Abstract
Aims
Heart failure is the main threat to long-term health in adults with transposition of the great arteries (TGA) corrected by an atrial switch operation (AtrSO). Current guidelines refrain from recommending heart failure medication in TGA-AtrSO, as there is insufficient data to support the hypothesis that it is beneficial. Medication is therefore prescribed based on personal judgements. We aimed to evaluate medication use in TGA-AtrSO patients and examine the association of use of renin–angiotensin–aldosterone system (RAAS) inhibitors and β-blockers with long-term survival.
Methods and results
We identified 150 TGA-AtrSO patients [median age 30 years (interquartile range 25–35), 63% male] included in the CONCOR registry from five tertiary medical centres with subsequent linkage to the Dutch Dispensed Drug Register for the years 2006–2014. Use of RAAS inhibitors, β-blockers, and diuretics increased with age, from, respectively, 21% [95% confidence interval (CI) 14–40], 12% (95% CI 7–21), and 3% (95% CI 2–7) at age 25, to 49% (95% CI 38–60), 51% (95% CI 38–63), and 41% (95% CI 29–54) at age 45. Time-varying Cox marginal structural models that adjusted for confounding medication showed a lower mortality risk with use of RAAS inhibitors and β-blockers in symptomatic patients [hazard ratio (HR) = 0.13 (95% CI 0.03–0.73); P = 0.020 and HR = 0.12 (95% CI 0.02–0.17); P = 0.019, respectively]. However, in the overall cohort, no benefit of RAAS inhibitors and β-blockers was seen [HR = 0.93 (95% CI 0.24–3.63); P = 0.92 and HR = 0.98 (0.23–4.17); P = 0.98, respectively].
Conclusion
The use of heart failure medication is high in TGA-AtrSO patients, although evidence of its benefit is limited. This study showed lower risk of mortality with use of RAAS inhibitors and β-blockers in symptomatic patients only. These findings can direct future guidelines, supporting use of RAAS inhibitors and β-blockers in symptomatic, but not asymptomatic patients.
Keywords: Mustard, Senning, Heart failure, Dispensed drugs, Transposition of the great arteries
Introduction
Adults with transposition of the great arteries (TGA) after an atrial switch operation (AtrSO) are at risk of gradual deterioration of systemic right ventricular (RV) function, congestive heart failure, and premature mortality.1 In patients with systemic left ventricular (LV) heart failure with reduced ejection fraction, angiotensin-converting enzyme inhibitors and β-blockers are the cornerstone of heart failure treatment as they have been shown to improve survival.2 However, current guidelines for TGA-AtrSO patients do not recommend the use of these drugs as primary or secondary prevention, as the limited and small trials do not provide sufficient data to support the use of these conventional heart failure drugs for systemic RV dysfunction.3,4
Recent long-term follow-up of the largest randomized controlled trial on renin–angiotensin–aldosterone system (RAAS) inhibition in unselected systemic RV patients failed to show a treatment effect of valsartan, but a post hoc analysis demonstrated favourable long-term outcomes in symptomatic patients.5,6 Additionally, observational data have suggested benefit from β-blockers on functional status and RV dimensions.7 However, cautious use of β-blockers is recommended considering concerns of bradycardia and the limited preload physiology due to reduced distensibility of the atrial baffles.4,8 Hence, evidence to select patients in whom treatment with RAAS inhibition and β-blockers would be justified remains inconclusive.
We therefore studied the use of cardiovascular medication in a cohort of TGA-AtrSO patients over time and with increasing age. Moreover, we analysed whether the use of RAAS inhibitors or β-blockers was associated with improved survival.
Methods
Study population and data collection
This study comprised adult patients (age ≥ 18 years) included in the Dutch nationwide CONCOR registry9 with simple or complex TGA (defined as TGA with concomitant ventricular septal defect, LV outflow tract obstruction, or coarctation of the aorta) after AtrSO from five tertiary medical centres. Data on diagnoses, clinical events, and procedures were retrieved from medical records and classified using the European Pediatric Cardiac Code (EPCC) short list after written informed consent for inclusion in the CONCOR registry. We collected data on diagnosis and occurrence of arrhythmias, pacemaker or implantable cardioverter-defibrillator (ICD) implantations, and baffle or tricuspid valve reinterventions (EPCC codes in Supplementary material online, Table S1). CONCOR was approved by the ethics boards of all participating centres and complies with the declaration of Helsinki.9
Patient-level data from the CONCOR registry were linked to the national Dispensed Drug Register (DDR) and Cause of Death Register (CDR) through Statistics Netherlands. The linkage of CONCOR to the DDR and CDR has been described in detail previously.10,11 For all Dutch residents, the DDR contains aggregated data on all types of dispensed outpatient drugs reimbursed by the compulsory basic Dutch health insurance per patient per year according to the Anatomical Therapeutic Chemical (ATC) classification, aggregated at the pharmacological level of the ATC classification. Specific drugs and their duration, timing, and daily doses within this 1-year window cannot be extracted. Receiving a drug is coded as dichotomous value for a full year, regardless of the amount of drugs dispensed. Data on dispensed cardiovascular medication, specifically RAAS inhibitors (i.e. angiotensin-converting enzyme inhibitors and angiotensin receptor blockers), diuretics (including thiazides, high-ceiling diuretics, and potassium-sparing agents), β-blockers, antiarrhythmics Classes I and III, and cardiac glycosides including digoxin (ATC codes in Supplementary material online, Table S2), were extracted from the DDR. Patients were followed from 2006 or CONCOR-inclusion, until end of study (2014) or death.
Definition of symptomatic heart failure
We used diuretics use as a proxy for symptomatic heart failure, as diuretics are prescribed to relieve symptoms and signs of congestion,2,3 and no data on functional status were available due to limited clinical detail inherent to the large administrative datasets used in this study. Patients with prior or concurrent diuretics use were defined as patients with symptomatic heart failure, and patients without diuretics as asymptomatic patients.
Statistical analysis
Data are summarized as n (%), mean ± standard deviation, and median [interquartile range (IQR)]. Medication use was described as number of patients with medication per total number of patients at risk. Generalized estimating equations (GEE) with exchangeable working correlation and robust variance estimators were used to model expected medication use in the population over age. Bivariable GEE were used to estimate the association between calendar year, patient characteristics, and medication use, corrected for age.
The association between use of heart failure medication, being RAAS inhibitors and β-blockers, and all-cause mortality was evaluated using time-varying Cox proportional hazard models. To adjust for indication bias, marginal structural Cox proportional hazard models were fit. These models were weighted by inverse probability-of-treatment weighting to correct for time-varying confounding related to the clinical decision to initiate RAAS inhibitors or β-blockers.12,13 Covariates included in the inverse probability-of-treatment weighting model were age, time-updated status of having a pacemaker/ICD device, and time-varying use of cardiac medication (including diuretics, digoxin, antiarrhythmics, and β-blockers for exposure to RAAS inhibition, and RAAS inhibition for exposure to β-blockers) (distribution of weights in Supplementary material online, Figure S1).
Due to the yearly aggregated nature of the data, medication used in a particular year was taken as medication present at the first of January of the following year. Therefore, we excluded patients who were included in 2014 or died in their year of inclusion in all survival analyses.
Subgroup analyses were performed in three subsets of the population hypothesized to be at highest risk of mortality, being symptomatic patients, patients with implanted pacemaker/ICD devices, and patients aged ≥30 years. For these subgroup analyses, patients were included in their year of first diuretics use, device implantation, or reaching the age of 30.
Statistical analyses were performed using R-version 3.5.2 (R Core Team, Vienna, Austria), the gee,14 survival,15 and ipw12,13 packages. Two-sided P-values of <0.05 were considered statistically significant.
Results
In total, 150 TGA-AtrSO patients were included (patient characteristics and medication use in Table 1). At baseline, the majority of patients did not use any cardiac medication, 51 (34%) used RAAS inhibitors, 35 (23%) β-blockers [19 patients (13%) used a combination of these two] and a minority used diuretics (n = 16, 11%), cardiac glycosides (n = 16, 11%), or antiarrhythmics (n = 13, 9%). At baseline, 11 patients (7%) used RAAS inhibitors, β-blockers, and diuretics concurrently.
Table 1.
Patient characteristics and medication use at baseline and during follow-up
| All patients (n = 150) | ||
|---|---|---|
| Baseline | Anya | |
| Patient characteristics | ||
| Age at baseline (years) | 30 (25–35) | |
| Female gender | 57 (38) | |
| Complex TGAb | 43 (29) | |
| Senning | 55 (37) | |
| Late repair (atrial switch > 1 year of age) | 76 (51) | |
| Age at atrial switch (years) | 1.02 (0.28–3.03) | |
| Clinical events | ||
| Arrhythmias | 59 (39) | 80 (53) |
| Pacemaker/ICD | 36 (24) | 43 (29) |
| Baffle/tricuspid reinterventions | 23 (15) | 31 (21) |
| Medication use | ||
| No medication usec | 77 (51) | 43 (29) |
| Cardiac glycosides | 16 (11) | 23 (15) |
| Antiarrhythmics Type I and III | 13 (9) | 21 (14) |
| Diuretics | 16 (11) | 43 (29) |
| β-blockers | 35 (23) | 75 (50) |
| RAAS inhibitors | 51 (34) | 78 (52) |
ICD, implantable cardioverter-defibrillator; RAAS, renin–angiotensin–aldosterone system; TGA, transposition of the great arteries.
Amount of patients with clinical events and medication use at baseline or during the study period.
Complex TGA was defined as TGA with concomitant ventricular septal defect, left ventricular outflow tract obstruction, or coarctation of the aorta.
None of the medications mentioned in the table. Data are described as frequency with percentage (%) or median with interquartile range.
A total of 1248 patient-years were analysed, with 124 patients (82%) followed for the complete 9-year study period. At the end of follow-up, at a median age of 38 (IQR 33–42) years, 80 patients (53%) had experienced arrhythmias, 43 (29%) had received a pacemaker or ICD, and 31 (21%) had undergone baffle or tricuspid valve reinterventions.
During the study period 78 patients (52%) used RAAS inhibitors, 75 (50%) β-blockers, 43 (29%) diuretics, 23 (15%) cardiac glycosides, and 21 (14%) antiarrhythmics at any time. Most patients (>60%) continuously used the medication following their first year of use. Only 43 patients (29%) did not use any of these drugs during follow-up. In symptomatic patients, the use of heart failure medication was high, with 35 (81%) using RAAS inhibitors and 33 (77%) using β-blockers. In asymptomatic patients, 59 (44%) used RAAS inhibitors and 49 (37%) used β-blockers.
Medication and age
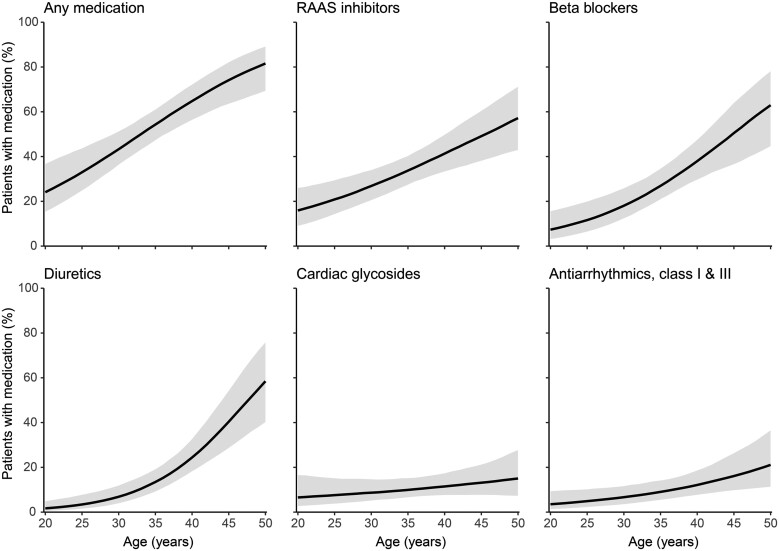
Medication use increased with age (Figure 1). RAAS inhibitors were already used by 21% [95% confidence interval (CI) 14–40] of patients at 25 years of age, increasing to 49% (95% CI 38–60) at 45 years of age [odds ratio (OR) = 1.07/year (95% CI 1.03–1.11)]. Use of β-blockers also increased, from 12% (95% CI 7–20) at 25 years of age to 51% (95% CI 37–64) at 45 years of age [OR = 1.11/year (95% CI 1.06–1.16)], as did use of antiarrhythmics, from 5% (95% CI 2–10) to 16% (95% CI 10–26) [OR = 1.07/year (95% CI 1.02–1.12)]. There was no significant increase in the use of cardiac glycosides with increasing age [OR = 1.03/year (95% CI 0.98–1.09)]. The largest difference was seen in use of diuretics, used by only 3% (95% CI 2–8) of patients at age 25, increasing to 40% (95% CI 29–54) at age 45 [OR = 1.16/year (95% CI 1.10–1.23)].
Figure 1.
Medication use according to age. Expected percentage of the population with dispensed medication according to age, with 95% confidence intervals. Any medication entails the percentage of patients with any of the five medication groups mentioned in the other five panels. RAAS, renin–angiotensin–aldosterone system.
With advanced age, the combined use of medication became more common (Figure 2). At age 25, 33% (95% CI 24–43) used any medication and only 7% (95% CI 3–16) used two or more different medication types, increasing to 54% (95% CI 40–67) at age 45. Thirty-two percent (95% CI 23–43) used three or more different medications simultaneously at age 45.
Figure 2.

Cumulative use of cardiac medication according to age. Expected percentage of the population with at least one, two, or three different types of dispensed cardiac medication according to age, with 95% confidence intervals. The different types of medication included are renin–angiotensin–aldosterone system inhibitors, β-blockers, diuretics, antiarrhythmics (Classes I and III), and cardiac glycosides.
Medication over time
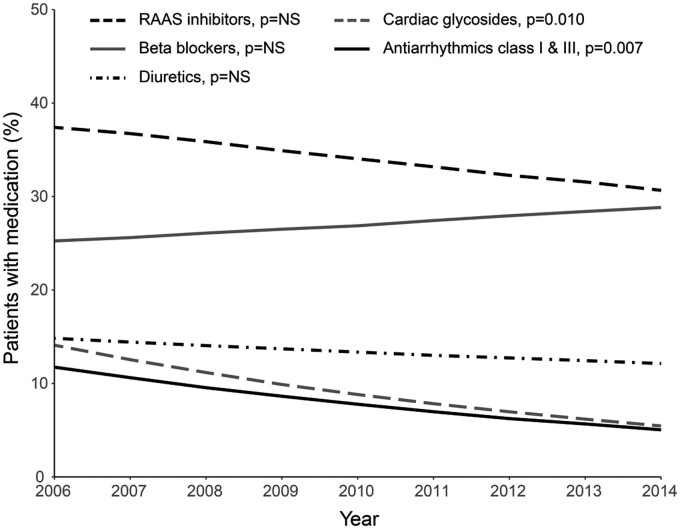
There was no significant association between calendar year and use of RAAS inhibitors, diuretics, and β-blockers after correction for age, suggesting no significant change in prescribing policy of these drugs over time (Figure 3, crude data in Supplementary material online, Figure S2). However, the age-corrected use of cardiac glycosides and antiarrhythmics significantly decreased over the years [OR = 0.88/year (95% CI 0.80–0.97); P = 0.010 and OR = 0.89 (95% CI 0.82–0.97); P = 0.007, respectively].
Figure 3.
Age-corrected medication use over the years. Expected percentage of patients with dispensed medication at 35 years of age during the different years of the study period. P-values for age-corrected association between calendar year and medication use (Supplementary material online, Table S3). NS, not significant; RAAS, renin–angiotensin–aldosterone system.
Factors associated with medication use
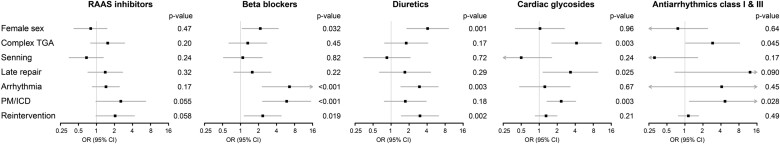
Patient factors associated with medication use—independent of age—are depicted in Figure 4. Women more commonly used diuretics and β-blockers than men [OR = 4.08 (95% CI 1.83–9.12) and OR = 2.14 (95% CI 1.07–4.28), respectively). Use of β-blockers was associated with arrhythmias [OR = 6.58 (95% CI 2.31–18.75)] and implanted devices [OR = 5.90 (95% CI 2.34–14.9)]. Patients with implanted devices also used antiarrhythmics more often than patients without devices [OR = 4.68 (95% CI 1.19–18.5)]. Baffle or tricuspid reinterventions were associated with higher risk of diuretics [OR = 3.04 (95% CI 1.49–6.20)] and β-blocker use [OR = 2.35 (1.15–4.77)]. Increased use of cardiac glycosides was seen in patients with complex TGA [OR = 4.20 (95% CI 1.62–10.9)], late AtrSO [>1 year after birth, OR = 3.33 (95% CI 1.16–9.54)], and implanted devices [OR = 2.33 (95% CI 1.35–4.05)]. None of the patient characteristics was significantly associated with higher use of RAAS inhibition.
Figure 4.
Age-corrected associations between patient characteristics and medication use. Associations between patient characteristics and the use of cardiac medications, corrected for age. Higher odds ratios indicate higher use of the medication in patients with the characteristic vs. patients without the characteristic. Late repairs are defined as atrial switch at >1 year of age. CI, confidence interval; ICD, implantable cardioverter-defibrillator; OR, odds ratio; PM, pacemaker; RAAS, renin–angiotensin–aldosterone system; TGA, transposition of the great arteries.
Survival
Survival analyses included 149 patients with a median of 8 years of follow-up, during which 10 patients died. Marginal structural models that adjusted for confounding medication showed that use of RAAS inhibitors or β-blockers was not associated with lower risk of mortality in the overall cohort of TGA-AtrSO patients [hazard ratio (HR) = 0.93 (95% CI 0.24–3.63); P = 0.92 and HR = 0.98 (0.23–4.17); P = 0.98, respectively]. No significant difference in mortality risk was seen with use of RAAS inhibitors or β-blockers in 42 patients with implanted devices [HR = 0.27 (95% CI 0.06–1.27); P = 0.097 and HR = 0.35 (95% CI 0.06–1.88); P = 0.22] or in 136 patients beyond the age of 30 years [HR = 0.57 (95% CI 0.14–2.32); P = 0.43 and HR = 0.66 (95% CI 0.16–2.76); P = 0.57]. In the 39 symptomatic patients, use of RAAS inhibitors and use of β-blockers was associated with a significantly lower risk of mortality [HR = 0.13 (95% CI 0.03–0.73); P = 0.020 and HR = 0.12 (95% CI 0.02–0.17); P = 0.019, respectively]. The treatment benefit of RAAS inhibitors was significantly greater in symptomatic vs. asymptomatic patients (Pinteraction = 0.045). For use of β-blockers, there was no significant difference in treatment benefit between symptomatic and asymptomatic patients (Pinteraction = 0.24).
Discussion
This is the largest study to date evaluating medication use in TGA-AtrSO patients. We showed that the use of conventional medication for left-sided heart failure, i.e. RAAS inhibitors and β-blockers, is high, with 52% of patients using RAAS inhibitors and 50% using β-blockers within the 9-year study period. Medication use increased strongly as the population aged, with 74% having cardiac medication at the age of 45 years. Cardiac glycosides and antiarrhythmics were prescribed less frequently in more recent years. Importantly, use of RAAS inhibitors and β-blockers was associated with improved survival in symptomatic patients only.
Medication burden
The high use of heart failure medication at baseline is in line with other smaller cohorts,16,17 but our study is the first to show the increasing and cumulative medication burden during adulthood, with 74% using cardiac medication at age of 45, and even 32% using three or more different cardiac drugs at that age. Although diuretics were sparsely used in early adulthood, its use steeply increased after the age of 30. As diuretics are essentially used to relieve symptoms in overt heart failure, this likely reflects deterioration of the systemic RV and the increasing prevalence of symptomatic heart failure in the population. Furthermore, 44% of asymptomatic patients used RAAS inhibition, suggesting that a large proportion of patients receive RAAS inhibition as primary prevention, possibly in the setting of asymptomatic reduced ventricular function. These data reveal that heart failure medication is broadly used in clinical practice, even though the speculative benefits mainly apply to symptomatic patients.3–6
Changes in medical management
The use of cardiac glycosides including digoxin and antiarrhythmics decreased over time, conforming to the downward temporal trend seen in patients with acquired cardiovascular disease.18,19 Although digoxin may be considered in patients with heart failure, cardiologists have become more selective in prescribing it, as data concerning its benefit remain controversial.2,20 The decrease in use of antiarrhythmics such as amiodarone may relate to growing safety concerns, switching from rhythm control to rate control strategies, and increasing use of catheter ablation to treat arrhythmias.21,22 Furthermore, suggestions to liberalize use of β-blockers, which may protect against ventricular arrhythmias and sudden cardiac death, may also play a role in the change in medical arrhythmia management.23,24 Reservations about the use of β-blockers due to risk of sinus node dysfunction and conduction disorders may be the main reason why the age-adjusted use of β-blockers has not significantly increased over the years.
Patients with higher medication use
Women were more likely to use diuretics and β-blockers than men, similar to findings in the general population.25 The rationale of these sex-differences is incompletely understood, but may include a lower likelihood of men to seek preventive healthcare than women26–28 and physical differences, as women may tolerate less weight gain from mild fluid retention and men may suffer sexual dysfunction.29 An increased risk for premature RV deterioration related to pregnancy may also play a role.30 The association between other patient characteristics and specific medication use may be due to an increased risk of complications or presence of symptoms in those patients. Complex TGA, late repair, and implanted devices have been reported as predictors for arrhythmias and heart failure.31–33 Patients with a prior baffle reintervention or tricuspid valve procedure will more often be symptomatic than those without need for reinterventions, as the interventions are particularly recommended when symptoms occur.3
Treatment benefit of conventional heart failure medication
Use of RAAS inhibitors and β-blockers was associated with reduced risk of mortality in a subset of symptomatic TGA-AtrSO patients, who used diuretics. Diuretics are prescribed to relieve symptoms and signs of congestion.2 Hence, it is reasonable to assume that the subgroup with benefit could be the more symptomatic patients. No significant association was seen in the overall cohort, thus providing no evidence for a possible effect of primary prevention in patients without symptomatic heart failure. As such, our data complement prior randomized data on RAAS inhibition that suggest reduced morbidity in symptomatic, but not in asymptomatic patients.5,6 Evidence on β-blockers is scarce, with only four small retrospective studies investigating their use.7,34 The lower mortality seen with use of β-blockers in symptomatic patients is in line with functional improvement seen with use of β-blockers in these prior studies.
The lack of an association of both RAAS inhibitors and β-blockers with survival in the overall cohort may reflect the heterogeneous clinical course and low event rate. Although we did not have access to data on RV ejection fraction, it seems likely that a large part of asymptomatic patients had only mild systolic dysfunction, as this is often the case in systemic RV cohorts.5,35,36 For acquired heart failure with preserved ejection fraction, evidence on treatment benefit of RAAS inhibitors and β-blockers also remains inconclusive.2 It is possible that the asymptomatic patients who did receive heart failure treatment had RV dysfunction or mild symptoms, but we could not differentiate between these patients and completely asymptomatic patients in the present study. Consequently, it is still not clear-cut whether treatment decisions should differ between asymptomatic patients with and without reduced systolic RV function.
Clinical implications
Current guidelines on medical therapy in TGA-AtrSO patients refrain from recommendations on heart failure medication as prevention in asymptomatic patients or as treatment in symptomatic patients.3,4,34 Therefore, clinicians have to base the decision to start medical therapy on personal judgement, resulting in high medication use in this cohort despite uncertain benefit. This study adds to the existing data of small studies, indicating that RAAS inhibitors and β-blockers may improve prognosis of symptomatic patients who require symptom relief by diuretics. Prescribing medication only in patients with possible benefit limits the risks of adverse drug events, especially regarding conduction disturbances with use of β-blockers in AtrSO patients. These findings can direct future guidelines that may suggest the use of RAAS inhibition and β-blockers as secondary prevention to decrease both morbidity and mortality in symptomatic patients. Additionally, more data on β-blockers are needed to further guarantee their beneficial effects, as only limited data exist to date. The small and heterogeneous population necessitates global research efforts to improve levels of evidence. It is, however, plausible that some uncertainty regarding treatment benefit in TGA-AtrSO will remain present. Nonetheless, the present study provides insight that helps to bridge the gap of knowledge.
Methodological issues
Strengths of this study include the complete follow-up, the time-updated data on dispensed medication, and the use of marginal structural models to correct for confounding by indication. Administrative databases are a powerful tool to assess patient management and outcomes.37 The automated collection of data on dispensed medication within a national administrative database provides more accurate information on actual medication consumption than medical records. However, limitations inherent to the use of administrative data include limited clinical detail, as no information on comorbidities, functional status, medication indications, and daily doses were available. This limited our definition of symptomatic patients to those on diuretic therapy. Additionally, privacy restrictions from Statistics Netherlands prohibited reporting on characteristics of small groups of patients. Differentiation between subgroups of medication classes (e.g. angiotensin-converting enzyme inhibitors and angiotensin receptor blockers) was therefore not possible. Although this is the largest study to date to evaluate medical therapy in TGA-AtrSO patients, with a long follow-up period and hard clinical endpoints, the amount of events was still small. Finally, as this was an observational study, there was a risk of additional unmeasured confounders that we could not adjust for in the models.
Conclusion
This study provides insight in the medical management of adult TGA-AtrSO patients. It showed that 74% of patients use cardiac medication by the age of 45. This mainly consists of heart failure therapy with 49% using RAAS inhibitors and 51% using β-blockers. In a subgroup of symptomatic patients, risk of mortality was lower with use of RAAS inhibitors and β-blockers. In the overall cohort, no benefit of RAAS inhibition and β-blockers was seen. These findings provide clinicians and future guidelines with directive data, supporting the use of RAAS inhibitors and β-blockers in symptomatic, but not in asymptomatic adult TGA-AtrSO patients.
Supplementary material
Supplementary material is available at European Heart Journal – Cardiovascular Pharmacotherapy online.
Supplementary Material
Acknowledgements
The authors thank all CONCOR participants, Lia Engelfriet, and Sylvia Mantels. CONCOR is part of Parelsnoer clinical biobanks at Health-RI. Results are based on calculations by the Amsterdam UMC—University of Amsterdam, using non-public microdata from Statistics Netherlands, which are accessible for statistical and scientific research under certain conditions.
Contributor Information
Joey M Kuijpers, Heart Center, Department of Clinical and Experimental Cardiology, Amsterdam UMC, University of Amsterdam, Amsterdam Cardiovascular Sciences, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Monique R M Jongbloed, Department of Cardiology, Leiden University Medical Center, Albinusdreef 2, 2333 ZA Leiden, The Netherlands; Department of Anatomy & Embryology, Leiden University Medical Center, Albinusdreef 2, 2333 ZA Leiden, The Netherlands.
Arie P J van Dijk, Department of Cardiology, Radboud University Medical Center, Geert Grooteplein Zuid 10, 6525 GA Nijmegen, The Netherlands.
Gertjan T Sieswerda, Department of Cardiology, University Medical Center Utrecht, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands.
Hubert W Vliegen, Department of Cardiology, Leiden University Medical Center, Albinusdreef 2, 2333 ZA Leiden, The Netherlands.
Anastasia D Egorova, Department of Cardiology, Leiden University Medical Center, Albinusdreef 2, 2333 ZA Leiden, The Netherlands.
Philippine Kiès, Department of Cardiology, Leiden University Medical Center, Albinusdreef 2, 2333 ZA Leiden, The Netherlands.
Anthonie L Duijnhouwer, Department of Cardiology, Radboud University Medical Center, Geert Grooteplein Zuid 10, 6525 GA Nijmegen, The Netherlands.
Daniëlle Robbers-Visser, Heart Center, Department of Clinical and Experimental Cardiology, Amsterdam UMC, University of Amsterdam, Amsterdam Cardiovascular Sciences, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Thelma C Konings, Department of Cardiology, Amsterdam UMC, VU University, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands.
Aeilko H Zwinderman, Department of Clinical Epidemiology, Biostatistics and Bioinformatics, Amsterdam UMC, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Folkert J Meijboom, Department of Cardiology, University Medical Center Utrecht, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands.
Barbara J M Mulder, Heart Center, Department of Clinical and Experimental Cardiology, Amsterdam UMC, University of Amsterdam, Amsterdam Cardiovascular Sciences, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Berto J Bouma, Heart Center, Department of Clinical and Experimental Cardiology, Amsterdam UMC, University of Amsterdam, Amsterdam Cardiovascular Sciences, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Funding
This work was supported by the Dutch Heart Foundation [CVON 2014-18 project CONCOR-genes to F.J.M. and B.J.B.] and the Amsterdam University Fund [8532 to O.I.W.].
Conflict of interest: B.J.M.M. and B.J.B. report grants from Actelion Pharmaceuticals, Bristol-Myers Squibb, Boehringer Ingelheim, Bayer, and Daiichi Sankyo outside this work. All other authors declared no conflict of interest.
References
- 1. Couperus LE, Vliegen HW, Zandstra TE, Kies P, Jongbloed MRM, Holman ER, Zeppenfeld K, Hazekamp MG, Schalij MJ, Scherptong RWC. Long-term outcome after atrial correction for transposition of the great arteries. Heart (British Cardiac Society) 2019;105:790–796. [DOI] [PubMed] [Google Scholar]
- 2. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 3. Baumgartner H, Bonhoeffer P, De Groot NMS, de Haan F, Deanfield JE, Galie N, Gatzoulis MA, Gohlke-Baerwolf C, Kaemmerer H, Kilner P, Meijboom F, Mulder BJM, Oechslin E, Oliver JM, Serraf A, Szatmari A, Thaulow E, Vouhe PR, Walma E; Task Force on the Management of Grown-up Congenital Heart Disease of the European Society of Cardiology (ESC); Association for European Paediatric Cardiology (AEPC); ESC Committee for Practice Guidelines (CPG). ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010;31:2915–2957. [DOI] [PubMed] [Google Scholar]
- 4. Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, Khairy P, Landzberg MJ, Saidi A, Valente AM, Van Hare GF. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation 2019;139:e698–e800. [DOI] [PubMed] [Google Scholar]
- 5. van Dissel AC, Winter MM, van der Bom T, Vliegen HW, van Dijk APJ, Pieper PG, Sieswerda GT, Roos-Hesselink JW, Zwinderman AH, Mulder BJM, Bouma BJ. Long-term clinical outcomes of valsartan in patients with a systemic right ventricle: follow-up of a multicenter randomized controlled trial. Int J Cardiol 2019;278:84–87. [DOI] [PubMed] [Google Scholar]
- 6. van der Bom T, Winter MM, Bouma BJ, Groenink M, Vliegen HW, Pieper PG, van Dijk AP, Sieswerda GT, Roos-Hesselink JW, Zwinderman AH, Mulder BJ. Effect of valsartan on systemic right ventricular function: a double-blind, randomized, placebo-controlled pilot trial. Circulation 2013;127:322–330. [DOI] [PubMed] [Google Scholar]
- 7. Doughan AR, McConnell ME, Book WM. Effect of beta blockers (carvedilol or metoprolol XL) in patients with transposition of great arteries and dysfunction of the systemic right ventricle. Am J Cardiol 2007;99:704–706. [DOI] [PubMed] [Google Scholar]
- 8. Tulevski II, Lee PL, Groenink M, van der Wall EE, Stoker J, Pieper PG, Romkes H, Hirsch A, Mulder BJ. Dobutamine-induced increase of right ventricular contractility without increased stroke volume in adolescent patients with transposition of the great arteries: evaluation with magnetic resonance imaging. Int J Cardiac Imaging 2000;16:471–478. [DOI] [PubMed] [Google Scholar]
- 9. van der Velde ET, Vriend JW, Mannens MM, Uiterwaal CS, Brand R, Mulder BJ. CONCOR, an initiative towards a national registry and DNA-bank of patients with congenital heart disease in the Netherlands: rationale, design, and first results. Eur J Epidemiol 2005;20:549–557. [DOI] [PubMed] [Google Scholar]
- 10. Woudstra OI, Kuijpers JM, Meijboom FJ, Post MC, Jongbloed MRM, Duijnhouwer AL, van Dijk APJ, van Melle JP, Konings TC, Zwinderman AH, Mulder BJM, Bouma BJ. High burden of drug therapy in adult congenital heart disease: polypharmacy as marker of morbidity and mortality. Eur Heart J Cardiovasc Pharmacother 2019;5:216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuijpers JM, Vaartjes I, Bokma JP, van Melle JP, Sieswerda GT, Konings TC, Bakker-de Boo M, van der Bilt I, Voogel B, Zwinderman AH, Mulder BJM, Bouma BJ. Risk of coronary artery disease in adults with congenital heart disease: a comparison with the general population. Int J Cardiol 2020;304:39–42. [DOI] [PubMed] [Google Scholar]
- 12. van der Wal WM, Geskus RB. ipw: an R package for inverse probability weighting. 2011;43:23. [Google Scholar]
- 13. Graffeo N, Latouche A, Geskus RB, Chevret S. Modeling time-varying exposure using inverse probability of treatment weights. Biom J 2018;60:323–332. [DOI] [PubMed] [Google Scholar]
- 14. Carey VJ. gee: Generalized Estimation Equation Solver. https://CRAN.R-project.org/package=gee (16 April 2020).
- 15. Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York: Springer; 2000. [Google Scholar]
- 16. Cuypers JAAE, Eindhoven JA, Slager MA, Opi P, Utens EMWJ, Helbing WA, Witsenburg M, van den Bosch AE, Ouhlous M, van Domburg RT, Rizopoulos D, Meijboom FJ, Bogers AJJC, Roos-Hesselink JW. The natural and unnatural history of the Mustard procedure: long-term outcome up to 40 years. Eur Heart J 2014;35:1666–1674. [DOI] [PubMed] [Google Scholar]
- 17. Morrison ML, Grant B, McCrossan BA, Sands AJ, Owens CG, Spence MS, Casey FA, Craig BG, Lockhart CJ. 32 year follow up of patients following atrial redirection surgery for transposition of the great arteries. Congenit Heart Dis 2019;14:846–853. [DOI] [PubMed] [Google Scholar]
- 18. Patel N, Ju C, Macon C, Thadani U, Schulte PJ, Hernandez AF, Bhatt DL, Butler J, Yancy CW, Fonarow GC. Temporal trends of digoxin use in patients hospitalized with heart failure: analysis from the American Heart Association get with the guidelines-heart failure registry. JACC Heart Fail 2016;4:348–356. [DOI] [PubMed] [Google Scholar]
- 19. Dalgaard F, Ruwald MH, Lindhardt TB, Gislason GH, Torp-Pedersen C, Pallisgaard JL. Patients with atrial fibrillation and permanent pacemaker: temporal changes in patient characteristics and pharmacotherapy. PLoS One 2018;13:e0195175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vamos M, Erath JW, Hohnloser SH. Digoxin-associated mortality: a systematic review and meta-analysis of the literature. Eur Heart J 2015;36:1831–1838. [DOI] [PubMed] [Google Scholar]
- 21. Hernández-Madrid A, Paul T, Abrams D, Aziz PF, Blom NA, Chen J, Chessa M, Combes N, Dagres N, Diller G, Ernst S, Giamberti A, Hebe J, Janousek J, Kriebel T, Moltedo J, Moreno J, Peinado R, Pison L, Rosenthal E, Skinner JR, Zeppenfeld K, Sticherling C, Kautzner J, Wissner E, Sommer P, Gupta D, Szili-Torok T, Tateno S, Alfaro A, Budts W, Gallego P, Schwerzmann M, Milanesi O, Sarquella-Brugada G, Kornyei L, Sreeram N, Drago F, Dubin A; ESC Scientific Document Group. Arrhythmias in congenital heart disease: a position paper of the European Heart Rhythm Association (EHRA), Association for European Paediatric and Congenital Cardiology (AEPC), and the European Society of Cardiology (ESC) Working Group on Grown-up Congenital heart disease, endorsed by HRS, PACES, APHRS, and SOLAECE. Europace 2018;20:1719–1753. [DOI] [PubMed] [Google Scholar]
- 22. Hayward C, Patel HC, Patel K, Di Mario C, Lyon AR, Ahsan SY, Rowland E. The evolving landscape of oral anti-arrhythmic prescriptions for atrial fibrillation in England: 1998-2014. Eur Heart J Cardiovasc Pharmacother 2016;2:90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khairy P, Van Hare GF, Balaji S, Berul CI, Cecchin F, Cohen MI, Daniels CJ, Deal BJ, Dearani JA, Groot N, Dubin AM, Harris L, Janousek J, Kanter RJ, Karpawich PP, Perry JC, Seslar SP, Shah MJ, Silka MJ, Triedman JK, Walsh EP, Warnes CA. PACES/HRS Expert Consensus Statement on the Recognition and Management of Arrhythmias in Adult Congenital Heart Disease: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology (ACC), the American Heart Association (AHA), the European Heart Rhythm Association (EHRA), the Canadian Heart Rhythm Society (CHRS), and the International Society for Adult Congenital Heart Disease (ISACHD). Heart Rhythm 2014;11:e102–e165. [DOI] [PubMed] [Google Scholar]
- 24. Khairy P, Harris L, Landzberg MJ, Fernandes SM, Barlow A, Mercier LA, Viswanathan S, Chetaille P, Gordon E, Dore A, Cecchin F. Sudden death and defibrillators in transposition of the great arteries with intra-atrial baffles: a multicenter study. Circ Arrhythm Electrophysiol 2008;1:250–257. [DOI] [PubMed] [Google Scholar]
- 25. Loikas D, Wettermark B, von Euler M, Bergman U, Schenck-Gustafsson K. Differences in drug utilisation between men and women: a cross-sectional analysis of all dispensed drugs in Sweden. BMJ Open 2013;3:e002378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pinkhasov RM, Wong J, Kashanian J, Lee M, Samadi DB, Pinkhasov MM, Shabsigh R. Are men shortchanged on health? Perspective on health care utilization and health risk behavior in men and women in the United States. Int J Clin Pract 2010;64:475–487. [DOI] [PubMed] [Google Scholar]
- 27. Billett J, Cowie MR, Gatzoulis MA, Vonder Muhll IF, Majeed A. Comorbidity, healthcare utilisation and process of care measures in patients with congenital heart disease in the UK: cross-sectional, population-based study with case-control analysis. Heart (British Cardiac Society) 2008;94:1194–1199. [DOI] [PubMed] [Google Scholar]
- 28. Willems R, Werbrouck A, De Backer J, Annemans L. Real-world healthcare utilization in adult congenital heart disease: a systematic review of trends and ratios. Cardiol Young 2019;29:553–563. [DOI] [PubMed] [Google Scholar]
- 29. La Torre A, Giupponi G, Duffy D, Conca A, Catanzariti D. Sexual dysfunction related to drugs: a critical review. Part IV: cardiovascular drugs. Pharmacopsychiatry 2015;48:1–6. [DOI] [PubMed] [Google Scholar]
- 30. Bowater SE, Selman TJ, Hudsmith LE, Clift PF, Thompson PJ, Thorne SA. Long-term outcome following pregnancy in women with a systemic right ventricle: is the deterioration due to pregnancy or a consequence of time? Congenit Heart Dis 2013;8:302–307. [DOI] [PubMed] [Google Scholar]
- 31. Schwerzmann M, Salehian O, Harris L, Siu SC, Williams WG, Webb GD, Colman JM, Redington A, Silversides CK. Ventricular arrhythmias and sudden death in adults after a Mustard operation for transposition of the great arteries. Eur Heart J 2009;30:1873–1879. [DOI] [PubMed] [Google Scholar]
- 32. Roubertie F, Thambo JB, Bretonneau A, Iriart X, Laborde N, Baudet E, Roques X. Late outcome of 132 Senning procedures after 20 years of follow-up. Ann Thorac Surg 2011;92:2206–2213; discussion 2213–2214. [DOI] [PubMed] [Google Scholar]
- 33. Moons P, Gewillig M, Sluysmans T, Verhaaren H, Viart P, Massin M, Suys B, Budts W, Pasquet A, De Wolf D, Vliers A. Long term outcome up to 30 years after the Mustard or Senning operation: a nationwide multicentre study in Belgium. Heart (British Cardiac Society) 2004;90:307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zaragoza-Macias E, Zaidi AN, Dendukuri N, Marelli A. Medical therapy for systemic right ventricles: a systematic review (part 1) for the 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:1564–1578. [DOI] [PubMed] [Google Scholar]
- 35. Venkatesh P, Evans AT, Maw AM, Pashun RA, Patel A, Kim L, Feldman D, Minutello R, Wong SC, Stribling JC, LaPar D, Holzer R, Ginns J, Bacha E, Singh HS. Predictors of late mortality in D-transposition of the great arteries after atrial switch repair: systematic review and meta-analysis. J Am Heart Assoc 2019;8:e012932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rydman R, Gatzoulis MA, Ho SY, Ernst S, Swan L, Li W, Wong T, Sheppard M, McCarthy KP, Roughton M, Kilner PJ, Pennell DJ, Babu-Narayan SV. Systemic right ventricular fibrosis detected by cardiovascular magnetic resonance is associated with clinical outcome, mainly new-onset atrial arrhythmia, in patients after atrial redirection surgery for transposition of the great arteries. Circ Cardiovasc Imaging 2015;8:e002628. [DOI] [PubMed] [Google Scholar]
- 37. Cohen S, Gilutz H, Marelli AJ, Iserin L, Benis A, Bonnet D, Burgun A. Administrative health databases for addressing emerging issues in adults with CHD: a systematic review. Cardiol Young 2018;28:844–853. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.