Abstract
Aims
To assess the cost-effectiveness of proprotein convertase subtilisin/kexin type 9 inhibition with evolocumab added to standard-of-care lipid-lowering treatment [maximum tolerated dose (MTD) of statin and ezetimibe] in Swedish patients with a history of myocardial infarction (MI).
Methods and results
Cost-effectiveness was evaluated using a Markov model based on Swedish observational data on cardiovascular event rates and efficacy from the FOURIER trial. Three risk profiles were considered: recent MI in the previous year; history of MI with a risk factor; and history of MI with a second event within 2 years. For each population, three minimum baseline low-density lipoprotein cholesterol (LDL-C) levels were considered: 2.5 mmol/L (≈100 mg/dL), based on the current reimbursement recommendation in Sweden; 1.8 mmol/L (≈70 mg/dL), based on 2016 ESC/EAS guidelines; and 1.4 mmol/L (≈55 mg/dL), or 1.0 mmol/L (≈40 mg/dL) for MI with a second event, based on 2019 ESC/EAS guidelines. Proprotein convertase subtilisin/kexin type 9 inhibition with evolocumab was associated with increased quality-adjusted life-years and costs vs. standard-of-care therapy. Incremental cost-effectiveness ratios (ICERs) were below SEK700 000 (∼€66 500), the generally accepted willingness-to-pay threshold in Sweden, for minimum LDL-C levels of 2.3 (recent MI), 1.7 (MI with a risk factor), and 1.7 mmol/L (MI with a second event). Sensitivity analyses demonstrated that base-case results were robust to changes in model parameters.
Conclusion
Proprotein convertase subtilisin/kexin type 9 inhibition with evolocumab added to MTD of statin and ezetimibe may be considered cost-effective at its list price for minimum LDL-C levels of 1.7–2.3 mmol/L, depending on risk profile, with ICERs below the accepted willingness-to-pay threshold in Sweden.
Keywords: Cost-effectiveness, Evolocumab, Low-density lipoprotein cholesterol, Myocardial infarction, PCSK9 inhibitors, Statins
Introduction
Standard-of-care (SoC) therapy for patients with elevated low-density lipoprotein cholesterol (LDL-C) levels includes a statin and ezetimibe.1 In recent years, the proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i) alirocumab and evolocumab have become available for the management of patients with atherosclerotic cardiovascular disease or familial hypercholesterolaemia with elevated LDL-C levels despite SoC treatment.2 This analysis focuses on evolocumab, a fully human monoclonal antibody against PCSK9 that has been shown in clinical trials to reduce LDL-C levels by ∼60%.3–6 Furthermore, the FOURIER cardiovascular (CV) outcomes trial showed that the addition of evolocumab to an optimized regimen of lipid-lowering therapy (LLT; moderate- to high-intensity statin therapy, with or without ezetimibe) in patients with established atherosclerotic CV disease (ASCVD) resulted in a 20% reduction in the key secondary endpoint of major CV events [MACE; i.e. a composite of myocardial infarction (MI), ischaemic stroke (IS), or CV death].7
A 2018 consensus statement from the European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS) recommended PCSK9i for high-risk patients with persistently high LDL-C despite being treated with a maximum tolerated dose (MTD) of statin in combination with ezetimibe, or in patients with statin intolerance.8 In Sweden, treatment with evolocumab is reimbursed in two patient populations: those with ASCVD and LDL-C ≥ 2.5 mmol/L while receiving MTD LLT; and those with heterozygous familial hypercholesterolaemia without ASCVD but with LDL-C ≥ 3.0 mmol/L while receiving MTD LLT.9,10
Updated guidelines published by the ESC/EAS in 2019 recommended the addition of a PCSK9i for secondary prevention patients who are at very high-CV risk and do not achieve their target LDL-C goal on a MTD of statin and ezetimibe (Class I, level A evidence).1 Moreover, for patients who present with acute coronary syndrome (ACS) and whose LDL-C levels are not at goal—despite MTD of statin and ezetimibe—the addition of a PCSK9i should be considered early after the ACS event (during hospitalization for the event if possible; Class IIa, level C evidence).
For the first time, we assessed the cost-effectiveness of PCSK9 inhibition added to SoC maximally tolerated LLT (i.e. MTD of statin and ezetimibe) in Swedish patients with a history of MI, based on risk profiles adapted from the 2019 ESC/EAS guidelines. For comparison, we also evaluated similar risk profiles based on the 2016 ESC/ESA guidelines and the current reimbursement conditions in Sweden.
Methods
Model structure
A Markov cohort-state transition model was used for the analysis, as used in all previous cost-effectiveness models for PCSK9i identified in a recent systematic literature review.11 The model was updated from previously published models12–18 and based on findings from Swedish observational data on baseline CV event rates18 and efficacy data from the FOURIER trial.7 The model was developed using Microsoft Excel (2019) and comprised six main health states (Supplementary material online, Figure S1): non-fatal MI; non-fatal IS; post-MI; post-IS; CV death; and non-CV death. The states for non-fatal MI and IS covered the first 1-year period after the respective event, with the post-event health states covering subsequent years. Coronary revascularisation was included in the model as a procedure (i.e. a cost) rather than a health state. The model also included composite health states that were a combination of two event health states. These were created to retain memory of previous CV events in patients who experienced more than one event, and to better model the increased risk, lower health-related quality of life, and higher costs associated with a history of multiple CV events. Further details of the model are presented in the Supplementary material online and in previous publications.12–18
Patient profiles
For this assessment of the cost-effectiveness of PCSK9 inhibition with evolocumab added to SoC LLT (i.e. MTD of statin and ezetimibe) in Sweden, three risk profiles were considered: (i) patients with an MI in the previous year (Recent MI); (ii) patients with a history of MI with a risk factor, illustrated by patients with diabetes and target organ damage (MI with a risk factor); and (iii) patients with a second CV event within 2 years, illustrated by a population with a second MI (MI with a second event). For each of the three risk profiles, three baseline LDL-C levels (while receiving SoC LLT) were considered: (i) 2.5 mmol/L (≈100 mg/dL), the minimum LDL-C level specified in the current reimbursement recommendation for evolocumab in Sweden; (ii) 1.8 mmol/L (≈70 mg/dL), the minimum LDL-C level specified in the 2016 ESC/EAS guidelines19; and (iii) 1.4 mmol/L (≈55 mg/dL), or 1.0 mmol/L (≈40 mg/dL) for patients with a second event within 2 years, the minimum LDL-C level specified in the 2019 ESC/EAS guidelines.1
Baseline patient characteristics
Baseline patient characteristics and CV event rates were derived from a retrospective study that included a cohort of patients meeting the inclusion criteria of the FOURIER trial,7 based on nationwide, linked Swedish population registry data.18 A summary of the inclusion criteria is included in the Supplementary material online. Patients were 72, 69, and 72 years old for the Recent MI, MI with a risk factor, and MI with a second event risk profiles, respectively. Cardiovascular event rates were calculated by dividing the number of first MACE observed since the index date by the number of patient-years of follow-up until censoring and expressed as MACE per 100 patient-years.18 Post-event rates of MACE per 100 patient-years at baseline were 6.2, 10.7, and 10.7 for the Recent MI, MI with a risk factor, and MI with a second event risk profiles, respectively. Throughout the simulation, baseline CV event rates were adjusted for age, LDL-C level, and CV event history using published standard methods.20
Treatment efficacy
In the FOURIER trial, the mean percentage reduction in LDL-C levels with evolocumab vs. placebo was 59% (intention-to-treat analysis)7 and a constant reduction over a lifetime treatment duration, consistent with long-term follow-up data,21 was assumed. Event-specific rate ratios used in our model were based on meta-analyses conducted by the Cholesterol Treatment Trialists’ Collaboration (CTTC), which are shown in Table 1.22 Cardiovascular event rates after treatment were calculated using the following formula:
where rtx, rate after treatment; r0, rate before treatment; RR, rate ratio per 1 mmol/L of LDL-C reduction; and ΔLDL-C, absolute LDL-C reduction.
Table 1.
Rate ratios of CV events per mmol/L of LDL-C reduction, utility values, and costs for CV events and procedures
| RR per mmol/L (≈39 mg/dL) LDL-C reduction | Utility values |
Direct costs, SEKa |
Indirect costs, SEKa | |||
|---|---|---|---|---|---|---|
| First year | Subsequent years | First year | Subsequent years | First year | ||
| Non-fatal MI | 0.73 | 0.672 | 0.824 | 86 014 | 23 406 | 32 447 |
| Non-fatal IS | 0.77 | 0.327 | 0.524 | 86 158 | 18 557 | 63 519 |
| CV death | 0.86 | 0.000 | – | 12 994 | – | – |
| Revascularization | 0.75 | – | – | 77 138 | – | – |
Exchange rate: 1 SEK ≈ 0.095 EUR.
CV, cardiovascular; IS, ischaemic stroke; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; RR, rate ratio; SEK, Swedish Kronor.
Costs were inflated to 2019 SEK using the consumer price index for health set by Statistics Sweden.
The rate ratios per mmol/L of LDL-C reduction observed in the FOURIER trial (after accounting for study duration) were aligned with those from the CTTC meta-analysis. It has been well documented that it takes time for the benefit of LLT to become evident.23–26 To account for this delayed treatment effect, prespecified landmark analyses were performed in FOURIER, in which patients who were alive and included in follow-up at the end of the first year formed the group at risk to estimate the effect of evolocumab on outcomes beyond the first year. These analyses showed that the magnitude of the relative risk reduction with regard to MACE grew over time, from 16% during the first year to 25% beyond the first year. Compared with the statin-based CTTC meta-analysis, treatment with evolocumab had very similar effects on the risk of MACE per 1 mmol/L of LDL-C reduction, as illustrated separately for years 0 to 1 and years 1 to 2.7 Furthermore, the results from the FOURIER trial are consistent with the results of a recent Mendelian randomization study showing that variants in the genes encoding PCSK9 and 3-hydroxy-3-methylglutaryl-coenzyme A reductase (the target of statins) were associated with nearly identical effects on the risk of CV events per unit decrease of LDL-C.27 In addition, a meta-analysis of 49 studies comparing the effects of statins and eight non-statin LLTs (including PCSK9i) demonstrated that lowering LDL-C level was associated with a consistent proportional improvement in CV outcomes.28 Importantly, the reduction in risk of MACE observed in the FOURIER trial, when adjusted for duration of follow-up, is superimposable with that of statins based on the CTTC meta-analysis.29 The treatment effect in our model was, therefore, based on the CTTC relationship between LDL-C reduction and reduced rates of CV events.
Utility values and costs
Cardiovascular disease health state utilities (Table 1) were derived from a time trade-off study based on a general population sample in the UK,30 as used in a previous submission to the Swedish Dental Care and Medicines Benefit Agency (TLV) that led to the current recommendation of evolocumab in Sweden. Costs associated with medication and ASCVD were also considered following the guidelines of the TLV.31 Annual medication costs based on list prices (before commercial discounts) were SEK48 759 for evolocumab 140 mg every 2 weeks, SEK206 for atorvastatin 20 or 40 mg/day and SEK454 for ezetimibe 10 mg (1 SEK = 0.095 EUR). An atorvastatin dose of 20 mg or 40 mg was used to reflect clinical practice in Sweden, where atorvastatin is the most prescribed statin in these intensity classifications. The assumptions used to generate medication costs are shown in Supplementary material online, Table S1. Therapy persistence for evolocumab, as observed in the FOURIER trial, was included in the model to adjust the cost of evolocumab over the modelled time horizon. Cardiovascular event and procedure costs were based on recent, retrospective studies from Swedish registries, including data from primary care, pharmaceutical prescriptions, inpatient care, and cause of death registries.32,33
In clinical trials of evolocumab in patients with hypercholesterolaemia, most reported adverse events were mild to moderate in severity, with infrequent reports of serious adverse events or adverse events leading to treatment discontinuation.34 For this reason, no disutility, cost, or increased CV event risk for adverse events were modelled.
Economic analysis
In line with TLV requirements31 and, as all patients in the present analysis were above retirement age, only costs associated with medication and ASCVD were considered. The analysis assumed a lifetime horizon, appropriate for evaluating the impact of an intervention on a chronic condition. The primary measure of health benefit was the quality-adjusted life-year (QALY), with the incremental cost-effectiveness ratio (ICER) calculated as the incremental cost per QALY gained. For transparency, the 10-year risk of MACE was also calculated using the model. In the base-case analyses, both costs and outcomes were discounted at an annual rate of 3.0%.
Sensitivity analyses
Univariate sensitivity analyses were conducted, in which one parameter was varied at a time relative to its base-case value. Efficacy parameters, baseline rates and their adjustment factors, health state utility values and costs were changed to the lower and upper bound of their 95% confidence interval. Probabilistic sensitivity analysis was also conducted to examine the combined effect of parameter uncertainty on the incremental cost per QALY gained. Appropriate probability distributions (Supplementary material online, Table S2) were assigned to model parameters based on their respective means and standard errors, and values for parameters were sampled by Monte‐Carlo simulation with 1000 iterations in each loop. Cost-effectiveness acceptability curves were generated to illustrate the probability that evolocumab is cost-effective over a range of willingness-to-pay thresholds.
Results
Base-case analysis
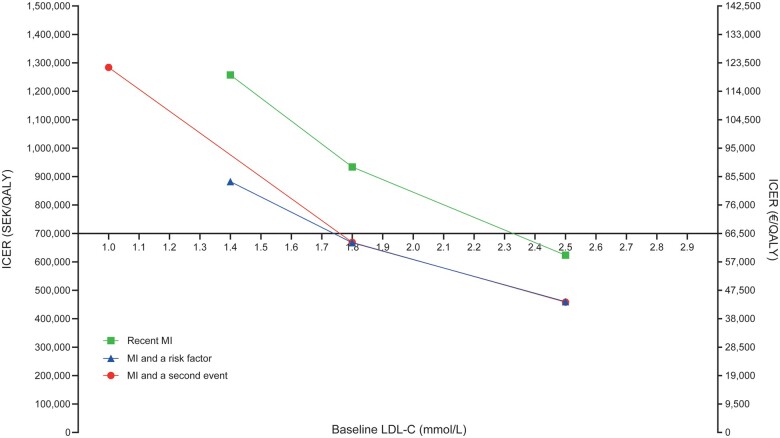
PCSK9 inhibition with evolocumab added to SoC was associated with QALY gains and increased costs compared with SoC therapy (Table 2). At the list price of evolocumab, ICERs were below SEK700 000 (∼€66 500) per QALY gained, the generally accepted willingness-to-pay threshold in Sweden,35 for minimum LDL-C levels of 2.3 (recent MI), 1.7 (MI with a risk factor), and 1.7 mmol/L (MI with a second event). Cost-effectiveness results improved for patients with high LDL-C levels and high 10-year risk of MACE (Figure 1).
Table 2.
Summary of cost-effectiveness results
|
Recent MI
|
MI with a risk factor
|
MI with a second event
|
||||
|---|---|---|---|---|---|---|
| Evo + SoC | SoC | Evo + SoC | SoC | Evo + SoC | SoC | |
| LDL-C of 2.5 mmol/L—minimum LDL-C level specified in the current reimbursement recommendation for evolocumab in Sweden | ||||||
| 10-year risk of first MACE (%) | 37 | 44 | 48 | 54 | 49 | 54 |
| Total cost (SEK) | 663 562 | 283 814 | 590 650 | 246 137 | 566 430 | 252 830 |
| Incremental cost (SEK) | 379 748 | — | 344 513 | — | 313 601 | — |
| Total QALY | 7.06 | 6.45 | 6.31 | 5.56 | 5.67 | 4.98 |
| QALY gained | 0.61 | — | 0.75 | — | 0.68 | — |
| ICER (SEK/QALY) [€/QALY] | 623 367 | — | 460 241 | — | 458 380 | — |
| [59 220] | [43 723] | [43 546] | ||||
| LDL-C of 1.8 mmol/L—minimum LDL-C specified in the 2016 ESC/EAS guidelines | ||||||
| 10-year risk of first MACE (%) | 36 | 41 | 47 | 51 | 47 | 52 |
| Total cost (SEK) | 679 934 | 289 919 | 611 491 | 254 233 | 585 841 | 260 509 |
| Incremental cost (SEK) | 390 014 | — | 357 258 | — | 325 332 | — |
| Total QALY | 7.27 | 6.86 | 6.56 | 6.03 | 5.91 | 5.42 |
| QALY gained | 0.42 | — | 0.54 | — | 0.49 | — |
| ICER (SEK/QALY) [€/QALY] | 933 748 | — | 667 456 | — | 668 512 | — |
| [88 706] | [63 408] | [63 509] | ||||
| LDL-C of 1.4 mmol/L for the Recent MI and MI with a risk factor profiles groups; LDL-C ≥1.0 mmol/L for the MI with a second event group—minimum LDL-C specified in the 2019 ESC/EAS guidelines | ||||||
| 10-year risk of first MACE (%) | 35 | 39 | 46 | 50 | 46 | 48 |
| Total cost (SEK) | 688 958 | 293 178 | 623 366 | 258 841 | 607 770 | 269 061 |
| Incremental cost (SEK) | 395 781 | — | 364 525 | — | 338 709 | — |
| Total QALY | 7.39 | 7.08 | 6.71 | 6.30 | 6.18 | 5.92 |
| QALY gained | 0.31 | — | 0.41 | — | 0.26 | — |
| ICER (SEK/QALY) [€/QALY] | 1 257 578 | — | 882 265 | — | 1 283 860 | — |
| [119 470] | [83 815] | [121 967] | ||||
Evo, evolocumab; ICER, incremental cost-effectiveness ratio; LDL-C, low-density lipoprotein cholesterol; MACE, major cardiovascular event; MI, myocardial infarction; QALY, quality-adjusted life-year; SEK, Swedish Kronor; SoC, standard of care.
Figure 1.
Incremental cost-effectiveness ratios as a function of 10-year risk while receiving standard-of-care lipid-lowering therapy. *≥1.0 mmol/L for the MI with a second event risk profile. ICER, incremental cost-effectiveness ratio; LDL-C, low-density lipoprotein cholesterol; MACE, major cardiovascular events; MI, myocardial infarction; SEK, Swedish Kronor; SoC, standard of care.
Sensitivity analyses
All univariate and probabilistic sensitivity analyses were conducted using the Recent MI risk profile (baseline LDL-C of 2.5 mmol/L) as an illustration. Univariate sensitivity analyses demonstrated that base-case results were generally robust to changes in model input parameters. Only three parameters affected the base-case ICER (SEK623 367) by more than 5% when varied: the rate ratio (per 1 mmol/L LDL-C reduction) for CV death22 (SEK530 187 to SEK763 600); the rate ratio for IS22 (SEK584 109 to SEK676 572); and the hazard ratio for patients with a history of both MI and IS20 (SEK591 596 to SEK660 870).
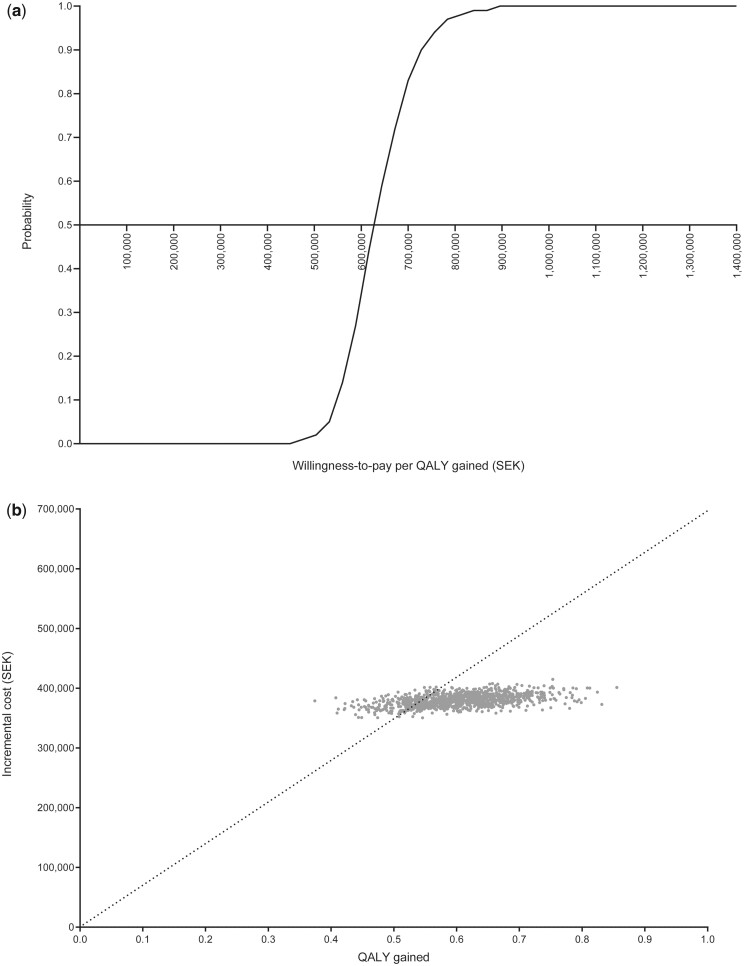
For the probabilistic sensitivity analysis, Figure 2A shows the cost‐effectiveness acceptability curves for PCSK9 inhibition with evolocumab added to SoC therapy compared with background LLT. The individual iterations plotted on the cost-effectiveness plane (Figure 2B) indicate that all incremental cost-QALY gained pairs are in the north-east quadrant, and thus adding evolocumab to MTD of statin with ezetimibe in the Recent MI risk profile (baseline LDL-C of 2.5 mmol/L) is both costlier and more effective than treatment without evolocumab. Overall, the probability that PCSK9 inhibition with evolocumab at its list price added to MTD of statin with ezetimibe is cost-effective at the generally accepted willingness-to-pay threshold of SEK700 000 (∼€66 500) per QALY gained was 82.5%. At this willingness-to-pay threshold and price of evolocumab, this probability becomes 0% for the Recent MI risk profile with baseline LDL-C of 1.8 mmol/L (Supplementary material online, Figure S2).
Figure 2.
Probabilistic sensitivity analysis for the Recent MI risk profile (baseline low-density lipoprotein cholesterol of 2.5 mmol/L): (A) cost-effectiveness acceptability curves; (B) cost-effectiveness plane. The cost-effectiveness acceptability curves represent the probability that the addition of evolocumab is cost-effective over a range of willingness-to-pay thresholds. The cost-effectiveness plane represents each individual iteration (incremental cost–QALY gained pairs) from the probabilistic sensitivity analysis. LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; QALY, quality-adjusted life-year; SEK, Swedish Kronor; SoC, standard-of-care.
Discussion
To our knowledge, our study is the first cost-effectiveness analysis of PCSK9 inhibition in the context of the 2019 ESC/EAS dyslipidaemia guidelines.1 In addition, we assessed the cost-effectiveness of PCSK9 inhibition added to SoC LLT in Swedish patients with a history of MI based on selected risk profiles adapted from the 2016 ESC/EAS dyslipidaemia guidelines19 and the current reimbursement conditions in Sweden. The addition of evolocumab to SoC was associated with QALY gains and increased costs compared with SoC LLT. Moreover, ICERs were below the generally accepted willingness-to-pay threshold in Sweden for minimum LDL-C levels of 2.3 (recent MI), 1.7 (MI with a risk factor), and 1.7 mmol/L (MI with a second event).
Consistent with the 2019 ESC/EAS guidelines, the cost-effectiveness of PCSK9 inhibition was improved in selected patients with high LDL-C levels and a history of MI with increased risk.1 Our results were also consistent with a recent cost-effectiveness analysis in the US context.17 Considering a list price similar to the one used in our analysis, Fonarow et al.17 showed that PCSK9 inhibition with evolocumab may be cost-effective in very high-risk patients with ASCVD as defined by the 2018 guidelines from the American College of Cardiology and American Heart Association.36 In Europe, Villa et al.14 had previously found that PCSK9 inhibition with evolocumab may be considered cost-effective in patients with ASCVD eligible for reimbursement in Spain. Other previously published European cost-effectiveness analyses in Germany,37 the Netherlands,38 and Norway39 considered higher PCSK9i base-case prices that are no longer relevant.
The results of our analysis can be extended to other lipid-lowering therapies with similar efficacy, safety, and price, and these data can be used to inform future European guidelines, which until now have mostly relied on US cost-effectiveness data.1,20 Our results should, however, be interpreted in the context of the data and modelling assumptions used. For example, the predictions of the model were based on extrapolation beyond the duration of the FOURIER trial. Furthermore, if levels of compliance with, and adherence to, evolocumab therapy and the components of SoC differed from those modelled based on the FOURIER trial, outcomes, and costs might be affected. It should also be noted that the analyses were conducted using the list price of evolocumab in Sweden. In practice, however, reimbursement agreements, including those in Sweden, usually involve payment of a confidential net price that is lower than the list price. Using such a net price in the model would have further improved the cost-effectiveness of treatment. Finally, it is important to note that cost-effectiveness results obtained in one country cannot necessarily be extrapolated to other countries.1 In the future, it will be informative to examine the cost-effectiveness of PCSK9 inhibition in other healthcare systems, and in other patient populations with similar, or even higher, risk profiles than those with a history of MI included in the current analysis.
In conclusion, our results indicate that the addition of PCSK9 inhibition with evolocumab to SoC treatment may be considered cost-effective at its list price for minimum LDL-C levels ranging from 1.7 mmol/L to 2.3 mmol/L, depending on the risk profile. The results may also be considered to be valid for other patient populations with similar or higher CV risk or LDL-C levels.
Supplementary material
Supplementary material is available at European Heart Journal – Quality of Care and Clinical Outcomes online.
Funding
This work was supported by Amgen.
Conflict of interest: U.L. has received lecture and advisory fees from Amgen, Sanofi, Medicines Company and Novartis. P.L. received grants from Amgen, BMS, EFPIA Merck, Novo Nordisk, Pfizer, and Sanofi. E.H. acted as expert committee member for and received lecture fees and institutional research grants from Sanofi and Amgen, and lecture fees from AstraZeneca, Bayer, and Novo Nordisk. B.v.H. consulted for Amgen. G.V., P.P.-R., J.A., M.E.S., and M.S. were employees and stockholders of Amgen. G.C.F. consulted for Abbott, Amgen, Bayer, Janssen, and Novartis.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Supplementary Material
Acknowledgements
Medical writing assistance was provided by Dan Booth PhD (Bioscript Medical Ltd, Macclesfield, UK) and funded by Amgen.
Contributor Information
Ulf Landmesser, Department of Cardiology, Medical Director Charité Cardiovascular Center (CC11), Campus Benjamin Franklin Charité – Universitätsmedizin Berlin, Hindenburgdamm 30, 12203, Berlin, Germany.
Peter Lindgren, Department of Learning, Informatics, Management and Ethics, Karolinska Institutet, 171 77, Stockholm, Sweden; Managing Director, The Swedish Institute for Health Economics, Box 2127, 220 02, Lund, Sweden.
Emil Hagström, Department of Medical Sciences, Cardiology, Uppsala University, 751 85, Uppsala, Sweden.
Ben van Hout, School of Health and Related Research, University of Sheffield, 30 Regent St, Sheffield, S1 4DA.
Guillermo Villa, Global Health Economics, Amgen (Europe) GmbH, Suurstoffi 22, 6343, Rotkreuz, Switzerland.
Peter Pemberton-Ross, Global Health Economics, Amgen (Europe) GmbH, Suurstoffi 22, 6343, Rotkreuz, Switzerland.
Jorge Arellano, Global Health Economics, Amgen Inc, 1 Amgen Center Drive, Thousand Oaks, CA, 91320, USA.
Maria Eriksson Svensson, Medical Affairs, Amgen AB, Gustav III: s Boulevard 54, 169 74, Solna, Sweden; Department of Medical Sciences, Uppsala University, 751 85, Uppsala, Sweden.
Mahendra Sibartie, Medical Affairs, Amgen (Europe) GmbH, Suurstoffi 22, 6343, Rotkreuz, Switzerland.
Gregg C Fonarow, Division of Cardiology, University of California Los Angeles, 10833 LeConte Avenue, Los Angeles, CA, 90095, USA.
References
- 1. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L et al. ; Scientific Document Group. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–188. [DOI] [PubMed] [Google Scholar]
- 2. Smith L, Mosley J, Yates J, Caswell L. The new face of hyperlipidemia management: proprotein convertase subtilisin/kexin inhibitors (PCSK-9) and their emergent role as an alternative to statin therapy. J Pharm Pharm Sci 2016;19:137–146. [DOI] [PubMed] [Google Scholar]
- 3. Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med 2014;370:1809–1819. [DOI] [PubMed] [Google Scholar]
- 4. Robinson JG, Nedergaard BS, Rogers WJ, Fialkow J, Neutel JM, Ramstad D et al.; LAPLACE-2 Investigators. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA 2014;311:1870–1882. [DOI] [PubMed] [Google Scholar]
- 5. Raal FJ, Stein EA, Dufour R, Turner T, Civeira F, Burgess L, et al. ; RUTHERFORD-2 Investigators. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet 2015;385:331–340. [DOI] [PubMed] [Google Scholar]
- 6. Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J et al. Open-label study of long-term evaluation against LDL cholesterol (OSLER) investigators. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1500–1509. [DOI] [PubMed] [Google Scholar]
- 7. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA et al. FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 8. Landmesser U, Chapman MJ, Stock JK, Amarenco P, Belch JJF, Borén J et al. 2017 Update of ESC/EAS Task Force on practical clinical guidance for proprotein convertase subtilisin/kexin type 9 inhibition in patients with atherosclerotic cardiovascular disease or in familial hypercholesterolaemia. Eur Heart J 2018;39:1131–1143. [DOI] [PubMed] [Google Scholar]
- 9. TLV. Repatha fortsätter att ingå i högkostnadsskyddet med ny bredare begränsning. 2018. https://.tlv.se/beslut/beslut-lakemedel/begransad-subvention/arkiv/2018-11-26-repatha-fortsatter-att-inga-i-hogkostnadsskyddet-med-ny-bredare-begransning.html (12 October 2020).
- 10. Sveriges Kommuner och Landsting. Repatha (evolocumab) och Praluent (alirokumab) för behandling av hyperkolesterolemi. 2018. https://janusinfo.se/download/18.3cc95cc2167c579a5a813e99/1545311218942/Repatha-och-Praluent-181220.pdf (12 October 2020).
- 11. Marquina C Zomer E Vargas-Torres S Zoungas S Ofori-Asenso R Liew D et al. . Novel Treatment Strategies for Secondary Prevention of Cardiovascular Disease: A Systematic Review of Cost-Effectiveness. PharmacoEconomics 2020;38:1095–1113. [DOI] [PubMed] [Google Scholar]
- 12. Gandra SR, Villa G, Fonarow GC, Lothgren M, Lindgren P, Somaratne R et al. Cost-effectiveness of LDL-C lowering with evolocumab in patients with high cardiovascular risk in the United States. Clin Cardiol 2016;39:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Toth PP, Danese M, Villa G, Qian Y, Beaubrun A, Lira A et al. Estimated burden of cardiovascular disease and value-based price range for evolocumab in a high-risk, secondary-prevention population in the US payer context. J Med Econ 2017;20:555–564. [DOI] [PubMed] [Google Scholar]
- 14. Villa G, Lothgren M, Kutikova L, Lindgren P, Gandra SR, Fonarow GC et al. Cost-effectiveness of evolocumab in patients with high cardiovascular risk in Spain. Clin Ther 2017;39:771–786. e773. [DOI] [PubMed] [Google Scholar]
- 15. Fonarow GC, Keech AC, Pedersen TR, Giugliano RP, Sever PS, Lindgren P, et al. Cost-effectiveness of evolocumab therapy for reducing cardiovascular events in patients with atherosclerotic cardiovascular disease. JAMA Cardiol 2017;2:1069–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Borissov B, Urbich M, Georgieva B, Tsenov S, Villa G. Cost-effectiveness of evolocumab in treatment of heterozygous familial hypercholesterolaemia in Bulgaria: measuring health benefit by effectively treated patient-years. J Mark Access Health Policy 2017;5:1412753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fonarow GC, van Hout B, Villa G, Arellano J, Lindgren P. Updated cost-effectiveness analysis of evolocumab in patients with very high-risk atherosclerotic cardiovascular disease. JAMA Cardiol 2019;4:691–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lindh M, Banefelt J, Fox KM, Hallberg S, Tai MH, Eriksson M, Villa G et al. Cardiovascular event rates in a high atherosclerotic cardiovascular disease risk population: estimates from Swedish population-based register data. Eur Heart J Qual Care Clin Outcomes 2019;5:225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, et al. ESC Scientific Document Group. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J 2016;37:2999–3058. [DOI] [PubMed] [Google Scholar]
- 20. Danese MD, Pemberton-Ross P, Catterick D, Villa G. Estimation of the increased risk associated with recurrent events or poly-vascular atherosclerotic cardiovascular disease in the United Kingdom. Eur J Prev Cardiol 2020. [DOI] [PubMed] [Google Scholar]
- 21. Koren MJ, Sabatine MS, Giugliano RP, Langslet G, Wiviott SD, Ruzza A et al. Long-term efficacy and safety of evolocumab in patients with hypercholesterolemia. J Am Coll Cardiol 2019;74:2132–2146. [DOI] [PubMed] [Google Scholar]
- 22. Cholesterol Treatment Trialists Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 24. Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016;388:2532–2561. [DOI] [PubMed] [Google Scholar]
- 25. Buchwald H, Varco RL, Matts JP, Long JM, Fitch LL, Campbell GS, et al. POSCH Group. Effect of partial ileal bypass surgery on mortality and morbidity from coronary heart disease in patients with hypercholesterolemia. Report of the Program on the Surgical Control of the Hyperlipidemias (POSCH). N Engl J Med 1990;323:946–955. [DOI] [PubMed] [Google Scholar]
- 26. Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med 1987;317:1237–1245. [DOI] [PubMed] [Google Scholar]
- 27. Ference BA, Robinson JG, Brook RD, Catapano AL, Chapman MJ, Neff DR et al. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med 2016;375:2144–2153. [DOI] [PubMed] [Google Scholar]
- 28. Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA 2016;316:1289–1297. [DOI] [PubMed] [Google Scholar]
- 29. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2017;38:2459–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Matza LS, Stewart KD, Gandra SR, Delio PR, Fenster BE, Davies EW et al. Acute and chronic impact of cardiovascular events on health state utilities. BMC Health Serv Res 2015;15:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tandvårds – och läkemedelsförmånsverkets allmänna råd. 2017. https://.tlv.se/download/18.467926b615d084471ac3230c/1510316374332/TLVAR_2017_1.pdf (12 October 2020).
- 32. Banefelt J, Hallberg S, Fox KM, Mesterton J, Paoli CJ, Johansson G et al. Work productivity loss and indirect costs associated with new cardiovascular events in high-risk patients with hyperlipidemia: estimates from population-based register data in Sweden. Eur J Health Econ 2016;17:1117–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hallberg S, Gandra SR, Fox KM, Mesterton J, Banefelt J, Johansson G et al. Healthcare costs associated with cardiovascular events in patients with hyperlipidemia or prior cardiovascular events: estimates from Swedish population-based register data. Eur J Health Econ 2016;17:591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Toth PD, Sattar N, Genest J, Descamps OS, Dent R, Djedjos C et al. A comprehensive safety analysis of 6026 patients from phase 2 and 3 short and long term clinical trials with evolocumab (AMG 145). J Am Coll Cardiol 2015;65:A1351. [Google Scholar]
- 35. Svensson M, Nilsson FO, Arnberg K. Reimbursement decisions for pharmaceuticals in Sweden: the impact of disease severity and cost effectiveness. Pharmacoeconomics 2015;33:1229–1236. [DOI] [PubMed] [Google Scholar]
- 36. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dressel A, Schmidt B, Schmidt N, Laufs U, Fath F, Chapman MJ et al. Cost effectiveness of lifelong therapy with PCSK9 inhibitors for lowering cardiovascular events in patients with stable coronary artery disease: insights from the Ludwigshafen Risk and Cardiovascular Health cohort. Vascul Pharmacol 2019;120:106566. [DOI] [PubMed] [Google Scholar]
- 38. Stam-Slob MC, van der Graaf Y, de Boer A, Greving JP, Visseren FLJ. Cost-effectiveness of PCSK9 inhibition in addition to standard lipid-lowering therapy in patients at high risk for vascular disease. Int J Cardiol 2018;253:148–154. [DOI] [PubMed] [Google Scholar]
- 39. Modelling the cost-effectiveness of PCSK9 inhibitors vs. ezetimibe through LDL-C reductions in a Norwegian setting. Eur Heart J Cardiovasc Pharmacother 2018;4:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.