conflict of interest: A.W.J.V.H. reports institutional fees and non-financial support from AstraZeneca as well as grants from Medtronic. D.J.A. reports receiving grant support, consulting fees, and honoraria from Amgen, Aralez, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Daiichi Sankyo, Eli Lilly, Janssen, Merck, and Sanofi, consulting fees and honoraria from Haemonetics, PhaseBio, PLx Pharma, Pfizer, and the Medicines Company, grant support and fees for review activities from CeloNova, fees for review activities from St. Jude Medical, and grant support from CSL Behring, Eisai, Gilead, Idorsia Pharmaceuticals, Matsutani Chemical Industry, Novartis, Osprey Medical, RenalGuard Solutions, and the Scott R. MacKenzie Foundation.
References
- 1. Franchi F, Rollini F, Angiolillo DJ. Antithrombotic therapy for patients with STEMI undergoing primary PCI. Nat Rev Cardiol 2017;14:361–379. [DOI] [PubMed] [Google Scholar]
- 2. Kubica J, Adamski P, Ostrowska M, Sikora J, Kubica JM, Sroka WD, Stankowska K, Buszko K, Navarese EP, Jilma B, Siller-Matula JM, Marszałł MP, Rość D, Koziński M. Morphine delays and attenuates ticagrelor exposure and action in patients with myocardial infarction: the randomized, double-blind, placebo-controlled IMPRESSION trial. Eur Heart J 2016;37:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McCarthy CP, Mullins KV, Sidhu SS, Schulman SP, McEvoy JW. The on- and off-target effects of morphine in acute coronary syndrome: a narrative review. Am Heart J 2016;176:114–121. [DOI] [PubMed] [Google Scholar]
- 4. Parodi G, Bellandi B, Xanthopoulou I, Capranzano P, Capodanno D, Valenti R, Stavrou K, Migliorini A, Antoniucci D, Tamburino C, Alexopoulos D. Morphine is associated with a delayed activity of oral antiplatelet agents in patients with ST-elevation acute myocardial infarction undergoing primary percutaneous coronary intervention. Circ Cardiovasc Interv 2014;8:e001593. [DOI] [PubMed] [Google Scholar]
- 5. Sibbing D, Aradi D, Alexopoulos D, Ten Berg J, Bhatt DL, Bonello L, Collet JP, Cuisset T, Franchi F, Gross L, Gurbel P, Jeong YH, Mehran R, Moliterno DJ, Neumann FJ, Pereira NL, Price MJ, Sabatine MS, So DYF, Stone GW, Storey FR, Tantry U, Trenk D, Valgimigli M, Waksman R, Angiolillo DJ. Updated expert consensus statement on platelet function and genetic testing for guiding P2Y12 receptor inhibitor treatment in percutaneous coronary intervention. JACC Cardiovasc Interv 2019;12:1521–1537. [DOI] [PubMed] [Google Scholar]
- 6. Nishikawa M, Takeda Y, Isomura N, Tanigawa T, Nanasato M, Tsukahara K, Kimura K, Takayama T, Hirayama A, Kato M, Nishikawa H, Nishimura Y, Isshiki T, Yokoi H; j-CHIPS group. Association between high platelet reactivity following dual antiplatelet therapy and ischemic events in Japanese patients with coronary artery disease undergoing stent implantation. J Atheroscler Thromb 2020;27:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 8. O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YL, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Stevenson WG, Yancy CS. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127:e362–e425. [DOI] [PubMed] [Google Scholar]
- 9. Pitt B, Pepine C, Willerson JT. Cyclooxygenase-2 inhibition and cardiovascular events. Circulation 2002;106:167–169. [DOI] [PubMed] [Google Scholar]
- 10. Shau WY, Chen HC, Chen ST, Chou HW, Chang CH, Kuo CW, Lai MS. Risk of new acute myocardial infarction hospitalization associated with use of oral and parenteral non-steroidal anti-inflammation drugs (NSAIDs): a case-crossover study of Taiwan's National Health Insurance claims database and review of current evidence. BMC Cardiovasc Disord 2012;12:4–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA 2001;286:954–959. [DOI] [PubMed] [Google Scholar]
- 12. Chiam E, Weinberg L, Bellomo R. Paracetamol: a review with specific focus on the haemodynamic effects of intravenous administration. Heart Lung Vessel 2015;7:121–132. [PMC free article] [PubMed] [Google Scholar]
- 13. Franchi F, Rollini F, Rivas A, Wali M, Briceno M, Agarwal M, Shaikh Z, Nawaz A, Silva G, Been L, Smairat R, Kaufman M, Pineda AM, Suryadevara S, Soffer D, Zenni MM, Bass TA, Angiolillo DJ. Platelet inhibition with cangrelor and crushed ticagrelor in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Circulation 2019;139:1661–1670. [DOI] [PubMed] [Google Scholar]
- 14. Rollini F, Franchi F, Hu J, Kureti M, Aggarwal N, Durairaj A, Park Y, Seawell M, Cox-Alomar P, Zenni MM, Guzman LA, Suryadevara S, Antoun P, Bass TA, Angiolillo DJ. Crushed prasugrel tablets in patients with STEMI undergoing primary percutaneous coronary intervention: the CRUSH study. J Am Coll Cardiol 2016;67:1994–2004. [DOI] [PubMed] [Google Scholar]
- 15. Parodi G, Xanthopoulou I, Bellandi B, Gkizas V, Valenti R, Karanikas S, Migliorini A, Angelidis C, Abbate R, Patsilinakos S, Baldereschi GJ, Marcucci R, Gensini GF, Antoniucci D, Alexopoulos D. Ticagrelor crushed tablets administration in STEMI patients: the MOJITO study. J Am Coll Cardiol 2015;65:511–512. [DOI] [PubMed] [Google Scholar]
- 16. Tavenier AH, Hermanides RS, Ottervanger JP, Rasoul S, Slingerland RJ, Tolsma R, van Workum S, Kedhi E, van ‘t Hof AWJ. A randomised, investigator-initiated, clinical trial of the effects of fentanyl on P2Y12-receptor inhibition in patients with ST-elevation myocardial infarction who are pre-treated with crushed ticagrelor: rationale and design of the Opioids aNd crushed Ticagrelor In Myocardial infarction Evaluation (ON-TIME 3) trial. Neth Heart J 2019;27:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McEvoy JW, Ibrahim K, Kickler TS, Clarke WA, Hasan RK, Czarny MJ, Keramati AR, Goli RR, Gratton TP, Brinker JA, Chacko M, Wang CW, Johnston PV, Miller JM, Trost JC, Herzog WR, Blumenthal RS, Thiemann DR, Resar JR, Schulman SP. Effect of intravenous fentanyl on ticagrelor absorption and platelet inhibition among patients undergoing percutaneous coronary intervention: the PACIFY randomized clinical trial (platelet aggregation with ticagrelor inhibition and fentanyl). Circulation 2018;137:307–309. [DOI] [PubMed] [Google Scholar]
- 18. Heestermans AA, van Werkum JW, Taubert D, Seesing TH, von Beckerath N, Hackeng CM, Schomig E, Verheugt FW, ten Berg JM. Impaired bioavailability of clopidogrel in patients with a ST-segment elevation myocardial infarction. Thromb Res 2008;122:776–781. [DOI] [PubMed] [Google Scholar]
- 19. Lapostolle F, van’t Hof AW, Hamm CW, Stibbe O, Ecollan P, Collet J-P, Silvain J, Lassen JF, Heutz WMJM, Bolognese L, Cantor WJ, Cequier A, Chettibi M, Goodman SG, Hammett CJ, Huber K, Janzon M, Merkely B, Storey RF, ten Berg J, Zeymer U, Licour M, Tsatsaris A, Montalescot G; ATLANTIC Investigators. Morphine and ticagrelor interaction in primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: ATLANTIC-morphine. Am J Cardiovasc Drugs 2019;19:173–183. [DOI] [PubMed] [Google Scholar]
- 20. Silvain J, Storey RF, Cayla G, Esteve JB, Dillinger JG, Rousseau H, Tsatsaris A, Baradat C, Salhi N, Hamm CW, Lapostolle F, Lassen JF, Collet JP, Ten Berg JM, Van ‘T Hof AWJ, Montalescot G. P2Y12 receptor inhibition and effect of morphine in patients undergoing primary PCI for ST-segment elevation myocardial infarction. The PRIVATE-ATLANTIC study. Thromb Haemost 2016;116:369–378. [DOI] [PubMed] [Google Scholar]
- 21. Montalescot G, van 't Hof AW, Lapostolle F, Silvain J, Lassen JF, Bolognese L, Cantor WJ, Cequier Á, Chettibi M, Goodman SG, Hammett CJ, Huber K, Janzon M, Merkely B, Storey RF, Zeymer U, Stibbe O, Ecollan P, Heutz WMJM, Swahn E, Collet J-P, Willems FF, Baradat C, Licour M, Tsatsaris A, Vicaut E, Hamm CW; ATLANTIC Investigators. Prehospital ticagrelor in ST-segment elevation myocardial infarction. N Engl J Med 2014;371:1016–1027. [DOI] [PubMed] [Google Scholar]
- 22. Farag M, Spinthakis N, Srinivasan M, Sullivan K, Wellsted D, Gorog DA. Morphine analgesia pre-PPCI is associated with prothrombotic state, reduced spontaneous reperfusion and greater infarct size. Thromb Haemost 2018;118:601–612. [DOI] [PubMed] [Google Scholar]
- 23. Puymirat E, Lamhaut L, Bonnet N, Aissaoui N, Henry P, Cayla G, Cattan S, Steg G, Mock L, Ducrocq G, Goldstein P, Schiele F, Bonnefoy-Cudraz E, Simon T, Danchin N. Correlates of pre-hospital morphine use in ST-elevation myocardial infarction patients and its association with in-hospital outcomes and long-term mortality: the FAST-MI (French Registry of Acute ST-elevation and non-ST-elevation Myocardial Infarction) programme. Eur Heart J 2016;37:1063–1071. [DOI] [PubMed] [Google Scholar]
- 24. Ari H, Ozkan H, Karacinar A, Ari S, Koca V, Bozat T. The EFFect of hIgh-dose ClopIdogrel treatmENT in patients with clopidogrel resistance (the EFFICIENT trial). Int J Cardiol 2012;157:374–380. [DOI] [PubMed] [Google Scholar]
- 25. Franchi F, Rollini F, Cho JR, Bhatti M, DeGroat C, Ferrante E, Dunn EC, Nanavati A, Carraway E, Suryadevara S, Zenni MM, Guzman LA, Bass TA, Angiolillo DJ. Impact of escalating loading dose regimens of ticagrelor in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: results of a prospective randomized pharmacokinetic and pharmacodynamic investigation. JACC Cardiovasc Interv 2015;8:1457–1467. [DOI] [PubMed] [Google Scholar]
- 26. Franchi F, Rollini F, Park Y, Hu J, Kureti M, Rivas Rios J, Faz G, Yaranov D, Been L, Pineda AM, Suryadevara S, Soffer D, Zenni MM, Bass TA, Angiolillo DJ. Effects of methylnaltrexone on ticagrelor-induced antiplatelet effects in coronary artery disease patients treated with morphine. JACC Cardiovasc Interv 2019;12:1538–1549. [DOI] [PubMed] [Google Scholar]
- 27. Sikora J, Niezgoda P, Barańska M, Buszko K, Skibińska N, Sroka W, Pstrągowski K, Siller-Matula J, Bernd J, Gorog D, Navarese E, Marszałł M, Kubica J. METoclopramide Administration as a Strategy to Overcome MORPHine-ticagrelOr Interaction in PatientS with Unstable Angina PectorIS—the METAMORPHOSIS trial. Thromb Haemost 2018;118:2126–2133. [DOI] [PubMed] [Google Scholar]
- 28. Valgimigli M, Tebaldi M, Campo G, Gambetti S, Bristot L, Monti M, Parrinello G, Ferrari R; FABOLUS PRO Investigators. Prasugrel versus tirofiban bolus with or without short post-bolus infusion with or without concomitant prasugrel administration in patients with myocardial infarction undergoing coronary stenting: the FABOLUS PRO (Facilitation through Aggrastat By drOpping or shortening Infusion Line in patients with ST-segment elevation myocardial infarction compared to or on top of PRasugrel given at loading dOse) trial. JACC Cardiovasc Interv 2012;5:268–277. [DOI] [PubMed] [Google Scholar]
- 29. Gargiulo G, Esposito G, Cirillo P, Nagler M, Minuz P, Campo G, Gragnano F, Manavifar N, Piccolo R, Avvedimento M, Tebaldi M, Wahl A, Hunziker L, Billinger M, Heg D, Windecker S, Valgimigli M. Facilitation through Aggrastat or Cangrelor Bolus and Infusion Over PrasugreL: a MUlticenter Randomized Open-label Trial in Patien-elevation Myocardial InFarction Referred for PrimAry PercutaneouS InTERvention (FABOLUS FASTER) trial: design and rationale: the FABOLUS FASTER trial. J Cardiovasc Transl Res 2020; 10.1007/s12265-020-09969-4. [DOI] [PubMed] [Google Scholar]
- 30. Vlachojannis GJ, Vogel RF, Wilschut JM, Lemmert ME, Delewi R, Diletti R, van Vliet R, van der Waarden N, Nuis RJ, Paradies V, Alexopoulos D, Zijlstra F, Montalescot G, Angiolillo DJ, Krucoff MW, Van Mieghem NM, Smits PC. COMPARison of pre-hospital CRUSHed vs. uncrushed Prasugrel tablets in patients with STEMI undergoing primary percutaneous coronary interventions: rationale and design of the COMPARE CRUSH trial. Am Heart J 2020;224:10–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.