Abstract
Introduction
Whereas interpatient heterogeneity in clinical characteristics and treatment outcomes of NSCLC harboring a KRAS mutation is recognized, the characterization of these patients in Asia has been limited.
Methods
A multicenter, retrospective cohort study was conducted in eight academic centers across Asia. Patients diagnosed with advanced NSCLC harboring a KRAS mutation and who had received at least one line of anticancer therapy between January 2014 and December 2018 were included. Modified time to next treatment (TTNT) was adopted as a proxy for progression-free survival.
Results
A total of 216 patients were analyzed. The median age at diagnosis of advanced NSCLC was 63.3 years, 70.8% were men and 89.8% had adenocarcinoma. KRAS G12D was the most common subtype (25.5%), followed by G12C (24.5%), and G12V (19.4%) The proportion of current or former smokers was 65.7% in the overall population, with 86.8% in G12C and 58.9% in non-G12C subgroups. For all treatments combined for the total population, the first-line duration of therapy, modified TTNT, and TTNT were 4.5 (95% confidence interval: 3.4–5.9), 6.2 (4.9–8.8), and 9.5 (7.1–11.4) months, respectively. The median overall survival for the total population was 10.3 (6.9–12.4) months and was prolonged in patients ever treated with immunotherapy (14.6 [8.6–19.1] versus 7.0 [5.9–10.6] mo, hazard ratio = 0.54, p < 0.001), with left truncation to account for the time of KRAS testing.
Conclusions
Whereas treatment outcomes with conventional anticancer therapy are reasonable and immunotherapy looks promising, the unmet need remains high for patients with KRAS-mutated NSCLC in Asia, underscoring the need for novel therapeutic approaches.
Keywords: KRAS, Non–small cell lung cancer, Asian, Time to next treatment, Overall survival, Immunotherapy
Introduction
Our approach to NSCLC has transformed dramatically with the discovery of numerous therapeutically targetable oncogenic alterations, and notable examples include EGFR, ALK, ROS1, and BRAF. In contrast, oncogenic KRAS mutations have long been considered undruggable, with many failed attempts to develop a targeted inhibitor because of the high binding affinity to guanosine triphosphate in its active state, and the smooth structure lacking a space for compounds to “hook” to exert their action.1
Another hindrance behind the emergence of an effective KRAS inhibitor has been the biologic and phenotypical heterogeneity of patients with KRAS mutations.2 Ethnically, the prevalence of KRAS mutations among NSCLC has been reported to be lower in Asian countries (5%–11%) compared with that of the West (20%–26%).3, 4, 5 Furthermore, there are at least nine different KRAS mutational subtypes, on the basis of amino acid substitutions discovered in lung cancer that have a distinct binding preference for downstream effector molecules.6 KRAS G12C, the subtype most often found in the West, is a transversion mutation, smoking-associated, and has a high addiction for RalGDS downstream pathway.6,7 The patterns reported in Asia are somewhat different. Whereas some studies similarly report G12C as the most common subtype, others report that G12D—which is a transition mutation associated with nonsmokers and has a high affinity for PI3K downstream pathway—is most often identified.8, 9, 10, 11, 12
Such interpatient heterogeneity within KRAS-mutated NSCLC may lead to varying clinical outcomes.6 The prognostic value of KRAS mutations in lung cancer remains controversial. Whereas earlier studies suggested a negative prognostic effect of KRAS mutations, subsequent studies have suggested no prognostic or predictive value for survival or treatment outcomes.13,14 The biological diversity in patients with lung cancer having KRAS mutations, which may account for these discrepancies, has not been investigated thoroughly in Asian patient populations owing to the lower prevalence of KRAS mutations in lung cancer compared with the West.
ATORG (Asian Thoracic Oncology Research Group)-005 is a multicenter, retrospective cohort study of patients with KRAS-mutated advanced NSCLC treated in real-world clinical practice in Asia. This study aimed to provide reliable and valid data on their characteristics and treatment outcomes and fill a knowledge gap in Asia on patients with KRAS-mutated advanced NSCLC.
Materials and Methods
Patients and Samples
Patients diagnosed with KRAS-mutated advanced NSCLC between January 1, 2014 and December 31, 2018 from eight academic centers across Asia (Korea, Singapore, People’s Republic of China, Japan, and India) were included. Relevant data were extracted from the electronic medical records. Patients had to have received at least one line of anticancer therapy. KRAS mutations were identified by local molecular testing including real-time polymerase chain reaction (RT-PCR), Sanger sequencing, or next-generation sequencing (NGS), on tumor tissues. The study was approved by the institutional ethics committees of each participating center. In accordance with each participating center’s guidelines, written informed consent has been provided by patients or a waiver of consent for retrospective review has been obtained.
Time to Event Measurements
Overall survival (OS) was defined as the time from initiation of therapy for advanced NSCLC to death from any cause and analyzed in two aspects—“classical OS” and “left truncated OS.” For classical OS, patients were at risk of death from the date of treatment initiation; for left truncated OS, patients were at risk of death from the date of KRAS testing, meaning the duration at risk of death before KRAS testing was left truncated. Left truncation was incorporated to account for the variable time of KRAS testing in clinical practice and often occurring after initiation of therapy.15, 16, 17 Patients alive at the time of analysis were censored on the date of the last follow-up. The time to next treatment (TTNT) was defined as the interval between the initiation of therapy and the initiation of the following therapy, and any patients who did not receive the following treatment were censored on the date of the last follow-up or death. The modified TTNT (mTTNT) was defined as the time from initiation of therapy to either initiation of the following therapy or death from any cause and was used as a real-world proxy for progression-free survival (PFS). Duration of therapy (DOT) was defined as the interval between initiation and termination of a therapy regimen. Patients were included for the analyses of TTNT, mTTNT, and DOT after their KRAS testing was performed.
Statistical Analysis
Left truncation was applied to account for immortal time bias. Patients were considered to be “at risk” in the survival analysis once they fulfilled their “entry date” (defined as KRAS testing date). Descriptive statistics are reported using proportions, medians, and ranges. Associations between categorical variables were evaluated with either chi-square tests or Fisher exact test, as appropriate. Associations between continuous variables were evaluated by means of the analysis of variance (ANOVA) tests, Mann-Whitney U test, or Kruskal-Wallis equality-of-populations rank test, as appropriate. Survival curves were estimated by the Kaplan-Meier method and compared using the log-rank test. The data cutoff date was November 5, 2020. All analyses were performed in R software version 3.6.3 and STATA version 16.0, with two-sided tests and a significance level of 0.05.
Results
Patient Demographics and Clinicopathologic Characteristics
Patient demographics and clinical characteristics are summarized in Table 1. A total of 216 patients with advanced NSCLC harboring a confirmed KRAS mutation were included from eight academic centers across Asia. In terms of ethnicity, the cohort consisted of 117 Korean (54.2%), 56 Chinese (25.9%), 28 Japanese (13.0%), 12 Indian (5.6%), and three Malay patients (1.4%). KRAS testing was performed using NGS (202 of 216, 93.5%), Sanger sequencing (12 of 216, 5.6%), and RT-PCR (2 of 216, 0.9%), and the time of testing relative to the line of therapy are summarized in Supplementary Table 1.
Table 1.
Baseline Characteristics
| Characteristic | Total N = 216 (%) |
KRAS G12C n = 53 (%) | KRAS Non-G12C n = 163 (%) | p |
|---|---|---|---|---|
| Age at advanced NSCLC Dx, y (range) | 63.3 (31.2–80.8) | 64.2 (41.0–80.8) | 62.4 (31.2–79.7) | 0.281 |
| Age at initial NSCLC Dx, y (range) | 63.1 (31.2–79.9) | 64.2 (41.0–79.9) | 61.9 (31.2–79.7) | 0.298 |
| Sex | 0.003 | |||
| Male | 153 (70.8) | 46 (86.8) | 107 (65.6) | |
| Female | 63 (29.2) | 7 (13.2) | 56 (34.4) | |
| Ethnicity | 0.949 | |||
| Chinese | 56 (25.9) | 15 (28.3) | 41 (25.2) | |
| Indian | 12 (5.6) | 2 (3.8) | 10 (6.1) | |
| Japanese | 28 (13.0) | 7 (13.2) | 21 (12.9) | |
| Korean | 117 (54.2) | 29 (54.7) | 88 (54.0) | |
| Malay | 3 (1.4) | 0 (0.0) | 3 (1.8) | |
| ECOG performance status | 0.726 | |||
| 0–1 | 199 (92.1) | 48 (90.6) | 151 (92.6) | |
| ≥2 | 14 (6.5) | 4 (7.5) | 10 (6.1) | |
| Unknown | 3 (1.4) | 1 (1.9) | 2 (1.3) | |
| Smoking status | <0.001 | |||
| Current or former smoker | 142 (65.7) | 46 (86.8) | 96 (58.9) | |
| Never smoker | 74 (34.3) | 7 (13.2) | 67 (41.1) | |
| Histology | 0.617 | |||
| Adenocarcinoma | 194 (89.8) | 48 (90.6) | 146 (89.6) | |
| Squamous cell carcinoma | 9 (4.2) | 1 (1.9) | 8 (4.9) | |
| Other | 13 (6.0) | 4 (7.5) | 9 (5.5) | |
| Disease status | 0.428 | |||
| De novo metastatic | 183 (84.7) | 42 (79.2%) | 141 (86.5) | |
| Relapse or recurrent | 33 (15.3) | 11 (20.8%) | 22 (13.5) | |
| Sites of metastases | ||||
| Brain | 55/204 (27.0) | 13/52 (25.0) | 42/152 (27.6) | 0.712 |
| Lung-to-lung | 79/216 (36.6) | 15/53 (28.3) | 64/163 (39.3) | 0.150 |
| Pleura | 68/216 (31.5) | 16/53 (30.2) | 52/163 (31.9) | 0.816 |
| Liver | 23/214 (10.7) | 8/53 (15.1) | 15/161 (9.3) | 0.239 |
| Bone | 72/214 (33.6) | 16/53 (30.2) | 56/161 (34.8) | 0.539 |
| Adrenal | 20/214 (9.3) | 6/53 (11.3) | 14/161 (8.7) | 0.590 |
| Co-mutations | ||||
| EGFR mutationa | 22/216 (10.2) | 4/53 (7.5) | 18/163 (11.0) | 0.775 |
| ALK rearrangement | 3/203 (1.5) | 0/49 (0.0) | 3/154 (1.9) | 1.000 |
| ROS1 rearrangement | 2/200 (1.0) | 1/48 (2.1) | 1/152 (0.7) | 0.423 |
| BRAF mutation | 5/200 (2.5) | 0/48 (0.0) | 5/152 (3.3) | 0.340 |
| PD-L1 TPSb | ||||
| ≥1 | 102/149 (68.5) | 29/39 (74.4) | 73/110 (66.4) | 0.356 |
| ≥50 | 49/149 (32.9) | 13/39 (33.3) | 36/110 (32.7) | 0.945 |
Dx, diagnosis; ECOG, Eastern Cooperative Oncology Group; PD-L1, programmed death-ligand 1; TPS, tumor proportion score.
EGFR exon 19 deletion and exon 21 L858R substitution only.
PD-L1 antibodies used were 22C3 (n = 73, 48.7%), SP263 (n = 48, 32.0%), SP142 (n = 15, 10.0%), and E1L3N (n = 14, 9.3%), excluding 67 patients who were not tested.
The median age at diagnosis of advanced NSCLC was 63.3 years old (range: 31.2–80.8) (Table 1). Most patients were men (70.8%) and had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1 (92.1%). Histologic subtypes comprised of adenocarcinoma (89.8%), squamous cell carcinoma (4.2%), and others (6.0%). Most (84.7%) were diagnosed with de novo metastatic disease, and brain metastases at initial diagnosis were detected in 27.0%.
The co-occurrences of genetic alterations were reported as follows; EGFR mutation (22 of 216, 10.2%), ALK fusion (3 of 203, 1.5%), ROS1 fusion (2 of 200, 1.0%), and BRAF mutation (5 of 200, 2.5%). Positive programmed death-ligand 1 (PD-L1) expression, defined as tumor proportion score (TPS) of greater than or equal to 1%, was found in 68.5% of patients (102 of 149), with TPS greater than or equal to 50% in 32.9% of patients (49 of 149).
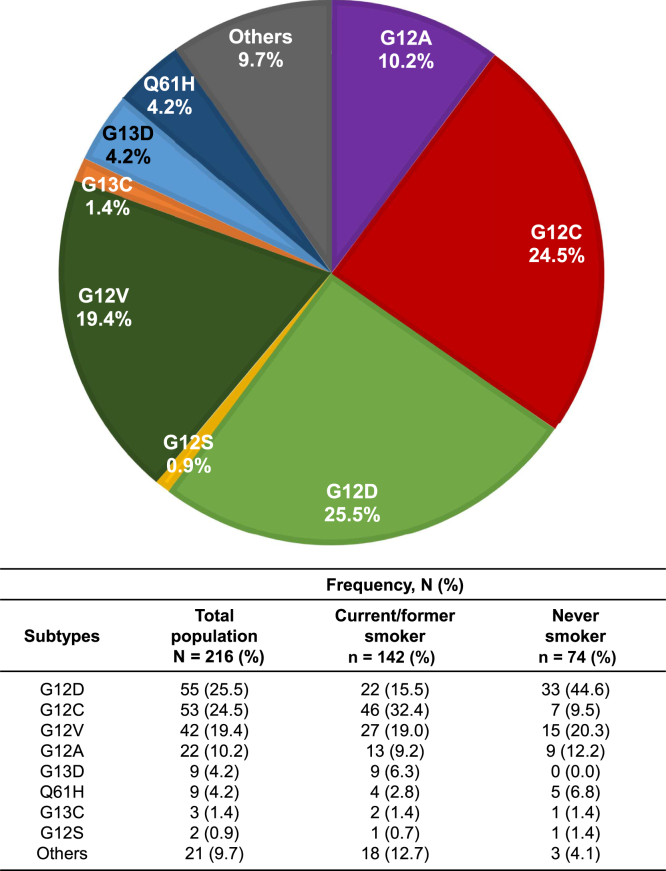
In the overall population, the most common KRAS mutational subtype was G12D with 25.5%, followed by G12C (24.5%) and G12V (19.4%) (Fig. 1). According to smoking status, KRAS G12C was the most common mutational subtype (32.4%) among current or former smokers, whereas KRAS G12D (44.6%) was the most frequent mutation among never-smokers, consistent with previous reports.7 There was no marked difference in clinicopathologic characteristics between KRAS G12C and non-G12C subtypes (Table 1).
Figure 1.
Proportion of KRAS mutational subtypes.
It is noteworthy that the proportion of current or former smokers was 142 of 216 (65.7%) in the total cohort, with 46 of 53 (86.8%) in KRAS G12C and 96 of 163 (58.9%) in non-G12C groups, which is considerably lower compared with that reported from the West.7,18
Treatment Approaches and Outcomes
We next evaluated the therapeutic approaches for KRAS-mutated patients to delineate real-world clinical practice. Whereas all of the 216 patients received their first-line treatment, 174 (80.6%), 102 (47.2%), and 51 (23.6%) received second-, third-, and fourth-line treatment, respectively (Table 2). Cytotoxic chemotherapy was the most common approach in first-line therapy (69.9%), followed by targeted therapy (13.0%), immunotherapy (6.5%), and chemotherapy plus immunotherapy combination therapy (2.8%).
Table 2.
Treatment Outcomes
| Treatments | 1L N = 216 (%) |
2L N = 174 (%) |
3L N = 102 (%) |
4L N = 51 (%) |
|---|---|---|---|---|
| Chemotherapy | 151 (69.9) | 70 (40.2) | 52 (51.0) | 27 (52.9) |
| Immunotherapy | 14 (6.5) | 60 (34.5) | 23 (22.5) | 6 (11.8) |
| CTx + IO combo | 6 (2.8) | 5 (2.9) | 1 (1.0) | 2 (3.9) |
| Targeted agent | 28 (13.0) | 21 (12.1) | 18 (17.6) | 9 (17.6) |
| Other | 17 (7.8) | 18 (10.3) | 8 (7.9) | 7 (13.8) |
| Median DOT, mo (95% CI) | Event/n | 1L DOT | Event/n | 2L DOT | Event/n | 3L DOT | Event/n | 4L DOT |
|---|---|---|---|---|---|---|---|---|
| Overall | 87/88 | 4.5 (3.4–5.9) | 106/106 | 2.1 (2.0–2.9) | 69/71 | 2.1 (1.4–3.6) | 43/44 | 1.9 (1.4–2.9) |
| KRAS | ||||||||
| G12C | 19/20 | 8.1 (1.5–11.3) | 21/21 | 2.1 (0.7–6.0) | 14/14 | 3.2 (1.5–7.3) | 11/11 | 1.9 (0.7–4.5) |
| Non-G12C | 68/68 | 4.2 (3.0–5.2) | 85/85 | 2.1 (1.8–3.0) | 55/57 | 1.8 (1.4–3.7) | 32/33 | 1.9 (1.0–2.9) |
| Smoking | ||||||||
| Current or former | 60/61 | 5.1 (3.4–7.9) | 69/69 | 2.1 (1.6–3.0) | 39/41 | 2.3 (1.4–4.1) | 23/23 | 1.6 (0.9–2.2) |
| Never | 27/27 | 4.1 (2.1–5.2) | 37/37 | 2.4 (1.4, 3.6) | 30/30 | 1.9 (1.4–3.9) | 20/21 | 2.2 (1.4–3.6) |
| Median mTTNT, mo (95% CI) | Event/n | 1L mTTNT | Event/n | 2L mTTNT | Event/n | 3L mTTNT | Event/n | 4L mTTNT |
|---|---|---|---|---|---|---|---|---|
| Overall | 81/88 | 6.2 (4.9–8.8) | 87/104 | 3.8 (2.8–5.6) | 63/71 | 5.7 (2.8–6.9) | 38/42 | 2.6 (2.1–3.9) |
| KRAS | ||||||||
| G12C | 19/20 | 8.8 (4.3–14.6) | 17/20 | 5.0 (1.6–7.7) | 13/14 | 6.9 (2.2–10.9) | 9/11 | 3.9 (1.2–NE) |
| Non-G12C | 62/68 | 5.9 (4.4–8.7) | 70/84 | 3.7 (2.7–5.3) | 50/57 | 3.8 (2.4–6.9) | 29/31 | 2.6 (2.1–3.8) |
| Smoking | ||||||||
| Current or former | 57/61 | 7.1 (4.9–10.1) | 55/67 | 4.0 (2.5–5.6) | 38/41 | 5.7 (3.1–7.6) | 18/22 | 3.4 (1.6–5.4) |
| Never | 24/27 | 5.5 (3.4–7.7) | 32/37 | 3.1 (2.6–6.5) | 25/30 | 4.3 (1.8–7.4) | 20/20 | 2.6 (1.5–5.3) |
| Median TTNT, mo (95% CI) | Event/n | 1L TTNT | Event/n | 2L TTNT | Event/n | 3L TTNT | Event/n | 4L TTNT |
|---|---|---|---|---|---|---|---|---|
| Overall | 56/88 | 9.5 (7.1–11.4) | 48/104 | 10.9 (6.5–12.7) | 27/71 | 11.0 (7.4–NE) | 20/42 | 5.4 (3.4–11.8) |
| KRAS | ||||||||
| G12C | 13/20 | 10.9 (7.1–22.7) | 8/20 | 11.0 (2.2–NE) | 6/14 | 11.0 (4.3–NE) | 5/11 | 5.5 (1.2–NE) |
| Non-G12C | 43/68 | 8.8 (5.3–11.4) | 40/84 | 9.4 (5.6–12.7) | 21/57 | 10.1 (6.7–NE) | 15/31 | 4.3 (2.5–6.4) |
| Smoking | ||||||||
| Current or former | 37/61 | 10.2 (7.3–15.2) | 30/67 | 10.9 (5.6–12.7) | 11/41 | 11.5 (9.9–NE) | 10/22 | 5.4 (3.4–NE) |
| Never | 19/27 | 6.2 (3.4–9.7) | 18/37 | 7.0 (3.0–19.0) | 16/30 | 6.7 (4.3–18.4) | 10/20 | 5.5 (2.5–NE) |
1L, first line; 2L, second line; 3L, third line; 4L, fourth line; CI, confidence interval; CTx, chemotherapy; DOT, duration of therapy; IO, immunotherapy; mTTNT, modified time to next treatment; NE, not estimable; TTNT, time to next treatment.
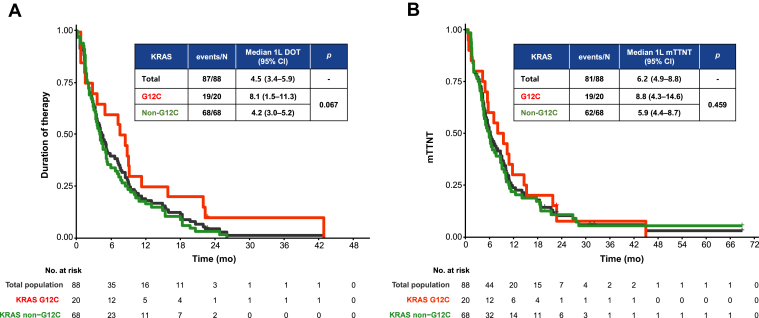
The median DOT, regardless of treatment received, was 4.5 (95% confidence interval [CI]: 3.4–5.9), 2.1 (2.0–2.9), 2.1 (1.4–3.6), and 1.9 (1.4–2.9) months for first-, second-, third-, and fourth-line treatment, respectively. The median mTTNT for all treatment regimens combined were 6.2 (4.9–8.8), 3.8 (2.8–5.6), 5.7 (2.8–6.9), and 2.6 (2.1–3.9) months for first-, second-, third-, and fourth-line treatment, respectively. Both DOT and mTTNT did not differ significantly according to KRAS subtypes (Table 2, Fig. 2A and B) or smoking status (Table 2 and Supplementary Fig. 1). For first-line cytotoxic chemotherapy, the most often adopted therapeutic approach, the DOT, mTTNT, and TTNT were 3.7 (2.8–5.3), 5.9 (4.4–8.0), and 8.8 (5.5–10.4) months, respectively.
Figure 2.
Kaplan-Meier estimates of the first-line (A) duration of therapy and (B) mTTNT for total population (black), KRAS G12C (red), and non-G12C (green) subgroups. 1L, first line; CI, confidence interval; DOT, duration of therapy; mTTNT, modified time to next treatment.
OS According to Immunotherapy
Classical OS was calculated for the total population and left truncated OS was calculated for 178 patients to account for the time of KRAS testing, excluding 3 patients whose testing dates were unavailable and 35 patients whose KRAS testing were done after the fourth line, as the last line of therapy analyzed for survival.
The median classical OS was 17.5 months (95% CI: 14.6–21.0) and the median left truncated OS was 10.3 months (95% CI: 6.9–12.4); they did not differ significantly according to KRAS subtypes or smoking status (Supplementary Figs. 1 and 2).
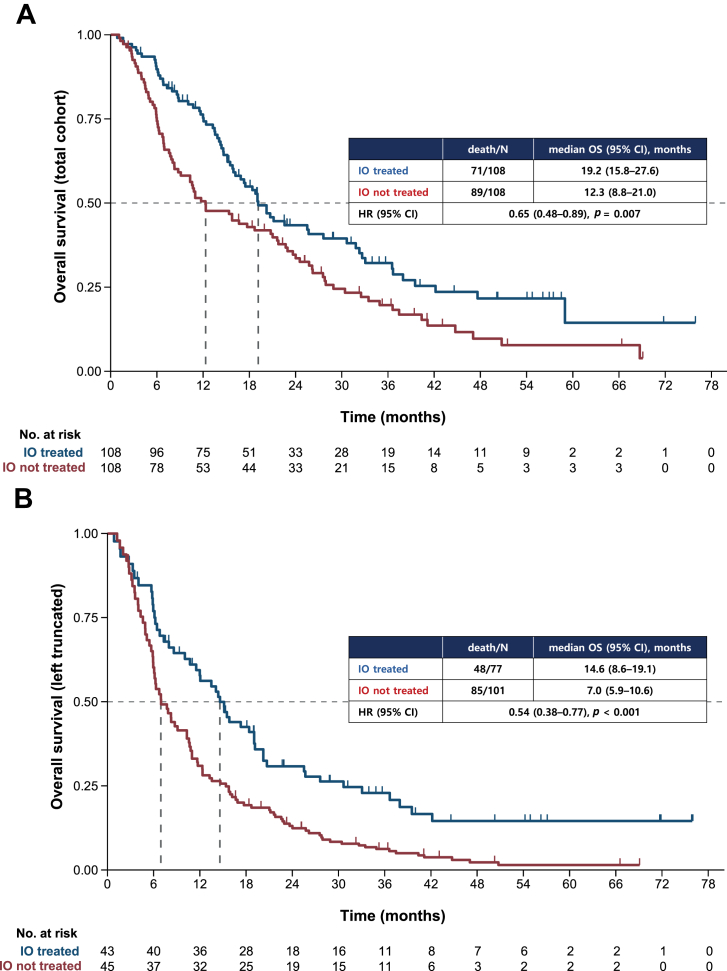
To further explore the factors associated with survival outcomes, we investigated the OS of patients who were ever treated with immunotherapy, alone or in combination with other drugs, compared with those who were not. During the course of treatment, 77 of 178 patients (43.3%) were treated with immunotherapy. There were no substantial differences in baseline characteristics between the two groups, although the patients who had received immunotherapy had a lower incidence of EGFR mutations, and a higher proportion of patients with PD-L1 had TPS greater than or equal to 50% (Supplementary Table 2).
For classical OS, the patients treated with immunotherapy had a median OS of 19.2 months (95% CI: 15.8–27.6) and those not treated with immunotherapy had a median OS of 12.3 months (95% CI: 8.8–21.0), exhibiting a hazard ratio (HR) of 0.65 (95% CI: 0.48–0.89, p = 0.007) (Fig. 3A). For left truncated OS, the median OS was 14.6 months (95% CI: 8.6–19.1) and 7.0 months (95% CI: 5.9–10.6) for patients treated and not treated with immunotherapy, respectively, with an HR of 0.54 (95% CI: 0.38–0.77, p < 0.001) (Fig. 3B). The multivariate analyses using relevant variables revealed that immunotherapy was associated with favorable survival outcome (classical OS HR = 0.58 [95% CI: 0.41–0.81], p = 0.001; left truncated OS HR = 0.53 [95% CI: 0.37–0.78], p = 0.001) (Supplementary Table 3). ECOG performance status and EGFR mutational status were also found to be associated with OS. To account for the immortal time bias that could arise from the fact that most immunotherapy uses were second line, we also analyzed the OS calculated from the initiation of the second line of therapy for patients who received immunotherapy (n = 60) versus chemotherapy (n = 70) in second line. The survival duration was prolonged in patients treated with immunotherapy, albeit statistically insignificant, with a median OS of 10.4 (95% CI: 6.5–12.4) versus 6.5 (95% CI: 5.3–9.3) months.
Figure 3.
Kaplan-Meier estimates of OS (A) for total population and (B) with left truncation to account for the time of KRAS testing, according to ever-receiving immunotherapy alone or in combination. CI, confidence interval; HR, hazard ratio; IO, immunotherapy; OS, overall survival.
Discussion
To the best of our knowledge, this is the largest study published to date that comprehensively analyzed patients with metastatic KRAS-mutated NSCLC from a multicenter cohort in Asia. KRAS mutations were more frequently found in men and smokers. However, despite the common association of KRAS mutations with a smoking history, we identified that the proportion of never-smokers in our cohort was considerably higher at 34.3%, compared with that reported from Western countries ranging between 6.4% to 7.1%.7,18 It was more dominantly represented in the non-G12C subgroup with 41.1% of never-smokers, compared with the G12C subgroup with 13.2% of never-smokers. The treatment or survival duration did not differ according to KRAS mutational subtypes or smoking status. Together with G12D being the most common KRAS mutational subtype, not only among never-smokers but in the overall population, these findings suggest that KRAS-mutated lung cancer in Asian patients may be driven by factors other than tobacco smoking and that the distinct biology of Asian patients with KRAS-mutated NSCLC warrants further investigation.
Another interesting characteristic of Asian patients with KRAS-mutated NSCLC was the high incidence of EGFR co-mutations. This is consistent with previous literature, which reported the KRAS and EGFR co-mutation incidence ranged between 1.3% and 4.0% for Western patients compared with 10.7% for Asian patients.8,18,19 Considering the close interaction between EGFR and RAS signaling pathways, an investigation on the effect of EGFR mutational status on KRAS targeted therapy, if any, is also warranted.
Although KRAS mutations are one of the most often identified genetic alterations in lung cancer, little progress has been made with novel targeted agents. Unlike other tractable targets, the standard treatment for patients with KRAS mutations remains platinum-based chemotherapy, or more recently, immunotherapy with or without cytotoxic chemotherapy. In our study, cytotoxic chemotherapy was the most often adopted approach as first-line therapy. This result was expected because our data was collected before the era of immunotherapy, some Asian countries still have limited access to immunotherapy owing to reimbursement issues, and the clinical efficacy of small molecule inhibitors against KRAS G12C has only recently been established.20 The first-line mTTNT of 5.9 months with cytotoxic chemotherapy was comparable to the historical median PFS with platinum-doublet chemotherapy ranging between 4.8 and 5.1 months.21 Nevertheless, the difference between TTNT and mTTNT indicates that a considerable proportion of patients do not receive subsequent treatment beyond the first-line, owing to poor performance status or death, presumably from rapid disease progression, and highlights the urgent need for improved therapeutic approaches.
With the lack of individualized treatments for KRAS-mutated patients, we discovered that patients who were ever treated with immunotherapy, alone or in combination with other drugs, revealed an improved survival outcome compared with those who were not. This survival benefit should be interpreted with caution considering the small sample size, the lack of stratification by lines of therapy, and potential bias from an imbalance of risk features, for example, higher incidence of brain metastasis, which is an unfavorable prognostic factor in immunotherapy-not-treated patients.22
Previously, many studies have concluded that immunotherapy confers superior clinical efficacy over chemotherapy regardless of KRAS mutational status, whereas a subgroup analysis described that the OS benefits were more evident in KRAS-mutated patients compared with KRAS-wild type patients.23, 24, 25, 26 The benefits of immunotherapy for patients with KRAS-mutated lung cancer have been largely attributed to their predilection to tobacco smoking. However, although approximately one-third of patients were never-smokers, a survival benefit with immunotherapy was observed in our patient cohort, suggesting a need to investigate this phenomenon outside the “smoking-associated” view.
First, PD-L1, an immunosuppressive protein that enables cancer cells to evade the host immune system, is widely adopted as a biomarker for sensitivity to immune checkpoint inhibitors, particularly in the context of high mutational load from tobacco exposure. PD-L1 expression was found to be intrinsically up-regulated by means of the MEK and ERK pathways in KRAS-mutated lung cancer cell lines and tissues, which may translate into clinical benefit from immunotherapy.27, 28, 29 Second, it is not only PD-L1 expression but the tumor microenvironment that is indispensable for an effective response after immune checkpoint blockade. Studies have reported that KRAS-mutated lung cancer cells modulate the tumor microenvironment to recruit CD8+ T cells, resulting in higher infiltration of CD8+ T cells compared with KRAS-wild type tumors, implying that the presence of a KRAS mutation itself results in an inflammatory phenotype.9,30 Third, KRAS mutations result in various defects in cell cycle checkpoints and DNA damage repair pathways, which potentially increases mutational errors and results in increased neoantigens.9,31 These findings suggest that KRAS mutations might be a potential driver to induce genetic instability and consequently lead to an augmented response from immunotherapy.
The efficacy of immune checkpoint inhibitors in patients with KRAS-mutated lung cancer deserves attention because a preclinical study has found that sotorasib, a first-in-class KRAS G12C inhibitor, induces a proinflammatory microenvironment along with synergized antitumor activity in murine models when combined with an anti–programmed cell death protein-1 (PD-1) inhibitor.32 With a modest overall response rate of 37.11% and median PFS of 6.88 months in patients with KRAS G12C-mutated NSCLC, these results highlight the potential role of combination approaches to improve efficacy.20,33
There are several limitations to our study. Given its retrospective nature, the study was subject to potential biases. Study data were collected from a period when NGS or KRAS testing was not reimbursed in many parts of Asia. The testing methods of molecular analysis were not standardized across different institutions, and as such, we were unable to evaluate the coexisting mutations such as TP53, STK11 or LKB1, or CDKN2A and CDKN2B, which compromise distinctive biologic subgroups within KRAS-mutated patients with different patterns of immune system engagement and therapeutic vulnerabilities.34 Most interestingly, patients with STK11 or LKB1 co-mutations are associated with lower T cell infiltration and PD-L1 expression, even in tumors with a high tumor mutational burden.35 They exhibited significantly shorter PFS and OS with immunotherapy compared with patients with TP53 or CDKN2A/B co-mutations, regardless of PD-L1 expression. This landscape of co-mutations within patients with KRAS-mutated NSCLC deserves further investigation in the Asian population.
In conclusion, KRAS-mutated NSCLC represents a heterogeneous group of patients with intrinsic molecular diversity, and our study revealed a lower predilection to tobacco smoking in an Asian patient population compared with the West. Although treatment outcomes with conventional chemotherapy were reasonable and the efficacy of immunotherapy looks promising, the unmet need remains high for Asian patients with KRAS-mutated NSCLC, emphasizing the need for improved biomarker stratification and novel therapeutic approaches.
CRediT Authorship Contribution Statement
Jiyun Lee: Conceptualization, Methodology, Investigation, Resources, Data curation, Writing - original draft, Writing - review & editing, Visualization, Project administration.
Aaron C. Tan: Conceptualization, Methodology, Investigation, Resources, Data curation, Writing – original draft, Writing - review & editing, Visualization, Project administration, Funding acquisition.
Siqin Zhou: Methodology, Validation, Formal analysis, Investigation, Resources, Data curation Writing – original draft, Writing- review & editing, Visualization.
Shin Kyo Yoon, Siyang Liu, Ken Masuda, Hidetoshi Hayashi, Ullas Batra, Dong-Wan Kim, Yasushi Goto, Sze Huey Tan, Yi-Long Wu: Conceptualization, Methodology, Investigation, Resources, Writing- review & editing, Project administration.
Dae Ho Lee: Conceptualization, Methodology, Investigation, Resources, Writing- Review & Editing, Project Administration, Supervision.
Daniel S. W. Tan: Conceptualization, Methodology, Investigation, Resources, Writing- review & editing, Project administration, Supervision, Funding acquisition.
Myung-Ju Ahn: Conceptualization, Methodology, Investigation, Resources, Writing- review & editing, Project administration, Supervision.
Acknowledgments
This trial was sponsored and coordinated by the Asian Thoracic Oncology Research Group and financed by a grant from Amgen. The authors thank the investigators from eight academic centers and their teams, and Amgen for supporting the study. Dr. AC Tan is the recipient of an International Association for the Study of Lung Cancer Fellowship from 2018 to 2020. Dr. DSW Tan is supported by National Medical Research Council Clinician-Scientist Award (MOH-CSAINV19nov-0005), and National Medical Research Council Open Fund - Large Collaborative Grant (NMRC/OFLCG/002/2018). Data collection, analysis, and interpretation of the data was undertaken by the investigators on the study.
Footnotes
Drs. Lee and AC Tan contributed equally as first authors.
Disclosure: Dr. Ahn reports receiving honoraria from AstraZeneca, Merck Sharp & Dohme, Roche, Bristol-Myers Squibb, Merck KGaA, and Alphas Pharmaceutical outside of the submitted work. Dr. Kim reports receiving grants from Alpha Biopharma, Amgen, AstraZeneca/Medimmune, Boehringer Ingelheim, Daiichi-Sankyo, Hanmi, Janssen, Merus, Mirati Therapeutics, Merck Sharp & Dohme, Novartis, Ono Pharmaceutical, Pfizer, Roche/Genentech, Takeda, TP Therapeutics, Xcovery, and Yuhan outside of the submitted work; and other fees from and Daiichi-Sankyo outside of the submitted work. Dr. AC Tan reports receiving honoraria from ThermoFisher; and consulting/advisory roles for Amgen outside of the submitted work. Dr. DSW Tan reports receiving honoraria from Takeda Pharmaceuticals, Novartis, Roche, Pfizer, Merck, Boehringer Ingelheim; consulting/advisory roles for Novartis, Merck, Loxo Oncology, AstraZeneca, Bayer, Eli Lilly, Boehringer Ingelheim; and receiving grants from Novartis, GlaxoSmithKline, AstraZeneca and Pfizer outside of the submitted work. The remaining authors declare no conflict of interest.
Cite this article as: Lee J, Tan AC, Zhou S, et al. Clinical characteristics and outcomes in advanced KRAS-mutated NSCLC: a multicenter collaboration in Asia (ATORG-005). JTO Clin Res Rep. 2022;3:100261.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2021.100261.
Supplementary Data
References
- 1.Lindsay C.R., Jamal-Hanjani M., Forster M., Blackhall F. KRAS: reasons for optimism in lung cancer. Eur J Cancer. 2018;99:20–27. doi: 10.1016/j.ejca.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Song P., Yang D., Wang H., et al. Relationship between the efficacy of immunotherapy and characteristics of specific tumor mutation genes in non-small cell lung cancer patients. Thorac Cancer. 2020;11:1647–1654. doi: 10.1111/1759-7714.13447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohno T., Nakaoku T., Tsuta K., et al. Beyond ALK-RET, ROS1 and other oncogene fusions in lung cancer. Transl Lung Cancer Res. 2015;4:156–164. doi: 10.3978/j.issn.2218-6751.2014.11.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dearden S., Stevens J., Wu Y.L., Blowers D. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap) Ann Oncol. 2013;24:2371–2376. doi: 10.1093/annonc/mdt205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu K.H., Ho C.C., Hsia T.C., et al. Identification of five driver gene mutations in patients with treatment-naïve lung adenocarcinoma in Taiwan. PLoS One. 2015;10 doi: 10.1371/journal.pone.0120852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nadal E., Beer D.G., Ramnath N. KRAS-G12C mutation is associated with poor outcome in surgically resected lung adenocarcinoma. J Thorac Oncol. 2015;10:e9–e10. doi: 10.1097/JTO.0000000000000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dogan S., Shen R., Ang D.C., et al. Molecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: higher susceptibility of women to smoking-related KRAS-mutant cancers. Clin Cancer Res. 2012;18:6169–6177. doi: 10.1158/1078-0432.CCR-11-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lei L., Wang W.X., Yu Z.Y., et al. A real-world study in advanced non-small cell lung cancer with KRAS mutations. Transl Oncol. 2020;13:329–335. doi: 10.1016/j.tranon.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu C., Zheng S., Jin R., et al. The superior efficacy of anti-PD-1/PD-L1 immunotherapy in KRAS-mutant non-small cell lung cancer that correlates with an inflammatory phenotype and increased immunogenicity. Cancer Lett. 2020;470:95–105. doi: 10.1016/j.canlet.2019.10.027. [DOI] [PubMed] [Google Scholar]
- 10.Liu S.Y., Sun H., Zhou J.Y., et al. Clinical characteristics and prognostic value of the KRAS G12C mutation in Chinese non-small cell lung cancer patients. Biomark Res. 2020;8:22. doi: 10.1186/s40364-020-00199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun J.M., Hwang D.W., Ahn J.S., Ahn M.J., Park K. Prognostic and predictive value of KRAS mutations in advanced non-small cell lung cancer. PLoS One. 2013;8 doi: 10.1371/journal.pone.0064816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu S.G., Liao W.Y., Su K.Y., et al. Prognostic characteristics and immunotherapy response of patients with nonsquamous NSCLC with kras mutation in East Asian populations: a single-center cohort study in Taiwan. JTO Clin Res Rep. 2020;2:100140. doi: 10.1016/j.jtocrr.2020.100140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan W., Yang Y., Zhu H., Zhang Y., Zhou R., Sun X. KRAS mutation is a weak, but valid predictor for poor prognosis and treatment outcomes in NSCLC: a meta-analysis of 41 studies. Oncotarget. 2016;7:8373–8388. doi: 10.18632/oncotarget.7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mascaux C., Iannino N., Martin B., et al. The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer. 2005;92:131–139. doi: 10.1038/sj.bjc.6602258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Candido-dos-Reis F.J., Song H., Goode E.L., et al. Germline mutation in BRCA1 or BRCA2 and ten-year survival for women diagnosed with epithelial ovarian cancer. Clin Cancer Res. 2015;21:652–657. doi: 10.1158/1078-0432.CCR-14-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips K.A., Milne R.L., Rookus M.A., et al. Tamoxifen and risk of contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2013;31:3091–3099. doi: 10.1200/JCO.2012.47.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chubak J., Boudreau D.M., Wirtz H.S., McKnight B., Weiss N.S. Threats to validity of nonrandomized studies of postdiagnosis exposures on cancer recurrence and survival. J Natl Cancer Inst. 2013;105:1456–1462. doi: 10.1093/jnci/djt211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El Osta B., Behera M., Kim S., et al. Characteristics and outcomes of patients with metastatic KRAS-mutant lung adenocarcinomas: the lung cancer mutation consortium experience. J Thorac Oncol. 2019;14:876–889. doi: 10.1016/j.jtho.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zer A., Ding K., Lee S.M., et al. Pooled analysis of the prognostic and predictive value of KRAS mutation status and mutation subtype in patients with non-small cell lung cancer treated with epidermal growth factor receptor tyrosine kinase inhibitors. J Thorac Oncol. 2016;11:312–323. doi: 10.1016/j.jtho.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Hong D.S., Fakih M.G., Strickler J.H., et al. KRASG12C inhibition with sotorasib in advanced solid tumors. N Engl J Med. 2020;383:1207–1217. doi: 10.1056/NEJMoa1917239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scagliotti G.V., Parikh P., von Pawel J., et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 22.Ali A., Goffin J.R., Arnold A., Ellis P.M. Survival of patients with non-small-cell lung cancer after a diagnosis of brain metastases. Curr Oncol. 2013;20:e300–e306. doi: 10.3747/co.20.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borghaei H., Paz-Ares L., Horn L., et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rittmeyer A., Barlesi F., Waterkamp D., et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herbst R.S., Lopes G., Kowalski D.M., et al. LBA4 Association of KRAS mutational status with response to pembrolizumab monotherapy given as first-line therapy for PD-L1-positive advanced non-squamous NSCLC in KEYNOTE-042. Ann Oncol. 2019;30(suppl 11):X163–X164. [Google Scholar]
- 26.Lee C.K., Man J., Lord S., et al. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non-small cell lung carcinoma: a systematic review and meta-analysis. JAMA Oncol. 2018;4:210–216. doi: 10.1001/jamaoncol.2017.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen N., Fang W., Lin Z., et al. KRAS mutation-induced upregulation of PD-L1 mediates immune escape in human lung adenocarcinoma. Cancer Immunol Immunother. 2017;66:1175–1187. doi: 10.1007/s00262-017-2005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coelho M.A., de Carné Trécesson S., Rana S., et al. Oncogenic RAS signaling promotes tumor immunoresistance by stabilizing PD-L1 mRNA. Immunity. 2017;47:1083–1099.e6. doi: 10.1016/j.immuni.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu H., Boyle T.A., Zhou C., Rimm D.L., Hirsch F.R. PD-L1 expression in lung cancer. J Thorac Oncol. 2016;11:964–975. doi: 10.1016/j.jtho.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dias Carvalho P., Guimarães C.F., Cardoso A.P., et al. KRAS oncogenic signaling extends beyond cancer cells to orchestrate the microenvironment. Cancer Res. 2018;78:7–14. doi: 10.1158/0008-5472.CAN-17-2084. [DOI] [PubMed] [Google Scholar]
- 31.Dong Z.Y., Zhong W.Z., Zhang X.C., et al. Potential predictive value of TP53 and KRAS mutation status for response to PD-1 blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res. 2017;23:3012–3024. doi: 10.1158/1078-0432.CCR-16-2554. [DOI] [PubMed] [Google Scholar]
- 32.Canon J., Rex K., Saiki A.Y., et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575:217–223. doi: 10.1038/s41586-019-1694-1. [DOI] [PubMed] [Google Scholar]
- 33.Li B., Skoulidis F., Falchook G., et al. PS01.07 registrational phase 2 trial of sotorasib in KRAS p.G12C mutant NSCLC: first disclosure of the codebreak 100 primary analysis. J Thorac Oncol. 2021;16(suppl):S61. [Google Scholar]
- 34.Skoulidis F., Byers L.A., Diao L., et al. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov. 2015;5:860–877. doi: 10.1158/2159-8290.CD-14-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skoulidis F., Goldberg M.E., Greenawalt D.M., et al. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov. 2018;8:822–835. doi: 10.1158/2159-8290.CD-18-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.