Abstract
The present study examined the effects of a proprietary Ashwagandha (Withania somnifera) root and leaf extract (NooGandha® Specnova LLC, USA) supplement for improving cognitive abilities, cortisol levels, and self-reported mood, stress, food cravings, and anxiety with adults who have perceived stress. Healthy adults (n = 43 women and n = 17 men; mean age = 34.41 years) who reported experiencing perceived stress were randomized to the following groups: Ashwagandha (400 mg/d), Ashwagandha (225 mg/d), and placebo for 30 days. The following outcomes were assessed at Day 0, Day 15, and Day 30: saliva cortisol levels, cognitive performance (i.e., CNS vital signs), and the self-reported measures of Trait Anxiety Inventory, Depression Anxiety Stress Scale, Perceived Stress Scale, and Food Cravings Questionnaire-15. For the self-report assessments, significant main effects for time were evidenced for anxiety, depression, perceived stress, and food cravings, p's < 0.01. The main effect for group and the interactions were non-significant. For the CNS vital signs, significant differences were observed in cognitive flexibility, visual memory, reaction time, psychomotor speed, and executive functioning, p's < 0.05, with the Ashwagandha groups often out-performing the placebo group. Both Ashwagandha groups had reductions in cortisol levels over time, with significant reductions evidenced for the Ashwagandha 225 mg/d group from Day 0 to Day 15 to Day 30. The placebo group had a non-significant increase in cortisol levels from Day 0 to Day 15–30. No adverse events were reported. In conclusion, Ashwagandha supplementation may improve the physiological, cognitive, and psychological effects of stress.
Keywords: Ashwagandha, Cognition, Stress, Cortisol, Withania somnifera
1. Introduction
Stress is associated with decreased cognitive performance, negative moods such as anxiety and depression, as well as exacerbating factors for chronic health conditions such as cardiovascular disease, obesity, and diabetes [1,2]. Ashwagandha (Withania somnifera) is an adaptogen herb that is purported to prevent and treat the effects of stress. Research reveals that Ashwagandha decreases cortisol levels, perceived stress, anxiety, and blood pressure in people under chronic stress [[3], [4], [5]]. Ashwagandha also improves cognitive function in adults with mild cognitive impairment [6].
The purpose of the present study was to assess the short-term effectiveness of Ashwagandha (NooGandha® Specnova LLC, USA) in improving cognitive performance, mood, anxiety, food cravings, and cortisol levels with healthy adults who reported high perceived stress. Using a randomized, double-blind, placebo-controlled design, we hypothesized that 30 days of supplementation with Ashwagandha, would result in improved cognitive performance, perceived stress/anxiety, depressive symptoms, food cravings, and cortisol levels compared to the placebo group, with a dose–response evidenced.
2. Material and methods
2.1. Participants
Participants were 58 healthy adults (n = 43 women and n = 17 men; M age = 34.41 years, SD = 11.78) who reported perceived stress. Participants were enrolled if they met the following inclusion criteria: (1) aged between 18 and 54 years, (2) free of health conditions, and (3) score of at least 14 on the Perceived Stress Scale. Participants were excluded if they met any of the following exclusion criteria: (1) suffering from a physical or psychiatric illness; (2) using hormonal birth-control; (3) taking medication; (4) taking herbal preparations or formulations containing Ashwagandha or related herbs; and (4) pregnant, lactating, or of childbearing potential.
2.2. Procedures and design
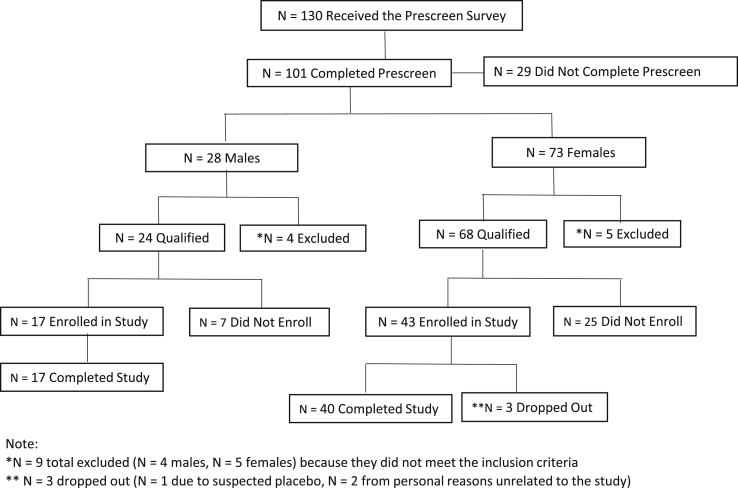
This study received Institutional Review Board (IRB) approval and the subjects signed the IRB consent form prior to participation. Using a placebo-controlled between-group design, participants were blinded and randomized into one of the following conditions: Group A which received 225 mg/d of the Ashwagandha supplement (n = 19), Group B which received 400 mg/d of the Ashwagandha supplement (n = 19), or Group C which received the Placebo supplement (n = 20) for 30 days. Placebo capsules contained rice flour, and the treatment capsules contained either 225 or 400 mg of Ashwagandha root and leaf extract powder called NooGandha®, which was originally sourced from the Neemuch district in the Indian state of Madhya Pradesh. All testing occurred in the exercise physiology laboratory at Day 0, Day 15, and Day 30; supplements were distributed at the Day 0 and Day 15 visits. At the Day 0 assessment, participants received a bottle labeled A, B, or C with the first 15 capsules for their respective group, and at the Day 15 assessment they received the final 15 capsules. During the 30 day trial period, participants were asked to take the supplement capsules at the same point during the day to keep dosing consistent, but each individual was permitted to self-select this daily time point. To ensure adherence to the protocol, participants received either daily email or text reminders to take the capsules, and supplement bottles were collected at the Day 15 and Day 30 lab assessments to determine if any capsules remained. At Day 0, Day 15, and Day 30 lab assessments, which took roughly 45 minutes to complete, were administered. During these testing sessions, participants completed self-report, neurocognitive, and hand grip strength assessments, and provided a saliva sample for cortisol level analysis. Sixty participants were enrolled and 57 completed the study (adherence rate = 95%, See Fig. 6). Reasons for dropout included suspected placebo (n = 1) and negative family events unrelated to the study (n = 2). No adverse events were reported by participants in either of the Ashwagandha root and leaf powder treatment groups.
Fig. 6.
Participant flow chart.
2.3. Measures
2.3.1. Self-report measures
The following psychometrically validated self-report measures were completed: Trait Anxiety Inventory which assesses trait anxiety levels [7]; Depression Anxiety Stress Scale (DASS-21) which measures the magnitude of depression, anxiety, and stress [8]; Perceived Stress Scale which measures the degree to which situations in one's life are appraised as stressful [9]; and the Food Cravings Questionnaire-15 which measures dimensions of food cravings [10].
2.3.2. CNS vital signs
To assess the cognitive ability, the participants completed the CNS Vital Signs, LLC., Morrisville, NC, USA, which is a psychometrically sound neurocognitive test [11]. The following seven CNS Vital Signs tests were administered: (1) Verbal Memory, (2) Visual Memory, (3) Finger Tapping, (4) Symbol Digit Coding, (5) Stroop Test, (6) Shifting Attention, and (7) Continuous Performance. The Stroop Test and Shifting Attention Tests are combined to compute a cognitive flexibility score.
2.3.3. Saliva cortisol
Saliva was collected to assess cortisol levels in the morning following an overnight fast (i.e., 8 h). The participants did not engage in exercise the morning of their assessment. Saliva samples were obtained by Salivette™ synthetic swabs. Synthetic swabs were placed in the oral cavity and chewed for 30 s for saliva collection. Saliva samples were then concentrated by centrifuge, aliquoted, and stored at −80 °C until analyzed by enzyme-linked immunosorbent assay (ELISA, Calbiotech™).
2.4. Data analysis
The data were examined for normality and then analyzed using SPSS and Excel to determine condition and time differences via 3 (Group: 400 mg, 225 mg, and Placebo) x 3 (Time: Day 0, Day 15, and Day 30) repeated measures ANOVAs. The significance level was p's ≤ .05.
3. Results
The daily supplement adherence was 90%. The participants did not report any adverse events.
For the self-report assessments, significant main effects for time were evidenced for anxiety, the DASS subscales of anxiety, stress, and depression, perceived stress, and food cravings, p's < .01. However, the main effect for group and the interactions were non-significant.
The hand grip strength measurements showed improvements in all 3 groups over time when mean scores from Day 0 was compared to both Day 15 and Day 30, but there were no statistically different time or group outcomes for this variable.
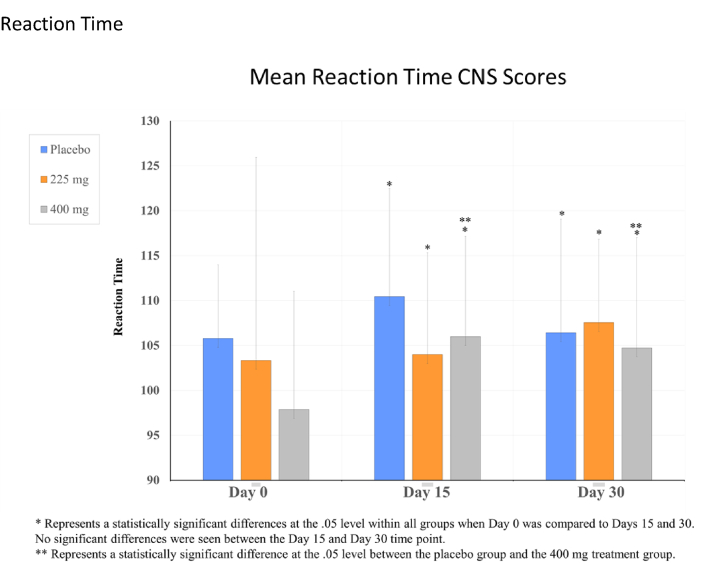
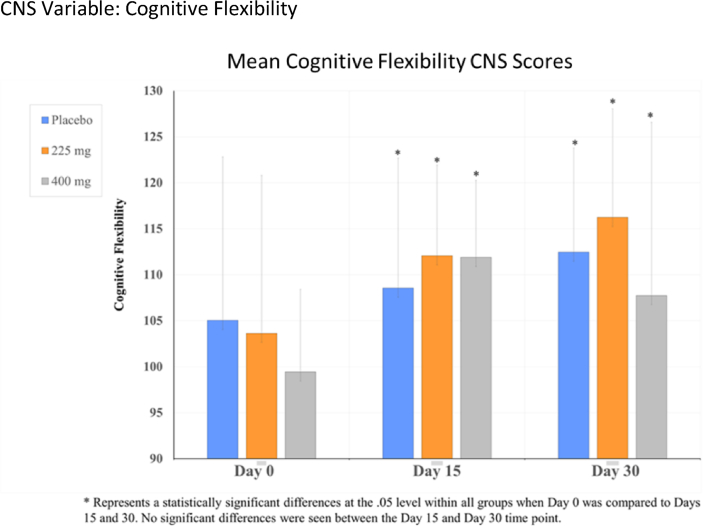
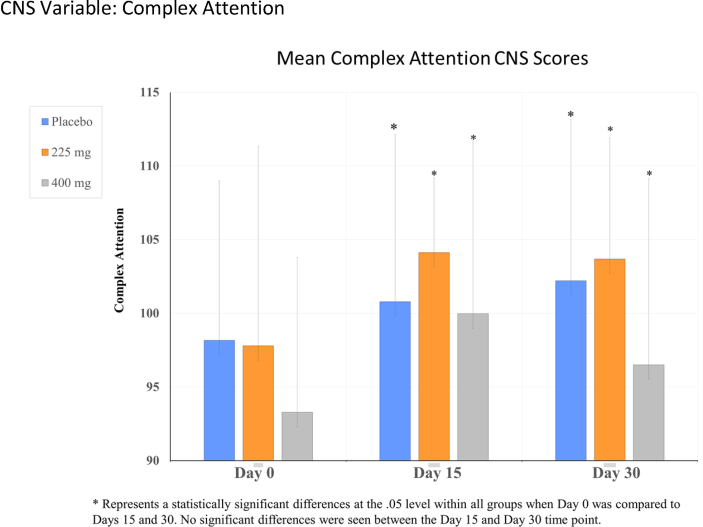
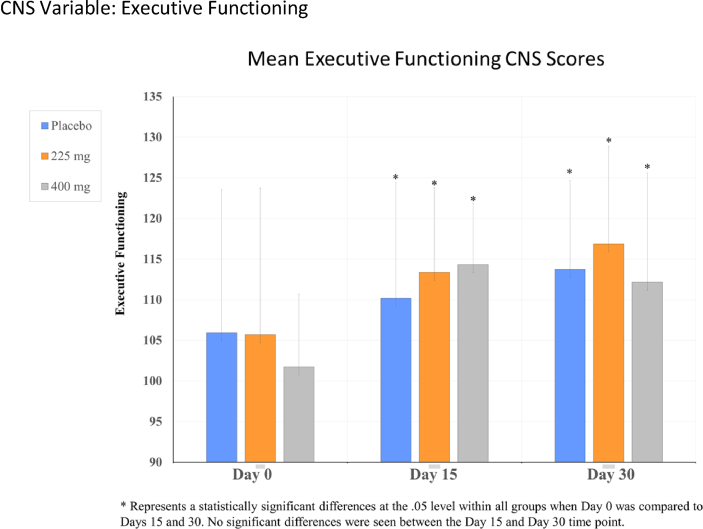
For the CNS vital signs, significant time effect differences were observed in reaction time, complex attention, cognitive flexibility, processing speed, executive functioning, and visual memory, p's < .05 (see Table 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5). Cognitive flexibility, complex attention, executive functioning, and processing speed improved significantly from Day 0 when compared to both Day 15 and Day 30 for all groups, including both Ashwagandha 225 mg and 400 mg/day groups, as well as in the placebo group. However, there was no significant time-effect noted for any variables when Day 15 scores were compared to those at Day 30. Reaction time scores show improvement when scores decrease; however, these increased in all groups from Day 0 to Day 15, then showed a decrease from Day 15 to Day 30 in the Ashwagandha 400 mg/day and placebo groups. A pair-wise comparison showed a significant difference in the scores for reaction time when the placebo group was compared to the Ashwaghanda 400 mg/day group, as scores were significantly lower in the latter group. Visual memory, which shows improvement when scores increase, did so from Day 0 to Day 15 in the placebo group only; however, these scores then decreased from Day 15 to Day 30. In both the Ashwagandha 225 mg and 400 mg groups, visual memory scores decreased from Day 0 to Day 15, and again from Day 15 to Day 30 (see Table 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5).
Table 1.
Descriptive statistics (mean/standard deviation [M/SD]) for CNS vital signs measures.
| Measure | Placebo |
Ashwagandha ± 225 mg |
Ashwagandha ± 400 mg |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 0 Mean ± sd | Day 15 Mean ± sd | Day 30 Mean ± sd | Day 0 Mean ± sd | Day 15 Mean ± sd | Day 30 Mean ± sd | Day 0 Mean ± sd | Day 15 Mean ± sd | Day 30 Mean ± sd | |
| Memory | 101.05 ± 13.1 | 108.75 ± 14.94 | 102.89 ± 18.06 | 106 ± 10.6 | 106.63 ± 8.52 | 103.73 ± 12.16 | 101.65 ± 14.66 | 102.06 ± 10.79 | 98.66 ± 12.81 |
| Psychomotor speed | 107.53 ± 14.52 | 105.76 ± 8.14 | 108.22 ± 10.71 | 99.41 ± 22.58 | 106.18 ± 11.32 | 107.5 ± 9.25 | 103.95 ± 13.15 | 106 ± 11.14 | 104.74 ± 12.29 |
| aReaction timeb | 105.78 ± 8.2 | 110.44 ± 12.04 | 106.42 ± 12.65 | 103.35 ± 11.48 | 104 ± 9.35 | 107.56 ± 9.38 | 97.89 ± 14 | 102.21 ± 6.82 | 102.37 ± 9.9 |
| aComplex attentionb | 98.17 ± 10.83 | 100.79 ± 11.34 | 102.21 ± 10.94 | 97.8 ± 13.52 | 104.13 ± 5.06 | 103.69 ± 8.22 | 93.29 ± 10.51 | 99.97 ± 11.71 | 96.5 ± 12.65 |
| Cognitive flexibilityb | 105.05 ± 17.75 | 108.56 ± 14.12 | 112.47 ± 11.32 | 103.63 ± 17.17 | 112.06 ± 9.85 | 116.24 ± 11.8 | 99.44 ± 8.98 | 111.89 ± 8.36 | 107.74 ± 16.84 |
| Processing speedb | 104.58 ± 12.8 | 109.11 ± 17.1 | 109.21 ± 13.51 | 100.12 ± 9.83 | 107.71 ± 11.08 | 105.18 ± 9.47 | 100.89 ± 13.42 | 106.97 ± 13.14 | 105.95 ± 11.3 |
| Executive functioningb | 105.95 ± 17.6 | 110.17 ± 14.24 | 113.74 ± 10.94 | 105.69 ± 18.02 | 113.38 ± 10.4 | 116.88 ± 12 | 101.72 ± 8.96 | 114.32 ± 7.52 | 112.17 ± 13.45 |
| Verbal memory | 105.24 ± 12.17 | 104.06 ± 15.78 | 102 ± 22.58 | 101.82 ± 14.07 | 104 ± 15.76 | 101.97 ± 13.78 | 98.67 ± 19.87 | 101.66 ± 13.89 | 103.45 ± 14.84 |
| Visual memoryb | 100.42 ± 9.61 | 108.94 ± 13.64 | 99.94 ± 19.59 | 105.69 ± 9.39 | 103 ± 16.1 | 100.94 ± 15.19 | 99.16 ± 13.35 | 98.37 ± 11.94 | 95.08 ± 13.54 |
| Simple attention | 100.41 ± 9.87 | 99.53 ± 13.42 | 103.5 ± 7.66 | 102 ± 8.37 | 99.31 ± 9.7 | 101.97 ± 6.91 | 97.71 ± 9.02 | 100.33 ± 9.88 | 96.72 ± 9.55 |
| Motor speed | 106.32 ± 17.94 | 108.89 ± 18.77 | 106.74 ± 16.12 | 99.47 ± 28.54 | 103.59 ± 12.02 | 106.03 ± 8.08 | 104.32 ± 12.97 | 103.29 ± 11.28 | 102 ± 11.53 |
Signifies that a lower score shows improvement.
Signifies a statistically significant difference at the .05 level.
Fig. 2.
Reaction time.
Fig. 3.
CNS variable: Cognitive flexibility.
Fig. 4.
CNS variable: Complex attention.
Fig. 5.
CNS variable: Executive functioning.
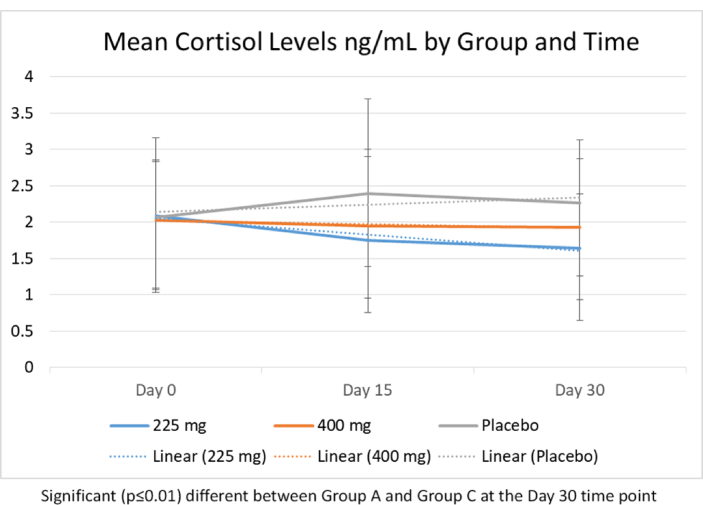
For cortisol levels, at Day 15 and Day 30 the Ashwagandha 225 mg/day group had significant reductions in cortisol levels compared to baseline. A time-effect was evidenced for the Ashwagandha 225 mg/day group with larger effects found at 30 Day compared to Day 15 (See Fig. 1). The Ashwagandha 400 mg/day group had a reduction in cortisol levels from baseline to Day 15 to Day 30, albeit non-significant. The placebo group had a non-significant increase in cortisol levels from baseline to Day 15 and 30.
Fig. 1.
Line graph of the mean cortisol levels by group and time.
4. Discussion
We found that one month of Ashwagandha supplementation (225 mg/day and 400 mg/day) was safe and it had a positive effects on the participants’ cortisol levels, cognitive ability, and self-reported stress, anxiety, depression, and food cravings. A placebo effect, however, on the self-reported measures prevented significant group and interaction effects to occur. The objective measures of cortisol and cognitive performance revealed significant positive effects of Ashwagandha compared to placebo. Research by Chandrasekhra et al. found significant improvements in perceived stress, mood, and cortisol levels in the Ashwagandha group compared to placebo over 8 weeks [3].
Similar to research by Panglio et al., we found significant improvements in cognitive performance with Ashwagandha supplementation [12]. Cognitive flexibility, which measures how well a person is able to adapt to rapidly changing and increasingly complex set of directions and/or to manipulate the information, improved significantly from Day 0 to both Day 15 and Day 30 for all groups. As there were no significant between-group differences noted, this indicates that there was a placebo effect. Executive functioning, which measures how well a person recognizes rules, categories, and manages or navigates rapid decision-making, also showed significant improvement in all groups from Day 0 when compared to both Day 15 and Day 30.
Reaction time, which assesses how quickly a person can react, in milliseconds, to a simple and increasingly complex direction set, increased in all groups over time. This may be attributed to “testing fatigue” as participants completed these assessments at all 3 site visits. However, scores were consistently lowest in the Ashwaghanda 400 mg/day group, and a significant between-group difference was found when this treatment group was compared to the placebo group. Visual memory, which measures how well a person can recognize, remember and retrieve geometric figures, (i.e., remembering graphic instructions, navigating, operating machines, recalling images, and/or remembering a calendar of events) decreased in both Ashwagandha 225 mg and 400 mg groups from Day 0 to Day 15, and in all groups from Day 15 to Day 30. Again, this may be a result of testing fatigue.
Despite the significant findings and similarities to previous research in this area, limitations of the study included a small sample size and acute time frame. Further research is needed to examine the longitudinal effects of Ashwagandha supplementation with a variety of populations.
5. Conclusion
In conclusion, stress has been associated with many health-related implications including decreases in cognitive ability, increases in mood disturbances like depression and anxiety, and an increased risk of developing certain chronic diseases. This study demonstrated that administration of Ashwagandha (Noogandha) supplement daily resulted in improvements in the physiological, cognitive, and psychological stress response over a 30 day period.
Source(s) of funding
SPECNOVA.
Conflict of interest
None.
Author contributions
Heather Hausenblas: Conceptualization, Methodology, Writing- Original draft. Abigail Remenapp: Data collection, Data curation, Writing- Original draft preparation. Stephanie Hooper: Data Curation, Data Analysis, Writing- Reviewing and Editing. Terrance Orange: Data collection. Kevin Coyle: Data collection. David Hooper: Data Analysis. Kara Conway: Cortisol sample analysis.
Acknowledgements
Thanks Drs. Christopher, Winters, and Mann for their assistance on this study.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Gujski M., Pinkas J., Witczak M., Owoc A., Bojar I. Models of cognitive functions with respect to selected parameters of functional state of the thyroid gland in post-menopausal women. Endokrynol Pol. 2017;68:290–298. doi: 10.5603/EP.2017.0022. [DOI] [PubMed] [Google Scholar]
- 2.Tafet G.E., Nemeroff C.B. The links between stress and depression: psychoneuroendocrinological, genetic, and environmental interactions. J Neuropsychiatry Clin Neurosci. 2016;28:77–88. doi: 10.1176/appi.neuropsych.15030053. [DOI] [PubMed] [Google Scholar]
- 3.Chandrasekhar K., Kapoor J., Anishetty S. A prospective, randomized double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of ashwagandha root in reducing stress and anxiety in adults. Indian J Psychol Med. 2012;34:255–262. doi: 10.4103/0253-7176.106022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choudhary D., Bhattacharyya S., Joshi K. Body weight management in adults under chronic stress through treatment with ashwagandha root extract: a double-blind, randomized, placebo-controlled trial. J Evid Based Complementary Altern Med. 2017;22:96–106. doi: 10.1177/2156587216641830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopresti A.L., Smith S.J., Malvi H., Kodgule R. An investigation into the stress-relieving and pharmacological actions of an ashwagandha (Withania somnifera) extract: a randomized, double-blind, placebo-controlled study. Medicine (Baltim) 2019;98 doi: 10.1097/MD.0000000000017186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choudhary D., Bhattacharyya S., Bose S. Efficacy and safety of ashwagandha (withania somnifera (L.) dunal) root extract in improving memory and cognitive functions. J Diet Suppl. 2017;14:599–612. doi: 10.1080/19390211.2017.1284970. [DOI] [PubMed] [Google Scholar]
- 7.Spielberger C.D., Gorsuch R.L., Lushene R., Vagg P.R., Jacobs G.A. Consulting Psychologists Press; Palo Alto, CA: 1983. Manual for the state-trait anxiety inventory. [Google Scholar]
- 8.Brown T.A., Chorpita B.F., Korotitsch W., Barlow D.H. Psychometric properties of the depression anxiety stress scales (DASS) in clinical samples. Behav Res Ther. 1997;35:79–89. doi: 10.1016/s0005-7967(96)00068-x. [DOI] [PubMed] [Google Scholar]
- 9.Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 10.Cepeda-Benito A., Gleaves D.H., Fernandez M.C., Vila J., Williams T.L., Reynoso J. The development and validation of Spanish versions of the state and trait food cravings questionnaires. Behav Res Ther. 2000;38:1125–1138. doi: 10.1016/s0005-7967(99)00141-2. [DOI] [PubMed] [Google Scholar]
- 11.Gualtieri C.T., Johnson L.G. Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Arch Clin Neuropsychol. 2006;21:623–643. doi: 10.1016/j.acn.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Pingali U., Pilli R., Fatima N. Effect of standardized aqueous extract of Withania somnifera on tests of cognitive and psychomotor performance in healthy human participants. Pharmacogn Res. 2014;6:12–18. doi: 10.4103/0974-8490.122912. [DOI] [PMC free article] [PubMed] [Google Scholar]