Abstract
Background
Empagliflozin, a sodium glucose cotransporter 2 inhibitor, is a medication to treat type 2 diabetes. The effect of empagliflozin in persons without diabetes has received less attention. Here we conducted a randomized, double-blind placebo-controlled clinical trial to examine the effect of empagliflozin on plasma triglycerides in obese non-diabetic adults.
Methods
Participants (n = 35; BMI ≥ 30 kg/m2) underwent body composition assessments using MRI, and were randomly assigned to either placebo or empagliflozin (10 mg/d) for three months. At the baseline and post-treatment visit, after an overnight fast, blood was drawn for biochemical analysis. Participants received [U–13C3]glycerol orally followed by multiple blood draws over 3 h to examine glycerol incorporation into triglycerides using NMR spectroscopy.
Results
The changes in blood triglyceride concentration with empagliflozin therapy related to the mass of baseline visceral adipose tissue (VAT; r = 0.53, p = 0.04). Empagliflozin slightly lowered triglycerides in obese subjects with low VAT, but increased triglycerides in the subjects with high VAT. Consistently, empagliflozin effectively suppressed triglyceride synthesis following [U–13C3]glycerol administration in the subjects with low VAT (p < 0.05), but not in the subjects with high VAT. The subjects with high VAT lost body weight after three months of empagliflozin treatment. In all subjects, about 20% of the triglyceride backbone originated from mitochondrial metabolism of glycerol.
Conclusions
The effect of empagliflozin on triglycerides in obese adults differed depending on VAT. Empagliflozin suppressed triglyceride synthesis in the subjects with low VAT, but tended to increase triglycerides in those with high VAT.
Keywords: SGLT2 inhibitor, Visceral fat, Glycerol, Triglycerides, Glyceroneogenesis, NMR
Highlights
-
•
Visceral fat modulates the effect of empagliflozin on triglycerides in obese adults.
-
•
Empagliflozin suppresses triglyceride synthesis in obese adults with low visceral fat.
-
•
Empagliflozin tends to increase triglycerides in obese adults with high visceral fat.
-
•
Empagliflozin induces weight loss in obese adults with high visceral fat.
Abbreviations
- ALT
alanine aminotransferase
- BMI
body mass index
- FFAs
free fatty acids
- FGF
fibroblast growth factor
- HbA1c
glycated hemoglobin
- GK
glycerol kinase
- G3P
glycerol 3-phosphate
- MRI
magnetic resonance imaging
- NMR
nuclear magnetic resonance
- SAT
subcutaneous adipose tissue
- SGLT2
sodium glucose cotransporter 2
- TCA
tricarboxylic acid
- T2DM
type 2 diabetes mellitus
- TG
triglycerides
- VAT
visceral adipose tissue
1. Introduction
Obesity threatens public health throughout the world because it is a risk factor for the development of multiple chronic diseases [[1], [2], [3]]. Visceral adipose tissue (VAT), triglyceride deposits lining internal organs, strongly associates with metabolic syndrome, type 2 diabetes mellitus (T2DM), hypertriglyceridemia, and cardiovascular disease [[4], [5], [6], [7], [8], [9]]. Plasma triglycerides, VAT, lipogenesis and lipolysis are tightly linked to glucose metabolism. Lipolysis occurs when blood glucose is low while excess glucose is converted to triglycerides and stored as fatty tissue [10]. VAT may play a particularly important role in glucose production because it is metabolically highly active, and lipolysis in VAT releases both glycerol and free fatty acids directly into the portal circulation [[11], [12], [13]]. Thus, VAT provides an excellent substrate for gluconeogenesis, glycerol, and a source of energy for the metabolically-expensive process of gluconeogenesis.
Sodium glucose cotransporter 2 (SGLT2) inhibitors were developed to treat T2DM in combination with metformin or other conventional antihyperglycemic drugs [14]. The inhibitors enhance glycosuria by preventing glucose reabsorption in the kidney [[15], [16], [17]]. The impact of SGLT2 inhibitors on glucose metabolism in diabetic patients has been extensively studied [[17], [18], [19], [20], [21], [22], [23], [24]]. They reduced fasting blood glucose and glycated hemoglobin (HbA1c), and improved insulin sensitivity and β cell function in diabetic patients. However, endogenous glucose production was increased to compensate for glycosuria [17]. Lipid metabolism with SGLT2 inhibitor therapy has been also investigated, but the results are controversial. The inhibitors led to body weight loss, and reduced VAT and liver fat in diabetic patients [[18], [19], [20], [21], [22],24]. The loss of blood glucose due to glycosuria is expected to increase glucagon secretion, which stimulates fatty acid oxidation and consequently reduces body fat content [[25], [26], [27]]. However, several earlier studies with diabetic patients also reported dyslipidemia associated with SGLT2 inhibitor therapy [[28], [29], [30]].
Empagliflozin is a highly selective inhibitor of SGLT2 [31]. Single doses of empagliflozin inhibited up to 60% of filtered glucose reabsorption in healthy subjects [32]. Empagliflozin also increased 13C-labeled glucose in blood after [U–13C3]glycerol ingestion in our recent study with non-diabetic obese adults, suggesting reduced gluconeogenesis from unlabeled glycerol derived from VAT [33]. However, little additional information is available regarding the effects of SGLT2 inhibitors in obese, non-diabetic subjects. Since we did not investigate the impact of empagliflozin on triglycerides previously, we acquired additional 13C nuclear magnetic resonance (NMR) spectra from the non-aqueous metabolites in plasma to determine whether there is an interaction between VAT and the effects of empagliflozin on plasma triglyceride concentration. MRI was used to measure baseline VAT and other aspects of body composition. NMR analysis was performed to monitor triglyceride synthesis from orally-administered [U–13C3]glycerol and to assess the glycerol metabolism through the TCA cycle in mitochondria.
2. Methods
2.1. Research design
This study was approved by the Institutional Review Board at the University of Texas Southwestern Medical Center, and it was registered on ClinicalTrials.gov (ClinicalTrials.gov Identifier: NCT02833415). Healthy adults with BMI ≥ 30 kg/m2 were recruited, but subjects with chronic illness including T2DM were excluded. Among 40 qualified participants initially, 35 volunteers (ages, 43–59 years; BMI, 32–40 kg/m2; 13 males and 22 females; 20 Caucasians, 12 African Americans and 3 others) completed this study (Fig. 1A and Table 1). The details of demographic and clinical characterization of participants were reported previously [33]. Each volunteer provided written informed consent prior to participation and visited four times spaced four weeks apart over three months (Fig. 1B). At the first visit after an overnight fast, body composition assessments were performed [33]. An intravenous catheter was positioned in an antecubital vein and blood was drawn for biochemical analysis at baseline. Participants ingested [U–13C3]glycerol (99%; Cambridge Isotope Laboratories, Tewksbury, MA; 50 mg/kg body weight) dissolved in water at 9:30 a.m. followed by a series of blood draws at 15, 30, 60, 90, 120, 150 and 180 min after the oral administration (Fig. 1C). At each time point, 40-mL of blood was drawn and blood was immediately centrifuged. Plasma was stored at −80 °C prior to further processing. Participants were randomly assigned to either empagliflozin (orally; 10 mg once daily; Boehringer Ingelheim, Germany) or placebo. The assignment was blinded to participants and investigators until all study procedures and data acquisition were completed. At the second and third visits, drug adherence and any adverse effects were assessed. At the fourth visit after an overnight fast, blood was drawn for analysis and the same procedures with [U–13C3]glycerol were performed completing the whole study.
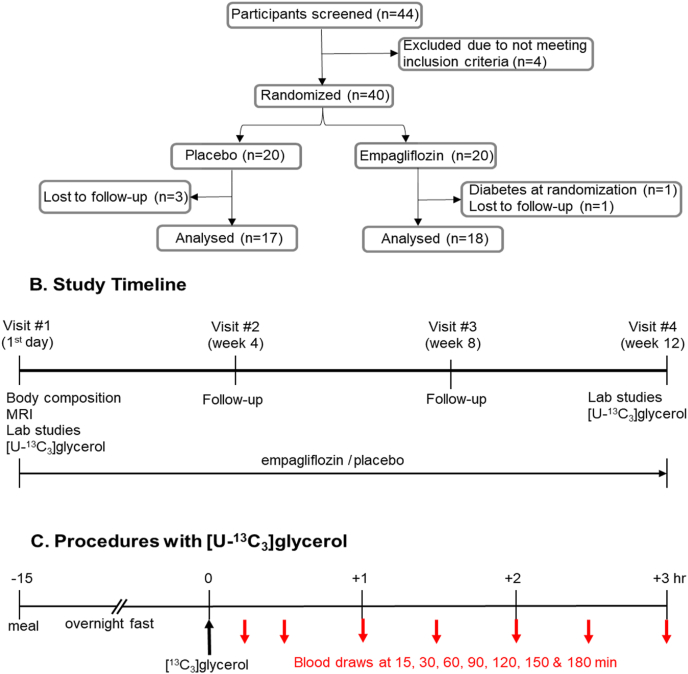
Fig. 1.
Study procedures. (A) Participants (n = 40) were randomly assigned to either placebo or empagliflozin. Three subjects from the placebo group and two from the empagliflozin group dropped out, and 17 volunteers in the placebo group and 18 in the empagliflozin group completed the study. (B) Participants made four visits with 4-week intervals. At the first visit after an overnight fast, MRI was performed for body composition measurement. Blood was drawn for biochemical analysis, and procedures with [U–13C3]glycerol were performed. Participants were assigned to either placebo or empagliflozin. At the second and the third follow-up visits, drug adherance and side effects were monitored. At the last visit after an overnight fast, blood was drawn for analysis and the procedures with [U–13C3]glycerol were performed. (C) At the first and the fourth visits, participants drank [U–13C3]glycerol-dissolved water and blood was drawn at multiple time points (15, 30, 60, 90, 120, 150 and 180 min) for NMR analysis of triglycerides.
Table 1.
Clinical characteristics of participants at baseline (1st visit).
| Characteristic | Placebo (n = 17) | Empagliflozin (n = 18) | p |
|---|---|---|---|
| Age (year) | 53.1 ± 2.2 | 50.4 ± 2.3 | 0.421 |
| Male/Female | 6/11 | 7/11 | |
| Race (Black/White/Other) | 7/9/1 | 5/11/2 | |
| Body weight (kg) | 104.0 ± 3.1 | 99.4 ± 2.4 | 0.246 |
| Body mass index (kg/m2) | 36.5 ± 1.1 | 35.6 ± 0.8 | 0.522 |
| Total body fat (%) | 44.9 ± 2.3 | 42.6 ± 2.1 | 0.467 |
| Total body lean mass (%) | 24.5 ± 1.1 | 25.8 ± 1.1 | 0.424 |
| Visceral fat (kg) | 5.4 ± 0.5 | 5.5 ± 0.6 | 0.902 |
| Abdominal subcutaneous fat (kg) | 14.4 ± 1.1 | 12.1 ± 1.1 | 0.150 |
| Liver fat (%) | 9.0 ± 1.8 | 10.5 ± 1.7 | 0.539 |
| Total thigh muscle (kg) | 11.8 ± 0.8 | 11.6 ± 0.7 | 0.882 |
Values are mean ± SE.
2.2. Body fat measurement
Body fat assessment was performed at the first visit and the details were reported previously [33]. Briefly, the assessment was performed using a Philips Achieva 3T MRI scanner (Philips Healthcare, Amsterdam, Netherlands) with the dual-echo Dixon Vibe protocol covering from the neck to knees, and multi-echo Dixon acquisition for fat assessment in the liver. A 16-channel SENSE XL Torso coil was used for the images of the liver and a body coil for the rest of the body. Image data were analyzed for VAT, liver fat and abdominal subcutaneous adipose tissue (SAT) using AMRA Profiler Research (AMRA Medical AB, Linköping, Sweden) [[34], [35], [36], [37]].
2.3. Lipid extraction and NMR spectroscopy
Plasma (3 mL) was transferred into a 20-mL glass vial containing a mixture of chloroform and methanol (2:1; 12 mL). The mixture was stirred for 30 min, centrifuged at a low speed, and the bottom chloroform layer was transferred to a new glass vial. The extraction was repeated after adding chloroform (8 mL) to the mixture in the first vial. After stirring and centrifugation, the chloroform layer was transferred and combined with the first one. Lipids extracted using chloroform were dried under vacuum with a liquid nitrogen trap. Dried lipids were dissolved in deuterated chloroform (CDCl3, 170 μL), and transferred to a 3-mm tube for NMR acquisition.
NMR spectra of lipids were collected using a 14.1T spectrometer (Varian INOVA, Agilent, Santa Clara, CA) equipped with a 3-mm broadband probe with the observe coil tuned to 13C (150 MHz). Spectra were acquired using a 60° pulse, a 36,765‐Hz sweep width, 110,294 data points and a 1.5‐sec acquisition time with 1.5‐sec interpulse delay at 25 °C. Proton decoupling was performed using a standard WALTZ-16 pulse sequence. Spectra were averaged at least 8000 scans requiring 7 h. All NMR spectra were analyzed using ACD/Labs NMR spectral analysis program (Advanced Chemistry Development, Inc., Toronto, Canada).
2.4. NMR analysis of triglycerides
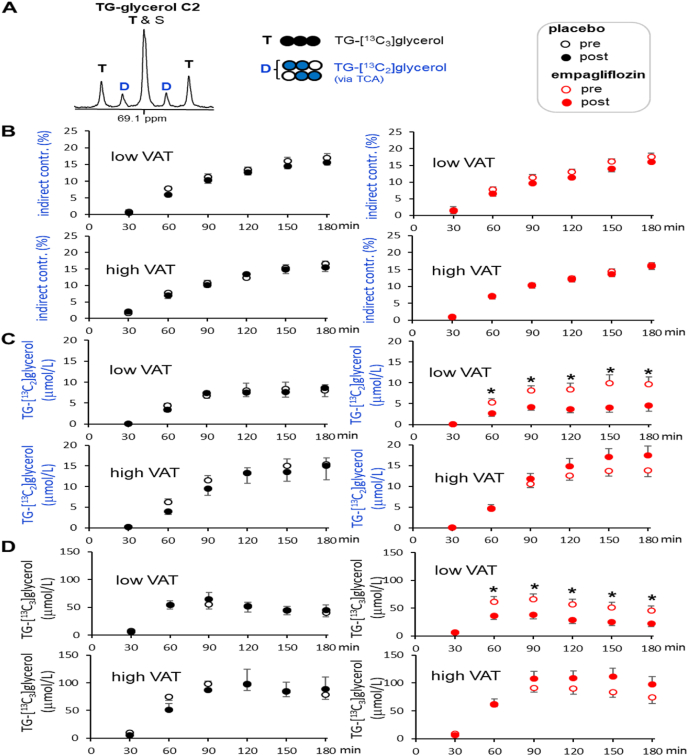
Plasma lipids were analyzed using 13C NMR focusing on the glycerol backbone of triglycerides (i.e., TG-glycerol), as reported previously [38]. Glycerol in the liver is phosphorylated via glycerol kinase to generate glycerol 3-phosphate, the required precursor for fatty acid esterification producing triglycerides. Thus, the appearance of 13C-labeled glycerol in the backbone of triglycerides in plasma after [U–13C3]glycerol administration is evidence of hepatic triglyceride synthesis from glycerol followed by triglyceride release into the circulation. The triglyceride backbone may be derived from glycerol through direct metabolism or indirectly via metabolism of glycerol through the TCA cycle followed by resynthesis to glycerol 3-phosphate. These pathways may be distinguished by 13C NMR spectroscopy as illustrate in Fig. 2.
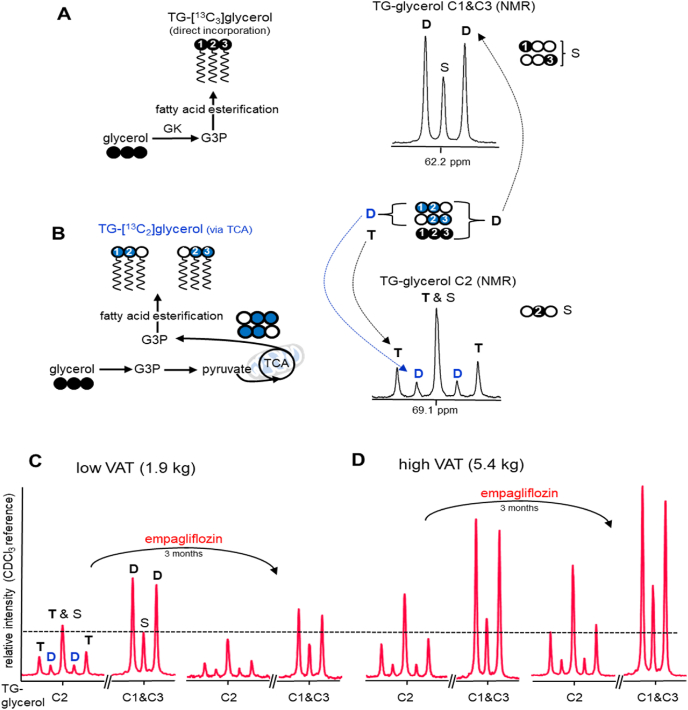
Fig. 2.
Glycerol incorporation to triglycerides. (A) Glycerol in the liver is converted to glycerol 3-phosphate (G3P), a necessary intermediate for fatty acid esterification. [U–13C3]glycerol direct incorporation through this process produces triglycerides containing triple-labeled glycerol backbone (i.e., TG-[13C3]glycerol). (B) A fraction of glycerol enters glycolytic pathways followed by the TCA cycle where carbons from [U–13C3]glycerol are scrambled, which generates 13C-double labeled trioses that can be further converted to G3P for fatty acid esterification. [U–13C3]glycerol indirect contribution to triglycerides through the TCA cycle produces double-labeled glycerol backbone (i.e., TG-[13C2]glycerol). A doublet (D) at TG-glycerol C1 & C3 (62.2 ppm) is the signal from both TG-[13C3]glycerol and TG-[13C2]glycerol. In contrast, a doublet at TG-glycerol C2 (69.1 ppm) is the signal from TG-[13C2]glycerol while a triplet (T) is the signal from TG-[13C3]glycerol only. (C-D) According to 13C NMR of TG-glycerol, empagliflozin treatment reduced plasma triglycerides in a subject with low VAT (1.9 kg) after [U–13C3]glycerol administration while it increased the triglycerides in a subject with high VAT (5.4 kg). A singlet (S) at TG-glycerol C1&C3 reflects the concentration of triglycerides and all spectra were derived from blood drawn at 180 min after oral administration of [U–13C3]glycerol. Signals can be directly compared because they are scaled to an internal standard. GK, glycerol kinase; open circle = 12C; black circle = 13C; blue circle = 13C after metabolism through the TCA cycle. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Total 13C-enrichment in the backbone of triglycerides was quantified by 13C NMR analysis of TG-glycerol carbons 1 and 3 (C1 & C3) at 62.2 ppm (Fig. 2A). A doublet (D) in this region is the signal from triglycerides containing [U–13C3]-, [1,2–13C2]- or [2,3–13C2]glycerol, and a singlet (S) is essentially from triglycerides with natural abundance backbone (i.e., TG-[1–13C1]glycerol or TG-[3–13C1]glycerol). The total 13C-enrichment in TG-glycerol was calculated using the peak area of the doublet assuming the singlet as natural abundance (1.1%). The absolute concentration of TG-[13C]glycerol (i.e., triglycerides containing [13C2]- or [13C3]glycerol) in plasma was calculated by multiplying fractional enrichment and the concentration of triglycerides. Relative triglyceride contents at different time points after [U–13C3]glycerol administration were obtained using the singlet from TG-glycerol C1 & C3 normalized by the peak area of CDCl3 solvent at 77.2 ppm in each spectrum.
Glycerol incorporation directly vs. indirectly through the TCA cycle can be distinguished based on 13C-labeling patterns in TG-glycerol carbon 2 (C2) at 69.1 ppm (Fig. 2B). Direct incorporation of [U–13C3]glycerol produces triglycerides with a triple-labeled backbone (i.e., TG-[13C3]glycerol). Glycerol metabolism through the TCA cycle prior to incorporation into triglycerides produces a double-labeled backbone (i.e., TG-[13C2]glycerol) due to 13C scrambling in the TCA cycle that produces double-labeled trioses [39]. In the 13C NMR spectrum of TG-glycerol C2, a doublet (D) is the signal from TG-[13C2]glycerol and a triplet (T) is from TG-[13C3]glycerol. Thus, the doublet and the triplet enable determination of glycerol metabolism to triglycerides via indirect and direct pathways, respectively. A double-labeled backbone in triglycerides is an index of mitochondrial biosynthetic function.
2.5. Biochemical assays
Assays for plasma insulin, HbA1c, TG, adiponectin, C-reactive protein, alanine aminotransferase (ALT), norepinephrine, free fatty acids and glycerol were performed by Quest Diagnostics (Irving, TX or San Juan Capistrano, CA). Glucose was measured using the glucose oxidase method (YSI 2300 Glucose Analyzer; GMI, Inc). Glucagon and fibroblast growth factor (FGF) 21 were measured using ELISA assay kits (Mercodia, Winston-Salem, NC, and Abcam, Cambrige, MA, respectively).
2.6. Statistical analysis
Linear correlation (two-tailed) between two variables was determined using Pearson correlation coefficient (r) and sample size. Comparisons between two data sets were made using a t-test (paired two sample for means), where p < 0.05 (two-tailed) was considered significant. Data were expressed as mean ± standard error.
3. Results
3.1. Biochemical variables in plasma
The average concentration of plasma triglycerides after empagliflozin treatment for 3 months remained unchanged. The concentrations of other common variables in plasma including metabolites, hormones and proteins were also essentially unchanged. There were no significant differences in insulin, glucagon, C-reactive protein, adiponectin and other biochemical assays (Table 2). Notably, however, HbA1c in the empagliflozin group at the final visit was lower compared to the placebo group (5.8 ± 0.1% versus 5.4 ± 0.1%, p = 0.012), but it was not significantly lower than that at the first visit in the same group (5.6 ± 0.1% versus 5.4 ± 0.1%, p = 0.229).
Table 2.
Biochemical characteristics of participants at baseline (1st visit, v1) and the final visit (4th visit, v4).
| Assay | Placebo (n = 17) | Empagliflozin (n = 18) | p | |
|---|---|---|---|---|
| Adiponectin (mcg/mL) | v1 | 7.1 ± 1.1 | 8.4 ± 1.7 | 0.512 |
| v4 | 8.1 ± 1.6 | 7.5 ± 1.0 | 0.708 | |
| ALT (U/L) | v1 | 23.6 ± 2.8 | 24.4 ± 3.3 | 0.866 |
| v4 | 20.9 ± 1.8 | 21.0 ± 2.4 | 0.953 | |
| C-reactive protein (mg/dL) | v1 | 1.2 ± 0.6 | 0.5 ± 0.1 | 0.213 |
| v4 | 2.9 ± 1.2 | 0.9 ± 0.5 | 0.118 | |
| FFAs (mmol/L) | v1 | 0.7 ± 0.1 | 0.6 ± 0.0 | 0.145 |
| v4 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.610 | |
| FGF21 (ng/mL)a | v1 | 1.02 ± 0.15 | 1.22 ± 0.11 | 0.265 |
| v4 | 1.45 ± 0.26 | 1.18 ± 0.20 | 0.423 | |
| Glucagon (pmol/L) | v1 | 3.5 ± 0.9 | 2.2 ± 0.9 | 0.467 |
| v4 | 3.9 ± 1.0 | 2.7 ± 0.6 | 0.278 | |
| Glucose (mmol/L)b | v1 | 5.3 ± 0.2 | 5.3 ± 0.1 | 0.977 |
| v4 | 5.4 ± 0.1 | 5.2 ± 0.1 | 0.178 | |
| Glycerol (ng/μL) | v1 | 15.4 ± 0.9 | 17.6 ± 2.4 | 0.386 |
| v4 | 15.7 ± 0.3 | 15.5 ± 0.3 | 0.909 | |
| HbA1c (%) | v1 | 5.9 ± 0.1 | 5.6 ± 0.1 | 0.073 |
| v4 | 5.8 ± 0.1 | 5.4 ± 0.1 | 0.012 | |
| HOMA-IR | v1 | 3.6 ± 0.6 | 2.6 ± 0.4 | 0.183 |
| v4 | 3.5 ± 0.6 | 2.7 ± 0.5 | 0.344 | |
| Insulin (μIU/mL) | v1 | 15.8 ± 2.4 | 11.0 ± 1.4 | 0.084 |
| v4 | 14.5 ± 2.5 | 11.4 ± 1.8 | 0.333 | |
| Norepinephrine (pg/mL) | v1 | 402 ± 50 | 400 ± 41 | 0.975 |
| v4 | 476 ± 102 | 439 ± 60 | 0.640 | |
| Triglycerides (mmol/L) | v1 | 1.31 ± 0.15 | 1.17 ± 0.13 | 0.474 |
| v4 | 1.13 ± 0.14 | 1.39 ± 0.23 | 0.346 | |
Values are mean ± SE. Abbreviations: ALT, alanine aminotransferase; FFAs, free fatty acids; FGF21, fibroblast growth factor 21; HbA1c, glycated hemoglobin; HOMA-IR, homeostatic model assessment of insulin resistance.
FGF21, n = 7–9 in each group.
Glucose was measured twice.
3.2. Changes in plasma triglycerides and body weight depending on VAT
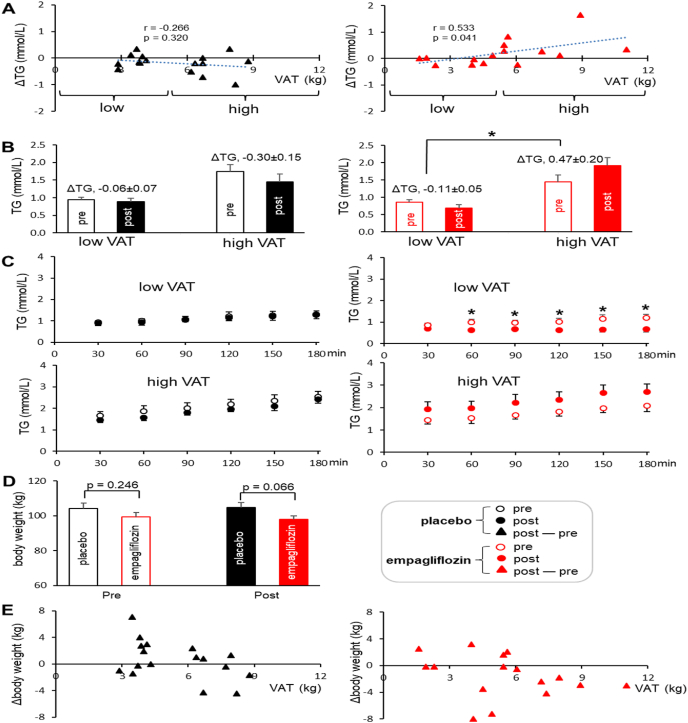
Though the average concentration of plasma triglycerides in the empagliflozin groups was similar with that of the placebo group at the first or the final visit (Table 2), the triglyceride contents in individuals were changed after empagliflozin treatment. Two examples are shown in Fig. 2 with 13C NMR of the glycerol backbone of triglycerides from two participants; one with 1.9-kg VAT (Fig. 2C) and the other with 5.4-kg VAT (Fig. 2D). As noted, the singlet at TG-glycerol C1 & C3 reflects a triglyceride pool. The peak area of the singlet from the subject with low VAT was reduced after empagliflozin treatment while the singlet from the other subject with high VAT was increased (Fig. 2C vs. 2D). Indeed, the difference of triglycerides between post and pre empagliflozin treatment (post – pre; ΔTG) positively correlated with baseline VAT (r = 0.533, p = 0.041; Fig. 3A). Such a correlation was not detected between ΔTG versus abdominal SAT or between ΔTG versus liver fat (Fig. S1). In the empagliflozin group, subjects with low VAT mostly lost triglycerides while subjects with high VAT gained triglycerides after three-month treatment. Consequently, ΔTG in the empagliflozin group with high VAT was significantly higher than ΔTG in the same group with low VAT (−0.11 ± 0.05 versus 0.47 ± 0.20 mmol/L; p = 0.031; Fig. 3B).
Fig. 3.
Effects of empagliflozin on triglycerides and body weight. (A) The difference of triglycerides between post- and pre-treatment (post – pre; ΔTG) correlated with baseline VAT in the empagliflozin group. (B) ΔTG in the empagliflozin group with low VAT was lower than that in the group with high VAT (−0.11 ± 0.05 vs. 0.47 ± 0.20 mmol/L). (C) The levels of triglycerides following [U–13C3]glycerol administration reduced in the empagliflozin group with low VAT after treatment, but tended to increase in the group with high VAT. (D) Body weight after treatment tended to be lower in the empagliflozin group than the placebo group (p = 0.066). (E) The graphs of VAT vs. the difference of body weight between post- and pre-treatment (post – pre; Δbody weight) show that most subjects in the empagliflozin group, especially subjects with high VAT, lost body weight. *, p < 0.05; n = 16–17 in the placebo group (8–9 with low VAT & 8 with high VAT); n = 15–18 in the empagliflozin group (7 with low VAT & 8–11 with high VAT).
Since the effect of empagliflozin on the concentration of plasma triglycerides correlated with VAT, data from low VAT (<5 kg) and high VAT (>5 kg) are separately presented in this study. The levels of triglycerides following [U–13C3]glycerol administration gradually increased over 3 h in most cases (Fig. 3C). However, triglycerides in the subjects with low VAT after empagliflozin treatment were not increased, and they were significantly lower compared to pre-treatment. Triglycerides in the empagliflozin group with high VAT tended to increase after treatment without statistical significance (Fig. 3C).
The body weight in the empagliflozin group after treatment tended to be lower than that of the placebo group (p = 0.066; Fig. 3D). Consistently, when VAT and the difference of body weight between post- and pre-treatment (Δbody weight) were plotted, many participants in the empagliflozin group, notably subjects with high VAT, lost body weight (Fig. 3E).
3.3. Effect of VAT on total glycerol metabolism to triglycerides
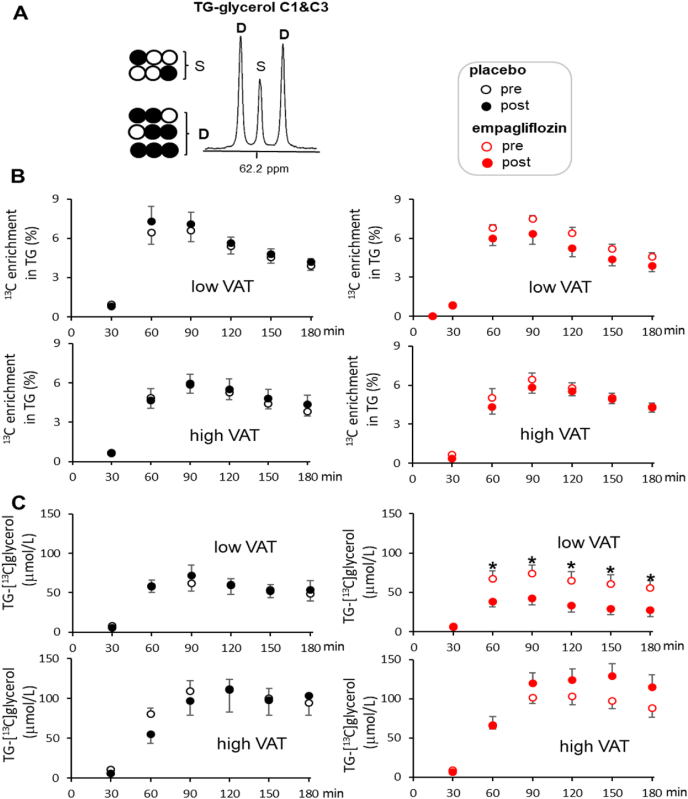
The total contribution of oral glycerol to triglycerides (i.e., TG-[13C]glycerol), the sum of indirect and direct pathways of glycerol metabolism, was not different between the placebo and empagliflozin groups. As noted, the total contribution was calculated based on 13C NMR analysis of TG-glycerol C1 and C3 (Fig. 4A). However, when stratified by VAT mass, the empagliflozin group with low VAT tended to have lower enrichment after treatment without statistical significance (Fig. 4B). Furthermore, the absolute concentrations of TG-[13C]glycerol after empagliflozin therapy substantially decreased among subjects with low VAT and tended to increase in the group with high VAT (Fig. 4C). TG-[13C]glycerol in the placebo group remained unchanged after three months regardless of VAT.
Fig. 4.
Total contribution of oral glycerol to plasma triglycerides. These results are based on analysis of TG-glycerol C1 & C3 following [U–13C3]glycerol administration, reflecting the sum of the direct and indirect contribution of labeled glycerol to the triglyceride backbone. In panels, data from the placebo group are on the left (data points in black) and data from the empagliflozin group are on the right (data points in red). (A) In 13C NMR of TG-glycerol C1 & C3, a doublet (D) is signal from both TG-[13C2]glycerol (indirect contribution) and TG-[13C3]glycerol (direct contribution), and a singlet (S) reflects TG-[13C1]glycerol with natural 13C abundance. (B) 13C enrichment in TG-glycerol over 3 h remained unchanged in all groups, but the enrichment tended to decrease in the empagliflozin group with low VAT after treatment. (C) The absolute concentrations of triglycerides with excess 13C-glycerol (i.e., TG-[13C]glycerol) decreased in the empagliflozin group with low VAT after treatment, but remained unchanged in the empagliflozin group with high VAT and in the placebo group regardless of VAT. *, p < 0.05; n = 16 in the placebo group (8 with low VAT & 8 with high VAT); n = 15 in the empagliflozin group (7 with low VAT & 8 with high VAT). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.4. Effect of VAT on indirect glycerol metabolism to triglycerides
The contribution of oral glycerol to the glycerol backbone through the TCA cycle was examined by analyzing the backbone C2 in triglycerides (Fig. 5A). In all subjects, regardless of therapy or VAT mass, a significant fraction of glycerol contributing to the triglyceride backbone underwent metabolism through the TCA cycle prior to fatty acid esterification. Specifically, the indirect glycerol contribution to triglycerides based on TG-[13C2]glycerol appearance reached up to ∼20% at 3 h after [U–13C3]glycerol administration in all groups (Fig. 5B). However, the absolute level of TG-[13C2]glycerol indicating [U–13C3]glycerol contribution to triglycerides through the TCA cycle was significantly reduced in the empagliflozin group with low VAT after treatment (Fig. 5C). Similarly, TG-[13C3]glycerol produced through glycerol direct incorporation was significantly suppressed in the empagliflozin group with low VAT after treatment (Fig. 5D). Such suppression was not detectable in the placebo group or in the empagliflozin group with high VAT.
Fig. 5.
Indirect contribution of oral glycerol to triglycerides. These results are based on analysis of TG-glycerol C2 that distinguishes glycerol indirect and direct contribution to triglycerides following [U–13C3]glycerol administration. (A) In 13C NMR of TG-glycerol C2, a doublet (D) is the signal from [13C2]glycerol backbone, reflecting labeled glycerol indirect contribution to triglycerides through the TCA cycle. A triplet (T) at this region is from [13C3]glycerol backbone, reflecting the glycerol direct incorporation to triglycerides. (B) The percentage of [U–13C3]glycerol indirect contribution to triglycerides remained unchanged in both groups. (C-D) Both indirect and direct glycerol incorporation to triglycerides decreased in the empagliflozin group with low VAT after treatment based on the quantitation of TG-[13C2]glycerol and TG-[13C3]glycerol, respectively. Open circle = 12C; black circle = 13C; blue circle = 13C after metabolism through the TCA cycle. *, p < 0.05; n = 16 in the placebo group (8 with low VAT & 8 with high VAT); n = 15 in the empagliflozin group (7 with low VAT & 8 with high VAT). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
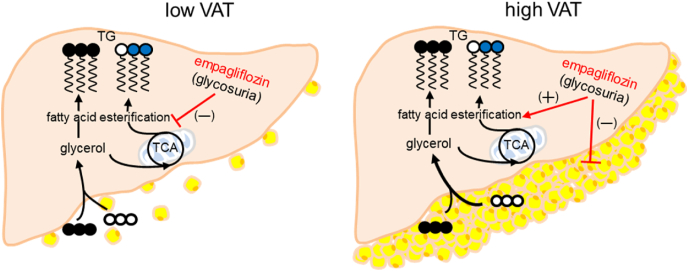
This study demonstrated that the effect of empagliflozin on triglyceride synthesis in non-diabetic adults with obesity differed depending on baseline VAT. Empagliflozin tended to decrease plasma triglyceride concentration in the subjects with low VAT, but to increase the concentration in those with high VAT. Notably, the subjects with high VAT lost body weight after empagliflozin treatment. Glycerol incorporation to triglycerides following [U–13C3]glycerol administration decreased in the empagliflozin group with low VAT while it tended to increase in the group with high VAT. Consistently, both glycerol direct incorporation and indirect incorporation through the TCA cycle to triglycerides decreased after empagliflozin treatment in the subjects with low VAT. Together these data demonstrated that treatment with empagliflozin for three months suppressed triglyceride synthesis in obese adults with low VAT and reduced lipid burden in the adults with high VAT evidenced by body weight loss (Fig. 6).
Fig. 6.
Indirect effects of empagliflozin on triglyceride (TG) synthesis. Empagliflozin enhances glycosuria by inhibiting glucose reabsorption. In obese adults after empagliflozin treatment, triglyceride synthesis following oral administration of [U–13C3]glycerol was suppressed in the subjects with low VAT while the synthesis tended to increase in those with high VAT. Since the subjects with high VAT lost body weight, the increased triglyceride synthesis was presumably associated with visceral fat lipolysis, releasing glycerol and fatty acids, to compensate for glycosuria caused by empagliflozin. Abbreviations: TCA, tricarboxylic acid cycle; VAT, visceral adipose tissue; open circle = 12C; black circle = 13C; blue circle = 13C after metabolism through the TCA cycle. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The reduction in plasma triglycerides after empagliflozin treatment was not a surprise because increased lipid utilization with SGLT2 inhibitor therapy is expected to compensate for glycosuria. From this perspective, the trend of triglyceride gain in the empagliflozin group with high VAT was unexpected and the finding seemed inconsistent with well-known beneficial effects of SGLT2 inhibitors. However, the loss of body weight was most noticeable in this population after treatment. Earlier studies with diabetic patients also reported body weight control and visceral fat decrease with empagliflozin therapy [18,23,40]. VAT is known to be metabolically active with a rich vascular supply and can be reduced by restricting caloric intake [[41], [42], [43]]. Since glycosuria also limits calorie availability, we may speculate that the triglyceride increase in this subgroup with high VAT was attributed to fat mobilization and consequent triglyceride release into the circulation. In such a case, the triglyceride gain in this study could be transient and extended empagliflozin treatment may eventually ameliorate hypertriglyceridemia once excess fat in the abdomen is mobilized.
The current finding, triglyceride or body weight reduction after empagliflozin treatment, is important for two reasons. First, participants were “healthy” adults with obesity, which are different from most earlier empagliflozin studies with diabetic patients. As a SGLT2 inhibitor, empagliflozin may lead to most noticeable changes with diabetic patients. Since the volunteers in this study were not hyperglycemic, metabolic changes were expected to be subtle. Nonetheless, this study found that empagliflozin relieved lipid burden in obese adults and its effect depended on baseline VAT. Second, this study provided a clue why many earlier studies reported negative results regarding the impact of empagliflozin on lipid metabolism [[28], [29], [30]]. The average concentration of triglycerides in this study remained unchanged with empagliflozin treatment, and even the tendency of increased triglycerides was observed in subjects with high VAT. Obesity and high VAT are risk factors for the development of T2DM [44], and the current finding from obese adults with high VAT was actually quite consistent with negative results from the earlier studies with diabetic patients. As noted, the tendency of increased triglycerides could be a consequence of lipid mobilization because the corresponding subjects lost body weight.
Monitoring 13C-labeling patterns in the backbone of triglycerides following [U–13C3]glycerol loading provided direct insight into a series of metabolic processes including hepatic triglyceride synthesis through fatty acid esterification and triglyceride release into the circulation. Triglyceride contents and the glycerol incorporation to triglycerides were consistently reduced in obese adults with low VAT after empagliflozin treatment. In addition, glycerol direct and indirect incorporation to triglycerides were conveniently distinguished based on TG-glycerol C2 analysis. As noted, double-labeled backbone in triglycerides (TG-[13C2]glycerol) reflects the glycerol metabolism through the TCA cycle prior to triglycerides. This process, termed glyceroneogenesis, shares metabolic pathways with gluconeogenesis including pyruvate carboxylase and phosphoenolpyruvate carboxykinase in the liver. Hepatic gluconeogenesis from the TCA cycle is the major contribution to endogenous glucose production in the body, and empagliflozin and other SGLT2 inhibitors were reported to increase the production in diabetic patients [45,46]. Here again we see some consistency between earlier studies with diabetic patients and the current study with obese adults with high VAT because of the tendency of increased glyceroneogenesis detected in the subjects with high VAT after empagliflozin treatment.
Interestingly, 13C-enrichments in triglycerides in the low VAT groups were high at 60–90 min while the enrichments in the high VAT groups peaked somewhat later, at 90–120 min after the administration of [U–13C3]glycerol (Fig. 4B). This could be attributed to slower [U–13C3]glycerol absorption in the high VAT groups. However, a delay in the uptake of glycerol by the intestine or the liver in the high VAT groups is not likely because plasma glycerol concentrations between the low versus high VAT groups were similar after the glycerol loading (Fig. S2). In addition, the absolute concentrations of 13C-labeled triglycerides at 60 min were actually similar between the low and the high VAT groups (Fig. 4C), and the concentrations after 90 min were higher in the high VAT groups. Consistent with this interpretation, an earlier rodent study found that glycerol uptake by the liver was actually increased in obese animals [47]. Thus, a slower uptake by the intestine or the liver does not explain the delay of peaks in 13C-labeled triglycerides. Related with the delay, changes of total triglyceride contents after the glycerol loading also differed between the low versus the high VAT groups (Fig. 3C). The high VAT groups showed steadily increasing total triglycerides up to 180 min while the low VAT groups reached plateau at ∼90 min. This kind of sluggish response in plasma triglycerides seems more likely a consequence of dysregulated triglyceride metabolism after the glycerol uptake. Obviously, as noted, fatty acid esterification in the high VAT groups tended to increase as evidenced with enhanced 13C-labeld triglycerides in plasma (Fig. 4C). The delay could be also related with reduced triglyceride utilization in the association with impaired fatty acid oxidation in periphery.
In summary, empagliflozin showed distinctive effects on lipid metabolism in obese non-diabetic adults depending on VAT. It suppressed triglyceride synthesis in the subjects with low VAT and led to body weight loss in those with high VAT. The tendency of increased triglycerides in the latter could be transient in association with VAT mobilization. In addition to the well-known role of empagliflozin in glycemic control for diabetic patients, this study demonstrated that it could benefit adults with obesity by ameliorating lipid burden. Three-month treatment in the current study was rather short and the long-term impact of empagliflozin on lipid metabolism in this population remains to be investigated.
Funding
This study was supported by the National Institutes of Health [DK099289 to ESJ, DK106520 to IJN, DK058398 and EB015908 to CRM, TR001105 to UT Southwestern], and UT Southwestern-Dedman Family Scholarship in Clinical Care to IJN.
CRediT authorship contribution statement
Min Hee Lee: Methodology, Investigation, Writing – original draft. Ian J. Neeland: Conceptualization, Writing – review & editing, Funding acquisition. Natalia de Albuquerque Rocha: Investigation. Connor Hughes: Investigation. Craig R. Malloy: Conceptualization, Writing – review & editing, Funding acquisition. Eunsook S. Jin: Conceptualization, Methodology, Investigation, Writing – review & editing, Funding acquisition.
Declaration of competing interest
All authors, except Dr. Neeland, declare no competing interests. Dr. Neeland is a former member of the scientific advisory board of AMRA Medical, and he has received honoraria, consulting, and speaker's bureau fees, and travel support from Boehringer-Ingelheim/Lilly Alliance, and a research grant from Novo Nordisk.
Acknowledgements
We thank Jeannie Baxter, Lucy Christie, Kelley Derner, Janet Jerrow, Carol Parcel and Maida Tai for technical support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.metop.2021.100161.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ormazabal V., Nair S., Elfeky O., Aguayo C., Salomon C., Zuñiga F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):122. doi: 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye X., Kong W., Zafar M.I., Chen L.L. Serum triglycerides as a risk factor for cardiovascular diseases in type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Cardiovasc Diabetol. 2019;18(1):48. doi: 10.1186/s12933-019-0851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neeland I.J., Poirier P., Després J.P. Cardiovascular and metabolic heterogeneity of obesity: clinical challenges and implications for management. Circulation. 2018;137:1391–1406. doi: 10.1161/CIRCULATIONAHA.117.029617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carr D.B., Utzschneider K.M., Hull R.L., Kodama K., Retzlaff B.M., Brunzell J.D., Shofer J.B., Fish B.E., Knopp R.H., Kahn S.E. Intra-abdominal fat is a major determinant of the national cholesterol education program adult treatment panel III criteria for the metabolic syndrome. Diabetes. 2004;53(8):2087–2094. doi: 10.2337/diabetes.53.8.2087. [DOI] [PubMed] [Google Scholar]
- 5.Morkedal B., Romundstad P.R., Vatten L.J. Informativeness of indices of blood pressure, obesity and serum lipids in relation to ischaemic heart disease mortality: the HUNT-II study. Eur J Epidemiol. 2011;26(6):457–461. doi: 10.1007/s10654-011-9572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stefan N., Kantartzis K., Machann J., Schick F., Thamer C., Rittig K., Balletshofer B., Machicao F., Fritsche A., Häring H.U. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 7.Fabbrini E., Magkos F., Mohammed B.S., Pietka T., Abumrad N.A., Patterson B.W., Okunade A., Klein S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA. 2009;106:15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldberg I.J. Clinical review 124: diabetic dyslipidemia: causes and consequences. J Clin Endocrinol Metab. 2001;86(3):965–971. doi: 10.1210/jcem.86.3.7304. [DOI] [PubMed] [Google Scholar]
- 9.Bitzur R., Cohen H., Kamari Y., Shaish A., Harats D. Triglycerides and HDL cholesterol: stars or second leads in diabetes? Diabetes Care. 2009;32(Suppl 2):S373–S377. doi: 10.2337/dc09-S343. Suppl 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alves-Bezerra M., Cohen D.E. Triglyceride metabolism in the liver. Compr Physiol. 2017;8(1):1–8. doi: 10.1002/cphy.c170012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czech M.P., Tencerova M., Pedersen D.J., Aouadi M. Insulin signalling mechanisms for triacylglycerol storage. Diabetologia. 2013;56(5):949–964. doi: 10.1007/s00125-013-2869-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cahill G.F., Jr. Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22. doi: 10.1146/annurev.nutr.26.061505.111258. [DOI] [PubMed] [Google Scholar]
- 13.Chait A., den Hartigh L.J. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med. 2020;7:22. doi: 10.3389/fcvm.2020.00022. eCollection 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madaan T., Akhtar M., Najmi A.K. Sodium glucose CoTransporter 2 (SGLT2) inhibitors: current status and future perspective. Eur J Pharmaceut Sci. 2016;93:244–252. doi: 10.1016/j.ejps.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 15.Shubrook J.H., Bokaie B.B., Adkins S.E. Empagliflozin in the treatment of type 2 diabetes: evidence to date. Drug Des Dev Ther. 2015;9:5793–5803. doi: 10.2147/DDDT.S69926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marx N., McGuire D.K. Sodium-glucose cotransporter-2 inhibition for the reduction of cardiovascular events in high-risk patients with diabetes mellitus. Eur Heart J. 2016;37(42):3192–3200. doi: 10.1093/eurheartj/ehw110. [DOI] [PubMed] [Google Scholar]
- 17.Ferrannini E., Muscelli E., Frascerra S., Baldi S., Mari A., Heise T., Broedl U.C., Woerle H.J. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124:499–508. doi: 10.1172/JCI72227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin Y., Moon J.H., Chin H.J., Ferrannini E., Lim S. Glycemic efficacy and metabolic consequences of an empagliflozin add-on versus conventional dose-increasing strategy in patients with type 2 diabetes inadequately controlled by metformin and sulfonylurea. Endocrinol Metab. 2020;35(2):329–338. doi: 10.3803/EnM.2020.35.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenstock J., Seman L.J., Jelaska A., Hantel S., Pinnetti S., Hach T., Woerle H.J. Efficacy and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, as add-on to metformin in type 2 diabetes with mild hyperglycaemia. Diabetes Obes Metabol. 2013;15:1154–1160. doi: 10.1111/dom.12185. [DOI] [PubMed] [Google Scholar]
- 20.Ozcelik S., Celik M., Vural A., Aydin B. The effect of low and high dose empagliflozin on HbA1c and lipid profile in type 2 diabetes mellitus: a real-world data. North Clin Istanb. 2020;7(2):167–173. doi: 10.14744/nci.2019.22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuchay M.S., Krishan S., Mishra S.K., Farooqui K.J., Singh M.K., Wasir J.S., Bansal B., Kaur P., Jevalikar G., Gill H.K., Choudhary N.S., Mithal A. Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (E-LIFT Trial) Diabetes Care. 2018;41(8):1801–1808. doi: 10.2337/dc18-0165. [DOI] [PubMed] [Google Scholar]
- 22.Sawada T., Uzu K., Hashimoto N., Onishi T., Takaya T., Shimane A., Taniguchi Y., Yasaka Y., Ohara T., Kawai H. Empagliflozin's ameliorating effect on plasma triglycerides: association with endothelial function recovery in diabetic patients with coronary artery disease. J Atherosclerosis Thromb. 2020;27(7):644–656. doi: 10.5551/jat.50807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovacs C.S., Seshiah V., Swallow R., Jones R., Rattunde H., Woerle H.J., Broedl U.C. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metabol. 2014;16(2):147–158. doi: 10.1111/dom.12188. [DOI] [PubMed] [Google Scholar]
- 24.Kahl S., Gancheva S., Straßburger K., Herder C., Machann J., Katsuyama H., Kabisch S., Henkel E., Kopf S., Lagerpusch M., Kantartzis K., Kupriyanova Y., Markgraf D., van Gemert T., Knebel B., Wolkersdorfer M.F., Kuss O., Hwang J.H., Bornstein S.R., Kasperk C., Stefan N., Pfeiffer A., Birkenfeld A.L., Roden M. Empagliflozin effectively lowers liver fat content in well-controlled type 2 diabetes: a randomized, double-blind, phase 4, placebo-controlled trial. Diabetes Care. 2020;43(2):298–305. doi: 10.2337/dc19-0641. [DOI] [PubMed] [Google Scholar]
- 25.Szekeres Z., Toth K., Szabados E. The effects of SGLT2 inhibitors on lipid metabolism. Metabolites. 2021;11(2):87. doi: 10.3390/metabo11020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu L., Nagata N., Nagashimada M., Zhuge F., Ni Y., Chen G., Mayoux E., Kaneko S., Ota T. SGLT2 inhibition by empagliflozin promotes fat utilization and browning and attenuates inflammation and insulin resistance by polarizing M2 macrophages in diet-induced obese mice. EBioMedicine. 2017;20:137–149. doi: 10.1016/j.ebiom.2017.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonner C., Kerr-Conte J., Gmyr V., Queniat G., Moerman E., Thévenet J., Beaucamps C., Delalleau N., Popescu I., Malaisse W.J., Sener A., Deprez B., Abderrahmani A., Staels B., Pattou F. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med. 2015;21:512–517. doi: 10.1038/nm.3828. [DOI] [PubMed] [Google Scholar]
- 28.Kohler S., Salsali A., Hantel S., Kaspers S., Woerle H.J., Kim G., Broedl U.C. Safety and tolerability of empagliflozin in patients with type 2 diabetes. Clin Therapeut. 2016;38:1299–1313. doi: 10.1016/j.clinthera.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 29.Halimi S., Vergès B. Adverse effects and safety of SGLT-2 inhibitors. Diabetes Metab. 2014;40:S28–S34. doi: 10.1016/S1262-3636(14)72693-X. [DOI] [PubMed] [Google Scholar]
- 30.Li D., Wu T., Wang T., Wei H., Wang A., Tang H., Song Y. Effects of sodium glucose cotransporter 2 inhibitors on risk of dyslipidemia among patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Pharmacoepidemiol Drug Saf. 2020;29(5):582–590. doi: 10.1002/pds.4985. [DOI] [PubMed] [Google Scholar]
- 31.Grempler R., Thomas L., Eckhardt M., Himmelsbach F., Sauer A., Sharp D.E., Bakker R.A., Mark M., Klein T., Eickelmann P. Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT2) inhibitor: characterisation and comparison with other SGLT2 inhibitors. Diabetes Obes Metabol. 2012;14:83–90. doi: 10.1111/j.1463-1326.2011.01517.x. [DOI] [PubMed] [Google Scholar]
- 32.Komoroski B., Vachharajani N., Boulton D., Kornhauser D., Geraldes M., Li L., Pfister M. Dapagliflozin, a novel SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Ther. 2009;85:520–526. doi: 10.1038/clpt.2008.251. [DOI] [PubMed] [Google Scholar]
- 33.Neeland I.J., de Albuquerque Rocha N., Hughes C., Ayers C.R., Malloy C.R., Jin E.S. Effects of empagliflozin treatment on glycerol-derived hepatic gluconeogenesis in adults with obesity: a randomized clinical trial. Obesity. 2020;28(7):1254–1262. doi: 10.1002/oby.22854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.West J., Dahlqvist Leinhard O., Romu T., Collins R., Garratt S., Bell J.D., Borga M., Thomas L. Feasibility of MR-based body composition analysis in large scale population studies. PLoS One. 2016;11 doi: 10.1371/journal.pone.0163332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borga M., Thomas E.L., Romu T., Rosander J., Fitzpatrick J., Dahlqvist Leinhard O., Bell J.D. Validation of a fast method for quantification of intra-abdominal and subcutaneous adipose tissue for large-scale human studies. NMR Biomed. 2015;28:1747–1753. doi: 10.1002/nbm.3432. [DOI] [PubMed] [Google Scholar]
- 36.Karlsson A., Rosander J., Romu T., Tallberg J., Gronqvist A., Borga M., Dahlqvist Leinhard O. Automatic and quantitative assessment of regional muscle volume by multi-atlas segmentation using whole-body water-fat MRI. J Magn Reson Imag. 2015;41:1558–1569. doi: 10.1002/jmri.24726. [DOI] [PubMed] [Google Scholar]
- 37.West J., Romu T., Thorell S., Lindblom H., Berin E., Holm A.S., Åstrand L.L., Karlsson A., Borga M., Hammar M., Leinhard O.D. Precision of MRI-based body composition measurements of postmenopausal women. PLoS One. 2018;13 doi: 10.1371/journal.pone.0192495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin E.S., Browning J.D., Murphy R.E., Malloy C.R. Fatty liver disrupts glycerol metabolism in gluconeogenic and lipogenic pathways in humans. J Lipid Res. 2018;59(9):1685–1694. doi: 10.1194/jlr.M086405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin E.S., Sherry A.D., Malloy C.R. An oral load of [13C3]glycerol and blood NMR analysis detect fatty acid esterification, pentose phosphate pathway, and glycerol metabolism through the tricarboxylic acid cycle in human liver. J Biol Chem. 2016;291(36):19031–19041. doi: 10.1074/jbc.M116.742262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neeland I.J., McGuire D.K., Chilton R., Crowe S., Lund S.S., Woerle H.J., Broedl U.C., Johansen O.E. Empagliflozin reduces body weight and indices of adipose distribution in patients with type 2 diabetes mellitus. Diabetes Vasc Dis Res. 2016;13(2):119–126. doi: 10.1177/1479164115616901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arner P. Differences in lipolysis between human subcutaneous and omental adipose tissue. Ann Med. 1995;27:435–438. doi: 10.3109/07853899709002451. [DOI] [PubMed] [Google Scholar]
- 42.Ibrahim M.M. Subcutaneous and visceral adipose tissue: structure and functional differences. Obes Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 43.Ross R., Rissanen J Mobilization of visceral and subcutaneous adipose tissue in response to energy restriction and exercise. Am J Clin Nutr. 1994;60(5):695–703. doi: 10.1093/ajcn/60.5.695. [DOI] [PubMed] [Google Scholar]
- 44.Anjana M., Sandeep S., Deepa R., Vimaleswaran K.S., Farooq S., Mohan V. Visceral and central abdominal fat and anthropometry in relation to diabetes in Asian Indians. Diabetes Care. 2004;27(12):2948–2953. doi: 10.2337/diacare.27.12.2948. [DOI] [PubMed] [Google Scholar]
- 45.Merovci A., Solis-Herrera C., Daniele G., Eldor R., Fiorentino T.V., Tripathy D., Xiong J., Perez Z., Norton L., Abdul-Ghani M.A., DeFronzo R.A. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124:509–514. doi: 10.1172/JCI70704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeFronzo R.A., Norton L., Abdul-Ghani M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol. 2017;13:11–26. doi: 10.1038/nrneph.2016.170. [DOI] [PubMed] [Google Scholar]
- 47.Maeda N., Funahashi T., Shimomura I. Metabolic impact of adipose and hepatic glycerol channels aquaporin 7 and aquaporin 9. Nat Clin Pract Endocrinol Metabol. 2008;4(11):627–634. doi: 10.1038/ncpendmet0980. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.